Introduction
Diffuse intrinsic pontine glioma (DIPG) is the most common brainstem tumor in pediatric patients, accounting for approximately 80%.1 This tumor remains one of the most deadly pediatric brain tumors, with mean survival of less than twelve months.2 The diagnosis primarily relies on clinical symptoms and imaging findings, as surgery is not routinely performed. Radiotherapy remains the mainstay of therapy. Despite attempts at new forms of chemotherapy, the survival time has not improved over many years.
In this article we provide an overview of DIPG and describe the typical imaging findings with a focus on advanced imaging techniques. These techniques will be described and examples will be provided from DIPG patients seen at our institution. Advanced imaging techniques complement conventional magnetic resonance imaging (MRI) and help to define the metabolic and physiologic make-up of these tumors.
Clinical Presentation
Brainstem tumors represent only 12.4% of all primary tumors of the central nervous system in children ≤14 years, but are responsible for the greatest proportion of deaths at 37.9%.3 Various types of brainstem gliomas have been described but they can be separated into three main categories: DIPG, posterior exophytic/cervicomedullary gliomas, and focal tectal gliomas.4 The most common of these primary brainstem tumors are DIPG at 80%. DIPGs can occur in any age group but are most common in children, affecting approximately 200–300 children/year in the United States. The median age at diagnosis is 6 to 7 years5.
Patients usually present with a classic triad of symptoms, consisting of cranial nerve deficits, long tract signs, and ataxia.1 These symptoms usually develop acutely (<1 month), but some patients may have more subtle complaints over several months. Patients may also demonstrate signs and symptoms from acute obstructive hydrocephalus due to compression of the fourth ventricle.
Pathology
Most patients do not undergo biopsy or resection of their tumor, due to its difficult location and essential functions. Some centers have begun incorporating stereotactic biopsy for pathologic diagnosis with low morbidity.6 The available histology has revealed that most of these DIPGs are fibrillary astrocytomas, with high-grade astrocytomas demonstrating anaplasia, necrosis, vascular proliferation and increased mitoses.7, 8 Less common are low-grade astrocytomas and pilocytic astrocytomas.
Unprecedented advances in genomic technology have made possible many modern improvements in cancer outcomes. Unlike other cancers, the scarcity of DIPG tissue available for analysis has thus far hindered efforts to better understand the molecular and genetic underpinnings of this deadly disease. The Pediatric Cancer Genome Project (PCGP) recently launched a major effort to obtain tumor tissue at autopsy for basic research. Researchers found that mutations in H3F3A or HIST1H3B (encoding histones H3.3 and H3.1, respectively) occur in 78% of DIPGs but only in 22% of non-brainstem pediatric glioblastomas.9 Histone H3 has complex roles that may influence gene expression and epigenetic regulations. These and other still to be discovered mutations may improve our understanding of DIPG biology and its differences from tumors located outside the brainstem or similar tumors in adults, and hopefully enable precision treatments to improve patient survival.
Imaging Findings
Patients presenting with the classic symptoms described above are usually sent for MRI as the next step in the diagnostic work-up. Conventional MRI provides a noninvasive accurate method of diagnosis of these tumors. DIPGs are expansile infiltrative tumors centered in the pons, often extending laterally to the middle cerebellar peduncles, cranially to the midbrain, and caudally to the medulla (Fig. 1). Exophytic growth of the tumors into the prepontine cistern occurs frequently, and the tumors can also engulf the basilar artery (Fig. 2). The tumors are hyperintense on T2-weighted and FLAIR images, and isointense or hypointense on T1-weighted images. Contrast-enhanced images usually show no enhancement or only mild heterogeneous enhancement (Fig. 3, Fig. 4). Contrast enhancement of the tumor has been shown to be correlated with survival. Poussaint et al. found that enhancement of DIPG, at initial imaging as well increased enhancement over time, was associated with reduced survival.10 Jansen and colleagues found similar results, and demonstrated that ring enhancement of the tumor was a negative predictor of overall survival.11
Fig. 1.
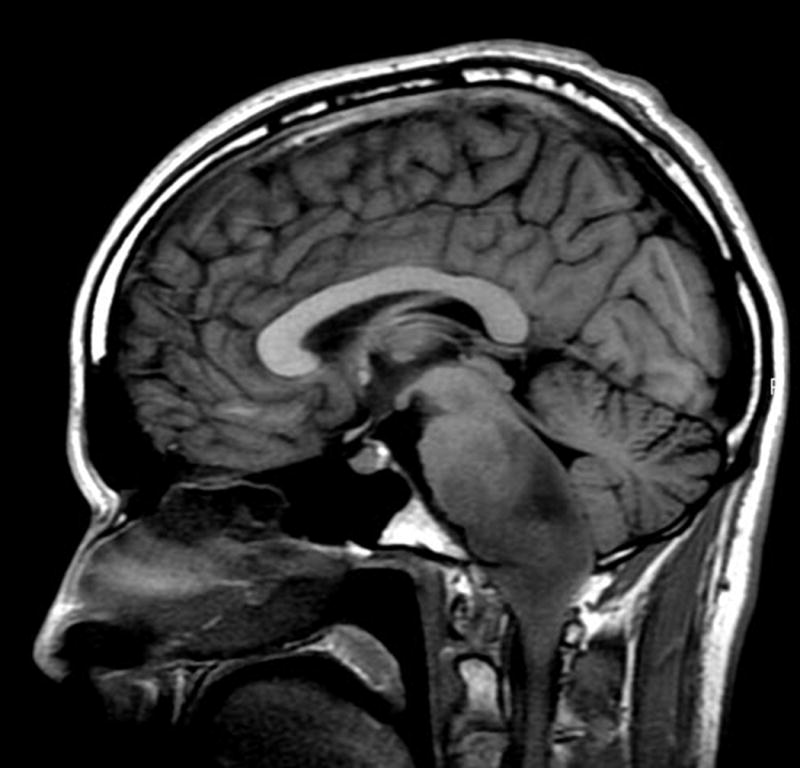
Sagittal T1-weighted image shows an expansile hypointense to isointense tumor in the pons, medulla, and cervicomedullary junction with partial effacement of the fourth ventricle and posterior displacement of the cerebellum.
Fig. 2.
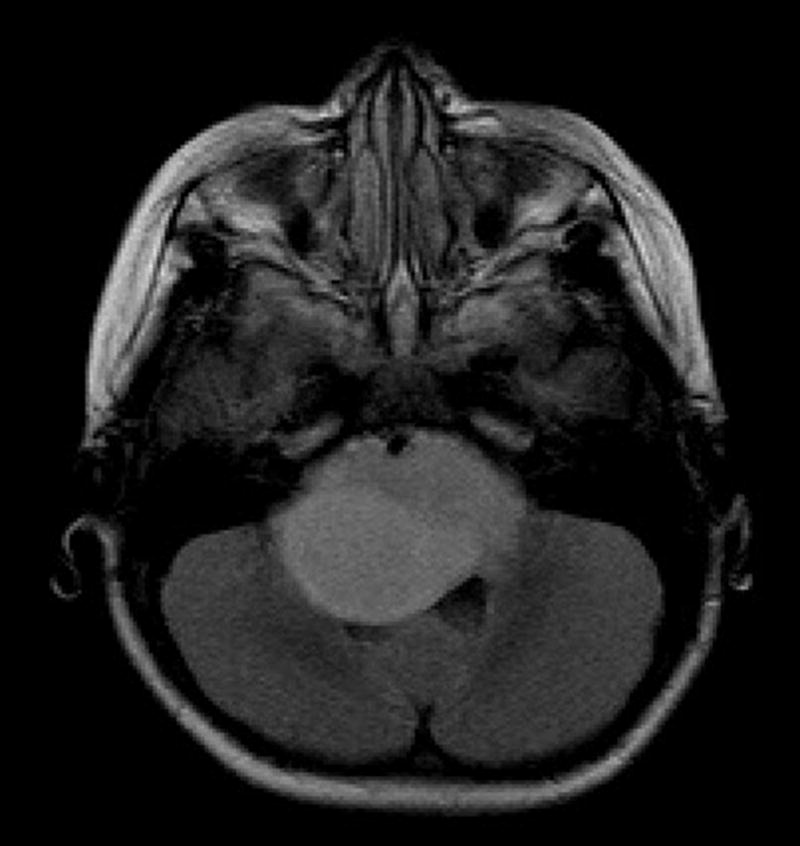
Axial fluid-attenuated inversion recovery image shows a large expansile hyperintense tumor in the pons and brachium pontis with partial encasement of the basilar artery anteriorly and partial effacement of the fourth ventricle posteriorly.
Fig. 3.
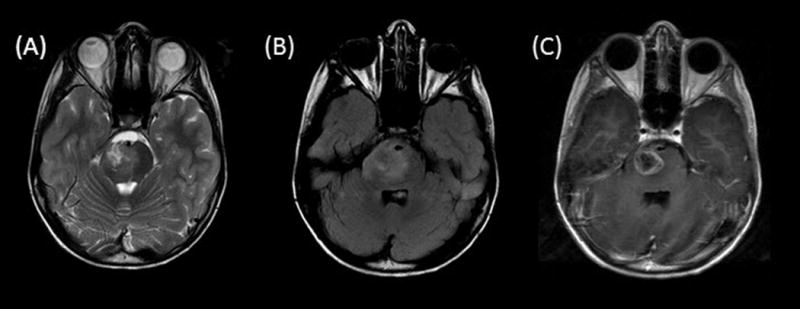
Axial T2-weighted (A), fluid-attenuated inversion recovery (B), and contrast T1-weighted (C) images demonstrate a hyperintense heterogeneously enhancing tumor centered in the right pons. Note that the exophytic partially cystic anterior portion engulfs the basilar artery flow void.
Fig. 4.
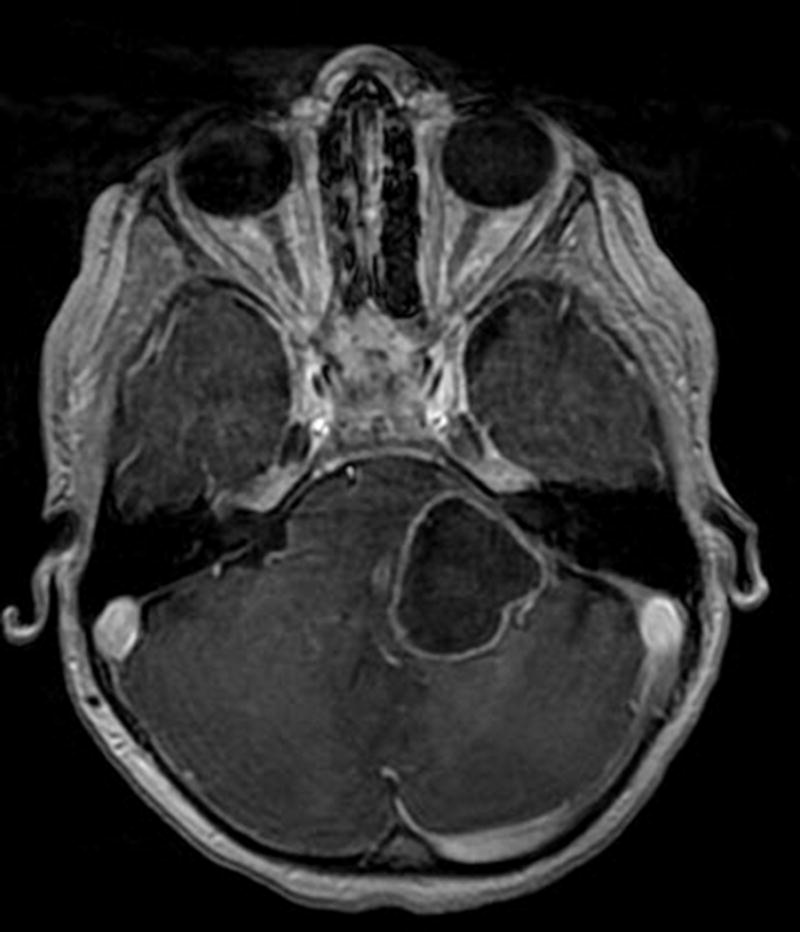
Axial contrast T1-weighted image shows a large ringenhancing component in the left pons and brachium pontis.
Other authors, however, have found that conventional MRI has not shown good correlation between imaging findings and prognosis.12 There is continued research into the use of advanced imaging techniques to assist in the noninvasive evaluation of DIPG patients at initial diagnosis, as well as in monitoring response to therapy. These techniques can assist in defining the metabolic characteristics and better define the anatomy of these tumors. With increased interest in obtaining biopsy of these tumors, advanced imaging has also been studied as a method to guide stereotactic biopsy to target the most malignant portions.
MR Spectroscopy (MRS)
MRS is an advanced imaging technique that measures concentrations of metabolites in brain tissue. The major metabolites measured include choline (Cho), creatine (Cr), N-acetyl-aspartate (NAA), lactate, and lipids. Choline is found in cell membranes and is usually increased in tumors due to increased cell turnover. Creatine is related to energy pathways in the cell and tends to decrease in tumors. NAA is found in neurons and is generally a marker of cell integrity and is therefore decreased in tumors. Lactate increases in hypoxic conditions, which can occur in tumors. Lipids can also increase in tumor.13 Citrate is a metabolite that has recently been examined with respect to pediatric brain tumors and may be associated with hypoxic conditions. Citrate has been shown to be elevated in DIPG.14, 15
This noninvasive imaging technique has been commonly used to evaluate adult brain tumors. MRS results can provide ratios between levels of the different metabolites. These ratios have been used to predict tumor grades. Cho:NAA and Cho:Cr ratios have been shown to be highly sensitive in differentiating between high-grade versus low-grade gliomas.16
There has been increased interest in the use of MRS to evaluate pediatric posterior fossa tumors. This technique has traditionally been limited in evaluation of posterior fossa tumors due to the small size of structures in the posterior fossa, as well as due to the interfaces between brain, air, bone, and fat which result in artifact. Recent advances in MRS have helped to overcome many of these technical limitations, and several recent studies have shown the utility of MRS in evaluating pediatric posterior fossa tumors. Steffen-Smith et al. evaluated DIPG patients with MRS and demonstrated that the Cho:NAA ratio has prognostic value and was able to determine which patients were at high-risk and likely to have shorter overall survival.17
MRS has also shown promise in monitoring DIPG tumors to evaluate for positive treatment response or treatment-related changes versus progression of disease. Thakur and colleagues showed how Cho:NAA and Cho:Cr ratios could be used to determine areas of disease progression versus radiation necrosis using MRS on follow-up studies after treatment. They also suggested that this technique could provide an estimate of tumor grade/aggressiveness,18 with more malignant areas demonstrating increased Cho ratios. (Fig. 6)
Fig. 6.
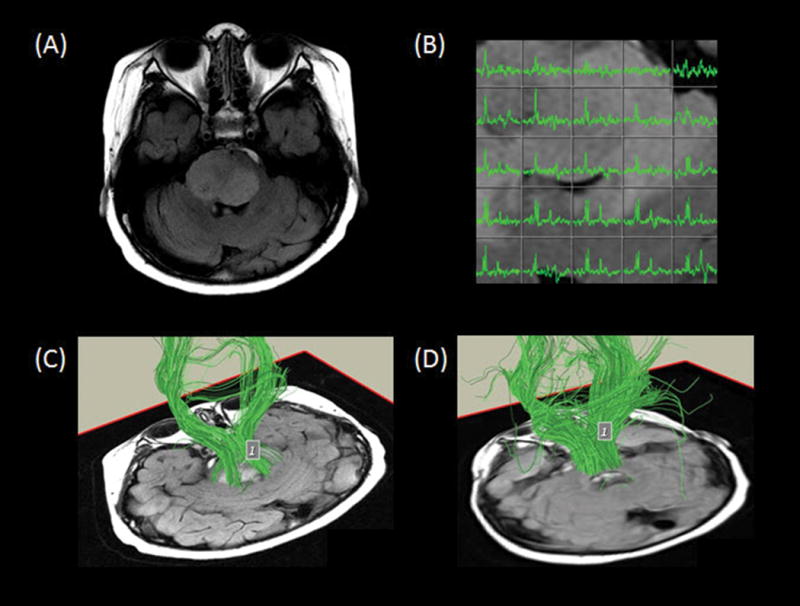
Axial fluid-attenuated inversion recovery image (A) demonstrates an expansile hyperintense tumor in the pons, eccentric to the right. Multivoxel spectroscopy (B) reveals increased choline, low N-acetyl-aspartate, and small lipid/lactate peak consistent with high-grade disease. Tractography superimposed on oblique axial T1-weighted images (C, D) demonstrate the descending corticospinal tracts and the transverse pontine fiber tracts traveling through the tumor region.
MR Perfusion (MRP)
MRP provides the noninvasive evaluation of hemodynamic parameters and vascularity of brain tumors such as DIPG. This technique measures variables such as blood volume and blood flow to the region of interest. The two main types of MRP are dynamic susceptibility contrast-enhanced (DSC) MRI, using a T2*-weighted sequence, and dynamic contrast-enhanced (DCE) MRI, using a T1-weighted sequence. In DSC imaging, successive images are acquired after gadolinium contrast administration and the decrease in T2*signal is measured over time. This decrease in signal is proportional to the concentration of gadolinium in tissue and the signal-time curve can be used to calculate relative cerebral blood volume. DSC imaging has historically been the more commonly used method for neuroimaging.19,20 This technique may be limited by factors that cause susceptibility artifact including blood, calcium, and brain-bone-air interfaces.21 In DCE imaging, successive images are acquired after gadolinium contrast is administered and allowed to extravasate into the extracellular space as a way to measure vessel permeability, in addition to perfusion. At our institution, we use the DCE technique as the benefits include better spatial resolution and less susceptibility artifact.22 The susceptibility artifact is most commonly due to hemorrhage and mineralization which frequently occurs in brain tumors, either as a function of the tumor itself or due to post treatment changes. This technique is also less susceptible to artifact from large vessels or osseous structures. Areas of high-grade tumor often demonstrate increased leakiness from altered blood-brain barrier and permeability on Ktrans maps and increased perfusion from neoangiogenesis on plasma volume maps (Fig. 5).
Fig. 5.
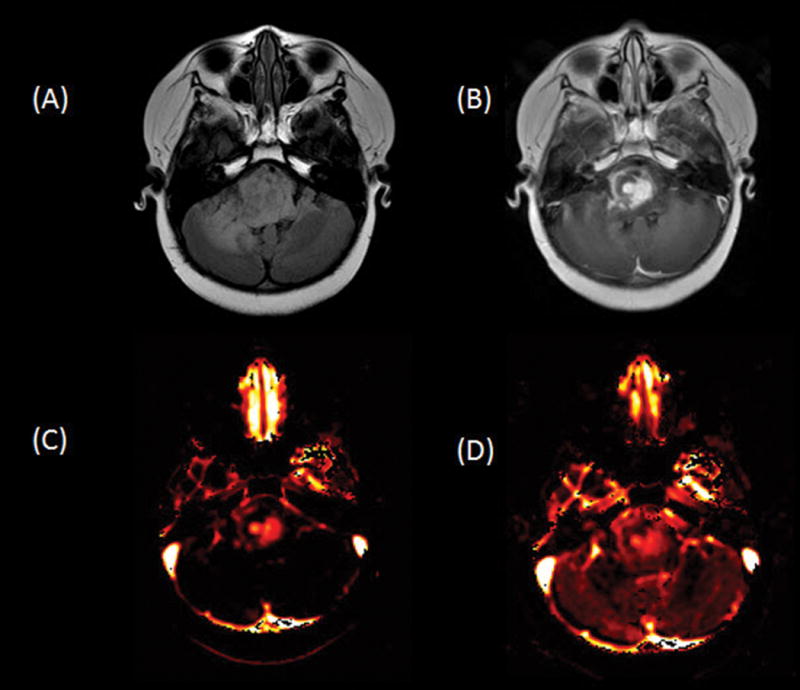
Axial fluid-attenuated inversion recovery (A) and contrast T1-weighted (B) images show an expansile hyperintense heterogeneously enhancing tumor in the pons and right greater than left cerebellum. Ktrans (C) and plasma volume (D) maps demonstrate heterogeneously increased leakiness and perfusion, respectively, in the areas of enhancing to suggest high-grade disease.
MRP has been used to help differentiate between higher and lower grade tumors as well as to help differentiate between recurrent tumor or areas of radiation necrosis or other posttreatment changes. MRP is also commonly used to follow treatment response of brain tumors. In a study by Hipp et al., increased perfusion of DIPG at baseline and at any time during follow-up was associated with reduced survival.23 In another study, Löbel and colleagues used MRP to evaluate heterogeneous areas within the DIPG. They found higher relative cerebral blood volume, corresponding to increased vascularity, in areas of potential anaplasia. The authors suggested that identifying these areas of focal anaplasia within a DIPG may be helpful in staging the disease, in guiding stereotactic biopsy, and also in following treatment response.24
Diffusion/Diffusion Tensor Imaging (DTI)
Diffusion MRI is a technique that evaluates the molecular motion of water in tissues, which can be abnormal in brain tumors.25 Diffusion imaging has been studied for use with DIPG. Areas of restricted diffusion within a tumor have shown correlation with regions of both elevated perfusion and contrast enhancement, respectively.10, 24 Yeom and colleagues found that diffusion MRI may help to identify subgroups of DIPG patients with clinically more aggressive tumors.26
DTI is an advanced imaging technique that evaluates white matter tracts in the brain. Infiltration or displacement of these fiber tracts by tumor can be detected by changes in the diffusion of water along the tracts, which is measured by fractional anisotropy. DTI is currently under investigation as an imaging technique for initial diagnosis of brain tumors, as well for monitoring treatment response.
In a recent study by Giussani et al., DTI was able to differentiate between DIPG and demyelinating disease in two groups of patients. The authors found that in the DIPG group, the white matter tracts in the brainstem were displaced, whereas in the demyelinating disease group, there was a paucity of white matter tracts which were truncated.27 Another group studied white matter tracts in the brainstem of DIPG patients before and after radiation and drug therapy. They found that DIPG initially infiltrated and displaced the fiber tracts without complete disruption. After treatment, the tracts demonstrated some improvement in infiltration. With disease progression, however, the fiber tracts were ultimately completely disrupted by tumor infiltration.28 Although more studies need to be performed, DTI may be useful in evaluating patients with DIPG. This technique can supplement conventional MRI during initial diagnosis and shows promise as a noninvasive method to follow treatment response (Fig. 6).
Positron Emission Tomography (PET)
PET is commonly used in oncologic imaging to evaluate metabolic activity of tumors. There has been increased interest in the use of PET to evaluate pediatric brainstem tumors. PET with 2-deoxy-2-[fluorine-18]fluoro-D-glucose (FDG), a glucose analogue, is the most commonly used technique. Kwon et al. studied a group of patients with brainstem gliomas of different histologic grades using FDG PET. They found that hypermetabolic tumors more likely reflected glioblastoma, compared to anaplastic astrocytoma or low-grade astrocytoma.29 Another group used FDG and 11C-methionine PET guidance in performing stereotactic biopsy of brainstem tumors in children. They found that PET guidance increased the diagnostic yield of biopsy. In addition, all tumors with increased metabolic activity were malignant and associated with shorter survival than tumors with absent or moderate activity.30 More recently, Zukotynski et al. found that increased FDG uptake in 50% or more of a DIPG correlated with shorter progression free survival and overall survival, compared to patients in which hypermetabolism was found in less than 50% of the tumor. These authors also found that increased metabolic activity in the tumor compared to gray matter suggested decreased survival.31 Therefore, PET is another imaging technique which shows promise in predicting tumor grade and prognosis, as well as in planning for stereotactic biopsy. The most commonly used radiotracer remains FDG, although its application remains limited by high background uptake in normal brain, which uses glucose as its preferred substrate. Further advances in PET are likely with the evolution of newer amino acid, protein, DNA and ischemia analog radiotracers.
Treatment
DIPGs are unresectable tumors due to their location in the brainstem and infiltrative nature. In addition, MRI is usually diagnostic so biopsy was often considered unnecessary and unlikely to change management. In recent years, however, there has been renewed interest in performing biopsy of these tumors with several groups increasingly advocated the safety and high diagnostic yield of biopsy.6, 32, 33 Potential benefits of biopsy include confirming the diagnosis in cases with indeterminate radiologic findings, determining specific histological characteristics to allow administration of targeted therapy, and to help in the research efforts of this tumor.
Conventional radiation therapy is the main form of treatment for DIPG. Trials using hyperfractionated radiation have not been shown to change overall survival or morbidity. Mandell et al. compared the effectiveness of conventional versus hyperfractionated radiotherapy in the treatment of DIPG. Patients were treated with either once a day treatment of 180 cGy per fraction to a total dose of 5400 cGy or a twice a day treatment of 117 cGy per fraction to a total dose of 7020 cGy. There was no reduction in the time to progression and no decrease in overall survival in either group.34
The use of temozolomide and other radiosensitizing chemotherapy agents have not shown much benefit to date in terms of increasing overall survival.35 No chemotherapeutic agent has yet to be shown more effective than radiation alone.36 Newer therapies such as convection-enhanced delivery (CED) of chemotherapeutic agents have shown promise. CED consists of the administration of medication directly into the tumor via a catheter placed surgically into the brainstem.37 This novel treatment is still undergoing trials and is not currently accepted as standard treatment. Another one of these novel treatment methods includes immunotherapy, which aims to induce an active systemic immune response against a tumor. Pollack and colleagues recently showed clinical response in a group of DIPG patients using a subcutaneous vaccination targeting glioma-associated antigen epitopes associated with pediatric gliomas.38
Conclusion
DIPG is a common brainstem tumor in children and remains one of the more aggressive tumors, with few patients surviving beyond one year after diagnosis. There has been little change in the poor prognosis of these tumors over the past 20 years. The combination of clinical symptoms and typical MRI findings allows a confident diagnosis in most cases. Classic findings on conventional MRI help to make the initial diagnosis. However, advanced MRI techniques are becoming more widely used and studied as additional noninvasive methods to assist clinicians in initial diagnosis and staging, monitoring disease, as well as in surgical and radiation planning. Conventional radiotherapy remains the mainstay of treatment, although novel methods of therapy such as administration of chemotherapy directly into the tumor with CED as well as immunotherapy are currently under investigation. The growing adoption of safe surgical sampling and treatment, and greater understanding of the genetic and molecular alterations that direct the biology of these tumors are likely to propel future advances in patient survival.
References
- 1.Freeman CR, Farmer JP. Pediatric brain stem gliomas: a review. International journal of radiation oncology, biology, physics. 1998;40:265–71. doi: 10.1016/s0360-3016(97)00572-5. [DOI] [PubMed] [Google Scholar]
- 2.Hargrave D, Bartels U, Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. The Lancet Oncology. 2006;7:241–8. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 3.Ostrom QT, de Blank PM, Kruchko C, et al. Alex’s Lemonade Stand Foundation Infant and Childhood Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2007–2011. Neuro-oncology. 2015;16(Suppl 10):x1–x36. doi: 10.1093/neuonc/nou327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillamo JS, Doz F, Delattre JY. Brain stem gliomas. Current opinion in neurology. 2001;14:711–5. doi: 10.1097/00019052-200112000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Chintagumpala M, Gajjar A. Brain Tumors. Pediatric Clinics of North America. 2015;62:167–78. doi: 10.1016/j.pcl.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Roujeau T, Machado G, Garnett MR, et al. Stereotactic biopsy of diffuse pontine lesions in children. Journal of neurosurgery. 2007;107:1–4. doi: 10.3171/PED-07/07/001. [DOI] [PubMed] [Google Scholar]
- 7.Schumacher M, Schulte-Monting J, Stoeter P, Warmuth-Metz M, Solymosi L. Magnetic resonance imaging compared with biopsy in the diagnosis of brainstem diseases of childhood: a multicenter review. Journal of neurosurgery. 2007;106:111–9. doi: 10.3171/ped.2007.106.2.111. [DOI] [PubMed] [Google Scholar]
- 8.Cage TA, Samagh SP, Mueller S, et al. Feasibility, safety, and indications for surgical biopsy of intrinsic brainstem tumors in children. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2013;29:1313–9. doi: 10.1007/s00381-013-2101-0. [DOI] [PubMed] [Google Scholar]
- 9.Wu G, Broniscer A, McEachron TA, et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nature genetics. 2012;44:251–3. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poussaint TY, Kocak M, Vajapeyam S, et al. MRI as a central component of clinical trials analysis in brainstem glioma: a report from the Pediatric Brain Tumor Consortium (PBTC) Neuro-oncology. 2011;13:417–27. doi: 10.1093/neuonc/noq200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen MH, Veldhuijzen van Zanten SE, Sanchez Aliaga E, et al. Survival prediction model of children with diffuse intrinsic pontine glioma based on clinical and radiological criteria. Neuro-oncology. 2015;17:160–6. doi: 10.1093/neuonc/nou104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hargrave D, Chuang N, Bouffet E. Conventional MRI cannot predict survival in childhood diffuse intrinsic pontine glioma. Journal of neuro-oncology. 2008;86:313–9. doi: 10.1007/s11060-007-9473-5. [DOI] [PubMed] [Google Scholar]
- 13.Soares DP, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clinical radiology. 2009;64:12–21. doi: 10.1016/j.crad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 14.Yeom KW, Lober RM, Nelson MD, Jr, Panigrahy A, Bluml S. Citrate concentrations increase with hypoperfusion in pediatric diffuse intrinsic pontine glioma. Journal of neuro-oncology. 2015;122:383–9. doi: 10.1007/s11060-015-1726-0. [DOI] [PubMed] [Google Scholar]
- 15.Seymour ZA, Panigrahy A, Finlay JL, Nelson MD, Jr, Bluml S. Citrate in pediatric CNS tumors? AJNR American journal of neuroradiology. 2008;29:1006–11. doi: 10.3174/ajnr.A1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR American journal of neuroradiology. 2003;24:1989–98. [PMC free article] [PubMed] [Google Scholar]
- 17.Steffen-Smith EA, Shih JH, Hipp SJ, Bent R, Warren KE. Proton magnetic resonance spectroscopy predicts survival in children with diffuse intrinsic pontine glioma. Journal of neuro-oncology. 2011;105:365–73. doi: 10.1007/s11060-011-0601-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakur SB, Karimi S, Dunkel IJ, Koutcher JA, Huang W. Longitudinal MR spectroscopic imaging of pediatric diffuse pontine tumors to assess tumor aggression and progression. AJNR American journal of neuroradiology. 2006;27:806–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Sourbron S. Technical aspects of MR perfusion. European journal of radiology. 2010;76:304–13. doi: 10.1016/j.ejrad.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Covarrubias DJ, Rosen BR, Lev MH. Dynamic magnetic resonance perfusion imaging of brain tumors. The oncologist. 2004;9:528–37. doi: 10.1634/theoncologist.9-5-528. [DOI] [PubMed] [Google Scholar]
- 21.Cha S, Knopp EA, Johnson G, Wetzel SG, Litt AW, Zagzag D. Intracranial mass lesions: dynamic contrast-enhanced susceptibility-weighted echo-planar perfusion MR imaging. Radiology. 2002;223:11–29. doi: 10.1148/radiol.2231010594. [DOI] [PubMed] [Google Scholar]
- 22.Cha S, Yang L, Johnson G, et al. Comparison of microvascular permeability measurements, K(trans), determined with conventional steady-state T1-weighted and first-pass T2*-weighted MR imaging methods in gliomas and meningiomas. AJNR American journal of neuroradiology. 2006;27:409–17. [PMC free article] [PubMed] [Google Scholar]
- 23.Hipp SJ, Steffen-Smith E, Hammoud D, Shih JH, Bent R, Warren KE. Predicting outcome of children with diffuse intrinsic pontine gliomas using multiparametric imaging. Neuro-oncology. 2011;13:904–9. doi: 10.1093/neuonc/nor076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobel U, Sedlacik J, Reddick WE, et al. Quantitative diffusion-weighted and dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging analysis of T2 hypointense lesion components in pediatric diffuse intrinsic pontine glioma. AJNR American journal of neuroradiology. 2011;32:315–22. doi: 10.3174/ajnr.A2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002;224:177–83. doi: 10.1148/radiol.2241010637. [DOI] [PubMed] [Google Scholar]
- 26.Lober RM, Cho YJ, Tang Y, et al. Diffusion-weighted MRI derived apparent diffusion coefficient identifies prognostically distinct subgroups of pediatric diffuse intrinsic pontine glioma. Journal of neuro-oncology. 2014;117:175–82. doi: 10.1007/s11060-014-1375-8. [DOI] [PubMed] [Google Scholar]
- 27.Giussani C, Poliakov A, Ferri RT, et al. DTI fiber tracking to differentiate demyelinating diseases from diffuse brain stem glioma. NeuroImage. 2010;52:217–23. doi: 10.1016/j.neuroimage.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 28.Prabhu SP, Ng S, Vajapeyam S, et al. DTI assessment of the brainstem white matter tracts in pediatric BSG before and after therapy: a report from the Pediatric Brain Tumor Consortium. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2011;27:11–8. doi: 10.1007/s00381-010-1323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwon JW, Kim IO, Cheon JE, et al. Paediatric brain-stem gliomas: MRI, FDG-PET and histological grading correlation. Pediatric radiology. 2006;36:959–64. doi: 10.1007/s00247-006-0256-5. [DOI] [PubMed] [Google Scholar]
- 30.Pirotte BJ, Lubansu A, Massager N, Wikler D, Goldman S, Levivier M. Results of positron emission tomography guidance and reassessment of the utility of and indications for stereotactic biopsy in children with infiltrative brainstem tumors. Journal of neurosurgery. 2007;107:392–9. doi: 10.3171/PED-07/11/392. [DOI] [PubMed] [Google Scholar]
- 31.Zukotynski KA, Fahey FH, Kocak M, et al. Evaluation of 18F-FDG PET and MRI associations in pediatric diffuse intrinsic brain stem glioma: a report from the Pediatric Brain Tumor Consortium. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52:188–95. doi: 10.2967/jnumed.110.081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pincus DW, Richter EO, Yachnis AT, Bennett J, Bhatti MT, Smith A. Brainstem stereotactic biopsy sampling in children. Journal of neurosurgery. 2006;104:108–14. doi: 10.3171/ped.2006.104.2.108. [DOI] [PubMed] [Google Scholar]
- 33.Phi JH, Chung HT, Wang KC, Ryu SK, Kim SK. Transcerebellar biopsy of diffuse pontine gliomas in children: a technical note. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2013;29:489–93. doi: 10.1007/s00381-012-1933-3. [DOI] [PubMed] [Google Scholar]
- 34.Mandell LR, Kadota R, Freeman C, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. International journal of radiation oncology, biology, physics. 1999;43:959–64. doi: 10.1016/s0360-3016(98)00501-x. [DOI] [PubMed] [Google Scholar]
- 35.Chassot A, Canale S, Varlet P, et al. Radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. Journal of neuro-oncology. 2012;106:399–407. doi: 10.1007/s11060-011-0681-7. [DOI] [PubMed] [Google Scholar]
- 36.Robison NJ, Kieran MW. Diffuse intrinsic pontine glioma: a reassessment. Journal of neuro-oncology. 2014;119:7–15. doi: 10.1007/s11060-014-1448-8. [DOI] [PubMed] [Google Scholar]
- 37.Luther N, Zhou Z, Zanzonico P, et al. The potential of theragnostic (1)(2)(4)I-8H9 convection-enhanced delivery in diffuse intrinsic pontine glioma. Neuro-oncology. 2014;16:800–6. doi: 10.1093/neuonc/not298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollack IF, Jakacki RI, Butterfield LH, et al. Antigen-specific immune responses and clinical outcome after vaccination with glioma-associated antigen peptides and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in children with newly diagnosed malignant brainstem and nonbrainstem gliomas. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2014;32:2050–8. doi: 10.1200/JCO.2013.54.0526. [DOI] [PMC free article] [PubMed] [Google Scholar]


