Abstract
Introduction
Next-generation sequencing and advances in ‘omics technology have rapidly increased our understanding of the molecular landscape of epithelial ovarian cancers.
Areas covered
Once characterized only by histologic appearance and clinical behavior, we now understand many of the molecular phenotypes that underlie the different ovarian cancer subtypes. While the current approach to treatment involves standard cytotoxic therapies after cytoreductive surgery for all ovarian cancers regardless of histologic or molecular characteristics, focus has shifted beyond a ‘one size fits all’ approach to ovarian cancer.
Expert commentary
Genomic profiling offers potentially ‘actionable’ opportunities for development of targeted therapies and a more individualized approach to treatment with concomitant improved outcomes and decreased toxicity.
Keywords: genomic profiling, ovarian cancer, targeted therapy, precision medicine
1.0 Introduction
The standard approach to treatment of the ovarian cancer patient continues to evolve. Within the past decade, our knowledge of this deadly disease has advanced in diagnostic imaging, treatment with platinum based and taxane combination chemotherapy [1–3] and aggressive cytoreduction [4] to no gross residual disease [5]. Technological advances, coupled with a better understanding of tumor biology, has piqued gynecologic oncologists’ interest toward more personalized therapy. The practice of precision medicine requires tailoring a specific treatment for a particular cancer type at a precise time point. Over the past decade in the treatment of malignancies, the paradigm of cancer treatment across many tumor lineages has shifted from the empiric use of cytotoxic therapies to genotype-directed therapy using targeted agents as well as to the implementation of immuno-oncology agents. In general, ovarian cancer therapy has lagged in this process.
Oncology is an ideal medical field for the development of targeted or precision medicine [6–9]. Like the genomic studies that have occurred in ovarian cancer, more recent trends have been to categorize oncology patients by their tumors’ genetic alterations rather than by the primary site of origin [10, 11]. The primary goal of molecular targeted therapy is to match patients with a specific therapy, based on their molecular aberrations, to increase the likelihood of a response and impact survival. This is also expected to decrease toxicity. Rather than take a blanket approach to therapy by treating all ovarian cancers with standard of care chemotherapy with a platinum and taxane after cytoreduction, this type of therapy would identify genomic alterations that were specific to the tissue removed by biopsy or surgery and integrate this information with other aspects of the patients tumor such as histology and spread [12, 13].
This step toward individualization of cancer therapies has been fueled by technological advances of high throughput molecular, proteomic, metabolomics and genomic techniques [14]. Next-generation sequencing has made genomic profiling of solid malignancies possible through whole-genome, exome, target sequencing of DNA as well as transcriptome sequencing of fresh, frozen, and fixed tumor samples widely accessible in all models of clinical practice [10, 15, 16]. Not only has the cost of performing these assays continued to decrease but they are now applicable to smaller tumors and also to more challenging conditions such as formalin fixed paraffin embedded samples. The advent of sequencing of circulating DNA including exosomal DNA offers the potential to bypass the need to obtain tumor tissue for analysis, making the ability to assess the tumor at the time of therapy or even during therapy more practical. How to best incorporate genomic profiling into our clinical practice and make informed treatment decisions that impact outcomes for women with ovarian cancer remains unknown [17] but is rapidly evolving. Mining the pre-existing genomic data that we have previously collected is associated with past and current challenges. However, we must also anticipate the future and how we navigate the inherent heterogeneity, relative lack of driver mutations, genomic instability, propensity for clonal evolution over time, and resistance that develops to chemo- and targeted therapies of ovarian cancers.
1.1 Genomic profiling of ovarian cancer
Previous clinicopathologic and molecular studies of epithelial ovarian cancer have classified tumors into two broad categories [18]: type I (low grade serous, mucinous, endometrioid, clear cell cancers, and Brenner tumors) and type II (high grade serous, high grade endometrioid, malignant mixed mesodermal tumors, and undifferentiated carcinomas) (Table 1) [19–21]. Type I tumors are characterized by specific mutations in KRAS, regulators of the mitogen-activated protein kinase (MAPK) pathway such as BRAF, receptor tyrosine kinases such as ERBB2, and PI3K abnormalities such as loss of function mutations in PTEN [20, 22]. Mutation of KRAS in type I ovarian cancers has been characterized as a “driver” mutation, and is thought to play a crucial role in tumorigenesis as well as disease progression and likely responsiveness to therapy. These molecular events confer an addiction of the tumor cell to a particular molecular pathway with specific dependencies despite other existing “passenger” alterations. High grade serous ovarian cancers frequently contain TP53 mutations and alterations of BRCA1/2 and associated homologous recombination (HR) genes either by mutation, promoter methylation, or loss of heterozygosity [23]. These mutations can be either germline contributing to cancer predisposition syndromes, or somatic. More recently, individual centers, genomic consortiums and initiatives such as The Cancer Genome Atlas (TCGA) and International Cancer Genomics Consortium have completed large scale studies of multiple tumor types to better understand and characterize alterations within pathways involved in the development of solid malignancies [24, 25]. These genomic studies have included glioblastoma, colon, ovarian, breast, lung, and uterine cancers.
Table 1.
Molecular characteristics of type 1 and 2 ovarian cancer neoplasms.
| Histology | Molecular characteristic | |
|---|---|---|
| Type 2 | High grade serous | TP53 mutation BRCA1/2 HRD WT1 KRAS mutation PIK3CA amplification |
| High grade endometrioid | PIK3CA mutation TP53 mutation WT1 |
|
| Type 1 | Low grade serous | KRAS mutation BRAF mutation ER+/PR+ EGFR amplification PIK3CA amplification/mutation PTEN mutation |
| Low grade endometrioid | CTTNB1 mutation PTEN mutation PIK3CA mutation ARID1A mutation BRAF mutation |
|
| Clear cell | IL6/JAK2/STAT3 pathway ARID1A mutation PTEN mutation or LOH PIK3CA amplification/mutation EGFR amplification /mutation |
|
| Mucinous | KRAS mutation BRAF mutation HER2 amplification |
TCGA evaluated 489 high grade serous ovarian cancers and identified four subtypes based on their molecular signatures: immunoreactive; differentiated; proliferative; and mesenchymal [26, 27]. However, these subsets appear to represent “gradients” rather than discreet tumor subtypes or cell of origin. Significant findings from this analysis included almost universal TP53 mutations as well as frequent germline or somatic mutations in BRCA1 and/or BRCA2 and other related HR DNA repair genes. Tumors that lacked a TP53 mutation within this TCGA analysis have recently been re-reviewed, and 93% of the tumors were either not a pure high grade serous ovarian carcinoma or represented a pathologic diagnosis other than a high grade serous ovarian carcinoma. This suggests that lack of TP53 molecular alterations within a tumor are inconsistent with the high grade serous ovarian cancer diagnosis, and an alternative diagnosis should be sought [28]. Also noted from the TCGA was overall lack of genomic stability, lack of driver mutations, and high frequency of loss-of-function events. These loss-of-functions events are related to abnormal homologous recombination, through dysfunction of proteins in the homologous recombination pathway or silencing of BRCA1 through methylation. Recurrent somatic mutations were identified in NF1, RB1, and CDK12, albeit at relatively low frequencies. These results largely recapitulated findings from our group on 235 ovarian cancers [29].
Another large scale analysis of germline mutations and tumor suppressor genes in 360 women with ovarian, peritoneal or fallopian tube cancers revealed that 24% carried loss-of-function mutations: 18% in BRCA1 or BRCA2 and 6% in other DNA repair genes BARD1, BRIP1, CHEK2, MRE11A, MSH6, NBN, PALB2, RAD50, RAD51C, or TP53. Six of these genes had not previously been implicated in inherited ovarian carcinoma [30]. Other profiling studies have reclassified ovarian carcinomas based on profiling characteristics. Tothill and colleagues performed microarray gene expression profiling on 285 serous and endometrioid tumors and identified six molecular subtypes, one of which was a high grade serous subtype reflecting mesenchymal features, and was characterized by N-cadherin and P-cadherin and low expression of CA125 and MUC1 [31]. Overall, this study demonstrated similar subtypes to the TCGA study with additional subtypes encompassing tumor types not included in the TCGA analysis. Although a less frequent histology pathologically, molecular profiles of characterized clear cell carcinoma and low grade endometrioid cancers were marked by somatic mutations in ARID1A, which encodes AF250a, a key component of the SWI–SNF chromatin remodeling complex [32, 33]. ARID1A performs a key function in DNA damage repair and gets recruited to DNA double strand breaks via its interactions with ATR, an upstream DNA damage checkpoint kinase, and enables sustained DNA damage signaling. The use of poly-ADP ribose polymerase (PARP) and EZH2 methyltransferase inhibitors in patients with ARID1A-mutant tumors may provide a potential therapeutic strategy, which has been demonstrated in pre-clinical models [34, 35]. ARID1A mutant tumors have also been demonstrated to be sensitive to EZH2 inhibitors offering an additional therapeutic opportunity for exploration.
Additionally, a small number of treated tumors have been profiled in the recurrent setting. Profiling tumors refractory to platinum-based therapy has identified amplification of 19q12, which contains cyclin E (CCNE1) in 15% of high grade serous cancers [36, 37]. CCNE1 has been identified as an amplified oncogene in high grade serous ovarian cancers and is strongly associated with treatment resistance to platinum-based chemotherapy [36, 37]. Knockdown of cyclin E resulted in G1/S phase arrest, reduced cell viability and apoptosis only in amplification-carrying cells [38]. Mucinous carcinomas, which typically do not respond to current platinum-based and taxane chemotherapy, have been noted to exhibit amplification of HER2 in 18.2% of mucinous carcinomas and 18.8% of mucinous borderline ovarian tumors [39].
Predictive biomarkers, descriptors that identify selective susceptibility to an intervention, have also emerged from big data profiling studies. Predictive biomarkers predict how a patient will respond to a particular intervention or the differential outcomes of two or more interventions, including toxicity. Recently, TP53 alterations have been found to predict sensitivity to VEGF/VEGFR inhibitors in clinic [40]. Prognostic biomarkers are those that characterize the outcome, such as survival or the probability of recurrence after adjuvant chemotherapy. Examples of prognostic biomarkers in ovarian cancer include CA125 [41] (post-operative levels of CA125 greater than 35 units/mL are independent prognostic factors for survival), EphA2 [42] (overexpression is associated with poor prognosis), and Dicer and Drosha mRNA levels (decreased Dicer expression correlates significantly with worse overall survival) [43]. Additionally, biomarker expression rates between subtypes have been evaluated by protein expression. For example, WT1, more frequently expressed in high grade serous carcinoma [44], and eight other markers including, CDKN2A, DKK1, HNF1B, MDM2, PGR, TFF3, TP53, VIM, can be used to predict one of five transcriptional subtypes in a retrospective cohort [45].
1.2 Heterogeneity and driver mutations in ovarian cancer
As with all therapies, intratumoral heterogeneity and how this contributes to ability of the tumor to overcome even the most precisely matched therapy will require further investigation. Conventional histopathology has shown that marked phenotypic heterogeneity is characteristic of some ovarian cancers. Primary and matched metastatic tumors have distinct phylogenetic branching patterns to suggest evolution of tumor clones through acquisition of additional mutations that underlie further tumor progression [46–48]. Mutational signatures from metastatic sites reflect subclones and populations that existed in the primary tumor, and some clones may acquire certain mutations that target metastases to specific organs [47, 48]. Finally, through further acquisition of passenger alterations, novel phenotypes may be created through these acquired genetic interactions. While these processes have been studied in multiple tumor lineages, their role in the resistance of ovarian cancers to therapy is less well characterized and requires further investigation. It is through this acquired resistance, that mixed responses to therapies may be seen [49]. Indeed, companion biomarkers that have been developed in parallel may become less reliable or useful in this situation. While initial cytoreduction offers the most tumor material from the primary and multiple metastatic sites, biopsy of a single site of metastatic disease at the time of recurrence will likely underestimate how the genomic tumor landscape has changed. Although protocols with targeted agents often delineate specifically where and when to re-biopsy, the necessity, whether or not tumoral evolution and heterogeneity is captured by a single point in time biopsy, and ethical considerations about acquisition of tissue without direct therapeutic benefit, must all be considered. Indeed, these concerns are fueling the development of analysis of circulating and exosomal DNA as surrogates for the tumor heterogeneity across multiple metastatic sites.
Recent historical success stories highlight therapies that match genetically defined tumor subtypes to improve treatment response and patient survival. Amplification of ERBB2/HER2 in breast cancer is successfully targeted by trastuzumab, a humanized monoclonal anti-HER2 antibody [50]. Imatinib, a tyrosine kinase inhibitor targets BCR-ABL, which is a constitutively activated tyrosine kinase that causes chronic myeloid leukemia (CML) [51]. In patients with non-small cell lung cancer, who received treatment with gefitinib and erlotinib, have enhanced susceptibility to these inhibitors only if their tumor harbors a specific epidermal growth factor receptor (EGFR) mutation [52]. Treatment of metastatic melanoma with vemurafenib in patients with tumors that carry the BRAFV600E mutation resulted in complete or partial tumor regression in the majority of patients [53], however as with most targeted therapies, these responses are transient. Recurrent translocations, such as those in non-small cell lung cancer including the EML4 anaplastic lymphoma kinase (ALK) rearrangement and c-ros oncogene 1 (ROS1) fusions, have been found to be targetable by an ALK/ROS1 inhibitor, crizotinib, and result in tumor shrinkage or stable disease [54, 55]. Also, treatment with vismodegib (a hedgehog pathway inhibitor) in patients with medulloblastoma or basal cell carcinomas (with a loss of function mutation in PTCH1) resulted in tumor regression and symptom regression [56, 57]. Identification of mutations have also identified and predicted those patients who will not respond to therapy. For example, the presence of RAS mutations in colorectal cancer is associated with decreased overall survival with panitumumab and chemotherapy [58]. Identification of these predictors can avoid treatment of patients who would not benefit, sparing them untoward toxicities and adverse events. However, high grade serous ovarian cancer does not offer opportunities for therapy with these agents due to the lack of recurrent mutations. A number of small molecules designed to normalize p53 function are in trial such as APR-246 and COTI-2, however, these trials are still at an early phase. Unfortunately, the mutations that occur in other types of ovarian cancer are not yet currently therapeutically targetable although ongoing studies are place to determine whether RAS and ARID1A mutant tumors can be targeted.
Not all molecular events that function as driver mutations in one cancer may do so in another cancer, and vice versa. In fact, specific alterations may engender different responses such as treatment of HER2-amplified breast and gastric cancer [50, 59] showing benefit to HER2 inhibitors but a lack of benefit for the same inhibitors in ovarian and uterine cancers [60, 61]. Indeed, colorectal carcinomas harboring the specific BRAFV600E mutation have been noted to have poor response rate to vemurafenib as compared to melanoma [62]. The success of imatinib in CML and vemurafenib in patients with tumors that carry the BRAFV600E mutation in melanoma reflects the reliance of these tumors on driver mutations [63] as well as a lack of bypass mechanisms in these lineages. However, advanced ovarian cancer and other solid malignancies have at least 30 to 60 mutated genes, but only 2 to 8 driver mutations [64]. This complexity, crosstalk between pathways, and feedback loops often yield unpredictable effects to therapy, and clinicians must realize the limitation of prescribing a targeted agent to match merely one mutation [65].
Microarray based gene expression studies have retrospectively demonstrated that ovarian cancer represents both a clinically diverse and molecularly heterogeneous cancer. These microarray based technologies permit analysis of thousands of transcripts in parallel, which allows for further classification into subgroups [66, 67]. Over the past ten years, prognostic and predictive molecular classifications have emerged, and their clinical impact remains promising, yet uncertain.
Early generation gene expression studies focused on identification of relevant genes that could serve as predictors of prognosis but lacked validation and were largely retrospective [68–71]. A systematic validation of gene expression based prognostic models published between 2007 and 2012 [72] identified 14 prognostic models for late stage ovarian cancer. These were validated in ten previously published data sets and showed a large range of accuracy. The top ranked including the TCGA consortium [26], the signature published by Yoshihara et al. [73]; and one by Bonome et al [74]. More recent technology including Illumina targeted RNA sequencing assays, NanoString assays, and trascriptome sequencing (RNAseq) have been used, but requires further validation [75, 76]. Use of next generation sequencing has highlighted several important areas that require further research including mutation reversions and upregulation of genes encoding multi-drug resistant proteins. These findings suggest that multiple serial samples must be obtained to best understand the changes that occur within a tumor as it progresses and develops resistance. However, the heterogeneity that exists within patient’s tumor may provide only a single snapshot in time of the biology within a tumor. Cancer represents an evolutionary process, making prediction of which driver mutations to target difficult. Attempts to trace the genetic alterations through stepwise processes has been difficult in ovarian cancer, and current molecular profiling often fails to address the high level of heterogeneity and chromosomal instability that contribute to ovarian cancer pathogenesis and evolution [77].
The clinical impact of the chromosomal instability in high grade serous ovarian carcinomas remains largely unknown. Several recent studies have addressed this issue [23, 78]. Using the 455 genomic profiles from ovarian cancer from TCGA, Cope and colleagues used a chromosomal disruption index to summarize the extent of copy number aberrations across the genome. A multivariate survival analysis showed that a higher chromosomal disruption index was associated with worse survival and expression of several genes was highly correlated a higher index.
Efforts from the multiple cancer genome sequence projects have challenged the preexisting framework of the current somatic gene mutation theory of cancer [79]. Chromosomal changes impact the genomic system to a greater extent than individual gene mutations. The heterogeneity seen within tumors is due to massive chromosomal instability, but one unifying theory to explain this mechanism has yet to be elucidated. Heterogeneous cancer types like ovarian cancer represent an evolutionary process and the identification of driver mutations limits practical application [80, 81]. This genomic chaos represents one new proposed mechanism for the development of drug resistance [82, 83] and includes chromothripsis, which describes a cellular catastrophe [84].
1.3 PARP and PARP inhibitors
Genomic profiling has yet to identify any approved molecular targets for ovarian cancer patients with the exception of germline and likely somatic BRCA1 and BRCA2 mutations [85]. Given that germline mutations in BRCA1 and BRCA2 account for approximately 15% of ovarian carcinomas, including women without a family history, the Society of Gynecologic Oncology recommended in a clinical practice statement: “Women diagnosed with epithelial ovarian, tubal, and peritoneal cancers should receive genetic counseling and be offered genetic testing, even in the absence of a family history [86–88].”
BRCA1/2 mutations function as both a predictive and prognostic biomarker. In general, patients with a BRCA mutation have more platinum sensitive disease and longer overall survival. The homologous recombination pathway, where BRCA1/2 are key components, is complex, and approximately half of all high grade serous ovarian cancers have some abnormality within this pathway [30, 89, 90]. Homologous recombination is the dominant DNA repair pathway for double strand DNA breaks, but when BRCA1/2 are mutated or otherwise dysfunctional, the base excision repair single strand DNA break pathway and the double stranded DNA repair nonhomologous end joining pathway are activated [91–93]. The rate limiting step within the base excision repair pathway is activation of PARP, which binds to the DNA and provides a structural change, which then activates subsequent repair proteins. This explains the success of PARP inhibitors and higher activity in BRCA mutated patients with ovarian cancer. PARP also functions to trigger apoptosis when it is inhibited through the low fidelity nonhomologous end joining repair pathways by creating more errors that are unable to be ultimately repaired [92]. The mechanism and success of PARP inhibitors in BRCA mutant tumors does not solely rest in their ability to block repair of single stranded DNA breaks. PARP trapping is the ability of PARP inhibitors to trap the PARP1 and PARP2 enzymes at the site of damaged DNA. This PARP-DNA complex is more cytotoxic than the unrepaired single strand breaks, which suggest that PARP inhibitors also act as a poison by trapping the PARP enzyme onto the DNA, which interferes with DNA replication. The available PARP inhibitors vary in their trapping abilities [94].
Olaparib was the first PARP inhibitor to be approved for use in recurrent ovarian cancer in patients (who had received three prior chemotherapy regimens) with BRCA1/2 mutations (Figure 1) [95, 96]. These data result from a phase II study of 298 patients that had germline mutations and had progressed after three lines of therapy. Tumor response rates were 31% and 54.6% of patients were progression free after six months of treatment. Median overall survival was 16 months [96]. The European Medicines Agency (EMA) has approved olaparib be used as a monotherapy for the maintenance treatment of adult patients with platinum-sensitive relapsed BRCA-mutated (germline and/or somatic) high grade serous epithelial ovarian, fallopian tube, or primary peritoneal cancer who have responded (complete response or partial response) to platinum-based chemotherapy [97, 98]. Veliparib, another PARP inhibitor has also been evaluated in women with germline BRCA mutations and recurrent ovarian cancer. The proportion of patients who responded was 26% overall, and 20% and 35% for platinum-resistant and platinum-sensitive patients, respectively. Overall, the therapy was well tolerated with only one grade 4 event (thrombocytopenia), and median progression free survival was 8.18 months [99].
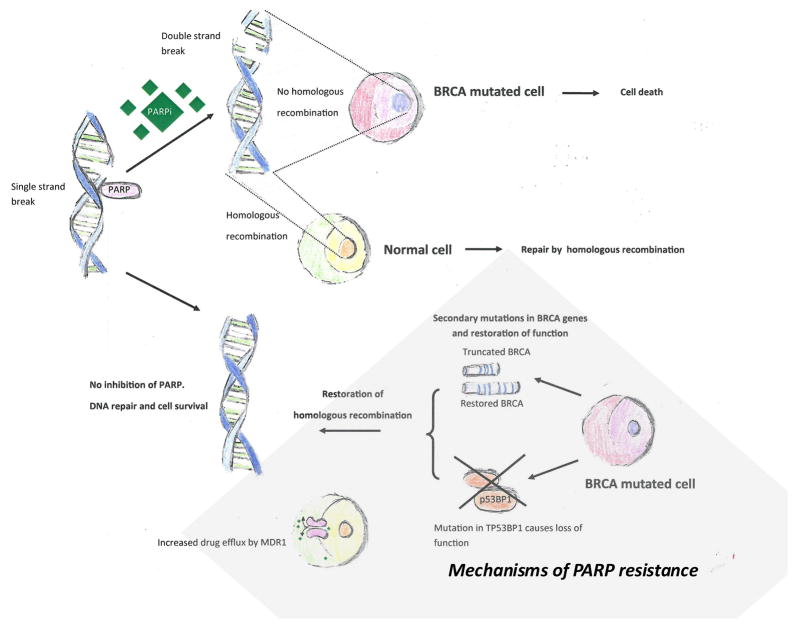
Figure 1.
In normal cells with intact BRCA, PARP functions to repair DNA normally. Treatment with PARP inhibitors inhibits a cell’s ability to repair its DNA and leads to cell death. There are multiple mechanisms of PARP inhibitor resistance.
The ARIEL2 trial sought to prospectively identify patients with non-germline BRCA1 or BRCA2 mutations who may have a homologous recombination deficiency by using a next-generation sequencing assay and analysis algorithm to predict rucaparib sensitivity by detecting tumor BRCA status and whether there is a high genomic loss of heterozygosity representing a DNA “scar” reflecting prior loss of HR repair. The association of loss of heterozygosity with defects in HR is based on a prior observation from our group [100]. Interim analysis for the first 61 patients were reported, and three subgroups were analyzed and included those with a BRCA1/2 mutation, a BRCA wild type with high LOH, and BRCA wild type with low LOH with objective response rates of 70%, 48%, and 8%, respectively [101].
The only effective biomarker-directed therapy in ovarian cancer consists of PARP inhibitors. However, resistance arises readily resulting in generally short term responses. Multiple resistance mechanisms to PARP inhibitors in ovarian cancer patients have been identified [76, 102]. The mechanisms of resistance include secondary mutations in the BRCA2 gene itself [103, 104] or loss of Tp53bp1 expression, a non-homologous end-joining factor [105, 106] amongst others. The mechanism of resistance induced by loss of Tp53bp1 was suggested to be deletion of Tp53bp1 promoting the processing of broken DNA ends to produce recombinogenic single stranded DNA that is competent for homologous recombination. Pharmacological effects also play a role in resistance to PARP inhibitors, such as increased expression of ATP-binding cassette transporters [107]. Increased expression leads to reduced drug efficacy by enhancing the intracellular to extracellular translocation of PARP inhibitors. The level of PARP protein may also influence resistance to PARP inhibitors through the differential trapping of PARP1 and PARP2 enzymes at the site of DNA damage. The different PARP inhibitors vary in their PARP trapping activity. An enhanced PARP1 trapping ability with greater amounts of the trapped PARP1-DNA complex leads to enhanced cytotoxicity. PARP1 loss of function mutants have been found to be more resistant to olaparib, suggesting that the PARP1 protein is necessary for olaparib-induced lethality [108, 109].
Resistance to other targeted therapies has been documented with EGFRT790M in gefitinib- and erlotinib-resistant lung cancers [110] and ALKL1196M in crizotinib-resistant ALK-rearranged lung cancer [111]. These newly found mutations, whether they are selected by the therapy or arise de novo, can affect the success of the targeted therapy by directly altering the target or through conformational changes of the targeted kinase. It remains unknown if these types of resistance mechanisms can be reliably predicted and if secondary resistance from a clonal selection process can be overcome.
1.4 Other possible targets and “actionable” mutations
An alteration can be considered “actionable” when identified through genomic testing if a drug can be used to target that particular alteration. Although results vary widely depending on the tissue type, 39–83% of patients with cancer are predicted to have a mutation for which matched therapy exists [112, 113]. Within ovarian cancer, rational combinations of therapy for typically chemoresistant forms of epithelial cancer can be conceived, and some of which have been previously evaluated in clinical trials (Figure 2 and Table 2).
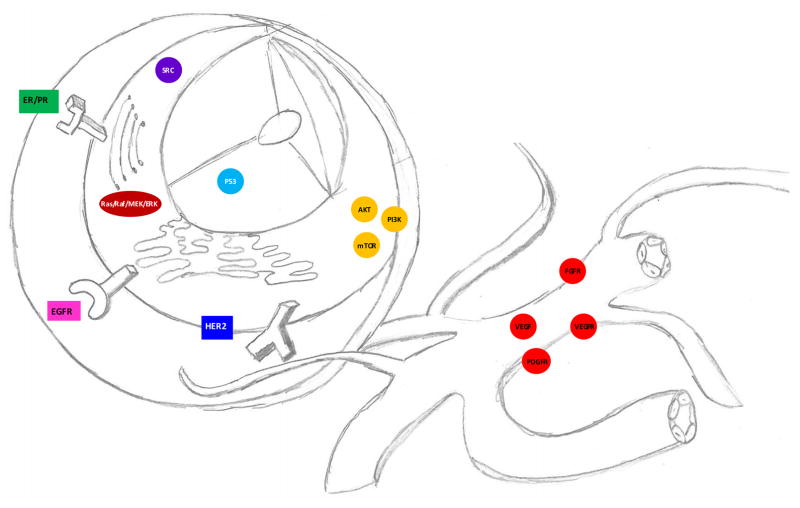
Figure 2.
Precision medicine or targeted therapies are treatments aimed at specific genes, proteins, or receptors on the tumor cell or within the microenvironment.
Table 2.
Clinical trials evaluating molecular therapeutics in ovarian cancer patients.
Low grade serous ovarian cancer is characterized by MAPK pathway mutations, which makes selumetinib, an inhibitor of MEK1/2, an informed targeted agent. In an early phase II trial of 52 patients with this therapy, 15% had objective responses reported, with one complete response and seven partial responses [114]. This led to the development of several randomized phase III trials to evaluate MEK inhibition compared to standard of care. Hormonal therapy and treatment of recurrent low grade serous ovarian cancer patients with agents including aromatase inhibitors, tamoxifen, and leuprolide acetate has also been evaluated. Estrogen and preceptor receptor expression can be assessed on paraffin-embedded sections of tumor and immunohistochemically analyzed. Gershenson et al. reported an experience with 64 women with recurrent low grade ovarian carcinoma treated with hormonal therapy, and the overall response rate was 9% with a stable disease rate of 61%. Median progression free survival was 7.4 months. Those patients whose tumors expressed estrogen and progesterone receptors had a longer median time to progression of 8.9 months than those whose tumors expressed the estrogen receptor and no progesterone receptor (6.2 months) [115].
Clear cell carcinoma, characterized by ARID1A mutations, has also been noted to be driven by hypoxia, similar to clear cell cancer of the kidney. Compared to high grade serous carcinomas, clear cell carcinomas have overexpression of the IL6-STAT3-HIF (interleukin 6-signal transducer and activator of transcription 3-hypoxia induced factor) pathway, suggesting that SRC family kinase inhibitors such as dasatinib or by other small molecules that target this pathway, may be effective [116]. Other ovarian cancers that have an ARID1A mutation such as low grade endometrioid ovarian cancers may benefit from treatment with a PARP inhibitor due to the role ARID1A plays in the DNA damage repair pathway [34] or as noted above to EZH2 inhibitors.
The phosphatidylinositol-3-kinase (PI3K) pathway is among one of the most commonly altered pathways in solid malignancies [117], especially ovarian cancer [118], which affects cell metabolism and survival [119]. Multiple pre-clinical studies have investigated direct inhibition of PI3K and mTOR in high grade serous, clear cell and mucinous ovarian carcinomas [120–123]. Various pathway inhibitors have been developed including PI3K, AKT, mTOR, and dual mTOR/PI3K inhibitors. A phase I trial involving an AKT inhibitor, perifosine and docetaxel has been completed in platinum resistant recurrent ovarian cancer patients that demonstrated acceptable safety and one patient with a partial response and four patients with stable disease [124]. A phase II trial GOG 170-1 has evaluated temsirolimus as a monotherapy in patients with recurrent ovarian cancer and reported a progression free survival of 3.1 months with a partial response rate of 9.3% [125]. Other phase II trials have evaluated the use of mTOR inhibitors and temsirolimus in combination with chemotherapy in the front line setting in patients with clear cell carcinoma. In this study, the combination of carboplatin and paclitaxel with temsirolimus was well tolerated, but when compared to historical controls, this regimen did not statistically increase progression free survival at 12 months [126]. Overall PI3K pathway targeted agents have been disappointing as monotherapy potentially due to lack of a therapeutic index. Combination therapy such as with PARP inhibitors has demonstrated intriguing activity in ovarian cancer and is the target of multiple clinical trials including ones at our center.
Small cell carcinoma of the ovary, hypercalemic type (SCCOHT), a highly aggressive undifferentiated ovarian cancer, has been characterized by a deleterious germline or somatic inactivating mutations in the SWI/SNF chromatin-remodeling gene in SMARCA4 (aka BRG1), which encodes the BRG1 protein [127–129]. Subsequent studies have suggested that loss of SMARCA4 and SMARCA2 protein expression is both sensitive and specific for SCCOHT [127, 130]. Identification of this variant through profiling and genetic testing could help improve the dismal prognosis of SCCOHT patients. Interestingly, mutations in SMARCA4 can also signal sensitivity to PARP inhibitors similar to ARID1A. This opportunity is being explored in early clinical trials in other tumor lineages.
Trastuzumab, pertuzumab (a recombinant, humanized monoclonal antibody directed against HER2 that inhibits ligand-activated heterodimerization with other HER receptors), and lapatinib (a small molecular dual tyrosine kinase inhibitor of HER2 and EGFR), are a potential treatment options for women with mucinous ovarian carcinoma when the tumor has HER2 amplification and overexpression. However, multiple trials evaluated the role of HER2-direct therapies in ovarian cancer have shown modest (or no) response and lack of relationships between tumor expression of HER2, clinical response or survival benefit [131–134]. Due to the relative rarity of mucinous tumors compared to other histologic subtypes, accrual to a trial with mucinous tumors only would be difficult. Poor response rates have been attributed to intratumoral heterogeneity, alternative signaling and survival pathways, and immune function [135–137].
2.0 Guidelines and recommendations
In March 2014, the Society of Gynecologic Oncology issued a “Clinical Practice Statement: Next Generation Cancer Gene Panels versus Gene by Gene Testing.” This statement cautions providers to consider both the limitations and advantages offered by cancer gene panels (and not germline testing for BRCA mutational status). Panels offer decreased cost, improved efficiency of testing, but the major drawback is the increased complexity of results. A primary concern is how to act on results of uncertain clinical significance, when a variant is identified and the impact on downstream function is unknown. At this time, the practice statement urges clinical management should not be dictated by uncertain variants in BRCA1/2 and other DNA repair genes, rather family history should guide current recommendations. Referrals to genetic counselors or other knowledgeable medical professionals should be used to help counsel patients on the acquisition of these tests or their interpretation [138].
The National Comprehensive Cancer Network does not address the use of genomic profiling in patients with ovarian cancer in its most recent version (1.2016) [139]. Although the American Society of Clinical Oncology (ASCO) has published guidelines on biomarkers and mutational testing of breast, colorectal, and lung cancers, none currently exist for ovarian cancer [139]. The Gynecologic Cancer InterGroup (GCIG) convened in 2010 and issued a consensus statement [140] on clinical trials in ovarian cancer, recommending collection of biological specimens to clarify the role of genomic aberrations in response to therapy.. The European Society for Medical Oncology indicates in their 2013 guidelines for newly diagnosed and recurrent epithelial ovarian cancer that more research is needed to identify molecular markers which could lead to advances in personalized medicine [141].
A number of commercially available tests have become available to physicians and patients in recent years, and the list of available tests continues to grow. Among these, Caris Life Sciences® offers Caris Molecular Intelligence®, which includes multiple testing technologies including immunohistochemistry, chromogenic in situ hybridization, fluorescence in situ hybridization, 46- and 592-gene next-generation sequencing, sanger sequencing, pyro Sequencing and fragment analysis [142]. FoundationOne from Foundation Medicine also offers a genomic profile on solid and liquid tumors using next-generation sequencing, which has been validated [143]. The Clearity Foundation offers a “tumor blueprint” to ovarian cancer patients, which measures a panel of protein biomarkers in over 300 genes [144]. Myriad Genetics as well as other companies offer comprehensive analysis of the spectrum of cancer predisposition genes that are likely to represent markers of sensitivity to PARP inhibitor.
3.0 Conclusion
The genomic architecture of ovarian cancer is heterogenous and complex. Our understanding of this genomically unstable disease has rapidly increased with comprehensive genomic profiling using next-generation sequencing. At this juncture, we continue to acquire more data to identify alterations within a tumor that could potentially be targeted with novel therapeutics to offer hope when current cytotoxic regimens fall short. Within the next decade, we predict that cancer therapy will begin to shift away from empiric chemotherapy in a one-sized fits all approach to treatment. Rather, we will begin to test agents less traditional clinical trial designs and approach treatment from a molecular phenotype as opposed to the tissue of origin. These opportunities will not only apply to targeted therapy but to immuno-oncology agents as well as rational combinations of the two. Importantly, the true utility of targeted and immunotherapy will only be realized in complex diseases such as ovarian cancer through the identification and implementation of rational combination therapies. It is unlikely that monotherapy approaches will demonstrate marked activity in complex epithelial cancers such as ovarian cancer that demonstrate a paucity of molecular drivers. Our current armamentarium of targeted therapies falls short, but with the possibilities of druggable targets, we will continue to accelerate development of therapeutics to offer more than hope to patients with ovarian cancer.
4.0 Expert commentary
Traditional clinical trials are giving way to newer trial designs, such as basket trials. Basket trials are designed to assess efficacy of a single drug in patients with various types of malignancies that contain a single genomic alteration. They are independent of histology and biomarker selected and driven, which is based on the hypothesis that biomarkers predict respond to targeted therapies across a variety of cancer types [156]. The National Cancer Institute’s (NCI) Molecular Analysis for Therapy Choice (MATCH) trial is an ongoing clinical trial that includes patients with any solid tumor or lymphoma that have a targetable genomic aberration. Patients are matched in a series of single arm Phase II studies with a targeted agent without regard to the cancer’s tissue of origin. This basket trial enables more efficient testing of potential targets [152]. Consideration to other trial types should be given including “window-of-opportunity” trials. In this trial design, women with newly diagnosed ovarian cancer (as confirmed by pathologic biopsy), would receive a study drug for one to two weeks between the time of biopsy and surgical resection. This allows for evaluation of the targets modulated by the study drug after a given period of exposure time and determine if additional modifications to clinical development should be considered. Additionally, pharmacokinetic assessments can be made on post-resection samples. Alternatively, if the woman received neoadjuvant therapy instead of upfront surgical resection, the biological agent can be added along with traditional chemotherapy for a longer period of time prior to surgery. One of the primary reasons alternative clinical trial designs must be considered is that traditional ways of evaluating response through Response Evaluation Criteria in Solid Tumors (RECIST) criteria, invalid conclusions may be reached about the potential benefit of an investigational agent. Pre-surgical studies such as these may further expedite approval of these agents because they shed light on the biologic effect of the drug early in the development, rather than waiting for the results of Phase II and III studies. Furthermore, they contribute to the validation of markers that will help clinicians identify which patients will best benefit from this type of therapy.
In ovarian cancer patients whose tumors possess actionable mutations, most are not treated on clinical trials with investigational agents. However, clinical trials are frequently open in only a subset of institutions, which limits the diffusion of these therapies, even if they appear to be efficacious. Whether or not insurance carriers will provide coverage to routine profiling of tumors and treatment with an agent off trial if an actionable target is uncovered, remains to be seen. Larger ethical questions of who should be tested and treated, when profiling should occur, and how cost influences these treatment decisions will need to be answered. Other ethical considerations to be addressed will include the possibility that a germline sequence variant is uncovered during a comprehensive genomic profile. How will this information be disseminated to patients and their families?
5.0 Five-year view
The modern clinician faces an emerging problem: how to utilize the sequencing data to select the best therapy for their patient with recurrent ovarian cancer. At the time of this review, targeted, biomarker direct therapy for the treatment of ovarian cancer, with the exception of PARP inhibitors, remains investigational. While most actionable alterations will continue to be treated on protocol, the emergence of multi-disciplinary tumor boards and changes in the way we design clinical trial are among trends in the near future.
The use of genomic profiling to inform therapeutic decisions is currently being undertaken in gynecologic and ovarian cancers through the creation of institutional molecular tumor boards. Another institutional experience with a formalized precision medicine program was published from the University of Oklahoma Health Sciences Center and 62 women were profiled with FoundationOne testing (31 of which were ovarian cancer tumors). Molecular profiling resulted in identification of only a few actionable mutations and so far, only four patients have had therapies matched to their actionable mutations [145].
Several studies have been published that suggest improved clinical benefit in patients with advanced cancer (including lung and breast cancer and melanoma) who received genomically targeted therapy in clinical trials over those patients who do not [146–149]. In 2007, the Initiative for Molecular Profiling and Advanced Cancer Therapy was launched for patients who were referred to the Phase I Program at The University of Texas MD Anderson Cancer Center (Houston, TX). This project sought to use specific targeted therapies to treat patients whose tumors contained targetable alterations on phase I trials. Previous studies found longer time to treatment failure in patients who received matched therapy, high overall response rates, and longer survival [150]. A validation analysis was performed confirming previous observations that in the matched therapy group, a two-month landmark analysis demonstrated that responders have longer survival and progression free survival [151]. However, few of the tumors in these studies were ovarian cancers due to the paucity of actionable mutations and the lack of access to PARP inhibitors for BRCA1/2 mutations during these trials.
Personalized and precision medicine will continue to impact the cancer treatment of patients over the next five years. A meta-analysis of 570 phase II studies (including 32,149 patients) of single agents over three years compared arms of personalized treatment strategies versus no personalization. A personalized strategy was an independent predictor of better outcomes and fewer deaths related to toxicities. Non-personalized targeted therapies had worse outcomes than standard chemotherapy [9]. Several worldwide randomized clinical trials are currently ongoing, including MATCH, I-SPY 2, and PROSPECT [152–154]. These trials are using genomic information to inform treatment decisions in addition to the feasibility and clinical utility of these tests. ASCO has also begun recruiting patients to the Targeted Agent and Profiling Utilization Registry (TAPUR) study, which is a non-randomized clinical trial evaluating the efficacy and safety of targeted therapies prescribed to patients whose tumors have an actionable variant. The primary endpoint for the study is objective response rate or stable disease at 16 weeks, but other endpoints include progression free survival, overall survival, time on treatment, and adverse events [142]. It is important to note that the only randomized phase II trial to date, SHIVA, failed to demonstrate a benefit for targeted therapy [155]. There are many potential reasons for this failure, however, it does supply a cautionary note.
6.0 Key issues
Tumors are undergoing reclassification from site of origin to their genetic criteria.
Genomic profiling should not guide decision making for therapy unless a patient is identified to have a BRCA1 or BRCA2 mutation or has enrolled on a clinical trial.
Not all somatic or germline mutations are druggable or actionable.
Companion biomarkers that predict whether a patient will respond to a current therapy should be developed in parallel with targeted agents.
The primary goal of molecular targeted therapy is to match tumors from patients that harbor specific alterations with a matched therapy to induce a response and impact survival.
Other types of clinical trial designs, such as basket trials, are designed to assess efficacy of a single drug in patients with various types of malignancies that contain a single genomic alteration.
Not all molecular events that function as driver mutations in one cancer may do so in another cancer, and vice versa. In fact, specific alterations in one type of cancer may have opposite behavior in another cancer type and may affect treatment.
Predictive biomarkers predict how a patient will respond to a particular intervention, while prognostic biomarkers are those than characterize the outcome, such as survival.
Acknowledgments
Funding
R.A. Previs is supported by NIH T32 Training Grant CA101642. S.N. Westin is supported by the Andrew Sabin Family Fellowship and NIH K12 Calabresi Scholar Award (K12 CA088084). This research was also supported in part by the NCI (P50 CA083639, UH3 TR000943, CA109298) to S.N. Westin, A.K. Sood, G.B. Mills and the NCI Cancer Center Support Grant (P30 CA016672) to MD Anderson Cancer Center.
Footnotes
Declaration of Interest
S.N. Westin is a consultant for AstraZeneca, Clovis, Medivation, and Roche/Genentech, and receives research support from AstraZeneca, Critical Outcomes Technologies Inc., and Novartis. G.B. Mills’ financial relationships include that he is an SAB/Consultant for AstraZeneca, Critical Outcome Technologies, Ionis Pharmaceuticals, Nuevolution, Symphogen, Takeda/Millennium Pharmaceuticals, and TTarveda. His stock/options/financial COI include ImmunoMet, a Licensed Technology HRD assay to Myriad Genetics. He has sponsored research with AstraZeneca, Critical Outcomes Technology, Illumina, Nanostring, Takeda/Millennium Pharmaceuticals, and Tesaro. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Young RC, et al. cis-Dichlorodiammineplatinum(II) for the treatment of advanced ovarian cancer. Cancer Treat Rep. 1979;63(9–10):1539–44. [PubMed] [Google Scholar]
- 2.McGuire WP, et al. Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer. N Engl J Med. 1996;334(1):1–6. doi: 10.1056/NEJM199601043340101. [DOI] [PubMed] [Google Scholar]
- 3.Katsumata N, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374(9698):1331–8. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 4.Young RC, et al. Staging laparotomy in early ovarian cancer. JAMA. 1983;250(22):3072–6. [PubMed] [Google Scholar]
- 5.Chang SJ, et al. Survival impact of complete cytoreduction to no gross residual disease for advanced-stage ovarian cancer: a meta-analysis. Gynecol Oncol. 2013;130(3):493–8. doi: 10.1016/j.ygyno.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 6.Garraway LA, Verweij J, Ballman KV. Precision oncology: an overview. J Clin Oncol. 2013;31(15):1803–5. doi: 10.1200/JCO.2013.49.4799. [DOI] [PubMed] [Google Scholar]
- 7.Werner HM, Mills GB, Ram PT. Cancer Systems Biology: a peek into the future of patient care? Nat Rev Clin Oncol. 2014;11(3):167–76. doi: 10.1038/nrclinonc.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubin EH, et al. Developing precision medicine in a global world. Clin Cancer Res. 2014;20(6):1419–27. doi: 10.1158/1078-0432.CCR-14-0091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Schwaederle M, et al. Impact of Precision Medicine in Diverse Cancers: A Meta-Analysis of Phase II Clinical Trials. J Clin Oncol. 2015;33(32):3817–25. doi: 10.1200/JCO.2015.61.5997. This meta-analysis of phase II studies included 570 studies and over 32,000 patients that were treated with a single agent, and showed that across malignancies, a personalized treatment strategy was an independent predict of better outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ciriello G, et al. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45(10):1127–33. doi: 10.1038/ng.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cancer Genome Atlas Research, N., et al. The Cancer Genome Atlas Pan-Cancer analysis project. Nat Genet. 2013;45(10):1113–20. doi: 10.1038/ng.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dienstmann R, et al. Genomic medicine frontier in human solid tumors: prospects and challenges. J Clin Oncol. 2013;31(15):1874–84. doi: 10.1200/JCO.2012.45.2268. [DOI] [PubMed] [Google Scholar]
- 13.Mendelsohn J. Personalizing oncology: perspectives and prospects. J Clin Oncol. 2013;31(15):1904–11. doi: 10.1200/JCO.2012.45.3605. [DOI] [PubMed] [Google Scholar]
- 14.Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–5. doi: 10.1056/NEJMp1500523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyerson M, Gabriel S, Getz G. Advances in understanding cancer genomes through second-generation sequencing. Nat Rev Genet. 2010;11(10):685–96. doi: 10.1038/nrg2841. [DOI] [PubMed] [Google Scholar]
- 16.Mardis ER. A decade’s perspective on DNA sequencing technology. Nature. 2011;470(7333):198–203. doi: 10.1038/nature09796. [DOI] [PubMed] [Google Scholar]
- 17.Meric-Bernstam F, et al. A decision support framework for genomically informed investigational cancer therapy. J Natl Cancer Inst. 2015;107(7) doi: 10.1093/jnci/djv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurman RJ. Origin and molecular pathogenesis of ovarian high-grade serous carcinoma. Ann Oncol. 2013;24(Suppl 10):16–21. doi: 10.1093/annonc/mdt463. [DOI] [PubMed] [Google Scholar]
- 19.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164(5):1511–8. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurman RJ, Shih Ie M. Molecular pathogenesis and extraovarian origin of epithelial ovarian cancer--shifting the paradigm. Hum Pathol. 2011;42(7):918–31. doi: 10.1016/j.humpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurman RJ, Shih Ie M. The Dualistic Model of Ovarian Carcinogenesis: Revisited, Revised, and Expanded. Am J Pathol. 2016;186(4):733–47. doi: 10.1016/j.ajpath.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singer G, et al. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95(6):484–6. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- 23.Wang ZC, et al. Profiles of genomic instability in high-grade serous ovarian cancer predict treatment outcome. Clin Cancer Res. 2012;18(20):5806–15. doi: 10.1158/1078-0432.CCR-12-0857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ledford H. Big science: The cancer genome challenge. Nature. 2010;464(7291):972–4. doi: 10.1038/464972a. [DOI] [PubMed] [Google Scholar]
- 25.International Cancer Genome, C., et al. International network of cancer genome projects. Nature. 2010;464(7291):993–8. doi: 10.1038/nature08987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Cancer Genome Atlas Research. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–15. doi: 10.1038/nature10166. The Cancer Genome Atlas project involved profiling of 489 high grade serous ovarian carcinoma, and delineated four ovarian cancer subtypes, reported of the frequency of TP53 mutations and other recurrent somatic mutations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bowtell DD. The genesis and evolution of high-grade serous ovarian cancer. Nat Rev Cancer. 2010;10(11):803–8. doi: 10.1038/nrc2946. [DOI] [PubMed] [Google Scholar]
- 28.Vang R, et al. Molecular Alterations of TP53 are a Defining Feature of Ovarian High-Grade Serous Carcinoma: A Rereview of Cases Lacking TP53 Mutations in The Cancer Genome Atlas Ovarian Study. Int J Gynecol Pathol. 2016;35(1):48–55. doi: 10.1097/PGP.0000000000000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hennessy BT, et al. Somatic mutations in BRCA1 and BRCA2 could expand the number of patients that benefit from poly (ADP ribose) polymerase inhibitors in ovarian cancer. J Clin Oncol. 2010;28(22):3570–6. doi: 10.1200/JCO.2009.27.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walsh T, et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proc Natl Acad Sci. 2011;108(44):18032–7. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tothill RW, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008;14(16):5198–208. doi: 10.1158/1078-0432.CCR-08-0196. [DOI] [PubMed] [Google Scholar]
- 32.Wiegand KC, et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N Engl J Med. 2010;363(16):1532–43. doi: 10.1056/NEJMoa1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones S, et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science. 2010;3306001:228–31. doi: 10.1126/science.1196333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen J, et al. ARID1A Deficiency Impairs the DNA Damage Checkpoint and Sensitizes Cells to PARP Inhibitors. Cancer Discov. 2015;5(7):752–67. doi: 10.1158/2159-8290.CD-14-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bitler BG, et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med. 2015;21(3):231–8. doi: 10.1038/nm.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etemadmoghadam D, et al. Integrated genome-wide DNA copy number and expression analysis identifies distinct mechanisms of primary chemoresistance in ovarian carcinomas. Clin Cancer Res. 2009;15(4):1417–27. doi: 10.1158/1078-0432.CCR-08-1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakayama N, et al. Gene amplification CCNE1 is related to poor survival and potential therapeutic target in ovarian cancer. Cancer. 2010;116(11):2621–34. doi: 10.1002/cncr.24987. [DOI] [PubMed] [Google Scholar]
- 38.Etemadmoghadam D, et al. Amplicon-dependent CCNE1 expression is critical for clonogenic survival after cisplatin treatment and is correlated with 20q11 gain in ovarian cancer. PLoS One. 2010;5(11):e15498. doi: 10.1371/journal.pone.0015498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McAlpine JN, et al. HER2 overexpression and amplification is present in a subset of ovarian mucinous carcinomas and can be targeted with trastuzumab therapy. BMC Cancer. 2009;9:433. doi: 10.1186/1471-2407-9-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wheler JJ, et al. TP53 Alterations Correlate with Response to VEGF/VEGFR Inhibitors: Implications for Targeted Therapeutics. Mol Cancer Ther. 2016;15(10):2475–2485. doi: 10.1158/1535-7163.MCT-16-0196. [DOI] [PubMed] [Google Scholar]
- 41.Makar AP, et al. Prognostic value of pre- and postoperative serum CA 125 levels in ovarian cancer: new aspects and multivariate analysis. Obstet Gynecol. 1992;79(6):1002–10. [PubMed] [Google Scholar]
- 42.Landen CN, Kinch MS, Sood AK. EphA2 as a target for ovarian cancer therapy. Expert Opin Ther Targets. 2005;9(6):1179–87. doi: 10.1517/14728222.9.6.1179. [DOI] [PubMed] [Google Scholar]
- 43.Merritt WM, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med. 2008;359(25):2641–50. doi: 10.1056/NEJMoa0803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobel M, et al. Ovarian carcinoma subtypes are different diseases: implications for biomarker studies. PLoS Med. 2008;5(12):e232. doi: 10.1371/journal.pmed.0050232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kalloger SE, et al. Calculator for ovarian carcinoma subtype prediction. Mod Pathol. 2011;24(4):512–21. doi: 10.1038/modpathol.2010.215. [DOI] [PubMed] [Google Scholar]
- 46.Campbell PJ, et al. The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature. 2010;467(7319):1109–13. doi: 10.1038/nature09460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47*.Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366(10):883–92. doi: 10.1056/NEJMoa1113205. Single tumor biopsy samples may underestimate a tumor’s heterogeneity and may present future challenges to personalized medicine and the development of biomarkers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashworth A, Lord CJ, Reis-Filho JS. Genetic interactions in cancer progression and treatment. Cell. 2011;145(1):30–8. doi: 10.1016/j.cell.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 49.Turner NC, Reis-Filho JS. Genetic heterogeneity and cancer drug resistance. Lancet Oncol. 2012;13(4):e178–85. doi: 10.1016/S1470-2045(11)70335-7. [DOI] [PubMed] [Google Scholar]
- 50.Slamon DJ, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 51.Druker BJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14):1031–7. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 52.Lynch TJ, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 53.Flaherty KT, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363(9):809–19. doi: 10.1056/NEJMoa1002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kwak EL, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.D’Arcangelo M, Wynes MW, Hirsch FR. The role of anaplastic lymphoma kinase inhibitors in the treatment of advanced nonsmall cell lung cancer. Curr Opin Oncol. 2013;25(2):121–9. doi: 10.1097/CCO.0b013e32835d8175. [DOI] [PubMed] [Google Scholar]
- 56.Rudin CM, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361(12):1173–8. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sekulic A, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366(23):2171–9. doi: 10.1056/NEJMoa1113713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Douillard JY, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 59.Bang YJ, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–97. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 60.Bookman MA, et al. Evaluation of monoclonal humanized anti-HER2 antibody, trastuzumab, in patients with recurrent or refractory ovarian or primary peritoneal carcinoma with overexpression of HER2: a phase II trial of the Gynecologic Oncology Group. J Clin Oncol. 2003;21(2):283–90. doi: 10.1200/JCO.2003.10.104. [DOI] [PubMed] [Google Scholar]
- 61.Fleming GF, et al. Phase II trial of trastuzumab in women with advanced or recurrent, HER2-positive endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2010;116(1):15–20. doi: 10.1016/j.ygyno.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kopetz S, et al. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J Clin Oncol. 2015;33(34):4032–8. doi: 10.1200/JCO.2015.63.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Westin JR, Kurzrock R. It’s about time: lessons for solid tumors from chronic myelogenous leukemia therapy. Mol Cancer Ther. 2012;11(12):2549–55. doi: 10.1158/1535-7163.MCT-12-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vogelstein B, et al. Cancer genome landscapes. Science. 2013;3396127:1546–58. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Westin JR. Busting robustness: using cancer’s greatest strength to our advantage. Future Oncol. 2015;11(1):73–7. doi: 10.2217/fon.14.49. [DOI] [PubMed] [Google Scholar]
- 66.Perou CM, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 67.Sorlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci. 2001;98(19):10869–74. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berchuck A, et al. Patterns of gene expression that characterize long-term survival in advanced stage serous ovarian cancers. Clin Cancer Res. 2005;11(10):3686–96. doi: 10.1158/1078-0432.CCR-04-2398. [DOI] [PubMed] [Google Scholar]
- 69.Jazaeri AA, et al. Gene expression profiles associated with response to chemotherapy in epithelial ovarian cancers. Clin Cancer Res. 2005;11(17):6300–10. doi: 10.1158/1078-0432.CCR-04-2682. [DOI] [PubMed] [Google Scholar]
- 70.Hartmann LC, et al. Gene expression profiles predict early relapse in ovarian cancer after platinum-paclitaxel chemotherapy. Clin Cancer Res. 2005;11(6):2149–55. doi: 10.1158/1078-0432.CCR-04-1673. [DOI] [PubMed] [Google Scholar]
- 71.Sabatier R, et al. A seven-gene prognostic model for platinum-treated ovarian carcinomas. Br J Cancer. 2011;105(2):304–11. doi: 10.1038/bjc.2011.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yoshihara K, et al. Gene expression profile for predicting survival in advanced-stage serous ovarian cancer across two independent datasets. PLoS One. 2010;5(3):e9615. doi: 10.1371/journal.pone.0009615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yoshihara K, et al. High-risk ovarian cancer based on 126-gene expression signature is uniquely characterized by downregulation of antigen presentation pathway. Clin Cancer Res. 2012;18(5):1374–85. doi: 10.1158/1078-0432.CCR-11-2725. [DOI] [PubMed] [Google Scholar]
- 74.Bonome T, et al. A gene signature predicting for survival in suboptimally debulked patients with ovarian cancer. Cancer Res. 2008;68(13):5478–86. doi: 10.1158/0008-5472.CAN-07-6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leong HS, et al. Efficient molecular subtype classification of high-grade serous ovarian cancer. J Pathol. 236(3):272–7. doi: 10.1002/path.4536. 201. [DOI] [PubMed] [Google Scholar]
- 76.Patch AM, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature. 2015;521(7553):489–94. doi: 10.1038/nature14410. [DOI] [PubMed] [Google Scholar]
- 77.Abdallah BY, et al. Ovarian cancer evolution through stochastic genome alterations: defining the genomic role in ovarian cancer. Syst Biol Reprod Med. 2014;60(1):2–13. doi: 10.3109/19396368.2013.837989. [DOI] [PubMed] [Google Scholar]
- 78.Cope L, et al. High level of chromosomal aberration in ovarian cancer genome correlates with poor clinical outcome. Gynecol Oncol. 2013;128(3):500–5. doi: 10.1016/j.ygyno.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Heng HH, et al. Chromosomal instability (CIN): what it is and why it is crucial to cancer evolution. Cancer Metastasis Rev. 2013;32(3–4): 325–40. doi: 10.1007/s10555-013-9427-7. [DOI] [PubMed] [Google Scholar]
- 80.Stepanenko AA, V, Kavsan M. Immortalization and malignant transformation of eukaryotic cells. Tsitol Genet. 2012;46(2):36–75. [PubMed] [Google Scholar]
- 81.Watson J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol. 2013;3(1):120144. doi: 10.1098/rsob.120144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heng HH, et al. Evolutionary mechanisms and diversity in cancer. Adv Cancer Res. 2011;112:217–53. doi: 10.1016/B978-0-12-387688-1.00008-9. [DOI] [PubMed] [Google Scholar]
- 83.Heng HH, et al. Genetic and epigenetic heterogeneity in cancer: the ultimate challenge for drug therapy. Curr Drug Targets. 2010;11(10):1304–16. doi: 10.2174/1389450111007011304. [DOI] [PubMed] [Google Scholar]
- 84.Stephens PJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144(1):27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kohn EC. Molecular profiling and commercial predication assays in ovarian cancer: still not ready for prime time? Am Soc Clin Oncol Educ Book. 2014:139–47. doi: 10.14694/EdBook_AM.2014.34.139. [DOI] [PubMed] [Google Scholar]
- 86.Pal T, et al. BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer. 2005;104(12):2807–16. doi: 10.1002/cncr.21536. [DOI] [PubMed] [Google Scholar]
- 87.Schrader KA, et al. Germline BRCA1 and BRCA2 mutations in ovarian cancer: utility of a histology-based referral strategy. Obstet Gynecol. 2012;120(2 Pt 1):235–40. doi: 10.1097/AOG.0b013e31825f3576. [DOI] [PubMed] [Google Scholar]
- 88**.SGO Clinical Practce Statement: Genetic Testing for Ovarian Cancer. 2014 Oct; Available at: https://www.sgo.org/clinical-practice/guidelines/genetic-testing-for-ovarian-cancer/. [cited 2016 July 6] This is a Clinical Practice Statement issued by the Society of Gynecologic Oncology about the role of genetic counseling and testing for all women who are diagnosed with epithelial ovarian, fallopian tube, and peritoneal cancers in the current practice of gynecologic oncology.
- 89.Walsh T, et al. Spectrum of mutations in BRCA1, BRCA2, CHEK2, and TP53 in families at high risk of breast cancer. JAMA. 2006;295(12):1379–88. doi: 10.1001/jama.295.12.1379. [DOI] [PubMed] [Google Scholar]
- 90.Turner N, Tutt A, Ashworth A. Hallmarks of ‘BRCAness’ in sporadic cancers. Nat Rev Cancer. 2004;4(10):814–9. doi: 10.1038/nrc1457. [DOI] [PubMed] [Google Scholar]
- 91.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 92.Patel AG, Sarkaria JN, Kaufmann SH. Nonhomologous end joining drives poly(ADP-ribose) polymerase (PARP) inhibitor lethality in homologous recombination-deficient cells. Proc Natl Acad Sci. 2011;108(8):3406–11. doi: 10.1073/pnas.1013715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nat Rev Genet. 2007;8(10):735–48. doi: 10.1038/nrg2159. [DOI] [PubMed] [Google Scholar]
- 94.Murai J, et al. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012;72(21):5588–99. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tutt A, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376(9737):235–44. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 96.Kaufman B, et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33(3):244–50. doi: 10.1200/JCO.2014.56.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ledermann J, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: a preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15(8):852–61. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 98.Matulonis UA, et al. Olaparib maintenance therapy in patients with platinum-sensitive, relapsed serous ovarian cancer and a BRCA mutation: Overall survival adjusted for postprogression poly(adenosine diphosphate ribose) polymerase inhibitor therapy. Cancer. 2016;122(12):1844–52. doi: 10.1002/cncr.29995. [DOI] [PubMed] [Google Scholar]
- 99.Coleman RL, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation - An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;137(3):386–91. doi: 10.1016/j.ygyno.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Abkevich V, et al. Patterns of genomic loss of heterozygosity predict homologous recombination repair defects in epithelial ovarian cancer. Br J Cancer. 2012;107(10):1776–82. doi: 10.1038/bjc.2012.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.McNeish IA, et al. Results of ARIEL2: A Phase 2 trial to prospectively identify ovarian cancer patients likely to respond to rucaparib using tumor genetic analysis. Journal Clin Oncol. 2015;33(15) [Google Scholar]
- 102.Lord CJ, Ashworth A. Mechanisms of resistance to therapies targeting BRCA-mutant cancers. Nat Med. 2013;19(11):1381–8. doi: 10.1038/nm.3369. [DOI] [PubMed] [Google Scholar]
- 103.Sakai W, et al. Secondary mutations as a mechanism of cisplatin resistance in BRCA2-mutated cancers. Nature. 2008;451(7182):1116–20. doi: 10.1038/nature06633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Edwards SL, et al. Resistance to therapy caused by intragenic deletion in BRCA2. Nature. 2008;451(7182):1111–5. doi: 10.1038/nature06548. [DOI] [PubMed] [Google Scholar]
- 105.Cao L, et al. A selective requirement for 53BP1 in the biological response to genomic instability induced by Brca1 deficiency. Mol Cell. 2009;35(4):534–41. doi: 10.1016/j.molcel.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bunting SF, et al. BRCA1 functions independently of homologous recombination in DNA interstrand crosslink repair. Mol Cel. 2012;46(2):125–35. doi: 10.1016/j.molcel.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choi YH, Yu AM. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des. 2014;20(5):793–807. doi: 10.2174/138161282005140214165212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pettitt SJ, et al. A genetic screen using the PiggyBac transposon in haploid cells identifies Parp1 as a mediator of olaparib toxicity. PLoS One. 2013;8(4):e61520. doi: 10.1371/journal.pone.0061520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Patel AG, et al. Enhanced killing of cancer cells by poly(ADP-ribose) polymerase inhibitors and topoisomerase I inhibitors reflects poisoning of both enzymes. J Biol Chem. 2012;287(6): 4198–210. doi: 10.1074/jbc.M111.296475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pao W, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Choi YL, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363(18):1734–9. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 112.Johnson DB, et al. Enabling a genetically informed approach to cancer medicine: a retrospective evaluation of the impact of comprehensive tumor profiling using a targeted next-generation sequencing panel. Oncologist. 2014;19(6):616–22. doi: 10.1634/theoncologist.2014-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meric-Bernstam F, et al. Feasibility of Large-Scale Genomic Testing to Facilitate Enrollment Onto Genomically Matched Clinical Trials. J Clin Oncol. 2015;33(25):2753–62. doi: 10.1200/JCO.2014.60.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Farley J, et al. Selumetinib in women with recurrent low-grade serous carcinoma of the ovary or peritoneum: an open-label, single-arm, phase 2 study. Lancet Oncol. 2013;14(2):134–40. doi: 10.1016/S1470-2045(12)70572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gershenson DM, et al. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol Oncol. 2012;125(3):661–6. doi: 10.1016/j.ygyno.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Anglesio MS, et al. IL6-STAT3-HIF signaling and therapeutic response to the angiogenesis inhibitor sunitinib in ovarian clear cell cancer. Clin Cancer Res. 2011;17(8):2538–48. doi: 10.1158/1078-0432.CCR-10-3314. [DOI] [PubMed] [Google Scholar]
- 117.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28(6):1075–83. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wu R, et al. Preclinical testing of PI3K/AKT/mTOR signaling inhibitors in a mouse model of ovarian endometrioid adenocarcinoma. Clin Cancer Res. 2011;17(23): 7359–72. doi: 10.1158/1078-0432.CCR-11-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8): 606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 120.Trinh XB, et al. The rationale for mTOR inhibition in epithelial ovarian cancer. Expert Opin Investig Drugs. 2009;18(12):1885–91. doi: 10.1517/13543780903321508. [DOI] [PubMed] [Google Scholar]
- 121.Kudoh A, et al. Dual inhibition of phosphatidylinositol 3′-kinase and mammalian target of rapamycin using NVP-BEZ235 as a novel therapeutic approach for mucinous adenocarcinoma of the ovary. Int J Gynecol Cancer. 2014;24(3):444–53. doi: 10.1097/IGC.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 122.Santiskulvong C, et al. Dual targeting of phosphoinositide 3-kinase and mammalian target of rapamycin using NVP-BEZ235 as a novel therapeutic approach in human ovarian carcinoma. Clin Cancer Res. 2011;17(8):2373–84. doi: 10.1158/1078-0432.CCR-10-2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mabuchi S, et al. mTOR is a promising therapeutic target both in cisplatin-sensitive and cisplatin-resistant clear cell carcinoma of the ovary. Clin Cancer Res. 2009;15(17):5404–13. doi: 10.1158/1078-0432.CCR-09-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fu S, et al. Perifosine plus docetaxel in patients with platinum and taxane resistant or refractory high-grade epithelial ovarian cancer. Gynecol Oncol. 2012;126(1): 47–53. doi: 10.1016/j.ygyno.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Behbakht K, et al. Phase II trial of the mTOR inhibitor, temsirolimus and evaluation of circulating tumor cells and tumor biomarkers in persistent and recurrent epithelial ovarian and primary peritoneal malignancies: a Gynecologic Oncology Group study. Gynecol Oncol. 2011;123(1):19–26. doi: 10.1016/j.ygyno.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Farley JH, BW, Fujiwara K. First-Line Therapy in the Treatment of Stage III–IV Clear Cell Carcinoma of the Ovary. J Clin Oncol. 2016;34(suppl) abstr 5531. [Google Scholar]
- 127.Ramos P, et al. Small cell carcinoma of the ovary, hypercalcemic type, displays frequent inactivating germline and somatic mutations in SMARCA4. Nat Genet. 2014;46(5): 427–9. doi: 10.1038/ng.2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Witkowski L, et al. Germline and somatic SMARCA4 mutations characterize small cell carcinoma of the ovary, hypercalcemic type. Nat Genet. 2014;46(5):438–43. doi: 10.1038/ng.2931. [DOI] [PubMed] [Google Scholar]
- 129.Jelinic P, et al. Recurrent SMARCA4 mutations in small cell carcinoma of the ovary. Nat Genet. 2014;46(5):424–6. doi: 10.1038/ng.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Conlon N, et al. Loss of SMARCA4 Expression Is Both Sensitive and Specific for the Diagnosis of Small Cell Carcinoma of Ovary, Hypercalcemic Type. Am J Surg Pathol. 2016;40(3):395–403. doi: 10.1097/PAS.0000000000000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gordon MS, et al. Clinical activity of pertuzumab (rhuMAb 2C4), a HER dimerization inhibitor, in advanced ovarian cancer: potential predictive relationship with tumor HER2 activation status. J Clin Oncol. 2006;24(26):4324–32. doi: 10.1200/JCO.2005.05.4221. [DOI] [PubMed] [Google Scholar]
- 132.Makhija S, et al. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. J Clin Oncol. 2010;28(7):1215–23. doi: 10.1200/JCO.2009.22.3354. [DOI] [PubMed] [Google Scholar]
- 133.Kaye SB, et al. A randomized phase II study evaluating the combination of carboplatin-based chemotherapy with pertuzumab versus carboplatin-based therapy alone in patients with relapsed, platinum-sensitive ovarian cancer. Ann Oncol. 2013;24(1):145–52. doi: 10.1093/annonc/mds282. [DOI] [PubMed] [Google Scholar]
- 134.Lheureux S, et al. Expected benefits of topotecan combined with lapatinib in recurrent ovarian cancer according to biological profile: a phase 2 trial. Int J Gynecol Cancer. 2012;22(9):1483–8. doi: 10.1097/IGC.0b013e31826d1438. [DOI] [PubMed] [Google Scholar]
- 135.English DP, Roque DM, Santin AD. HER2 expression beyond breast cancer: therapeutic implications for gynecologic malignancies. Mol Diagn Ther. 2013;17(2):85–99. doi: 10.1007/s40291-013-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lanitis E, et al. Primary human ovarian epithelial cancer cells broadly express HER2 at immunologically-detectable levels. PLoS One. 2012;7(11):e49829. doi: 10.1371/journal.pone.0049829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tuefferd M, et al. HER2 status in ovarian carcinomas: a multicenter GINECO study of 320 patients. PLoS One. 2007;2(11):e1138. doi: 10.1371/journal.pone.0001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. [Last accessed 6 July 2016];SGO Clinical Practice Statement: Next Generation Cancer Gene Panels Versus Gene by Gene Testing. Available from: https://www.sgo.org/clinical-practice/guidelines/genetic-testing-for-ovarian-cancer/
- 139. [Last accessed 17 October 2016];NCCN Clinical Practice Guidelines in Oncology, Ovarian Cancer including Fallopian Tube Cancer and Primary Peritoneal Cancer. Version 1.2016. Available from: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf.
- 140.Stuart GC, et al. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer. 2011;21(4):750–5. doi: 10.1097/IGC.0b013e31821b2568. [DOI] [PubMed] [Google Scholar]
- 141.Ledermann JA, et al. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):vi24–32. doi: 10.1093/annonc/mdt333. [DOI] [PubMed] [Google Scholar]
- 142. [Last accessed 4 September 2016];Caris Molecular Intelligence. Available at: http://www.carismolecularintelligence.com/
- 143.Frampton GM, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–31. doi: 10.1038/nbt.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.The Clearity Foundation. [Last accessed 4 September 2016]; Available at: http://www.clearityfoundation.org/
- 145.Gunderson CC, et al. Initiation of a formalized precision medicine program in gynecologic oncology. Gynecol Oncol. 2016;141(1): 24–8. doi: 10.1016/j.ygyno.2016.02.024. [DOI] [PubMed] [Google Scholar]
- 146.Kris MG, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311(19):1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lovly CM, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PLoS One. 2012;7(4):e35309. doi: 10.1371/journal.pone.0035309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Andre F, et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: a multicentre, prospective trial (SAFIR01/UNICANCER) Lancet Oncol. 2014;15(3):267–74. doi: 10.1016/S1470-2045(13)70611-9. [DOI] [PubMed] [Google Scholar]
- 149.Tafe LJ, et al. Implementation of a Molecular Tumor Board: The Impact on Treatment Decisions for 35 Patients Evaluated at Dartmouth-Hitchcock Medical Center. Oncologist. 2015;20(9):1011–8. doi: 10.1634/theoncologist.2015-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tsimberidou AM, et al. Personalized medicine in a phase I clinical trials program: the MD Anderson Cancer Center initiative. Clin Cancer Res. 2012;18(22):6373–83. doi: 10.1158/1078-0432.CCR-12-1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tsimberidou AM, et al. Personalized medicine for patients with advanced cancer in the phase I program at MD Anderson: validation and landmark analyses. Clin Cancer Res. 2014;20(18): 4827–36. doi: 10.1158/1078-0432.CCR-14-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McNeil C. NCI-MATCH launch highlights new trial design in precision-medicine era. J Natl Cancer Inst. 2015;107(7) doi: 10.1093/jnci/djv193. [DOI] [PubMed] [Google Scholar]
- 153.Printz C. I-SPY 2 may change how clinical trials are conducted: researchers aim to accelerate approvals of cancer drugs. Cancer. 2013;119(11):1925–7. doi: 10.1002/cncr.28172. [DOI] [PubMed] [Google Scholar]
- 154.Weiser MR, et al. Progress in the PROSPECT trial: precision treatment for rectal cancer? Bull Am Coll Surg. 2015;100(4):51–2. [PubMed] [Google Scholar]
- 155.Le Tourneau C, et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): a multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015;16(13):1324–34. doi: 10.1016/S1470-2045(15)00188-6. [DOI] [PubMed] [Google Scholar]
- 156.Kou T, et al. The possibility of clinical sequencing in the management of cancer. J Clin Oncol. 2016;46(5):399–406. doi: 10.1093/jjco/hyw018. [DOI] [PubMed] [Google Scholar]
- 157.Domchek SM, et al. Efficacy and safety of olaparib monotherapy in germline BRCA1/2 mutation carriers with advanced ovarian cancer and three or more lines of prior therapy. Gynecol Oncol. 2016;140(2):199–203. doi: 10.1016/j.ygyno.2015.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Del Conte G, et al. Phase I study of olaparib in combination with liposomal doxorubicin in patients with advanced solid tumours. Br J Cancer. 2014;111(4):651–9. doi: 10.1038/bjc.2014.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lee JM, et al. Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2 mutation-associated breast or ovarian cancer with biomarker analyses. J Natl Cancer Inst. 2014;106(6):dju089. doi: 10.1093/jnci/dju089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Balmana J, et al. Phase I trial of olaparib in combination with cisplatin for the treatment of patients with advanced breast, ovarian and other solid tumors. Ann Oncol. 2014;25(8): 1656–63. doi: 10.1093/annonc/mdu187. [DOI] [PubMed] [Google Scholar]
- 161.Liu JF, et al. A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor olaparib (AZD2281) in combination with the anti-angiogenic cediranib (AZD2171) in recurrent epithelial ovarian or triple-negative breast cancer. Eur J Cancer. 2013;49(14):2972–8. doi: 10.1016/j.ejca.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ledermann J, et al. Olaparib maintenance therapy in platinum-sensitive relapsed ovarian cancer. N Engl J Med. 2012;366(15):1382–92. doi: 10.1056/NEJMoa1105535. [DOI] [PubMed] [Google Scholar]
- 163.Kaye SB, et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J Clin Oncol. 2012;30(4): 372–9. doi: 10.1200/JCO.2011.36.9215. [DOI] [PubMed] [Google Scholar]
- 164.Gelmon KA, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol. 2011;12(9):852–61. doi: 10.1016/S1470-2045(11)70214-5. [DOI] [PubMed] [Google Scholar]
- 165.Sandhu SK, et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;14(9): 882–92. doi: 10.1016/S1470-2045(13)70240-7. [DOI] [PubMed] [Google Scholar]
- 166.Drew Y, et al. Phase 2 multicentre trial investigating intermittent and continuous dosing schedules of the poly(ADP-ribose) polymerase inhibitor rucaparib in germline BRCA mutation carriers with advanced ovarian and breast cancer. Br J Cancer. 2016;114(7): 723–30. doi: 10.1038/bjc.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kummar S, et al. Randomized Trial of Oral Cyclophosphamide and Veliparib in High-Grade Serous Ovarian, Primary Peritoneal, or Fallopian Tube Cancers, or BRCA-Mutant Ovarian Cancer. Clin Cancer Res. 2015;21(7):1574–82. doi: 10.1158/1078-0432.CCR-14-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Reiss KA, et al. A Phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy in patients with advanced solid malignancies and peritoneal carcinomatosis. Clin Cancer Res. 2015;21(1): 68–76. doi: 10.1158/1078-0432.CCR-14-1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kummar S, et al. A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res. 2012;18(6): 1726–34. doi: 10.1158/1078-0432.CCR-11-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schilder RJ, et al. Phase II study of gefitinib in patients with relapsed or persistent ovarian or primary peritoneal carcinoma and evaluation of epidermal growth factor receptor mutations and immunohistochemical expression: a Gynecologic Oncology Group Study. Clin Cancer Res. 2005;11(15):5539–48. doi: 10.1158/1078-0432.CCR-05-0462. [DOI] [PubMed] [Google Scholar]
- 171.Pautier P, et al. Phase II study of gefitinib in combination with paclitaxel (P) and carboplatin (C) as second-line therapy for ovarian, tubal or peritoneal adenocarcinoma (1839IL/0074) Gynecol Oncol. 2010;116(2): 157–62. doi: 10.1016/j.ygyno.2009.10.076. [DOI] [PubMed] [Google Scholar]
- 172.Posadas EM, et al. A phase II and pharmacodynamic study of gefitinib in patients with refractory or recurrent epithelial ovarian cancer. Cancer. 2007;109(7): 1323–30. doi: 10.1002/cncr.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wagner U, et al. Gefitinib in combination with tamoxifen in patients with ovarian cancer refractory or resistant to platinum-taxane based therapy--a phase II trial of the AGO Ovarian Cancer Study Group (AGO-OVAR 2.6) Gynecol Oncol. 2007;105(1):132–7. doi: 10.1016/j.ygyno.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 174.Gordon AN, et al. Efficacy and safety of erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR) tyrosine kinase inhibitor, in patients with advanced ovarian carcinoma: results from a phase II multicenter study. Int J Gynecol Cancer. 2005;15(5):785–92. doi: 10.1111/j.1525-1438.2005.00137.x. [DOI] [PubMed] [Google Scholar]
- 175.Nimeiri HS, et al. Efficacy and safety of bevacizumab plus erlotinib for patients with recurrent ovarian, primary peritoneal, and fallopian tube cancer: a trial of the Chicago, PMH, and California Phase II Consortia. Gynecol Oncol. 2008;110(1): 49–55. doi: 10.1016/j.ygyno.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Blank SV, et al. Erlotinib added to carboplatin and paclitaxel as first-line treatment of ovarian cancer: a phase II study based on surgical reassessment. Gynecol Oncol. 2010;119(3): 451–6. doi: 10.1016/j.ygyno.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Chambers SK, et al. Overexpression of tumor vascular endothelial growth factor A may portend an increased likelihood of progression in a phase II trial of bevacizumab and erlotinib in resistant ovarian cancer. Clin Cancer Res. 2010;16(21):5320–8. doi: 10.1158/1078-0432.CCR-10-0974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hirte H, et al. A phase II study of erlotinib (OSI-774) given in combination with carboplatin in patients with recurrent epithelial ovarian cancer (NCIC CTG IND.149) Gynecol Oncol. 2010;118(3): 308–12. doi: 10.1016/j.ygyno.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 179.Vergote IB, et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: a European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study. J Clin Oncol. 2014;32(4): 320–6. doi: 10.1200/JCO.2013.50.5669. [DOI] [PubMed] [Google Scholar]
- 180.Schilder RJ, et al. Phase II trial of single agent cetuximab in patients with persistent or recurrent epithelial ovarian or primary peritoneal carcinoma with the potential for dose escalation to rash. Gynecol Oncol. 2009;113(1): 21–7. doi: 10.1016/j.ygyno.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Secord AA, et al. Phase II trial of cetuximab and carboplatin in relapsed platinum-sensitive ovarian cancer and evaluation of epidermal growth factor receptor expression: a Gynecologic Oncology Group study. Gynecol Oncol. 2008;108(3): 493–9. doi: 10.1016/j.ygyno.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wong KK, et al. Significantly greater expression of ER, PR, and ECAD in advanced-stage low-grade ovarian serous carcinoma as revealed by immunohistochemical analysis. Int J Gynecol Pathol. 2007;26(4):404–9. doi: 10.1097/pgp.0b013e31803025cd. [DOI] [PubMed] [Google Scholar]
- 183.Kimball KJ, et al. A phase I study of lapatinib in combination with carboplatin in women with platinum sensitive recurrent ovarian carcinoma. Gynecol Oncol. 2008;111(1): 95–101. doi: 10.1016/j.ygyno.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 184.Elser C, HH, Kaizer L, et al. Phase II study of MKC-1 in patients with metastatic or resistant epithelial ovarian cancer or advanced endometrial cancer. J Clin Oncol. 2009;27(15s) [Google Scholar]
- 185.Secord AA, et al. A phase I trial of dasatinib, an SRC-family kinase inhibitor, in combination with paclitaxel and carboplatin in patients with advanced or recurrent ovarian cancer. Clin Cancer Res. 2012;18(19): 5489–98. doi: 10.1158/1078-0432.CCR-12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.McNeish IA, et al. A randomised, placebo-controlled trial of weekly paclitaxel and saracatinib (AZD0530) in platinum-resistant ovarian, fallopian tube or primary peritoneal cancerdagger. Ann Oncol. 2014;25(10): 1988–95. doi: 10.1093/annonc/mdu363. [DOI] [PubMed] [Google Scholar]
- 187.Poole C, LA, Rodenhuis S, et al. A randomized phase II clinical trial of the SRC inhibitor saracatinib (AZD0530) and carboplatin plus paclitaxel (C plus P) versus C plus P in patients (pts) wiht advanced platinum-sensitive epithelial ovarian cancer (eoc) Ann Oncol. 2010;21(8):2. [Google Scholar]
- 188.Do K, et al. Phase I Study of Single-Agent AZD1775 (MK-1775), a Wee1 Kinase Inhibitor, in Patients With Refractory Solid Tumors. J Clin Oncol. 2015;33(30): 3409–15. doi: 10.1200/JCO.2014.60.4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Burger RA, et al. Phase II trial of bevacizumab in persistent or recurrent epithelial ovarian cancer or primary peritoneal cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(33): 5165–71. doi: 10.1200/JCO.2007.11.5345. [DOI] [PubMed] [Google Scholar]
- 190.Cannistra SA, et al. Phase II study of bevacizumab in patients with platinum-resistant ovarian cancer or peritoneal serous cancer. J Clin Oncol. 2007;25(33): 5180–6. doi: 10.1200/JCO.2007.12.0782. [DOI] [PubMed] [Google Scholar]
- 191.Garcia AA, et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: a trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J Clin Oncol. 2008;26(1):76–82. doi: 10.1200/JCO.2007.12.1939. [DOI] [PubMed] [Google Scholar]
- 192.del Carmen MG, et al. A phase II clinical trial of pegylated liposomal doxorubicin and carboplatin plus bevacizumab in patients with platinum-sensitive recurrent ovarian, fallopian tube, or primary peritoneal cancer. Gynecol Oncol. 2012;126(3): 369–74. doi: 10.1016/j.ygyno.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 193.Burger RA, et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med. 2011;365(26): 2473–83. doi: 10.1056/NEJMoa1104390. [DOI] [PubMed] [Google Scholar]
- 194.Oza AM, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16(8): 928–36. doi: 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Aghajanian C, et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J Clin Oncol. 2012;30(17): 2039–45. doi: 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Brown J, et al. Efficacy and safety of bevacizumab in recurrent sex cord-stromal ovarian tumors: results of a phase 2 trial of the Gynecologic Oncology Group. Cancer. 2014;120(3):344–51. doi: 10.1002/cncr.28421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Coleman RL, et al. Phase 1–2 study of docetaxel plus aflibercept in patients with recurrent ovarian, primary peritoneal, or fallopian tube cancer. Lancet Oncol. 2011;12(12):1109–17. doi: 10.1016/S1470-2045(11)70244-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Tew WP, et al. Intravenous aflibercept in patients with platinum-resistant, advanced ovarian cancer: results of a randomized, double-blind, phase 2, parallel-arm study. Cancer. 2014;120(3): 335–43. doi: 10.1002/cncr.28406. [DOI] [PubMed] [Google Scholar]
- 199.Hirte H, et al. A phase 2 study of cediranib in recurrent or persistent ovarian, peritoneal or fallopian tube cancer: a trial of the Princess Margaret, Chicago and California Phase II Consortia. Gynecol Oncol. 2015;138(1):55–61. doi: 10.1016/j.ygyno.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 200.Ledermann JA, et al. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;387(10023): 1066–74. doi: 10.1016/S0140-6736(15)01167-8. [DOI] [PubMed] [Google Scholar]
- 201.Matulonis UA, et al. Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol. 2009;27(33): 5601–6. doi: 10.1200/JCO.2009.23.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bodnar L, Gornas M, Szczylik C. Sorafenib as a third line therapy in patients with epithelial ovarian cancer or primary peritoneal cancer: a phase II study. Gynecol Oncol. 2011;123(1): 33–6. doi: 10.1016/j.ygyno.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 203.Hainsworth JD, et al. Paclitaxel/carboplatin with or without sorafenib in the first-line treatment of patients with stage III/IV epithelial ovarian cancer: a randomized phase II study of the Sarah Cannon Research Institute. Cancer Med. 2015;4(5): 673–81. doi: 10.1002/cam4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Herzog TJ, et al. A randomized phase II trial of maintenance therapy with Sorafenib in front-line ovarian carcinoma. Gynecol Oncol. 2013;130(1):25–30. doi: 10.1016/j.ygyno.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 205.Matei D, et al. Activity of sorafenib in recurrent ovarian cancer and primary peritoneal carcinomatosis: a gynecologic oncology group trial. J Clin Oncol. 2011;29(1): 69–75. doi: 10.1200/JCO.2009.26.7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Polcher M, et al. Sorafenib in combination with carboplatin and paclitaxel as neoadjuvant chemotherapy in patients with advanced ovarian cancer. Cancer Chemother Pharmacol. 2010;66(1):203–7. doi: 10.1007/s00280-010-1276-2. [DOI] [PubMed] [Google Scholar]
- 207.Ramasubbaiah R, et al. Sorafenib in combination with weekly topotecan in recurrent ovarian cancer, a phase I/II study of the Hoosier Oncology Group. Gynecol Oncol. 2011;123(3): 499–504. doi: 10.1016/j.ygyno.2011.08.033. [DOI] [PubMed] [Google Scholar]
- 208.Schwandt A, et al. Randomized phase II trial of sorafenib alone or in combination with carboplatin/paclitaxel in women with recurrent platinum sensitive epithelial ovarian, peritoneal, or fallopian tube cancer. Invest New Drugs. 2014;32(4): 729–38. doi: 10.1007/s10637-014-0078-5. [DOI] [PubMed] [Google Scholar]
- 209.Welch SA, et al. Sorafenib in combination with gemcitabine in recurrent epithelial ovarian cancer: a study of the Princess Margaret Hospital Phase II Consortium. Int J Gynecol Cancer. 2010;20(5):787–93. doi: 10.1111/IGC.0b013e3181e273a8. [DOI] [PubMed] [Google Scholar]
- 210.Baumann KH, et al. A phase II trial (AGO 2.11) in platinum-resistant ovarian cancer: a randomized multicenter trial with sunitinib (SU11248) to evaluate dosage, schedule, tolerability, toxicity and effectiveness of a multitargeted receptor tyrosine kinase inhibitor monotherapy. Ann Oncol. 2012;23(9): 2265–71. doi: 10.1093/annonc/mds003. [DOI] [PubMed] [Google Scholar]
- 211.Biagi JJ, et al. A phase II study of sunitinib in patients with recurrent epithelial ovarian and primary peritoneal carcinoma: an NCIC Clinical Trials Group Study. Ann Oncol. 2011;22(2): 335–40. doi: 10.1093/annonc/mdq357. [DOI] [PubMed] [Google Scholar]
- 212.Campos SM, et al. A phase II trial of Sunitinib malate in recurrent and refractory ovarian, fallopian tube and peritoneal carcinoma. Gynecol Oncol. 2013;128(2):215–20. doi: 10.1016/j.ygyno.2012.07.126. [DOI] [PubMed] [Google Scholar]
- 213.du Bois A, et al. Incorporation of pazopanib in maintenance therapy of ovarian cancer. J Clin Oncol. 2014;32(30):3374–82. doi: 10.1200/JCO.2014.55.7348. [DOI] [PubMed] [Google Scholar]
- 214.Friedlander M, et al. A Phase II, open-label study evaluating pazopanib in patients with recurrent ovarian cancer. Gynecol Oncol. 2010;119(1):32–7. doi: 10.1016/j.ygyno.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 215.Pignata S, et al. Pazopanib plus weekly paclitaxel versus weekly paclitaxel alone for platinum-resistant or platinum-refractory advanced ovarian cancer (MITO 11): a randomised, open-label, phase 2 trial. Lancet Oncol. 2015;16(5):561–8. doi: 10.1016/S1470-2045(15)70115-4. [DOI] [PubMed] [Google Scholar]