Abstract
Introduction
Patients with breast cancer (bca) who overexpress her2 (the human epidermal growth factor receptor 2) are at risk for cardiotoxicity when treated with anthracycline-based chemotherapy and her2-targeted agents. The Framingham risk score (frs) is a validated tool that stratifies patients into high-, intermediate-, or low-risk groups and calculates their 10-year risk of developing cardiovascular disease (cvd) based on past medical history, systolic blood pressure, and measurement of serum lipids. We retrospectively analyzed patients with her2-positive bca to determine whether the frs predicts adverse cardiovascular (CV) events or cardiotoxicity in patients treated using anthracyclines or her2-targeted therapy, or both.
Methods
The frs was determined for patients with bca referred to The Ottawa Hospital Cardiology–Oncology Clinic from October 2008 to August 2014. The patients were stratified into high (≥20%), intermediate (10%–20%), and low (<10%) 10-year cv risk groups. Primary outcomes included cvd-related hospitalizations and deaths, and cardiotoxicity [drop in left ventricular ejection fraction (lvef) of >10% to a lvef ≤50%].
Results
Of the 152 patients included in the analysis (median follow-up: 40.7 months; range: 3.5–263 months), 47 (31%) were classified as high risk; 36 (24%), as intermediate risk; and 69 (45%), as low-risk. The number of cvd-related hospitalizations and deaths was 22, for an overall prevalence of 14%, with significantly more events occurring in high-risk than in low-risk patients (odds ratio: 4.18; 95% confidence limits: 1.47, 11.89). The frs predicted a 10-year risk of any cv event of 11.2% and underestimated the actual rate of cv events in the entire cohort. High frs was not associated with cardiotoxicity (p = 0.82).
Conclusions
In a population of patients with her2-positive bca referred to a cardiology–oncology clinic, the frs does not accurately predict the risk of cv events or cardiotoxicity.
Keywords: Cardio-oncology, breast cancer, her2, trastuzumab, cardiotoxicity, Framingham risk score
INTRODUCTION
Breast cancer (bca) is the most common malignancy in Canadian women, with an estimated 25,700 new cases diagnosed in Canada in 20161. For patients diagnosed with bca, 5-year survival is 87%1. Given the increasing number of bca survivors, managing serious cancer treatment–related toxicities, including cardiotoxicity, is important.
The human epidermal growth factor receptor 2 (her2) is overexpressed in 20%–25% of all bca patients. Trastuzumab, a humanized monoclonal antibody against her2, has significantly improved overall survival in patients with her2-positive (her2+) bca, but is associated with an increased risk of cardiotoxicity, which can manifest as heart failure, a decrease in left ventricular ejection fraction (lvef), or both2. Despite numerous efforts, no validated tools are currently available to reliably predict cardiotoxicity from trastuzumab-based therapies.
Trastuzumab-induced cardiotoxicity (tic) can be defined as a drop in lvef of more than 10% to a value at or below 50%, or having symptoms of heart failure3, which is often reversible. In patients with early-stage her2+ bca receiving sequential anthracycline-based chemotherapy and trastuzumab, the cumulative incidence of heart failure over 5 years is 6%–20%4. Within 1 year of starting trastuzumab, 19% of patients with node-positive her2+ bca in the National Surgical Adjuvant Breast and Bowel Project B-31 trial experienced asymptomatic reductions in lvef5. A recent study in Ontario reported that bca patients receiving sequential therapy had a 6.6% cumulative risk over 5 years of developing major cardiac events, including hospitalizations or emergency room visits for heart failure, pulmonary edema, or cardiomyopathy; outpatient diagnosis of heart failure; and cv death6. Risk factors linked to tic include age greater than 75 years, history of cv disease (cvd), diabetes, renal disease, and lower baseline lvef7. Patients receiving non-anthracycline-based chemotherapy or radiotherapy are also at risk of developing cv complications such as pericarditis, cardiac arrhythmia, coronary artery disease, and hypertension8.
The Framingham risk score (frs) is a validated tool for assessing global cv risk that stratifies patients into a high-, intermediate-, or low-risk group and calculates their 10-year risk of developing cvd based on age and CV risk factors9. The frs was originally derived in a population consisting largely of white men of European descent, 30–74 years of age, to determine the risk of cvd and the patients who would benefit from more aggressive lipid targets for primary prevention. Since then, the frs has been validated in individuals of various ethnicities10 and in women11.
Several attempts have been made to develop a tool that can predict the risk of treatment-related cardiotoxicity in cancer patients. Herrmann et al.12 explored chemotherapy- and patient-related risk factors leading to cardiotoxicity. Ezaz et al.7 developed a mathematical model specifically for heart failure and cardiomyopathy among older women who received trastuzumab. Despite those efforts, a standardized risk score to predict tic has yet to be implemented in routine clinical practice.
The objectives of the present study were to determine whether the frs predicts either or both of adverse CV events leading to hospitalization or death, or of cardiotoxicity, in patients with her2+ bca treated with trastuzumab-based therapy. Given the overlap of risk factors for tic and global cv risk assessment, we hypothesized that the frs would predict those patients at increased risk of cvd, but might underestimate the absolute cv risk in patients with her2+ bca receiving potentially cardiotoxic cancer treatment.
METHODS
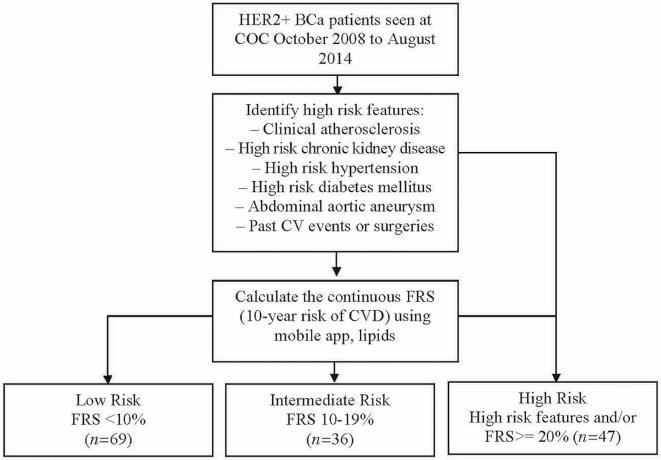
We included patients with early- and advanced-stage her2+ bca referred to The Ottawa Hospital Cardiology–Oncology Clinic (coc) from October 2008 to August 2014. Patients with anthracycline exposure before their bca diagnosis and those who had missing data or had been lost to follow-up were excluded. Data were collected retrospectively from the coc database as previously described13, including age at diagnosis, sex, bca stage, past medical history, premature family history of cvd, cv history, medications, and imaging. Baseline renal function, fasting lipid levels, and systolic blood pressures before the cancer diagnosis were collected retrospectively through the eHealth Ontario portal (http://www.ehealthontario.on.ca/en). Cardiovascular outcomes and cancer treatment regimens (chemotherapy, targeted agents, and endocrine therapy) were obtained through electronic hospital medical records. The frs was calculated according to the Canadian Cardiovascular Society 2012 guidelines, using the mobile Lipids application [now replaced by the iCCS application (https://www.ccs.ca/en/resources/mobile-apps)]. Using the same guideline approach as that used for routine risk-stratification of patients with coronary artery disease, our patients were stratified into high-, intermediate-, and low-risk frs groups (Figure 1). Cardiovascular outcomes of interest included cv events (heart failure– and cvd-related hospitalizations and deaths) and cardiotoxicity (lvef drop of >10% from baseline imaging to lvef of ≤50%)14.
FIGURE 1.
Patients with HER2-positive (HER2+) breast cancer (BCa) seen at the Cardiology–Oncology Clinic (COC) from October 2008 to August 2014 were stratified into low-, intermediate-, and high-risk groups based on high-risk features and the Framingham Risk Score (FRS). CV = cardiovascular; CVD = cardiovascular disease.
An analysis of variance was conducted to evaluate differences between the frs groups. Univariate and correlation analyses were conducted between the frs groups for the cv outcomes of interest. Survivor curves were completed using the Cox proportional hazards model for cvd-related hospitalizations and deaths, comparing the low- and intermediate-risk groups with the high-risk group. All statistical analyses were performed using the R software application (version 3.1.3: The R Foundation, Vienna, Austria).
The study was approved by the Ottawa Health Sciences Network Research Ethics Board.
RESULTS
Between October 2008 and August 2014, 206 patients with her2+ bca were seen consecutively at The Ottawa Hospital coc. Of those patients, 54 were excluded because of any one or a combination of prior exposure to anthracyclines, incomplete data for the frs calculation, or inadequate follow-up. Table i shows the baseline characteristics of the 152 patients included in the analysis.
TABLE I.
Baseline characteristics of the study population
| Characteristic | Value |
|---|---|
| Patients (n) | 152 |
| Age at diagnosis (years) | |
| Median | 57 |
| Range | 33–87 |
| Sex [n (%) women] | 147 (97) |
| Body mass index > 25 [n (%)] | 89 (57) |
| Follow-up (months) | |
| Median | 40.7 |
| Range | 3.5–263 |
| Early-stage diseasea [n (%)] | 129 (85) |
| Receptor status [n (%)] | |
| ER-positive | 97 (64) |
| PR-positive | 75 (49) |
| HER2-positive | 152 (100) |
| Positive nodal status [n (%)] | 79 (52) |
| Chemotherapy [n (%)] | |
| Adjuvant | 152 (100) |
| Trastuzumab | 152 (100) |
| Anthracycline plus trastuzumab | 115 (76) |
| Hormonal therapy [n (%)] | 95 (62) |
I, II, IIIA.
ER = estrogen receptor; PR = progesterone receptor; HER2 = human epidermal growth factor receptor 2.
Median age at diagnosis was 57 years (range: 33–87 years), with a median follow-up of 40.7 months (range: 3.5–263 months). Most patients were women (n = 147, 97%), and most had early-stage (i–iiia) bca (n = 129, 85%). All patients received trastuzumab, and 115 (76%) received sequential therapy (anthracyclines followed by trastuzumab). More than half the patients (n = 95, 63%) were prescribed endocrine therapy after completion of chemotherapy and trastuzumab.
As outlined in Table ii, all patients had 1 or more frs risk factors: 143 (94%) were more than 40 years of age; 5 (3%) were men; 117 (77%) had an unfavourable fasting lipid profile (total cholesterol > 5 mmol, high-density lipoprotein < 1.0 mmol, or both); 55 (36%) had a smoking history; 89 (59%) had hypertension (systolic blood pressure > 130 mmHg), of whom 39 (42%) were untreated; 23 (15%) had diabetes mellitus; and 16 (11%) had a family history of premature cvd.
TABLE II.
Cardiovascular risk factors in study group
| Risk factor | All patients | Framingham risk group | p Value | ||
|---|---|---|---|---|---|
|
| |||||
| Low | Intermediate | High | |||
| Patients (n) | 152 | 69 | 36 | 47 | — |
| Framingham risk factor [n (%)] | |||||
| Age ≥40 years | 143 (94) | 61 (88) | 36 (100) | 46 (98) | <0.05 |
| Male sex | 5 (3) | 0 | 1 (3) | 4 (8) | <0.05 |
| Dyslipidemia | 117 (77) | 46 (67) | 30 (83) | 41 (87) | <0.05 |
| Smoking history | 55 (36) | 16 (23) | 15 (42) | 24 (51) | <0.01 |
| Hypertension | 89 (59) | 26 (38) | 28 (78) | 35 (81) | <0.0001 |
| Diabetes mellitus | 23 (15) | 0 | 0 | 23 (49) | <0.0001 |
| Family history of premature CVD | 16 (11) | 1 (1) | 6 (17) | 9 (19) | <0.01 |
| Other risk factors | |||||
| Newly added cardiac medication [n (%)] | 57 (38) | 25 (36) | 9 (25) | 23 (49) | <0.0001 |
| Baseline LVEF (%) | 0.86 | ||||
| Median | 58.5 | 58 | 59 | 59 | |
| Range | 35–80 | 35–72 | 46–80 | 35–78 | |
| Follow-up (months) | 0.57 | ||||
| Median | 40.7 | 34.7 | 49.5 | 40.7 | |
| Range | 3.5–263 | 5.4–283 | 3.5–170 | 4.5–142 | |
| Clinical stagea [n (%)] | 129 (85) | 60 (87) | 33 (92) | 36 (76) | 0.13 |
I, II, IIIA.
CVD = cardiovascular disease; LVEF = left ventricular ejection fraction.
Consistent with recommendations for coronary artery disease stratification in a non-cardiotoxicity context, patients with 1 or more high-risk clinical features or a frs of 20% or greater were placed in the high-risk group9. High-risk clinical features include clinical atherosclerosis in any artery, high-risk chronic kidney disease, diabetes mellitus, abdominal aortic aneurysm, and past cv event or surgery. Based on those clinical features, 37 patients (24%) were categorized as high risk. An additional 10 patients (7%) were categorized as high risk based on age and risk factors (frs of at least 20%). Overall, 47 patients (31%) were categorized as high risk; 36 patients (24%), as intermediate risk; and 69 (45%), as low risk. The average (expected) 10-year risk of cvd for the high-risk group was 21.1% ± 2.46%, compared with 11.2% ± 1.35% for the entire cohort. As shown in Table iii, the groups also had significant variance for the number of newly added cardiac medications (p < 0.05).
TABLE III.
Actual cardiovascular events leading to hospitalizations and deaths in the study population
| Event | Outcome | Framingham risk group [n (%)] | ||
|---|---|---|---|---|
|
| ||||
| Low (n=69) | Intermediate (n=36) | High (n=47) | ||
| Heart failure | Hospitalization | 3 | 0 | 4 |
| Death | 0 | 0 | 1 | |
| Arrhythmia | Hospitalization | 0 | 2 | 2 |
| Death | 0 | 0 | 1 | |
| Pericardial disease | Hospitalization | 1 | 0 | 0 |
| Death | 1 | 0 | 1 | |
| Myocardial infarction | Hospitalization | 0 | 0 | 1 |
| Death | 0 | 1 | 0 | |
| Enlarged abdominal aortic aneurysm | Hospitalization | 0 | 0 | 1 |
| Death | 0 | 0 | 0 | |
| Nonspecific cardiac symptoms | Hospitalization | 1 | 0 | 2 |
| Death | 0 | 0 | 0 | |
| TOTAL | 6 (8.7) | 3 (8.3) | 13 (28) | |
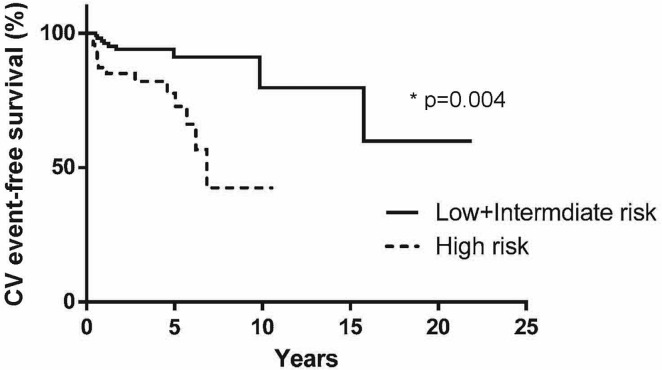
Heart failure–related and all cvd-related events numbered 8 and 22 respectively, accounting for 5.3% and 14% of the study population (Table iii). The most common events were heart failure (7 hospitalizations, 1 death), cardiac arrhythmia (4 hospitalizations, 1 death), pericardial disease (1 hospitalization, 2 deaths), myocardial infarction (1 hospitalization, 1 death), enlarged aortic aneurysm (1 hospitalization), and nonspecific cardiac symptoms (3 hospitalizations). Of the patients in the high-risk group, 13 (28%) experienced a cv event, a proportion that was significantly greater than that in the lower-risk groups (hazard ratio: 4.18; 95% confidence limits: 1.47, 11.9). A plot of time to cvd-related hospitalizations and deaths (Figure 2) shows that the high-risk group experienced a greater rate of severe cv events overall than did the low- and intermediate-risk groups (hazard ratio: 3.77; 95% confidence limits: 1.53, 9.35).
FIGURE 2.
A Cox proportional hazards model of time to hospitalization or death attributable to cardiovascular (CV) disease for low- and intermediate-risk patients compared with high-risk patients showed a hazard ratio of 3.77 (95% confidence limits: 1.53, 9.35; p = 0.004). Compared with patients at low and intermediate risk, high-risk patients experienced severe CV events more quickly.
Cardiotoxicity occurred slightly more frequently in patients who received anthracycline-based chemotherapy than in patients who did not. Of 115 and 37 patients respectively, 11 (10%) and 3 (8.1%) underwent a drop in lvef of more than 10% from baseline imaging, reaching a final lvef of 50% or less. No association between cardiotoxicity and frs group was evident in either population (p = 0.82).
DISCUSSION
Cardiovascular health is important for the well-being of bca survivors. Our study shows that a high frs is associated with a greater number of cv events; however, the frs underestimates the true incidence of cv events in the overall cohort despite management by the coc. The predicted mean 10-year risk of cvd in our population was 11.2% ± 1.35%, which is lower than the observed number of cv-related hospitalizations and deaths (14% in our total population). The mean 10-year risk of cvd in our cohort was similar to that for cancer survivors, but greater than for the general population. Population studies have shown that the anticipated 10-year cv risk is 12.5% ± 0.36% in cancer survivors compared with 6.7% ± 0.07% in a non-cancer control group13.
If referrals to the coc had included only patients with a high frs, 9 of 22 patients who went on to experience serious cv events would not have been appropriately managed for cv risk factors. That observation suggests that the frs cannot be used in isolation to identify patients at risk for cv events and that patients at low clinical risk might require additional monitoring to predict and detect cardiotoxicity. A high frs was not associated with cardiotoxicity as defined by left ventricular dysfunction in patients who received trastuzumab alone or anthracyclines followed by trastuzumab (p = 0.82).
Although the frs did not predict cardiotoxicity, the association between frs and cv events in this patient population is clinically important. In patients with a high frs, we observed an increased rate of serious cv events despite more aggressive management, as suggested by the greater number of new cardiac medications prescribed for those patients at the coc. Further clinical research is needed to determine strategies that might mitigate the increased risk.
The frs has previously been studied in cancer populations15–18; however, to our knowledge, use of the frs to evaluate cv outcomes and cardiotoxicity in a her2+ bca population has not been examined. Studies have examined the utility of cancer-specific clinical risk scores for patients with bca. Using clinical risk factors and anthracycline exposure, Ezaz et al.7 derived and validated a risk prediction model for heart failure and cardiomyopathy in patients receiving adjuvant trastuzumab for bca. Observed rates of cardiotoxicity in low-, intermediate-, and high-risk patients were 16%, 26%, and 43% respectively. As in our study, low-risk patients in the Ezaz study still experienced cardiotoxicity, which suggests that even patients at low clinical risk would require monitoring to detect tic. Other tools currently used to evaluate the risk of cardiotoxicity include biomarkers such as cardiac troponin and brain natriuretic peptide19, global longitudinal strain imaging using echocardiography20, and lifestyle factors21. A potential avenue for future research is to determine whether clinical risk scores can be used to modulate the intensity of cardiac imaging and biomarker testing, with monitoring being less frequent in low-risk patients compared with high-risk patients. The role of preventive management to reduce the high cv event rate in this population should be a priority for future clinical research in cardio-oncology.
There are some limitations to this study. First, confounding factors such as radiotherapy22 and anthracycline dose or interval23 might have affected the rates of tic. Because there was no significant variance between the three frs groups in observed clinical stage (p = 0.13), it would be unlikely that substantially different treatment regimens were used in one group compared with another. Second, the median follow-up time in our study (40.7 months) was less than 10 years, which is the period for which the frs is used to predict risk. However, even within the current follow-up period, more cv events occurred than expected, which furthers our argument that the frs underestimated the cv risk in a population that was already being carefully monitored in the coc. Third, this was a single-centre pilot study with a modest sample size and limited external validity. We intend to validate the results of this study prospectively and to propose a method that assigns appropriate follow-up for patients. Fourth, selection and detection bias was present in our study population, which was entirely composed of patients with her2+ bca referred by health care providers. Our population represented an enriched sample of those at higher risk of cvd and actively managed at the coc, such that the observed incidence rates of cvd would likely be different from rates in patients not seen at the coc. Future prospective studies are required to evaluate the role of clinical risk factors in predicting cv complications from cancer therapy and to determine whether optimizing cv risk factors mitigates risk against cancer therapy–induced cardiac dysfunction.
CONCLUSIONS
In our study, the frs was successfully applied to a real-life her2+ bca cohort referred to a cardio-oncology clinic. Compared with low-risk patients, high-risk patients were more likely to experience hospital admission or mortality from cv causes. Those patients could benefit from early referral to a cardiology–oncology clinic for evaluation and optimization of cv risk factors. However, in our population, the frs underestimated the true incidence of cv events in the entire cohort and did not predict tic (decline in lvef by >10% to a final lvef ≤50%). Future research should explore the value of the frs in combination with other risk scores to tailor the intensity of cardiac imaging and the biomarkers used for monitoring cardiotoxicity. Further studies might address the question of which patients would benefit from cardiac medical therapy for primary prevention of cardiotoxicity.
ACKNOWLEDGMENTS
We are grateful for the University of Ottawa Health Sciences Library, whose staff assisted with the literature search. We also acknowledge Freya Crawley for her contribution to collecting data for the coc database.
This project was supported by the University of Ottawa Undergraduate Research Opportunity Program research award.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Breast Cancer Statistics [Web page] Toronto, ON: Canadian Cancer Society; 2017. [Available at: http://www.cancer.ca/en/cancer-information/cancer-type/breast/statistics/?region=on; cited 17 March 2017] [Google Scholar]
- 2.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97:2869–79. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 4.Bowles EJA, Wellman R, Feigelson HS, et al. on behalf of the Pharmacovigilance Study Team Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst. 2012;104:1293–305. doi: 10.1093/jnci/djs317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2–overexpressing breast cancer: nsabp B-31. J Clin Oncol. 2005;23:7811–19. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 6.Thavendiranathan P, Abdel-Qadir H, Fischer HD, et al. Breast cancer therapy–related cardiac dysfunction in adult women treated in routine clinical practice: a population-based cohort study. J Clin Oncol. 2016;34:2239–46. doi: 10.1200/JCO.2015.65.1505. [DOI] [PubMed] [Google Scholar]
- 7.Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472. doi: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bird BR, Swain SM. Cardiac toxicity in breast cancer survivors: review of potential cardiac problems. Clin Cancer Res. 2008;14:14–24. doi: 10.1158/1078-0432.CCR-07-1033. [DOI] [PubMed] [Google Scholar]
- 9.Anderson TJ, Gregoire J, Hegele RA, et al. 2012 Update of the Canadian Cardiovascular Society guidelines for the diagnosis and treatment of dyslipidemia for the prevention of cardiovascular disease in the adult. Can J Cardiol. 2013;29:151–67. doi: 10.1016/j.cjca.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 10.D’Agostino RB, Sr, Grundy S, Sullivan LM, Wilson P, on behalf of the chd Risk Prediction Group Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA. 2001;286:180–7. doi: 10.1001/jama.286.2.180. [DOI] [PubMed] [Google Scholar]
- 11.Kivimäki M, Batty GD, Singh-Manoux A, et al. Validating the Framingham Hypertension Risk Score: results from the Whitehall ii study. Hypertension. 2009;54:496–501. doi: 10.1161/HYPERTENSIONAHA.109.132373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrmann J, Lerman A, Sandhu NP, Villarraga HR, Mulvagh SL, Kohli M. Evaluation and management of patients with heart disease and cancer: cardio-oncology. Mayo Clin Proc. 2014;89:1287–306. doi: 10.1016/j.mayocp.2014.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rushton M, Crawley F, Sulpher J, Johnson C, Dent S. Cardiotoxicity in breast cancer patients: a single center, retrospective review. Prog Pediatr Cardiol. 2015;39:67–9. doi: 10.1016/j.ppedcard.2015.10.002. [DOI] [Google Scholar]
- 14.Nousiainen T, Jantunen E, Vannienen E, Hartikainen J. Early decline in left ventricular ejection fraction predicts doxorubicin cardiotoxicity in lymphoma patients. Br J Cancer. 2002;86:1697–700. doi: 10.1038/sj.bjc.6600346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.So JH, Lee JK, Shin JY, Park W. Risk of cardiovascular disease using Framingham Risk Score in Korean cancer survivors. Korean J Fam Med. 2016;37:235–41. doi: 10.4082/kjfm.2016.37.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poullis M, McShane J, Shaw M, Page R, Shackcloth M, Mediratta N. Framingham risk–based survival of non-small-cell lung cancer. Asian Cardiovasc Thorac Ann. 2012;20:30–5. doi: 10.1177/0218492311432801. [DOI] [PubMed] [Google Scholar]
- 17.Bardia A, Arieas ET, Zhang Z, et al. Comparison of breast cancer recurrence risk and cardiovascular disease incidence risk among postmenopausal women with breast cancer. Breast Cancer Res Treat. 2012;131:907–14. doi: 10.1007/s10549-011-1843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daher IN, Daigle TR, Bhatia N, Durand JB. The prevention of cardiovascular disease in cancer survivors. Tex Heart Inst J. 2012;39:190–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Romano S, Fratini S, Ricevuto E, et al. Serial measurements of nt-probnp are predictive of not-high-dose anthracycline cardiotoxicity in breast cancer patients. Br J Cancer. 2011;105:1663–8. doi: 10.1038/bjc.2011.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoodley PW, Richards DA, Boyd A, et al. Altered left ventricular longitudinal diastolic function correlates with reduced systolic function immediately after anthracycline chemotherapy. Eur Heart J Cardiovasc Imaging. 2013;14:228–34. doi: 10.1093/ehjci/jes139. [DOI] [PubMed] [Google Scholar]
- 21.Jones SB, Thomas GA, Hesselsweet SD, Alvarez-Reeves M, Yu H, Irwin ML. Effect of exercise on markers of inflammation in breast cancer survivors: the Yale Exercise and Survivorship Study. Cancer Prev Res (Phila) 2013;6:109–18. doi: 10.1158/1940-6207.CAPR-12-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–98. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 23.Yeh ETH, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109:3122–31. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]