Abstract
In this article, we review our multidisciplinary approach for patients with pancreatic cancer. Specifically, we review the epidemiology, diagnosis and staging, biliary drainage techniques, selection of patients for surgery, chemotherapy, radiation therapy, and discuss other palliative interventions. The areas of active research investigation and where our knowledge is limited are emphasized.
INTRODUCTION
Pancreatic ductal adenocarcinoma (PDAC) is a highly lethal malignancy. It is the fourth leading cause of cancer-related death in the United States and second only to colorectal cancer as a cause of digestive cancer-related death (1). Surgical resection is the only potentially curative treatment. Unfortunately, because of the late presentation, only 15–20% of patients are candidates for surgical intervention. Furthermore, prognosis is poor, even after a complete resection. Five-year survival after pancreaticoduodenectomy is ∼ 25–30% for node-negative and 10% for node-positive disease. On the other hand, advancements in radiologic and endoscopic ultrasound (EUS) imaging have improved our ability to detect and stage pancreatic cancer allowing for more selective surgical intervention for patients with “resectable disease”. In most patients, palliative therapy and efforts to prolong life and maximize the quality of life (QOL) are the major goals.
In this article, we will review our multidisciplinary approach for patients with pancreatic cancer. Specifically, we will review the epidemiology, diagnosis and staging, biliary drainage techniques, selection of patients for surgery, chemotherapy, radiation therapy, and discuss other palliative interventions. The areas of active research investigation and where our knowledge is limited will be emphasized.
BACKGROUND AND EPIDEMIOLOGY
PDAC is the fourth leading cause of cancer-related mortality in the United States. Over 45,000 patients are diagnosed each year in the United States, and the majority of these patients succumb to their disease. Eighty percent of patients are diagnosed with advanced, unresectable disease. According to the latest statistics from the American Cancer Society (2), only 7% of patients survive 5 years after diagnosis. While the 5-year survival rate improves to 25% in patients presenting with stage 1 or localized disease, only 9% of patients are identified at this early stage (2). The majority of patients (53%) presents with distant, metastatic disease, and have a 5-year survival of 2% (3). Identification of risk factors and establishing earlier detection methods are therefore of paramount importance. Smoking and obesity are modifiable risk factors. However, an increased incidence of PDAC is also noted with advanced age, as two-thirds of patients are aged >65 years. Men are 30% more likely to develop PDAC than women, with African Americans more commonly affected than Caucasians (2). Chronic pancreatitis also increases this risk (4), and a recent meta-analysis (3) found that the pooled relative risk for PDAC in chronic pancreatitis was 13.3 (95% confidence interval: 6.1–28.9). This relative risk is even higher in hereditary pancreatitis and tropical pancreatitis, suggesting that patients who present earlier with chronic pancreatitis are at increased risk for PDAC. Patients with long-standing type 2 diabetes have an approximate twofold increase in PDAC (5–7), and Helicobacter pylori infection (8) has also been found to associate with PDAC. While the majority of patients have sporadic disease, recent advances in genome sequencing have identified mutations in PALB2, BRCA2, STK11/LKB1, and P16 and an increased incidence of PDAC (9–11). Despite this improvement in knowledge of PDAC and its risk factors, no therapy has been identified that significantly alters the course of the disease, particularly since early diagnosis remains problematic. The absence of reliable blood markers for PDAC reduces the potential effectiveness of a screening strategy in high-risk patients. The discovery of a biomarker that would facilitate identification of PDAC would greatly affect patient management and prognosis. To date, there is no established screening test, although patients with significant family history might undergo close observation with abdominal magnetic resonance imaging (MRI) and EUS (12). Further study is clearly needed in this area.
DIAGNOSIS AND STAGING
Radiology
In most institutions, computed tomography (CT) is the primary modality for staging of suspected PDAC. Pancreas protocol CT entails several alterations to routine abdominal CT. CT should be performed with rapid injection of intravenous iodinated contrast, ideally at a rate of at least 4 ml/s. Slices should be reconstructed at less than or equal to 3 mm with overlap. At least two postcontrast acquisitions, in the late arterial (or parenchymal) and venous phases, are useful to assess the arteries (celiac, common hepatic, peripancreatic, and superior mesenteric arteries) and veins (portal, splenic, and superior mesenteric veins). The parenchymal phase best shows the tumor as an ill-defined hypodense mass in the pancreatic parenchyma (Figure 1), whereas the venous phase is best for detecting liver metastases. Neutral enteral contrast such as water is helpful, as this allows for better identification of the duodenal wall compared with positive enteral contrast, and results in artifact-free reformations. In many centers no oral contrast is used. Coronal and sagittal reformations in both arterial and venous phases increase the sensitivity for determining local invasion. Maximum intensity projection images are helpful in identifying variant vascular anatomy. Curved reformations may be helpful in staging PDAC, although we do not produce these on a routine basis.
Figure 1.
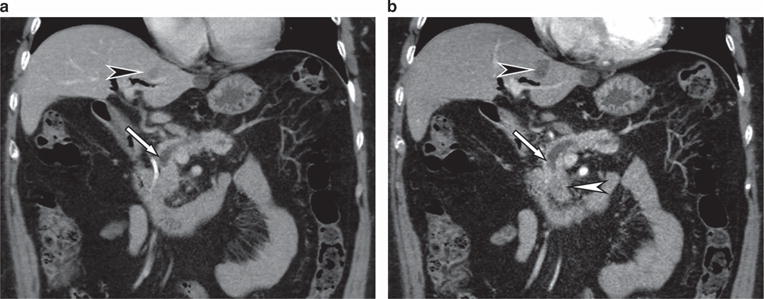
A 67-year-old female presents with persistent abdominal pain and weight loss. (a) Axial computed tomography (CT) performed with thick slices (5 mm) at an outside institution shows an abrupt cutoff of the main pancreatic duct (arrow). No obvious pancreatic head mass was seen. In addition, an ill-defined liver lesion was seen (black arrowhead). (b) Axial CT at our institution performed as per the pancreas protocol with thin slices (3 mm) in parenchymal phase shows the duct cutoff (arrow) caused by an ill-defined low-density pancreatic head mass (white arrowhead). The liver lesion (black arrowhead) is shown to have a clear outline and was diagnosed as a benign cyst. The patient underwent pancreaticoduodenectomy for resectable ductal adenocarcinoma.
MRI is also useful for diagnosis and staging of PDAC, although not proven to be superior to CT. The most important sequence is dynamic postgadolinium series.
Diagnosis of PDAC
CT is reported to have a sensitivity of 89–97% for PDAC, although it is less effective in diagnosing small (<2 cm) lesions with a sensitivity of 65–75% (13). In this respect EUS is superior. About 10% of pancreas cancers are not discerned as a mass (i.e., they are isodense to pancreas). The only clue to the presence of PDAC may be an abrupt cutoff of the pancreatic duct, particularly if there is upstream glandular atrophy (14). Other secondary imaging features may include a distal bile duct stricture and abnormal bulge to the contour of the gland, although these features are nonspecific and may be seen in benign pathology (e.g., chronic pancreatitis). Accuracy of diagnosis is improved if CT is performed before biliary stenting, as an artifact from a stent may make it difficult to identify the tumor in the pancreatic head.
PDAC is hypointense to adjacent parenchyma on precontrast and initial postcontrast MRI. In late postcontrast MRI, the tumor may show delayed enhancement and may become isointense. The finding of a non-occluded duct within a mass-like lesion in the pancreas is termed the “duct penetrating sign”. This sign is thought to be specific for a benign lesion, such as chronic pancreatitis or autoimmune pancreatitis.
Staging of PDAC
Surgical resection with negative margins (R0 resection) is the only potentially curative treatment for PDAC, but only 15–20% of patients present with potentially resectable disease (see Surgical Approaches to PDAC below). To increase the chances of R0 resection, venous resection and reconstruction are increasingly performed (13,15). As a result, the American Joint Commission on Cancer considers isolated venous invasion as T3 disease (locally advanced but potentially resectable), while arterial invasion is deemed unresectable (T4 disease) (13,14). The concept of borderline resectability has also been raised recently. This entity includes patients with advanced venous invasion or early invasion of the hepatic artery in whom a trial of chemoradiotherapy is initiated (16,17). Surgery is only considered if there is a good response to the neoadjuvant therapy.
Current criteria for resectability include the absence of distant metastases, the absence of tumor involvement of major arteries, and no venous invasion. If there is venous involvement, the vein should be patent and important venous branches such as jejunal veins should not be involved. Criteria of venous resectability may vary based on the surgeon’s performance and experience.
In general, a vessel is said to be invaded if >180° circumference is contiguous with the tumor. Such a CT or MRI finding is 45–84% sensitive and 98–100% specific for vascular invasion (Figure 2) (13,14). Vessel deformity (teardrop sign) is also considered a sign of invasion, even if the tumor has <180° footprint on the vessel. A sign that is relatively underutilized is the dilation of peripancreatic veins. The anterior and posterior superior pancreaticoduodenal veins and the gastrocolic trunk form an arcade around the pancreatic head and invasion of any part of this arcade may lead to venous engorgement.
Figure 2.
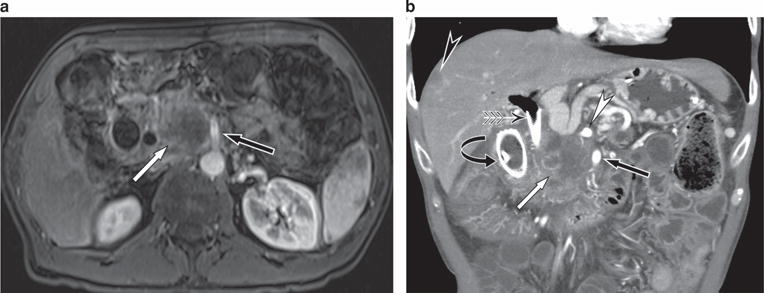
A 53-year-old male presents with painless jaundice. (a) Axial postgadolinium magnetic resonance imaging (MRI) shows a large mass in the pancreatic head (white arrow) closely applied to the superior mesenteric artery (SMA) (black arrow). The tumor was deemed unresectable because of the long length of contact with the SMA. (b) Coronal computed tomography (CT) performed 2 months later shows metal biliary (winged arrow) and duodenal (curved arrow) stents in place. There remains a large tumor (white arrow) that is closely applied to the SMA (black arrow) and celiac artery (white arrowhead). In addition, a liver metastases (black arrowhead) is seen.
CT is not sensitive for detecting nodal metastases. Using a short axis dimension of 10 mm as the cutoff, CT has a sensitivity of only 15% for detecting nodal metastases (13,14). Using a 5 mm threshold, sensitivity increases to ∼ 70% but specificity drops to 65%. On the other hand, enlarged nodes may be secondary to pancreatitis, chronic liver disease, or other benign processes.
CT is also inaccurate in detecting small hepatic and peritoneal metastases. Up to a third of patients with no obvious metastases on a high-quality CT may be found to have small liver or peritoneal metastases at surgery. Hepatic metastases are seen as ill-defined, low-density lesions on the venous phase of contrast-enhanced CT or MRI. Even when seen, lesions <10 mm may not be characterized with certainty on CT. These should not be considered metastases, as the majority of such small lesions, even in a patient with PDAC, are benign. In this respect, MRI is superior to differentiating a small cyst or hemangioma from metastases.
Endoscopic ultrasound
EUS is the most sensitive nonoperative imaging test for the detection of benign or malignant pancreatic lesions with reported sensitivities of over 95% in most studies (18). This excellent sensitivity has provided the rationale for its use (along with MRI) in screening high-risk individuals for PDAC (12,19–23). EUS is particularly useful for identification of small tumors (≤20 mm in diameter) that have been undetected by other imaging modalities (24,25). We recommend, therefore, that EUS should be performed in all patients with non-calculous obstructive jaundice in whom CT or MRI does not definitively identify a pancreatic lesion, both to detect any tumor and to exclude non-neoplastic diseases. A normal pancreas by EUS examination essentially rules out PDAC, but follow-up EUS or other study should be undertaken when EUS demonstrates chronic pancreatitis without a definite mass (26,27). EUS may also fail to identify true pancreatic masses in patients with a diffusely infiltrating carcinoma, recent episode (<4 weeks) of acute pancreatitis or indwelling biliary stent (28). Imaging-based technologies such as contrast-enhanced EUS and elastography have been used widely in Europe and Asia to aid in differentiation of pancreatic masses (29–32). These techniques are not used in our center and much in the United States due to high cost, lack of both contrast agent and elastography software availability, and minimal expertise.
EUS and multidetector CT are equivalent at determining surgical resectability of PDAC (24,25). Nevertheless we perform EUS and cross-sectional imaging (CT or MRI) at our center to stage most patients with known or suspected PDAC. Although the TNM (tumor node metastasis) staging system is widely used for staging of PDAC, we believe that dividing these patients into resectable, borderline resectable, locally advanced, and metastatic categories is more clinically useful. Resectable cancers have no vascular or regional spread, which would contraindicate surgery. Borderline cancers have regional spread into vessels (i.e., portal vein) or other organs (i.e., stomach), which would make surgery difficult but not impossible (i.e., with vein removal and reconstruction or partial gastrectomy, respectively—see Figure 3). Locally invasive cancers are not metastatic but have invasion into structures (e.g., celiac artery), which make curative surgery impossible. Metastatic tumors are surgically incurable because of the spread to distant sites (i.e., lung, liver). EUS may detect and sample metastatic liver masses, ascites, or distant lymph nodes missed by other imaging studies and therefore meticulous search for these lesions should be carried out during these exams (33,34).
Figure 3.
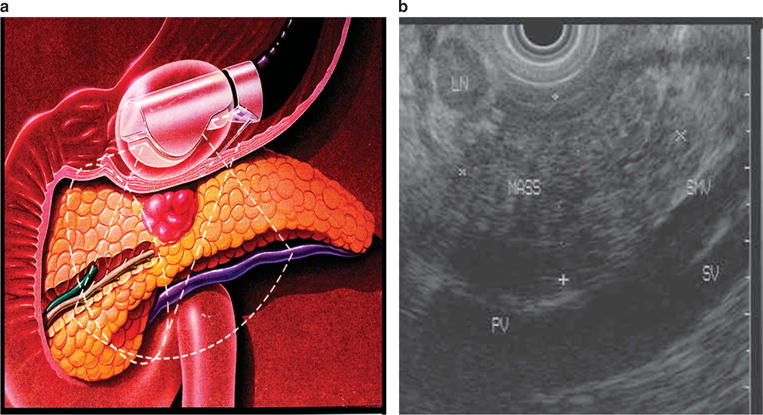
(a) Schematic illustration of a mass in the body of the pancreas. Endoscopic ultrasound evaluation of a patient with epigastric pain and weight loss, with computed tomography (CT) demonstrating a pancreas mass. (b) The pancreas mass is abutting the portal confluence.
Role of tissue diagnosis before decompression
The presence of a mass in the head of the pancreas on cross-sectional imaging with a “double duct sign” (dilated bile and pancreatic ducts), intra-hepatic lesions, and/or lymphadenopathy is specific for PDAC in the appropriate clinical context. However, a degree of uncertainty will remain as benign disease at the time of pancreaticoduodenectomy is found in up to 15% of patients. Chronic pancreatitis with inflammatory pseudotumor, sequelae of severe acute pancreatitis and type I autoimmune pancreatitis all may display radiographic features that overlap PDAC and can also present with jaundice. The incidence of autoimmune pancreatitis approaches 40% in resected specimens ultimately found to have benign disease. In addition, lymphoma localized to the pancreas, pancreatic neuroendocrine tumor, and metastatic disease to the pancreas from distant primary lesions (renal cell carcinoma, breast, and lung cancer) are conditions with natural histories that diverge from PDAC. We perform EUS-guided fine-needle aspiration (FNA) or occasionally fine-needle biopsy of almost all suspicious pancreatic masses to aid in diagnosing these lesions, if therapeutic decisions may be altered knowing the pathology result. In some patients with suspected resectable PDAC, it is reasonable to proceed directly to surgery without EUS or a tissue diagnosis. However, if the diagnosis remains uncertain, alternative diagnoses are considered, or neoadjuvant therapy (and therefore biopsy) is required, then EUS-FNA should be performed. Specimens are obtained using a linear array echoendoscope and a 19-, 22-, or 25-gauge needle. The sensitivity of EUS-FNA for the diagnosis of pancreatic malignancy is 85–90%, with a specificity approaching 100% (35). Complications are infrequent but may include pancreatitis in 1% (36). EUS-FNA is now the standard of care for establishing a diagnosis when uncertainty remains and can be performed at the same session as endoscopic retrograde cholangiopancreatography (ERCP) to render a tissue diagnosis. We use onsite, in-room cytopathologic confirmation at our institution, a practice that aids the endosonographer in determining the number of passes required. Consensus guidelines (37) recommend preliminary tissue diagnosis in patients that are borderline resectable or if type I autoimmune pancreatitis is suspected. A diagnosis before or at the time of ERCP also has a role in a cost-effective decompression strategy as discussed below.
Stage and surgical candidacy
In select cases, early-stage, resectable PDAC identified in patients with a pancreas head/uncinate mass that are surgical candidates may proceed directly to pancreaticoduodenectomy even in the presence of symptomatic biliary obstruction. Procedure-related complications of post-ERCP pancreatitis, hemorrhage (early), and stent occlusion with cholangitis (late) can further delay pancreaticoduodenectomy. Studies have failed to demonstrate that routine preoperative biliary decompression improves operative outcomes in resectable patients with PDAC. More importantly, routine preoperative decompression is associated with greater pre- and postoperative morbidity in prospective studies. A recent prospective, randomized controlled trial (RCT) demonstrated a higher rate of serious complications (74% vs. 39%, P <0.001), greater frequency of hospitalizations, with an average of 2 additional hospital days in patients who underwent preoperative ERCP decompression for a period of 4–6 weeks compared with patients who proceeded directly to surgery at <1 week (38). However, biliary decompression was achieved with plastic stents in this study, and patients with deep jaundice (bilirubin >14.6 mg/dl) were excluded. We proceed directly to surgery without biliary decompression in surgically fit, resectable patients without deep jaundice or in those who are minimally symptomatic (e.g., without intense pruritus), in the absence of plans for preoperative neoadjuvant therapy (see below). Preoperative ERCP with biliary decompression offers metabolic benefit for surgical candidates with deep jaundice, warranting the procedure. The majority of patients (>75%) with PDAC, however, are not resectable at presentation and biliary decompression is appropriate for the palliation of symptoms and/or to facilitate chemotherapy and/or radiation. One caveat is for patients with intrahepatic metastatic disease, as jaundice should be carefully assessed for radiographic evidence of biliary obstruction (i.e., duct dilation). In the setting of overwhelming intrahepatic metastatic disease and absence of duct dilation, jaundice is more likely due to compromised hepatic synthetic function and biliary decompression would be unlikely to benefit the patient.
ESTABLISHING BILIARY DRAINAGE FOR THE JAUNDICED PATIENT
Jaundice (often painless) is the most common symptom at presentation (>50%) in patients with a new diagnosis of PDAC. The mechanism is due to compression/invasion of the bile duct from a periampullary/head mass that is found in >60% of patients (39,40). Pruritus, fatigue, and fat malabsorption follow from an obstructed bile duct and endoscopic decompression translates to an improved QOL (39–45). Resolution of jaundice (<2.5 mg/dl) is also requisite for chemotherapy, as unacceptable chemotoxicity may result without adequate biliary excretion of metabolites (41). However, selecting an approach for decompression of malignant biliary obstruction is complex and must take into consideration: (i) an available confirmatory tissue diagnosis, (ii) both surgical candidacy and anticipated timing of resection, and (iii) life expectancy of the patient. This decision is best undertaken with input from a multidisciplinary team of specialists (gastroenterology, surgery, and oncology).
ERCP
ERCP for decompression of malignant biliary strictures has a technical success rate of >90% and risk of complications <5% in experienced hands and is considered the standard of care (41). Predominantly performed in the outpatient setting, ERCP has a shorter recovery period, lower relative expense, rate of complications, and morbidity when compared with surgical or radiologic/percutaneous interventions for biliary decompression.
Polyethylene stents (plastic) are an inexpensive, effective means of biliary decompression when placed at ERCP. They are easily extracted endoscopically and at the time of resection if subsequently performed. When plastic stent placement is pursued, 10 Fr stents are considered optimal, as comparative studies have failed to demonstrate greater advantage with 11.5 Fr stents (46), and smaller 7 Fr and 8.5 Fr stents offer negligible technical advantage in terms of ease of deployment (47).
Plastic stent exchanges are often necessary, as these stents inevitably occlude because of bacterial biofilm formation. When these plastic stents are placed, we follow an “on-demand” stent exchange protocol, repeating ERCP only when the patient develops recurrent signs or symptoms of biliary obstruction, as scheduled stent exchanges offer no additional advantage (40). Recent data suggest that stent failure may occur at even earlier time intervals in patients with locally advanced/borderline resectable PDAC receiving preoperative chemotherapy. A study evaluating patency in this population reported a “premature” stent failure rate of 35% (median patency 49 days, interquartile range 25–91), with 45% of patients having an unplanned hospitalization (48). Stent lengths >7 cm were also associated with failure in this study cohort (48% vs. 24%, P <0.01).
Self-expandable metallic stents (SEMS) are mesh (steel or nitinol) prostheses that range from 6 to 10 mm in diameter. However, 6 mm stents are rarely used because of suboptimal patency rates. Proprietary designs vary by the manufacturer in terms of mesh cell configuration and deployment systems. Covered (cSEMS) and uncovered SEMS (uSEMS) designs offer important advantages and disadvantages, with the principal differentiation being that uSEMS embed within biliary epithelium and typically cannot be removed. A tissue diagnosis is therefore desired before deployment of uSEMS, but may not be considered imperative, if patient management and decision making will not be affected (e.g., proceeding with surgery). Prospective studies and meta-analyses of jaundiced patients with PDAC treated by SEMS demonstrate superior duration of patency and need for fewer subsequent interventions compared with plastic stents (40,49–54). Figure 4 illustrates SEMS placement in a patient with PDAC causing pancreatic and bile duct obstruction. Limited data suggest that cSEMS may have slightly longer patency rates than uSEMS; however, this is not definitive and the increased cost of cSEMS may nullify this slight benefit (55–57). Universal use of SEMS are tempered by the cost added to the procedure, as metallic stents carry orders of magnitude greater expense than plastic stents. However, a recent prospective, randomized study evaluating plastic, uncovered, and partially covered stents in patients within unresectable PDAC found no difference in mean total costs between groups (6,906, 7,039, and 5,801€, respectively, P =0.28) when accounting for follow-up procedures and subsequent hospitalizations (49). Subgroup analysis for patients with shorter survival (<3 months) and metastatic disease also showed no differences in total costs. Based on these data, the upfront expense of SEMS may be offset by superior stent patency and lower costs associated with subsequent hospitalizations and procedures (48,49,58–60). Currently, we tend to place uSEMS as first-line therapy in most cases, as suggested in Figure 5. In addition, when preoperative neoadjuvant chemotherapy is being considered (see Surgical Approaches to PDAC below), compelling evidence indicates that self-expanding metal, not plastic stents should be used, as complication rates (particularly time to first stent occlusion) are much higher in the plastic group.
Figure 4.
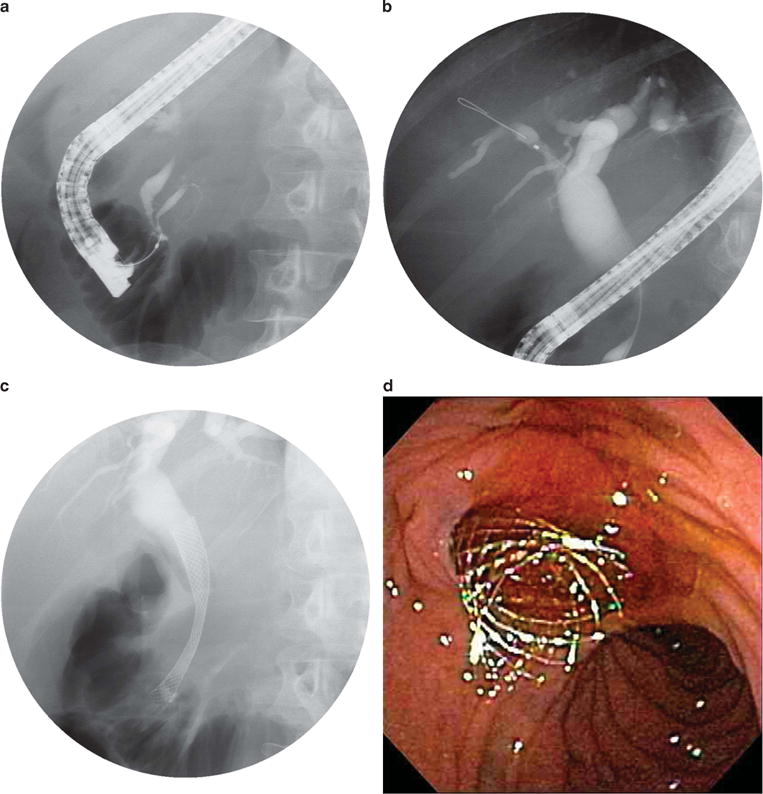
Endoscopic retrograde cholangiopancreatography (ERCP) images obtained in a 70-year-old man with abdominal pain, diarrhea, jaundice, and weight loss. Computed tomography (CT) scan reveals a head of pancreas mass. (a) Contrast injection reveals an obstructed pancreatic duct and distal bile duct stricture (“double-duct sign”). (b) Biliary dilation proximal to the biliary stricture. (c) Fluoroscopic image of a metal stent placed through the biliary stricture. (d) Endoscopic image of a metal stent placed through the biliary stricture, with subsequent bile flow.
Figure 5.
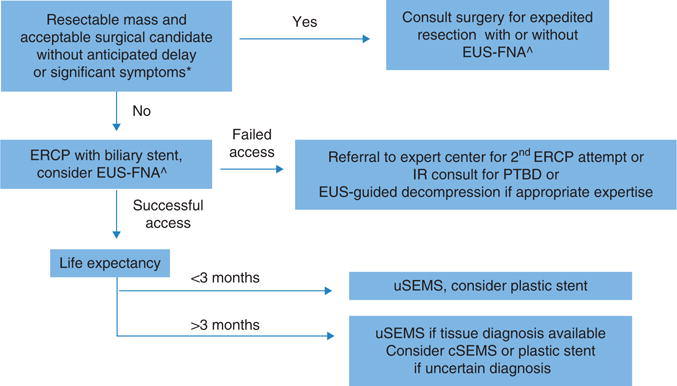
Algorithm for management of malignant biliary obstruction. *Patients with anticipated delays of surgery >2 weeks for work-up/stabilization of comorbidities should undergo preoperative decompression; significant symptoms=deep jaundice, refractory pruritus. ^Endoscopic ultrasound fine-needle aspiration (EUS-FNA) should be performed in patients with elevated suspicion for autoimmune pancreatitis or otherwise benign disease-causing obstruction, or for tissue confirmation before planned chemoradiotherapy. cSEMS, covered self-expandable metallic stent; PTBD, percutaneous transhepatic biliary decompression; uSEMS, uncovered self-expandable metallic stent.
Endoscopic options when an attempted ERCP decompression fails
Rates of adequate biliary drainage at first ERCP range from 70 to >90%. Factors associated with success include a patent gastric outlet, procedure volume at the performing center (61,62) and familiarity with advanced techniques for ERCP biliary access (e.g., precut sphincterotomy) (62–65). A repeat ERCP attempt at a tertiary center is an appropriate next step that meets with clinical success in the majority of repeat procedures (64,66,67). Percutaneous transhepatic biliary drainage (PTBD, see further discussion below) remains an established option when ERCP fails, with high levels of clinical success in the setting of dilated biliary radicals, and the possibility to convert to internal drainage with subsequent radiologic or endoscopic procedures.
EUS-guided biliary drainage (hepaticogastrostomy, choledochoduodenostomy) is an emerging technique encompassing endosonographic-guided transluminal stenting of the biliary tree and has reported rates of clinical success of (>90%) in some series. However, rates of complications can approach 10% and a substantial learning curve exists for performing these procedures effectively and safely. EUS-guided rendezvous procedures using the sonographic antegrade advancement of a guidewire across the papilla following needle puncture of the dilated biliary tree to facilitate ERCP cannulation also reports clinical success rates between 70 and 100%. However, rates of complications also are similarly high, 3–15% (68). Preliminary experience at expert centers suggests possible advantages of these techniques over PTBD with fewer follow-up procedures and lower total cost for biliary decompression (68–77). Further studies are required to establish the utility of these EUS-guided interventions.
Percutaneous transhepatic biliary drainage
PTBD provides biliary decompression for patients with malignant biliary obstruction who are not candidates for ERCP because of anatomy-altering surgical procedures or have failed attempted endoscopic stent placement. As the volume of the right liver is usually greater than that of the left, PTBD to treat extrahepatic or hilar biliary obstruction (as from metastatic PDAC) is usually attempted from the right. In several instances, a left-sided approach is preferred. The right hepatic duct is typically shorter than the left and thus is more susceptible to isolation of the right anterior and posterior divisions when malignant obstruction extends above the hepatic hilum. In these cases, left-sided access may allow drainage of a greater volume of functional liver with a single catheter (78). Similarly, if unilateral right hepatic atrophy occurs due to portal vein attenuation or thrombosis, drainage of the contralateral still functional left liver will provide greater benefit (79). Patients with ascites may experience leakage around a percutaneous drainage catheter that can cause skin irritation. Left-sided drainage using an anterior rather than a right midaxillary line transhepatic approach will minimize gravity-dependent leakage of ascites. Last, colon overriding the right liver may necessitate left-sided access.
PTBD is performed using fluoroscopic guidance and initial percutaneous transhepatic cholangiography to evaluate biliary anatomy and the location of the obstruction. Ultrasound may be used to guide initial bile duct puncture, especially if ducts are dilated by downstream obstruction. Contraindications for percutaneous transhepatic cholangiography and PTBD include bleeding diatheses, severe ascites, intrahepatic ductal obstructions due to diffuse hepatic parenchymal metastases, and chronic liver disease. PTBD can be accomplished using one of three types of devices: an external biliary drainage catheter, an internal–external biliary drainage catheter, or an internal SEMS.
Percutaneous external biliary drainage catheters traverse liver parenchyma to enter a bile duct and place drainage holes above the obstruction to allow bile to flow via gravity into an external drainage bag. These drains are used for high-grade biliary obstructions that cannot be crossed by a wire, as a means of initial decompression before staged internalization once edema resolves, or as temporary decompression before definitive surgical treatment.
Percutaneous internal–external biliary drainage catheters are longer and therefore more stable. These catheters traverse liver parenchyma to enter a bile duct and place drainage holes above the obstruction, and also traverse the obstruction and major papilla or biliary-enteric anastomosis to allow bile to flow via the catheter into bowel and reestablish normal enterohepatic circulation. Internal–external catheters can also provide external drainage into a gravity bag. Except in cases of high bile output, sepsis, or enteric obstruction, efforts are made to “cap” the external portion of the catheter to force antegrade internal flow of bile into bowel. When capped, a patient needs to be monitored for fever, leakage, or elevation of the serum bilirubin, in which case the internal-external drainage catheter is uncapped to external gravity drainage (80).
Following PTBD, patients should be monitored for signs of bleeding or infection. Despite prophylactic antibiotic coverage, sepsis may be seen during or within hours after PTBD (81). As bile ducts travel alongside hepatic arteries and portal veins in portal triads, transient hemobilia during catheter exchange may result when blood enters a bile duct. Sudden onset of bleeding following PTBD or any demonstrated abnormality of a hepatic arterial branch such as a pseudoaneurysm adjacent to a catheter should be considered presumptive evidence of arterial injury and should be treated with selective bracketing embolization of the artery across the level of injury (82). Leakage of bile around the percutaneous catheter back to the skin is commonly due to catheter sidehole occlusion or malposition due to internal migration leaving no holes above the obstruction or respiratory variation retracting holes out of the bile ducts into hepatic parenchyma. This usually resolves following exchange for a properly positioned catheter. Additional complications of PTBD can include hemoperitoneum, hemothorax, pneumothorax, bile peritonitis, pancreatitis, and cholangitis.
Percutaneous internal–external and external drainage catheters maximize biliary flow or diversion, but require maintenance, including emptying of drainage bags and flushing to maintain patency (83). Outpatient exchanges for occlusion, migration or malposition, or leakage are easily performed with moderate intravenous sedation. Following percutaneous access to the obstructed biliary tree, a lower maintenance option is placement of an internal SEMS across the obstruction. SEMS placement re-establishes drainage of bile into the bowel without the need for an external device, improving QOL. SEMS occlusion (by tumor ingrowth, proximal or distal tumor overgrowth, biliary sludge) can be treated endoscopically, reserving additional percutaneous intervention for endoscopic failures (84,85).
Percutaneously placed SEMS should only be used when placement leaves no effectively or impendingly isolated ducts that would be at risk for cholangitis. Ductal systems are completely isolated when percutaneous transhepatic cholangiography contrast does not opacify the ducts. Ducts are effectively isolated when opacified with contrast that does not empty on delayed imaging. Ducts may show impending isolation if central stenosis allows opacification and delayed emptying but is likely to progress to complete isolation. Unlike completely isolated ducts, ducts with effective or impending isolation are at increased risk of cholangitis because their poorly draining bile ducts may become colonized when contrast material enters during percutaneous transhepatic cholangiography. To decrease the likelihood that isolated ducts require additional intervention, SEMS should not be placed until all contaminated segments of the biliary tree are successfully drained or until patients are afebrile for at least 48 h after discontinuing antibiotics. Percutaneous internal–external catheters can be used to treat remaining isolated segments (86).
While both percutaneous internal–external drainage catheter and SEMS placement are safe and effective when initial ERCP is unsuccessful, an alternative approach to percutaneous drainage alone is the combined interventional radiology and ERCP rendezvous procedure (87). Similar to the EUS-ERCP rendezvous procedure described above, antegrade cholangiography defines the level of biliary obstruction, followed by advancement of the guidewire across the obstruction into the duodenum. Using endoscopic guidance, the wire is then snared and used for retrograde access to the common bile duct, facilitating plastic or metal stent placement. This multidisciplinary approach is effective in patients with severe obstruction or complex anatomy.
SURGICAL APPROACHES TO PDAC
Proper selection for operative resection remains paramount in the modern era of multidisciplinary management of patients with PDAC. High-resolution CT with dual-phase contrast enhancement is the ideal imaging modality for staging PDAC and determining the surgeon’s ability to achieve a complete resection with negative tissue margins on final pathologic assessment. Figure 6 lists the CT criteria for determining PDAC resectability based on preoperative imaging. Historically, patients with resectable CT criteria were offered operation as the first modality of therapy followed by adjuvant chemotherapy or chemoradiotherapy. The dismal long-term disease-free survival rates for patients who undergo an operation-first strategy for resectable cancer have reinforced the need for clinical trials and the development of more effective systemic and targeted therapies for PDAC. Recent advances in the efficacy of systemic chemotherapy agents have further supported the hypothetical benefits of preoperative neoadjuvant chemotherapy even for resectable PDAC (Table 1). Patients with borderline or locally advanced PDAC based on CT criteria should receive preoperative chemotherapy or chemoradiotherapy. Ideally, patients in these high-risk categories of disease should be offered enrollment in clinical trials investigating novel treatment agents. Despite newer systemic regimens which carry response rates nearing 40%, the vast majority of patients with locally advanced cancer will not be eligible for operative resection (88,89). Figure 7 outlines our institution’s approach to patients with PDAC after complete cancer staging and determination of resectability based on local vascular involvement according to CT findings.
Figure 6.
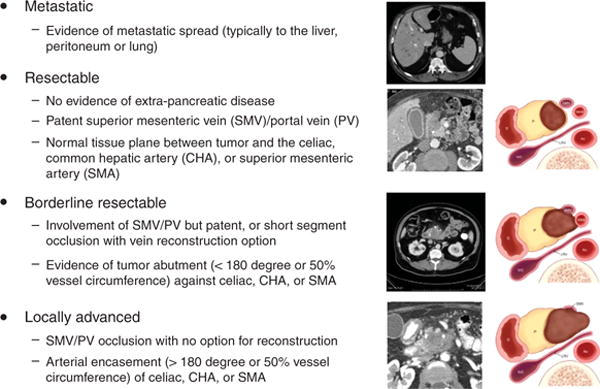
Computed tomography (CT) criteria for determining resectability of pancreatic ductal adenocarcinoma.
Table 1.
Rationale for neoadjuvant therapy
| • | Increase likelihood of truly negative surgical margins |
| • | Increase likelihood of completion of all intended multimodality therapy |
| • | Declaration of distant metastases and early progression of disease |
| • | Declaration of patient’s functional status and inability to tolerate operation |
| • | Opportunities for in vivo and in vitro testing for chemoresponsiveness |
Figure 7.
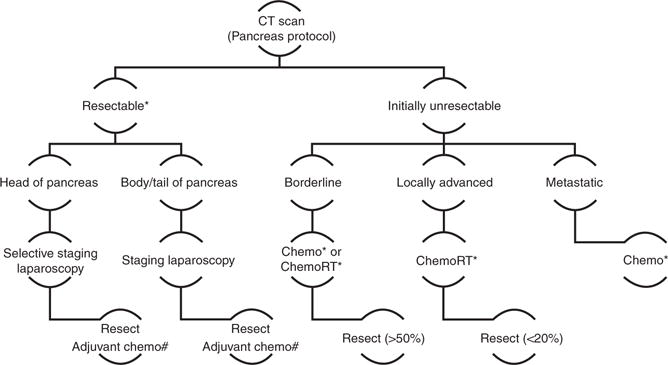
Approach to patients with pancreatic ductal adenocarcinoma after complete staging by computed tomography (CT). # Substitute ChemoRT for Chemo if R1 resection on final pathology. * Clinical trial offered for patients.
Once a patient with PDAC comes to resection, the surgeon’s goal is to achieve R0 resection-complete tumor extirpation with no evidence of macroscopic or microscopic disease at the resection margins. With pancreatic head resection, the most challenging margin to clear in general is the so-called retroperitoneal soft tissue margin (superior mesenteric artery margin). Systematic review of margin status is challenging; however, most studies suggest that R1 resection (microscopic disease remains in situ) or R2 resection (gross evidence of disease) is associated with decreased long-term survival (90). With the primary goal of tumor clearance in mind, pancreatic surgeons have looked to extended lymphadenectomy and vascular resection.
Attempts to improve survival by extending lymphadenectomy have failed. One Italian and two United States prospective, randomized trials of extended lymphadenectomy vs. standard lymphadenectomy failed to show survival benefit in the extended lymphadenectomy group (91–93). This extended lymphadenectomy group did manifest substantially increased perioperative complications. Therefore, our current practice includes standard lymphadenectomy with pancreatic resection.
Vascular resection—specifically of the superior mesenteric vein and portal vein—is now being applied commonly by experienced pancreatic surgeons. This degree of extended local resection permits operative treatment of what had previously been considered locally advanced and unresectable disease. Early enthusiasm for arterial (superior mesenteric artery and hepatic artery) resection has been tempered. Single center analysis from high volume, experienced pancreatic surgery programs document no difference in mortality with venous resection, although these patients routinely have greater perioperative morbidity than those without vascular reconstruction. More recently, nationwide survey data from the United States National Surgical Quality Improvement Project suggest that venous resection may indeed be accompanied by higher mortality (94). Most authorities agree that this technique should be practiced in higher-volume centers with surgeons experienced in both pancreatic surgery and vascular reconstructive techniques. Our current practice applies venous resection and reconstruction to ∼ 20% of patients undergoing pancreatic resection (95).
Mortality after pancreatectomy has decreased substantially in recent years. Advances in operative technique, critical care, and perhaps most importantly the ability of high-volume centers to rescue patients after major complication have led to contemporary postoperative mortality rates consistently in the 2–3% range (95). Despite decreases in mortality, postpancreatectomy morbidity remains relatively high, on the order of 30–40% (96,97) even at many high-volume centers, including our own (90,95,98). Common complications after pancreatic resection include bleeding, delayed gastric emptying (99), and fistula from any of the three enteric anastomoses: gastrojejunostomy, hepaticojejunostomy, and pancreaticojejunostomy (99–102). The international study group for pancreatic surgery has published consensus definitions for many of these complications, facilitating standardized communication among reporting centers (103–105). Among all postpancreatectomy complications, pancreatic fistula remains the most common, and the Achilles’ heel of the operation. Pancreatic fistula occurs in ∼ 15% of patients after pancreatic head resection (pancreatoduodenectomy) and in ∼ 25% of patients after left-sided pancreatic resection (distal pancreatectomy) (102,106). The greatest risk factor for pancreatic fistula development is a normal pancreas, with soft parenchyma and a small pancreatic duct (107). Fortunately, most pancreatic fistulae will heal with minimal additional intervention. However, these fistulae may lead to significant problems including intra-abdominal abscess, hemorrhage, and death. In fact, more than half of the mortality after pancreatectomy can be related directly to pancreatic fistula. The key to managing pancreatic fistula is controlled external drainage. To this end, prospective, randomized data highlight the utility of intraoperative drain placement in pancreatectomy (108). Patients with externally controlled pancreatic fistulae may continue along common postoperative management pathway—i.e., have diet advanced liberally, and are discharged from the hospital with the drain in situ. After a few weeks’ time to permit intra-abdominal adhesions around the drain, the drain may be “cracked” or withdrawn a few centimeters. Drainage typically slows, and the drain may then be removed. Some surgeons prefer to perform a “sinogram”—injecting contrast through the drain—before removal. Occasionally, persistent pancreatic fistula after distal pancreatectomy may require ERCP with stenting to assist closure. Uncontrolled pancreatic fistula may present with abdominal distention, vomiting, fever, and signs of SIRS. Cross-sectional imaging (CT) will diagnose undrained intra-abdominal collections, many of which are amenable to percutaneous interventional radiology drainage. Occasionally, reoperation is required to gain control of pancreatic fistula.
Despite substantial technical improvements in the conduct of pancreatic resection, patients continue to die from recurrent disease even after a “perfect” operation. These disappointing observations underscore the need for improved systemic therapy.
SYSTEMIC THERAPY FOR PATIENTS WITH PANCREATIC CANCER
Management of resectable disease
Only a small fraction of patients with PDAC present with resectable disease. Median overall survival (OS) for these patients is 20–22 months (109,110). These patients are offered surgical resection followed by adjuvant chemotherapy or chemoradiotherapy (the radiotherapy being controversial and generally carried out more in the United States than in most of Europe). However, owing to the fact that 5-year survival of this group is 10% with surgery alone and 25% with the addition of adjuvant therapy (111), preoperative neoadjuvant chemotherapy has been proposed (112,113) (as outlined in Surgical Approaches to PDAC, and Table 1). The challenge to this approach is a lack of effective systemic therapies. A meta-analysis summarized several neoadjuvant studies and demonstrated an actual resection rate of 70% in patients with initially radiographically resectable PDAC (113). Resection after neoadjuvant therapy appears to be safe (114). In a prospective phase II study, patients who underwent neoadjuvant, gemcitabine-based, chemoradiotherapy had a median OS of 34 months and a 5-year survival of 36% (115). Adjuvant chemotherapy with gemcitabine in comparison with observation after surgical resection improves disease-free survival, OS, and 5-year OS. 5-fluorouracil (5-FU) with leucovorin has been compared with gemcitabine in the adjuvant setting and was equally effective (110). However, because of the more manageable toxicity profile associated with gemcitabine, it is the preferred adjuvant chemotherapy choice throughout much of the world. Currently, at Indiana University, we are conducting a prospective phase II study of neoadjuvant FOLFIRINOX (5-FU, leucovorin, irinotecan, and oxaliplatin) for 2 months in patients with apparently resectable disease by radiographic criteria. Patients are initially discussed by our multidisciplinary tumor board, which involves colleagues from several disciplines (medical and radiation oncology, surgery, gastroenterology). This helps stratify patients based on their stage, with discussion regarding the possibility of surgical resection, should downstaging be accomplished. Furthermore, patient eligibility for therapeutic or biomarker clinical trials may also be coordinated.
Management of borderline resectable and locally advanced unresectable PDAC
The optimal management of this group is controversial. Up to 70% of patients with locally advanced disease die of metastatic disease (116), and therefore a systemic approach is more favorable. Combination chemotherapy with radiation therapy has yielded controversial results, especially in the absence of predictive biomarkers (117–119). Although highly selected patients with borderline or locally advanced PDAC have gone on to surgical resection (120), this phenomenon remains uncommon. One goal of therapy in borderline resectable disease cancer is to induce significant response to allow surgical resection. A recent pilot study by the US Alliance cooperative group evaluated the feasibility and safety of modified FOLFIRINOX, followed by external beam radiotherapy in combination with capecitabine (121). This strategy appears to be feasible, and resulted in a high rate of R0 resection in patients who completed preoperative therapy without evidence of progression. Several questions remain to be addressed regarding the role of radiation therapy, the duration of chemotherapy, and the optimal regimen of systemic therapy.
Management of metastatic disease
For the past two decades, gemcitabine was the only real option for the management of patients with PDAC. This was based on early data demonstrating improved pain control and decreased rate of weight loss with gemcitabine (122). The survival benefit of gemcitabine over 5-FU was quite modest. More recently, combination cytotoxic chemotherapy has shown improved progression-free survival and OS over gemcitabine alone. FOLFIRINOX has demonstrated improvement in progression-free survival from 3.4 to 6.6 months (88). Similarly, OS was improved with FOLFIRINOX (6.7–11.1 months). This improvement was associated with increased toxicity, but despite this, QOL was preserved for a longer duration in patients treated with FOLFIRINOX (123). The first combination therapy to be approved by the Food and Drug Administration was the combination of nanoparticle albumin-bound paclitaxel (nab-paclitaxel) in combination with gemcitabine. Similarly, this combination improved progression-free survival (3.3–5.5 months) and OS (6.7–8.5 months) (89). QOL studies were not conducted with this combination therapy.
At our institution, the approach to systemic therapy depends on the patient’s symptoms, performance status, and comorbidities. For patients with good ECOG (Eastern Cooperative Oncology Group) performance status, clinical trials are preferred when available. If not, FOLFIRINOX or nab-paclitaxel/gemcitabine is commonly offered. At the time of progression on first-line therapy, patients with maintained performance status are typically treated with 5-FU based-therapy or FOLFOX if they received initial gemcitabine-based therapy (124), or gemcitabine-based therapy if 5-FU-based therapy was used first line.
RADIATION THERAPY FOR PDAC
Radiation treatment (RT) paradigms for PDAC are constantly evolving with the development of more active chemotherapy regimens and continuing advances in radiation planning and delivery techniques. Treatment decision-making in patients with PDAC has been shaped by a series of ongoing controversies regarding the role of RT in managing this disease as well as by technological advances in imaging, radiation planning, and treatment delivery.
Vigorous debate continues within gastrointestinal oncology regarding the optimal adjuvant therapy for patients with PDAC. Although early results published in the 1980s reported a survival benefit with the postoperative delivery of 40 Gy split-course adjuvant RT plus 5-FU (125), subsequent European studies suggested that at best, no clinical benefit was associated with postoperative treatment, and that at worst, adjuvant RT was associated with worse outcomes. ESPAC-1 tested postoperative chemotherapy alone, chemoradiation, chemotherapy, followed by chemoradiation, and observation in patients with resected PDAC and reported shorter survival in patients treated with chemoradiation compared with those who received chemotherapy (126). This was not truly an RCT, as clinicians were allowed to select the randomization arm that the patients would enter; furthermore, there was considerable crossover between arms, and the study lacked centralized quality assurance practices. EORTC 40891 tested 40 Gy split-course RT plus 5-FU vs. observation in patients with both pancreatic and periampullary cancers (127). This study showed a nonsignificant trend toward improved OS in the treatment group, with a suggestion that patients with pancreatic head tumors may have derived a greater benefit from treatment than those with ampullary tumors. Similar to ESPAC-1, the results of this trial should be interpreted cautiously, given its use of an outdated radiation regimen and lack of radiation quality assurance procedures. Furthermore, the inclusion of only patients with relatively early-stage (T1–2/N0–1) pancreatic tumors and the pooling of patients with pancreatic and periampullary lesions in the same study may also have reduced the likelihood of observing a significant benefit from adjuvant RT in this trial.
Several more recent studies have attempted to answer the questions raised by these earlier trials. RTOG 9704 randomized patients to receive either induction gemcitabine or 5-FU plus adjuvant chemoradiation therapy (50.4 Gy/5-FU) and reported no significant difference in OS between the two treatment arms (128). At virtually the same time, a German study (CONKO-001) investigated adjuvant gemcitabine alone (vs. placebo) and reported an absolute benefit in 5-year OS of ∼ 10% (with 5-year OS times of 20.7% vs. 10.4% in the gemcitabine and placebo arms, respectively) (111). Survival outcomes in RTOG 9704 and CONKO-001 were comparable, supporting the contention that chemotherapy alone (rather than chemoradiation) may be an acceptable adjuvant therapy option in patients with resected PDAC. Nonetheless, postoperative RT should still be considered in selected patients, given the restrictive eligibility criteria of CONKO-001 (which required postoperative CA19-9 levels to be under 90 IU/ml), the relatively high local failure rates when RT is omitted, and pooled data from high-volume US centers, suggesting that, in experienced hands, adjuvant radiation therapy does indeed provide a survival benefit to well-selected patients with resected PDAC (129). Ongoing efforts to improve patient selection for adjuvant treatment are exemplified by the ongoing US cooperative group study, RTOG 0848, which delivers RT only in patients whose disease does not progress after five cycles of adjuvant chemotherapy (plus or minus erlotinib). Our present approach is to consider postoperative RT in patients with high-risk features such as positive resection margins or multiple positive lymph nodes who have not developed metastatic progression during the first few cycles of postoperative chemotherapy.
Patients with locally advanced PDAC have high rates of occult metastatic disease and generally benefit from a course of induction chemotherapy before radiation therapy can be considered. In a recent prospective series testing this approach (130), patients were initially randomized to gemcitabine or gemcitabine plus erlotinib; those without progression on this regimen were then randomized to capecitabine-based chemoradiation vs. chemotherapy alone. Forty percent of initially randomized patients developed progressive disease and did not proceed to the second randomization. Although the study did not demonstrate a benefit in OS for RT, local failure rates and time to treatment resumption were both significantly improved in the RT arm. We generally recommend a similar approach (i.e., chemotherapy followed by chemoradiation) in patients with borderline resectable disease, with surgical exploration planned 4–6 weeks after chemoradiation is completed. However, long-term tumor control and OS outcomes in this group of patients remain unsatisfactory, especially in patients with unresectable tumors, and innovative approaches to intensifying treatment are clearly needed.
Stereotactic body radiation therapy permits radiation dose intensification via hypofractionated treatments that are delivered to highly focused targets using daily image guidance and respiratory motion control. Although phase III studies of this technique are not yet available, mature phase II studies have reported encouraging local control rates with a favorable acute toxicity profile (131). The primary side effect associated with stereotactic body radiation therapy to the upper abdomen is late gastrointestinal bleeding/ulceration. Risk factors for stereotactic body radiation therapy-related upper gastrointestinal bleeding include the use of large fraction sizes and the presence of tumor invasion into the lumen of the bowel or stomach, which should be considered an absolute contraindication to the use of this technique in patients with PDAC (132). Presently, we recommend that stereotactic body radiation therapy be used for PDAC only in the context of a clinical trial. However, the therapy is promising and merits investigation for multiple indications in patients with PDAC, including as adjuvant therapy, downstaging for patients with borderline to locally advanced disease, and as an adjunct to chemotherapy and a substitute for long-course radiation in patients with unresectable tumors.
Additional avenues for research into optimizing outcomes in patients receiving RT for PDAC include evaluating neoadjuvant therapy, which has significant theoretical advantages over postoperative treatment, even in patients with resectable disease; testing particle therapy, particularly proton-beam therapy; and further study of the integration of immunotherapy, such as immune checkpoint inhibitors and antitumor vaccines, with RT and conventional chemotherapy.
PANCREATIC EXOCRINE INSUFFICIENCY AND NUTRITIONAL SUPPORT
Exocrine pancreatic insufficiency is a well-described phenomenon in patients with PDAC in both the preoperative and postoperative setting. Rates of exocrine insufficiency, lipid maldigestion and malabsorption as measured by a Lundh test, fecal elastase testing, and 13C-trioctanoin breath testing approach 80–90% in patients with PDAC (133–136). Likely related to obstruction of the main pancreatic duct, maldigestion may be further exacerbated by biliary obstruction (134,136). Pancreatic exocrine insufficiency is also prevalent following resection, more frequently noted with pancreaticoduodectomy (>70%) than distal pancreatectomy (30–60%). Indeed, pancreaticoduodenectomy and preoperative duct diameter >10 mm have been found to be predictors for maldigestion (136,137). Mechanisms for exocrine insufficiency following pancreaticoduodenectomy, beyond loss of pancreatic parenchyma, include poor mixing of chyme with pancreatic enzymes, impairment of exocrine pancreatic secretion from disruption of vagus branches, and diminished cholecystokinin and secretin stimulation after duodenal resection (136). It is a misconception that steatorrhea is always associated with exocrine insufficiency. Studies have demonstrated poor correlation between symptoms and exocrine insufficiency (136). Consequently relying on symptoms alone for decision-making regarding pancreatic enzyme supplementation is not appropriate. Randomized studies are now available demonstrating weight gain and superior survival in patients with PDAC managed with pancreatic enzyme supplementation. However, benefit of systematic supplementation following pancreaticoduodectomy has not been as clearly demonstrated (136,138). This is likely due to the additional mechanisms for maldigestion outlined above. Bartel et al. (136) offer a thorough review of exocrine insufficiency in the context of PDAC. The authors offer a cogent argument for pancreatic enzyme supplementation in all unresectable patients with pancreatic duct obstruction and treatment in patients following pancreaticoduodenectomy dictated by 72 h fecal fat testing. Comanagement of patients with a dietitian, palliation of gastric outlet obstruction (GOO) and pain, and micronutrient supplementation are also elements of the multidisciplinary approach to management of malnutrition in patients with PDAC. However, beyond pancreatic enzyme supplementation, the optimal approach for nutritional support in patients with PDAC is a subject that is rarely discussed in available societal guidelines and requires further study.
PALLIATION FOR UNRESECTABLE PANCREATIC CANCER
Palliation remains the cornerstone of management of patients with unresectable PDAC and is primarily directed at relief of symptoms and improvement in QOL. A tailored therapeutic approach based on the patient’s preferences, prognosis, life expectancy, and on the local expertise should be followed.
Palliative care consultation
Referral to a specialized palliative care service is often delayed because of the patients’ (and often the physicians’) misconception about palliative care being an alternative (rather than an additional) resource for anticancer treatment (139,140). Specialized palliative care service includes home-based hospice care programs, in-patient hospices, palliative care units, and palliative care teams. Several trials suggest that a combined early introduction of the specialized palliative care service, providing on-demand specialized palliative care, and the routine use of screening tools and feedback to the treating physicians of QOL measurements or symptom assessment scales, contribute to a better QOL for cancer patients receiving active cancer treatment (141–143). We recommend consideration of an early referral to a specialized palliative care service as part of the multidisciplinary approach to patients with unresectable PDAC.
Pain management
Up to 90% of patients with PDAC experience significant abdominal pain during the course of their illness. The celiac ganglion is responsible for pain transmission, and interventions targeted here (percutaneous, endoscopic, or surgical) may offer pain relief. We favor endoscopic over percutaneous access to the celiac ganglion at our institution, primarily due to availability. Furthermore, limited data from a single RCT favored the endoscopic approach (144). Surgical intervention typically occurs in those patients who undergo a planned resective procedure but are found to have unresectable disease intraoperatively. When analgesia is suboptimal with oral/topical agents alone, or these are not tolerated, EUS-guided celiac plexus neurolysis (EUS-CPN) and EUS-celiac ganglion neurolysis are achieved by injection of local anesthetic (e.g., bupivacaine) followed by absolute alcohol into the celiac plexus or celiac ganglia. Early EUS-CPN, at the time of diagnostic and staging EUS, provides better pain relief and may prevent pain escalation while moderating narcotic use (145). Pain reduction can be expected in up to 80% of patients within 2 weeks of the procedure (146). There are no predictive factors for pain relief after EUS-CPN, although direct tumor invasion of the celiac plexus is associated with reduced efficacy (147). EUS-CPN has little to no impact on survival compared with controls (148).
Bilateral (both sides of the celiac plexus) injection allows a wider distribution of the neurolytic solution in the area of the celiac axis. While there has been discordance between studies evaluating effectiveness of bilateral vs. unilateral injections (149,150), a meta-analysis suggested superiority of bilateral injection over unilateral injection (151). In addition, a recent multicenter RCT showed superiority of EUS-celiac ganglion neurolysis over EUS-CPN for the palliation of pain; however, the comparison was not against bilateral injection and the response rate with celiac ganglion neurolysis vs. unilateral was similar to the bilateral vs. unilateral technique (152).
Data on adverse events of EUS-CPN are limited to small retrospective series and case reports (146). Mild complications include transient diarrhea (4–15%), hypotension (1%), and increase in pain (9%). Major complications (2.5%) include retroperitoneal bleeding and abscess formation. In our practice and in view of the relative efficacy and safety, we recommend early consideration of EUS-CPN for patients with unresectable PDAC with opiate-requiring abdominal pain.
Gastric outlet obstruction
GOO is a surrogate marker for poor survival in patients with unresectable PDAC, occurring in 10–25% of patients. It can cause significant morbidity through persistent intractable nausea and vomiting, malnutrition, and weight loss. Historically, treatment has consisted of an open or laparoscopic surgical bypass, i.e., gastro jejunostomy. However, some patients are not surgical candidates, either because of their overall physical status or limited life expectancy. Similar to biliary SEMS, enteral SEMS have emerged as a non-surgical alternative in these patients (Figure 8). A recent systematic review and meta-analysis of 3 RCTs and 17 non-RCTs of endoscopic stenting vs. operative gastrojejunostomy (open or laparoscopic) was conducted (153). Taking all studies as a whole, patients who received endoscopic SEMS had significantly fewer major and minor complications, a shorter time to tolerance of oral intake, and a shorter hospital stay. In the non-RCTs, there was also a trend towards lower medical costs (mean US$8,629 vs. US $17,842; P =0.09) in the SEMS group. There was no difference in median survival time among RCTs and non-RCTs and in QOL (153,154).
Figure 8.
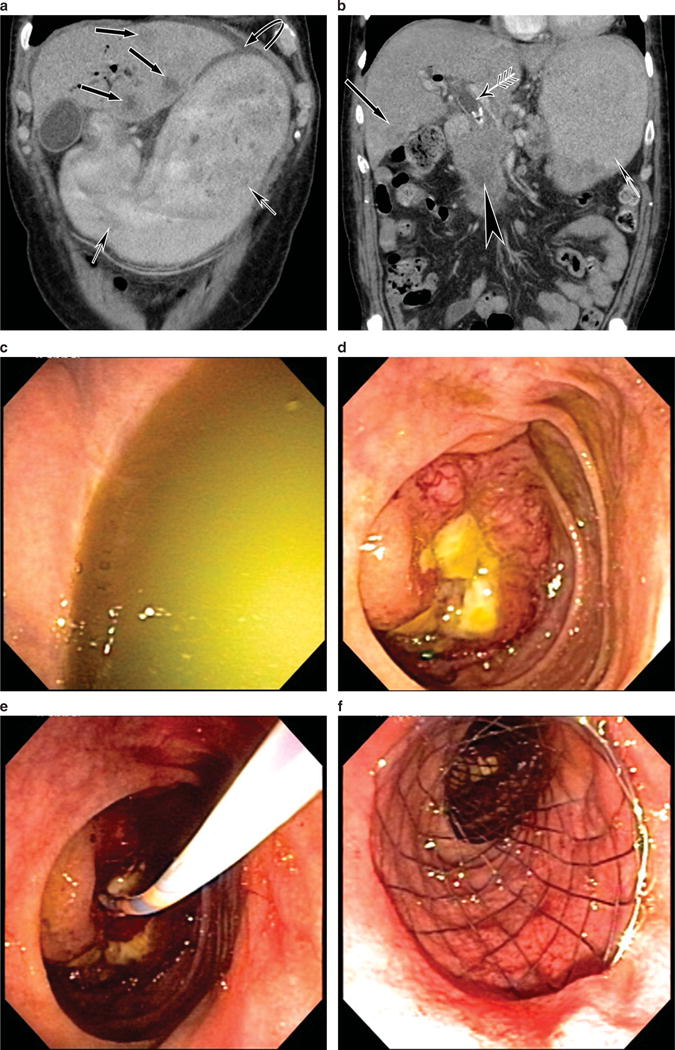
This patient with metastatic pancreas cancer presents with nausea and vomiting. (a) Computed tomography (CT) scan reveals liver metastases and a distended stomach. (b) CT scan reveals the pancreas mass, a dilated bile duct with stent in place, liver metastasis, and a distended fluid-filled stomach. (c) Endoscopic image of the fluid-filled stomach. (d) Duodenal obstruction by tumor. (e) Catheter and guidewire passage beyond the obstruction. (f) Duodenal stent placed.
Mild early adverse events of gastroduodenal SEMS include abdominal discomfort, nausea, and vomiting. These symptoms can be related to a delayed full-expansion of the stent, which may take up to 1 week, and are typically treated conservatively. As early stent obstruction may occur from food particles, we typically ask patients to follow a liquid diet for a few days poststent placement, to be advanced as tolerated to a low residue diet indefinitely. Major adverse events are rare but may be early or delayed. Those occurring within the first week include severe pain, bleeding, perforation, and stent migration. Late adverse events include fistula formation, delayed perforation, biliary obstruction, and stent migration (41,80). Stent obstruction occurs in 15–20% of patients and typically results from tumor in- or overgrowth. This can be treated with placement of a second stent, either within or overlapping the first stent. Partially covered, fully covered, and conformable stents represent an alternative to uncovered SEMS (155–157). These are not available in the United States and are associated with higher migration rates.
If possible, the distal end of the SEMS should be left proximal to the major papilla, allowing for future biliary interventions if needed. The specific location of the GOO and gastroduodenal anatomy, however, impact the length of SEMS necessary and this may not be possible. In this case, to treat biliary obstruction, either present or impending at the time of GOO diagnosis, we usually attempt endoscopic biliary drainage before deployment of duodenal stents. If simultaneous biliary and duodenal SEMS placement is not possible, and the distal end of the duodenal SEMS crosses the major papilla, subsequent biliary decompression via ERCP may be very difficult, as the papilla is obscured and distorted by the SEMS. While it may be possible to attempt endoscopic biliary drainage through the interstices of the enteral stent or by fenestration of the stent (using a rat-toothed forceps or argon plasma coagulation), these maneuvers frequently fail. In these cases, percutaneous or EUS-guided (transhepatic or transduodenal without accessing the papilla or via rendezvous with ERCP) options are available (158), pending local expertise (see Establishing Biliary Drainage for the Jaundiced Patient above), with surgical biliary drainage less desirable. An informed patient and discussion with the multidisciplinary team is mandatory in these cases.
In summary, significant developments continue to be made in the diagnosis and management of patients with PDAC. These patients are best managed by a multidisciplinary team, consisting of gastroenterologists, pancreatobiliary surgeons, radiologists, and oncologists. Advances in cross-sectional imaging have led to more accurate diagnosis and staging. When needed, endoscopists are able to provide tissue diagnosis and further staging at EUS, as well as biliary decompression at ERCP with a high level of success. Use of self-expandable metal stents has proven to be a cost-effective strategy. Furthermore, palliation of GOO and debilitating pain can often be managed endoscopically, with enteral stent placement and EUS-guided celiac neurolysis, respectively. Surgical resection remains the only chance for cure in this disease, and improved surgical techniques including venous reconstruction may lead to an increase in the percentage of resectable patients. New chemotherapy protocols, with or without radiation therapy, have led to an increase in OS, although this strategy remains palliative. On the other hand, preliminary data evaluating the use of neoadjuvant chemotherapy in the preoperative setting appear promising. Despite these advances and innovations, pancreatic cancer remains the silent killer, as most patients are not candidates for curative therapy at presentation. Further study is desperately needed, particularly in early detection. The discovery of a biomarker that would facilitate earlier identification of pancreas cancer would greatly affect patient management and prognosis.
Acknowledgments
Financial support: None.
Footnotes
Guarantor of the article: Evan L. Fogel, MSc, MD.
Specific author contributions: Each of the contributing authors listed played a role in drafting of the manuscript. In addition, Drs Fogel and Sherman also served as editors of the article. I have been in contact with all of the authors, and all have approved the final draft of the manuscript submitted for publication.
Potential competing interests: None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2013. National Cancer Institute; Bethesda, MD: available at http://seer.cancer.gov/csr/1975_2013/, based on November 2015. SEER data submission, posted to the SEER website, April 2016. [Google Scholar]
- 3.Raimondi S, Lowenfels AB, Morselli-Labate AM, et al. Pancreatic cancer in chronic pancreatitis: aetiology, incidence, and early detection. Best Pract Res Clin Gastro. 2010;24:349–58. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Lowenfels AB, Maisonneuve P, Cavallini G, et al. Pancreatitis and the risk of pancreatic cancer. N Engl J Med. 1993;328:1433–7. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 5.Ben Q, Xu M, Ning X, et al. Diabetes mellitus and risk of pancreatic cancer: a meta-analysis of cohort studies. Eur J Cancer. 2011;47:1928–37. doi: 10.1016/j.ejca.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Li D, Tang H, Hassan MM, et al. Diabetes and risk of pancreatic cancer: a pooled analysis of three large case–control studies. Cancer Causes Control. 2011;22:189–97. doi: 10.1007/s10552-010-9686-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1252–61. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trikudanathan G, Philip A, Dasanu CA, et al. Association between Helicobacter pylori infection and pancreatic cancer. A cumulative meta-analysis JOP. 2011;12:26–31. [PubMed] [Google Scholar]
- 9.Waddell N, Pajic M, Patch AM, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hruban RH, Petersen GM, Goggins M, et al. Familial pancreatic cancer. Ann Oncol. 1999;4:69–73. [PubMed] [Google Scholar]
- 11.Jones S, Hruban RH, Kamiyama M, et al. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canto M, Harinck F, Hruban RH, et al. International cancer of the pancreas screening (CAPS) consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 2013;62:339–47. doi: 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wong JC, Lu DS. Staging of pancreatic adenocarcinoma by imaging studies. Clin Gastroenterol Hepatol. 2008;6:1301–8. doi: 10.1016/j.cgh.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Tamm EP, Balachandran A, Bhosale PR, et al. Imaging of pancreatic adenocarcinoma: update on staging/resectability. Radiol Clin N Am. 2012;50:407–28. doi: 10.1016/j.rcl.2012.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Marcal LP, Fox PS, Evans DB, et al. Analysis of free-form radiology dictations for completeness and clarity for pancreatic cancer staging. Abdom Imag. 2015;40:2391–7. doi: 10.1007/s00261-015-0420-1. [DOI] [PubMed] [Google Scholar]
- 16.Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol. 2014;20:7864–77. doi: 10.3748/wjg.v20.i24.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tempero MA, Arnoletti JP, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2012: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2012;10:703–13. doi: 10.6004/jnccn.2012.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Haddad M, DeWitt J. EUS and pancreatic tumors. In: Hawes R, Fockens P, Varadarajulu S, editors. Endosonography. 2nd. Elsevier Press; London, UK: 2011. pp. 148–65. [Google Scholar]
- 19.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol. 2004;2:606–21. doi: 10.1016/s1542-3565(04)00244-7. [DOI] [PubMed] [Google Scholar]
- 20.Langer P, Kann PH, Fendrich V, et al. Five years of prospective screening of high-risk individuals from families with familial pancreatic cancer. Gut. 2009;58:1410–8. doi: 10.1136/gut.2008.171611. [DOI] [PubMed] [Google Scholar]
- 21.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175–81. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 22.Zubarik R, Gordon SR, Lidofsky SD, et al. Screening for pancreatic cancer in a high-risk population with serum CA 19-9 and targeted EUS: a feasibility study. Gastrointest Endosc. 2011;74:87–95. doi: 10.1016/j.gie.2011.03.1235. [DOI] [PubMed] [Google Scholar]
- 23.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. quiz e714–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeWitt J, Devereaux B, Chriswell M, et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann Int Med. 2004;141:753–63. doi: 10.7326/0003-4819-141-10-200411160-00006. [DOI] [PubMed] [Google Scholar]
- 25.Dewitt J, Devereaux BM, Lehman GA, et al. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol. 2006;4:717–25. doi: 10.1016/j.cgh.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 26.Catanzaro A, Richardson S, Veloso H, et al. Long-term follow-up of patients with clinically indeterminate suspicion of pancreatic cancer and normal EUS. Gastrointest Endosc. 2003;58:836–40. doi: 10.1016/s0016-5107(03)02301-0. [DOI] [PubMed] [Google Scholar]
- 27.Klapman JB, Chang KJ, Lee JG, et al. Negative predictive value of endoscopic ultrasound in a large series of patients with a clinical suspicion of pancreatic cancer. Am J Gastroenterol. 2005;100:2658–61. doi: 10.1111/j.1572-0241.2005.00315.x. [DOI] [PubMed] [Google Scholar]
- 28.Bhutani MS, Gress FG, Giovannini M, et al. The No Endosonographic Detection of Tumor (NEST) Study: a case series of pancreatic cancers missed on endoscopic ultrasonography. Endoscopy. 2004;36:385–9. doi: 10.1055/s-2004-814320. [DOI] [PubMed] [Google Scholar]
- 29.Săftoiu A, Vilmann P. Differential diagnosis of focal pancreatic masses by semiquantitative EUS elastography: between strain ratios and strain histograms. Gastrointest Endosc. 2013;78:188–9. doi: 10.1016/j.gie.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 30.Mei M, Ni J, Liu D, et al. EUS elastography for diagnosis of solid pancreatic masses: a meta-analysis. Gastrointest Endosc. 2013;77:578–89. doi: 10.1016/j.gie.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 31.Săftoiu A, Iordache SA, Gheonea DI, et al. Combined contrast-enhanced power Doppler and real-time sonoelastography performed during EUS, used in the differential diagnosis of focal pancreatic masses (with videos) Gastrointest Endosc. 2010;72:739–47. doi: 10.1016/j.gie.2010.02.056. [DOI] [PubMed] [Google Scholar]
- 32.Săftoiu A, Vilmann P, Dietrich CF, et al. Quantitative contrast-enhanced harmonic EUS in differential diagnosis of focal pancreatic masses (with videos) Gastrointest Endosc. 2015;82:59–69. doi: 10.1016/j.gie.2014.11.040. [DOI] [PubMed] [Google Scholar]
- 33.DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound guided-fine needle aspiration cytology of solid liver lesions: a large single center experience. Am J Gastroenterol. 2003;98:1976–81. doi: 10.1111/j.1572-0241.2003.07638.x. [DOI] [PubMed] [Google Scholar]
- 34.DeWitt J, LeBlanc J, McHenry L, et al. Endoscopic ultrasound-guided fine needle aspiration of ascites. Clin Gastroenterol Hepatol. 2007;5:609–15. doi: 10.1016/j.cgh.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 35.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 36.Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: a systematic review. Gastrointest Endosc. 2011;73:283–90. doi: 10.1016/j.gie.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 37.Asbun HJ, Conlon K, Fernandez-Cruz L, et al. When to perform a pancreatoduodenectomy in the absence of positive histology? A consensus statement by the International Study Group of Pancreatic Surgery Surgery. 2014;155:887–92. doi: 10.1016/j.surg.2013.12.032. [DOI] [PubMed] [Google Scholar]
- 38.van der Gaag NA, Rauws EA, van Eijck CH, et al. Preoperative biliary drainage for cancer of the head of the pancreas. N Engl J Med. 2010;362:129–37. doi: 10.1056/NEJMoa0903230. [DOI] [PubMed] [Google Scholar]
- 39.Keane MG, Horsfall L, Rait G, et al. A case–control study comparing the incidence of early symptoms in pancreatic and biliary tract cancer. BMJ Open. 2014;4:e005720. doi: 10.1136/bmjopen-2014-005720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moss AC, Morris E, Mac Mathuna P. Palliative biliary stents for obstructing pancreatic carcinoma. Cochrane Database Syst Rev. 2006:CD004200. doi: 10.1002/14651858.CD004200.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Cote GA, Sherman S. Endoscopic palliation of pancreatic cancer. Cancer J. 2012;18:584–90. doi: 10.1097/PPO.0b013e3182745ad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barkay O, Mosler P, Schmitt CM, et al. Effect of endoscopic stenting of malignant bile duct obstruction on quality of life. J Clin Gastroenterol. 2013;47:526–31. doi: 10.1097/MCG.0b013e318272440e. [DOI] [PubMed] [Google Scholar]
- 43.Ballinger AB, McHugh M, Catnach SM, et al. Symptom relief and quality of life after stenting for malignant bile duct obstruction. Gut. 1994;35:467–70. doi: 10.1136/gut.35.4.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abraham NS, Barkun JS, Barkun AN. Palliation of malignant biliary obstruction: a prospective trial examining impact on quality of life. Gastrointest Endosc. 2002;56:835–41. doi: 10.1067/mge.2002.129868. [DOI] [PubMed] [Google Scholar]
- 45.Luman W, Cull A, Palmer KR. Quality of life in patients stented for malignant biliary obstructions. Eur J Gastroenterol Hepatol. 1997;9:481–4. doi: 10.1097/00042737-199705000-00013. [DOI] [PubMed] [Google Scholar]
- 46.Wagh MS, DeBellis M, Fogel EL, et al. Multicenter randomized trial of 10-French versus 11.5-French plastic stents for malignant biliary obstruction. Diag Ther Endosc. 2013;89:1915. doi: 10.1155/2013/891915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dumonceau J-M, Tringali A, Blero D, et al. Biliary stenting: indications, choice of stents and results: European Society of Gastrointestinal Endoscopy (ESGE) clinical guideline. Endoscopy. 2012;44:277–98. doi: 10.1055/s-0031-1291633. [DOI] [PubMed] [Google Scholar]
- 48.Ge PS, Hamerski CM, Watson RR, et al. Plastic biliary stent patency in patients with locally advanced pancreatic adenocarcinoma receiving downstaging chemotherapy. Gastrointest Endosc. 2015;81:360–6. doi: 10.1016/j.gie.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 49.Walter D, van Boeckel PG, Groenen MJ, et al. Cost efficacy of metal stents for palliation of extrahepatic bile duct obstruction in a randomized controlled trial. Gastroenterology. 2015;149:130–8. doi: 10.1053/j.gastro.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 50.Tol JA, van Hooft JE, Timmer R, et al. Metal or plastic stents for preoperative biliary drainage in resectable pancreatic cancer. Gut. 2015;308762 doi: 10.1136/gutjnl-2014-308762. [DOI] [PubMed] [Google Scholar]
- 51.Moss AC, Morris E, Leyden J, et al. Do the benefits of metal stents justify the cost? A systematic review and meta-analysis of trials comparing endoscopic stents for malignant biliary obstruction. Eur J Gastroenterol Hepatol. 2007;19:1119–24. doi: 10.1097/MEG.0b013e3282f16206. [DOI] [PubMed] [Google Scholar]
- 52.Schmassmann A, von Gunten E, Knuchel J, et al. Wallstents versus plastic stents in malignant biliary obstruction: effects of stent patency of the first and second stent on patient compliance and survival. Am J Gastroenterol. 1996;91:654–9. [PubMed] [Google Scholar]
- 53.Yeoh KG, Zimmerman MJ, Cunningham JT, et al. Comparative costs of metal versus plastic biliary stent strategies for malignant obstructive jaundice by decision analysis. Gastrointest Endosc. 1999;49:466–71. doi: 10.1016/s0016-5107(99)70044-1. [DOI] [PubMed] [Google Scholar]
- 54.Yoon W, Ryu J, Yang K, et al. A comparison of metal and plastic stents for the relief of jaundice in unresectable malignant biliary obstruction in Korea: an emphasis on cost-effectiveness in a country with a low ERCP cost. Gastrointest Endosc. 2009;70:284–9. doi: 10.1016/j.gie.2008.12.241. [DOI] [PubMed] [Google Scholar]
- 55.Saleem A, Leggett CL, Murad MH, et al. Meta-analysis of randomized trials comparing the patency of covered and uncovered self-expandable metal stents for palliation of distal malignant bile duct obstruction. Gastrointest Endosc. 2011;74:321–7. doi: 10.1016/j.gie.2011.03.1249. [DOI] [PubMed] [Google Scholar]
- 56.Almadi MA, Barkun AN, Martel M. No benefit of covered vs. uncovered self-expandable metal stents in patients with malignant distal biliary obstruction: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11:27–37. doi: 10.1016/j.cgh.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 57.Kitano M, Yamashita Y, Tanaka K, et al. Covered self-expandable metal stents with an anti-migration system improve patency duration without increased complications compared with uncovered stents for distal biliary obstruction caused by pancreatic carcinoma: a randomized multicenter trial. Am J Gastroenterol. 2013;108:1713–22. doi: 10.1038/ajg.2013.305. [DOI] [PubMed] [Google Scholar]
- 58.Aoun E, Hashash JG, Moser J, et al. Biliary decompression during neo-adjuvant chemoradiation for pancreatic cancer: plastic vs. metal stents. Gastrointest Endosc. 2010;(71):AB165. [Google Scholar]
- 59.Wang AY. Is plastic stenting for pancreatic cancer still relevant or obsolete in 2015? Gastrointest Endosc. 2015;81:367–9. doi: 10.1016/j.gie.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 60.Boulay BR, Gardner TB, Gordon SR. Occlusion rate and complications of plastic biliary stent placement in patients undergoing neoadjuvant chemoradiotherapy for pancreatic cancer with malignant biliary obstruction. J Clin Gastroenterol. 2010;44:452–6. doi: 10.1097/MCG.0b013e3181d2ef06. [DOI] [PubMed] [Google Scholar]
- 61.Kapral C, Duller C, Wewalka F, et al. Case volume and outcome of endoscopic retrograde cholangiopancreatography: results of a nationwide Austrian benchmarking project. Endoscopy. 2008;40:625–30. doi: 10.1055/s-2008-1077461. [DOI] [PubMed] [Google Scholar]
- 62.Cote GA, Imler TD, Xu H, et al. Lower provider volume is associated with higher failure rates for endoscopic retrograde cholangiopancreatography. Med Care. 2013;51:1040–7. doi: 10.1097/MLR.0b013e3182a502dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cote GA, Keswani RN, Jackson T, et al. Individual and practice differences among physicians who perform ERCP at varying frequency: a national survey. Gastrointest Endosc. 2011;74:65–73 e12. doi: 10.1016/j.gie.2011.01.072. [DOI] [PubMed] [Google Scholar]
- 64.Swan MP, Bourke MJ, Williams SJ, et al. Failed biliary cannulation: clinical and technical outcomes after tertiary referral endoscopic retrograde cholangiopancreatography. World J Gastroenterol. 2011;17:4993–8. doi: 10.3748/wjg.v17.i45.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sundaralingam P, Masson P, Bourke MJ. Early precut sphincterotomy does not increase risk during endoscopic retrograde cholangiopancreatography in patients with difficult biliary access: a meta-analysis of randomized controlled trials. Clin Gastroenterol Hepatol. 2015;13:1722–9. doi: 10.1016/j.cgh.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 66.Choudari CP, Sherman S, Fogel EL, et al. Success of ERCP at a referral center after a previously unsuccessful attempt. Gastrointest Endosc. 2000;52:478–83. doi: 10.1067/mge.2000.108972. [DOI] [PubMed] [Google Scholar]
- 67.Ramirez FC, Dennert B, Sanowski RA. Success of repeat ERCP by the same endoscopist. Gastrointest Endosc. 1999;49:58–61. doi: 10.1016/s0016-5107(99)70446-3. [DOI] [PubMed] [Google Scholar]
- 68.Sharma V, Rana SS, Bhasin DK. Endoscopic ultrasound guided interventional procedures. World J Gastrointest Endosc. 2015;7:628–42. doi: 10.4253/wjge.v7.i6.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dhir V, Artifon EL, Gupta K, et al. Multicenter study on endoscopic ultrasound-guided expandable biliary metal stent placement: choice of access route, direction of stent insertion, and drainage route. Dig Endosc. 2014;26:430–5. doi: 10.1111/den.12153. [DOI] [PubMed] [Google Scholar]
- 70.Kawakubo K, Isayama H, Kato H, et al. Multicenter retrospective study of endoscopic ultrasound-guided biliary drainage for malignant biliary obstruction in Japan. J Hepatobiliary Pancreat Sci. 2014;21:328–34. doi: 10.1002/jhbp.27. [DOI] [PubMed] [Google Scholar]
- 71.Khashab MA, Valeshabad AK, Modayil R, et al. EUS-guided biliary drainage by using a standardized approach for malignant biliary obstruction: rendezvous versus direct transluminal techniques (with videos) Gastrointest Endosc. 2013;78:734–41. doi: 10.1016/j.gie.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 72.Artifon EL, Aparicio D, Paione JB, et al. Biliary drainage in patients with unresectable, malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768–74. doi: 10.1097/MCG.0b013e31825f264c. [DOI] [PubMed] [Google Scholar]
- 73.Song TJ, Hyun YS, Lee SS, et al. Endoscopic ultrasound-guided choledochoduodenostomies with fully covered self-expandable metallic stents. World J Gastroenterol. 2012;18:4435–40. doi: 10.3748/wjg.v18.i32.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vila JJ, Perez-Miranda M, Vazquez-Sequeiros E, et al. Initial experience with EUS-guided cholangiopancreatography for biliary and pancreatic duct drainage: a Spanish national survey. Gastrointest Endosc. 2012;76:1133–41. doi: 10.1016/j.gie.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Gupta K, Perez-Miranda M, Kahaleh M, et al. Endoscopic ultrasound-assisted bile duct access and drainage: multicenter, long-term analysis of approach, outcomes, and complications of a technique in evolution. J Clin Gastroenterol. 2014;48:80–7. doi: 10.1097/MCG.0b013e31828c6822. [DOI] [PubMed] [Google Scholar]
- 76.Iwashita T, Doi S, Yasuda I. Endoscopic ultrasound-guided biliary drainage: a review. Clin J Gastroenterol. 2014;7:94–102. doi: 10.1007/s12328-014-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khashab MA, Valeshabad AK, Afghani E, et al. A comparative evaluation of EUS-guided biliary drainage and percutaneous drainage in patients with distal malignant biliary obstruction and failed ERCP. Dig Dis Sci. 2015;60:557–65. doi: 10.1007/s10620-014-3300-6. [DOI] [PubMed] [Google Scholar]
- 78.Inal M, Akgul E, Aksungur A, et al. Percutaneous placement of biliary metallic stents in patients with malignant hilar obstruction: unilobar versus bilobar drainage. J Vasc Interv Radiol. 2003;14:1409–16. doi: 10.1097/01.rvi.0000096762.74047.a6. [DOI] [PubMed] [Google Scholar]
- 79.Hann LE, Getrajdman GI, Brown KT, et al. Hepatic lobar atrophy: association with ipsilateral portal vein obstruction. Am J Roentgenol. 1996;167:1017–21. doi: 10.2214/ajr.167.4.8819404. [DOI] [PubMed] [Google Scholar]
- 80.Boulay BR, Parepally M. Managing malignant biliary obstruction in pancreas cancer: choosing the appropriate strategy. World J Gastroenterol. 2014;20:9345–53. doi: 10.3748/wjg.v20.i28.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith TP, Ryan JM, Niklason LE. Sepsis in the interventional radiology patient. J Vasc Interv Radiol. 2004;15:317–25. doi: 10.1097/01.rvi.0000116188.30591.e4. [DOI] [PubMed] [Google Scholar]
- 82.Winick AB, Waybill PN, Vanbrux AC. Complications of percutaneous transhepatic biliary interventions. Tech Vasc Inter Radiol. 2001;4:200–6. doi: 10.1016/s1089-2516(01)90026-5. [DOI] [PubMed] [Google Scholar]
- 83.Robson PC, Heffernan N, Gonen M, et al. Prospective study of outcomes after percutaneous biliary drainage for malignant biliary obstruction. Ann Surg Oncol. 2010;17:2303–11. doi: 10.1245/s10434-010-1045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee JH, Krishna SG, Singh A, et al. Comparison of the utility of covered metal stents versus uncovered metal stents in the management of malignant biliary strictures in 749 patients. Gastrointest Endosc. 2013;78:312–24. doi: 10.1016/j.gie.2013.02.032. [DOI] [PubMed] [Google Scholar]
- 85.Wasan SM, Ross WA, Staerkel GA, et al. Use of expandable metallic biliary stents in resectable pancreatic cancer. Am J Gastroenterol. 2005;100:2056–61. doi: 10.1111/j.1572-0241.2005.42031.x. [DOI] [PubMed] [Google Scholar]
- 86.Convey AM, Brown KT. Palliative percutaneous drainage in malignant biliary obstruction part 2: mechanisms and postprocedure management. J Support Oncol. 2006;4:329–35. [PubMed] [Google Scholar]
- 87.Liu YD, Wang ZQ, Wang XD, et al. Stent implantation through rendezvous technique of PTBD and ERCP: the treatment of obstructive jaundice. J Dig Dis. 2007;8:198–202. doi: 10.1111/j.1751-2980.2007.00305.x. [DOI] [PubMed] [Google Scholar]
- 88.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 89.Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Howard TJ, Krug JE, Yu J, et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon’s contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006;10:1338–45. doi: 10.1016/j.gassur.2006.09.008. discussion 1345-1346. [DOI] [PubMed] [Google Scholar]
- 91.Farnell MB, Pearson RK, Sarr MG, et al. A prospective randomized trial comparing standard pancreatoduodenectomy with pancreatoduodenectomy with extended lymphadenectomy in resectable pancreatic head adenocarcinoma. Surgery. 2005;138:618–28. doi: 10.1016/j.surg.2005.06.044. discussion 628–630. [DOI] [PubMed] [Google Scholar]
- 92.Pedrazzoli S, DiCarlo V, Dionigi R, et al. Standard versus extended lymphadenectomy associated with pancreatoduodenectomy in the surgical treatment of adenocarcinoma of the head of the pancreas: a multicenter, prospective, randomized study. Lymphadenectomy Study Group. Ann Surg. 1998;228:508–17. doi: 10.1097/00000658-199810000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy with or without distal gastrectomy and extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma, part 2: randomized controlled trial evaluating survival, morbidity, and mortality. Ann Surg. 2002;236:355–66. doi: 10.1097/00000658-200209000-00012. discussion 366–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Castelberry AW, White RR, De La Fuente SG, et al. The impact of vascular resection on early postoperative outcomes after pancreaticoduodenectomy: an analysis of the American College of bSurgeons National Surgical Quality Improvement Program database. Ann Surg Oncol. 2012;19:4068–77. doi: 10.1245/s10434-012-2585-y. [DOI] [PubMed] [Google Scholar]
- 95.Ziegler KM, Nakeeb A, Pitt HA, et al. Pancreatic surgery: evolution at a high-volume center. Surgery. 2010;148:702–9. doi: 10.1016/j.surg.2010.07.029. discussion 709–710. [DOI] [PubMed] [Google Scholar]
- 96.Tzeng CW, Katz MH, Fleming JB, et al. Morbidity and mortality after pancreaticoduodenectomy in patients with borderline resectable type C clinical classification. J Gastrointest Surg. 2014;18:146–55. doi: 10.1007/s11605-013-2371-6. [DOI] [PubMed] [Google Scholar]
- 97.Greenblatt DY, Kelly KJ, Rajamanickam V, et al. Preoperative factors predict perioperative morbidity and mortality after pancreaticoduodenectomy. Ann Surg Oncol. 2011;18:2126–35. doi: 10.1245/s10434-011-1594-6. [DOI] [PubMed] [Google Scholar]
- 98.Ceppa EP, Pitt HA, Nakeeb A, et al. Reducing readmissions after pancreatectomy: limiting complications and coordinating the care continuum. J Am Coll Surg. 2015;221:708–16. doi: 10.1016/j.jamcollsurg.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 99.Beane JD, House MG, Miller A, et al. Optimal management of delayed gastric emptying after pancreatectomy: an analysis of 1,089 patients. Surgery. 2014;156:939–46. doi: 10.1016/j.surg.2014.06.024. [DOI] [PubMed] [Google Scholar]
- 100.Zyromski NJ, Vieira C, Stecker M, et al. Improved outcomes in postoperative and pancreatitis-related visceral pseudoaneurysms. J Gastrointest Surg. 2007;11:50–5. doi: 10.1007/s11605-006-0038-2. [DOI] [PubMed] [Google Scholar]
- 101.McMillan MT, Vollmer CM, Jr, Asbun HJ, et al. The characterization and prediction of ISGPF grade C fistulas following pancreaticoduodenectomy. J Gastrointest Surg. 2016;20:262–76. doi: 10.1007/s11605-015-2884-2. [DOI] [PubMed] [Google Scholar]
- 102.Ceppa EP, McCurdy RM, Becerra DC, et al. Does pancreatic stump closure method influence distal pancreatectomy outcomes? J Gastrointest Surg. 2015;19:1449–56. doi: 10.1007/s11605-015-2825-0. [DOI] [PubMed] [Google Scholar]
- 103.Bassi C, Dervenis C, Butturini G, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 104.Wente MN, Veit JA, Bassi C, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. 2007;142:20–25. doi: 10.1016/j.surg.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 105.Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS) Surgery. 2007;142:761–8. doi: 10.1016/j.surg.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 106.Schmidt CM, Choi J, Powell ES, et al. Pancreatic fistula following pancreaticoduodenectomy: clinical predictors and patient outcomes. HPB Surg. 2009;404520 doi: 10.1155/2009/404520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mathur A, Pitt HA, Marine M, et al. Fatty pancreas: a factor in postoperative pancreatic fistula. Ann Surg. 2007;246:1058–64. doi: 10.1097/SLA.0b013e31814a6906. [DOI] [PubMed] [Google Scholar]
- 108.Van Buren G, 2nd, Bloomston M, Hughes SJ, et al. A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann Surg. 2014;259:605–12. doi: 10.1097/SLA.0000000000000460. [DOI] [PubMed] [Google Scholar]
- 109.Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA. 2013;310:1473–81. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 110.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs. gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–81. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 111.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 112.Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8:123–32. doi: 10.1007/s10434-001-0123-4. [DOI] [PubMed] [Google Scholar]
- 113.Gillen S, Schuster T, zum Büschenfelde CM, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Heinrich S, Schäfer M, Weber A, et al. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: results of a prospective phase II trial. Ann Surg. 2008;248:1014–22. doi: 10.1097/SLA.0b013e318190a6da. [DOI] [PubMed] [Google Scholar]
- 115.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 116.Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol. 2009;27:1806–13. doi: 10.1200/JCO.2008.17.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Loehrer PJ, Sr, Feng Y, Cardenes HR, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol. 2011;29:4105–12. doi: 10.1200/JCO.2011.34.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hammel P, Huguet F, Van Laethem J-L, et al. Comparison of chemo-radiotherapy (CRT) and chemotherapy (CT) in patients with a locally advanced pancreatic cancer (LAPC) controlled after 4 months of gemcitabine with or without erlotinib: final results of the international phase III LAP 07 study. J Clin Oncol. 2013;31(Suppl) abstr LBA4003. [Google Scholar]
- 119.Chauffert B, Mornex F, Bonnetain F, et al. Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol. 2008;19:1592–9. doi: 10.1093/annonc/mdn281. [DOI] [PubMed] [Google Scholar]
- 120.Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist. 2013;18:543–8. doi: 10.1634/theoncologist.2012-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Katz MHG, Shi Q, Ahmad SA, et al. Preoperative modified FOLFIRINOX (mFOLFIRINOX) followed by chemoradiation (CRT) for borderline resectable (BLR) pancreatic cancer (PDAC): initial results from Alliance Trial A021101. J Clin Oncol. 2015;33(Suppl) abstr 4008. [Google Scholar]
- 122.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 123.Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol. 2013;31:23–9. doi: 10.1200/JCO.2012.44.4869. [DOI] [PubMed] [Google Scholar]
- 124.Oettle H, Riess H, Stieler J, et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J Clin Oncol. 2014;32:2423–9. doi: 10.1200/JCO.2013.53.6995. [DOI] [PubMed] [Google Scholar]
- 125.Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985;120:899–903. doi: 10.1001/archsurg.1985.01390320023003. [DOI] [PubMed] [Google Scholar]
- 126.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;18:350 1200–10. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 127.Smeenk HG, van Eijck CH, Hop WC, et al. Long-term survival and metastatic pattern of pancreatic and periampullary cancer after adjuvant chemoradiation or observation: long-term results of EORTC trial 40891. Ann Surg. 2007;246:734–40. doi: 10.1097/SLA.0b013e318156eef3. [DOI] [PubMed] [Google Scholar]
- 128.Regine WF, Winter KA, Abrams R, et al. Fluorouracil-based chemoradiation with either gemcitabine or fluorouracil chemotherapy after resection of pancreatic adenocarcinoma: 5-year analysis of the U.S. Intergroup/RTOG 9704 phase III trial. Ann Surg Oncol. 2011;18:1319–26. doi: 10.1245/s10434-011-1630-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hsu CC, Herman JM, Corsini MM, et al. Adjuvant chemoradiation for pancreatic adenocarcinoma: the Johns Hopkins Hospital-Mayo Clinic collaborative study. Ann Surg Oncol. 2010;17:981–90. doi: 10.1245/s10434-009-0743-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Huguet F, Hammel P, Vernerey D, et al. Impact of chemoradiotherapy (CRT) on local control and time without treatment in patients with locally advanced pancreatic cancer (LAPC) included in the international phase III LAP 07 study. J Clin Oncol. 2014;32(Suppl):4001. [Google Scholar]
- 131.Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer. 2015;121:1128–37. doi: 10.1002/cncr.29161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiation therapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2008;72:678–86. doi: 10.1016/j.ijrobp.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 133.Keller J, Layer P. Human pancreatic exocrine response to nutrients in health and disease. Gut. 2005;54(Suppl 6):vi, 1–28. doi: 10.1136/gut.2005.065946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kato H, Nakao A, Kishimoto W, et al. 13C-labeled trioctanoin breath test for exocrine pancreatic function test in patients after pancreatoduodenectomy. Am J Gastroenterol. 1993;88:64–9. [PubMed] [Google Scholar]
- 135.Ihse I, Arnesjo B, Kugelberg C, et al. Intestinal activities of trypsin, lipase, and phospholipase after a test meal. An evaluation of 474 examinations. Scand J Gastroenterol. 1977;12:663–8. doi: 10.3109/00365527709181700. [DOI] [PubMed] [Google Scholar]
- 136.Bartel MJ, Asbun H, Stauffer J, et al. Pancreatic exocrine insufficiency in pancreatic cancer: a review of the literature. Dig Liver Dis. 2015;47:1013–20. doi: 10.1016/j.dld.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 137.Sato N, Yamaguchi K, Yokohata K, et al. Short-term and long-term pancreatic exocrine and endocrine functions after pancreatectomy. Dig Dis Sci. 1998;43:2616–21. doi: 10.1023/a:1026686824173. [DOI] [PubMed] [Google Scholar]
- 138.Bruno MJ, Haverkort EB, Tijssen GP, et al. Placebo controlled trial of enteric coated pancreatin microsphere treatment in patients with unresectable cancer of the pancreatic head region. Gut. 1998;42:92–6. doi: 10.1136/gut.42.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Costantini M, Toscani F, Gallucci M, et al. Terminal cancer patients and timing of referral to palliative care: a multicenter prospective cohort study. J Pain Symptom Manage. 1999;18:243–52. doi: 10.1016/s0885-3924(99)00084-6. [DOI] [PubMed] [Google Scholar]
- 140.Morita T, Akechi T, Ikenaga M, et al. Late referrals to specialized palliative care service in Japan. J Clin Oncol. 2005;23:2637–44. doi: 10.1200/JCO.2005.12.107. [DOI] [PubMed] [Google Scholar]
- 141.Morita T, Fujimoto K, Namba M, et al. Palliative care needs of cancer outpatients receiving chemotherapy: an audit of a clinical screening project. Support Care Cancer. 2008;16:101–7. doi: 10.1007/s00520-007-0271-6. [DOI] [PubMed] [Google Scholar]
- 142.Velikova G, Booth L, Smith AB, et al. Measuring quality of life in routine oncology practice improves communication and patient well-being: a randomized controlled trial. J Clin Oncol. 2004;22:714–24. doi: 10.1200/JCO.2004.06.078. [DOI] [PubMed] [Google Scholar]
- 143.Strasser F, Sweeney C, Willey J, et al. Impact of a half-day multidisciplinary symptom control and palliative care outpatient clinic in a comprehensive cancer center on recommendations, symptom intensity, and patient satisfaction: a retrospective descriptive study. J Pain Symptom Manage. 2004;27:481–91. doi: 10.1016/j.jpainsymman.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 144.Gress F, Schmitt C, Sherman S, et al. A prospective randomized comparison of endoscopic ultrasound and computed tomography guided celiac plexus block for the management of chronic pancreatitis. Am J Gastroenterol. 1999;94:900–5. doi: 10.1111/j.1572-0241.1999.01042.x. [DOI] [PubMed] [Google Scholar]
- 145.Wyse JM, Carone M, Paquin SC, et al. Randomized, double-blind, controlled trial of early endoscopic ultrasound-guided celiac plexus neurolysis to prevent pain progression in patients with newly diagnosed, painful, inoperable pancreatic cancer. J Clin Oncol. 2011;29:3541–6. doi: 10.1200/JCO.2010.32.2750. [DOI] [PubMed] [Google Scholar]
- 146.Arcidiacono PG, Calori G, Carrara S, et al. Celiac plexus block for pancreatic cancer pain in adults. Cochrane Database Syst Rev. 2011:CD007519. doi: 10.1002/14651858.CD007519.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Iwata K, Yasuda I, Enya M, et al. Predictive factors for pain relief after endoscopic ultrasound-guided celiac plexus neurolysis. Dig Endosc. 2011;23:140–5. doi: 10.1111/j.1443-1661.2010.01046.x. [DOI] [PubMed] [Google Scholar]
- 148.Fujii-Lau LL, Bamlet WR, Eldrige JS, et al. Impact of celiac neurolysis on survival in patients with pancreatic cancer. Gastrointest Endosc. 2015;82:46–56. doi: 10.1016/j.gie.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.LeBlanc JK, Al-Haddad M, McHenry L, et al. A prospective, randomized study of EUS-guided celiac plexus neurolysis for pancreatic cancer: one injection or two? Gastrointest Endosc. 2011;74:1300–7. doi: 10.1016/j.gie.2011.07.073. [DOI] [PubMed] [Google Scholar]
- 150.Téllez-Ávila FI, Romano-Munive AF, Herrera-Esquivel J, et al. Central is as effective as bilateral endoscopic ultrasound-guided celiac plexus neurolysis in patients with unresectable pancreatic cancer. Endosc Ultrasound. 2013;2:153–6. doi: 10.7178/eus.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Puli SR, Reddy JB, Bechtold ML, et al. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330–7. doi: 10.1007/s10620-008-0651-x. [DOI] [PubMed] [Google Scholar]
- 152.Doi S, Yasuda I, Kawakami H, et al. Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy. 2013;45:362–9. doi: 10.1055/s-0032-1326225. [DOI] [PubMed] [Google Scholar]
- 153.Nagaraja V, Eslick GD, Cox MR. Endoscopic stenting versus operative gastrojejunostomy for malignant gastric outlet obstruction—a systematic review and meta-analysis of randomized and non-randomized trials. J Gastrointest Oncol. 2014;5:92–8. doi: 10.3978/j.issn.2078-6891.2014.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jeurnink SM, Steyerberg EW, van Hooft JE, et al. Surgical gastrojejunostomy or endoscopic stent placement for the palliation of malignant gastric outlet obstruction (SUSTENT study): a multicenter randomized trial. Gastrointest Endosc. 2010;71:490–9. doi: 10.1016/j.gie.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 155.Kim JW, Jeong JB, Lee KL, et al. Comparison between uncovered and covered self-expandable metal stent placement in malignant duodenal obstruction. World J Gastroenterol. 2015;21:1580–7. doi: 10.3748/wjg.v21.i5.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Oh D, Lee SS, Song TJ, et al. Efficacy and safety of a partially covered duodenal stent for malignant gastroduodenal obstruction: a pilot study. Gastrointest Endosc. 2015;82:32–6. doi: 10.1016/j.gie.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 157.Lim SG, Kim JH, Lee KM, et al. Conformable covered versus uncovered self-expandable metallic stents for palliation of malignant gastroduodenal obstruction: a randomized prospective study. Dig Liver Dis. 2014;46:603–8. doi: 10.1016/j.dld.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 158.Khashab MA, Fujii LL, Baron TH, et al. EUS-guided biliary drainage for patients with malignant biliary obstruction with an indwelling duodenal stent (with videos) Gastrointest Endosc. 2012;76:209–13. doi: 10.1016/j.gie.2012.03.170. [DOI] [PubMed] [Google Scholar]


