Abstract
Electrochemical detection method allowing to detect prostate-specific antigen (PSA), a biomarker of prostate cancer (PCa), with PSA glycoprofiling was applied in an analysis of PCa serum samples for the first time. Electrochemical impedance spectroscopy (EIS) as a label-free method with immobilized anti-PSA was applied for PSA detection and lectins to glycoprofile captured PSA on the same surface. A proper choice of blocking agent providing high selectivity of biosensor detection with the immobilized anti-PSA antibody was done. The biosensor could detect PSA down to 100 ag/mL with a linear concentration working range from 100 ag/mL up to 1 mg/mL, i.e. 10 orders of concentration magnitude and the sensitivity of (5.5 ± 0.2)%/decade. The results showed that a commercial carbo-free blocking solution was the best one, reducing non-specific binding 55-fold when compared to the immunosensor surface without any blocking agent applied, while allowing to detect PSA. The biosensor response obtained after addition of lectin (i.e. proportional to the amount of a particular glycan on PSA) divided by the biosensor response obtained after incubation with a sample (i.e. proportional to the PSA level in the sample) was applied to distinguish serum samples of PCa patients from those of healthy individuals. The results showed that Maackia amurensis agglutinin (MAA) recognizing α-2,3-terminal sialic acid can be applied to distinguish between these two sets of samples since the MAA/PSA response obtained from the analysis of the PCa samples was significantly higher (5.3-fold) compared to the MAA/PSA response obtained by the analysis of samples from healthy individuals. Thus, combined analysis of serological PSA levels together with PSA glycoprofiling of aberrant glycosylation of PSA (i.e. increase in the level of α-2,3-terminal sialic acid) has a potential to improve detection of PCa.
Keywords: Biosensors, Electrochemical impedance spectroscopy, Lectins, Prostate cancer, Prostate-specific antigen
1. Introduction
Altered glycosylation might indicate various diseases, including cancer, autoimmune diseases and congenital defects of glycosylation [1,2]. Since carbohydrates are frequently involved in many biological processes (e.g. cell-cell recognition, cell-matrix, host-pathogen interaction, immune modulation, etc.), it is not surprising that changes in glycan moieties reflect the physiological and pathological state of the organism [3]. Alterations in both glycan types (N- and O-glycans) often influence the aggressive potential of tumor cells and their interactions with stromal cell types, including leukocytes, platelets, fibroblasts and endothelial cells [4,5]. Moreover, changed glycoforms are closely linked to tumor progression and metastatic spread [6,7].
Prostate cancer (PCa, adenocarcinoma or glandular cancer of the prostate gland) represents the second most common cancer in men worldwide, with an estimated 1.1 million cases diagnosed in 2012 alone [8]. The principal screening methods for PCa diagnosis are digital rectal examination (DRE) and determination of the level of prostate-specific antigen (PSA) in serum [9]. However, positive results of the DRE do not necessarily mean presence of PCa and such final diagnosis should be supported by PSA level screening [10]. The level of PSA in serum can also rise as a result of benign prostate hypertrophy (BPH), suggesting some limitations in early-stage detection of PCa.
Currently, PSA is widely used as the gold standard in PCa diagnostics. PSA belongs to tissue kallikrein-related family of peptidases and is also known as γ-seminoprotein, kallikrein-3 or KLK3 [11]. PSA is a 28.4 kDa glycoprotein containing approximately 8% (by weight) of N-glycan with a single glycosylation site [12,13]. A free form of PSA is most commonly found in seminal fluid, urine and tissue, whereas PSA in serum is complexed with several extracellular proteinase inhibitors, such as α-1-antichymotrypsin (ACM) and α-2-macroglobulin (A2M) [14]. Due to disruption of basal cells, basement membrane and ductal lumen architecture, serum PSA concentration is elevated in prostate diseases, including PCa [15]. Although serum PSA screening is routinely used for diagnostic purposes, there are many limitations associated with a substantial amount of false-positive results, extensive overtreatment and unnecessary biopsies [16]. Therefore, in order to enhance sensitivity and specificity of PCa detection, novel screening/diagnostic strategies need to be developed [17–20].
Here we present lectin-based electrochemical immunoassay of cancer associated glycosylation status of PSA present in human serum samples performed in a sensitive and patient-friendly way. Our approach is based on electrochemical impedance spectroscopy (EIS), enabling detection of analyte down to attomolar level with a possibility to directly glycoprofile the analyte [21,22]. Electrochemical impedance spectroscopy (EIS) is a label-free method which can detect analytes down to a single molecule level [21]. The method is based on the application of a small sinusoidal voltage signal to an electrode with the resulting current measured. The impedance is then calculated as the ratio between voltage and current with complex impedance being a sum of the real (Z´) and imaginary (Z´´) impedance [23]. Electrochemical processes on the electrode surface can be modelled using equivalent circuits (with Randles and Ershler circuit model most frequently applied) to extract circuit components such as charge transfer resistance (Rct) and double layer capacitance [23]. Charge transfer resistance Rct is detected across the interface by a redox probe present in the solution and is influenced by the potential of the redox reaction on the electrode surface, the activation barrier generated by this interface and the electrostatic and/or steric hindrance by adsorbed species. Charge transfer resistance is very sensitive to changes at the electrode-solution interface i.e. during the build-up of the bioreceptive layer and, subsequently, in the recruitment of targets from the solution bulk [24]. Changes in Rct as a function of a biorecognition event can be specifically analyzed [24] and applied to quantify the analyte concentration. Electrochemical (including EIS-based) biosensors represent low-cost, quick and miniaturizable platforms of detection [21,23] applicable in clinical settings [21,24]. EIS-based immunosensors were successfully applied for detection of PSA [25–28] and free PSA or total PSA complexed with other proteins were determined in human serum [27,28].
Lectin-based electrochemical biosensors can be applied in analysis of a specific sub-glycoproteome (e.g. the presence of various glycans and even the indication of various linkages between sialic acids and the rest of glycan) [21]. Although a glycan analysis could be performed employing traditional instrumental mass spectrometric techniques, analysis of glycosidic linkages (i.e. the differentiation between α-2,3- and α-2,6-terminated sialic acids) is quite challenging [21]. Furthermore, advanced spectrometric methods are time consuming, requiring extensive sample pre-treatment, chemical/enzymatic release of glycans, glycan derivatization and subsequent manual data interpretation by skilled operators [29].
2. Materials and methods
2.1. Materials
11-mercaptoundecanoic acid (MUA), 6-mercapto-1-hexanol (MH), ethanolamine hydrochloride (ETA), gelatin from porcine skin (type A), hydrogen peroxide solution 30% (w/w), phosphate buffered saline (PBS) tablets, potassium chloride, potassium hexacyanoferrate (III), potassium hexacyanoferrate (II) trihydrate, 1-ethyl-3-(3-(dimethylamino)propyl)carbodiimide (EDC), N-hydroxysuccinimide (NHS) and Tween 20 were obtained from Sigma Aldrich (St. Louis, MO, USA). Monoclonal antibody against PSA was purchased from Abcam (Cambridge, UK). Sambucus nigra agglutinin type I (SNA, specific for α-2,6-sialic acid) from elderberry was obtained from EY Laboratories (San Mateo, CA, USA). Maackia amurensis agglutinin II (MAA, recognizing α-2,3-sialic acid) and carbo-free (CF) blocking solution were purchased from Vector Laboratories (Burlingame, CA, USA). Ultrapure ethanol (for UV/VIS spectroscopy) was received from Slavus (Bratislava, SK). Phosphate buffer saline (PBS) solution (10 mM, pH 7.4) was prepared by dissolving 1 tablet in 200 mL of ultra-pure deionized water (DW). All solutions were filtered prior to use (0.2 mm sterile filters) and working solutions of anti-PSA antibody and lectins were diluted in freshly prepared 10 mM PBS, pH 7.4.
2.2. Serum samples
Patient PCa serum samples and control sera (without PCa and BPH) were obtained from The Private urological ambulance, Trenčín (SK), and its Ethics Committee approved the use of the samples. All participants signed an informed consent document prior to sample collection. Untreated serum samples were obtained from three PCa patients and three healthy donors. Serum samples were taken during the morning fasted state into a gel and clot activator tube (Vacutest Kima, Piove di Sacco, IT). After 30 min but within 2 h, the tubes were centrifuged at 25 °C for 10 min at 2500-3000 rpm. The sera were transferred into sterile plastic vials and stored at -20 °C before use. Before electrochemical analysis, human serum samples (50 μL) were depleted from IgG and HAS according to the manufacturer's instructions (Multiple Affinity Removal Spin Cartridge HSA/IgG, Agilent Technologies, CA, USA) and prepared in 10 mM PBS buffer, pH 7.4. All stock solutions (human sera, antibodies, lectins) were stored at -20 °C in aliquots. None of the samples underwent more than two freeze-thaw cycles.
2.3. Assay procedures and apparatus
All electrochemical measurements were performed on a laboratory potentiostat/galvanostat PGSTAT 128 N (Metrohm Autolab, NL) run under Nova Software 1.10 (Ecochemie, The Netherland), with investigation of changes in charge transfer resistance (Rct). A three electrode cell system, using a modified gold disk working electrode (d = 1.6 mm, Bioanalytical systems, USA), an auxiliary platinum electrode and a reference Ag/AgCl electrode (Bioanalytical systems, USA) was used in all experiments. The EIS measurements were recorded at 50 different frequencies (from 0.1 Hz up to 100 kHz), applying a 200 mV a.c. voltage in a freshly prepared and filtered electrolyte containing 5 mM potassium hexacyanoferrate (III), 5 mM potassium hexacyanoferrate (II) and 10 mM PBS (pH 7.4). The data acquired were shown in a Nyquist diagram with a Randles-Erschler equivalent circuit (R (Q [RW]) applied for data fitting from which Rct values were extracted. The change in charge-transfer resistance (Rct) relative to the reference surface (e.g. a biosensor surface after the lectin immobilization and stabilization expressed in %) was used as the output signal. All measurements were performed at RT (25 °C) and each sample was analyzed at least in triplicate (±SD), with an independent biosensor device to ensure assay reproducibility.
2.4. Preparation of the impedimetric biosensor
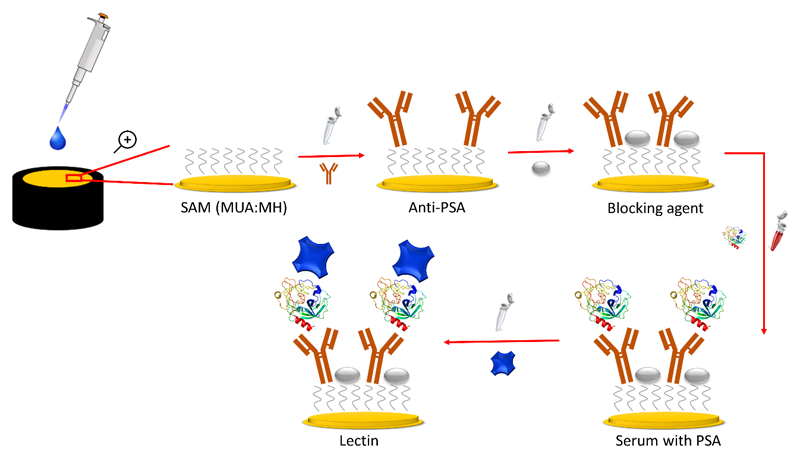
The gold electrodes were first cleaned by electrochemical reductive desorption under anaerobic conditions using cyclic voltammetry measurements in 0.1 M NaOH. Subsequently, the electrodes were polished for 5 min each by MicroPolish alumina slurry with the particle size of 1.0 mm and 0.3 mm (Buehler, USA), and afterwards rinsed thoroughly with DW and cleaned ultrasonically for 5 min. In the next step, the polished electrodes were treated with a freshly prepared piranha solution (H2SO4:H2O2, v/v 3:1) for 15 min, and rinsed with DW and further sonicated. Afterwards, the electrodes were electrochemically polished in 0.1 M H2SO4 by running 50 scans in the potential window from -200 mV to +1500 mV. The Au electrodes were then cleaned with gold oxide stripping procedure (20 scan run from +750 mV to +200 mV at a scan rate of 100 mV/s) [30]. Finally, the electrodes were rinsed with DW and ultra-pure ethanol and then dried in a stream of pure nitrogen gas. The clean electrodes were immediately immersed into a mixed ethanolic solution of 1 mM MUA and 1 mM MH in a ratio of 1:3 v/v (MUA:MH) [20] and incubated overnight at RT in the dark. The SAM-modified electrodes with terminal carboxyl groups were activated with aqueous solution containing 1:1 mixture of 0.2 M EDC and 0.05 M NHS for 15 min. The anti-PSA antibody was then covalently immobilized on the activated SAM layer from 40 μL stock solution (20 ng/mL) by 30 min incubation. After that, the electrodes were incubated with a blocking agent (i.e. CF blocking solution if not mentioned otherwise) for 30 min. In the next step, PSA from 40 μL IgG/HSA depleted serum samples (as indicated above) for analysis of PCa samples or standard PSA solution for biosensor calibration was incubated for 30 min. The serum samples from the PCa patients were finally glycoprofiled using two lectins (SNA and MAA) incubated with the biosensor for 30 min (40 μL droplet of 0.5 mg/mL). After each procedure, the electrodes were rinsed thoroughly with 10 mM PBS (pH 7.4) to remove unbound lectin molecules. The overall process of the biosensor construction and PSA sensing is provided in Scheme 1.
Scheme 1.
Construction of the biosensor device showing sensing of PSA and glycoprofiling of PSA by application of lectin.
3. Results and discussion
3.1. Reduction of non-specific interactions
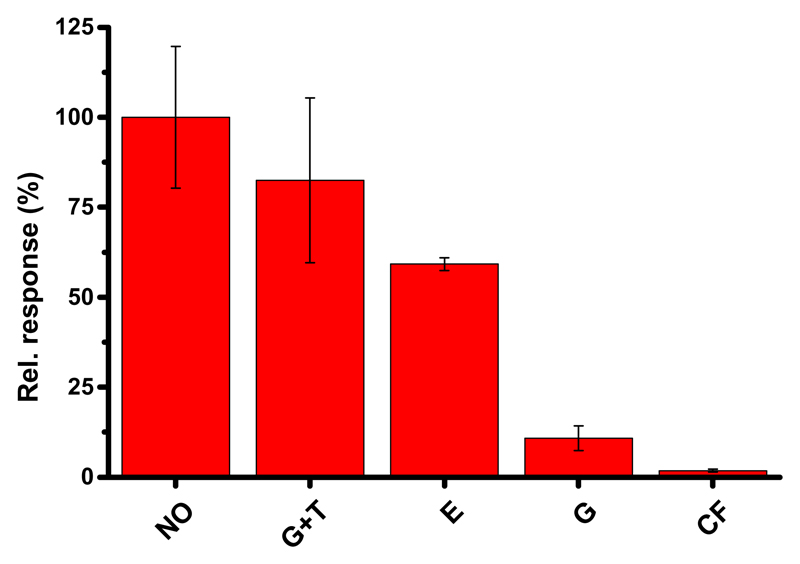
In order to create an appropriate immunosensor platform for glycoprofiling of human serum samples and in order to minimize non-specific interactions, the choice of a blocking agent was optimized. We compared four blocking agents (1 M ETA; 0.8% gelatin + 0.05% Tween 20; 0.1% gelatin and a carbo-free blocking solution) by monitoring a non-specific binding of SNA lectin towards the biosensor surface with an immobilized antibody. When SNA lectin was incubated onto the surface with immobilized antibody (without any blocking agent, without PSA analyte), a significant increase of the Rct value was observed (labelled as “NO” in Fig. 1).
Figure 1.
Performance of various blocking agents (G+T=0.8% gelatin + 0.05% Tween 20; E=1 M ETA; G=0.1% gelatin and CF= a CF blocking solution) applied for 30 min on the electrode modified with covalently immobilized antibody (from 40 μL of 20 ng/mL stock solution incubated for 30 min) to resist non-specific SNA lectin interactions compared to the biosensor without any blocking agent applied (NO). In this work, SNA lectin with concentration of 0.5 mg/mL was applied for 30 min to test performance of the blocking agents. 100% was set to the response value obtained without addition of any blocking agent, i.e. labelled as NO. The EIS measurements were recorded at 50 different frequencies (from 0.1 Hz up to 100 kHz), applying a 200 mV a.c. voltage in a freshly prepared and filtered electrolyte containing 5 mM potassium hexacyanoferrate(III), 5 mM potassium hexacyanoferrate(II) and 10 mM PBS (pH 7.4). All measurements were performed at RT (~25°C) and each sample was analyzed at least in triplicate, showing results with standard deviation (SD).
This suggests either substantial non-specific binding of SNA lectin with the biosensor surface or binding of SNA to the antibody's glycan exposed to solution phase since an antibody can contain α-2,6-linked sialic acid recognized by SNA. Similarly to our observations, Haab's group found out that particular attention has to be paid to block interactions between an immobilized antibody and lectins applied to complete a sandwich configuration [31]. A decrease of non-specific binding was found with application of all examined blocking agents when compared to the biosensor response from the device prepared without any blocking agent, i.e. from (83 ± 23)% for gelatin + Tween 20 to (11 ± 3)% for gelatin. The best blocking behavior was nevertheless exhibited by the CF buffer (i.e. a relative binding response of (1.8 ± 0.4)%) as shown in Fig. 1 (100% was set to the response value obtained without addition of any blocking agent). Thus, by application of the CF blocking buffer, non-specific binding was suppressed 55-fold and this buffer was used in the following electrochemical experiments.
3.2. Validation of the immunosensor specificity
The biosensor with the immobilized anti-PSA antibody and blocked by the CF buffer was calibrated with PSA in the concentration window (~0.001-1000 ng/mL), including the physiological concentration of PSA in the serum. The calibration curve showed that the application of the CF buffer helped to resist non-specific interactions from the SNA lectin, but allowed specific interaction with its analyte e PSA. The calibration curve was fitted using the following linear equation: ΔRct (%) = (41.6 ± 0.4) + (5.5 ± 0.2)*log(cPSA) with R2 = 0.994. The limit of detection (LOD) was calculated from the calibration curve taking into account 3x average SD (standard deviation) as the assay noise as described previously [32], with an extrapolated LOD of 100 ag/mL and a linear working concentration range spanning 10 concentration magnitude from 100 ag/mL up to 1 mg/mL. In Table 1, the performance of our immunosensor for detection of PSA is compared with literature data, indicating that our approach could detect PSA with the highest sensitivity, i.e. with the lowest LOD. The reproducibility of the immunosensor preparation expressed as average RSD was 4.5% (RSD in the range from 1.5% to 8.6%).
Table 1.
Analytical characteristics of PSA electrochemical immunosensors
| Immobilization | Technique | LOD | LR | Ref. |
|---|---|---|---|---|
| GCE/MWCNTs-ILs/Thi)/anti-PSA/PSA/HRP-labeled PSA | DPV | 20 pg/mL | 0.2 – 40 ng/mL | [33] |
| AuNWs/anti-PSA doped polypyrole/BSA/PSA | DPV | 0.3 pg/mL | 10 fg/mL – 10 ng/mL | [34] |
| GCE/GO/Chi/anti-PSA+Thi/PSA/AuNPs–PAMAM/aptamer | DPV EIS |
10 fg/mL 5 pg/mL |
0.1 pg/mL– 90ng/mL 0.05 – 35 ng/mL |
[35] |
| GCE/MWCNTs/IL/Chi-AuNPs-PAMAM)/ PhCl/anti-PSA+Thi/PSA/HRP- anti-PSA | DPV EIS |
1 pg/mL 0.5 ng/mL |
0.05 – 80 ng/mL 5 – 25 ng/mL |
[36] |
| AuNano/OEGMA-co-GMA/anti-PSA/PSA/Si-NPs-Ab2 | DPV | 2 pg/mL | 5 pg/mL – 1000 ng/mL | [37] |
| GCE/NH2-GS–FcCOH/anti-PSA/PSA/Ab2 | EIS | 2 pg/mL | 0.01 – 10 ng/mL | [38] |
| GCE/GS-AuNPs/anti-PSA/PSA | CV | 0.59 ng/mL | 0 – 10 ng/mL | [39] |
| GCE/Ag@MSNs/anti-PSA/BSA/PSA | CV | 15 pg/mL | 0.05 – 50 ng/mL | [40] |
| Au/SAM1/NH2-PEG3-biotin/avidin/biotin-anti-PSA/PSA/HRP-anti-PSA | EIS | 0.51 ng/mL* | 2 – 24 ng/mL | [27] |
| Au/anti-PSA/PSA/anti-PSA - coated with enzyme-encapsulated liposomes | LSV | 7 pg/mL | 0.01 – 100 ng/mL | [41] |
| Au/SAM2/anti-PSA | EIS | 10 pg/mL | 1.1 – 53 pg/mL | [25] |
| SPE/array of holes in a polymer/polyaniline/biotin/avidin/biotin-anti-PSA | EIS | 1 ng/mL # 1 pg/mL # |
1 – 100 ng/mL # 1 – 100 pg/mL # |
[26] |
| Au/SAM3/protein A/BSA/anti-PSA(total) Au/SAM3/protein A/BSA/anti-PSA(free) |
EIS EIS |
1 ng/mL 1 ng/mL |
1 – 10 ng/mL 1 – 10 ng/mL |
[28] |
| Au/SAM4/anti-PSA | EIS | 130 ag/mL | 130 ag/mL – 1 ug/mL • | [20] |
| Au/SAM5/anti-PSA/CF/PSA from human serum/lectin | EIS | 100 ag/mL & | 100 ag/mL – 1 ug/mL • | This study |
LOD: limit of detection, LR: linear range, GCE: glassy carbon electrode, IL: ionic liquids, Thi: thionine, PSA: prostate-specific antigen, anti-PSA: antibody against PSA, MWCNTs: multi-walled carbon nanotubes, HRP: horseradish peroxidase, DPV: differential pulse voltammetry, AuNWs: gold nanowires, BSA: bovine serum albumin, GO: graphene oxide, Chi: chitosan, AuNPs–PAMAM: polyamidoamine dendrimer encapsulated gold nanoparticles, PhCl: phtaloyl chloride, AuNano/OEGMA-co-GMA: nanostructured-Au/polymer brush of [oligo(ethylene glycol)methacrylate-co-glycidyl methacrylate, SiNP: silica nanoparticles, Ab2: secondary antibody, FcCOH: ferrocenecarboxaldehyde, GS: graphene sheets, Ag@NH2-MCM48: silver hybridized mesoporous silica, AuNPs: gold nanoparticles, CV: cyclic voltammetry, Ag@MSNs: silver hybridized mesoporous silica nanoparticles, SAM1: self-assembled monolayer HS-C16-COOH/EG3SH, LSV: linear sweep voltammetry, SAM2: various dithiols with –COOH terminations, SPE: screen printed carbon electrode, SAM3: HS--C11-COOH/HS-C2-OH, SAM4: HS--C11-COOH/HS-C6-OH, SAM5: HS-C11-COOH/HS-C6-OH, CF: carbo-free buffer,* - total PSA, # - depending on the way anti-PSA is immobilized, & - this value was extrapolated, •- semilogarithmic calibration curve.
3.3. Glycoprofiling of serum samples with the impedimetric bi sensors
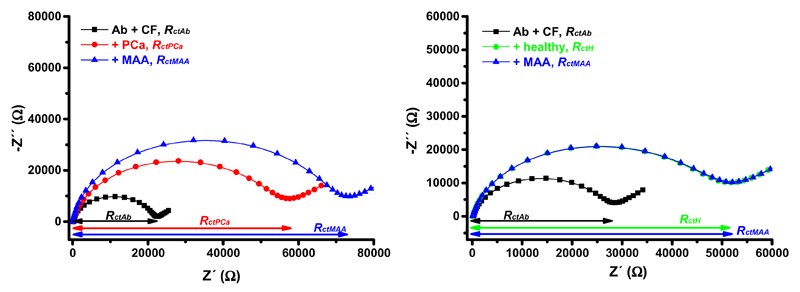
Three samples from healthy individuals and three samples from PCa patients were analyzed. Moreover, two different lectins - SNA and MAA - were applied in the study, since these two lectins can distinguish between different linkages attaching terminal sialic acid to the glycan backbone on PSA (i.e. SNA recognizing terminal α-2,6-linked sialic acid and MAA recognizing terminal α-2,3-linked sialic acid). The lectin-based immunosensor in a sandwich configuration was applied for glycoprofiling of IgG/HSA depleted serum samples. In Fig. 2, the response after incubation of the immunosensor with the serum sample (i.e. proportional to the level of PSA in the serum) and the response after incubation with MAA (i.e. proportional to the level of terminal α-2,3-linked sialic acid within the glycan present on PSA) for both types of samples (i.e. from PCa patients and from healthy individuals) is depicted. The Nyquist plots shown in Fig. 2 indicated a shift of Rct = 32.1 kΩ after addition of the serum sample from a PCa patient (Fig. 2 left), while a shift of Rct = 26.6 kΩ was observed when the serum sample from a healthy individual was incubated with the biosensor surface (Fig. 2 right). Moreover, the Nyquist plots presented in Fig. 2 pointed out to a large shift of Rct = 15.3 kΩ after addition of MAA lectin to the biosensor surface, which interacted with the serum sample from the PCa patient (Fig. 2 left), while almost no change in Rct was found (i.e. only 0.3 kΩ) when MAA lectin interacted with the biosensor surface previously incubated with the serum sample from the healthy individual (Fig. 2 right). Impedimetric analyses of all 6 serum samples analyzed by the immunosensor involving MAA lectin are shown in Table 2.
Figure 2.
Nyquist plots of the immunosensor before incubation with a sample (i.e. immunosensor with immobilized antibody (Ab) and blocked by a CF buffer, i.e. Ab + CF, black line and symbols), after incubation with a serum sample from a healthy individual (right, +healthy, green line and symbols) or after incubation with a serum sample from a PCa patient (left, +PCa, red line and symbols), and after interaction with MAA lectin (blue line and symbols). Please note that for examination of the serum sample from the healthy individual blue line and symbols overlap green line and symbols. Both serum samples were diluted with an optimal dilution fold 1+1,000 in a PBS buffer prior to analysis by the biosensor. EIS assay conditions are described in Fig. 1.
Table 2.
Impedimetric analysis of serum samples from PCa patients (highlighted in light red) and healthy individuals (highlighted in light green).
| A | B | C | D | E | F | G | H | I | |
|---|---|---|---|---|---|---|---|---|---|
| Patient # | Biosensor Rct (kΩ) | + serum Rct (kΩ) | + MAA Rct (kΩ) | + serum Rel. (%) a | + MAA Rel. (%) b | + serum Aver. (%) ± SD | + MAA Aver. (%) ± SD | MAA/serum | MAA/serum Aver. (%) ± SD |
| 1 | 7.0 | 16.0 | 27.4 | 128 | 71.3 | 120 ± 37 | 43.4 ± 24.1 | 0.554 | 0.374 ± 0.183 |
| 1 | 18.5 | 33.3 | 43.4 | 80 | 30.3 | 0.379 | |||
| 1 | 21.1 | 53.2 | 68.5 | 152 | 28.8 | 0.189 | |||
| 2 | 7.5 | 22.4 | 33.3 | 199 | 48.7 | 156 ± 48 | 54.2 ± 6.0 | 0.245 | 0.374 ± 0.135 |
| 2 | 8.4 | 17.1 | 26.2 | 103 | 53.2 | 0.514 | |||
| 2 | 5.8 | 15.5 | 24.9 | 167 | 60.6 | 0.363 | |||
| 3 | 12.3 | 28.8 | 54.6 | 134 | 89.6 | 162 ± 36 | 98.5 ± 38.7 | 0.668 | 0.601 ± 0.142 |
| 3 | 19.1 | 57.7 | 139.0 | 202 | 140.9 | 0.697 | |||
| 3 | 18.9 | 47.0 | 77.6 | 149 | 65.1 | 0.438 | |||
| 4 | 22.2 | 32.1 | 32.2 | 45 | 0.312 | 62 ± 32 | 0.543 ± 0.223 | 0.007 | 0.011 ± 0.006 |
| 4 | 37.1 | 52.9 | 53.3 | 43 | 0.756 | 0.018 | |||
| 4 | 26.9 | 53.5 | 53.8 | 99 | 0.561 | 0.006 | |||
| 5 | 6.3 | 9.4 | 10.4 | 49 | 10.6 | 68 ± 27 | 8.45 ± 6.87 | 0.216 | 0.159 ± 0.133 |
| 5 | 17.1 | 26.5 | 30.2 | 55 | 14.0 | 0.254 | |||
| 5 | 26.9 | 53.5 | 53.9 | 99 | 0.75 | 0.008 | |||
| 6 | 22.7 | 41.6 | 42.6 | 83 | 2.40 | 69 ± 18 | 5.64 ± 3.57 | 0.029 | 0.086 ± 0.051 |
| 6 | 19.4 | 33.8 | 37.0 | 74 | 9.47 | 0.128 | |||
| 6 | 29.3 | 43.7 | 45.9 | 49 | 5.03 | 0.102 |
Legend: a = ((B-A)/A*100)-100; b = ((C-B)/B*100)-100; samples 1-3 were from PCa patients and samples 4-6 were from healthy individuals; Aver. = an average value; SD = standard deviation; MAA = after incubation with Maackia amurensis agglutinin. All six samples were measured at least in triplicate.
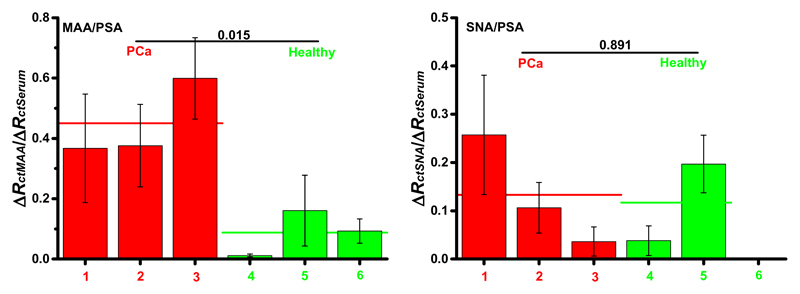
Standard techniques (i.e. ELISA reading of PSA) revealed the concentration of PSA in the range 7.1-77.0 ng/mL for PCa patients and in the range 1.1-2.5 ng/mL for healthy individuals. The biosensor response obtained after the addition of lectin (i.e. proportional to the amount of a particular glycan on PSA) was divided by the biosensor response obtained after incubation with a serum sample (i.e. proportional to the PSA level in the sample) and the relative responses (MAA/PSA or SNA/PSA) using six samples (3 from PCa patients and 3 from healthy individuals) and two lectins (SNA and MAA) are plotted in Fig. 3.
Figure 3.
The biosensor response obtained after the addition of lectin (i.e. proportional to the amount of a particular glycan on PSA) was divided by the biosensor response obtained after incubation with a serum sample (i.e. proportional to the PSA level in the serum sample) and the relative responses (MAA/PSA or SNA/PSA) using 6 samples (3 from PCa patients and 3 from healthy individuals) and two lectins (SNA and MAA) are shown here. Two different lectins, i.e. SNA recognizing terminal α-2,6-linked sialic acid and MAA recognizing terminal α-2,3-linked sialic acid, were applied in the study. A t-test was employed to determine whether the differences between these two sets of samples are significant, with t-values indicated in the figures. Moreover, the average values for each data set are indicated by the horizontal line. EIS assay conditions are described in Fig. 1.
When MAA lectin was applied for glycoprofiling of PSA bound to the immobilized anti-PSA antibody from serum samples, it was possible to see much higher relative response of the biosensor after incubation with the samples from PCa patients (i.e. relative response MAA/PSA from 0.37 ± 0.18 to 0.60 ± 0.14 with an average value of 0.45 ± 0.18) compared to the biosensor response after incubation with the samples from healthy individuals (i.e. relative response MAA/PSA from 0.010 ± 0.007 to 0.16 ± 0.11 with an average value of 0.086 ± 0.010). Moreover, a t-test showed a significant difference between these two sets of samples with the t value of 0.015 (Fig. 3 left). Thus, a relative response MAA/PSA was 5.3-fold higher when PCa samples were analyzed, compared to the analysis of samples from healthy individuals.
When SNA lectin was applied for glycoprofiling of two sets of samples only a moderate increase of the average relative signal SNA/PSA obtained after incubation of serum samples – from 0.117 ± 0.112 for healthy individuals to 0.133 ± 0.112 for PCa patients - was observed. Application of the t-test to data obtained from the analysis of these two sets of samples revealed an insignificant difference between these two sets of samples, with t = 0.891 (Fig. 3 right). A relative response SNA/PSA was 1.3-fold higher when PCa samples were analyzed compared to the analysis of samples from healthy individuals.
Our results revealed a significant increase of terminal α-2,3-linked sialic acid on PSA recognizable by MAA lectin and insignificant, minute increase of the level of terminal α-2,6-linked sialic acid on PSA recognizable by SNA lectin present in the PCa patient serum samples when compared to the analysis of serum samples from healthy individuals. Both observations are in agreement with a previous study detecting glycans on PSA by instrumental-based approaches, when a slight increase of terminal α-2,6-linked sialic acid on PSA in serum samples from PCa patients compared to serum from healthy individuals was attributed to elevated glycan branching on PSA present in PCa serum samples [42]. The study showed an increased level of terminal α-2,3-linked sialic acid present on PSA in the serum samples from PCa patients compared to the serum samples from healthy individuals [42]. An increased sialylation of PSA is a result of up-regulation of the enzymes responsible for increased sialylation of proteins by α-2,3- and α-2,6- sialyltransferases in PCa [43–45].
3.4. Comparison with literature data
Our results showed 5.3-fold difference in response MAA/PSA between samples from PCa patients and healthy individuals (H) i.e. PCa/H ratio. Aberrant glycosylation of PSA has been determined in the past in various ways. A method using three different antibodies (anti-PSA, Ab1 recognizing terminal α-2,3-sialic acid and anti-Ab1 antibody) with magnetic microparticles revealed a response ratio PCa/H of 1.8 [46]. Mass spectrometry used to get the ratio between sialylated and non-sialylated peptides released from PSA isolated from serum sample offered a response ratio PCa/H of 3.6 [47]. When an enzyme-linked lectin assay with immobilized anti-PSA antibody and glycoprofiling with MAA lectin was applied, a response ratio PCa/H of 1.1 was obtained [48]. 2-D electrophoresis employed for separation/quantification of subforms (glycoforms) of PSA revealed a response ratio PCa/H of 1.4 [49]. From literature data, only the approach described by Li et al. [47] relativized glycan abundance to PSA level as in our case, but such approach employed quite expensive instrumentation such as mass spectrometry (MS).
4. Conclusions
The impedimetric biosensor device presented here could detect PSA down to 100 ag/mL with a linear concentration working range from 100 ag/mL up to 1 mg/mL, i.e. 10 orders of concentration magnitude and the sensitivity of (5.5 ± 0.2)% decade-1. From a literature survey it is clear that our approach for glycoprofiling of PSA offered the most sensitive discrimination between samples from PCa patients and those of healthy individuals, with the ratio PCa/H = 5.3, while in three publications a much lower ratio PCa/H in the range 1.1-1.8 [46,48,49] was obtained. Moreover, such ratio was not relativized to the PSA level in the serum. There has been only one study, in which relativized glycan response to PSA level was calculated with the ratio PCa/H = 3.6, only slightly lower when compared to our results, but fairly expensive equipment (i.e. MS instrument) was needed to perform such analyses. Thus, in this study, the electrochemical lectin-based immunosensor is described to obtain MAA/PSA ratio with a potential to apply such ratio to a more specific diagnosis of PCa than the diagnosis based on determination of the serological level of PSA alone. The main limitations of the current platform were quite tedious electrode preparation, and low reproducibility of electrode to electrode preparation. These can be addressed by utilization of disposable biochips/electrodes working in an array format. Moreover, more samples have to be analyzed to prove the viability of the electrochemical platform of detection for potential diagnostic applications in PCa detection.
Highlights.
Construction of an electrochemical lectin-based immunosensor working in a sandwich configuration is described.
Optimal blocking agent suppressed non-specific protein binding 55-fold compared to the device without any blocking.
The device detected a prostate specific antigen reliably in a clinically relevant concentration range (1-400 ng/mL).
The biosensor could effectively discriminate serum samples from healthy individuals and patients with prostate cancer.
Acknowledgements
This publication was made possible by NPRP grant # NPRP-6-381-1-078 from the Qatar National Research Fund (a member of Qatar Foundation). The statements made herein are solely the responsibility of the authors. The research leading to these results received funding from the European Research Council under the European Union's Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement no. 311532 and this work has received funding from the European Union's Seventh Framework Program for research, technological development and demonstration under grant agreement no. 317420. We are grateful to Natalia Kopuncova (Department of Urology, University Medicine Greifswald, Germany) for her help.
References
- [1].Meany DL, Chan DW. Aberrant glycosylation associated with enzymes as cancer biomarkers. Clin Proteom. 2011;8 doi: 10.1186/1559-0275-1188-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Saldova R, St€ockmann H, O'Flaherty R, Lefeber DJ, Jaeken J, Rudd PM. N-glycosylation of serum IgG and total glycoproteins in Man1b1 deficiency. J Proteome Res. 2015;14:4402–4412. doi: 10.1021/acs.jproteome.5b00709. [DOI] [PubMed] [Google Scholar]
- [3].Christiansen MN, Chik J, Lee L, Anugraham M, Abrahams JL, Packer NH. Cell surface protein glycosylation in cancer. Proteomics. 2014;14:525–546. doi: 10.1002/pmic.201300387. [DOI] [PubMed] [Google Scholar]
- [4].Freire-de-Lima L. Sweet and sour: the impact of differential glycosylation in cancer cells undergoing epithelial-mesenchymal transition. Front Oncol. 2014;4:59. doi: 10.3389/fonc.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mueller MM, Fusenig NE. Friends or foes - bipolar effects of the tumour stroma in cancer. Nat Rev Cancer. 2004;4:839–849. doi: 10.1038/nrc1477. [DOI] [PubMed] [Google Scholar]
- [6].Dube DH, Bertozzi CR. Glycans in cancer and inflammation -potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–488. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- [7].Adamczyk B, Tharmalingam T, Rudd PM. Glycans as cancer biomarkers, Biochim. Biophys Acta-Gen Subj. 2012;1820:1347–1353. doi: 10.1016/j.bbagen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- [8].Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in globocan 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- [9].Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid H-P, van der Kwast T, Wiegel T, Zattoni F. Eau guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61–71. doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- [10].Yamamoto T, Ito K, Ohi M, Kubota Y, Suzuki K, Fukabori Y, Kurokawa K, Yamanaka H. Diagnostic significance of digital rectal examination and transrectal ultrasonography in men with prostate-specific antigen levels of 4 ng/ml or less. Urology. 2001;58:994–998. doi: 10.1016/s0090-4295(01)01409-1. [DOI] [PubMed] [Google Scholar]
- [11].Kirwan A, Utratna M, _ODwyer ME, Joshi L, Kilcoyne M. Glycosylation-based serum biomarkers for cancer diagnostics and prognostics. Biomed Res Int. 2015;2015 doi: 10.1155/2015/490531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Belanger A, van Halbeek H, Graves HC, Grandbois K, Stamey TA, Huang L, Poppe I, Labrie F. Molecular mass and carbohydrate structure of prostate specific antigen: studies for establishment of an international PSA standard. Prostate. 1995;27:187–197. doi: 10.1002/pros.2990270403. [DOI] [PubMed] [Google Scholar]
- [13].Vermassen T, Speeckaert MM, Lumen N, Rottey S, Delanghe JR. Glycosylation of prostate specific antigen and its potential diagnostic applications. Clin Chim Acta. 2012;413:1500–1505. doi: 10.1016/j.cca.2012.06.007. [DOI] [PubMed] [Google Scholar]
- [14].Lilja H, Christensson A, Dahlen U, Matikainen MT, Nilsson O, Pettersson K, Lovgren T. Prostate-specific antigen in serum occurs predominantly in complex with alpha 1-antichymotrypsin. Clin Chem. 1991;37:1618–1625. [PubMed] [Google Scholar]
- [15].Balk SP, Ko YJ, Bubley GJ. Biology of prostate-specific antigen. J Clin Oncol. 2003;21:383–391. doi: 10.1200/JCO.2003.02.083. [DOI] [PubMed] [Google Scholar]
- [16].Moyer VA. Screening for prostate cancer: us preventive services task force recommendation statement. Ann Intern Med. 2012;157:120i134. doi: 10.7326/0003-4819-157-2-201207170-00459. [DOI] [PubMed] [Google Scholar]
- [17].Gronberg H, Adolfsson J, Aly M, Nordstrom T, Wiklund P, Brandberg Y, Thompson J, Wiklund F, Lindberg J, Clements M, Egevad L, et al. Prostate cancer screening in men aged 50e69 years (sthlm3): a prospective population-based diagnostic study. Lancet Oncol. 2015;16:1667–1676. doi: 10.1016/S1470-2045(15)00361-7. [DOI] [PubMed] [Google Scholar]
- [18].Rabin RC. New Diagnostic Tools for Prostate Cancer. The New York Times, The New York Times Company; New York: Dec 22, 2015. p. D3. [Google Scholar]
- [19].Hodson R. Prostate cancer: 4 big questions. Nature. 2015;528:S137–S137. doi: 10.1038/528S137a. [DOI] [PubMed] [Google Scholar]
- [20].Pihikova D, Belicky S, Kasak P, Bertok T, Tkac J. Sensitive detection and glycoprofiling of a prostate specific antigen using impedimetric assays. Analyst. 2016;141:1044–1051. doi: 10.1039/c5an02322j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Paleček E, Tkáč J, Bartošík M, Bertók T, Ostatná V, Paleček J. Electrochemistry of nonconjugated proteins and glycoproteins. Toward sensors for biomedicine and glycomics. Chem Rev. 2015;115:2045–2108. doi: 10.1021/cr500279h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bertok T, Sediva A, Katrlik J, Gemeiner P, Mikula M, Nosko M, Tkac J. Label-free detection of glycoproteins by the lectin biosensor down to attomolar level using gold nanoparticles. Talanta. 2013;108:11–18. doi: 10.1016/j.talanta.2013.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Katz E, Willner I. Probing biomolecular interactions at conductive and semiconductive surfaces by impedance spectroscopy: routes to impedimetric immunosensors, DNA-sensors, and enzyme biosensors. Electroanalysis. 2003;15:913–947. [Google Scholar]
- [24].Xu Q, Davis JJ. The diagnostic utility of electrochemical impedance. Electroanalysis. 2014;26:1249–1258. [Google Scholar]
- [25].Fragoso A, Laboria N, Latta D, O'Sullivan CK. Electron permeable self-assembled monolayers of dithiolated aromatic scaffolds on gold for biosensor applications. Anal Chem. 2008;80:2556–2563. doi: 10.1021/ac702195v. [DOI] [PubMed] [Google Scholar]
- [26].Barton AC, Davis F, Higson SPJ. Labeless immunosensor assay for prostate specific antigen with picogram per milliliter limits of detection based upon an ac impedance protocol. Anal Chem. 2008;80:6198–6205. doi: 10.1021/ac800491m. [DOI] [PubMed] [Google Scholar]
- [27].Gutiérrez-Zúňiga GG, Hernández-López JL. Sensitivity improvement of a sandwich-type ELISA immunosensor for the detection of different prostate-specific antigen isoforms in human serum using electrochemical impedance spectroscopy and an ordered and hierarchically organized interfacial supramolecular architecture. Anal Chim Acta. 2016;902:97–106. doi: 10.1016/j.aca.2015.10.042. [DOI] [PubMed] [Google Scholar]
- [28].Chiriac_o MS, Primiceri E, Montanaro A, de Feo F, Leone L, Rinaldi R, Maruccio G. On-chip screening for prostate cancer: an EIS microfluidic platform for contemporary detection of free and total PSA. Analyst. 2013;138:5404–5410. doi: 10.1039/c3an00911d. [DOI] [PubMed] [Google Scholar]
- [29].Alley WR, Mann BF, Novotny MV. High-sensitivity analytical approaches for the structural characterization of glycoproteins. Chem Rev. 2013;113:2668–2732. doi: 10.1021/cr3003714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Tkac J, Davis JJ. An optimised electrode pre-treatment for SAM formation on polycrystalline gold. J Electroanal Chem. 2008;621:117–120. [Google Scholar]
- [31].Chen S, LaRoche T, Hamelinck D, Bergsma D, Brenner D, Simeone D, Brand RE, Haab BB. Multiplexed analysis of glycan variation on native proteins captured by antibody microarrays. Nat Methods. 2007;4:437–444. doi: 10.1038/nmeth1035. [DOI] [PubMed] [Google Scholar]
- [32].Bertok T, Sediva A, Filip J, Ilcikova M, Kasak P, Velic D, Jane E, Mravcová M, Rovenský J, Kunzo P. Carboxybetaine modified interface for electrochemical glycoprofiling of antibodies isolated from human serum. Langmuir. 2015;31:7148–7157. doi: 10.1021/acs.langmuir.5b00944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Salimi A, Kavosi B, Fathi F, Hallaj R. Highly sensitive immunosensing of prostate-specific antigen based on ionic liquid-carbon nanotubes modified electrode: application as cancer biomarker for prostate biopsies. Biosens Bioelectron. 2013;42:439–446. doi: 10.1016/j.bios.2012.10.053. [DOI] [PubMed] [Google Scholar]
- [34].Moon J-M, Hui Kim Y, Cho Y. A nanowire-based label-free immunosensor: direct incorporation of a PSA antibody in electropolymerized polypyrrole. Biosens Bioelectron. 2014;57:157–161. doi: 10.1016/j.bios.2014.02.016. [DOI] [PubMed] [Google Scholar]
- [35].Kavosi B, Salimi A, Hallaj R, Moradi F. Ultrasensitive electrochemical immunosensor for PSA biomarker detection in prostate cancer cells using gold nanoparticles/pamam dendrimer loaded with enzyme linked aptamer as integrated triple signal amplification strategy. Biosens Bioelectron. 2015;74:915–923. doi: 10.1016/j.bios.2015.07.064. [DOI] [PubMed] [Google Scholar]
- [36].Kavosi B, Salimi A, Hallaj R, Amani K. A highly sensitive prostate-specific antigen immunosensor based on gold nanoparticles/PAMAM dendrimer loaded on MWCNTs/chitosan/ionic liquid nanocomposite. Biosens Bioelectron. 2014;52:20–28. doi: 10.1016/j.bios.2013.08.012. [DOI] [PubMed] [Google Scholar]
- [37].Rafique S, Bin W, Bhatti AS. Electrochemical immunosensor for prostate-specific antigens using a label-free second antibody based on silica nanoparticles and polymer brush. Bioelectrochemistry. 2015;101:75–83. doi: 10.1016/j.bioelechem.2014.08.001. [DOI] [PubMed] [Google Scholar]
- [38].Li Y, Han J, Chen R, Ren X, Wei Q. Label electrochemical immunosensor for prostate-specific antigen based on graphene and silver hybridized mesoporous silica. Anal Biochem. 2015;469:76–82. doi: 10.1016/j.ab.2014.09.022. [DOI] [PubMed] [Google Scholar]
- [39].Jang HD, Kim SK, Chang H, Choi J-W. 3d label-free prostate specific antigen (PSA) immunosensor based on graphene-egold composites. Biosens Bioelectron. 2015;63:546–551. doi: 10.1016/j.bios.2014.08.008. [DOI] [PubMed] [Google Scholar]
- [40].Wang H, Zhang Y, Yu H, Wu D, Ma H, Li H, Du B, Wei Q. Label-free electrochemical immunosensor for prostate-specific antigen based on silver hybridized mesoporous silica nanoparticles. Anal Biochem. 2013;434:123–127. doi: 10.1016/j.ab.2012.11.012. [DOI] [PubMed] [Google Scholar]
- [41].Qu B, Guo L, Chu X, Wu D-H, Shen G-L, Yu R-Q. An electrochemical immunosensor based on enzyme-encapsulated liposomes and biocatalytic metal deposition. Anal Chim Acta. 2010;663:147–152. doi: 10.1016/j.aca.2010.01.050. [DOI] [PubMed] [Google Scholar]
- [42].Gilgunn S, Conroy PJ, Saldova R, Rudd PM, O'Kennedy RJ. Aberrant PSA glycosylation - a sweet predictor of prostate cancer. Nat Rev Urol. 2013;10:99–107. doi: 10.1038/nrurol.2012.258. [DOI] [PubMed] [Google Scholar]
- [43].Dall'Olio F, Malagolini N, Trinchera M, Chiricolo M. Sialosignaling: sialyltransferases as engines of self-fueling loops in cancer progression. BBA-Gen Subj. 2014;1840:2752–2764. doi: 10.1016/j.bbagen.2014.06.006. [DOI] [PubMed] [Google Scholar]
- [44].Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–555. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- [45].Wang XC, Chen J, Li QK, Peskoe SB, Zhang B, Choi C, Platz EA, Zhang H. Overexpression of alpha (1,6) fucosyltransferase associated with aggressive prostate cancer. Glycobiology. 2014;24:935–944. doi: 10.1093/glycob/cwu051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Yoneyama T, Ohyama C, Hatakeyama S, Narita S, Habuchi T, Koie T, Mori K, Hidari KIPJ, Yamaguchi M, Suzuki T, Tobisawa Y. Measurement of aberrant glycosylation of prostate specific antigen can improve specificity in early detection of prostate cancer. Biochem Biophys Res Commun. 2014;448:390–396. doi: 10.1016/j.bbrc.2014.04.107. [DOI] [PubMed] [Google Scholar]
- [47].Li Y, Tian Y, Rezai T, Prakash A, Lopez MF, Chan DW, Zhang H. Simultaneous analysis of glycosylated and sialylated prostate-specific antigen revealing differential distribution of glycosylated prostate-specific antigen isoforms in prostate cancer tissues. Anal Chem. 2011;83:240–245. doi: 10.1021/ac102319g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Meany DL, Zhang Z, Sokoll LJ, Zhang H, Chan DW. Glycoproteomics for prostate cancer detection: changes in serum PSA glycosylation patterns. J Proteome Res. 2009;8:613–619. doi: 10.1021/pr8007539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sarrats A, Comet J, Tabares G, Ramirez M, Nuria Aleixandre R, de Llorens R, Peracaula R. Differential percentage of serum prostate-specific antigen subforms suggests a new way to improve prostate cancer diagnosis. Prostate. 2010;70:1–9. doi: 10.1002/pros.21031. [DOI] [PubMed] [Google Scholar]