Summary
Rab small GTPases control membrane trafficking through effectors that recruit downstream mediators, such as motor proteins. Typically subcellular trafficking involves multiple Rabs, with each specific step mediated by a distinct Rab protein. We describe a collaboration between two distinct Rab protein-orchestrated trafficking circuits in bladder epithelial cells (BECs) that expels intracellular Uropathogenic E.coli (UPEC) from their intracellular niche. RAB11a and RAB27b and their trafficking circuitry are simultaneously involved in UPEC expulsion. While RAB11a recruits its effector RAB11FIP3 and cytoskeletal motor Dynein, RAB27b mobilizes the effector MyRIP and motor Myosin VIIa to mediate bacterial expulsion. This collaboration is coordinated by deposition of the exocyst complex on bacteria-containing vesicles (BCVs), an event triggered by the innate receptor Toll-like Receptor (TLR) 4. Both RAB11a and RAB27b are recruited and activated by the exocyst complex components SEC6/SEC15. Thus, the cell autonomous defense system can mobilize and coalesce multiple subcellular trafficking circuitries to combat infections.
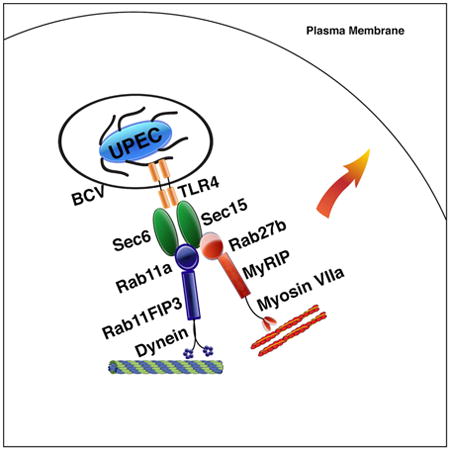
Graphical abstract
Bacterial expulsion is a powerful immune defense mechanism that rapidly eliminates Uropathogenic E.coli infections in the bladder epithelium. Miao et al. demonstrate that the concerted actions of both RAB11a and RAB27b along with their respective effectors are mobilized by innate immune signaling, resulting in the prompt expulsion of bacteria.
Introduction
Rab proteins represent the largest subfamily of Ras-like small GTPases (Zerial and McBride, 2001). In mammalian cells, more than 60 Rab proteins regulate almost every aspect of membrane trafficking, including vesicle transport, cargo selection, vesicle tethering, docking and fusion (Rodriguez-Boulan et al., 2005). These small GTPases control membrane trafficking by orchestrating a host of effectors, whose function is centered on recruiting downstream mediators, such as motor proteins, to achieve specific tasks (Stenmark, 2009). In a typical subcellular trafficking circuit, multiple Rabs are usually involved, and each specific step of the trafficking circuit is typically mediated by a distinct Rab protein (Zerial and McBride, 2001). In each case, different Rab proteins regulating different steps function in a sequential manner, during which the preceding Rab is replaced by another Rab protein at the intersection of a different trafficking step and where the subsequent activity of a Rab protein is critically regulated by the preceeding Rab protein in the series (Rink et al., 2005; Stenmark, 2009). As the primary organizers of subcellular trafficking events, the role of Rab small GTPase have been extensively documented in many cellular activities, yet how Rab proteins contribute to immune defense remains poorly understood.
Bacterial expulsion is a cell autonomous immune defense strategy recently described in infected BECs to rapidly reduce intracellular bacterial burden via regulated exocytosis (Abraham and Miao, 2015; Bishop et al., 2007; Miao et al., 2015). Bacterial expulsion is an innate immune signaling-orchestrated process and appears to occur in two waves, with a TLR4 initiated early wave of expulsion occurring between 4-6 h post infection (Miao et al., 2016; Song et al., 2009) followed by a second TRPML3 channel-activated wave occurring around 8 h post infection (Miao et al., 2015). So far, the immune surveillance molecules involved in initiating the early stages of bacterial expulsion have been identified. For example, it is known that following entry of UPEC into BECs, the TLR4 molecules, localized in BCVs that encase the bacteria, sense the pathogen in the BCV and recruit the compartmental signaling adaptors TRIF/TRAM which in turn recruit the signaling protein TRAF3 that subsequently becomes K33-linked polyubiquitinated (Miao et al., 2016). This unique form of post-translational modification on TRAF3, a critical immune modulator, is then sensed by a Guanine Exchange Factor (GEF) protein, RalGDS, which activates the assembly of the Exocyst complex, a group of proteins involved in tethering and spatial targeting of vesicles (Hsu et al., 2004). The deposition of the exocyst complex on the cytosolic surface of BCVs is thought to respond to the innate immune signaling, and initiate the polarized trafficking of the BCVs to the plasma membrane, but the intracellular trafficking pathway that is subsequently recruited by the exocyst complex to transport the BCV-bound bacteria is still unknown. In view of its central role in intracellular trafficking, we suspect that BCV trafficking is also mediated by a Rab small GTPase. Identification of this putative Rab protein should not only fill a critical void in our understanding of the molecular aspects of bacterial expulsion as a powerful immune defense strategy, but also provide valuable information on how Rab small GTPases contribute to cell autonomous immunity in the host cell.
Here, we present evidence showing that, surprisingly, instead of being governed by a single Rab protein, the peripheral transport step of BCV requires the simultaneous collaboration of two distinct Rab small GTPases, and their specific effector-orchestrated motor proteins.
Results
RAB11a and RAB27b associate with Exocyst complex components in BECs upon UPEC infections
Following TLR4 activation in infected BECs, the critical trigger activated by innate immune signaling for the trafficking of BCVs to plasma membrane is likely to be the deposition of the exocyst complex on the cytosolic surface of the bacteria-containing vesicles (BCVs) (Miao et al., 2016). We reasoned that the putative Rab protein responsible for BCV trafficking would be recruited and directly bind to components of the exocyst. Therefore, in order to identify the putative BCV-trafficking Rab small GTPase, we performed a proximity ligation assay (PLA)-based protein interaction screen (Fredriksson et al., 2002). This approach has been employed before to successfully construct the interactome for all the Rab GTPase in dendritic cells (Li et al., 2016). Here we used exocyst complex proteins as bait to identify Rab proteins that specifically interact with exocyst complex subunits following exposure of the human bladder epithelium line 5637 to the UPEC CI5 strain (Figure 1A). To improve our success rate, we narrowed our targets in the screen to a list of 11 Rab small GTPases that are known to mediate exocytic events. As we expected, most of the Rab proteins we tested showed no specific interaction with exocyst complex under steady state conditions (Supplementary Figure 1). Interestingly, we consistently found RAB11a and RAB27b and not other Rab proteins as positive interacting partners with exocyst complex subunits, such as SEC6 in infected but not steady state BECs (Figure 1B). To validate the PLA screening result, we probed extracts of isolated BCVs for these Rab proteins. The BCVs were isolated by a recently described method involving pre-tagging infecting bacteria with magnetic beads before they were introduced to BECs (Lonnbro et al., 2008; Miao et al., 2016). Following bacterial infection, the BEC membranes were disrupted mechanically and BCVs were isolated using a magnet. Consistent with the PLA results, we observed both RAB11a and RAB27b to be co-associated with BCVs (Figure 1C). That RAB11a and RAB27b associate with the intracellular vesicles harboring UPEC was also confirmed by confocal microscopy (Figure1 D). Thus, two Rab small GTPases, RAB11a and RAB27b, were found to associate with BCVs and potentially contribute to trafficking and expulsion of infecting bacteria from BECs.
Figure 1. Identification of RAB11a and RAB27b as binding partners for exocyst complex and their co-association with intracellular UPEC.
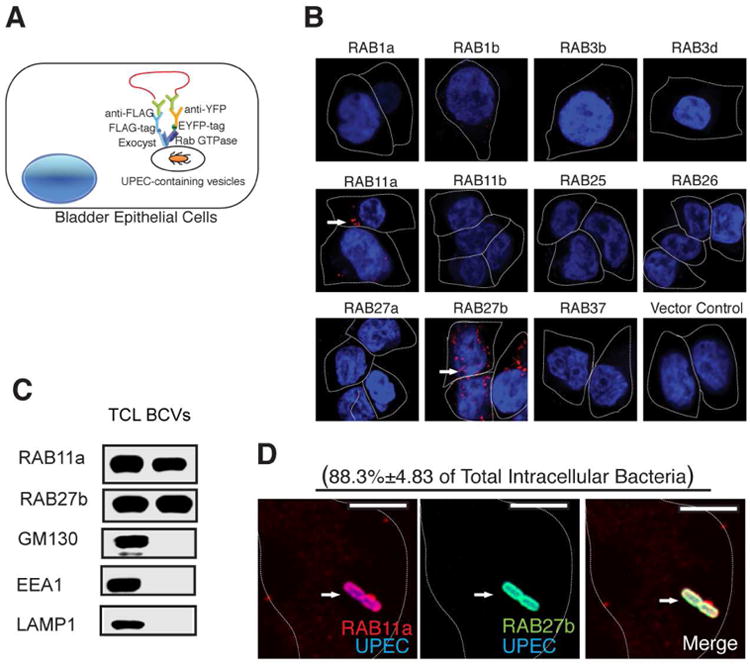
(A) Diagram showing PLA based assay for the identification of Rab proteins in BECs that bind Exocyst complex upon UPEC infection. 5637 BECs were transfected with lentivirus carrying various Rab-EYFP, as well as FLAG tagged exocyst complex components. The cells were then infected with UPEC, fixed and incubated with primary antibodies against EYFP or FLAG followed by Duolink in situ PLA probe-linked secondary antibodies. After ligation, the signals were amplified and detected by confocal microscopy.
(B) Proximity Ligation Assay detecting RAB11a and RAB27b (signals are indicated by an arrow) as binding partners for the Exocyst complex upon UPEC infection.
(C) Detection of RAB11a and RAB27b in total cell lysates (TCL) or bacteria-containing vesicle (BCV) fractions isolated from BECs infected with UPEC by western blot. Unrelated organelle markers (GM130 for Golgi, EEA1 for early endosome, LAMP1 for late endosome and lysosome) were used to confirm the purity of the isolated BCV fraction.
(D) Immunofluorescence staining of infected BECs revealing the co-association of RAB27b (green) and RAB11a (red) with UPEC (blue). The percentage of intracellular bacteria that stain positive for RAB11a and RAB27b (double positive) over total intracellular UPEC counted (approximately 50 bacteria on each slide) is indicated in parenthesis. n=3 slides Scale bar: 5 μm. See also Figure SI
RAB11a and RAB27b are implicated in expulsion of infecting UPEC
That RAB27b was associated with the BCV is not surprising as it has previously been shown to be hijacked for the entry of UPEC into BECs (Bishop et al., 2007). However, it was surprising that, two distinct Rab small GTPases, including the same Rab proteins exploited by pathogen, simultaneously exhibit specific binding to exocyst complex, the critical machinery initiating the bacterial expulsion in UPEC infected BECs. Therefore, we examined if both RAB11a and RAB27b were equally critical for bacterial expulsion. Since RAB27b is also important for bacterial entry (Bishop et al., 2007), we adjusted infecting MOI to ensure the initial bacterial load is comparable between control and RAB27b siRNA transfected BECs. Indeed, when we knocked down either RAB11a or RAB27b employing siRNA, bacterial expulsion in the infected BECs was significantly reduced compared to control siRNA transfected BECs (Figure 2A). Temporal analysis revealed that this reduction was mainly manifested within the first 4 to 6 hours post infection (h.p.i), which corresponded to when the early wave of expulsion mediated by TLR4 and the exocyst complex occurred (Miao et al., 2016). No difference in bacterial expulsion activity was observed at 8 h.p.i, when a completely different expulsion process mediated by TRPML3 becomes activated (Miao et al., 2015) (data not shown).
Figure 2. RAB11a and RAB27b regulate intracellular bacterial load by promoting bacterial expulsion.
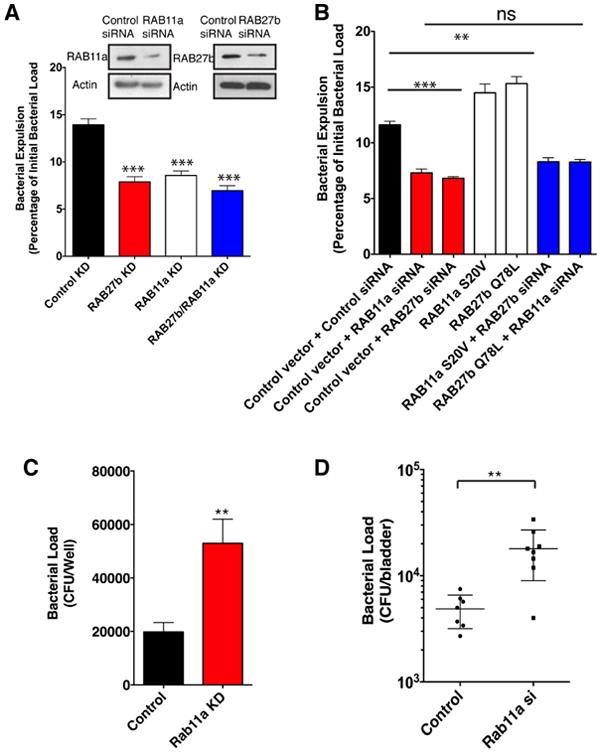
(A) Bacterial expulsion from infected BECs transfected with control siRNA or siRNA targeting either RAB11a or RAB27b or a combination of siRNA targeting both RAB11a and RAB27b. Knockdown efficiency is indicated in the adjoining western blots. Error bars represent SEM. The experiments were repeated three times, where each experiment employed n=6 wells.
(B) Bacterial expulsion from infected BECs transfected with 1) empty vector plus control siRNA; 2) empty vector plus siRNA targeting RAB11a or RAB27b; 3) dominant active RAB11a (S20V) or RAB27b (Q78L) vector plus control siRNA; 4) dominant active RAB11a vector plus siRNA targeting RAB27b; 5) dominant active RAB27b vector plus siRNA targeting RAB11a. Error bars represent SEM. The experiments were repeated three times, where each experiment employed n=6 wells.
(C) Intracellular bacterial load measured in vitro cultured BEC line receiving control siRNA or siRNA targeting RAB11a at 2 h.p.i. Error bars represent SEM. The experiments were repeated three times, where each experiment employed n=6 wells.
(D) Bacterial burden at 6 h.p.i in UPEC infected bladder tissue in mice intravesicularly treated with modified siRNA targeting a scrambled control or Rab11a 72 hours before infection. Error bars represent SEM. The experiments were repeated three times, where each experiment employed n=3 mice, and the data were pooled.
To further demonstrate the relevance of RAB11a and RAB27b in bacterial expulsion, we undertook gain-of -function assays. For this, we over-expressed a constitutively GTP-bound form of RAB11a or RAB27b to enhance their function in BECs. In both cases, we observed that compared to controls, bacterial expulsion was dramatically augmented in BECs over-expressing a dominant active form of either RAB11a or RAB27b (Figure 2B). Thus, both RAB11a and RAB27b appear to functionally contribute to bacterial expulsion.
The next important question to address was whether RAB11a and RAB27b function separately or are components of the same expulsion pathway. To answer this question, we compared reduction in expulsion activity in cultured BECs silenced in either RAB11a or RAB27b or simultaneously silenced in both RAB11a and RAB27b. Although BECs with combined siRNA treatment were more efficient at blocking expulsion than BECs with a single siRNA treatment, there did not appear to be clear additive effects in the double knock down cells (Figure 2A). To further substantiate that RAB11a and RAB27b function cooperatively during expulsion, we silenced RAB11a in BECs that over-express the dominant active mutant of RAB27b and examined bacterial exocytosis. Conversely, we knocked down RAB27b in BECs over-expressing dominant active RAB11a and examined bacterial expulsion. In both cases, the augmentation of the bacterial expulsion activity from overexpression of the dominant active mutant of RAB11a or RAB27b was completely negated by knocking down the other Rab protein (Figure 2B). These results support the idea that RAB11a and RAB27b function in the same expulsion pathway and both are required for effective bacterial exocytosis. It is possible that the two Rab proteins are involved in different steps of the same expulsion pathway, rather than function simultaneously together. However, our microscopy data seemed to exclude this possibility, as intracellular bacteria always appeared to be labeled with both RAB27b and RAB11a at all time points post-infection that we examined (Figure 1D). At no time did we visualize one Rab protein around intracellular bacteria and not the other. Based on these data, we conclude that RAB11a and RAB27b collaborate and do so simultaneously to achieve bacterial expulsion.
We next sought to investigate the physiological contribution of Rab protein mediated bacterial exocytosis in BECs in vitro and then during urinary tract infections (UTI) in a mouse model. When we blocked this by silencing RAB11a in the cultured BEC line, the intracellular bacterial load increased dramatically in these cells (Figure 2C). We next investigated how abrogation of bacterial expulsion from BECs impacted UTIs in mice. Since Rab11a knockout mice are nonviable, we opted to silence Rab11a in the superficial bladder epithelium using a recently described in vivo siRNA transfection technique (Miao et al., 2016). As we expected, in vivo silencing of Rab11a in the bladder epithelium resulted in significant defect in bacterial clearance, resulting in a large increase in bacterial load in UPEC infected bladder tissue (Figure 2D). It is noteworthy that the increase in bacterial burden observed in RAB11a KD BECs was similar in size to that observed with knocking down of other bacterial expulsion components in BECs, such as the individual exocyst complex subunits or specific proteins involved in activating exocyst complex (Miao et al., 2016). All the data provided so far support the conclusion that both RAB11a and RAB27b are required for efficient bacterial clearance from infected BECs via a TLR4 initiated, and exocyst complex-mediated expulsion.
Simultaneous involvement of the RAB11a/FIP3/Dynein and RAB27b/MyRIP/Myosin VIIa axes in bacterial expulsion
Having implicated RAB11a and RAB27b in bacterial exocytosis we next sought to identify the trafficking circuitry that each of these Rab proteins direct. Rab small GTPases organize subcellular trafficking events by orchestrating a network of effector proteins, which then mobilize a variety of motor proteins to transport their target cargos which are encapsulated in membrane-bound vesicles (Stenmark, 2009). Based on this scenario, we carried out an RNAi based screen, silencing each of the effectors or motor proteins known to be associated with RAB11a or RAB27b in human bladder epithelial cell line, with the purpose of identifying the downstream modulators recruited by these two Rab proteins to mediate bacterial expulsion. Interestingly, among five different RAB11a effectors examined (Guichard et al., 2014), only knocking down RAB11FIP3 effectively blocked bacterial expulsion, resulting in a similar phenotype as the RAB11a knockdown (Figure 3A and Supplementary Figure 2A). In addition, RAB11FIP3 could be induced to interact with RAB11a in BECs upon exposure to UPEC (Figure 3B, Supplementary Figure 2B), and this interaction depended on the GTP-binding status of RAB11a (Figure 3B, Supplementary Figure 2B). Furthermore, RAB11FIP3 was found to specifically deposit on the BCVs isolated from WT UPEC infected BECs (Figure 3C) and this localization depended on the prior recruitment and activation of RAB11a (Figure 3C). Importantly, using a mutant E.coli ΔmsbB strain which has a mutation in the msbB gene and so lacks the myristic acid moiety of lipidA and is poorly recognized by TLR4 (Somerville et al., 1996), we demonstrated that the recruitment of both RAB11a and RAB11FIP3 onto the BCVs depends on the activation of TLR4 signaling (Figure 3D). Confocal microscopy also confirmed the co-localization of RAB11a and RAB11FIP3 on BCVs (Figure 3E). Since RAB11FIP3 mediated trafficking of subcellular vesicles requires the involvement of cellular microtubules (Welz et al., 2014), we reasoned that the RAB11FIP3-RAB11a complex might be recruiting the motor protein Dynein, which mediates BCV trafficking along microtubules (Horgan et al., 2010). When we knocked down the light chain of Dynein to disrupt its function, or treated UPEC infected BECs with a Dynein-specific inhibitor (Firestone et al., 2012), we observed significant reduction in bacterial expulsion, which was comparable in magnitude to that observed in RAB11a or RAB11FIP3 knockdown BECs (Figure 3F, G). We were also able to localize Dynein to BCVs in infected BECs, and we observed silencing RAB11a in BECs significantly blocked its recruitment into BECs (Figure 3H). Immunofluorescent staining of infected BECs also revealed that Dynein colocalized with RAB11a on BCVs (Figure 3I). These observations suggest that RAB11FIP3 and Dynein are the downstream effector molecules recruited by RAB11a to mediate trafficking of BCVs.
Figure 3. The RAB11a effector RAB11FIP3 and Dynein are involved in bacterial expulsion.
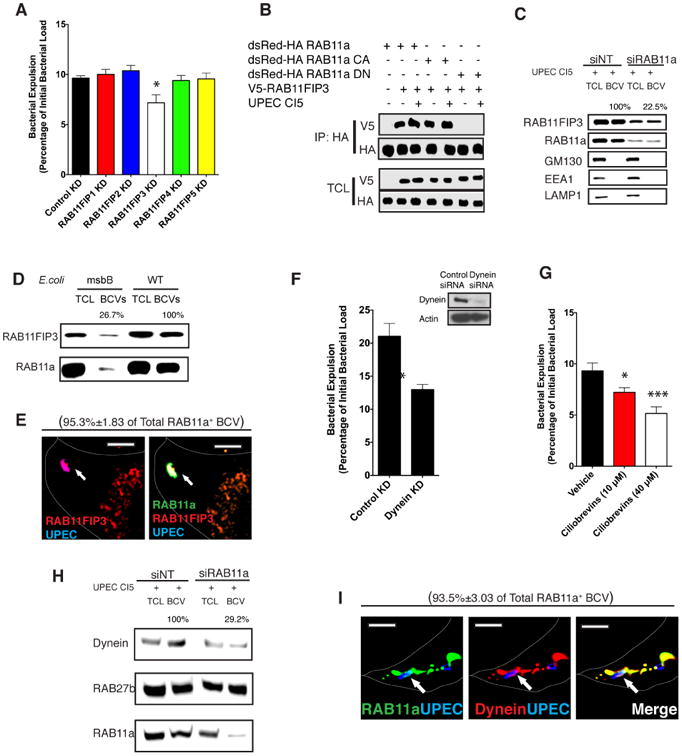
(A) Bacterial expulsion from infected BECs transfected with control siRNA or siRNA targeting RAB11FIP1, RAB11FIP2, RAB11FIP3, RAB11FIP4 orRAB11FIP5 respectively. Error bars represent SEM. The experiments were repeated three times, where each experiment employed n=6 wells.
(B) HA tagged WT RAB11a and constitutively active (CA) or dominant negative (DN) mutants of RAB11a were immunoprecipitated from naïve or infected BECs. The association between RAB11a and RAB11FIP3 in different conditions was examined by western blotting of the IP fractions. The protein of interests in the total cell lysates (TCL) was also depicted to indicate that similar levels of RAB11 or RAB11FIP3 protein was present in each fraction.
(C) Immunoblots of RAB11FIP3 in total cell lysates (TCL) or bacteria-containing vesicle (BCV) fractions isolated from infected BECs which were pre-transfected with control siRNA or siRNA targeting RAB11a. Unrelated organelle markers (GM130 for Golgi, EEA1 for early endosome, LAMP1 for late endosome and lysosome) were employed to demonstrate the purity of BCV isolation.
(D) Protein immunoblots of RAB11FIP3 and RAB11a in total cell lysate (TCL) or bacteria-containing vesicle (BCV) fractions isolated from BECs infected with WT or msbB mutant E.coli which cannot activate TLR4 signaling. Unrelated organelle markers (GM130 for Golgi, EEA1 for early endosome, LAMP1 for late endosome and lysosome) were used to demonstrate the purity of BCV isolation.
(E) Immunofluorescence staining of infected BECs revealing the co-association of RAB11a (green) and RAB11FIP3 (red) with UPEC (blue). Scale bar: 5 μm.
(F) Bacterial expulsion from infected BECs transfected with control siRNA or siRNA targeting the light chain of Dynein. Error bars represent SEM. The experiments were repeated three times, where each experiment employed n=6 wells.
(G) Bacterial expulsion from infected BECs treated with vehicle control or a specific inhibitor of Dynein. Error bars represent SEM. The experiments were repeated three times, where each experiment employed n=6 wells.
(H) Immunoblots of Dynein in total cell lysates (TCL) or bacteria-containing vesicle (BCV) fractions isolated from infected BECs which are pre-transfected with control siRNA or siRNA targeting RAB11a.
(I) Immunofluorescence staining of infected BECs revealing the co-association of RAB11a (green) and Dynein (red) with UPEC (blue). Scale bar: 5 μm. See also Figure S2
To complement these studies, we sought to identify the downstream effectors mobilized by RAB27b to expel the BCVs. Compared to other effectors tested, specifically silencing the RAB27b effector MyRIP result in significant blockade of bacterial expulsion activity in BECs (Figure 4A). Although MyRIP has previously been implicated in bacterial expulsion (Song et al., 2009), the downstream motor proteins remain unknown. Two motor proteins, Myosin Va and VIIa have been reported to be mobilized by RAB27b (Desnos et al., 2003) but only silencing Myosin VIIa was found to block expulsion activity in BECs (Figure 4B). Additionally, Myosin VIIa was found to colocalize with RAB27b on BCVs (Figure 4C). For further evidence that these two distinct Rab small GTPase-mediated trafficking pathways were functioning collaboratively to achieve expulsion, we individually silenced Myosin VIIa and Dynein or silenced both in BECs and observed no additive effect to the expulsion activity in the double knock down cells (Figure 4D). Furthermore, we observed that Myosin VIIa and Dynein appeared to be co-associated on the same intracellular UPEC at all time points examined, confirming that both motor proteins work together rather than at different stages of bacterial expulsion. (Figure 4E).
Figure 4. RAB27b effectors MyRIP and MyosinVIIa are critical for bacterial expulsion.
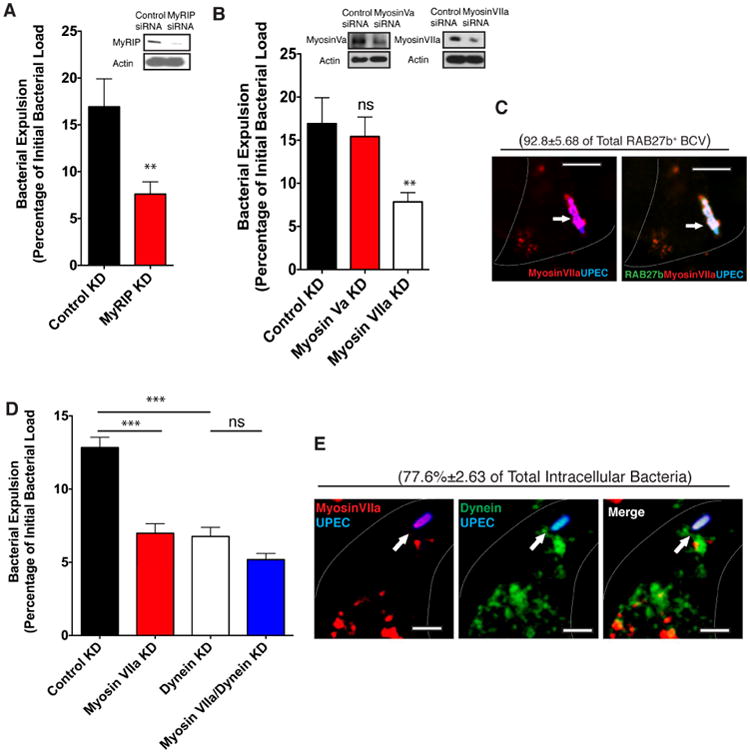
(A) Bacterial expulsion from infected BECs transfected with control siRNA or siRNA targeting MyRIP. Knockdown efficiency is indicated by western blots. Error bars represent SEM. The experiments were repeated three times, where each experiment employed n=6 wells.
(B) Bacterial expulsion from infected BECs transfected with control siRNA or siRNA targeting Myosin Va or MyosinVila. Knockdown efficiency is indicated by western blots. Error bars represent SEM. The experiments were repeated three times, where each experiment employed n=6 wells.
(C) Immunofluorescence staining of infected BECs revealing the co-association of RAB27b (green) and MyosinVIIa (red) with UPEC (blue). The percentage of intracellular bacteria that stain positive for MyosinVIIa over total intracellular UPEC stained positive for RAB27b is indicated in parenthesis. n=3 slides Scalebar: 5 μm.
(D) Bacterial expulsion from infected BECs transfected with control siRNA or siRNA targeting MyosinVIIa or Dynein respectively, or combined siRNA targeting both MyosinVIIa and Dynein. Error bars represent SEM. The experiments were repeated three times, where each experiment employed n=6wells.
(E) Immunofluorescence staining of infected BECs revealing the co-association of MyosinVIIa (red) and Dynein (green) with UPEC (blue). The percentage of intracellular bacteria that stain positive for MyosinVIIa and Dynein (double positive) over total intracellular UPEC counted is indicated in parenthesis. n=3 slides Scale bar: 5 μm.
In sum, the RAB11a/RAB11FIP3/Dynein and the RAB27b/MyRIP/Myosin VIIa trafficking axes are simultaneously activated on BCVs to mediate bacterial expulsion.
Exocyst complex components promote collaboration between RAB11a and RAB27b-mediated trafficking circuits
Although most subcellular trafficking processes involve multiple Rab proteins, they typically work sequentially, with each Rab protein regulating only a single step. Thus, it is uncommon for a single vesicular trafficking step to simultaneously require two distinct sets of trafficking machinery working cooperatively. Here, we investigated how this collaborative effort was coordinated. Since earlier we had observed the exocyst complex proteins depositing on BCVs and simultaneously binding RAB11a and RAB27b in infected BECs (Figure 1), we hypothesized that the exocyst complex was the signaling hub integrating the activity of the two distinct trafficking circuits. To test this hypothesis, we sought to identify which of the exocyst complex proteins bound RAB11a and RAB27b, and whether any of their respective effectors were also bound by exocyst complex proteins in UPEC-infected BECs. Interestingly, we found that RAB11a bound both SEC6 and SEC15 of the exocyst complex (Figure 5A, B, Supplementary Figure 3A, C, D, E, F), whereas RAB27b bound only SEC15 (Figure 5C, E), and both interactions between RAB11a or RAB27b with exocyst complex were markedly enhanced upon infection (Figure 5A, B) and were highly dependent on the GTP binding status of the Rab protein (Supplementary Figure 3B). These interactions have been confirmed by both expressing tagged proteins (Figure 5 A, C) or pulling down endogenous proteins (Figure 5B,D). While the RAB11a effector RAB11FIP3 doesn't interact with any of the exocyst proteins (data not shown), the RAB27b effector MyRIP was found to specifically bind SEC6 (Figure 5D, F). Having identified which exocyst proteins bind Rab proteins or their effectors we next sought to establish the functional relevance of each of these interactions. We found that silencing SEC15 dramatically diminished the binding of SEC6 to RAB11a whereas silencing SEC6 did not appear to diminish the binding of RAB11a to SEC15 (Supplementary Figure 4), suggesting that the interaction between RAB11a and SEC15 was more relevant in localizing RAB11a to the BCV. Importantly, when we silenced SEC15, the recruitment of RAB11a to the BCV is significantly reduced (Figure 5G). While SEC15 is more important to localize RAB11a to the BCVs, the RAB27b –associated trafficking circuitry seems to depend more on SEC6. Whereas the RAB27b itself is not affected by the absence of SEC6, the effectors of RAB27b, such as MyRIP and Myosin VIIa required SEC6 to be deposited on BCVs (Figure 5 H). Since the exocyst complex recruits both Rab proteins to the BCVs, we investigated how this complex impacted the activity of each of the Rab pathways. We assessed the GTP binding abilities of RAB11a and RAB27b as an indicator of their activation status. For these studies, we employed pull down assays using the RAB11 binding domain cloned from its effector RAB11FIP3 or the RAB27 binding domain amplified from its effector Slac2 to isolate GTP-bound RAB11a or RAB27b (Fukuda and Kuroda, 2002). We found that the activation of RAB11a or RAB27b following UPEC infection was significantly blunted by silencing exocyst complex component SEC15 (Figure 5I). Consistent with these results, we observed that any defects in exocyst complex resulted in deficient binding between RAB11a or RAB27b and their respective effector proteins (Figure 5J, K). Cumulatively, these observations suggest that different exocyst complex components contribute to localizing components of the two distinct trafficking circuitry to the BCVs, and in activating the GTP binding capacity of RAB11a and RAB27b, thereby facilitating their collaboration in the trafficking of the BCV.
Figure 5. Exocyst complex form a molecular bridge linking RAB11a and RAB27b-associated trafficking circuits.
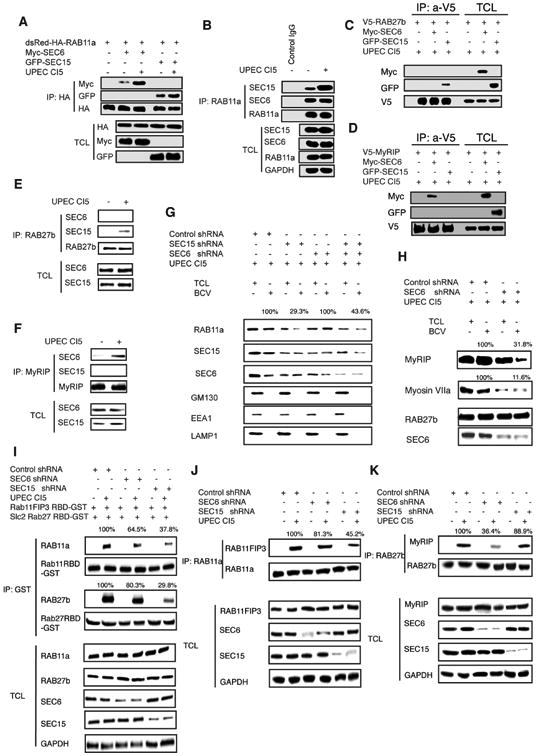
(A) HA tagged WT RAB11a immunoprecipitated from uninfected or infected BECs. The association between RAB11a and Exocyst complex protein SEC6 or SEC15 in different conditions was examined by western blots of the IP fractions. The proteins of interests in the total cell lysate (TCL) was depicted to indicate that similar levels of RAB11a, SEC6 or SEC15 protein were present in each fraction.
(B) Endogenous RAB11a immunoprecipitated from uninfected or infected BECs. The association between RAB11a and endogenous Exocyst complex protein SEC6 or SEC15 in different conditions was examined by western blotting of the IP fractions. The proteins of interests in the total cell lysates (TCL) was also depicted to indicate that similar levels of RAB11a, SEC6 or SEC15 protein was present in each fraction.
(C) V5 tagged WT RAB27b immunoprecipitated from infected BECs. The association between RAB27b and Exocyst complex protein SEC6 or SEC15 was examined by western blotting of these IP fractions. The protein of interests in the total cell lysates (TCL) was depicted to demonstrate that similar levels of RAB27b protein was present in each fraction.
(D) V5 tagged MyRIP immunoprecipitated from infected BECs. The association between MyRIP and Exocyst complex protein SEC6 or SEC15 was examined by western blotting of the IP fractions. The proteins of interest in the total cell lysates (TCL) was depicted to indicate that similar levels of RAB27b protein was present in each fraction.
(E) Endogenous RAB27b immunoprecipitated from uninfected or infected BECs. The association between RAB27b and endogenous Exocyst complex proteins SEC6 or SEC15 in uninfected or infected BECs were examined by western blotting of the IP fractions. The proteins of interest in the total cell lysates (TCL) were also depicted to indicate that similar levels of RAB27b, SEC6 or SEC15 protein were present in each fraction.
(F) Endogenous MyRIP immunoprecipitated from uninfected or infected BECs. The associations between MyRIP and endogenous Exocyst complex protein SEC6 or SEC15 in naïve or infected BECs were examined by western blotting of the IP fractions. The proteins of interest in the total cell lysates (TCL) were also depicted to indicate that similar levels of MyRIP, SEC6 or SEC15 protein were present in each fraction.
(G) Protein immunoblot analysis of RAB11a in total cell lysates (TCL) or bacteria-containing vesicle (BCV) fractions isolated from infected BECs which are pre-transfected with control shRNA or shRNA targeting Exocyst complex protein SEC6 or SEC15.
(H) Protein immunoblot analysis of MyRIP or MyosinVIIa in total cell lysates (TCL) or bacteria-containing vesicle (BCV) fractions isolated from infected BECs which are pre-transfected with control shRNA or shRNA targeting Exocyst complex protein SEC6.
(I) Rab11-binding domain (R11BD) from the RAB11a effector RAB11FIP3 and the Rab27b-binding domain (R27BD) from the RAB27b effector SLAC2 were cloned and fused to GST. The purified R11BD-GST or R27BD-GST proteins were immobilized and used to pulled down GTP-bound active RAB11a or RAB27b from uninfected or infected BECs transfected with control shRNA or shRNA targeting SEC6 or SEC15. The proteins of interest in the total cell lysates (TCL) were depicted to demonstrate that similar levels of RAB11a or RAB27b protein were present in each fraction.
(J) Immunoprecipitation of RAB11a from uninfected or infected BECs transfected with control shRNA or shRNA targeting SEC6 or SEC15. The association between RAB11a with its effector RAB11FIP3 in control or KD cells were examined by western blotting of the IP fractions. The proteins of interests in the total cell lysates (TCL) were depicted to demonstrate that similar levels of RAB11FIP3 protein was present in each fraction.
(K) Immunoprecipitation of RAB27b from uninfected or infected BECs transfected with control shRNA or shRNA targeting SEC6 or SEC15. The association between RAB27b with its effector MyRIP in control or KD cells were examined by western blotting of the IP fractions. The proteins of interests in the total cell lysates (TCL) were depicted to demonstrate that similar levels of MyRIP protein were present in each fraction. See Figure S3 and S4
The nature of the codependency of RAB11a and RAB27b-mediated trafficking pathways
So far, we have revealed a unique mode of cell autonomous defense, which simultaneously requires two sets of subcellular trafficking machinery orchestrated by two distinct Rab small GTPases. We sought to gain further insights into the nature of the cooperation between these two Rab protein mediated pathways. We found that, although these two Rab protein-orchestrated trafficking pathways depend on different exocyst complex subunits to be localized on BCVs, their activity also appear to depend on each other. Supporting this notion, when we silenced RAB11a, the RAB27b failed to be effectively activated and RAB27b-related exporting machinery failed to be assembled on the BCVs. For example, we found that the GTP binding to RAB27b induced by UPEC infection was markedly reduced by RAB11a knockdown (Figure 6A). Additionally, the binding of RAB27b to its effectors was diminished (Figure 6B) in these RAB11a deficient BECs. Further, although RAB27b was still present on BCVs, its effector proteins MyRIP and Myosin VIIa failed to efficiently deposit on these structures (Figure 6C). Conversely, when the RAB27b was silenced, significant defects in the RAB11a associated pathway were observed. These included defects in GTPase activation of RAB11a (Figure 6D), reduced binding to the effector RAB11FIP3 (Figure 6E), as well as reduced recruitment of RAB11FIP3 to the BCVs (Figure 6F). These remarkable results indicate that these two Rab proteins not only regulate the activity of their own effectors, but also promote the assembly of the complex formed with the other Rab small GTPase. We sought to further explore the underlying basis of how this cooperative activity occurred. Since the two critical Rab proteins appear to be bridged and activated by the exocyst complex (Figure 5), we hypothesized that both RAB11a and RAB27b may be promoting the other's activity by enhancing their corresponding interaction with the exocyst complex. To validate this notion, we examined the interactions between exocyst complex components with each of the two Rab proteins when the other Rab was silenced. As expected, each of the two Rab proteins failed to complex with the exocyst complex if the other Rab is missing (Figure 6 G, H). Therefore, only when both sets of Rab trafficking machinery are assembled on the BCV, by the bridging activities of the exocyst complex, are BCVs effectively exported from the cytosol to the plasma membrane for expulsion.
Figure 6. Co-dependency of the RAB11a and RAB27b-associated trafficking circuits for efficient bacterial expulsion.
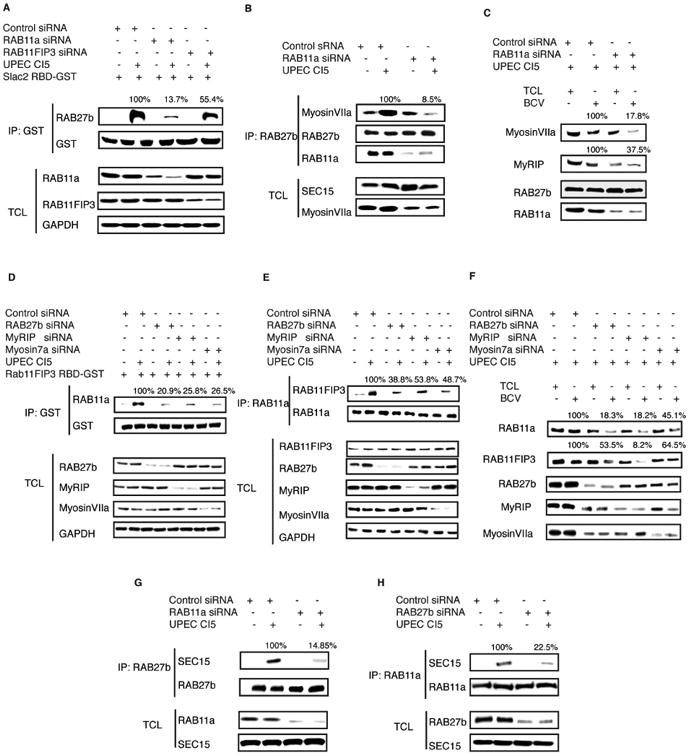
(A) Rab27b-binding domain (R27BD) from the RAB27b effector SLAC2 was cloned and fused to GST. The purified R27BD-GST protein was immobilized and used to pulled down GTP-bound active RAB27b from uninfected or infected BECs transfected with control siRNA or siRNA targeting RAB11a. The proteins of interest in the total cell lysates (TCL) were depicted to demonstrate that similar levels of RAB27b protein were present in each fraction.\
(B) RAB27b immunoprecipitated from uninfected or infected BECs transfected with control siRNA or siRNA targeting RAB11a. The association between RAB27b with its effector MyosinVIIa in RAB11a-expressing or deficient cells was examined by western blotting of the IP fractions. The proteins of interest in the total cell lysates (TCL) were depicted to demonstrate that similar levels of MyosinVIIa protein were present in each fraction.
(C) Imunoblots of MyRIP or MyosinVIIa in total cell lysates (TCL) or bacteria-containing vesicle (BCV) fractions isolated from infected BECs which are pre-transfected with control siRNA or siRNA targeting RAB11a.
(D) Rab11-binding domain (R11BD) from the RAB11a effector RAB11FIP3 was cloned and fused to GST. The purified R11BD-GST protein was used to pulled down GTP-bound active Rab11a from uninfected or infected BECs transfected with control siRNA or siRNA targeting RAB27b or RAB27b effector MyRIP or MyosinVIIa. The proteins of interest in the total cell lysates (TCL) were depicted to demonstrate that similar levels of RAB11a protein were present in each fraction.
(E) Immunoprecipitation of RAB11a from uninfected or infected BECs transfected with control siRNA or siRNA targeting RAB27b or its effector MyRIP or MyosinVIIa. The association between RAB11a with its effector RAB11FIP3 in control or KD cells were examined by western blotting of the IP fractions. The proteins of interests in the total cell lysates (TCL) were depicted to demonstrate that similar levels of RAB11FIP3 protein were present in each fraction.
(F) Immunoblots of RAB11FIP3 in total cell lysates (TCL) or bacteria-containing vesicle (BCV) fractions isolated from infected BECs which are pre-transfected with control siRNA or siRNA targeting RAB27b or its effector MyRIP or MyosinVIIa.
(G) RAB27b immunoprecipitated from naïve or infected BECs transfected with control siRNA or siRNA targeting RAB11a. The association between RAB27b with Exocyst complex protein SEC15 in RAB11a expressing or deficient cells were examined by western blotting of the IP fractions. The proteins of interest in the total cell lysates (TCL) were depicted to demonstrate that similar levels of SEC15 protein were present in each fraction.
(H) RAB11a was immunoprecipitated from uninfected or infected BECs transfected with control siRNA or siRNA targeting RAB27b. The association of RAB11a with Exocyst complex protein SEC15 in control or KD cells was examined by western blotting of the IP fractions. The proteins of interest in the total cell lysates (TCL) were depicted to demonstrate that similar levels of SEC15 protein were present in each fraction.
Discussion
Here, we describe two Rab small GTPases that are simultaneously mobilized by the innate immune signaling circuitry of the host cells to clear the infections. We have found that unlike most membrane trafficking processes where each step in the pathway is effectively mediated by a single Rab protein, this mode of bacterial clearance requires the collaboration of two distinct Rab trafficking circuits working in unison to achieve the same trafficking step. It appears that the simultaneous presence of both RAB11a and RAB27b is required before the downstream effectors for each Rab protein deposit and assemble on the BCV. Absence of any one of the Rab proteins results in the failure of the other Rab protein to be activated and interact with its own complementary effector proteins. This collaborative action seems to be mediated by the exocyst complex, which not only promotes the recruitment of RAB11a and RAB27b, but also brings about their activation. Presumably the exocyst complex, recruits the guanine nucleotide exchange factors (GEFs) for both RAB11a and RAB27b, while activating and stabilizing the interactions between each of these two Rab proteins with its respective effector proteins. A similar scenario has been reported before where during lumen formation, Rab11a was found to regulate Rab8 activity through recruiting Rabin8, the GEF protein for Rab8a (Bryant et al., 2010). Since Rabin8 is also able to interact with Sec15 (Feng et al., 2012), it is unknown whether Rab11a activates Rab8a through recruiting Sec15 which subsequently brings Rabin8, or if Rab8a activates Rab11a in a similar manner as shown here in the Rab11a/Rab27b/Exocyst complex. Further studies are also required to reveal the identity of the GEF proteins for RAB11a and RAB27b which could shed light on how these Rab pathways collaborate to achieve bacterial expulsion. The involvement of multiple Rabs in the transport of large intracellular membrane bound particles have previously been noted. For example, two distinct Rab proteins have been implicated in the peripheral transport of melanosomes, which are relatively large particulates (∼500 nm in diameter) found in melanocyte (Raposo and Marks, 2007). The transport of melanosome toward plasma membrane was found to require two Rab small GTPases, Rab27a (Hume et al., 2007) and Rab1a (Ishida et al., 2015). Whereas Rab27a recruited Myosin Va through its effector Mlph (Hume et al., 2007), Rab1a recruited the motor protein Kinesin through SKIP (Ishida et al., 2015). It is not clear in the case of melanosome transport, whether any collaborative interaction between Rab27a and Rab1a was needed. Nevertheless, it would appear that mobilization of multiple Rab-orchestrated motor proteins is a common cellular strategy to export relatively large particulate cargo such as bacteria and melanosomes. Future structural studies of these composite “transport complexes” formed by multiple Rab pathways should provide valuable insights on if and how collaboration occurs. Another future avenue of investigation is elucidating how the transport of the BCVs is achieved when two distinct types of motor proteins are required. It is well established that Dynein traffics their cargo along microtubules (Bhabha et al., 2016), whereas Myosin utilizes the actin network (Udovichenko et al., 2002). Clearly, for bacterial expulsion to occur both microtubule and actin-based subcellular trafficking needs to be coordinated. It is noteworthy that such intimate cooperation between microtubule and F-actin-mediated subcellular transport has been described during the intracellular transport of melanosome. Indeed, components of the microtubule and actin-mediated trafficking machinery such as myosin Va and Dynein were implicated in the trafficking of melanosomes to the periphery of the cell (Byers et al., 2000; Rogers et al., 1999). Interestingly, interfering with the myosin-based movement also slows down the microtubule motor-based transport and vise versa (Bridgman, 1999; Wu et al., 1998). Apparently, the microtubule-based and actin-linked motor proteins function coordinately in a “tug-of-war” fashion to promote the movement of the large melanosome (Gross et al., 2002). Whether similar mechanisms also drive the transport of bacteria-containing vesicle remains to be determined.
Another key finding in our study is that the exocyst complex functions as a platform for the assembly of the “export complex” which consists of RAB11a and RAB27b, as well as their respective effectors. A previous study has shown that the exocyst complex senses and responds to activation signals from of innate immune sensing molecule TLR4 and initiates the bacterial expulsion program (Miao et al., 2016). However, the underlying mechanism of how this occurs was not clear. Here, we reveal that exocyst complex orchestrates the expulsion process by directly recruiting RAB11a and RAB27b onto the surface of BCVs, as well as promoting their activation for engaging their respective motor proteins to mediate BCV transport. The current understanding of the contribution of the exocyst complex to regulated exocytosis is that when exocyst subunits on the transported vesicle dock with other subunits located on the plasma membrane, they provide spatial targets for the vesicles as well as a tether to the plasma membrane (Heider and Munson, 2012). Here, we report another function for the exocyst complex. Namely, mediating the orchestration of multiple exocytic Rab proteins. Since the exocyst complex is directly responding to activation signals emanating from TLR4, our studies reveal that the exocyst complex serves as a signaling hub, integrating upstream activation signals with appropriate downstream trafficking machineries. Based on this finding, we speculate that the exocyst complex plays a similar role in other regulated exocytic processes. If this is the case, it would be interesting to determine exactly how the exocyst complex discerns different signals and selects an appropriate Rab protein to bind.
Many pathogens exploit the compartmental and membranous nature of eukaryotic cells to invade and establish intracellular niches in these membranes. Therefore, it is not surprising that the subcellular membrane is also the site where the immune surveillance system of the host cell initiates its response. As the major modulator of subcellular membrane, Rab small GTPases are the natural targets of innate immune signaling following microbial invasion, yet relatively little is known of how Rab proteins contribute to the host defense. Here, we have uncovered two especially interesting Rab proteins specifically mobilized by TLR4 to transport and expel vesicle-encased UPEC. In the bladder epithelium, RAB27b is coopted by the UPEC to invade the BECs and becomes co-associated with UPEC harboring vesicles (Bishop et al., 2007). Therefore, RAB27b appears to be a prime target for innate immune signaling to expel bacteria. RAB11a is an especially intriguing mediator of innate signaling. Previous studies have reported that RAB11a is critical for evoking a robust type I interferon response, following compartmental TRIF/TRAM and TLR4 signaling (Husebye et al., 2010). Similar roles have also been reported for the exocyst complex (Chien et al., 2006). Although there is strong evidence that bacterial expulsion and interferon secretion are distinct cellular responses (Miao et al., 2016), the involvement of both RAB11a and the Exocyst complex in both of these cellular responses reveal an intimate connection between these distinct autonomous defense mechanisms. Conceivably, bacterial expulsion is an initial clearance strategy evoked upon detection of intracellular bacteria. If the expulsion is incomplete, the same machinery (i.e. Exocyst and RAB11a) re-organize their functions and shift to mediate interferon regulated cellular defense. In any case, our study should provide early insights for future studies directed at exploring the cell autonomous defense network, especially to investigate whether Rab small GTPases indeed function as the critical node connecting the various intracellular host defense mechanisms.
Experimental Model and Subject Details
Mice
Eight- to ten-week old female C57BL/6 mice were obtained from the Jackson Laboratory. Upon arrival, the mice were maintained in specific pathogen-free facilities at Duke University with cages changed every week. All the mice were monitored and under care by veterinarians and were immunocompetent, healthy and with no drugs given prior to experiments. The mice were randomly assigned to experimental groups by the investigators in a non-blinded fashion. At the end of each experiments, mice were humanely euthanized and all mouse experiments were performed in accordance with protocol approved by the Duke University Animal Care and Use Committee.
Bacterial strain and cell line
Uropathogenic E. coli clinical strain CI5 is routinely used for establish infection in in vitro cultured BECs, and a more virulent strain J96 was used for infection in mouse UTI model. Wild type and ΔmsbB mutant E.coli K12 strain have been described before. The bacteria were statically grown overnight in Luria-Bertani broth 24 h prior to infect BECs. The human bladder epithelial cell line 5637 (ATCC HTB-9) was derived from a male bladder cancer patient and was grown in RPMI 1640 with 10% fetal bovine serum (HyClone). Primary human bladder epithelial cells were purchased from CellnTec, and was grown in defined Keratinocyte SFM and incubated at 37°C with 5% C02.
Method Details
PLA based Rab-Exocyst interaction screen
5637 BECs were transfected with lentivirus carry various Rab-EYFP. Rab stable expressing cells were selected based on fluorescence. Then, those cells were transfected by plasmid carrying FLAG tagged exocyst complex component. The cells were then infected with UPEC, fixed and blocked using blocking buffer provided by Sigma. Then, cells were incubated with primary antibodies (mouse anti-FLAG, sigma; goat anti-EGFP, abeam) at 4°C overnight. After washing, secondary antibodies (Duolink in situ PLA probe anti-Goat plus and Duolink In Situ PLA probe anti-mouse minus) were added, followed by the addition of the ligation mixture. After ligation, the signals were amplified with Texas red-labeled oligonucleotide detection probes (Olink Bioscience) and detected by confocal microscopy.
Bacterial expulsion assay
Human 5637 BECs cells were infected with UPEC strain CI5 for one hour, and then the extracellular bacteria were washed and killed with 100μg/mL gentamicin. At this time point, initial bacteria load was determined by adding 0.1% Triton X-100 to release intracellular bacteria. In the meantime, BEC culture medium containing 100 mM methyl α-D-mannopyranoside and 25 μg/mL bacteriostatic agent trimethoprim was added. After two hours, 1/10 of the culture supernatant was plated to quantify the exocytosed bacteria, and this number was normalized against initial intracellular bacterial load at 0 h and expressed as percentage of bacterial expulsion. To be noticed, the bacterial expulsion appears to be initiated as soon as bacterial invasion, therefore, higher initial bacterial load was always observed when components important for expulsion, such as RAB11a, were knocked down, and lower bacterial load was observed in BECs with enhanced expulsion (RAB11a DA overexpression). Therefore, the control KD BECs was usually infected with MOI at 100:1, while RAB11a, Dynein or FIP3 KD BECs were always infected with MOI at 50:1, and the RAB11a DA over expression was infected at MOI of 300:1, in order to adjust the initial bacterial load at 0 h. p. i to the comparable level.
In vitra RNA silencing
RAB27b (Silencer® Pre-Designed siRNA, Thermo Fisher), MyRIP (Silencer® PreDesigned siRNA, Thermo Fisher), MyosinVIIa (SMARTpool, Dharmacon Inc.), Myosin Va (SMARTpool, Dharmacon Inc.), Rab11FIP1 to 5 (FlexiTube siRNA, Qiagen) were knocked down by siRNA targeting four non-overlapping regions, and the siRNAs were transfected into BECs using Lipofectamine 2000 (Invitrogen). To knockdown other components, shRNA targeting RAB11a (UGGUUUGUCAUUCAUUGAAAC), Sec6 (GGCCUCCGUGGAGGCCAGA), Sec15 (CCAAAUGCGCACAAGAAGUU) or DYNC1L1 (GCACUUAUUUACACUUCAGUA) was inserted into pLKO.1 vector and transfected in human BECs
In vivo knockdown and UPEC infection in mice
Accel siRNA with sequences simultaneously targeting four different regions of mouse RAB11a (SMARTpool, Dharmacon Inc.) were transurethrally inoculated into the bladder at 96 hours before infection, and the treatment was repeated 24 hours later. 72 hours after the second treatment, the treated bladder were transurethrally inoculated with UPEC strain J96 using polyethylene catheters. After one hour infection, the gentamicin was inoculated to kill any un-invaded bacteria. After 4 hours, before the recruitment of neutrophils, the mice were sacrificed, the bladder tissue was collected, extensively washed, and homogenized to quantify bacterial burden in the bladder tissue.
Isolation of intracellular BCVs
To isolate BCVs, UPEC cell were conjugated with BioMag carboxyl magnetite nanoparticles (BM570, Bangs Laboratories), and the magnetic bacteria were added to 2×107 BECs at different MOI (WT: 300:1; ΔmsbB mutant: 100:1 to ensure initial bacterial load in different strain infected BECs were comparable). One hour later, the plasma membrane of infected cells were mechanically disrupted by passage of the cell suspension through Gauge30 needles. The BCVs were isolated from the supernatant on a magnetic cell separation rack (BD IMagnet™, BD Biosciences, 552311).
Immunoprecipitation
The plasmids were transfected into 3×106 BECs by X-fect, and 24 hours later, the transfected cells were infected for one hour and lysed. The immunoprecipitations were performed using Co-immunoprecipitation kit (Thermo Scientific), following the instruction from the manufacturer. Briefly, different antibodies were conjugated onto the coupling resin, and mixed with pre-cleared cell lysate. After overnight incubation at 4 degree, the resin was extensively washed with lysis buffer, and the bound proteins were eluted and analyzed by western blot.
Immunofluorescence microscopy
BECs were seeded on 22×22 mm glass coverslips and cultured for 48h. After one hour infection of BECs with different bacterial strains, the cells were fixed in 4% paraformaldehyde and permeabilized in blocking buffer (0.1% saponin, 1% fish gelatin, 10% goat serum in PBS) for 30 min at RT. The cells were then incubated with primary antibodies diluted in blocking buffer (100:1) overnight at 4 °C, followed by staining with conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) for 30 min at RT. Coverslips were mounted with Prolong Gold antifade reagent (Molecular Probes) and examined using a Leica SP5 confocal microscope or a Nikon Eclipse TE200 confocal laser scanning instrument.
Quantification and Statistical Analysis
Image Analysis
Immunofluorescent images or intensity of western gel were analyzed using ImageJ. At least 150 intracellular bacteria from three different slides were manually counted. The association of any markers to UPEC was defined as its location around the bacterium and the positively associated bacteria were calculated as the percentage of total counted intracellular bacteria.
Statistical analysis
Quantitative data were presented as mean ± standard error of mean (SEM) of number (n) of replicates or animals (indicated in each figure) from at least three independent experiments. Statistical analyses were performed using Prism v.6 (GraphPad Software, La Jolla, CA, USA). Unpaired Student's t-tests were used to determine statistical significance in in vitro assays and Mann-Witney-U test was used for in vivo experiments. p<0.05 was considered statistically significant. Post-test p values are as follows: * p<0.05; ** p<0.01; *** p<0.001.
Supplementary Material
Highlights.
RAB11a and RAB27b are simultaneously involved in bacterial expulsion
RAB11a recruits RAB11FIP3/Dynein, while RAB27b mobilizes MyRIP/MyosinVIIa
Exocyst components SEC6/SEC15 recruit and activate RAB11a and RAB27b
RAB11a and RAB27b mutually regulate the activity of each other during expulsion
Acknowledgments
We thank Duke Light Microscopy Core Facility (LMCF), especially Dr. Yasheng Gao, for their expertise and advice in light microscopy imaging. The authors' work is supported by the US National Institutes of Health grants R01 AI96305, R01 AI35678, R01 DK077159, R01 AI50021, R37 DK50814 and R21 AI056101 and a block grant from Duke-NUS Graduate Medical School, Singapore.
Footnotes
Supplemental Information: Supplemental Information includes four figures and can be found with this article online
Author Contributions: Studies were designed by Y.M., P.B., J.W. and S.N.A.. Y. M., P. B., and J. W. carried out experiments. Data was analyzed by Y.M., P.B. and J.W. with advice from S.N.A. Y.W. and Q.L., assisted with the PLA screen assays, and Q.Z. assisted with in vitro expulsion assay. The manuscript was primarily written by Y.M. and S.N.A. All authors contributed to discussions and manuscript review.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham SN, Miao Y. The nature of immune responses to urinary tract infections. Nature reviews Immunology. 2015;15:655–663. doi: 10.1038/nri3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhabha G, Johnson GT, Schroeder CM, Vale RD. How Dynein Moves Along Microtubules. Trends Biochem Sci. 2016;41:94–105. doi: 10.1016/j.tibs.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop BL, Duncan MJ, Song J, Li G, Zaas D, Abraham SN. Cyclic AMP-regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat Med. 2007;13:625–630. doi: 10.1038/nm1572. [DOI] [PubMed] [Google Scholar]
- Bridgman PC. Myosin Va movements in normal and dilute-lethal axons provide support for a dual filament motor complex. J Cell Biol. 1999;146:1045–1060. doi: 10.1083/jcb.146.5.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant DM, Datta A, Rodriguez-Fraticelli AE, Peranen J, Martin-Belmonte F, Mostov KE. A molecular network for de novo generation of the apical surface and lumen. Nature cell biology. 2010;12:1035–1045. doi: 10.1038/ncb2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers HR, Yaar M, Eller MS, Jalbert NL, Gilchrest BA. Role of cytoplasmic dynein in melanosome transport in human melanocytes. J Invest Dermatol. 2000;114:990–997. doi: 10.1046/j.1523-1747.2000.00957.x. [DOI] [PubMed] [Google Scholar]
- Chien Y, Kim S, Bumeister R, Loo YM, Kwon SW, Johnson CL, Balakireva MG, Romeo Y, Kopelovich L, Gale M, Jr, et al. RalB GTPase-mediated activation of the IkappaB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell. 2006;127:157–170. doi: 10.1016/j.cell.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Desnos C, Schonn JS, Huet S, Tran VS, El-Amraoui A, Raposo G, Fanget I, Chapuis C, Menasche G, de Saint Basile G, et al. Rab27A and its effector MyRIP link secretory granules to F-actin and control their motion towards release sites. J Cell Biol. 2003;163:559–570. doi: 10.1083/jcb.200302157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S, Knodler A, Ren J, Zhang J, Zhang X, Hong Y, Huang S, Peranen J, Guo W. A Rab8 guanine nucleotide exchange factor-effector interaction network regulates primary ciliogenesis. J Biol Chem. 2012;287:15602–15609. doi: 10.1074/jbc.M111.333245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone AJ, Weinger JS, Maldonado M, Barlan K, Langston LD, O'Donnell M, Gelfand VI, Kapoor TM, Chen JK. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature. 2012;484:125–129. doi: 10.1038/nature10936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson S, Gullberg M, Jarvius J, Olsson C, Pietras K, Gustafsdottir SM, Ostman A, Landegren U. Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol. 2002;20:473–477. doi: 10.1038/nbt0502-473. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kuroda TS. Slac2-c (synaptotagmin-like protein homologue lacking C2 domains-c), a novel linker protein that interacts with Rab27, myosin Va/VIIa, and actin. J Biol Chem. 2002;277:43096–43103. doi: 10.1074/jbc.M203862200. [DOI] [PubMed] [Google Scholar]
- Gross SP, Tuma MC, Deacon SW, Serpinskaya AS, Reilein AR, Gelfand VI. Interactions and regulation of molecular motors in Xenopus melanophores. J Cell Biol. 2002;156:855–865. doi: 10.1083/jcb.200105055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guichard A, Nizet V, Bier E. RAB11-mediated trafficking in host-pathogen interactions. Nat Rev Microbiol. 2014;12:624–634. doi: 10.1038/nrmicro3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heider MR, Munson M. Exorcising the exocyst complex. Traffic. 2012;13:898–907. doi: 10.1111/j.1600-0854.2012.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horgan CP, Hanscom SR, Jolly RS, Futter CE, McCaffrey MW. Rab11-FIP3 links the Rab11 GTPase and cytoplasmic dynein to mediate transport to the endosomal-recycling compartment. J Cell Sci. 2010;123:181–191. doi: 10.1242/jcs.052670. [DOI] [PubMed] [Google Scholar]
- Hsu SC, TerBush D, Abraham M, Guo W. The exocyst complex in polarized exocytosis. International review of cytology. 2004;233:243–265. doi: 10.1016/S0074-7696(04)33006-8. [DOI] [PubMed] [Google Scholar]
- Hume AN, Ushakov DS, Tarafder AK, Ferenczi MA, Seabra MC. Rab27a and MyoVa are the primary Mlph interactors regulating melanosome transport in melanocytes. J Cell Sci. 2007;120:3111–3122. doi: 10.1242/jcs.010207. [DOI] [PubMed] [Google Scholar]
- Husebye H, Aune MH, Stenvik J, Samstad E, Skjeldal F, Halaas O, Nilsen NJ, Stenmark H, Latz E, Lien E, et al. The Rab11a GTPase controls Toll-like receptor 4-induced activation of interferon regulatory factor-3 on phagosomes. Immunity. 2010;33:583–596. doi: 10.1016/j.immuni.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida M, Ohbayashi N, Fukuda M. Rab1A regulates anterograde melanosome transport by recruiting kinesin-1 to melanosomes through interaction with SKIP. Sci Rep. 2015;5:8238. doi: 10.1038/srep08238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang Y, Zou L, Tang X, Yang Y, Ma L, Jia Q, Ni Q, Liu S, Tang L, et al. Analysis of the Rab GTPase Interactome in Dendritic Cells Reveals Anti-microbial Functions of the Rab32 Complex in Bacterial Containment. Immunity. 2016;44:422–437. doi: 10.1016/j.immuni.2016.01.027. [DOI] [PubMed] [Google Scholar]
- Lonnbro P, Nordenfelt P, Tapper H. Isolation of bacteria-containing phagosomes by magnetic selection. BMC cell biology. 2008;9:35. doi: 10.1186/1471-2121-9-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Li G, Zhang X, Xu H, Abraham SN. A TRP Channel Senses Lysosome Neutralization by Pathogens to Trigger Their Expulsion. Cell. 2015;161:1306–1319. doi: 10.1016/j.cell.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Wu J, Abraham SN. Ubiquitination of Innate Immune Regulator TRAF3 Orchestrates Expulsion of Intracellular Bacteria by Exocyst Complex. Immunity. 2016;45:94–105. doi: 10.1016/j.immuni.2016.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raposo G, Marks MS. Melanosomes--dark organelles enlighten endosomal membrane transport. Nature reviews Molecular cell biology. 2007;8:786–797. doi: 10.1038/nrm2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink J, Ghigo E, Kalaidzidis Y, Zerial M. Rab conversion as a mechanism of progression from early to late endosomes. Cell. 2005;122:735–749. doi: 10.1016/j.cell.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nature reviews. Molecular cell biology. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- Rogers SL, Karcher RL, Roland JT, Minin AA, Steffen W, Gelfand VI. Regulation of melanosome movement in the cell cycle by reversible association with myosin V. J Cell Biol. 1999;146:1265–1276. doi: 10.1083/jcb.146.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville JE, Jr, Cassiano L, Bainbridge B, Cunningham MD, Darveau RP. A novel Escherichia coli lipid A mutant that produces an anti-inflammatory lipopolysaccharide. J Clin Invest. 1996;97:359–365. doi: 10.1172/JCI118423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Bishop BL, Li G, Grady R, Stapleton A, Abraham SN. TLR4-mediated expulsion of bacteria from infected bladder epithelial cells. Proc Natl Acad Sci U S A. 2009;106:14966–14971. doi: 10.1073/pnas.0900527106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nature reviews Molecular cell biology. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- Udovichenko IP, Gibbs D, Williams DS. Actin-based motor properties of native myosin VIIa. J Cell Sci. 2002;115:445–450. doi: 10.1242/jcs.115.2.445. [DOI] [PubMed] [Google Scholar]
- Welz T, Wellbourne-Wood J, Kerkhoff E. Orchestration of cell surface proteins by Rab11. Trends Cell Biol. 2014;24:407–415. doi: 10.1016/j.tcb.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Wu X, Bowers B, Rao K, Wei Q, Hammer JA., 3rd Visualization of melanosome dynamics within wild-type and dilute melanocytes suggests a paradigm for myosin V function In vivo. J Cell Biol. 1998;143:1899–1918. doi: 10.1083/jcb.143.7.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial M, McBride H. Rab proteins as membrane organizers. Nature reviews Molecular cell biology. 2001;2:107–117. doi: 10.1038/35052055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


