Abstract
In order to investigate bean-nodulating rhizobia in different types of soil, 41 nodule isolates from acid and alkaline soils in Mexico were characterized. Based upon the phylogenetic studies of 16S rRNA, atpD, glnII, recA, rpoB, gyrB, nifH and nodC genes, the isolates originating from acid soils were identified as the phaseoli symbiovar of the Rhizobium leguminosarum-like group and Rhizobium grahamii, whereas the isolates from alkaline soils were defined as Ensifer americanum sv. mediterranense and Rhizobium radiobacter. The isolates of “R. leguminosarum” and E. americanum harbored nodC and nifH genes, but the symbiotic genes were not detected in the four isolates of the other two species. It was the first time that “R. leguminosarum” and E. americanum have been reported as bean-nodulating bacteria in Mexico. The high similarity of symbiotic genes in the Rhizobium and Ensifer populations showed that these genes had the same origin and have diversified recently in different rhizobial species. Phenotypic characterization revealed that the “R. leguminosarum” population was more adapted to the acid and low salinity conditions, while the E. americanum population preferred alkaline conditions. The findings of this study have improved the knowledge of the diversity, geographic distribution and evolution of bean-nodulating rhizobia in Mexico.
Keywords: Rhizobia, Biogeography, Phylogeny, Soil pH, Mobility, Phaseolus
Introduction
Phaseolus vulgaris (L), commonly known as bean or common bean, is a legume species that originated in Mexico and the Andes Mountains. It has been cultivated worldwide as a grain or vegetable crop, and forms root nodules in different regions with a wide range of symbiotic nitrogen-fixing bacteria, including the Rhizobium species R. etli, R. gallicum, R. giardinii, R. leguminosarum, R. lusitanum, R. phaseoli, R. vallis, R. leucaenae [36], R. tropici [4], R. mesoamericanum [25], R. freirei [11] and R. azibense [29], as well as Ensifer meliloti [50], Ensifer americanum [30], and Bradyrhizobium sp. [17]. In addition, P. vulgaris can be nodulated by several symbiotic or endophytic Rhizobium species originally isolated from other plants, including R. mongolense and R. oryzae [32], as well as some strains of the phytopathogenic species Rhizobium rhizogenes [43].
Among the bean nodulating rhizobia, only R. etli, R. gallicum, R. mesoamericanum and R. leucaenae have been isolated in Mexico. It is believed that most of the species of bean-nodulating rhizobia have acquired their nodulation ability through lateral transfer of symbiotic genes from R. etli because they harbor symbiotic genes (nifH and nodC) identical or very similar to those of R. etli in phylogenetic analyses [1,30,43,50]. However, the existence of two symbiovars in the bean-nodulating rhizobia, sv. phaseoli in Rhizobium species and sv. mediterranense in Ensifer species, has demonstrated that their symbiotic genes may have the same origin, although they have evolved independently. These data have shown that bean is a relatively promiscuous host for rhizobia which strongly prefers a symbiotic genomic background rather than a genomic background. In this case, it can be hypothesized that the introduction and cultivation of bean plants in different regions have forced the evolution of some local bacteria to be bean-nodulating rhizobia, so that bean plants can form symbiosis with rhizobia adapted to diverse conditions.
It has been estimated that the center of origin for a legume species is also the center of diversification of rhizobia associated with the same plant [27]. However, the lower species number of bean rhizobia in Mexico compared to other regions does not fit this estimation. Previous studies have shown the biogeographic patterns of bean rhizobia: R. etli is dominant in the South and Middle Americas, Europe and Jordan [12,39]; R. leguminosarum is the main species in the Andean region and Nepal [1,7,35]; R. tropici is more abundant in regions with acid soils and high temperature [4,14]; R. phaseoli, R. etli and a novel Rhizobium group are found in Africa [5]; while the Ensifer spp. are unique for alkaline-saline soils [28,30,50]. These data, as well as the studies on soybean rhizobia, have shown that the geographic distribution and diversity of rhizobia are mainly determined by the distribution of the host legume and soil parameters such as pH, salinity and nutrient content [16,49]. Therefore, we hypothesized that more species might be found when bean nodules were collected from different soil types in Mexico.
The present study was performed because the diversity and richness of bean-nodulating rhizobial species in Mexico provide important information for the biogeography and evolution of rhizobia, and there was a lack of a comparative study on the relationship between rhizobial diversity and the soil types in Mexico. Therefore, the aim of the study was to investigate whether distinct rhizobia exist in different Mexican soils.
Materials and methods
Soil sampling and characterization
Based upon the climate types, geographic distance and soil types, five soil samples were collected from plots (approximately 1 ha each) in fields with a history of recent bean cultivation (within one to two years), except in the Xochimilco location. The sampling sites were: (1) Xochimilco, Mexico City, where the soils were sampled from an uncultured “chinampa”, an artificial island made of lake sediment and dead vegetation, located in the Canal de Apatlaco, Community of Puente de Alvarado; (2) General Cepeda, Coahuila: located in Buenos Aires/Narihua, at Ejido El Gabillero, where the soil was taken in a field with bean cultivation; (3) Parras de la Fuente, Coahuila: located in Ejido 28 de Agosto where the soil was sampled from a field with bean cultivation (cv. Pinto Americano); (4) Acaxochitlán, Hidalgo: located at Carretera Tulancingo-Tuxpan 170 km, in the Community of Tepepan, with an intercropping history of bean and maize; (5) Ocozocuautla, Chiapas: located on the road from Arriaga to Domingo Chanona-El Cielito at the Community of La Frailesca, Ejido Domingo Chanona, where the soil was sampled from a field used to test the acid resistance of bean. Soils were sampled from five points (the four corners and the center) in each site, which were mixed in the same ratio to form a compiled sample, and they were stored at 4°C in black plastic bags until use.
The physicochemical features of the soil samples were determined according to the NMX-EC-17025-IMNC-2006 (ISO/IEC17025:2005) standard, and included pH, nutrient content.
Rhizobial counting and trapping in the soil
To determine the abundance of rhizobia in the soil samples and to isolate them, seeds of two bean cultivars commonly cultivated in Mexico were used: Flor de Mayo and Negro Querétaro. Flor de Mayo is the most common seed for cultivation and for consumption in Mexico, and it is adapted to adverse conditions such as high temperature, draught and poor soils. Negro Querétaro is a cultivar more adapted to acidic soils.
For trapping the rhizobia from soils, the bean seeds were surface-sterilized by 1% (w/v) sodium hypochlorite for 5min and washed six times with sterilized distilled water, as mentioned elsewhere [44], and germinated in Petri dishes with water-agar under aseptic conditions at 28 °C in the dark. Four of the germinated seeds were sown in a Leonard jar (Styrofoam cups with a volume of 1 L) [23] filled with a mixture of vermiculite-peat moss-soil (volume ratio 2:2:1) moistened with N-free Faraheus plant nutrient solution [44]. Ten jars were involved in each treatment (cultivar vs. soil sample). The seedlings were grown in a greenhouse under sunlight at room temperature during March and May 2010 for four weeks.
The abundance of bean-nodulating rhizobia in the soil samples was estimated by the most probable number method described by Vincent [44] who used a similar procedure for rhizobia trapping. Two seeds were sown in each Leonard jar (200 mL Styrofoam cup) filled with a mixture of vermiculite-peat moss (1:1, v/v). The seeds in each jar were inoculated with 1 mL of diluted soil (10−1, 10−2 and 10−3), and five repetitions were used. Positive or negative nodulation was observed after four weeks growth.
Isolation of rhizobia
Rhizobia were isolated with a standard procedure [44] from the nodules of trap plants grown in the mixture of vermiculite-peat moss-soil sample (2:2:1, v/v). Briefly, the root nodules were cut from the plant, surface-sterilized in the same way as for seed preparation mentioned above, and then approximately 20 nodules from each treatment were crushed and streaked separately on peptone yeast agar (PY) (peptone, 5g; yeast-extract, 3g; CaCl2, 0.6g; agar, 18g; pH 7.0) plates. The streaked plates were incubated at 28 °C for 3–14 days. After single colonies occurred, one colony for each nodule isolate was picked and repeatedly streaked on the same medium until the morphology was homogenous for all the colonies on the plate. The purity of the isolates was confirmed by microscopic observation after Gram-staining. Only the pure cultures of Gram-negative rods were used in the subsequent studies and they were stored in PY broth containing 20% (w/v) glycerol at −70 °C for long-term storage or on PY plates at 4°C for short-time storage.
Sequence analysis of the 16S rRNA gene
In the present study, analysis of amplified 16S rDNA restriction fragment length polymorphism (ARDRA) was used to group the isolates [19,21]. Then, the representative isolates for each ARDRA group (rDNA type) were selected for 16S rDNA sequencing. The genomic DNA of each isolate was extracted from 5 mL of a tryptone yeast (TY) broth culture (peptone in PY replaced with tryptone) by the guanidine thiocyanate (GuTC) method [41] and it was used as template for amplification of the gene fragments. The 16S rRNA gene was amplified with the primers fD1 and rD1 using the protocol described by Weisburg et al. [48]. The PCR products were checked by electrophoresis in 1% (w/v) agarose gel (0.5 × TBE as electron buffer), and they were visualized under UV light after staining with ethidium bromide (0.5 |μgmL−1) [21]. ARDRA was performed by digesting 10 μL of the PCR products separately with the restriction endonucleases MspI, AluI, HaeIII and HinfI, as described previously [21]. Isolates sharing the same RFLP patterns were defined as an rDNA type. Representative isolates for each rDNA type were randomly selected for genus identification by sequence analysis. The 16S rDNA was amplified from each of the representative isolates with primers P1 and P6 [16] and it was sequenced directly, as described previously [18]. The acquired nucleotide sequences were compared with those in GenBank by Blast searching [2]. The most related sequences extracted from the database were aligned together with the acquired sequences using the CLUSTAL W program [42] and edited with SeaView 4.2.5 [13]. The search for an appropriate substitution model was conducted using jModel test [34]. The phylogenetic trees were constructed in the PhyML 3.0 online server (http://www.atgc-montpellier.fr/phyml/) and visualized in the MEGA 6 software package [40]. Phylogenetic trees were reconstructed with the neighbor-joining (NJ) method and the Jukes-Cantor distance [40]. Bootstrap analysis based on 1000 replications was performed in order to check the stability of the grouping results.
Identification of rhizobia by multilocus sequence analysis (MLSA)
Currently, MLSA is used widely in bacterial species definition [45] and the atpD, glnII, gyrB, recA and ropB genes are those that can differentiate defined rhizobial species [15,37,45]. In the present study, these five genes were amplified from the representative isolates with the previously reported methods and primer sets atpD255F/atpD782R, glnII12F/glnII6810R, gryB343F/gryB1043R, recA41F/recA640R and rpoB454F/rpoB1364R, respectively [37,45]. The acquired sequences were used in phylogenetic analyses with methods similar to 16S rDNA analysis, but the Kimura two-parameter distance [20] was calculated and used in the phylogenetic analysis of the housekeeping genes.
Symbiotic performance of the isolates
The nifH symbiotic gene was amplified for all isolates with primer pair nifHF/nifHI and the protocol described by Laguerre et al. [22]. Then, the nifH gene with the same primers and nodC genes (coding for N-acetylglucosaminyltransferase) with primers nodC540/nodC1160 [38] were amplified for the representative isolates. The phylogenetic analysis of these two genes was the same as for 16S rDNA, and the phylogenetic trees were constructed with the amino acid sequences. The nodulation test was performed with two isolates from each rhizobial group defined by the phylogenetic analyses mentioned above. The method was the same as that for rhizobial trapping, but the vermiculite-peat moss-soil (2:2:1, v/v) mixture was sterilized and each seedling was inoculated with 0.1 mL (approximately 107 CFU) of a 24 h old PY broth culture.
Phenotypic characterization of the isolates
Phenotypic characterization of the isolates focused on the adaptation features, including growth in PY medium supplied with 0.5–3.0% NaCl in intervals of 0.5%, and growth in PY medium at pH 4.0–10.0 with intervals of 0.5 units, as described previously [46]. In addition, the mobility (swimming) of the isolates was determined in semisolid PY medium (0.4% agar), as described previously [9,26]. All the assays were performed at 28 °C and they were incubated for 2 or 3 days.
Results
Soil characterization and rhizobial abundance
In general, the five soil samples had a Fraco or Arcillo Limoso texture with various pH values and nutrient contents (Table 1). No nodules were observed on the seedlings of bean cv. Flor de Mayo or Negro Querétaro grown in soils collected from Xochimilco, Mexico City and from Ocozocuautla, Chiapas State. The reason for absence of nodulation in the chinampa soils (xochimilco) may be related to their high nutrient content, especially the high N content (1068.1 mgkg−1), which may inhibit nodulation and nitrogen fixation. In this field, it was observed that both the bean and alfalfa plants did not form nodules. The absence of nodulation in the Ocozocuautla soil may be related to its strong acid feature (pH 4.25), which might be outside the limit for survival and activity of rhizobia. In the remaining three soil samples, rhizobia were trapped by both bean cultivars (Table 1), in which the General Cepeda soil (pH 7.84) harbored a major abundance of rhizobia (MPN = 104 g−1 of soil), while the MPNs in the other two soils from Parras de la Fuente (pH 7.86) and Acaxochitlán (pH 4.92) were 82-620 and 320-450, respectively, with the two cultivars. In all soils, cv. Negro Querétaro showed stronger nodulation ability than cv. Flor de Mayo.
Table 1. Location, physicochemical characteristics and rhizobial abundance of the soil samples used in this study.
| Soil character | Sample site | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Xochimilco, Mexico City | Ocozocuautla, Chiapas | General Cepeda, Coahuila | Parras de la Fuente, Coahuila | Acaxochitlán, Hidalgo | ||
| Texture | Sandy loam | Sandy loam | Silty clay loam | Silty clay | Loam | |
| Field capacity (g kg−1) | 750.0 | 225.0 | 232.5 | 337.5 | 517.5 | |
| Electric conductivity (dS m−1) | 4.193 | 0.883 | 0.420 | 0.629 | 1.059 | |
| Organic material (g kg−1) | 157.70 | 17.40 | 7.50 | 9.50 | 77.90 | |
| Total nitrogen (mg kg−1) | 1068.10 | 39.44 | 10.57 | 39.62 | 204.89 | |
| Total phosphor Olsen (mg kg−1) | 258.74 | 28.59 | 12.90 | 6.85 | 16.28 | |
| Total phosphor Bray (mg kg−1) | 239.26 | 58.06 | 10.46 | 7.63 | 8.00 | |
| Potassium (mg kg−1) | 2345.79 | 175.20 | 194.80 | 288.41 | 598.54 | |
| Calcium (mgkg−1) | 4513.85 | 1400.95 | 4807.15 | 5108.19 | 2377.13 | |
| Magnesium (mg kg−1) | 2784.10 | 109.89 | 5.26 | 229.61 | 127.19 | |
| Sodium (mgkg−1) | 782.58 | 105.46 | 60.65 | 94.57 | 81.47 | |
| Iron (mgkg−1) | 26.26 | 247.66 | 0.87 | 0 | 58.87 | |
| Zinc (mgkg−1) | 8.01 | 1.40 | 0.17 | 0 | 0.42 | |
| Manganese (mg kg−1) | 0 | 16.84 | 0 | 0 | 4.83 | |
| Copper (mgkg−1) | 0.86 | 0.85 | 0.73 | 0.74 | 1.25 | |
| Boron (mgkg−1) | 17.42 | 1.00 | 1.00 | 1.34 | 0.80 | |
| Sulfur (mgkg−1) | 293.88 | 139.20 | 61.25 | 77.84 | 33.54 | |
| pH | Value | 8.72 | 4.25 | 7.84 | 7.86 | 4.92 |
| Definitiona | Strong alkaline | Strong acid | Intermediate alkaline | Intermediate alkaline | Strong acid | |
|
| ||||||
| MPN of rhizobiab | Flor de Mayo | 0 | 0 | 1.1 ×104 | 82 | 320 |
| Negro Querétaro | 0 | 0 | >1.8×104 | 620 | 450 | |
According to official Mexican standard NOM-021-RECNAT-2000.
Most probable number of rhizobia was estimated by the plant trapping method using the two cultivars, respectively.
Isolation of rhizobia and genus definition by 16S rDNA phylogeny
A total of 41 isolates were obtained from both the trap plant cultivars, of which 24 were from the Hidalgo acid soil and 17 were from the alkaline soils of Coahuila (Table 2). In the ARDRA, six rDNA types were defined. All the isolates in rDNA types 1-3 originated from the acid soil of Hidalgo State, while all the isolates from alkaline soils were grouped in rDNA types 4-6 (Table 2). In the 16S rRNA gene phylogenetic tree (available as Supplementary Fig. S1), isolates in rDNA type 1 showed almost identical sequences with those of R. leguminosarum and R. laguerreae, whereas two isolates of rDNA types 2 and 3 were most similar to Rhizobium grahamii, two isolates in rDNA type 6 were grouped with Rhizobium radiobacter, and the fifteen isolates in rDNA types 4 and 5 were most similar to Ensifer fredii.
Table 2. Rhizobial isolates from bean plants and their relevant characters.
| Isolatea | nifHb | RNA RFLPc | Growth in TY with NaCl (%) | Growth in TY medium at pH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||||
| Pattern | Type | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 4.5 | 5.0 | 5.5 | 8.0 | 8.5 | 9.0 | ||
| R. leguminosarum–like group (22) | ||||||||||||||
| FH14 | + | + | + | + | + | + | + | + | + | + | + | + | ||
| FH19 | + | + | + | + | + | + | + | + | + | + | + | + | ||
| FH31 | + | W | − | − | − | − | − | + | + | − | − | − | ||
| FH32 | + | + | − | − | − | − | W | + | + | + | + | W | ||
| FH20 | + | + | − | − | − | − | + | + | + | + | − | − | ||
| FH23 | + | + | W | − | − | − | + | + | + | + | + | W | ||
| FH25 | + | + | + | − | − | − | − | + | + | + | + | + | ||
| FH27 | + | + | + | − | − | − | − | + | + | + | + | + | ||
| FH34 | + | + | − | − | − | − | W | + | + | + | W | W | ||
| NH01 | + | + | W | − | − | − | W | + | + | + | W | − | ||
| NH05 | + | + | W | − | − | − | − | + | + | + | W | − | ||
| NH06 | + | AAAA | 1 | + | W | − | − | − | + | + | + | + | + | + |
| NH07 | + | ND | ND | ND | ND | ND | − | − | ND | ND | ND | ND | ||
| NH10 | + | + | − | − | − | − | − | + | + | + | + | − | ||
| NH15 | + | + | − | − | − | − | − | + | + | + | + | − | ||
| NH16 | + | + | − | − | − | − | − | + | + | + | + | + | ||
| NH37 | + | + | W | W | − | − | − | + | + | + | + | + | ||
| FH02 | ND | ++ | + | − | − | − | − | + | + | + | + | + | ||
| FH08 | ND | ++ | W | − | − | − | − | + | + | + | + | + | ||
| FH11 | ND | + | W | − | − | − | − | + | + | + | + | + | ||
| FH12 | ND | + | W | − | − | − | − | + | + | + | + | + | ||
| FH13 | ND | + | W | − | − | − | − | + | + | + | + | + | ||
| R. grahamii (2) | ||||||||||||||
| FH29-1 | + | AABA | 2 | + | − | − | − | − | + | + | + | + | + | + |
| NH18 | + | AADA | 3 | + | + | − | − | − | W | + | + | + | + | − |
| Ensifer americanum (15) | ||||||||||||||
| FG01 | + | + | + | W | − | − | − | + | + | + | + | + | ||
| FG04 | + | + | + | W | − | − | − | + | + | + | + | + | ||
| FG08 | + | + | + | + | W | − | − | + | + | + | + | + | ||
| FG03 | ND | + | + | + | W | − | − | + | + | + | + | + | ||
| FG09 | + | + | + | W | W | − | − | + | + | + | + | + | ||
| FG10 | + | EAAH | 4 | + | + | W | W | − | − | + | + | + | + | + |
| NG13 | ND | + | + | + | W | − | − | + | + | + | + | + | ||
| NG07A | + | + | + | + | − | − | − | + | + | + | + | + | ||
| NG07B | + | + | + | + | W | − | − | + | + | + | + | + | ||
| NG12 | + | + | + | + | − | − | − | + | + | + | + | + | ||
| NG16 | + | + | + | + | − | − | − | + | + | + | + | + | ||
| NG35 | ND | + | + | W | − | − | − | + | + | + | + | + | ||
| FG17B | + | + | + | W | − | − | − | + | + | + | + | + | ||
| FG20 | + | EADH | 5 | + | + | W | − | − | − | + | + | + | + | + |
| FG25 | + | + | + | W | W | − | − | + | + | + | + | + | ||
| Rhizobium radiobacter (2) | ||||||||||||||
| NF20A | + | HADA | 6 | ++ | ++ | ++ | ++ | ++ | + | + | + | + | + | + |
| NF22 | + | ++ | ++ | ++ | ++ | ++ | + | + | + | + | + | + | ||
| Reference strains | ||||||||||||||
| E. meliloti Rm1021 | + | + | + | + | + | − | + | + | + | + | + | |||
| R. giardinii H152T | + | W | − | − | − | − | W | + | + | + | W | |||
| R. gallicum USDA2918T | + | W | W | − | − | − | + | + | + | + | W | |||
| R. leguminosarum USDA2370T | + | W | − | − | − | − | + | + | + | + | W | |||
| R. etli CFN42T | + | W | − | − | − | − | + | + | + | + | + | |||
| R. phaseoli ATCC14482T | + | + | − | − | − | − | + | + | + | + | + | |||
| R. lusitanum LMG22705T | + | W | − | − | − | + | + | + | + | + | + | |||
+, growth; ++, vigorous growth; −, no growth; ND, not determined; W, weak growth.
The first letter in the isolate number represents the origin of the cultivar (e.g. F = Flor de Mayo and N = Negro Querétaro), whereas the second letter corresponds to the soil sample site (e.g. H = Hidalgo, G = General Cepeda, Coahuila, F=Parras de la Fuente, Coahuila). Boldface isolates were isolates used in sequence analysis.
The nifH gene was detected by PCR after isolation.
PCR-based RFLP of 16S rRNA gene digested seperately with the restriction endonucleases MspI, AluI, HaeIII and HinfI.
Species identification of the isolates by MLSA
In the phylogenetic tree of the concatenated sequences (Fig. 1), the isolates of type 1 formed a distinct group most related to R. leguminosarum, R. phaseoli and R. etli; and the separated nucleotide sequences of recA, atpD and glnII (available as Supplementary Figs. S2-S4) showed that these isolates were more similar to different subgroups of R. leguminosarum which may have intermingled with other species. In this case, they were defined as the R. leguminosarum-like group. The representative isolates of types 2 and 3, and type 6 always showed identical or high similarity (>97%) with the reference strains of R. grahamii and R. radiobacter, respectively (Fig. 1 and Supplementary Figs. S2-S4). Therefore, they were identified as members of these two species. The isolates in rDNA types 4 and 5 showed the highest similarities (98%) with reference strains of E. fredii in the atpD tree, but they presented the highest similarities (99-100%) with strains ofE. americanum in the concatenated tree, and recA and glnII trees. In this case, the Ensifer isolates were identified as E. americanum. The sequences of gyrB and rpoB were not included in the concatenated analysis since these genes were not available for all the reference strains. The gyrB and rpoB phylogenetic trees (available as Supplementary Figs. S5 and S6) supported the above identifications: the isolates in rDNA types 1,4 and 6 were grouped with the R. leguminosarum complex (including R. etli, R. phaseoli, R. fabae, R. pisi, etc.), R. radiobacter and E. americanum, respectively, or appeared as distinct groups for FH210-1 (rDNA type 2) and for the isolates of rDNA types 5 and 6 when the R. grahamii and/or E. americanum reference strains were absent.
Fig. 1.
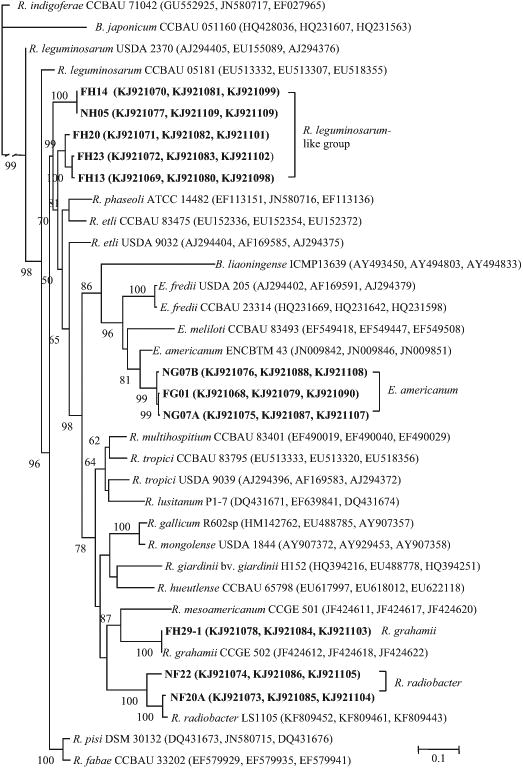
Neighbor-joining (NJ) tree of concatenated sequences of atpD, glnII and recA genes (accession numbers are in brackets) reconstructed with the Kimura two-parametertest, showing the phylogenetic relationships of the bean nodule bacteria from acid and alkaline soils in Mexico (in boldface). Bootstrap values greater than 50% are shown at the nodes. The scale bar indicates 10% nucleotide substitution. B. japonicum CCBAU 051160 was used as an out group.
Symbiotic gene phylogeny and the nodulation test
In the present study, the nodC and nifH genes were amplified and sequenced successfully for the representative isolates of the E. americanum and R. leguminosarum group. The amplification of the symbiotic genes was unsuccessful for the R. radiobacter and R. grahamii isolates. In the nodC phylogenetic tree (Fig. 2), the representative isolates were grouped within two closely related subclusters: (1) three R. leguminosarum reference isolates showed nodC genes very similar or identical to those of R. etli, R. phaseoli and R. gallicum sv. phaseoli reference strains, which formed a subcluster that was defined as symbiovar phaseoli; (2) the representative isolates of E. americanum formed a subcluster with those of several bean-nodulating E. meliloti strains, and they were defined as symbiovar mediterranense. These two subclusters were closely related to each other and were linked to the Mimosa-nodulating R. etli and R. giardinii strains. The close relationships between our isolates and R. etli for the symbiotic genes were also confirmed by the nifH phylogeny (available as Supplementary Fig. S7). The three representative isolates of the R. leguminosarum group showed high similarities with the bean nodulating strains of R. etli, R. phaseoli, R. gallicum, and R. vallis. The three isolates representing E. americanum showed high similarities with the bean nodulating strain of E. fredii bv. mediterranense and strains of E. chiapanecum, E. mexicanus and E. americanum nodulating Acaciella and Acacia species in Mexico.
Fig. 2.
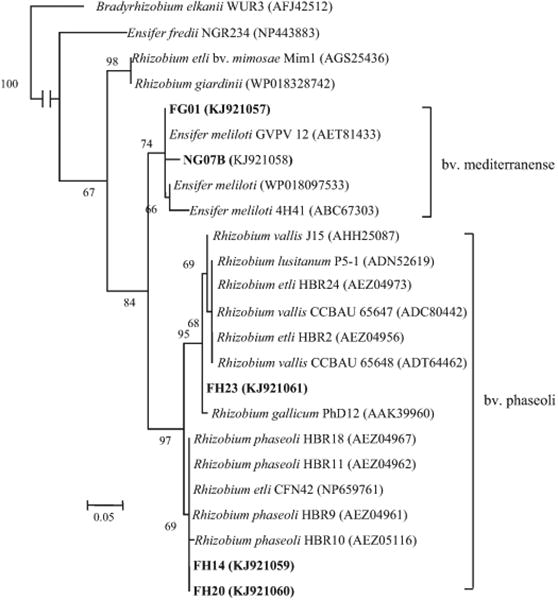
Neighbor-joining (NJ) tree of nodC genes (accession numbers are in brackets) reconstructed with the Jukes-Cantor distance, showing the phylogenetic relationships of the bean nodule bacteria from acid and alkaline soils in Mexico (in boldface). Bootstrap values greater than 50% are shown at the nodes. The scale bar indicates 5% nucleotide substitution. B. elkanii WUR3 was used as an out group.
The two R. radiobacter isolates were not included in the nodulation test. The R. leguminosarum-like strains FH02 and NH05 formed nodules with plants of cv. Negro Querétaro in alkaline soil, while only FH02 occasionally nodulated with plants of cv. Negro Querétaro in acid soil. No nodules were found on plants of cv. Flor de Mayo grown in either the acid or alkaline soil inoculated with these two isolates. The two E. americanum isolates FG17B and NG07B formed nodules on most seedlings of both cultivars in both the acid and alkaline soils. R. grahamii FH29-1 formed nodules on the seedlings of both cultivars in both the acid and alkaline soils, but NH18 did not nodulate at all.
Growth and mobility of the isolates in different conditions
The results presented in Table 2 demonstrated that the tested isolates grew well most of all in medium supplied with 0.5% NaCl. The 1.0% NaCl supplement inhibited the growth of most of the Rhizobium isolates, and the growth of the Ensifer isolates was inhibited by 1.5% NaCl. However, two R. leguminosarum-like isolates, FH14 and FH19, and the two R. radiobacter isolates grew well at up to 2.5% NaCl.
No growth was observed in medium with pH 4.0 for all the isolates and in medium with pH 4.5 for the E. americanum isolates. At pH 5.0-8.0, most of the R. leguminosarum and R. grahamii isolates grew well and some of them were inhibited by pH 8.5 and pH 9.0. All the E. americanum isolates grew well in medium with pH 5.0-9.0.
The data in Table 3 showed that the mobility (swimming) of R. leguminosarum-like and R. grahamii isolates increased when the NaCl concentration was increased from 0.5% to 1.0% and 1.5%. The E. americanum isolates swam weakly in the presence of 0.5% and 1.0% NaCl, but they did not grow in the semisolid medium with 1.5% NaCl. For both the Rhizobium and Ensifer isolates, maximum mobility was observed at neutral pH. At pH 4.5 and 5.0 the mobility of Rhizobium isolates decreased dramatically, compared with that at neutral and alkaline pH values. No growth was observed at pH 4.5 and very weak growth/mobility was observed at pH 5.0 for the Ensifer isolates in the semisolid medium. Mobility was observed in the Ensifer isolates only between pH 6.0-8.0 and, although they could grow, they did not express mobility when the pH was increased further.
Table 3. Mobility data (diameter of colony in mm) in soft PY medium (0.4% agar) incubated at 30°Ca.
| Isolate | With NaClb | With pHc | Incubation time (h) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| 0.5% | 1.0% | 1.5% | 4.5 | 5.0 | 6.0 | 7.0 | 8.0 | 8.5 | 9.0 | ||
| R. leguminosarum-like group | |||||||||||
| FH14 | 5.0 ± 0.0 | 6.0 ± 0.0 | 12.5 ±5.5 | 6.0 ±0.0 | 6.0 ± 1.0 | 23.3 ±1.5 | >40 | 22.0 ±2.0 | 12.0 ±3.0 | 14.5 ±4.5 | 48 |
| FH19 | 5.0 ± 0.5 | 8.0 ± 1.0 | 23.0 ±2.0 | 5.2 ±0.8 | 5.5 ± 0.5 | 30.0 ± 0.0 | >40 | 30.0 ±0.0 | 12.5 ±4.5 | 14.0 ±4.0 | 48 |
| FH20 | 4.0 ± 0.0 | 5.5 ± 0.5 | 16.0 ±6.0 | 4.0 ±0.0 | 4.5 ± 0.5 | 19.0 ± 1.0 | 27.5 ± 2.5 | 17.0±1.0 | 12.0 ±4.0 | 12.5 ±5.5 | 48 |
| FH23 | 7.0 ± 1.0 | 10.0± 1.0 | 29.0 ±3.0 | 6.5 ±0.5 | 5.5 ± 0.5 | >45.0 | >40 | 35.0 ±0.0 | 12.5 ±3.5 | 22.0 ± 2.0 | 48 |
| FH31 | 5.0 ± 0.5 | 7.0 ± 0.0 | 15.0±1.0 | 4.5 ±1.0 | 5.0 ± 0.0 | 21.0 ±0.0 | >40 | 25.0 ±1.0 | 12.5 ±5.5 | 14.5 ±4.5 | 48 |
| FH32 | 5.0 ±5.0 | 5.0 ± 0.0 | 12.5 ±2.5 | − | 4.0 ± 1.0 | 22.0 ± 1.0 | >40 | 27.0 ±1.0 | 12.5 ±0.5 | 16.5 ±2.5 | 48 |
| FH34 | 6.5 ±1.0 | 9.0 ± 0.0 | 30.5 ± 2.5 | 6.0 ±0.0 | 5.0 ± 0.0 | >40.0 | >40 | 37.01.0 | 15.5 ±2.5 | 24.5 ±3.5 | 48 |
| R. grahamii | |||||||||||
| FH29-1 | 5.5 ± 0.5 | 8.0 ± 1.0 | 21.0± 1.0 | 5.0 ±0.0 | 5.5 ±1.0 | 25.0 ± 0.0 | >40 | 28.0 ± 1.0 | 14.0±3.0 | 16.5 ±2.5 | 48 |
| E. americanum | |||||||||||
| FG01 | w | w | − | − | w | 9.5 ±1.5 | 11.0 ±0.0 | 6.0 ±0.0 | + | + | 36 |
| FG04 | w | w | − | − | w | + | 4.5 ± 0.5 | + | + | + | 36 |
| FG17B | w | w | − | − | w | 8.0 ± 0.0 | 9.0 ± 0.0 | 6.0 ±0.0 | + | + | 36 |
| FG20 | w | w | − | − | w | 9.5 ± 0.5 | 11.0 ±0.0 | 8.0 ±0.0 | + | + | 36 |
| FG25 | w | w | − | − | w | 6.0 ± 0.0 | 9.0 ± 0.0 | + | + | + | 36 |
| NG07A | w | w | − | − | w | 7.5 ±1.5 | 10.0 ±0.0 | 6.0 ±0.0 | + | + | 36 |
| NG12 | w | w | − | − | w | + | + | + | w | w | 36 |
| NG16 | w | w | − | − | w | 6.0 ± 0.0 | 8.0 ± 0.0 | 5.00.0 | w | w | 36 |
Data are mean ± variation, n = 2; W, weak mobility; −, no growth; +, grow without mobility.
2.0% NaCl was also tested, but no isolate grew under this condition.
At pH 10, the Rhizobium isolates grew and moved weakly, while no Ensifer isolate grew.
Discussion
Currently, the phylogenetic relationships of the 16S rRNA gene and the MLSA of housekeeping genes, such as atpD, glnII and recA, are used as the basis for defining rhizobial genus and species, respectively. Using 97% MLSA similarity as the threshold of species [24,35], the 41 isolates used in the present study were identified as R. leguminosarum-like group, R. grahamii, R. radiobacter and E. americanum (Table 2 and Fig. 1). Previously, R. leguminosarum and E. americanum have been described as bean-nodulating rhizobia in other regions [1,7,30,36,47]. However, our study provides the first set of clear evidence that bean-nodulating R. leguminosarum-like group, R. grahamii and E. americanum populations were native to Mexico, and that no R. etli were isolated in the acid and alkaline soils in Mexico. Similar to a previous study [10], the isolates of R. radiobacter could be regarded as nodule endophytes.
Symbiotic gene phylogeny has been used to define the symbiovars among the rhizobia and to estimate their host range [30]. According to the nodC and nifH phylogenetic relationships (Fig. 2 and Supplementary Fig. S7), the isolates of E. americanum could be defined as symbiovar mediterranense, which covers some strains of E. meliloti, E.fredii and E. americanum nodulating with bean, as well as Leucaena and Acacia [30]. The isolates of the R. leguminosarum-like group from this study might be defined as symbiovar phaseoli, based upon their high similarity with R. etli, R. phaseoli, R. vallis and R. gallicum in the nodC and nifH phylogenetic trees (Fig. 2 and Supplementary Fig. S7). The failure to amplify symbiotic genes in the sequence analysis and the fact that one isolate showed nodulation ability, but another did not, demonstrated that the symbiotic gene clusters for these two isolates might be unstable without host selection.
These findings added new and important information concerning the distribution and evolution of bean-nodulating bacteria, as discussed subsequently.
Firstly, the absence of R. etli in the acid and alkaline soils sampled in the present study (Tables 1 and 2) implied that R. etli strains might be symbiotic bacteria adapted to neutral and slightly acid soils, since it has been reported as the dominant group in soils with pH 6.4 in Mexico [8] and pH 5.3-5.5 in China [47]. In addition, the predominance of E. americanum in alkaline soil and the R. leguminosarum-like group in acid soil implied that they were bean-nodulating rhizobia native to Mexico and were more competent in these soils than the previously reported R. mesoamericanum and R. leucaenae.
Secondly, the dominance of E. americanum in alkaline soils and R. leguminosarum in acid soils (Tables 1 and 2) [1,7,30,35], as well as the absence of R. etli in this study, showed that the distribution and evolution of bean-nodulating bacteria have been affected by both soil characters, such as pH values, and the host plant, which is similar to the situation for soybean rhizobia [16,49]. Furthermore, the nodulation ability of representative isolates of these two rhizobial groups in the sterilized soils might indicate that soil biological factors, such as the microbial communities, also affected the nodulation of bean plants with different rhizobia. It is possible that the microflora in soils helped the R. leguminosarum-like group and E. americanum to be predominant bean rhizobia in acid and alkaline soils, respectively.
Thirdly, the results of this study showed that the symbiotic genes of different bean-nodulating rhizobial species in Mexico had the same origin and they were less diversified. However, with our results, it cannot be concluded that R. etli is the original source of bean-nodulating genes, and it is questionable that the species found in other regions, but not in Mexico, might have obtained their bean-nodulating ability by lateral transfer of symbiotic genes from R. etli [3,12,50]. It is possible that the bean-nodulating R. leguminosarum and E. americanum populations in other regions were also introduced from Mexico, as estimated for R. etli [33].
Fourthly, the existence of R. radiobacter, and possibly also R. grahamii, as endophytes in bean nodules implied that they were potential bean-nodulating rhizobia which could emerge in the future by acquiring the symbiotic genes via lateral gene transfer, as reported in other cases [6,12,31].
The phenotypic characterization was performed mainly for estimating the adaptation ability of the isolates. The growth data (Table 2) revealed that the E. americanum isolates were resistant to a higher NaCl concentration (1.5%) and a greater alkaline condition (pH 9.0) than the R. leguminosarum-like isolates, while some R. leguminosarum-like isolates were more resistant to acid conditions (pH 4.5). These features demonstrated that these two rhizobial populations possessed mechanisms for adapting to their original soil types.
The mobility analysis (Table 3) gave some interesting information and may support the growth data. In general, the mobility of bacteria is considered as a result of chemotaxis, and bacteria may move away from a chemical stimulus when conditions in their habitat are not adequate. Therefore, it could be assumed that the bacteria should move less when they grow under their optimal conditions. For both the R. leguminosarum-like group and R. grahamii, greater mobility in neutral and alkaline conditions, and less mobility in semisolid medium with pH 4.5 and 5.0 were observed. For isolates of E. americanum, the apparent mobility was only observed at pH 6.0-8.0, but not at pH 8.5 and 9.0. These data might provide evidence to show that the R. leguminosarum-like population was adapted to acid soils and the E. americanum population was adapted to alkaline conditions. The increased mobility of R. leguminosarum-like isolates associated with enhancement of the NaCl concentration and the weak mobility of E. americanum in semisolid medium with 0.5% and 1.0% NaCl demonstrated that the latter might be more adapted to the saline soils. Furthermore, in the mobility analysis, R. leguminosarum-like isolates presented growth in a wider range of pH and NaCl concentrations than E. americanum, implying that the R. leguminosarum-like group might have greater adaptation ability than E. americanum, which might be the reason why R. leguminosarum has a wide geographic distribution.
In conclusion, isolates of sv. phaseoli from a R. leguminosarum-like group and E. americanum sv. mediterranense were identified for the first time as the dominant rhizobia associated with bean plants in the acid and alkaline soils in Mexico, respectively. The Rhizobium and Ensifer populations showed different but very similar symbiotic genes, demonstrating the same origin for these genes. Phenotypic characterization revealed that the Rhizobium populations were adapted to the acid and low salinity conditions of the study, while the Ensifer population preferred alkaline conditions. These findings extended the knowledge of the diversity, geographic distribution and evolution of bean-nodulating rhizobia in Mexico.
Supplementary Material
Acknowledgments
This study was financially supported by the projects SIP 20130828 and a sabbatical program authorized by IPN, Mexico; and by the National Institute of Health, USA (SGM081147 to H.-P.C). We thank the Instituto Nacional de Investigaciones Fore-stales, Agrícolas y Pecuarias (INIFAP), Campos Saltillo and Centro de Chiapas for helping with soil sampling.
Footnotes
The gene sequences acquired in this study have been deposited in the GenBank database under the accession numbers of KJ921024–KJ921045 for the 16S rRNA genes, KJ921046–KJ921056 for gyrB; KJ921057–KJ921061 for nodC; KJ921062–KJ921067 for nifH; KJ921068–KJ921078 for atpD; KJ921070–KJ921089 for glnII; KJ921090–KJ921110 for recA; KJ921111–KJ921121 for rpoB.
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.syapm. 2014.08.005.
References
- 1.Adhikari D, Itoh K, Suyama K. Genetic diversity of common bean (Phaseolus vulgaris L.) nodulating rhizobia in Nepal. Plant Soil. 2013;368:341–353. [Google Scholar]
- 2.Altschul SF, Gish W, Miller W, Myers E, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Amarger N, Macheret V, Laguerre G. Rhizobium gallicum sp. nov. and Rhizobium giardinii sp. nov., from Phaseolus vulgaris nodules. Int J Syst Bacteriol. 1997;47:996–1006. doi: 10.1099/00207713-47-4-996. [DOI] [PubMed] [Google Scholar]
- 4.Anyango B, Wilson KJ, Beynon JL, Giller KE. Diversity of rhizobia nodulating Phaseolus vulgaris L. in two Kenyan soils with contrasting pHs. Appl Environ Microbiol. 1995;61:4016–4021. doi: 10.1128/aem.61.11.4016-4021.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aserse AA, Räsänen LA, Assefa F, Hailemariam A, Lindström K. Phylogeny and genetic diversity of native rhizobia nodulating common bean (Phaseolus vulgaris L.) in Ethiopia. Syst Appl Microbiol. 2012;35:120–131. doi: 10.1016/j.syapm.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Barcellos FG, Menna P, da Silva Batista JS, Hungria M. Evidence of horizontal transfer of symbiotic genes from a Bradyrhizobium japonicum inocu-lant strain to indigenous diazotrophs Ensifer(Ensifer) fredii and Bradyrhizobium elkanii in a Brazilian savannah soil. Appl Environ Microbiol. 2007;73:2635–2643. doi: 10.1128/AEM.01823-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernal G, Graham PH. Diversity in the rhizobia associated with Phaseolus vulgaris L. in Ecuador, and comparisons with Mexican bean rhizobia. Can J Microbiol. 2001;47:526–534. doi: 10.1139/w01-037. [DOI] [PubMed] [Google Scholar]
- 8.Caballero-Mellado J, Martínez-Romero E. Soil fertilization limits the genetic diversity of Rhizobium in bean nodules. Symbiosis. 1999;26:111–121. [Google Scholar]
- 9.Cheng HP, Walker GC. Succinoglycan production by Rhizobium meliloti is regulated through the ExoS-ChvI two-component regulatory system. J Bacteriol. 1998;180:20–26. doi: 10.1128/jb.180.1.20-26.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chihaoui SA, Mhadhbi H, Mhamdi R. The antibiosis of nodule-endophytic agrobacteria and its potential effect on nodule functioning of Phaseolus vulgaris. Arch Microbiol. 2012;194:1013–1021. doi: 10.1007/s00203-012-0837-7. [DOI] [PubMed] [Google Scholar]
- 11.Dall'Agnol RF, Ribeiro RA, Ormeño-Orrillo E, Rogel MA, Delamuta JR, Andrade DS, Martínez-Romero E, Hungria M. Rhizobium freirei sp. nov., a symbiont of Phaseolus vulgaris that is very effective at fixing nitrogen. Int J Syst Evol Microbiol. 2013;63:4167–4173. doi: 10.1099/ijs.0.052928-0. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Fraile P, Mulas-Garcia D, Peix A, Rivas R, Gonzalez-Andres F, Velazquez E. Phaseolus vulgaris is nodulated in northern Spain by Rhizobium leguminosarum strains harboring two nodC alleles present in American Rhizobium etli strains: biogeographical and evolutionary implications. Can J Microbiol. 2010;56:657–666. doi: 10.1139/w10-048. [DOI] [PubMed] [Google Scholar]
- 13.Gouy M, Guindon S, Gascuel O. SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol Biol Evol. 2010;27:221–224. doi: 10.1093/molbev/msp259. [DOI] [PubMed] [Google Scholar]
- 14.Grange L, Hungria M. Genetic diversity of indigenous common bean (Phaseolus vulgaris) rhizobia in two Brazilian ecosystems. Soil Biol Biochem. 2004;36:1389–1398. [Google Scholar]
- 15.Gurkanli CT, Ozkoc I, Gunduz I. Genetic diversity of rhizobia nodulating common bean (Phaseolus vulgaris L.) in the Central Black Sea Region of Turkey. Ann Microbiol. 2013;63:971–987. [Google Scholar]
- 16.Han LL, Wang ET, Han TX, Liu J, Sui XH, Chen WF, Chen WX. Unique community structure and biogeography of soybean rhizobia in the saline-alkaline soils of Xinjiang, China. Plant Soil. 2009;324:291–305. [Google Scholar]
- 17.Han SZ, Wang ET, Chen WX. Diverse bacteria isolated from root nodules of Phaseolus vulgaris and species within the genera Campylotropis and Cassia grown in China. Syst Appl Microbiol. 2005;28:265–276. doi: 10.1016/j.syapm.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Hurek T, Wagner B, Reihold-Hurek B. Identification of N-fixing plant-and fungus-associated Azoarcus species by PCR-based genomic fingerprints. Appl Environ Microbiol. 1997;63:4331–4339. doi: 10.1128/aem.63.11.4331-4339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jarabo-Lorenzo A, Velázquez E, Pérez GR, Vega-Hernández M, Martínez-Molina E, Mateos PE, Vinuesa P, Martínez-Romero E, León-Barrios M. Restriction fragment length polymorphism analysis of PCR-amplified 16S rDNA and low molecular weight RNA profiling in the characterization of rhizobial isolates from shrubby legumes endemic to the Canary Islands. Syst Appl Microbiol. 2000;23:418–425. doi: 10.1016/s0723-2020(00)80073-9. [DOI] [PubMed] [Google Scholar]
- 20.Kimura M. A simple method of estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1983;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 21.Laguerre G, Allard M, Revoy F, Amarger N. Rapid identification of rhizobia by restriction fragment length polymorphism analysis of PCR-amplified 16S rRNA genes. Appl Environ Microbiol. 1994;61:56–63. doi: 10.1128/aem.60.1.56-63.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laguerre G, Nour SM, Macheret V, Sanjuan J, Drouin P, Amarger N. Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology. 2001;147:981–993. doi: 10.1099/00221287-147-4-981. [DOI] [PubMed] [Google Scholar]
- 23.Leonard LT. A simple assembly for use in the testing for cultures of rhizobia. J Bacteriol. 1943;45:523–527. doi: 10.1128/jb.45.6.523-527.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López-Guerrero MG, Ormeño-Orrillo E, Velázquez E, Rogel MA, Acosta JL, Gónzalez V, Martínez J, Martínez-Romero E. Rhizobium etli taxonomy revised with novel genomic data and analyses. Syst Appl Microbiol. 2012;35:353–358. doi: 10.1016/j.syapm.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 25.López-López A, Rogel-Hernández MA, Barois I, Ortiz Ceballos AI, Martínez J, Ormeño-Orrillo E, Martínez-Romero E. Rhizobium grahamii sp. nov., from nodules of Dalea leporina, Leucaena leucocephala and Clitoria ternatea, and Rhizobium mesoamericanum sp. nov., from nodules of Phaseolus vulgaris, siratro, cowpea and Mimosa pudica. Int J Syst Evol Microbiol. 2012;62:2264–2271. doi: 10.1099/ijs.0.033555-0. [DOI] [PubMed] [Google Scholar]
- 26.Lu HY, Luo L, Yang MH, Cheng HP. Ensifer meliloti ExoR is the target of periplasmic proteolysis. J Bacteriol. 2012;194:4029–4040. doi: 10.1128/JB.00313-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez-Romero E. Coevolution in Rhizobium-legume symbiosis? DNA Cell Biol. 2009;28:361–370. doi: 10.1089/dna.2009.0863. [DOI] [PubMed] [Google Scholar]
- 28.Mhamdi R, Laguerre G, Aouani ME, Mars M, Amarger N. Different species and symbiotic genotypes of field rhizobia can nodulate Phaseolus vulgaris in Tunisian soils. FEMS Microbiol Ecol. 2002;41:77–84. doi: 10.1111/j.1574-6941.2002.tb00968.x. [DOI] [PubMed] [Google Scholar]
- 29.Mnasri B, Liu TY, Saidi S, Chen WF, Chen WX, Zhang XX, Mhamdi R. Rhizobium azibense sp. nov., a nitrogen fixing bacterium isolated from root-nodules of Phaseolus vulgaris. Int J Syst Evol Microbiol. 2014;64:1501–1516. doi: 10.1099/ijs.0.058651-0. [DOI] [PubMed] [Google Scholar]
- 30.Mnasri B, Saidi S, Chihaoui SA, Mhamdi R. Ensifer americanum symbiovar mediterranense is a predominant symbiont that nodulates and fixes nitrogen with common bean (Phaseolus vulgaris L.) in a Northern Tunisian field. Syst Appl Microbiol. 2012;35:263–269. doi: 10.1016/j.syapm.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Moulin L, Béna G, Boivin-Masson C, Stȩpkowski T. Phylogenetic analyses of symbiotic nodulation genes support vertical and lateral gene co-transfer within the Bradyrhizobium genus. Mol Phylogenet Evol. 2004;30:720–732. doi: 10.1016/S1055-7903(03)00255-0. [DOI] [PubMed] [Google Scholar]
- 32.Peng G, Yuan Q, Li H, Zhang W, Tan Z. Rhizobium oryzae sp. nov., isolated from the wild rice Oryza alta. Int J Syst Evol Microbiol. 2008;58:2158–2163. doi: 10.1099/ijs.0.65632-0. [DOI] [PubMed] [Google Scholar]
- 33.Pérez-Ramirez NO, Rogel-Hernández MA, Wang ET, Martínez-Romero E. Seeds of Phaseolus vulgaris bean carry Rhizobium etli. FEMS Microbiol Ecol. 1998;26:289–296. [Google Scholar]
- 34.Posada D. jModel Test: phylogenetic model averaging. Mol Biol Evol. 2008;25:1253–1256. doi: 10.1093/molbev/msn083. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro RA, Ormeno-Orrillo E, Dall'Agnol RF, Graham PH, Martínez-Romero E, Hungria M. Novel Rhizobium lineages isolated from root nodules of the common bean (Phaseolus vulgaris L.) in Andean and Mesoamerican areas. Res Microbiol. 2013;164:740–748. doi: 10.1016/j.resmic.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Ribeiro RA, Rogel MA, López-López A, Ormeño-Orrillo E, Barcellos FG, Martínez J, Thompson FL, Martínez-Romero E, Hungria M. Reclassification of Rhizobium tropici type A strains as Rhizobium leucaenae sp. nov. Int J Syst Evol Microbiol. 2012;62:1179–1184. doi: 10.1099/ijs.0.032912-0. [DOI] [PubMed] [Google Scholar]
- 37.Rivas R, Martens M, de Lajudie P, Willems A. Multilocus sequence analysis of the genus Bradyrhizobium. Syst Appl Microbiol. 2009;32:101–110. doi: 10.1016/j.syapm.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Sarita S, Sharma PK, Priefer UB, Prell J. Direct amplification of rhizobial nodC sequences from soil total DNA and comparison to nodC diversity of root nodule isolates. FEMS Microbiol Ecol. 2005;54:1–11. doi: 10.1016/j.femsec.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 39.Tamimi SA, Young JPW. Rhizobium etli is the dominant common bean nodulating rhizobia in cultivated soils from different locations in Jordan. Appl Soil Ecol. 2004;26:193–200. [Google Scholar]
- 40.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Terefework Z, Kaijalainen S, Lindstrom K. AFLP fingerprinting as a tool to study the genetic diversity of Rhizobium galegae isolated from Galega orientalis and Galega officinalis. J Biotechnol. 2001;101:169–180. doi: 10.1016/s0168-1656(01)00338-8. [DOI] [PubMed] [Google Scholar]
- 42.Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velázquez E, Peix A, Zurdo-Pineiro JL, Palomo JL, Mateos PF, Rivas R, Muñoz-Adelantado E, Toro N, García-Benavides P, Martínez-Molina E. The coexistence of symbiosis and pathogenicity-determining genes in Rhizobium rhizogenes strains enables them to induce nodules and tumors or hairy roots in plants. Mol Plant-Microbe Interact. 2005;18:1325–1332. doi: 10.1094/MPMI-18-1325. [DOI] [PubMed] [Google Scholar]
- 44.Vincent JM. International Biological Programme IBP Handbook. Vol. 15. Blackwell Scientific Publications; Oxford, UK: 1970. Manual for the Practical Study of the Root Nodule Bacteria. [Google Scholar]
- 45.Vinuesa P, Silva C, Lorite MJ, Izaguirre-Mayoral ML, Bedmar EJ, Martínez-Romero E. Molecular systematics of rhizobia based on maximum likelihood and Bayesian phylogenies inferred from rrs, atpD, recA and nifH sequences, and their use in the classification of Sesbania microsymbionts from Venezuelan wetlands. Syst Appl Microbiol. 2005;28:702–716. doi: 10.1016/j.syapm.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 46.Wang ET, van Berkum P, Beyene D, Sui XH, Chen WX, Martínez-Romero E. Diversity of rhizobia associated with Amorpha fruticosa isolated from Chinese soils and description of Mesorhizobium amorphae sp. nov. Int J Syst Bacteriol. 1999;49:51–65. doi: 10.1099/00207713-49-1-51. [DOI] [PubMed] [Google Scholar]
- 47.Wang H, Man CX, Wang ET, Chen WX. Diversity of rhizobia and interactions among the host legumes and rhizobial genotypes in an agricultural-forestry ecosystem. Plant Soil. 2009;314:169–182. [Google Scholar]
- 48.Weisburg WG, Barns SM, Pelletior DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang YM, Li Y, Jr, Chen WF, Wang ET, Tian CF, Li QQ, Zhang YZ, Sui XH, Chen WX. Biodiversity and biogeography of rhizobia associated with soybean plants grown in the North China Plain. Appl Environ Microbiol. 2011;77:6331–6342. doi: 10.1128/AEM.00542-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zurdo-Pineiro JL, García-Fraile P, Rivas R, Peix A, León-Barrios M, Willems A, Mateos PF, Martínez-Molina E, Velázquez E, van Berkum P. Rhizobia from Lanzarote, the Canary Islands, that nodulate Phaseolus vulgaris have characteristics in common with Ensifer meliloti isolates from mainland Spain. Appl Environ Microbiol. 2009;75:2354–2359. doi: 10.1128/AEM.02811-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


