Abstract
T cell antigen receptor (TCR) signaling in the thymus initiates positive selection but CD8 lineage fate is thought to be induced by cytokines after TCR signaling has ceased, although this remains controversial and unproven. We now identify four non-common gamma chain (γc) receptor-signaling cytokines (IL-6, IFN-γ, TSLP, TGF-β) that, like IL-7 and IL-15, induce expression of the lineage-specifying transcription factor Runx3d and signal the generation of CD8 T cells. Remarkably, elimination of in vivo signaling by all ‘lineage-specifying cytokines’ during positive selection eliminated Runx3d expression and completely abrogated CD8 single-positive thymocyte generation. Thus, this study proves that signaling during positive selection by lineage-specifying cytokines is responsible for all CD8 lineage fate decisions in the thymus.
Introduction
T cells develop in the thymus from hematopoietic precursors via an ordered sequence of events that generate αβT cells reactive against foreign pathogens but tolerant to self-ligands1. Differentiation of immature CD4+CD8+ (double positive, DP) thymocytes into mature single positive (SP) T cells is initiated by TCR signaling and is referred to as positive selection1,2. The lineage direction of positive selection is determined with remarkable accuracy by the MHC- specificity of the TCRs that DP thymocytes express, such that MHC class II (MHCII)-specific TCRs direct differentiation into CD4+ helper-lineage T cells and MHC class I (MHCI)-specific TCRs direct differentiation into CD8+ cytotoxic-lineage T cells3. How TCR specificity determines thymocyte lineage fate during positive selection is best described by the kinetic signaling model4-7 which proposes that lineage fate is determined by whether TCR signaling persists throughout positive selection or is disrupted, allowing positively selected thymocytes to then be signaled by cytokines. In this perspective, persistent TCR signaling induces expression of ThPOK8-10, the CD4-helper lineage-specifying transcription factor, whereas cytokine signaling induces expression of Runx3d11-13, the CD8-cytotoxic lineage-specifying factor4,11-15. Thus, the kinetic signaling model stipulates that CD8 lineage fate is signaled by cytokines and not by TCRs which instead signal CD4 lineage fate.
That CD8 lineage fate is signaled by cytokines and not by TCRs is central to current understanding, but remains controversial and unproven16,17. Thus far, two common gamma chain (γc) receptor-dependent cytokines (interleukin 7 (IL-7) and IL-15) have been identified whose signals induce Runx3d and promote differentiation of developing thymocytes into CD8SP (SP8) cells18. However, elimination of IL-7 and IL-15 signaling during positive selection did not eliminate SP8 generation, but reduced it by 70% which was the same reduction obtained by conditionally deleting γc expression and eliminating signaling by all γc cytokines18. In fact germline γc-deficiency also reduced but did not eliminate SP8 generation, with remaining γc-deficient SP8 cells displaying anti-viral cytolytic effector function if their survival in the periphery was maintained by a pro-survival Bcl-2 transgene19. Consequently, it is possible for developing thymocytes to differentiate into SP8-cytotoxic lineage cells in the absence of γc cytokine signals, but it is not known if CD8-lineage fate is then signaled by cytokines that use receptors other than γc or if it is signaled by a different stimulus entirely.
The present study was undertaken to determine if CD8 lineage fate decisions in the thymus are signaled exclusively by cytokines or not. To address this issue, we identified non-γc cytokines that, like IL-7 and IL-15, could signal developing thymocytes to express Runx3d and we assessed the consequences of eliminating in vivo signaling by these cytokines during positive selection. Four non-γc cytokines (IL-6, IFN-γ, TSLP, and TGF-β) were identified that induced Runx3d but, unlike IL-7 and IL-15, did not substantially upregulate pro-survival genes. We considered the non-γc and γc cytokines that induced Runx3d to be ‘lineage-specifying cytokines’. Remarkably, elimination of in vivo signaling by all six lineage-specifying cytokines during positive selection eliminated Runx3d expression and limited Runx1 function to abrogate all CD8 T cell generation, proving that signaling by lineage-specifying cytokines was strictly required for CD8 lineage fate decisions. We conclude that CD8 lineage fate decisions are exclusively signaled by lineage-specifying cytokines during positive selection in the thymus.
Results
The present study was undertaken to identify the signals that promote the differentiation of γc-deficient thymocytes into CD8 T cells, and to evaluate the concept that all CD8-lineage fate decisions in the thymus are signaled by cytokines. We began by assessing the generation of SP8 cells in mice whose γc genes (Il2rg) were conditionally deleted in pre-selection DP thymocytes18. The mice we used were γcfl/flE8III-CreTg (referred to as γccKO) that delete Il2rg just prior to positive selection and are devoid of γc proteins during positive selection and thereafter18. In γccKO mice SP8 frequency was only ∼30% of that in normal B6 mice (Fig. 1a). To determine if development of the remaining SP8 cells had been signaled by non-γc cytokines, we generated γccKO mice expressing a transgene encoding the cytokine signaling inhibitor protein SOCS1 (SOCS1Tg)20 that binds Jak proteins and specifically inhibits Jak-STAT-mediated signaling. Expression of SOCS1Tg further reduced SP8 frequency in γccKOSOCS1Tg mice to ∼10% of that in B6, indicating that most SP8 cells in γc-deficient mice were generated by non-γc cytokines that signaled via the Jak-STAT transduction pathway (Fig. 1a). Notably, SP8 cells in γccKO mice up-regulated Runx3d but did not up-regulate any pro-survival gene (i.e. Bcl2, Mcl1 and Bcl2l1), unlike SP8 cells in γc-sufficient B6 mice which up-regulated Runx3d and Bcl2 genes (Fig. 1b). Failure to upregulate pro-survival genes specifically diminished survival of the very most mature (CD69−Qa2hiCD24lo) SP8 thymocytes in γccKO mice, reducing overall SP8 expression of cytotoxicity-related proteins (Supplementary Fig. 1a) and genes18.
Figure 1. Runx3d induction by non-γc cytokine signals.
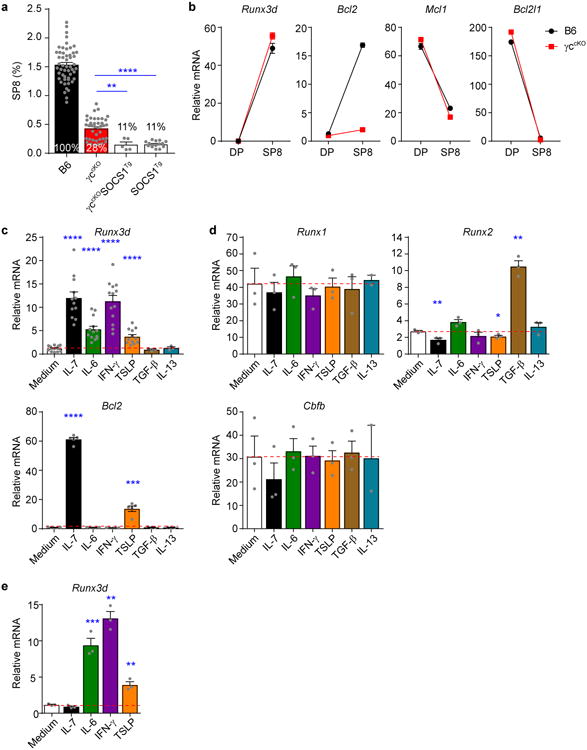
(a) CD8 T cell generation in the thymus by non-γc cytokine signals. Frequencies of TCRβhiCD8SP (SP8) thymocytes in the indicated mouse strains are displayed as a bar graph and numbers within the bars display SP8 frequencies relative to that in normal B6 mice (set equal to 100%). In γccKO mice SP8 cells that had failed to delete γc were excluded from the analysis. Data are from 5-52 mice combined from 3-44 experiments. (b) Expression of mRNA encoding the indicated genes in pre-selection DP (CD69-CD4+CD8+) and SP8 thymocytes was determined by quantitative PCR and normalized to Rpl13a. Data are technical triplicates representative of two experiments. (c,d) B6 pre-selection DP thymocytes were stimulated with various cytokines during the DP stimulation assay (schematized in Supplementary Fig. 1e) and their subsequent expression of Runx3d and Bcl2 mRNA displayed in c, and their subsequent expression of Runx1, Runx2 and Cbfb mRNA displayed in d, and compared to medium alone (horizontal red dashed line). Data are combined from 3-13 experiments in c and 2-3 experiments in d. (e) γccKO pre-selection DP thymocytes were stimulated with the indicated cytokines during the DP stimulation assay and their subsequent expression of Runx3d mRNA determined. Data are technical triplicates representative of two experiments. Mean and s.e.m. are shown. *P < 0.05, ** P < 0.01, ***P < 0.001, ****P < 0.0001.
To identify non-γc cytokines that might signal SP8 thymocyte generation, we examined the cytokine receptor proteins on intermediate thymocytes undergoing MHCI-specific positive selection into SP8 cells in MHCIIKOCD1dKO mice which lack unconventional NKT cells (Supplementary Fig. 1b-d). MHCI-specific intermediate thymocytes were phenotypically CD4+CD8loCD69+ and expressed receptor components for five different non-γc cytokines (IL-6, IFN-γ, TSLP, IL-13, and TGF-β) (Supplementary Fig. 1b-d). These five non-γc cytokines became the focus of our study.
IL-6, IFN-γ and TSLP signaling induce Runx3d expression
Because Runx3d specifies CD8 cytotoxic lineage fate, we sought to determine which non-γc cytokines could signal developing thymocytes to express Runx3d. To do so, we developed a two-step in vitro assay, called the ‘DP stimulation assay’, in which electronically sorted pre-selection DP thymocytes (CD4+CD8+CD69−) were transiently stimulated with PMA + Ionomycin to convert them into cytokine-responsive cells, transferred to second cultures containing either cytokine or medium, and then assessed for Runx3d mRNA expression (Fig. 1c-e and Supplementary Fig. 1e). We validated the specificity of the DP stimulation assay by demonstrating that: IL-7 induced Runx3d mRNA only in γc-sufficient B6 but not γc-deficient γccKO cells (Fig. 1c,e); and IL-7 upregulated Runx3d but not other Runx or Cbfb mRNAs (the latter encoding the Runx binding partner protein CBFβ) (Fig. 1d). We then tested various non-γc cytokines in the DP stimulation assay and identified three non-γc cytokines (IL-6, IFN-γ, and TSLP) that upregulated Runx3d mRNA in both B6 and γccKO thymocytes (Fig. 1c,e). Signaling by these three non-γc cytokines up-regulated Runx3d but not other Runx or Cbfb mRNAs (Fig. 1d). Notably the three non-γc cytokines (IL-6, IFN-γ, TSLP) stimulated little or no expression of the pro-survival gene Bcl2 (Fig. 1c bottom), concordant with our in vivo observation that γc-deficient SP8 cells expressed Runx3d but little Bcl2 (Fig. 1b). Curiously, the non-γc cytokine TGF-β did not induce Runx3d but was unique in upregulating Runx2, a Runx family transcription factor expressed in extremely low amounts in thymocytes where it has no known function21,22 (Fig. 1d). A complete list of cytokines tested for Runx3d induction in the DP stimulation assay is provided (Supplementary Fig. 1f). Thus, IL-6, IFN-γ, and TSLP are non-γc cytokines that can signal developing thymocytes to express Runx3d.
IL-6, IFN-γ and TSLP generate SP8 cells in FTOCs
We assessed the three non-γc cytokines that up-regulated Runx3d expression for their ability to signal thymocytes to differentiate into SP8 cells. For these experiments, we established fetal thymus organ cultures (FTOCs) with 16.5d embryonic thymus lobes and cultured them for 5d, at which point SP8 cells had arisen (Fig. 2a and Supplementary Fig. 2a). Notably, SP8 cell frequency and number in B6 FTOCs were 10-15 fold higher than in medium cultures of γccKO FTOCs (Fig. 2a-c), with γccKO FTOCs generating fewer than 500 SP8 cells/lobe (Fig. 2c). Importantly, addition of exogenous IL-6, IFN-γ, or TSLP to γccKO FTOCs increased frequency and number of SP8 cells by 3-10 fold (Fig. 2b,c and Supplementary Fig. 2b). Addition of all three non-γc cytokines together (IL-6+IFN-γ+TSLP) increased SP8 numbers further, but the increase was not strictly additive, suggesting that they signaled overlapping thymocyte populations (Fig. 2c and Supplementary Fig. 2b). IL-13, which did not induce Runx3d in the DP stimulation assay, also did not induce SP8 cells in γccKO FTOCs (Supplementary Fig. 2c). Interestingly, addition of each non-γc cytokine to B6 FTOCs increased SP8 cell generation as did addition of IL-7, indicating that γc and non-γc cytokines were limiting in FTOCs so that addition of any one CD8-promoting cytokine quantitatively increased SP8 cell generation (Supplementary Fig. 2d).
Figure 2. Non-γc cytokines generate SP8 cells in FTOCs.
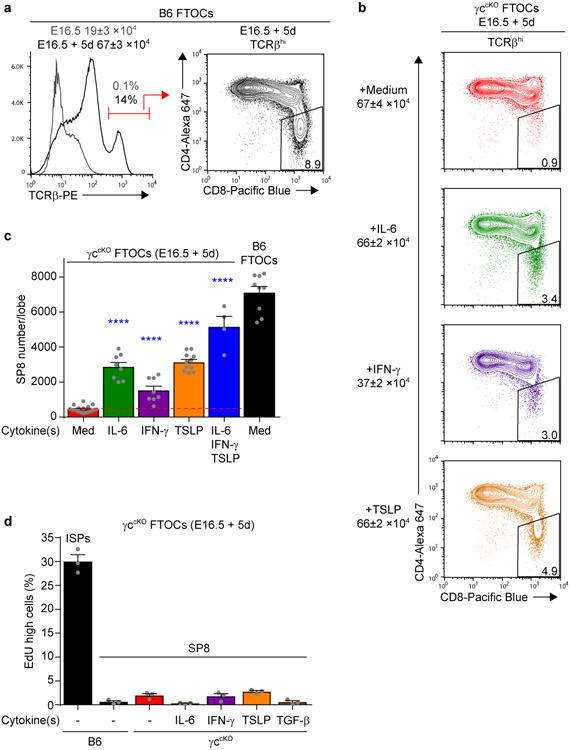
(a) Thymocyte profiles of E16.5 thymic lobes from B6 mice on d0 (gray line) and d5 (black line) of culture. Cell numbers/lobe before and after 5d culture are shown above the TCRβ histogram (left). A profile of TCRβhi thymocytes after 5 days of culture is shown on the right. Data are from 3-9 individual lobes combined from 2-4 experiments. (b) Profiles of TCRβhi thymocytes from γccKO FTOCs after 5 days in culture with the indicated cytokines are displayed. Numbers of total cells/lobe are indicated on the left and SP8 cell frequencies among TCRβhi thymocytes are displayed in boxes within the profiles. Data are from 8-11 individual lobes combined from 3 experiments. (c) Bar graph of SP8 cell numbers from γccKO FTOCs cultured with the indicated cytokines, with SP8 cell numbers in each cytokine group compared to medium alone (horizontal red dashed line). As an additional comparison, SP8 cell numbers from B6 FTOCs are also shown. Data are from 4-17 individual lobes combined from 2-6 experiments. (d) Frequencies of EdUhi cells among SP8 cells from B6 and γccKO FTOCs cultured with the indicated cytokines. EdU was added during the last 14h of culture. ISP (CD4-CD8+TCRβ-) thymocytes from B6 FTOCs are shown as a positive control for EdU incorporation. Data are from 3 individual lobes combined from 2 experiments. Mean and s.e.m. are shown. ****P < 0.0001.
The increase in SP8 cell numbers by exogenous non-γc cytokines was not due to extensive proliferation of a small number of endogenously generated SP8 cells because SP8 thymocytes (unlike immature ISP thymocytes) incorporated little EdU, a thymidine analog, added during the final 14h of FTOCs (Fig. 2d). We conclude that non-γc cytokines IL-6, IFN-γ and TSLP can signal developing thymocytes to differentiate into SP8 cells.
Non-γc cytokines signal SP8 cell generation in vivo
To determine if endogenously produced IL-6, IFN-γ, and TSLP actually signaled SP8 cell generation in the thymus, we eliminated in vivo signaling by these cytokines either by deleting the gene encoding the cytokine itself (i.e. IL-6) or by deleting a component of their surface cytokine receptor (e.g. IFN-γR, TSLPR) in γccKO mice (Fig. 3). We found that the effect on SP8 generation of eliminating signaling by one cytokine was modest, as elimination of TSLP signaling had no significant effect and elimination of signaling by IL-6 or IFN-γ managed to just achieve statistical significance (Fig. 3a). In contrast, elimination of signaling by any two of these cytokines in individual γccKO mice (e.g. γccKOIL-6KOIFN-γRKO mice) caused highly significant reductions in both SP8 frequency and number (Fig. 3b), suggesting that these cytokines had overlapping effects in vivo.
Figure 3. In vivo signaling by non-γc cytokines is required for generation of γc-independent CD8 T cells.
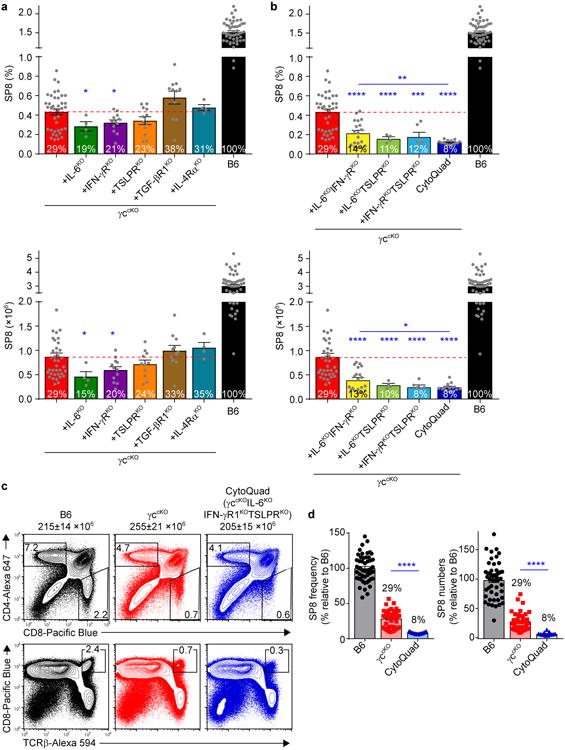
(a,b) SP8 thymocyte frequencies (top) and numbers (bottom) in γccKO mice (red bars) were compared with those in γccKO mice additionally deficient in cytokine and/or cytokine receptor genes (other colored bars). Numbers within bars display SP8 cell frequencies and numbers relative to those in normal B6 mice (set equal to 100%). For simplicity, γccKOIL6KOIFN-γRKOTSLPRKO mice are designated CytoQuad mice. Data are from 4-50 combined mice from 2-39 experiments. (c) Thymocyte profiles from the indicated mice are shown with total thymocyte numbers indicated. Numbers within or adjacent to boxes in the profiles indicate frequency of cells in that box. Data are from 8-13 mice combined from 5 experiments. (d) SP8 thymocyte frequencies (left) and numbers (right) in the indicated mice were compared and are shown relative to B6 which was set equal to 100%. Data are from 13-50 mice combined from 7-39 experiments. Mean and s.e.m. are shown. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Because of the potential for cytokine redundancy, we thought that full appreciation of the contribution of non-γc cytokine signaling to SP8 cell generation might require elimination of in vivo signaling by all three non-γc cytokines (IL-6, IFN-γ, TSLP). Accordingly, we generated γccKOIL6KOIFN-γRKOTSLPRKO mice that we refer to simply as CytoQuad mice in which signaling by γc and the three non-γc cytokines was eliminated. Notably, CytoQuad thymocytes differed from γccKOIL6KOIFN-γRKO thymocytes in that TSLP signaling was only eliminated in CytoQuad mice. Comparing these two mouse strains demonstrated that SP8 frequency and number were both significantly reduced in CytoQuad mice, revealing the contribution of TSLP signaling to SP8 generation (Fig. 3b). Thus, signaling by TSLP, as well as by IL-6 and IFN-γ, contributes to SP8 cell generation but the contribution of each is obscured by cytokine redundancy.
Importantly, comparing thymus profiles from γccKO and CytoQuad mice revealed that elimination of signaling by all three non-γc cytokines (IL-6, IFN-γ, TSLP) affected the generation of SP8 cells but not other thymocyte subsets (Fig. 3c). Quantifying the impact of non-γc cytokines on SP8 generation revealed that SP8 frequency and number were both significantly reduced in CytoQuad compared to γccKO mice (Fig. 3b), and revealed that the CytoQuad thymus contained only 8% of the SP8 cells in the B6 thymus (Fig. 3d). Notably the SP8 cells that were generated in the thymus of both γccKO and CytoQuad mice had low or absent Bcl-2 expression and failed to survive in the lymphoid periphery (Supplementary Fig. 3). We conclude that in vivo signaling by the non-γc cytokines IL-6, IFN-γ, and TSLP promotes SP8 cell generation, and that in vivo signaling by these three cytokines accounts for most - but not quite all - SP8 thymocytes generated in γc-deficient mice.
γc and non-γc cytokines signal overlapping SP8 subsets
Having documented that non-γc cytokines (IL-6, IFN-γ, and TSLP) induce Runx3d and signal SP8 generation in γc-deficient mice, we considered these cytokines to be lineage-specifying cytokines. We then wondered if γc and non-γc lineage-specifying cytokines signaled generation of the same or different SP8 cells, such that some were generated by γc cytokines and others by non-γc cytokines. Three experimental observations suggested that γc and non-γc lineage-specifying cytokines generated overlapping, not distinct, SP8 cells. First, non-γc cytokine signaling did not generate additional SP8 cells in γc-sufficient mice, as SP8 frequencies were no higher in B6 than in IL-6KOIFN-γRKOTSLPRKO mice (Fig. 4a). Second, γc and non-γc cytokines generated SP8 cells with similar TCR-Vβ repertoires, as the pattern of TCR-Vβ usage was similar in γc-sufficient (B6) and γc-deficient (γccKO) mice (Fig. 4b). Third, γc and non-γc cytokines did not preferentially generate high-versus low-affinity SP8 cells as assessed by TCR-transgenic mouse strains, as the number of SP8 thymocytes with high affinity OT-I TCRs was essentially equal to those with low affinity HY TCRs in both γc-sufficient and γc-deficient mice (Fig. 4c). We conclude that, in normal mice, γc and non-γc cytokine signals do not generate distinct SP8 populations so that the contribution of non-γc cytokines to SP8 generation is best appreciated in mice lacking γc cytokine signals (schematized in Supplementary Fig. 4).
Figure 4. Non-γc cytokines do not generate a unique subset of CD8 T cells.
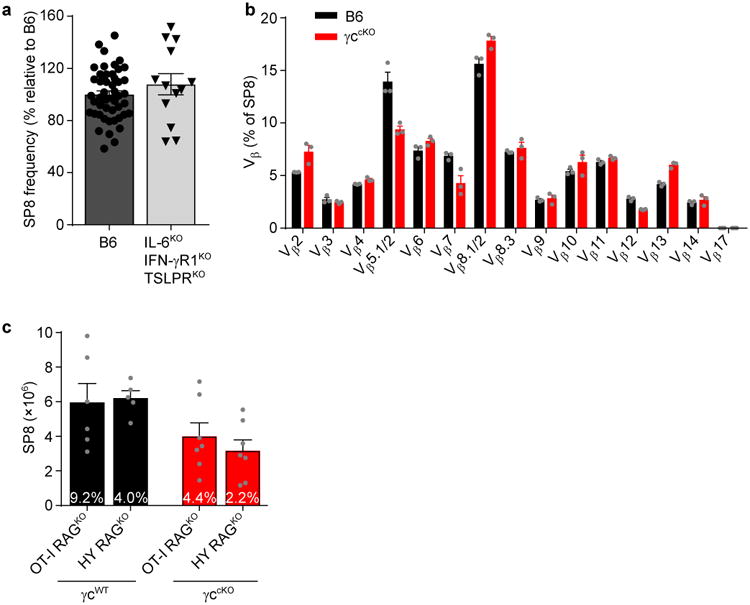
(a) SP8 thymocyte frequencies in γc-sufficient experimental (IL6KOIFN-γRKOTSLPRKO) mice were compared to that in B6 mice which was set equal to 100%. Symbols indicate individual mice. Data are 13-50 mice combined from 3-39 experiments. (b) TCR-Vβ usage among SP8 (CD4-CD8+CCR7+) thymocytes from B6 (black) and γccKO (red) mice. Data are from 3 mice combined from 2 experiments. (c) SP8 thymocyte numbers in the indicated TCR transgenic γc-sufficient (γcWT) and γc-deficient (γccKO) mice were compared. Numbers within the bars display mean SP8 thymocyte frequencies for each strain. Data are from 5-7 mice combined from 4 experiments. Mean and s.e.m. are shown.
Identification of TGF-β as a lineage-specifying cytokine
Analysis of CytoQuad mice revealed that γc and non-γc lineage-specifying cytokines together account for ∼92% of the SP8 cells generated in the B6 thymus (Fig. 3d). Interestingly, each of these lineage-specifying cytokines utilizes Jak-STAT proteins to transduce intracellular signals. Consequently, the few SP8 thymocytes arising in CytoQuad mice, like those in γccKOSOCS1Tg mice, appeared to be generated by an as yet unknown lineage-specifying cytokine that signaled independently of Jak-STAT proteins23. To identify such a cytokine, we performed computational analyses to search for binding motifs for cytokine signal transducing molecules in Runx3d regulatory sequences conserved between humans and mice (Supplementary Fig. 5a). As expected from our current observations, we found multiple STAT binding motifs in Runx3d conserved sequences. In addition, we found multiple SMAD binding motifs (Supplementary Fig. 5a) which suggested that TGF-β signaling might also contribute to Runx3d regulation.
To assess whether the few SP8 cells in CytoQuad mice might have been signaled by TGF-β, we carefully analyzed the molecular profile of CytoQuad SP8 cells and found it to resemble that of B6 SP8 cells - except that Runx2 mRNA was significantly higher in CytoQuad SP8 cells (Fig. 5a). Even though Runx2 exerts no known function in the normal thymus, TGF-β up-regulates Runx2 in bone24 and TGF-β signaling upregulated Runx2 in the DP stimulation assay (Fig. 1d). TGF-β also upregulates CD103 expression on peripheral CD8 T cells25 and we found that CytoQuad SP8 thymocytes were CD103hi (Fig. 5a right). Consequently these observations, together with previous reports that TGF-β signals precursors of intraepithelial lymphocytes to express CD8α26, regulates IL-7Rα expression on SP8 cells27, and signals B cells to express Runx328, led us to further assess the possibility that TGF-β was the remaining Jak-STAT-independent lineage-specifying cytokine.
Figure 5. TGF-β contributes to generation of γc-independent CD8 T cells.
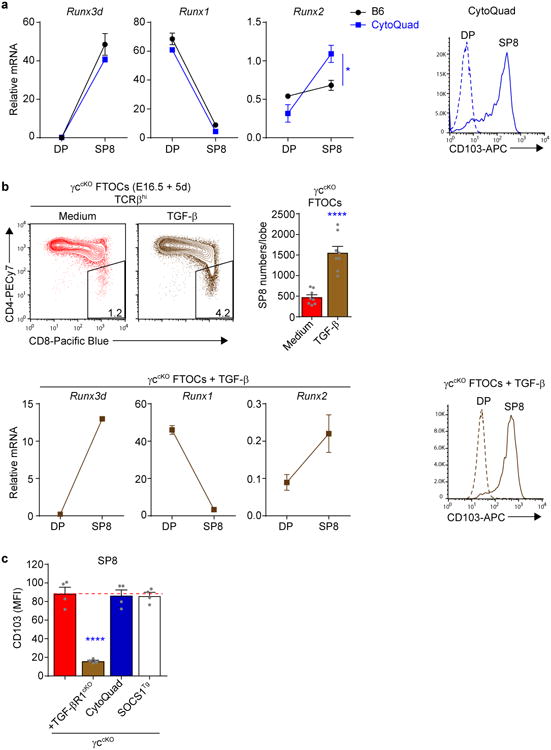
(a) Molecular profiles of pre-selection DP and SP8 thymocytes from CytoQuad mice. Expression of mRNA encoding Runx3d, Runx1 and Runx2 were compared in pre-selection DP and SP8 thymocytes from B6 and CytoQuad mice (left). Histograms of CD103 expression on DP and SP8 thymocytes from CytoQuad mice are shown (right). Data are technical triplicates from 1-6 pooled mice and one of two experiments is shown. (b) Impact of TGF-β on SP8 thymocyte generation. Profiles of TCRβhi thymocytes from γccKO FTOCs cultured with TGF-β or medium are shown, and numbers in boxes within the profiles indicate frequency of SP8 cells among TCRβhi thymocytes (top left). Numbers of SP8 cells generated in γccKO FTOCs cultured with and without TGF-β were compared (top right). DP and SP8 thymocytes from γccKO FTOCs cultured with TGF-β were profiled as in (a), with quantitative PCR of mRNA encoding Runx3d, Runx1, and Runx2 shown (bottom left) and surface CD103 histograms displayed (bottom right). Data are from 8-12 individual lobes combined from 2 experiments. (c) Comparison of CD103 expression on SP8 thymocytes from various mice. Data are from 4 mice combined from 2 experiments. Mean and s.e.m. are shown. ****P < 0.0001.
To do so, we first assessed the ability of TGF-β to signal SP8 generation in γccKOFTOCs. Exogenous TGF-β significantly increased both SP8 frequency and number (Fig. 5b, top), and the molecular profile of these SP8 cells bore a striking resemblance to SP8 thymocytes in adult CytoQuad mice, including upregulated (but still very low) Runx2 mRNA expression and CD103hi surface expression (Fig. 5b, bottom, compare to panel a). Thus, TGF-β signaling did upregulate Runx3d and generate SP8 cells in γccKO FTOCs, even though it had not upregulated Runx3d in the DP stimulation assay. As explanation, we think that Smad-mediated transduction of TGF-βsignaling may require more time to upregulate Runx3d than was available in the in vitro DP stimulation assay.
Having determined that TGF-β signaling generated SP8 cells in FTOCs, we wanted to then assess the impact on SP8 cells of eliminating in vivo TGF-β signaling during positive selection. However, elimination of in vivo TGF-β signaling triggers a devastating lympho-proliferative disorder that is usually fatal29,30. Fortunately, we found that γc-deficiency prevented disease and that γccKOTGF-βR1cKO mice remained healthy and disease-free. As confirmation that in vivo TGF-β signaling had been eliminated in these healthy mice, SP8 cells in γccKOTGF-βR1cKO mice were CD103lo whereas SP8 cells from related mice with intact TGF-β signaling were all CD103hi (Fig. 5c and Supplementary Fig. 5b). Thus, these results identify TGF-β as a fourth non-γc lineage-specifying cytokine.
Elimination of all lineage-specifying cytokine signals
To determine that TGF-β was the Jak-STAT-independent cytokine responsible for SP8 cell generation, we eliminated in vivo TGF-β signaling during positive selection by generating γccKOSOCS1TgTGF-βR1cKO mice. Notably γccKOSOCS1Tg and γccKOSOCS1TgTGF-βR1cKO mice expressed E8III-Cre so the only difference between them was the potential for TGF-β signaling during positive selection. Remarkably, whereas γccKOSOCS1Tg mice generated a few (∼120,000) SP8 thymocytes, γccKOSOCS1TgTGF-βR1cKO appeared to be devoid of SP8 cells (Fig. 6). Careful quantitation revealed ∼6,000 total SP8 cells in γccKOSOCS1TgTGF-βR1cKO mice, of which 2000 were documented escapees from Cre-mediated Tgf-br1 deletion (as revealed by high CD103 expression) or from failure to express the SOCS1Tg (as revealed by absent expression of the Myc epitope tag on transgenic SOCS1 protein). In fact, the very few remaining SP8 cells in γccKOSOCS1TgTGF-βR1cKO mice represent a remarkable 99.9% depletion relative to the ∼3.2×106 SP8 cells in B6 mice, and we think these few SP8 cells arose only because they were signaled by cytokines before their surface cytokine receptors were completely deleted. In comparison, CBFβcKO thymocytes contained even more SP8 escapees (Fig. 6).
Figure 6. Elimination of in vivo signaling by all lineage-specifying cytokines.
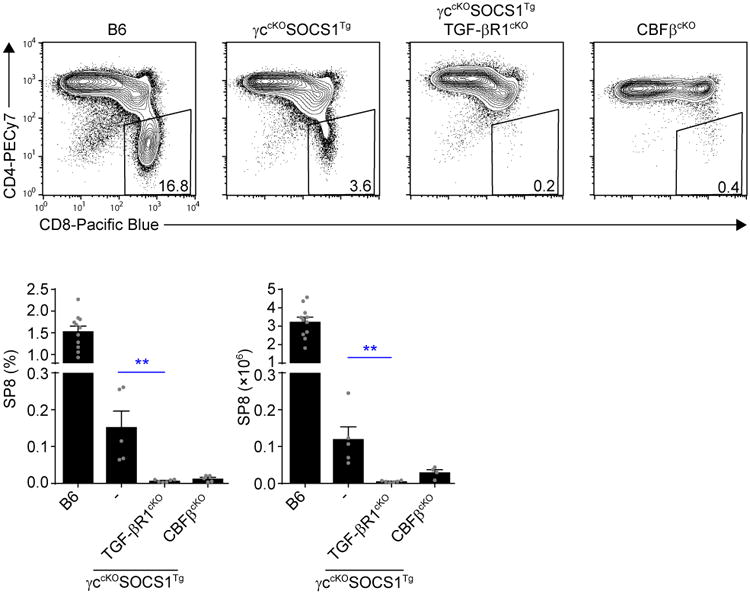
Profiles of TCRβhi thymocytes from the indicated mice are shown, and numbers in boxes within the profiles indicate frequency of SP8 cells among TCRβhi thymocytes (top). Bar graphs display SP8 thymocyte frequencies (bottom left) and numbers (bottom right) from the indicated mice. Data are from 4-11 mice combined from 2-5 experiments. Mean and s.e.m. are shown. **P < 0.01.
We conclude that TGF-β is a Jak-STAT-independent lineage-specifying cytokine that promotes SP8 cell generation. More importantly, we conclude that all CD8 lineage fate decisions in the thymus require cytokine signaling.
SP8 cell generation by Runx1 requires cytokine signaling
Because SP8 cell generation can be mediated by either Runx3d or Runx1 (ref.31), we were surprised that SP8 cells were not generated by Runx1 in the absence of cytokine-signaled Runx3d expression (Fig. 6). To understand why this was the case, we first confirmed that Runx1 and Runx3d could each promote SP8 generation in mice deficient for the other, in that SP8 cells were generated in Runx1-deficient (Runx1cKO) and Runx3d-deficient (Runx3dYFP/YFP) but not double deficient (Runx1cKORunx3dYFP/YFP) mice (Fig. 7a). These results confirm that Runx1 and Runx3d can each promote SP8 cell generation and they exclude any significant contribution by Runx2, since double deficient mice were essentially devoid of SP8 cells (Fig. 7a).
Figure 7. In vivo cytokine signaling is always required for SP8 cell generation.
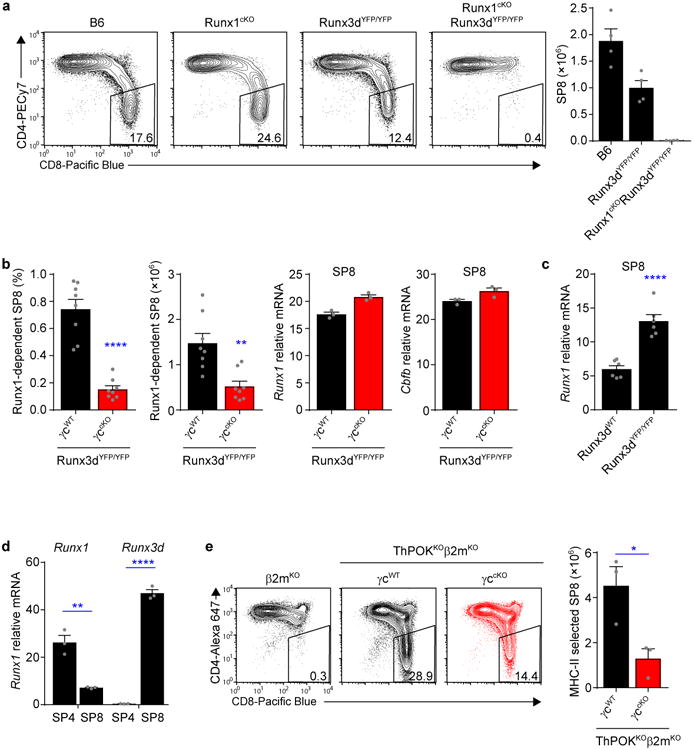
(a) Profiles of TCRβhiCCR7+ thymocytes from the indicated mice are shown, and numbers in boxes within the profiles indicate frequency of SP8 cells among TCRβhi thymocytes (left). Bar graph displays SP8 thymocyte numbers in each mouse strain (right). Data are from 2-5 mice combined from 2-5 experiments. (b) Comparisons of SP8 thymocyte frequencies (left) and numbers (middle) in γc-sufficient (γcWT) and γc-deficient (γccKO) Runx3dYFP/YFP mice, and Runx1 and Cbfb mRNA abundance in SP8 cells from the same mice (right). Cell numbers and frequencies are from 8 mice combined from 5 experiments, quantitative PCR data are technical triplicates and one of two experiments is shown. (c) Comparison of Runx1 mRNA expression in electronically sorted SP8 thymocytes from Runx3d-sufficient (Runx3dWT) and Runx3d-deficient (Runx3dYFP/YFP) mice. Data are replicates from two representative experiments out of a total of four experiments done. (d) Runx1 and Runx3d mRNA expression in electronically sorted SP4 and SP8 thymocytes from B6 mice. Data are technical triplicates and one of two experiments is shown. (e) Profiles of MHC class II-selected TCRβhi thymocytes from γc-sufficient (γcWT) and γc-deficient (γccKO) ThPOKKOβ2mKO mice are shown, and numbers in boxes within the profiles indicate frequency of SP8 cells among TCRβhi thymocytes (left). The bar graph compares the number of MHC class II-selected SP8 thymocytes in γc-sufficient and γc-deficient ThPOKKOβ2mKO mice (right). Data are from 3 mice combined from 3 experiments. Mean and s.e.m. are shown. *P < 0.05, **P < 0.01, ****P < 0.0001.
To explain why Runx1 did not promote SP8 generation in cytokine-unsignaled thymocytes, we wondered if Runx1 might require cytokine signaling to generate SP8 cells. To assess this possibility, we determined the impact of eliminating γc-cytokine signaling in Runx3d-deficient mice (Fig. 7b). Indeed γc-deficiency markedly reduced Runx1-mediated SP8 cell generation in γccKORunx3dYFP/YFP mice by 3-5 fold (Fig. 7b left), indicating that signaling by γc cytokines was in fact required for Runx1-mediated SP8 generation. The simplest explanation was that γc-cytokine signaling upregulated Runx1 (or possibly Cbfb) expression, but this was not the case as γc-sufficient and γc-deficient SP8 cells in Runx3dYFP/YFP mice contained the same amount of Runx1 and Cbfb mRNA (Fig. 7b right). Together, these findings indicate that cytokine signaling is required for Runx1-mediated SP8 generation.
Runx1 does not normally contribute to SP8 cells
Because cytokine-signaled SP8 cells expressed high amounts of Runx3d but low amounts of Runx1 mRNA (Fig. 5a,b), we wondered if Runx3d down-regulated Runx1 expression. To address this issue, we compared Runx1 amounts in Runx3d-deficient and -sufficient SP8 thymocytes (Fig. 7c). We found that Runx1 mRNA expression was 2.5 fold greater in Runx3d-deficient (Runx3dYFP/YFP) than Runx3d-sufficient B6 SP8 thymocytes (Fig. 7c), indicating that Runx3d does inhibit Runx1 expression, as was observed in Runx3 transgenic mice32,33.
We then compared Runx1 mRNA expression in SP8 and SP4 thymocytes from B6 mice because SP8 thymocytes express cytokine-signaled Runx3d, whereas SP4 thymocytes are not cytokine-signaled. Runx1 mRNA expression was 4-fold higher in SP4 thymocytes compared to SP8 thymocytes in the same mice (Fig. 7d), consistent with inhibition of Runx1 by cytokine-induced Runx3d. Because cytokine-induced Runx3d inhibited Runx1 expression, we wondered if cytokines might upregulate Runx1 expression in thymocytes lacking Runx3d. To address this possibility, we performed the DP stimulation assay with Runx3d-deficient thymocytes but found that cytokine signals still failed to up-regulate Runx1 mRNA expression (Supplementary Fig. 6a). All of these experimental findings together indicate that SP8 cell generation in Runx3d-sufficient mice is not mediated by Runx1 but, instead, is mediated by cytokine-induced Runx3d which then inhibits Runx1 expression (schematized in Supplementary Fig. 7).
Cytokine signaling of MHCII-selected SP8 cells
Lastly, we wondered if cytokine signaling was also required for misdirected differentiation of MHCII-selected thymocytes into SP8 cells. Misdirected differentiation occurs in ThPOK-deficient mice such that MHCII-selected thymocytes incorrectly differentiate into SP8 cells8, 34, which is especially evident in ThPOKKOβ2mKO mice (Fig. 7e and Supplementary Fig. 6b). To assess a requirement for cytokine signaling, we compared γc-sufficient and γc-deficient ThPOKKOβ2mKO mice. In fact γc-deficiency resulted in 2-3-fold fewer misdirected SP8 thymocytes (Fig. 7e right and Supplementary Fig. 6b), demonstrating that cytokine signaling is even important for SP8 generation by developmental misdirection. We conclude that signaling by lineage-specifying cytokines is required for all CD8 lineage fate decisions in the thymus (schematized in Supplementary Fig. 7).
Discussion
The present study proves that signaling by lineage-specifying cytokines during positive selection is responsible for all CD8 lineage fate decisions in the thymus. Lineage-specifying cytokines are a diverse group of cytokines that consist of two γc cytokines (previously identified18 as IL-7 and IL-15) and four non-γc cytokines (IL-6, IFN-γ, TSLP, TGF-β) with disparate immune functions in the periphery35-39. However, in the thymus, all of these cytokines induce Runx3d and signal SP8 cell generation, regardless of whether they induce pro-survival genes or not. Most importantly, elimination of signaling by all six lineage-specifying cytokines during positive selection abrogated SP8 cell generation, revealing that the cytokine requirement for CD8 lineage determination could not be replaced by other signals in the thymus, including TCR signals.
CD4-CD8 lineage fate determination in the thymus is currently best described by the kinetic signaling model4-6,13,15. A unique requirement of this model is that CD8 lineage fate must be signaled by cytokines and not by TCRs which signal CD4 lineage fate. While many predictions of the kinetic signaling model have been fulfilled13,18,40, this key precept has remained controversial and unproven. In the present study, abrogation of SP8 cell generation by elimination of cytokine signaling documents that CD8 lineage fate decisions require cytokine signaling during positive selection and that the requirement for cytokine signaling is not circumvented by signaling from other receptors in the thymus, including TCRs. In fact cytokine signaling was even required for the generation of SP8 cells during MHCII-specific positive selection by misdirected differentiation in ThPOK-deficient mice.
Prior to the present study, it was difficult to distinguish the impact of cytokine signaling on CD8 lineage fate decisions from an impact of cytokine signaling on thymocyte survival. However, the non-γc lineage-specifying cytokines (IL-6, IFN-γ, TSLP, TGF-β) identified here lacked pro-survival function but signaled SP8 generation anyway, which effectively excludes any possibility that cytokine signaling simply promotes survival of otherwise CD8-committed cells. These non-γc cytokines display diverse functions in the periphery, as IL-6, IFN-γ, and TSLP are pro-inflammatory35-37 and TGF-β is anti-inflammatory41. In the thymus IFN-γ is produced by NKT1 cells42,43, TSLP by medullary thymic epithelial cells44, IL-6 by epithelial cells and fibroblasts35, and TGF-β by thymic epithelial cells45 and macrophages46. It is also conceivable that non-γc cytokines produced in the periphery can enter the thymus and contribute to CD8 lineage determination. For example, the misdirected differentiation of MHCII-selected AND TCR transgenic cells into SP8 cells observed in SOCS1-deficient mice is thought to be due to elevated serum levels of the pro-inflammatory cytokines IFN-γ and IL-6 as a consequence of SOCS1 deficiency47. And, we observed during this study that SP8 thymocyte numbers in IL-6-deficient neonatal γccKO mice were greater with an IL-6+/- mother than an IL-6-/- mother, suggesting that peripheral IL-6 from the mother can augment SP8 thymocyte generation.
The present study delineates the cytokine signaling requirements for SP8 differentiation in the thymus from the cytokine signaling requirements for survival of SP8 cells in the periphery. Six different lineage-specifying cytokines (IL-7, IL-15, IL-6, IFN-γ, TSLP, TGF-β) were found to signal SP8 thymocyte generation, but maintenance of naïve SP8 T cells in the periphery requires IL-7 signaling48. As a result, γc-deficient SP8 cells cannot survive in the periphery, but this does not mean that non-γc cytokines do not normally contribute to the generation of peripheral CD8 T cells. In fact we think SP8 cells may normally be signaled by several cytokines (e.g. γc and non-γc) during their differentiation in the thymus. As an example, B6 SP8 thymocytes are mostly generated by γc cytokines but they are also CD103hi, indicating that they were signaled by TGF-β as well.
This study also provides new insights into the impact of cytokine signaling on Runx1- and Runx3d-mediated SP8 cell generation. Cytokine signaling was required to induce Runx3d expression and was also required for Runx1 function in developing SP8 cells. However, the molecular basis for the Runx1 cytokine requirement remains uncertain, but one possibility is that γc cytokine signaling is necessary to induce expression of molecular co-factors such as AP4 (ref.49) which contributes to Cd4 gene silencing50. In any event, Runx1 and Runx3d are thought to be redundant for SP8 cell generation because each generates SP8 cells in mice genetically deficient for the other31, and it has been presumed that both Runx factors participate in SP8 generation in normal (i.e. Runx-sufficient) mice. However, the fact that cytokine-induced Runx3d inhibits Runx1 expression makes it difficult for both Runx factors to participate in SP8 generation in normal (i.e. Runx-sufficient) thymocytes. Instead, we think that Runx3d is the CD8 lineage-specifying transcription factor and that Runx1 is not, and that Runx1 mediates SP8 generation only in Runx3d-deficient mice - because cytokine signaling of Runx3d-deficient thymocytes cannot result in Runx3d-mediated inhibition of Runx1 expression.
Finally, redundancy among lineage-specifying cytokines in the thymus caused difficulty in appreciating the contribution of individual non-γc cytokines to SP8 generation. However, we think that another result of cytokine redundancy is that SP8 thymocyte number is determined by the total amount, rather than the identity, of CD8 lineage-specifying cytokines available to positively selected thymocytes in vivo. Consequently, exogenous addition of any one lineage-specifying cytokine would be expected to increase total numbers of SP8 thymocytes in vivo, as we observed in fetal thymic organ cultures.
In conclusion, the present study has identified a diverse group of lineage-specifying cytokines whose signaling is strictly required during positive selection for all CD8 lineage fate decisions in the thymus. In addition, this study has identified an interplay between lineage-specifying cytokines and the Runx proteins that transcriptionally specify CD8 lineage fate and mediate SP8 cell differentiation.
Methods
Animals
Mice with γcfl alleles18 were bred to E8III-Cre mice to obtain γccKO mice as previously described18. SOCS1Tg mice20 were provided by M. Kubo; Tslpr−/− (TSLPRKO) mice51 by W. Leonard; Tgfbr1fl/fl mice52 by W. Chen; Runx3dYFP/YFP mice53 by D. Littman; and Zbtb7b−/− (ThPOKKO) mice34 were provided by R. Bosselut. Tgfbr1fl/fl mice were bred to E8III-Cre mice to obtain TGF-βR1cKO mice, while Cbfbfl/fl and Runx1fl/fl mice were obtained from The Jackson Laboratory and bred to CD4-Cre mice to obtain CBFβcKO and Runx1cKO mice. Il6−/− (IL-6KO), Ifngr1−/− (IFN-γRKO), Il4r−/− (IL-4RαKO), β2mKO, HY RagKO, OT-I RagKO, and H2-Ab−/−Cd1d−/− (MHCIIKOCD1dKO) mice were bred in our own animal colony. C57BL/6 (B6) mice were obtained from the Frederick National Laboratory for Cancer Research. All animal experiments were approved by the National Cancer Institute Animal Care and Use Committee, and mice were cared for in accordance with National Institutes of Health guidelines.
Flow cytometry
Monoclonal antibodies with the following specificities were used: CD132 (4G3), CD103 (M290), CD126 (D7715A7), CD24 (M1/69), CD25 (7D4), CD5 (53-7.3), CD122 (TM-Beta 1), T-bet (04-46) and CD4 (RM4-4) from Becton Dickinson; CD119 (2E2), CD127 (A7R34), CD197 (4B12), CD4 (RM4-5), CD130 (KGP130), CD213a1 (13MOKA), Qa2 (69H1-9-9), CD28 (37.51), Eomes (Dan11mag), Perforin (OMAK-D) and TCRβ (H57-597) from eBioscience; CD69 (H1.2F3), CD124 (I015F8), Granzyme B (GB11), CD8β (YTS156.7.7) and IFN-γRβ (MOB-47) from BioLegend; CD8 (5H10) from Invitrogen; and c-Myc (9E10) from R&D Systems. Polyclonal antibodies specific for TSLPR and TGF-βRII were from R&D Systems. The anti-mouse TCR-Vβ screening panel from Becton Dickinson was used to analyze TCR-Vβ usage.
Single cell suspensions of thymus and lymph nodes were obtained by tweezing the organs with forceps. Cells were stained at 4°C in HBSS supplemented with 0.5% BSA and 0.5% NaN3 and first incubated with anti-FcR (2.4G2) followed by staining with fluorochrome-conjugated antibodies. For intracellular staining of cells, surface staining was performed first followed by fixation and permeabilization with either the Fixation/Permeabilization Solution Kit (BD) or the Transcription Factor Staining Buffer Set (eBioscience) followed by intracellular staining. Cells were acquired using an LSRII or LSRFortessa (Becton Dickinson). Doublets and dead cells were excluded from analysis by forward light-scatter and propidium iodide gating. Data were analyzed using EIB-Flow Control software developed at the National Institutes of Health and FlowJo software (TreeStar).
Note that pre-selection DP thymocytes were identified as CD4+CD8+CD69- thymocytes; SP8 cells from γc-sufficient mice were identified as CD4-CD8+TCRβhi cells, while SP8 cells from γccKO mice were identified as γc-CD4-CD8+TCRβhi cells to exclude any escapees from γc deletion.
Electronic cell sorting
Cells were electronically sorted on a FACSAria II. To obtain pre-selection DP thymocytes, whole thymocytes were stained for CD4, CD8, and CD69 and electronically sorted to obtain purified CD4+CD8+CD69- cells. To obtain SP8 thymocytes, whole thymocytes were depleted of CD4+ cells with anti-CD4 microbeads on MACS columns (Miltenyi Biotec); then stained for CD4, CD8, TCRβ, and CD132, and then electronically sorted to obtain either purified CD4-CD8+TCRβ+ cells (B6) or purified γc-CD4-CD8+TCRβ+ cells (γccKO).
Quantitative real time PCR
Total RNA was isolated using RNeasy Mini or RNeasy Micro kits (Qiagen), genomic DNA was removed from samples using TURBO DNA-free Kit (Applied Biosystems) and cDNA was synthesized using SuperScript III with oligo(dT) primers (Invitrogen). TaqMan primers and probes (Applied Biosystems) or QuantiTect SYBR Green detection system (Qiagen) reagents were used for real time PCR and samples were analyzed on a ABI PRISM 7900HT Sequence Detection System or QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems). The following TaqMan assays were used: Bcl2 Mm00477631_m1, Mcl-1 Mm00725832_s1, Bcl2l1 Mm00437783_m1, Runx1 Mm01213405_m1 and Rpl13a Mm01612986_gH. The following primers were used for SYBR green assays: Runx1 F: 5′‐CCGCAGCATGGTGGAGGTA‐3′; R: 5′‐AGCGATGGGCAGGGTCTTG-3′, Runx2 F: 5′-CCGTGGCCTTCAAGGTTGT‐3′; R: 5′‐CGGCCATGACGGTAACCA-3′, Runx3d F: 5′‐GCGACATGGCTTCCAACAGC‐3′; R: 5′‐CTTAGCGCGCCGCTGTTCTCGC‐3′, CBFb F: TGTGGCTACAGGAACCAATCTG‐3′; R: 5′‐TTGTCGCTGTTCTCCCTGC-3′ and Rpl13a F: 5′‐CGAGGCATGCTGCCCCACAA‐3′, R: 5′‐AGCAGGGACCACCATCCGCT‐3′.
All mRNA values were determined by quantitative PCR and are expressed relative to Rpl13a.
DP stimulation assay
Pre-selection DP thymocytes (CD4+CD8+CD69−) were electronically sorted and cultured with 0.3 ng/ml PMA (Sigma) and 0.3 μg/ml Ionomycin (Sigma) for 16 h, washed and cultured in medium alone for 10 h. Cells were then co-cultured with cytokines for 16-20 hours, harvested and assayed for mRNA expression. Note that all mRNA values were determined by quantitative PCR and are expressed relative to Rpl13a.
The following cytokines were used: IL-7 (10 ng/ml, Peprotech), IL-6 (45 ng/ml, eBioscience), IFN-γ (25 ng/ml, Peprotech), TSLP (25 ng/ml, eBioscience), TGF-β (10 ng/ml, eBioscience), IL-13 (25 ng/ml, eBioscience), IL-4 (40 ng/ml, Peprotech), IFN-α (50 ng/ml, eBioscience) and IFN-β (50 ng/ml, Peprotech).
Fetal thymic organ cultures
Thymic lobes were harvested from embryonic day 16.5 (E16.5) fetuses from B6 or γccKO mice and cultured on collagen sponge-supported filter membranes (Helistat and Whatman). On day 1 of culture cytokines were added to the culture medium: IL-6 (200 ng/ml, eBioscience), IFN-γ (200 ng/ml, Peprotech), TSLP (200 ng/ml, eBioscience), TGF-β (100 ng/ml, eBioscience), IL-13 (200 ng/ml, eBioscience), or IL-7 (200 ng/ml, Peprotech). FTOCs were analyzed on day 5 of culture. To assess proliferation in FTOCs, 50-100 μM EdU was added to FTOCs for the last 14 h of culture. EdU incorporation was analyzed following surface staining of single cell suspensions from FTOCs by using a Click-iT Plus EdU Flow Cytometry Assay Kit (ThermoFisher).
Alignment of Runx3d distal promoter region
VISTA (http://genome.lbl.gov/vista/index.shtml) was used for analysis of conservation between human and mouse genomic Runx3d regulatory elements. The Genomatix MatInspector software package was used to predict nuclear factor binding motifs in these conserved regions.
Statistical analysis
Statistical analysis was performed with GraphPad Prism 7 software using two-tailed unpaired t test. P values of 0.05 or less were considered significant.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
Supplementary Material
Acknowledgments
We thank T. McCaughtry for his initial contributions to the project; J.-H. Park, V. Lazarevic, D. Singer, X. Zhou and M. Kimura for critical readings of the manuscript; M. Kubo (Tokyo University of Science, Japan) for SOCS1Tg mice; W. Leonard (National Heart, Lung and Blood Institute) for TSLPRKO mice; W. Chen (National Institute of Dental and Craniofacial Research) for Tgfbr1fl mice; D. Littman (NYU) for Runx3dYFP mice; R. Bosselut (National Cancer Institute) for ThPOKKO mice; and S. Sharrow, A. Adams and L. Granger for expert flow cytometry. This research was supported by the Intramural Research Program of the US National Institutes of Health, National Cancer Institute, Center for Cancer Research and the US National Institutes of Health grant R01AI097244-01A1 to TE.
Footnotes
Author Contributions: R.E. designed the study, performed experiments, analyzed data and contributed to the writing of the manuscript; T.K., X.T., T.I.G. and T.E. performed experiments, analyzed data and provided helpful discussions; A.A. and B.E. generated experimental mice; and A.S. designed and supervised the study, analyzed data and wrote the manuscript.
Competing Financial Interests Statement: The authors declare no competing financial interests.
References
- 1.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter AC, Bosselut R. Decision checkpoints in the thymus. Nat Immunol. 2010;11:666–673. doi: 10.1038/ni.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teh HS, et al. Thymic major histocompatibility complex antigens and the alpha beta T-cell receptor determine the CD4/CD8 phenotype of T cells. Nature. 1988;335:229–233. doi: 10.1038/335229a0. [DOI] [PubMed] [Google Scholar]
- 4.Brugnera E, et al. Coreceptor reversal in the thymus: signaled CD4+8+ thymocytes initially terminate CD8 transcription even when differentiating into CD8+ T cells. Immunity. 2000;13:59–71. doi: 10.1016/s1074-7613(00)00008-x. [DOI] [PubMed] [Google Scholar]
- 5.Singer A. New perspectives on a developmental dilemma: the kinetic signaling model and the importance of signal duration for the CD4/CD8 lineage decision. Curr Opin Immunol. 2002;14:207–215. doi: 10.1016/s0952-7915(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 6.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8:788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Littman DR. How Thymocytes Achieve Their Fate. J Immunol. 2016;196:1983–1984. doi: 10.4049/jimmunol.1600032. [DOI] [PubMed] [Google Scholar]
- 8.He X, et al. The zinc finger transcription factor Th-POK regulates CD4 versus CD8 T-cell lineage commitment. Nature. 2005;433:826–833. doi: 10.1038/nature03338. [DOI] [PubMed] [Google Scholar]
- 9.Kappes DJ, He X, He X. Role of the transcription factor Th-POK in CD4:CD8 lineage commitment. Immunol Rev. 2006;209:237–252. doi: 10.1111/j.0105-2896.2006.00344.x. [DOI] [PubMed] [Google Scholar]
- 10.Xiong Y, Bosselut R. The enigma of CD4-lineage specification. Eur J Immunol. 2011;41:568–574. doi: 10.1002/eji.201041098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins A, Littman DR, Taniuchi I. RUNX proteins in transcription factor networks that regulate T-cell lineage choice. Nat Rev Immunol. 2009;9:106–115. doi: 10.1038/nri2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taniuchi I, et al. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- 13.Park JH, et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol. 2010;11:257–264. doi: 10.1038/ni.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Q, Erman B, Bhandoola A, Sharrow SO, Singer A. In vitro evidence that cytokine receptor signals are required for differentiation of double positive thymocytes into functionally mature CD8+ T cells. J Exp Med. 2003;197:475–487. doi: 10.1084/jem.20021765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura MY, et al. Timing and duration of MHC I positive selection signals are adjusted in the thymus to prevent lineage errors. Nat Immunol. 2016;17:1415–1423. doi: 10.1038/ni.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiong Y, Bosselut R. CD4-CD8 differentiation in the thymus: connecting circuits and building memories. Curr Opin Immunol. 2012;24:139–145. doi: 10.1016/j.coi.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurd N, Robey EA. T-cell selection in the thymus: a spatial and temporal perspective. Immunol Rev. 2016;271:114–126. doi: 10.1111/imr.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McCaughtry TM, et al. Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus. J Exp Med. 2012;209:2263–2276. doi: 10.1084/jem.20121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Decaluwe H, et al. Gamma(c) deficiency precludes CD8+ T cell memory despite formation of potent T cell effectors. Proc Natl Acad Sci U S A. 2010;107:9311–9316. doi: 10.1073/pnas.0913729107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanada T, et al. A mutant form of JAB/SOCS1 augments the cytokine-induced JAK/STAT pathway by accelerating degradation of wild-type JAB/CIS family proteins through the SOCS-box. J Biol Chem. 2001;276:40746–40754. doi: 10.1074/jbc.M106139200. [DOI] [PubMed] [Google Scholar]
- 21.Voon DC, Hor YT, Ito Y. The RUNX complex: reaching beyond haematopoiesis into immunity. Immunology. 2015;146:523–536. doi: 10.1111/imm.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vaillant F, et al. A full-length Cbfa1 gene product perturbs T-cell development and promotes lymphomagenesis in synergy with myc. Oncogene. 1999;18:7124–7134. doi: 10.1038/sj.onc.1203202. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–465. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 24.Lee KS, et al. Runx2 is a common target of transforming growth factor beta1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol Cell Biol. 2000;20:8783–8792. doi: 10.1128/mcb.20.23.8783-8792.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Asady R, et al. TGF-{beta}-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Konkel JE, et al. Control of the development of CD8alphaalpha+ intestinal intraepithelial lymphocytes by TGF-beta. Nat Immunol. 2011;12:312–319. doi: 10.1038/ni.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ouyang W, et al. TGF-beta cytokine signaling promotes CD8+ T cell development and low-affinity CD4+ T cell homeostasis by regulation of interleukin-7 receptor alpha expression. Immunity. 2013;39:335–346. doi: 10.1016/j.immuni.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi MJ, Stavnezer J. CBF alpha3 (AML2) is induced by TGF-beta1 to bind and activate the mouse germline Ig alpha promoter. J Immunol. 1998;161:6751–6760. [PubMed] [Google Scholar]
- 29.Kulkarni AB, Karlsson S. Transforming growth factor-beta 1 knockout mice. A mutation in one cytokine gene causes a dramatic inflammatory disease. Am J Pathol. 1993;143:3–9. [PMC free article] [PubMed] [Google Scholar]
- 30.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egawa T, Tillman RE, Naoe Y, Taniuchi I, Littman DR. The role of the Runx transcription factors in thymocyte differentiation and in homeostasis of naive T cells. J Exp Med. 2007;204:1945–1957. doi: 10.1084/jem.20070133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohu K, et al. Overexpression of the Runx3 transcription factor increases the proportion of mature thymocytes of the CD8 single-positive lineage. J Immunol. 2005;174:2627–2636. doi: 10.4049/jimmunol.174.5.2627. [DOI] [PubMed] [Google Scholar]
- 33.Grueter B, et al. Runx3 regulates integrin alpha E/CD103 and CD4 expression during development of CD4-/CD8+ T cells. J Immunol. 2005;175:1694–1705. doi: 10.4049/jimmunol.175.3.1694. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, et al. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4(+) T cells. Nat Immunol. 2008;9:1122–1130. doi: 10.1038/ni.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 36.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 37.Ziegler SF, et al. The biology of thymic stromal lymphopoietin (TSLP) Adv Pharmacol. 2013;66:129–155. doi: 10.1016/B978-0-12-404717-4.00004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Travis MA, Sheppard D. TGF-beta activation and function in immunity. Annu Rev Immunol. 2014;32:51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rochman Y, et al. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A. 2010;107:19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adoro S, et al. Coreceptor gene imprinting governs thymocyte lineage fate. EMBO J. 2012;31:366–377. doi: 10.1038/emboj.2011.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen W, et al. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pobezinsky LA, et al. Let-7 microRNAs target the lineage-specific transcription factor PLZF to regulate terminal NKT cell differentiation and effector function. Nat Immunol. 2015;16:517–524. doi: 10.1038/ni.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dewas C, et al. TSLP expression: analysis with a ZsGreen TSLP reporter mouse. J Immunol. 2015;194:1372–1380. doi: 10.4049/jimmunol.1400519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahama Y, Letterio JJ, Suzuki H, Farr AG, Singer A. Early progression of thymocytes along the CD4/CD8 developmental pathway is regulated by a subset of thymic epithelial cells expressing transforming growth factor beta. J Exp Med. 1994;179:1495–1506. doi: 10.1084/jem.179.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fadok VA, et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Catlett IM, Hedrick SM. Suppressor of cytokine signaling 1 is required for the differentiation of CD4+ T cells. Nat Immunol. 2005;6:715–721. doi: 10.1038/ni1211. [DOI] [PubMed] [Google Scholar]
- 48.Fry TJ, Mackall CL. Interleukin-7: master regulator of peripheral T-cell homeostasis? Trends Immunol. 2001;22:564–571. doi: 10.1016/s1471-4906(01)02028-2. [DOI] [PubMed] [Google Scholar]
- 49.Chou C, et al. The Transcription Factor AP4 Mediates Resolution of Chronic Viral Infection through Amplification of Germinal Center B Cell Responses. Immunity. 2016;45:570–582. doi: 10.1016/j.immuni.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egawa T, Littman DR. Transcription factor AP4 modulates reversible and epigenetic silencing of the Cd4 gene. Proc Natl Acad Sci U S A. 2011;108:14873–14878. doi: 10.1073/pnas.1112293108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Shami A, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Larsson J, et al. TGF-beta signaling-deficient hematopoietic stem cells have normal self-renewal and regenerative ability in vivo despite increased proliferative capacity in vitro. Blood. 2003;102:3129–3135. doi: 10.1182/blood-2003-04-1300. [DOI] [PubMed] [Google Scholar]
- 53.Egawa T, Littman DR. ThPOK acts late in specification of the helper T cell lineage and suppresses Runx-mediated commitment to the cytotoxic T cell lineage. Nat Immunol. 2008;9:1131–1139. doi: 10.1038/ni.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


