Abstract
Objective
Photosensitivity is common in patients with systemic lupus erythematosus, although the mechanisms linking ultraviolet (UV) light to flares are not well understood. We undertook this study to determine whether repetitive UVB exposure could induce type I interferon (IFN) production in normal mouse skin, and to investigate the roles of inflammatory monocytes and plasmacytoid dendritic cells (PDCs) in type I IFN production and development of UVB irradiation–induced inflammation.
Methods
Mice were irradiated with UVB at 100 mJ/cm2 for 5 days, and cutaneous manifestations were examined by messenger RNA expression of inflammatory and type I IFN response genes, histology, and flow cytometry. Inflammatory monocyte and PDC depletion experiments were performed in CCR2–diphtheria toxin receptor (DTR)–transgenic mice and blood dendritic cell antigen 2–DTR–transgenic mice. The roles of type I IFN and of the adaptor protein stimulator of IFN genes (STING) in UVB irradiation–induced inflammation were investigated using IFN-α/β/ω receptor (IFNAR)–knockout mice and STING-knockout mice.
Results
Repeated UVB irradiation stimulated an inflammatory cell infiltrate and induction of type I IFN and proinflammatory cytokines. Interestingly, the type I IFN response was independent of PDCs but dependent on inflammatory monocytes, which were recruited following UVB irradiation. The adaptor protein STING was necessary for both type I IFN and proinflammatory cytokine expression in the skin. UVB-irradiated IFNAR-knockout mice showed increased levels of proinflammatory genes and more severe inflammation by histology, suggesting a protective role for type I IFN.
Conclusion
In wild-type mice, repeated doses of UVB irradiation induce monocyte-dependent and PDC-independent expression of type I IFN together with expression of other proinflammatory cytokines. Induction is dependent on the adaptor protein STING. Surprisingly, studies using IFNAR-deficient mice revealed that type I IFN protects against UVB irradiation–induced skin inflammation, in part by attenuating proinflammatory cytokine expression and limiting tissue damage.
Skin disease and photosensitivity are common manifestations of systemic lupus erythematosus (SLE) and are observed in more than 70% of patients at some point during disease (1). Ultraviolet (UV) light is a well-identified trigger of both localized cutaneous disease (cutaneous lupus erythematosus [CLE]) and systemic disease (SLE). In addition, patients with CLE develop cutaneous lupus lesions when challenged with UV phototesting (2). Photosensitivity is thought to occur when lupus autoantibodies bind to nucleoprotein-containing antigens (e.g., Ro/SSA) exposed upon UV-mediated cell death (3). The in situ–formed immune complexes (ICs) induce inflammation in the skin through activation of complement, engagement of activating Fcγ receptors (FcγR), and other less-defined pathways. While this model of photosensitivity was formulated following the analysis of human SLE skin biopsy samples, the role of innate immune cells in UV-stimulated normal skin injury has not been well studied.
Two events, keratinocyte apoptosis and plasmacytoid dendritic cell (PDC) activation, have been linked to UV-induced skin lesions in CLE (4). Apoptotic keratinocytes are observed in biopsy samples from CLE lesions, and keratinocyte apoptosis is thought to be the source of nuclear autoantigens in CLE (3,5,6). PDCs, innate-type immune cells, are proposed to be central to the pathogenic response to ICs seen in lupus (7). PDCs are present in CLE lesions on biopsy (8) and are known to secrete large amounts of type I interferon (IFN). The IFN family includes cytokines likely responsible for the type I IFN signature seen both systemically and locally in CLE lesions (9). PDCs are stimulated to secrete type I IFN when DNA- and RNA-containing ICs are phagocytosed by FcγRIIa and delivered to the endosomal compartment, triggering the activation and signaling of the endosomal pattern-recognition receptors Toll-like receptor 7 (TLR-7) and TLR-9 (10). However, DNA and RNA released by dying cells are also potent inducers of the innate immune response (11), so it is not known whether and how repetitive UVB-mediated cell damage stimulates type I IFN in the skin prior to development of autoantibodies. To address these questions, we exposed normal mice as well as mice deficient in type I IFN receptor and mice deficient in stimulator of IFN genes (STING; an adaptor that responds to intracytoplasmic DNA by stimulating the production of type I IFN) to UVB irradiation, and we assessed inflammation and type I IFN production.
In this study, we observed that repeated UV irradiation of normal mouse skin induced a modest expression of type I IFN and IFN-stimulated genes (ISGs) together with proinflammatory cytokine expression. We observed that expression of proinflammatory cytokines and ISGs was dependent on STING, indicating a prominent role for DNA in the initiation of type I IFN stimulation. While depletion of PDCs did not abrogate the type I IFN response, depletion of CCR2+ monocytes, which were prominently recruited to the skin, reduced type I IFN expression. Surprisingly, in the absence of IFN signaling, cutaneous infiltrates, inflammation, and interleukin-6 (IL-6) expression were increased, suggesting a protective role for type I IFN in UVB irradiation–induced inflammation and skin damage.
MATERIALS AND METHODS
Mice
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Washington, Seattle. All animal experiments were conducted using 8–20-week-old C57BL/6 (B6) mice unless indicated otherwise. Animals were maintained and bred in specific pathogen–free facilities. Transgenic mice that express the diphtheria toxin receptor (DTR) under the control of the highly specific human PDC gene promoter blood dendritic cell antigen 2 (BDCA-2–DTR–transgenic mice) were purchased from The Jackson Laboratory. Mice transgenic for CCR2 and green fluorescent protein (CCR2-GFP–transgenic mice) and CCR2-DTR–transgenic mice were kindly donated by Dr. Tobias Hohl (Memorial Sloan Kettering Cancer Center, New York, NY).
UV irradiation and tape stripping
UVB light was delivered by FS40T12/UVB bulbs (National Biological Corporation) with peak emission between 300 and 315 nm and rapid decrease in emission beyond the UVB range (according to the manufacturer’s data, emittance below 280 nm was undetectable). The UVB dose was measured with a Photolight IL1400A radiometer equipped with a SEL240/UVB detector (International Light Technologies) and calibrated prior to each experiment. The backs of the mice were shaved prior to irradiation. Mice were allowed to move freely in their cage during UVB exposure. For subacute UVB irradiation, the back was irradiated with UVB at a dose of 100 mJ/cm2 for 5 consecutive daily doses totaling 500 mJ/cm2. Time points were in relation to the fifth day of UVB exposure, which was used as the zero time point. For tape stripping, backs of mice were shaved and depilated (Nair; Church and Dwight) immediately before cutaneous injury. Injury was induced by using 15 strokes of meditape (Scotch; 3M Company) across the back (as described in ref. 12), and skin was examined at 24 hours.
Cell depletion
For depletion experiments, DT (Sigma) was injected intraperitoneally at 125 ng (for PDC depletion) or at 250 ng (for CCR2+ monocyte depletion). PDC-depleted and wild-type (WT) mice were injected 24 hours before tape stripping or the first UVB dose. In UVB experiments, DT injection was repeated every 2–3 days for the duration of the experiment (a total of 3 doses per mouse).
Immunohistochemistry/immunofluorescence
Skin was preserved in 10% formalin and embedded in paraffin or snap-frozen on dry ice and embedded in TissueTek OCT compound (Sakura Finetek) and stored at −70°C. Paraffin-embedded sections were stained with hematoxylin and eosin (H&E) for examination by a comparative pathologist (DL) who was blinded to the treatment protocol. An aggregate lesion severity score ranging from 0 (normal) to 4+ (greatest severity) was generated based on assessment of several parameters including epidermal thickness and relative degree of intraepithelial, dermal, and hypodermal inflammatory change. Nucleated cells were quantified in H&E-stained sections in a blinded manner using ImageJ software (National Institutes of Health).
Quantitative polymerase chain reaction (qPCR) of messenger RNA (mRNA)
RNA was isolated from full-thickness skin samples using an RNeasy Fibrous Tissue Mini Kit with on-column DNase treatment (Qiagen). First-strand complementary DNA (cDNA) was generated using 100 ng RNA with a High-Capacity cDNA Reverse Transcription Kit using random primers (Applied Biosystems). Reactions (20 μl) were run in duplicate on a StepOnePlus real-time PCR instrument (Applied Biosystems) using gene-specific primers. Ct values were determined with constant threshold at 0.2. Ct values were standardized to the house-keeping gene 18S, and fold change was calculated against a separate cohort of nonirradiated mice using the 2−ΔΔCt method. The standard curve showed similar amplification efficiencies for each gene, and template concentrations were within the linear dynamic range for each primer set.
The following primer sequences were used: for 18S, 5′-GAGGGAGCCTGAGAAACGG-3′ (forward) and 5′-GTCGGGAGTGGGTAATTTGC-3′ (reverse); for cyclooxygenase 2 (COX-2), 5′-GCAGGAAGTCTTTGGTCTGG-3′ (forward) and 5′-AAGTGGTAACCGCTCAGGTG-3′ (reverse); for CXCL10, 5′-GCCGTCATTTTCTGCCTCAT-3′ (forward) and 5′-GCTTCCCTATGGCCCTCATT-3′ (reverse); for IFN-induced protein with tetratricopeptide repeats 1 (IFIT-1), 5′-TGCTGAGATGGACTGTGAGG-3′ (forward) and 5′-CTCCACTTTCAGAGCCTTCG-3′ (reverse); for IFNα1, 5′-AGTGAGCTGACCCAGCAGAT-3′ (forward) and 5′-AGTGAGCTGACCCAGCAGAT-3′ (reverse); for IFNα2, 5′-ATCCAGAAGGCTCAAGCCATCC-3′ (forward) and 5′-GGAGGGTTGTATTCCAAGCAGC-3′ (reverse); for IL-1β, 5′-CAACCAACAAGTGATATTCTCCATG-3′ (forward) and 5′-GATCCACACTCTCCAGCTGCA-3′ (reverse); for IL-6, 5′-CTACCCCAATTTCCAATGCTCT-3′ (forward) and 5′-TGAATTGGATGGTCTTGGTCC-3′ (reverse); for IFN regulatory factor 7 (IRF-7), 5′-GTCTCGGCTTGTGCTTGTCT-3′ (forward) and 5′-CCAGGTCCATGAGGAAGTGT-3′ (reverse); for ISG-15, 5′-AAGCAGCCAGAAGCAGACTC-3′ (forward) and 5′-CACCAATCTTCTGGGCAATC-3′ (reverse); for matrix metalloproteinase 13 (MMP-13), 5′-CATCCATCCCGTGACCTTAT-3′ (forward) and 5′-GTCTTCCCCGTGTTCTCAAA-3′ (reverse); for Mx-1 protein, 5′-CCTCAGGCTAGATGGCAAG-3′ (forward) and 5′-CCTCAGGCTAGATGGCAAG-3′ (reverse); and for tumor necrosis factor (TNF), 5′-GTCAGGTTGCCTCTGTCTCA-3′ (forward) and 5′-GTCAGGTTGCCTCTGTCTCA-3′ (reverse).
Isolation of skin cells and flow cytometry
Skin samples were minced and digested with 0.28 units/ml Liberase TM (Roche) and 0.1 mg/ml of Deoxyribonuclease I (Worthington) for 60 minutes at 37°C, then passed through a 70-μM filter and washed with 1% bovine serum albumin in phosphate buffered saline. Fluorescence-activated cell sorting analysis was performed using a BD FACSCanto flow cytometer and analyzed using FlowJo software (Tree Star). T cells (CD45+CD3+), neutrophils (CD45+ CD11b+Ly-6C+Ly-6G+), dermal monocytes (CD45+CD11b+ Ly-6ChighLy-6G−), dermalmacrophages (CD45+CD11b+CD64high and major histocompatibility complex class II positive), and PDCs (CD45+Ly-6C+CD11c+, PDC antigen 1 positive [PDCA-1+], and Siglec H positive) (13) were identified using monoclonal antibodies from eBioscience, Miltenyi Biotec, or BioLegend.
RESULTS
Repeated skin exposure to UVB induces cutaneous inflammation and mixed inflammatory cell infiltrate
Since our pilot qPCR studies did not reveal a type I IFN response after a high, single-dose UVB exposure of 500 mJ/cm2 (data not shown), we used a lower-dose UVB irradiation regimen developed by Sharma et al (14). This UVB regimen, referred to herein as “subacute UVB irradiation,” consisted of 5 consecutive days of UVB irradiation at 100 mJ/cm2, which should be more representative of repetitive UVB exposure in humans. We performed biopsies after the final (fifth) UVB dose, isolated cells from full-thickness skin sections, and performed flow cytometry analysis.
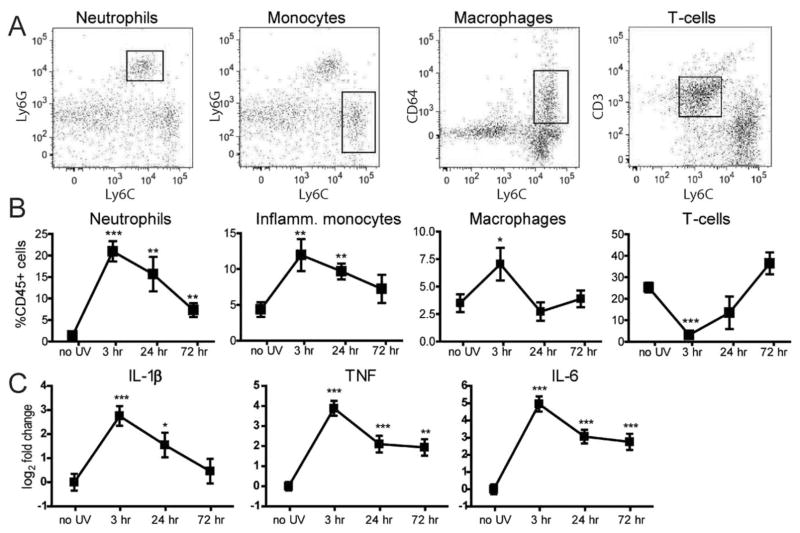
Subacute UVB irradiation induced a robust tissue response with a large influx of neutrophils and monocytes comprising 20% and 12%, respectively, of CD45+ cells; this response peaked at 3 hours before decreasing to near-baseline levels by 72 hours (Figure 1B). The large influx of neutrophils following subacute UVB irradiation was distinctly different from that resulting from a single UVB dose of 500 mJ/cm2, which induced an influx of monocytes but much lower numbers of neutrophils (data not shown). Following subacute UVB irradiation, there was an increase in macrophages at 3 hours that returned to baseline levels by 24 hours. In nonirradiated skin, T cells comprised ~25% of skin immune cells. Following UVB irradiation, the proportion of T cells decreased to <5% at 3 hours before returning to pre–UVB irradiation levels at 72 hours (Figure 1B). Quantitative PCR analysis of RNA isolated from full-thickness skin sections showed up-regulation of several proinflammatory genes. IL-1β was significantly up-regulated, with an 8-fold increase by 3 hours that gradually decreased toward baseline, while expression levels of TNF and IL-6 showed a more moderate increase and declined at 24 hours, suggesting active inhibition (Figure 1C).
Figure 1.
Repeated exposure to ultraviolet B (UVB) irradiation induces cutaneous inflammation and mixed inflammatory (inflamm.) cell response in skin. C57BL/6 mice were exposed to subacute UVB irradiation (100 mJ/cm2 for 5 days). A, Representative flow cytometry plots from UVB-irradiated skin following the final UVB dose for neutrophils (CD45+CD11b+Ly-6C+Ly-6G+), inflammatory monocytes (CD45+CD11b+Ly-6C+Ly-6G−), macrophages (CD45+CD11b+CD64+), and T cells (CD45+CD3+). B, Flow cytometry analysis showing the percentage of neutrophils, inflammatory monocytes, macrophages, and T cells in the skin 3, 24, and 72 hours following UVB irradiation. C, Cutaneous expression of mRNA for proinflammatory cytokine genes 3, 24, and 72 hours following subacute UVB injury in B6 mice, as determined by quantitative polymerase chain reaction. Fold change was calculated against a separate cohort of nonirradiated mice, using the 2−ΔΔCt method. Data are compiled from at least 3 independent experiments (n =20 mice per group). Values in B and C are the mean ±SD. * = P <0.05; ** = P <0.01; *** =P <0.001 versus no UV irradiation, by Student’s unpaired t-test.
Repeated UVB irradiation induces a type I IFN response in the skin
Because of the interest in the role of type I IFN in SLE, including cutaneous lupus, we focused on the type I IFN response resulting from UVB irradiation. Quantitative PCR evaluation of subacute UVB–irradiated skin showed a modest, although statistically significant, increase in multiple ISGs (Figure 2A). Expression of mRNA for Mx-1 protein, IRF-7, and IFIT-1 was increased up to ~4-fold at 3 hours. Generally, we noted that the peaks were bimodal, as ISG expression decreased from 3 hours to 24 hours before increasing again at 72 hours. Subacute UVB irradiation induced modest increases in IFNα gene expression, with peaks at 3 hours and 72 hours, similar to ISG expression (Figure 2B). IFNβ expression was evaluated in irradiated and nonirradiated samples; however, the mRNA expression was below the limit of detection by qPCR. To evaluate whether repeated as opposed to single dosing of UVB irradiation preferentially induced type I IFN, we compared exposure to subacute UVB irradiation with exposure to a single dose of UVB irradiation (100 mJ/cm2). While subacute UVB irradiation was effective at increasing ISG expression, the single-dose exposure caused a down-regulation of mRNA for Mx-1 protein, IRF-7, and IFIT-1 (Figure 2C).
Figure 2.
Repeated exposure to ultraviolet B (UVB) irradiation induces a modest type I interferon (IFN) response in the skin. C57BL/6 (B6) mice were exposed to subacute UVB irradiation and biopsy specimens were obtained at the time points shown. Their RNA was isolated and quantitative polymerase chain reaction (qPCR) was performed. A, Cutaneous expression of mRNA for IFN-stimulated genes following subacute UVB injury in B6 mice is shown. Fold change was calculated against a separate cohort of nonirradiated mice using the 2−ΔΔCt method. Data are compiled from at least 3 independent experiments (n =20 mice per group). B, Fold change in expression of mRNA for type I IFN genes after UVB exposure is shown. Data are compiled from at least 3 independent experiments (n =20 mice per group). C, B6 mice were exposed to either a single 100-mJ/cm2 dose of UVB irradiation or five 100-mJ/cm2 doses of UVB irradiation and examined for expression of mRNA for type I IFN response genes by qPCR, expressed as the fold change in expression relative to that in nonirradiated control mice. Data are compiled from 2 independent experiments (n =20 mice per group). Values are the mean ±SD. * = P <0.05; ** = P <0.01; *** = P <0.001 versus no UV irradiation or single-dose UVB irradiation, by Student’s unpaired t-test.
PDCs are dispensable for UVB irradiation–induced type I IFN
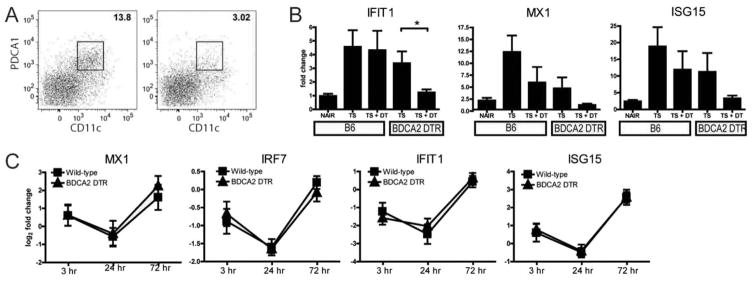
PDCs have been observed in skin biopsy samples from patients with CLE (8). To determine whether PDCs are recruited and generate type I IFN following exposure to UVB irradiation, we used BDCA-2–DTR–transgenic mice, which express the DTR under the control of the PDC-specific BDCA-2 promoter; PDCs in these mice are efficiently depleted following DT administration (15). To validate the experimental approach, we examined PDC numbers in the skin following tape stripping in BDCA-2–DTR–transgenic mice, a protocol that was previously shown to induce PDC recruitment and PDC-dependent type I IFN expression (12).
PDCs (CD45+Ly-6C+CD11c+PDCA-1+ and Siglec H positive), which were not detected in unstimulated skin (data not shown), were effectively depleted in BDCA-2–DTR–transgenic mice injected with DT but not in WT mice injected with DT 24 hours following tape stripping (Figure 3A). Quantitative PCR analysis of skin samples obtained 24 hours after tape stripping for type I IFN genes (for IFIT-1, Mx-1 protein, and ISG-15) showed abrogation of the type I IFN response in the skin in BDCA-2–DTR–transgenic mice but not in control mice (Figure 3B). To evaluate the role of PDCs in UVB irradiation–induced type I IFN, BDCA-2–DTR–transgenic mice and control mice were next subjected to the 5-day subacute irradiation protocol as described above. Mice were injected with DT every 2–3 days during the irradiation and biopsy period. Expression of mRNA for the ISGs Mx-1 protein, IRF-7, IFIT-1, and ISG-15 by qPCR was similar between the 2 groups (Figure 3C), suggesting that PDCs were not required for ISG production following UVB irradiation. Thus, while PDCs appear to be necessary for the IFN response seen in tape stripping (12), our data suggest that PDCs are dispensable for type I IFN signaling following repeated exposure of normal skin to UVB irradiation.
Figure 3.
Plasmacytoid dendritic cells (PDCs) are dispensable for ultraviolet B (UVB) irradiation–induced type I interferon (IFN) production. Transgenic mice that express the diphtheria toxin receptor (DTR) under the control of the highly specific human PDC gene promoter blood dendritic cell antigen 2 (BDCA-2–DTR–transgenic mice) as well as C57BL/6 (B6) mice were subjected to tape stripping and were injected with 125 ng of DT at −48 hours and at the time of tape stripping. A, Representative flow cytometry of cutaneous PDCs (CD45+Ly-6C+CD11c+ and PDC antigen 1 [PDCA-1] positive) in wild-type (B6) mice (left) and BDCA-2–DTR–transgenic mice (right) 24 hours following tape stripping is shown. B, Cutaneous expression of mRNA for type I IFN genes evaluated as fold change 24 hours following tape stripping is shown. NAIR =depilated with Nair only; TS =depilated with Nair and tape stripped; TS +DT =depilated with Nair, tape stripped, and injected with DT. Data are compiled from 3 independent experiments (n =8 mice per group). C, BDCA-2–DTR–transgenic mice and B6 mice were exposed to subacute UVB irradiation. Both groups were injected with 125 ng of DT every 2–3 days beginning at the first UVB exposure and through the duration of the experiment. Cutaneous expression of mRNA for IFN-stimulated genes was quantified by quantitative polymerase chain reaction and expressed as fold change. Data are compiled from 2 experiments (n =5–7 mice per group). Values in B and C are the mean ±SD. * = P <0.05 by Student’s unpaired t-test.
CCR2+ cells are recruited to skin following UVB irradiation and contribute to type I IFN signaling
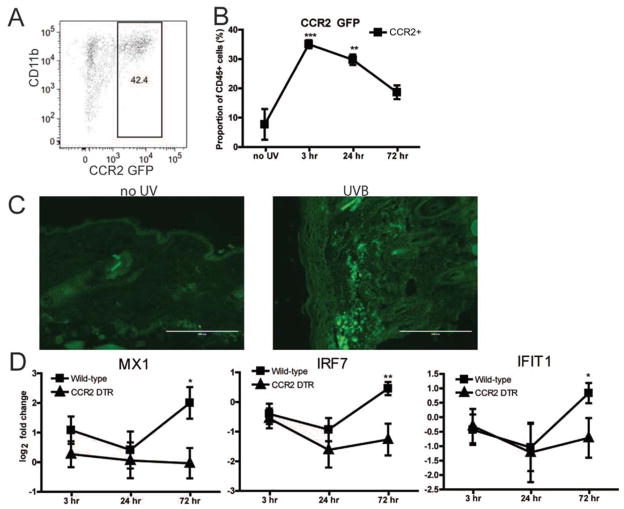
Inflammatory monocytes are recruited to tissue in response to inflammation, and they express DNA and RNA sensors capable of generating type I IFN (16,17). Since we observed cells with the phenotype of inflammatory macrophages (CD11bhighLy-6ChighLy-6Glow) in the skin following subacute UVB irradiation, we sought to determine whether this cell population was increased and could contribute to inflammation. To specifically follow this cell population, we used CCR2-GFP–transgenic mice (18) and exposed them to UVB irradiation using the subacute protocol.
UVB irradiation induced an ~5-fold increase in the number of inflammatory monocytes (CD11b+CCR2+) in the skin (Figure 4B). Serial biopsy samples showed that CCR2+ monocytes represented more than one-third of CD45+ cells in the skin 3 hours following subacute UVB irradiation, with the numbers steadily declining over the next 3 days (Figure 4B). Immunofluorescence of skin samples revealed that CCR2+ monocytes were recruited predominantly to the dermis (Figure 4C). To determine whether CCR2+ monocytes were implicated in UVB irradiation–induced expression of type I IFN, we used CCR2-DTR–transgenic mice, which express the DTR under the control of the CCR2 promoter; inflammatory monocytes are depleted in these mice following DT injection (19). After we confirmed that DT depleted inflammatory monocytes from both the spleen and skin (data not shown), CCR2-DTR–transgenic mice and WT mice were injected intraperitoneally with 250 ng DT every 2–3 days (maximum of 3 injections) and exposed to subacute UVB irradiation. In contrast to our previous experiments performed with PDC depletion, we observed that inflammatory monocyte–depleted mice had reduced expression of the ISGs for Mx-1 protein, IRF-7, and IFIT-1 24 hours and, particularly, 72 hours following subacute UVB irradiation (Figure 4D). Interestingly, expression of ISGs at 3 hours was similar between monocyte-depleted and nondepleted mice, which may suggest that an additional cell type is responsible for early type I IFN production in response to UVB irradiation.
Figure 4.
CCR2+ cells recruited to skin contribute to ultraviolet B (UVB) irradiation–induced type I interferon (IFN) production. Mice transgenic for CCR2 and green fluorescent protein (GFP) were exposed to subacute UVB irradiation. A, Representative flow cytometry plot of collagenase-digested skin 24 hours after final UVB irradiation is shown. B, Flow cytometry analysis shows the percentage of CCR2+ cells in the skin following UVB irradiation (n =6 mice). C, Representative immunofluorescence of CCR2-GFP expression in the skin before and after subacute UVB irradiation (24 hours after the final UVB dose) is shown. Bars =200 μm. D, CCR2–diphtheria toxin receptor (DTR)–transgenic mice and wild-type mice were exposed to subacute UVB irradiation. Both groups were injected with 250 ng of DT every 2–3 days beginning at the first UVB exposure and through the duration of the experiment. Cutaneous expression of mRNA for IFN-stimulated genes was assessed by quantitative polymerase chain reaction and expressed as fold change. Data are compiled from 2 experiments (n =6–8 mice per group). Values in B and D are the mean ±SD. * = P <0.05; ** = P <0.01; *** = P <0.001 versus no UV irradiation or versus CCR2–DTR–transgenic mice, by Student’s unpaired t-test.
STING mediates both the inflammatory and type I IFN responses to UVB irradiation
UVB irradiation causes cell injury and death with extensive DNA damage (20). Numerous intracellular DNA sensors converge on the core adaptor protein STING to induce the expression of type I IFN and other cytokines through IRF-3 and NF-κB pathways (11). To determine whether the STING pathway contributed to UVB irradiation–induced immune responses in vivo, we compared UVB irradiation–induced skin responses in mice lacking the gene encoding STING (STING-knockout mice) with UVB irradiation–induced skin responses in WT mice. In response to subacute UVB irradiation, we observed a marked decrease in Mx-1 protein, IRF-7, and IFIT-1 mRNA transcripts in STING-knockout mice compared to WT mice (Figure 5B), which suggests that STING is required for type I IFN production. We observed that expression of mRNA for the proinflammatory cytokines IL-1β, TNF, and IL-6 was also attenuated in STING-knockout mice (Figure 5C). Taken together, these results indicate that cytosolic nucleic acid sensor(s) and the adaptor protein STING play an important role in sensing damage and mediating immune responses following UVB irradiation.
Figure 5.
Deficiency in stimulator of interferon (IFN) genes (STING) attenuates the inflammatory cytokine response to ultraviolet B (UVB) irradiation. C57BL/6 (B6) and STING-knockout (KO) mice were exposed to subacute UVB irradiation. A, Cutaneous expression of mRNA for proinflammatory cytokine genes and IFN-stimulated genes after UVB irradiation. B and C, Expression of mRNA for IFN-stimulated genes (B) and proinflammatory cytokine genes (C) 3, 24, and 72 hours after UVB irradiation. Data are compiled from at least 3 independent experiments (n=11–20 mice per group). Values are the mean ±SD. *** = P <0.001 by Student’s unpaired t-test.
Type I IFN attenuates the UVB irradiation–induced inflammatory response in the skin
In the model of mechanical skin injury induced by tape stripping, type I IFN was observed to play a physiologic role in wound healing, since loss of IFN signaling led to delayed reepithelialization following injury (12). To evaluate whether type I IFN exerted a pathologic or beneficial role following UVB irradiation, we compared cytokine responses in WT mice with those in mice lacking the common murine receptor for IFNα/IFNβ (IFN-α/β/ω receptor [IFNAR]–knockout mice). Following subacute UVB irradiation, IFNAR-knockout mice were observed to have increased inflammatory cell infiltrates compared to WT B6 mice on light microscopic analysis (Figure 6A). Compared to WT mice, IFNAR-knockout mice consistently showed increased skin thickness and inflammatory changes, including increased tissue edema, as well as increased numbers of infiltrating cells in both the reticular dermis and subcutis. Quantification of nucleated cells in the dermis and subcutis of H&E-stained sections using image-processing software revealed increased numbers of cells in IFNAR-knockout mice at 3 hours (Figure 6B) as well as increases in expression of mRNA for CD45 and CD11b by qPCR (Figure 6D). These results suggest that type I IFN plays a role in limiting the extent of the clinical sunburn reaction in normal mice, and that in the absence of type I IFN signaling, more severe inflammation occurs following UVB irradiation.
Figure 6.
Type I interferon (IFN) attenuates ultraviolet B (UVB) irradiation–induced cell recruitment and inflammatory response. C57BL/6 (B6) and IFN-α/β/ω receptor (IFNAR)–knockout (KO) mice were exposed to subacute UVB irradiation. A, Representative images of hematoxylin and eosin staining of skin 3 hours following the final UVB dose, showing increased thickness, edema, and inflammatory cell infiltration in IFNAR-knockout mice. Original magnification × 10. B, Nucleated cell count in the dermis and subcutis at 3 hours, as measured using ImageJ software analysis of images in A (n =14–15 mice per group). C, Cutaneous expression of mRNA for inflammatory cytokine genes following subacute UVB injury in B6 and IFNAR-knockout mice. D, Cutaneous expression of mRNA for tissue mediators of inflammation genes at 3 hours. Fold change was calculated against a separate cohort of nonirradiated mice, using the 2−ΔΔCt method. Data are compiled from at least 3 independent experiments (n =15–20 mice per group). Values in B–D are the mean ±SD. * = P <0.05; ** = P <0.01, by Student’s unpaired t-test.
To evaluate whether type I IFN exerts a protective effect through binding to IFNAR and subsequent down-regulation of proinflammatory cytokines (21), we examined expression of mRNA for IL-1β, TNF, and IL-6 in IFNAR-knockout and WT mice (Figure 6C). IL-6 expression was significantly increased in IFNAR-knockout mice at 3 hours before decreasing to levels similar to those in WT mice at later time points. Although expression of both IL-1β and TNF was higher at some time points in IFNAR-knockout mice after UVB irradiation, the results were not statistically significant compared to WT mice. Three hours after UVB irradiation, IFNAR-knockout mice had significantly increased expression of mRNA for COX-2 (which generates prostaglandin E2) and tissue collagenase MMP-13 (a mediator of tissue damage following UVB irradiation), possibly implicating type I IFN in regulation of these inflammatory pathways (Figure 6D). Thus, type I IFN appears to play a physiologic role in reducing skin inflammation and plays a regulatory role in limiting expression of some proinflammatory cytokines and mediators of inflammation, thereby limiting tissue damage in normal B6 mice.
DISCUSSION
Type I IFN is strongly implicated in the pathogenesis of SLE and cutaneous lupus, and it is classically thought to be important as an adjuvant promoting pro-inflammatory responses during viral infections and in various autoimmune disorders (7). Our study identifies a novel role for type I IFN in regulating inflammatory responses in normal skin in response to repeated UVB irradiation. This was demonstrated by increased skin inflammation and enhanced expression of the proinflammatory cytokine IL-6 as well as COX-2 and MMP-13 in mice that were exposed to UVB irradiation but were unable to respond to type I IFN because they lacked the common IFN receptor. Antiinflammatory properties of type I IFN have also been shown in other contexts. For example, apoptotic cells in the spleen stimulate IFNβ production followed by induction of the immunosuppressive molecule indoleamine 2,3-dioxygenase and by recruitment of Treg cells (22). In addition, type I IFN appears to be immunosuppressive in murine models of malaria and tuberculosis (23,24) and can stimulate IL-10 production during certain viral infections (25). Our study suggests an important antiinflammatory role for UVB irradiation–induced type I IFN production in the skin, as evidenced by a reduction in inflammatory cell recruitment and reduced expression of genes mediating inflammation and tissue damage.
While both tape stripping and UVB irradiation induced type I IFN in normal skin, UVB irradiation–induced IFN appeared to play a notably different role in the skin following injury. First, the type I IFN response was much higher following tape stripping, compared to the low-dose response obtained after UVB irradiation. Further, while we observed increased levels of IL-6 mRNA in IFNAR-knockout mice following UVB irradiation, tape stripping of IFNAR-knockout mice causes significantly decreased levels of IL-6 mRNA and slightly lower levels of TNF mRNA (12). Thus, UVB irradiation–induced type I IFN had an antiinflammatory effect that limited the extent of inflammation, while tape stripping induced a proinflammatory effect that was important for wound healing (12). The differences between UVB irradiation and tape stripping suggest that the type or duration of injury is important in determining the inflammatory response. In addition to differences in PDC involvement (see below), different nucleic acid sensing pathways could be implicated. Consistent with a role for PDCs, TLR-7 and TLR-9 pathways were necessary for type I IFN stimulation in the tape stripping model, while TLR-3 (26) and, in our studies, the STING pathway are implicated in mediating responses to UV irradiation.
Yin et al (27) recently reported stimulation of a transient type I IFN response in mouse skin after 2 high doses of UVB irradiation (500 mJ/cm2). The authors suggested that PDCs were the source of type I IFN, although this was not tested directly by depletion studies. In contrast, we were not able to demonstrate PDC recruitment to UVB-irradiated skin in our subacute UVB irradiation model by flow cytometry (data not shown). Furthermore, while PDC depletion attenuated ISG responses in tape-stripped mice, PDC depletion in mice exposed to UVB irradiation neither altered ISG mRNA levels nor recapitulated the phenotype seen in IFNAR-knockout mice. While it is possible that differences in the UVB irradiation regimens explains the altered outcomes, the CD11c+PDCA-1+ population seen by Yin et al (27) may not be typical PDCs, considering the lack of specificity of many traditional cell markers in skin populations (28).
In contrast to the dearth of PDCs, we observed that more than one-third of cells in the skin examined early after the subacute protocol were inflammatory monocytes. Furthermore, when these cells were depleted, ISG expression was diminished. Inflammatory monocytes were previously shown to produce type I IFN following viral infection (16,17), but their role in type I IFN production in the skin following UVB irradiation in the skin, whether direct or indirect, is a novel observation. We noticed that the reduction of inflammatory monocyte–mediated type I IFN response was maximal at the 72-hour time point, leaving open the possibility that other cells such as keratinocytes and/or fibroblasts participate in the earlier (at ~3 hours) type I IFN response. Further investigation is underway to identify the IFN-producing populations following subacute UV irradiation, as many resident immune (dermal dendritic cells, mast cells) and nonimmune (keratinocytes, fibroblasts) cell populations have been reported to produce type I IFN (13,29,30).
The striking decrease of both ISG and proinflammatory gene levels by qPCR in STING-deficient mice suggests that STING plays an important role in transmitting cytokine responses in response to UVB irradiation. STING-mediated TANK-binding kinase 1 (TBK-1) activity may explain the effects of STING deficiency observed in our model. STING-induced TBK-1 is required for type I IFN production via IRF-3, but it also promotes canonical NF-κB activity and generation of the inflammatory cytokines TNF and IL-6 (31,32). Interestingly, TBK-1–deficient mice spontaneously develop inflammatory skin lesions and activation of monocytes (32). Taken together with our data showing similar features in IFNAR-deficient mice, this may suggest a critical role for TBK-1–mediated type I IFN in limiting harmful inflammatory responses to environmental triggers in normal skin.
Using cells in vitro, Gehrke et al (33) reported that UV irradiation induced type I IFN through activation of the STING pathway. UVB and UVC are well known to oxidize guanine to generate 8-hydroxydeoxyguanosine (8-OHdG). Interestingly, Gehrke et al showed that oxidized DNA is more resistant to the 3–5′ repair exonuclease 1 (TREX-1)–mediated degradation, resulting in increased type I IFN production in TREX-1–deficient cells (33). In addition, it has been previously demonstrated that 8-OHdG is readily detected in the skin lesions of lupus patients as well as in the UV-irradiated skin of healthy individuals (33,34). Our observation that STING-knockout mice have markedly reduced levels of ISGs in response to UVB irradiation suggests that oxidation of DNA by UVB irradiation, with sequential activation of cyclic GMP-AMP cyclase and STING (35), is a key pathway in initiation of type I IFN production in normal skin. However, other pathways contributing to nucleic acid sensing and production of inflammatory cytokines such as TLR-3 (26) may also be involved.
In summary, we identified a novel role for a STING-dependent low-dose type I IFN response in protection against UVB irradiation–induced inflammation and tissue damage. Inflammatory monocytes, as opposed to PDCs, were required for type I IFN responses. Given the heightened sensitivity of lupus patients to UVB exposure–induced skin damage, further studies are needed to assess whether the initial type I IFN pathway is altered in lupus patients and whether autoantibody binding to cell death antigens to generate ICs that activate FcγRIIa on PDCs overwhelms regulatory processes and leads to uncontrolled type I IFN production.
Acknowledgments
Dr. Sontheimer’s work was supported by T32 Training Grants in rheumatology and dermatology from the NIH (grants AR-007108-35 and T32-AR-056969), a Rheumatology Research Foundation Scientist Development Award, and a Research Fellowship Award from Mallinckrodt Pharmaceuticals. Dr. Elkon’s work was supported by the Lupus Research Institute and the NIH (National Institute of Environmental Health Sciences grant R21-ES-024437).
The authors would like to thank Thomas Teal and Nalini Agrawal for technical assistance and Lucrezia Colonna and Christian Lood for comments and discussion of the manuscript.
Footnotes
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Elkon had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Sontheimer, Elkon.
Acquisition of data. Sontheimer, Elkon.
Analysis and interpretation of data. Sontheimer, Liggitt, Elkon.
References
- 1.Pistiner M, Wallace DJ, Nessim S, Metzger AL, Klinenberg JR. Lupus erythematosus in the 1980s: a survey of 570 patients. Semin Arthritis Rheum. 1991;21:55–64. doi: 10.1016/0049-0172(91)90057-7. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann P, Holzle E, Kind P, Goerz G, Plewig G. Experimental reproduction of skin lesions in lupus erythematosus by UVA and UVB radiation. J Am Acad Dermatol. 1990;22:181–7. doi: 10.1016/0190-9622(90)70020-i. [DOI] [PubMed] [Google Scholar]
- 3.LeFeber WP, Norris DA, Ryan SR, Huff JC, Lee LA, Kubo M, et al. Ultraviolet light induces binding of antibodies to selected nuclear antigens on cultured human keratinocytes. J Clin Invest. 1984;74:1545–51. doi: 10.1172/JCI111569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Achtman JC, Werth VP. Pathophysiology of cutaneous lupus erythematosus. Arthritis Res Ther. 2015;17:182. doi: 10.1186/s13075-015-0706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowson AN, Magro C. The cutaneous pathology of lupus erythematosus: a review. J Cutan Pathol. 2001;28:1–23. doi: 10.1034/j.1600-0560.2001.280101.x. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa F, Kashihara-Sawami M, Lyons MB, Norris DA. Binding of antibodies to the extractable nuclear antigens SS-A/Ro and SS-B/La is induced on the surface of human keratinocytes by ultraviolet light (UVL): implications for the pathogenesis of photosensitive cutaneous lupus. J Invest Dermatol. 1990;94:77–85. doi: 10.1111/1523-1747.ep12873930. [DOI] [PubMed] [Google Scholar]
- 7.Elkon KB, Wiedeman A. Type I IFN system in the development and manifestations of SLE. Curr Opin Rheumatol. 2012;24:499–505. doi: 10.1097/BOR.0b013e3283562c3e. [DOI] [PubMed] [Google Scholar]
- 8.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon-α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–43. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Braunstein I, Klein R, Okawa J, Werth VP. The interferon-regulated gene signature is elevated in subacute cutaneous lupus erythematosus and discoid lupus erythematosus and correlates with the cutaneous lupus area and severity index score. Br J Dermatol. 2012;166:971–5. doi: 10.1111/j.1365-2133.2012.10825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bave U, Magnusson M, Eloranta ML, Perers A, Alm GV, Ronnblom L. FcγRIIa is expressed on natural IFN-α-producing cells (plasmacytoid dendritic cells) and is required for the IFN-α production induced by apoptotic cells combined with lupus IgG. J Immunol. 2003;171:3296–302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 11.Barrat FJ, Elkon KB, Fitzgerald KA. Importance of nucleic acid recognition in inflammation and autoimmunity. Annu Rev Med. 2016;67:323–36. doi: 10.1146/annurev-med-052814-023338. [DOI] [PubMed] [Google Scholar]
- 12.Gregorio J, Meller S, Conrad C, Di Nardo A, Homey B, Lauerma A, et al. Plasmacytoid dendritic cells sense skin injury and promote wound healing through type I interferons. J Exp Med. 2010;207:2921–30. doi: 10.1084/jem.20101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamoutounour S, Guilliams M, Montanana Sanchis F, Liu H, Terhorst D, Malosse C, et al. Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity. 2013;39:925–38. doi: 10.1016/j.immuni.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Sharma MR, Werth B, Werth VP. Animal models of acute photodamage: comparisons of anatomic, cellular and molecular responses in C57BL/6J, SKH1 and Balb/c mice. Photochem Photobiol. 2011;87:690–8. doi: 10.1111/j.1751-1097.2011.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8+ T cell accrual. Immunity. 2010;33:955–66. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu RH, Wong EB, Rubio D, Roscoe F, Ma X, Nair S, et al. Sequential activation of two pathogen-sensing pathways required for type I interferon expression and resistance to an acute DNA virus infection. Immunity. 2015;43:1148–59. doi: 10.1016/j.immuni.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbalat R, Lau L, Locksley RM, Barton GM. Toll-like receptor 2 on inflammatory monocytes induces type I interferon in response to viral but not bacterial ligands. Nat Immunol. 2009;10:1200–7. doi: 10.1038/ni.1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satpathy AT, Briseno CG, Lee JS, Ng D, Manieri NA, Kc W, et al. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol. 2013;14:937–48. doi: 10.1038/ni.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hohl TM, Rivera A, Lipuma L, Gallegos A, Shi C, Mack M, et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–81. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Laethem A, Garmyn M, Agostinis P. Starting and propagating apoptotic signals in UVB irradiated keratinocytes. Photochem Photobiol Sci. 2009;8:299–308. doi: 10.1039/b813346h. [DOI] [PubMed] [Google Scholar]
- 21.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–23. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Ravishankar B, Liu HY, Shinde R, Chandler P, McGaha T. Innate and adaptive tolerance to apoptotic cells is controlled by an IDO-dependent mechanism [abstract] J Immunol. 2012;(Suppl 123):49. [Google Scholar]
- 23.Haque A, Best SE, Ammerdorffer A, Desbarrieres L, de Oca MM, Amante FH, et al. Type I interferons suppress CD4+ T-cell-dependent parasite control during blood-stage plasmodium infection. Eur J Immunol. 2011;41:2688–98. doi: 10.1002/eji.201141539. [DOI] [PubMed] [Google Scholar]
- 24.Manca C, Tsenova L, Freeman S, Barczak AK, Tovey M, Murray PJ, et al. Hypervirulent M. tuberculosis W/Beijing strains upregulate type I IFNs and increase expression of negative regulators of the Jak-Stat pathway. J Interferon Cytokine Res. 2005;25:694–701. doi: 10.1089/jir.2005.25.694. [DOI] [PubMed] [Google Scholar]
- 25.Arimori Y, Nakamura R, Yamada H, Shibata K, Maeda N, Kase T, et al. Type I interferon limits influenza virus-induced acute lung injury by regulation of excessive inflammation in mice. Antiviral Res. 2013;99:230–7. doi: 10.1016/j.antiviral.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, et al. Ultraviolet radiation damages self non-coding RNA and is detected by TLR3. Nat Med. 2012;18:1286–90. doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin Q, Xu X, Lin Y, Lv J, Zhao L, He R. Ultraviolet B irradiation induces skin accumulation of plasmacytoid dendritic cells: a possible role for chemerin. Autoimmunity. 2014;47:185–92. doi: 10.3109/08916934.2013.866105. [DOI] [PubMed] [Google Scholar]
- 28.Malissen B, Tamoutounour S, Henri S. The origins and functions of dendritic cells and macrophages in the skin. Nat Rev Immunol. 2014;14:417–28. doi: 10.1038/nri3683. [DOI] [PubMed] [Google Scholar]
- 29.Farina GA, York MR, Di Marzio M, Collins CA, Meller S, Homey B, et al. Poly(I:C) drives type I IFN- and TGFβ-mediated inflammation and dermal fibrosis simulating altered gene expression in systemic sclerosis. J Invest Dermatol. 2010;130:2583–93. doi: 10.1038/jid.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lappalainen J, Rintahaka J, Kovanen PT, Matikainen S, Eklund KK. Intracellular RNA recognition pathway activates strong anti-viral response in human mast cells. Clin Exp Immunol. 2013;172:121–8. doi: 10.1111/cei.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abe T, Barber GN. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-κB activation through TBK1. J Virol. 2014;88:5328–41. doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchlik E, Thakker P, Carlson T, Jiang Z, Ryan M, Marusic S, et al. Mice lacking Tbk1 activity exhibit immune cell infiltrates in multiple tissues and increased susceptibility to LPS-induced lethality. J Leukoc Biol. 2010;88:1171–80. doi: 10.1189/jlb.0210071. [DOI] [PubMed] [Google Scholar]
- 33.Gehrke N, Mertens C, Zillinger T, Wenzel J, Bald T, Zahn S, et al. Oxidative damage of DNA confers resistance to cytosolic nuclease TREX1 degradation and potentiates STING-dependent immune sensing. Immunity. 2013;39:482–95. doi: 10.1016/j.immuni.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed NU, Ueda M, Nikaido O, Osawa T, Ichihashi M. High levels of 8-hydroxy-2′-deoxyguanosine appear in normal human epidermis after a single dose of ultraviolet radiation. Br J Dermatol. 1999;140:226–31. doi: 10.1111/j.1365-2133.1999.02653.x. [DOI] [PubMed] [Google Scholar]
- 35.Lood C, Blanco LP, Purmalek MM, Carmona-Rivera C, de Ravin SS, Smith CK, et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat Med. 2016;22:146–53. doi: 10.1038/nm.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]