Abstract
Objective
To estimate the risk of adverse perinatal outcomes among women with isolated fetal growth restriction from 17 to 22 weeks of gestation.
Methods
This was a retrospective cohort study of all singleton, non-anomalous pregnancies undergoing ultrasound to assess fetal anatomy between 17 and 22 weeks of gestation at a single center from 2010 to 2014. After excluding patients with fetal structural malformations, chromosomal abnormalities, or identified infection etiologies, we compared perinatal outcomes between pregnancies with and without fetal growth restriction, defined as estimated fetal weight <10th percentile for gestational age. Our primary outcome was small for gestational age (SGA) at birth, defined as birthweight < 10th percentile. Secondary outcomes included preterm delivery <37 weeks and <28 weeks, preeclampsia, abruption, stillbirth, neonatal death, neonatal intensive care unit (NICU) admission, intraventricular hemorrhage, need for respiratory support, and necrotizing enterocolitis.
Results
Of 12,783 eligible patients, 355 (2.8%) had early second-trimester fetal growth restriction. Risk factors for growth restriction were African American race and tobacco use. Early second-trimester growth restriction was associated with over a fivefold increase in risk of SGA at birth (36.9% vs 9.1%, aOR 5.5, 95% CI 4.3, 7.0), stillbirth (2.5% vs 0.4%, OR 6.2, 95% CI 2.7, 12.8), and neonatal death (1.4% vs 0.3%, OR 5.2, 95% CI 1.6, 13.5). Rates of indicated preterm birth <37 weeks (7.3% vs 3.3%, OR 2.3, 95% CI 1.5,3.5) and <28 weeks (2.5% vs 0.2%, OR 10.8, 95% CI 4.5,23.4), neonatal need for respiratory support (16.9% vs 7.8%, aOR 1.6, 95% CI 1.1,2.2), and necrotizing enterocolitis (1.4% vs 0.2%, OR 7.7, 95% CI 2.3, 20.9) were also significantly higher for those with growth restriction. Rates of preeclampsia, abruption, and other neonatal outcomes were not significantly different.
Conclusion
Although fetal growth restriction in the early second trimester occurred in less than 3% of our cohort and most of those with isolated growth restriction did not have adverse outcomes, it is a strong risk factor for SGA, stillbirth, neonatal death, and indicated preterm birth.
Introduction
Fetal growth restriction, defined as estimated fetal weight <10th percentile for gestational age, is a common pregnancy complication which is associated with increased perinatal morbidity and mortality. (1, 2) Most of the data on the association of growth restriction with these outcomes are based on growth restriction diagnosed in the late second or third trimester. (2–4) Some studies have evaluated earlier onset growth restriction; however, these analyses are largely limited to those with growth restriction diagnosed after 24 weeks. (5–7)
Little data exist to guide counseling and management of women when growth restriction is diagnosed before 24 weeks of gestation in the absence of an identifiable cause. We therefore aimed to estimate the risk of adverse perinatal outcomes among women with isolated fetal growth restriction diagnosed at the time of ultrasound to assess fetal anatomy.
Materials and Methods
We conducted a retrospective cohort study of women undergoing routine ultrasound to assess fetal anatomy at a single institution between January 1, 2010, and December 31, 2014. Patients were included if they had singleton, non-anomalous pregnancies and were between 17 0/7 and 22 6/7 weeks gestational age with an estimated fetal weight performed at the time of ultrasound. Patients were excluded if they had fetal structural malformations, chromosomal abnormalities, identified infectious etiologies, or if the pregnancy did not continue past 20 weeks of gestation. They were also excluded if outcome data were missing or not available. Pregnancies with ultrasonographic soft markers for aneuploidy including echogenic intracardiac focus, single umbilical artery, and pyelectasis were included.(8) Gestational age was determined based on last menstrual period (LMP) if first trimester ultrasound agreed with the estimated due date (EDD) within seven days or second-trimester ultrasound agreed with EDD within 14 days. If the due date differed by more than seven days in the first trimester or 14 days in the second trimester, or if the LMP was unknown, the EDD was changed to that calculated by earliest available ultrasound. Any dating discrepancy was resolved after review of the medical record by attending maternal-fetal medicine physician. For the purposes of this study, if the patient had more than one pregnancy during the study period, only the first pregnancy was included. The study was conducted after approval from the Washington University School of Medicine Human Research Protection Office.
Details regarding maternal and obstetric history, antepartum complications, as well as obstetric and neonatal outcomes are obtained and entered into a database by dedicated perinatal research nurses in a prospective, ongoing manner for all women having ultrasounds at our institution. Maternal demographic and pregnancy information are entered at the time of the ultrasound. Perinatal research nurses obtain information about antepartum complications, obstetric and neonatal outcomes using electronic medical records, records from referring providers, and telephone contact with the patient. This database was used to identify study participants.
We first calculated the incidence of fetal growth restriction at the time of ultrasound to assess fetal anatomy in our cohort with 95% confidence intervals based on the binomial exact method. Fetal growth restriction was defined as estimated fetal weight <10th percentile using Warsof growth curves before 20 0/7 weeks gestation and Hadlock growth curves from20 0/7 weeks gestation onward.(9–12) Patients underwent standardized management of fetal growth restriction with serial growth ultrasounds beginning at viability, antenatal surveillance including umbilical artery Dopplers, and timing of delivery based on the NICHD/SMFM recommendations. (13)
We compared maternal demographic information and outcomes between women with and without fetal growth restriction. The primary outcome for this analysis was small for gestational age (SGA) at birth, which was defined as <10th percentile birthweight on the Alexander growth curves. (14) Secondary outcomes included SGA based on the newer Oken and Fenton growth curves(15, 16), birthweight, gestational age at delivery, indicated preterm birth, stillbirth, abruption, preeclampsia, neonatal death, neonatal intensive care unit (NICU) admission, need for neonatal respiratory support, neonatal intraventricular hemorrhage and necrotizing enterocolitis in the neonate. We performed a stratified analysis on estimated fetal weight <5th percentile and from the 5–10th percentile for the primary outcome of small for gestational age.
Baseline characteristics were compared between the two groups using the unpaired Student t test or the Wilcoxon rank-sum test as appropriate for continuous variables, and the χ2 test or Fisher’s exact test as appropriate for categorical variables. We calculated rates and unadjusted odds ratios (OR) and 95% confidence intervals (CI) for the primary and secondary outcomes. Multivariable logistic regression was used to adjust for confounders and estimate the association between isolated growth restriction and adverse outcomes. Factors adjusted for were chosen based on results of bivariate analysis, as well as biologically plausible risk factors associated with adverse outcomes. Model fit for the final model was assessed with the Hosmer-Lemeshow goodness-of-fit test.
No a priori sample size estimation was performed and all consecutive patients meeting inclusion criteria were included. All tests were two-tailed with a p value of greater than 0.05 considered significant. Statistical analysis were completed with STATA software package (version 12, Stata Corporation, College Station, TX.)
Results
Of 12,783 eligible women, 355 (2.8%, 95% CI 2.5%, 3.1%) had growth restriction at the time of survey to assess fetal anatomy, of whom 131 (1.0%, 95% CI 0.9%, 1.2%) had estimated fetal weight <5th percentile for gestational age and 224 (1.8%, 95% CI 1.5%, 2.0%) had estimated fetal weight between the 5th and 10th percentile. (Figure 1) Women with fetal growth restriction had ultrasound performed at a slightly later gestational age and were more likely to be African American and to smoke. Other baseline characteristics, including parity, chronic hypertension, body mass index, and diabetes were similar between those with and without fetal growth restriction.. (Table 1)
Figure 1.
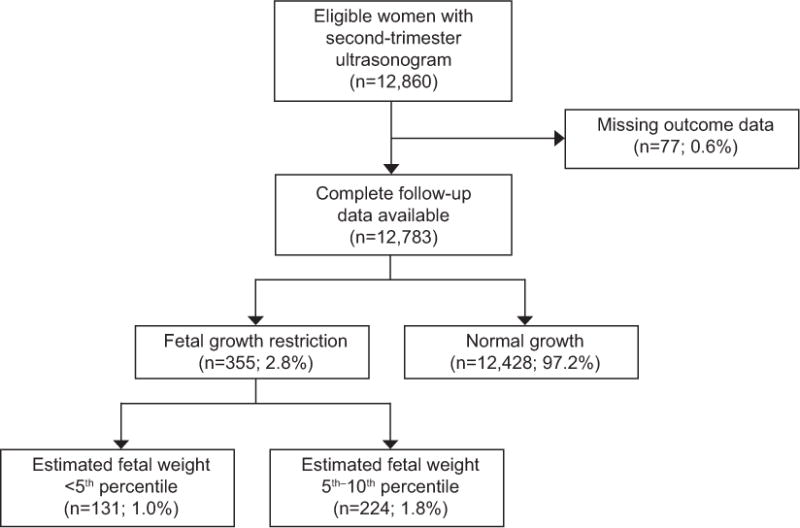
Flow Diagram of Study Participants
Table 1.
Baseline characteristics for patients with and without fetal growth restriction at ultrasound between 17 and 22 weeks gestation.
| Characteristic | FGR at anatomy scan n=355 | AGA at anatomy scan n= 12428 | P value |
|---|---|---|---|
| Age (years) | 28.1 ± 6.3 | 28.7 ± 5.8 | 0.05 |
| Age over 35 | 66 (18.6) | 2083 (16.8) | 0.36 |
| Gestational Age at ultrasound (weeks) | 19.5± 0.9 | 19.3 ± 1.0 | <0.001 |
| African American Race | 152 (42.8) | 4128 (33.2) | <0.001 |
| Gravidity | 2 (1,3) | 2 (1,3) | 0.06 |
| Tobacco Use | 46 (13.0) | 910 (7.3) | <0.001 |
| Maternal Body Mass Index (BMI kg/m2) | 26.8 ± 7.3 | 26.3 ± 8.2 | 0.28 |
| Obese (BMI ≥ 30.0 kg/m2) | 110 (31.0) | 3559 (28.6) | 0.34 |
| Morbid Obesity (BMI ≥ 40.0 kg/m2) | 47 (13.2) | 1508 (12.1) | 0.53 |
| Chronic Hypertension | 4 (1.1) | 155 (1.3) | 0.84 |
| Drug Use | 12 (3.4) | 240 (1.9) | 0.05 |
| Pregestational Diabetes Mellitus | 22 (6.2) | 610 (4.9) | 0.27 |
Data presented as n(%), mean ± standard deviation or median(IQR), significant values are bolded
IQR=Interquartile Range
Fetal growth restriction at the early second trimester was associated with an over five-fold increased risk of SGA at birth (36.9% vs 9.1%, aOR 5.5, 95% CI 4.3, 7.0). This remained true using the Oken and Fenton growth curves (Table 2). When stratified by degree of growth restriction, those with estimated fetal weight <5 % had an over seven-fold increased risk of SGA compared to those with normally grown fetuses (46.6% vs 9.1%, aOR 7.9, 95% CI 5.5, 11.4) and those with estimated fetal weight 5–10% had an over four-fold increased risk of SGA (31.3% vs 9.1%, 4.4 95% CI 3.2, 5.9).
Table 2.
Obstetric and neonatal outcomes for patients with and without fetal growth restriction between 17 and 22 weeks gestation.
| Outcome n=12783 | FGR at anatomy scan n=355 | AGA at anatomy scan n=12428 | OR (95% Confidence Interval) | aOR (95% Confidence Interval |
|---|---|---|---|---|
| Gestational Age at delivery (weeks) | 37.2 ± 3.4 | 38.3 ± 2.4 | p<0.001 | |
| Small for Gestational Age (SGA) (Alexander criteria) | 131 (36.9) | 1132 (9.1) |
5.8 (4.6–7.3) |
5.5 (4.3–7.0)* |
| SGA (Fenton criteria) | 109 (30.7) | 975 (7.9) |
5.2 (4.1–6.6) |
4.8 (3.8–6.2)* |
| SGA (Oken criteria) | 134 (37.8) | 1268 (10.2) |
5.3 (4.2–6.7) |
5.0 (4.0–6.3)* |
| Birthweight (grams) | 2725 ± 763 | 3267 ± 775 | p<0.001 | – |
| Stillbirth | 9 (2.5) | 52 (0.4) |
6.2 (2.7–12.8) |
– |
| Mean gestational age at stillbirth (weeks) | 25.1 ± 5.2 | 27.7 ± 6.9 | p=0.29 | – |
| Indicated Preterm Delivery <37 | 26 (7.3) | 413 (3.3) |
2.3 (1.5–3.5) |
– |
| Indicated Preterm delivery <28 | 9 (2.5) | 30 (0.2) |
10.8 (4.5–23.4) |
– |
| Abruption | 4 (1.1) | 69 (0.6) | 2.0 (0.5–5.5) |
– |
| Preeclampsia | 43 (12.1) | 1135 (9.1) | 1.4 (1.0–1.9) |
1.3 (0.9–1.8)* |
| Neonatal Death | 5 (1.4) | 34 (0.3) |
5.2 (1.6–13.5) |
|
| NICU admission | 32 (9.0) | 503 (4.1) | 2.4 (1.6–3.4) |
1.5 (0.9–2.2)† |
| Need for respiratory support | 60 (16.9) | 966 (7.8) |
2.4 (1.8–3.2) |
1.6 (1.1–2.2)† |
| Intraventricular Hemorrhage | 1 (0.3) | 43 (0.4) | 0.8 (0.4–4.8) |
– |
| Necrotizing Enterocolitis | 5 (1.4) | 23 (0.2) |
7.7 (2.3–20.9) |
– |
Data presented as n(%), mean ± standard deviation, significant values are bolded
Adjusted for African American race and tobacco use
Adjusted for race, tobacco use, and gestational age at delivery
Women with growth restriction were more likely to deliver at earlier gestational ages (37.2 weeks ± 3.4 vs 38.3 weeks ± 2.4, p<0.001), deliver neonates with lower birth weights (2725 grams ± 763 vs 3267 grams ± 775, p <0.001), and undergo indicated delivery before 37 weeks and 28 weeks (Table 2). Rates of abruption and preeclampsia were not significantly different between those with and without early second-trimester fetal growth restriction. Although rates of NICU admission and intraventricular hemorrhage were not significantly different between groups, neonates born to those with fetal growth restriction were more likely to need respiratory support and to develop necrotizing enterocolitis. (Table 2).
Rates of neonatal death (1.4% vs 0.3%, OR 5.2, 95% CI 1.6, 13.5) and stillbirth were significantly higher in those with fetal growth restriction (2.5% vs 0.4%, OR 6.2, 95% CI 2.7, 12.8). The gestational age at stillbirth was not significantly different between those with and without fetal growth restriction at the ultrasound to assess anatomy25.1 weeks ± 5.2 vs 27.7 weeks ± 6.9, p=0.29). Among those with fetal growth restriction, the rate of stillbirth was significantly higher among those with estimated fetal weight <5th percentile than those with estimated fetal weight from 5–10th percentile (6.1% vs 0.5%, OR 14.5, 95% CI 1.9, 646.3). (Appendix 1, available online at http://links.lww.com/xxx).
Discussion
Fetal growth restriction between 17 and 22 weeks of gestation, in the absence of those anomalies, aneuploidy, or infection, was significantly associated with SGA at birth. Although the rate of adverse perinatal outcomes among those with the diagnosis was low, it was still elevated and significantly associated with stillbirth, neonatal death, indicated preterm birth, neonatal need for respiratory support, and necrotizing enterocolitis. It was not associated with increased rates of abruption or preeclampsia.
Although it intuitively would seem that earlier diagnosis of fetal growth restriction would lead to worse consequences, estimated fetal weights are known to be less accurate at the lower and higher extremes of estimated fetal size. (17) Additionally, at earlier gestational ages, small changes in measurements can result in large changes in growth percentiles. (9) Given the relative lack of clinical data in this context, clinicians may wonder if growth restriction at the time of ultrasound to assess fetal anatomy without an identifiable cause represents true pathology or measurement imprecision.
Few studies have evaluated outcomes when growth restriction is diagnosed before 24 weeks. A large, population based study conducted by Nakling and colleagues found that lagging growth at the time of 18 week utlrasound of fetal anatomy was associated with increased risks of preterm birth, perinatal death, low birthweight, and SGA. (18) This study included fetuses with known anomalies, infections, and aneuploidy, which makes its results difficult to generalize their findings to fetuses without these conditions. A study by Fox et al compared outcomes between 252 patients with an estimated fetal weight <25th percentile to unmatched controls with normal growth between 18–24 weeks gestation and found increased rates of stillbirth, neonatal death, indicated preterm birth, SGA, and admission to the NICU.
Our findings are consistent with and build on these previous studies. We excluded patients with clear etiologies for growth restriction and used the commonly accepted definition of growth restriction of <10th percentile. We used a large cohort of patients with detailed, prospectively collected data, which enabled us to examine a comprehensive set of outcomes. We had birthweight and outcome data available on 99.4% of those eligible for the study and used three growth curves to assess the diagnosis of SGA. We used both the traditional Alexander growth curve and the more modern Oken and Fenton growth curves. The Oken growth curve is based on US birth certificate data from 1999–2000, while the Fenton growth curve may be more sensitive for use in preterm infants. The use of three curves increases the generalizability and strength of our conclusions regarding SGA.
There are potential limitations of our study. Although the data were collected prospectively, the study design was retrospective in nature and therefore subject to selection bias, confounding, and errors in data collection. However, the database utilized has been validated in several previous studies.(19, 20) Although we controlled for multiple confounders, there is the potential for residual or unmeasured confounding. Additionally, some of the adverse outcomes, including abruption and intraventricular hemorrhage, occurred infrequently and thus we were likely underpowered to detect associations with these outcomes and fetal growth restriction. Finally, detailed information on postnatal diagnosis of anomalies or genetic disorders were not available in the database. This allows for the possibility of inclusion of cases where a cause for growth restriction was discovered postnatally, which could bias the results towards an association with adverse outcomes. However, our approach which included those with early second-trimester fetal growth restriction without an antenatally identifiable cause is consistent with the way growth restriction is diagnosed and managed in clinical practice.
In summary, although fetal growth restriction between 17 and 22 weeks occurred in less than 3% of our cohort and most of those with isolated growth restriction did not have adverse outcomes, it is a strong risk factor for SGA, stillbirth, neonatal death and indicated preterm birth.
Supplementary Material
Acknowledgments
Dr. Temming is supported by a NIH T32 training grant (5T32HD055172-07). This publication was also made possible by Grant Number UL1 TR000448 from the NIH National Center for Advancing Translational Sciences (NCATS), components of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCATS or NIH.
Footnotes
Financial Disclosure
The authors did not report any potential conflicts of interest.
Each author has indicated that he or she has met the journal’s requirements for authorship.
Presented as a poster at the 36th annual meeting of the Society for Maternal and Fetal Medicine, Atlanta, GA, February 1st–6th, 2016.
References
- 1.ACOG Practice bulletin no. 134: fetal growth restriction. Obstetrics and gynecology. 2013 May;121(5):1122–33. doi: 10.1097/01.AOG.0000429658.85846.f9. [DOI] [PubMed] [Google Scholar]
- 2.Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. American journal of obstetrics and gynecology. 2000 Jan;182(1 Pt 1):198–206. doi: 10.1016/s0002-9378(00)70513-8. [DOI] [PubMed] [Google Scholar]
- 3.Boers KE, Vijgen SM, Bijlenga D, van der Post JA, Bekedam DJ, Kwee A, et al. Induction versus expectant monitoring for intrauterine growth restriction at term: randomised equivalence trial (DIGITAT) BMJ (Clinical research ed) 2010:341. doi: 10.1136/bmj.c7087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resnik R. Intrauterine growth restriction. Obstetrics and gynecology. 2002 Mar;99(3):490–6. doi: 10.1016/s0029-7844(01)01780-x. [DOI] [PubMed] [Google Scholar]
- 5.Lees C, Marlow N, Arabin B, Bilardo CM, Brezinka C, Derks JB, et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE) Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2013 Oct;42(4):400–8. doi: 10.1002/uog.13190. [DOI] [PubMed] [Google Scholar]
- 6.Baschat AA, Cosmi E, Bilardo CM, Wolf H, Berg C, Rigano S, et al. Predictors of neonatal outcome in early-onset placental dysfunction. Obstetrics and gynecology. 2007;109(2 PART 1):253–61. doi: 10.1097/01.AOG.0000253215.79121.75. [DOI] [PubMed] [Google Scholar]
- 7.A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG : an international journal of obstetrics and gynaecology. 2003 Jan;110(1):27–32. doi: 10.1046/j.1471-0528.2003.02014.x. [DOI] [PubMed] [Google Scholar]
- 8.Reddy UM, Abuhamad AZ, Levine D, Saade GR. Fetal imaging: executive summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in Ultrasound Fetal Imaging Workshop. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2014 May;33(5):745–57. doi: 10.7863/ultra.33.5.745. [DOI] [PubMed] [Google Scholar]
- 9.Hadlock FP, Harrist RB, Martinez-Poyer J. In utero analysis of fetal growth: a sonographic weight standard. Radiology. 1991 Oct;181(1):129–33. doi: 10.1148/radiology.181.1.1887021. [DOI] [PubMed] [Google Scholar]
- 10.Hadlock FP, Harrist RB, Sharman RS, Deter RL, Park SK. Estimation of fetal weight with the use of head, body, and femur measurements–a prospective study. American journal of obstetrics and gynecology. 1985 Feb 01;151(3):333–7. doi: 10.1016/0002-9378(85)90298-4. [DOI] [PubMed] [Google Scholar]
- 11.Warsof SL, Gohari P, Berkowitz RL, Hobbins JC. The estimation of fetal weight by computer-assisted analysis. American journal of obstetrics and gynecology. 1977 Aug 15;128(8):881–92. doi: 10.1016/0002-9378(77)90058-8. [DOI] [PubMed] [Google Scholar]
- 12.Mirghani HM, Weerasinghe S, Ezimokhai M, Smith JR. Ultrasonic estimation of fetal weight at term: an evaluation of eight formulae. The journal of obstetrics and gynaecology research. 2005 Oct;31(5):409–13. doi: 10.1111/j.1447-0756.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 13.Spong CY, Mercer BM, D’Alton M, Kilpatrick S, Blackwell S, Saade G. Timing of indicated late-preterm and early-term birth. Obstetrics and gynecology. 2011 Aug;118(2 Pt 1):323–33. doi: 10.1097/AOG.0b013e3182255999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstetrics and gynecology. 1996 Feb;87(2):163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 15.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC pediatrics. 2013 Apr 20;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC pediatrics. 2003 Jul 08;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esinler D, Bircan O, Esin S, Sahin EG, Kandemir O, Yalvac S. Finding the best formula to predict the fetal weight: comparison of 18 formulas. Gynecologic and obstetric investigation. 2015;80(2):78–84. doi: 10.1159/000365814. [DOI] [PubMed] [Google Scholar]
- 18.Nakling J, Backe B. Adverse obstetric outcome in fetuses that are smaller than expected at second trimester routine ultrasound examination. Acta Obstet Gynecol Scand. 2002 Sep;81(9):846–51. doi: 10.1034/j.1600-0412.2002.810908.x. [DOI] [PubMed] [Google Scholar]
- 19.Odibo AO, Singla A, Gray DL, Dicke JM, Oberle B, Crane J. Is chorionic villus sampling associated with hypertensive disorders of pregnancy? Prenatal diagnosis. 2010 Jan;30(1):9–13. doi: 10.1002/pd.2410. [DOI] [PubMed] [Google Scholar]
- 20.Carbone JF, Tuuli MG, Dicke JM, Macones GA, Odibo AO. Revisiting the risk for aneuploidy in fetuses with isolated pyelectasis. Prenatal diagnosis. 2011 Jun;31(6):566–70. doi: 10.1002/pd.2749. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


