Abstract
Highly pathogenic avian influenza virus (HPAIV) infections are frequently associated with systemic disease and high mortality in domestic poultry, particularly in chickens and turkeys. Clade 2.3.4.4 represents a genetic cluster within the Asian HPAIV H5 Goose/Guangdong lineage that has transmitted through migratory birds and spread throughout the world. In 2014, clade 2.3.4.4 strains entered the U.S. via the Pacific flyway, reassorted with local strains of the North American lineage, and produced novel HPAIV strains of the H5N1, H5N2, and H5N8 subtypes. By 2015, the H5N2 HPAIVs disseminated eastwards within the continental U.S. and Canada and infected commercial poultry, causing the largest animal health outbreak in recent history in the U.S. The outbreak was controlled by traditional mass depopulation methods, but the outbreak was of such magnitude that it led to the consideration of alternative control measures, including vaccination. In this regard, little information is available on the long-term protection of turkeys vaccinated against avian influenza. In this report, a vaccination study was carried out in turkeys using 3 prime-boost approaches with a combination of 2 different vaccines, an alphavirus-based replicon vaccine and an adjuvanted-inactivated reverse genetics vaccine. Vaccine efficacy was assessed at 6 and 16 weeks of age following challenge with a prototypic novel clade 2.3.4.4 H5N2 HPAIV. All three vaccines protocols were protective with significantly reduced virus shedding and mortality after challenge at 6 weeks of age. In contrast, significant variations were seen in 16-week old turkeys after challenge: priming with the alphavirus-based replicon followed by boost with the adjuvanted-inactivated vaccine conferred the best protection, whereas the alphavirus-based replicon vaccine given twice provided the least protection. Our study highlights the importance of studying not only different vaccine platforms but also vaccination strategies to maximize protection against HPAIV especially with regards to the longevity of vaccine-induced immune response.
Introduction
Infections with highly pathogenic avian influenza virus (HPAIV) are recognized as a serious threat to the domestic poultry industry and can cause devastating socio-economic burden [1]. During 2014–2015, unprecedented intercontinental outbreaks of H5 HPAIVs from the Asian clade 2.3.4.4 were reported [2–8]. In North America, cases of the clade 2.3.4.4 HPAI H5Nx viruses were reported in Canada and the US [6, 7] which was followed by rapid reassortment with at least two local low pathogenic avian influenza virus (LPAIV) strains. The resulting novel reassortant H5N1 and H5N2 HPAIVs spread to 21 states in the continental U.S [7, 9, 10]. These viruses spilled over to commercial poultry [1] with more than 48 million birds that died or were culled with an estimated economic loss of $3.3 billion [11]. Of the 232 farms affected, 160 were turkey farms indicating high susceptibility of these poultry species to the reassortant H5N2 HPAIV [12]. The extent of the H5N2 HPAIV outbreak and the associated risk of reintroduction of the virus in commercial poultry by migratory wild birds has led to the establishment of an emergency stockpile of approved vaccines against clade 2.3.4.4 H5N2 by the U.S. government [1, 13].
Numerous vaccines strategies have been developed for controlling HPAIVs in domestic poultry [14–20]. Only a few of these strategies have been systematically tested in turkeys [14, 19]. Differences in disease susceptibility among relevant poultry species highlight the importance of studies aimed at specifically evaluating vaccine-induced immunity and protection in turkeys [21, 22].
We evaluated the efficacy of 2 vaccines in 3 different prime-boost regimes against challenge with a prototypical clade 2.3.4.4 H5N2 HPAIV in turkeys at 6 and 16 weeks of age. Both vaccine strategies, an adjuvanted-inactivated reverse genetics vaccine (ΔH5N1) and a recombinant alphavirus-based replicon vaccine (α-replicon), regardless of regime used, were immunogenic in turkeys and reduced virus shedding and mortality after challenge compared to unvaccinated control birds. The longevity of the immune protective status revealed important differences depending on the vaccine regime analyzed. Our study highlights the importance of studying not only different vaccine platforms but also vaccination regime and strategies to maximize protection against HPAIV especially with regards to age and duration of vaccine-induced immune responses in different bird species.
Materials and Methods
Ethics statement
All animal studies were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Georgia (UGA) and the Southeast Poultry Research Laboratory, Agricultural Research Service, USDA. Vaccination studies were conducted under BSL-2 conditions at the Southeast Poultry Research Laboratory and the Poultry Diagnostic and Research Center (PDRC) at UGA. Challenge studies were carried out in a BSL-3 Ag containment facility at the Animal Health Research Center (AHRC) at UGA.
Vaccines and Viruses
The adjuvanted-inactivated ΔH5N1 vaccine was produced by expressing the hemagglutinin (HA) from a representative virus strain of clade 2.3.4.4, A/GyrFalcon/Washington/41088-6/2014 (H5N2) with the NA (N1 subtype) and internal genes of A/Puerto Rico/08/1934 (H1N1) strain. The HA polybasic cleavage site was replaced with a monobasic cleavage site (Δ) by site-directed mutagenesis and confirmed by sequencing prior to and after virus rescue [23]. The low pathogenic ΔH5N1 virus was authorized for deselection by APHIS for handling under BSL-2 conditions. Virus stock was prepared in Specific Pathogen Free (SPF) embryonated chicken eggs (ECE) followed by inactivation with beta-propiolactone (BPL) and diluted to provide a concentration of 512 HA units per 0.2 ml when mixed (70/30) with Montanide ISA VG70 oil emulsion (SEPPIC Inc., Fairfield, NJ) according to the manufacturers’ recommendations [24]. The recombinant α-replicon (SirraVax, a conditional-USDA-approved vaccine from Harrisvaccines, Inc., Ames, IA) [25, 26] carries the Δ HA gene from A/GyrFalcon/Washington/41088-6/2014 (H5N2). The A/turkey/Minnesota/12582/2015 (H5N2) HPAIV strain (HPAIV H5N2) was handled under BSL3-enhanced conditions and used in challenge studies. Virus stock was amplified in SPF ECE and virus titer was determined by 50% Egg Infectious Dose (EID50).
Vaccination
2-day old turkey poults were purchased from a commercial farm and co-housed in a single open floor barn to mimic commercial production conditions. Food and water were provided ad libitum. Birds were randomly divided into 4 groups (n=40/group). Group 1 served as unvaccinated control (Un). Birds in Groups 2–4 were vaccinated subcutaneously (SQ) with either 0.2 mL of the ΔH5N1 vaccine (512 HA units per dose) or 0.2 mL of the α-replicon vaccine. Three weeks post vaccination (wpv), a boost was administered SQ. Group 2 (I-I) was primed and boosted with the ΔH5N1 vaccine. Group 3 (A-I) was primed with the α-replicon vaccine and boosted with the ΔH5N1 vaccine. Group 4 (A-A) received two doses of the recombinant α-replicon vaccine. Unvaccinated, unchallenged turkeys showed lack of avian influenza virus (AIV) antibodies throughout the entire study, providing strong evidence of an AIV-free status in turkey poults prior to vaccination.
Challenge
One week prior to challenge, birds were placed in BSL-3 Ag containment and housed in open floor rooms. At 6 or 16 weeks of age (3 or 13 weeks post-boost (wpb), respectively), each bird was challenged intranasally (I.N.) with 106.5 EID50 of HPAIV H5N2 in 0.2mL of phosphate-buffered saline (PBS). Birds were monitored daily for disease signs and mortality. Animals displaying severe signs of disease were humanely euthanized and recorded as dead in the survival curve. At 2 and 4 days post challenge (dpc), tracheal swabs were collected and stored in 1 mL of 3.7% Brain Heart Infusion (BHI) (Becton Dickinson, Franklin Lakes, NJ) containing 1X gentamicin (Sigma-Aldrich, St. Louis, MO) and 1X antibiotic-antimycotic solution (Sigma-Aldrich, St. Louis, MO). For birds challenged at 6-weeks of age (n=20 birds per group), animals randomly selected or displaying severe signs of disease (n=3 per group) were euthanized at 3 and 6 dpc to collect tissues for virus titration (and histopathology and immunohistochemistry, not shown). For birds challenged at 16-weeks of age [n=19 (Un), n=20 (I-I), n=19 (A-I) and n=18 (A-A)], tissue collections were performed in a subset of birds (up to 3 birds/group) as they displayed severe signs of disease or succumbed to infection. Differences in number of birds per group in 16-week old birds reflects unexpected loses unrelated to the vaccination procedures or vaccine responses. In both challenge studies, tracheal swabs were collected at time of necropsy.
Quantification of virus shedding in biological samples
Virus RNA was isolated using the MagMAX-96 AI/ND Viral RNA Isolation Kit (Thermo Fisher Scientific, Waltham, MA). Viral RNA was reverse transcribed using SuperScript® III First-Strand Synthesis SuperMix kit (Thermo Fisher Scientific, Waltham, MA) and random hexamers. Quantitative polymerase chain reaction (qPCR) based on the avian influenza matrix gene as surrogate of virus shedding was carried out as described [27]. The qPCR was performed in a LightCycler 480 Real Time PCR instrument (Roche Diagnostics, Rotkreuz, Switzerland) using the LightCycler 480 Probes Master kit (Roche Life Science, Mannheim, Germany). A standard curve was generated using 10-fold serial dilutions from a virus stock of the HPAIV H5N2 strain of known titer to correlate qPCR crossing point (Cp) values with the amount of virus shedding from each bird, and expressed as log10 EID50/ml equivalents, as previously described [28]. For lung and trachea homogenates, qPCR Cp values were adjusted by tissue weight and expressed as log10 EID50/gr equivalents.
Analysis of antibody responses
Sera were obtained from birds at 3, 6, and 16 wpv. The antibody responses were evaluated by the hemagglutination inhibition (HI) assay using the ΔH5N1 virus as described [29] with minor modifications: Sera were pre-treated by mixing with a suspension of 10% chicken red blood cell (RBC), and 1X PBS (1:1:2 ratio) to remove non-specific inhibitors. Sera were also analyzed for anti-NP antibodies using a commercial ELISA kit (IDEXX Laboratories, Westbrook, ME).
Statistical analysis
All data analyses were performed using GraphPad Prism Software Version 7 (GraphPad Software Inc., San Diego, CA). For multiple comparisons, either one-way or two-way analysis of variance (ANOVA) was performed followed by a post-hoc Tukey test. Differences in survival curves were analyzed using the log-rank test. A p value below 0.05 (P<0.05) was considered significant.
Results
Prime-Boost vaccination strategy and antibody responses
Turkey poults were primed at 2 days of age and boosted at 3 wpv (Fig. 1). The ΔH5N1 and α-replicon vaccines were well tolerated with no apparent adverse effects. Low HI antibody titers were observed at 3 wpv in 10 birds (out 40) in Group 2 (I-I), whereas in other groups such response remained below limit of detection (Fig. 2A). At 6 wpv (3 wpb), birds in Group 3 (A-I) exhibited the best overall HI antibody response (16–512, average 140.6) compared to birds in either Group 2 (I-I) (P<0.05) or Group 4 (A-A) (P<0.001)(Fig. 2A). Birds in Group 2 (I-I) displayed moderate levels of HI antibody titers (8–128, average 48.4). In Group 4 (A-A), birds had relatively low antibody titers (4–64, average 13.6); 4 birds (out of 20) did not show seroconversion by HI assay and 10 birds had an HI titer of 8 post-boost. At 16 wpv (13 wpb), HI antibody titers were drastically reduced compared to the 6-week old birds, irrespective of the vaccine regime used (Fig. 2A). Differences in antibody responses among vaccine groups in 16-week old turkeys were not significant; however, HI antibody titers were still observed in individual birds from Groups 2 (I-I) and 3 (A-I).
Figure 1. Vaccination strategy in turkeys.
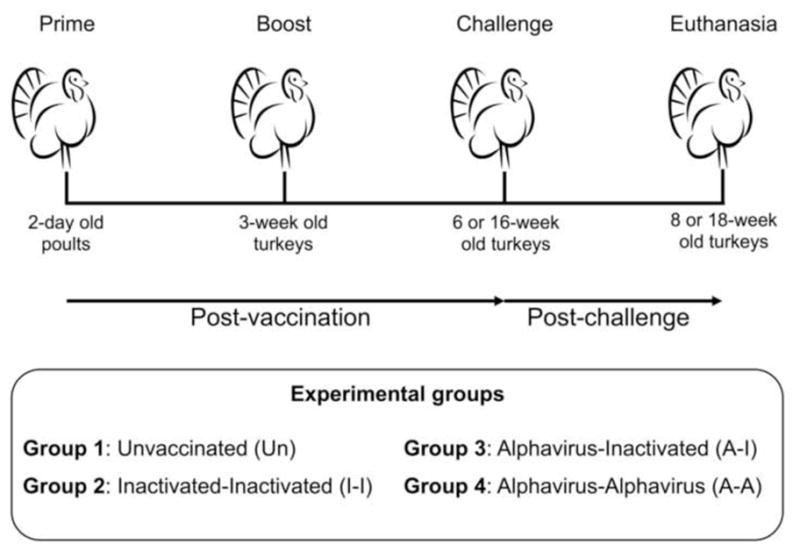
Turkey poults were divided into four groups (n=40 birds/group) and vaccinated using a prime-boost strategy. At 6 or 16 wpv, birds (n=20/group) were challenged with HPAIV H5N2 and monitored daily for disease signs and mortality.
Figure 2. Immunogenicity of different prime-boost vaccine regimes in turkeys.
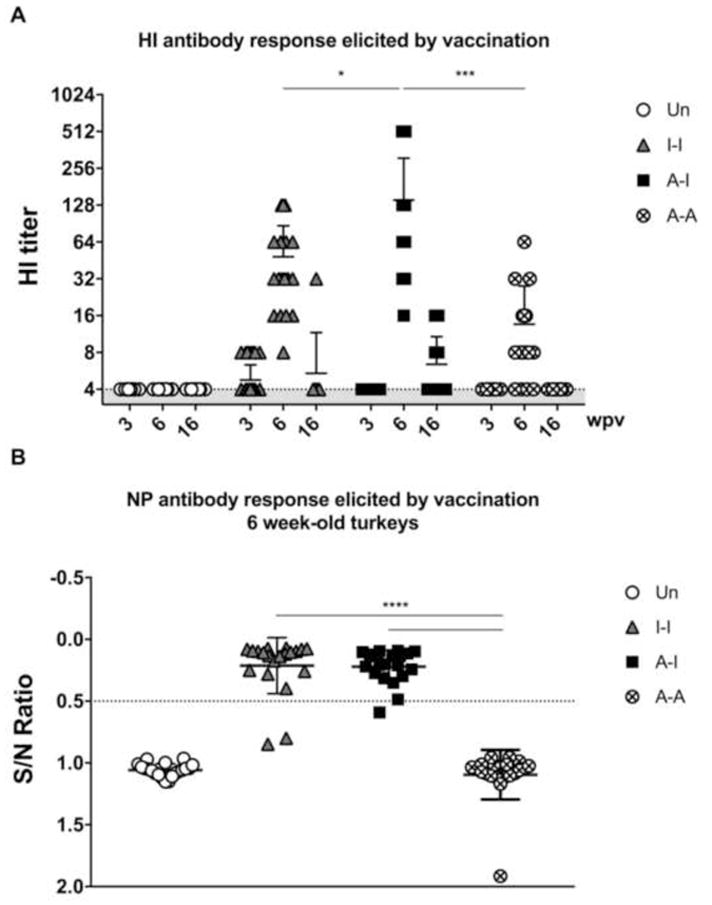
Sera were used in HI assays against the ΔH5N1 virus and in competitive NP-ELISA assays. (A) HI titers at 3 (n=40/group), 6 (n=20/group), and 16 (n=20/group) wpv. (B) Antibodies to NP measured at 6 wpv (n=20 per group). Plotted data: means + standard deviation (SD). Statistical analyses using one-way or two-way ANOVA. *, P<0.05; ***, P<0.001; ****, P<0.0001.
Post-boost sera from 6-week-old turkeys were also tested for the presence of nucleoprotein (NP) antibodies. As expected, only birds that received at least one dose of inactivated ΔH5N1 vaccine (birds from either Group 2 or 3) developed antibodies against NP (Fig. 2B). No statistical differences were observed in the levels of NP antibodies between these two groups.
Vaccine protection in 6-week old turkeys
The 6-week old birds received a lethal dose of the HPAIV H5N2 strain. Unvaccinated birds, Group 1 (Un), showed no apparent signs of disease by 2 dpc but by 3 dpc they had either succumbed to the infection or were humanely euthanized due to the severity of clinical signs (severe depression, lethargy, diarrhea, and nervous signs) (Fig. 3A). Birds in Groups 2 (I-I) and 3 (A-I) showed no disease signs and 100% survived the challenge. In contrast, some birds in Group 4 (4 out of 20, 93% survival) exhibited severe signs of disease: 3 birds were euthanized at 6 dpc and the fourth bird was euthanized at 7 dpc (Fig. 3A).
Figure 3. Protective efficacy in 6-week old turkeys.
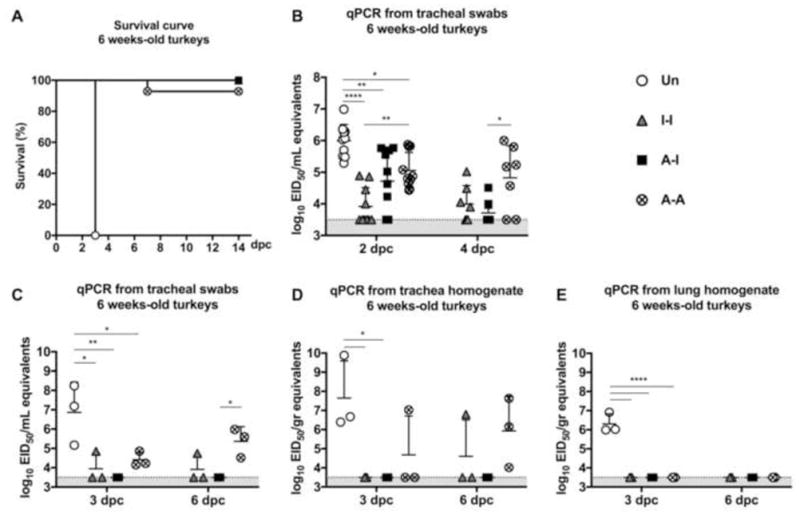
(A) Survival curve after challenge. Virus shedding in (B) tracheal swabs collected at 2 dpc (n=10/group) and 4 dpc (n=7/group); and from euthanized animals at 3 dpc (n=3/group) and 6 dpc, (C) tracheal swabs, (D) tracheal homogenates, and (E) lung homogenates. Plotted data: means + SD. One-way or Two-way ANOVA was performed. *, P<0.05; **, P<0.01; ****, P<0.0001.
Consistent with the poor survival outcome, high virus levels were present in tracheal swabs of Group 1 (Un) birds at 2 dpc (Fig. 3B and C) and trachea and lung homogenates at 3 dpc (n=3) (Fig. 3D and E). Despite significant protection, none of the vaccine strategies completely prevented virus shedding following challenge. Birds in Group 2 (I-I) showed low virus levels in tracheal swabs at 2 dpc (P<0.0001) and at 4 dpc, compared to Group 1 (Un) challenged birds (Fig. 3B). Likewise, birds from Group 3 (AI) showed low virus shedding at 2 dpc (P<0.01) and indication of rapid virus clearance by 4 dpc (Fig. 3B). Birds from Group 4 (A-A) experienced the highest levels of virus shedding among vaccinated groups in tracheal swabs at 2 dpc although significantly lower than Group 1 (Un) challenged birds (P<0.05). Unlike the birds in Groups 2 (I-I) and 3 (A-I), Group 4 (A-A) birds showed no decrease in virus shedding by 4 dpc compared to 2 dpc (Fig. 3B).
At 3 dpc and 6 dpc, a subset of birds from Groups 2 (I-I), 3 (A-I), and 4 (A-A) were euthanized and tracheal and tissues samples collected. Tracheal swabs from Group 3 (A-I) birds showed no detectable virus, whereas those from Group 2 (I-I) birds exhibited low virus levels in 1 out of 3 animals at 3 dpc and at 6 dpc (Fig. 3C). Virus levels were below limit of detection in tissue homogenates from either the trachea or lung of Groups 2 (I-I) and 3 (A-I), except for one bird in Group 2 at 6 dpc (Fig. 3D and E). Virus shedding in Group 4 (A-A) birds was detected in both tracheal swabs and trachea homogenates, but not in lung homogenates (Fig. 3C–E).
Protective efficacy in 16-week old turkeys
After intranasal challenge at 16 wpv (13 wpb), Group 1 (Un) showed 100% mortality by 5 dpc from either death or euthanasia due to disease severity (Fig. 4A). The I-I prime-boost regime (Group 2) resulted in 75% survival (15 out 20 birds) with unnoticeable or mild clinical disease signs, except in 5 birds that either died (n=1) or were euthanized (n=4). The A-I prime-boost regime (Group 3) afforded the best overall protection (90%, 17 out of 19) with no apparent signs of disease in surviving birds. Interestingly, Group 4 (A-A) showed poor protection (16%, 3 out of 18) after challenge.
Figure 4. Protective efficacy in 16-week old turkeys.
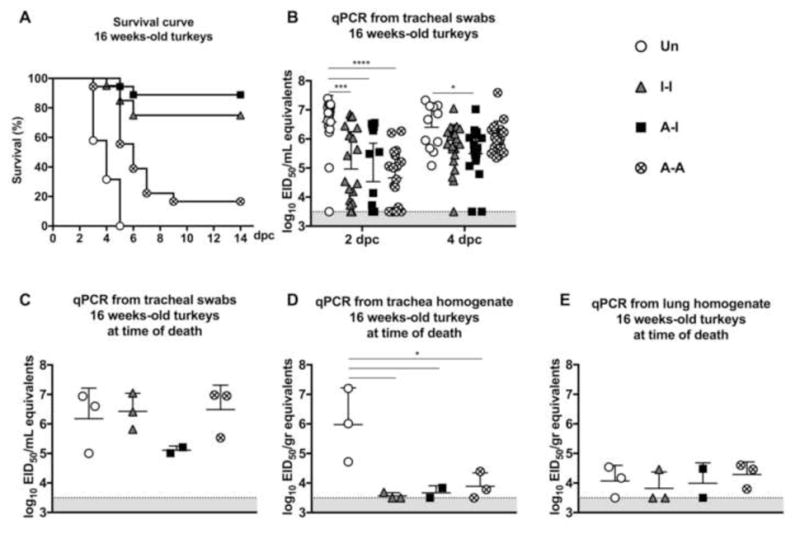
(A) Survival curve following challenge Virus shedding in (B) tracheal swabs collected at 2 dpc [n=19 (Un), n=20 (I-I), n=19 (A-I) and n=18 (A-A)] and 4 dpc [n=11 (Un), n=20 (I-I), n=19 (A-I) and n=17 (A-A)]; and from subset of birds displaying severe disease signs or succumbed to infection at the time of necropsy [n=3 (Un), n=3 (I-I), n=2 (A-I) and n=3 (A-A)], (C) tracheal swabs, (D) tracheal homogenates, and (E) lung homogenates. Plotted data: means + SD. Statistical analyses as in Fig 3. *, P<0.05; ***, P<0.001; ****, P<0.0001.
Group 1 (Un) birds showed elevated virus levels in the trachea at 2 dpc and in surviving birds at 4 dpc (n=11) (Fig. 4B). The tracheal swabs of Group 2 (I-I) 16-week old turkeys showed significantly lower virus titers than Group 1 (Un) at 2 dpc (P<0.001) (Fig. 4B). Likewise, tracheal virus shedding in Group 3 (A-I) birds was significantly lower than in unvaccinated birds at 2 dpc (P<0.0001) and 4 dpc (P<0.05) (Fig. 4B). Birds from Group 4 (A-A) showed diminished tracheal virus shedding in comparison to unvaccinated birds at 2 dpc (P<0.0001), but by 4 dpc virus shedding in tracheal swabs showed titers similar to those in Group 1 (Un) (P=ns) (Fig 4B). Overall, following challenge, the amount of virus shedding was significantly higher in the 16-week old birds than the respective groups at 6 weeks of age and consistent with differences in survival (Fig. 3A and B vs. Fig. 4A and B).
Tracheal swabs collected at the time of necropsy from animals that displayed severe disease signs (n=3 for Groups 1, 2, and 4, n=2 for Group 3) showed high virus levels indistinguishable among groups regardless of vaccination status (Fig. 4C). Virus levels in lung homogenates were lower than in tracheal swabs but also indistinguishable among groups (Fig. 4D and E). In contrast, trachea homogenates of Group 1 (Un) birds showed higher virus levels (P<0.05) than other groups (Fig. 4D).
Discussion
We evaluated the short- and long-term protective efficacy against a prototypical clade 2.3.4.4. H5N2 HPAIV in turkeys vaccinated SQ with 2 different vaccines in 3 different prime-boost regimes. All 3 prime-boost regimes were immunogenic as measured by HI assays. Consistent with previous reports, HI antibody titers at 3 wpv were low or undetectable [14, 19]. Boosting with either the same (I-I or A-A) or a different vaccine (A-I) strategy significantly increased HI titers by 6 wpv. However, the HI response was drastically reduced by 16 wpv. Not surprisingly, the prime-boost regimes with a single (A-I) or two doses (I-I) of the adjuvanted inactivated ΔH5N1 vaccine resulted in antibodies against the NP. There were not statistically significant differences in the anti-NP response comparing the group with two doses (I-I) versus one dose (A-I) of ΔH5N1 vaccine. It must be noted that we did not measure NP responses at 3 wpv and therefore we cannot establish whether there was an increase in NP responses post-boost in the I-I group. However, the apparent lack of a boosting effect to NP responses in the I-I group (compared to the A-I group) is not necessarily surprising in the context of the limited antibody responses observed at 3 wpv, which are reflected by the low HI titers. Thus, the immaturity of the immune system in young birds at priming (2 days of age) precluded the development of a robust immune response [30, 31]. In this case, it is highly plausible that the NP antibodies measured at 6 wpv are largely due to the second dose of the inactivated vaccine in the I-I group and, therefore, limited boost responses would be expected.
Following challenge, all 3 prime-boost strategies reduced virus shedding and lung pathology (not shown) in vaccinated birds. The reduction in virus titer would have most likely limited virus transmission as suggested by previous reports [32]. Unvaccinated turkeys shed slightly higher levels of virus and exhibited a shorter mean death time compared to a previous study using the same challenge strain [11]. Differences in age at time of challenge between studies may account for such discrepancy, which warrant further investigation. It is well established that the age of birds at infection can influence the severity of disease caused by influenza viruses in different avian species [33–35]. Likewise, age-dependent resistance to disease may be associated to differences in immune function and early host responses [33–35]. The Group 1 (Un) 16-week old turkeys showed higher virus levels in tracheal swabs at 2 dpc than the same group at 6-weeks of age. The onset of mortality was different depending on age (the unvaccinated old turkeys died by 5 dpc, whereas the unvaccinated young turkeys succumbed by 3 dpc). Considering that most affected turkeys during the recent outbreak in the U.S. were 12 weeks of age or older, the extended window of virus shedding in older birds observed in our study may help explain the rapid spread of the virus reported in the field [11].
All 3 prime-boost regimes offered adequate protection against challenge with the clade 2.3.4.4. H5N2 HPAIV at 6 wpv (3wpb), with no statistically significant differences in survival despite some birds succumbing to the challenge in Group 4 (A-A). However, turkeys challenged at 16 weeks of age (13 wpb) showed decreased protection when compared to turkeys challenged at 6 weeks of age, consistent with the reduction in the HI antibody response. The protection against HPAIV is influenced by both the humoral and cell mediated immune responses. While HI antibodies titers induced by inactivated vaccines to the challenge strain strongly correlate with protection, vectored vaccines lack a definite correlate of protection [36]. The strong protection in 6-week old turkeys correlated well with the HI antibody levels. As expected HI responses decreased over time resulting in less protection. The group with the worst survival outcome was the A-A group, which also had undetectable HI antibody titers at 16 weeks. Nevertheless, some birds with undetectable HI titers, regardless of age, did survive the lethal challenge. This is consistent with previous observations using other viral vectored vaccines that induced HI responses below limit of detection but resulted in turkeys that were still protected from virulent challenge [19]. The cytotoxic cell mediated immune response is also known to provide additive protection in challenge. Adjuvanted killed vaccines provide a strong antibody response, but little to no cell mediated immunity [36, 37]. The recombinant alphavirus-based replicon vaccine, although replication incompetent, does infect cells and stimulates humoral, cellular and mucosal immune response [25, 26, 38–40]. The best protection observed in this study was the prime with the “live” alphavirus replicon vaccine and a boost with the killed vaccine. The ability to stimulate both mucosal and systemic immunity and prime a strong recall antibody response contributed to a more robust and sustained protective immune response observed in the heterologous prime-boost group [36].
The challenges in generating robust and sustained immunity against H5 HPAIVs are not restricted to turkeys or other domestic poultry species [14, 19, 41, 42]. Pandemic H5N1 influenza vaccines are often poorly immunogenic in humans, requiring multiple doses and the use of adjuvants to induce detectable HI antibody titers [43–45]. Combining different vaccine platforms in heterologous prime-boost regimes has been critical to potentiate immune responses and improve long-term performance of vaccine candidates [46]. Previous vaccination studies in chickens and ducks have shown sustained HI antibody levels for up to a year following prime-boost immunization protocols [47–49]. Although the vaccines, vaccination protocols and the timing of vaccination evaluated differ among studies, those results are in contrast with the findings in the present report in which vaccine-induced immunity in turkeys was short-lived with marked decrease in HI antibody levels over time. Short-live vaccine-induced immunity was reported in geese [49], highlighting the complexities associated with immune responses in Galliformes as well as Anseriformes. Together, these results emphasize the need to avoid extrapolations and to careful plan vaccination programs tailored to specific avian species [49]. The extent of protection over time evaluated in this study offers important insights and practical guidelines for future evaluation of vaccine candidates against H5 HPAIVs. Our findings reinforce the need to monitor the long-term protection of vaccine-elicited immunity against HPAIVs and LPAIVs in multiple relevant poultry species. Caution should be exercised against making broad generalizations of vaccine studies from one poultry species to another.
Highlights.
A prime-boost vaccination strategy resulted in protective responses in turkeys against a prototypical clade 2.3.4.4 H5N2 HPAIV.
Responses against the HA of a prototypical clade 2.3.4.4 H5N2 HPAIV were stimulated using either a recombinant alphavirus replicon or an adjuvanted-inactivated influenza virus reverse genetics vaccine.
In turkey sera, hemagglutination inhibition titers were clearly discernible by 6 weeks post-vaccination (3 weeks post-boost), but were drastically reduced by 16 weeks post-vaccination (13 weeks post-boost).
Best overall immunological and protective responses were achieved by priming with the alphavirus replicon followed by boost with inactivated vaccine, particularly with respect to long-term protection.
Acknowledgments
Mention of trade names or commercial products is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA. USDA is an equal opportunity provider and employer. The authors would like to thank Animal Resources personnel at the PDRC and AHRC and Dr. Mark Mogler of Harrisvaccines for supplying the alphavirus vaccine used in the study. The studies were funded by: Cooperative agreement between the ARS-USDA and the University of Georgia; start-up funds from the University of Georgia and Caswell S Eidson Chair in Poultry Medicine; the National Institute of Allergy and Infectious Diseases (NIAID) Center for Research on Influenza Pathogenesis (CRIP) (contract HHSN272201400008C); and the Animal and Plant Health Inspection Service Project 16-9419-0378 HPAIV Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Swayne DE, Hill RE, Clifford J. Safe application of regionalization for trade in poultry and poultry products during highly pathogenic avian influenza outbreaks in the USA. Avian Pathol. 2016:1–6. doi: 10.1080/03079457.2016.1257775. [DOI] [PubMed] [Google Scholar]
- 2.Lee YJ, Kang HM, Lee EK, Song BM, Jeong J, Kwon YK, et al. Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerging infectious diseases. 2014;20:1087–9. doi: 10.3201/eid2006.140233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanehira K, Uchida Y, Takemae N, Hikono H, Tsunekuni R, Saito T. Characterization of an H5N8 influenza A virus isolated from chickens during an outbreak of severe avian influenza in Japan in April 2014. Arch Virol. 2015;160:1629–43. doi: 10.1007/s00705-015-2428-9. [DOI] [PubMed] [Google Scholar]
- 4.Fan S, Zhou L, Wu D, Gao X, Pei E, Wang T, et al. A novel highly pathogenic H5N8 avian influenza virus isolated from a wild duck in China. Influenza Other Respir Viruses. 2014;8:646–53. doi: 10.1111/irv.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adlhoch C, Gossner C, Koch G, Brown I, Bouwstra R, Verdonck F, et al. Comparing introduction to Europe of highly pathogenic avian influenza viruses A(H5N8) in 2014 and A(H5N1) in 2005. Euro Surveill. 2014;19:20996. doi: 10.2807/1560-7917.es2014.19.50.20996. [DOI] [PubMed] [Google Scholar]
- 6.Global Consortium for H5N8 and Related Influenza Viruses T. Role for migratory wild birds in the global spread of avian influenza H5N8. Science. 2016;354:213–7. doi: 10.1126/science.aaf8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ip HS, Torchetti MK, Crespo R, Kohrs P, DeBruyn P, Mansfield KG, et al. Novel Eurasian highly pathogenic avian influenza A H5 viruses in wild birds, Washington, USA, 2014. Emerg Infect Dis. 2015;21:886–90. doi: 10.3201/eid2105.142020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verhagen JH, Herfst S, Fouchier RA. Infectious disease. How a virus travels the world. Science. 2015;347:616–7. doi: 10.1126/science.aaa6724. [DOI] [PubMed] [Google Scholar]
- 9.Pasick J, Berhane Y, Joseph T, Bowes V, Hisanaga T, Handel K, et al. Reassortant Highly Pathogenic Influenza A H5N2 Virus Containing Gene Segments Related to Eurasian H5N8 in British Columbia, Canada, 2014. Sci Rep-Uk. 2015:5. doi: 10.1038/srep09484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee DH, Torchetti MK, Winker K, Ip HS, Song CS, Swayne DE. Intercontinental Spread of Asian-Origin H5N8 to North America through Beringia by Migratory Birds. J Virol. 2015;89:6521–4. doi: 10.1128/JVI.00728-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spackman E, Pantin-Jackwood MJ, Kapczynski DR, Swayne DE, Suarez DL. H5N2 Highly Pathogenic Avian Influenza Viruses from the US 2014–2015 outbreak have an unusually long pre-clinical period in turkeys. BMC Vet Res. 2016;12:260. doi: 10.1186/s12917-016-0890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dargatz D, Beam A, Wainwright S, McCluskey B. Case Series of Turkey Farms from the H5N2 Highly Pathogenic Avian Influenza Outbreak in the United States During 2015. Avian Dis. 2016;60:467–72. doi: 10.1637/11350-121715-Reg. [DOI] [PubMed] [Google Scholar]
- 13.Lee DH, Torchetti MK, Killian ML, DeLiberto TJ, Swayne DE. Reoccurrence of Avian Influenza A(H5N2) Virus Clade 2.3.4.4 in Wild Birds, Alaska, USA, 2016. Emerg Infect Dis. 2017;23:365–7. doi: 10.3201/eid2302.161616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kilany WH, Abdelwhab EM, Arafa AS, Selim A, Safwat M, Nawar AA, et al. Protective efficacy of H5 inactivated vaccines in meat turkey poults after challenge with Egyptian variant highly pathogenic avian influenza H5N1 virus. Vet Microbiol. 2011;150:28–34. doi: 10.1016/j.vetmic.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 15.Song H, Nieto GR, Perez DR. A new generation of modified live-attenuated avian influenza viruses using a two-strategy combination as potential vaccine candidates. J Virol. 2007;81:9238–48. doi: 10.1128/JVI.00893-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cai Y, Song H, Ye J, Shao H, Padmanabhan R, Sutton TC, et al. Improved hatchability and efficient protection after in ovo vaccination with live-attenuated H7N2 and H9N2 avian influenza viruses. Virol J. 2011;8:31. doi: 10.1186/1743-422X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qiao C, Jiang Y, Tian G, Wang X, Li C, Xin X, et al. Recombinant fowlpox virus vector-based vaccine completely protects chickens from H5N1 avian influenza virus. Antiviral Res. 2009;81:234–8. doi: 10.1016/j.antiviral.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Ge J, Deng G, Wen Z, Tian G, Wang Y, Shi J, et al. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J Virol. 2007;81:150–8. doi: 10.1128/JVI.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapczynski DR, Dorsey K, Chrzastek K, Moraes M, Jackwood M, Hilt D, et al. Vaccine Protection of Turkeys Against H5N1 Highly Pathogenic Avian Influenza Virus with a Recombinant Turkey Herpesvirus Expressing the Hemagglutinin Gene of Avian Influenza. Avian Dis. 2016;60:413–7. doi: 10.1637/11267-090115-Reg. [DOI] [PubMed] [Google Scholar]
- 20.Hikke MC, Pijlman GP. Veterinary Replicon Vaccines. Annu Rev Anim Biosci. 2016 doi: 10.1146/annurev-animal-031716-032328. [DOI] [PubMed] [Google Scholar]
- 21.Pantin-Jackwood MJ, Stephens CB, Bertran K, Swayne DE, Spackman E. The pathogenesis of H7N8 low and highly pathogenic avian influenza viruses from the United States 2016 outbreak in chickens, turkeys and mallards. PLoS One. 2017;12:e0177265. doi: 10.1371/journal.pone.0177265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spackman E, Gelb J, Jr, Preskenis LA, Ladman BS, Pope CR, Pantin-Jackwood MJ, et al. The pathogenesis of low pathogenicity H7 avian influenza viruses in chickens, ducks and turkeys. Virol J. 2010;7:331. doi: 10.1186/1743-422X-7-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jadhao SJ, Suarez DL. New approach to delist highly pathogenic avian influenza viruses from BSL3+ Select Agents to BSL2 non-select status for diagnostics and vaccines. Avian Dis. 2010;54:302–6. doi: 10.1637/8926-051509-ResNote.1. [DOI] [PubMed] [Google Scholar]
- 24.King DJ. Evaluation of different methods of inactivation of Newcastle disease virus and avian influenza virus in egg fluids and serum. Avian Dis. 1991;35:505–14. [PubMed] [Google Scholar]
- 25.Hooper JW, Ferro AM, Golden JW, Silvera P, Dudek J, Alterson K, et al. Molecular smallpox vaccine delivered by alphavirus replicons elicits protective immunity in mice and non-human primates. Vaccine. 2009;28:494–511. doi: 10.1016/j.vaccine.2009.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vander Veen RL, Loynachan AT, Mogler MA, Russell BJ, Harris DL, Kamrud KI. Safety, immunogenicity, and efficacy of an alphavirus replicon-based swine influenza virus hemagglutinin vaccine. Vaccine. 2012;30:1944–50. doi: 10.1016/j.vaccine.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 27.Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, et al. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–60. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das A, Spackman E, Pantin-Jackwood MJ, Suarez DL. Removal of real-time reverse transcription polymerase chain reaction (RT-PCR) inhibitors associated with cloacal swab samples and tissues for improved diagnosis of Avian influenza virus by RT-PCR. J Vet Diagn Invest. 2009;21:771–8. doi: 10.1177/104063870902100603. [DOI] [PubMed] [Google Scholar]
- 29.Webster R, Cox N, Stohr K. WHO Manual on Animal Influenza Diagnosis and Surveillance. World Health Organization Department of Communicable Disease Surveillance and Response; 2005. [Google Scholar]
- 30.Schultz-Cherry S, Dybing JK, Davis NL, Williamson C, Suarez DL, Johnston R, et al. Influenza virus (A/HK/156/97) hemagglutinin expressed by an alphavirus replicon system protects chickens against lethal infection with Hong Kong-origin H5N1 viruses. Virology. 2000;278:55–9. doi: 10.1006/viro.2000.0635. [DOI] [PubMed] [Google Scholar]
- 31.Cagle C, Wasilenko J, Adams SC, Cardona CJ, To TL, Nguyen T, et al. Differences in pathogenicity, response to vaccination, and innate immune responses in different types of ducks infected with a virulent H5N1 highly pathogenic avian influenza virus from Vietnam. Avian Dis. 2012;56:479–87. doi: 10.1637/10030-120511-Reg.1. [DOI] [PubMed] [Google Scholar]
- 32.Ellis TM, Leung CY, Chow MK, Bissett LA, Wong W, Guan Y, et al. Vaccination of chickens against H5N1 avian influenza in the face of an outbreak interrupts virus transmission. Avian Pathol. 2004;33:405–12. doi: 10.1080/03079450410001724012. [DOI] [PubMed] [Google Scholar]
- 33.Pantin-Jackwood MJ, Suarez DL, Spackman E, Swayne DE. Age at infection affects the pathogenicity of Asian highly pathogenic avian influenza H5N1 viruses in ducks. Virus Res. 2007;130:151–61. doi: 10.1016/j.virusres.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Londt BZ, Nunez A, Banks J, Alexander DJ, Russell C, Richard-Londt AC, et al. The effect of age on the pathogenesis of a highly pathogenic avian influenza (HPAI) H5N1 virus in Pekin ducks (Anas platyrhynchos) infected experimentally. Influenza Other Respir Viruses. 2010;4:17–25. doi: 10.1111/j.1750-2659.2009.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reemers SS, van Leenen D, Koerkamp MJ, van Haarlem D, van de Haar P, van Eden W, et al. Early host responses to avian influenza A virus are prolonged and enhanced at transcriptional level depending on maturation of the immune system. Mol Immunol. 2010;47:1675–85. doi: 10.1016/j.molimm.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 36.Suarez DL, Pantin-Jackwood MJ. Recombinant viral-vectored vaccines for the control of avian influenza in poultry. Vet Microbiol. 2017;206:144–51. doi: 10.1016/j.vetmic.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 37.Loving CL, Vincent AL, Pena L, Perez DR. Heightened adaptive immune responses following vaccination with a temperature-sensitive, live-attenuated influenza virus compared to adjuvanted, whole-inactivated virus in pigs. Vaccine. 2012;30:5830–8. doi: 10.1016/j.vaccine.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hubby B, Talarico T, Maughan M, Reap EA, Berglund P, Kamrud KI, et al. Development and preclinical evaluation of an alphavirus replicon vaccine for influenza. Vaccine. 2007;25:8180–9. doi: 10.1016/j.vaccine.2007.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thompson JM, Nicholson MG, Whitmore AC, Zamora M, West A, Iwasaki A, et al. Nonmucosal alphavirus vaccination stimulates a mucosal inductive environment in the peripheral draining lymph node. J Immunol. 2008;181:574–85. doi: 10.4049/jimmunol.181.1.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalil SM, Tonkin DR, Snead AT, Parks GD, Johnston RE, White LJ. An alphavirus-based adjuvant enhances serum and mucosal antibodies, T cells, and protective immunity to influenza virus in neonatal mice. J Virol. 2014;88:9182–96. doi: 10.1128/JVI.00327-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spackman E, Swayne DE, Pantin-Jackwood MJ, Wan XF, Torchetti MK, Hassan M, et al. Variation in protection of four divergent avian influenza virus vaccine seed strains against eight clade 2.2.1 and 2.2.1.1. Egyptian H5N1 high pathogenicity variants in poultry. Influenza Other Respir Viruses. 2014;8:654–62. doi: 10.1111/irv.12290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cha RM, Smith D, Shepherd E, Davis CT, Donis R, Nguyen T, et al. Suboptimal protection against H5N1 highly pathogenic avian influenza viruses from Vietnam in ducks vaccinated with commercial poultry vaccines. Vaccine. 2013;31:4953–60. doi: 10.1016/j.vaccine.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 43.Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, Ypma E, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–43. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 44.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 45.Stephenson I, Nicholson KG, Colegate A, Podda A, Wood J, Ypma E, et al. Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. Vaccine. 2003;21:1687–93. doi: 10.1016/s0264-410x(02)00632-1. [DOI] [PubMed] [Google Scholar]
- 46.Luke CJ, Subbarao K. Improving pandemic H5N1 influenza vaccines by combining different vaccine platforms. Expert Rev Vaccines. 2014;13:873–83. doi: 10.1586/14760584.2014.922416. [DOI] [PubMed] [Google Scholar]
- 47.Beato MS, Toffan A, De Nardi R, Cristalli A, Terregino C, Cattoli G, et al. A conventional, inactivated oil emulsion vaccine suppresses shedding and prevents viral meat colonisation in commercial (Pekin) ducks challenged with HPAI H5N1. Vaccine. 2007;25:4064–72. doi: 10.1016/j.vaccine.2007.02.042. [DOI] [PubMed] [Google Scholar]
- 48.Boltz DA, Douangngeun B, Sinthasak S, Phommachanh P, Midouangchanh P, Walker D, et al. Field assessment of an H5N1 inactivated vaccine in chickens and ducks in Lao PDR. Arch Virol. 2009;154:939–44. doi: 10.1007/s00705-009-0385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tian G, Zhang S, Li Y, Bu Z, Liu P, Zhou J, et al. Protective efficacy in chickens, geese and ducks of an H5N1-inactivated vaccine developed by reverse genetics. Virology. 2005;341:153–62. doi: 10.1016/j.virol.2005.07.011. [DOI] [PubMed] [Google Scholar]


