1. Introduction
The world’s population is aging at an unprecedented rate. According to the WHO, the number of people aged over 60 is set to rise to 2 billion by 2050, a significant proportion of whom will have to cope with normative or pathological cognitive decline. While a large of body of research into cognitive aging has focused on functions such as memory and attention, relatively few studies have investigated changes in spatial navigation. This is surprising because key structures of the brain’s navigation circuit are particularly vulnerable to the deleterious consequences of aging. Hence, detection of navigational deficits should provide a distinct metric for assessing age-related cognitive decline. Furthermore, recent breakthroughs in understanding the cellular components of basic navigational circuits in a variety of species, including rodents, bats and nonhuman primates, provide the opportunity to distinguish those network changes that occur as a result of normative aging processes independent from those imposed by specific neuropathological conditions.
In surveys, older people indeed report substantial declines in navigational capabilities (Burns, 1999), which can severely restrict mobility and social participation. Self-reported difficulties and objective tests are consistent with the observations of altered spatial cognition in all species examined. For example, older humans show deficits in tasks designed to test spatial navigation (Moffat, 2009) as do nonhuman primates (Rapp et al., 1997), dogs (Head et al., 1995), rats (Barnes, 1979) and mice (Bach et al., 1999). Given that these problems cannot be explained by general, age-related declines in cognitive (e.g., reduced processing speed) or motor functioning, the following sections will provide an in-depth discussion of the behavioral and neural mechanisms that cause specific navigational impairments in old age.
2. Mechanisms of Spatial Navigation
Navigation – one of the most fundamental behaviors in humans and other animals – involves a multitude of cognitive functions and processes. First of all, spatial navigation can be based on dynamic self-motion cues and/or static environmental cues. The former comprise body based self-motion cues derived from motor efference copy, vestibular feedback and proprioceptive cues, all of which can be used to keep track of one’s position and orientation. Following primary sensory processing, these cues are first integrated in brainstem nuclei to yield estimates of angular and linear movement velocity. Similarly, optic flow arising from visual perception also provides self-motion information to the organism. In primates, both types of self-motion cues are further processed in parietal/superior temporal cortices (i.e., areas MST, VIP and 7a), thus providing an integrated percept of self-motion. The second type of cues, referred to here as environmental cues, mainly comprise stable objects such as landmarks and extended boundaries that can be used to determine one’s position and orientation relative to the environment. In primates, these cues are predominantly derived from visual perception, but other species also make heavy use of non-visual cues (i.e., auditory, olfactory and tactile stimuli). Perception of environmental cues involves, beyond primary sensory cortices, various structures in posterior parietal cortex (Kravitz et al., 2011). Importantly, self-motion and environmental cues can inform both allocentric and egocentric reference frames (Figure 1A, B). An allocentric reference frame is independent of the position of the navigator and does not change as the navigator moves through space. An egocentric reference frame, on the other hand, involves representations of locations that are encoded relative to the navigator.
Figure 1. Navigation within different spatial reference frames and spatial scales.
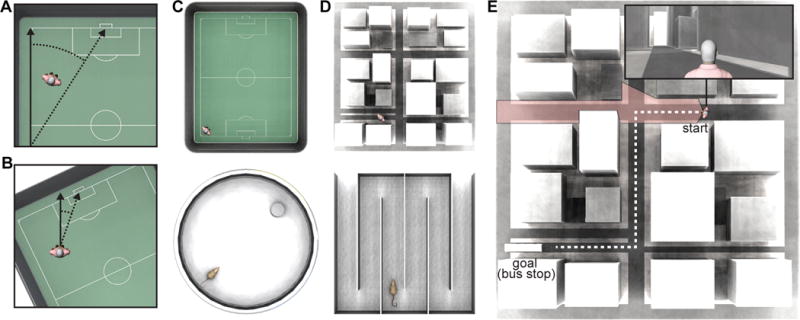
(A) An example of an Allocentric Reference Frame. Spatial information, such as the position of a landmark, is encoded with respect to other objects in the environment, i.e., the edge of the soccer field. The solid vertical arrow represents the allocentric reference direction that is fixed with respect to the dominant geometric boundary, and hatched lines represent the allocentric direction to other features in the space (e.g., the soccer goal). (B) An example of an Egocentric Reference Frame. The solid vertical arrow represents the egocentric reference direction, which is aligned to the orientation of the observer, and hatched lines denote the egocentric self-to-object distance and direction. (C) An example of a typical Vista Space for humans (top) and rats (bottom). Vista space refers to the space that is visible from a single location with little or no movement (Wolbers and Wiener, 2014). Both the person standing in the corner of the open soccer stadium and the rat performing the Morris Water Maze task have nearly full visual access to their surroundings. (D) An example of a typical Environmental Space for humans (top) and rats (bottom). Environmental space refers to large-scale spaces that require substantial movement or exploration to be sampled and comprehended. For humans city environments such as the example shown (top), or complex mazes with multiple hallways qualify as environmental spaces. In animal studies of navigation, multicompartment environments, such as the Hairpin Maze shown (bottom), would qualify as an environmental space. (E) The visual field of view is shown for an observer within an example environmental space. The pink shaded region represents the visual horizon for the observer. For any location within an environmental space the visual horizon is limited, requiring the navigator to move through multiple connected (vista) spaces and to integrate information over extended space and time to create a complete representation of that space. Furthermore, target locations may lie outside the sensory horizon, such as the bus stop in in the example shown, requiring the planning of more complex routes with multiple decision points.
Secondly, the mammalian brain uses self-motion and environmental cues to build up a variety of spatial representations. The first window into such representations came from O’Keefe and Dostrovsky’s observation (1971) that the firing properties of rodent hippocampal principal cells are tuned to the region of space an animal is in. These place cells inspired ideas about how space provides a scaffold for integrating multimodal features of a given experience or episode. Cells with similar properties have subsequently been found in a number of mammalian species including mice (e.g., McHugh et al., 1996), bats (e.g., Ulanovsky and Moss, 2007), humans (e.g., Ekstrom et al., 2003) and non-human primates (e.g., Feigenbaum and Rolls, 1991). O’Keefe and Nadel (1978) went on to describe how space and navigation through it anchors our daily experiences within the hippocampus to create a cognitive map. Since this time, a number of other functionally classified spatially modulated cell types have been identified including grid cells, head direction cells, border cells and speed cells. All are thought to contribute to the computations required for successful navigation and the generation of an integrated cognitive map. While the firing of all these cell subtypes is likely influenced by both self-motion and by environmental cues, each may utilize these cues differently to perform distinct computational functions. For environmental cues, both distal landmarks and environmental boundaries (e.g., walls) are important. Distance and direction to boundaries are signaled by border cells, which have been found in entorhinal cortex, subiculum, pre- and parasubiculum (Boccara et al., 2010; Lever et al., 2009; Solstad et al., 2008).
For self-motion cues, body orientation and linear and angular velocity are important. Head direction cells signal the orientation of an organism’s head in the horizontal plane. These cells were first observed in the dorsal presubiculum and later in a network of structures including thalamic nuclei, mammillary bodies, parasubiculum, retrosplenial and entorhinal cortices (Taube, 2007). Neuroimaging studies have provided corresponding evidence for head direction coding in humans (Shine et al., 2016). Similarly, speed cells, recently discovered in the hippocampus and medial entorhinal cortex, vary their firing rate based on an animal’s running speed (Kropff et al., 2015). Grid cells have been found in entorhinal cortex, subiculum, pre- and parasubiculum in several species, including humans (Hafting et al., 2008; Jacobs et al., 2013; Killian et al., 2012). These cells fire in multiple locations in the environment to form a repeating hexagonal grid-like pattern. Various features of the grid field are preserved regardless of what environment the animal occupies (Fyhn et al., 2007), and these cells often also show heading and velocity modulation (Kropff et al., 2015; Sargolini et al., 2006). Together, speed and head direction cells may support a path integration function whereby an animal’s current position can be continually updated – on the basis of self-motion cues through the integration of distance and heading information – and fed forward to the grid cell network (Kropff et al., 2015). Importantly, most behavioral paradigms used to study path integration tasks also require an estimate of a vector towards a goal location (i.e., the origin of a journey), which has been linked to hippocampal/entorhinal and parietal computations (Chadwick et al., 2015; Sarel et al., 2017; Wolbers et al., 2007). Moreover, the function of head direction (Taube et al., 1990) and grid cells (Chen et al., 2016; Hafting et al., 2005; Pérez-Escobar et al., 2016) is still dependent on environmental cues, which serve to align their internal representation to an allocentric reference frame and to correct for path integration errors that accumulate over time and space (McNaughton et al., 1996, 2006; O’Keefe, 1976).
Finally, it is important to note that humans and other animals face a variety of navigation tasks on a daily basis. The navigation strategies and the neural computations employed to solve these tasks critically depend on the scale of the environment (Figure 1C–E), the availability of environmental cues and the agent’s knowledge of the space, the location of the destination, and the path to the destination (Wiener et al., 2009; Wolbers and Wiener, 2014). Multiple terms have evolved to describe navigation strategies (e.g., egocentric versus allocentric vs. beacon; non-spatial versus spatial; response versus place; route versus survey; objects versus geometry; language-based code versus visual imagery), and these can be sources of confusion as different researchers may use different nomenclature to describe the same cognitive process. In the present review, we rely on Tolman’s description of an allocentric or place strategy (Tolman, 1948), which utilizes the configuration of environmental cues to determine position, and is thought to be mediated by temporal lobe structures, contrasted with the route strategy which involves learning habitual routes. Route knowledge consists of associations between local views of an environment and associated movements (e.g., turn right at the bakery), which is critically dependent on striatal structures such as the caudate nucleus (Hartley et al., 2003). Despite its egocentric, procedural nature, however, there is evidence that route knowledge may also depend on hippocampal computations, for example information about the temporal order in which landmarks have been encountered (Rondi-Reig et al., 2006). Importantly, because strategies can be considered dynamic interactions between the characteristics of the spatial environment/task and of the individual, it is difficult to design a task that is inherently egocentric or allocentric without taking into account the behavior and cognitive processes of the navigator (Ekstrom et al., 2014). Older humans and other animals will, for example, differ from younger individuals in strategic approaches in solving even the simplest two choice T-maze or Y-maze (Barnes et al., 1980; Rodgers et al., 2012). Conclusive evidence of the strategy utilized typically requires post-test manipulation or testing to discern strategy preferences. Nevertheless, some tasks may lend themselves to allocentric/egocentric strategies such that the adoption of one strategy over the other will more readily result in success.
To conclude, successful everyday navigation is a particularly complex behavior relying on a range of perceptual, mnemonic and executive computations. It requires the integration of different types of spatial information, the selection of the appropriate navigation strategy and, if circumstances change, switching between strategies. Moreover, navigation typically involves coordinating a number of different sub-tasks or processes. Given this complexity, it is unlikely that age-related declines in navigation abilities can solely be explained by general declines in learning and memory. Rather, such deficits can result from a multitude of mechanisms, which will be discussed in the following sections.
3. How does aging affect our ability to compute online spatial information?
We begin our review of age-related changes in spatial navigation by discussing the processes involved in computing online or transient representations of spatial information. Even though older adults are known to experience various changes in visual perception (e.g., visual acuity; contrast sensitivity), perceiving the position of static external objects is unimpaired. For example, older adults outperform younger participants in the perception of egocentric distances (4–12m) in an outdoor environment, because they show less compression of space at longer distances (Bian and Andersen, 2013). These results are in line with other studies showing intact perception of surface slant in old age (Norman et al., 2009), which is an important cue for distance perception. Similarly, other studies found no impairment when older participants estimated the distance between two external objects (Norman et al., 2015), demonstrating that neither egocentric nor allocentric distance perception are significantly affected by age.
In contrast to the preserved perception of static spatial relationships, older adults exhibit subtle deficits in perceiving the direction and speed of self-motion. For example, Warren and colleagues (Warren et al., 1989) found a small but significant age-related decline of about 1–2° in the ability to perceive heading direction, and subsequent studies have reported similar results with a range of optic flow stimuli (Kavcic et al., 2011; Lich and Bremmer, 2014). Importantly, such deficits are also observed when self-motion is computed from body-based cues and used to regulate walking speed (Lalonde-Parsi and Lamontagne, 2015; Roditi and Crane, 2012). Note, however, that self-motion perception in everyday navigation is normally based on body-based and visual cues simultaneously, and older adults appear to at least partially compensate for the unisensory deficits by recalibrating the relative weights given to each cue type (Bates and Wolbers, 2014; Lalonde-Parsi and Lamontagne, 2015).
Age-related deficits in self-motion perception have been linked to several mechanisms. First, neuron loss or reduced GABAergic inhibition in areas VIP and MT/MST (Lich and Bremmer, 2014) increase the level of noise and decrease the directional tuning of motion sensitive neurons (Liang et al., 2010). Such deficits can be further enhanced by altered eye movement behavior (Dowiasch et al., 2015), which changes the way older adults sample the outside world. Secondly, epidemiological, physiological and histopathological studies have shown that vestibular function declines with age (Anson and Jeka, 2015). While the vestibular system is known for its role in maintaining balance and postural control, increasing evidence demonstrates important connections between the vestibular system and spatial navigation in humans (Brandt et al., 2005) as previously reported in rodents (Potegal et al., 1977). In rodent model studies, disruption of vestibular inputs results in reduced place cell spatial tuning (Stackman et al., 2002) and a complete loss of directional tuning of anterior thalamic head direction cells (Stackman and Taube, 1997). In addition, areas VIP and MT/MST also receive vestibular input, hence vestibular damage could also alter the computation of self-motion speed and direction in cortical structures.
Even though age-related deficits in space perception appear to be absent for static cues and relatively subtle for self-motion cues, older adults often show striking difficulties in path integration tasks). For example, after moving about in an unfamiliar environment, they underestimate travelled distances and turns, and are less accurate when returning to the start of a journey. This has been shown when navigation is based either on visual or non-visual cues (Adamo et al., 2012; Allen et al., 2004; Harris and Wolbers, 2012; Mahmood et al., 2009). Given that both self-motion and environmental cues are used to compute position and orientation in all the spatially-modulated cells described above, the particular vulnerability of the hippocampus and other medial temporal lobe structures to the deleterious consequences of aging (Barnes et al., 1997; Burke and Barnes, 2006; Lister and Barnes, 2009; Stranahan and Mattson, 2010) could result in compromised computations of position and orientation. In fact, even though there is mixed evidence regarding an association between age-related changes in hippocampal volume and memory function (van Petten, 2004), several studies have reported changes in place cell firing characteristics. For example, old rats show differences in the stability of their cognitive maps as assessed by ensemble recordings of hippocampal neurons (Barnes et al., 1997; Schimanski et al., 2013). CA1 hippocampal pyramidal cells in aged rats occasionally generate a new and distinct place cell representation for a familiar environment (Barnes et al., 1997; Schimanski et al., 2013), and these cells take significantly longer to begin to show consistent spatial firing, even in familiar environments (Hok et al., 2012).
Changes in spatial working memory are a further likely source of impaired positional coding in old age. For example, older adults are impaired when revisiting a location that was memorized relative to external landmarks (Bates and Wolbers, 2014), which is consistent with rodent studies employing working memory versions of the Morris Water Maze (Bizon et al., 2009; Frick et al., 1995). Given the intact perception of spatial relationships (see above), these findings demonstrate that adding a working memory component – i.e., having to remember a location from an outbound trajectory – leads to substantial performance drops in older adults. Such deficits in navigational working memory are not related to changes in long-term memory (Bizon et al., 2009; Laczó et al., 2017), arguing against a role of hippocampal dysfunction. Rather, numerous studies have established medial prefrontal cortex as a key structure for monitoring hippocampal output and for using stored location information to guide navigational behavior (Jones and Wilson, 2005; Wolbers et al., 2007). Neurons in medial PFC undergo several changes in the course of normal aging, including a loss of NMDA receptors and increased inhibition of pyramidal cells due to GABAergic changes (Carpenter et al., 2016; McQuail et al., 2016). As a consequence, compromised computations in medial prefrontal circuits can cause deficits in maintaining information about position, and navigational goals in working memory, thus contributing to the navigational errors observed in both rodent and human studies.
Finally, changes in vestibular processing can affect the coding for position and orientation in old age. Age-related degeneration of the vestibular system (i.e., progressive neuronal loss) is a multifactorial process, affecting the peripheral end-organs, the brainstem, the cerebellum and the cerebral cortex (Arshad and Seemungal, 2016). Age-related neuronal loss in the vestibular nucleus has been reported in both humans (Lopez et al., 1997) and mice (Sturrock, 1989). The vestibular nuclei of aged rats also show a number of abnormal structural changes (Johnson and Miquel, 1974) as well as reduced levels of glutamate which could indicate a reduction in excitatory afferent drive (Liu et al., 2010). Given that vestibular signals convey information about translation and rotation of the body, age-related changes of vestibular functioning will render self-motion perception imprecise. This could explain why, for example, older adults are impaired when they are transported in a wheelchair and have to return to the starting location at the end of an outbound journey (Adamo et al., 2012; Allen et al., 2004). Importantly, such deficits are attenuated when the outbound path is experienced via active walking (Adamo et al., 2012; Allen et al., 2004), showing that motor efference copy and/or proprioceptive feedback can at least partially compensate for impaired vestibular processing. Furthermore, vestibular damage can induce pathology in downstream structures such as the hippocampus (Brandt et al., 2005), thus impacting the precision of positional computations in the place and grid cell systems. Finally, vestibular changes often induce problems with balance and postural control, which makes avoiding obstacles more difficult and increases the risk of falling. To counteract such deficits, older adults often employ more conservative adaptation strategies by taking slow, short and more frequent steps (Caetano et al., 2016) and by prioritizing walking control over concurrent cognitive tasks (Simieli et al., 2015). As a consequence, a reduced amount of attentional resources can be allocated to keeping track of both self-motion and environmental cues, ultimately resulting in reduced navigation performance.
4. How does aging affect our ability to create, consolidate and retrieve memory traces of spatial information?
Beyond the deficits with creating online representations of spatial information, older adults have difficulties with turning such representations into enduring long-term memory traces and with retrieving them when necessary. For example, in large environmental-scale spaces which require substantial movement to be fully sampled and comprehended (Figure 1D, E; Wolbers & Wiener, 2014), advanced age is often associated with substantial problems in learning the position of distal targets located beyond the current sensory horizon (Head and Isom, 2010; Lövden et al., 2012). Similarly,, studies using a virtual analog of the Morris Water Maze, a vista-scale space that is visible in its entirety from a single viewpoint with little or no movement (Figure 1C; Wolbers & Wiener, 2014), have shown that older adults generally show slower learning rates (Daugherty et al., 2015), are less accurate in locating the hidden platform and are less able to reproduce a map of the environment (Moffat and Resnick, 2002). Similar effects have been observed in studies with aged rodents (Gage et al., 1984), and the mechanisms underlying such deficits will be discussed in this section.
Place learning and cognitive mapping
In human navigation, the role of the hippocampus and associated medial-temporal lobe structures has been of considerable interest, in part because of the findings from animal models of spatial navigation noted above. Place learning and cognitive mapping have been shown to critically rely on hippocampus and retrosplenial computations (Goodrich-Hunsaker et al., 2010; Hartley et al., 2003; Kolarik et al., 2016; Morris et al., 1982; Wolbers and Büchel, 2005), hence, in human aging there has been great interest in the role of these structures in mediating age-related navigation impairments. For example, three studies have now reported quite consistent results demonstrating reduced or absent hippocampal/parahippocampal and retrosplenial activation (Figure 2B) in older as compared to younger adults during virtual navigation (Antonova et al., 2009; Meulenbroek et al., 2004; Moffat et al., 2006), despite quite different task demands across studies. This may suggest a lack of hippocampal recruitment or switching to extra-hippocampal strategies (see section 5 below) during spatial navigation in older participants, but could also result from sub-threshold activations or possibly from sparse coding (activations in a relatively smaller set of neurons) in older participants.
Figure 2. Correspondence of age-related navigational deficits in rodents and humans: Cognitive mapping.
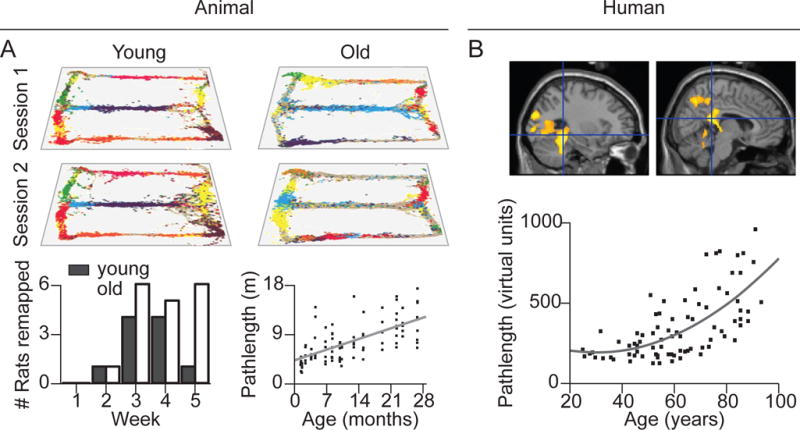
(A) Aged rats periodically generate new and distinct cognitive maps after repeated exposure to a familiar environment. Place field distributions are shown for one young and one old rat recorded over two consecutive episodes of running on a figure-8 maze (Barnes et al., 1997). Individual place cells are denoted with colored points. Place-field maps of young animals are highly correlated between consecutive exposures while aged animals occasionally showed uncorrelated firing, indicative of remapping. When the proportion of rats that remapped between morning and afternoon sessions was tracked over 31 days (Schimanski et al., 2013), maps were stable between sessions until day 14, when both age groups began to show periodic remapping episodes, with aged animals remapping more frequently. Aged rats also show reduced spatial navigation accuracy in the Morris Water Maze task (Lindner, 1997) (bottom right, shows performance for rats from age 1.3 to 26.3 months old). (B) In a virtual Morris Water Maze task, there was a non-linear relationship between age and total distance traveled in the virtual environment (Moffat and Resnick, 2002) in humans. As with a number of rodent aging studies (e.g., A, bottom right), older adults took a longer time and traversed a greater linear distance in locating the hidden platform compared to younger adults (bottom). In a neuroimaging study (top), older adults showed reduced activation in the hippocampus and parahippocampal gyrus and in the retrosplenial cortex compared to younger participants (Moffat et al., 2006).
One key mechanism for storing spatial memories in the hippocampus is long-term potentiation (LTP), a sustained increase in synaptic strength (Bliss and Lomo, 1973). Donald Hebb first postulated that memories may be stored within a neural network, or cell assembly, through the selective modification of synapses between co-activated neurons (Hebb, 1949), and David Marr developed a theoretical model suggesting that the anatomical configuration of the hippocampus is well-suited to perform a rapid mnemonic function (Marr, 1971). The first empirical validation of these theories was accomplished by delivering patterned electrical stimulation to axons afferent to dentate gyrus granule cells, which resulted in strengthened synaptic weights (Bliss and Gardner-Medwin, 1973; Bliss and Lomo, 1973). It is now well established that defects in plasticity mechanisms underlying LTP in hippocampal and other circuits across the brain result in learning and memory deficits.
Aged animals undergo a number of changes in synaptic communication and plasticity regulation (Figure 3). We focus here on changes in transmitter systems and intracellular signaling pathways within the hippocampus as they relate to LTP and long-term depression (LTD). Acetycholine is an important neuromodulator for memory encoding and has been shown to facilitate hippocampal LTP (Huerta and Lisman, 1995). In aged rats, cholinergic drive from the medial septum is reduced by approximately half in all three primary areas of the hippocampus, including the dentate gyrus, CA3 and CA1 (Shen and Barnes, 1996). The catecholamine transmitter systems have also been found to modify LTP in the hippocampus. Age-related spatial memory deficits have been linked to hippocampal dopamine D1 receptor signaling deficits (Hersi et al., 1995), altered serotonergic 5HT receptors within the hippocampus (Gozlan et al., 1990) and altered adrenergic receptor density and binding in the hippocampus (Topic et al., 2007). Glutamate is the predominant excitatory neurotransmitter in the mammalian nervous system. There are alterations in both AMPA and NMDA receptor function in the aging rodent (Sonntag et al., 2000; Yang et al., 2008) and nonhuman primate (Gazzaley et al., 1996). Additionally, glutamate binding to metabotropic glutamate receptors can facilitate the release of calcium through their action on inositol trisphosphate receptors. Memory-impaired aged rats also show reduced expression levels of postsynaptic metabotropic glutamate receptor 5 in CA1, accompanied by a loss in related downstream signaling molecules involved in LTP maintenance (Ménard and Quirion, 2012). Memory-impaired aged rats also have significantly smaller postsynaptic density areas in CA1 perforated synapses, suggesting some of these synapses are not functional during aging (Nicholson et al., 2004). Finally, aged rats show elevated voltage-dependent calcium channel mediated LTP (Shankar et al., 1998) and a higher density of L-type calcium channels (Thibault and Landfield, 1996). This overall disruption of calcium homeostasis may actually shift the threshold for synaptic modification and bias aged synapses towards LTD and away from LTP (Foster and Norris, 1997; Norris et al., 1996).
Figure 3. Neurophysiological changes with age.
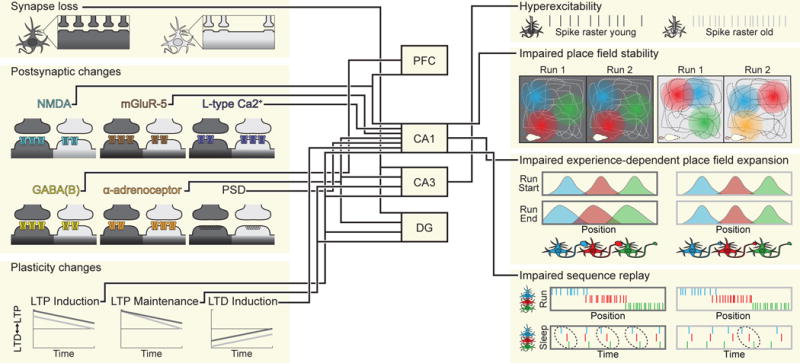
Graphical overview of the major age-related neurophysiological changes discussed in the text. Examples from young animals are indicated in dark gray and those from age animals are indicated with light gray. NMDA, N-methyl-D-aspartate; mGluR-5, metabotropic glutamate receptor 5; GABA(B), gamma-amino butyric acid receptor B-type; PSD, postsynaptic density; LTP, long- term potentiation; LTD, long- term depression.
Assessing the functional properties of synapses is one way to examine how spatial memory is altered in the aged nervous system. Older animals show reduced elevations of synaptic strength following tetanic stimulation-induced frequency potentiation (Landfield et al., 1978). This reduced synaptic depolarization likely results from a defect in synaptic integration of coincident inputs in old animals (Rosenzweig et al., 1997) and may contribute to degraded capacity to induce a synaptic weight change. When high frequency and high amplitude stimulation protocols were used that resulted in LTP induction, no age-related deficit was evident in the ability to modify synaptic strength (Barnes, 1979; Dieguez and Barea-Rodriguez, 2004; Landfield et al., 1978). Weaker stimulation protocols, however, unmasked reliable LTP induction deficits in aged animals (Moore et al., 1993; Rosenzweig et al., 1997). Using the same robust stimulus protocols in which no LTP induction differences were observed, aged animals do show a more rapid decay of LTP (Barnes, 1979; Dieguez and Barea-Rodriguez, 2004), and this LTP maintenance deficit is significantly correlated with spatial memory accuracy (Barnes, 1979). In contrast, when stimulation protocols that induce LTD (Lynch et al., 1977) are applied, older rats actually show more robust depression than do young rats (Norris et al., 1996). This suggests that the bidirectionality of synaptic modification is compromised in the aged brain – i.e., it is more difficult to increase synaptic strength and easier to decrease synaptic strength in older animals. Both of these mechanisms could, at least in part, account for the observations that older animals show faster forgetting of a spatial memory task (Barnes and McNaughton, 1985). Additionally, one particular NMDA-dependent form of plasticity, which is known to be impaired in aged animals, is experience-dependent place field expansion plasticity (Ekstrom et al., 2001, Shen et al., 1997; Figure 3). Normally, even in familiar environments, the size of a CA1 place field is smaller on initial exposure to the environment on a given day. As the rat makes repeated traversals of a route, the place field becomes larger, and shifts backwards from the direction of movement. For aged rats this phenomenon is attenuated. Because NMDA receptor blockade impairs both stimulation-induced LTP (Collingridge et al., 1983) and experience-dependent place field expansion plasticity in young rats, dysregulation of NMDA receptor function likely plays a key role in age-related plasticity and spatial navigation deficits (Figure 3).
One potential contribution to the LTP deficits discussed above is the observation that granule cells in aged rats have a quarter fewer synaptic contacts from layer II medial entorhinal cortex (MEC) (Geinisman et al., 1992; Figure 3). The same MEC cells also project to the lacunosum moleculare layer of CA3, and older rats with spatial learning deficits show decreased levels of the presynaptic marker synaptophysin, suggesting reduced synaptic input in CA3 as well (Smith et al., 2000). Because there is no loss of layer II MEC projection cells in old rats (Merrill et al., 2001), axons from these cells are thought to undergo pruning of their extensive collateral projections. In support of this model, the extracellularly recorded presynaptic fiber potential is smaller in old rats (Barnes and McNaughton, 1980). In addition, the size of the extracellularly recorded EPSP is smaller in old animals (Barnes, 1979; Barnes and McNaughton, 1980; Yang et al., 2008) for equivalent stimulation strengths, which is consistent with reduced synaptic input. In line with the rodent data suggesting axon pruning, older, memory-impaired human participants show reduced white matter volume in the vicinity of the perforant pathway (Rogalski et al., 2012). The dentate gyrus with its disproportionately large population of granule cells receives minimally overlapping projections from the entorhinal cortex that could serve to expand the representational space in downstream CA3 principal cells (Marr, 1971). Specifically, this process of pattern separation decorrelates cortical inputs in order to minimize the overlap between neurons recruited to encode successive memory traces. The drastic reduction in perforant path synapses observed in aged rats could, therefore, contribute to changes in pattern separation mechanisms. Consistent with this idea, aged humans with degraded perforant path integrity as assessed through DTI imaging (Yassa et al., 2011a), and a patient with dentate gyrus damage show poorer performance on discrimination tasks used to assess pattern separation (Baker et al., 2016).
In addition to deficits with forming spatial representations, inefficient retrieval from long-term memory can also contribute to a decline of navigational abilities (Head and Isom, 2010; Iaria et al., 2009). One specific mechanism by which spatial/mnemonic representations may be reinstantiated in the hippocampus is pattern completion (Marr, 1971; McNaughton and Morris, 1987). Pattern completion is achieved when recall of a complete representation is accomplished in the presence of partial or degraded input. The anatomical implementation of this process involves the autoassociative connections of CA3 principal cells. These connections functionally couple coactive cells at the time of encoding so that later activation of a sufficient subset will propagate to all members of the cell assembly resulting in a complete reinstantiation of the learned pattern. As mentioned above, old rats show distinct differences in the stability of their cognitive maps as assessed by ensemble recordings of CA1 hippocampal neurons (Barnes et al., 1997; Schimanski et al., 2013). Aged rats occasionally generate a new and distinct place cell representations for a familiar environment (Barnes et al., 1997; Schimanski et al., 2013) – that is, they occasionally retrieve ‘the wrong map’ (Figure 2A; Figure 3). The inability to represent an identical environment as the same place may indicate excessive pattern separation in the aged CA1 region. In CA3, the opposite change occurs – when old rats are exposed to distinct environments, they often retrieve the same map for these multiple places – this may suggest excessive pattern completion mechanisms in this structure (Wilson et al., 2006). There is evidence of hyperactivity (Figure 3) of CA3 cells in older rats (Wilson et al., 2005) and non-human primates (Thomé et al., 2016), as well as increased CA3 and dentate gyrus activity linked to pattern separation/completion deficits in aged humans (Yassa et al., 2011a). Given that the auto-associative connections in stratum radiatum of the CA3 network are unaffected by age (Smith et al., 2000), the hyperactivity of CA3 principal cells could bias this auto-associative network to incorrectly re-instantiate a stored memory trace.
Finally, aged rats show a decline in cholinergic modulation, which might reduce the influence of new information arriving through the perforant pathway (Hasselmo et al., 1995). Collectively, these changes would result in the network favoring the reactivation of stored cognitive maps, particularly under conditions in which new stimuli have a high degree of feature overlap with learned stimuli. On the behavioral level, corresponding data have been reported in older adults, who show increased pattern completion in situations when only partial cues of an environment can be seen (Head and Isom, 2010; Liu et al., 2011). Using a scene recognition task, Vieweg et al. (2015) observed a bias in older adults towards falsely classifying novel scenes as familiar ones, in particular when the scenes were partially masked. This bias could not be explained by an overall tendency to produce more false alarms, suggesting that new partial information triggered the recognition of learned items. Together with fMRI studies pointing to a reduced ability of the aging hippocampus to distinguish between similar stimuli (Yassa et al., 2011a, 2011b), these data support the rodent literature in terms of a heightened, age-related tendency for pattern completion in CA3 (Wilson et al., 2005).
Response-learning and route-based learning
In contrast to the wealth of findings on age-related deficits in cognitive mapping or place-based navigation, fewer studies have investigated how aging affects route navigation and response-based navigation. Aged rats and primates preferentially use response-based strategies over place-based strategies (Barnes et al., 1980; Rapp et al., 1997; Rodgers et al., 2012). Nevertheless, route learning deficits are detected in older animals when using multi-compartment mazes, but can be partially ameliorated by cholinergic treatment in both rats and mice (Ingram, 1988; Pistell et al., 2012). In humans, arguably the most frequently used navigation task is route navigation, and several studies have demonstrated declines in route learning in older adults (Head and Isom, 2010; Moffat et al., 2001; Wiener et al., 2012; Wilkniss et al., 1997). Successful route learning depends on a number of processes and mechanisms, most of which are affected by aging. Specifically, route navigation requires the recognition of landmarks and places encountered during learning, knowledge about the sequence in which places/landmarks were encountered, the selection of landmarks that are navigationally relevant (i.e., those at decision points), and typically also the association of directional information with these landmarks. Moreover, successful navigation often requires one to find their way back, that is, to retrace a recently travelled path. Aging has been associated with less accurate binding of directional knowledge to landmarks (Head and Isom, 2010; Liu et al., 2011; Wiener et al., 2012; Zhong and Moffat, 2016) and impaired memory of the sequence in which landmarks were encountered (Head and Isom, 2010; Wiener et al., 2012; Wilkniss et al., 1997).
One mechanism that has already been discussed and could contribute to age-dependent sequence learning deficits is experience-dependent place field expansion plasticity. As described previously, this plasticity mechanism increases the size of place fields in the direction opposite to the animal’s trajectory of movement, which could provide a means of encoding sequences within cell assemblies (Blum and Abbott, 1996). Specifically, when place fields enlarge, the fields adjacent to each other will overlap to a greater degree following this expansion. The resultant longer periods of concurrent firing lead to synaptic weight changes favoring activation of cells in sequential order, which may act to more effectively link ‘places’ together (Figure 3). Older rats show deficits in this plasticity mechanism which could result in less effective storage of spatial sequences. This idea is also consistent with observations in aged human navigation behavior that shows older adults require longer acquisition periods to reduce their search path length in a virtual Morris Water test (Daugherty et al., 2016).
Finally, older adults are also more likely to point out salient landmarks than turns as providing the most useful information when learning novel routes (Lipman, 1991), and their ability to retrace a recently travelled route is impaired as compared to younger participants (Liu et al., 2011; Wiener et al., 2012). In terms of the underlying neural mechanisms, route learning and route navigation has been associated with caudate activation (Hartley et al., 2003) and caudate volume (Head and Isom, 2010). Furthermore, the caudate shows age-related neurodegenerative changes (Betts et al., 2016) at similar rates to the hippocampus, which may explain aging-related declines in route navigation performance. In contrast to deficits with sequence learning and with associating directional information with landmarks, the ability to freely recall or recognize objects/landmarks encountered along a route is comparatively spared in older adults (Cushman et al., 2008; Head and Isom, 2010; Zhong and Moffat, 2016). This demonstrates that aging-related declines in route learning performance do not exclusively result from general memory decline.
Consolidation of spatial memories
Newly encoded spatial memories are typically fragile and, because they may decay, require additional maintenance processes. The concept of memory consolidation, originally proposed by Muller and Pilzecker (Müller and Pilzecker, 1900), refers, in the broadest sense, to the process by which memories are stabilized over time. At the network level, one such process is the offline reactivation of assembly firing patterns that occurred during previous active behavior. The recurrent collaterals of CA3 have long been thought to subserve an auto-associative function in the hippocampus, allowing for spontaneous replay of memories even in the absence of external input (Marr, 1971). As previously mentioned, place fields with spatially-adjacent firing fields undergo an associative, temporally-asymmetric synaptic weight change that bias these cells toward firing in a specific sequence. These sequences are preserved even when an animal is not actively exploring an environment, such that place cells which are activated sequentially during waking exploration will be more likely to fire in the same sequence during off-line periods such as sleep and wakeful resting (Figure 3). Such replay events could help strengthen a given spatial representation within the hippocampus as well as coordinate the reactivation of multiple neocortical networks in order to consolidate new long-term memory traces (Wilson and McNaughton, 1994). Reactivation of ensemble activity predominantly occurs during episodes of sharp wave ripple activity, which are hypothesized to coordinate the hippocampal-cortical transfer of memories (Buzsáki, 1996). While cell pair reactivation in CA1 ensembles is equivalent in young and old rats (Gerrard et al., 2001), the temporal sequence of cell pair firing is disrupted in aging animals and is correlated with the fidelity of spatial memory (Gerrard et al., 2008; Figure 3). This age-related sequence reactivation deficit may be linked to changes in behaviorally induced place field expansion plasticity that is implicated in sequence encoding (Mehta et al., 2000; Shen et al., 1997). This is also consistent with observed deficits in sequence learning (Oler and Markus, 2000), and may account for impairments in memory consolidation more generally. Sleep-dependent memory consolidation is also diminished in older humans (Spencer et al., 2007), but whether this deficit also affects the consolidation of spatial memories is unknown at present.
More recently, it has been found that reactivation also occurs during waking periods (Foster and Wilson, 2006). This waking replay is implicated in memory consolidation (Jadhav et al., 2012), real time action selection (Johnson and Redish, 2007) and generation of novel un-experienced paths during spatial exploration (Gupta et al., 2010). It remains to be determined if aging also results in compromised waking replay, and if so, what the implications might be for spatial memory. Evidence from the human literature suggest spatial memory for landmark locations in a virtual navigation task is poorer in older compared with younger adults, but both age groups improved to a similar degree following rest periods that might foster waking replay. This suggests that waking replay may be similarly efficient in older and younger adults (Craig et al., 2016).
5. How does aging change the use of spatial information during navigational behavior?
Successful navigation not only requires the formation of spatial representations and their retrieval from long-term memory, it often involves the computation of novel information, recombining information, or switching between different strategies or representations. For example, planning a novel route requires both the retrieval of cognitive maps from long-term memory as well as the computation of novel and viable path options from the current position to the destination. Aging humans are less efficient in using cognitive map-like knowledge for such route planning tasks, even if they have first successfully learned an environment (Harris and Wolbers, 2014; Iaria et al., 2009; Liu et al., 2011).
With respect to navigation strategies, aged rodents not only show more severe performance deficits in allocentric than in egocentric navigation tasks (Barnes et al., 1980), but they also exhibit spontaneous preferences for egocentric response strategies (Figure 4A) after being successfully trained on both allocentric and egocentric strategies (Barnes et al., 1980; Nicolle et al., 2003). This strategy bias is also reflected in the firing behavior of place cells. For example, when the starting position on a linear track is systematically varied with respect to the distance from the end of the track, CA1 place cells of both old and young rats tend to dynamically shift from being anchored to the starting location on the track (egocentric reference frame; Figure 1B), to aligning with the external room cues (Rosenzweig et al., 2003) (allocentric reference frame; Figure 1A). In older rats, this reference shift is delayed, suggesting the network itself is biased toward maintaining an egocentric reference frame (Rosenzweig et al., 2003). Similar to rodents, aged humans have more difficulties in allocentric than in egocentric navigation tasks (Head and Isom, 2010; Iaria et al., 2009; Moffat et al., 2007) and accordingly show preferences for egocentric navigation strategies (Figure 4B; Rodgers et al., 2012) even if these strategies are maladaptive and result in navigational errors (Figure 4C; Wiener et al., 2013). Shifts in navigation strategy use and preferences, away from allocentric strategies towards egocentric strategies, are accompanied by the use of different environmental cues during navigation. In the virtual navigation Morris Water Maze task, for example, older adults tend to rely more on proximal cues rather than distal cues or boundaries (Moffat and Resnick, 2002; Schuck et al., 2013). Thus, age-related shifts in preferences for navigation strategies are now well established for a number of species including humans.
Figure 4. Correspondence of age-related navigational deficits in rodents and humans: Strategy preferences.
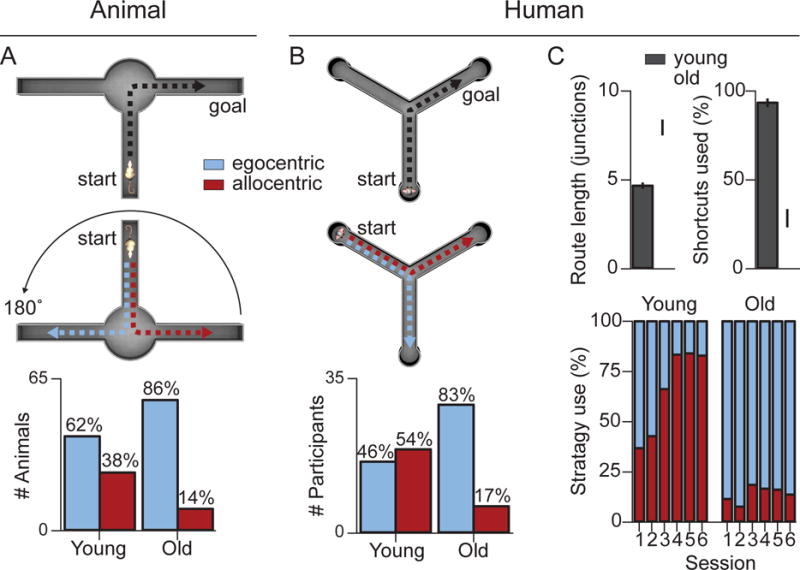
(A) In the T-maze task, an egocentric strategy was coded when a rat ‘turned right’ following 180° rotation of the start location and an allocentric strategy was coded when an animal moved to the same learned goal location relative to the external cues. Older rats overwhelmingly revealed an egocentric strategy on the probe trials (Barnes et al., 1980). (B) In the virtual Y-maze task, an egocentric strategy was coded when a human participant ‘turned right’ following displacement to a new starting location and an allocentric strategy was coded when the participant moved to the same learned goal location in absolute space (Rodgers et al., 2012). As with the rodent study (A), older adults spontaneously chose an egocentric strategy over an allocentric strategy compared to younger adults. (C) Similar results are observed in an environmental space task (top), in which older adults were impaired at switching from a learned route to a more optimal allocentric strategy, leading to increased route lengths and a reduced use of shortcuts (Harris and Wolbers, 2014). Critically, in a task that distinguishes between allocentric and two egocentric (beacon/associative cue, not shown) strategies (bottom), older adults remain biased toward using an egocentric navigation strategy (Wiener et al., 2013). These results suggest that egocentric strategy preferences are difficult to overcome for older adults, even when they are maladaptive and lead to suboptimal task performance.
In many situations, successful navigation also requires switching between different navigation strategies or frames of reference, for example when the available cues or task demands change. This ability is particularly problematic for aged humans and other animals when required to switch from an egocentric to an allocentric strategy (Figure 4C), whereas switches from allocentric to egocentric strategies are less affected (Harris and Wolbers, 2012, 2014). Age-related deficits with navigational strategy switching are assumed to be a special case of general strategy switching impairments, which are caused by changes in prefrontal and locus coeruleus (LC) functioning. In the LC, noradrenaline (NA) levels increase in response to changes in rewards associated with the current behavioral strategy (Aston-Jones and Cohen, 2005). Depletion of prefrontal NA by lesioning noradrenergic fibers projecting from LC to prefrontal cortex (PFC) or by infusion of a NA receptor antagonist into medial PFC produces deficits in switching between different strategies (Caetano et al., 2012; Tait et al., 2007). Given that aging degrades LC and disrupts NA function (Grudzien et al., 2007; Manaye et al., 1995), older adults can experience problems with detecting when a given behavioral strategy is no longer appropriate and with adjusting their behavior accordingly. Moreover, the frontal aging hypothesis suggests that various aspects of age-related cognitive decline may be attributable to PFC degradation (Pfefferbaum et al., 2005; West, 1996). While there are currently no animal model studies specifically exploring aged PFC function in the context of navigation strategy switching, both aged monkeys (Gray et al., 2017; Rapp, 1990) and rats (Beas et al., 2017) show reliable impairments on tasks assessing set shifting. Like navigation strategy switching, these set shifting paradigms require an animal to modify their behavior in response to changing task demands and are PFC dependent. Rodent studies show that these impairments are directly linked to decreased GABA(B) receptor expression in medial PFC (Beas et al., 2017; Figure 3), but may also be a result of age-related decreases in PFC gray matter volume that is present in rats (Alexander et al., in press), non-human primates (Alexander et al., 2008) and humans (Storsve et al., 2014).
6. Clinical potential of spatial navigation
In the previous sections, the behavioral manifestations and putative neural mechanisms of spatial navigation deficits have been reviewed with a focus on normal aging – that is, cognitive and brain aging that occurs in the absence of diagnosed neuropathology. Both neuroscientists and the lay public are intensely interested in pathological aging, with Alzheimer’s disease (AD) being the second most feared disease of aging worldwide (Alzheimer’s Association Worldwide Survey, 2014). Behaviorally, AD manifests as a progressive cognitive decline often beginning with memory and progressing to other cognitive and perceptual domains as the disease advances (Weintraub et al., 2012). A common, though understudied aspect of AD is that of topographical disorientation. Even very early AD patients may become disoriented in their environment, a phenomenon that is more colloquially referred to as ‘getting lost’ or ‘wandering’.
The early emergence of topographical disorientation in AD would be expected from the overlap of neural mechanisms of spatial computation with AD pathology, which prominently affects the perirhinal and entorhinal cortex, the hippocampus (Braak and Braak, 1995) and the retrosplenial cortex (Pengas et al., 2010). Motivated by this observation and also by the prospect of early detection and early intervention, there has been considerable recent interest in studying spatial navigation deficits in AD and in mild cognitive impairment (MCI), a condition hypothesized to be prodromal to AD. Serino et al. (2014) reviewed the extant literature and reported substantial navigation impairments in AD and MCI in tasks that emphasized both allocentric and egocentric processing. Interestingly, one study showed relatively specific deficits in translating an allocentric reference frame to an egocentric reference frame (Pai and Yang, 2013), an ability thought to rely on the retrosplenial cortex (Byrne et al., 2007). Based in part on these findings, the earliest deficit in preclinical AD may be in the conversion between allocentric to egocentric coordinate systems (Serino and Riva, 2013). Moreover, deficits in route learning performance in early AD have been associated with hypometabolism in the retrosplenial cortex, thalamus and parietal cortex (Pengas et al., 2012). Mokrisova et al. (2016) showed that deficits in path integration in AD and MCI were mediated in part by changes in hippocampal, entorhinal and parietal cortex volumes. Cumulatively, these findings suggest that changes in the ‘navigation circuit’ may be a predominant and early consequence of AD and manifest as behavioral deficiencies in spatial navigation.
With respect to entorhinal cortex, tau deposition has been found to impair grid cell function and to cause navigation deficits in a transgenic mouse model of AD (Fu et al., 2017). Impaired grid cell-like properties have also been reported in young adults who were carriers of the APOE E4 allele (Kunz et al., 2015), suggesting that physiological changes in grid cell networks may appear very early in adulthood (Figure 5). In addition to the APOE E4 gene, a negative influence of other genetic risk factors for AD on navigation skill has been observed among carriers of the VL variant of the Tomm40 gene (Laczó et al., 2015) and the T polymorphism of the KIBRA gene (Schuck et al., 2013). Given that old, memory-deficient rats show improved memory following pharmacological treatments that manipulate KIBRA activity (Huentelman et al., 2009), KIBRA pathways may be promising therapeutic targets for cognitive enhancement in normative aging, and potentially in attenuating AD-related memory deficits.
Figure 5. Alzheimer’s disease-related grid cell dysfunction in mice and humans.
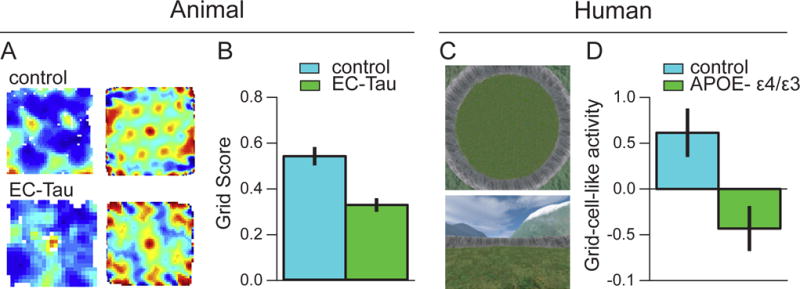
(A) Firing rate map (left panels) and spatial autocorrelograms (right panels) are shown for an example grid cell recorded from a control mouse and a grid cell from an age-matched mouse expressing a human tau mutation (EC-Tau; Fu et al., 2017). (B) The EC-Tau mice who formed mature tangles also had impaired grid cell function at 30+ months of age. (C) Top-down (top panel) and first-person (bottom panel) view of a virtual reality environment used to test memory performance and grid cell function in young controls and young human adults at risk of developing AD (i.e., those carrying the APOE- ε4 allele; Kunz et al., 2015). (D) fMRI was used to assess a correlate of grid cell activity in the entorhinal cortex, and the pattern of activity suggested disrupted grid cell-like representations in the at risk population.
Given that healthy older individuals show impairments in various navigational computations, it is not surprising that those individuals with MCI, early AD or who are at genetic risk for AD show still more profound impairments. A major limitation in the current literature, however, is the dearth of prospective longitudinal studies examining whether navigation assessment in healthy elderly can be used as a predictor of future AD or MCI onset on an individual basis, as this is critical to early diagnosis and intervention. One such study (Laczo et al., 2010) demonstrated that amnestic MCI converters (compared with the nonconverters) showed higher deficits in both ego- and allocentric navigation tasks, suggesting that spatial navigation testing may help predict the conversion to AD among MCI patients.
A third aspect of potential clinical utility of navigation assessment is for purposes of treatment and intervention. In this domain, spatial navigation tasks have been used as both an outcome variable (to assess the efficacy of clinical/pharmacologic intervention) and also as an intervention for possible cognitive/neural training. Pharmacological treatment of early AD with cholinergic agonists to reverse AD-related acetylcholine depletion has been found to enhance navigation performance in early AD (Hort et al., 2014) and to increase navigation-related hippocampal activation in MCI (Grön et al., 2006). In addition, behavioral interventions in the form of cognitive training may enhance cognitive performance in healthy elderly and also delay the onset or ameliorate the symptoms of dementia. The utility of cognitive training to enhance cognitive function is an area of some controversy as it is clear that participants improve on virtually all trained domains, but generalization of this training to other cognitive domains or to real world tasks (i.e., transfer) has been less consistent (Noack et al., 2014). Lövdén et al. (2012) found that navigation training improved navigation performance in younger and older adults and also prevented the age-related hippocampal volume loss that would have occurred over the training interval. In another study of the same participants, increases in cortical thickness in the precuneus and parietal lobe were observed only in younger trained participants, with no effect of navigation training on cortical thickness observed in the older participants (Wenger et al., 2012). The navigation training in this study showed little transfer to other, non-trained cognitive domains. In contrast, Mitolo et al. (2016) found that route learning training enhanced route learning performance up to 3 months post-training and also improved visuo-spatial working memory, indicating transfer to an important cognitive domain. Finally, a one month spatial training in middle aged adults (40–55 years) has been shown to improved performance on a maze learning task and to decrease activation in the hippocampus and parahippocampal gyrus (Hötting et al., 2013), a finding which the authors attributed to reduced cognitive effort required post-training.
Researchers and clinicians have just begun to incorporate navigation tasks into clinical research and practice. Navigation assessment has potential utility as a predictor of future AD, as an outcome measure in behavioral and pharmacological intervention studies, and as a cognitive training task. Spatial navigation may prove to be particularly useful in these domains because of its known dependence on the highly plastic brain systems that may be both benefitted by training but also vulnerable to AD pathology. Currently, however, this field is hampered by the absence of agreement on ‘gold standard’ navigation task(s) to be used in clinical settings that are quick to administer, require minimal training, have established population norms, and meet quality criteria for diagnostic tests.
7. Summary
A progressive loss of navigational abilities in old age is now a well-established fact both in rodents and humans. Given that spatial navigation is a complex cognitive operation, deficits can arise at multiple processing stages that are summarized in Figure 6. These include difficulties with computing spatial information from incoming sensory cues, deficits with forming stable and distinct memory traces of spatial information, and challenges arising during planning and control of navigational behavior. Importantly, the research discussed in this review clearly shows that the deficits go beyond general impairments in learning and memory, because highly specialized, spatial computations (e.g. self-motion processing) are also altered in old age. As a consequence, age-related navigational deficits are best understood as reflecting a combination of impaired spatial coding as well as other learning and memory problems, which will also affect non-spatial behaviors.
Figure 6. Mechanisms underlying navigational deficits in old age.
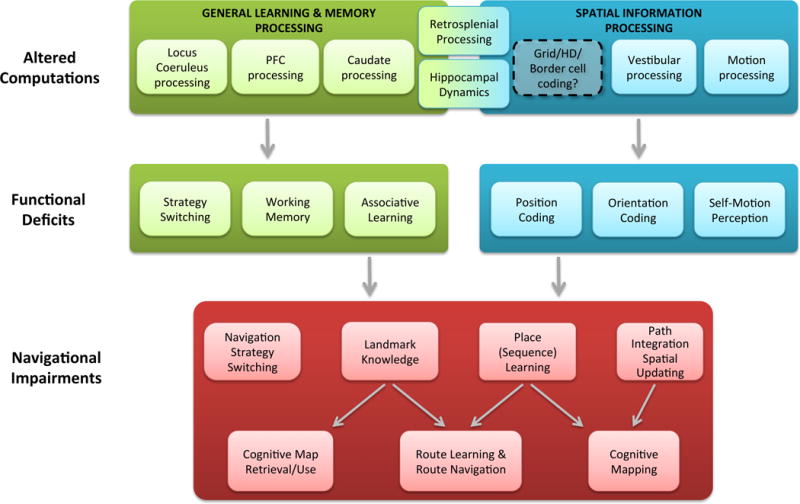
The age-related neurophysiological changes described in Figure 3 affect neural computations in multiple (sub-)cortical structures, thus leading to changes in general learning and memory processes (upper left box) as well as changes in processes that are more specific to spatial cognition (upper right box). Given that aberrant retrosplenial processing and altered hippocampal dynamics (which include aberrant pattern separation/completion, delayed spatial firing, etc.) are thought to also play a more general role in episodic memory, these processing deficits are likely to contribute to navigational deficits at both levels. Potential changes in grid, border or head direction cell coding are highlighted to indicate that direct evidence is missing at present. Collectively, both general learning and memory deficits as well as spatially specific changes can give rise to multiple functional deficits (middle boxes). These functional deficits will in turn affect various navigational processes (lower box), thereby causing the everyday navigational difficulties (e.g., impaired cognitive mapping) often seen in older adults.
Despite the wealth of studies discussed in this review, several important problems remain to be addressed. For example, the almost complete absence of longitudinal studies in humans makes it difficult to separate true age-related change from confounding cohort effects and complicates the precise identification of the variables driving potential decline. Moreover, while we know that the scale of space in which navigation takes place affects the exact cognitive processes involved in navigation (Meilinger et al., 2016; Wolbers & Wiener, 2014), it is currently unknown whether aging differentially affects navigation in, and spatial representations of, vista and environmental scale spaces. It is also unknown if and how key systems such as the entorhinal grid cell system are affected in healthy aging, which makes it difficult to pinpoint the exact reasons why older adults may find it difficult to keep track of their position. Critically, such studies are also needed if we are to harness the clinical potential of spatial navigation.
Given the current interest in developing functional biomarkers to detect people at risk for dementia, and the fact that key structures of the navigation circuit are among the first cortical structures affected by AD pathology, assessing navigational abilities might contribute to the detection of preclinical stages of AD. Moreover, navigational indicators could prove to be sensitive outcome measures – or cognitive endpoints – in clinical trials testing novel therapeutic approaches. This, however, requires the development of standardized, validated assessment protocols, which are not available at present. Excitingly, the recent advent of affordable, high quality virtual reality technology opens up completely new possibilities for developing ecologically valid assessments of navigational functions and for training navigational computations.
Acknowledgments
T.W. was supported by a Starting Investigator Grant of the European Research Council (AGESPACE 335090). J.W. was supported by a grant of the Economic and Social Research Council (ES/M009254/1). C.A.B. and A.W.L. were supported by McKnight Brain Research Foundation and National Institutes of Health RO1 AG003376.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamo DE, Briceño EM, Sindone JA, Alexander NB, Moffat SD. Age differences in virtual environment and real world path integration. Front Aging Neurosci. 2012;4:26. doi: 10.3389/fnagi.2012.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Lin L, Yoshimaru E, Pradyumna BK, Bergfield KL, Hoang L, Chawla M, Chen K, Moeller JR, Barnes CA, et al. Age-related regional network covariance of magnetic resonance imaging gray matter in the rat. J Neurosci. doi: 10.3389/fnagi.2020.00267. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Chen K, Aschenbrenner M, Merkley TL, Santerre-Lemmon LE, Shamy JL, Skaggs WE, Buonocore MH, Rapp PR, Barnes CA. Age-related regional network of magnetic resonance imaging gray matter in the rhesus macaque. J Neurosci. 2008;28:2710–2718. doi: 10.1523/JNEUROSCI.1852-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GL, Kirasic KC, Rashotte MA, Haun DB. Aging and path integration skill: kinesthetic and vestibular contributions to wayfinding. Percept Psychophys. 2004;66:170–179. doi: 10.3758/bf03194870. [DOI] [PubMed] [Google Scholar]
- Anson E, Jeka J. Perspectives on Aging Vestibular Function. Front Neurol. 2015;6:269. doi: 10.3389/fneur.2015.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonova E, Parslow D, Brammer M, Dawson GR, Jackson SH, Morris RG. Age-related neural activity during allocentric spatial memory. Memory. 2009;17:125–143. doi: 10.1080/09658210802077348. [DOI] [PubMed] [Google Scholar]
- Arshad Q, Seemungal BM. Age-Related Vestibular Loss: Current Understanding and Future Research Directions. Front Neurol. 2016;7:231. doi: 10.3389/fneur.2016.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc Natl Acad Sci USA. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S, Vieweg P, Gao F, Gilboa A, Wolbers T, Black SE, Rosenbaum RS. The Human Dentate Gyrus Plays a Necessary Role in Discriminating New Memories. Curr Biol. 2016;26:2629–2634. doi: 10.1016/j.cub.2016.07.081. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. An age comparison of the rates of acquisition and forgetting of spatial information in relation to long-term enhancement of hippocampal synapses. Behav Neurosci. 1985;99:1040–1048. doi: 10.1037//0735-7044.99.6.1040. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Nadel L, Honig WK. Spatial memory deficit in senescent rats. Can J Psychol. 1980;34:29–39. doi: 10.1037/h0081022. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- Bates SL, Wolbers T. How cognitive aging affects multisensory integration of navigational cues. Neurobiol Aging. 2014;35:2761–2769. doi: 10.1016/j.neurobiolaging.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Beas BS, McQuail JA, Ban Uelos C, Setlow B, Bizon JL. Prefrontal cortical GABAergic signaling and impaired behavioral flexibility in aged F344 rats. Neuroscience. 2017;345:274–286. doi: 10.1016/j.neuroscience.2016.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betts MJ, Acosta-Cabronero J, Cardenas-Blanco A, Nestor PJ, Düzel E. High-resolution characterisation of the aging brain using simultaneous quantitative susceptibility mapping (QSM) and R2* measurements at 7T. Neuroimage. 2016;138:43–63. doi: 10.1016/j.neuroimage.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Bian Z, Andersen GJ. Aging and the perception of egocentric distance. Psychol Aging. 2013;28:813–825. doi: 10.1037/a0030991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bizon JL, LaSarge CL, Montgomery KS, McDermott AN, Setlow B, Griffith WH. Spatial reference and working memory across the lifespan of male Fischer 344 rats. Neurobiol Aging. 2009;30:646–655. doi: 10.1016/j.neurobiolaging.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum KI, Abbott LF. A model of spatial map formation in the hippocampus of the rat. Neural Comput. 1996;8:85–93. doi: 10.1162/neco.1996.8.1.85. [DOI] [PubMed] [Google Scholar]
- Boccara CN, Sargolini F, Thoresen VH, Solstad T, Witter MP, Moser EI, Moser MB. Grid cells in pre- and parasubiculum. Nat Neurosci. 2010;13:987–994. doi: 10.1038/nn.2602. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–278–284. doi: 10.1016/0197-4580(95)00021-6. [DOI] [PubMed] [Google Scholar]
- Brandt T, Schautzer F, Hamilton DA, Bruning R, Markowitsch HJ, Kalla R, Darlington C, Smith P, Strupp M. Vestibular loss causes hippocampal atrophy and impaired spatial memory in humans. Brain. 2005;128:2732–2741. doi: 10.1093/brain/awh617. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Burns PC. Navigation and the mobility of older drivers. J Gerontol B Psychol Sci Soc Sci. 1999;54:S49–55. doi: 10.1093/geronb/54b.1.s49. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. The hippocampo-neocortical dialogue. Cereb Cortex. 1996;6:81–92. doi: 10.1093/cercor/6.2.81. [DOI] [PubMed] [Google Scholar]
- Byrne P, Becker S, Burgess N. Remembering the past and imagining the future: a neural model of spatial memory and imagery. Psychol Rev. 2007;114:340–375. doi: 10.1037/0033-295X.114.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caetano MJD, Lord SR, Schoene D, Pelicioni PHS, Sturnieks DL, Menant JC. Age-related changes in gait adaptability in response to unpredictable obstacles and stepping targets. Gait Posture. 2016;46:35–41. doi: 10.1016/j.gaitpost.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Caetano MS, Jin LE, Harenberg L, Stachenfeld KL, Arnsten AFT, Laubach M. Noradrenergic control of error perseveration in medial prefrontal cortex. Front Integr Neurosci. 2012;6:125. doi: 10.3389/fnint.2012.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter HE, Kelly KB, Bizon JL, Frazier CJ. Age-related changes in tonic activation of presynaptic versus extrasynaptic γ-amniobutyric acid type B receptors in rat medial prefrontal cortex. Neurobiol Aging. 2016;45:88–97. doi: 10.1016/j.neurobiolaging.2016.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick MJ, Jolly AEJ, Amos DP, Hassabis D, Spiers HJ. A Goal Direction Signal in the Human Entorhinal/Subicular Region. Curr Biol. 2015;25:87–92. doi: 10.1016/j.cub.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, Manson D, Cacucci F, Wills TJ. Absence of Visual Input Results in the Disruption of Grid Cell Firing in the Mouse. Curr Biol. 2016;26:2335–2342. doi: 10.1016/j.cub.2016.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Kehl SJ, McLennan H. Excitatory amino acids in synaptic transmission in the Schaffer collateral-commissural pathway of the rat hippocampus. J Physiol (Lond) 1983;334:33–46. doi: 10.1113/jphysiol.1983.sp014478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig M, Wolbers T, Harris MA, Hauff P, Della Sala S, Dewar M. Comparable rest-related promotion of spatial memory consolidation in younger and older adults. Neurobiol Aging. 2016;48:143–152. doi: 10.1016/j.neurobiolaging.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman LA, Stein K, Duffy CJ. Detecting navigational deficits in cognitive aging and Alzheimer disease using virtual reality. Neurology. 2008;71:888–895. doi: 10.1212/01.wnl.0000326262.67613.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Yuan P, Dahle CL, Bender AR, Yang Y, Raz N. Path Complexity in Virtual Water Maze Navigation: Differential Associations with Age, Sex, and Regional Brain Volume. Cereb Cortex. 2015;25:3122–3131. doi: 10.1093/cercor/bhu107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty AM, Bender AR, Yuan P, Raz N. Changes in Search Path Complexity and Length During Learning of a Virtual Water Maze: Age Differences and Differential Associations with Hippocampal Subfield Volumes. Cereb Cortex. 2016;26:2391–2401. doi: 10.1093/cercor/bhv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieguez D, Barea-Rodriguez EJ. Aging impairs the late phase of long-term potentiation at the medial perforant path-CA3 synapse in awake rats. Synapse. 2004;52:53–61. doi: 10.1002/syn.20004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowiasch S, Marx S, Einhäuser W, Bremmer F. Effects of aging on eye movements in the real world. Front Hum Neurosci. 2015;9:46. doi: 10.3389/fnhum.2015.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom AD, Meltzer J, McNaughton BL, Barnes CA. NMDA receptor antagonism blocks experience-dependent expansion of hippocampal “place fields”. Neuron. 2001;31:631–638. doi: 10.1016/s0896-6273(01)00401-9. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Kahana MJ, Caplan JB, Fields TA, Isham EA, Newman EL, Fried I. Cellular networks underlying human spatial navigation. Nature. 2003;425:184–188. doi: 10.1038/nature01964. [DOI] [PubMed] [Google Scholar]
- Ekstrom AD, Arnold AEGF, Iaria G. A critical review of the allocentric spatial representation and its neural underpinnings: toward a network-based perspective. Front Hum Neurosci. 2014;8 doi: 10.3389/fnhum.2014.00803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenbaum JD, Rolls ET. Allocentric and egocentric spatial information processing in the hippocampal formation of the behaving primate. Psychobiology. 1991;19:21–40. [Google Scholar]
- Foster TC, Norris CM. Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7:602–612. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Foster DJ, Wilson MA. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature. 2006;440:680–683. doi: 10.1038/nature04587. [DOI] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Fu H, Rodriguez GA, Herman M, Emrani S, Nahmani E, Barrett G, Figueroa HY, Goldberg E, Hussaini SA, Duff KE. Tau Pathology Induces Excitatory Neuron Loss, Grid Cell Dysfunction, and Spatial Memory Deficits Reminiscent of Early Alzheimer’s Disease. Neuron. 2017;93:533–541e5. doi: 10.1016/j.neuron.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhn M, Hafting T, Treves A, Moser MB, Moser EI. Hippocampal remapping and grid realignment in entorhinal cortex. Nature. 2007;446:190–194. doi: 10.1038/nature05601. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Björklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gazzaley AH, Siegel SJ, Kordower JH, Mufson EJ, Morrison JH. Circuit-specific alterations of N-methyl-D-aspartate receptor subunit 1 in the dentate gyrus of aged monkeys. Proc Natl Acad Sci USA. 1996;93:3121–3125. doi: 10.1073/pnas.93.7.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Gerrard JL, Kudrimoti H, McNaughton BL, Barnes CA. Reactivation of hippocampal ensemble activity patterns in the aging rat. Behav Neurosci. 2001;115:1180–1192. [PubMed] [Google Scholar]
- Gerrard JL, Burke SN, McNaughton BL, Barnes CA. Sequence reactivation in the hippocampus is impaired in aged rats. J Neurosci. 2008;28:7883–7890. doi: 10.1523/JNEUROSCI.1265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Livingstone SA, Skelton RW, Hopkins RO. Spatial deficits in a virtual water maze in amnesic participants with hippocampal damage. Hippocampus. 2010;20:481–491. doi: 10.1002/hipo.20651. [DOI] [PubMed] [Google Scholar]
- Gozlan H, Daval G, Verge D, Spampinato U, Fattaccini CM, Gallissot MC, el Mestikawy S, Hamon M. Aging associated changes in serotoninergic and dopaminergic pre- and postsynaptic neurochemical markers in the rat brain. Neurobiol Aging. 1990;11:437–449. doi: 10.1016/0197-4580(90)90011-n. [DOI] [PubMed] [Google Scholar]
- Gray DT, Smith AC, Burke SN, Gazzaley A, Barnes CA. Attentional updating and monitoring and affective shifting are impacted independently by aging in macaque monkeys. Behav Brain Res. 2017;322:329–338. doi: 10.1016/j.bbr.2016.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grön G, Brandenburg I, Wunderlich AP, Riepe MW. Inhibition of hippocampal function in mild cognitive impairment: targeting the cholinergic hypothesis. Neurobiol Aging. 2006;27:78–87. doi: 10.1016/j.neurobiolaging.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Grudzien A, Shaw P, Weintraub S, Bigio E, Mash DC, Mesulam MM. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol Aging. 2007;28:327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Gupta AS, van der Meer MAA, Touretzky DS, Redish AD. Hippocampal replay is not a simple function of experience. Neuron. 2010;65:695–705. doi: 10.1016/j.neuron.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436:801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Bonnevie T, Moser MB, Moser EI. Hippocampus-independent phase precession in entorhinal grid cells. Nature. 2008;453:1248–1252. doi: 10.1038/nature06957. [DOI] [PubMed] [Google Scholar]
- Harris MA, Wolbers T. Ageing effects on path integration and landmark navigation. Hippocampus. 2012;22:1770–1780. doi: 10.1002/hipo.22011. [DOI] [PubMed] [Google Scholar]
- Harris MA, Wolbers T. How age-related strategy switching deficits affect wayfinding in complex environments. Neurobiol Aging. 2014;35:1095–1102. doi: 10.1016/j.neurobiolaging.2013.10.086. [DOI] [PubMed] [Google Scholar]
- Hartley T, Maguire EA, Spiers HJ, Burgess N. The well-worn route and the path less traveled. Distinct neural bases of route following and wayfinding in humans. Neuron. 2003;37:877–888. doi: 10.1016/s0896-6273(03)00095-3. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E, Barkai E. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J Neurosci. 1995;15:5249–5262. doi: 10.1523/JNEUROSCI.15-07-05249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head D, Isom M. Age effects on wayfinding and route learning skills. Behav Brain Res. 2010;209:49–58. doi: 10.1016/j.bbr.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Head E, Mehta R, Hartley J, Kameka M, Cummings BJ, Cotman CW, Ruehl WW, Milgram NW. Spatial learning and memory as a function of age in the dog. Behav Neurosci. 1995;109:851–858. doi: 10.1037//0735-7044.109.5.851. [DOI] [PubMed] [Google Scholar]
- Hebb DJ. The Organization of Behavior. New York: Wiley; 1949. [Google Scholar]
- Hersi AI, Rowe W, Gaudreau P, Quirion R. Dopamine D1 receptor ligands modulate cognitive performance and hippocampal acetylcholine release in memory-impaired aged rats. Neuroscience. 1995;69:1067–1074. doi: 10.1016/0306-4522(95)00319-e. [DOI] [PubMed] [Google Scholar]
- Hok V, Chah E, Reilly RB, O’Mara SM. Hippocampal dynamics predict interindividual cognitive differences in rats. J Neurosci. 2012;32:3540–3551. doi: 10.1523/JNEUROSCI.6449-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hort J, Andel R, Mokrisova I, Gazova I, Amlerova J, Valis M, Coulson EJ, Harrison J, Windisch M, Laczó J. Effect of donepezil in Alzheimer disease can be measured by a computerized human analog of the Morris water maze. Neurodegener Dis. 2014;13:192–196. doi: 10.1159/000355517. [DOI] [PubMed] [Google Scholar]
- Hötting K, Holzschneider K, Stenzel A, Wolbers T, Röder B. Effects of a cognitive training on spatial learning and associated functional brain activations. BMC Neurosci. 2013;14:73. doi: 10.1186/1471-2202-14-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huentelman MJ, Stephan DA, Talboom J, Corneveaux JJ, Reiman DM, Gerber JD, Barnes CA, Alexander GE, Reiman EM, Bimonte-Nelson HA. Peripheral delivery of a ROCK inhibitor improves learning and working memory. Behav Neurosci. 2009;123:218–223. doi: 10.1037/a0014260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- Iaria G, Palermo L, Committeri G, Barton JJ. Age differences in the formation and use of cognitive maps. Behav Brain Res. 2009;196:187–191. doi: 10.1016/j.bbr.2008.08.040. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Complex maze learning in rodents as a model of age-related memory impairment. Neurobiol Aging. 1988;9:475–485. doi: 10.1016/s0197-4580(88)80101-5. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Weidemann CT, Miller JF, Solway A, Burke JF, Wei XX, Suthana N, Sperling MR, Sharan AD, Fried I, et al. Direct recordings of grid-like neuronal activity in human spatial navigation. Nat Neurosci. 2013;16:1188–1190. doi: 10.1038/nn.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science. 2012;336:1454–1458. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JE, Miquel J. Fine structural changes in the lateral vestibular nucleus of aging rats. Mech Ageing Dev. 1974;3:203–224. doi: 10.1016/0047-6374(74)90017-7. [DOI] [PubMed] [Google Scholar]
- Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27:12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavcic V, Vaughn W, Duffy CJ. Distinct visual motion processing impairments in aging and Alzheimer’s disease. Vision Res. 2011;51:386–395. doi: 10.1016/j.visres.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killian NJ, Jutras MJ, Buffalo EA. A map of visual space in the primate entorhinal cortex. Nature. 2012;491:761–764. doi: 10.1038/nature11587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolarik BS, Shahlaie K, Hassan A, Borders AA, Kaufman KC, Gurkoff G, Yonelinas AP, Ekstrom AD. Impairments in precision, rather than spatial strategy, characterize performance on the virtual Morris Water Maze: A case study. Neuropsychologia. 2016;80:90–101. doi: 10.1016/j.neuropsychologia.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nat Rev Neurosci. 2011;12:217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropff E, Carmichael JE, Moser MB, Moser EI. Speed cells in the medial entorhinal cortex. Nature. 2015;523:419–424. doi: 10.1038/nature14622. [DOI] [PubMed] [Google Scholar]
- Kunz L, Schröder TN, Lee H, Montag C, Lachmann B, Sariyska R, Reuter M, Stirnberg R, Stöcker T, Messing-Floeter PC, et al. Reduced grid-cell-like representations in adults at genetic risk for Alzheimer’s disease. Science. 2015;350:430–433. doi: 10.1126/science.aac8128. [DOI] [PubMed] [Google Scholar]
- Laczo J, Andel R, Vyhnalek M, Vlcek K, Magerova H, Varjassyova A, Tolar M, Hort J. Human analogue of the morris water maze for testing subjects at risk of Alzheimer’s disease. Neurodegener Dis. 2010;7:148–152. doi: 10.1159/000289226. [DOI] [PubMed] [Google Scholar]
- Laczó J, Andel R, Vyhnalek M, Matoska V, Kaplan V, Nedelska Z, Lerch O, Gazova I, Moffat SD, Hort J. The effect of TOMM40 on spatial navigation in amnestic mild cognitive impairment. Neurobiol Aging. 2015;36:2024–2033. doi: 10.1016/j.neurobiolaging.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Laczó J, Andel R, Nedelska Z, Vyhnalek M, Vlcek K, Crutch S, Harrison J, Hort J. Exploring the contribution of spatial navigation to cognitive functioning in older adults. Neurobiol Aging. 2017;51:67–70. doi: 10.1016/j.neurobiolaging.2016.12.003. [DOI] [PubMed] [Google Scholar]
- Lalonde-Parsi MJ, Lamontagne A. Perception of Self-Motion and Regulation of Walking Speed in Young-Old Adults. Motor Control. 2015;19:191–206. doi: 10.1123/mc.2014-0010. [DOI] [PubMed] [Google Scholar]
- Landfield PW, McGaugh JL, Lynch G. Impaired synaptic potentiation processes in the hippocampus of aged, memory-deficient rats. Brain Res. 1978;150:85–101. doi: 10.1016/0006-8993(78)90655-8. [DOI] [PubMed] [Google Scholar]
- Lever C, Burton S, Jeewajee A, O’Keefe J, Burgess N. Boundary vector cells in the subiculum of the hippocampal formation. J Neurosci. 2009;29:9771–9777. doi: 10.1523/JNEUROSCI.1319-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Yang Y, Li G, Zhang J, Wang Y, Zhou Y, Leventhal AG. Aging affects the direction selectivity of MT cells in rhesus monkeys. Neurobiol Aging. 2010;31:863–873. doi: 10.1016/j.neurobiolaging.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Lich M, Bremmer F. Self-motion perception in the elderly. Front Hum Neurosci. 2014;8:681. doi: 10.3389/fnhum.2014.00681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindner MD. Reliability, distribution, and validity of age-related cognitive deficits in the Morris water maze. Neurobiol Learn Mem. 1997;68:203–220. doi: 10.1006/nlme.1997.3782. [DOI] [PubMed] [Google Scholar]
- Lipman PD. Age and exposure differences in acquisition of route information. Psychol Aging. 1991;6:128–133. doi: 10.1037//0882-7974.6.1.128. [DOI] [PubMed] [Google Scholar]
- Lister JP, Barnes CA. Neurobiological changes in the hippocampus during normative aging. Arch Neurol. 2009;66:829–833. doi: 10.1001/archneurol.2009.125. [DOI] [PubMed] [Google Scholar]
- Liu I, Levy RM, Barton JJ, Iaria G. Age and gender differences in various topographical orientation strategies. Brain Res. 2011;1410:112–119. doi: 10.1016/j.brainres.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Liu P, Zhang H, Devaraj R, Ganesalingam GS, Smith PF. A multivariate analysis of the effects of aging on glutamate, GABA and arginine metabolites in the rat vestibular nucleus. Hear Res. 2010;269:122–133. doi: 10.1016/j.heares.2010.06.019. [DOI] [PubMed] [Google Scholar]
- Lopez I, Honrubia V, Baloh RW. Aging and the Human Vestibular Nucleus. J Vestib Res. 1997;7:77–85. [PubMed] [Google Scholar]
- Lövden M, Schaefer S, Noack H, Bodammer NC, Kuhn S, Heinze HJ, Duzel E, Backman L, Lindenberger U. Spatial navigation training protects the hippocampus against age-related changes during early and late adulthood. Neurobiol Aging. 2012;33:620 e9–620 e22. doi: 10.1016/j.neurobiolaging.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Lynch GS, Dunwiddie T, Gribkoff V. Heterosynaptic depression: a postsynaptic correlate of long-term potentiation. Nature. 1977;266:737–739. doi: 10.1038/266737a0. [DOI] [PubMed] [Google Scholar]
- Mahmood O, Adamo D, Briceno E, Moffat SD. Age differences in visual path integration. Behav Brain Res. 2009;205:88–95. doi: 10.1016/j.bbr.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Manaye KF, McIntire DD, Mann DM, German DC. Locus coeruleus cell loss in the aging human brain: a non-random process. J Comp Neurol. 1995;358:79–87. doi: 10.1002/cne.903580105. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond, B, Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Blum KI, Tsien JZ, Tonegawa S, Wilson MA. Impaired Hippocampal Representation of Space in CA1-Specific NMDAR1 Knockout Mice. Cell. 1996;87:1339–1349. doi: 10.1016/s0092-8674(00)81828-0. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Morris RGM. Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci. 1987;10:408–415. [Google Scholar]
- McNaughton BL, Barnes CA, Gerrard JL, Gothard K, Jung MW, Knierim JJ, Kudrimoti H, Qin Y, Skaggs WE, Suster M, et al. Deciphering the hippocampal polyglot: the hippocampus as a path integration system. J Exp Biol. 1996;199(Pt 1):173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Battaglia FP, Jensen O, Moser EI, Moser MB. Path integration and the neural basis of the “cognitive map”. Nat Rev Neurosci. 2006;7:663–678. doi: 10.1038/nrn1932. [DOI] [PubMed] [Google Scholar]
- McQuail JA, Beas BS, Kelly KB, Simpson KL, Frazier CJ, Setlow B, Bizon JL. NR2A-Containing NMDARs in the Prefrontal Cortex Are Required for Working Memory and Associated with Age-Related Cognitive Decline. J Neurosci. 2016;36:12537–12548. doi: 10.1523/JNEUROSCI.2332-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MR, Barnes CA, McNaughton BL. Experience-dependent, asymmetric expansion of hippocampal place fields. Proc Natl Acad Sci USA. 1997;94:8918–8921. doi: 10.1073/pnas.94.16.8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MR, Quirk MC, Wilson MA. Experience-dependent asymmetric shape of hippocampal receptive fields. Neuron. 2000;25:707–715. doi: 10.1016/s0896-6273(00)81072-7. [DOI] [PubMed] [Google Scholar]
- Meilinger T, Strickrodt M, Bülthoff HH. Qualitative differences in memory for vista and environmental spaces are caused by opaque borders, not movement or successive presentatio. Cognition. 2016;155:77–95. doi: 10.1016/j.cognition.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Ménard C, Quirion R. Successful cognitive aging in rats: a role for mGluR5 glutamate receptors, homer 1 proteins and downstream signaling pathways. PLoS ONE. 2012;7:e28666. doi: 10.1371/journal.pone.0028666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill DA, Chiba AA, Tuszynski MH. Conservation of neuronal number and size in the entorhinal cortex of behaviorally characterized aged rats. J Comp Neurol. 2001;438:445–456. doi: 10.1002/cne.1327. [DOI] [PubMed] [Google Scholar]
- Meulenbroek O, Petersson KM, Voermans N, Weber B, Fernández G. Age differences in neural correlates of route encoding and route recognition. Neuroimage. 2004;22:1503–1514. doi: 10.1016/j.neuroimage.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Mitolo M, Borella E, Meneghetti C, Carbone E, Pazzaglia F. How to enhance route learning and visuo-spatial working memory in aging: a training for residential care home residents. Aging Ment Health. 2016:1–9. doi: 10.1080/13607863.2015.1132673. [DOI] [PubMed] [Google Scholar]
- Moffat SD. Aging and spatial navigation: what do we know and where do we go? Neuropsychol Rev. 2009;19:478–489. doi: 10.1007/s11065-009-9120-3. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Resnick SM. Effects of age on virtual environment place navigation and allocentric cognitive mapping. Behav Neurosci. 2002;116:851–859. doi: 10.1037//0735-7044.116.5.851. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Zonderman AB, Resnick SM. Age differences in spatial memory in a virtual environment navigation task. Neurobiol Aging. 2001;22:787–796. doi: 10.1016/s0197-4580(01)00251-2. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Elkins W, Resnick SM. Age differences in the neural systems supporting human allocentric spatial navigation. Neurobiol Aging. 2006;27:965–972. doi: 10.1016/j.neurobiolaging.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Moffat SD, Kennedy KM, Rodrigue KM, Raz N. Extrahippocampal contributions to age differences in human spatial navigation. Cereb Cortex. 2007;17:1274–1282. doi: 10.1093/cercor/bhl036. [DOI] [PubMed] [Google Scholar]
- Mokrisova I, Laczo J, Andel R, Gazova I, Vyhnalek M, Nedelska Z, Levcik D, Cerman J, Vlcek K, Hort J. Real-space path integration is impaired in Alzheimer’s disease and mild cognitive impairment. Behav Brain Res. 2016;307:150–158. doi: 10.1016/j.bbr.2016.03.052. [DOI] [PubMed] [Google Scholar]
- Moore CI, Browning MD, Rose GM. Hippocampal plasticity induced by primed burst, but not long-term potentiation, stimulation is impaired in area CA1 of aged Fischer 344 rats. Hippocampus. 1993;3:57–66. doi: 10.1002/hipo.450030106. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Müller G, Pilzecker A. Experimental contributions to the theory of memory. Z Psychol Z Angew Psychol. 1900;1:1–288. [Google Scholar]
- Nicholson DA, Yoshida R, Berry RW, Gallagher M, Geinisman Y. Reduction in Size of Perforated Postsynaptic Densities in Hippocampal Axospinous Synapses and Age-Related Spatial Learning Impairments. J Neurosci. 2004;24:7648–7653. doi: 10.1523/JNEUROSCI.1725-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle MM, Prescott S, Bizon JL. Emergence of a cue strategy preference on the water maze task in aged C57B6 x SJL F1 hybrid mice. Learn Mem. 2003;10:520–524. doi: 10.1101/lm.64803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noack H, Lövdén M, Schmiedek F. On the validity and generality of transfer effects in cognitive training research. Psychol Res. 2014;78:773–789. doi: 10.1007/s00426-014-0564-6. [DOI] [PubMed] [Google Scholar]
- Norman JF, Crabtree CE, Bartholomew AN, Ferrell EL. Aging and the perception of slant from optical texture, motion parallax, and binocular disparity. Atten Percept Psychophys. 2009;71:116–130. doi: 10.3758/APP.71.1.116. [DOI] [PubMed] [Google Scholar]
- Norman JF, Adkins OC, Pedersen LE, Reyes CM, Wulff RA, Tungate A. The visual perception of exocentric distance in outdoor settings. Vision Res. 2015;117:100–104. doi: 10.1016/j.visres.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O’Keefe J. Place units in the hippocampus of the freely moving rat. Exp Neurol. 1976;51:78–109. doi: 10.1016/0014-4886(76)90055-8. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Oxford University Press; 1978. [Google Scholar]
- Oler JA, Markus EJ. Age-related deficits in the ability to encode contextual change: a place cell analysis. Hippocampus. 2000;10:338–350. doi: 10.1002/1098-1063(2000)10:3<338::AID-HIPO14>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Pai MC, Yang YC. Impaired translation of spatial representation in young onset Alzheimer’s disease patients. Curr Alzheimer Res. 2013;10:95–103. [PubMed] [Google Scholar]
- Pengas G, Hodges JR, Watson P, Nestor PJ. Focal posterior cingulate atrophy in incipient Alzheimer’s disease. Neurobiol Aging. 2010;31:25–33. doi: 10.1016/j.neurobiolaging.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Pengas G, Williams GB, Acosta-Cabronero J, Ash TW, Hong YT, Izquierdo-Garcia D, Fryer TD, Hodges JR, Nestor PJ. The relationship of topographical memory performance to regional neurodegeneration in Alzheimer’s disease. Front Aging Neurosci. 2012;4:17. doi: 10.3389/fnagi.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Escobar JA, Kornienko O, Latuske P, Kohler L, Allen K. Visual landmarks sharpen grid cell metric and confer context specificity to neurons of the medial entorhinal cortex. Elife. 2016;5 doi: 10.7554/eLife.16937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Sullivan EV. Frontal circuitry degradation marks healthy adult aging: Evidence from diffusion tensor imaging. Neuroimage. 2005;26:891–899. doi: 10.1016/j.neuroimage.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Spangler EL, Kelly-Bell B, Miller MG, de Cabo R, Ingram DK. Age-associated learning and memory deficits in two mouse versions of the Stone T-maze. Neurobiol Aging. 2012;33:2431–2439. doi: 10.1016/j.neurobiolaging.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potegal M, Day MJ, Abraham L. Maze orientation, visual and vestibular cues in two-maze spontaneous alternation of rats. Physiological Psychology. 1977;5:414–420. [Google Scholar]
- Rapp PR. Visual discrimination and reversal learning in the aged monkey (Macaca mulatta) Behav Neurosci. 1990;104:876–884. doi: 10.1037//0735-7044.104.6.876. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Kansky MT, Roberts JA. Impaired spatial information processing in aged monkeys with preserved recognition memory. Neuroreport. 1997;8:1923–1928. doi: 10.1097/00001756-199705260-00026. [DOI] [PubMed] [Google Scholar]
- Rodgers MK, Sindone JA, Moffat SD. Effects of age on navigation strategy. Neurobiol Aging. 2012;33:202.e15–22. doi: 10.1016/j.neurobiolaging.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roditi RE, Crane BT. Directional asymmetries and age effects in human self-motion perception. J Assoc Res Otolaryngol. 2012;13:381–401. doi: 10.1007/s10162-012-0318-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogalski E, Stebbins GT, Barnes CA, Murphy CM, Stoub TR, George S, Ferrari C, Shah RC, deToledo-Morrell L. Age-related changes in parahippocampal white matter integrity: a diffusion tensor imaging study. Neuropsychologia. 2012;50:1759–1765. doi: 10.1016/j.neuropsychologia.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondi-Reig L, Petit GH, Tobin C, Tonegawa S, Mariani J, Berthoz A. Impaired sequential egocentric and allocentric memories in forebrain-specific-NMDA receptor knock-out mice during a new task dissociating strategies of navigation. J Neurosci. 2006;26:4071–4081. doi: 10.1523/JNEUROSCI.3408-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Rao G, McNaughton BL, Barnes CA. Role of temporal summation in age-related long-term potentiation-induction deficits. Hippocampus. 1997;7:549–558. doi: 10.1002/(SICI)1098-1063(1997)7:5<549::AID-HIPO10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Redish AD, McNaughton BL, Barnes CA. Hippocampal map realignment and spatial learning. Nat Neurosci. 2003;6:609–615. doi: 10.1038/nn1053. [DOI] [PubMed] [Google Scholar]
- Sarel A, Finkelstein A, Las L, Ulanovsky N. Vectorial representation of spatial goals in the hippocampus of bats. Science. 2017;355:176–180. doi: 10.1126/science.aak9589. [DOI] [PubMed] [Google Scholar]
- Sargolini F, Fyhn M, Hafting T, McNaughton BL, Witter MP, Moser MB, Moser EI. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science. 2006;312:758–762. doi: 10.1126/science.1125572. [DOI] [PubMed] [Google Scholar]
- Schimanski LA, Lipa P, Barnes CA. Tracking the course of hippocampal representations during learning: when is the map required. J Neurosci. 2013;33:3094–3106. doi: 10.1523/JNEUROSCI.1348-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck NW, Doeller CF, Schjeide BMM, Schröder J, Frensch PA, Bertram L, Li SC. Aging and KIBRA/WWC1 genotype affect spatial memory processes in a virtual navigation task. Hippocampus. 2013;23:919–930. doi: 10.1002/hipo.22148. [DOI] [PubMed] [Google Scholar]
- Serino S, Riva G. Getting lost in Alzheimer’s disease: a break in the mental frame syncing. Med Hypotheses. 2013;80:416–421. doi: 10.1016/j.mehy.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Serino S, Cipresso P, Morganti F, Riva G. The role of egocentric and allocentric abilities in Alzheimer’s disease: a systematic review. Ageing Res Rev. 2014;16:32–44. doi: 10.1016/j.arr.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Shankar S, Teyler TJ, Robbins N. Aging differentially alters forms of long-term potentiation in rat hippocampal area CA1. J Neurophysiol. 1998;79:334–341. doi: 10.1152/jn.1998.79.1.334. [DOI] [PubMed] [Google Scholar]
- Shen B, McNaughton BL. Modeling the spontaneous reactivation of experience-specific hippocampal cell assembles during sleep. Hippocampus. 1996;6:685–692. doi: 10.1002/(SICI)1098-1063(1996)6:6<685::AID-HIPO11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Shen J, Barnes CA. Age-related decrease in cholinergic synaptic transmission in three hippocampal subfields. Neurobiol Aging. 1996;17:439–451. doi: 10.1016/0197-4580(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Shen J, Barnes CA, McNaughton BL, Skaggs WE, Weaver KL. The effect of aging on experience-dependent plasticity of hippocampal place cells. J Neurosci. 1997;17:6769–6782. doi: 10.1523/JNEUROSCI.17-17-06769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine JP, Valdés-Herrera JP, Hegarty M, Wolbers T. The Human Retrosplenial Cortex and Thalamus Code Head Direction in a Global Reference Frame. J Neurosci. 2016;36:6371–6381. doi: 10.1523/JNEUROSCI.1268-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simieli L, Barbieri FA, Orcioli-Silva D, Lirani-Silva E, Stella F, Gobbi LTB. Obstacle crossing with dual tasking is a danger for individuals with Alzheimer’s disease and for healthy older people. J Alzheimers Dis. 2015;43:435–441. doi: 10.3233/JAD-140807. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser MB, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Bennett SA, Khan AS, Thornton PL, Xu X, Ingram RL, Brunso-Bechtold JK. Age and insulin-like growth factor-1 modulate N-methyl-D-aspartate receptor subtype expression in rats. Brain Res Bull. 2000;51:331–338. doi: 10.1016/s0361-9230(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Spencer RMC, Gouw AM, Ivry RB. Age-related decline of sleep-dependent consolidation. Learn Mem. 2007;14:480–484. doi: 10.1101/lm.569407. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Taube JS. Firing Properties of Head Direction Cells in the Rat Anterior Thalamic Nucleus: Dependence on Vestibular Input. J Neurosci Off J Soc Neurosci. 1997;17:4349–4358. doi: 10.1523/JNEUROSCI.17-11-04349.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman RW, Clark AS, Taube JS. Hippocampal spatial representations require vestibular input. Hippocampus. 2002;12:291–303. doi: 10.1002/hipo.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone CP. The age factor in animal learning: I. Rats in the problem box and the maze. Genetic Psychology Monographs. 1929:1–130. [Google Scholar]
- Sturrock RR. Age related changes in neuron number in the mouse lateral vestibular nucleus. J Anat. 1989;166:227–232. [PMC free article] [PubMed] [Google Scholar]
- Storsve AB, Fjell AM, Tamnes CK, Westlye LT, Overbye K, Aasland HW, Walhovd KB. Differential longitudinal changes in cortical thickness, surface area and volume across the adult life span: regions of accelerating and decelerating change. J Neurosci. 2014;34:8488–8498. doi: 10.1523/JNEUROSCI.0391-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Mattson MP. Selective vulnerability of neurons in layer II of the entorhinal cortex during aging and Alzheimer’s disease. Neural Plast. 2010;2010:108190. doi: 10.1155/2010/108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait DS, Brown VJ, Farovik A, Theobald DE, Dalley JW, Robbins TW. Lesions of the dorsal noradrenergic bundle impair attentional set-shifting in the rat. Eur J Neurosci. 2007;25:3719–3724. doi: 10.1111/j.1460-9568.2007.05612.x. [DOI] [PubMed] [Google Scholar]
- Taube JS. The head direction signal: origins and sensory-motor integration. Annu Rev Neurosci. 2007;30:181–207. doi: 10.1146/annurev.neuro.29.051605.112854. [DOI] [PubMed] [Google Scholar]
- Taube JS, Muller RU, Ranck JB., Jr Head-direction cells recorded from the postsubiculum in freely moving rats. II. Effects of environmental manipulations. J Neurosci. 1990;10:436–447. doi: 10.1523/JNEUROSCI.10-02-00436.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272:1017–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- Thomé A, Gray DT, Erickson CA, Lipa P, Barnes CA. Memory impairment in aged primates is associated with region-specific network dysfunction. Mol Psychiatry. 2016;21:1257–1262. doi: 10.1038/mp.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman CE. Cognitive maps in rats and man. Psych Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- Topic B, Willuhn I, Palomero-Gallagher N, Zilles K, Huston JP, Hasenöhrl RU. Impaired maze performance in aged rats is accompanied by increased density of NMDA, 5-HT1A, and alpha-adrenoceptor binding in hippocampus. Hippocampus. 2007;17:68–77. doi: 10.1002/hipo.20246. [DOI] [PubMed] [Google Scholar]
- Ulanovsky N, Moss CF. Hippocampal cellular and network activity in freely moving echolocating bats. Nat Neurosci. 2007;10:224–233. doi: 10.1038/nn1829. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Vieweg P, Stangl M, Howard LR, Wolbers T. Changes in pattern completion–a key mechanism to explain age-related recognition memory deficits? Cortex. 2015;64:343–351. doi: 10.1016/j.cortex.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WH, Jr, Blackwell AW, Morris MW. Age differences in perceiving the direction of self-motion from optical flow. J Gerontol. 1989;44:P147–53. doi: 10.1093/geronj/44.5.p147. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Wicklund AH, Salmon DP. The neuropsychological profile of Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006171. doi: 10.1101/cshperspect.a006171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger E, Schaefer S, Noack H, Kuhn S, Martensson J, Heinze HJ, Duzel E, Backman L, Lindenberger U, Lovden M. Cortical thickness changes following spatial navigation training in adulthood and aging. Neuroimage. 2012;59:3389–3397. doi: 10.1016/j.neuroimage.2011.11.015. [DOI] [PubMed] [Google Scholar]
- West RL. An application of prefrontal cortex function theory to cognitive aging. Psychol Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wiener JM, Büchner SJ, Hölscher C. Towards a Taxonomy of Wayfinding Tasks: A Knowledge-Based Approach. Spatial Cognition and Computation. 2009;9:152–165. [Google Scholar]
- Wiener JM, Kmecova H, de Condappa O. Route repetition and route retracing: effects of cognitive aging. Front Aging Neurosci. 2012;4:7. doi: 10.3389/fnagi.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener JM, de Condappa O, Harris MA, Wolbers T. Maladaptive bias for extrahippocampal navigation strategies in aging humans. J Neurosci. 2013;33:6012–6017. doi: 10.1523/JNEUROSCI.0717-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkniss SM, Jones MG, Korol DL, Gold PE, Manning CA. Age-related differences in an ecologically based study of route learning. Psychol Aging. 1997;12:372–375. doi: 10.1037//0882-7974.12.2.372. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbers T, Büchel C. Dissociable retrosplenial and hippocampal contributions to successful formation of survey representations. J Neurosci. 2005;25:3333–3340. doi: 10.1523/JNEUROSCI.4705-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbers T, Wiener JM. Challenges for identifying the neural mechanisms that support spatial navigation: the impact of spatial scale. Front Hum Neurosci. 2014;8:571. doi: 10.3389/fnhum.2014.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolbers T, Wiener JM, Mallot HA, Büchel C. Differential recruitment of the hippocampus, medial prefrontal cortex, and the human motion complex during path integration in humans. J Neurosci. 2007;27:9408–9416. doi: 10.1523/JNEUROSCI.2146-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Krause M, Rao G, McNaughton BL, Barnes CA. Synaptic commitment: developmentally regulated reciprocal changes in hippocampal granule cell NMDA and AMPA receptors over the lifespan. J Neurophysiol. 2008;99:2760–2768. doi: 10.1152/jn.01276.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Mattfeld AT, Stark SM, Stark CEL. Age-related memory deficits linked to circuit-specific disruptions in the hippocampus. Proc Natl Acad Sci USA. 2011a;108:8873–8878. doi: 10.1073/pnas.1101567108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CEL. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011b;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong JY, Moffat SD. Age-Related Differences in Associative Learning of Landmarks and Heading Directions in a Virtual Navigation Task. Front Aging Neurosci. 2016;8:122. doi: 10.3389/fnagi.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]


