Abstract
Kenya belongs to a high incidence region known as Africa’s esophageal cancer (EC) corridor. It has one of the highest incidence rates of EC worldwide, but research on EC in Kenya has gone highly unnoticed. EC in Kenya is unique in its high percentage of young cases (< 30 years of age). In this review, we show the current status of EC in the country. We mainly focus on significant risk factors such as alcohol drinking, genetic factors, malnutrition and hot food/drink. Future directions in the study and prevention of EC in Kenya are also discussed.
Keywords: Esophageal cancer, zinc, selenium, Kenya, alcohol
Introduction
Kenya has one of the highest incidence rates of esophageal cancer (EC) in the continent with a rate of 17.6 per 100,000 [1, 2]. It is one of a few countries that lie on Africa’s EC corridor, which is a region situated in the geographic area of the Eastern and Western rift-valley and is reported to have the highest EC incidences in Africa [1]. Squamous cell carcinoma (SCC) accounts for 90% of EC cases in Kenya [3–7]. Western and Central Kenya represent regions with the highest number of EC cases in Kenya (Figure 1) [3, 5–7]. As seen in Table 1, weighted EC incidence in Kenya is much higher than that in other well-known high-incidence countries, such as China, Iran, and South Africa. The ratio between men and women in Kenya is ~1.5:1 as opposed to 3:1 in other high-incidence countries indicating that men and women in Kenya may be exposed to the same risk factors. EC in Kenya was first reported to make up 30% of all tumors in Western Kenya with higher incidence rates near Lake Victoria [8]. Currently, EC is a major concern in Kenya as 11% of new cancer cases are EC, which is the second most prevalent cancer in the country [2].
Figure 1.
Geographic maps showing regions of interest. A: Africa’s EC corridor; B: Map of Kenya highlighting high-incidence regions and hospitals that treat EC. Maps outlines were adapted from: https://clipartfest.com/categories/view/f2a48eb6e7df5a11e1327a2914da4055293c6687/outline-of-africa-map-clipart-without-background.html and http://www.enchantedlearning.com/africa/kenya/outlinemap/.
Table 1.
Comparison of age-standardized rates and prevalence based on GLOBOCAN data [2]
| Country | Men
|
Women
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incidence ASR (W) |
Incidence (%) |
Mortality ASR (W) |
Mortality (%) |
5-yr prevalence (%) |
Incidence ASR (W) |
Incidence (%) |
Mortality ASR (W) |
Mortality (%) |
5-yr prevalence (%) |
|
| Kenya | 20.5 | 10.7 | 19.4 | 12.6 | 5.7 | 15.1 | 6.6 | 14.1 | 9.5 | 2.9 |
| South Africa | 13.9 | 6.1 | 13.0 | 9.0 | 3.2 | 6.9 | 4.0 | 6.3 | 6.0 | 1.6 |
| Iran | 9.0 | 6.5 | 8.8 | 8.3 | 3.2 | 8.0 | 6.1 | 7.4 | 9.7 | 2.5 |
| China | 18.6 | 8.8 | 16.2 | 9.8 | 6.5 | 6.7 | 5.1 | 5.8 | 7.4 | 2.4 |
| France | 5.5 | 1.7 | 5.2 | 3.3 | 0.8 | 1.7 | 0.4 | 1.0 | 1.3 | 0.2 |
| UK | 10.0 | 3.6 | 8.7 | 6.3 | 1.4 | 3.5 | 1.7 | 2.9 | 3.4 | 0.6 |
| USA | 6.1 | 1.6 | 5.1 | 3.9 | 0.7 | 1.1 | 0.7 | 1.0 | 1.1 | 0.3 |
ASR (W) is the weighted age standard incidence rate or mortality rate for EC in each country, i.e., the number of new cases per 100,000 people. Incidence is a percentage of the number of new EC cases divided by the total number of new cancer cases. Mortality is the percentage of the number of deaths from EC compared to the total number of cancer deaths. The 5-yr prevalence is a percentage of the number of survivors of EC divided by the total number of cancer survivors within a 5-year period.
Unlike EC in Europe and Asia, 11% of EC in Western Kenya affects individuals below the age of 30 [6, 7]. Tenwek and Eldoret (areas in Western Kenya) have a significantly high number of patients below 40 years of age (Figure 2). The mean age of EC patients in Kenya is approximately 50 years, as opposed to 65 years in Western countries and high-incidence East Asian countries [2, 3].
Figure 2.
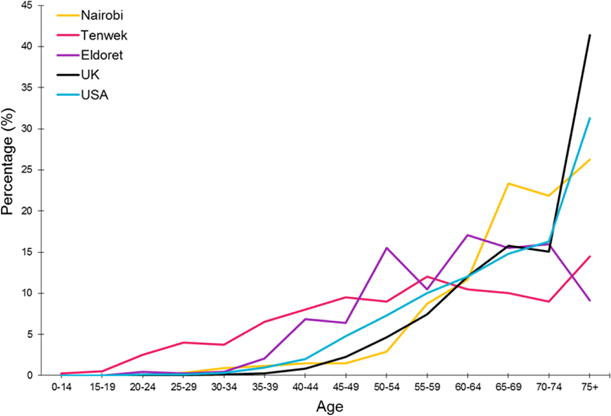
Percentage of EC cases at each age interval in three regions of Kenya compared to Western countries (yellow for Nairobi, pink for Tenwek, purple for Eldoret, black for the UK, and blue for the United States) [3, 6, 7]. UK data are adapted from http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oesophageal-cancer/incidence#ref-1.
Endoscopy and histopathology are the most common diagnostic tools for EC in Kenya [3, 9–14]. Only a few hospitals in Kenya treat EC patients, some of which include Kenyatta National Teaching and Referral Hospital, Moi Teaching Referral Hospital, Tenwek Mission Hospital, Kijabe Mission Hospital, M.P. Shah Hospital/ Cancer Care Kenya (Figure 1B). Esophagectomy remains the most commonly used procedure, despite the limitations of the procedure [12, 15]. Self-expandable metallic stents applied under endoscopy are the most frequently used form of palliation [13, 14, 16–20]. Chemoradiotherapy and endoscopic intubation are offered for inoperable tumors. Patients who underwent DXT radiotherapy alone had a maximum survival of 1 year. When used together with chemotherapy, there was a median survival of 12–20 months with a maximum survival of 2 years. Endoscopic intubation, on the other hand, has reported a median survival of 6 months [12]. Ultimately, EC accounts for the highest rate of cancer death in Kenya [2].
In Kenya, 70–80% of cancer cases are diagnosed in late stages due to lack of awareness amongst patients and healthcare workers, poor access to health facilities and insufficient diagnostic facilities. The high use of stents in Western Kenya as a palliative treatment for EC is largely due to diagnosis at very late stages where curative treatment is not possible. In high-incidence areas of the country, most people have insufficient knowledge of risk factors leading to EC [21]. In a study conducted to evaluate awareness of EC in Bomet district, approximately 35% of participants thought EC was contagious or was virally transmitted. Close to half of the participants believed that herbal therapy was the best treatment option for EC, and none of the participants had any knowledge of cancer statistics in the country. In essence, knowledge of cancer is limited to individuals or families affected by cancer within the village or surrounding areas; this is disturbing as cancer is the third leading cause of mortality in the country [21, 22]. Moreover, Kenya still lacks the necessary expertise to keep up with the current medical needs of cancer cases which are about 40,000 new cases each year in a population of approximately 48 million. Based on information published in 2014, there are only 25 oncology specialists in the country: 14 physicians, 4 radiation oncologists, 6 medical oncologists, and 4 pediatric oncologists. In addition, there are very few thoracic surgeons [21]. To tackle the current needs, the Kenyan government has developed strategic plans to control cancer. Nevertheless, much still remains to be done in understanding mechanisms involved in esophageal cancer development and devise strategies for prevention and treatment [21].
Risk factors & mechanisms of action
Tobacco smoking
A positive relationship between tobacco smoking and development of EC in Kenya has been shown to be statistically significant in one epidemiology study in which smokers had 2.51 odds of developing EC than non-smokers [23]. Other Kenyan studies did not show any significance between tobacco smoking and EC [6, 24, 25]. Moreover, esophageal dysplasia was also not associated with tobacco smoking in Kenya [25]. Passing of the Tobacco Control Act to the law in 2007 has substantially reduced the number of smokers in Kenya from 10.9% in 2007 to 8.6% in 2012 [26]. The population of smokers has further decreased to 7.8% in 2014 [27].
Hot food or drink
Consumption of very hot tea and porridge is common in Kenya and has been suspected as a risk factor for EC. A study conducted at the Moi Teaching Referral Hospital found a high association between hot food/drink consumption and an increased risk of developing EC (odds ratio, OR=12.3) [23]. Another study conducted in nearby Tanzania showed a strong correlation between drinking of hot milky tea (≥ 70°C) and EC [28]. Similar results were found in Northern Iran and Southern China, where individuals who drank tea at ≥ 70°C had 8-fold increased risk of EC [29, 30]. Moreover, mouse studies have shown that thermal injury to the esophagus due to hot food or drink may lead to the development of hyperproliferative premalignant lesions in the esophagus [31].
Viral infection
Oncogenic human papillomavirus (HPV) infection is especially high among women in Kenya, causing an annual mortality of 2,451 women from HPV-related cervical cancer [32]. The association between HPV and EC has shown mixed results, with some studies supporting HPV as a risk factor for EC, while other data do not support this hypothesis [33–39]. Patel et al at Moi Teaching Referral Hospital failed to detect 17 types of HPV in 28 samples of EC. None of the 27 known HPV types was detected in samples from the Tenwek Mission Hospital [18, 40]. Although small sample sizes may be a major limitation in these studies, similar results have been reported from other countries within Africa’s EC corridor such as Malawi and Zambia [41, 42]. Next-generation sequencing of samples from Malawi showed the absence of viral genome in the genome of EC patients again supporting the opinion that HPV infection is not a major risk factor in Kenya [42].
Malnutrition and food preparation
Malnutrition is a major risk factor for EC in Kenya and other areas of the EC corridor. Adoption of Western diets and food preparation contribute to malnutrition. Food preparation on its own is a risk factor as certain methods may increase exposure to carcinogens such as heterocyclic amines and polycyclic aromatic hydrocarbons. Nutritional deficiencies associated with EC were zinc (Zn) and selenium (Se) intake [1]. The daily required intake of Zn is 11 mg for men and 8 mg for women. The average Zn intake in Eastern Africa is estimated as 8.6 mg, thus the plausibility for Zn deficiency in Eastern Africa is quite high [1, 43]. Based on findings from Kenya, Uganda, and Malawi, Zn deficiency in children is a ubiquitous challenge in rural settings within the EC corridor [44–47]. Zn deficiency is known to promote EC progression through inflammation whereas replenishment of Zn induces apoptosis in esophageal epithelial cells in rodents in vivo [48–50].
As an important micronutrient involved in anti-oxidative functions, Se is important for glutathione peroxidase and thioredoxin reductase [51, 52]. Se compounds may also impact cell cycle by inhibition of cyclin-dependent kinases involved in mid-to-late G1 phase. Selenite rapidly blocks DNA synthesis and arrests cells in S phase. Some Se-based compounds such as methaneselenol/methylselenol induce caspase-mediated apoptosis in p53 mutant cancer cells [52]. Recommended daily intake for Se is 50–70 μg, with a minimum of 40 μg and a maximum of 400 μg daily for adults. Daily intake of 100–200 μg has shown inhibition of genetic damage and cancer development in humans [53]. Individuals in the African EC corridor take an average of 36.5 μg of Se, 1.5 to 2 times lower than the daily intake in West and Central Africa, and much lower than the recommended daily intake [1].
Major reasons for deficiencies in nutrition may be related to types of foods taken, dietary changes and food preparation [54]. High-incidence countries such as Kenya, Malawi, and South Africa have a high maize diet lacking Zn and Se, as compared to brown sorghum which is high in Zn and Se and a staple grain in West Africa-a low-incidence region [1, 4]. In Kenya, the change to a Westernized diet has led to monotonous food types, a lack of food variety, and low nutritional quality of food [54]. In Western Kenya, replacement of traditional cereals and vegetables such as brown sorghum, Achak Achak (Laurnea cornuta), Akeyo (Cleome gynandra) and Osuga (Solanum spp.) by foreign cereals and vegetables such as maize, cabbage and kales has led to a decrease in Zn and Se intake [54, 55]. Maize currently makes up 50% of energy intake within Africa’s EC corridor [1, 55]. Boiling of bitter tasting Achak Achak and Akeyo to improve taste leads to a decrease in Se and Zn [54, 55]. Moreover, traditional modes of food preparation such as soaking, fermentation, and drying are looked down upon and are seen as primitive, yet they preserve vitamin and micronutrients in foods [54, 56].
The increase of legumes in the Kenyan diet has led to increased phytate intake in food, which inhibits micronutrient uptake [1, 54]. Maize and rice are rich sources of phytates within the EC corridor [57]. Traditional methods of food preparation such as fermentation and malting of cereals reduce phytate content unlike Western food processing methods that do not reduce the amount of phytates in foods [54, 56].
Certain cooking fuels such as charcoal contain polycyclic aromatic hydrocarbons [58]. Charcoal use in food preparation, a prime fuel source in Kenya, Malawi and Zambia especially in rural areas and poor urban families, is highly correlated with EC development in those countries [23, 41, 59]. Grilling or roasting of red meat leads to the formation of mutagenic heterocyclic amines, especially when red meat is well done. These mutagens have previously been associated with an increased risk of EC in studies conducted in Sweden, USA, and Uruguay [60]. Well-done Nyama Choma (roasted meat) is fairly popular throughout Kenya and has been suspected to be a risk factor for EC in Western Kenya, although it has not been well studied [23].
Alcohol and acetaldehyde
McGlashan et al first suspected alcoholic spirits as a risk factor for EC in East and Central Africa [61, 62]. A review on EC in Africa pointed out a definite correlation between maize alcohol and high EC incidence [4]. In Western Kenya, alcohol drinkers had a 45% higher risk of developing EC than non-drinkers [23]. Additionally, there is a 13% higher prevalence of esophageal dysplasia among alcohol drinkers compared to non-drinkers in an older patient population (> 50 years) [25].
Studies have shown that the risk of EC from alcohol consumption depends on the average daily intake and not the duration of the habit, thus binge drinking increases EC risk [63]. In an Australian study, it was shown that intake of alcohol containing a mass volume of ≥ 170 g/ wk of ethanol showed a significantly higher risk of developing EC. The risk increased 4 fold for individuals who drank ≥ 420 g/wk [64]. A case-control study on the risk of developing EC conducted by Morita et al showed higher OR for heavy drinkers (OR=10) compared to tobacco smokers (OR=5.8) [65]. They further showed that heavy drinking led to an increased number of cancer lesions [65]. Likewise, individuals who consume ≥ 50 or more grams of alcohol per day or roughly ≥ 3.5 drinks per day, have at least a two to three times greater risk of developing head and neck cancers than nondrinkers [66]. Moreover, among EC patients, non-drinkers have been shown to have significantly greater survival than alcohol drinkers [67].
Binge drinking is common in Western Kenya. In a study conducted by Lo et al, 60.3% of participants (3,019/5,010) were more likely to be drunk half the time they drank alcohol [68]. Of these 3,019 individuals, 66.9% of them were drunk for more than 18 days in a month [68]. Chang’aa, a distilled grain-based alcoholic drink usually of a concentration ≥ 50% ethanol, is the most commonly consumed drink in rural Western Kenya [69]. Daily intake of ethanol in the form of Chang’aa may be as high as 106.5 g [70]. This dose is highly correlated with EC as reported by Morita et al [65].
Four main chemicals have been suspected to be involved in alcohol drinking-related EC in Kenya, namely, ethanol, acetaldehyde, nitrosamines, and aflatoxin. Ethanol may contribute to carcinogenesis of squamous epithelial cells primarily via its metabolism to acetaldehyde [71]. Inside the squamous epithelial cells, ethanol metabolism causes oxidative damage to DNA, proteins, and lipids due to the production of reactive oxygen species, and affects fatty acid metabolism. Ethanol and intracellular acetaldehyde stimulate cell proliferation, inflammation, and angiogenesis, and suppress squamous cell differentiation, therefore promoting EC development [71, 72]. Acetaldehyde production increases with increased alcohol intake, and high concentrations of acetaldehyde can react with DNA bases to form ethylidene adducts. These adducts interfere with DNA repair mechanism, leading to errors in replication and/or mutations in oncogenes and tumor suppressor genes, ultimately leading to cancer [73, 74]. The current theory may explain how ethanol contributes to gene mutations as shown in a Chinese population where mutation analysis showed unique clustering for drinking EC patients. Drinkers had a much higher frequency of G→T transversions than non-drinkers [75].
In addition to intracellular acetaldehyde, acetaldehyde from extracellular sources is also carcinogenic and may contribute to EC development. The esophageal epithelium is exposed to acetaldehyde from extracellular sources when ethanol is broken down by oral microflora, or when acetaldehyde is contained in foods or drink. Acetaldehyde may perturb the lipid bilayer of the cell membrane, affects the functions of intrinsic membrane proteins, such as SHH, WNT, TLR4, and NOTCH pathways and thus activates or inhibits downstream signaling [72]. In Kenya, fermented milk known as mursik has been shown to be a rich source of acetaldehyde. Out of 8 mursik samples that were tested, 7 starter cultures produced mutagenic levels of acetaldehyde (> 100 μM), and 4 starter cultures produced more than 1,000 μM of acetaldehyde [76].
Nitrosamine was suspected to be a major carcinogen in alcoholic beverages in Kenya, contributing to EC development [62]. However, further testing did not find a significant amount of nitrosamines in grain-based alcohol from Kenya and Zambia [77].
Aflatoxins in grain-based alcoholic drinks such as Busaa (a cereal based alcoholic beverage) may also be a risk factor. Aflatoxins were first suspected to be a risk factor for EC in Kenya in 1972 [78]. A study conducted in Bomet county, Western Kenya, showed that 57 out of 61 samples of Busaa had a mean of 5.2 μg of aflatoxin [79]. The same region suffered from a crisis of aflatoxicosis in 2004, resulting in several cases of liver cancer [80].
Apart from the four chemicals shown to be risk factors for EC development, alcoholic drink is often adulterated with other chemicals that are or may be carcinogenic such as methanol and formaldehyde in Chang’aa. Many times the adulterant is not known to the public. Effects of these in the development of EC have not been explored [81, 82].
Genetics factors
In a case series of young EC (< 30 yrs of age) at the Tenwek Mission Hospital, Western Kenya, it was found that 45 out of 60 patients had a family history of cancer and 21 out of 60 had a family history of EC. Additionally, 25 patients had a first-degree relative suffering from cancer, and 16 patients had multiple relatives suffering from cancer. Moreover, five patients had multiple cases of EC in their families. Most of these patients came from the nearby Kipsigis community, and therefore share a conserved gene pool. EC cases as young as 12 years and their family history suggest possible heritable germline mutations in EC [24]. Similar to this study in Kenya, a case-control study conducted in China also demonstrated family history as a risk factor of EC. In this Chinese cohort, 34.7% of EC cases had first-degree relatives with the disease compared to 21.9% of controls. Individuals with both parents affected by EC were 8 times more likely to develop EC compared to those whose parents were not affected. A positive family history of any cancer was found to be associated with an excess risk of EC [83]. Strong contribution of familial factors in EC development suggests genetic factors associated with genome maintenance as seen in genetic disorders, such as Fanconi Anemia (FA) and Howel-Evans syndrome/tylosis with esophageal cancer (TOC), which may contribute to EC [84].
FA is a recessively inherited disease characterized by congenital abnormalities, bone marrow failure, and development of certain cancers such as EC. In a study measuring cancer incidence in FA patients, it was found that EC has the second highest observed-to-expected ratio among solid tumors [85]. This ratio for EC was even higher in a German FA cohort [86]. FA patients have a 700–1,000 fold increased the likeliness of developing EC and head and neck SCC [84]. It is now known that chromosomal changes in FA SCC are similar to those in sporadic SCC; the same genes appear to be targeted in both forms. In fact, FA SCC and sporadic SCC have the same pattern of allelic loss [87].
As an autosomal dominant trait with complete penetrance, TOC is characterized by yellowish thickened plaque in soles of feet and palms of hands or weight-bearing areas of the body. In a study conducted in Liverpool, UK, 21 out of 89 TOC patients from two related families consisting of 345 individuals died from EC [88]. TOC results from mutations in RHBDF2 which impacts N-terminal loop domain of iRHOM proteins, iRHOM1 and iRHOM2. The iRHOM proteins have been shown to affect ADAM17 maturation, which would then impact EGF signaling. It has been proposed that the dysregulated production of EGF may lead to uncontrolled growth of squamous epithelial cells [89].
Studies have shown that cell cycle genes are frequently mutated in EC. Of these genes, only P53 has been studied in Kenyan EC patients. In most high-incidence EC regions, P53 is mutated in ~90% of EC patients. However, in Kenya, P53 was only mutated in 40% EC according to a study conducted at the Moi Teaching Referral Hospital [40]. The difference may account for a unique etiology, however, a larger cohort would be required to validate such a finding. Liu et al have recently sequenced EC tissue samples from the Kamuzu Central Hospital, Malawi, which is on the African EC corridor. Whole exome sequencing suggested an unidentified etiology when compared to Chinese EC. The unidentified signature was characterized by C→A trans-versions as well as C→T transitions [42]. Other than such differences, frequently mutated genes in Malawi samples were similar to those identified in Chinese and Japanese populations, P53, NOTCH1, CDKN2A, PIK3CA, NFE2L2 [42, 75, 90].
Conclusions
Alcohol drinking, genetic factors, dietary change/food preparation, and consumption of hot food or drink are the main risk factors for EC in Kenya. There is a need to investigate the causal relationship between these four major risk factors and the development of EC in Kenya. The effect of alcohol drinking on the development of EC in Kenyans warrants experimental studies on the possible molecular mechanisms. Many genetic studies on EC conducted in Iran and China have focused on the effect of gene polymorphisms associated with ethanol metabolism, cell cycle, or DNA repair. Genotyping requires a large patient population. Unfortunately, Kenya currently does not have strong cancer reporting abilities, and thus current work should be focused on understanding major genetic changes leading to EC development in order to identify the genetic etiology of EC in Kenya [21]. Kenya currently has the highest reported percentage of young patients (< 30 years of age) globally, and thus it is important to establish the reasons underlying so many young cases [24]. In this review, we suggest the possibility of heritable diseases as a predisposing condition. Likewise, the impact of dietary change, malnutrition and the use of “modern” food preparation methods requires further inquiry. Furthermore, there is a need to develop studies that investigate the relationship between hot food or drink and EC in a Kenyan cohort.
Several steps may be taken to prevent EC in Kenya including reducing alcohol intake, eating traditional foods high in micronutrients and vitamins, low-temperature cooking of red meat, consuming food and drinks at lower temperatures, use of traditional food preparation methods, as well as screening in high-incidence areas or in predisposed individuals. Clinically, it is urgent to train more medical specialists who can diagnose and treat EC. Community health educators in high-incidence regions should disseminate knowledge of EC within their communities, leading to greater awareness of the disease. Furthermore, there is a need for a population-based national cancer registry in order to attain a more accurate rate of EC in the country. To improve the current knowledge of cancer incidence and prevalence, the Kenyan government has recently announced an allocation of KSh 17 million (~$170,000) towards the development of four more cancer registries in Kisumu, Mombasa, Nakuru and Nyeri (Elizabeth Merab: “Government allocates Sh17m for national cancer registry launch”, Daily Nation Kenya 2016 http://www.nation.co.ke/news/Government-national-cancerregistry-launch/1056-3072334-qcvrme/index. html). Even so, there is still a great need for funding cancer research in Kenya.
Acknowledgments
The authors are supported by NIH/NCI U54 CA156735 and NIH/NIAAA U54 AA019765.
Abbreviations
- EC
esophageal cancer
- HPV
human papillomavirus
- OR
odds ratio
- Zn
zinc
- SCC
squamous cell carcinoma
- Se
selenium
- FA
Fanconi anemia
- TOC
tylosis with esophageal cancer
Footnotes
Disclosure of conflict of interest
None.
References
- 1.Schaafsma T, Wakefield J, Hanisch R, Bray F, Schuz J, Joy EJ, Watts MJ, McCormack V. Africa’s oesophageal cancer corridor: geographic variations in incidence correlate with certain micronutrient deficiencies. PLoS One. 2015;10:e0140107. doi: 10.1371/journal.pone.0140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Cheng ML, Zhang L, Borok M, Chokunonga E, Dzamamala C, Korir A, Wabinga HR, Hiatt RA, Parkin DM, Van Loon K. The incidence of oesophageal cancer in Eastern Africa: identification of a new geographic hot spot? Cancer Epidemiol. 2015;39:143–149. doi: 10.1016/j.canep.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook P. Cancer of the oesophagus in Africa. A summary and evaluation of the evidence for the frequency of occurrence, and a preliminary indication of the possible association with the consumption of alcoholic drinks made from maize. Br J Cancer. 1971;25:853–880. doi: 10.1038/bjc.1971.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gatei DG, Odhiambo PA, Orinda DA, Muruka FJ, Wasunna A. Retrospective study of carcinoma of the esophagus in Kenya. Cancer Res. 1978;38:303–307. [PubMed] [Google Scholar]
- 6.Parker RK, Dawsey SM, Abnet CC, White RE. Frequent occurrence of esophageal cancer in young people in Western Kenya. Dis Esophagus. 2010;23:128–135. doi: 10.1111/j.1442-2050.2009.00977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakhisi J, Patel K, Buziba N, Rotich J. Esophageal cancer in north rift valley of Western Kenya. Afr Health Sci. 2005;5:157–163. [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed N, Cook P. The incidence of cancer of the oesophagus in West Kenya. Br J Cancer. 1969;23:302–312. doi: 10.1038/bjc.1969.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodenyo H, Rana F, Mutuma GZ, Kabanga JM, Kuria JK, Okoth FA. Patterns of upper gastrointestinal diseases based on endoscopy in the period 1998-2001. Afr J Health Sci. 2005;12:49–54. doi: 10.4314/ajhs.v12i1.30800. [DOI] [PubMed] [Google Scholar]
- 10.Makanga W, Nyaoncha A. Upper gastrointestinal disease in Nairobi and Nakuru counties, Kenya; a two year comparative endoscopy study. The Annals of African Surgery. 2014;11:35–39. [Google Scholar]
- 11.Ogendo SW. Surgery of the oesophagus: a Nairobi experience. East Afr Med J. 1993;70:307–309. [PubMed] [Google Scholar]
- 12.Ogendo SW. Follow up of oesophageal cancer therapy at the Kenyatta national hospital, Nairobi. East Afr Med J. 2001;78:650–654. doi: 10.4314/eamj.v78i12.8935. [DOI] [PubMed] [Google Scholar]
- 13.White RE, Parker RK. Oesophageal cancer: an overview of a deadly disease. The Annals of African Surgery. 2007;1:34–48. [Google Scholar]
- 14.White RE, Parker RK, Fitzwater JW, Kasepoi Z, Topazian M. Stents as sole therapy for oesophageal cancer: a prospective analysis of outcomes after placement. Lancet Oncol. 2009;10:240–246. doi: 10.1016/S1470-2045(09)70004-X. [DOI] [PubMed] [Google Scholar]
- 15.Ogendo SW. Weight change post oesophagectomy for carcinoma of oesophagus. East Afr Med J. 2007;84:271–278. doi: 10.4314/eamj.v84i6.9536. [DOI] [PubMed] [Google Scholar]
- 16.Parker RK, White RE, Topazian M, Chepkwony R, Dawsey S, Enders F. Stents for proximal esophageal cancer: a case-control study. Gastrointest Endosc. 2011;73:1098–1105. doi: 10.1016/j.gie.2010.11.036. [DOI] [PubMed] [Google Scholar]
- 17.White RE, Chepkwony R, Mwachiro M, Burgert SL, Enders FT, Topazian M. Randomized trial of small-diameter versus large-diameter esophageal stents for palliation of malignant esophageal obstruction. J Clin Gastroenterol. 2015;49:660–665. doi: 10.1097/MCG.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 18.White RE, Mungatana C, Mutuma G, Robert ME, Daniel RW, Topazian MD, Shah KV. Absence of human papillomavirus in esophageal carcinomas from Southwestern Kenya. Dis Esophagus. 2005;18:28–30. doi: 10.1111/j.1442-2050.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- 19.White RE, Mungatana C, Topazian M. Esophageal stent placement without fluoroscopy. Gastrointest Endosc. 2001;53:348–351. doi: 10.1016/s0016-5107(01)70415-4. [DOI] [PubMed] [Google Scholar]
- 20.White RE, Mungatana C, Topazian M. Expandable stents for iatrogenic perforation of esophageal malignancies. J Gastrointest Surg. 2003;7:715–719. doi: 10.1016/s1091-255x(03)00064-7. discussion 719-720. [DOI] [PubMed] [Google Scholar]
- 21.Topazian H, Cira M, Dawsey SM, Kibachio J, Kocholla L, Wangai M, Welch J, Williams MJ, Duncan K, Galassi A. Joining forces to overcome cancer: the Kenya cancer research and control stakeholder program. J Cancer Policy. 2016;7:36–41. doi: 10.1016/j.jcpo.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duron V, Bii J, Mutai R, Ngetich J, Harrington D, Parker R, White R. Esophageal cancer awareness in Bomet district, Kenya. Afr Health Sci. 2013;13:122–128. doi: 10.4314/ahs.v13i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel K, Wakhisi J, Mining S, Mwangi A, Patel R. Esophageal cancer, the topmost cancer at MTRH in the Rift Valley, Kenya, and its potential risk factors. ISRN Oncol. 2013;2013:503249. doi: 10.1155/2013/503249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawsey SP, Tonui S, Parker RK, Fitzwater JW, Dawsey SM, White RE, Abnet CC. Esophageal cancer in young people: a case series of 109 cases and review of the literature. PLoS One. 2010;5:e14080. doi: 10.1371/journal.pone.0014080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mwachiro MM, Burgert SL, Lando J, Chepkwony R, Bett C, Bosire C, Abnet CC, Githanga J, Waweru W, Giffen CA, Murphy G, White RE, Topazian MD, Dawsey SM. Esophageal squamous dysplasia is common in asymptomatic Kenyans: a prospective, community-based, cross-sectional study. Am J Gastroenterol. 2016;111:500–507. doi: 10.1038/ajg.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abuse NAftCAAaD. Rapid situation assessment of the status of drug and substance abuse in Kenya, 2012. 2012 [Google Scholar]
- 27.(WHO) WHO. WHO Report on the global tobacco epidemic. Kenya: 2015. [Google Scholar]
- 28.Munishi MO, Hanisch R, Mapunda O, Ndyetabura T, Ndaro A, Schuz J, Kibiki G, McCormack V. Africa’s oesophageal cancer corridor: do hot beverages contribute? Cancer Causes Control. 2015;26:1477–1486. doi: 10.1007/s10552-015-0646-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Islami F, Pourshams A, Nasrollahzadeh D, Kamangar F, Fahimi S, Shakeri R, Abedi-Ardekani B, Merat S, Vahedi H, Semnani S, Abnet CC, Brennan P, Moller H, Saidi F, Dawsey SM, Malekzadeh R, Boffetta P. Tea drinking habits and oesophageal cancer in a high risk area in northern Iran: population based case-control study. BMJ. 2009;338:b929. doi: 10.1136/bmj.b929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J, Zeng R, Cao W, Luo R, Chen J, Lin Y. Hot beverage and food intake and esophageal cancer in Southern China. Asian Pac J Cancer Prev. 2011;12:2189–2192. [PubMed] [Google Scholar]
- 31.Rapozo DC, Blanco TC, Reis BB, Gonzaga IM, Valverde P, Canetti C, Barja-Fidalgo C, Simao TA, Albano RM, Kruel CD, Pinto LF. Recurrent acute thermal lesion induces esophageal hyperproliferative premalignant lesions in mice esophagus. Exp Mol Pathol. 2016;100:325–331. doi: 10.1016/j.yexmp.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Bruni L, Barrionuevo-Rosas L, Albero G, Serrano B, Mena M, Gómez D, Muñoz J, Bosch FX, de Sanjosé S. Human papillomavirus and related diseases in Kenya. ICO Information Centre on HPV and Cancer (HPV Information Centre); 2016. pp. 1–71. [Google Scholar]
- 33.Antunes LC, Prolla JC, Lopes AD, da Rocha MP, Fagundes RB. No evidence of HPV DNA in esophageal squamous cell carcinoma in a population of Southern Brazil. World J Gastroenterol. 2013;19:6598–6603. doi: 10.3748/wjg.v19.i39.6598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bucchi D, Stracci F, Buonora N, Masanotti G. Human papillomavirus and gastrointestinal cancer: a review. World J Gastroenterol. 2016;22:7415–7430. doi: 10.3748/wjg.v22.i33.7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao F, Han H, Zhang F, Wang B, Ma W, Wang Y, Sun G, Shi M, Ren Y, Cheng Y. HPV infection in esophageal squamous cell carcinoma and its relationship to the prognosis of patients in Northern China. ScientificWorldJournal. 2014;2014:804738. doi: 10.1155/2014/804738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koshiol J, Kreimer AR. Lessons from Australia: human papillomavirus is not a major risk factor for esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2010;19:1889–1892. doi: 10.1158/1055-9965.EPI-10-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koshiol J, Wei WQ, Kreimer AR, Chen W, Gravitt P, Ren JS, Abnet CC, Wang JB, Kamangar F, Lin DM, von Knebel-Doeberitz M, Zhang Y, Viscidi R, Wang GQ, Gillison ML, Roth MJ, Dong ZW, Kim E, Taylor PR, Qiao YL, Dawsey SM. No role for human papillomavirus in esophageal squamous cell carcinoma in China. Int J Cancer. 2010;127:93–100. doi: 10.1002/ijc.25023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liyanage SS, Segelov E, Garland SM, Tabrizi SN, Seale H, Crowe PJ, Dwyer DE, Barbour A, Newall AT, Malik A, Macintyre CR. Role of human papillomaviruses in esophageal squamous cell carcinoma. Asia Pac J Clin Oncol. 2013;9:12–28. doi: 10.1111/j.1743-7563.2012.01555.x. [DOI] [PubMed] [Google Scholar]
- 39.Ludmir EB, Stephens SJ, Palta M, Willett CG, Czito BG. Human papillomavirus tumor infection in esophageal squamous cell carcinoma. J Gastrointest Oncol. 2015;6:287–295. doi: 10.3978/j.issn.2078-6891.2015.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel K, Mining S, Wakhisi J, Gheit T, Tommasino M, Martel-Planche G, Hainaut P, Abedi-Ardekani B. TP53 mutations, human papilloma virus DNA and inflammation markers in esophageal squamous cell carcinoma from the Rift Valley, a high-incidence area in Kenya. BMC Res Notes. 2011;4:469. doi: 10.1186/1756-0500-4-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kayamba V, Bateman AC, Asombang AW, Shibemba A, Zyambo K, Banda T, Soko R, Kelly P. HIV infection and domestic smoke exposure, but not human papillomavirus, are risk factors for esophageal squamous cell carcinoma in Zambia: a case-control study. Cancer Med. 2015;4:588–595. doi: 10.1002/cam4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W, Snell JM, Jeck WR, Hoadley KA, Wilkerson MD, Parker JS, Patel N, Mlombe YB, Mulima G, Liomba NG, Wolf LL, Shores CG, Gopal S, Sharpless NE. Subtyping sub-Saharan esophageal squamous cell carcinoma by comprehensive molecular analysis. JCI Insight. 2016;1:e88755. doi: 10.1172/jci.insight.88755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Institutes of Health OoDS. Zinc fact sheet for consumers. 2016 [Google Scholar]
- 44.Ferguson E, Chege P, Kimiywe J, Wiesmann D, Hotz C. Zinc, iron and calcium are major limiting nutrients in the complementary diets of rural Kenyan children. Matern Child Nutr. 2015;11(Suppl 3):6–20. doi: 10.1111/mcn.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manary MJ, Hotz C, Krebs NF, Gibson RS, Westcott JE, Broadhead RL, Hambidge KM. Zinc homeostasis in Malawian children consuming a high-phytate, maize-based diet. Am J Clin Nutr. 2002;75:1057–1061. doi: 10.1093/ajcn/75.6.1057. [DOI] [PubMed] [Google Scholar]
- 46.May T, Westcott C, Thakwalakwa C, Ordiz MI, Maleta K, Westcott J, Ryan K, Hambidge KM, Miller LV, Young G, Mortimer E, Manary MJ, Krebs NF. Resistant starch does not affect zinc homeostasis in rural Malawian children. J Trace Elem Med Biol. 2015;30:43–48. doi: 10.1016/j.jtemb.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tidemann-Andersen I, Acham H, Maage A, Malde MK. Iron and zinc content of selected foods in the diet of schoolchildren in Kumi district, east of Uganda: a cross-sectional study. Nutr J. 2011;10:81. doi: 10.1186/1475-2891-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fong LY, Nguyen VT, Farber JL. Esophageal cancer prevention in zinc-deficient rats: rapid induction of apoptosis by replenishing zinc. J Natl Cancer Inst. 2001;93:1525–1533. doi: 10.1093/jnci/93.20.1525. [DOI] [PubMed] [Google Scholar]
- 49.Wan SG, Taccioli C, Jiang Y, Chen H, Smalley KJ, Huang K, Liu XP, Farber JL, Croce CM, Fong LY. Zinc deficiency activates S100A8 inflammation in the absence of COX-2 and promotes murine oral-esophageal tumor progression. Int J Cancer. 2011;129:331–345. doi: 10.1002/ijc.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guo W, Zou YB, Jiang YG, Wang RW, Zhao YP, Ma Z. Zinc induces cell cycle arrest and apoptosis by upregulation of WIG-1 in esophageal squamous cancer cell line EC109. Tumour Biol. 2011;32:801–808. doi: 10.1007/s13277-011-0182-5. [DOI] [PubMed] [Google Scholar]
- 51.El-Bayoumy K, Sinha R. Molecular chemoprevention by selenium: a genomic approach. Mutat Res. 2005;591:224–236. doi: 10.1016/j.mrfmmm.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 52.Zeng HW, Combs GF. Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasion. J Nutr Biochem. 2008;19:1–7. doi: 10.1016/j.jnutbio.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 53.El-Bayoumy K. The protective role of selenium on genetic damage and on cancer. Mutat Res. 2001;475:123–139. doi: 10.1016/s0027-5107(01)00075-6. [DOI] [PubMed] [Google Scholar]
- 54.Walingo MK. Indigenous food processing methods that improve zinc absorption and bio-availability of plant diets consumed by the Kenyan population. African Journal of Food, Agriculture, Nutrition and Development. 2009;9:523–535. [Google Scholar]
- 55.Otieno SB, Were F, Kabiru E, Waza K. Selenium levels in foods in a high HIV prevalence community: a case study in pala-bondo district, Kenya. East African Journal of Public Health. 2013;10:516–520. [Google Scholar]
- 56.Makokha AO, Oniang’o RK, Njoroge SM, Kamar OK. Effect of traditional fermentation and malting on phytic acid and mineral availability from sorghum (sorghum bicolor) and finger millet (eleusine coracana) grain varieties grown in Kenya. Food Nutr Bull. 2002;23:241–245. [PubMed] [Google Scholar]
- 57.Joy EJ, Louise Ander E, Broadley MR, Young SD, Chilimba AD, Hamilton EM, Watts MJ. Elemental composition of Malawian rice. Environ Geochem Health. 2016 doi: 10.1007/s10653-016-9854-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 58.Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38:27–57. vii. doi: 10.1016/j.gtc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mlombe YB, Rosenberg NE, Wolf LL, Dzamalala CP, Chalulu K, Chisi J, Shaheen NJ, Hosseinipour MC, Shores CG. Environmental risk factors for oesophageal cancer in malawi: a case-control study. Malawi Med J. 2015;27:88–92. doi: 10.4314/mmj.v27i3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zheng W, Lee SA. Well-done meat intake, heterocyclic amine exposure, and cancer risk. Nutr Cancer. 2009;61:437–446. doi: 10.1080/01635580802710741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McGlashan ND. Oesophageal cancer and alcoholic spirits in central Africa. Gut. 1969;10:643–650. doi: 10.1136/gut.10.8.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGlashan ND, Walters CL, McLean AE. Nitrosamines in African alcoholic spirits and oesophageal cancer. Lancet. 1968;2:1017. doi: 10.1016/s0140-6736(68)91303-2. [DOI] [PubMed] [Google Scholar]
- 63.Castellsague X, Munoz N, De Stefani E, Victora CG, Castelletto R, Rolon PA, Quintana MJ. Independent and joint effects of tobacco smoking and alcohol drinking on the risk of esophageal cancer in men and women. Int J Cancer. 1999;82:657–664. doi: 10.1002/(sici)1097-0215(19990827)82:5<657::aid-ijc7>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 64.Pandeya N, Williams G, Green AC, Webb PM, Whiteman DC, Australian Cancer S Alcohol consumption and the risks of adenocarcinoma and squamous cell carcinoma of the esophagus. Gastroenterology. 2009;136:1215–1224. e1211–1212. doi: 10.1053/j.gastro.2008.12.052. [DOI] [PubMed] [Google Scholar]
- 65.Morita M, Kumashiro R, Kubo N, Nakashima Y, Yoshida R, Yoshinaga K, Saeki H, Emi Y, Kakeji Y, Sakaguchi Y, Toh Y, Maehara Y. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: epidemiology, clinical findings, and prevention. Int J Clin Oncol. 2010;15:126–134. doi: 10.1007/s10147-010-0056-7. [DOI] [PubMed] [Google Scholar]
- 66.Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, Altieri A, Cogliano V, WHO International Agency for Research on Cancer Monograph Working Group Carcinogenicity of alcoholic beverages. Lancet Oncol. 2007;8:292–293. doi: 10.1016/s1470-2045(07)70099-2. [DOI] [PubMed] [Google Scholar]
- 67.Ma Q, Liu W, Jia R, Long H, Zhang L, Lin P, Zhao H, Ma G. Alcohol and survival in ESCC: pre-diagnosis alcohol consumption and postoperative survival in lymph node-negative esophageal carcinoma patients. Oncotarget. 2016;7:38857–38863. doi: 10.18632/oncotarget.8754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lo TQ, Oeltmann JE, Odhiambo FO, Beynon C, Pevzner E, Cain KP, Laserson KF, Phillips-Howard PA. Alcohol use, drunkenness and tobacco smoking in rural Western Kenya. Trop Med Int Health. 2013;18:506–515. doi: 10.1111/tmi.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Papas RK, Sidle JE, Wamalwa ES, Okumu TO, Bryant KL, Goulet JL, Maisto SA, Braithwaite RS, Justice AC. Estimating alcohol content of traditional brew in Western Kenya using culturally relevant methods: the case for cost over volume. AIDS Behav. 2010;14:836–844. doi: 10.1007/s10461-008-9492-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Korir ED, Ochieng D, Ndiritu D. Comparative genetics of alcoholism in the Kenyan populations. African Journal of Biotechnology. 2004;3:152–155. [Google Scholar]
- 71.Ohashi S, Miyamoto S, Kikuchi O, Goto T, Amanuma Y, Muto M. Recent advances from basic and clinical studies of esophageal squamous cell carcinoma. Gastroenterology. 2015;149:1700–1715. doi: 10.1053/j.gastro.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 72.Liu Y, Chen H, Sun Z, Chen X. Molecular mechanisms of ethanol-associated oro-esophageal squamous cell carcinoma. Cancer Lett. 2015;361:164–173. doi: 10.1016/j.canlet.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Espina N, Lima V, Lieber CS, Garro AJ. In vitro and in vivo inhibitory effect of ethanol and acetaldehyde on O6-methylguanine transferase. Carcinogenesis. 1988;9:761–766. doi: 10.1093/carcin/9.5.761. [DOI] [PubMed] [Google Scholar]
- 74.Seitz HK, Homann N. The role of acetaldehyde in alcohol-associated cancer of the gastrointestinal tract. Novartis Found Symp. 2007;285:110–119. doi: 10.1002/9780470511848.ch8. discussion 119-114, 198-119. [DOI] [PubMed] [Google Scholar]
- 75.Song Y, Li L, Ou Y, Gao Z, Li E, Li X, Zhang W, Wang J, Xu L, Zhou Y, Ma X, Liu L, Zhao Z, Huang X, Fan J, Dong L, Chen G, Ma L, Yang J, Chen L, He M, Li M, Zhuang X, Huang K, Qiu K, Yin G, Guo G, Feng Q, Chen P, Wu Z, Wu J, Ma L, Zhao J, Luo L, Fu M, Xu B, Chen B, Li Y, Tong T, Wang M, Liu Z, Lin D, Zhang X, Yang H, Wang J, Zhan Q. Identification of genomic alterations in oesophageal squamous cell cancer. Nature. 2014;509:91–95. doi: 10.1038/nature13176. [DOI] [PubMed] [Google Scholar]
- 76.Nieminen MT, Novak-Frazer L, Collins R, Dawsey SP, Dawsey SM, Abnet CC, White RE, Freedman ND, Mwachiro M, Bowyer P, Salaspuro M, Rautemaa R. Alcohol and acetaldehyde in African fermented milk mursik–a possible etiologic factor for high incidence of esophageal cancer in Western Kenya. Cancer Epidemiol Biomarkers Prev. 2013;22:69–75. doi: 10.1158/1055-9965.EPI-12-0908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Collis CH, Cook PJ, Foreman JK, Palframan JF. Cancer of the oesophagus and alcoholic drinks in East Africa. Lancet. 1972;1:441. doi: 10.1016/s0140-6736(72)90896-3. [DOI] [PubMed] [Google Scholar]
- 78.Cook P, Collis CH. Cancer of the oesophagus and alcoholic drinks in East Africa. Lancet. 1972;1:1014. doi: 10.1016/s0140-6736(72)91183-x. [DOI] [PubMed] [Google Scholar]
- 79.Kirui MC, Alakonya AE, Talam KK, Tohru G, Bii CC. Total aflatoxin, fumonisin and deoxynivalenol contamination of busaa in Bomet county, Kenya. African Journal of Biotechnology. 2014;13:2675–2678. [Google Scholar]
- 80.Centers for Disease Control and Prevention. Outbreak of aflatoxin poisoning–eastern and central provinces, Kenya, January–July 2004. MMWR Morb Mortal Wkly Rep. 2004;53:790–793. [PubMed] [Google Scholar]
- 81.Carey K, Kinney J, Eckman M, Nassar A, Mehta K. Chang’aa culture and process: detecting contamination in a killer brew. Humanitarian technology: science, systems and global impact 2015. Humtech2015. 2015;107:395–402. [Google Scholar]
- 82.Rostrup M, Edwards JK, Abukalish M, Ezzabi M, Some D, Ritter H, Menge T, Abdelrahman A, Rootwelt R, Janssens B, Lind K, Paasma R, Hovda KE. The methanol poisoning outbreaks in Libya 2013 and Kenya 2014. PLoS One. 2016;11:e0152676. doi: 10.1371/journal.pone.0152676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen T, Cheng H, Chen X, Yuan Z, Yang X, Zhuang M, Lu M, Jin L, Ye W. Family history of esophageal cancer increases the risk of esophageal squamous cell carcinoma. Sci Rep. 2015;5:16038. doi: 10.1038/srep16038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van Monsjou HS, Wreesmann VB, van den Brekel MW, Balm AJ. Head and neck squamous cell carcinoma in young patients. Oral Oncol. 2013;49:1097–1102. doi: 10.1016/j.oraloncology.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 85.Rosenberg PS, Greene MH, Alter BP. Cancer incidence in persons with Fanconi anemia. Blood. 2003;101:822–826. doi: 10.1182/blood-2002-05-1498. [DOI] [PubMed] [Google Scholar]
- 86.Rosenberg PS, Alter BP, Ebell W. Cancer risks in Fanconi anemia: findings from the German Fanconi anemia registry. Haematologica. 2008;93:511–517. doi: 10.3324/haematol.12234. [DOI] [PubMed] [Google Scholar]
- 87.van Zeeburg HJ, Snijders PJ, Wu T, Gluckman E, Soulier J, Surralles J, Castella M, van der Wal JE, Wennerberg J, Califano J, Velleuer E, Dietrich R, Ebell W, Bloemena E, Joenje H, Leemans CR, Brakenhoff RH. Clinical and molecular characteristics of squamous cell carcinomas from Fanconi anemia patients. J Natl Cancer Inst. 2008;100:1649–1653. doi: 10.1093/jnci/djn366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ellis A, Risk JM, Maruthappu T, Kelsell DP. Tylosis with oesophageal cancer: diagnosis, management and molecular mechanisms. Orphanet J Rare Dis. 2015;10:126. doi: 10.1186/s13023-015-0346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee MY, Nam KH, Choi KC. iRhoms; its functions and essential roles. Biomol Ther (Seoul) 2016;24:109–114. doi: 10.4062/biomolther.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sawada G, Niida A, Uchi R, Hirata H, Shimamura T, Suzuki Y, Shiraishi Y, Chiba K, Imoto S, Takahashi Y, Iwaya T, Sudo T, Hayashi T, Takai H, Kawasaki Y, Matsukawa T, Eguchi H, Sugimachi K, Tanaka F, Suzuki H, Yamamoto K, Ishii H, Shimizu M, Yamazaki H, Yamazaki M, Tachimori Y, Kajiyama Y, Natsugoe S, Fujita H, Mafune K, Tanaka Y, Kelsell DP, Scott CA, Tsuji S, Yachida S, Shibata T, Sugano S, Doki Y, Akiyama T, Aburatani H, Ogawa S, Miyano S, Mori M, Mimori K. Genomic landscape of esophageal squamous cell carcinoma in a Japanese population. Gastroenterology. 2016;150:1171–1182. doi: 10.1053/j.gastro.2016.01.035. [DOI] [PubMed] [Google Scholar]


