Abstract
Body composition measurements from DXA have been available since DXA technology was developed 30 years ago, but are historically underutilized. Recently, there have been rapid developments in body composition assessment including the analysis and publication of representative data for the US, official usage guidance from the International Society for Clinical Densitometry, and development of regional body composition measures with clinical utility. DXA body composition is much more than whole body percent fat. In this paper celebrating 30 years of DXA for body composition, we will review the principles of DXA soft tissue analysis, practical clinical and research applications, and what to look for in the future.
Keywords: Total Body DXA in Athletes, Bariatrics, Sarcopenia, Current and Future Use
Why use DXA to measure body composition?
DXA is a special imaging modality that is not typically available on general use x-ray systems because of the need for special beam filtering and near-perfect spatial registration of the two attenuations. The whole body can be scanned to measure whole body bone mass and soft tissue composition (1, 2). DXA is the preferred method for bone and body composition for several reasons. First, there are few assumptions required for DXA composition measurements. The two X-ray attenuations passing through the body can be used to accurately calculate the mass of two different materials given simple algebra and the physical properties of those materials (3). There were details to work out, such as how to quantify the soft tissue mass in a divergent fan-beam geometry (4), but the fundamental nature of DXA gives it the promise of accuracy over a wide range of body sizes and body types. Second, DXA can measure regional body composition by subdividing the body using specific well-defined cut lines. Third, DXA is precise and stable for years. Using phantoms, it is straightforward to verify measurement stability of better than 0.5% change in body composition accuracy over decades of operation for a single DXA system.
DXA does expose the patient and operator to ionizing radiation but the dose is very small to both. The effective radiation dose from a single whole body DXA (< 10 microSieverts) is similar to the normal background radiation received over one day at sea level. However, most states in the US and other countries require some sort of licensing of the technologist and a designated X-ray site supervisor.
One of the early papers on body composition and DXA was Mazess et al. (5). See Figure 1. Mazess showed that DXA was accurate to reference materials of lard and water representing fat and lean tissues. The system in that study was a Lunar DPX that created images with 5 × 10 mm pixel dimensions and took 10 to 20 minutes for a whole-body scan depending on subject size. The active scan area of the table was 60 cm × 200 cm, the system could accurately penetrate 25 to 30 cm body thickness, and the table was rated to support up to 250 lbs. The DPX could measure total body BMD with a precision of better than 1%. In a demonstration of system stability, one skeleton was measured on 37 systems and the coefficient of variation (intrascanner) was 0.96%. The body was subdivided by cut lines into arms, legs, trunk and head, and fat, lean soft tissue, and bone mass composition were reported for each subregion. If all of this was true 30 years ago, what has changed in DXA performance and capabilities regarding body composition over the last few decades? We will examine this question from various different perspectives that surround body composition: metabolic conditions, musculoskeletal health, sports and fitness, and disease-specific indications.
Figure 1.
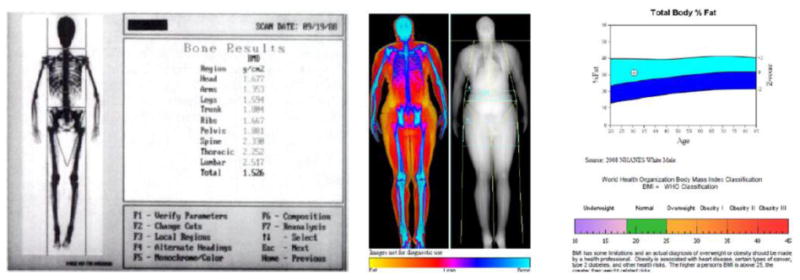
(left) Screenshot from a Lunar DPX pencil beam system circa 1990. (from (5)). (right) Screenshot from Hologic Horizon circa 2016 (courtesy of J. Shepherd).
Body Size
DXA systems are used to quantify body composition from the smallest to largest humans. Infants as small as 1 kg are routinely scanned for studies of bone and soft tissue. This has been an active area of research for DXA including regions of interest development and studies on the precision and accuracy of infant scanning (6). A recent review of infant DXA body composition was completed in 2014 (7).
Large individuals pose two challenges for DXA scanning: The length, width, and rated load capacity may all be exceeded. Table dimensions were set to the maximum size that allowed the system to fit in a small 8′ × 8′ room knowing that some patients would be too tall or wide to fit completely on the table. These design decisions were made in observation of the primary market for DXA systems: bone density measurements of the spine and hip, where it is not necessary to fit the entire body on the table. Steadily over the years, the systems were improved to support higher weights with current specifications for widely used systems being 500 lbs. For body composition assessment, it is desirable to scan the entire body. Instead of increasing the tabletop width and height, which would make the unit more expensive for the primary osteoporosis market, clever vertical and horizontal offset scanning techniques were devised with matching analysis techniques to piece together complete whole body scans from partial scans. For very wide participants, the participant was offset horizontally such that in one scan, all of the body can be estimated even though one of the arms, legs, or both were not acquired. This scanning method is called offset scanning in general, reflection mode on Hologic systems, and mirror mode on GE systems. Rothney et al. (8) showed that the resulting scan from mirrored scanning agreed well with no significant difference in whole body results when compared to standard scans. Shepherd et al. (6) also showed the reflection method can be used for infant scans to exclude regions with motion artifacts. For patients too tall to fit in the scan field, two scans can be acquired: one where the feet are included at the expense of including the head, and a second partial scan down through the torso that includes the head. Then the head values would be manually substituted for the measures from the first scan (9). Only recently did a DXA system come to the market specifically designed for body composition. The Norland Elite can scan individuals up to 283.5 kg (625 lb), 137 cm (54”) wide and 228 cm (7′6”) tall. Figure 2 summarizes the differences in table widths, lengths, and weight limits. Thus, the overall flexibility to complete DXA body composition assessments on subjects of all different heights, widths, and weights has substantially improved in the past 30 years.
Figure 2.
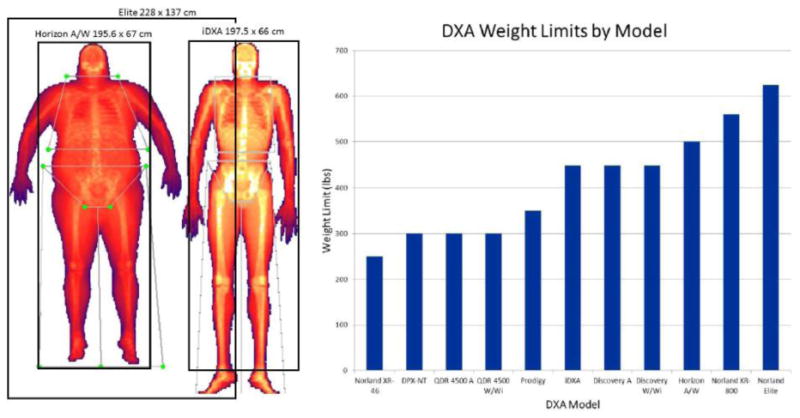
Comparisons of table scan areas of three current DXA systems (LEFT). Comparison of table weight limits across makes and models (RIGHT).
Reference Data
Until 2009, reference data for DXA body composition had been proprietary and specific to each make system. However, in 2009, the National Health and Nutrition Examination Survey (NHANES) published representative data for the US population (10). Both Hologic and GE systems have the NHANES data integrated into their software to generate Z-scores for various adiposity and lean mass measures (11, 12). Localized Z-scores for arms, legs, and trunk have also been reported using the NHANES data (13). The Official Position of the International Society for Clinical Densitometry (ISCD) (14) states that Z-scores for body composition should be derived using the NHANES DXA data (15). There have also been similar studies to NHANES for representative DXA data in other countries such as the KNHANES (16). However, these representative datasets do not necessarily represent optimal health or fitness. Some attempts have been made to characterize body composition characteristics by sport (17), but how generally these are applicable to different age groups and level of competition is uncertain. Reference data can be collected on one make of DXA and applied to another using universal standardization equations (18).
Indices and special subcompartments
There are many ways to represent body composition but a popular approach in recent years has been the use of indices normalized by height. For example, fat mass index is defined as the total DXA fat mass normalized by height squared (FMI = Fat mass / Height2). FMI has a distinct advantage over BMI for defining obesity status since it is independent of lean mass status. Kelly et al. (11) suggested sex-specific cutpoints for normal, excess fat, obesity I, obesity II, and obesity III. DXA system also report estimates for visceral adipose tissue (VAT) for either Hologic (19) or GE systems (20). DXA VAT has a similar relationship to other biomarkers of metabolic health as does VAT measured by computed tomography (CT). Lean mass indices have received significant attention in the past few years as metrics to define sarcopenia. We will discuss lean mass indices, including appendicular lean mass index (ALMI = [arms + legs lean mass] / Height2) in the next section.
Phenotype Descriptors
Another advancement that has happened over the past 30 years is the development of phenotypical descriptors for different disease states. Examples include normal status, sarcopenic, sarcopenic obese, osteosarcopenic, and ostesarcopenic obese. After age 50, muscle mass decreases 1-2% annually, and those with sarcopenia are at 3 times greater risk of falls than their peers after adjustment for other risk factors (21). In the New Mexico Study, prevalence of sarcopenia was 12% for persons 60 to 70 years of age and nearly 30% for persons over 80 years (22, 23). Drey et al. (24) showed that women with osteosarcopenia had the lowest grip strength, low chair rise, low sit-to-stand (STS) power compared to osteopenic, sarcopenic, and control women. Ilich et al. (25) added to this story by showing that a similar loss of function is observed in postmenopausal obese women as they have a combination of poor bone, muscle, and fat status. Weber et al. (26) suggested that ALMI may need to be adjusted by FMI since they found that virtually no individuals in the NHANES sample were sarcopenic if their FMI Z-score was over +2. Prado et al. suggested a working definition of sarcopenic obesity as being in the top 50th percentile of both ALMI and FMI in the top 50th percentile by age and sex (27). This definition requires age and sex reference values for ALMI and FMI from a large representative population. Prado et al. used the 1999-2004 NHANES DXA dataset made up of 16,383 men and women of mixed ethnicity. Using this definition, it was found that 10% of the women and 15% of the men were classified as sarcopenic obese. However, much controversy exists on how best to represent sarcopenia (28). The reason for the controversy is that muscle mass measures alone often do not predict functional strength and health in older adults. For example Newman et al. (29) showed that strength, but not muscle mass, predicted mortality in older men and women. Bischoff-Ferrari et al. (30) compared nine different definitions of sarcopenia varying by threshold values for appendicular lean mass index (ALMI) combined with different strength measures. She showed that the simple definition by Baumgartner for sarcopenia of ALMI < 7.26 kg/m2 (men) and 5.45 kg/m2 (women) gave the best prevalence and probability of falls in a prospective study of community dwelling men and women. But these thresholds might not be appropriate for obese individuals.
DXA and fitness and sports
Much of the advancement in the use of DXA for sports and fitness has been application of the technology that has been available for years. Yet, there is a unique place for DXA in evaluating the success of sports, diet, and fitness interventions because of its unique ability to simultaneously measure bone, lean, and fat mass status. In athletes, Relative Energy Deficiency in Sport (RED-S) is a condition that impacts bone health due to overtraining and poor nutrition (31). It can be seen in both men and women but in women it may be associated with amenorrhea where it is called the Female Athlete Triad. In 1997, the American college of sports medicine (ACSM) released the Female Athlete Triad Position Stand where they outlined the components of the Female Triad: Eating disorder, amenorrhea, and low bone mass (32). A reasonable DXA protocol for young athletes includes hip, spine, and total body assessment for osteopenia, low fat, and low muscle mass evaluation. DXA can also be used to evaluate recovery from injury and performance by looking at the differences between left and right limbs. Examples include the effects of lean mass symmetry on performance of skiers with ACL injuries (33) and Australian football kickers (34).
Challenges that still exists in the field
The accuracy of DXA to show equivalence to actual muscle and fat is difficult to perform in the field. The way to validate absolute calibration is to image whole or sectioned cadavers and then perform chemical analysis to definitively quantify composition. Accuracy validations have been performed over the years using fetal pigs and still-born infants (35, 36), adult cadavers (37), and lamb carcasses (38, 39). Overall, the agreement of DXA-measured mass with scale weight is typically within 1%. Agreement between DXA and whole-body CT fat mass has been found to be very high as well with correlations of 0.99 but with DXA underestimating whole body fat mass by as much as 5 kg on average (40). However, there is still a lack of reference phantoms that can be used in the field that assure absolute calibration.
Future Directions
To date, DXA measures have been defined as cumulative or average values for large regions of interests such as arms, legs, and trunk. An alternative approach is to study the variations of fat, lean, and bone mass using shape and appearance modeling (41). There is growing evidence that body shape and body composition distribution are strong indicators of metabolic health. Shape and appearance models are statistical models that accurately describe holistic body shape, thickness, and leanness. These models allow for spatial registration to remove shape and pose variation across subject images such that each pixel in a DXA image is precisely aligned across a population. Afterwards, these models can be used to visualize images of risk-based phenotypes. Figure 3 shows an example visualization of metabolic syndrome risk (41). Models derived exclusively from DXA body shape were able to predict 6-year mortality in an older multi-ethnic population of men and women with an AUC=0.66 (41).
Figure 3.
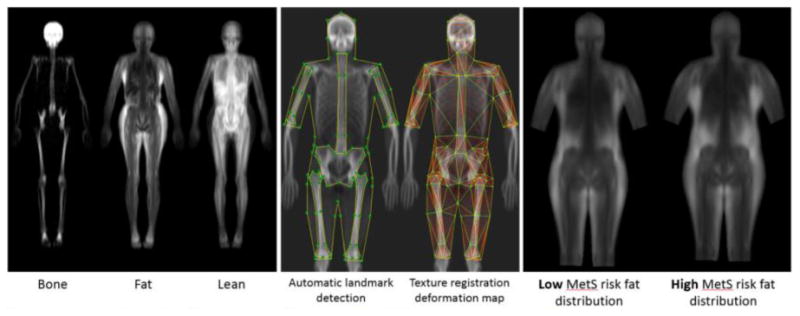
Examples of UCSF algorithms to separate DXA images into bone, fat, and lean tissues (left). These images are used to construct statistical appearance models (SAM) that compactly describe tissue variance across individuals (middle). We have used SAMs to identify tissue shape and distribution phenotypes that are associated with adverse metabolic outcomes (right).
In summary, DXA is a mature technology for measuring body composition and there have been major advances in the technology over the past 30 years. However, it is not a static method. New and novel approaches to the interpretation of DXA body composition keep DXA at the forefront of applications ranging from infants to the morbidly obese, and athletes to the elderly.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laskey M, Phil D. Dual-energy x-ray absorptiometry and body composition. Nutrition. 1996;12(1):45–51. doi: 10.1016/0899-9007(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 2.Kelly TL, Berger N, Richardson TL. DXA body composition: theory and practice. Appl Radiat Isot. 1998;49(5-6):511–3. doi: 10.1016/s0969-8043(97)00226-1. [DOI] [PubMed] [Google Scholar]
- 3.Blake GM, Wahner HW, Fogelman I. The evaluation of osteoporosis: Dual energy X-ray absorptiometry and ultrasound in clinical practice. 2nd ed. London: Martin Dunitz; 1999. [Google Scholar]
- 4.Determining body composition using fan beam dual-energy x-ray absorptiometry. In: USA2001. 2001. [Google Scholar]
- 5.Mazess RB, Barden HS, Ohlrich ES. Skeletal and body-composition effects of anorexia nervosa. Am J Clin Nutr. 1990;52(3):438–41. doi: 10.1093/ajcn/52.3.438. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd JA, Sommer MJ, Fan B, Powers C, Stranix-Chibanda L, Zadzilka A, Basar M, George K, Mukwasi-Kahari C, Siberry G. Advanced Analysis Techniques Improve Infant Bone and Body Composition Measures by Dual-Energy X-Ray Absorptiometry. The Journal of pediatrics. 2017;181:248–53e3. doi: 10.1016/j.jpeds.2016.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demerath EW, Fields DA. Body composition assessment in the infant. American Journal of Human Biology. 2014;26(3):291–304. doi: 10.1002/ajhb.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rothney MP, Brychta RJ, Schaefer EV, Chen KY, Skarulis MC. Body composition measured by dual-energy X-ray absorptiometry half-body scans in obese adults. Obesity. 2009;17(6):1281–6. doi: 10.1038/oby.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva A, Heymsfield S, Sardinha L. Assessing body composition in taller or broader individuals using dual-energy X-ray absorptiometry: a systematic review. European journal of clinical nutrition. 2013;67(10):1012–21. doi: 10.1038/ejcn.2013.148. [DOI] [PubMed] [Google Scholar]
- 10.Borrud L, Flegal K, Looker A, Everhart J, Harris T, Shepherd J. Body composition data for individuals 8 years of age and older: US population, 1999-2004. Vital and health statistics Series 11, Data from the national health survey. 2010;(250):1–87. [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. Epub 2009/09/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fan B, Shepherd JA, Levine MA, Steinberg D, Wacker W, Barden HS, Ergun D, Wu XP. National Health and Nutrition Examination Survey Whole-Body Dual-Energy X-Ray Absorptiometry Reference Data for GE Lunar Systems. Journal of Clinical Densitometry. 2013;17(3):344–77. doi: 10.1016/j.jocd.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 13.Hinton BJ, Fan B, Ng BK, Shepherd JA. Dual energy X-ray absorptiometry body composition reference values of limbs and trunk from NHANES 1999-2004 with additional visualization methods. PloS one. 2017;12(3):e0174180. doi: 10.1371/journal.pone.0174180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schousboe JT, Shepherd JA, Bilezikian JP, Baim S. Executive Summary of the 2013 ISCD Position Development Conference on Bone Densitometry. J Clin Densitom. 2013 doi: 10.1016/j.jocd.2013.08.004. XXX(XXX):XXX. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd JA, Baim S, Bilezikian JP, Schousboe JT. Executive Summary of the 2013 International Society for Clinical Densitometry Position Development Conference on Body Composition. Journal of Clinical Densitometry. 2013;16(4):489–95. doi: 10.1016/j.jocd.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Hong S, Oh HJ, Choi H, Kim JG, Lim SK, Kim EK, Pyo EY, Oh K, Kim YT, Wilson K. Characteristics of body fat, body fat percentage and other body composition for Koreans from KNHANES IV. Journal of Korean medical science. 2011;26(12):1599–605. doi: 10.3346/jkms.2011.26.12.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santos DA, Dawson JA, Matias CN, Rocha PM, Minderico CS, Allison DB, Sardinha LB, Silva AM. Reference values for body composition and anthropometric measurements in athletes. PloS one. 2014;9(5):e97846. doi: 10.1371/journal.pone.0097846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shepherd JA, Fan B, Lu Y, Wu XP, Wacker WK, Ergun DL, Levine MA. A multinational study to develop universal standardization of whole-body bone density and composition using GE Healthcare Lunar and Hologic DXA systems. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2012;27(10):2208–16. doi: 10.1002/jbmr.1654. Epub 2012/05/25. [DOI] [PubMed] [Google Scholar]
- 19.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-Energy X-Ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity. 2012;20(5):1109–14. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, Ergun DL. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity. 2012;20(6):1313–8. doi: 10.1038/oby.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E, Bernabei R, Onder G. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clinical nutrition. 2012;31(5):652–8. doi: 10.1016/j.clnu.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 23.Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. Journal of Laboratory and Clinical Medicine. 2001;137(4):231–43. doi: 10.1067/mlc.2001.113504. [DOI] [PubMed] [Google Scholar]
- 24.Drey M, Sieber CC, Bertsch T, Bauer JM, Schmidmaier R. Osteosarcopenia is more than sarcopenia and osteopenia alone. Aging clinical and experimental research. 2016;28(5):895–9. doi: 10.1007/s40520-015-0494-1. [DOI] [PubMed] [Google Scholar]
- 25.Ilich J, Inglis J, Kelly O, McGee D. Osteosarcopenic obesity is associated with reduced handgrip strength, walking abilities, and balance in postmenopausal women. Osteoporosis International. 2015;26(11):2587–95. doi: 10.1007/s00198-015-3186-y. [DOI] [PubMed] [Google Scholar]
- 26.Weber D, Long J, Leonard MB, Zemel B, Baker JF. Development of Novel Methods to Define Deficits in Appendicular Lean Mass Relative to Fat Mass. PloS one. 2016;11(10):e0164385. doi: 10.1371/journal.pone.0164385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prado CM, Siervo M, Mire E, Heymsfield SB, Stephan BC, Broyles S, Smith SR, Wells JC, Katzmarzyk PT. A population-based approach to define body-composition phenotypes. The American journal of clinical nutrition. 2014;99(6):1369–77. doi: 10.3945/ajcn.113.078576. [DOI] [PubMed] [Google Scholar]
- 28.Shepherd J. Evaluation of Sarcopenia by DXA. Clinical Reviews in Bone and Mineral Metabolism. 2016;14(1):45–9. [Google Scholar]
- 29.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–7. doi: 10.1093/gerona/61.1.72. Epub 2006/02/04. doi:61/1/72 [pii] [DOI] [PubMed] [Google Scholar]
- 30.Bischoff-Ferrari H, Orav J, Kanis J, Rizzoli R, Schlögl M, Staehelin H, Willett W, Dawson-Hughes B. Comparative performance of current definitions of sarcopenia against the prospective incidence of falls among community-dwelling seniors age 65 and older. population. 2015;9:14. doi: 10.1007/s00198-015-3194-y. [DOI] [PubMed] [Google Scholar]
- 31.Joy E, Kussman A, Nattiv A. 2016 update on eating disorders in athletes: A comprehensive narrative review with a focus on clinical assessment and management. British journal of sports medicine. 2016;50(3):154–62. doi: 10.1136/bjsports-2015-095735. [DOI] [PubMed] [Google Scholar]
- 32.Otis CL, Drinkwater B, Johnson M, Loucks A, Wilmore J. American College of Sports Medicine position stand. The Female Athlete Triad. Medicine and Science in Sports and Exercise. 1997;29(5):i–ix. doi: 10.1097/00005768-199705000-00037. [DOI] [PubMed] [Google Scholar]
- 33.Jordan M, Aagaard P, Herzog W. Lower limb asymmetry in mechanical muscle function: A comparison between ski racers with and without ACL reconstruction. Scandinavian journal of medicine & science in sports. 2015;25(3) doi: 10.1111/sms.12314. [DOI] [PubMed] [Google Scholar]
- 34.Hart NH, Nimphius S, Spiteri T, Newton RU. Leg strength and lean mass symmetry influences kicking performance in Australian Football. Journal of sports science & medicine. 2014;13(1):157. [PMC free article] [PubMed] [Google Scholar]
- 35.Andres A, Mitchell A, Badger T. Validation of a new body composition method for infant and children using piglets. Int J Obes. 2010;34:775–80. doi: 10.1038/ijo.2009.284. [DOI] [PubMed] [Google Scholar]
- 36.Brunton JA, Bayley HS, Atkinson SA. Validation and application of dual-energy x-ray absorptiometry to measure bone mass and body composition in small infants. Am J Clin Nutr. 1993;58(6):839–45. doi: 10.1093/ajcn/58.6.839. [DOI] [PubMed] [Google Scholar]
- 37.Scafoglieri A, Deklerck R, Tresignie J, De Mey J, Clarys JP, Bautmans I. Assessment of regional adipose tissue depots: a DXA and CT comparison in cadavers of elderly persons. Exp Gerontol. 2013;48(9):985–91. doi: 10.1016/j.exger.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 38.Dunshea F, Suster D, Eason P, Warner R, Hopkins D, Ponnampalam E. Accuracy of dual energy X-ray absorptiometry, weight, longissimus lumborum muscle depth and GR fat depth to predict half carcass composition in sheep. Animal Production Science. 2007;47(10):1165–71. [Google Scholar]
- 39.Mercier J, Pomar C, Marcoux M, Goulet F, Thériault M, Castonguay F. The use of dual-energy X-ray absorptiometry to estimate the dissected composition of lamb carcasses. Meat science. 2006;73(2):249–57. doi: 10.1016/j.meatsci.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 40.Kullberg J, Brandberg J, Angelhed JE, Frimmel H, Bergelin E, Strid L, Ahlstrom H, Johansson L, Lonn L. Whole-body adipose tissue analysis: comparison of MRI, CT and dual energy X-ray absorptiometry. The British journal of radiology. 2009;82(974):123–30. doi: 10.1259/bjr/80083156. Epub 2009/01/27. [DOI] [PubMed] [Google Scholar]
- 41.Shepherd JA, Ng BK, Fan B, Schwartz AV, Cawthon P, Cummings SR, Kritchevsky S, Nevitt M, Santanasto A, Cootes TF. Modeling the shape and composition of the human body using dual energy X-ray absorptiometry images. PloS one. 2017;12(4):e0175857. doi: 10.1371/journal.pone.0175857. [DOI] [PMC free article] [PubMed] [Google Scholar]


