Abstract
How epithelial tissues are able to self-renew to maintain homeostasis and regenerate in response to injury remains a persistent question. The transcriptional effectors YAP and TAZ are increasingly being recognized as central mediators of epithelial stem cell biology, and a wealth of recent studies have been directed at understanding the control and activity of these factors. Recent work by Hu et al. [1] has added to this knowledge, as they identify an Integrin-FAK-CDC42-PP1A signaling cascade that directs nuclear YAP/TAZ activity in stem cell populations of the mouse incisor, and define convergence on mTORC1 signaling as an important mediator of the proliferation of these cells. Here, we review recent studies on YAP/TAZ function and regulation in epithelial tissue-specific stem cells, merging the Hu et al. study together with our current knowledge of YAP/TAZ.
Keywords: stem cells, YAP/TAZ
Graphical abstract
The Hippo pathway effectors YAP and TAZ broadly regulate adult stem cells, and have recently been implicated in dental epithelial stem cell proliferation and restricted differentiation via an Integrin-FAK-CDC42-PP1A cascade. Here we discuss the extracellular cues which control YAP/TAZ activity and examine downstream pathways that direct stem cell fate.
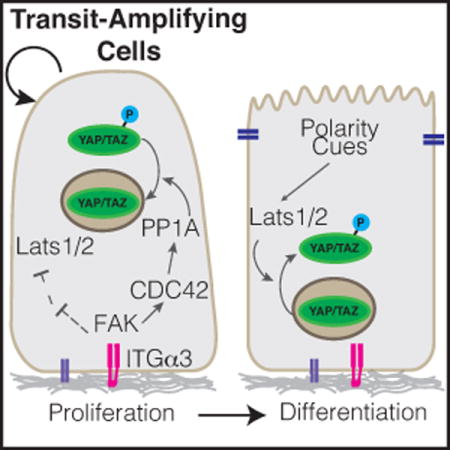
Introduction
The ability of epithelial tissues to balance cell proliferation and differentiation is essential for proper organ development and homeostasis, and is critical for coordinating regenerative responses following injury. One signaling pathway that has emerged as central in transducing cues that impact stem/progenitor cell proliferation and differentiation is the Hippo pathway. The Hippo signaling pathway is composed of a set of factors that direct the action of a kinase cascade controlling the function of two paralogous transcriptional regulators: YAP (Yes-associated protein; YAP1) and TAZ (transcriptional co-activator with a PDZ-binding domain; also known as WW domain containing transcription regulator 1, or WWTR1) (hereafter referred to together as YAP/TAZ). First identified in Drosophila melanogaster for its roles in tissue growth control [2–8], the Hippo pathway has emerged as an important mediator of various developmental processes, as a regulator of tissue regeneration, and as a tumor suppressor [9]. In brief, the core mammalian Hippo pathway is comprised of Mammalian Ste20-like (MST) serine/threonine kinase family that phosphorylate and activate the Nuclear Dbf (NDR) family kinases LATS1 and LATS2 (hereafter referred to together as LATS1/2), which subsequently phosphorylate YAP/TAZ to direct their localization, stability and activity [2,10–12]. Hypo-phosphorylated YAP/TAZ accumulate in the nucleus, where they regulate gene expression. Upon phosphorylation, YAP/TAZ are excluded from the nucleus and generally targeted for degradation, consequently resulting in low YAP/TAZ-mediated transcriptional activity [10].
Several phosphorylation sites are described to control YAP/TAZ function, the best characterized being the phosphorylation of a serine residue that promotes binding to the 14-3-3 family proteins (Ser127 in human YAP; S89 in human TAZ) [13,14]. A canonical phosphodegron motif (DSGXXS) within YAP/TAZ also plays important regulatory roles, as the phosphorylation of this motif is implicated in YAP/TAZ recognition and ubiquitination by the β-TRCP/SCF ubiquitin ligase complex, and subsequent targeting for proteasomal degradation [15,16]. LATS1/2-mediated phosphorylation of YAP/TAZ on a conserved serine residue (Ser397 in human YAP-isoform 1; S311 in human TAZ) has been shown to prime the neighboring phosphodegron motif for phosphorylation by CK1δ/ε kinases, providing multi-level control of YAP/TAZ levels. Several other phosphorylation sites have been shown to control YAP/TAZ activity, some of which direct divergent regulation. For example, YAP has recently been shown to be phosphorylated by AMPK on Ser94 under conditions of energy stress, consequently disrupting YAP binding to the TEAD family of transcription factors [17], which is one of the major family of transcription factors regulated by YAP/TAZ. Osmotic stress has also recently been shown to lead to Nemo-like kinase (NLK)-mediated phosphorylation of YAP on Ser128, which increases nuclear YAP levels [18,19], possibly by disrupting interactions with or modification of the neighboring Ser127 residue. Phosphorylation of TAZ by glycogen synthase kinase 3β (GSK3β) on Ser58 and Ser62 in human TAZ has been shown to recruit the β-TrCP ubiquitin ligase, targeting TAZ for degradation [20]. Similar GSK3β-mediated regulation of YAP has not been described, offering an explanation for why TAZ is more unstable than YAP in many cell culture models. Genetic studies in mice have shown that dysregulation of YAP/TAZ phosphorylation has severe consequences on the development and homeostasis of various organs. Thus, the control of YAP/TAZ phosphorylation serves as a key mode of regulation for the localization and stability of these factors (key phosphorylation sites are outlined in Figure 1), and understanding the context-specific mechanisms regulating these modifications becomes important for potential interception of disease and our ability to control regenerative events.
Figure 1.
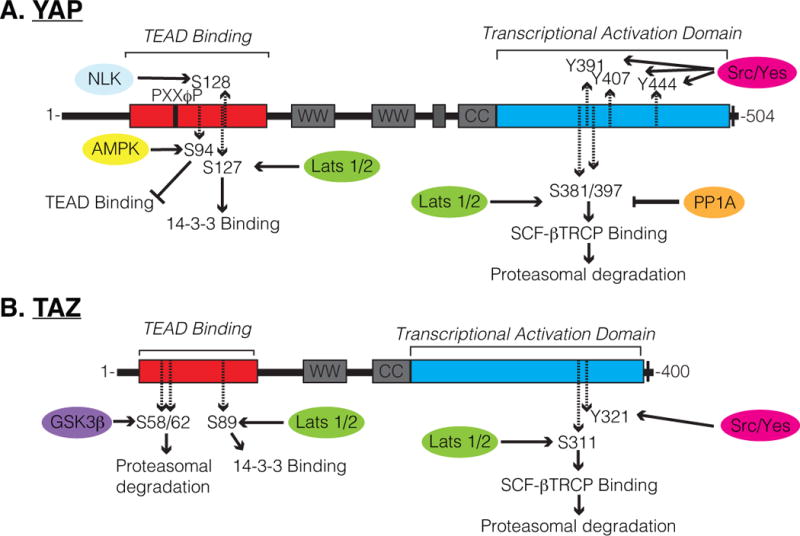
Schematic of (A) YAP and (B) TAZ regulatory domains and phosphorylation sites. Several residues described to be phosphorylated are highlighted, including S397 within human YAP-Isoform1, at which phosphorylation by Lats1/2 is known to prime YAP for SCF-βTRCP-mediated proteasomal degradation, and is now shown by Hu et al. to be dephosphorylated by FAK-CDC42-PP1A signaling cascade.
Hu et al. [1] have recently added to our knowledge of YAP/TAZ regulation and function by studying YAP/TAZ-directed stem cell regulation in the adult mouse incisor. Using a combination of genetic models and explant culture experiments the group showed an active role for YAP/TAZ in promoting mTORC1-mediated proliferation of transit-amplifying cells in the inner enamel epithelium. Deletion of YAP/TAZ in adult dental epithelium with the use of a Cre-recombinase under the control of the Keratin14 (Krt14) promoter led to dramatic tissue loss in the incisor epithelium in locations of high cellular turnover, where stem and transit-amplifying cells are actively proliferating and differentiating. They further showed that as quiescent stem cells shift to become transit-amplifying cells, an integrin-FAK-CDC42-PP1A cascade acts to dephosphorylate YAP, driving nuclear accumulation and consequent upregulation of target genes that direct transit-amplifying cell proliferation. A notable target identified was Rheb, which is a positive regulator of the mTORC1 complex. Signaling via the mTORC1 pathway is essential for coordinating nutrient availability with cell growth and plays important roles in stem cell proliferation control [21], thus the study by Hu et al. [1] offers insight into how Hippo-mTORC1 signaling may converge to direct stem cell dynamics. Below, we briefly review our current knowledge of YAP/TAZ in epithelial stem cell biology, comparing the recent findings by Hu et al. [1] in the mouse dental epithelium with our knowledge of YAP/TAZ in other organs.
Transcriptional regulation by YAP and TAZ
Though they lack a direct DNA binding domain, YAP/TAZ are able to exert transcriptional control via a C-terminal transcriptional activation domain, a N-terminal PxxΦP motif that mediates binding with the TEAD family of transcription factors, and a WW domain that mediates binding with various other transcription factors [22–25]. Chromatin immunoprecipitation experiments have revealed that YAP and TAZ generally occupy enhancer regulatory regions [26–29], and function to recruit chromatin remodeling complexes, such as the Mediator complex [27], p300 [30,31], the SWI/SNF complex [32], and the NuRD [26] and NCoR1 [33] repressor complexes. In doing so, YAP/TAZ direct both the activation and repression of gene expression.
Context for YAP/TAZ-regulated gene expression in stem/progenitor cell control is dictated by associated transcription factor complexes. For example, YAP interacts with the transcription factor p63 in bronchial epithelium to direct gene expression required to maintain the basal stem cell state [34]. In the intestinal epithelium, YAP cooperativity with different transcription factors modulates the balance between proliferation and cell fate commitment: in intestinal stem cells, YAP acts in concert with TEADs to promote proliferation, while cooperation with Klf4 can direct differentiation to the goblet cell fate [35]. Binding to Smad transcription factors direct TGF-beta-regulated processes important for human embryonic stem cell maintenance and differentiation [26,36], and interaction with beta-catenin controls Wnt-regulated processes during cardiomyocyte proliferation [37]. Further, interaction with the TEAD transcription factors and convergence with Activator Protein-1 transcriptional complex is emerging as important for driving pro-tumorigenic events in various cancers [29,38]. Thus, increasing our knowledge into YAP/TAZ-binding transcription factor complexes will undoubtedly offer better insight into the context of YAP/TAZ activity.
YAP/TAZ direct tissue specific stem/progenitor cell dynamics
Analyses of YAP/TAZ activity in a variety of tissues have demonstrated common and differing roles for YAP and TAZ in stem/progenitor cell expansion in development and regeneration. Conditional deletion studies of YAP/TAZ in developing and adult animals have shown the depletion of stem/progenitor cell populations in several organs [39], including the lung [34,40], salivary gland [41], pancreas [42,43], epidermis [44,45], and, most recently, the incisors, as demonstrated by Hu et al. [1]. The potency of YAP/TAZ for establishing and maintaining lineage specific stem cells has been further demonstrated by the ability of transient YAP or TAZ expression to reprogram differentiated mammary, neural, and pancreatic cells into local tissue specific stem cells with self-renewal and differentiation capacity [46]. Whether divergent or functional redundancy between YAP and TAZ exists in these various contexts has been explored in some studies. Observations reported by Hu et al. [1] indicate that functional redundancy exists in the dental epithelium, as severe defects, such as the complete loss of the labial cervical loop, a region that contains dental epithelial progenitor cells, are observed only following YAP/TAZ deletion. Interestingly, in the dental epithelium TAZ appears to shift localization following YAP deletion or forced nuclear YAP exclusion, indicating the existence of regulated mechanisms that compensate for the loss of nuclear YAP activity. YAP levels in cell culture are reported to have an inverse correlation to TAZ levels, with reduced YAP levels increasing TAZ stability and conversely increased YAP levels reducing TAZ stability in a GSK3-dependent manner [47], providing a potential mechanism for how YAP/TAZ functional redundancy is regulated. However, such mechanisms remain to be explored in an in vivo context. YAP and TAZ exhibit similar redundancy in the epidermis, as deletion of both YAP/TAZ in Keratin5 (Krt5)-positive epidermal stem cells results in severe hypoplasia and defective skin repair following wounding, phenotypes that are more apparent than those observed following the individual deletion of YAP or TAZ [48]. Despite this redundancy, YAP appears to play a more dominant role than TAZ, as lineage specific deletion of YAP is sufficient to reduce the numbers of epidermal stem cells, and lead to hypoplasia [49,50].
Epidermal stem cells exhibit elevated nuclear YAP levels, and YAP exits the nucleus in differentiating and mature keratinocytes [48,49]. Recent observations indicate that in epidermal stem cells nuclear YAP/TAZ promotes the expression of Delta-like ligands, which serve as ‘in cis’ inhibitors of Notch signaling and consequently promote the stem cell state [51]. Hyper-activation of nuclear YAP activity through induced expression of the YAP-S127A mutant has been shown to promote the expansion of epidermal stem cells into the suprabasal compartment in vivo. Expression of a YAP-TEAD binding mutant (S79A) phenocopies the YAP knockout in the epidermis, indicating that in this context YAP function is dependent on transcriptional regulation of the TEAD family of transcription factors [50,52]. Much like the dental epithelium, the mature epidermis represents a prototypical example of a tissue undergoing continuous cycles of stem cell self-renewal and differentiation to maintain homeostasis and therefore based on these observations it is logical to speculate that YAP/TAZ are critical in tissues with high cellular turnover. However, arguing against this idea, YAP/TAZ are dispensable in the homeostatic intestinal epithelium, another tissue with high turnover rates, and only are required under regenerative conditions following injury [53–55]. Thus, the question of why the homeostasis of only some epithelial tissues rely on YAP/TAZ remains to be better understood.
YAP is also essential for the control of various other somatic stem cell populations, including those found in the airway epithelium of the lung and trachea. Like the epidermis and the dental epithelium, airway epithelial stem cells express Krt5 and the transcription factor p63, suggesting common features between these cells that may relate to YAP/TAZ activity (e.g. regulation of similar transcriptional factors, such as p63, and similar target genes). The airway epithelium is arranged as a pseudostratified layer of cells, with YAP exhibiting predominantly cytoplasmic localization in the luminal positioned differentiated cells, and pronounced nuclear localization in the basal positioned stem cells [34,40]. Expression of the YAP-S127A mutant in Krt5-positive basal stem cells promotes hyperplastic overgrowth, while deletion of YAP leads to aberrant differentiation of this cell population [34]. Though manipulation of YAP expression within the airway leads to severe epithelial defects, TAZ activity has not yet been well studied in this region of the lung. Developing lung epithelium relies predominantly on YAP activity, as deletion of YAP results in severe branching and patterning defects that are not observed following the deletion of TAZ [40,56,57]. However, TAZ does play important roles in lung development, as TAZ null mice exhibit alveolarization defects and the onset of emphysema-like phenotypes [56,57]. Similar to the lung, deletion of YAP in the developing salivary gland epithelium results in branching and patterning defects [41]. In these branching organs, deletion of YAP results in the failure to specify progenitors that give rise to the ductal epithelium, indicating that YAP serves to integrate the converging signals required for ductal patterning during the branching morphogenesis program.
The relationship between YAP/TAZ and mTOR signaling
While the transcriptional regulatory activity of YAP/TAZ is clearly important for stem/progenitor cell maintenance and expansion, the precise targets and mechanisms by which YAP/TAZ regulate “stemness” are not well defined. Hu et al. [1] provide evidence that YAP/TAZ mediated induction of mTOR signaling plays a role in the proliferation of transit-amplifying cells in the inner enamel epithelium, and that this is achieved in part by the YAP/TAZ-directed increases in the expression of Rheb, which encodes a GTP binding protein that activates mTORC1. Given the intimate relationship between mTOR signaling, nutrient sensing, and metabolic control, convergence with mTOR offers a logical downstream mechanism for how YAP/TAZ direct stem/progenitor cell survival and expansion. Consistent with such a relationship, mTOR contributes, at least in part, to YAP-induced basal cell hyperplasia in the epidermis, as inhibition with LY294002 suppresses the hyperplastic phenotype [58].
Given the transcriptional data provided by Hu et al. [1], it is difficult to discern whether this positive regulation of mTOR signaling occurs solely as a result of Rheb regulation. Indeed, several points of convergence between mTOR and YAP/TAZ signaling have been described. YAP increases miR-29 levels, which negatively regulate the mTOR antagonist PTEN, thereby promoting active mTOR signaling [58]. HEK-293A cell culture growth is regulated by YAP/TAZ-induced expression of amino acid uptake receptors, such as the leucine transporter, which increases intracellular amino acid levels and consequently activates mTORC1 signaling [59]. Further, YAP/NuRD cooperativity in breast epithelial cells has been shown to directly suppress the expression of DDIT4, a known inhibitor of mTOR signaling [60]. Thus, it is likely the relationship between YAP/TAZ and mTOR signaling is multilayered, and likely defined by the local cellular context.
While the data provided by Hu et al. [1] support a role for mTOR signaling in transit-amplifying cell expansion, it is worth noting that observations in keratinocytes and hematopoietic stem cells indicate that mTOR paradoxically directs the quiescence of these local stem cells [61–63]. Thus, additional tissue specific cues may modulate the roles of mTOR signaling in directing progenitor cell dynamics. Further work remains to determine the mechanisms by which YAP/TAZ transcriptionally regulate the signaling cascade, and establish how YAP/TAZ restrain and balance the antagonistic proliferative and quiescence cues that mTOR may exert within local tissue progenitors.
Independent signals control the phosphorylation and de-phosphorylation of YAP/TAZ
The Hippo pathway, encompassing the kinase cascade of MST1/2 and LATS1/2, is the best studied YAP/TAZ regulatory pathway. Through inducible lineage specific Cre-mediated knockout experiments, the MST1/2 kinases have been shown to participate in the nuclear exclusion of YAP and TAZ in a number of organs including the lung [64,65], intestine [66], liver [67–70] and pancreas [42]. Although the core Hippo pathway presents a mechanism of YAP/TAZ control, the roles of several effectors of the pathway are not conserved across different organs. For example, in the epidermis, YAP is regulated via MST1/2 independent mechanisms, as deletion of both MST1/2 has no effect on epidermal organization or YAP S127 phosphorylation [50]. Similarly, Hu et al. [1] demonstrated that the activity of YAP in the dental epithelial stem cells and transit-amplifying cells is independent of MST1/2 activity. LATS1/2 activity, however, appears to be central for YAP/TAZ localization control in most in vivo contexts, including the dental epithelium, highlighting that alternate upstream cues, such as those potentially mediated by the MAP4K family of kinases [71,72], direct LATS1/2 kinase activation to control of YAP/TAZ activity.
In addition to kinases, emerging evidence indicates important roles for phosphatases in the positive regulation of YAP/TAZ transcriptional activity [50,73,74], however, relatively little attention has been dedicated to such regulation in an in vivo context. Hu et al. [1] now provide evidence that the dephosphorylation of YAP may be equally as important as phosphorylation by the LATS1/2 kinases for controlling YAP activity, and consequently regulation of progenitors in the dental epithelium. They showed that a signaling axis composed of ITGA3-FAK-CDC42 is activated specifically in transit-amplifying cells of the dental epithelium, and that this network promotes the binding of the PP1A phosphatase complex to YAP, driving increased nuclear YAP activity. Interestingly, PP1A was shown to dephosphorylate Ser397 within YAP, doing so independently of LATS1/2 activity. Given that this residue has been shown to prime the phosphodegron for phosphorylation [15], an obvious molecular mechanism for YAP nuclear activation is that ITGA3-FAK-CDC42 signaling promotes YAP stability and consequently results in its increased nuclear levels. However, evidence presented by Hu et al. [1] suggests that the modification of Ser397 may impact YAP localization independent of alterations in protein levels. Therefore, further molecular studies are required to clarify the mechanisms behind the regulation and functional consequences of this modification.
Integrin and extracellular matrix-regulated cues control YAP and TAZ
Integrin-FAK signaling as a general mechanism for controlling YAP/TAZ activity in stem cells is appealing, as many YAP/TAZ-regulated stem cell populations exhibit direct contact with the basement membrane and thus are in a position to receive integrin-activating instructive cues to maintain the stem cell state (Figure 2). The epidermis provides perhaps the most striking example of this model, as stem cell populations reside at the basal layer of a stratified epithelium, and differentiating daughter cells are completely separated from the basement membrane via asymmetric cell division [75]. Indeed, recent work by Thompson and colleagues [48] has demonstrated that integrin-mediated signals activated by the basement membrane function in the epidermis to direct YAP/TAZ activity. In the epidermis, Integrin-FAK-SRC signaling was shown to direct nuclear YAP/TAZ localization in basal cells via a positive feedback loop involving the expression of YAP/TAZ target genes that induce integrin signaling, such as Integrins α3, α6, β1, β2, β4, and CYR61, CTGF, and AREG. Interestingly, β4 integrin-mediated activation of SRC tyrosine kinase has been shown to promote the direct phosphorylation of tyrosine residues within the transcriptional activation domain of YAP, leading to increased YAP transcriptional activity [76]. Positive feedback signaling between integrin-SRC and YAP is proposed to be disrupted as differentiating keratinocytes lose contact with the basement membrane enabling increased phosphorylation and a consequent nuclear exit of YAP/TAZ [48].
Figure 2.
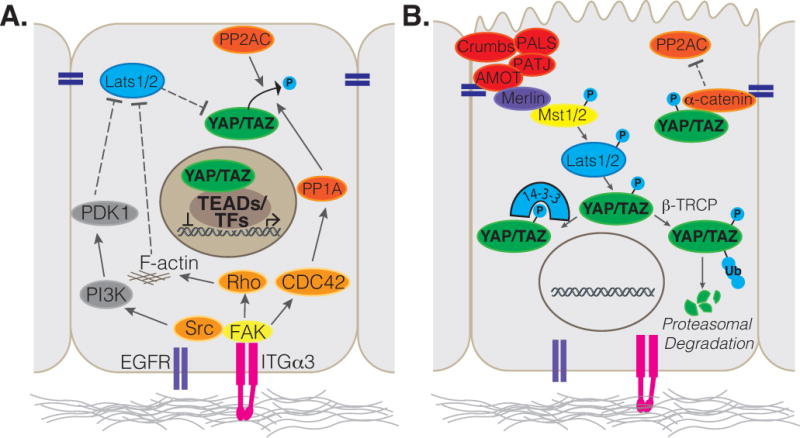
Illustration depicting (A) basal signals that are induced by Integrins to promote YAP/TAZ nuclear localization and activity, and (B) apical cues described to restrict YAP/TAZ from the nucleus.
Studies in epithelial cell lines have also demonstrated that YAP nuclear localization is promoted by a FAK-SRC-PI3K-PDK1 cascade that acts to inhibit LATS1/2 in response to integrin mediated adhesion to fibronectin [77]. The intestinal epithelium presents an in vivo view of YAP regulation through SRC family tyrosine kinases, where activated SRC promotes the phosphorylation of YAP by the tyrosine kinase, YES, and additionally leads to tyrosine phosphorylation and activation of FAK in the intestinal stem cells, stabilizing YAP levels with reduced phosphorylation at S127 and S397 [78,79]. A direct mechanism for YAP regulation by FAK is not described in this context, and it will therefore be interesting to learn whether this is mediated through activation of phosphatases that direct YAP activity.
Given the central role for mechanical stimuli in regulating the subcellular localization of YAP/TAZ [80,81], the underlying matrix stiffness may additionally direct YAP/TAZ activity. Recent work has described a role for Integrin-FAK signaling in the regulation of YAP via mechanical cues in hepatocellular carcinoma [82]. Further work has also described that in addition to receiving and transducing mechanical stimuli, YAP and TAZ execute a feedback loop to direct focal adhesion formation and thereby regulate cell stiffness and mechanosensing [83]. Additionally, while Integrins and FAK serve as basal cues to activate nuclear YAP/TAZ activity, it is likely that extracellular signaling molecules and growth factors relay Hippo pathway signals or control YAP/TAZ phosphatase activation. This includes secreted growth factor-mediated activation of SRC via stimulation of the EGFR, and extracellular lipids, such as LPA, which act through GPCRs to activate PI3K [84]. An addition GPCRs, such as GNAS, the gene coding for the Gαs heterotrimeric G protein, has been shown to restrict the nuclear activity of YAP in the epidermis, has been shown to restrict the nuclear activity of YAP in the epidermis, with induced expression of active Gαs driving basal stem cell exhaustion [85]. These signaling cascades that control YAP/TAZ localization may therefore function in concert with mechanical cues from the basement membrane, or serve as alternate mechanisms for YAP/TAZ activation in tissues where basal signals exert less control over progenitor cell fate.
Apical-Basal polarity and junctional complexes restrain YAP/TAZ nuclear activity
In several organs, the loss of basement membrane contact is not a requirement for stem cell differentiation. For example, the lung and tracheal epithelium is arranged as a pseudostratifed layer of cells that all contact the basal membrane. Similarly, the columnar epithelial cells of the small intestine are all in contact with the basement membrane. Thus, it is likely that alternate cues are required to mediate epithelial cell differentiation. Such cues may be initiated by the establishment of a polarized apical domain, which generates a negative regulatory signal that counterbalances the ‘pro-nuclear YAP/TAZ’ signaling from the basement membrane. The transmembrane Crumbs proteins, which direct the assembly of a protein complex required for apical domain identity [86], is implicated in YAP/TAZ regulation, as the loss of Crumbs complex proteins induce nuclear YAP/TAZ [87,88]. The Crumbs complex is crucial for establishing and maintaining the apical epithelial domain, and thus likely has important roles in directing epithelial differentiation in various contexts. In the airway epithelium, for example, deletion of Crumbs3 (Crb3), a major Crumbs isoform expressed in this tissue, results in dysregulated nuclear YAP localization and consequent failure for the airway epithelium to mature [87]. Apical polarity complexes serve as signaling hubs that facilitate LATS1/2-YAP interactions, thereby promoting YAP phosphorylation and nuclear exit. Notably, in addition to providing a polarization signal during differentiation, the Crumbs complex may also serve as a mechanosensor via the spectrin cytoskeleton, which is known to direct Hippo pathway activity [89,90]. Stretch of the epithelium may therefore have the potential to reduce the potency of the Crumbs complex to activate Hippo kinases thereby relieving YAP/TAZ from inhibitory signaling [91]. Other proteins associated with cell-cell junctions in the apical domain have also been implicated in negative regulation of YAP transcriptional activity. In the epidermis, for example, α-catenin binds and stabilizes phosphorylated YAP [50,92], in part by protecting it from PP2Ac mediated dephosphorylation, presenting another potential parallel mechanism of LATS1/2 independent inhibition of nuclear YAP. Similarly, α-catenin has been shown to regulate the tooth signaling center, the enamel knot formation, by controlling YAP/TAZ activity during tooth development [93]. Thus, as epithelial cells differentiate they negatively regulate YAP/TAZ nuclear activity through the establishment of a polarized apical domain and cell-cell junctions, which activate Hippo signaling and buffer YAP from phosphatases. It will be interesting, in future in vivo studies, to examine whether alterations in the polarized state of differentiated epithelial cells liberates the pool of cytoplasmic YAP/TAZ to provide instructive cues and promote progenitor cell expansion in response to injury.
Conclusions
In their recent study [1], Klein and colleagues have demonstrated the persistence of YAP/TAZ activity as a potent regulator of epithelial progenitor cell fate and expansion. However, while this recent study has defined regulatory roles for YAP in the transit-amplifying population, a core question still remains: What signals a quiescent stem/progenitor cell to self-renew versus transition into a transit-amplifying cell state? Both the transit-amplifying cells and the stem cells appear to be lost following the deletion of YAP/TAZ, suggesting that YAP/TAZ have roles in sustaining stem cell renewal or survival in addition to driving transit-amplifying cell expansion. The low levels of nuclear YAP localization in the stem cells suggest potential non-nuclear roles in these cells. The proposed model by Hu et al. [1] for transit-amplifying cell expansion relies on the specific expression of integrin α-3 to drive YAP nuclear localization. But integrin α-3 is a transcriptional target of YAP. So, whether the Integrin-FAK-PP1A-YAP cascade serves as the initiator of progenitor expansion or as a positive feedback loop to sustain expansion remains unresolved. In addition to describing YAP/TAZ regulation in transit-amplifying cells, this work also points out further intricacies of the downstream roles of YAP/TAZ that would be well worth pursuing in models of injury and regeneration in other tissues.
Acknowledgments
X.V. is supported by the March of Dimes Foundation and NIH R01HL124392. J.B is supported by NIH F31HL13250601.
Footnotes
The authors declare no conflict of interest.
References
- 1.Hu JK, Du W, Shelton SJ, Oldham MC, et al. An FAK-YAP-mTOR Signaling Axis Regulates Stem Cell-Based Tissue Renewal in Mice. Cell Stem Cell. 2017;21:91–106.e6. doi: 10.1016/j.stem.2017.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang J, Wu S, Barrera J, Matthews K, et al. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell. 2005;122:421–34. doi: 10.1016/j.cell.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Jia J, Zhang W, Wang B, Trinko R, et al. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17:2514–9. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Justice RW, Zilian O, Woods DF, Noll M, et al. The Drosophila tumor suppressor gene warts encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9:534–46. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 5.Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–7. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- 6.Tapon N, Harvey KF, Bell DW, Wahrer DC, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell. 2002;110:467–78. doi: 10.1016/s0092-8674(02)00824-3. [DOI] [PubMed] [Google Scholar]
- 7.Udan RS, Kango-Singh M, Nolo R, Tao C, et al. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914–20. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 8.Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–56. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 9.Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163:811–28. doi: 10.1016/j.cell.2015.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B, Wei X, Li W, Udan RS, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao Y, Chun A, Cheung K, Rashidi B, et al. Tumor Suppressor LATS1 Is a Negative Regulator of Oncogene YAP. J Biol Chem. 2008;283:5496–509. doi: 10.1074/jbc.M709037200. [DOI] [PubMed] [Google Scholar]
- 12.Zhao B, Wei X, Li W, Udan RS, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong J, Feldmann G, Huang J, Wu S, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanai F, Marignani PA, Sarbassova D, Yagi R, et al. TAZ: a novel transcriptional co-activator regulated by interactions with 14-3-3 and PDZ domain proteins. EMBO J. 2000;19:6778–91. doi: 10.1093/emboj/19.24.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao B, Li L, Tumaneng K, Wang CY, et al. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24:72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CY, Zha ZY, Zhou X, Zhang H, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010;285:37159–69. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mo JS, Meng Z, Kim YC, Park HW, et al. Cellular energy stress induces AMPK-mediated regulation of YAP and the Hippo pathway. Nat Cell Biol. 2015;17:500–10. doi: 10.1038/ncb3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong AW, Meng Z, Yuan HX, Plouffe SW, et al. Osmotic stress-induced phosphorylation by NLK at Ser128 activates YAP. EMBO Rep. 2017;18:72–86. doi: 10.15252/embr.201642681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moon S, Kim W, Kim S, Kim Y, et al. Phosphorylation by NLK inhibits YAP-14-3-3-interactions and induces its nuclear localization. EMBO Rep. 2017;18:61–71. doi: 10.15252/embr.201642683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang W, Lv X, Liu C, Zha Z, et al. The N-terminal phosphodegron targets TAZ/WWTR1 protein for SCFbeta-TrCP-dependent degradation in response to phosphatidylinositol 3-kinase inhibition. J Biol Chem. 2012;287:26245–53. doi: 10.1074/jbc.M112.382036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saxton RA, Sabatini DM. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;169:361–71. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 22.Chen L, Chan SW, Zhang X, Walsh M, et al. Structural basis of YAP recognition by TEAD4 in the hippo pathway. Genes Dev. 2010;24:290–300. doi: 10.1101/gad.1865310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z, Zhao B, Wang P, Chen F, et al. Structural insights into the YAP and TEAD complex. Genes & Development. 2010;24:235–40. doi: 10.1101/gad.1865810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao B, Ye X, Yu J, Li L, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–71. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauviel A, Nallet-Staub F, Varelas X. Integrating developmental signals: a Hippo in the (path)way. Oncogene. 2012;31:1743–56. doi: 10.1038/onc.2011.363. [DOI] [PubMed] [Google Scholar]
- 26.Beyer TA, Weiss A, Khomchuk Y, Huang K, et al. Switch Enhancers Interpret TGF-beta and Hippo Signaling to Control Cell Fate in Human Embryonic Stem Cells. Cell Rep. 2013;5:1611–24. doi: 10.1016/j.celrep.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 27.Galli GG, Carrara M, Yuan WC, Valdes-Quezada C, et al. YAP Drives Growth by Controlling Transcriptional Pause Release from Dynamic Enhancers. Mol Cell. 2015;60:328–37. doi: 10.1016/j.molcel.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein C, Bardet AF, Roma G, Bergling S, et al. YAP1 Exerts Its Transcriptional Control via TEAD-Mediated Activation of Enhancers. PLoS Genet. 2015;11:e1005465. doi: 10.1371/journal.pgen.1005465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanconato F, Forcato M, Battilana G, Azzolin L, et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat Cell Biol. 2015;17:1218–27. doi: 10.1038/ncb3216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujii M, Toyoda T, Nakanishi H, Yatabe Y, et al. TGF-beta synergizes with defects in the Hippo pathway to stimulate human malignant mesothelioma growth. J Exp Med. 2012;209:479–94. doi: 10.1084/jem.20111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strano S, Monti O, Pediconi N, Baccarini A, et al. The transcriptional coactivator Yes-associated protein drives p73 gene-target specificity in response to DNA Damage. Mol Cell. 2005;18:447–59. doi: 10.1016/j.molcel.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 32.Skibinski A, Breindel JL, Prat A, Galvan P, et al. The Hippo transducer TAZ interacts with the SWI/SNF complex to regulate breast epithelial lineage commitment. Cell reports. 2014;6:1059–72. doi: 10.1016/j.celrep.2014.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rashidian J, Le Scolan E, Ji X, Zhu Q, et al. Ski regulates Hippo and TAZ signaling to suppress breast cancer progression. Sci Signal. 2015;8:ra14. doi: 10.1126/scisignal.2005735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao R, Fallon TR, Saladi SV, Pardo-Saganta A, et al. Yap tunes airway epithelial size and architecture by regulating the identity, maintenance, and self-renewal of stem cells. Dev Cell. 2014;30:151–65. doi: 10.1016/j.devcel.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imajo M, Ebisuya M, Nishida E. Dual role of YAP and TAZ in renewal of the intestinal epithelium. Nat Cell Biol. 2015;17:7–19. doi: 10.1038/ncb3084. [DOI] [PubMed] [Google Scholar]
- 36.Varelas X, Sakuma R, Samavarchi-Tehrani P, Peerani R, et al. TAZ controls Smad nucleocytoplasmic shuttling and regulates human embryonic stem-cell self-renewal. Nat Cell Biol. 2008;10:837–48. doi: 10.1038/ncb1748. [DOI] [PubMed] [Google Scholar]
- 37.Heallen T, Zhang M, Wang J, Bonilla-Claudio M, et al. Hippo pathway inhibits Wnt signaling to restrain cardiomyocyte proliferation and heart size. Science. 2011;332:458–61. doi: 10.1126/science.1199010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu X, Li H, Rajurkar M, Li Q, et al. Tead and AP1 Coordinate Transcription and Motility. Cell Rep. 2016;14:1169–80. doi: 10.1016/j.celrep.2015.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141:1614–26. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- 40.Mahoney JE, Mori M, Szymaniak AD, Varelas X, et al. The hippo pathway effector Yap controls patterning and differentiation of airway epithelial progenitors. Dev Cell. 2014;30:137–50. doi: 10.1016/j.devcel.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Szymaniak AD, Mi R, McCarthy SE, Gower AC, et al. The Hippo pathway effector YAP is an essential regulator of ductal progenitor patterning in the mouse submandibular gland. Elife. 2017;6:e23499. doi: 10.7554/eLife.23499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao T, Zhou D, Yang C, Singh T, et al. Hippo signaling regulates differentiation and maintenance in the exocrine pancreas. Gastroenterology. 2013;144:1543–53, 53 e1. doi: 10.1053/j.gastro.2013.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.George NM, Day CE, Boerner BP, Johnson RL, et al. Hippo signaling regulates pancreas development through inactivation of Yap. Mol Cell Biol. 2012;32:5116–28. doi: 10.1128/MCB.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JH, Kim TS, Yang TH, Koo BK, et al. A crucial role of WW45 in developing epithelial tissues in the mouse. EMBO J. 2008;27:1231–42. doi: 10.1038/emboj.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, et al. Yap1 acts downstream of alpha-catenin to control epidermal proliferation. Cell. 2011;144:782–95. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panciera T, Azzolin L, Fujimura A, Di Biagio D, et al. Induction of Expandable Tissue-Specific Stem/Progenitor Cells through Transient Expression of YAP/TAZ. Cell Stem Cell. 2016;19:725–37. doi: 10.1016/j.stem.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finch-Edmondson ML, Strauss RP, Passman AM, Sudol M, et al. TAZ Protein Accumulation Is Negatively Regulated by YAP Abundance in Mammalian Cells. J Biol Chem. 2015;290:27928–38. doi: 10.1074/jbc.M115.692285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Elbediwy A, Vincent-Mistiaen ZI, Spencer-Dene B, Stone RK, et al. Integrin signalling regulates YAP and TAZ to control skin homeostasis. Development. 2016;143:1674–87. doi: 10.1242/dev.133728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang H, Pasolli HA, Fuchs E. Yes-associated protein (YAP) transcriptional coactivator functions in balancing growth and differentiation in skin. Proc Natl Acad Sci USA. 2011;108:2270–5. doi: 10.1073/pnas.1019603108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlegelmilch K, Mohseni M, Kirak O, Pruszak J, et al. Yap1 Acts Downstream of α-Catenin to Control Epidermal Proliferation. Cell. 2011;144:782–95. doi: 10.1016/j.cell.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Totaro A, Castellan M, Battilana G, Zanconato F, et al. YAP/TAZ link cell mechanics to Notch signalling to control epidermal stem cell fate. Nat Commun. 2017;8:15206. doi: 10.1038/ncomms15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Walko G, Woodhouse S, Pisco AO, Rognoni E, et al. A genome-wide screen identifies YAP/WBP2 interplay conferring growth advantage on human epidermal stem cells. Nat Commun. 2017;8:14744. doi: 10.1038/ncomms14744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gregorieff A, Liu Y, Inanlou MR, Khomchuk Y, et al. Yap-dependent reprogramming of Lgr5(+) stem cells drives intestinal regeneration and cancer. Nature. 2015;526:715–8. doi: 10.1038/nature15382. [DOI] [PubMed] [Google Scholar]
- 54.Barry ER, Morikawa T, Butler BL, Shrestha K, et al. Restriction of intestinal stem cell expansion and the regenerative response by YAP. Nature. 2013;493:106–10. doi: 10.1038/nature11693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cai J, Zhang N, Zheng Y, de Wilde RF, et al. The Hippo signaling pathway restricts the oncogenic potential of an intestinal regeneration program. Genes Dev. 2010;24:2383–8. doi: 10.1101/gad.1978810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makita R, Uchijima Y, Nishiyama K, Amano T, et al. Multiple renal cysts, urinary concentration defects, and pulmonary emphysematous changes in mice lacking TAZ. Am J Physiol Renal Physiol. 2008;294:F542–53. doi: 10.1152/ajprenal.00201.2007. [DOI] [PubMed] [Google Scholar]
- 57.Mitani A, Nagase T, Fukuchi K, Aburatani H, et al. Transcriptional coactivator with PDZ-binding motif is essential for normal alveolarization in mice. Am J Respir Crit Care Med. 2009;180:326–38. doi: 10.1164/rccm.200812-1827OC. [DOI] [PubMed] [Google Scholar]
- 58.Tumaneng K, Schlegelmilch K, Russell RC, Yimlamai D, et al. YAP mediates crosstalk between the Hippo and PI(3)K–TOR pathways by suppressing PTEN via miR-29. Nat Cell Biol. 2012;14:1322–9. doi: 10.1038/ncb2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansen CG, Ng YL, Lam WL, Plouffe SW, et al. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. 2015;25:1299–313. doi: 10.1038/cr.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim M, Kim T, Johnson Randy L, Lim D-S. Transcriptional Co-repressor Function of the Hippo Pathway Transducers YAP and TAZ. Cell Rep. 2015;11:270–82. doi: 10.1016/j.celrep.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 61.Castilho RM, Squarize CH, Chodosh LA, Williams BO, et al. mTOR Mediates Wnt-Induced Epidermal Stem Cell Exhaustion and Aging. Cell Stem Cell. 2009;5:279–89. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, et al. mTOR Inhibition Prevents Epithelial Stem Cell Senescence and Protects from Radiation-Induced Mucositis. Cell Stem Cell. 2012;11:401–14. doi: 10.1016/j.stem.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen C, Liu Y, Liu R, Ikenoue T, et al. TSC–mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chung C, Kim T, Kim M, Kim M, et al. Hippo-Foxa2 signaling pathway plays a role in peripheral lung maturation and surfactant homeostasis. Proc Natl Acad Sci USA. 2013;110:7732–7. doi: 10.1073/pnas.1220603110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lange AW, Sridharan A, Xu Y, Stripp BR, et al. Hippo/Yap signaling controls epithelial progenitor cell proliferation and differentiation in the embryonic and adult lung. J Mol Cell Biol. 2014;7:35–47. doi: 10.1093/jmcb/mju046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou D, Zhang Y, Wu H, Barry E, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc Natl Acad Sci USA. 2011;108:E1312–20. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee KP, Lee JH, Kim TS, Kim TH, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci USA. 2010;107:8248–53. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lu L, Li Y, Kim SM, Bossuyt W, et al. Hippo signaling is a potent in vivo growth and tumor suppressor pathway in the mammalian liver. Proc Natl Acad Sci USA. 2010;107:1437–42. doi: 10.1073/pnas.0911427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Song H, Mak KK, Topol L, Yun K, et al. Mammalian Mst1 and Mst2 kinases play essential roles in organ size control and tumor suppression. Proc Natl Acad Sci USA. 2010;107:1431–6. doi: 10.1073/pnas.0911409107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou D, Conrad C, Xia F, Park JS, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–38. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Meng Z, Moroishi T, Mottier-Pavie V, Plouffe SW, et al. MAP4K family kinases act in parallel to MST1/2 to activate LATS1/2 in the Hippo pathway. Nat Commun. 2015;6:8357. doi: 10.1038/ncomms9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng Y, Wang W, Liu B, Deng H, et al. Identification of Happyhour/MAP4K as Alternative Hpo/Mst-like Kinases in the Hippo Kinase Cascade. Dev Cell. 2015;34:642–55. doi: 10.1016/j.devcel.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu C-Y, Lv X, Li T, Xu Y, et al. PP1 Cooperates with ASPP2 to Dephosphorylate and Activate TAZ. J Biol Chem. 2011;286:5558–66. doi: 10.1074/jbc.M110.194019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Couzens AL, Knight JDR, Kean MJ, Teo G, et al. Protein Interaction Network of the Mammalian Hippo Pathway Reveals Mechanisms of Kinase-Phosphatase Interactions. Sci Signal. 2013;6:rs15–rs. doi: 10.1126/scisignal.2004712. [DOI] [PubMed] [Google Scholar]
- 75.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–80. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li P, Silvis MR, Honaker Y, Lien WH, et al. alphaE-catenin inhibits a Src-YAP1 oncogenic module that couples tyrosine kinases and the effector of Hippo signaling pathway. Genes Dev. 2016;30:798–811. doi: 10.1101/gad.274951.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim NG, Gumbiner BM. Adhesion to fibronectin regulates Hippo signaling via the FAK-Src-PI3K pathway. J Cell Biol. 2015;210:503–15. doi: 10.1083/jcb.201501025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Imada S, Murata Y, Kotani T, Hatano M, et al. Role of Src family kinases in regulation of intestinal epithelial homeostasis. Mol Cell Biol. 2016 doi: 10.1128/MCB.00311-16. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Taniguchi K, Wu L-W, Grivennikov SI, de Jong PR, et al. A gp130-Src-YAP module links inflammation to epithelial regeneration. Nature. 2015;519:57–62. doi: 10.1038/nature14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aragona M, Panciera T, Manfrin A, Giulitti S, et al. A Mechanical Checkpoint Controls Multicellular Growth through YAP/TAZ Regulation by Actin-Processing Factors. Cell. 2013;154:1047–59. doi: 10.1016/j.cell.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 81.Dupont S, Morsut L, Aragona M, Enzo E, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–83. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 82.Chakraborty S, Njah K, Pobbati AV, Lim YB, et al. Agrin as a Mechanotransduction Signal Regulating YAP through the Hippo Pathway. Cell Rep. 2017;18:2464–79. doi: 10.1016/j.celrep.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 83.Nardone G, Oliver-De La Cruz J, Vrbsky J, Martini C, et al. YAP regulates cell mechanics by controlling focal adhesion assembly. 2017;8:15321. doi: 10.1038/ncomms15321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fan R, Kim N-G, Gumbiner BM. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc Natl Acad Sci USA. 2013;110:2569–74. doi: 10.1073/pnas.1216462110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Iglesias-Bartolome R, Torres D, Marone R, Feng X, et al. Inactivation of a Galpha(s)-PKA tumour suppressor pathway in skin stem cells initiates basal-cell carcinogenesis. Nat Cell Biol. 2015;17:793–803. doi: 10.1038/ncb3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Whiteman EL, Fan S, Harder JL, Walton KD, et al. Crumbs3 Is Essential for Proper Epithelial Development and Viability. Mol Cell Biol. 2014;34:43–56. doi: 10.1128/MCB.00999-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Szymaniak AD, Mahoney JE, Cardoso WV, Varelas X. Crumbs3-Mediated Polarity Directs Airway Epithelial Cell Fate through the Hippo Pathway Effector Yap. Dev Cell. 2015;34:283–96. doi: 10.1016/j.devcel.2015.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Varelas X, Samavarchi-Tehrani P, Narimatsu M, Weiss A, et al. The Crumbs complex couples cell density sensing to Hippo-dependent control of the TGF-beta-SMAD pathway. Dev Cell. 2010;19:831–44. doi: 10.1016/j.devcel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 89.Fletcher GC, Elbediwy A, Khanal I, Ribeiro PS, et al. The Spectrin cytoskeleton regulates the Hippo signalling pathway. EMBO J. 2015;34:940–54. doi: 10.15252/embj.201489642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Medina E, Williams J, Klipfell E, Zarnescu D, et al. Crumbs interacts with moesin and beta(Heavy)-spectrin in the apical membrane skeleton of Drosophila. J Cell Biol. 2002;158:941–51. doi: 10.1083/jcb.200203080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fletcher GC, Elbediwy A, Khanal I, Ribeiro PS, et al. The Spectrin cytoskeleton regulates the Hippo signalling pathway. EMBO J. 2015;34:940–54. doi: 10.15252/embj.201489642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Silvis MR, Kreger BT, Lien WH, Klezovitch O, et al. alpha-catenin is a tumor suppressor that controls cell accumulation by regulating the localization and activity of the transcriptional coactivator Yap1. Sci Signal. 2011;4:ra33. doi: 10.1126/scisignal.2001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li CY, Hu J, Lu H, Lan J, et al. alphaE-catenin inhibits YAP/TAZ activity to regulate signalling centre formation during tooth development. Nat Commun. 2016;7:12133. doi: 10.1038/ncomms12133. [DOI] [PMC free article] [PubMed] [Google Scholar]


