Abstract
Four members of the Ixodes ricinus species complex, Ixodes pacificus, Ixodes persulcatus, Ixodes ricinus and Ixodes scapularis, have, between them, a worldwide distribution within the northern hemisphere. They are responsible for the transmission of several animal and human pathogens, including the causal agents of Lyme borreliosis, tick-borne encephalitis, human granulocytic anaplasmosis and human babesiosis. Despite the importance of these ticks as vectors, the knowledge and understanding of the role that diapause plays in their complex life cycles are confused and incomplete. In view of the continuing geographic spread of these tick species, as well as the effects of climate change on vector-borne diseases, it is timely encourage research on diapause phenomena to improve understanding of their biology and of pathogen transmission dynamics. In our review we seek to clarify thinking on the topic and to address gaps in our knowledge that require the attention of researchers.
Keywords: Diapause, Quiescence, Ixodes, Ecology, Life cycles
1. Introduction
The Ixodes ricinus complex of ticks (Acari: Ixodidae), which comprises at least 15 species distributed globally, has become particularly well known because four species, I. ricinus and Ixodes persulcatus in Eurasia, Ixodes scapularis in eastern and midwestern North America, and Ixodes pacificus in western North America, transmit many pathogens of both medical and veterinary significance. These include the causal agents of Lyme borreliosis, Borrelia burgdorferi sensu lato, considered to be the commonest and most widely distributed vector-borne disease of humans in the temperate northern hemisphere (Stanek et al., 2012).
These four tick species are closely related and occupy similar niches in their respective ecosystems. They are three-host ticks having catholic feeding habits, with the adults occurring on medium-sized and large animals such as hedgehogs, hares, deer and domestic livestock, and the immature stages mainly parasitizing lizards, birds and small to medium-sized mammals, in addition to large animals (Fig. 1). Since they readily attach to and feed on humans they are significant bridging vectors of zoonotic agents. In the case of I. persulcatus, the adult females are the main vectors, whereas in the other three species all parasitic stages bite humans, particularly the nymphs. They are exophilic, so unlike most Ixodes spp., which seek their hosts in burrows and nests, they ambush passing hosts in the open. Both developing and host-seeking (questing) ticks are very vulnerable to desiccation, and therefore require microclimates where the relative humidity remains above 80% for prolonged periods (Gray, 1998). The ticks can quest for several months, but must replenish their reserves of water by frequent journeys from the surface vegetation down to the base, where they obtain water from sub-saturated air by secreting hygroscopic fluid produced by the salivary glands onto the external mouthparts and then re-ingesting the water-enriched fluid (Kahl and Knülle, 1988a). The length of time a tick might remain in this questing phase is determined by its host-finding success, the extent of its energy reserves available for water replenishment and local micro-climatic conditions. Questing by individual ticks can persist for several weeks or months in suitable habitat, and is stage-dependent with larvae questing for the shortest periods and adults for the longest.
Fig. 1.
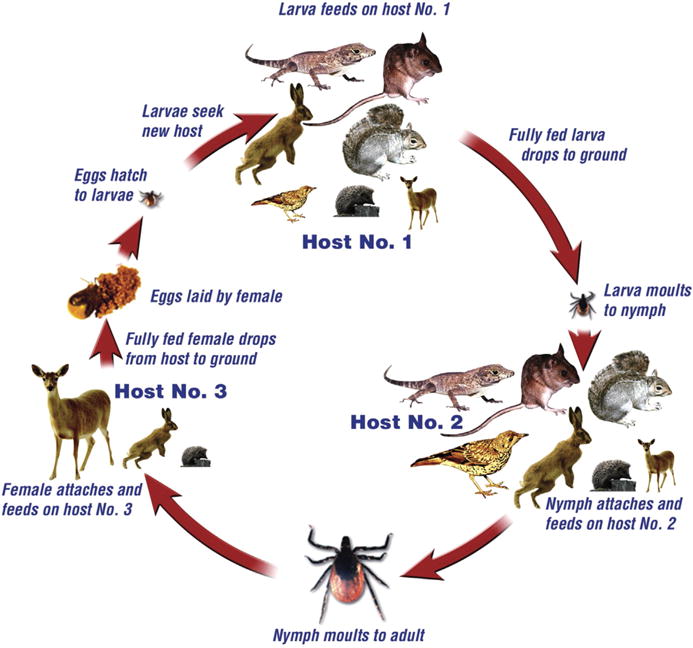
Ixodes ricinus species-complex life cycle. The four species considered in this review (I. pacificus, I. persulcatus, I. ricinus and I. scapularis) have similar life cycles and utilize the same range of hosts. Diapause can delay host-seeking following the moult, or delay development of one stage to the next.
2. Patterns of host-seeking behaviour
In all four species, host-seeking behaviour is distinctly seasonal, which is a major determinant of the relative risk of tick bite. The nature of this seasonality not only varies among tick species and life stages, but also according to the latitude in which they occur. In I. ricinus, the most studied species, peak questing of adult and nymphal ticks usually occurs in spring and early summer in northern and central Europe, followed by comparatively low-level activity in mid-summer, though questing may persist throughout the summer in cool and humid conditions, especially in relatively protected habitats. In many areas, a second, usually lesser, peak of activity occurs in autumn. Larval ticks typically begin questing slightly later than the adults and nymphs, appearing in large numbers in early summer and reaching their highest activity peaks in mid-summer prior to the second adult and nymphal peaks (Gray, 1991). In parts of southern Europe and the southeastern extremity of the tick’s range in Iran, the autumn/early winter peaks, especially of the adults, tend to predominate and questing may occur throughout the winter (Yousfi-Monod and Aeschlimann, 1986; Estrada-Peña et al., 2004; Vahedi-Noori et al., 2012; Dantas-Torres and Otranto, 2013). Notably, some historical questing records from southern Europe might have been compromised by the recent identification of a new species, I. inopinatus (Estrada-Peña et al., 2014), which hitherto was considered to be I. ricinus.
The seasonal activity of I. persulcatus varies significantly in different parts of its geographical range. The main difference from that of I. ricinus is that a second peak of adults in autumn, often seen in I. ricinus, is uncommon in I. persulcatus (Balashov, 1972; Korenberg, 2000). Adult I. persulcatus have a clearly defined spring-summer activity period with a single peak in May or early June. The first active I. persulcatus adults appear in late March (Korenberg, 2000); the lower threshold of their questing activity is approximately 1 °C (Kheisin et al., 1955). In most cases, adult activity ceases in July/early August, depending on the weather conditions (Korenberg, 2000; Babenko, 1985a). However under the maritime climate in the south of the Russian Far East, limited adult I. persulcatus activity may persist into early November (Babenko, 1985a). Larvae capable of infesting animal hosts usually emerge in May, but in the maritime regions this sometimes occurs in late April (Babenko, 1985a). The earliest collections of unfed larvae by flagging from vegetation have also been reported to be May (Babenko, 1985a; Levin, 1987). Although each individual larva can survive for only a few days after becoming active, they can be collected from both hosts and vegetation until September/October due to asynchronous activation (Babenko, 1985a; Levin, 1987; Korenberg, 2000). The first seasonal peak of larval abundance occurs in June, and the second smaller peak, when present, in late August or September. The seasonal dynamics of nymphal I. persulcatus, assessed by flagging from vegetation and by collecting from a wide range of vertebrate hosts, suggest that nymphs are usually active from April/May until late summer, with the peak of seasonal activity recorded in early or late summer depending on the climatic conditions (Levin, 1984; Babenko, 1985b). In the Baltic region, a second peak may be present in the autumn when nymphs of the new generation, moulting from larvae fed in the spring, complete post-moulting development in the same season. This second peak is always much smaller and shorter in duration than the first one.
The seasonal activity of the Nearctic species, I. scapularis, differs in certain respects from that of I. ricinus and varies both latitudinally and longitudinally. In northeastern USA, the autumn adult peak tends to be larger than the spring peak, with nymphal activity in spring and early summer, and larval activity in mid-summer (Spielman et al., 1985). In the northcentral region, the relative sizes of the autumn and spring peaks may be more comparable, and the nymphal and larval activity overlap in late spring and early summer (Gatewood et al., 2009; Hamer et al., 2012; Tsao, unpublished data). In southern areas, just as in southern Europe for I. ricinus, autumnal and winter tick activity is predominant for adults (Harris, 1959; Cilek and Olson, 2000; Mackay and Foil, 2005) with larvae and nymphs active throughout the spring and summer (Kollars et al., 1999; Clark et al., 1998; Tsao, unpublished data).
In the other Nearctic species, I. pacificus, the activity of northern Californian populations resembles that of northeastern populations of I. scapularis, with adult activity from late autumn to early spring, nymphs peaking in mid-spring, and larvae peaking in spring to early summer (Westrom et al., 1985; Lane and Stubbs, 1990; Clover and Lane, 1995; Eisen et al., 2001; Salkeld et al., 2014). In southern California, the temporal abundance and seasonality of I. pacificus adults in the Santa Monica Mountains are similar to those reported for some northern Californian populations (Lane et al., 2013). Limited sampling by dragging litter or removal from lizards in the same general study area revealed that the nymphs are active from early March to late May and the larvae in April and May. MacDonald and Briggs (2016) reported that the seasonal activity periods of immature stages are highly truncated in southern California compared to those in the north of the state, and suggested that this brief window for horizontally transmitted pathogens has important implications for pathogen transmission dynamics. The situation in more northerly areas (Oregon, Washington, British Columbia) is unclear because few systematic studies have been conducted, but it seems likely that the adults are most active in spring and autumn, the nymphs in spring and early summer, and the larvae in mid-summer (Arthur and Snow, 1968). Thus, superficially northern I. pacificus seasonal activity resembles that of I. ricinus in central and northern Europe.
To summarise, these four closely related Ixodes species exhibit very similar biologies and seasonalities, despite occupying widely separated geographical areas, spanning several degrees of latitude, and display similar patterns of host-seeking seasonality, though these differ in some minor respects. Reported differences could be caused by several factors, including intrinsic biological differences and differences in habitats, but also lack of suitable comparative data, so that in some cases any disparity may be more apparent than real. If the underlying mechanisms determining seasonality could be clarified, a level of predictability in risk should be attainable, which would inform practical preventive measures against the tick-borne pathogens they transmit.
3. Explanations of seasonal activity patterns
Several theories have been advanced to explain patterns of seasonal questing activity in I. ricinus-complex ticks. At one time it was suggested that the timing of development and questing activity of I. ricinus was driven entirely by temperature. Bimodal activity was explained as the result of two successive broods in the same year (Milne, 1945). Such interpretations were based largely on the assumption that activity peaks of different stages reflect successive development, i.e. larva to nymph to adult. However, when the development of engorged ticks was followed in the field in several geographical areas, it was discovered that successive peaks are not necessarily related to each other and that the life cycle is rarely completed in less than two years, usually three, and may take as long as six (Babenko, 1956; Chmela, 1969; Bauch, 1972; Gray, 1982; Shashina, 1985; Kahl, 1989; Estrada-Peña et al., 2004; Korotkov, 2008).
There is now general agreement that the mechanisms regulating I. ricinus seasonal activity are determined by a biological strategy in which ticks avoid questing at unfavourable times of the year, such as mid-summer when the temperature is too high and humidity too low for survival, or winter when temperatures are too low for efficient questing activity or for development of engorged stages. There is no doubt that to a certain extent the tick maximizes survival by responding to ambient conditions. Thus, MacLeod (1935) reported that I. ricinus ceases questing when temperatures exceed 35 °C, ticks avoid direct sunshine (Babenko, 1974; Kahl and Knülle, 1988b), and activity is correlated with saturation deficit (Perret et al., 2000). Temperature, degree and amount of sunshine, rainfall and relative humidity have been correlated either negatively or positively with questing behaviour of Ixodes spp. in several studies (Zemskaya 1984; Goddard, 1992; Mejlon, 1997; Vail and Smith, 1998; Eisen et al., 2002; Hubálek et al., 2003; Lane et al., 2007; Kiewra et al., 2014; Berger et al., 2014; Daniel et al., 2015), but the importance of these factors vary in different habitats and are mainly relevant in that they impact the water balance and energy depletion of the tick, and thus its survival. Whereas responses to ambient conditions can best be described as tactical, anticipation of unfavourable conditions well in advance of predictable seasonal climate changes is more of a strategic response, and the mechanisms involved are various forms of diapause that are incorporated into the developmental biology of these tick species. Diapause mechanisms therefore have a role in determining the fundamental phenology of a tick species. Activity peaks of a particular stage may consist of individuals of different generational cohorts, so observations of the seasonal occurrence of larvae, nymphs and adults on vegetation or hosts, as illustrated in Fig. 2A, a hypothetical representation of seasonal activity within one year, cannot determine their origin. The data required for such a determination can only be acquired when the development of groups of ticks of each life stage, deposited at intervals in the vegetation, is followed under quasi-natural conditions. The origin of the peaks displayed in Fig. 2A, and the role of diapause in regulating the timing of their appearance, are illustrated in Fig. 2B. Ticks feeding before the critical photoperiod moult in the same year and overwinter as unfed ticks, possibly in behavioural diapause; those feeding thereafter enter a developmental diapause, overwinter in the engorged state, and moult only in the summer of the following year. Note that Fig. 2 does not illustrate the life cycle of I. ricinus, but is a hypothetical representation of how these two developmental groups relate to each other in regions where significant autumn activity occurs. In other parts of the distribution range of I. ricinus, where little autumn activity is evident, I. ricinus behaves more like I. persulcatus, in which activity is postponed until the following spring. The two developmental groups are flexible and some interchange of individuals between them always occurs, depending on whether ticks feed before or after the critical seasonal point of diapause induction. The life cycle may therefore be significantly lengthened when ticks enter diapause or shortened when they feed prior to this period.
Fig. 2.
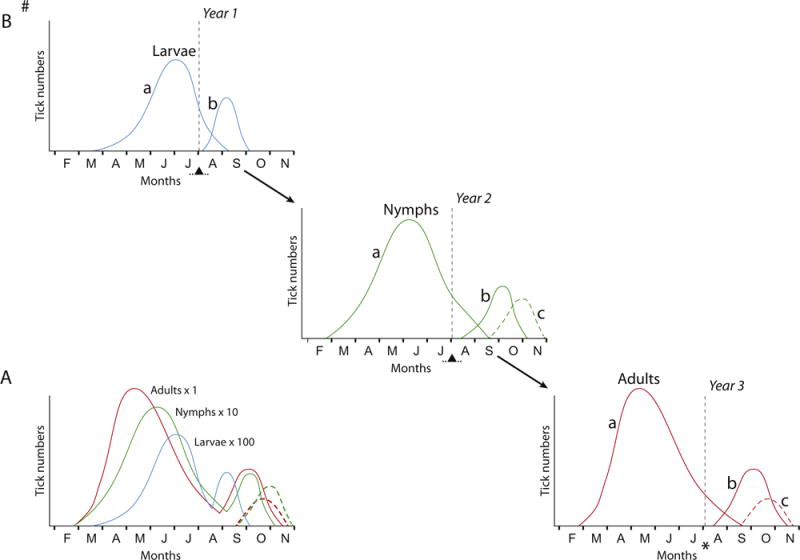
Stylized Ixodes ricinus questing activity in deciduous woodland (central and western Europe) in an area where appreciable autumn activity occurs, depicting the relationships of seasonal larval (L), nymphal (N) and adult (A) peaks, and the critical diapause period (▲).
(A) Seasonal activity peaks of the three active stages (larva, nymph, adult) as observed within one year.
(B) Chronological representation of diapause regulation of seasonal activity, showing the origin of the activity peaks of the different stages
# Although this diagram may appear to illustrate a 3–year cycle, in reality the flexibility of I. ricinus development and the influence of diapause means that 2–6 years may be taken to complete the life cycle.
a Overwintering as unfed stages or non-diapausing eggs, most nymphs and adults in Behavioural Diapause.
b Overwintering as fed stages or eggs in Developmental Diapause.
In this scenario the majority of ticks in a particular category, a or b, appear as the next life cycle stage in the same season a year later, regulated by developmental or behavioural diapause. However, considerable variation on this basic pattern can occur.
c Derived from the previous life stage that fed in spring/summer of the same year.
…▲… Critical diapause period (indicating the approximate photoperiod threshold after which date the majority of ticks enter diapause).
* The precise timing of the critical diapause period for adults is uncertain; the diapause manifests as delayed embryogenesis in the egg, but the induction stimulus is perceived by the questing mother tick.
4. Nature of dormancy phenomena
Dormancy in biology is generally regarded as a state of minimal metabolic activity with cessation of growth and development, either as a reaction to adverse conditions or as part of an organism’s normal annual rhythm. Most authors further subdivide the phenomenon by distinguishing between quiescence, a state of torpidity, which is an immediate response to prevailing hazardous environmental conditions, usually low temperatures, and diapause, a period of hormonally-controlled arrested development. Diapause usually occurs seasonally before environmental conditions become unfavourable, and has a fixed latency period that must be completed before development can resume (Tauber et al., 1986). In contrast, quiescence ceases with the disappearance of adverse conditions. If, in some years, the length of the diapause period is insufficient to avoid unfavourable conditions, the organism may enter a post-diapause quiescence and will remain torpid until favourable conditions occur (Belozerov, 2009). Under field conditions it can be very difficult to determine precisely when such a transition from diapause to quiescence has occurred.
The study of tick diapause is in its infancy compared with insect diapause, but nevertheless there are many similarities between the two, including the use of similar terminology. Since, in the view of some authorities published studies of insect diapause contain many misstatements and misunderstandings (Kostál, 2006), it is hardly surprising that there is considerable confusion about tick diapause. Few reviews of tick diapause have been published, the most complete being those of Belozerov (1982, 2009) on both argasid and ixodid ticks, and another more focused review restricted to the prostriate (Ixodes spp.) ticks and to one form of diapause only, developmental diapause (Belozerov et al., 2002). Although pro viding much information, especially on environmental triggers of diapause initiation/termination, these reviews do not appear to have resulted in much clarity and understanding of how diapause phenomena regulate Ixodes spp. life cycles in the field.
Most authors distinguish two main types of tick diapause – developmental (morphogenetic), here including reproductive, and behavioural. Developmental diapause describes a cessation of the development of engorged ticks at a predictable time owing to the blocking of essential steps in the developmental process, which presumably are under hormonal control (Belozerov, 1982). This form of diapause may manifest either as delays in oviposition or in the development of eggs, engorged larvae or engorged nymphs. Behavioural diapause involves temporary suppression of host seeking and/or attachment by unfed ticks (Belozerov, 1982). In some of the literature this term is loosely applied to overwintering ticks that are not necessarily in true diapause, but may be in a temperature-regulated quiescence. It is impossible to distinguish between the two by simple observation under field conditions, especially with the added complication of post-diapause quiescence. Behavioural diapause may best be detected by attempting artificial stimulation of activity and feeding in individual specimens, or by extrapolation from laboratory experiments.
Genuine behavioural diapause is a well-documented phenomenon in metastriate ticks (Wilkinson, 1968; McEnroe, 1985; Addison and McLaughlin, 1988; Pegram and Banda, 1990; Fujimoto, 1995, 1998, 1999; Madder et al., 1999; Cabrera and Labruna, 2009), but is less well known in the prostriate ticks (Ixodes spp.).
Together, the two forms of diapause synchronize tick development and host-seeking activity with the seasons, so that vulnerable ticks are less exposed to the dangers of dehydration or freezing. In I. ricinus in central and northern Europe, diapause also ensures that the nymphal and adult moults occur during the warmest part of the year, which minimizes the length of the pharate phases and maximizes the rate of metabolic water gain by the ticks (Kahl, 1991).
However, since both forms of diapause cause delays in the life cycle, they tend to reduce reproductive fitness, and so are only brought into play when the nature of the seasonal climate threatens tick survival (Randolph, 2013).
5. Induction, maintenance and termination of diapause in ticks
Diapause occurs after ticks are exposed to an induction stimulus (i.e., conditioned), typically at a particular time of year. The primary stimulus for diapause induction appears to be photoperiod, with temperature having an important modifying influence in some cases. The threshold period or critical diapause period (defined by a critical photoperiod), after which more than 50% of ticks enter diapause, varies with latitude, life stage and probably the genetics of the local tick population.
One of the seminal studies examining the role of photoperiod as a regulating factor in ticks was undertaken in North America by Smith and Cole (1941) working on the seasonal activity of Dermacentor variabilis. By the 1960s, photoperiod had been implicated as a regulator of the metamorphosis of I. ricinus and I. persulcatus (Belozerov, 1964; Babenko and Platonova, 1965). Since then, physiological responses to photoperiod have been found to be widespread in ticks. In northern regions, a change from long to short days is necessary to induce both behavioural and developmental diapause. Through perception of this reliable environmental change, the ticks can anticipate the onset of winter, ensuring that oviposition and moulting occur at favourable summer temperatures. In some metastriate species, such as Rhipicephalus appendiculatus, a behavioural diapause occurs as a result of prior exposure to long-day signals, resulting in the avoidance of questing during mid-summer, the warmest time of the year (Madder et al., 1999). There is no evidence in northern regions for such a ‘summer diapause’ among the prostriate ticks (Ixodes spp.), which apparently utilize tactical behavioural responses, such as retreating to cool humid microclimates, to overcome the dehydration risks that are posed by mid-summer temperatures and high saturation deficits. However, life cycle studies on I. pacificus in northern California suggest that a behavioural diapause may occur in immature ticks to enable them to survive the hot dry summers and become active in late winter or early spring (Padgett and Lane, 2001). A similar situation may occur in southern races of I. ricinus, for example in North Africa and southern Italy, and in I. scapularis in southern USA.
The precise phases of the developmental cycle where ticks are sensitive to diapause induction stimuli have received some attention, but this topic requires further elucidation. According to Belozerov (1982), tick diapause is unlike diapause in many insects in that sensitivity to photoperiod occurs in some developmental phases throughout the development cycle (pre-feeding, feeding, post-feeding), but the precise nature of the response depends on the species and population involved. In early laboratory experiments on I. persulcatus, Babenko and Platonova (1965) demonstrated that in ticks of Far Eastern and Siberian origin, development of engorged larvae depends on the photoperiodic conditions both before and after feeding and moreover, the percentage of larvae developing with or without a diapause was affected by both the direction of the change and length of the period before the change (Babenko, 1969). For example, fed larvae kept in a long photoperiod for up to 10 days after engorgement and then transferred to a short photoperiod entered diapause, while most of those kept under long-day conditions for 15–20 days post-engorgement developed into nymphs without a delay, despite being transferred to a short photoperiod subsequently. Ixodes ricinus reportedly shows similar sensitivities to photoperiod in its larval and nymphal stages (Belozerov, 1964).
An informal survey of 18 laboratories in which one or more of the four Ixodes spp. under consideration are reared under artificial conditions, provided further evidence that ticks respond variably to diapause induction stimuli (Table 1). Nevertheless, a pattern emerges and it can be concluded that in these laboratories diapauses are avoided by exposing ticks to a transition of short-day to long-day photoperiods during one or more phases of development, and/or by the provision of high temperatures (21–24 °C). It should be noted, however, that high temperatures might eventually lead to reduced feeding rates and shortened life-expectancy. It is also possible that prolonged storage under continuous dark or light conditions may compromise the tick’s biological clock. Nevertheless, storage of nymphs and adults at 4 °C in the dark before feeding (i.e. simulating winter) appears to benefit colonies by improving both feeding and moulting rates.
Table 1.
Light and temperature conditions for laboratory rearing of Ixodes pacificus, I. persulcatus, I. ricinus and I. scapularis.
| Category (no. of labs) | Tick species | Feeding | Development | Storage |
|---|---|---|---|---|
| I. pacificus | LD, M-H T °C | LD, M-H T °C | LD (or dark for longterm-one lab), | |
| I. ricinus | ||||
| A (8) | I. scapularis | M-H T °C | ||
| B (3) | I. ricinus | LD, M-H T °C | LD, M-H T °C | SD/dark, L T °C |
| C (2) | I. ricinus | LD, M T °C | LD, M T °C | LD, M T °C |
| D (1) | I. persulcatus | LD, M T °C; adults in dark | LD, M T °C | nymphs/adults dark, L T °C; larvae LD, M T °C |
| I. ricinus | ||||
| I. scapularis | ||||
| E (1) | I. ricinus | LD, M T °C | SD, M T °C | SD, M T °C or dark, L T °C |
| F (1) | I. pacificus | SD, M T °C | LD, H T °C | nymphs/adults dark, L T °C; larvae LD, H T °C |
| I. persulcatus | ||||
| I. ricinus | ||||
| I. scapularis | ||||
| G (1) | I. persulcatus | SD, M T °C | SD, M T °C | dark, L T °C |
| H (1) | I. ricinus | SD, H T °C | SD, H T °C | SD, H T °C |
Long-day (LD) = >12 h light per 24-h period; Short-day (SD) = <12 h light/24-h period.
High (H) temperature (T °C) = >20 °C; medium (M) temperature = 10–20 °C; low (L) temperature = 4–9 °C.
Few studies have specifically addressed the factors responsible for terminating diapause in Ixodes spp. ticks, which may occur as a result of the cessation of diapause maintenance stimuli, exposure to particular diapause termination stimuli or even as an essentially spontaneous change over time from a diapausing to a non-diapausing state. The most likely situation is that all three models apply to some extent.
Belozerov (2009) lists several factors for the induction of developmental and behavioural diapause in I. ricinus, I. persulcatus and I. scapularis, primarily rising temperatures and increasing day-length, but how these interact with each other is uncertain. Fujimoto (2003), concluded that behavioural diapause in I. persulcatus could be terminated by exposure to a photoperiod of 12L–12D for 7 months, following rearing and maintenance at 16L–8D and 24 °C. However, this very high temperature makes it difficult to relate the study to natural conditions.
The gradual increase in the abundance of active I. ricinus and I. persulcatus in spring (Fig. 2) suggests that temperature changes, while not necessarily responsible for terminating behavioural diapause, have a major role in determining the readiness of the ticks to commence questing. Indeed, it is likely that a post-diapause quiescence occurs immediately following the termination of diapause, as discussed by Belozerov (2009), so that regardless of the role of photoperiod, the appearance of questing ticks in the spring becomes primarily temperature-driven. It is also probable that the threshold for activity, whether driven by temperature, photoperiod, or both, varies for individual ticks.
Little is known about the physiology of diapause in ticks, but Belozerov (1982) suggested that, as in insects, there are three main steps in the processing of seasonal information by ticks. Firstly, the perception of extrinsic signals such as photoperiod (or scotoperiod), secondly the accumulation of this information in the brain and thirdly the transformation of this information via neurosecretory cells into hormonal signals that block or initiate metamorphosis. It has been reported that the administration of ecdysone or juvenile hormone analogues can terminate diapause in argasids (Bassal and Roshdy, 1974) and metastriate ixodids (Sannasi and Subramoniam, 1972), but this model of the physiological mechanisms responsible for tick diapause remains speculative until further data on the topic can be obtained.
It is still uncertain whether tick diapause is induced by a physiological switch activated when a certain day-length threshold is reached, or whether the tick responds to a gradual change in day length. Most laboratory studies on tick diapause appear to have been conducted on the assumption that a specific day length by itself is enough to induce diapause (Belozerov, 1982). Although such experimental designs resulted in observable diapause effects, accumulation of sensory information due to gradual changes in day length may be more relevant in nature and, ideally, should be incorporated into the design of future laboratory experiments.
6. Intraspecific differences between populations
It is intuitive that, given the extensive geographical distribution of the four Ixodes spp. considered here, phenotypic differences would occur among populations, including responsiveness to diapause-conditioning stimuli. Although environmental conditions probably give rise to regional differences, genetically-determined responses may also differ, as demonstrated for behavioural diapause in Rhipicephalus appendiculatus (Madder et al., 1999). Studies on the population genetics of the four tick species considered here are in their infancy, but the identification of genetically distinct strains of ticks are necessary before phenotypic differences can be considered in context.
Although no population genetic studies appear to have been undertaken on I. persulcatus, there are interesting data on regional differences in diapause-induction dates for I. persulcatus larvae and nymphs. According to Korotkov (2009), these differences are due to adaptation of I. persulcatus populations to regional climatic conditions. In the European part of the distribution range (latitude 56–57°N), the critical diapause period for engorged larvae occurs between July 25 and August 2. In West Siberia (latitude 53–56°N), with its harsh continental climate, this date is between July 10 and July 29, an average of 8 days earlier than in central Russia. Conversely, the average critical diapause date for larval diapause in Far Eastern populations (latitude 43–49°N) is delayed until August 4–16, or by approximately 24 days, compared to the West Siberian populations. These data suggest that in eastern Europe 50% of engorged larvae enter diapause when the day length shortens to approximately 16.0–16.2 h; in Western Siberia to 16.0–16.5 h; and in Russian Far East to 14.0–14.5 h. The critical diapause date for larvae occurs earlier as the distance from the Pacific Ocean increases and the climate becomes harsher and more continental. These variations in diapause-induction dates may be caused by genetic adaptations of different populations of I. persulcatus to local conditions.
Similar, though limited, observations on I. ricinus were made by Belozerov (1966), who reported that the photoperiod reactions of northern (Leningrad, 59.6°N) and southern (Moldavia, 46.3°N) larval populations differed by a few hours. Regional differences between other I. ricinus populations also occur, as described by Estrada-Peña et al. (1996) for cuticular hydrocarbon variations, and Gilbert et al. (2014) for questing temperatures of ticks from north-east Scotland (56.5°N) compared with central France (45.5°N).
Population genetic studies have been conducted on I. pacificus (Kain et al. (1997) and I. scapularis (Qiu et al., 2002), and for both species it has been observed that northern ticks tend to quest from surface vegetation, whereas further south they quest beneath the vegetation (Lane et al., 2013; Arsnoe et al., 2015). In the case of I. scapularis, this difference cannot be explained by environmental conditions alone and probably has a genetic basis (Arsnoe et al., 2015). These north-south regional differences suggest that the relative susceptibility of tick populations to diapause-induction stimuli may also differ.
7. Diapause in Ixodes ricinus
There are more studies addressing diapause in I. ricinus than in any other tick species, mainly because of the large body of work by Belozerov (e.g., 1964; Belozerov (1966); Belozerov et al. (1966); 1967a,b; 1971; 1973), primarily involving ticks from the Leningrad/St. Petersburg area in Russia. The laboratory experiments described in these publications consisted of manipulating the photoperiod and/or temperature during various phases of the tick life cycle, mainly the post-engorgement development phase, but also before and during feeding. Unfortunately, these remain the only laboratory-based studies of diapause phenomena in I. ricinus, and since they have almost all appeared in the Russian literature, many important details are difficult to access. Their complex design was apparently intended to address the mechanisms of diapause, and Belozerov (1998) concluded from his experiments that a two-step process occurred, involving separate responses to long- and short-day photoperiods. Furthermore, ticks apparently remain sensitive to these photoperiods throughout their life cycle including both questing and engorged stages. Laboratory studies are undoubtedly very useful, but highly relevant data can also be obtained from studies of tick development under natural conditions. The deposition of tubes containing engorged specimens within the natural habitat has furnished us with such data for I. ricinus (Campbell, 1948; Chmela, 1969; Bauch, 1972; Gray, 1982; Kahl, 1989; Estrada-Peña et al., 2004) including in some cases, the onset of activity (Gray, 1982). Additional indirect evidence for the role and importance of diapause has been obtained from several modelling studies of the I. ricinus life cycle, in which the best theoretical fit with the observed data occurred when both developmental and behavioural diapauses were built into the models (Gardiner and Gray, 1986; Walker, 2001; Dobson et al., 2011; Hancock et al., 2011).
7.1. Developmental diapause
The many laboratory studies on developmental diapause in Ixodes spp. (summarized in Belozerov et al., 2002) clearly demonstrated its existence in I. ricinus larvae and nymphs and there is some evidence that an egg diapause occurs (Belozerov, 1973; Gray, 1982). However, a pre-ovipositional diapause, which features in the life cycles of many metastriate ticks and some other Ixodes spp. (see below), does not seem to occur in I. ricinus (Belozerov et al., 1966; Belozerov, 1973; Belozerov, 1982; Gray, 1981, 1982).
Belozerov’s experiments suggest that if larval and nymphal ticks are exposed to increasing hours of light from the unfed phase through feeding and into the post-engorgement phase, they will develop without any developmental diapause. On the other hand, if exposed to decreasing hours of light in the laboratory, diapause will ensue and development will not commence in the engorged specimens for at least 90 days. The same phenomenon occurs in adult ticks, but although the diapause-induction stimulus is perceived by the unfed female, the developmental diapause manifests as delayed embryogenesis within the eggs, rather than delayed oogenesis (Belozerov, 1973).
Although photoperiod is regarded as the primary environmental signal that induces developmental diapause, tick age and ambient temperature have important modifying effects. For example, it was found that the older the larvae are at the time of feeding, the greater the proportion that enter developmental diapause (Belozerov, 1967a). In the same paper Belozerov reported that when larvae were maintained under short-day conditions, which would be expected to induce developmental diapause in the following post-engorgement phase, temperatures above 25 °C before feeding prevented subsequent diapause in young larvae (<1 month after hatching). High temperatures may also terminate developmental diapause in larvae under laboratory conditions (Campbell, 1948). Temperature effects (accelerated development of eggs and high temperature termination of diapausing engorged larvae) were postulated to affect tick-seasonal dynamics in Ireland for two years following the very hot year of 1976 (Gray, 1984; Gardiner and Gray, 1986). Interestingly, Dautel and Knülle (2009) reported that in the laboratory, continuous cold temperatures such as 4 °C for at least 6 weeks, or −10 and −20 °C for 24 h, completely eliminated egg diapause which suggests that in regions that experience harsh winters, few larvae are likely to join the diapausing part of the population, because most of them will probably become active before the diapause threshold period in late summer.
7.2. Behavioural diapause
Nearly 70 years ago, it was noted that in Scotland, ticks that moult in late summer from spring and early-summer feeding ticks, were reluctant to become active and feed, a manifestation of behavioural diapause, and usually overwintered as unfed stages before becoming active in the spring (Campbell, 1948). However, I. ricinus nymphs have been reported questing in mild weather in winter months (Dautel et al., 2008) and some researchers view winter dormancy as merely a form of temperature-controlled quiescence. Such a quiescence probably applies to those individuals that feed as larvae and nymphs in the autumn of the previous year, overwinter as diapausing engorged stages, moult in late summer and become active in early autumn, without entering a behavioural diapause (Gray, 1982). Their numbers are usually small compared with nymphs and adults that are active only in spring and early summer, but this can depend on the relative density of hosts in spring compared with autumn (Gray, 1984). However, in many parts of the I. ricinus distribution range, almost no autumn activity occurs, despite the availability, by late summer, of moulted nymphs and adults derived from the developmental diapause cohort. These ticks are presumably in a form of behavioural diapause, but so far this has not been investigated.
Despite the necessity for the inclusion of behavioural diapause in the explanatory models of Walker (2001), Hancock et al. (2011) and Dobson et al. (2011), few controlled experiments have been conducted to demonstrate its existence. However, there is good evidence for behavioural diapause in nymphs from a Russian (Leningrad/St. Petersburg) population (Belozerov, 1971). In that study, recently moulted nymphs reared in a short-day regime were exposed to long-day (20 h light) or short-day (12 h light) conditions and after several months were tested for attachment rates on laboratory mice. It was found that ticks maintained as unfed nymphs in long-day conditions fed 3–10 times more readily than those maintained under short-day conditions. Switching ticks in diapause from short-day to long-day regimens could terminate diapause and raising the temperature to 25 °C could produce the same effect, demonstrating the influence of an extrinsic factor other than photoperiod. However, if switched from a long-day to a short-day regimen, the nymphs did not become less aggressive, showing that once the ticks become active they cannot be induced to enter behavioural diapause. This is in contrast to the metastriate tick, Dermacentor marginatus, in which repeated induction of behavioural diapause in unfed adults is possible (Belozerov, 1967b). A less strongly developed behavioural diapause was demonstrated in nymphs in Ireland (Gray, unpublished) where unfed nymphs maintained at 4 °C in the dark for three months (i.e. overwintered), all became active, when exposed to long-day conditions compared with less than two-thirds of the nymphs maintained previously at room temperature in long-day conditions (i.e. not overwintered). These latter nymphs had attachment rates of only 27% on gerbils compared with 65% for the ‘overwintered’ group.
The only relevant published study on adults was incidental to an investigation into the effects of off-host mating on questing activity (Gray, 1987). Two groups of laboratory-reared unfed females, mated and unmated, were tested for activity in vertical tubes placed in the natural habitat in early autumn, following maintenance at room temperature and a long-day photoperiod. These conditions were designed to simulate a cohort that had fed in the spring and moulted in late summer and were therefore expected to exhibit behavioural diapause. The majority (63%) of the unmated ticks remained inactive, demonstrating the expected behavioural diapause, albeit at a modest rate. However, all the mated females became active, suggesting that behavioural diapause can be eliminated by a change in tick physiological condition.
It is uncertain whether a true behavioural diapause occurs in I. ricinus larvae. Belozerov (1966) maintained that there is no evidence for a photoperiod-regulated behavioural diapause, though this is contradicted in tables published later (Belozerov, 1982, 2009). However, no experimental evidence appears to have been published. The lack of larval activity in late autumn and early winter may be mainly due to the onset of low temperatures combined with the need for recently hatched larvae to consume the residual lipids from the egg before becoming active. In spring, the slight delay in larval activity relative to nymphs and adults in Ireland (Gray, 1982) and the UK (Randolph et al., 2002) is thought to result from the larval requirement for slightly higher approximate threshold temperatures for the commencement of activity (10 °C) compared with nymphs and adults (7 °C) (Randolph, 2004).
To summarise, there is strong evidence for developmental and behavioural diapauses in I. ricinus, the former affecting eggs, larvae and nymphs, and the latter nymphs and adults. Both forms of diapause are induced when ticks are exposed to decreasing day length. Thus, engorged immature ticks overwinter without further development until early summer, and ticks that moult in the second half of the year, when days are shortening, are relatively reluctant to quest and feed before winter, though some individuals are evidently capable of becoming active. Studies on behavioural diapause are scarce and this topic needs further investigation, particularly in an experimental laboratory setting. Both forms of diapause can be influenced by elevated temperatures, but more data on this interaction are also required.
8. Diapause in Ixodes persulcatus
8.1. Developmental diapause
Systematic studies on diapause in this tick species began with the work of Babenko (1956) and was followed by many laboratory-and field-based experiments on ticks from different parts of the geographical range of this species. Much information regarding the dynamics of development of engorged ticks under natural conditions and the ability of different life stages to survive through the winter has been generated by depositing ticks in containers in various tick habitats (Babenko and Rubina, 1968; Babenko, 1985b; Korotkov and Kislenko, 1991). This approach established that an overwinter delay in development of engorged larvae and nymphs is typical for I. persulcatus throughout its range (Kheisin et al., 1955; Babenko, 1956; Babenko and Rubina, 1968; Zhmaeva, 1969). Moulting was still delayed for several months when late-engorged immature ticks were exposed to temperatures as high as 16–23 °C in the laboratory (Kheisin et al., 1955).
Laboratory experiments on I. persulcatus larvae demonstrated that the development of engorged larvae depends on the photoperiodic conditions of their maintenance. All larvae held under a long photophase (≥18 h) both before and after feeding moulted to nymphs without a diapause. Conversely, when exposed to a short photophase (6 h), 95% larvae showed delayed development (Babenko and Platonova, 1965). Similarly, I. persulcatus nymphs also developed without diapause under long-day conditions, but showed a developmental diapause when exposed to a short-day photoperiod during and after engorgement (Fujimoto, 1993; Belozerov, 1995).
Neither engorged females nor eggs of I. persulcatus are capable of a diapause, and all invariably die during the winter (Babenko and Rubina, 1968; Kachanko, 1978). Therefore, engorged females must oviposit early enough for the eggs to hatch within the same season. This notable difference from I. ricinus is interpreted as an adaptation of I. persulcatus to harsher climatic conditions and shorter warm seasons in the taiga region (Belozerov, 1981).
8.2. Behavioural diapause
In Russia, behavioural diapause in I. persulcatus prevents most adult and nymphal ticks from feeding in late autumn (as observed for some populations of I. ricinus – see above), so that they do not become active until the following spring (Belozerov, 1985). Similar observations were made in a Japanese population of I. persulcatus in which it was confirmed that behavioural diapause is triggered by a transition from long-day to short-day photoperiods in both nymphs (Fujimoto, 2002) and adults (Fujimoto, 2001, 2003). This overwintering of unfed adults in behavioural diapause correlates with the inability of either engorged females or eggs to undergo developmental diapause.
The existence of behavioural diapause in I. persulcatus larvae is debatable. Larval behavioural diapause is referred to by Belozerov (1985), but experimental evidence is lacking, and behavioural diapause is not listed in a table presented by Belozerov (1982), but is listed in Belozerov (2009). The absence of larval activity in late autumn and early winter may be explained by the onset of low temperatures, as in I. ricinus, but experimental investigations are required.
I. persulcatus behavioural diapause in adults is probably obligatory under natural conditions (Belozerov, 1985) and may be more strongly developed than in I. ricinus (Balashov, 1972). Not only do adults that have fed as nymphs in the spring and summer of the same year enter a behavioural diapause, but so do those that overwintered in diapause as engorged nymphs the previous winter. In contrast, in Ireland most I. ricinus adults developed from such nymphs start questing within two or three weeks of moulting in the autumn (Gray, 1982). The well-developed behavioural diapauses of nymphal and adult I. persulcatus appear to be adaptations to the harsh winters characteristic of the geographical range of this tick species.
9. Diapause in Ixodes scapularis
The first authors to refer to diapause in I. scapularis (then known as I. dammini) were Yuval and Spielman (1990). They placed engorged stages collected from mice (Peromyscus leucopus) or deer (Odocoileus virginianus) in nylon mesh tubes that were partially buried in litter, and then made observations on development times and mortality. They suggested that a developmental diapause occurred in nymphs fed in August or later in the season and in larvae fed in September or later, and that a pre-ovipositional diapause occurred in adults that fed during the autumn and which did not start laying eggs until June of the following year. A behavioural diapause in nymphs was reported to extend from their moult in summer to May of the following year, and adults that moulted from nymphs in June/July apparently exhibited a short behavioural diapause until September of the same year. According to these studies, therefore, I. scapularis in Massachusetts differs from I. ricinus in Europe in that it shows no egg diapause, but unlike I. ricinus undergoes a pre-oviposition diapause, which seemed to be confirmed in similar field studies by Daniels et al. (1996) in New York State. Pre-ovipositional diapause is also evident further north in Ontario, Canada, according to the observations of Lindsay et al. (1998). Additionally, their data suggest larval and nymphal developmental diapauses, a nymphal behavioural diapause and possibly an egg diapause, not previously observed in I. scapularis. The observed behavioural diapause of I. scapularis nymphs seems to resemble that of I. ricinus, but the very short behavioural diapause in adults reported by Yuval and Spielman (1990) (if it is indeed a classic diapause) does not prevent questing in the autumn of the year that they moulted–unlike I. persulcatus and to some extent, I. ricinus.
Some of these field observations may be explicable by responses to ambient temperatures rather than diapause phenomena. However, Kahl (1989) observed developmental diapause in larvae and nymphs of I. scapularis (I. dammini) from Massachusetts, USA, at 90% RH and 15 and 20 °C in the laboratory, and Belozerov and Naumov (2002) reported developmental diapause in I. scapularis nymphs in laboratory studies of a mid-Atlantic population. They concluded that, as in I. ricinus, a two-step response to first long-day and then short-day conditions were required. According to Yuval and Spielman (1990), the importance of nymphal I. scapularis developmental diapause in nature is questionable because the overwintering survival of diapausing individuals was very poor and pre-empted by behavioural diapause in the nymphs. Nevertheless, Ogden et al. (2004, 2005), who constructed a temperature-based development model using laboratory data and also field data from Ontario, concluded that development of engorged I. scapularis nymphs to adults is regulated to a considerable extent by temperature-independent diapause phenomena.
It is evident that similar controlled laboratory studies to those of Belozerov and Naumov (2002), as well as further field studies, are necessary to confirm the existence and importance of developmental and other diapause phenomena in this tick species.
10. Diapause in Ixodes pacificus
Ixodes pacificus is distributed over a considerable range of latitudes, extending along the west coast of North America from Baja California north to British Columbia; disjunct populations have been detected in Oregon, Nevada, Utah and Arizona (Peavey and Lane, 1996; Kain et al., 1997). Photoperiod and climate might therefore be expected to significantly impact its life cycle, though the biology of this tick has been poorly studied except in California (Furman and Loomis, 1984; Peavey and Lane, 1996; Padgett and Lane, 2001). Peavey and Lane (1996) constructed a degree-day model for oviposition and hatching based on data obtained from temperature regimes in the laboratory, and then compared predicted development times with those observed during field observations of confined engorged females placed within suitable habitat in northern California. The model predicted the onset of oviposition accurately, but not that of hatching, which occurred approximately a month later than predicted. These observations suggest an absence of a pre-oviposition diapause, and the existence of an egg diapause, though this was manifest as only a one-month delay in hatching. However, the authors suggested that either inherent limitations of the model or the influence of low humidity on egg development were the probable causes of the discrepancies in egg hatch. They drew attention to the possible influence of photoperiod, but this variable was not built into the experimental design.
In a similar study in northern California, Padgett and Lane (2001) fed all stages of both colony and wild-caught ticks on rabbits (adults) or western fence lizards (larvae and nymphs), and then deposited the engorged stages within silk packets in the habitat at times of the year that corresponded with observed tick feeding in nature. No pre-oviposition or developmental diapauses were observed, but larvae and nymphs appeared to exhibit a distinct behavioural diapause. The absence of developmental diapauses presumably reflects the fact that overwintering of developing stages in this region is not particularly hazardous because temperatures are high enough to permit development. The behavioural diapauses in larvae and nymphs are a summer-survival strategy since they prevent unfed ticks from questing during the hottest and driest time of year.
Although mentioned in both papers considered here, photoperiod was not investigated as a possible diapause-inducing factor in either study and, clearly, the effects of photoperiod on tick development and activity under controlled conditions are required to separate genuine diapause mechanisms from tick responses to ambient conditions.
11. Comparison of diapause manifestations in I. ricinus, I. persulcatus, I. scapularis and I. pacificus
The occurrence in the four Ixodes spp. of developmental and behavioural diapauses supported by experimental data, is summarized in Table 2.
Table 2.
Occurrence of diapause in Ixodes pacificus, I. persulcatus, I. ricinus and I. scapularis.
| Tick species | Developmental diapause | Behavioural diapause |
|---|---|---|
| I. pacificus | unknown | L, N |
| I. persulcatus | L, N | N, A |
| I. ricinus | E, L, N | N, A |
| I. scapularis | N, Pre-ov | N |
E = egg, L = larva, N = nymph, A = adult, Pre-ov = pre-ovipositional.
Developmental diapause in I. ricinus, I. persulcatus and I. scapularis is a mechanism that enhances overwintering survival. In the two Palaearctic species, I. ricinus and I. persulcatus, developmental diapauses are evident in engorged larvae and nymphs. An egg diapause occurs in I. ricinus, but not in I. persulcatus. In I. scapularis, a Nearctic species, no egg diapause has been reported, but a pre-ovipositional diapause occurs. Although nymphal developmental diapause in the field was reported in I. scapularis by Yuval and Spielman (1990), they concluded that it may be of limited significance because of poor survival of diapausing engorged individuals, an interpretation that is apparently at odds with the observations of Kahl (1989) and Belozerov and Naumov (2002) that good survival occurred under laboratory conditions.
In the other Nearctic species under consideration, I. pacificus, the most complete studies have been conducted in northern California, where summer rather than winter is the major seasonal obstacle to completion of the life cycle. Developmental diapauses were not observed in this tick, which evidently uses behavioural diapause in the larval and nymphal stages to survive hot, dry summer conditions. No diapause studies have been conducted in more northerly populations of I. pacificus, so developmental diapauses may await discovery in this species. Similarly, southern populations of I. ricinus and I. scapularis exposed to very mild winters and hot summers have not been investigated for behavioural diapauses that may help unfed stages to survive the summer.
Behavioural diapause has been reported in all four Ixodes spp. In I. pacificus, it is an adaptation for summer survival, whereas in the other three species it is evidently an overwintering mechanism. The phenomenon is strongly developed in I. persulcatus; both nymphs and adults enter a behavioural diapause in late summer or early autumn that prevents them from becoming active until the following spring. The same is true for I. scapularis nymphs, which in northern regions typically commence activity in autumn, become inactive in winter and resume activity in spring. The situation in I. ricinus is both more complex and more confused. Distinct behavioural diapause of a varying proportion of individuals has been reported in both nymphs and adults, but some studies have reported its absence and have interpreted low activity levels in late autumn and winter as being due only to quiescence in response to low ambient temperatures. There are contradictory reports on larval behavioural diapause in I. ricinus and I. persulcatus. Although reported in both species (Belozerov, 2009), detailed published experimental data seem to be lacking.
12. Conclusions and future research
Diapause is evidently a widespread phenomenon in the life cycles of the tick species considered here. Clearly, it has an important role in determining their seasonal activity, and the consequent risk of infection and pathogen maintenance within tick populations. An understanding of the precise role that diapause plays in the life cycle of each species is particularly important in the context of global climate change which may affect the timing of critical diapause periods, thus influencing the activity, survival/longevity, and relative abundance of questing ticks (Gray, 2008). Intraspecific differences in responses to diapause induction and termination stimuli almost certainly exist, but have so far received little attention.
Although the four species are closely related and occupy similar niches in their respective ecosystems, there are marked differences among them in the occurrence and expression of both developmental and behavioural diapause. While such differences may provide insight into their life cycles, some may be more apparent than real because of the difficulty of genuine comparisons. Studies have been conducted in different geographical regions, often in widely differing latitudes with different seasonal climate characteristics, and the methods utilized have differed considerably. For example, some conclusions were based solely on controlled laboratory studies, lacking natural context and with ticks from just one geographical area, whereas others were based on field studies conducted under quasi-natural conditions. In the latter case, it can be difficult to distinguish diapause phenomena from responses of the ticks to ambient conditions. A combination of the two approaches seems best, but unfortunately substantial data are only available for I. ricinus, and even in this species there remain many unanswered questions.
There is a good case for researching all aspects of diapause in all four species of ticks considered here, but the most pressing diapause questions to be addressed are as follows:
I. ricinus
Controlled laboratory studies on behavioural diapause of all three active stages involving variable photoperiods and temperatures.
Quasi-natural studies involving observation of development and questing of confined ticks, especially in southern regions of its range.
I. persulcatus
Controlled laboratory studies on behavioural diapause of all three active stages involving variable photoperiods and temperatures.
Determination of presence or absence of egg diapause.
I. scapularis
Controlled laboratory studies on behavioural diapause of all three active stages involving variable photoperiods and temperatures.
Controlled laboratory studies on developmental diapause of engorged larvae and eggs.
Quasi-natural studies involving observation of development and questing of confined ticks in different geographical areas, particularly in southern regions of its range.
I. pacificus
Controlled laboratory studies on behavioural and possible developmental diapause of all three active stages involving variable photoperiods and temperatures.
Quasi-natural studies involving observation of development and questing of confined ticks in northern regions of its range.
All species
Laboratory investigations on the interaction between temperature and photoperiod in inducing and terminating diapause.
Tick-plot studies consisting of observations on activity of unconfined ticks deposited as engorged stages in small plots within the natural habitat (Dautel et al., 2008).
Comparisons under controlled conditions of genetic susceptibility to diapause of geographically distinct populations of each species, including transplant studies of different genetic types.
Acknowledgments
We are very grateful to the colleagues in 18 laboratories, who supplied information on their tick-rearing methods for use in Table 1. Thanks are also due to Bernard Kaye (University College Dublin) for his excellent work on the figures. JIT acknowledges Funding provided by the National Science Foundation (Emerging Infectious Disease Award EF-0914476).
References
- Addison EM, McLaughlin RF. Growth and development of winter tick, Dermacentor albipictus, on moose, Alces alces. J Parasitol. 1988;74:670–678. [PubMed] [Google Scholar]
- Arsnoe IM, Hickling GJ, Ginsberg HS, McElreath R, Tsao JI. Different populations of blacklegged tick nymphs exhibit differences in questing behavior that have implications for human Lyme disease risk. PLoS One. 2015;10(5):e0127450. doi: 10.1371/journal.pone.0127450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur DR, Snow KR. Ixodes pacificus Cooley and Kohls, 1943: its life-history and occurrence. Parasitology. 1968;58:893–906. doi: 10.1017/s0031182000069663. [DOI] [PubMed] [Google Scholar]
- Babenko LV, Platonova VF. On diapause of larvae of Ixodes ricinus L. and Ixodes persulcatus P. Sch. (Parasitiformes, Ixodidae) 1 Experimental data on the effect of the photoperiod on hungry and fed larvae. Med Parazitol Parazit Bolezn. 1965;34:69–73. Russian. [PubMed] [Google Scholar]
- Babenko LV, Rubina MA. Patterns of development of the taiga tick in the vicinity of Kremenchug station. In: Pospelova-Shtrom MV, Rashina MG, editors. Problems of Epidemiology of Tick-Borne Encephalitis and Biological Patterns in Its Natural Focus. Meditsina; Moscow: 1968. pp. 138–168. Russian. [Google Scholar]
- Babenko LV. Problem of seasonal variations and life of Ixodes ricinus L. and Ixodes persulcatus P. Sch. Med Parazitol Parazit Bolezn. 1956;25:346–352. Russian. [PubMed] [Google Scholar]
- Babenko LV. Diapause in larvae of Ixodes persulcatus (Parasitiformes: ixodidae) effect of some biotic and non-biotic factors on tick development. Proceedings of the 2d International Congress of Acarology; Budapest. 1969. pp. 447–453. [Google Scholar]
- Babenko LV. Diurnal variations in the activity of unfed nymphs: Ixodes ricinus and I. persulcatus. Med Parazitol Parazit Bolezn. 1974;43:520–527. Russian. [PubMed] [Google Scholar]
- Babenko LV. Seasonal dynamics of activity. In: Filippova NA, editor. Taiga Tick Ixodes persulcatus Schulze (Acarina, Ixodidae): Morphology, Systematics, Ecology, and Medical Significance. Nauka; Leningrad: 1985a. pp. 220–230. Russian. [Google Scholar]
- Babenko LV. Duration of development of engorged larvae and nymphs. In: Filippova NA, editor. Taiga Tick Ixodes persulcatus Schulze (Acarina, Ixodidae): Morphology, Systematics, Ecology, and Medical Significance. Nauka; Leningrad: 1985b. pp. 265–273. Russian. [Google Scholar]
- Balashov YS. Vectors of Diseases of Man and Animals. Medical Zoology Department USNMR Unit 3; Cairo, Egypt: 1972. Blood Sucking Ticks (Ixodoidea) [Google Scholar]
- Bassal TT, Roshdy MA. Argas (Persicargas) arboreus: juvenile hormone analog termination of diapause and oviposition control. Exp Parasitol. 1974;36:34–39. doi: 10.1016/0014-4894(74)90110-6. [DOI] [PubMed] [Google Scholar]
- Bauch RJ. Bionomy of Ixodes ricinus. II. Population and seasonal dynamics in several localities of the district of Magdeburg in the GDR. Angew Parasitol. 1972;13:141–154. German. [PubMed] [Google Scholar]
- Belozerov VN, Naumov RL. Nymphal diapause and its photoperiodic control in the tick Ixodes scapularis (Acari: Ixodidae) Folia Parasitol (Praha) 2002;49:314–318. doi: 10.14411/fp.2002.058. [DOI] [PubMed] [Google Scholar]
- Belozerov VN, Bogdanov VE, Kvitko NV. Seasonal changes in temperature reaction of engorged Ixodes ricinus L. (Ixodidae) females. Vest Leningr Univ S Biol. 1966;3:37–44. [Google Scholar]
- Belozerov VN, Fourie LJ, Kok DJ. Photoperiodic control of developmental diapause in nymphs of prostriate ixodid ticks (Acari: Ixodidae) Exp Appl Acarol. 2002;28:163–168. doi: 10.1023/a:1025377829119. [DOI] [PubMed] [Google Scholar]
- Belozerov VN. Larval diapause of the tick Ixodes ricinus L. and its dependence on external conditions. Zool Zhurn. 1964;43:1626–1637. [Google Scholar]
- Belozerov VN. Photoperiodic regulation of development and behavior of Ixodes ricinus L. larvae and nymphs from different populations and its changes owing to age of the ticks. Tezisy Dokl. 1. Akarol Soveshch. 1966:26–27. [Google Scholar]
- Belozerov VN. Larval diapause in the tick Ixodes ricinus L. and its relation to external conditions. IV. Interactions between exogenous and endogenous factors in the control of the larval diapause. Entomol Rev. 1967a;46:447–451. [Google Scholar]
- Belozerov VN. Dependence of aggressiveness in adult Dermacentor marginatus upon photoperiodic conditions. Parazitol Leningrad. 1967b;9:13–18. [Google Scholar]
- Belozerov VN. Nymphal diapause in the tick Ixodes ricinus. IV. Influence of changes in photoperiodic regime of unfed nymphs on their aggressiveness. Parazitologiia. 1971;5:3–6. [Google Scholar]
- Belozerov VN. Egg diapause in Ixodes ricinus and its relation to the photoperiodic conditions of maintenance of unfed females. Vest Leningr Skogos Univ Biol. 1973;9:33–37. Russian. [Google Scholar]
- Belozerov VN. Ecological rhythms in ixodid ticks and their regulation. Parazitol Sb (Leningrad) 1981;30:22–46. Russian. [Google Scholar]
- Belozerov VN. Diapause and biological rhythms in ticks. In: Obenchain FD, Galun R, editors. Physiology of Ticks. Pergamon Press; Oxford: 1982. pp. 469–500. [Google Scholar]
- Belozerov VN. Diapause, its role in the life cycle, mechanism. In: Filippova NA, editor. Taiga Tick Ixodes persulcatus Schulze (Acarina, Ixodidae): Morphology, Systematics, Ecology, and Medical Significance. Nauka; Leningrad: 1985. pp. 214–219. Russian. [Google Scholar]
- Belozerov VN. The photoperiodic regulation of the development and diapause of the nymphs of the taiga tick Ixodes persulcatus (Ixodidae) Parazitologiya. 1995;29:101–104. Russian. [PubMed] [Google Scholar]
- Belozerov VN. Participation of two-step photoperiodic reaction in control of development and diapause in nymphs of Ixodes persulcatus. Zool Zh. 1998;77:885–890. [Google Scholar]
- Belozerov VN. Diapause and quiescence as two main kinds of dormancy and their significance in life cycles of mites and ticks (Chelicerata: Arachnida: Acari). Part 2 Parasitiformes. Acarina. 2009;17:3–32. [Google Scholar]
- Berger KA, Ginsberg HS, Gonzalez L, Mather TN. Relative humidity and activity patterns of Ixodes scapularis (Acari: ixodidae) J Med Entomol. 2014;51:769–776. doi: 10.1603/me13186. [DOI] [PubMed] [Google Scholar]
- Cabrera RR, Labruna ML. Influence of photoperiod and temperature on the larval behavioral diapause of Amblyomma cajennense (Acari: ixodidae) J Med Entomol. 2009;46:1303–1309. doi: 10.1603/033.046.0608. [DOI] [PubMed] [Google Scholar]
- Campbell JA. PhD Thesis. University of Edinburgh; UK: 1948. The life history and development of the sheep tick Ixodes ricinus L. in Scotland under natural and controlled conditions. [Google Scholar]
- Chmela J. On the developmental cycle of the common tick (Ixodes ricinus L.) in the North Moravian natural focus of tick-borne encephalitis. Folia Parasitol (Praha) 1969;16:313–319. [Google Scholar]
- Cilek JE, Olson MA. Seasonal distribution and abundance of ticks (Acari: Ixodidae) in northwestern Florida. J Med Entomol. 2000;37:439–444. doi: 10.1093/jmedent/37.3.439. [DOI] [PubMed] [Google Scholar]
- Clark KL, Oliver JH, McKechnie DB, Williams DC. Distribution, abundance, and seasonal activities of ticks collected from rodents and vegetation in South Carolina. J Vector Ecol. 1998;23:89–105. [PubMed] [Google Scholar]
- Clover JR, Lane RS. Evidence implicating nymphal Ixodes pacificus (Acari: Ixodidae) in the epidemiology of Lyme disease in California. Am J Trop Med Hyg. 1995;53:237–240. doi: 10.4269/ajtmh.1995.53.237. [DOI] [PubMed] [Google Scholar]
- Daniel M, Malý M, Danielová V, Kříž B, Nuttall P. Abiotic predictors and annual seasonal dynamics of Ixodes ricinus, the major disease vector of Central Europe. Parasit Vectors. 2015;18(478) doi: 10.1186/s13071-015-1092-y. http://dx.doi.org/10.1186/s13071-015-1092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels TJ, Falco RC, Curran KL, Fish D. Timing of Ixodes scapularis (Acari: Ixodidae) oviposition and larval activity in southern New York. J Med Entomol. 1996;33:140–147. doi: 10.1093/jmedent/33.1.140. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F, Otranto D. Seasonal dynamics of Ixodes ricinus on ground level and higher vegetation in a preserved wooded area in southern Europe. Vet Parasitol. 2013;192:253–258. doi: 10.1016/j.vetpar.2012.09.034. [DOI] [PubMed] [Google Scholar]
- Dautel H, Dippel C, Kämmer D, Werkhausen A, Kahl O. Winter activity of Ixodes ricinus in a Berlin forest. Int J Med Microbiol. 2008;298(Suppl 1):50–54. [Google Scholar]
- Dautel H, Knülle W. Embryonic diapause and cold hardiness of Ixodes ricinus eggs (Acari: Ixodidae) In: Sabelis MW, Bruin J, editors. Trends in Acarology. 2009. pp. 327–331. [Google Scholar]
- Dobson ADM, Finnie TJR, Randolph SE. A modified matrix model to describe the seasonal population ecology of the European tick, Ixodes ricinus. J Appl Ecol. 2011;48:1017–1028. [Google Scholar]
- Eisen RJ, Eisen L, Lane RS. Prevalence and abundance of Ixodes pacificus immatures (Acari: Ixodidae) infesting western fence lizards (Sceloporus occidentalis) in northern California: temporal trends and environmental correlates. J Parasitol. 2001;87:1301–1307. doi: 10.1645/0022-3395(2001)087[1301:PAAOIP]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Eisen L, Eisen RJ, Lane RS. Seasonal activity patterns of Ixodes pacificus nymphs in relation to climatic conditions. Med Vet Entomol. 2002;16:235–244. doi: 10.1046/j.1365-2915.2002.00372.x. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A, Gray JS, Kahl O. Variability in cuticular hydrocarbons and phenotypic discrimination of Ixodes ricinus populations (Acarina: Ixodidae) from Europe. Exp Appl Acarol. 1996;20:457–467. [Google Scholar]
- Estrada-Peña A, Martinez JM, Sanchez Acedo C, Quilez J, Del Cacho E. Phenology of the tick, Ixodes ricinus, in its southern distribution range (central Spain) Med Vet Entomol. 2004;18:387–397. doi: 10.1111/j.0269-283X.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- Estrada-Peña A, Nava S, Petney T. Description of all the stages of Ixodes inopinatus n. sp. (Acari: Ixodidae) Ticks Tick Borne Dis. 2014;5:734–743. doi: 10.1016/j.ttbdis.2014.05.003. [DOI] [PubMed] [Google Scholar]
- Fujimoto K. Effect of photoperiod on the attachment and development of immature Ixodes persulcatus Schulze. Jpn J Sanit Zool. 1993;44:271–277. [Google Scholar]
- Fujimoto K. Effect of photoperiod on the host attachment and development in the tick: Haemaphysalis longicornis. Jpn J Sanit Zool. 1995;46:345–348. [Google Scholar]
- Fujimoto K. Effects of photoperiod on the host-feeding and development of Haemaphysalis flava nymphs (Acari: Ixodidae) Med Entomol Zool. 1998;49:235–238. [Google Scholar]
- Fujimoto K. The host-feeding activity of Haemaphysalis flava females observed under experimental conditions. Med Entomol Zool. 1999;50:57–59. [Google Scholar]
- Fujimoto K. A preliminary study on the termination of the behavioural diapause of Ixodes persulcatus adults (Acari: Ixodidae) Med Entomol Zool. 2001;52:73–75. [Google Scholar]
- Fujimoto K. Effect of exposure to short-day photoperiods on the host feeding activity of Ixodes persulcatus Schulze nymphs (Acari: Ixodidae) reared in a long-day photoperiod before and after molting. Med Entomol Zool. 2002;53:187–189. [Google Scholar]
- Fujimoto K. Preliminary studies on the termination of behavioral diapause of Ixodes persulcatus Schulze adults (Acari: Ixodidae): effects of the exposure to a medium-day photoperiod. Med Entomol Zool. 2003;54:305–307. [Google Scholar]
- Furman DP, Loomis EC. The ticks of California (Acari: Ixodida) Bull Calif Insect Surv. 1984;25:1–239. [Google Scholar]
- Gardiner WP, Gray JS. A computer simulation of the effects of specific environmental factors on the development of the sheep tick Ixodes ricinus L. Vet Parasitol. 1986;19:133–144. doi: 10.1016/0304-4017(86)90041-5. [DOI] [PubMed] [Google Scholar]
- Gatewood AG, Liebman KA, Vourc’h G, Bunikis J, Hamer SA, Cortinas R, Melton F, Cislo P, Kitron U, Tsao J, Barbour AG, Fish D, Diuk-Wasser MA. Climate and tick seasonality are predictors of Borrelia burgdorferi genotype distribution. Appl Environ Microbiol. 2009;75:2476–2483. doi: 10.1128/AEM.02633-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert L, Aungier J, Tomkins JL. Climate of origin affects tick (Ixodes ricinus) host-seeking behavior in response to temperature: implications for resilience to climate change? Ecol Evol. 2014;4:1186–1198. doi: 10.1002/ece3.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddard J. Ecological studies of adult Ixodes scapularis in central Mississippi: questing activity in relation to time of year, vegetation type, and meteorologic conditions. J Med Entomol. 1992;29:501–506. doi: 10.1093/jmedent/29.3.501. [DOI] [PubMed] [Google Scholar]
- Gray JS. The fecundity of Ixodes ricinus L. and the mortality of developmental stages under field conditions. Bull Entomol Res. 1981;71:533–542. [Google Scholar]
- Gray JS. The development and questing activity of Ixodes ricinus L. under field conditions in Ireland. Bull Entomol Res. 1982;72:263–270. [Google Scholar]
- Gray JS. Studies on the dynamics of active populations of the sheep tick Ixodes ricinus L. in Co. Wicklow, Ireland. Acarologia. 1984;25:167–178. [PubMed] [Google Scholar]
- Gray JS. Mating and behavioural diapause in Ixodes ricinus L. Exp Appl Acarol. 1987;3:61–71. doi: 10.1007/BF01200414. [DOI] [PubMed] [Google Scholar]
- Gray JS. The development and seasonal activity of the tick, Ixodes ricinus: a vector of Lyme borreliosis. Rev Med Vet Entomol. 1991;79:323–333. [Google Scholar]
- Gray JS. The ecology of ticks transmitting Lyme borreliosis. Exp Appl Acarol. 1998;22:249–258. [Google Scholar]
- Gray JS. Ixodes ricinus seasonal activity: implications of global warming indicated by revisiting tick and weather data. Int J Med Microbiol. 2008;298(Suppl 1):19–24. [Google Scholar]
- Hamer SA, Hickling GJ, Sidge JL, Walker ED, Tsao JI. Synchronous phenology of juvenile Ixodes scapularis vertebrate host relationships, and associated patterns of Borrelia burgdorferi ribotypes in the midwestern United States. Ticks Tick Borne Dis. 2012;3:65–74. doi: 10.1016/j.ttbdis.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Hancock PA, Brackley R, Palmer SC. Modelling the effect of temperature variation on the seasonal dynamics of Ixodes ricinus tick populations. Int J Parasitol. 2011;41:513–522. doi: 10.1016/j.ijpara.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Harris RL. Biology of the black-legged tick. J Kans Entomol Soc. 1959;32:61–68. [Google Scholar]
- Hubálek Z, Halouzka J, Juricová Z. Host-seeking activity of ixodid ticks in relation to weather variables. J Vector Ecol. 2003;28:159–165. [PubMed] [Google Scholar]
- Kachanko NI. Development of ixodid ticks in the northern limits of their geographic range in Amur Province. Parazitologiia. 1978;20:218–225. Russian. [PubMed] [Google Scholar]
- Kahl O, Knülle W. Water vapour uptake from subsaturated atmosphere by engorged immature ixodid ticks. Exp Appl Acarol. 1988a;4:73–88. doi: 10.1007/BF01213843. [DOI] [PubMed] [Google Scholar]
- Kahl O, Knülle W. Seasonal and diurnal activity pattern of Ixodes ricinus (Acari, Ixodidae) and its infection with Lyme spirochetes and tick-borne encephalitis (TBE) virus in Berlin (West) Mitt Dtsch Ges Allg Angew Entomol. 1988b;6:223–225. German. [Google Scholar]
- Kahl O. Dissertation. Free University of Berlin; Germany: 1989. Investigations on the water balance of ticks (Acari, Ixodoidea) in the course of their postembryonic development with special reference to active water vapour uptake in the engorged phases; p. 356. German. [Google Scholar]
- Kahl O. Water management of the non-parasitic phases of Ixodes ricinus in the course of its post-embryonic development. In: Dusábek F, Bukva V, editors. Modern Acarology. Vol. 2. Academia Prague and SPB Academic Publishing bv; The Hague: 1991. pp. 371–374. [Google Scholar]
- Kain DE, Sperling FAH, Lane RS. Population genetic structure of Ixodes pacificus (Acari: Ixodidae) using allozymes. J Med Entomol. 1997;34:441–450. doi: 10.1093/jmedent/34.4.441. [DOI] [PubMed] [Google Scholar]
- Kheisin EM, Pavlovskaya O, Malakhova RP, Rybak VF. Duration of the developmental cycle of Ixodes persulcatus under natural conditions of the Karelo-Finnish SSR. Proceedings of the Karelo-Finnish University. 1955;6:102–123. Russian. [Google Scholar]
- Kiewra D, Kryza M, Szymanowski M. Influence of selected meteorological variables on the questing activity of Ixodes ricinus ticks in Lower Silesia, SW Poland. J Vector Ecol. 2014;39:138–145. doi: 10.1111/j.1948-7134.2014.12080.x. [DOI] [PubMed] [Google Scholar]
- Kollars TM, Oliver JH, Kollars PG, Durden LA. Seasonal activity and host associations of Ixodes scapularis (Acari: Ixodidae) in southeastern Missouri. J Med Entomol. 1999;36:720–726. doi: 10.1093/jmedent/36.6.720. [DOI] [PubMed] [Google Scholar]
- Korenberg EI. Seasonal population dynamics of Ixodes ticks and tick-borne encephalitis virus. Exp Appl Acarol. 2000;24:665–681. doi: 10.1023/a:1010798518261. [DOI] [PubMed] [Google Scholar]
- Korotkov YS, Kislenko GS. The morphogenetic diapause of the taiga tick and the methods for its quantitative assessment in a field experiment. Parazitologiia (St Petersburg) 1991;25:494–503. Russian. [PubMed] [Google Scholar]
- Korotkov YS. Variability of the life cycle duration in the taiga tick from mixed coniferous-broad-leaved forests of the Udmurt Republic. Parazitologiia. 2008;42:264–270. Russian. [PubMed] [Google Scholar]
- Korotkov YS. Abstract of a Dissertation for the Degree of Dr Biol Sci Moscow. Chumakov Institute of Poliomyelitis & Viral Encephalitides; 2009. Ecology of the taiga ticks (Ixodes persulcatus Schulze, 1930) under conditions of climate change in Eurasia; p. 46. Russian. [Google Scholar]
- Kostál V. Eco-physiological phases of insect diapause. J Insect Physiol. 2006;52:113–127. doi: 10.1016/j.jinsphys.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Lane RS, Stubbs HA. Host-seeking behavior of adult Ixodes pacificus (Acari: Ixodidae) as determined by flagging vegetation. J Med Entomol. 1990;27:282–287. doi: 10.1093/jmedent/27.3.282. [DOI] [PubMed] [Google Scholar]
- Lane RS, Mun J, Peribáñez MA, Stubbs HA. Host-seeking behavior of Ixodes pacificus (Acari: Ixodidae) nymphs in relation to environmental parameters in dense-woodland and woodland-grass habitats. J Vector Ecol. 2007;32:342–357. doi: 10.3376/1081-1710(2007)32[342:hboipa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lane RS, Fedorova N, Kleinjan JE, Maxwell M. Eco-epidemiological factors contributing to the low risk of human exposure to ixodid tick-borne borreliae in southern California, USA. Ticks Tick Borne Dis. 2013;4:377–385. doi: 10.1016/j.ttbdis.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Levin ML. Characteristics of the spatial distribution of unfed taiga tick nymphs. In: Kucheruk VV, editor. Proceedings of XI All-Union Conference on Natural Focality of Diseases. Tyumen, USSR: 1984. pp. 94–96. Russian. [Google Scholar]
- Levin ML. Biology of the unfed larvae of the taiga tick Ixodes persulcatus under natural conditions. Zool Zh. 1987;66:348–359. Russian. [Google Scholar]
- Lindsay LR, Barker IK, Surgeoner GA, McEwen SA, Gillespie TJ, Addison EM. Survival and development of the different life stages of Ixodes scapularis (Acari: Ixodidae) held within four habitats on Long Point, Ontario, Canada. J Med Entomol. 1998;35:189–199. doi: 10.1093/jmedent/35.3.189. [DOI] [PubMed] [Google Scholar]
- MacDonald AJ, Briggs CJ. Truncated seasonal activity patterns of the western blacklegged tick (Ixodes pacificus) in central and southern California. Ticks Tick Borne Dis. 2016;7:234–242. doi: 10.1016/j.ttbdis.2015.10.016. [DOI] [PubMed] [Google Scholar]
- MacLeod J. Ixodes ricinus in relation to its physical environment: II. The factors governing survival and activity. Parasitology. 1935;27:123–144. [Google Scholar]
- Mackay A, Foil L. Seasonal and geographical distribution of adult Ixodes scapularis say (Acari: Ixodidae) in Louisiana. J Vector Ecol. 2005;30:168–170. [PubMed] [Google Scholar]
- Madder M, Speybroeck N, Brandt J, Berkvens D. Diapause induction in adults of three Rhipicephalus appendiculatus stocks. Exp Appl Acarol. 1999;23:961–968. doi: 10.1023/a:1006363316638. [DOI] [PubMed] [Google Scholar]
- McEnroe WD. Adaptions in the life cycle of Dermacentor variabilis (Say) and Ixodes dammini (Spielman, Clifford, Piesman, and Corwin) marginal populations (Acari: Ixodidae) Exp Appl Acarol. 1985;1:179–184. doi: 10.1007/BF01198514. [DOI] [PubMed] [Google Scholar]
- Mejlon HA. Diel activity of Ixodes ricinus (Acari:Ixodidae) at two locations near Stockholm. Sweden Exp Appl Acarol. 1997;21:247–255. doi: 10.1023/a:1018446921644. [DOI] [PubMed] [Google Scholar]
- Milne A. The ecology of the sheep tick, Ixodes ricinus L. The seasonal activity in Britain with particular reference to Northern England. Parasitology. 1945;36:141–152. [Google Scholar]
- Ogden NH, Lindsay LR, Beauchamp G, Charron D, Maarouf A, O’Callaghan CJ, Waltner-Toews D, Barker IK. Investigation of relationships between temperature and developmental rates of tick Ixodes scapularis (Acari: Ixodidae) in the laboratory and field. J Med Entomol. 2004;41:622–633. doi: 10.1603/0022-2585-41.4.622. [DOI] [PubMed] [Google Scholar]
- Ogden NH, Bigras-Poulin M, O’Callaghan CJ, Barker IK, Lindsay LR, Maarouf A, Smoyer-Tomic KE, Waltner-Toews D, Charron D. A dynamic population model to investigate effects of climate on geographic range and seasonality of the tick Ixodes scapularis. Int J Parasitol. 2005;35:375–839. doi: 10.1016/j.ijpara.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Padgett KA, Lane RS. Life cycle of Ixodes pacificus (Acari: Ixodidae): timing of developmental processes under field and laboratory conditions. J Med Entomol. 2001;38:684–693. doi: 10.1603/0022-2585-38.5.684. [DOI] [PubMed] [Google Scholar]
- Peavey CA, Lane RS. Field and laboratory studies on the timing of oviposition and hatching of the western black-legged tick, Ixodes pacificus (Acari: Ixodidae) Exp Appl Acarol. 1996;20:695–711. doi: 10.1007/BF00051555. [DOI] [PubMed] [Google Scholar]
- Pegram RG, Banda DS. Ecology and phenology of cattle ticks in Zambia: development and survival of free-living stages. Exp Appl Acarol. 1990;8:291–301. doi: 10.1007/BF01202139. [DOI] [PubMed] [Google Scholar]
- Perret JL, Guigoz E, Rais O, Gern L. Influence of saturation deficit and temperature on Ixodes ricinus tick questing activity in a Lyme borreliosis-endemic area (Switzerland) Parasitol Res. 2000;86:554–557. doi: 10.1007/s004360000209. [DOI] [PubMed] [Google Scholar]
- Qiu WG, Dykhuizen DE, Acosta MS, Luft BJ. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the Northeastern United States. Genetics. 2002;160:833–849. doi: 10.1093/genetics/160.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph SE, Green RM, Hoodless AN, Peacey MF. An empirical quantitative framework for the seasonal population dynamics of the tick Ixodes ricinus. Int J Parasitol. 2002;32:979–989. doi: 10.1016/s0020-7519(02)00030-9. [DOI] [PubMed] [Google Scholar]
- Randolph SE. Tick ecology: processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology. 2004;129:S37–S66. doi: 10.1017/s0031182004004925. [DOI] [PubMed] [Google Scholar]
- Randolph SE. Is expert opinion enough? A critical assessment of the evidence for potential impacts of climate change on tick-borne diseases. Anim Health Res Rev. 2013;14:133–137. doi: 10.1017/S1466252313000091. [DOI] [PubMed] [Google Scholar]
- Salkeld DJ, Castro MB, Bonilla D, Kjemtrup A, Kramer VL, Lane RS, Padgett KA. Seasonal activity patterns of the western black-legged tick, Ixodes pacificus, in relation to onset of human Lyme disease in northwestern California. Ticks Tick Borne Dis. 2014;5:790–796. doi: 10.1016/j.ttbdis.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Sannasi A, Subramoniam T. Hormonal rupture of larval diapause in the tick Rhipicephalus sanguineus (Lat) Experientia. 1972;28:666–667. doi: 10.1007/BF01944966. [DOI] [PubMed] [Google Scholar]
- Shashina NI. Total duration of life cycle (Russian) In: Filippova NA, editor. Taiga Tick Ixodes persulcatus Schulze (Acarina, Ixodidae): Morphology, Systematics, Ecology, and Medical Significance. Nauka; Leningrad: 1985. pp. 275–277. [Google Scholar]
- Smith CN, Cole MM. Effect of length of day on the activity and hibernation of the American dog tick: Dermacentor variabilis. Ann Ent Soc Am. 1941;34:426–431. [Google Scholar]
- Spielman A, Wilson ML, Levine JF, Piesman J. Ecology of Ixodes dammini-borne human babesiosis and Lyme disease. Annu Rev Entomol. 1985;30:439–460. doi: 10.1146/annurev.en.30.010185.002255. [DOI] [PubMed] [Google Scholar]
- Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379:461–473. doi: 10.1016/S0140-6736(11)60103-7. [DOI] [PubMed] [Google Scholar]
- Tauber MJ, Tauber CA, Masaki S. Seasonal Adaptations of Insects. Oxford University Press; New York: 1986. p. 411. [Google Scholar]
- Vahedi-Noori N, Rahbari S, Bokaei S. The seasonal activity of Ixodes ricinus tick in Amol, Mazandaran Province, Northern Iran. J Arthropod Borne Dis. 2012;6:129–135. [PMC free article] [PubMed] [Google Scholar]
- Vail SG, Smith G. Air temperature and relative humidity effects on behavioral activity of blacklegged tick (Acari: Ixodidae) nymphs in New Jersey. J Med Entomol. 1998;35:1025–1028. doi: 10.1093/jmedent/35.6.1025. [DOI] [PubMed] [Google Scholar]
- Walker AR. Age structure of a population of Ixodes ricinus (Acari: Ixodidae) in relation to its seasonal questing. Bull Entomol Res. 2001;91:69–78. [PubMed] [Google Scholar]
- Westrom DR, Lane RS, Anderson JR. Ixodes pacificus Ixodes pacificus (Acari: Ixodidae): population dynamics and distribution on Columbian blacktailed deer (Odocoileus hemionus columbianus) J Med Entomol. 1985;12:507–511. doi: 10.1093/jmedent/22.5.507. [DOI] [PubMed] [Google Scholar]
- Wilkinson PR. Phenology, behavior, and host-relations of Dermacentor andersoni Stiles in outdoor rodentaria and in nature. Can J Zool. 1968;46:677–689. doi: 10.1139/z68-095. [DOI] [PubMed] [Google Scholar]
- Yousfi-Monod R, Aeschlimann A. Studies on ticks (Acarina, Ixodidae), parasites of cattle in West Algeria. I. Systematic survey and seasonal activity. Ann Parasitol Hum Comp. 1986;61:341–358. doi: 10.1051/parasite/1986613341. French. [DOI] [PubMed] [Google Scholar]
- Yuval B, Spielman A. Duration and regulation of the developmental cycle of Ixodes dammini (Acari: Ixodidae) J Med Entomol. 1990;27:196–201. doi: 10.1093/jmedent/27.2.196. [DOI] [PubMed] [Google Scholar]
- Zemskaya AA. Seasonal activity of adult ticks Ixodes persulcatus P. Sch. in the eastern part of the Russian Plain. Folia Parasitol (Praha) 1984;31:260–276. [PubMed] [Google Scholar]
- Zhmaeva ZM. Tick-Borne Encephalitis in Udmurtia and Adjacent Regions. Udmurtia; Izhevsk: 1969. About development of Ixodes persulcatus P. Sch. in European south-taiga forests; pp. 118–141. Russian. [Google Scholar]


