Abstract
Lysosomes are the central organelles responsible for macromolecule recycling in the cell. Lysosomal dysfunction is the primary cause of lysosomal storage diseases (LSDs), and contributes significantly to the pathogenesis of common neurodegenerative diseases. The lysosomes are also intracellular stores for calcium ions, one of the most common second messenger in the cell. Lysosomal Ca2+ is required for diverse cellular processes including signal transduction, vesicular trafficking, autophagy, nutrient sensing, exocytosis, and membrane repair. In this review, we first summarize some recent progresses in the studies of lysosome Ca2+ regulation, with a focus on the newly discovered lysosomal Ca2+ channels and the mechanisms of lysosomal Ca2+ store refilling. We then discuss how defects in lysosomal Ca2+ release and store maintenance cause lysosomal dysfunction and neurodegeneration.
Keywords: Lysosomes, Calcium Channels, TRPML1, Refilling, Lysosomal Storage Diseases, Neurodegenration, NPC
INTRODUCTION
Lysosome is the cell’s recycling center that mediates the degradation of macromolecules from intracellular (via autophagy) or extracellular (via endocytosis and phagocytosis) sources (Luzio et al., 2007). Armed with more than 60 hydrolases and 50 membrane proteins, lysosomes degrade proteins, polysaccharides, and lipids into their “building blocks” which are transported out of lysosomes via specific transporters or vesicular membrane trafficking, for energy or re-use (Kolter and Sandhoff 2005; Schwake et al., 2013; Xu and Ren 2015). Lysosome function thus plays a pivotal role in maintaining cellular homeostasis. Defects in lysosomal degradation, exporting, or trafficking can cause accumulation of undigested biomaterials in the lysosome. This represents lysosomal storage diseases (LSDs) (Platt et al., 2012; Boustany 2013; Xu and Ren 2015). Most LSDs exhibit neurological pathologies, suggesting that the nervous system is particularly vulnerable to lysosome dysfunction (Boustany 2013). On the other hand, common neurodegenerative diseases such as Alzhermer’s Disease (AD), Parkinson’s Disease (PD), and Huntington’s Disease (HD) are intimately associated with lysosome dysfunction (Fraldi et al. 2016).
Ca2+ is one of the most common second messenger involved in a variety of intracellular signaling events, which in turn control a vast array of cellular processes, including gene expression, exocytosis, cell growth, proliferation, differentiation, cell motility, cell death, and muscle contraction (Clapham 2007; Berridge 2012). Hence mammalian cells are equipped with specific channel and transporter proteins to tightly control intracellular Ca2+ levels ([Ca2+]i). Inappropriate Ca2+ signaling and abnormal Ca2+ levels are found to be associated with many cellular defects, contributing to a broad spectrum of clinical disorders, including heart disease, AD, and stroke (Bojarski et al., 2008; Berridge 2012). [Ca2+]i undergoes dynamic changes in response to stimulation by cellular and environmental cues in animal cells. At resting conditions, free cytosolic [Ca2+]i is maintained at a very low level (~100 nM), which is about 20,000-fold lower than extracellular [Ca2+], and about 5,000-fold lower than the intracellular Ca2+ stores such as endoplasmic reticulum (ER) and lysosomes (Berridge et al., 2000; Christensen et al., 2002; Clapham 2007). Upon stimulation by hormones, neurotransmitters, or membrane depolarization, [Ca2+]i increases dramatically through passive Ca2+ flow down the electrochemical gradient from extracellular space and/or from intracellular Ca2+ stores. Intracellular Ca2+ signals display spectacular spatiotemporal complexity, which may encode the nature and intensity of the stimulus. The information carried by Ca2+ signaling is reliably decoded by a long list of Ca2+ effector proteins, such as calmodulin and synaptotagmins, relaying to fulfill diverse cellular functions. Upon signal termination, cytosolic Ca2+ returns to the resting value, mainly through Ca2+ pumps and transporters localized at the plasma membrane (e.g., PMCA) and on intracellular membranes (e.g., SERCA in the ER) (Berridge 2012).
Besides ER, lysosomes have also emerged as intracellular Ca2+ stores (Christensen et al., 2002; Churchill et al. 2002; Patel and Docampo 2010). While the importance of Ca2+ homeostasis in neurodegeneration is well recognized (Marambaud et al., 2009; Bezprozvanny 2010), the role of lysosomal Ca2+ homeostasis in neurodegeneration is largely unexplored. In this review, we will summarize the current knowledge on the key players and mechanisms in the regulation of lysosomal Ca2+ signaling. We will then discuss how de-regulation of lysosomal Ca2+ causes lysosomal dysfunction and neurodegeneration.
LYSOSOMAL CALCIUM HOMEOSTASIS
The Ca2+ concentration in lysosome lumen ([Ca2+]iy): is estimated to be about 500 µM (Christensen et al., 2002; Patel and Cai 2015), which could be released by intracellular cues, for example, nicotinic acid adenine dinucleotide phosphate (NAADP), one of the most potent Ca2+-mobilizing second messenger (Christensen et al., 2002; Calcraft et al., 2009). Like the plasma membrane and ER, lysosomes are also believed to be equipped with ion channels and transporters to control Ca2+ flux across lysosome membranes (Dong et al., 2010; Xu et al., 2015; Xu and Ren 2015). The local Ca2+ release from lysosomes is crucial for lysosome function, but may also be involved in global Ca2+ signaling by interacting with ER Ca2+ signaling (Kilpatrick et al., 2013; Morgan et al., 2013; Kilpatrick et al., 2016; Raffaello et al., 2016) With the development of organelle-specific physiological and imaging approaches, for example, the whole-lysosomal patch-clamp technique, the single-organelle planar patch-clamp and live imaging with genetically encoded Ca2+ indicators (Dong et al., 2008; Dong et al., 2010; Schieder et al., 2010; Shen et al., 2012, Wang et al., 2012, Cang et al., 2015; Cao et al., 2015), an expanding number of lysosomal ion channels, including some Ca2+-permeable ones, have been identified recently. We will briefly review these channels as well as their physiological importance. In addition, we will also discuss the mechanisms by which lysosomal Ca2+ is taken up or refilled after release.
Lysosomal Ca2+ Channels
TRPMLs
TRPML proteins belong to the mucolipin subgroup of TRP ion channel family (Ramsey et al., 2006; Nilius et al., Scientific Publishers 2007). There are three TRPML isoforms in mammals: TRPML1, TRPML2, and TRPML3. Whereas TRPML1 is widely expressed and predominantly localized on the late endosomes and lysosomes, TRPML2 and TRPML3 are expressed on the early/recycling endosomes in addition to late endosomes and lysosomes (see Fig. 1). TRPML1 is a Ca2+-permeable, non-selective cation channel that can be activated by phosphatidylinositol 3,5-bisphosphate [PI(3,5)P2], a phospholipid enriched on the late endosomal and lysosomal membranes (Dong et al., 2010). Mutations in human TRPML1 have been associated with Mucolipidosis type IV disease (ML-IV), an LSD characterized by accumulation of various lipids in lysosomes. TRPML1-mediated lysosomal Ca2+ release may fulfill diverse physiological functions, which includes lysosomal exocytosis (Medina et al., 2011; Samie et al., 2013; Park et al., 2016), membrane repair (Cheng et al., 2014), autophagy (Medina et al., 2015), nutrient sensing (Wang et al., 2015), oxidative stress sensing (Zhang et al., 2016), lysosome motility, and lysosome tubulation and reformation (Li et al., 2016).
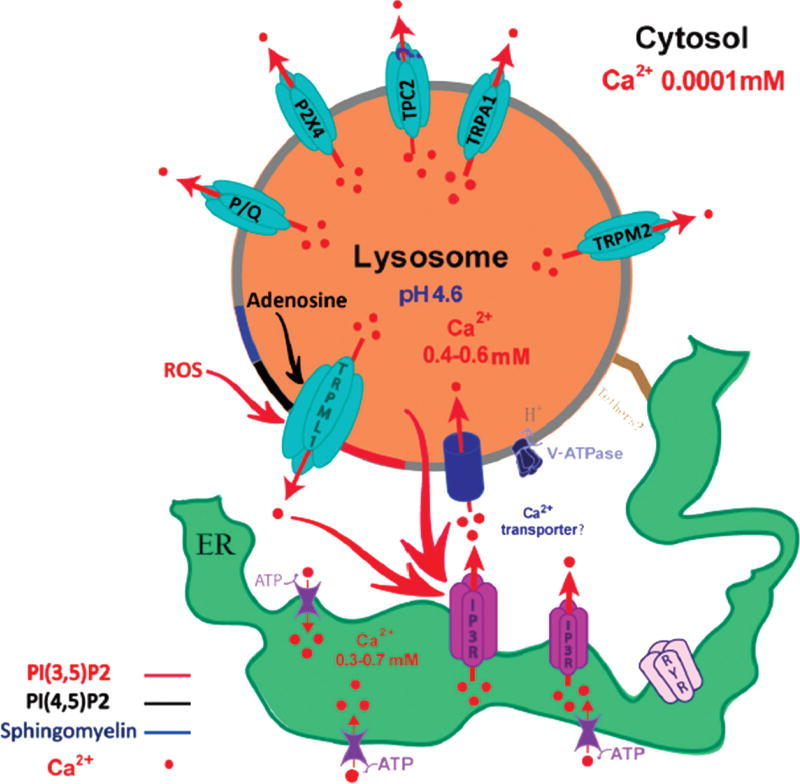
Figure 1.
Lysosomal Ca2+ release channels and store refiling mechanisms. Several Ca2+-permeable channels may be present on the lysosomal membranes. Activation of the channels trigger Ca2+ release from lysosomal Ca2+ stores. Among them, the gating mechanisms are most well-established for TRPML1. The lysosome-enriched phosphoinositide PI(3,5)P2 (red) can activate the channel, while plasma-membrane-enriched phosphoinositide PI(4,5)P2 (black) can inhibit TRPML1. Additionally, TRPML1 can be activated (red arrow) by reactive oxygen species (ROS), and ROS-induced lysosomal Ca2+ release may induce lysosomal biogenesis (Zhang et al., 2016). In addition, accumulated materials such as sphingomyelin (blue) and adenosine may inhibit (black arrow) TRPML1. While TPC-mediated lysosomal Ca2+ release can be triggered by NAADP or sphingosine (Calcraft et al., 2009; Zong et al., 2009; Lloyd-Evans et al., 2010; Hoglinger et al., 2015) (whether TPC2 serves as the Ca2+ channel still remains convtroversial, indicated by the question mark), activation mechanisms for other lysosomal Ca2+ channels are less clear. Upon lysosomal Ca2+ channel activation, lysosome stores are largely depleted, which is expected to trigger a refilling mechanism. In refilling mechanism 1, a putative Ca2+–H+ exchanger may transport Ca2+ ions into lysosome lumen in an H+-dependent manner (Morgan et al., 2011). Alternatively, Garrity et al. suggest that in the ER-lysosome membrane contact sites, IP3Rs–mediated ER release Ca2+ may allow an unidentified Ca2+ transporter to transport Ca2+ to lysosomes. In this model, possible tether proteins may link ER membrane proteins directly with lysosomal membrane proteins at ER-lysosome membrane contact sites. Reprinted with permission from Garrity, A. G., et al. (2016). The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. Elife 5, e15887. © 2016, eLife/CC BY 4.0.
P2X4
P2X4 is an ATP-gated cation channel that belongs to the ionotropic P2X–family ATP receptors (North 2002). In addition to its expression on the plasma membrane, P2X4 was also found on the lysosomal membranes (Qureshi et al., 2007). Whole-lysosome patch-clamp studies revealed that lysosomal P2X4 was functional (Huang et al., 2014) (see Fig. 1). Interestingly, ATP, the natural ligand of P2X4 was found to accumulate in lysosomes (Cao et al., 2015; Zhong et al., 2016). However, even with the presence of ATP, P2X4 activity was inhibited by acidic pH of lysosome lumen (Huang et al., 2014). Luminal alkalization could counteract this inhibition thus allow lysosomal Ca2+ release through P2X4 (Huang et al., 2014), which was shown to be involved in lysosomal membrane fusion in a calmodulin-dependent manner (Cao et al., 2015).
TPCs (Two-Pore Channels)
TPCs contain two tandem six-transmembrane domains (2X:6 TM) which might be evolutionary intermediates between 6 TM and 4X 6TM voltage-gated ion channels (Rahman et al., 2014). Whereas TPC1 is expressed in both early endosomes and lysosomes, TPC2 is predominantly present on the lysosomal membranes (see Fig. 1) (Calcraft et al., 2009). TPCs were proposed to be the long-sought NAADP receptor that mediates NAADP-induced Ca2+ release from the lysosome (Brailoiu et al., 2009; Calcraft et al., 2009; Zong et al., 2009; Lloyd-Evans et al., 2010; Penny and Patel 2015). Consistent with this hypothesis, TPC overexpression increased NAADP binding (Calcraft et al., 2009). The Ca2+-permeability of TPC2 was also supported by calcium imaging experiments and confirmed by electrophysiological studies (Brailoiu et al., 2010; Pitt et al., 2010; Schieder et al., 2010; Grimm et al., 2014, Ruas et al., 2015; Penny et al., 2016). However, several recent studies showed that TPC2 is a Na+-selective channel with a low Ca2+ permeability (Wang et al., 2012; Cang et al., 2013). The crystal structures of Arabidopsis thaliana TPC1 (AtTPC1), a non-selective cation channel, have been resolved recently (Guo et al., 2016; Kintzer and Stroud 2016). Structure-guided mutagenesis studies revealed the molecular determinants for the Na+-selectivity (Guo et al., 2017).
Despite the controversies on the biophysical properties of TPCs, they are suggested to have important physiological roles (Patel 2015). For example, TPC2 may regulate Ebola virus infection (Sakurai et al., 2015), pigmentation in melanocytes (Lin-Moshier et al., 2014; Ambrosio et al., 2016), autophagy regulation in cardiomyocytes (Pereira et al., 2011; Garcia-Rua et al., 2016), and physical endurance during nutrition restriction (Cang et al. 2013).
TRPM2
TRPM2 is a member of the melastatin family of the TRP family (Ramsey et al., 2006; Nilius et al., 2007). TRPM2 is a Ca2+-permeable channel gated by ADPR, ADPR-2′-phosphate (ADPRP), and Ca2+ (Toth et al., 2015). In addition to acting as a channel on the plasma membrane, TRPM2 is also functional as a lysosomal channel in pancreatic β-cells and dendritic cells. The Ca2+ release through TRPM2 contributes to H2O2-induced β-cell death, and is critical for the maturation and chemotaxis of dendritic cells (Lange et al. 2009; Sumoza-Toledo et al., 2011).
P/Q Type Voltage-Gated Ca2+ Channels (VGCCs)
VGCCs are a group of voltage-gated ion channels predominately reside on the plasma membrane of excitable cells (e.g., muscle cells, neurons, etc.) with a high permeability to Ca2+ (Catterall et al., 2005). VGCCs are activated by depolarization, allowing Ca2+ influx and coupling of electrical activity with intracellular Ca2+ signaling (Catterall 2011; Buraei and Yang 2015). Activation of P/Q-type VGCCs triggers neurotransmitter release in neurons (Luebke et al., 1993; Takahashi and Momiyama 1993). Unexpectedly, a recent study reported the presence of P/Q-type VGCCs in the lysosomes of neurons, in both fruit flies and mice (Tian et al., 2015). Loss of pore-forming α1A subunit or auxiliary α2δ (encoded by Cacna1a and Cacna2d2 respectively), leads to defects in autophagosome-lysosome fusion (Tian et al., 2015).
TRPA1
TRPA1 is a Ca2+ permeable nonselective cation channels mainly expressed on primary afferent nociceptors and could be activated by diverse noxious compounds, such as mustard oil and tear gas (Bandell et al., 2004; Jordt et al., 2004; Bautista et al., 2006; Julius 2013). Recently, Shang et al., reported the functional expression of TRPA1 in the peripheral lysosomes of dorsal root ganglion (DRG) neurons (Shang et al., 2016). Ca2+ imaging studies has shown before that allyl isothiocyanate (AITC) could induce a TRPA1-dependent intracellular Ca2+ release (Bandell et al., 2004; Jordt et al., 2004). With pharmacological and immunohistochemical analyses, Shang et al., found the presence of TRPA1 proteins in the lysosomes of DRG neurons. Electrophysiology and immunoassay also showed that lysosomal Ca2+ efflux through TRPA1 could trigger vesicle exocytosis and neuropeptides (NPY, CGRP) release. Furthermore, behavior analysis indicated the physiological function of intracellular TRPA1 channel in nocifensive sensation.
In summary, multiple Ca2+ channels have been found on the lysosomal membrane in the past few years. It should be noted, however, that direct electrophysiological studies of TRPM2, P/Q VGCCs, and TRPA1 are still lacking. Outstanding questions such as what their endogenous activation mechanisms are, and what the roles of these channels are in lysosomal physiology and diseases remain to be answered.
Lysosomal Ca2+ Refilling
The storage capacity of intracellular stores is limited. Thus Ca2+ release from stores must be followed by Ca2+ refilling. ER Ca2+ refilling is mediated by a process termed the store-operated calcium entry (SOCE) (Putney 2007), the molecular mechanism of which have been delineated recently (Clapham 2007; Berridge 2012). In contrast, the refilling mechanisms for lysosomal stores are largely unknown. It’s been proposed that lysosome Ca2+ refilling is a pH-dependent process based on two observations (Morgan et al., 2011). First, lysosomal pH gradient dissipation, either by v-ATPase inhibitors or by alkalizing reagents such as NH4Cl, lead to the loss of lysosomal Ca2+ stores (Christensen et al., 2002; Lloyd-Evans et al., 2008; Calcraft et al., 2009; Dickson et al., 2012; Shen et al., 2012). Second, in yeast and plant vacuoles, the lysosome equivalents in these organisms, the Ca2+–H+ exchanger (CAX) is required for their Ca2+ store maintenance (Morgan et al., 2011; Pittman 2011). This hypothesis was further emphasized by recently cloned CAXs from two non-placental mammalian: the platypus and the Tasmanian devil (Melchionda et al., 2016).
However, a recent study by Garrity et al. suggested an alternative mechanism (Garrity et al., 2016). In this study, a physiological assay was developed using GCaMP3-tagged TRPML1 and its specific cell-permeable agonist ML-SA1 (Shen et al., 2012). The lysosomal Ca2+ stores of TRPML1 stable cells were depleted by a brief ML-SA1 application. Unexpectedly, pH dissipation by v-ATPase inhibitors bafilomycin-A or concanamycin-A did not abolish the refilling, suggesting a dispensable role of lysosomal pH gradient. Instead, the ER store was found to be required for lysosomal Ca2+ refilling. Depleting ER Ca2+ store by either thapsgargin (a SERCA inhibitor) or TPEN (a luminal Ca2+ chelator operating at neutral pH) abolished the refilling. Furthermore, IP3-receptors (IP3Rs) but not Ryanodine receptors (RyRs) on the ER are required for the refilling. Inhibition of IP3Rs caused lysosome dysfunction and LSD-like phenotype. Furthermore, the critical role of ER for lysosomal calcium refilling is also supported by the intimate spatial localization of ER and lysosomes (also see (Kilpatrick et al., 2013; Aston et al., 2017; Kilpatrick et al., 2017)). Overall, this new study provided a new platform to study the mechanism of lysosomal Ca2+ refilling. Further test of the ER-refilling hypothesis may require the identification of new players including the involved lysosomal Ca2+ sensor(s) and pump(s), as well as the ER-lysosome tether(s) (see Fig. 1).
LYSOSOMAL CALCIUM HOMEOSTASIS DEFECTS AND NEURODEGENERATION
Diminished Lysosome Ca2+ Store in Neurodegeneration
Several studies suggest a correlation between diminished lysosomal Ca2+ stores and neurodegeneration. Niemann-Pick disease type C (NPC) is an autosomal recessive LSD caused by loss of function mutations in NPC1 or NPC2 genes (Carstea et al., 1997; Sleat et al., 2004). NPC1 is a membrane protein that resides on the limited membrane of lysosomes, while NPC2 is a soluble protein in the lysosome lumen (Infante et al., 2008; Kwon et al., 2009). NPC1 and NPC2 transport un-esterified cholesterol outside of lysosome lumen through lysosome membranes (Kwon et al., 2009). It was reported that in NPC−/− cells have reduced lysosomal Ca2+ stores, presumably due to inhibition of Ca2+ uptake or refilling by sphingosine accumulation in NPC cells (Lloyd-Evans et al., 2008).
A role of lysosomal Ca2+ was suggested in Familial Alzheimer’s disease (FAD), which is caused by mutations in presenilin1 (PS1), presenilin2 (PS2) or amyloid precursor protein (APP) (Campion et al., 1999). PS1 and PS2 are key components of γ-secretase, a protein complex resides on plasma membrane that cleaves APP to amyloid-β (Aβ peptides (Bertram and Tanzi 2008). Two recent studies both showed that deletion of PS(s) caused a decrease in lysosome Ca2+ stores (Coen et al. 2012; Lee et al., 2015). Whether this is linked with lysosomal acidification defect, as reported earlier (Lee et al., 2010), still remains controversial. In Coen et al.’s study, PS(s) knock-out cells appear to have similar pH as Wt cells (Coen et al., 2012). Lee et al., however, reported that impaired lysosomal acidification leads to lysosomal Ca2+ homeostasis in a TRPML1-dependent manner (Lee et al., 2015). Further investigation is thus needed to clarify whether lysosomal acidification is defected and contribute to the decreased lysosomal Ca2+ store in PS(s)-deficient cells.
One of the genetic risk factors for Parkinson’s disease (PD) is GBA1, a gene that encodes lysosomal hydrolase β-glucocerebrosidase that degrades glucocerebroside to glucose and ceramide (Gan-Or et al., 2015). Recessive mutations of GBA1 cause Gaucher disease (GD), either neuropathic (type II and III) or non-neuropathic (type I) depending on mutations (Nagral 2014). A recent study showed that fibroblasts from GD type I patients or PD patients carring GBA1 mutation have enlarged lysosomes and reduced Ca2+ release induced by GPN (Kilpatrick et al., 2016), suggesting a decreased lysosomal Ca2+ store. Besides GBA1, mutation of Leucine-rich repeat kinase 2 (LRRK2) is another most common genetic risk factor of PD, that may also play a role in lysosome function and autophagy (Gan-Or et al., 2015). A recent study reported that primary fibroblasts from an LRRK2-G2019S PD patient contained enlarged and clustered lysosomes with elevated NAADP-induced Ca2+ release (Hockey et al., 2015). Very intriguingly, the abnormal lysosome morphology can be reversed by inhibiting TPC2 or applying a fast Ca2+ chelator, BAPTA-AM, but not a slow Ca2+ chelator, EGTA-AM, suggesting a dysregulation of lysosomal Ca2+.
Defects in key elements of lysosomal Ca2+ refilling, such as IP3Rs and proteins that mediate ER-lysosome contact, may also potentially affect lysosomal Ca2+ stores. For example, in HD, an autosomal recessive genetic disease caused by the expansion of a trinucleotide CAG repeat in HTT gene (MacDonald et al., 1993; Bates et al., 2015), mutant Htt (mHtt) but not the wild type Htt sensitize IP3Rs’ response to IP3 (Tang et al., 2003). Reduced ER Ca2+ store was also observed in an HD mouse model (Wu et al., 2016). Similarly, spinocerebellar ataxia (SCA), another neurodegenerative disease caused by CAG repeat expansion in SCA genes, often features altered IP3Rs expression and IP3 responses (Brown and Loew 2014). Given that IP3Rs are required for lysosome Ca2+ refilling, it is possible that impaired lysosomal Ca2+ signaling may underlie neurodegenerative diseases with defective IP3R function (Takada et al., 2016).
ER-lysosome contact sites are still poorly understood in mammalian cells (Kilpatrick et al., 2013; Kilpatrick et al., 2017). The yeast counterparts, ER-vacuole contact sites, are much more clearly defined in the involving players, which may therefore shed light on disorders caused by mutation in their human homolog genes (Hariri et al., 2016). For example, deficiency in human sorting nexin 14 (Snx14) is linked with pediatric cerebellar ataxia and intellectual disability (Thomas et al., 2014; Akizu et al., 2015). Study in its yeast homolog Mdm1, an ER-vacuole tether protein, revealed that the patient allele of SNX14 disturbs sphingolipid synthesis when expressed in yeast (Henne et al., 2015). It is possible that impaired lysosomal Ca2+ signaling may underlie related human diseases.
Impaired Lysosome Ca2+ Release and Neurodegeneration
Besides diminished lysosomal Ca2+ store, defects in key components of Ca2+ releasing machineries such as Ca2+ channels could also lead to lysosomal Ca2+ dysregulation. The most direct link between lysosomal Ca2+ release defects and human disease came from ML-IV, an autosomal recessive LSD that manifests severe neurodegeneration caused by loss of function of TRPML1 (Berman et al., 1974; Slaugenhaupt 2002). In ML-IV cells, lysosomal Ca2+ store might remain unchanged (Lloyd-Evans and Platt 2011) or accumulated (Wong et al., 2012; Cao et al., 2015), and NAADP-induced Ca2+ release also not affected (Yamaguchi et al., 2011). However, enlarged lysosomes lysosomal storage are observed in TRPML1-deficient cells (Dong et al., 2008; Venkatachalam et al., 2015), suggestive of lysosomal dysfunction. Given the roles of TRPML1 in autophagy, lysosomal exocytosis, and oxidative stress sensing, it is of no surprise that ML-IV is a neurodegenerative disease (Andersen 2004; Menzies et al., 2015). Neurodegeneration in ML-IV patients is likely a result of defects in multiple ML1-dependent processes required for maintaining cellular homeostasis.
Defective TRPML1 signaling might be a common feature for many LSDs. In NPC cells, TRPML1-mediated lysosomal Ca2+ release is much reduced, which may be caused by Sphingomyelins (SMs) storage (see Fig. 1). Boosting TRPML1 activity genetically or pharmacologically may reduce lysosome storage and cholesterol accumulation (Shen et al., 2012). Similarly, TRPML1-:m ediated Ca2+ release is also compromised in Niemann-Pick disease type A (NPA) and Fabry disease (Shen et al., 2012; Zhong et al., 2016).
In HD, mHtt expression can cause increased perinuclear lysosome accumulation and decreased lysosome mobility (Erie et al., 2015). Acute activation and chronic inhibition of TRPML1 can both result in lysosome perinuclear accumulation (Li et al., 2016). It remains to be investigated whether altered TRPML1 activity underlies HD.
Cacna1a mutant neurons are defective in autophagosome-lysosome fusion. Inhibition of CACNA1A Ca2+ channel function in the lysosome but not PM led to the lysosomal fusion defects (Tian et al., 2015). Human mutations in Cacna1a are also associated with neurological diseases including Episodic ataxia type 2 (EA2), Familial hemiplegic migraine-1 (FHM1), and pinocerebellar ataxia type 6 (SCA6) (Rajakulendran et al., 2012). It is possible the lysosomal Ca2+ defects may contribute to these diseases. P2X4 is involved in epilepsy and amyotrophic lateral sclerosis (ALS) (Saez-Orellana et al., 2015). Whether these functions are mediated by lysosomal P2X4 is still unclear.
Other than mutations of the channels, the impairment of lysosomal Ca2+ release could also be the result of other causes such as decreased activation or increased inhibition of channel activity, or other environmental cues (summarized in Fig. 1). Taking TRPML1 as the example, mutations in key components of PI(3,5)P2 synthesis, such as FIG4 and PIKfyve, are associated with neurological disorders (Jin et al., 2016). Inhibition of TRPML1 is observed in NPC cells (by SMs) (Shen et al., 2012) or adenosine deaminase (ADA)-deficient cells (by adenosine) (Zhong et al., 2017). TRPML1 can also be activated by ROS (Zhang et al., 2016), which is closely linked with neurodegenerative diseases (Barnham et al., 2004; Niedzielska et al., 2016).
CONCLUDING REMARKS
Diverse biological processes crucial for maintaining cellular homeostasis require Ca2+ release from lysosomes. Given the importance of lysosomal Ca2+ in lysosomal functions and the importance of lysosome function in neuronal health, studies on direct links between lysosomal Ca2+ and neurodegeneration are surprisingly few (summarized in Table I, II). The ER-refilling studies suggest that Ca2+ dysregulation in ER may subsequently affect Ca2+ homeostasis in lysosomes. Common defects in TRPML1-mediated lysosomal Ca2+ release in multiple LSDs suggest that pharmacologically manipulating its functions may be a potential general strategy in alleviating lysosome storage and neurodegeneration.
Table I.
Summary of neurodegenration with defected calcium store.
| Disease | Model | store | Ref. |
|---|---|---|---|
| NPC | Human B | Decreased | Lloyd-Evans et al., 2008 |
| lymphoblasts & Human fibroblast | Decreased | ||
| FAD | PSENdKO MEF | Decreased | Coen et al., 2012 |
| PSEN −/− neuron | Decreased | ||
| PSEN1 KO murine blastocyst | Decreased | Lee et al., 2015 | |
| PD + GBA1 | Human fibroblast | Decreased | Kilpatrick et al., 2016 |
Table II.
Summary of neurodegenration with impaired lysosomal calcium release.
| Disease | Model | efflux | Ref. |
|---|---|---|---|
| NPA | Human fibroblast | TRPML1-mediated, decreased GPN-induced, unchanged | Shen et al., 2012 |
| Human fibroblast | TRPML1-mediated, decreased GPN-induced, unchanged | Zhong et al., 2016 | |
| NPC | Human B lymphoblasts & Human fibroblast | GPN-induced, decreased | Lloyd-Evans et al., 2008 |
| NPC−/− CHO & Humam fibroblast | TRPML1-mediated, decreased GPN-induced, unchanged | Shen et al., 2012 | |
| ML IV | Unpublishied SKBR3 cell transfected with channel dead TRPML1 | TRPML1-mediated, missing NAADP-induced, unchanged | Peterneva et al., Yamaguchi et al., 2011 |
| Fabry disease | Human fibroblast | TRPML1-mediated, decreased GPN-induced, unchanged | Zhong et al., 2016 |
| FAD | PSENdKO MEF & PSEN−/− neuron | GPN-induced, decreased | Coen et al., 2012 |
| PSEN1 KO murine blastocyst | GPN-induced, decreased | Lee et al., 2015 | |
| PD + GBA1 | Human fibroblast | GPN-induced, decreased | Kilpatrick et al., 2016 |
| PD | LRRK2 G2019S human fibroblast | NAADP-induced, increased | Hockey et al., 2015 |
| EA2, FHM1, SCA6 | Cacna1a mutant mouse | VGCCly-mediated, missing | Tian et al., 2015 |
Acknowledgments
We apologize to colleagues whose works are not cited due to space limitations. The authors are supported by funds from the Collaborative Innovation Center of Yangtze River Delta Region Green Pharmaceuticals at ZJUT. J. Y. is also supported by NIH grant (NS062792). We appreciate the helpful comments from Dr. Haoxing Xu from University of Michigan.
Biographies

Xinghua Feng is member of collaborative innovation center in Zhejiang University of Technology. He obtained a Ph.D. from Shanghai Institutes for Biological Sciences, CAS. He did post-doctoral works with Professor Dejian Ren at UPENN, then with Professor Michael Zhu at UThealth. His works focus on the lysosomal ion channels and their physiological functions.

Junsheng Yang obtained a B.S. in Biochemistry and Molecular Biology from Peking University and a Ph.D. in Molecular Biology from University of Southern California under the supervision of Dr. John Tower. He worked with Dr. Thomas Nyström as a post-doc fellow in the University of Gothenburg. His research mainly focuses on aging and age-related diseases. Junsheng recently joined the Collaborative Innovation Center of Green Pharmaceuticals in Zhejiang University of Technology as a member of an innovation team.
References
- Akizu N, Cantagrel V, Zaki MS, Al-Gazali L, Wang X, Rosti RO, Dikoglu E, Gelot AB, Rosti B, Vaux KK, Scott EM, Silhavy JL, Schroth J, Copeland B, Schaffer AE, Gordts PL, Esko JD, Buschman MD, Field SJ, Napolitano G, Abdel-Salam GM, Ozgul RK, Sagiroglu MS, Azam M, Ismail S, Aglan M, Selim L, Mahmoud IG, Abdel-Hadi S, Badawy AE, Sadek AA, Mojahedi F, Kayserili H, Masri A, Bastaki L, Temtamy S, Muller U, Desguerre I, Casanova JL, Dursun A, Gunel M, Gabriel SB, de Lonlay P, Gleeson JG. Biallelic mutations in SNX14 cause a syndromic form of cerebellar atrophy and lysosome-autophagosome dysfunction. Nat. Genet. 2015;47:528–534. doi: 10.1038/ng.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio AL, Boyle JA, Aradi AE, Christian KA, Di Pietro SM. TPC2 controls pigmentation by regulating melanosome pH and size. Proc. Natl. Acad. Sci. USA. 2016;113:5622–5627. doi: 10.1073/pnas.1600108113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen JK. Oxidative stress in neurodegeneration: Cause or consequence. Nat. Med. 2004;10 Suppl:S18–25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- Aston D, Capel RA, Ford KL, Christian HC, Mirams GR, Rog-Zielinska EA, Kohl P, Galione A, Burton RA, Terrar DA. High resolution structural evidence suggests the Sarcoplasmic Reticulum forms microdomains with Acidic Stores (lysosomes) in the heart. Sci Rep. 2017;7:40620. doi: 10.1038/srep40620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Bates GP, Dorsey R, Gusella JF, Hayden MR, Kay C, Leavitt BR, Nance M, Ross CA, Scahill RI, Wetzel R, Wild EJ, Tabrizi SJ. Huntington disease. Nat. Rev. Dis. Primers. 2015;1:15005. doi: 10.1038/nrdp.2015.5. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Berman ER, Livni N, Shapira E, Merin S, Levij IS. Congenital corneal clouding with abnormal systemic storage bodies: A new variant of mucolipidosis. J. Pediatr. 1974;84:519–526. doi: 10.1016/s0022-3476(74)80671-2. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Calcium signalling remodelling and disease. Biochem. Soc. Trans. 2012;40:297–309. doi: 10.1042/BST20110766. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. Thirty years of Alzheimer’s disease genetics: The implications of systematic meta-analyses. Nat. Rev. Neurosci. 2008;9:768–778. doi: 10.1038/nrn2494. [DOI] [PubMed] [Google Scholar]
- Bezprozvanny IB. Calcium signaling and neurodegeneration. Acta Naturae. 2010;2:72–82. [PMC free article] [PubMed] [Google Scholar]
- Bojarski L, Herms J, Kuznicki J. Calcium dysregulation in Alzheimer’s disease. Neurochem. Int. 2008;52:621–633. doi: 10.1016/j.neuint.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Boustany RM. Lysosomal storage diseases—the horizon expands. Nat. Rev. Neurol. 2013;9:583–598. doi: 10.1038/nrneurol.2013.163. [DOI] [PubMed] [Google Scholar]
- Brailoiu E, Churamani D, Cai X, Schrlau MG, Brailoiu GC, Gao X, Hooper R, Boulware MJ, Dun NJ, Marchant JS, Patel S. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brailoiu E, Rahman T, Churamani D, Prole DL, Brailoiu GC, Hooper R, Taylor CW, Patel S. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J. Biol. Chem. 2010;285:38511–38516. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Loew LM. Integration of modeling with experimental and clinical findings synthesizes and refines the central role of inositol 1,4,5-trisphosphate receptor 1 in spinocerebellar ataxia. Front Neurosci. 8:453. doi: 10.3389/fnins.2014.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buraei Z, Yang J. Inhibition of Voltage-Gated Calcium Channels by RGK Proteins. Curr. Mol. Pharmacol. 2015;8:180–187. doi: 10.2174/1874467208666150507105613. [DOI] [PubMed] [Google Scholar]
- Calcraft PJ, Ruas M, Pan Z, Cheng X, Arredouani A, Hao X, Tang J, Rietdorf K, Teboul L, Chuang KT, Lin P, Xiao R, Wang C, Zhu Y, Lin Y, Wyatt CN, Parrington J, Ma J, Evans AM, Galione A, Zhu MX. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459:596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campion D, Dumanchin C, Hannequin D, Dubois B, Belliard S, Puel M, Thomas-Anterion C, Michon A, Martin C, Charbonnier F, Raux G, Camuzat A, Penet C, Mesnage V, Martinez M, Clerget-Darpoux F, Brice A, Frebourg T. Early-onset autosomal dominant Alzheimer disease: Prevalence, genetic heterogeneity, and mutation spectrum. Am. J. Hum. Genet. 1999;65:664–670. doi: 10.1086/302553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang C, Aranda K, Seo YJ, Gasnier B, Ren D. TMEM175 Is an Organelle K(+) Channel Regulating Lysosomal Function. Cell. 2015;162:1101–1112. doi: 10.1016/j.cell.2015.08.002. [DOI] [PubMed] [Google Scholar]
- Cang C, Zhou Y, Navarro B, Seo YJ, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell. 2013;152:778–790. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Zhong XZ, Zou Y, Murrell-Lagnado R, Zhu MX, Dong XP. Calcium release through P2X4 activates calmodulin to promote endolysosomal membrane fusion. J. Cell. Biol. 2015;209:879–894. doi: 10.1083/jcb.201409071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q, Zhong XZ, Zou Y, Zhang Z, Toro L, Dong XP. BK Channels Alleviate Lysosomal Storage Diseases by Providing Positive Feedback Regulation of Lysosomal Ca2+ Release. Dev. Cell. 2015;33:427–441. doi: 10.1016/j.devcel.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Carstea ED, Morris JA, Coleman KG, Loftus SK, Zhang D, Cummings C, Gu J, Rosenfeld MA, Pavan WJ, Krizman DB, Nagle J, Polymeropoulos MH, Sturley SL, Ioannou YA, Higgins ME, Comly M, Cooney A, Brown A, Kaneski CR, Blanchette-Mackie EJ, Dwyer NK, Neufeld EB, Chang TY, Liscum L, Strauss JF, 3rd, Ohno K, Zeigler M, Carmi R, Sokol J, Markie D, O’Neill RR, van Diggelen OP, Elleder M, Patterson MC, Brady RO, Vanier MT, Pentchev PG, Tagle DA. Niemann-Pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science. 1997;277:228–231. doi: 10.1126/science.277.5323.228. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Voltage-gated calcium channels. Cold Spring Harb. Perspect. Biol. 2011;3:a003947. doi: 10.1101/cshperspect.a003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall WA, Goldin AL, Waxman SG. International Union of Pharmacology. XLVII. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005;57:397–409. doi: 10.1124/pr.57.4.4. [DOI] [PubMed] [Google Scholar]
- Cheng X, Zhang X, Gao Q, Ali Samie M, Azar M, Tsang WL, Dong L, Sahoo N, Li X, Zhuo Y, Garrity AG, Wang X, Ferrer M, Dowling J, Xu L, Han R, Xu H. The intracellular Ca(2)(+) channel MCOLN1 is required for sarcolemma repair to prevent muscular dystrophy. Nat. Med. 2014;20:1187–1192. doi: 10.1038/nm.3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen KA, Myers JT, Swanson JA. pH-dependent regulation of lysosomal calcium in macrophages. J. Cell Sci. 2002;115(Pt 3):599–607. doi: 10.1242/jcs.115.3.599. [DOI] [PubMed] [Google Scholar]
- Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. NAADP mobilizes Ca(2+) from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- Clapham DE. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- Coen K, Flannagan RS, Baron S, Carraro-Lacroix LR, Wang D, Vermeire W, Michiels C, Munck S, Baert V, Sugita S, Wuytack F, Hiesinger PR, Grinstein S, Annaert W. Lysosoml calcium homeostasis defects, not proton pump defects, cause endolysosomal dysfunction in PSEN-deficient cells. J. Cell Biol. 2212;198:23–35. doi: 10.1083/jcb.201201076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson EJ, Duman JG, Moody MW, Chen L, Hille B. Orai-STIM-mediated Ca2+ release from secretory granules revealed by a targeted Ca2+ and pH probe. Proc. Natl. Acad. Sci. USA. 2012;109:E3539–3548. doi: 10.1073/pnas.1218247109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Cheng X, Mills E, Delling M, Wang F, Kurz T, Xu H. The type IV mucolipidosis-associated protein TRPML1 is an endolysosomal iron release channel. Nature. 2008;455:992–996. doi: 10.1038/nature07311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Shen D, Wang X, Dawson T, Li X, Zhang Q, Cheng X, Zhang Y, Weisman LS, Delling M, Xu H. PI(3,5)P(2) controls membrane trafficking by direct activation of mucolipin Ca(2+) release channels in the endolysosome. Nat. Commun. 2010;1:38. doi: 10.1038/ncomms1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong XP, Wang X, Xu H. TRP channels of intracellular membranes. J. Neurochem. 2010;113:313–328. doi: 10.1111/j.1471-4159.2010.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erie C, Sacino M, Houle L, Lu ML, Wei J. Altered lysosomal positioning affects lysosomal functions in a cellular model of Huntington’s disease. Eur. J. Neurosci. 2015;42:1941–1951. doi: 10.1111/ejn.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraldi A, Klein AD, Medina DL, Settembre C. Brain Disorders due to Lysosomal Dysfunction. Annu. Rev. Neurosci. 2016;39:277–295. doi: 10.1146/annurev-neuro-070815-014031. [DOI] [PubMed] [Google Scholar]
- Gan-Or Z, Dion PA, Rouleau GA. Genetic perspective on the role of the autophagy-lysosome pathway in Parkinson disease. Autophagy. 2015;11:1443–1457. doi: 10.1080/15548627.2015.1067364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rua V, Feijoo-Bandin S, Rodriguez-Penas D, Mosquera-Leal A, Abu-Assi E, Beiras A, Maria Seoane L, Lear P, Parrington J, Portoles M, Rosello-Lleti E, Rivera M, Gualillo O, Parra V, Hill JA, Rothermel B, Gonzalez-Juanatey JR, Lago F. Endolysosomal two-pore channels regulate autophagy in cardiomyocytes. J. Physiol. 2016;594:3061–3077. doi: 10.1113/JP271332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrity AG, Wang W, Collier CM, Levey SA, Gao Q, Xu H. The endoplasmic reticulum, not the pH gradient, drives calcium refilling of lysosomes. Elife. 2016;5:e15887. doi: 10.7554/eLife.15887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm C, Holdt LM, Chen CC, Hassan S, Muller C, Jors S, Cuny H, Kissing S, Schroder B, Butz E, Northoff B, Castonguay J, Luber CA, Moser M, Spahn S, Lullmann-Rauch R, Fendel C, Klugbauer N, Griesbeck O, Haas A, Mann M, Bracher F, Teupser D, Saftig P, Biel M, Wahl-Schott C. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat. Commun. 2014;5:4699. doi: 10.1038/ncomms5699. [DOI] [PubMed] [Google Scholar]
- Guo J, Zeng W, Chen Q, Lee C, Chen L, Yang Y, Cang C, Ren D, Jiang Y. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:196–201. doi: 10.1038/nature16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Zeng W, Jiang Y. Tuning the ion selectivity of two-pore channels. Proc. Natl. Acad. Sci. USA. 2017;114:1009–1014. doi: 10.1073/pnas.1616191114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri H, Ugrankar R, Liu Y, Henne WM. Inter-organelle ER-endolysosomal contact sites in metabolism and disease across evolution. Commun. Integr. Biol. 2016;9:e1156278. doi: 10.1080/19420889.2016.1156278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Zhu L, Balogi Z, Stefan C, Pleiss JA, Emr SD. Mdm1/Snx13 is a novel ER-endolysosomal interorganelle tethering protein. J. Cell Biol. 2015;210:541–551. doi: 10.1083/jcb.201503088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hockey LN, Kilpatrick BS, Eden ER, Lin-Moshier Y, Brailoiu GC, Brailoiu E, Futter CE, Schapira AH, Marchant JS, Patel S. Dysregulation of lysosomal morphology by pathogenic LRRK2 is corrected by TPC2 inhibition. J. Cell Sci. 201;128:232–238. doi: 10.1242/jcs.164152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger D, Haberkant P, Aguilera-Romero A, Riezman H, Porter FD, Platt FM, Galione A, Schultz C. Intracellular sphingosine releases calcium from lysosomes. Elife. 2015;4:e10616. doi: 10.7554/eLife.10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang P, Zou Y, Zhong XZ, Cao Q, Zhao K, Zhu MX, Murrell-Lagnado R, Dong XP. P2X4 forms functional ATP-activated cation channels on lysosomal membranes regulated by luminal pH. J. Biol. Chem. 2014;289:17658–17667. doi: 10.1074/jbc.M114.552158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Infante RE, Wang ML, Radhakrishnan A, Kwon HJ, Brown MS, Goldstein JL. NPC2 facilitates bidirectional transfer of cholesterol between NPC1 and lipid bilayers, a step in cholesterol egress from lysosomes. Proc. Natl. Acad. Sci. USA. 2008;105:15287–15292. doi: 10.1073/pnas.0807328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin N, Lang MJ, Weisman LS. Phosphatidylinositol 3,5-bisphosphate: Regulation of cellular events in space and time. Biochem. Soc. Trans. 2016;44:177–184. doi: 10.1042/BST20150174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Julius D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- Kilpatrick BS, Eden ER, Hockey LN, Yates E, Futter CE, Patel S. An Endosomal NAADP-Sensitive Two-Pore Ca2+ Channel Regulates ER-Endosome Membrane Contact Sites to Control Growth Factor Signaling. Cell Rep. 2017;18:1636–1645. doi: 10.1016/j.celrep.2017.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick BS, Eden ER, Schapira AH, Futter CE, Patel S. Direct mobilisation of lysosomal Ca2+ triggers complex Ca2+ signals. J. Cell Sci. 2013;126(Pt 1):60–66. doi: 10.1242/jcs.118836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick BS, Magalhaes J, Beavan MS, McNeill A, Gegg ME, Cleeter MW, Bloor-Young D, Churchill GC, Duchen MR, Schapira AH, Patel S. Endoplasmic reticulum and lysosomal Ca(2)(+) stores are remodelled in GBA1-linked Parkinson disease patient fibroblasts. Cell Calcium. 2016;59:12–20. doi: 10.1016/j.ceca.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick BS, Yates E, Grimm C, Schapira AH, Patel S. Endo-lysosomal TRP mucolipin-1 channels trigger global ER Ca2+ release and Ca2+ influx. J. Cell Sci. 2016;129:3859–3867. doi: 10.1242/jcs.190322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintzer AF, Stroud RM. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:258–262. doi: 10.1038/nature17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolter T, Sandhoff K. Principles of lysosomal membrane digestion: Stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu. Rev. Cell Dev. Biol. 2005;21:81–103. doi: 10.1146/annurev.cellbio.21.122303.120013. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Abi-Mosleh L, Wang ML, Deisenhofer J, Goldstein JL, Brown MS, Infante RE. Structure of N-terminal domain of NPC1 reveals distinct subdomains for binding and transfer of cholesterol. Cell. 2009;137:1213–1224. doi: 10.1016/j.cell.2009.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange I, Yamamoto S, Partida-Sanchez S, Mori Y, Fleig A, Penner R. TRPM2 functions as a lysosomal Ca2+-release channel in beta cells. Sci. Signal. 2009;2:ra23. doi: 10.1126/scisignal.2000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, McBrayer MK, Wolfe DM, Haslett LJ, Kumar A, Sato Y, Lie PP, Mohan P, Coffey EE, Kompella U, Mitchell CH, Lloyd-Evans E, Nixon RA. Presenilin 1 Maintains Lysosomal Ca(2+) Homeostasis via TRPML1 by Regulating vATPase-Mediated Lysosome Acidification. Cell Rep. 2015;12:1430–1444. doi: 10.1016/j.celrep.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM, Nixon RA. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rydzewski N, Hider A, Zhang X, Yang J, Wang W, Gao Q, Cheng X, Xu H. A molecular mechanism to regulate lysosome motility for lysosome positioning and tubulation. Nat. Cell Biol. 2016;18:404–417. doi: 10.1038/ncb3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin-Moshier Y, Keebler MV, Hooper R, Boulware MJ, Liu X, Churamani D, Abood ME, Walseth TF, Brailoiu E, Patel S, Marchant JS. The Two-pore channel (TPC) interactome unmasks isoform-specific roles for TPCs in endolysosomal morphology and cell pigmentation. Proc. Natl. Acad. Sci. USA. 2014;111:13087–13092. doi: 10.1073/pnas.1407004111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, Churchill GC, Schuchman EH, Galione A, Platt FM. Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat. Med. 2008;14:1247–1255. doi: 10.1038/nm.1876. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Platt FM. Lysosomal Ca(2+) homeostasis: role in pathogenesis of lysosomal storage diseases. Cell Calcium. 2011;50:200–205. doi: 10.1016/j.ceca.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Lloyd-Evans E, Waller-Evans H, Peterneva K, Platt FM. Endolysosomal calcium regulation and disease. Biochem. Soc. Trans. 2010;38:1458–1464. doi: 10.1042/BST0381458. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Dunlap K, Turner TJ. Multiple calcium channel types control glutamatergic synaptic transmission in the hippocampus. Neuron. 1993;11:895–902. doi: 10.1016/0896-6273(93)90119-c. [DOI] [PubMed] [Google Scholar]
- Luzio JP, Pryor PR, Bright NA. Lysosomes: Fusion and function. Nat. Rev. Mol. Cell Biol. 2007;8:622–632. doi: 10.1038/nrm2217. [DOI] [PubMed] [Google Scholar]
- MacDonald ME, Ambrose CM, Duyao MP, Myers RH, Lin C, Srinidhi L, Barnes G, Taylor SA, James M, Groot N, MacFarlane H, Jenkins B, Anderson MA, Wexler NS, Gusella JF, Bates GP, Baxendale S, Hummerich H, Kirby S, North M, Youngman S, Mott R, Zehetner G, Sedlacek Z, Poustka A, Frischauf A-M, Lehrach H, Buckler AJ, Church D, Doucette-Stamm L, O’Donovan MC, Riba-Ramirez L, Shah M, Stanton VP, Strobel SA, Draths KM, Wales JL, Dervan P, Housman DE, Altherr M, Shiang R, Thompson L, Fielder T, Wasmuth JJ, Tagle D, Valdes J, Elmer L, Allard M, Castilla L, Swaroop M, Blanchard K, Collins FS, Snell R, Holloway T, Gillespie K, Datson N, Shaw D, Harper PS. A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- Marambaud P, Dreses-Werringloer U, Vingtdeux V. Calcium signaling in neurodegeneration. Mol. Neurodegener. 2009;4:20. doi: 10.1186/1750-1326-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Di Paola S, Peluso I, Armani A, De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso C, Forrester A, Settembre C, Wang W, Gao Q, Xu H, Sandri M, Rizzuto R, De Matteis MA, Ballabio A. Lysosomal calcium signalling regulates autophagy through calcineurin and TFEB. Nat. Cell Biol. 2015;17:288–299. doi: 10.1038/ncb3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina DL, Fraldi A, Bouche V, Annunziata F, Mansueto G, Spampanato C, Puri C, Pignata A, Martina JA, Sardiello M, Palmieri M, Polishchuk R, Puertollano R, Ballabio A. Transcriptional activation of lysosomal exocytosis promotes cellular clearance. Dev. Cell. 2011;21:421–430. doi: 10.1016/j.devcel.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchionda M, Pittman JK, Mayor R, Patel S. Ca2+/H+ exchange by acidic organelles regulates cell migration. in vivo. J. Cell Biol. 2016;212:803–813. doi: 10.1083/jcb.201510019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies FM, Fleming A, Rubinsztein DC. Compromised autophagy and neurodegenerative diseases. Nat. Rev. Neurosci. 2015;16:345–357. doi: 10.1038/nrn3961. [DOI] [PubMed] [Google Scholar]
- Morgan AJ, Davis LC, Wagner SK, Lewis AM, Parrington J, Churchill GC, Galione A. Bidirectional Ca(2)(+) signaling occurs between the endoplasmic reticulum and acidic organelles. J. Cell Biol. 2013;200:789–805. doi: 10.1083/jcb.201204078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ, Platt FM, Lloyd-Evans E, Galione A. Molecular mechanisms of endolysosomal Ca2+ signalling in health and disease. Biochem. J. 2011;439:349–374. doi: 10.1042/BJ20110949. [DOI] [PubMed] [Google Scholar]
- Nagral A. Gaucher disease. J. Clin. Exp. Hepatol. 2014;4:37–50. doi: 10.1016/j.jceh.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzielska E, Smaga I, Gawlik M, Moniczewski A, Stankowicz P, Pera J, Filip M. Oxidative Stress in Neurodegenerative Diseaases. Mol. Neurobiol. 2016;53:4094–4125. doi: 10.1007/s12035-015-9337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiol. Rev. 2007;87(1):165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Park S, Ahuja M, Kim MS, Brailoiu GC, Jha A, Zeng M, Baydyuk M, Wu LG, Wassif CA, Porter FD, Zerfas PM, Eckhaus MA, Brailoiu E, Shin DM, Muallem S. Fusion of lysosomes with secretory organelles leads to uncontrolled exocytosis in the lysosomal storage disease mucolipidosis type IV. EMBO Rep. 2016;17:266–278. doi: 10.15252/embr.201541542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. Function and dysfunction of two-pore channels. Sci. Signal. 2015;8:re7. doi: 10.1126/scisignal.aab3314. [DOI] [PubMed] [Google Scholar]
- Patel S, Cai X. Evolution of acidic Ca(2)(+) stores and their resident Ca(2)(+)-permeable channels. Cell Calcium. 2015;57:222–230. doi: 10.1016/j.ceca.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Patel S, Docampo R. Acidic calcium stores open for business: Expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny CJ, Patel S. Poring over two-pore channel pore mutants. Messenger (Los Angel) 2015;4:46–52. doi: 10.1166/msr.2015.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penny CJ, Rahman T, Sula A, Miles AJ, Wallace BA, Patel S. Isolated pores dissected from human two-pore channel 2 are functional. Sci. Rep. 2016;6:38426. doi: 10.1038/srep38426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira GJ, Hirata H, Fimia GM, do Carmo LG, Bincoletto C, Han SW, Stilhano RS, Ureshino RP, Bloor-Young D, Churchill G, Piacentini M, Patel S, Smaili SS. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J. Biol. Chem. 2011;286:27875–27881. doi: 10.1074/jbc.C110.216580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt SJ, Funnell TM, Sitsapesan M, Venturi E, Rietdorf K, Ruas M, Ganesan A, Gosain R, Churchill GC, Zhu MX, Parrington J, Galione A, Sitsapesan R. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+ J. Biol. Chem. 2010;285:35039–35046. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittman JK. Vacuolar Ca(2+) uptake. Cell Calcium. 2011;50:139–146. doi: 10.1016/j.ceca.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Platt FM, Boland B, van der Spoel AC. The cell biology of disease: Lysosomal storage disorders: The cellular impact of lysosomal dysfunction. J. Cell Biol. 2012;199:723–734. doi: 10.1083/jcb.201208152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney JW., Jr Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi OS, Paramasivam A, Yu JC, Murrell-Lagnado RD. Regulation of P2X4 receptors by lysosomal targeting, glycan protection and exocytosis. J. Cell Sci. 2007;120(Pt 21):3838–3849. doi: 10.1242/jcs.010348. [DOI] [PubMed] [Google Scholar]
- Raffaello A, Mammucari C, Gherardi G, Rizzuto R. Calcium at the Center of Cell Signaling: Interplay between Endoplasmic Reticulum, Mitochondria, and Lysosomes. Trends Biochem. Sci. 2016;41:1035–1049. doi: 10.1016/j.tibs.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman T, Cai X, Brailoiu GC, Abood ME, Brailoiu E, Patel S. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci. Signal. 2014;7:ra109. doi: 10.1126/scisignal.2005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakulendran S, Kaski D, Hanna MG. Neuronal P/Q-type calcium channel dysfunction in inherited disorders of the CNS. Nat. Rev. Neurol. 2012;8:86–96. doi: 10.1038/nrneurol.2011.228. [DOI] [PubMed] [Google Scholar]
- Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu. Rev. Physiol. 2006;68:619–647. doi: 10.1146/annurev.physiol.68.040204.100431. [DOI] [PubMed] [Google Scholar]
- Ruas M, Davis LC, Chen CC, Morgan AJ, Chuang KT, Walseth TF, Grimm C, Garnham C, Powell T, Platt N, Platt FM, Biel M, Wahl-Schott C, Parrington J, Galione A. Expression of Ca(2)(+)-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015;34:1743–1758. doi: 10.15252/embj.201490009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez-Orellana F, Godoy PA, Silva-Grecchi T, Barra KM, Fuentealba J. Modulation of the neuronal network activity by P2X receptors and their involvement in neurological disorders. Pharmacol. Res. 2015;101:109–115. doi: 10.1016/j.phrs.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Sakurai Y, Kolokoltsov AA, Chen CC, Tidwell MW, Bauta WE, Klugbauer N, Grimm C, Wahl-Schott C, Biel M, Davey RA. Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347:995–998. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samie M, Wang X, Zhang X, Goschka A, Li X, Cheng X, Gregg E, Azar M, Zhuo Y, Garrity AG, Gao Q, Slaugenhaupt S, Pickel J, Zolov SN, Weisman LS, Lenk GM, Titus S, Bryant-Genevier M, Southall N, Juan M, Ferrer M, Xu H. A TRP channel in the lysosome regulates large particle phagocytosis via focal exocytosis. Dev. Cell. 2013;26:511–524. doi: 10.1016/j.devcel.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieder M, Rotzer K, Bruggemann A, Biel M, Wahl-Schott C. Planar patch clamp approach to characterize ionic currents from intact lysosomes. Sci. Signal. 2010;3:13. doi: 10.1126/scisignal.3151pl3. [DOI] [PubMed] [Google Scholar]
- Schieder M, Rotzer K, Bruggemann A, Biel M, Wahl-Schott CA. Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes. J. Biol. Chem. 2010;285:21219–21222. doi: 10.1074/jbc.C110.143123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake M, Schroder B, Saftig P. Lysosomal membrane proteins and their central role in physiology. Traffic. 2013;14:739–748. doi: 10.1111/tra.12056. [DOI] [PubMed] [Google Scholar]
- Shang S, Zhu F, Liu B, Chai Z, Wu Q, Hu M, Wang Y, Huang R, Zhang X, Wu X, Sun L, Wang Y, Wang L, Xu H, Teng S, Liu B, Zheng L, Zhang C, Zhang F, Feng X, Zhu D, Wang C, Liu T, Zhu MX, Zhou Z. Intracellular TRPA1 mediates Ca2+ release from lysosomes in dorsal root ganglion neurons. J. Cell Biol. 2016;215:369–381. doi: 10.1083/jcb.201603081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen D, Wang X, Li X, Zhang X, Yao Z, Dibble S, Dong XP, Yu T, Lieberman AP, Showalter HD, Xu H. Lipid storage disorders block lysosomal trafficking by inhibiting a TRP channel and lysosomal calcium release. Nat. Commun. 2012;3:731. doi: 10.1038/ncomms1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaugenhaupt SA. The molecular basis of mucolipidosis type IV. Curr Mol. Med. 2002;2:445–450. doi: 10.2174/1566524023362276. [DOI] [PubMed] [Google Scholar]
- Sleat DE, Wiseman JA, El-Banna M, Price SM, Verot L, Shen MM, Tint GS, Vanier MT, Walkley SU, Lobel P. Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport. Proc. Natl. Acad. Sci. USA. 2004;101:5886–5891. doi: 10.1073/pnas.0308456101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumoza-Toledo A, Lange I, Cortado H, Bhagat H, Mori Y, Fleig A, Penner R, Partida-Sanchez S. Dendritic cell maturation and chemotaxis is regulated by TRPM2-mediated lysosomal Ca2+ release. FASEB J. 2011;25:3529–3542. doi: 10.1096/fj.10-178483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada SH, Ikebara JM, de Sousa E, Cardoso DS, Resende RR, Ulrich H, Ruckl M, Rudiger S, Kihara AH. Determining the Roles of Inositol Trisphosphate Receptors in Neurode-generation: Interdisciplinary Perspectives on a Complex Topic. Mol. Neurobiol. 2016 doi: 10.1007/s12035-016-0205-8. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Momiyama A. Different types of calcium channels mediate central synaptic transmission. Nature. 1993;366:156–158. doi: 10.1038/366156a0. [DOI] [PubMed] [Google Scholar]
- Tang TS, Tu H, Chan EY, Maximov A, Wang Z, Wellington CL, Hayden MR, Bezprozvanny I. Huntingtin and huntingtin-associated protein 1 influence neuronal calcium signaling mediated by inositol-(1,4,5) triphosphate receptor type 1. Neuron. 2003;39:227–239. doi: 10.1016/s0896-6273(03)00366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AC, Williams H, Seto-Salvia N, Bacchelli C, Jenkins D, O’Sullivan M, Mengrelis K, Ishida M, Ocaka L, Chanudet E, James C, Lescai F, Anderson G, Morrogh D, Ryten M, Duncan AJ, Pai YJ, Saraiva JM, Ramos F, Farren B, Saunders D, Veray B, Gissen P, Straatmaan-Iwanowska A, Baas F, Wood NW, Hersheson J, Houlden H, Hurst J, Scott R, Bitner-Glindzicz M, Moore GE, Sousa SB, Stanier P. Mutations in SNX14 cause a distinctive autosomal-recessive cerebellar ataxia and intellectual disability syndrome. Am. J. Hum. Genet. 2014;95:611–621. doi: 10.1016/j.ajhg.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian X, Gala U, Zhang Y, Shang W, Nagarkar Jaiswal S, di Ronza A, Jaiswal M, Yamamoto S, Sandoval H, Duraine L, Sardiello M, Sillitoe RV, Venkatachalam K, Fan H, Bellen HJ, Tong C. A voltage-gated calcium channel regulates lysosomal fusion with endosomes and autophagosomes and is required for neuronal homeostasis. PLoS Biol. 2015;13:e1002103. doi: 10.1371/journal.pbio.1002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth B, Iordanov I, Csanady L. Ruling out pyridine dinucleotides as true TRPM2 channel activators reveals novel direct agonist ADP-ribose-2’-phosphate. J. Gen. Physiol. 2015;145:419–430. doi: 10.1085/jgp.201511377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam K, Wong CO, Zhu MX. The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium. 2015;58:48–56. doi: 10.1016/j.ceca.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Gao Q, Yang M, Zhang X, Yu L, Lawas M, Li X, Bryant-Genevier M, Southall NT, Marugan J, Ferrer M, Xu H. Up-regulation of lysosomal TRPML1 channels is essential for lysosomal adaptation to nutrient starvation. Proc. Natl. Acad. Sci. USA. 2015;112:E1373–1381. doi: 10.1073/pnas.1419669112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Zhang X, Dong XP, Samie M, Li X, Cheng X, Goschka A, Shen D, Zhou Y, Harlow J, Zhu MX, Clapham DE, Ren D, Xu H. TPC proteins are phosphoinositide-activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151:372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong CO, Li R, Montell C, Venkatachalam K. Drosophila TRPML is required for TORC1 activation. Curr. Biol. 2012;22:1616–1621. doi: 10.1016/j.cub.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Ryskamp DA, Liang X, Egorova P, Zakharova O, Hung G, Bezprozvanny I. Enhanced Store-Operated Calcium Entry Leads to Striatal Synaptic Loss in a Huntington’s Disease Mouse Model. J. Neurosci. 2016;36:125–141. doi: 10.1523/JNEUROSCI.1038-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Martinoia E, Szabo I. Organellar channels and transporters. Cell Calcium. 2015;58:1–10. doi: 10.1016/j.ceca.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Ren D. Lysosomal physiology. Annu. Rev. Physiol. 2015;77:57–80. doi: 10.1146/annurev-physiol-021014-071649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S, Jha A, Li Q, Soyombo AA, Dickinson GD, Churamani D, Brailoiu E, Patel S, Muallem S. Transient receptor potential mucolipin 1 (TRPML1) and two-pore channels are functionally independent organellar ion channels. J. Biol. Chem. 2011;286:22934–22942. doi: 10.1074/jbc.M110.210930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Cheng X, Yu L, Yang J, Calvo R, Patnaik S, Hu X, Gao Q, Yang M, Lawas M, Delling M, Marugan J, Ferrer M, Xu H. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat. Commun. 2016;7:12109. doi: 10.1038/ncomms12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XZ, Cao Q, Sun X, Dong XP. Activation of lysosomal P2X4 by ATP transported into lysosomes via VNUT/SLC17A9 using V-ATPase generated voltage gradient as the driving force. J. Physiol. 2016;594:4253–4266. doi: 10.1113/JP271893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XZ, Sun X, Cao Q, Dong G, Schiffmann R, Dong XP. BK channel agonist represents a potential therapeutic approach for lysosomal storage diseases. Sci. Rep. 2016;6:33684. doi: 10.1038/srep33684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong XZ, Zou Y, Sun X, Dong G, Cao Q, Pandey A, Rainey JK, Zhu X, Dong XP. Inhibition of TRPML1 by lysosomal adenosine involved in severe combined immunodeficiency diseases. J. Biol. Chem. 2017 doi: 10.1074/jbc.M116.743963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X, Schieder M, Cuny H, Fenske S, Gruner C, Rotzer K, Griesbeck O, Harz H, Biel M, Wahl-Schott C. The two-pore channel TPCN2 mediates NAADP-dependent Ca(2+)-release from lysosomal stores. Pflugers Arch. 2009;458:891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]