Abstract
Background
The efficacy of carboplatin–paclitaxel in the trimodality setting was demonstrated in the cross trial. Because of better tolerance, that regimen has been adopted as an alternative for patients receiving definitive chemoradiation (dcrt). The purpose of our study was to compare outcomes in patients with localized esophageal and gastroesophageal junction (gej) cancer who received dcrt using either platinum–5-fluorouracil (5fu) or carboplatin–paclitaxel.
Methods
Medical records and outcomes for all patients diagnosed with localized carcinoma of the esophagus and gej at our centre between 2008 and 2015 were reviewed. All patients who underwent dcrt using cisplatin–5fu, carboplatin–5fu, or carboplatin–paclitaxel were included.
Results
The 73 identified patients (34 cisplatin–5fu, 13 carboplatin–5fu, 26 carboplatin–paclitaxel) were all prescribed concomitant radiotherapy of 50 Gy in 25 daily fractions. The diagnosis was adenocarcinoma in 64% and squamous cell carcinoma in 36%. Median overall survival (os) duration for the cisplatin–5fu group was 28 months [95% confidence interval (ci): 19 to 41 months], with a 3-year os rate of 44%, in contrast to the 15 months (95% ci: 11 to 17 months) and 15% in the carboplatin–paclitaxel group (log-rank p = 0.0047). Median os duration for the carboplatin–5fu group was 17 months (95% ci: 11 to 68 months) with a 3-year os rate of 31%. Adjusting for patient and disease factors, better os durations and rates were associated with cisplatin–5fu (hazard ratio: 0.34; p = 0.0016) and carboplatin–5fu (hazard ratio: 0.55; p = 0.20) than with carboplatin–paclitaxel.
Conclusions
In a dcrt regimen, a better os is associated with cisplatin–5fu than with carboplatin–paclitaxel. Clinical trials to determine optimal chemotherapy regimens are warranted for patients who are not suitable for surgery.
Keywords: Esophageal cancer, gastroesophageal junction tumours, definitive chemoradiation, practice patterns
INTRODUCTION
Esophageal carcinoma is the 8th most common cancer and the 6th leading cause of cancer-related mortality worldwide1. It was estimated that, in 2012, 455,800 new esophageal cancer cases occurred, and the incidence of the disease is increasing rapidly2,3. Overall prognosis remains poor, with a 5-year survival rate of 15%–25%2.
Trimodality management is the standard of care for localized esophageal and gastroesophageal junction (gej) cancer in patients who are medically fit, have resectable disease, and are willing to undergo surgery4,5. The cross trial demonstrated efficacy for carboplatin–paclitaxel together with radiotherapy (rt) at 41.4 Gy in the preoperative setting6. In patients who do not undergo surgery, the Radiation Therapy Oncology Group 8501 trial established the current standard of care: cisplatin–5-fluorouracil (5fu) concomitant with rt at 50 Gy in the definitive setting, with a median survival duration of 12.5 months7. In recent years, because of better tolerance, carboplatin–paclitaxel has also been adopted as an alternative for patients receiving definitive chemoradiation (dcrt) despite no direct comparison with cisplatin–5fu4,6.
No prospective trial has assessed the efficacy of carboplatin–paclitaxel in the definitive setting. To our knowledge, only one retrospective study has compared carboplatin–paclitaxel with cisplatin–5fu, reporting comparable outcomes for the two groups in that setting8.
The objective of the present study was to evaluate tolerability, toxicity, and outcomes in patients with localized esophageal and gej cancer who received dcrt using any of cisplatin–5fu, carboplatin–5fu, or carboplatin–paclitaxel at our institute.
METHODS
Study Population
We retrospectively reviewed all patients diagnosed with localized esophageal or gej cancer (or both) referred to the Cancer Centre of Southeastern Ontario in Kingston, Ontario, between January 2008 and March 2015. All eligible patients who were seen at the regional hospital by medicine, gastroenterology, surgical oncology, radiation oncology, medical oncology, or other services were screened. Patients who underwent dcrt using cisplatin–5fu, carboplatin–5fu, or carboplatin–paclitaxel were included. Patients with small-cell carcinoma or neuroendocrine histology were excluded.
Staging
All patients underwent endoscopic evaluations and had a tissue diagnosis. Endoscopic ultrasonography was not routinely performed. All patients underwent computed tomography of chest and abdomen (and the neck in patients with upper esophageal cancer) as well as 18F–fluorode-oxyglucose positron-emission tomography. Patients were clinically staged according to the staging manual of the American Joint Committee on Cancer, 7th edition.
Chemoradiation Treatment
At our institute, a treatment regimen consisting of cisplatin–5fu with concomitant rt was the standard treatment; however, a shift to a carboplatin–paclitaxel chemotherapy regimen with concomitant rt evolved in more recent years. The cisplatin–5fu regimen consisted of a continuous intravenous infusion of 5fu (1000 mg/m2 daily for 4 days) and cisplatin (75 mg/m2 at 1 mg/min) administered concomitantly during weeks 1 and 5 of rt, with an additional 2 cycles in weeks 8 and 11 when possible7. Carboplatin was used instead of cisplatin in some patients with significant renal insufficiency or hearing impairment. The carboplatin–paclitaxel was administered weekly (carboplatin: area under the curve 2 mg/mL/ min; paclitaxel: 50 mg/m2) for 5 weeks6. Dose adjustments, deferrals, or substitutions of the chemotherapy agent were at the treating physician’s discretion.
The standard dose for concurrent rt with all three chemotherapy regimens was 50 Gy in 25 fractions 5 days per week, using 3-dimensional conformal rt.
Data Collection
We retrospectively collected patient demographics, cancer diagnosis details, symptoms, medical comorbidities, score on the Charlson comorbidity index9, and baseline Eastern Cooperative Oncology Group performance status10 based on the initial oncology consultation and clinic notes. Treatment details, toxicities, response, recurrence, and survival outcomes were obtained from several documented assessments during treatment and follow-up. Toxicity was graded based on the criteria jointly published by the Radiation Therapy Oncology Group and the European Organisation for Research and Treatment of Cancer11. The database was reviewed by a trained reviewer for quality assurance.
Statistical Analysis
The Kaplan–Meier method was used for survival and recurrence analysis. The reverse Kaplan–Meier method was used to estimate median follow-up time. Time-to-event outcomes were defined as the time from pathology diagnosis to the event. In the absence of an event, over-all survival (os) and local and distant recurrence were censored on the date of the last clinic visit. Patients with residual disease on post-treatment endoscopic biopsy were excluded from the disease-free survival analysis; patients who did not undergo endoscopic evaluation were included.
The log-rank test was used to compare survival and recurrence outcomes between the patient groups. Comparisons having a p value less than 0.1 on univariate analysis were selected for a multivariate Cox proportional hazards model. Age was preselected for face validity. Backward elimination was used in the multivariate analysis, and variables with a p value less than 0.2 were retained in the model for adjustment. A 2-sided p value less than 0.05 was considered statistically significant. Data were analyzed using the SAS software application (version 9.3: SAS Institute, Cary, NC, U.S.A.).
The study was approved by the Queen’s University Health Sciences and Affiliated Teaching Hospitals Research Ethics Board. We followed the strobe guideline in conducting and reporting the study.
RESULTS
Patient Characteristics
A total of 73 patients met the inclusion criteria and were included in the analysis. Overall, median age at diagnosis was 74 years (range: 48–86 years), and men constituted 79% of the cohort (n = 58). At presentation, 89% of the patients (n = 65) had an Eastern Cooperative Oncology Group performance status of 0–1, and 52% (n = 38) scored 0–1 on the Charlson comorbidity index. Adenocarcinoma was the most common histology (n = 47, 64%). Most patients (n = 44, 60%) had lower-third or gej cancer (or both). Table i shows the patient and disease characteristics by chemotherapy regimen received. Patients in the cisplatin–5fu group were younger (median age: 72 vs. 76–77, p = 0.032), and compared with the other two groups, the carboplatin–5fu group had a higher proportion of patients with adenocarcinoma (92% vs. 53%–65%, p = 0.041). Other patient and disease characteristics were similar in the 3 groups. The common reasons for not undergoing surgery were comorbidities (n = 24, 33%), patient preference (n = 23, 32%), and unresectable disease (n = 17, 23%).
TABLE I.
Patient and disease characteristics
| Characteristic | Patient group | p Value | ||
|---|---|---|---|---|
|
| ||||
| Cisplatin–5FU (n=34) | Carboplatin–5FU (n=13) | Carboplatin–paclitaxel (n=26) | ||
| Age at diagnosis (years) | 0.032 | |||
| Median | 72 | 77 | 76 | |
| Range | 48–86 | 49–85 | 54–82 | |
| Sex [n (%)] | 0.96 | |||
| Women | 7 (21) | 3 (23) | 5 (19) | |
| Men | 27 (79) | 10 (77) | 21 (81) | |
| Histology [n (%)] | 0.041 | |||
| Adenocarcinoma | 18 (53) | 12 (92) | 17 (65) | |
| Squamous cell carcinoma | 16 (47) | 1 (8) | 9 (35) | |
| Grade [n (%)] | 0.66 | |||
| 1 | 0 (0) | 0 (0) | 2 (8) | |
| 2 | 18 (53) | 7 (54) | 13 (50) | |
| 3 | 13 (38) | 5 (38) | 8 (31) | |
| Unknown | 3 (9) | 1 (8) | 3 (12) | |
| Clinical T stage [n (%)] | 0.73 | |||
| T2 | 10 (29) | 3 (23) | 7 (27) | |
| T3 | 21 (62) | 10 (77) | 18 (69) | |
| T4 | 3 (9) | 0 (0) | 1 (4) | |
| Clinical N stage [n (%)] | 0.45 | |||
| N0 | 16 (47) | 9 (69) | 14 (54) | |
| N1 | 11 (32) | 3 (23) | 10 (38) | |
| N2 | 7 (21) | 1 (8) | 2 (8) | |
| Clinical anatomic stage [n (%)] | 0.27 | |||
| I | 2 (6) | 1 (8) | 4 (15) | |
| II | 14 (41) | 9 (69) | 11 (42) | |
| III | 18 (53) | 3 (23) | 11 (42) | |
| Primary tumour location [n (%)] | 0.29 | |||
| Lower third or GEJ | 19 (56) | 10 (77) | 15 (58) | |
| Middle third | 11 (32) | 2 (15) | 4 (15) | |
| Upper third | 2 (6) | 0 (0) | 5 (19) | |
| Overlap | 2 (6) | 1 (8) | 2 (8) | |
| Tumour length [n (%)] | 0.35 | |||
| ≤5 cm | 14 (41) | 8 (62) | 10 (38) | |
| >5 cm | 20 (59) | 5 (38) | 16 (62) | |
| ECOG performance status [n (%)] | 0.36 | |||
| 0 | 13 (38) | 2 (15) | 6 (23) | |
| 1 | 16 (47) | 9 (69) | 18 (69) | |
| 2 | 5 (15) | 2 (15) | 2 (8) | |
| Score on the CCI [n (%)] | 0.20 | |||
| 0–1 | 21 (62) | 5 (38) | 12 (46) | |
| ≥2 | 13 (38) | 8 (62) | 14 (54) | |
5FU = 5-fluorouracil; GEJ = gastroesophageal junction; ECOG = Eastern Cooperative Oncology Group; CCI = Charlson comorbidity index.
Treatment Characteristics and Tolerability
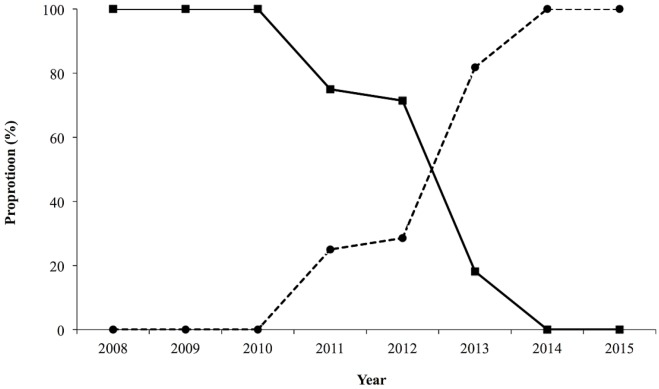
Cisplatin–5fu was given to 47% of the patients (n = 34); carboplatin–paclitaxel, to 36% (n = 26); and carboplatin–5fu, to 18% (n = 13). In this group of patients, the chemotherapy practice pattern shifted to carboplatin–paclitaxel from platinum–5fu during the last 8 years at our centre (Figure 1).
FIGURE 1.
Practice pattern of chemotherapy over an 8-year period in patients undergoing definitive chemoradiation for localized esophageal or gastroesophageal junction cancer. Solid line = cisplatin or carboplatin with 5-fluorouracil; broken line = carboplatin-paclitaxel.
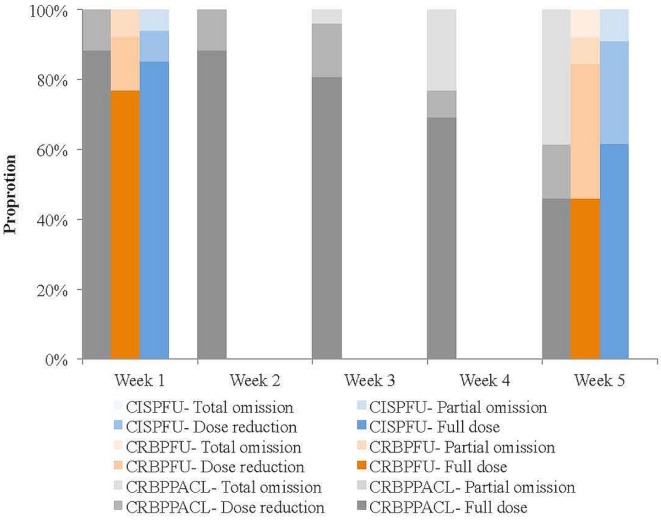
Chemotherapy tolerance was similar for the three regimens (Figure 2). Most patients started with a full chemotherapy dose (carboplatin–paclitaxel: 88%, n = 23; cisplatin–5fu: 85%, n = 29; carboplatin–5fu: 77%, n = 10; p = 0.62). The proportion of patients who continued to tolerate a full chemotherapy dose at the end of the 5-week period was not significantly different between the regimens (carboplatin–paclitaxel: 46%, n = 12; cisplatin–5fu: 62%, n = 21; carboplatin–5fu: 46%, n = 6; p = 0.45).
FIGURE 2.
Chemotherapy tolerance. CISPFU = cisplatin–5-fluorouracil (5FU); CRBPFU = carboplatin–5FU; CRBPPACL = carboplatin–paclitaxel.
Dose reductions ranged from 10% to 25%. Common reasons for an initial dose reduction included pre-existing comorbidities and older age; dose reductions or omissions in subsequent treatment cycles were related mainly to mucositis or neutropenia. An additional 1–2 cycles of chemotherapy after dcrt were given in 21% of the cisplatin–5fu group (n = 7) and in 8% of the carboplatin–5fu group. No additional chemotherapy was given in patients who received the carboplatin–paclitaxel regimen.
All patients received rt, with 99% of patients (n = 72) receiving at least 95% of the prescribed rt dose of 50 Gy. In 2 patients, 1 fraction was omitted, and in 1 patient, treatment was discontinued because of a sudden stroke after 72% of the prescribed dose had been delivered. When residual disease was identified on endoscopic evaluation, 2 patients—both in the carboplatin–paclitaxel group—received intraluminal brachytherapy (18 Gy in 3 fractions) after dcrt.
Treatment Toxicities
Table ii lists acute treatment-related toxicities. Overall, 36% of the patients (n = 26) experienced grade 3 or greater toxicities, and 10% of the patients (n = 7) experienced grade 4 or greater toxicities, without any significant differences between the three chemotherapy groups. Within 90 days of treatment, 4 patients (5%) died from failure to thrive, and 1 patient died from metastatic disease. Significantly more grade 3 esophagitis was observed in the carboplatin–paclitaxel group.
TABLE II.
Acute and late toxicities
| Toxicity | Patient group | p Value | ||
|---|---|---|---|---|
|
| ||||
| Cisplatin–5FU (n=34) | Carboplatin–5FU (n=13) | Carboplatin–paclitaxel (n=26) | ||
| Any grade 3 or greater | 11 (32) | 5 (38) | 10 (38) | 0.85 |
| Any grade 4 or greater | 3 (9) | 1 (8) | 3 (12) | 1.0 |
| Any grade 5a | 1 (3) | 1 (8) | 2 (8) | 0.52 |
| Grade 3 | ||||
| Weight loss | 3 (9) | 0 (0) | 0 (0) | 0.29 |
| Vomiting | 1 (3) | 1 (8) | 5 (19) | 0.098 |
| Esophagitis | 1 (3) | 0 (0) | 6 (23) | 0.021 |
| Mucositis | 1 (3) | 1 (8) | 1 (4) | 0.77 |
| Infection | 0 (0) | 0 (0) | 2 (8) | 0.15 |
| Neutropenia | 1 (3) | 2 (15) | 3 (12) | 0.22 |
| Thrombocytopenia | 1 (3) | 3 (23) | 1 (4) | 0.067 |
| Febrile neutropenia | 1 (3) | 0 (0) | 1 (4) | 1.0 |
| Grade 3 or greaterb | ||||
| Hematologic | 5 (15) | 4 (31) | 3 (12) | 0.37 |
| Nonhematologic | 6 (18) | 1 (8) | 9 (35) | 0.14 |
| Grade 4 | ||||
| Neutropenia | 1 (3) | 0 (0) | 0 (0) | 1.0 |
| Febrile neutropenia | 1 (3) | 0 (0) | 0 (0) | 1.0 |
| Cardiac | 0 (0) | 0 (0) | 1 (4)c | 0.53 |
Death from failure to thrive occurred in 4 patients 2–3 months after concurrent chemoradiation.
No grade 3 or greater diarrhea, pneumonitis, renal failure, or anemia was documented.
In 1 patient, an abdominal aortic aneurysm ruptured after 1 fraction of radiation and 1 dose of chemotherapy.
5FU = 5-fluorouracil.
A gastrostomy tube was placed before treatment in 3 patients (4%). Strictures requiring dilatation later developed in 9 patients (12%). Fistula with the major airway occurred in 2 patients (3%) after they developed a local recurrence. No other late toxicities were documented.
Treatment Response
After dcrt, 70% of the patients (n = 51) underwent endoscopic evaluation and biopsy, with no significant differences observed between the three groups. Treatment response was similar in the three groups (no residual cancer: cisplatin–5fu, 59%, n = 20; carboplatin–5fu, 62%, n = 8; carboplatin–paclitaxel, 62%, n = 16; p = 0.70).
Survival and Recurrence
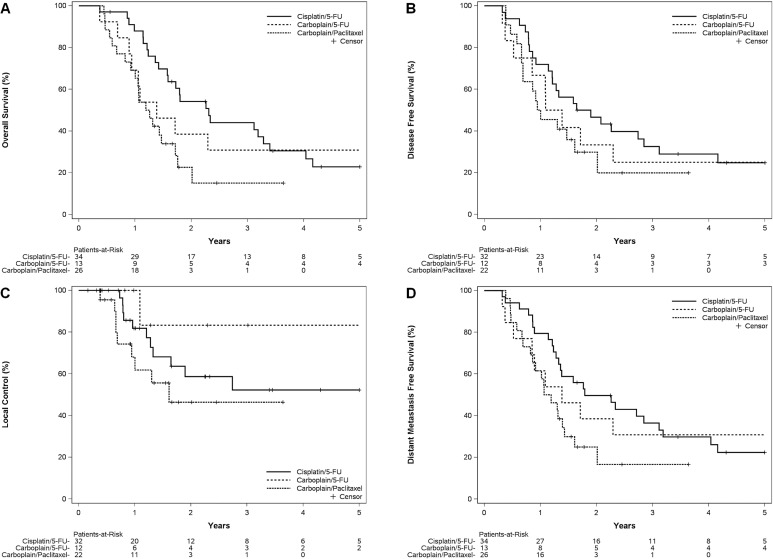
The median follow-up for the cisplatin–5fu, carboplatin–5fu, and carboplatin–paclitaxel groups was 62, 101, and 30 months respectively. At the time of analysis, only 9 patients (12%) were known to be living; 8 patients (11%) had been lost to follow-up. The os was significantly better for the cisplatin–5fu group than for the carboplatin–paclitaxel group [log-rank p = 0.0047, Figure 3(A)]. The median os duration for the cisplatin–5fu group was 28 months (95% ci: 19 to 41 months), with a 3-year os rate of 44% (95% ci: 27% to 61%) and a 5-year os rate of 23% (95% ci: 14% to 38%). The median os duration for the carboplatin–paclitaxel group was 15 months (95% ci: 11 to 21 months), with a 3-year os rate of 15% (95% ci: 2% to 32%). The median os duration for the carboplatin–5fu group was 17 months (95% ci: 11 to 68 months), with a 3-year os rate of 31% [95% ci: 6% to 56%; Figure 3(A)].
FIGURE 3.
Survival and recurrence by treatment groups. (A) Overall survival. (B) Disease-free survival. (C) Local control. (D) Distant metastasis-free survival. 5-FU = 5-fluorouracil.
Figure 3(B–D) shows disease-free survival, local control, and distant metastasis-free survival by chemotherapy group. Compared with the carboplatin–paclitaxel group, the cisplatin–5fu group experienced significantly better distant metastasis-free survival (p = 0.021) and a trend toward better disease-free survival (p = 0.11) and local control (p = 0.25).
Table iii presents the univariate and multivariate Cox proportional hazards models for os. On univariate analysis, cisplatin–5fu chemotherapy, stage i disease, better Eastern Cooperative Oncology Group performance status, squamous cell histology, shorter tumour length, and low-grade disease were found to be significantly associated with better os. In multivariate analysis, grade and score on the Charlson comorbidity index were initially tested; however, they did not meet the pre-set criteria to be selected in the final model. Compared with the carboplatin–paclitaxel group, the cisplatin–5fu and carboplatin–5fu groups experienced better os after adjustment for patient and disease factors (respective hazard ratios: 0.34, p = 0.0016; 0.55, p = 0.20).
TABLE III.
Univariate and multivariate analysis for overall survival
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Chemotherapy | ||||||
| Carboplatin–paclitaxel | Reference | Reference | ||||
| Cisplatin–5FU | 0.44 | 0.24 to 0.82 | 0.0092 | 0.34 | 0.17 to 0.67 | 0.0016 |
| Carboplatin–5FU | 0.59 | 0.27 to 1.28 | 0.18 | 0.55 | 0.23 to 1.36 | 0.20 |
| Age (per 10-year increment) | 0.98 | 0.75 to 1.27 | 0.87 | 0.73 | 0.52 to 1.02 | 0.062 |
| Anatomic stage | ||||||
| I | 0.26 | 0.078 to 0.87 | 0.028 | 0.16 | 0.045 to 0.58 | 0.0049 |
| II | 0.74 | 0.42 to 1.28 | 0.28 | 0.55 | 0.26 to 0.93 | 0.029 |
| III | Reference | Reference | ||||
| ECOG performance status (1–2 vs. 0) | 2.03 | 1.07 to 3.85 | 0.030 | 3.02 | 1.38 to 6.61 | 0.0056 |
| Adenocarcinoma (vs. squamous cell carcinoma) | 1.85 | 1.02 to 3.35 | 0.042 | 2.11 | 1.09 to 4.06 | 0.026 |
| Tumour length (>5 cm vs. ≤5 cm) | 2.10 | 1.18 to 3.72 | 0.011 | 1.58 | 0.84 to 2.97 | 0.15 |
| Grade 2 to 3 (vs. 1 or unknown) | 1.93 | 0.77 to 4.84 | 0.016 | Not selected | ||
| Score on the CCI (per 1-point increment) | 1.10 | 0.99 to 1.22 | 0.073 | Not selected | ||
| Lower or GEJ (vs. middle, upper, or overlapping) | 1.24 | 0.71 to 2.16 | 0.45 | |||
| Female sex (vs. male sex) | 1.17 | 0.62 to 2.23 | 0.63 | |||
HR = hazard ratio; CI = confidence interval; 5FU = 5-fluorouracil; ECOG = Eastern Cooperative Oncology Group; CCI = Charlson comorbidity index; GEJ = gastroesophageal junction.
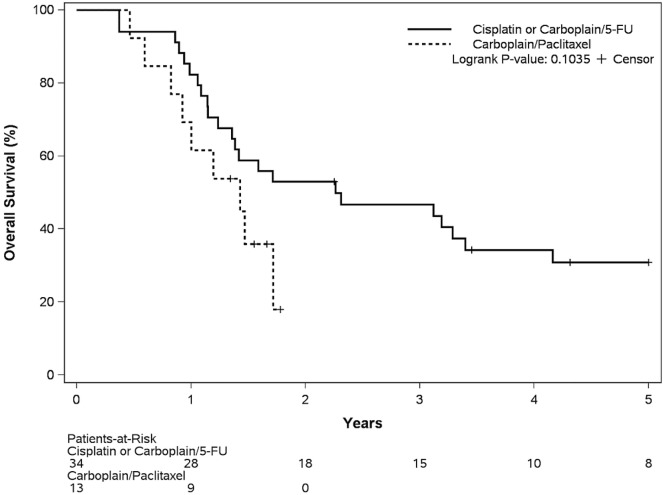
To further minimize the influence of patient selection and potential bias, we examined a subset of 47 patients, 34 of whom had been diagnosed during 2008–2010, when all patients received cisplatin (or carboplatin) with 5fu, and 13 of whom had been diagnosed during 2014–2015 when all patients received carboplatin–paclitaxel. Baseline disease and patient characteristics in the groups were similar (Table iv). Median os duration was 27 months (95% ci: 17 to 68 months) in the platinum–5fu group and 17 months (95% ci: 10 months to not reached) in the carboplatin–paclitaxel group (Figure 4; hazard ratio: 0.51; log-rank p = 0.10).
TABLE IV.
Patient and disease characteristics in subgroup analysis
| Variable | 2008–2010 | 2014–2015 | p Value | |
|---|---|---|---|---|
|
|
|
|||
| Platinum–5FUa (n=34) | Carboplatin–paclitaxel (n=13) | |||
| Age at diagnosis (years) | 0.6 | |||
| Median | 73.5 | 76 | ||
| Range | 49–85 | 54–82 | ||
| Sex [n (%)] | 0.69 | |||
| Women | 7 (21) | 2 (15) | ||
| Men | 27 (79) | 11 (85) | ||
| Histology [n (%)] | 0.49 | |||
| Adenocarcinoma | 22 (65) | 7 (54) | ||
| Squamous cell carcinoma | 12 (35) | 6 (46) | ||
| Grade [n (%)] | 0.11 | |||
| 1 | 0 (0) | 2 (15) | ||
| 2 | 17 (50) | 7 (54) | ||
| 3 | 13 (38) | 3 (23) | ||
| Unknown | 4 (12) | 1 (8) | ||
| Clinical T stage [n (%)] | 0.7 | |||
| T2 | 9 (26) | 2 (15) | ||
| T3 | 22 (65) | 10 (77) | ||
| T4 | 3 (9) | 1 (8) | ||
| Clinical N stage [n (%)] | 0.75 | |||
| N0 | 20 (59) | 9 (69) | ||
| N1 | 9 (26) | 3 (23) | ||
| N2 | 5 (15) | 1 (8) | ||
| Clinical anatomic stage [n (%)] | 0.58 | |||
| I | 2 (6) | 2 (15) | ||
| II | 18 (53) | 6 (46) | ||
| III | 14 (41) | 5 (38) | ||
| Primary tumour location [n (%)] | 0.008 | |||
| Lower third or GEJ | 23 (68) | 5 (38) | ||
| Middle third | 9 (26) | 3 (23) | ||
| Upper third | 2 (6) | 1 (8) | ||
| Overlap | 0 (0) | 4 (31) | ||
| Tumour length [n (%)] | 0.4 | |||
| ≤5 cm | 15 (44) | 4 (31) | ||
| >5 cm | 19 (56) | 9 (69) | ||
| ECOG performance status [n (%)] | 0.41 | |||
| 0 | 9 (26) | 6 (46) | ||
| 1 | 20 (59) | 6 (46) | ||
| 2 | 5 (15) | 1 (8) | ||
| Score on the CCI [n (%)] | 0.72 | |||
| 0–1 | 20 (59) | 9 (69) | ||
| ≥2 | 2 (6) | 1 (8) |
Cisplatin or carboplatin.
5FU = 5-fluorouracil; GEJ = gastroesophageal junction; ECOG = Eastern Cooperative Oncology Group; CCI = Charlson comorbidity index.
FIGURE 4.
Overall survival by treatment group in a subset of patients: 2008–2010, platinum–5-fluorouracil (5-FU); and 2014–2015, carboplatin–paclitaxel.
DISCUSSION
In the present study, we compared commonly used concomitant chemoradiation regimens that had a standardized rt component in patients who were not planned for surgery. We note a shift toward the use of carboplatin–paclitaxel (per the cross trial) in the definitive setting for managing localized esophageal or gej cancer. We report that, compared with carboplatin–paclitaxel, cisplatin–5fu is associated with better os. We observed no significant differences in treatment tolerance or treatment-related toxicities between those regimens.
Worldwide, the use of carboplatin–paclitaxel in esophageal cancer management has increased12,13. The evidence comparing carboplatin–paclitaxel and cisplatin– 5fu is scant. A retrospective review by Blom et al.14 reported that, in trimodality management, outcomes were comparable but treatment-related toxicity was less for carboplatin–paclitaxel and a rt dose of 41.4 Gy compared with cisplatin–5fu and a rt dose of 50.4 Gy. Honing et al.8 did not find a significant difference in survival between the two chemotherapy regimens in the definitive setting, reporting a median survival duration of 16.1 months (95% ci: 11.8 to 20.5 months) in the cisplatin–5fu group and 13.8 months (95% ci: 10.8 to 16.9 months) in carboplatin–paclitaxel group (p = 0.879). The current U.S. National Comprehensive Cancer Network guideline recommends carboplatin–paclitaxel as one of the preferred regimens in dcrt4. Nevertheless, to our knowledge, little new evidence about the use of carboplatin–paclitaxel in the dcrt setting has emerged to support the shift. We also observed a gradual switch to the use of carboplatin–paclitaxel in dcrt at our centre after the results of the cross trial became available. A large population-based study could potentially assess this change in practice and evaluate the associated out-comes to help expedite clinical decisions.
Carboplatin–paclitaxel with concomitant rt is the current standard of care in the neoadjuvant setting6. This chemotherapy regimen has also started to be preferred for patients receiving dcrt because of better tolerance and less toxicity6,8,14. In the present study, we report a slightly higher incidence of grade 3 toxicities than was reported in the cross trial (hematologic: 12% vs. 8%; nonhematologic: 35% vs. 13%) and a higher incidence of esophagitis (23% vs. 1%). Furthermore, the incidence of grade 3 toxicities in the cisplatin–5fu group was lower at our institution than in the Radiation Therapy Oncology Group 8501 and int 0123 trials (32% vs. 66%–71%). We did not, as expected, observe a significantly better toxicity profile for carboplatin–paclitaxel. In addition to the limitations associated with assessing toxicity retrospectively, bias in the reported crude event rate could result from fitter patients being treated with cisplatin–5fu.
A few other modifications to the standard chemotherapy regimens have been tested when chemoradiation is used as definitive treatment without surgery. The scope1 trial showed that adding cetuximab to cisplatin–capecitabine with an rt dose of 50 Gy adversely affected outcome, with significantly worse os and treatment-related toxicities15. Yang et al.16 randomized 68 patients to either paclitaxel–lobaplatin or cisplatin–5fu with 60–70 Gy and observed better progression-free survival in the paclitaxel–lobaplatin group; however, median follow-up was 9 months, and os was not reported. Two retrospective studies comparing cisplatin–paclitaxel and cisplatin–5fu showed comparable results in one study and a better outcome in terms of survival for cisplatin–paclitaxel in the other study17,18. A comparison of the efficacy and toxicities of various dcrt regimens will be best conducted in a properly designed clinical trial.
We report a median survival duration of 15 months in the group receiving carboplatin–paclitaxel, which is similar to the duration seen in previous reports8,19. The median os duration for dcrt with cisplatin–5fu reported in phase iii trials ranged from 14 to 18 months7,20,21. We observed a median os duration of 28 months in the cisplatin–5fu group, which is similar to the 25–26 months reported in more recent literature15,22,23. Known prognostic factors such as performance status, tumour length, clinical stage, histology, and age were considered in the multivariate model that confirmed the association of a survival benefit with cisplatin–5fu16,24. Although not statistically significant, a trend toward better local control and less distant metastasis was also observed for the cisplatin–5fu group, which supported our finding that the survival benefit observed in the cisplatin–5fu group was likely attributable to a better treatment outcome rather than to selection of fitter patients. Interestingly, the complete response rate by endoscopic evaluation did not differ between the two treatment groups (59% and 62%) and was comparable to the rate reported from the scope1 trial (61%)20.
Our study is limited by its retrospective nature and small sample size. Intraluminal ultrasonography was not routinely performed for staging purposes in this cohort. Similarly, information about performance status and medical comorbidities was collected retrospectively based on documentation in medical records. The choice of chemotherapy in the transitional period was at the physician’s discretion, and the reason for the chemotherapy regimen choice was not always clear. Choices between carboplatin–5fu and carboplatin–paclitaxel were made for less-fit patients, but the reasons were not always documented. Residual confounding by indication might be present even after careful adjustment. We performed a sensitivity analysis by time period to further minimize patient selection bias. Although no statistically significant os difference was detected in that subgroup analysis (p = 0.10), a clear trend for a better outcome with a platinum–5fu regimen, with a large effect size (hr: 0.51), was observed. Despite the limitations posed by the retrospective nature of our study, it represents one of few esophageal cancer studies to compare two commonly used chemotherapy regimens in the dcrt setting. Our report might provide insight for practicing oncologists, in that better evidence is likely required before a paradigm shift in the existing standard of care is accepted. It also highlights the need for a prospective clinical trial to address this particular question.
CONCLUSIONS
Our series demonstrates that, compared with carboplatin–paclitaxel, cisplatin–5fu is associated with a survival benefit in the treatment, by dcrt, of patients with esophageal and gej cancer. The practice pattern of chemotherapy in this setting has shifted from platinum–5fu to carboplatin–paclitaxel, which might be premature. Clinical trials to determine the optimal chemotherapy regimen for patients who are not suitable for surgery are warranted.
ACKNOWLEDGMENTS
The authors thank Ms. L. Tremblay for data acquisition.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81. doi: 10.1016/j.ejca.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Pennathur A, Gibson MK, Jobe BA, Luketich JD. Oesophageal carcinoma. Lancet. 2013;381:400–12. doi: 10.1016/S0140-6736(12)60643-6. [DOI] [PubMed] [Google Scholar]
- 3.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancers. Fort Washington, PA: NCCN; 2016. Ver. 2.2016. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf (free registration required); cited 20 October 2016] [Google Scholar]
- 5.Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92. doi: 10.1016/S1470-2045(11)70142-5. [DOI] [PubMed] [Google Scholar]
- 6.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 7.Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–8. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 8.Honing J, Smit JK, Muijs CT, et al. A comparison of carboplatin and paclitaxel with cisplatinum and 5-fluorouracil in definitive chemoradiation in esophageal cancer patients. Ann Oncol. 2014;25:638–43. doi: 10.1093/annonc/mdt589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Sax FL, MacKenzie CR, Braham RL, Fields SD, Douglas RG., Jr Morbidity during hospitalization: can we predict it? J Chronic Dis. 1987;40:705–12. doi: 10.1016/0021-9681(87)90107-X. [DOI] [PubMed] [Google Scholar]
- 10.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (rtog) and the European Organization for Research and Treatment of Cancer (eortc) Int J Radiat Oncol Biol Phys. 1995;31:1341–6. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 12.Suntharalingam M, Moughan J, Coia LR, et al. The national practice for patients receiving radiation therapy for carcinoma of the esophagus: results of the 1996–1999 Patterns of Care Study. Int J Radiat Oncol Biol Phys. 2003;56:981–7. doi: 10.1016/S0360-3016(03)00256-6. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd S, Chang BW. Current strategies in chemoradiation for esophageal cancer. J Gastrointest Oncol. 2014;5:156–65. doi: 10.3978/j.issn.2078-6891.2014.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blom RL, Sosef MN, Nap M, et al. Comparison of two neoadjuvant chemoradiotherapy regimens in patients with potentially curable esophageal carcinoma. Dis Esophagus. 2014;27:380–7. doi: 10.1111/dote.12110. [DOI] [PubMed] [Google Scholar]
- 15.Crosby T, Hurt CN, Falk S, et al. Chemoradiotherapy with or without cetuximab in patients with oesophageal cancer (scope1): a multicentre, phase 2/3 randomised trial. Lancet Oncol. 2013;14:627–37. doi: 10.1016/S1470-2045(13)70136-0. [DOI] [PubMed] [Google Scholar]
- 16.Yang JS, Wang T, Qiu MQ, Li QL. Comparison of efficacy and toxicity profiles between paclitaxel/lobapoatin– and cisplatin/5-fluorouracil–based concurrent chemoradiotherapy of advanced inoperable oesophageal cancer. Intern Med J. 2015;45:757–61. doi: 10.1111/imj.12773. [DOI] [PubMed] [Google Scholar]
- 17.Hu G, Wang Z, Wang Y, et al. Comparison of cisplatinum/paclitaxel with cisplatinum/5-fluorouracil as first-line therapy for nonsurgical locally advanced esophageal squamous cell carcinoma patients. Drug Des Devel Ther. 2016;10:2129–36. doi: 10.2147/DDDT.S105441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun X, Han S, Gu F, et al. A retrospective comparison of taxane and fluorouracil-based chemoradiotherapy in patients with inoperable esophageal squamous cell carcinoma. J Cancer. 2016;7:1066–73. doi: 10.7150/jca.13547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Courrech Staal EF, Aleman BM, van Velthuysen ML, et al. Chemoradiation for esophageal cancer: institutional experience with three different regimens. Am J Clin Oncol. 2011;34:343–9. doi: 10.1097/COC.0b013e3181dbbafe. [DOI] [PubMed] [Google Scholar]
- 20.Conroy T, Galais MP, Raoul JL, et al. Definitive chemoradiotherapy with folfox versus fluorouracil and cisplatin in patients with oesophageal cancer (prodige5/accord17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15:305–14. doi: 10.1016/S1470-2045(14)70028-2. [DOI] [PubMed] [Google Scholar]
- 21.Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase iii trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20:1167–74. doi: 10.1200/JCO.2002.20.5.1167. [DOI] [PubMed] [Google Scholar]
- 22.Crosby TDL, Brewster AE, Borley A, et al. Definitive chemoradiation in patients with inoperable oesophageal carcinoma. Br J Cancer. 2013;90:70–5. doi: 10.1038/sj.bjc.6601461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shridhar R, Chuong MD, Weber J, et al. Outcomes of definitive or preoperative imrt chemoradiation for esophageal cancer. J Clin Oncol. 2012;1:347–54. [Google Scholar]
- 24.Di Fiore F, Lecleire S, Rigal O, et al. Predictive factors of survival in patients treated with definitive chemoradiotherapy for squamous cell esophageal carcinoma. World J Gastroenterol. 2006;12:4185–90. doi: 10.3748/wjg.v12.i26.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]