Abstract
The effects of biodiversity on ecosystem functioning generally increase over time but the underlying processes remain unclear. Using 26 long-term grassland and forest experimental ecosystems we demonstrate that biodiversity-ecosystem functioning relationships strengthen mainly by greater increases in functioning in high-diversity communities in grasslands and forests. In grasslands, biodiversity effects also strengthen due to decreases in functioning in low-diversity communities. Contrasting trends across grasslands are associated with differences in soil characteristics.
More than two decades of research have revealed that biodiversity is a significant driver of ecosystem functioning1,2. Positive biodiversity effects on ecosystem functioning have been found in grassland and forest biodiversity experiments3,4 with growing evidence showing that biodiversity–ecosystem functioning relationships may become stronger over time5,6 7. Moreover, several recent studies suggest that long-term biodiversity effects in experiments better mirror natural conditions than short-term studies and likely help explain biodiversity–ecosystem functioning relationships in real-world ecosystems8,9,10,11.
Temporal increases in plant diversity effects on ecosystem functioning may result from an increase in functioning in high-diversity communities7, a decrease in functioning in low-diversity communities12 or both. However, it remains unknown which of the above trends drive temporal increases in diversity effects on ecosystem functioning, whether these trends are consistent across experiments and ecosystems, and if not, whether context-dependency in temporal trends may be attributed to site conditions. For instance, soil characteristics likely influence the biodiversity–ecosystem functioning relationship10,13,14 and may influence temporal trajectories as well, but whether or not they do so is unclear.
Understanding temporal trends of biodiversity effects on ecosystem functioning is critical for providing insights into biodiversity–ecosystem functioning relationships9,16 and predicting the potential consequences that progressive biodiversity change18,19 and management20,21 have on ecosystem functioning and service provisioning over time. Further, examining these temporal trends is fundamental for guiding research on understanding the underlying mechanisms, e.g. a variety of niche-differentiation processes such as complementary resource use and facilitation, which can have positive effects on the functioning of high-diversity communities6,17, and the impact of pest and diseases, which can have negative effects on the functioning of low-diversity communities9.
In this study, we examined temporal shifts in biodiversity effects on ecosystem functioning in terrestrial ecosystems, specifically plant diversity effects on plant aboveground biomass in grassland and on basal area in forest experimental ecosystems. We used data from 26 long-term biodiversity experiments that manipulated plant-species richness in grasslands and forests (14 and 12 experiments, respectively; Supplementary Table 1). We investigated whether the strength of the biodiversity–ecosystem functioning relationship increases with time and whether temporal divergence across plant richness levels is driven by an increase in function in high-diversity communities, a decrease in function in low-diversity communities, or a combination of both. Finally, if temporal trends differed across experiments, we assessed the potential role of soil characteristics in shaping these temporal trends.
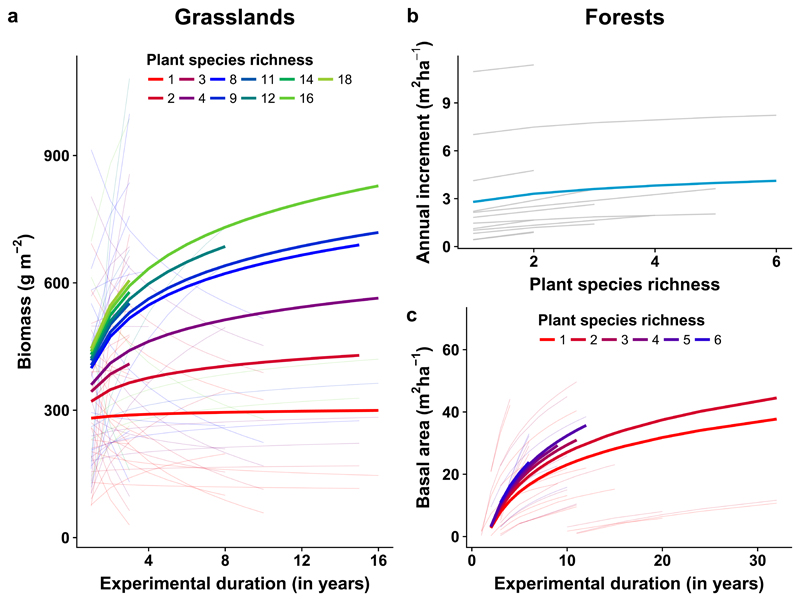
In grasslands, the relationship between plant species richness and plant aboveground biomass was positive and became significantly stronger over time (Supplementary Table 2, Fig. 1a). Temporal divergence across plant richness levels was observed in 10 out of 14 grassland experiments (Supplementary Fig. 1). Although temporal divergence was frequently associated with more diverse communities showing stronger increases in plant aboveground biomass over time (Fig. 1a), the temporal increase of diversity effects was not determined by a consistent trend across studies (see variance components in Supplementary Table 2): temporal divergence was driven by a decrease in function in low-diversity communities in one experiment, by an increase in function in high-diversity communities in six experiments, or a combination of both in three experiments (Supplementary Fig. 1).
Figure 1. Ecosystem functioning in grassland and forest experimental ecosystems.
In grasslands, trajectories of aboveground biomass (g m-2) among plant species richness levels diverge over time (a). In forests, significant plant species richness effects on periodic annual increment of basal area (m2 ha-1) are consistent over time (b). The consistent positive effect of high-diversity communities on periodic annual increment of basal area may explain the temporal divergence in total basal area among plant species richness levels (c). For panels a and c, lines are mixed-effects model fits for each plant species richness level within each study (thin lines) or across all studies (thick lines). For panel b, lines are mixed-effects model fits for each study (gray lines) or across studies (blue line). For grasslands, aboveground biomass was significant affected by species richness (F1,5754.7 = 14.21, p-value <0.001) and the species richness × time interaction (F1,5754.7 = 8.53, p-value <0.01). For forests, periodic annual increment of basal area was significantly affected by species richness (F1,1433.1 = 10.07, p-value <0.01), and total basal area was significantly affected by time (F1,291.9 = 24.32, p-value <0.001) and the species richness × time interaction (F1,291.9 = 18.39, p-value <0.001). See extended information in Supplementary Tables 2 and 4. Data from 14 grassland (1,045 plots n = 7,886 measurements (plot by age combination)) and from 12 forest experimental ecosystems were entered in the analyses (370 plots, n = 1,887 measurements (plot by age combination)).
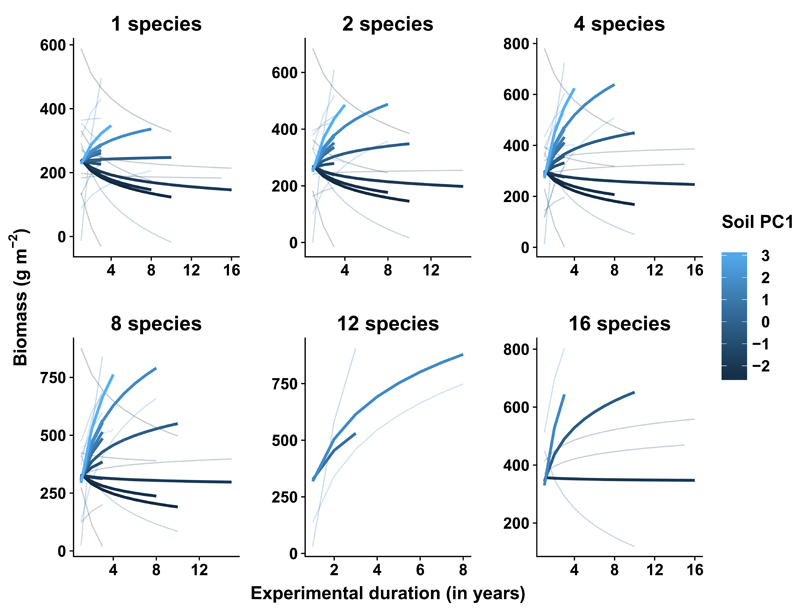
The context-dependency underlying biodiversity–ecosystem functioning relationships in grasslands was strongly associated with variation in soil characteristics across experiments (Supplementary Table 3). Soils influenced biodiversity–ecosystem functioning relationships in two ways. First, the interaction between soil characteristics related to soil texture and pH (soil PC2) and plant species richness shaped the overall richness effect (significant richness × soil PC2 interaction; Supplementary Table 3 and Supplementary Figs. 2 and 3). Second, soil characteristics, such as cation-exchange capacity (CEC), soil organic carbon (C), water content at wilting point, and bulk density (soil PC1) contributed to driving temporal divergence (significant richness × time × soil PC1 interaction; Supplementary Table 3, Fig. 2, and Supplementary Fig. 2). Temporal divergence driven by an increase in function in high-diversity communities was associated with studies located in areas with higher CEC, soil organic C, water content, and lower bulk density, while a decrease in function in low-diversity communities was associated with the inverse pattern (that is lower cation-exchange capacity, soil organic carbon and water content and higher bulk density; Fig. 2).
Figure 2. Influence of soil characteristics on temporal divergence in grasslands.
Lines are mixed-effects model fits for each plant species richness level and soil characteristics within each study (thin lines) or across all studies (thick lines). Plots only show temporal trajectories of plant species richness levels present in at least two experimental grasslands. Soil characteristics are based on a principal component analysis; the first principal axis (Soil PC1) explained 48% of variation where positive values were associated with higher cation-exchange capacity, soil organic carbon content, and volumetric water content at wilting point and lower soil bulk density. See extended information in Supplementary Table 3 and Supplementary Fig. 2.
The general increase of the biodiversity–ecosystem functioning relationship through time was due to contrasting trajectories across grassland studies, showing the importance of context-dependency of the biodiversity–ecosystem functioning relationship in this ecosystem. Our analyses reveal that soil characteristics contribute to strengthening plant species richness effects on ecosystem functioning in general and through time in multiple ways. First, variability in ecosystem functioning across plant species richness levels was generally lower in experiments with sandy soils. Second, temporal divergence was explained by stronger increases in ecosystem function in high-diversity than in low-diversity communities in experimental sites with higher soil organic C, whereas temporal divergence in experimental sites with low soil organic C was explained by a decrease in ecosystem function in low-diversity communities. Therefore, the influence of resource availability on plant-plant interactions as well as multi-trophic interactions15 may underlie temporal changes of biodiversity effects10,13 and related mechanisms14,22. It is also likely that other abiotic and biotic factors play a role in shaping the biodiversity–ecosystem functioning relationship through time. For instance, most of the grassland biodiversity experiments are perennial–dominated (more than 75% of the species were perennial), except for BIODEPTH Greece and Portugal sites (less than 30% of the species were perennial), where there was no evidence of temporal divergence. Grassland experiments dominated by annual plants may be strongly affected by processes related to recruitment, such as seed availability (either from their own plot or surrounding plots) and microsites23. Recruitment may influence diversity effects in grasslands, mainly due to changes in plant density rather than changes in plant size24.
In forests, plant richness effects on periodic annual increment of basal area were consistently positive across studies (see variance components in Supplementary Table 2, Fig. 1b, Supplementary Fig. 4), and, in contrast to grasslands, we did not find evidence that they changed over time (neither time nor richness × time were significant; Supplementary Table 2, Fig. 1b). Consequently, the temporal divergence of total basal area among tree species richness levels depended on consistently positive diversity effects on periodic annual increment of basal area. (Supplementary Tables 2 and 4, Fig. 1c, and Supplementary Fig. 5). The absence of context-dependency in forests could not be explained by overall differences in soil characteristics between forest and grassland studies, which are located along similar soil gradients (Supplementary Figs. 6 and 7) that exhibit moderate differences in soil cation-exchange capacity (p-value = 0.06) and pH (p-value = 0.02; Supplementary Fig. 8).
Our results show that positive tree diversity effects started early and accumulated through time. Thus, mechanisms associated with positive biodiversity effects on ecosystem functioning like complementarity may play a key role even during the early stages of community assembly25. Decreases in ecosystem functioning in forests, e.g. due to tree mortality28, appear to be offset by higher growth of surviving trees. This differs from grasslands, in which community-level biomass is highly dependent on plant density24. Temporal divergence may continue to increase not only due to cumulative processes (detected in our study), but also due to strengthening of competitive interactions26. The importance of niche partitioning over time also may increase at smaller spatial scales27, and thus may require longer to be detected at the plot level. Data availability from long-term studies and from more diverse forest systems remain one of the main challenges for understanding temporal dynamics in forest experimental ecosystems. For example, the longest-running forest biodiversity experiments in this study usually had communities with only one and two species. Moreover, longer and multi-generation forest experiments may provide a better understanding of the effects that pathogen and herbivore attacks and the accumulation of soil pathogens may have on biodiversity effects through time. It is possible that temporal dynamics of biodiversity effects in forest ecosystems become increasingly similar to those of grasslands when compared at similar stages in terms of generations of the study organisms or under different soil characteristics, e.g. sites with lower CEC and higher pH (Supplementary Fig. 8).
In conclusion, our results show a consistent temporal divergence of ecosystem functioning across plant diversity levels in both grassland and forest experimental ecosystems. In grasslands, temporal divergence was the result of a variety of patterns, all ultimately causing an increase in biodiversity effects over time. In forests, by contrast, temporal divergence was not detected when ecosystem functioning was measured as a rate (periodic annual increment of basal area) but rather as an amount (total basal area). Therefore, the increasing strength of the biodiversity–ecosystem functioning relationship in forests was related to an increase in function of high-diversity communities driven by a consistent positive effect of high-diversity communities on periodic annual increment of basal area. Temporal divergence in ecosystem functioning found in our analysis may have multiple implications for the provisioning of vital ecosystem services in managed ecosystems. For instance, we need to determine other potential biotic and abiotic factors that drive either an increase of ecosystem function in high-diversity communities or a decrease in low-diversity communities over time. Such mechanistic understanding is fundamental as low-diversity plant communities are widely used in productive landscapes20,21. Overall, our results support the importance of management practices that reinforce the functional and structural complexity of ecosystems at different spatial and temporal scales20 and, crucially, either attenuate decreases in function in grasslands or increase function in grassland and forest ecosystems.
Methods
Data acquisition and description
Long-term experiments that have manipulated plant species richness in grasslands and forests were identified using published meta-analyses, review papers on related topics and experimental platforms for biodiversity research (Supplementary methods). Long-term experiments were included if: a) plant species richness was directly manipulated through sowing or planting and included monocultures of all species present in the mixtures, b) raw data at least at the plot level were available, c) aboveground plant biomass (in grassland) or basal area (in forest) data from at least three points in time from different years were available, and d) the experiment was conducted for at least 3 years in grasslands and 5 years in forests. For forests, the required experimental duration was higher than for grasslands because the establishment of tree-dominated experimental studies and the biodiversity effects on ecosystem functions are expected to take longer in forests.
Data from 26 long-term biodiversity experiments met these criteria (Supplementary Table 1) including 12 forest experiments (370 plots, n = 1,887 measurements (plot by age combination) across experiments) and 14 grassland experiments (1,045 plots, n = 7886 measurements (plot by age combination) across experiments). Annual peak aboveground biomass (g/m2) and basal area (m2/ha) were used in grassland and forests, respectively. In forests, we included two types of ecosystem functions: – periodic annual increment of basal area – is a rate and is therefore more comparable to annual peak aboveground biomass in grasslands (see Supplementary methods) and – total basal area – is an amount that captures cumulative tree growth. Both measures were used to quantify ecosystem functioning following the definition in Hooper3, i.e. ecosystem functioning includes ecosystem properties such as process rates and the size of the compartments.
Temporal divergence
We used linear mixed-effect models to assess the temporal dynamics of ecosystem function among plant species richness levels using either plant aboveground biomass in grassland or basal area in forest experiments. We fitted a separate model for grassland experiments using annual peak aboveground biomass and two separate models for forest experiments, one using total basal area and the other using periodic annual increment of basal area. The initial model included plant species richness, time, and the interaction between richness and time as fixed effects in both grassland and forest experiments. We then simplified models by excluding non-significant fixed effects and interactions (p-value > 0.1). Plant richness was the sown or planted richness (natural logarithm), and time was experimental age in years (natural logarithm). The natural logarithm transformation was used based on the expectation of fast, initial increases in ecosystem function, followed by constant growth in the later years of the experiment. Using a random slope and intercept structure, random effects were included for: study, study × richness, study × time, study × richness × time interaction, and a term for plot within study for grasslands and for total basal area in forests. The random structure for periodic annual increment of basal area included study, study × richness interaction, and a term for plot within study. We accounted for repeated measurements within plots by using a first-order autoregressive covariance structure, which fitted the data better than a compound symmetry covariance structure based on the Akaike information criterion. The best covariance structure was first-order autoregressive. Models were fitted with asreml function in the asreml package in R, and the results were extracted using the test.asreml function in the pascal package in R. Analyses were run in R version 3.2.429.
Effects of soil characteristics on temporal divergence
To explore the variation in temporal trends among grassland studies, an additional model was tested that included species richness, time, soil characteristics, and their interactions (Supplementary methods). Because a consistent set of soil variables was not available across studies, we used data from SoilGrids25030 to provide a general and consistent description of the study area. However, these data are proxies for site-specific quantitative information and need to be interpreted with caution. The soil characteristics were used to perform a principal component analysis, in which the first and second axes explained 48 and 40% of the variation across grassland experiments, respectively (Supplementary Fig. 2). We did not analyze the effects of soil characteristics in forest experiments because we did not find evidence of multiple trends underlying the temporal divergence (Supplementary Table 2, Fig. 1b). To compare the potential differences in the range of soil characteristics between experimental ecosystems, we performed an additional principal component analysis including both forest and grassland studies (Supplementary methods and Supplementary Figs. 6, 7, and 8).
Supplementary Material
Acknowledgements
This study was supported by the German Research Foundation through the Emmy Noether research group (Ei 862/2) the European Research Council (ERC Starting Grant; grant agreement no 677232) provided to N.E. and through the financial support of the German Centre for Integrative Biodiversity Research (iDiv) Halle-Jena-Leipzig (FZT 118). The Jena Experiment is funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; FOR 1451). N.G-R. thanks Daniel Binkley, Alexandra Weigelt, and Enrica De Luca for contributing data, Simon Bilodeau-Gauthier for his support with the database and Petr Keil for help with data analysis. Support for BioCON came from from the U.S. National Science Foundation (NSF) Long-Term Ecological Research (DEB-9411972, DEB-0080382, DEB-0620652, and DEB-1234162), Biocomplexity Coupled Biogeochemical Cycles (DEB-0322057), Long-Term Research in Environmental Biology (DEB-0716587, DEB-1242531), and Ecosystem Sciences (NSF DEB- 1120064) Programs; as well as the U.S. Department of Energy Programs for Ecosystem Research (DE-FG02-96ER62291), and National Institute for Climatic Change Research (DE-FC02-06ER64158).
Footnotes
Author contributions
N.E conceived the idea; N.E and N.G-R developed the idea; A.H, B.W, C.P, C.Pot, C.R, D.F, D.P, D.T, F.M, H.A, H.E, J.E, J.J, J.K, J.P. JvR, P.R contributed experimental data, N.G-R assembled the data; N.G-R and D.C analysed the data with the input from F.I, J.K, and A.H; N.G-R wrote the paper with substantial input from all authors.
Competing interests
The authors have no competing interests.
Data availability
The data that support the findings of this study are available from the authors upon request.
Code availability
R code of linear mixed-effects models is provided in the Supplementary methods section.
References
- 1.Isbell F, et al. Nature. 2017;546:65–72. doi: 10.1038/nature22899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilman D, Isbell F, Cowles JM. Annu Rev Ecol Evol Syst. 2014;45:471–493. [Google Scholar]
- 3.Hooper DU, et al. Ecological Monographs. 2005;75:3–35. [Google Scholar]
- 4.Balvanera P, et al. Ecology Letters. 2006;9:1146–1156. doi: 10.1111/j.1461-0248.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 5.Reich PB, et al. Science. 2012;336:589–592. doi: 10.1126/science.1217909. [DOI] [PubMed] [Google Scholar]
- 6.Cardinale BJ, et al. PNAS. 2007;104:18123–18128. doi: 10.1073/pnas.0709069104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ewel JJ, Celis G, Schreeg L. Biotropica. 2015;47:162–171. [Google Scholar]
- 8.Flombaum P, Sala OE. PNAS. 2008;105:6087–6090. doi: 10.1073/pnas.0704801105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenhauer N, et al. Journal of Vegetation Science. 2016;27:1061–1070. [Google Scholar]
- 10.Grace JB, et al. Nature. 2016;529:390–393. doi: 10.1038/nature16524. [DOI] [PubMed] [Google Scholar]
- 11.Hautier Y, et al. Nature. 2014;508:521–525. doi: 10.1038/nature13014. [DOI] [PubMed] [Google Scholar]
- 12.Marquard E, et al. Plos One. 2013;8:e75599. doi: 10.1371/journal.pone.0075599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fridley JD. Oecologia. 2002;132:271–277. doi: 10.1007/s00442-002-0965-x. [DOI] [PubMed] [Google Scholar]
- 14.Boyden S, Binkley D, Senock R. Ecology. 2005;86:992–1001. [Google Scholar]
- 15.De Deyn GB. Oikos. 2017;126:497–507. [Google Scholar]
- 16.Forrester DI, Bauhus J. Curr Forestry Rep. 2016;2:45–61. [Google Scholar]
- 17.Fargione J, et al. Proc R Soc B. 2007;274:871–876. doi: 10.1098/rspb.2006.0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newbold T, et al. Nature. 2015;520:45–50. doi: 10.1038/nature14324. [DOI] [PubMed] [Google Scholar]
- 19.Urban MC. Science. 2015;348:571–573. doi: 10.1126/science.aaa4984. [DOI] [PubMed] [Google Scholar]
- 20.Paquette A, Messier C. Fron Ecol Environ. 2010;8:27–34. [Google Scholar]
- 21.Tilman D, et al. Science. 2001;292:281–284. doi: 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- 22.Craven D, et al. Philosophical Transactions B. 2016;371:1–8. [Google Scholar]
- 23.Eriksson O, Ehrlén J. Oecologia. 1992;91:360–364. doi: 10.1007/BF00317624. [DOI] [PubMed] [Google Scholar]
- 24.Marquard E, Weigelt A, Roscher C, Gubsch M, Lipowsky A, Schmid B. Journal of Ecology. 2009;97:696–704. [Google Scholar]
- 25.Williams LJ, Paquette A, Cavender-Bares J, Messier C, Reich PB. Nature Ecology and Evolution. 2017;1:1–7. doi: 10.1038/s41559-016-0063. [DOI] [PubMed] [Google Scholar]
- 26.Ewel JJ, Mazzarino MJ. PNAS. 2008;105:18836–18841. doi: 10.1073/pnas.0807216105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potvin C, Dutilleul P. Ecology. 2009;90:321–327. doi: 10.1890/08-0353.1. [DOI] [PubMed] [Google Scholar]
- 28.Binkley D, Senock R, Bird S, Cole TG. Forest Ecology and Management. 2003;182:93–102. [Google Scholar]
- 29.R Core Development Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2015. URL: http://www.R-project.org. [Google Scholar]
- 30.Hengl T, et al. Plos One. 2017;12:e0169748. doi: 10.1371/journal.pone.0169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.