Abstract
Background
Although many studies have investigated the association of a single nucleotide polymorphism in TNF-α G-308A gene with depression, their association is still controversial. To clarify this, we performed a meta-analysis.
Method
Studies related to TNF-α G-308A and depression were retrieved from PubMed, Medline, Embase, and Scopus (up to April 18, 2017). The odds ratios (ORs) and 95% confidence intervals (CIs) were estimated in the models of allele comparison (G vs A), homozygote comparison (GG vs AA), dominant (GG vs GA + AA), and recessive (GG + GA vs AA) to estimate the strength of the associations.
Results
A total of 10 case–control studies were included in this meta-analysis. Overall, no significant association between TNF-α G-308A and depression was found (G vs A: OR [95% CI] =1.09 [0.92, 1.29]; GG vs AA: 1.24 [0.71, 2.15]; GG vs GA + AA: 1.01 [0.76, 1.35]; GG + GA vs AA: 1.22 [0.70, 2.13]). In subgroup analyses by ethnicity or age group, no statistically significant association between TNF-α G-308A polymorphisms and depression was shown.
Conclusion
This meta-analysis revealed that TNF-α G-308A polymorphism is not associated with susceptibility to depression.
Keywords: TNF-α G-308A, rs1800629, genetic polymorphism, depressive disorder
Introduction
Depression is a common psychiatric disorder and is one of the leading causes of disability along with various other chronic diseases worldwide.1 Recently, a large number of studies have reported that patients diagnosed with depression have higher levels of pro-inflammatory cytokines in their blood or in their cerebrospinal fluid,2–6 and these findings have been supported by subsequent meta-analyses.7,8 Elevated pro-inflammatory cytokines can cause alterations in neuronal transmissions leading to neuropsychiatric disorders, especially depression.9,10 Peripheral blood interleukin-1 beta, interleukin-6, tumor necrosis factor-alpha (TNF-α), and C-reactive protein have been reported as reliable biomarkers in patients with depression.11
In the year 2000, increased levels of TNF-α in patients with depression was reported for the first time.12 While its biological role in the pathogenesis or progression of depression is yet to be defined, increasing amount of evidence from clinical studies shows that the blood levels of TNF-α correlate with the progression of depressive symptoms.13 Moreover, blood levels of TNF-α reportedly normalized following the treatment with antidepressants.14 Genetic variants of pro-inflammatory cytokines can alter the gene transcription and thereby protein expression and biological action of these cytokines. Regarding the genetic variants of TNF-α, a single nucleotide polymorphism G-308A is linked to increased production of TNF-α compared with the wild-type allele.15–17 Many studies based on different ethnicities and different pathogenesis of depression have reported conflicting evidence regarding the association of G-308A polymorphism in TNF-α gene (rs1800629) with the development of depression. While some studies have shown that A-allele of TNF-α G-308A was associated with depression,18,19 another study found that the frequency of A-carrying subjects was lower in patients with depression than that of healthy subjects.20 A study has reported that the GG genotype was a risk factor for suicide attempt.21
In this study, we performed a meta-analysis to evaluate the association of TNF-α G-308A polymorphism with depression.
Methods
Search strategy
We searched PubMed, Medline, Embase, and Scopus (up to April 18, 2017) for studies that have evaluated the association of TNF-α G-308A polymorphism with depression in humans using the following search terms: (“mood disorders” OR “depressive disorder” OR “depressive episode” OR “depression”) AND (“genetic polymorphism” OR “genetic variation” OR “genetic variant” OR “polymorphism” OR “variant”) AND (“tumor necrosis factor” OR “TNF-alpha”) AND (“rs1800629” OR “308”). A manual search was also conducted to find more studies based on references listed in the individual articles.
Study selection
The inclusion criteria of literature were as follows: 1) case–control design; 2) investigating the association between TNF-α G-308A polymorphisms and depression; 3) patients diagnosed with depressive disorder by adequate diagnostic tool; 4) providing available information about the genotype frequencies of TNF-α genetic polymorphisms for calculating the value of odds ratio (OR) and 95% confidence interval (CI); 5) full-text article; and 6) published in English. We excluded the article if 1) it is not an original investigation, 2) it is not the study of the association between TNF-α G-308A and depression, 3) it does not provide reusable data, or 4) it does not have control data. Duplicate reports were also excluded. In case where the article contains the genotype information of the patient with other psychiatric disorders, we used only the information of patients with depressive disorder.
Data extraction
In accordance with the PRISMA guidance,22 the data were checked and extracted by two investigators independently. Using a standardized form, we extracted the following information: name of the first author, publication year, country of the study, diagnostic tools, the number of cases and controls, and genotype frequency. Disagreements were resolved by discussion among all authors until consensus was reached.
Statistical analysis
The meta-analysis was performed using the Review Manager (RevMan) 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The relationship between TNF-α G-308A polymorphism and depression was assessed by determining the pooled ORs and 95% CIs for allele (G vs A) and homozygote comparisons (GG vs AA) and for dominant (GG vs GA + AA) and recessive (GG + GA vs AA) models. Statistical heterogeneity among studies was estimated using the Q-test and I2 statistics. We used I2 statistics to measure the degree of heterogeneity (I2=0%–20%, no heterogeneity; I2=20%–50%, moderate heterogeneity; I2>50%, obvious heterogeneity). A random-effects model was used to estimate the pooled ORs and 95% CIs, as heterogeneity was found with P<0.10 or I2>50%. Potential publication bias was evaluated using Begg’s funnel plot.
Results
Literature search
Figure 1 displays our strategy for literature search. After a comprehensive literature search, a total of 76 studies were initially retrieved including 19 duplicate records. After reviewing the abstracts of 57 non-duplicate studies, we removed 39 studies due to the following reasons: the study was not about TNF-α G-308A or depression; they did not have an abstract; or they were not the original research articles, that is, if they were reviews, meeting reports, or protocols. Of the remaining 18 studies, one study was excluded as the full-text article was not available. We further removed 7 studies because of the following reasons: they did not provide detailed information on cases and controls (n=1); they did not study the association between TNF-α G-308A and depression (n=3); or they did not provide reusable data (n=3). Finally, 10 studies were selected for this meta-analysis.
Figure 1.
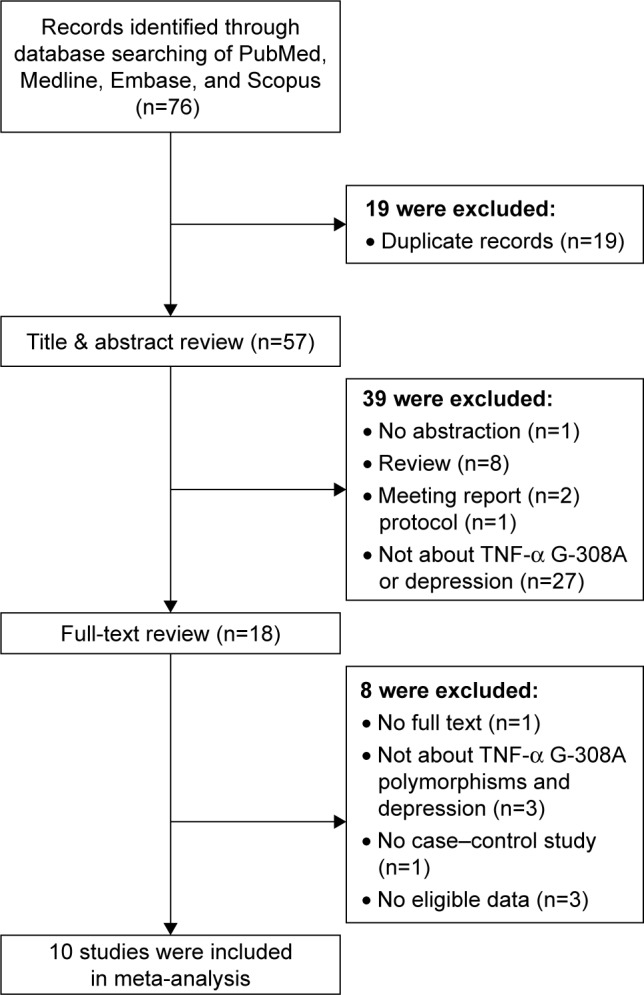
Flow diagram of the selection of studies and reason for exclusion from the present meta-analysis
Characteristics of the studies
Table 1 summarizes the characteristics of the studies included in our analysis. These studies were reported from 2009 to 2016, encompassing 917 cases and 2,678 controls. Geographically, 5 were conducted in Korea; 2 in Italy; and 1 each in Bulgaria, Denmark, and South Africa. To make the diagnosis of depressive disorder, each study used the following diagnostic criteria: Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV, International Classification of Diseases (ICD)-10, Schedules for Clinical Assessment in Neuropsychiatry (SCAN) interview, and Geriatric Mental State diagnostic schedule. In three studies, the mean ages of cases and controls were found to be above 70 years.
Table 1.
Main characteristics of studies included
| Author | Year | No of cases of depression | No of controls | Region | Diagnostic tools | Age (mean ± SD [range])
|
Gender (M/F)
|
||
|---|---|---|---|---|---|---|---|---|---|
| Cases | Controls | Cases | Controls | ||||||
| Cerri et al20 | 2010 | 50 | 240 | Italian | DSM-IV, GDS | 81.42±7.45 [65–102] |
82.0±6.0 [67–99] |
13/37 | – |
| Clerici et al23 | 2009 | 31 | 363 | Italian | DSM-IV | – | 39 [24–54] |
– | 262/101 |
| Haastrup et al27 | 2012 | 288 | 335 | Danish | ICD-10, SCAN | 43.0 [18.3–70.5] |
42.5 [18–66] |
99/189 | 176/159 |
| Jun et al18 | 2003 | 108 | 125 | Korean | DSM-IV | 50.4±14.2 | 37.6±6.9 | 47/61 | 48/77 |
| Kang et al44 | 2015 | 101 | 631 | Korean | GMS, DSM-IV | 77.6±6.8 | 72.0±5.3 | 28/73 | 272/359 |
| Kim et al24 | 2012 | 77 | 199 | Korean | DSM-IV | 66.5±9.5 | 63.5±9.4 | 43/34 | 121/78 |
| Kim et al25 | 2013 | 19 | 164 | Korean | DSM-IV | 51.6±8.2 | 49.8±10.3 | 0/19 | 0/164 |
| Kim et al45 | 2013 | 63 | 458 | Korean | GMS, DSM-IV | 71.9±4.5 | 72.5±5.7 | 33/30 | 201/257 |
| Lückhoff et al19 | 2014 | 94 | 111 | South African | DSM-V | 41.1±10.9 (Male) 42.2±9.8 (Female) |
43.3±12.4 (Male) 41.0±11.5 (Female) |
22/72 | 44/67 |
| Mihailova et al46 | 2016 | 80 | 52 | Bulgarian | ICD-10, HAM-D | 45.5 | 36.7 | 30/50 | 22/30 |
Abbreviations: MDD, major depressive disorder; DSM, Diagnostic and Statistical Manual of Mental Disorders; GDS, geriatric depression scale; ICD, international classification of diseases; SCAN, schedules for clinical assessment in neuropsychiatry; HAM-D, Hamilton Depression Scale; GMS, geriatric mental state diagnostic schedule; SD, standard deviation.
Average male-to-female ratios were found to be 1:1.79 for cases and 1:1.13 for controls, respectively. Cerri et al20 used age-matched controls in their analysis and did not provide gender information. Clerici et al23 did not specifically report on the mean age and gender of patients with major depressive disorder because their study also included patients with bipolar disorders. Two studies differ from the others regarding gender ratio: Kim et al24 and Kim et al25 involved patients who developed depressive disorder following the diagnosis of stroke and cancer, respectively. Kim et al24 included more male patients than the other studies, whereas Kim et al25 only included female patients with depression.
Results of meta-analysis
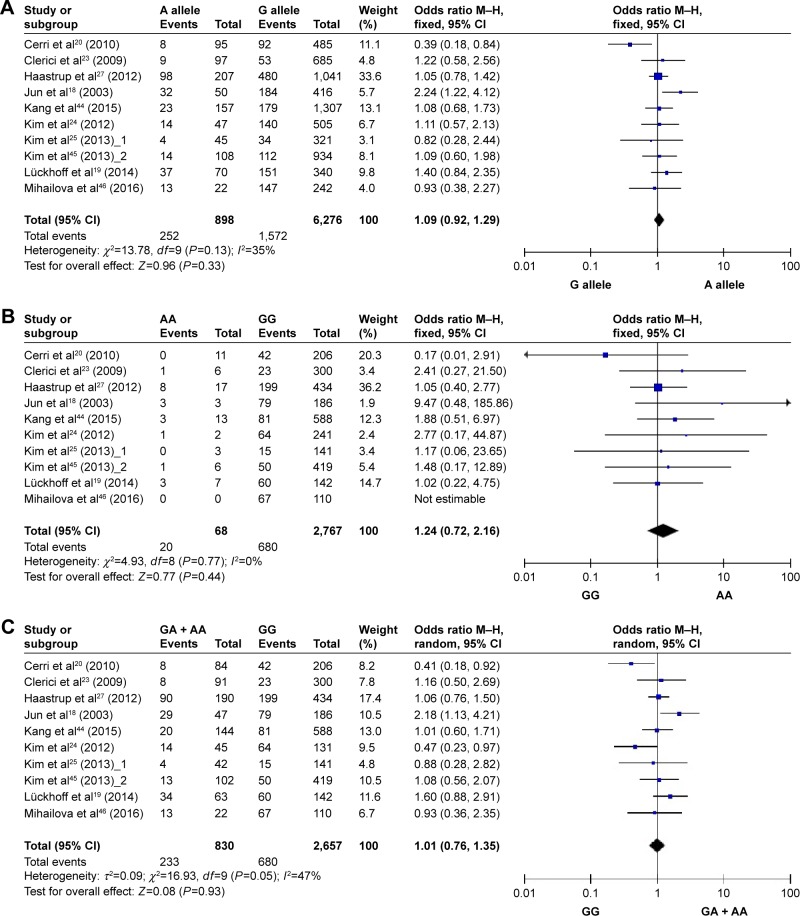
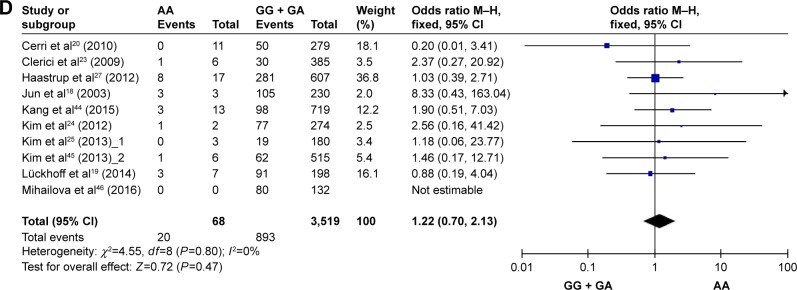
Figure 2 and Table 2 summarize the result of the meta-analysis. Overall, no significant association was found between TNF-α G-308A polymorphisms and depression in any of the comparison models we used. The OR and 95% CI for each model were as follows: 1.09 [0.92, 1.29] in the allele comparison model (G vs A); 1.24 [0.71, 2.15] in the homozygote comparison model (GG vs AA); 1.01 [0.76, 1.35] in the dominant genetic model (GG vs GA + AA); and 1.22 [0.70, 2.13] in the recessive genetic model (GG + GA vs AA).
Figure 2.
Forest plots of TNF-α G-308A polymorphisms and depression. (A) G allele vs A allele, (B) GG vs AA, (C) GG vs GA + AA, and (D) GG + GA vs AA.
Table 2.
Main results in the total and subgroup analysis
| Study groups | G-allele vs A-allele
|
GG vs AA
|
GG vs GA + AA
|
GG + GA vs AA
|
||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Overall | 1.09 (0.92, 1.29) | 0.33 | 1.24 (0.71, 2.15) | 0.44 | 1.01 (0.76, 1.35) | 0.93 | 1.22 (0.70, 2.13) | 0.47 |
| By ethnicity | ||||||||
| Asian | 1.25 (0.95, 1.63) | 0.11 | 2.34 (0.98, 5.57) | 0.06 | 1.02 (0.63, 1.67) | 0.93 | 2.29 (0.96, 5.49) | 0.06 |
| Non-Asian | 1.00 (0.80, 1.24) | 0.97 | 0.87 (0.42, 1.77) | 0.70 | 1.02 (0.79, 1.31) | 0.89 | 0.86 (0.42, 1.76) | 0.68 |
| By mean age | ||||||||
| <70 years | 1.20 (0.98, 1.47) | 0.08 | 1.45 (0.73, 2.85) | 0.29 | 1.12 (0.79, 1.59) | 0.54 | 1.37 (0.69, 2.68) | 0.37 |
| ≥70 years | 0.82 (0.46, 1.47) | 0.51 | 0.91 (0.33, 2.46) | 0.85 | 0.81 (0.47, 1.39) | 0.45 | 0.97 (0.36, 2.64) | 0.95 |
Abbreviations: OR, odds ratio; CI, confidence interval.
To rule out the effect of ethnicity and age, we subsequently performed subgroup analyses. There was no statistically significant association between TNF-α G-308A polymorphisms and depression in any of the ethnic and age subgroups (Table 2), except that there was a trend that the frequency of depression was higher in genotype AA than that of other genotypes with respect to homozygote comparison model (GG vs AA: OR [95% CI] =2.34 [0.98, 5.57]) and recessive model (GG + GA vs AA: OR [95% CI] =2.29 [0.96, 5.49]) among Asians.
Figure 3 shows Begg’s funnel plots estimating publication bias. The shape of the funnel plots does not show any evidence of obvious publication bias.
Figure 3.
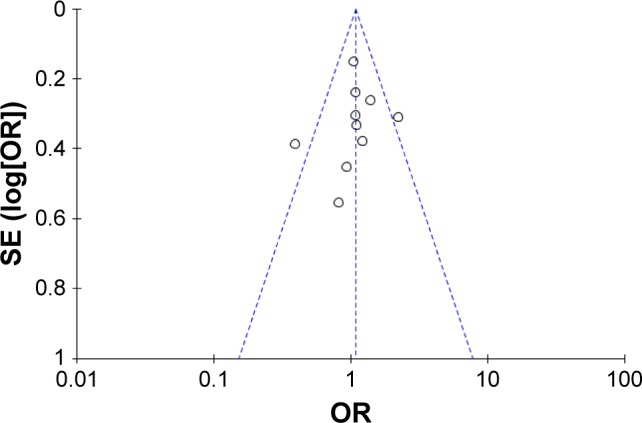
Funnel plots of publication bias.
Discussion
Association of TNF-α polymorphism with various diseases such as autoimmune diseases, infectious diseases, and cancer have been previously reported.26 Given the fact that the levels of TNF-α in blood or in cerebrospinal fluid were higher in patients with depression than that of healthy controls, multiple studies have reported conflicting results regarding the association of TNF-α G-308A polymorphism with depression. Jun et al18 were the first to report the role of TNF-α G-308A polymorphism in depression. They found that the proportion of subjects with an A allele was significantly higher in depression group than that of control group subjects (P=0.024). Furthermore, Lückhoff et al19 demonstrated that minor A-allele of TNF-α G-308A is associated with onset of depression at an early age. However, several other studies could not find any association of TNF-α G-308A polymorphism with depression. Moreover, Cerri et al20 reported the association of GG genotype with depression at a greater risk (OR [95% CI] =2.433 [1.09, 5.43]). Despite these controversial results, to the best our knowledge, till date, there is no comprehensive meta-analysis to clarify the association of TNF-α G-308A polymorphism with depression. Therefore, in this study, we conducted a comprehensive meta-analysis for the first time.
The relationship between TNF-α G-308A polymorphism and risk of depression was evaluated with a total of 10 independent studies encompassing 917 cases and 2,678 controls. We did not find any evidence that this polymorphism is significantly associated with depression. This inconsistency might be attributed to the small sample sizes of the studies included in this meta-analysis. The maximum number of patients with depression was 289.27 In case of the report by Kim et al published in the year 2013,25 data of only 19 patients with persistent depression were used in their analysis. Furthermore, the frequency of the A allele of TNF-α was found to be low, which is reported as about 7% in Chinese, 11% in Korean, 11%–15% in European and also 11%–15% in U.S. populations,28–34 resulting in a very small number of patients carrying the minor A-allele. This inconsistent findings for TNF-α G-308A might be attributed to the polygenic contribution of the development of depression. For example, the association of Val66Met polymorphism with brain-derived neurotrophic-factor and depression has been confirmed by a number of studies.35–37 Furthermore, it has been identified that polymorphism of serotonin transporter gene promoter (5-HTTLPR) is a modulator of depression.38,39 Other genetic polymorphisms such as COMT Val158Met,40 G-Protein β3 C825T,41 and MTHFR C677T42 have also been reported as potential risk factors for depression. As the frequencies of these genetic variants might be different from study to study, the effect of TNF-α G-308A on depression could not be evaluated consistently.
We performed subgroup analyses on ethnicity and age to exclude potential sources of heterogeneity among the included studies. However, we could not find any statistically significant associations in the subgroup analysis. A significantly higher frequency of depression was observed among Asians (Koreans) with AA genotype (P=0.06), which might be attributed to a single study that had reported a strong association,18 rather than a high frequency of this genotype in Asians in general.
There are some limitations in our meta-analysis. There exists a substantial degree of heterogeneity among included studies with regard to gender. Differences in gender ratio are of particular importance, since women are about twice as likely as men to develop depression.43 Owing to the lack of genotype information by gender, we were unable to do a subgroup analysis. There is also some heterogeneity regarding severity of depression. Some articles included in this study described their patients as having “depression” or “depressive disorder” rather than “major depressive disorder.” The difference in the severity of depression might limit the evaluation of the effect of TNF-α polymorphism. However, many studies on the association between depressive disorder and genetic polymorphisms of cytokine genes did not limit their analysis to the patients with major depressive disorder.10 In this regard, we thought it would be worthwhile to address this question in terms of depressive disorder in general.
In conclusion, our study found that the existing literature does not suggest the association of TNF-α G-308A polymorphisms with depression. Considering the polygenic effect on depression, a large number of case–control studies with the information on other related genetic polymorphisms could provide reliable evidence for the role of TNF-α G-308A polymorphism with respect to susceptibility to depression.
Acknowledgments
Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science, ICT & Future Planning (Grant no NRF-015-M3A9E1028327). Dr Kim was supported by a training program grant from the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (HI14C2339).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vos T, Barber RM, Bell B, et al. Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743–800. doi: 10.1016/S0140-6736(15)60692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez JM, Garakani A, Yehuda R, Gorman JM. Proinflammatory and “resiliency” proteins in the CSF of patients with major depression. Depress Anxiety. 2012;29(1):32–38. doi: 10.1002/da.20876. [DOI] [PubMed] [Google Scholar]
- 3.Maes M. Major depression and activation of the inflammatory response system. Adv Exp Med Biol. 1999;461:25–46. doi: 10.1007/978-0-585-37970-8_2. [DOI] [PubMed] [Google Scholar]
- 4.Sluzewska A. Indicators of immune activation in depressed patients. Adv Exp Med Biol. 1999;461:59–73. doi: 10.1007/978-0-585-37970-8_4. [DOI] [PubMed] [Google Scholar]
- 5.Levine J, Barak Y, Chengappa KN, Rapoport A, Rebey M, Barak V. Cerebrospinal cytokine levels in patients with acute depression. Neuropsychobiology. 1999;40(4):171–176. doi: 10.1159/000026615. [DOI] [PubMed] [Google Scholar]
- 6.Lindqvist D, Janelidze S, Hagell P, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66(3):287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 7.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71(2):171–186. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 8.Dowlati Y, Herrmann N, Swardfager W, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 9.Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience. 2013;246:199–229. doi: 10.1016/j.neuroscience.2013.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–741. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22(4):370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 13.Ma K, Zhang H, Baloch Z. Pathogenetic and therapeutic applications of tumor necrosis factor-alpha (TNF-alpha) in major depressive disorder: a systematic review. Int J Mol Sci. 2016;17(5) doi: 10.3390/ijms17050733. pii: E733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36(12):2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroeger KM, Carville KS, Abraham LJ. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Mol Immunol. 1997;34(5):391–399. doi: 10.1016/s0161-5890(97)00052-7. [DOI] [PubMed] [Google Scholar]
- 16.Louis E, Franchimont D, Piron A, et al. Tumour necrosis factor (TNF) gene polymorphism influences TNF-alpha production in lipopolysaccharide (LPS)-stimulated whole blood cell culture in healthy humans. Clin Exp Immunol. 1998;113(3):401–406. doi: 10.1046/j.1365-2249.1998.00662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cuenca J, Cuchacovich M, Perez C, et al. The -308 polymorphism in the tumour necrosis factor (TNF) gene promoter region and ex vivo lipopolysaccharide-induced TNF expression and cytotoxic activity in Chilean patients with rheumatoid arthritis. Rheumatology. 2003;42(2):308–313. doi: 10.1093/rheumatology/keg092. [DOI] [PubMed] [Google Scholar]
- 18.Jun TY, Pae CU, Hoon H, et al. Possible association between -G308A tumour necrosis factor-alpha gene polymorphism and major depressive disorder in the Korean population. Psychiatr Genet. 2003;13(3):179–181. doi: 10.1097/00041444-200309000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Lückhoff HK, Van Rensburg SJ, Botha K, Kidd M, Kotze MJ. The pro-inflammatory TNFA -308G.A (rs1800629) polymorphism is associated with an earlier age at onset in patients with major depressive disorder. J Psychiatry. 2014;17(3):1000124. [Google Scholar]
- 20.Cerri AP, Arosio B, Viazzoli C, Confalonieri R, Vergani C, Annoni G. The -308 (G/A) single nucleotide polymorphism in the TNF-alpha gene and the risk of major depression in the elderly. Int J Geriatr Psychiatry. 2010;25(3):219–223. doi: 10.1002/gps.2323. [DOI] [PubMed] [Google Scholar]
- 21.Kim YK, Hong JP, Hwang JA, et al. TNF-alpha -308G.A polymorphism is associated with suicide attempts in major depressive disorder. J Affect Disord. 2013;150(2):668–672. doi: 10.1016/j.jad.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 23.Clerici M, Arosio B, Mundo E, et al. Cytokine polymorphisms in the pathophysiology of mood disorders. CNS Spectr. 2009;14(8):419–425. doi: 10.1017/s1092852900020393. [DOI] [PubMed] [Google Scholar]
- 24.Kim JM, Stewart R, Kim SW, et al. Associations of cytokine gene polymorphisms with post-stroke depression. World J Biol Psychiatry. 2012;13(8):579–587. doi: 10.3109/15622975.2011.588247. [DOI] [PubMed] [Google Scholar]
- 25.Kim JM, Stewart R, Kim SY, et al. A one year longitudinal study of cytokine genes and depression in breast cancer. J Affect Disord. 2013;148(1):57–65. doi: 10.1016/j.jad.2012.11.048. [DOI] [PubMed] [Google Scholar]
- 26.Hajeer AH, Hutchinson IV. TNF-alpha gene polymorphism: clinical and biological implications. Microsc Res Tech. 2000;50(3):216–228. doi: 10.1002/1097-0029(20000801)50:3<216::AID-JEMT5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 27.Haastrup E, Bukh JD, Bock C, et al. Promoter variants in IL18 are associated with onset of depression in patients previously exposed to stressful-life events. J Affective Disord. 2012;136(1–2):134–138. doi: 10.1016/j.jad.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 28.Lee JY, Kim HY, Kim KH, et al. Association of polymorphism of IL-10 and TNF-A genes with gastric cancer in Korea. Cancer Lett. 2005;225(2):207–214. doi: 10.1016/j.canlet.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Li C, Xia B, Yang Y, Li J, Xia HH. TNF gene polymorphisms and Helicobacter Pylori infection in gastric carcinogenesis in Chinese population. Am J Gastroenterol. 2005;100(2):290–294. doi: 10.1111/j.1572-0241.2005.40806.x. [DOI] [PubMed] [Google Scholar]
- 30.Rad R, Dossumbekova A, Neu B, et al. Cytokine gene polymorphisms influence mucosal cytokine expression, gastric inflammation, and host specific colonisation during Helicobacter pylori infection. Gut. 2004;53(8):1082–1089. doi: 10.1136/gut.2003.029736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perri F, Piepoli A, Bonvicini C, et al. Cytokine gene polymorphisms in gastric cancer patients from two Italian areas at high and low cancer prevalence. Cytokine. 2005;30(5):293–302. doi: 10.1016/j.cyto.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 32.Machado JC, Figueiredo C, Canedo P, et al. A proinflammatory genetic profile increases the risk for chronic atrophic gastritis and gastric carcinoma. Gastroenterology. 2003;125(2):364–371. doi: 10.1016/s0016-5085(03)00899-0. [DOI] [PubMed] [Google Scholar]
- 33.Hänninen K, Katila H, Rontu R, Mattila KM, Hurme M, Lehtimäki T. Tumor necrosis factor-alpha -G308A polymorphism in schizophrenia in a Finnish population. Neurosci Lett. 2005;385(1):76–81. doi: 10.1016/j.neulet.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 34.El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of non-cardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology. 2003;124(5):1193–1201. doi: 10.1016/s0016-5085(03)00157-4. [DOI] [PubMed] [Google Scholar]
- 35.Pei Y, Smith AK, Wang Y, et al. The brain-derived neurotrophic-factor (BDNF) Val66Met polymorphism is associated with geriatric depression: a meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(5):560–566. doi: 10.1002/ajmg.b.32062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niitsu T, Fabbri C, Bentini F, Serretti A. Pharmacogenetics in major depression: a comprehensive meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2013;45:183–194. doi: 10.1016/j.pnpbp.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Zou YF, Ye DQ, Feng XL, Su H, Pan FM, Liao FF. Meta-analysis of BDNF Val66Met polymorphism association with treatment response in patients with major depressive disorder. Eur Neuropsychopharmacol. 2010;20(8):535–544. doi: 10.1016/j.euroneuro.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Gao Z, Yuan H, Sun M, Wang Z, He Y, Liu D. The association of serotonin transporter gene polymorphism and geriatric depression: a meta-analysis. Neurosci Lett. 2014;578:148–152. doi: 10.1016/j.neulet.2014.06.046. [DOI] [PubMed] [Google Scholar]
- 39.Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68(5):444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang M, Ma Y, Yuan W, Su K, Li MD. Meta-analysis of the COMT Val158Met polymorphism in major depressive disorder: effect of ethnicity. J Neuroimmune Pharmacol. 2016;11(3):434–445. doi: 10.1007/s11481-016-9651-3. [DOI] [PubMed] [Google Scholar]
- 41.Fang L, Zhou C, Bai S, et al. The C825T polymorphism of the G-protein β3 gene as a risk factor for depression: a meta-analysis. PLoS One. 2015;10(7):e0132274. doi: 10.1371/journal.pone.0132274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang W, Xu J, Lu XJ, Sun Y. Association between MTHFR C677T polymorphism and depression: a meta-analysis in the Chinese population. Psychol Health Med. 2016;21(6):675–685. doi: 10.1080/13548506.2015.1120327. [DOI] [PubMed] [Google Scholar]
- 43.Albert PR. Why is depression more prevalent in women? J Psychiatry Neurosci. 2015;40(4):219–221. doi: 10.1503/jpn.150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang HJ, Kim JM, Kim SW, et al. Associations of cytokine genes with Alzheimer’s disease and depression in an elderly Korean population. J Neurol Neurosurg Psychiatry. 2015;86(9):1002–1007. doi: 10.1136/jnnp-2014-308469. [DOI] [PubMed] [Google Scholar]
- 45.Kim JM, Stewart R, Kim SW, et al. Physical health and incident late-life depression: modification by cytokine genes. Neurobiol Aging. 2013;34(1):356.e1–e9. doi: 10.1016/j.neurobiolaging.2012.01.111. [DOI] [PubMed] [Google Scholar]
- 46.Mihailova S, Ivanova-Genova E, Lukanov T, Stoyanova V, Milanova V, Naumova E. A study of TNF-α, TGF-β, IL-10, IL-6, and IFN-γ gene polymorphisms in patients with depression. J Neuroimmunol. 2016;293:123–128. doi: 10.1016/j.jneuroim.2016.03.005. [DOI] [PubMed] [Google Scholar]