Abstract
Postpartum depression (PPD) adversely affects the health and well being of many new mothers, their infants, and their families. A comprehensive understanding of biopsychosocial precursors to PPD is needed to solidify the current evidence base for best practices in translation. We conducted a systematic review of research published from 2000 through 2013 on biological and psychosocial factors associated with PPD and postpartum depressive symptoms. Two hundred fourteen publications based on 199 investigations of 151,651 women in the first postpartum year met inclusion criteria. The biological and psychosocial literatures are largely distinct, and few studies provide integrative analyses. The strongest PPD risk predictors among biological processes are hypothalamic-pituitary-adrenal dysregulation, inflammatory processes, and genetic vulnerabilities. Among psychosocial factors, the strongest predictors are severe life events, some forms of chronic strain, relationship quality, and support from partner and mother. Fully integrated biopsychosocial investigations with large samples are needed to advance our knowledge of PPD etiology.
Keywords: mental health, biopsychosocial, pregnancy, postnatal, risk factors
INTRODUCTION
The birth of a child most often evokes maternal feelings of happiness and joy, but much less attention is paid to the fact that postpartum depression (PPD) is also present for many new mothers (Gavin et al. 2005). The conflict between the positive emotions that new mothers often think they should feel and the reality of depressed mood and anxiety that many of them actually experience can be confusing and overwhelming. Women may expect that these symptoms will subside without treatment, and this is generally the case for postpartum blues, a milder mood disruption within the first 10 days after delivery (Grigoriadis & Romans 2006, O’Hara & Wisner 2014). However, PPD is a clinical condition that lasts for at least two weeks, creates significant impairment in functioning, and typically requires professional treatment [Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR); Am. Psychiatr. Assoc. 2000].
A large body of empirical studies has examined risk factors for PPD. Current evidence can be categorized into two categories, biological and psychosocial. For the most part, the biological research addresses the endocrine system, the immune system, and genetic factors (for reviews, see Bloch et al. 2003, Brummelte & Galea 2010, Corwin et al. 2010, Corwin & Pajer 2008, Skalkidou et al. 2012), and the psychosocial literature addresses stressors and interpersonal relationships (for reviews, see Beck 2001, O’Hara 2009, O’Hara & Swain 1996, Robertson et al. 2004). Review papers typically concern only one of these bodies of research (for an exception, see Hopkins et al. 1984). The downside of this bifurcated literature on PPD is that biopsychosocial processes and interactions are neglected, and integrative models remain underdeveloped and untested. The present review incorporates the empirical evidence from both research domains in an effort to provide an expanded perspective on PPD risk, to foster integrative work, to provide insight into biobehavioral mechanisms in PPD etiology, and to identify potential avenues for interventions.
We begin by defining PPD, describing its symptoms and assessment, and specifying how a diagnosis is determined for research purposes. We then introduce models of the etiological pathways leading to PPD. The main body of the review has three sections: (a) biological processes/factors, including endocrine, immune/inflammatory, and genetic/epigenetic risk factors; (b) psychosocial processes/factors, including stress and interpersonal risk factors; and (c) integrative studies. We conclude with an evaluation of the current state of the literature and suggest promising directions for future research.
Definitions, Classifications, and Assessment
The same criteria used to diagnose major depressive disorder apply in the postpartum period (DSM-IV-TR; Am. Psychiatr. Assoc. 2000). A postpartum specifier can be attached to the diagnosis if symptom onset occurred within four weeks after delivery. In the DSM-5 (Am. Psychiatr. Assoc. 2013), this has been expanded to a “peripartum specifier,” indicating onset at any time during pregnancy or the first four weeks post partum. The International Statistical Classification of Diseases and Related Health Problems, 10th revision (ICD-10) (World Health Organ. 1992) lists criteria for postpartum depressive episodes similar to those in the DSM-IV-TR, but it specifies symptom onset within six weeks post partum. In empirical research, definitions of the postpartum period are more variable, including anywhere from the first few hours after delivery up to one year. Symptoms of postpartum blues, a risk factor for PPD (Dennis 2004), have therefore been included in the present review.
A complexity given this definition is that PPD can be a first episode of depression (de novo), or it may be preceded by depressive symptoms in the present pregnancy, in previous pregnancies, or in previous postpartum periods, and it may also occur within the context of a lifetime history of depression. In particular, a history of puerperal depression—that is, depression in this or previous pregnancies and PPD following previous pregnancies—and nonpuerperal depression, anxiety, and stress typically have moderate to strong associations with PPD (Beck 2001, Grigoriadis & Romans 2006, O’Hara & Swain 1996, Robertson et al. 2004). Thus, although disagreement exists about whether PPD is a disorder distinct from other depressions, it is unique in at least two ways. First, it is preceded and accompanied by major biological adaptations that may also affect mood. Second, PPD not only affects the mother but also adversely affects the newborn’s cognitive, behavioral, and emotional development (Feldman et al. 2009, Fihrer et al. 2009), with effects potentially lasting into adolescence (Verbeek et al. 2012).
Most reports indicate that 10% to 15% of new mothers experience PPD, with the most recent meta-analysis estimating the prevalence within three months after delivery at 19.2% for minor and 7.1% for major PPD (Gavin et al. 2005) (see The Importance of Culture sidebar). Comorbidity studies suggest that PPD is likely to co-occur with other psychiatric disorders that affect women after birth, most commonly anxiety disorders (Figueira et al. 2009, Le Strat et al. 2011).
THE IMPORTANCE OF CULTURE.
Most studies of PPD report PPD incidence of 10% to 15% (Gavin et al. 2005). However, Halbreich & Karkun’s (2006) review of 143 studies from 40 countries suggests a range of prevalence from almost nonexistent to well above 50%, which the authors partially attribute to cultural factors including variability in the definition and expression of depressive symptoms. Other cultural factors are dietary proscriptions and restrictions, sources and types of stress, social support, parental gender roles, religious customs, and attitudes and norms about mental health in general. For example, PPD rates are low and symptom onset is delayed in cultures that emphasize family support to the mother over the first month post partum (Halbreich 2005). Cultural beliefs and values surrounding childbearing, family structure and function, and the maternal role are also particularly important in shaping postpartum maternal mental health (Stern & Kruckman 1983). Protective factors, such as cultural rituals that help mothers, and vulnerability factors, such as cultural devaluing of female children, have been highlighted as well (Bina 2008). Overall, examinations of a woman’s experience of cultural traditions and her family’s observation of them have been neglected and are important to include in any complete analysis of PPD.
The gold standard for diagnosing PPD is a clinical interview, the most well known of which is the Structured Clinical Interview for the DSM-IV (First et al. 2002). Shorter screening tools, most prominently the Edinburgh Postnatal Depression Scale (EPDS; Cox et al. 1987) are also commonly used in research because they are reliable, well validated, and often more practical and cost-effective in widely screening for PPD risk. In particular, the EPDS has demonstrated a sensitivity of 95% and specificity of 93% compared to DSM-III criteria (Harris et al. 1989). In this review, we reserve the term PPD for studies in which a categorical designation was made based on a diagnostic interview or scores above a specified cutoff on a screener and we refer to PPD symptoms when a measure of depressive symptoms was used to produce a continuous score reflecting number and degree of symptoms. In summary statements we default to the broader term “PPD symptoms.”
Etiological Models of Postpartum Depression
Classic biological models of PPD can be conceptualized as withdrawal models that concern the fact that reproductive hormones (Chrousos et al. 1998, Douma et al. 2005) and stress hormones (Glynn et al. 2013, Kammerer et al. 2006) rise dramatically prior to delivery and then drop suddenly at delivery, which is hypothesized to trigger system dysregulation and depressive symptoms in a subset of vulnerable women. Support for these theories comes from observations of a reproductive subtype of depression related to hormonal fluctuations during the menstrual cycle, puberty, pregnancy, the postpartum period, and menopause (e.g., Payne et al. 2009) and from treatment studies (for a critical review, see Moses-Kolko et al. 2009) and case reports (e.g., Ahokas et al. 1999) documenting a rapid improvement in symptoms after estradiol administration. (See Evolutionary Perspectives sidebar.)
EVOLUTIONARY PERSPECTIVES.
Why does PPD exist at all? Theoretical perspectives from evolutionary science offer explanations for PPD as a psychological adaptation or a byproduct of modern civilization. Hagen (1999) proposed that PPD facilitates maternal disinvestment in offspring that are unlikely to survive and later reproduce, and it also broadcasts the mother’s need for support. In line with this view, the present review points to poor infant and maternal health and lack of social support (father abandonment, poor family support) as predictors of PPD. More recently, Hahn-Holbrook & Haselton (2014) proposed that current high rates of PPD are a byproduct of major changes in motherhood over the last century, leading to the conclusion that PPD may be a “disease of civilization.” For example, early weaning, low omega-3 fatty acid consumption, vitamin D deficiency, sedentary lifestyles, and isolation from family are more prevalent today than in the past. Each has been associated with increased risk of PPD and with elevated inflammation.
These two evolutionary perspectives are complementary: The adaptationist perspective is a framework for understanding why PPD initially served an adaptive purpose, whereas civilization byproduct accounts explain why PPD is common today even among women with healthy babies and supportive partners.
In contrast, psychological models such as the stress process model (Pearlin et al. 1981) and the cognitive behavioral model of PPD (O’Hara et al. 1982) emphasize the deleterious role of psychological stressors (e.g., father abandonment, financial strain) and underlying cognitive vulnerabilities (e.g., negative attributional style) and the ameliorating role of psychosocial resources (e.g., social support, self-esteem). These theories posit that pregnancy, childbirth, and new parenthood are stressors for many mothers, helping to explain why women may be especially vulnerable to depression at this life stage. Psychological models have consistently found support in the psychological literature and are still quite influential, as is evident from our systematic review.
Biological and psychological theories have guided research and provided insight into an important piece of the PPD puzzle, but they do not help us understand how psychosocial stress processes are instantiated in women’s brains and bodies, nor how genetic or epigenetic changes interact with psychosocial risk factors to influence PPD risk. To bridge this divide, integrated models have been developed, including the stress vulnerability model, which proposes that stress can trigger PPD in women with genetic, hormonal, and cognitive vulnerabilities (O’Hara et al. 1991). There is evidence to support this theory (e.g., Ross et al. 2004), although most studies have relied on family and personal history of depression as proxies for underlying genetic risk. More recently, Halbreich’s (2005) bio-psycho-social-cultural model overlaid the stress vulnerability model with additional biological sophistication and added cultural aspects to the list of moderating variables. By this account, biological vulnerability is conceptualized as a genetically derived hypersensitivity to hormonal changes and to dysregulation or impaired adaptation mechanisms in the central nervous system. This vulnerability is thought to interact reciprocally with the environment, both shaping the organism’s responses to environmental challenges and being shaped by stressors and positive experiences over the life span. Very few studies have attempted to test these integrated models.
METHODS
Search Strategy
A systematic literature search was performed according to guidelines in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (Moher et al. 2009). Searches were conducted in PubMed and PsycINFO. Keywords included combinations of the words postpartum or postnatal and depression. These terms were combined with broader search words: biological, psychological, social, and psychosocial, as well as more specific search words: endocrine, hormone, immune, inflammatory, cytokine, genetic, stress, demands, events, couple relationship, partner, marital, marriage, close relationship, interpersonal, social, family, social network, social support, and integration.
Inclusion and Exclusion Criteria
Studies were considered if they were published between January 2000 and December 2013 and appeared in peer-reviewed, English language journals. For a study to be eligible for inclusion, four criteria had to be met. First, participants in a study gave birth within the past year. Second, assessments of PPD symptoms by clinical interview or questionnaire were done any time between one day and one year after delivery. Third, a biological measure (reproductive, stress or thyroid hormones, immune/inflammatory, genetic factors) or psychosocial measure (stress or interpersonal factor) was administered at least once during pregnancy and/or within the first year post partum. Finally, a statistical result indicating the relationship between the predictor (biological or psychosocial variables) and the outcome (PPD or symptoms thereof) was reported. Single case studies, treatment and intervention studies, studies on depression during pregnancy, and abortion studies were not considered. Studies on other psychiatric conditions including postpartum psychosis, anxiety disorders, and posttraumatic stress disorders were excluded, as were mixed samples when results were not available for PPD as a separate outcome. Studies on nonhuman animals were also considered to be beyond the scope of this review.
Selected Studies
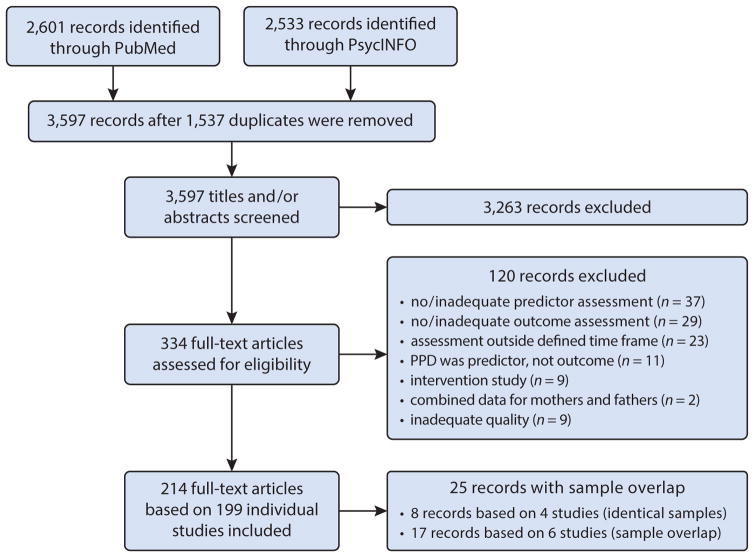
The search strategy yielded 2,601 hits in PubMed and 2,533 hits in PsycINFO, resulting in 3,597 records after removal of 1,537 duplicates (see Figure 1 for details). Titles and/or abstracts of these records were screened, and 3,263 did not meet eligibility criteria. Of the remaining 334 full-text articles, 120 were excluded for various reasons. Among the remaining 214 publications, four pairs of records provided findings that were based on identical samples. The two papers were treated as a single publication and reported in a combined table entry, resulting in a total of 210 table entries. If samples were overlapping but not identical, each publication received an individual table entry. This affected 17 publications from six studies. Thus, our systematic review includes 214 publications from 199 studies, reported in 210 table entries.
Figure 1.
Flow chart following guidelines in the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (Moher et al. 2009).
The 199 studies report on a total of 151,424 women who were assessed at least once within the first year post partum (the total includes the highest reported n for each set of overlapping samples). Sample sizes varied from 16 participants in one influential study (Bloch et al. 2000) to 15,389 in a report on three large cohorts of women from Australia (Eastwood et al. 2013). On average, the 48 studies in the biological section included fewer participants (M = 238.3, SD = 357.2; range: 16–1,804) than the 150 studies in the psychosocial section (M = 959.7, SD = 2,124.4; range: 35–15,389); the average sample size was smallest among the 11 integrative studies (M = 183.8, SD = 123.2; range: 41–419). Studies were predominantly conducted in North America (42.4%) and Europe (24.7%), with the remaining studies from Oceania (13.6%), Asia (13.1%), South America (4.5%), and Africa (1.5%). Considering individual countries, more than one-third of all studies were conducted in the United States (38.2%), followed by Australia (8.5%), Canada (7.5%), and the United Kingdom and Brazil (both 3.5%). Almost all studies relied on convenience samples. Because of space constraints, the detailed table reporting, for each paper, the study design (longitudinal versus cross-sectional), participant characteristics, predictor(s) including the timing of their assessment, control variables, measure(s) of PPD including the timing of their assessment, and relevant results, is presented in Supplement A (follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org).
BIOLOGICAL PREDICTORS OF POSTPARTUM DEPRESSION
Normal human pregnancy is characterized by substantial biological changes designed to maintain the pregnancy, support fetal development, and promote labor, delivery, and lactation. To meet the often-conflicting needs of the mother and the quickly developing fetus, the female body is equipped with considerable adaptive capacity. After delivery of the baby and the placenta, the intricate balance that sustained the maternal-placental-fetal unit throughout gestation is suddenly obsolete, and the maternal systems undergo dramatic biological changes within the first postnatal days. Depending in part on how long a woman breastfeeds, the new nonpregnant biological balance may take many months to establish. Ultimately, these biological adjustments may also impact maternal mental health.
Partial reviews are available that cover biological processes broadly (Skalkidou et al. 2012, Wisner & Stowe 1997, Zonana & Gorman 2005), with emphasis on the endocrine system (Bloch et al. 2003, Brummelte & Galea 2010, McCoy et al. 2003), the immune system (Corwin & Pajer 2008, Kendall-Tackett 2007) or genetic factors (Corwin et al. 2010). Our systematic review of all qualified studies on endocrine, immune/inflammatory, and genetic predictors of PPD provides an up-to-date and comprehensive review of the state of the biological evidence and highlights intersections with the psychosocial literature.
Review of Endocrine Studies
Within this literature, we reviewed reproductive, stress, and thyroid hormones. Because of space constraints, and because this literature is conceptually distinct, we provide the review of thyroid hormones in Supplement B (follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org). The Thyroid System sidebar provides a brief summary of the findings.
THE THYROID SYSTEM.
Symptoms of PPD overlap with those of postpartum thyroiditis, a typically temporary dysregulation of the thyroid affecting 5% to 7% of women in the first year after delivery (Kennedy et al. 2010). This observation has led to the development of theories implicating the thyroid system in PPD. For example, it has been suggested that pregnancy-related changes in the thyroid system may impair the activity of the serotonin system (Hendrick et al. 1998, Upadhyaya et al. 1992) or alter estrogen receptor sensitivity (Sylvén et al. 2013, Vasudevan et al. 2002). A systematic review of thyroid factors implicated in PPD risk is provided in Supplement B. We identified 11 studies that tested the link between thyroid antibodies or thyroid hormones and PPD symptoms. Whereas some studies linked positive antibody status with PPD symptoms, others reported null findings. Similarly, some studies suggest links between higher TSH and lower T3 and T4 levels with PPD symptoms, as theorized, but some larger studies do not provide evidence of such links. Thyroid markers should not be studied in isolation but rather in interaction with closely related biological factors, most prominently estrogen.
Reproductive hormones
Reproductive hormones play an important role in orchestrating pregnancy, labor, and birth. They have also been implicated in nonpuerperal depression. A review of 30 years of literature finds that mood disturbance is associated with the sudden withdrawal of estrogen, estrogen fluctuations, and sustained estrogen deficiencies (Douma et al. 2005). Likewise, progesterone is thought to be protective against depression because of its anxiolytic and anesthetic properties (Herrmann & Beach 1978, Itil et al. 1974) and because it modulates serotonergic receptors (Biegon et al. 1983). Thus, shifts in estrogen and progesterone during pregnancy and post partum may contribute to PPD (Bloch et al. 2000).
Reproductive hormones increase over the course of pregnancy to a degree that is unparalleled by any other neuroendocrine events (e.g., menstruation, puberty, menopause) in the life span of a healthy female. Most prominently, estriol increases by approximately 1,000-fold (Speroff et al. 1983). Estradiol increases approximately 50-fold, progesterone 10-fold, (Tulchinsky et al. 1972), and prolactin 7-fold (Bloch et al. 2003); testosterone shows modest increases compared to prepregnancy levels (Bammann et al. 1980), and oxytocin increases just before parturition (Leake et al. 1981). Most hormones return to prepregnancy levels within one to two weeks (Bloch et al. 2003). However, in breastfeeding women, prolactin remains elevated, and breastfeeding bouts trigger acute increases in both prolactin and oxytocin, while estradiol and progesterone levels are suppressed during lactation amenorrhea (Battin et al. 1985).
Estrogens
The strongest evidence that estrogen withdrawal plays a causal role in PPD comes from Bloch et al.’s (2000) double-blind pregnancy-simulation study in which synthetic estradiol and progesterone were administered and then withdrawn, triggering symptoms of depression in the eight women with a history of PPD but not in the eight women without a history of PPD. Of note, at no time were group differences in estradiol or progesterone levels observed, nor was either hormone correlated with EPDS scores. This small but influential landmark study suggests that women with a history of PPD may be differentially sensitive to the mood-destabilizing effects of changes in gonadal steroids and that the assessment of estradiol and progesterone levels may not be an appropriate measure to adequately reflect the processes through which these hormones impact PPD development.
Accordingly, little support exists for the association of the magnitude of the perinatal estrogen drop or perinatal estrogen levels with PPD. In a study of 70 mothers without a psychiatric history, O’Keane et al. (2011) found no link between the severity of postpartum blues symptoms and the magnitude of the estriol drop between 36 weeks’ gestational age (GA) and the first week post partum. Two smaller studies not selecting for psychiatric history also report the lack of an association between late-pregnancy levels of estradiol (Chatzicharalampous et al. 2011, Ingram et al. 2003) or the magnitude of the estradiol drop (Chatzicharalampous et al. 2011) and PPD symptoms within six months post partum.
All nine studies on endogenous estrogen and PPD symptoms obtained a measure postpartum, hypothesizing that if estradiol is psycho-protective, lower naturally occurring estrogen levels should be associated with PPD symptoms. However, the majority of studies report null results or point to higher estradiol as a risk factor. The largest study of 192 new mothers at five days post partum found that mothers with a PPD diagnosis had higher levels of plasma estradiol on the third but not the first day after birth compared to healthy controls (Klier et al. 2007). Another study found a link between higher estradiol and a concurrent PPD diagnosis within the first six months post partum (Epperson et al. 2006). Neither study controlled for breastfeeding, which is a viable confounding variable associated with both lower rates of PPD (Dennis & McQueen 2009) and lower levels of estradiol (Battin et al. 1985). The one study that did find evidence for a psycho-protective effect of postpartum estrogen was also the lone study measuring the estrogen subtype estriol (O’Keane et al. 2011). In that study, blues symptoms within the first week post partum were inversely correlated with estriol levels. All other studies failed to detect a link between postpartum levels of estradiol and PPD symptoms (Chatzicharalampous et al. 2011, Hohlagschwandtner et al. 2001, Ingram et al. 2003, Nappi et al. 2001, Okun et al. 2011, Stuebe et al. 2013). In sum, little evidence supports estrogen withdrawal theories, and biological vulnerability models remain largely untested.
Progesterone
Similarly, few studies implicate progesterone withdrawal in PPD risk. Progesterone levels after 36 weeks’ GA were not associated with PPD symptoms in three small studies (Chatzicharalampous et al. 2011, Ingram et al. 2003, O’Keane et al. 2011). Moreover, two of these studies found no evidence that the magnitude of the perinatal progesterone drop predicted PPD symptoms (Chatzicharalampous et al. 2011, O’Keane et al. 2011).
If progesterone is psycho-protective, women with higher naturally occurring levels of progesterone post partum may experience lower rates of PPD symptoms. Consistent with this hypothesis, a longitudinal study of 54 mothers found that progesterone levels within 12 to 48 hours after birth, but not at 1 or 4 weeks post partum, were inversely related to PPD symptoms 6 months after delivery (Ingram et al. 2003). Three small studies report on the absence of a link between progesterone and concurrent symptoms of depression between 1 and 17 weeks post partum (Nappi et al. 2001, Okun et al. 2011, Stuebe et al. 2013). In sum, little evidence suggests that progesterone in late pregnancy or post partum predicts PPD symptoms, but studies have been small, and moderators associated with vulnerability to hormone changes remain untested.
Prolactin
Few studies addressed the role of prolactin for PPD risk, and most assessed prolactin post partum. One exception is the Ingram et al. (2003) study of 54 mothers that reports lower prolactin at 36 weeks’ GA with higher likelihood of PPD symptoms at six months post partum. However, this association did not hold after controlling for progesterone and stressful life events. Moreover, no significant association between PPD symptoms and the magnitude of the perinatal prolactin drop was observed.
Prolactin has anxiolytic properties and is thought to contribute to the stress-buffering effects of lactation consistently observed in studies of humans and rats (Torner & Neumann 2002). Therefore, higher basal levels post partum may be protective against PPD onset. In line with this view, two studies with partially overlapping samples found that women in the highest decile on the Profile of Mood States-Depression subscale between four and six weeks post partum had lower levels of prolactin compared to the other women in the sample (Groër 2005, Groer & Morgan 2007). However, a study of 48 women found no association between PPD symptoms and prolactin at baseline and in response to breastfeeding at two and eight weeks post partum (Stuebe et al. 2013). One study suggests no prospective link between prolactin in the first week post partum and PPD symptoms at six months (Ingram et al. 2003). In sum, although data are mixed, it is noteworthy that the two largest studies suggest an inverse association between PPD and prolactin.
Oxytocin
One prospective study of 73 Swiss women who were symptom free at the time of recruitment found that lower oxytocin levels between 21 and 32 weeks’ GA predicted more PPD symptoms within the first two weeks post partum (Skrundz et al. 2011). Lower baseline oxytocin at two and eight weeks was also associated with concurrent symptoms of PPD in a study of 48 US women (Stuebe et al. 2013). In that study, lower oxytocin released in association with breastfeeding or pumping was also linked with more symptoms at eight weeks but not at two weeks post partum. A cross-sectional study of 35 women with and without cocaine use during pregnancy found no evidence for such a link between 1 and 11 months post partum (Light et al. 2004), but these latter findings should be considered in light of the small sample, the unique population, and the large window for PPD assessment. In sum, this small literature suggests that lower levels of oxytocin in pregnancy or post partum may be a risk factor for PPD.
Testosterone
A prospective study of 57 women provided no evidence that testosterone levels late in pregnancy or the magnitude of the perinatal drop were associated with PPD symptoms in the first four postpartum days or at six weeks post partum (Chatzicharalampous et al. 2011). That study also reports on the absence of a link between concurrently assessed testosterone and PPD symptoms within the first four days and at six weeks post partum. In contrast, a correlational study of 193 women suggests a positive association between testosterone and PPD symptoms within the first three postpartum days (Hohlagschwandtner et al. 2001).
Summary
The empirical evidence does not support a role for estrogen or progesterone withdrawal in the development of PPD symptoms, but studies have not tested moderators of biological vulnerability such as a history of depression or life stress. Furthermore, associations of estrogen or progesterone post partum with PPD symptoms were either significant in the unexpected direction or nonsignificant, with one notable exception for estriol. Conclusions about prolactin and testosterone are limited by the paucity of available studies, but a small but fairly consistent literature links lower perinatal oxytocin to more PPD symptoms. For prolactin, significant inverse correlations only emerged when assessments were made approximately four to six weeks post partum. Breastfeeding was not carefully controlled in most studies.
Stress hormones
The negative mood, cognitive difficulties, and heightened anxiety that are characteristic of depressive disorders are hypothesized to involve dysregulation of the body’s stress response systems such that affective and biological stress responses occur in disproportion to events or persist for extended periods of time (Ehlert et al. 2001). Stress hormones, in particular those of the hypothalamic-pituitary-adrenal (HPA) axis, have been implicated in nonpuerperal depression (Bao et al. 2008, Gold & Chrousos 2002). In principle, stress hormones follow a pattern similar to reproductive hormones, such that they increase over the course of pregnancy and then drop after delivery. However, the neuropeptide corticotropin-releasing hormone (CRH) increases exponentially over the course of pregnancy (McLean et al. 1995, Sandman et al. 2006), reaching levels observed only under conditions of stress in the median eminence, a local portal system connecting the hypothalamus with the pituitary gland (Lowry 1993). This exponential increase occurs because CRH, which is typically released by the hypothalamus, is also produced by the placenta (Sasaki et al. 1984). Because cortisol stimulates placental CRH production (Sandman et al. 2006), a positive feed-forward loop is established. Thus, (stress-related) cortisol increases early in pregnancy may result in accelerated CRH increases throughout pregnancy.
Corticotropin-releasing hormone
A prospective study of 100 women found accelerated CRH trajectories between 23 and 26 weeks’ GA and higher CRH levels between 18 weeks’ GA and the end of pregnancy among women with PPD symptoms at 9 weeks post partum (Yim et al. 2009). Similarly, a prospective study of 210 pregnant women found more pronounced increases in CRH from 29 to 37 weeks’ GA and higher absolute levels at 37 weeks’ GA with PPD symptoms at 8 weeks post partum (Hahn-Holbrook et al. 2013). An earlier finding by Rich-Edwards et al. (2008), however, suggests a lack of association between CRH levels between 25 and 37 weeks’ GA and PPD symptoms at 6 months post partum. Glynn & Sandman (2014) reconcile these seemingly contradictory findings by showing a significant association of CRH levels and trajectories with PPD symptoms at 3 months but not at 6 months post partum. Meltzer-Brody et al. (2011) report the absence of a link between CRH before 20 weeks’ and between 24 and 29 weeks’ GA and PPD symptoms at 12 weeks and 1 year post partum. However, their study used a less validated assay technique, and, as Glynn & Sandman (2014) point out, that study also failed to detect the well-established link between CRH and gestational length (McLean et al. 1995, Sandman et al. 2006). Only one study assessed the magnitude of the perinatal CRH drop and found larger CRH decreases from 36 weeks’ GA to the first week post partum with less pronounced PPD symptoms (O’Keane et al. 2011), contradicting the withdrawal hypothesis. None of the studies assessing CRH post partum report a significant link with PPD symptoms (O’Keane et al. 2011, Stuebe et al. 2013).
Adrenocorticotropic hormone
The emerging link between CRH in pregnancy and PPD symptoms does not replicate for adrenocorticotropic hormone (ACTH). Yim et al. (2009) found higher baseline levels of ACTH at 25 (but not at 15, 19, 31, and 37) weeks’ GA in women with PPD symptoms at 9 weeks post partum compared to those without, but this association did not hold after controlling for CRH. Similarly, Glynn & Sandman (2014) report on the absence of a link between ACTH assessed repeatedly in pregnancy and PPD symptoms at 3 and 6 months post partum. One study tested the link between PPD symptoms and the magnitude of the perinatal ACTH drop and reports null findings (O’Keane et al. 2011). Two studies assessed baseline levels of ACTH after birth, with one confirming null findings at 4 to 6 weeks post partum (Groër 2005), and the other reporting higher ACTH with more symptoms in the first postnatal week (O’Keane et al. 2011).
Finally, a study using treadmill exercise as a stressor to elicit ACTH responses did not find differences in ACTH reactivity between women with and without PPD symptoms at 6 and 12 weeks post partum (Jolley et al. 2007). However, the ACTH-to-cortisol regression line differed, such that cases showed higher ACTH with lower cortisol levels at each assessment.
β-Endorphin
One study tested the link between β-endorphin in pregnancy and PPD symptoms and suggests the absence of an overall effect (Yim et al. 2010). However, among women who report being euthymic at 25 weeks’ GA, those with higher β-endorphin levels across pregnancy were more likely to experience symptoms at 9 weeks post partum. This finding highlights the importance of studying subgroups of women and lends support to the CRH literature that found the time around 25 weeks’ GA to be a crucial one in predicting PPD risk.
Cortisol
Studies of cortisol fairly consistently report null findings1. Only two studies, both conceptualizing PPD as a categorical variable and both assessing cortisol post partum, found significant associations with PPD symptoms. Groer & Morgan (2007) found that salivary (but not serum) cortisol was positively associated with PPD symptoms at four to six weeks post partum. Taylor et al. (2009) documented the absence of an expected free cortisol increase within 30 minutes after waking in symptomatic women, indicating an impairment in HPA axis sensitivity to endogenous stimuli (i.e., waking up) in symptomatic women.
Two studies tested the link between cortisol responses to external stimuli and PPD symptoms. Women with more pronounced cortisol responses to an acute psychosocial laboratory stressor between 13 and 31 weeks’ GA had greater PPD symptoms 2 to 27 days following delivery (Nierop et al. 2006). The same study yielded null findings for baseline cortisol levels, suggesting that stress reactivity may be more important in the pathophysiology leading to PPD than baseline hormone levels. However, a small study of 22 new mothers using treadmill exercise as a stressor did not find differences in cortisol reactivity between women with and without PPD symptoms at 6 and 12 weeks post partum (Jolley et al. 2007).
Catecholamines
One study suggests a link between higher peripheral norepinephrine (but not epinephrine) levels and PPD symptoms assessed concurrently between 1 and 11 months post partum in 35 new mothers, 10 of whom used cocaine during pregnancy (Light et al. 2004). The small sample size, the unique population, and the large postpartum time frame in this study make it impossible to draw inferences at this time.
Summary
Emerging evidence indicates that accelerated CRH trajectories and higher levels of CRH in mid-to-late pregnancy may be predictive of PPD symptoms during the first few postpartum months. This association does not seem to extend to other HPA axis hormones. The finding from the β-endorphin literature that associations may emerge for subgroups of individuals holds promise. The majority of studies on postpartum stress hormones yielded null findings. The few studies assessing stimulated HPA axis activity suggest that stress reactivity may be an important area for future research.
Review of Immune/Inflammatory Studies
The immune system’s function is to protect the body from pathogenic organisms and foreign substances by attacking what it identifies as foreign while recognizing and protecting what it identifies as self. During pregnancy, this task is complicated by the genetically distinct fetus, which carries paternal antigens that are foreign to the maternal immune system but that nevertheless should not be attacked by it (Zenclussen 2013). The exact mechanism by which the developing fetus is tolerated by the maternal immune system is not completely understood, but evidence suggests that this process involves a shift in the inflammatory balance of the innate immune system (Corwin & Pajer 2008, Elenkov & Chrousos 2002).
The innate immune system is regulated by an intricate balance of proinflammatory cytokines [e.g., interleukin (IL)-6, IL-1β, tumor necrosis factor-alpha (TNF-α)] that initiate the inflammatory response and anti-inflammatory cytokines (e.g., IL-10) counteracting these effects. Pregnancy is characterized by moderate increases in IL-6 and unchanged levels of IL-10 until 35 weeks’ GA, followed by a sevenfold increase in IL-6 and an approximately 50% increase in IL-10 by the time of delivery (Kronborg et al. 2011). In the first few days after delivery, this proinflammatory state is further enhanced (Maes et al. 2000) and is not unlike that characteristic of depression. Heightened inflammation associated with psychological or biological stressors or sickness is consistently associated with depressive symptoms in nonpuerperal cases of depression (Raison et al. 2006). Because of these parallels, the proinflammatory state that is characteristic of late pregnancy and the early postpartum period has been argued to be of relevance for PPD (Kendall-Tackett 2007). We identified 11 studies investigating the link between immune/inflammatory risk factors and PPD.
Cytokines
Most studies assessed proinflammatory cytokines around the time of delivery and PPD symptoms within the first six weeks post partum. For example, a study of 91 women showed that those whose depressive symptoms increased over the first few days after delivery had higher levels of IL-6 and the IL-6 receptor (Maes et al. 2000). Another small study linked depressive symptoms with increased levels of IL-1β and with the absence of the expected declines in IL-6 concentrations over the first four weeks after birth (Corwin et al. 2008). Boufidou et al. (2009) report in 56 mothers that IL-6 and TNF-α at the time of labor are positively correlated with EPDS scores within the first four days and six weeks post partum, although some associations were significant in cerebrospinal fluid but not in maternal peripheral blood. Although these three studies suggest an exaggerated inflammatory response with PPD symptoms, others, including some with larger sample sizes, suggest no link between PPD symptoms and proinflammatory cytokines during labor (Skalkidou et al. 2009) or within the first months post partum (Groer & Morgan 2007, Okun et al. 2011, Scrandis et al. 2008).
Two publications with overlapping samples, both reporting on approximately 200 women, tested the association between IL-10 (an anti-inflammatory cytokine) and IFN-γ (interferon gamma, a facilitator of the inflammatory response) and symptoms of PPD at four to six weeks post partum. Women scoring in the highest decile of the Profile of Mood States-Depression subscale, an unspecific measure of PPD symptoms, had lower IFN-γ levels and a lower IFN-γ:IL-10 ratio, the latter indicating suppressed cellular immunity among symptomatic mothers (Groer & Morgan 2007). Findings further highlight the importance of breastfeeding, as IFN-γ levels were correlated with depressive symptoms among exclusively formula-feeding mothers but not among exclusively breastfeeding mothers (Groer & Davis 2006). IL-10 was not associated with PPD symptoms in either study.
C-reactive protein
Two studies assessed C-reactive protein (CRP), an overall marker of systemic inflammation. The larger study of 1,053 women found no link between CRP on the second postnatal day and PPD risk assessed concurrently and at 8 and 32 weeks post partum (Albacar et al. 2010). In contrast, a study of 27 women at high risk for PPD showed a positive association between CRP and the likelihood of developing PPD in the first five postnatal days but not between five and six weeks post partum (Scrandis et al. 2008).
Summary
Studies examining inflammatory processes in the context of PPD have yielded inconsistent results. Among the studies yielding significant findings, most provide evidence for an exaggerated proinflammatory response in women with depressive symptoms. However, many studies had very small sample sizes and used unspecific screeners for depression. We surmise that future work on inflammatory processes in the context of PPD symptoms may be one of the most exciting areas of empirical investigation.
Review of Genetic and Epigenetic Studies
Many studies and meta-analytic reviews have investigated the role of genetics in major depression in the general population (Flint & Kendler 2014), and studies on the contribution of epigenetic factors have gained much momentum (Sun et al. 2013, Toyokawa et al. 2012). In comparison, studies addressing genetic and epigenetic contributions to PPD remain rare. The largest of these studies is an association study of 508 polymorphisms in 44 genes conducted on 1,804 new mothers from Spain (Costas et al. 2010). Genes were selected for their proposed role in PPD symptoms, including those involved in the regulation of the HPA axis, in sex hormones, and in the effects of stress on the prefrontal cortex. A haplotype analysis found an association between three single-nucleotide polymorphisms (SNPs) at protein kinase C with PPD during the first 32 weeks after delivery. When depressive symptoms were screened with the EPDS, only a SNP at the transcriptional start site of kininogen 1 remained significant. These sites may be interesting candidates for future research in this area. The remaining studies tested a smaller number of SNPs as possible predictors of PPD symptoms.
Serotonin
The role of the serotonin transporter (5-HTT) is to remove serotonin from the synaptic cleft, thereby determining the magnitude and duration of the postsynaptic serotonin signal and implicating it in psychiatric disease, including depression (Lesch & Mössner 1998). Polymorphisms of the serotonin transporter gene studied in the context of PPD include 5-HTTLPR (5HTT-linked polymorphic region), a functional polymorphism affecting transcriptional activity of the serotonin promoter, and STin2 VNTR, a variable number tandem repeat in the second intron region. The short allele of the 5-HTTLPR has been associated with reduced transcriptional efficiency and lower serotonin expression, implying that carriers of the short allele may be at higher risk of developing depression. For STin2 VNTR, a rare allele with nine repeats has been associated with major depression in the general population (Ogilvie et al. 1996).
Accordingly, in a study of 188 women with a psychiatric history, carriers of the short allele were at increased PPD risk at less than 8 weeks but not at 9 to 24 weeks post partum (Binder et al. 2010). Similarly, in a study of 419 women, Mehta et al. (2012) reported more symptoms among carriers of the short allele at 6 to 8 months post partum when negative life events were present, but at 2 to 3 days no effects were detected. However, other studies suggest that the long allele may confer risk. Sanjuan et al. (2008) report increased PPD risk among carriers of the high-expression genotypes (long allele of 5-HTTLPR, high-activity variant of STin2 VNTR) at 8 weeks but not at 32 weeks among 1,407 women. In another study, the long allele variant of the 5-HTTLPR was also associated with PPD symptoms at 6 weeks but not at 6 months post partum, and only among women who reported previous contact with a psychiatrist (Comasco et al. 2011a,b). In a study of 207 women, Pinheiro et al. (2013) found that PPD risk, but not a PPD diagnosis, between 45 and 90 days post partum was higher among women with the long allele, and this effect was more pronounced in the presence of a stressful life event during pregnancy. Two studies report the absence of a link between the 5-HTTLPR and PPD symptoms (Doornbos et al. 2009, Khabour et al. 2013).
The discrepancy between studies linking the short versus the long allele with symptoms might be reconciled by a study of 1,206 women reporting a crossover interaction between the 5-HTTLPR gene variant and socioeconomic status (SES) (Mitchell et al. 2011). In that study, homozygous carriers of the short allele had higher levels of PPD if they were also low in SES, whereas short allele carriers high in SES showed a low incidence of PPD, similar to that of carriers of the long allele of both high and low SES. The authors suggest that whether or not interactive effects were found in previous studies may have depended on the socioeconomic distribution in that sample.
Three studies measured tryptophan hydroxylase, an enzyme involved in the synthesis of serotonin. One of these studies (Fasching et al. 2012) documented in 361 women that a haplotype block in the promoter region of the tryptophan hydroxylase type 2 isoform, but not a haplotype block in intron 8, was associated with PPD symptoms at 6 to 8 months post partum but not immediately after birth. In contrast, the absence of a link for the tryptophan hydroxylase type 1 (Khabour et al. 2013) and type 2 (Khabour et al. 2013, Pinsonneault et al. 2013) isoforms was reported in studies with similar sample sizes.
COMT and MAO-A
Allelic variations in two other genes of the monoaminergic system that have been associated with major depression in nonpuerperal populations are catechol-O-methyltransferase (COMT), which is involved in dopamine and noradrenalin metabolism, and monoamine oxidase-A (MAO-A), which is involved in serotonin and noradrenaline degradation in the brain (Mandelli & Serretti 2013). Doornbos et al. (2009) reported that the low-activity variants of the MAO-A (uVNTR) and COMT (Val158Met; Met carriers) polymorphisms were associated with increased depressive symptoms at 6 but not 12 weeks post partum, an effect that was particularly pronounced among carriers of both low-activity variants. The key finding was replicated by Comasco et al. (2011b) and further extended such that a multivariate model indicated that 30% of the variance in PPD could be explained by COMT Val158Met (Met/Met genotype), previous contact with a psychiatrist, and maternity stressors. Another study provides further confirmation that the low-activity (Met/Met) genotype is associated with higher PPD risk (Alvim-Soares et al. 2013). Finally, an association of MAO-A and COMT with PPD symptoms was observed in an additive model (Pinsonneault et al. 2013).
Estrogen receptor
As discussed in the section on endocrine factors, estrogens are implicated in depression, in part by influencing serotonin transmission (Bethea et al. 2002). Two studies tested the role of the estrogen receptor gene (ESR1) in PPD, one of which is the above-mentioned study of 1,804 women that examined the role of 508 SNPs from 44 genes in PPD (Costas et al. 2010). Of the seven polymorphisms that initially emerged as significant, four were located on introns four and five of the ESR1 receptor; however, findings did not hold after statistical correction for multiple comparisons. Another study found a link between the ESR1 (TA repeat) and a PPD diagnosis within the first 12 weeks after birth, as well as an interaction between the ESR1 (TA repeat) and the 5-HTT (5-HTTLPR) with PPD symptoms (Pinsonneault et al. 2013).
Oxytocin
One study investigated the association between PPD and three polymorphisms on the oxytocin peptide gene and the oxytocin receptor gene (Mileva-Seitz et al. 2013). Women who as children perceived their own care to be of high quality scored lower on depression at six months post partum, in particular if they were also carriers of the C/C variant (rs2740210) and G/G variant (rs4813627) of the two oxytocin peptide gene polymorphisms.
Glucocorticoids and CRH
One study of 140 women investigated the role of two polymorphisms of the glucocorticoid receptor gene and three polymorphisms of the CRH receptor 1 gene at two to eight weeks post partum (Engineer et al. 2013). Findings suggest higher PPD risk exists among carriers of the glucocorticoid receptor BclI (C/G) and CRH receptor CRHR1 (A/G) minor allele carriers, with more pronounced effects for carriers of both high-risk alleles. Findings also indicate an overrepresentation of the C-G-T haplotype of the CRHR1 among women with EPDS scores suggestive of PPD.
Brain-derived neurotropic factor
The brain-derived neurotrophic factor (BDNF) system plays an important role in many neuronal functions including the regulation of synaptic plasticity. It interacts with the serotonin system, and the BDNF polymorphism Val66Met has been associated with nonpuerperal depression (Martinowich & Lu 2008). Two studies tested the link between the Val66Met polymorphism and PPD. Neither Figueira et al. (2010) in a sample of 227 women nor Comasco et al. (2011a) in a sample of 219 women detected differences in the genotype distribution between women with and without depressive symptoms. However, the latter study suggests a seasonal effect, such that Met allele carriers were more likely to develop symptoms at six weeks post partum if they delivered in fall or winter, which may hint toward an involvement of BDNF in seasonal affective disorders.
Period 2
The Period 2 (Per2) gene plays an important role in regulating the circadian rhythm, which is often disrupted in patients with depression (Monteleone & Maj 2008). The only study we identified that tested a link between the polymorphism Per2 10870 and PPD suggests the absence of an association (Comasco et al. 2011a).
Epigenetic studies
Epigenetics refers to the study of traits that are heritable but environmentally modifiable by mechanisms that are not DNA encoded, such as DNA methylation and histone modification (Kim et al. 2009, Toyokawa et al. 2012). Recent epigenetic work has implicated epigenetic processes in the pathophysiology of major depression and is beginning to reshape our understanding of depression by incorporating epigenetic expressions of complex life experiences into our research designs (Sun et al. 2013). This approach has not yet been taken much advantage of in research on PPD, but it has valuable integrative potential. One elegant translational study cross-referenced DNA methylation changes observed in mice responsive to estrogen treatment with DNA methylation characteristics of 51 human pregnant women (Guintivano et al. 2014). Findings indicate increased estrogen-mediated DNA methylation change in women who receive a PPD diagnosis within four weeks post partum and point to two genes related to estrogen signaling (promoter regions of HP1BP3 and TTC9B) as significantly associated biomarkers. The authors suggest that women with PPD may have an increased sensitivity to epigenetic change related to estrogen but may well have comparable circulating estrogen levels, an observation that is in line with the findings in our review.
Summary
Findings point to possible effects of polymorphic variations in candidate genes within the monoaminergic system, but also for the estrogen receptor, the oxytocin peptide, the glucocorticoid receptor, and the CRH receptor 1 genes. There is no definitive answer as to whether the short or long allele of the 5-HTTLPR is associated with PPD risk and under which conditions. The study of epigenetic factors in the pathophysiology of PPD holds exceptional promise in further elucidating these complex associations.
PSYCHOSOCIAL PREDICTORS OF POSTPARTUM DEPRESSION
Psychosocial research on PPD has a much longer history than research on biological factors has and is thus a much larger literature as well. The many studies on psychological factors tend to cluster into two groups, those on stress and those on interpersonal factors. Our search resulted in the identification of 151 studies with psychosocial predictors of PPD. Because of the size of this literature, we focus this section on the methodologically stronger studies and on areas of research that have been less well described previously. We summarize all other findings briefly and provide study ID numbers that refer the reader to the detailed description of each study in the table in online Supplement A.
Review of Stress Studies
In parallel to the abrupt changes occurring in maternal physiology after delivery are numerous sudden changes in a woman’s roles and responsibilities, including the demands of caring for a newborn. Past perspectives on PPD emphasize stress as a risk factor for depression after the birth of a child, particularly when stress is paired with other psychological, social, and biological vulnerabilities (Halbreich 2005, O’Hara et al. 1991). Previous reviews have identified psychosocial stress as one of the most consistent predictors of PPD (Beck 2001, O’Hara 2009, Robertson et al. 2004). A meta-analysis of publications between 1990 and 2000 concluded that childcare stress and life stress had moderate effects on PPD (Beck 2001). Between 2000 and 2013, 44 longitudinal and 50 cross-sectional studies tested the association between psychosocial stress and PPD or depressive symptoms.
Stress is defined as demands that are appraised as personally significant and as taxing or exceeding the resources of the individual (Lazarus & Folkman 1984). Researchers have most frequently measured major life events but have also studied daily hassles, catastrophic events, parenting or childcare stress, chronic strain, and general “perceived stress.” Some studies included more than one stress measure, and many tested multivariate models using a broader set of psychosocial variables (e.g., social support, self-esteem, marital quality). To organize our review, studies were divided into those measuring episodic stressors (life events, catastrophic events, daily hassles) and those measuring chronic stress (parenting stress, perceived stress, chronic strain).
Episodic stressors
The literature on episodic stressors and PPD symptoms includes studies on stressful life events, catastrophic events, and daily hassles.
Stressful life events
By far, the largest number of psychosocial studies focuses on life events. We found 21 well-controlled longitudinal studies2 of stressful life events and PPD, of which about half reported significant associations of prenatal stressful life events with subsequent PPD or depressive symptoms (#87, 118, 165, 168, 196, 197, 208) or between stressful life events assessed during the early postpartum period and later depressive symptoms (#57, 77, 129). The other half of the studies, however, reported nonsignificant effects of stressful life events or found univariate associations that did not hold up in multivariate, controlled analyses (e.g., #61, 105, 133, 135, 149, 195, 201). Notably, three of those studies examined clinically diagnosed PPD as the outcome variable (#107, 154, 179), and three had large samples (#148, 154, 179). The absence of consistent results in this large group of well-controlled studies suggests that confounding variables may account for the associations between stressful life events and PPD in many studies, which is a conclusion missed in prior reviews.
Another possibility is that different types of life events may have differential effects on the onset of PPD. A prospective study of 1,035 urban South African women indicated that the association of life events with probable PPD depended upon the severity of the event such that exposure to extreme societal stressors (e.g., witnessing a violent crime, being in danger of being killed) was a significant predictor, whereas milder marital and economic stressors were not (Ramchandani et al. 2009). No other studies disaggregated different types of stressful life events.
Catastrophic events
Fewer studies tested the impact of catastrophic events such as hurricanes or earthquakes on the occurrence of PPD symptoms. All reported a significant association between more severe experiences of a natural disaster and greater likelihood of concurrent (Qu et al. 2012) or subsequent elevated PPD symptoms (Ehrlich et al. 2010, Harville et al. 2009, Hibino et al. 2009). From these, we may conclude that long-term consequences of catastrophic events (e.g., loss of income, property damage) are more closely associated with PPD than exposure to the event itself, a conclusion worth noting for future studies on this issue.
Daily hassles
Only three studies assessed minor episodic stressors termed daily hassles. A well-controlled study of 12,361 Australian women showed that women reporting moderate levels of daily hassles around 25 weeks’ GA had higher EPDS scores at 6 weeks post partum compared to women reporting minimal daily hassles (Milgrom et al. 2008). The other two studies were much smaller and reported either positive correlations (Honey et al. 2003a,b) or null findings (Da Costa et al. 2000).
Chronic stressors
The literature on chronic stressors and PPD symptoms includes studies on parenting stress, perceived stress, and chronic strain.
Parenting stress
Parenting stress and childcare stress refer to a perceived imbalance between the demands of parenting or caring for young children and available resources. The vast majority of cross-sectional studies on general parenting stress reported significant associations with concurrent depressive symptoms (#53, 58, 73, 89, 102, 113, 133, 134, 143, 169, 175, 184) or a PPD diagnosis (#154), whereas one study of 139 women found that mothers with elevated EPDS scores did not report greater childcare stress than nondepressed mothers reported (McGrath et al. 2008). Only two studies assessed the prospective effect of parenting or childcare stress on a subsequent clinical diagnosis of PPD. Parenting stress at 6 weeks post partum in 106 adolescent mothers was a risk factor for both PPD and subclinical depression at 3 and 6 months post partum (Venkatesh et al. 2014), but in a smaller study, no such effects were found (Kim et al. 2008).
Some studies investigated specific parenting stressors, such as caring for an infant with temperamental difficulties, colic, or inconsolable or excessive crying. Most found significant associations between mother reports of infant temperamental difficulty and greater depressive symptoms (#58, 71, 92, 102, 144, 147), although a few found none (#156, 184). Two other studies reported significant associations between infant colic or excessive crying and elevated maternal depressive symptoms (Radesky et al. 2013, Stock et al. 2013). Finally, a large study of US mothers reported that infant colic was associated with greater odds of elevated PPD symptoms between two and six weeks post partum in Hispanic and white mothers but not in African American mothers (Howell et al. 2005, 2006). This last study highlights the importance of taking race/ethnicity into account to get a fully biopsychosocial picture of PPD.
Perceived stress
Perceived stress refers to feeling generally overwhelmed and unable to cope (Cohen et al. 1983). The majority of cross-sectional studies find associations between perceived stress and levels of PPD symptoms (#55, 64, 74, 95, 131, 138, 140, 204), although null findings exist (#130, 199). The results of the five existing prospective investigations of perceived stress are more equivocal with three studies reporting significant effects in well-controlled analyses (Corwin et al. 2005, Dennis & Ross 2006a, Rodríguez et al. 2010) and two with null findings (Leung et al. 2005, Monk et al. 2008).
Chronic strain
Another set of studies explored the effects of chronic strain on PPD symptoms. For example, a study of 817 employed women reported a cross-sectional association between greater psychological work demands and greater depressive symptoms at 11 weeks post partum (Dagher et al. 2009). In follow-up prospective analyses, Dagher and colleagues (2011) found associations of higher total workload and lower job flexibility with higher depressive symptoms. In three additional studies, unfavorable job conditions including lack of job security and short family leave times were associated with greater odds of elevated depressive symptoms (Chatterji & Markowitz 2012, Cooklin et al. 2011, Dennis et al. 2004).
Chronic strains resulting from low SES have also been linked to PPD and PPD symptoms in a handful of studies. For instance, financial stress has been associated with a greater likelihood of PPD (Rich-Edwards et al. 2006) and higher levels of depressive symptoms (Choi et al. 2013) in cross-sectional designs (for an exception, see Horowitz et al. 2005). In a longitudinal study of 179 married women, financial stress assessed during pregnancy predicted higher levels of depressive symptoms at 6 and 12 months post partum, but only among women scoring low on optimism (Grote & Bledsoe 2007). Two other studies associated low subjective SES (Dolbier et al. 2013) and food insecurity (Dewing et al. 2013) with PPD symptoms.
Three studies examined multiple chronic stressors. In one study of 1,376 Pacific Island mothers measuring 30 possible predictors, stress due to insufficient food, household income less than $40,000, and difficulty with transportation was associated cross-sectionally with elevated EPDS scores at 6 weeks post partum in controlled analyses (Abbott & Williams 2006). In a smaller study, life difficulties related to finances, marriage, other relationships, and entrapment predicted greater risk of elevated EPDS scores at 6 months post partum (Husain et al. 2012). Finally, in a longitudinal study of 510 urban US pregnant women, three or more of 19 chronic psychosocial stressors such as abuse, financial problems, or homelessness predicted elevated EPDS scores at 6 weeks post partum (Silverman & Loudon 2010).
Summary
Overall, there is mixed evidence that symptoms or diagnoses of PPD are preceded by stressful life events in controlled analyses. However, it appears that the type and severity of major life events are relevant. Relatively few studies have examined interactions of events with predispositions to test a stress diathesis perspective. Perceived stress and chronic strains such as work demands and financial stress more consistently predict PPD. Finally, women with higher parenting stress appear to be at risk of PPD, but more prospective studies are needed to address the directionality of these associations.
Review of Interpersonal Studies
Poor quality interpersonal relationships contribute to risk for depression across the life span (Hammen 2003) and have been implicated in the etiology of PPD (Hopkins et al. 1984). Specifically, there is consensus among systematic and meta-analytic reviews that social support and marital functioning influence postpartum distress (Beck 2001, O’Hara & Swain 1996, Robertson et al. 2004). However, there is a lack of specificity regarding the interrelated components of interpersonal functioning underlying these associations, and the mechanisms by which they act are still unclear. Furthermore, there is great variability in conceptualization, measurement, and analytic approach across studies. To address this gap, we differentiate various aspects of interpersonal relationships (both protective and risk producing) involved in PPD: marital relationship status, several aspects of social support, relationship quality, adult attachment style, and family relationships. Although a number of studies have also focused on network size and PPD symptoms, we do not review them here (but a brief summary of the findings is provided in the Network Size sidebar) (see also Supplement C; follow the Supplemental Material link from the Annual Reviews home page at http://www.annualreviews.org).
NETWORK SIZE.
Thirteen studies tested whether the number of people in a woman’s social network is related to PPD symptoms, and these studies are described in detail in Supplement C. Some studies suggested that a larger social network and a larger number of relationships that are close in nature are protective. Many results are attributable to the dichotomy between none versus one or more supportive others. A few preliminary studies indicated that specific subgroups of women, for example, pregnant adolescents and women living with extended family, may benefit most from larger networks. Still, findings overall were mixed, and some associations became null after controlling for relevant variables such as stress or self-esteem. Quality and quantity of network connections are most likely to interact in their effects on postpartum affect such that when women are satisfied with the support they receive, a larger network is protective.
Marital relationship status3
The most commonly studied predictor of PPD is maternal partner relationship status including legal marital status, “defacto” marriage (usually cohabiting), and the presence of any partner relationship with the baby’s biological father. Although often thought to be a critical factor in PPD, marital status in and of itself is of little importance according to two meta-analyses (Beck 1996, O’Hara & Swain 1996), although an update (Beck 2001) found small but significant effects across three studies. In our review, more than one dozen studies found no univariate associations between marital or partner status and depressive symptoms or diagnosis (#53, 69, 77, 79, 80, 91, 100, 115, 117, 119, 125, 154, 159, 166). Nearly as many studies found that women who were married or cohabiting with the baby’s father had fewer PPD symptoms or a lower risk of PPD compared to women separated from their partner or not in a relationship during the first postpartum year (#49, 54, 56, 70, 94, 108, 116, 124, 139, 148, 164, 168, 179), and the effect reversed direction in one study (Kozinszky et al. 2011). None of these effects remained after controlling for potential confounds such as maternal education, employment, parity, relationship quality, stress, and social support.
However, studies on specific subgroups and diverse samples contradict this. For example, being married or in a stable partner relationship was protective in mothers of high-risk infants (Stock et al. 2013), Latina women (Kuo et al. 2004), and ethnically and socioeconomically diverse women (Segre et al. 2007), especially in the early postpartum period and after controlling for other demographic factors. In a study of inner-city ethnic minority women, cohabitating with a partner was protective against continued elevation of symptoms in the first month post partum (Yonkers et al. 2001). Another large, population-based study of marital and cohabitation status found that being unmarried and not cohabiting conferred risk for PPD at five to nine months (Urquia et al. 2013). Thus, relationship status might confer stronger protection for ethnic minority women or those of lower SES. Consistent with this, a representative cohort study of more than 4,000 largely poor and ethnic-minority urban women found that married women had lower rates of PPD at one year post partum in comparison with cohabiting but unmarried women (Akincigil et al. 2010); cohabiting women, in turn, had lower rates of PPD than did women who were noncohabiting, and those not in a relationship with the baby’s father had the highest rates of PPD. Of note, these effects were fully mediated by relationship quality. Thus, relationship status may be a distal predictor of PPD and a proxy for more specific relational factors such as cohabitation and quality. To this point, longer relationship length predicted lower PPD risk and PPD symptoms in a few studies (Schachman & Lindsey 2013, Seimyr et al. 2013, Urquia et al. 2013), with one nonreplication (Honey et al. 2003a). Worth adding is that relationship status sometimes works the opposite way. A study of 108 high-risk adolescents and adults (Figueiredo et al. 2007) found that mothers who lived with the baby’s father were at greater risk for PPD at two to three months post partum after controlling for demographics and prenatal depressive symptoms (see also Rogers et al. 2013).
Social support
Several previous reviews have examined social support as a predictor of PPD and found evidence for a moderate protective effect of higher support levels for adolescents (Reid & Meadows-Oliver 2007) and adult women (Beck 1996, 2001; O’Hara 2009; Robertson et al. 2004). However, the type and source of support examined vary widely across studies, and different components of support appear to have differential influence on PPD with partner support among the strongest predictors (Beck 2001, O’Hara & Swain 1996). Altogether, we identified 87 studies (47 longitudinal, 40 cross-sectional) on this topic.
Perceived support
By far the most commonly studied aspect of social support is perceived support—the belief that support is available if needed—which is thought to be a stable individual difference. Out of 50 studies on perceived support, 23 reported significant negative associations between perceived support and postpartum outcomes that were robust after controlling for demographics, general depression, and relevant other factors. These 23 studies included a large set of longitudinal studies (#63, 82, 88, 113, 125, 135, 154, 166) and nearly twice as many cross-sectional ones (#68, 69, 74, 83, 95, 127, 130, 140, 151, 152, 159, 169, 182, 189, 193). In contrast, no significant associations existed between general social network perceived support and postpartum outcomes in six longitudinal (#76, 92, 117, 180, 186, 196) and two cross-sectional (#54, 167) studies. One anomalous cross-sectional study even reported a significant positive association (#146). Another set of controlled studies with longitudinal (#87, 133, 139, 148, 166) or cross-sectional (#55, 67, 91, 100, 110, 175) designs reported negative associations between perceived support and PPD, although the significant effects diminished with controls such as prenatal depression, stress, or partner violence in the model. These findings suggest that future research should focus on confounds and pathways linking perceived social support to lower PPD since it is well established that it is a major protective factor.
Enacted or received support
The literature on how much support has actually been received by women in the perinatal period is smaller, and results are inconsistent. Several cross-sectional studies with respectable sample sizes found negative associations between received support and depressive symptoms after controlling for stress or support need (#58, 64, 65, 135, 138), but a few other cross-sectional studies found effects that disappeared after controlling for demographics or reported null findings (#115, 116, 138, 171).
Need for support and support satisfaction
A complementary group of studies assessed perceived need for support and whether the support received was satisfactory to meet that need. Two longitudinal and three cross-sectional studies with small-to-moderate sample sizes identified increased need for support as a significant risk factor for PPD (#64, 134, 138, 141, 197). Regarding satisfaction with support and depression severity, nine studies found negative associations (#55–57, 113, 134, 141, 146, 149, 165), five of which remained significant after controlling for prenatal mood (#113, 134, 141, 149, 165). Four additional studies examining support satisfaction had null findings (#71, 117, 143, 197), although these had smaller, relatively homogenous samples and/or methodological limitations. Of note, unmet support needs are also associated with increased likelihood of PPD (#103).
Sources of support
Other studies have examined different specific sources of social support. Global perceived support did not predict PPD after considering the effect of specific relationships when they were compared (Dennis & Letourneau 2007). Further, a woman’s perceptions of reliable tangible assistance from her partner, opportunities to provide support to her partner, and emotional closeness with other mothers were the key factors that predicted PPD at eight weeks post partum. The majority of studies on perceived partner support found significant protective effects (#75–78, 112, 142, 155, 161, 164; but see #108, 205). Received partner support (Fagan & Lee 2010, Leung et al. 2005), satisfaction with partner support (Boyce 2003, Husain et al. 2012, Iles et al. 2011, Kruse et al. 2013, Sheng et al. 2010), and unmet partner support expectations during the transition to parenting (Gremigni et al. 2011) have also been consistently associated with increased PPD risk. Additionally, effective partner support during pregnancy was protective against increases in depressive symptoms from pregnancy to post partum in a diverse sample (Tanner Stapleton et al. 2012). Multivariate models have shown declines in partner support over time (Simpson et al. 2003) and the quality of partner support (Tanner Stapleton et al. 2012) to mediate effects of other social and relationship factors on PPD in moderately sized samples. Thus, fairly consistent evidence from many studies indicates partner support in various forms is beneficial.
One large population study found both partner and family support predicted lower PPD risk at six months after birth controlling for partner status and sociodemographics (Rich-Edwards et al. 2006). Support from the woman’s family, especially her mother, may have a protective influence. Out of 13 studies addressing family support (including mother, mother-in-law, and general family), 10 found significant negative associations (#51, 77, 104, 105, 108, 117, 134, 161, 171, 176) and 3 found no effect on PPD risk despite relatively large samples (#75, 123, 142). Three studies combining low perceived support from family and friends also found significant univariate associations with elevated PPD symptoms and risk (Ho et al. 2013, Ramchandani et al. 2009, Rich-Edwards et al. 2006). However, social support from other women with children or from friends was not associated with PPD symptoms after controlling for other risk factors (Dennis et al. 2004, Secco et al. 2007, Siu et al. 2012). Of note, in some cultures that differentially value male versus female children, female infant sex (Xie et al. 2009) and in-law preference for a male child (Gao et al. 2009) have also been associated with increased PPD risk, possibly due in part to lower family support.
Partner relationship quality
Partner relationship quality includes both measures of relationship satisfaction and other measures of relationship quality. Findings are mixed for the influence of partner relationship satisfaction during pregnancy on PPD symptoms. Four large studies (Escribà-Agüir & Artazcoz 2011, Kruse et al. 2013, Ramchandani et al. 2009, Siu et al. 2012) found that greater relationship satisfaction predicted fewer PPD symptoms, after adjusting for demographic and psychosocial covariates. Also, low partner satisfaction in the third trimester has been linked to PPD at 6 to 8 months post partum (Mehta et al. 2012). Other studies, however, found no effect of partner relationship satisfaction during pregnancy on PPD in the first three months after delivery (DaCosta et al. 2000, Feeney et al. 2003, Gremigni et al. 2011, Grussu & Quatraro 2009, Mohammad et al. 2011) or effects did not withstand controlled analyses (Kim et al. 2008, Oppo et al. 2009).
Many additional studies examined postpartum partner relationship quality in general or specific aspects such as communication, relationship depth, and low control or constraints by the partner. This work has consistently linked stronger partner relationships with PPD risk or symptoms across the first year after birth (#49, 51, 53, 56, 57, 69, 87, 106, 112, 123, 137, 145, 154, 155, 169, 175, 191, 197; but see 147 for an exception). These associations remained significant after controlling for maternal prenatal mood and demographic factors in most of these studies.
Perinatal declines in relationship satisfaction and quality may contribute to increases in maternal PPD symptoms over time. Two prospective studies (Diaz et al. 2007, Zelkowitz et al. 2008) and one retrospective cross-sectional study (McVey & Tuohy 2007) showed that women with declines on these relationship variables from pregnancy to post partum had increased depressive symptoms compared to women whose relationship quality was stable or increased (see also Whisman et al. 2011). More research on temporal ordering of effects permitting causal inferences is needed.
Adult attachment style
Adult attachment style is defined as the tendency to feel more or less secure about close relationships and is a relatively stable individual difference (Pietromonaco et al. 2013) that has been thought to influence PPD or depressive symptoms. Close to one dozen studies report significant positive associations between less secure attachment style (anxious/fearful, preoccupied, or ambivalent attachment or interpersonal sensitivity) and increased PPD symptoms (#54, 92, 98, 119, 128, 142, 145, 157, 178, 183, 191). Women’s attachment security has also been studied in conjunction with partner support in predicting PPD. Two studies found that the effect of lower attachment security on depressive symptoms at six to eight weeks post partum was at least partially mediated by lower satisfaction with partner support and ratings of support effectiveness (Iles et al. 2011, Tanner Stapleton et al. 2012). During the transition to parenthood, women’s relationship-specific anxiety predicted more depressive symptoms at six weeks, independent of the effects of many related relationship, infant, and maternal factors (Feeney et al. 2003). Ambivalent attachment also may interact with particular negative spouse behaviors to predict depressive symptoms in married couples during the transition to parenthood (Simpson et al. 2003). Thus, adult attachment style appears to influence risk for PPD both directly, perhaps due to underlying negative cognitions, and via contemporaneous relationship functioning.
A few studies have investigated precursors to women’s attachment style or later family support such as parental caregiving during a mother’s own childhood. Low levels of maternal and/or paternal care and emotional support during childhood have been consistently associated with PPD symptoms (#66, 142, 145, 148, 161), but these associations are sometimes attenuated when considering more proximal factors such as prenatal depression or current social support. One study suggested that early family relationship experiences may contribute to a more anxious attachment style, which in turn increases PPD risk (McMahon et al. 2005). Regarding the effects of parental overprotection, a study from Australia suggests that higher maternal overprotection is a risk factor (Matthey et al. 2000), whereas a study from Japan linked higher paternal but lower maternal overprotection with risk (Choi et al. 2013).
Relationship conflict and violence
Five studies found significant associations between higher partner conflict or disagreement and increased depressive symptoms in medium-to-large adolescent samples (Fagan & Lee 2010) and adult samples (Boyce 2003; Dennis & Ross 2006a,b; Giardinelli et al. 2012), but conflict was no longer predictive in one study after considering partner support and involvement (Dennis & Ross 2006b, Fagan & Lee 2010). Two studies also linked arguments with family (Page & Wilhelm 2007) and conflicts with other mothers (Dennis & Ross 2006a) to increases or persistence in PPD symptoms over time.
Intimate partner violence, family violence, and other forms of abuse are more frequent targets of inquiry. Ten longitudinal studies (#51, 79, 88, 126, 139, 153, 163, 166, 185, 186) and eight cross-sectional studies (#60, 91, 96, 97, 100, 101, 109, 136) found intimate partner violence or abuse to be predictive of increased PPD risk and symptoms, with the strongest results for abuse in the past year or during pregnancy rather than past abuse. Experiences of violence from the broader family network also appear to increase PPD risk (Ali et al. 2009, Certain et al. 2008, Cohen et al. 2002), as do lifetime abuse history and other interpersonal violence and trauma experiences (#68, 79, 91, 97, 121, 148, 161–163, 166, 174, 177, 189). These associations were often attenuated when more proximal psychosocial risk factors, such as perinatal experiences, were included.
Summary
Interpersonal risk and protective factors are popular topics of research on PPD. The strongest evidence reviewed here suggests that the quality of a woman’s relationships in the perinatal period is associated with her risk of PPD, with high-quality relationships and social support offering protection against PPD, and conflict-ridden, abusive, or unsupportive relationships conferring risk. A woman’s partner and her own mother emerge as most critical with respect to PPD, whereas evidence is mixed for the roles of friends and other family. In contrast to measures of quality, structural indicators such as partner status and network size (see Supplement C) are less consistently associated with PPD, likely because they are mere proxies of specific relationship processes.
INTEGRATIVE STUDIES INCLUDING BIOLOGICAL AND PSYCHOSOCIAL PREDICTORS OF POSTPARTUM DEPRESSION
Rigorous studies on PPD symptoms assessing both biological and psychosocial predictors are rare. Of the 11 studies identified, five reported results for biological and psychosocial variables in separate statistical analyses (Cheng & Pickler 2009, Corwin et al. 2005, Groer & Morgan 2007, Ingram et al. 2003, Nierop et al. 2006) and thus are not taking full advantage of the data. Such studies can enable us to compare effect sizes with sufficient statistical detail, but they cannot tell us whether or how biological and psychological processes work in concert to predict PPD, nor can they tell us if they represent different levels of the same stress vulnerability pathway.
One study that addressed both psychosocial and biological factors followed 291 women from early pregnancy until 36 weeks post partum (Kuijpens et al. 2001; see Supplement B) and found that both thyroid antibody status and major life events independently predicted PPD at 4 and 12 weeks post partum, but the effects of antibody status did not extend to 20, 28, or 36 weeks post partum, whereas the effects of life events did. An additional study (Figueira et al 2010) found that life stressors, but not the BDNF polymorphism Val66Met, were predictive of PPD in univariate models. However, when life stressors and Val66Met were included in a multivariate regression model, neither emerged as a significant predictor of PPD, and interactive effects were not reported.
Three additional studies on polymorphisms in the serotonin transporter gene tested the stress vulnerability model. Pinheiro et al. (2013) showed that carriers of the long allele of the serotonin transporter gene 5-HTTLPR were more likely to receive a PPD diagnosis and that this association was stronger in the presence of stressful life events during pregnancy. Similarly, significant associations between variations of the 5-HTTLPR and COMT genes and PPD differed depending on the presence of maternity stressors and psychiatric treatment in a study of 275 mothers in Sweden (Comasco et al. 2011a,b). There was also seasonal moderation, which may be tied to seasonal depressive diseases. Mehta et al. (2012) confirmed a link between the 5-HTTLPR gene and PPD in the presence of life events, although in their study short allele carriers emerged as the high-risk group. Altogether, these studies confirm evidence from studies in the general population (Caspi et al. 2003) regarding interactions between polymorphisms in the serotonin transporter gene and stressors in posing risk of depression.
A single study was integrative in the sense that it investigated how social factors become instantiated biologically, raising women’s PPD risk. Hahn-Holbrook and colleagues (2013) followed 210 pregnant women from mid-pregnancy to 8 weeks post partum. They found that changes in placental CRH between 29 and 37 weeks and family social support at 29 weeks of pregnancy independently predicted PPD symptoms at 8 weeks, and the changes in CRH fully mediated the effect of family social support on PPD. In other words, family support appeared to protect against PPD by dampening increases in CRH during pregnancy. These results demonstrate that psychological and biological predictors are not likely to be distinct etiological factors in PPD as commonly thought, but rather, they likely represent different levels of the same etiological pathway.
In sum, very few studies considered both biological and psychosocial predictors of PPD symptoms, and even fewer examined interrelationships among them. Nonetheless, a small, emerging literature shows promise. In particular, the four studies testing gene-environment interactions support the stress vulnerability model, and one further study supports the biopsychosocial model emphasizing the utility of testing mediational relationships.
DISCUSSION OF SYSTEMATIC REVIEW
We systematically reviewed the literature published between 2000 and 2013 on biological (hormones, immune/inflammatory, genetic) and psychosocial (stress, interpersonal) risk factors for PPD. These two literatures have evolved separately and remain remarkably distinct, with rare examples of rigorous, integrative empirical research. Below, we draw conclusions for both literatures and enumerate challenges in advancing the field henceforth.
Lessons Learned from the Biological Literature
The theoretical rationale for the role of various biological factors in the etiology of PPD is fairly convincing (e.g., Halbreich 2005, Hochberg et al. 2003), but empirical support is lacking. At best, results are equivocal, and at worst, the predominance of findings does not support the premises. The key question in evaluating the biological literature is therefore a rather provocative one: Are biological factors largely inconsequential in the etiology of PPD? And, in moving forward, where should researchers focus their attention? We argue that the real challenge facing this field is not the absence of biological effects but rather the need to take a more sophisticated approach in testing theoretical premises. It is not surprising that most studies have failed to yield significant results, and the emerging overall picture is one of inconsistency if one takes into account issues related to PPD theory and research design.
One reason for equivocal results is that most empirical work on the role of biological factors in PPD risk does not carefully test theoretical propositions. For example, withdrawal theories imply that the intricate system of hormonal adaptations established over the course of pregnancy is suddenly disrupted, which challenges the maternal organism to restore a new endocrine balance (e.g., Hochberg et al. 2003). Hormone trajectories during and after pregnancy may well be an important piece of the puzzle, but other factors, in particular receptor sensitivity to the hormonal signal in the target tissue, are at least as relevant but remain largely unstudied. This is of concern because insensitivity to glucocorticoid signals has been suggested as a central mechanism in the development of depression and other stress-related disorders (Raison & Miller 2003). Moving forward, studies testing withdrawal theories should address levels of circulating hormones in combination with measures of tissue sensitivity. Promising approaches may include testing tissue sensitivity using in vitro models (Rohleder 2012), using positron emission tomography scanning to access the hormonal signal received by receptors in key brain areas (see Sacher et al. 2010), or assessing gene transcriptional activity in peripheral blood (Segman et al. 2010).
Furthermore, empirical tests of stress vulnerability models are rare in this literature. These models propose that associations between biological variables and PPD should be more pronounced among high-risk populations, whereas links may be absent in low-risk or mixed-risk samples. The few studies that have tested this model confirm that these moderated associations do exist (Comasco et al. 2011a,b; Mehta et al. 2012; Pinheiro et al. 2013). However, most studies in this field rely on mixed-risk samples. Future work might focus on high-risk populations or consider risk status as a moderator. It should also address genetic variations in other relevant biological systems (e.g., CRH, glucocorticoid, and gonadal systems as well as immune factors, including receptor variations) and psychological risk factors (e.g., life stress, lack of social support, psychiatric history). The inclusion of epigenetic changes related to pregnancy and to various types of stressors also holds great promise in furthering this line of research.
Another issue that may contribute to the many null results is the piecemeal approach commonly taken in biological studies where each measure is tested individually for associations with depression, limiting our ability to examine how biological risk factors interact to contribute to PPD risk. For instance, cortisol may affect the function of other biological systems implicated in depression, for example by altering gonadal and inflammatory processes (Torpy & Chrousos 1996), stimulating increases in placental CRH (Sandman et al. 2006), and by impairing serotonin function (Cowen 2002, Dinan 1994). Systems approaches that take into account interactive processes might help to integrate this fragmented literature and resolve some of the inconsistencies. In a related vein, there is a relative lack of appreciation that pregnancy and the postpartum period are periods of dynamic change. Our review suggests that studies that took a process approach by investigating change were more likely to yield significant findings compared to studies that treated biological measures as a static construct.
Insufficient attention has also been paid to the issue of timing. There is some indication that the most promising time to assess biological measures is not at the end of pregnancy when concentrations are uniformly high in preparation for delivery, but rather earlier in pregnancy (first and second trimesters) when the rate of change in biological measures may be more sensitive to individual differences in vulnerability. Regarding the timing of the assessment of PPD, it has been argued that pregnancy-related biological measures can affect PPD symptoms only as long as they still exert physiological effects (Halbreich 2005). Our review supports the notion that associations between biological measures and PPD are more likely to be detected when symptoms are assessed earlier in the postpartum period. For example, significant familial aggregation was detected when depression onset was defined within four weeks but not when defined within six months of delivery (Forty et al. 2006). The emerging literature on the link between placental CRH trajectories and PPD suggests associations only until three months post partum (Glynn & Sandman 2014). Thus, research design decisions related to the timing of predictor and outcome assessment may obscure true associations.
Few studies have used structural clinical interviews to establish the diagnosis of PPD or at least categorized their sample based on cutoff scores in validated screening instruments. This is likely due to the relatively low sample size in biological studies that typically involve higher financial cost and invasive data collection. To ameliorate the resulting power issue, studies have often treated depressive symptoms as a continuous variable and conducted analyses that presume linear effects even though a threshold model may be superior for understanding PPD etiology. In a related vein, most studies conceptualized PPD as a unidimensional construct instead of distinguishing between subtypes. There is accumulating evidence that HPA axis and inflammatory dysregulations differ between subtypes of depression, such as typical and atypical depression, which may suggest differences in pathophysiological processes (e.g., Gold & Chrousos 2002, Halbreich 2005).
With reference to studies testing the withdrawal model, we propose that the deluge of null findings may be the result of a ceiling effect. Late in pregnancy, hormone levels are extraordinarily high in all women, and all experience a massive perinatal hormonal drop. As such, subtle differences in the magnitude of the decline may not be the primary force driving these effects. Instead, women who are vulnerable to the mood effects of hormonal fluctuations will tend to experience associated mood effects, whereas women without this vulnerability will not, irrespective of where they rank among all women undergoing this transition.
Amid the paucity of significant findings, it is important to note emerging patterns such as the relatively small literatures reporting relationships between PPD and peripheral levels of oxytocin, studies testing interactions between serotonin transporter gene polymorphisms and psychological stressors, and studies assessing CRH trajectories in mid-pregnancy. Looking toward the next decade of PPD research, studies ideally would be designed to explicitly test various iterations of the proposed biopsychosocial models, using prospective study designs and testing genetic, epigenetic, and life history moderators of the relationships between changes in endocrine and inflammatory measures, psychosocial risk and resilience factors, and PPD.
Lessons Learned from the Psychosocial Literature
The psychosocial literature is not a new literature, but it is one that has become much stronger in recent years, permitting much greater scrutiny and elaboration of the findings of earlier reviews. In particular, the nature of this updated review permitted a more refined examination of the specific facets of stress and personal relationships that are most predictive of PPD. Research on stress suggests that women who experience particular types of more severe major stressful life events or catastrophes during pregnancy or after the birth of a child are more likely to develop PPD. Relatively few well-controlled, longitudinal studies have examined the effects of a wide range of chronic strains as yet, but available evidence suggests that two forms, parenting stress and financial strain, contribute to increased risk. Prospective studies of perceived stress report mixed results, and evidence from cross-sectional studies may be due, in part, to confounding with other factors such as negative affect. Future studies should test theory-driven hypotheses about the types of life events that might confer the greatest vulnerability to PPD due to their severity or their potential to create or exacerbate chronic strains (e.g., the stressful event of losing one’s job could result in enduring financial stress). We must also continue to hone in on the specific forms of stress most likely to increase PPD risk, and to determine the physiological, psychological, and interpersonal factors that moderate stress effects. A reasonable candidate is chronic stress.
Regarding interpersonal relationships, there is persuasive evidence that social support satisfaction, partner support, and postpartum relationship satisfaction are protective factors and that high support need, adult attachment anxiety and ambivalence, intimate partner or family violence, and substance abuse in the family confer increased vulnerability to PPD. Preliminary or slightly mixed evidence with modest effect sizes exists for perceived and received social support, family support, childhood relationships with caregivers, nonabusive partner conflict, and lifetime abuse history. Finally, there is little indication that marital or relationship status, network size, or support from peers have any influence on PPD, beyond associations with other indicators of the quality of mothers’ interpersonal relationships. Theoretical formulations suggest that high-quality close relationships serve specific functions during the perinatal transition, such as preserving or enhancing the mother’s self-esteem and confidence in the wake of role transitions and buffering overwhelming daily demands and stress. Focused research on these potential protective functions is much needed.
The study designs and methodologies of psychosocial research have become much more sophisticated in recent years. For example, the use of advanced statistical techniques has provided greater insight into how multiple psychosocial factors work additively and interactively to increase PPD risk. Effects were often reduced when controlling for prenatal depression, suggesting that many factors contributing to PPD risk are not unique to the postpartum period. Indeed, among three studies examining differential prediction of de novo PPD (Giardinelli et al. 2012, Husain et al. 2012, Phillips et al. 2010), only recent life events were associated with postpartum onset.
Researchers have also begun to consider moderators of the effects of stress exposure on postpartum mental health such as social support, optimism, and a few other resilience resources (Cheng & Pickler 2009, Grote & Bledsoe 2007, Hibino et al. 2009). However, these potential protective factors are more frequently conceptualized and tested as separate predictors, and interactive effects have rarely been explored. Too many studies test a large swath of psychosocial factors either as isolated correlates of PPD or in tandem with a host of other demographic, socioeconomic, and obstetric indicators without a clear rationale for inclusion. These studies offer little insight into the potential causal relations between variables. In fact, sophisticated cross-lagged analyses across pregnancy and post partum suggest that, for example, poor partner relationship quality may be a consequence rather than cause of PPD (Whisman et al. 2011). Greater attention to the timing of predictors and outcomes would help to clarify the development or mitigation of risk across the dynamic context of the perinatal period.
A great deal of methodological variability exists with respect to measures, samples, and methods across psychosocial studies—perhaps even more so than in biological paradigms—which greatly hinders conclusions about the predictive utility of various psychosocial risk measures. Nearly all of the reviewed studies relied on questionnaire screeners rather than clinical diagnoses, and widely varying cutoff points for dichotomizing continuous symptom scores limit conclusions about PPD as a diagnostic construct. Researchers also often fail to select well-validated measures to assess psychosocial variables. The use of the best available measures (e.g., interview methods for episodic stress measurement) in future studies may help to reconcile inconsistent evidence regarding the effects of psychosocial factors on PPD.
The co-occurrence of maternal and paternal PPD has not been studied much, nor has the influence of having a partner who is depressed or has other psychopathology. One meta-analysis (Paulson & Bazemore 2010) identified a moderate overall concordance between paternal and maternal incidence of depression in the prenatal or postpartum period. Certainly, the many interpersonal contributors to and consequences of depression such as assortative mating, negative interaction patterns, depression contagion, or shared life stressors that operate in close relationships (e.g., Hammen 2003) apply to the postpartum context and may be exacerbated due to the many challenges couples face during this time. Additionally, despite documented declines in marital satisfaction across the transition to parenthood (e.g., Lawrence et al. 2008), only recently have researchers examined directly the interrelationships of longitudinal changes between relationship satisfaction and PPD, and these studies have used primarily white, married samples.
Although our review presented studies examining stress and interpersonal factors in separate sections, these psychosocial factors are clearly intertwined. For example, divorce or relationship strain can result in decreased social support, leading to both multiple life stressors and depleted interpersonal resources. Additionally, the impact of stressful life events may depend on the availability of other resources (friends and family, SES and income) that can moderate the interpersonal effects of the stressor. Furthermore, interpersonal risk factors for PPD might also be considered sources of stress and are indeed conceptualized in this way by some researchers. For example, the stress of social isolation that was found to predict PPD (Ramchandani et al. 2009) is analogous to network size. There is also measurement confounding between the two sets of constructs; for instance, some measures that purport to measure stress include items intended to assess the level of social support received from the spouse and other individuals. Thus, stress and interpersonal factors are distinct but sometimes overlapping constructs and should be studied with great care to avoid conceptual and measurement confounding.
Nevertheless, substantial evidence suggests that high levels of stress, low levels of social resources, and strained personal or close relationships increase a woman’s risk of PPD. These risk factors have implications for maternal well being and infant and child development regardless of whether they result in the onset of clinical PPD. Therefore, the important question at this point is not whether psychosocial factors influence postpartum mental health, as undoubtedly they do, but rather why and how, and what can be done to provide women and families with the support and resources they need during the first year of their children’s lives. Among the steps that can be taken to promote use of these basic findings are to identify, assess, and build up resilience resources that allow women to better manage increasing demands and changing relationships during the transition to parenthood (Dunkel Schetter & Dolbier 2011). We hope this review provides a stronger basis for translating current knowledge regarding psychosocial risk factors into evidence-based interventions to prevent and treat PPD. (See Interpersonal Relationship Intervention Studies sidebar.)
INTERPERSONAL RELATIONSHIP INTERVENTION STUDIES.
Randomized controlled intervention studies in relationship science support some of the observational findings reviewed here. For example, individual and group-based interpersonal therapy, which focuses on navigating role transitions and resolving conflicts with close others, has shown moderate success in reducing PPD (Cuijpers et al. 2008). Similarly, focused efforts to prevent PPD among high-risk women by interventions that increase social support from significant others or paraprofessionals have shown promising results (e.g., Fraser et al. 2000), whereas broader intervention efforts to increase social support have shown little benefit (Dennis 2005). More efforts should be directed toward theory building in this area (Pietromonaco et al. 2013) and translating theoretically and methodologically strong observational studies of stress and social relationships into experimental designs that can shed light on causal paths.
A Call for Integration
Two distinct literatures exist on PPD—a biological one and a psychosocial one. Although promising integrative conceptual frameworks exist (Halbreich 2005, O’Hara et al. 1991), integrative empirical studies are rare. This is not surprising insomuch as integrative research is much more challenging to conduct than discipline-specific work. Obstacles can also be of a more pragmatic nature, such that the cost of collecting and assaying biological measures may be prohibitive regarding sample sizes needed for adequate power to test interactions with psychosocial factors.
In light of these challenges, it is fair to ask: Do the benefits of integrative research outweigh this substantial list of obstacles? Our answer is unequivocally “yes” because when cross-disciplinary perspectives are applied to complex problems of a biopsychosocial nature, then novel and useful findings are more likely to emerge. Equally important, understanding how psychosocial factors interact with biological causes of PPD within sociocultural contexts can improve the quality of any translational work. For example, if family support is protective against PPD because it acts as a buffer against increases in CRH at the end of pregnancy (Hahn-Holbrook et al. 2013), then support interventions designed to prevent PPD may need to be undertaken in early pregnancy to be effective.
A fruitful place to begin the next stage of integrative efforts is to examine existing theories to determine how useful they are in guiding integrative work and to develop them further. Second, we recommend that future studies test more complex models of moderated and mediated effects. For example, one might build upon the studies reporting effects of both stress biology and stressful life events on PPD symptomatology to test theory-driven hypotheses regarding mechanisms. Third, as we suggested, research teams able to mount such studies are most likely to be interdisciplinary. Finally, these efforts are sure to further elucidate the biopsychosocial processes underlying PPD symptoms and disorders and thereby will contribute to their future prevention and treatment.
Supplementary Material
Acknowledgments
Drs. Tanner Stapleton, Guardino, and Hahn-Holbrook were supported by NIMH training fellowships as pre- or postdoctoral fellows through NIMH T32 15750 funding. The authors are grateful to Jacqueline O’Brien for administrative assistance.
Footnotes
These nonsignificant findings are not further discussed here, but studies are included in the table in Supplement A (see table entries #7, 13, 14, 34, 47, 199, 205 for cortisol in pregnancy, entries #34, 202 for the perinatal cortisol drop, and entries #13, 15, 19, 25, 26, 33–35, 43, 199, 202 for cortisol post partum).
Some additional studies met inclusion criteria but were either cross-sectional or longitudinal and not well controlled (#54, 56, 58, 67, 69, 75, 80, 85, 90, 124, 132, 151, 152, 157, 167, 170, 189, 198, 203, 204, 206, 210). Most of these studies suggest positive associations between stressful life events and PPD symptoms. They are included in the table in Supplement A, but because they are less conclusive than well-controlled prospective studies, they are not further discussed here.
As marital or partner status is often included as a covariate in studies addressing a wide variety of topics, studies were included here if they examined this variable explicitly (e.g., mentioned partner status in title, abstract, or keywords) or included marital status as a possible covariate in conjunction with other psychosocial variables of interest.
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Abbott MW, Williams MM. Postnatal depressive symptoms among Pacific mothers in Auckland: prevalence and risk factors. Aust N Z J Psychiatry. 2006;40:230–38. doi: 10.1080/j.1440-1614.2006.01779.x. [DOI] [PubMed] [Google Scholar]
- Ahokas A, Kaukoranta J, Aito M. Effect of oestradiol on postpartum depression. Psychopharmacology (Berl) 1999;146:108–10. doi: 10.1007/s002130051095. [DOI] [PubMed] [Google Scholar]
- Akincigil A, Munch S, Niemczyk K. Predictors of maternal depression in the first year postpartum: marital status and mediating role of relationship quality. Soc Work Health Care. 2010;49:227–44. doi: 10.1080/00981380903213055. [DOI] [PubMed] [Google Scholar]
- Albacar G, Sans T, Martin-Santos R, Garcia-Esteve L, Guillamat R, et al. Thyroid function 48h after delivery as a marker for subsequent postpartum depression. Psychoneuroendocrinology. 2010;35:738–42. doi: 10.1016/j.psyneuen.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Ali NS, Ali BS, Azam IS. Post partum anxiety and depression in peri-urban communities of Karachi, Pakistan: a quasi-experimental study. BMC Public Health. 2009;9:384. doi: 10.1186/1471-2458-9-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvim-Soares A, Miranda D, Campos SB, Figueira P, Romano-Silva MA, Correa H. Postpartum depression symptoms associated with Val158Met COMT polymorphism. Arch Women’s Ment Health. 2013;16:339–40. doi: 10.1007/s00737-013-0349-8. [DOI] [PubMed] [Google Scholar]
- Am. Psychiatr. Assoc. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: Am. Psychiatr. Publ; 2000. text rev. [Google Scholar]
- Am. Psychiatr. Assoc. Diagnostic and Statistical Manual of Mental Disorders. 5 Washington, DC: Am. Psychiatr. Publ; 2013. [Google Scholar]
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293–98. doi: 10.1016/0002-9378(80)90912-6. [DOI] [PubMed] [Google Scholar]
- Bao AM, Meynen G, Swaab DF. The stress system in depression and neurodegeneration: focus on the human hypothalamus. Brain Res Rev. 2008;57:531–53. doi: 10.1016/j.brainresrev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Battin DA, Marrs RP, Fleiss PM, Mishell DR., Jr Effect of suckling on serum prolactin, luteinizing hormone, follicle-stimulating hormone, and estradiol during prolonged lactation. Obstet Gynecol. 1985;65:785–88. [PubMed] [Google Scholar]
- Beck CT. A meta-analysis of predictors of postpartum depression. Nurs Res. 1996;45:297–303. doi: 10.1097/00006199-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Beck CT. Predictors of postpartum depression: an update. Nurs Res. 2001;50:275–85. doi: 10.1097/00006199-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Biegon A, Reches A, Snyder L, McEwen BS. Serotonergic and noradrenergic receptors in the rat brain: modulation by chronic exposure to ovarian hormones. Life Sci. 1983;32:2015–21. doi: 10.1016/0024-3205(83)90053-x. [DOI] [PubMed] [Google Scholar]
- Bina R. The impact of cultural factors upon postpartum depression: a literature review. Health Care Women Int. 2008;29:568–92. doi: 10.1080/07399330802089149. [DOI] [PubMed] [Google Scholar]
- Binder EB, Newport JD, Zach EB, Smith AK, Deveau TC, et al. A serotonin transporter gene polymorphism predicts peripartum depressive symptoms in an at-risk psychiatric cohort. J Psychiatr Res. 2010;44:640–46. doi: 10.1016/j.jpsychires.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch M, Daly RC, Rubinow DR. Endocrine factors in the etiology of postpartum depression. Compr Psychiatry. 2003;44:234–46. doi: 10.1016/S0010-440X(03)00034-8. [DOI] [PubMed] [Google Scholar]
- Bloch M, Schmidt PJ, Danaceau M, Murphy J, Nieman L, Rubinow DR. Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry. 2000;157:924–30. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- Boufidou F, Lambrinoudaki I, Argeitis J, Zervas IM, Pliatsika P, et al. CSF and plasma cytokines at delivery and postpartum mood disturbances. J Affect Disord. 2009;115:287–92. doi: 10.1016/j.jad.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Boyce PM. Risk factors for postnatal depression: a review and risk factors in Australian populations. Arch Women’s Ment Health. 2003;6(Suppl 2):S43–50. doi: 10.1007/s00737-003-0005-9. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:766–76. doi: 10.1016/j.pnpbp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–89. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Certain HE, Mueller M, Jagodzinski T, Fleming M. Domestic abuse during the previous year in a sample of postpartum women. J Obstet Gynecol Neonatal Nurs. 2008;37:35–41. doi: 10.1111/j.1552-6909.2007.00200.x. [DOI] [PubMed] [Google Scholar]
- Chatterji P, Markowitz S. Family leave after childbirth and the mental health of new mothers. J Ment Health Policy Econ. 2012;15:61–76. [PubMed] [Google Scholar]
- Chatzicharalampous C, Rizos D, Pliatsika P, Leonardou A, Hasiakos D, et al. Reproductive hormones and postpartum mood disturbances in Greek women. Gynecol Endocrinol. 2011;27:543–50. doi: 10.3109/09513590.2010.501886. [DOI] [PubMed] [Google Scholar]
- Cheng C-Y, Pickler RH. Effects of stress and social support on postpartum health of Chinese mothers in the United States. Res Nurs Health. 2009;32:582–91. doi: 10.1002/nur.20356. [DOI] [PubMed] [Google Scholar]
- Choi H, Yamashita T, Wada Y, Kohigashi M, Mizuhara Y, et al. Predictors for exacerbation/improvement of postpartum depression—a focus on anxiety, the mothers’ experiences of being cared for by their parents in childhood and borderline personality: a perspective study in Japan. J Affect Disord. 2013;150:507–12. doi: 10.1016/j.jad.2013.04.051. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann Intern Med. 1998;129:229–40. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Schei B, Ansara D, Gallop R, Stuckless N, Stewart DE. A history of personal violence and postpartum depression: Is there a link? Arch Women’s Ment Health. 2002;4:83–92. [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- Comasco E, Sylvén SM, Papadopoulos FC, Oreland L, Sundström-Poromaa I, Skalkidou A. Postpartum depressive symptoms and the BDNF Val66Met functional polymorphism: effect of season of delivery. Arch Women’s Ment Health. 2011a;14:453–63. doi: 10.1007/s00737-011-0239-x. [DOI] [PubMed] [Google Scholar]
- Comasco E, Sylvén SM, Papadopoulos FC, Sundström-Poromaa I, Oreland L, Skalkidou A. Postpartum depression symptoms: a case-control study on monoaminergic functional polymorphisms and environmental stressors. Psychiatr Genet. 2011b;21:19–28. doi: 10.1097/YPG.0b013e328341a3c1. [DOI] [PubMed] [Google Scholar]
- Cooklin AR, Canterford L, Strazdins L, Nicholson JM. Employment conditions and maternal postpartum mental health: results from the Longitudinal Study of Australian Children. Arch Women’s Ment Health. 2011;14:217–25. doi: 10.1007/s00737-010-0196-9. [DOI] [PubMed] [Google Scholar]
- Corwin EJ, Brownstead J, Barton N, Heckard S, Morin K. The impact of fatigue on the development of postpartum depression. J Obstetr Gynecol Neonatal Nurs. 2005;34:577–86. doi: 10.1177/0884217505279997. [DOI] [PubMed] [Google Scholar]
- Corwin EJ, Johnston N, Pugh L. Symptoms of postpartum depression associated with elevated levels of interleukin-1 beta during the first month postpartum. Biol Res Nurs. 2008;10:128–33. doi: 10.1177/1099800408323220. [DOI] [PubMed] [Google Scholar]
- Corwin EJ, Kohen R, Jarrett M, Stafford B. The heritability of postpartum depression. Biol Res Nurs. 2010;12:73–83. doi: 10.1177/1099800410362112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin EJ, Pajer K. The psychoneuroimmunology of postpartum depression. J Women’s Health. 2008;17:1529–34. doi: 10.1089/jwh.2007.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costas J, Gratacos M, Escaramis G, Martin-Santos R, de Diego Y, et al. Association study of 44 candidate genes with depressive and anxiety symptoms in post-partum women. J Psychiatr Res. 2010;44:717–24. doi: 10.1016/j.jpsychires.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Cowen PJ. Cortisol, serotonin and depression: All stressed out? Br J Psychiatry. 2002;180:99–100. doi: 10.1192/bjp.180.2.99. [DOI] [PubMed] [Google Scholar]
- Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–86. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Brannmark JG, van Straten A. Psychological treatment of postpartum depression: a meta-analysis. J Clin Psychol. 2008;64:103–18. doi: 10.1002/jclp.20432. [DOI] [PubMed] [Google Scholar]
- Da Costa D, Larouche J, Dritsa M, Brender W. Psychosocial correlates of prepartum and postpartum depressed mood. J Affect Disord. 2000;59:31–40. doi: 10.1016/s0165-0327(99)00128-7. [DOI] [PubMed] [Google Scholar]
- Dagher RK, McGovern PM, Alexander BH, Dowd BE, Ukestad LK, McCaffrey DJ. The psychosocial work environment and maternal postpartum depression. Int J Behav Med. 2009;16:339–46. doi: 10.1007/s12529-008-9014-4. [DOI] [PubMed] [Google Scholar]
- Dagher RK, McGovern PM, Dowd BE, Lundberg U. Postpartum depressive symptoms and the combined load of paid and unpaid work: a longitudinal analysis. Int Arch Occup Environ Health. 2011;84:735–43. doi: 10.1007/s00420-011-0626-7. [DOI] [PubMed] [Google Scholar]
- Dennis CL. Can we identify mothers at risk for postpartum depression in the immediate postpartum period using the Edinburgh Postnatal Depression Scale? J Affect Disord. 2004;78:163–69. doi: 10.1016/s0165-0327(02)00299-9. [DOI] [PubMed] [Google Scholar]
- Dennis CL. Psychosocial and psychological interventions for prevention of postnatal depression: systematic review. BMJ. 2005;331:15. doi: 10.1136/bmj.331.7507.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis CL, Janssen PA, Singer J. Identifying women at-risk for postpartum depression in the immediate postpartum period. Acta Psychiatr Scand. 2004;110:338–46. doi: 10.1111/j.1600-0447.2004.00337.x. [DOI] [PubMed] [Google Scholar]
- Dennis CL, Letourneau N. Global and relationship-specific perceptions of support and the development of postpartum depressive symptomatology. Soc Psychiatry Psychiatr Epidemiol. 2007;42:389–95. doi: 10.1007/s00127-007-0172-5. [DOI] [PubMed] [Google Scholar]
- Dennis CL, McQueen K. The relationship between infant-feeding outcomes and postpartum depression: a qualitative systematic review. Pediatrics. 2009;123:e736–51. doi: 10.1542/peds.2008-1629. [DOI] [PubMed] [Google Scholar]
- Dennis CL, Ross LE. Depressive symptomatology in the immediate postnatal period: identifying maternal characteristics related to true- and false-positive screening scores. Can J Psychiatry. 2006a;51:265–73. doi: 10.1177/070674370605100501. [DOI] [PubMed] [Google Scholar]
- Dennis CL, Ross LE. Women’s perceptions of partner support and conflict in the development of postpartum depressive symptoms. J Adv Nurs. 2006b;56:588–99. doi: 10.1111/j.1365-2648.2006.04059.x. [DOI] [PubMed] [Google Scholar]
- Dewing S, Tomlinson M, le Roux IM, Chopra M, Tsai AC. Food insecurity and its association with co-occurring postnatal depression, hazardous drinking, and suicidality among women in peri-urban South Africa. J Affect Disord. 2013;150:460–65. doi: 10.1016/j.jad.2013.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz MA, Le HN, Cooper BA, Muñoz RF. Interpersonal factors and perinatal depressive symptomatology in a low-income Latina sample. Cult Divers Ethnic Minor Psychol. 2007;13:328–36. doi: 10.1037/1099-9809.13.4.328. [DOI] [PubMed] [Google Scholar]
- Dinan TG. Glucocorticoids and the genesis of depressive illness. A psychobiological model. Br J Psychiatry. 1994;164:365–71. doi: 10.1192/bjp.164.3.365. [DOI] [PubMed] [Google Scholar]
- Dolbier CL, Rush TE, Sahadeo LS, Shaffer ML, Thorp J. Relationships of race and socioeconomic status to postpartum depressive symptoms in rural African American and non-Hispanic white women. Matern Child Health J. 2013;17:1277–87. doi: 10.1007/s10995-012-1123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doornbos B, Dijck-Brouwer DA, Kema IP, Tanke MA, van Goor SA, et al. The development of peripartum depressive symptoms is associated with gene polymorphisms of MAOA, 5-HTT and COMT. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1250–54. doi: 10.1016/j.pnpbp.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Douma SL, Husband C, O’Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders: reproductive life cycle factors. Adv Nurs Sci. 2005;28:364–75. doi: 10.1097/00012272-200510000-00008. [DOI] [PubMed] [Google Scholar]
- Dunkel Schetter C, Dolbier C. Resilience in the context of chronic stress and health in adults. Soc Personal Psychol Compass. 2011;5:634–52. doi: 10.1111/j.1751-9004.2011.00379.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood JG, Kemp LA, Jalaludin BB, Phung HN. Neighborhood adversity, ethnic diversity, and weak social cohesion and social networks predict high rates of maternal depressive symptoms: a critical realist ecological study in South Western Sydney, Australia. Int J Health Serv. 2013;43:241–66. doi: 10.2190/HS.43.2.d. [DOI] [PubMed] [Google Scholar]
- Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus-pituitary-adrenal axis. Biol Psychol. 2001;57:141–52. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Ehrlich M, Harville E, Xiong X, Buekens P, Pridjian G, Elkind-Hirsch K. Loss of resources and hurricane experience as predictors of postpartum depression among women in southern Louisiana. J Women’s Health. 2010;19:877–84. doi: 10.1089/jwh.2009.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Chrousos GP. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann N Y Acad Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- Engineer N, Darwin L, Nishigandh D, Ngianga-Bakwin K, Smith SC, Grammatopoulos DK. Association of glucocorticoid and type 1 corticotropin-releasing hormone receptors gene variants and risk for depression during pregnancy and post-partum. J Psychiatr Res. 2013;47:1166–73. doi: 10.1016/j.jpsychires.2013.05.003. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Gueorguieva R, Czarkowski KA, Stiklus S, Sellers E, et al. Preliminary evidence of reduced occipital GABA concentrations in puerperal women: a 1H-MRS study. Psychopharmacology (Berl) 2006;186:425–33. doi: 10.1007/s00213-006-0313-7. [DOI] [PubMed] [Google Scholar]
- Escriba-Agüir V, Artazcoz L. Gender differences in postpartum depression: a longitudinal cohort study. J Epidemiol Community Health. 2011;65:320–26. doi: 10.1136/jech.2008.085894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan J, Lee Y. Perceptions and satisfaction with father involvement and adolescent mothers’ postpartum depressive symptoms. J Youth Adolesc. 2010;39:1109–21. doi: 10.1007/s10964-009-9444-6. [DOI] [PubMed] [Google Scholar]
- Fasching PA, Faschingbauer F, Goecke TW, Engel A, Haberle L, et al. Genetic variants in the tryptophan hydroxylase 2 gene (TPH2) and depression during and after pregnancy. J Psychiatr Res. 2012;46:1109–17. doi: 10.1016/j.jpsychires.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Feeney J, Alexander R, Noller P, Hohaus L. Attachment insecurity, depression, and the transition to parenthood. Pers Relatsh. 2003;10:475–93. [Google Scholar]
- Feldman R, Granat A, Pariente C, Kanety H, Kuint J, Gilboa-Schechtman E. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry. 2009;48:919–27. doi: 10.1097/CHI.0b013e3181b21651. [DOI] [PubMed] [Google Scholar]
- Figueira P, Fernandes Malloy-Diniz L, Aurelio Romano-Silva M, Silva Neves F, Correa H. Postpartum depression and comorbid disorders: frequency and relevance to clinical management. Arch Women’s Ment Health. 2009;12:451. doi: 10.1007/s00737-009-0108-z. [DOI] [PubMed] [Google Scholar]
- Figueira P, Malloy-Diniz L, Campos SB, Miranda DM, Romano-Silva MA, et al. An association study between the Val66Met polymorphism of the BDNF gene and postpartum depression. Arch Women’s Ment Health. 2010;13:285–89. doi: 10.1007/s00737-010-0146-6. [DOI] [PubMed] [Google Scholar]
- Figueiredo B, Pacheco A, Costa R. Depression during pregnancy and the postpartum period in adolescent and adult Portuguese mothers. Arch Women’s Ment Health. 2007;10:103–9. doi: 10.1007/s00737-007-0178-8. [DOI] [PubMed] [Google Scholar]
- Fihrer I, McMahon CA, Taylor AJ. The impact of postnatal and concurrent maternal depression on child behaviour during the early school years. J Affect Disord. 2009;119:116–23. doi: 10.1016/j.jad.2009.03.001. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P) New York: Biom. Res., N. Y. State Psychiatr. Inst; 2002. [Google Scholar]
- Flint J, Kendler KS. The genetics of major depression. Neuron. 2014;81:484–503. doi: 10.1016/j.neuron.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forty L, Jones L, Macgregor S, Caesar S, Cooper C, et al. Familiality of postpartum depression in unipolar disorder: results of a family study. Am J Psychiatry. 2006;163:1549–53. doi: 10.1176/ajp.2006.163.9.1549. [DOI] [PubMed] [Google Scholar]
- Fraser JA, Armstrong KL, Morris JP, Dadds MR. Home visiting intervention for vulnerable families with newborns: follow-up results of a randomized controlled trial. Child Abuse Negl. 2000;24:1399–429. doi: 10.1016/s0145-2134(00)00193-9. [DOI] [PubMed] [Google Scholar]
- Gao L-L, Chan SW-C, Mao Q. Depression, perceived stress, and social support among first-time Chinese mothers and fathers in the postpartum period. Res Nurs Health. 2009;32:50–58. doi: 10.1002/nur.20306. [DOI] [PubMed] [Google Scholar]
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106:1071–83. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- Giardinelli L, Innocenti A, Benni L, Stefanini MC, Lino G, et al. Depression and anxiety in perinatal period: prevalence and risk factors in an Italian sample. Arch Women’s Ment Health. 2012;15:21–30. doi: 10.1007/s00737-011-0249-8. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Sandman CA. New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides. 2013;47:363–70. doi: 10.1016/j.npep.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Glynn LM, Sandman CA. Evaluation of the association between placental corticotrophin-releasing hormone and postpartum depressive symptoms. Psychosom Med. 2014;76:355–62. doi: 10.1097/PSY.0000000000000066. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high versus low CRH/NE states. Mol Psychiatry. 2002;7:254–75. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gremigni P, Mariani L, Marracino V, Tranquilli AL, Turi A. Partner support and postpartum depressive symptoms. J Psychosom Obstetr Gynaecol. 2011;32:135–40. doi: 10.3109/0167482X.2011.589017. [DOI] [PubMed] [Google Scholar]
- Grigoriadis S, Romans S. Postpartum psychiatric disorders: What do we know and where do we go? Curr Psychiatry Rev. 2006;2:151–58. [Google Scholar]
- Groër MW. Differences between exclusive breastfeeders, formula-feeders, and controls: a study of stress, mood, and endocrine variables. Biol Res Nurs. 2005;7:106–17. doi: 10.1177/1099800405280936. [DOI] [PubMed] [Google Scholar]
- Groer MW, Davis MW. Cytokines, infections, stress, and dysphoric moods in breastfeeders and formula feeders. J Obstetr Gynecol Neonatal Nurs. 2006;35:599–607. doi: 10.1111/j.1552-6909.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- Groer MW, Morgan K. Immune, health and endocrine characteristics of depressed postpartum mothers. Psychoneuroendocrinology. 2007;32:133–39. doi: 10.1016/j.psyneuen.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Grote NK, Bledsoe SE. Predicting postpartum depressive symptoms in new mothers: the role of optimism and stress frequency during pregnancy. Health Soc Work. 2007;32:107–18. doi: 10.1093/hsw/32.2.107. [DOI] [PubMed] [Google Scholar]
- Grussu P, Quatraro RM. Prevalence and risk factors for a high level of postnatal depression symptomatology in Italian women: a sample drawn from ante-natal classes. Eur Psychiatry. 2009;24:327–33. doi: 10.1016/j.eurpsy.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Guintivano J, Arad M, Gould TD, Payne JL, Kaminsky ZA. Antenatal prediction of postpartum depression with blood DNA methylation biomarkers. Mol Psychiatry. 2014;19:560–67. doi: 10.1038/mp.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen EH. The functions of postpartum depression. Evol Hum Behav. 1999;20:325–59. [Google Scholar]
- Hahn-Holbrook J, Dunkel Schetter C, Chander A, Hobel C. Placental corticotropin-releasing hormone mediates the association between prenatal social support and postpartum depression. Clin Psychol Sci. 2013;1:253–65. doi: 10.1177/2167702612470646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn-Holbrook J, Haselton M. Is postpartum depression a disease of modern civilization? Curr Dir Psychol Sci. 2014;23:395–400. doi: 10.1177/0963721414547736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbreich U. Postpartum disorders: multiple interacting underlying mechanisms and risk factors. J Affect Disord. 2005;88:1–7. doi: 10.1016/j.jad.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Halbreich U, Karkun S. Cross-cultural and social diversity of prevalence of postpartum depression and depressive symptoms. J Affect Disord. 2006;91:97–111. doi: 10.1016/j.jad.2005.12.051. [DOI] [PubMed] [Google Scholar]
- Hammen CL. Interpersonal stress and depression in women. J Affect Disord. 2003;74:49–57. doi: 10.1016/s0165-0327(02)00430-5. [DOI] [PubMed] [Google Scholar]
- Harris B, Huckle P, Thomas R, Johns S, Fung H. The use of rating scales to identify post-natal depression. Br J Psychiatry. 1989;154:813–17. doi: 10.1192/bjp.154.6.813. [DOI] [PubMed] [Google Scholar]
- Harville EW, Xiong X, Pridjian G, Elkind-Hirsch K, Buekens P. Postpartum mental health after Hurricane Katrina: a cohort study. BMC Pregnancy Childbirth. 2009;9:21. doi: 10.1186/1471-2393-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick V, Altshuler LL, Suri R. Hormonal changes in the postpartum and implications for postpartum depression. Psychosomatics. 1998;39:93–101. doi: 10.1016/S0033-3182(98)71355-6. [DOI] [PubMed] [Google Scholar]
- Herrmann WM, Beach RC. Experimental and clinical data indicating the psychotropic properties of progestogens. Postgrad Med J. 1978;54(Suppl 2):82–87. [PubMed] [Google Scholar]
- Hibino Y, Takaki J, Kambayashi Y, Hitomi Y, Sakai A, et al. Health impact of disaster-related stress on pregnant women living in the affected area of the Noto Peninsula earthquake in Japan. Psychiatry Clin Neurosci. 2009;63:107–15. doi: 10.1111/j.1440-1819.2008.01911.x. [DOI] [PubMed] [Google Scholar]
- Ho CL, Chang LI, Wan KS. The relationships between postpartum adaptation and postpartum depression symptoms of first pregnancy mothers in Taiwan. Int J Psychiatry Med. 2013;45:1–13. doi: 10.2190/PM.45.1.a. [DOI] [PubMed] [Google Scholar]
- Hochberg Z, Pacak K, Chrousos GP. Endocrine withdrawal syndromes. Endocr Rev. 2003;24:523–38. doi: 10.1210/er.2001-0014. [DOI] [PubMed] [Google Scholar]
- Hohlagschwandtner M, Husslein P, Klier C, Ulm B. Correlation between serum testosterone levels and peripartal mood states. Acta Obstet Gynecol Scand. 2001;80:326–30. doi: 10.1034/j.1600-0412.2001.080004326.x. [DOI] [PubMed] [Google Scholar]
- Honey KL, Bennett P, Morgan M. Predicting postnatal depression. J Affect Disord. 2003a;76:201–10. doi: 10.1016/s0165-0327(02)00085-x. [DOI] [PubMed] [Google Scholar]
- Honey KL, Morgan M, Bennett P. A stress-coping transactional model of low mood following childbirth. J Reprod Infant Psychol. 2003b;21:129–43. [Google Scholar]
- Hopkins J, Marcus M, Campbell SB. Postpartum depression: a critical review. Psychol Bull. 1984;95:498–515. [PubMed] [Google Scholar]
- Horowitz JA, Damato EG, Duffy ME, Solon L. The relationship of maternal attributes, resources, and perceptions of postpartum experiences to depression. Res Nurs Health. 2005;28:159–71. doi: 10.1002/nur.20068. [DOI] [PubMed] [Google Scholar]
- Howell EA, Mora PA, Horowitz CR, Leventhal H. Racial and ethnic differences in factors associated with early postpartum depressive symptoms. Obstet Gynecol. 2005;105:1442–50. doi: 10.1097/01.AOG.0000164050.34126.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell EA, Mora PA, Leventhal H. Correlates of early postpartum depressive symptoms. Matern Child Health J. 2006;10:149–57. doi: 10.1007/s10995-006-0116-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain N, Cruickshank K, Husain M, Khan S, Tomenson B, Rahman A. Social stress and depression during pregnancy and in the postnatal period in British Pakistani mothers: a cohort study. J Affect Disord. 2012;140:268–76. doi: 10.1016/j.jad.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles J, Slade P, Spiby H. Posttraumatic stress symptoms and postpartum depression in couples after childbirth: the role of partner support and attachment. J Anxiety Disord. 2011;25:520–30. doi: 10.1016/j.janxdis.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Ingram JC, Greenwood RJ, Woolridge MW. Hormonal predictors of postnatal depression at 6 months in breastfeeding women. J Reprod Infant Psychol. 2003;21:61–68. [Google Scholar]
- Itil TM, Cora R, Akpinar S, Herrmann WM, Patterson CJ. “Psychotropic” action of sex hormones: computerized EEG in establishing the immediate CNS effects of steroid hormones. Curr Ther Res Clin Exp. 1974;16:1147–70. [PubMed] [Google Scholar]
- Jolley SN, Elmore S, Barnard KE, Carr DB. Dysregulation of the hypothalamic-pituitary-adrenal axis in postpartum depression. Biol Res Nurs. 2007;8:210–22. doi: 10.1177/1099800406294598. [DOI] [PubMed] [Google Scholar]
- Kammerer M, Taylor A, Glover V. The HPA axis and perinatal depression: a hypothesis. Arch Women’s Ment Health. 2006;9:187–96. doi: 10.1007/s00737-006-0131-2. [DOI] [PubMed] [Google Scholar]
- Kendall-Tackett KA. A new paradigm for depression in new mothers: the central role of inflammation and how breastfeeding and anti-inflammatory treatments protect maternal mental health. Int Breastfeed J. 2007;2:6. doi: 10.1186/1746-4358-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy RL, Malabu UH, Jarrod G, Nigam P, Kannan K, Rane A. Thyroid function and pregnancy: before, during and beyond. J Obstet Gynaecol. 2010;30:774–83. doi: 10.3109/01443615.2010.517331. [DOI] [PubMed] [Google Scholar]
- Khabour O, Amarneh B, Bani Hani E, Lataifeh I. Associations between variations in TPH1, TPH2 and SLC6A4 genes and postpartum depression: a study in the Jordanian population. Balkan J Med Genet. 2013;16:41–8. doi: 10.2478/bjmg-2013-0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JK, Samaranayake M, Pradhan S. Epigenetic mechanisms in mammals. Cell Mol Life Sci. 2009;66:596–612. doi: 10.1007/s00018-008-8432-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YK, Hur JW, Kim KH, Oh KS, Shin YC. Prediction of postpartum depression by sociodemographic, obstetric and psychological factors: a prospective study. Psychiatry Clin Neurosci. 2008;62:331–40. doi: 10.1111/j.1440-1819.2008.01801.x. [DOI] [PubMed] [Google Scholar]
- Klier CM, Muzik M, Dervic K, Mossaheb N, Benesch T, et al. The role of estrogen and progesterone in depression after birth. J Psychiatr Res. 2007;41:273–79. doi: 10.1016/j.jpsychires.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Kozinszky Z, Dudas RB, Csatordai S, Devosa I, Toth E, et al. Social dynamics of postpartum depression: a population-based screening in South-Eastern Hungary. Soc Psychiatry Psychiatr Epidemiol. 2011;46:413–23. [Google Scholar]
- Kronborg CS, Gjedsted J, Vittinghus E, Hansen TK, Allen J, Knudsen UB. Longitudinal measurement of cytokines in pre-eclamptic and normotensive pregnancies. Acta Obstet Gynecol Scand. 2011;90:791–96. doi: 10.1111/j.1600-0412.2011.01134.x. [DOI] [PubMed] [Google Scholar]
- Kruse JA, Low LK, Seng JS. Validation of alternative indicators of social support in perinatal outcomes research using quality of the partner relationship. J Adv Nurs. 2013;69:1562–73. doi: 10.1111/jan.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuijpens JL, Vader HL, Drexhage HA, Wiersinga WM, van Son MJ, Pop VJ. Thyroid peroxidase antibodies during gestation are a marker for subsequent depression postpartum. Eur J Endocrinol. 2001;145:579–84. doi: 10.1530/eje.0.1450579. [DOI] [PubMed] [Google Scholar]
- Kuo WH, Wilson TE, Holman S, Fuentes-Afflick E, O’Sullivan MJ, Minkoff H. Depressive symptoms in the immediate postpartum period among Hispanic women in three US cities. J Immigr Health. 2004;6:145–53. doi: 10.1023/B:JOIH.0000045252.10412.fa. [DOI] [PubMed] [Google Scholar]
- Lawrence E, Cobb RJ, Rothman AD, Rothman MT, Bradbury TN. Marital satisfaction across the transition to parenthood. J Fam Psychol. 2008;22:41–50. doi: 10.1037/0893-3200.22.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus RS, Folkman S. Stress, Appraisal, and Coping. New York: Springer; 1984. [Google Scholar]
- Le Strat Y, Dubertret C, Le Foll B. Prevalence and correlates of major depressive episode in pregnant and postpartum women in the United States. J Affect Disord. 2011;135:128–38. doi: 10.1016/j.jad.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Leake RD, Weitzman RE, Glatz TH, Fisher DA. Plasma oxytocin concentrations in men, nonpregnant women, and pregnant women before and during spontaneous labor. J Clin Endocrinol Metab. 1981;53:730–33. doi: 10.1210/jcem-53-4-730. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Mössner R. Genetically driven variation in serotonin uptake: Is there a link to affective spectrum, neurodevelopmental, and neurodegenerative disorders? Biol Psychiatry. 1998;44:179–92. doi: 10.1016/s0006-3223(98)00121-8. [DOI] [PubMed] [Google Scholar]
- Leung SS, Martinson IM, Arthur D. Postpartum depression and related psychosocial variables in Hong Kong Chinese women: findings from a prospective study. Res Nurs Health. 2005;28:27–38. doi: 10.1002/nur.20053. [DOI] [PubMed] [Google Scholar]
- Light KC, Grewen KM, Amico JA, Boccia M, Brownley KA, Johns JM. Deficits in plasma oxytocin responses and increased negative affect, stress, and blood pressure in mothers with cocaine exposure during pregnancy. Addict Behav. 2004;29:1541–64. doi: 10.1016/j.addbeh.2004.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry PJ. Corticotropin-releasing factor and its binding protein in human plasma. Ciba Found Symp. 1993;172:108–15. discussion 115–28. [PubMed] [Google Scholar]
- Maes M, Lin AH, Ombelet W, Stevens K, Kenis G, et al. Immune activation in the early puerperium is related to postpartum anxiety and depressive symptoms. Psychoneuroendocrinology. 2000;25:121–37. doi: 10.1016/s0306-4530(99)00043-8. [DOI] [PubMed] [Google Scholar]
- Mandelli L, Serretti A. Gene environment interaction studies in depression and suicidal behavior: an update. Neurosci Biobehav Rev. 2013;37:2375–97. doi: 10.1016/j.neubiorev.2013.07.011. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- Matthey S, Barnett B, Ungerer J, Waters B. Paternal and maternal depressed mood during the transition to parenthood. J Affect Disord. 2000;60:75–85. doi: 10.1016/s0165-0327(99)00159-7. [DOI] [PubMed] [Google Scholar]
- McCoy SJ, Beal JM, Watson GH. Endocrine factors and postpartum depression. A selected review. J Reprod Med. 2003;48:402–8. [PubMed] [Google Scholar]
- McGrath JM, Records K, Rice M. Maternal depression and infant temperament characteristics. Infant Behav Dev. 2008;31:71–80. doi: 10.1016/j.infbeh.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–63. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- McMahon C, Barnett B, Kowalenko N, Tennant C. Psychological factors associated with persistent postnatal depression: past and current relationships, defence styles and the mediating role of insecure attachment style. J Affect Disord. 2005;84:15–24. doi: 10.1016/j.jad.2004.05.005. [DOI] [PubMed] [Google Scholar]
- McVey C, Tuohy A. Differential effects of marital relationship and social support on three subscales identified within the Edinburgh Postnatal Depression Scale. J Reprod Infant Psychol. 2007;25:203–9. [Google Scholar]
- Mehta D, Quast C, Fasching PA, Seifert A, Voigt F, et al. The 5-HTTLPR polymorphism modulates the influence on environmental stressors on peripartum depression symptoms. J Affect Disord. 2012;136:1192–97. doi: 10.1016/j.jad.2011.11.042. [DOI] [PubMed] [Google Scholar]
- Meltzer-Brody S, Stuebe A, Dole N, Savitz D, Rubinow D, Thorp J. Elevated corticotropin releasing hormone (CRH) during pregnancy and risk of postpartum depression (PPD) J Clin Endocrinol Metab. 2011;96:E40–47. doi: 10.1210/jc.2010-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileva-Seitz V, Steiner M, Atkinson L, Meaney MJ, Levitan R, et al. Interaction between oxytocin genotypes and early experience predicts quality of mothering and postpartum mood. PLOS ONE. 2013;8:e61443. doi: 10.1371/journal.pone.0061443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom J, Gemmill AW, Bilszta JL, Hayes B, Barnett B, et al. Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord. 2008;108:147–57. doi: 10.1016/j.jad.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Mitchell C, Notterman D, Brooks-Gunn J, Hobcraft J, Garfinkel I, et al. Role of mother’s genes and environment in postpartum depression. Proc Natl Acad Sci USA. 2011;108:8189–93. doi: 10.1073/pnas.1014129108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammad KI, Gamble J, Creedy DK. Prevalence and factors associated with the development of antenatal and postnatal depression among Jordanian women. Midwifery. 2011;27:e238–45. doi: 10.1016/j.midw.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Monk C, Leight KL, Fang Y. The relationship between women’s attachment style and perinatal mood disturbance: implications for screening and treatment. Arch Women’s Ment Health. 2008;11:117–29. doi: 10.1007/s00737-008-0005-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone P, Maj M. The circadian basis of mood disorders: recent developments and treatment implications. Eur Neuropsychopharmacol. 2008;18:701–11. doi: 10.1016/j.euroneuro.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Berga SL, Kalro B, Sit DK, Wisner KL. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52:516–29. doi: 10.1097/GRF.0b013e3181b5a395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum “blues. Obstet Gynecol. 2001;97:77–80. doi: 10.1016/s0029-7844(00)01112-1. [DOI] [PubMed] [Google Scholar]
- Nierop A, Bratsikas A, Zimmermann R, Ehlert U. Are stress-induced cortisol changes during pregnancy associated with postpartum depressive symptoms? Psychosomat Med. 2006;68:931–37. doi: 10.1097/01.psy.0000244385.93141.3b. [DOI] [PubMed] [Google Scholar]
- O’Hara MW. Postpartum depression: what we know. J Clin Psychol. 2009;65:1258–69. doi: 10.1002/jclp.20644. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Rehm LP, Campbell SB. Predicting depressive symptomatology: cognitive-behavioral models and postpartum depression. J Abnorm Psychol. 1982;91:457–61. doi: 10.1037//0021-843x.91.6.457. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Schlechte JA, Lewis DA, Varner MW. Controlled prospective study of postpartum mood disorders: psychological, environmental, and hormonal variables. J Abnorm Psychol. 1991;100:63–73. doi: 10.1037//0021-843x.100.1.63. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Swain AM. Rates and risk of postpartum depression—a meta-analysis. Int Rev Psychiatry. 1996;8:37–54. [Google Scholar]
- O’Hara MW, Wisner KL. Perinatal mental illness: definition, description and aetiology. Best Pract Res Clin Obstet Gynaecol. 2014;28:3–12. doi: 10.1016/j.bpobgyn.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keane V, Lightman S, Patrick K, Marsh M, Papadopoulos AS, et al. Changes in the maternal hypothalamic-pituitary-adrenal axis during the early puerperium may be related to the postpartum “blues. J Neuroendocrinol. 2011;23:1149–55. doi: 10.1111/j.1365-2826.2011.02139.x. [DOI] [PubMed] [Google Scholar]
- Ogilvie AD, Battersby S, Bubb VJ, Fink G, Harmar AJ, et al. Polymorphism in serotonin transporter gene associated with susceptibility to major depression. Lancet. 1996;347:731–33. doi: 10.1016/s0140-6736(96)90079-3. [DOI] [PubMed] [Google Scholar]
- Okun ML, Luther J, Prather AA, Perel JM, Wisniewski S, Wisner KL. Changes in sleep quality, but not hormones predict time to postpartum depression recurrence. J Affect Disord. 2011;130:378–84. doi: 10.1016/j.jad.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppo A, Mauri M, Ramacciotti D, Camilleri V, Banti S, et al. Risk factors for postpartum depression: the role of the Postpartum Depression Predictors Inventory-Revised (PDPI-R) Arch Women’s Ment Health. 2009;12:239–49. doi: 10.1007/s00737-009-0071-8. [DOI] [PubMed] [Google Scholar]
- Page M, Wilhelm MS. Postpartum daily stress, relationship quality, and depressive symptoms. Contemp Fam Ther. 2007;29:237–51. [Google Scholar]
- Paulson JF, Bazemore SD. Prenatal and postpartum depression in fathers and its association with maternal depression: a meta-analysis. JAMA. 2010;303:1961–69. doi: 10.1001/jama.2010.605. [DOI] [PubMed] [Google Scholar]
- Payne JL, Palmer JT, Joffe H. A reproductive subtype of depression: conceptualizing models and moving toward etiology. Harvard Rev Psychiatry. 2009;17:72–86. doi: 10.1080/10673220902899706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J Health Soc Behav. 1981;22:337–56. [PubMed] [Google Scholar]
- Phillips J, Sharpe L, Matthey S, Charles M. Subtypes of postnatal depression? A comparison of women with recurrent and de novo postnatal depression. J Affect Disord. 2010;120:67–75. doi: 10.1016/j.jad.2009.04.011. [DOI] [PubMed] [Google Scholar]
- Pietromonaco PR, Uchino B, Dunkel Schetter C. Close relationship processes and health: Implications of attachment theory for health and disease. Health Psychol. 2013;32:499–513. doi: 10.1037/a0029349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro RT, Coelho FM, Silva RA, Pinheiro KA, Oses JP, et al. Association of a serotonin transporter gene polymorphism (5-HTTLPR) and stressful life events with postpartum depressive symptoms: a population-based study. J Psychosom Obstet Gynaecol. 2013;34:29–33. doi: 10.3109/0167482X.2012.759555. [DOI] [PubMed] [Google Scholar]
- Pinsonneault JK, Sullivan D, Sadee W, Soares CN, Hampson E, Steiner M. Association study of the estrogen receptor gene ESR1 with postpartum depression—a pilot study. Arch Women’s Ment Health. 2013;16:499–509. doi: 10.1007/s00737-013-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Z, Wang X, Tian D, Zhao Y, Zhang Q, et al. Posttraumatic stress disorder and depression among new mothers at 8 months later of the 2008 Sichuan earthquake in China. Arch Women’s Ment Health. 2012;15:49–55. doi: 10.1007/s00737-011-0255-x. [DOI] [PubMed] [Google Scholar]
- Radesky JS, Zuckerman B, Silverstein M, Rivara FP, Barr M, et al. Inconsolable infant crying and maternal postpartum depressive symptoms. Pediatrics. 2013;131:e1857–64. doi: 10.1542/peds.2012-3316. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–65. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Ramchandani PG, Richter LM, Stein A, Norris SA. Predictors of postnatal depression in an urban South African cohort. J Affect Disord. 2009;113:279–84. doi: 10.1016/j.jad.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Reid V, Meadows-Oliver M. Postpartum depression in adolescent mothers: an integrative review of the literature. J Pediatr Health Care. 2007;21:289–98. doi: 10.1016/j.pedhc.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Kleinman K, Abrams A, Harlow BL, McLaughlin TJ, et al. Sociodemographic predictors of antenatal and postpartum depressive symptoms among women in a medical group practice. J Epidemiol Community Health. 2006;60:221–27. doi: 10.1136/jech.2005.039370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich-Edwards JW, Mohllajee AP, Kleinman K, Hacker MR, Majzoub J, et al. Elevated midpregnancy corticotropin-releasing hormone is associated with prenatal, but not postpartum, maternal depression. J Clin Endocrinol Metab. 2008;93:1946–51. doi: 10.1210/jc.2007-2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289–95. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Rodríguez MA, Valentine J, Ahmed SR, Eisenman DP, Sumner LA, et al. Intimate partner violence and maternal depression during the perinatal period: a longitudinal investigation of Latinas. Violence Against Women. 2010;16:543–59. doi: 10.1177/1077801210366959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers CE, Kidokoro H, Wallendorf M, Inder TE. Identifying mothers of very preterm infants at-risk for postpartum depression and anxiety before discharge. J Perinatol. 2013;33:171–76. doi: 10.1038/jp.2012.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohleder N. Acute and chronic stress induced changes in sensitivity of peripheral inflammatory pathways to the signals of multiple stress systems—2011 Curt Richter Award Winner. Psychoneuroendocrinology. 2012;37:307–16. doi: 10.1016/j.psyneuen.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Ross LE, Sellers EM, Gilbert Evans SE, Romach MK. Mood changes during pregnancy and the postpartum period: development of a biopsychosocial model. Acta Psychiatr Scand. 2004;109:457–66. doi: 10.1111/j.1600-0047.2004.00296.x. [DOI] [PubMed] [Google Scholar]
- Sacher J, Wilson AA, Houle S, Rusjan P, Hassan S, et al. Elevated brain monoamine oxidase A binding in the early postpartum period. Arch Gen Psychiatry. 2010;67:468–74. doi: 10.1001/archgenpsychiatry.2010.32. [DOI] [PubMed] [Google Scholar]
- Sandman CA, Glynn L, Dunkel Schetter C, Wadhwa P, Garite T, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–63. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Sanjuan J, Martin-Santos R, Garcia-Esteve L, Carot JM, Guillamat R, et al. Mood changes after delivery: role of the serotonin transporter gene. Br J Psychiatry. 2008;193:383–88. doi: 10.1192/bjp.bp.107.045427. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Liotta AS, Luckey MM, Margioris AN, Suda T, Krieger DT. Immunoreactive corticotropin-releasing factor is present in human maternal plasma during the third trimester of pregnancy. J Clin Endocrinol Metab. 1984;59:812–14. doi: 10.1210/jcem-59-4-812. [DOI] [PubMed] [Google Scholar]
- Schachman K, Lindsey L. A resilience perspective of postpartum depressive symptomatology in military wives. J Obstet Gynecol Neonatal Nurs. 2013;42:157–67. doi: 10.1111/1552-6909.12007. [DOI] [PubMed] [Google Scholar]
- Scrandis DA, Langenberg P, Tonelli LH, Sheikh TM, Manogura AC, et al. Prepartum depressive symptoms correlate positively with C-reactive protein levels and negatively with tryptophan levels: a preliminary report. Int J Child Health Hum Dev. 2008;1:167–74. [PMC free article] [PubMed] [Google Scholar]
- Secco LM, Profit S, Kennedy E, Walsh A, Letourneau N, Stewart M. Factors affecting postpartum depressive symptoms of adolescent mothers. J Obstet Gynecol Neonatal Nurs. 2007;36:47–54. doi: 10.1111/j.1552-6909.2006.00114.x. [DOI] [PubMed] [Google Scholar]
- Segman RH, Goltser-Dubner T, Weiner I, Canetti L, Galili-Weisstub E, et al. Blood mononuclear cell gene expression signature of postpartum depression. Mol Psychiatry. 2010;15:93–100. doi: 10.1038/mp.2009.65. [DOI] [PubMed] [Google Scholar]
- Segre LS, O’Hara MW, Arndt S, Stuart S. The prevalence of postpartum depression: the relative significance of three social status indices. Soc Psychiatry Psychiatr Epidemiol. 2007;42:316–21. doi: 10.1007/s00127-007-0168-1. [DOI] [PubMed] [Google Scholar]
- Seimyr L, Welles-Nystrom B, Nissen E. A history of mental health problems may predict maternal distress in women postpartum. Midwifery. 2013;29:122–31. doi: 10.1016/j.midw.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Sheng X, Le HN, Perry D. Perceived satisfaction with social support and depressive symptoms in perinatal Latinas. J Transcult Nurs. 2010;21:35–44. doi: 10.1177/1043659609348619. [DOI] [PubMed] [Google Scholar]
- Silverman ME, Loudon H. Antenatal reports of pre-pregnancy abuse is associated with symptoms of depression in the postpartum period. Arch Women’s Ment Health. 2010;13:411–15. doi: 10.1007/s00737-010-0161-7. [DOI] [PubMed] [Google Scholar]
- Simpson JA, Rholes WS, Campbell L, Tran S, Wilson CL. Adult attachment, the transition to parenthood, and depressive symptoms. J Personal Soc Psychol. 2003;84:1172–87. doi: 10.1037/0022-3514.84.6.1172. [DOI] [PubMed] [Google Scholar]
- Siu BW, Leung SS, Ip P, Hung SF, O’Hara MW. Antenatal risk factors for postnatal depression: a prospective study of Chinese women at maternal and child health centres. BMC Psychiatry. 2012;12:22. doi: 10.1186/1471-244X-12-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalkidou A, Hellgren C, Comasco E, Sylven S, Sundstrom Poromaa I. Biological aspects of postpartum depression. Women’s Health (Lond Engl) 2012;8:659–72. doi: 10.2217/whe.12.55. [DOI] [PubMed] [Google Scholar]
- Skalkidou A, Sylven SM, Papadopoulos FC, Olovsson M, Larsson A, Sundstrom-Poromaa I. Risk of postpartum depression in association with serum leptin and interleukin-6 levels at delivery: a nested case-control study within the UPPSAT cohort. Psychoneuroendocrinology. 2009;34:1329–37. doi: 10.1016/j.psyneuen.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology. 2011;36:1886–93. doi: 10.1038/npp.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speroff L, Glass RH, Kase NG. Clinical Gynecologic Endocrinology and Infertility. Baltimore, MD: Williams & Wilkins; 1983. [Google Scholar]
- Stern G, Kruckman L. Multi-disciplinary perspectives on postpartum depression: an anthropological critique. Soc Sci Med. 1983;17:1027–41. doi: 10.1016/0277-9536(83)90408-2. [DOI] [PubMed] [Google Scholar]
- Stock A, Chin L, Babl FE, Bevan CA, Donath S, Jordan B. Postnatal depression in mothers bringing infants to the emergency department. Arch Dis Childhood. 2013;98:36–40. doi: 10.1136/archdischild-2012-302679. [DOI] [PubMed] [Google Scholar]
- Stuebe AM, Grewen K, Meltzer-Brody S. Association between maternal mood and oxytocin response to breastfeeding. J Women’s Health (Larchmt) 2013;22:352–61. doi: 10.1089/jwh.2012.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Kennedy PJ, Nestler EJ. Epigenetics of the depressed brain: role of histone acetylation and methylation. Neuropsychopharmacology. 2013;38:124–37. doi: 10.1038/npp.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvén SM, Elenis E, Michelakos T, Larsson A, Olovsson M, et al. Thyroid function tests at delivery and risk for postpartum depressive symptoms. Psychoneuroendocrinology. 2013;38:1007–13. doi: 10.1016/j.psyneuen.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Tanner Stapleton LR, Dunkel Schetter C, Westling E, Rini C, Glynn LM, et al. Perceived partner support in pregnancy predicts lower maternal and infant distress. J Fam Psychol. 2012;26:453–63. doi: 10.1037/a0028332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A, Glover V, Marks M, Kammerer M. Diurnal pattern of cortisol output in postnatal depression. Psychoneuroendocrinology. 2009;34:1184–88. doi: 10.1016/j.psyneuen.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Torner L, Neumann ID. The brain prolactin system: involvement in stress response adaptations in lactation. Stress. 2002;5:249–57. doi: 10.1080/1025389021000048638. [DOI] [PubMed] [Google Scholar]
- Torpy DJ, Chrousos GP. The three-way interactions between the hypothalamic-pituitary-adrenal and gonadal axes and the immune system. Baillieres Clin Rheumatol. 1996;10:181–98. doi: 10.1016/s0950-3579(96)80014-8. [DOI] [PubMed] [Google Scholar]
- Toyokawa S, Uddin M, Koenen KC, Galea S. How does the social environment “get into the mind”? Epigenetics at the intersection of social and psychiatric epidemiology. Soc Sci Med. 2012;74:67–74. doi: 10.1016/j.socscimed.2011.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I Normal pregnancy. Am J Obstet Gynecol. 1972;112:1095–100. doi: 10.1016/0002-9378(72)90185-8. [DOI] [PubMed] [Google Scholar]
- Upadhyaya I, Agrawal JK, Dubey GP, Udupa KN. Biogenic amines and thyrotoxicosis. Acta Endocrinol (Copenh) 1992;126:315–18. doi: 10.1530/acta.0.1260315. [DOI] [PubMed] [Google Scholar]
- Urquia ML, O’Campo PJ, Ray JG. Marital status, duration of cohabitation, and psychosocial well-being among childbearing women: a Canadian nationwide survey. Am J Public Health. 2013;103:e8–15. doi: 10.2105/AJPH.2012.301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan N, Ogawa S, Pfaff D. Estrogen and thyroid hormone receptor interactions: physiological flexibility by molecular specificity. Physiol Rev. 2002;82:923–44. doi: 10.1152/physrev.00014.2002. [DOI] [PubMed] [Google Scholar]
- Venkatesh KK, Phipps MG, Triche EW, Zlotnick C. The relationship between parental stress and postpartum depression among adolescent mothers enrolled in a randomized controlled prevention trial. Matern Child Health J. 2014;18:1532–39. doi: 10.1007/s10995-013-1394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeek T, Bockting CL, van Pampus MG, Ormel J, Meijer JL, et al. Postpartum depression predicts offspring mental health problems in adolescence independently of parental lifetime psychopathology. J Affect Disord. 2012;136:948–54. doi: 10.1016/j.jad.2011.08.035. [DOI] [PubMed] [Google Scholar]
- Whisman MA, Davila J, Goodman SH. Relationship adjustment, depression, and anxiety during pregnancy and the postpartum period. J Fam Psychol. 2011;25:375–83. doi: 10.1037/a0023790. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Stowe ZN. Psychobiology of postpartum mood disorders. Semin Reprod Endocrinol. 1997;15:77–89. doi: 10.1055/s-2008-1067970. [DOI] [PubMed] [Google Scholar]
- World Health Organ. International Statistical Classification of Diseases and Related Health Problems. Geneva, Switz: World Health Organ; 1992. 10th rev. [Google Scholar]
- Xie RH, He G, Koszycki D, Walker M, Wen SW. Fetal sex, social support, and postpartum depression. Can J Psychiatry. 2009;54:750–56. doi: 10.1177/070674370905401105. [DOI] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Dunkel Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA. Risk of postpartum depressive symptoms with elevated corticotropin-releasing hormone in human pregnancy. Arch Gen Psychiatry. 2009;66:162–69. doi: 10.1001/archgenpsychiatry.2008.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Glynn LM, Dunkel Schetter C, Hobel CJ, Chicz-DeMet A, Sandman CA. Prenatal beta-endorphin as an early predictor of postpartum depressive symptoms in euthymic women. J Affect Disord. 2010;125:128–33. doi: 10.1016/j.jad.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonkers KA, Ramin SM, Rush AJ, Navarrete CA, Carmody T, et al. Onset and persistence of postpartum depression in an inner-city maternal health clinic system. Am J Psychiatry. 2001;158:1856–63. doi: 10.1176/appi.ajp.158.11.1856. [DOI] [PubMed] [Google Scholar]
- Zelkowitz P, Saucier JF, Wang T, Katofsky L, Valenzuela M, Westreich R. Stability and change in depressive symptoms from pregnancy to two months postpartum in childbearing immigrant women. Arch Women’s Ment Health. 2008;11:1–11. doi: 10.1007/s00737-008-0219-y. [DOI] [PubMed] [Google Scholar]
- Zenclussen AC. Adaptive immune responses during pregnancy. Am J Reprod Immunol. 2013;69:291–303. doi: 10.1111/aji.12097. [DOI] [PubMed] [Google Scholar]
- Zonana J, Gorman JM. The neurobiology of postpartum depression. CNS Spectr. 2005;10:792–99. 805. doi: 10.1017/s1092852900010312. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.