Abstract
Stem cell delivery by local injection has tremendous potential as a regenerative therapy but has seen limited clinical success. Several mechanical challenges hinder therapeutic efficacy throughout all stages of cell transplantation, including mechanical forces during injection and loss of mechanical support post-injection. Recent studies have begun exploring the use of biomaterials, in particular hydrogels, to enhance stem cell transplantation by addressing the often-conflicting mechanical requirements associated with each stage of the transplantation process. This review explores recent biomaterial approaches to improve the therapeutic efficacy of stem cells delivered through local injection, with a focus on strategies that specifically address the mechanical challenges that result in cell death and/or limit therapeutic function throughout the stages of transplantation.
Introduction
Stem cell transplantation through systemic or local injection is a promising regenerative approach for injury and disease treatment. Despite the relative clinical success of systemic stem cell delivery, this strategy often relies on cell homing to the injury or disease site for increased efficacy. While local injection strategies do not require cell homing, the clinical application of this therapy is limited by low cell viability and poor cell function. Locally transplanted cells face several challenges at each stage of the transplantation process. This review explores the design of hydrogel systems for improving the therapeutic potential of locally injected stem cells with a focus on the role of mechanics throughout the transplantation process.
In their native environment, mammalian cells are surrounded by an extracellular matrix (ECM), which acts as a structural support and provides biochemical and biomechanical signals to regulate cell function. Cells are known to respond to mechanical cues in their microenvironment by altering their proliferation rate [1,2], migration speed [3,4], differentiation potential [5], and secretory function [6]. Similarly, the behavior of locally injected stem cells is influenced by interactions with their microenvironment. This microenvironment can include the native, host ECM as well as an engineered biomaterial. In injured or diseased tissues, the host ECM often becomes dysfunctional and may not be sufficient to support healing and therapeutic efficacy of transplanted stem cells. Engineered biomaterials have the potential to modify the local environment to improve the transplantation process. In addition, engineered biomaterials have the potential to improve cell viability and function during the local injection process.
Cell transplantation through local injection can be divided into three stages: injection, acute post-injection, and long-term survival and function. At each stage of transplantation, cells experience mechanical and structural challenges that can result in cell death and compromise cell function. For example, during the injection process, cells may experience mechanical forces that can damage the cell membrane, while post-injection, cells may experience a loss of structural support and hence an absence of mechanical cues. The relative importance of these different challenges can vary dramatically depending on the specific clinical application [7]. Consequently, engineered biomaterial strategies have been developed to address the specific mechanical challenges at each delivery stage. While all cell therapies (whether transplantation of stem, progenitor, immature or terminally differentiated cells) experience these same mechanical challenges, stem and progenitor cell therapies have the additional consideration that mechanical cues can influence their differentiation and maturation. Hydrogels have received significant interest as ECM mimics due to their high water content and water-swollen networks that allow for facile transport of water-soluble biomolecules [8,9] Additionally, these materials have tunable mechanical properties that span the range of physiological tissues [10]. While several injectable hydrogels have shown significant benefits in stem cell transplantation, there is no current material that is able to address all of the mechanical challenges of each transplantation stage in succession.
In the first section of this review, we discuss the challenges for each stage of the transplantation process with a focus on the mechanical requirements that can be addressed by biomaterials. Several studies have demonstrated that hydrogel mechanics play a critical role in successful cell transplantation, and careful consideration of the distinct mechanical features of the selected biomaterial can significantly improve therapeutic efficacy. In the second section we discuss biomaterial design strategies for stem cell transplantation focusing on several new materials designed to address distinct mechanical challenges at different stages of the transplantation process. We end with future directions for the design of injectable hydrogels focusing on materials that change their properties during the stages of stem cell transplantation.
Mechanical Challenges to Successful Stem Cell Transplantation
Stem cells face several distinct mechanical challenges during transplantation that have the potential to drastically reduce their viability and therapeutic efficacy. Current protocols for local injection generally result in poor cell viability, often with as few as 1–20% of cells surviving the transplantation process [11-14]. In this section, the transplantation process is divided into three distinct stages: injection, acute post-injection and long-term survival and function to better elucidate the specific mechanical and structural challenges stem cells face throughout transplantation (Figure 1a).
Figure 1. Comparison of stem cell delivery using a liquid or hydrogel carrier at each stage of transplantation.
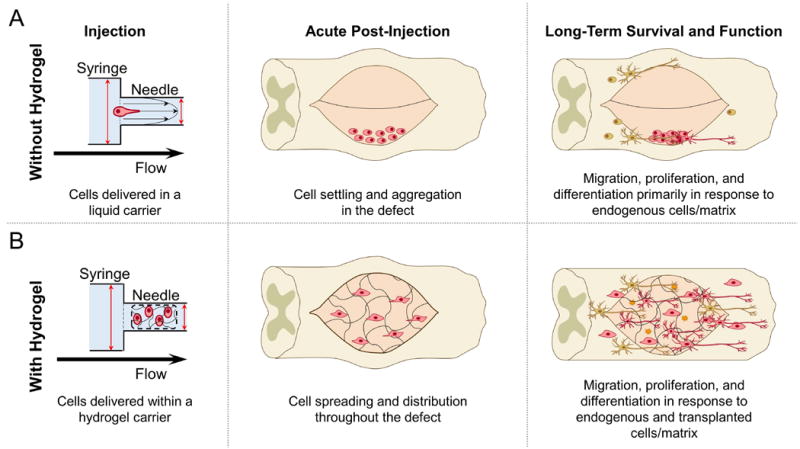
A) During injection, cells in a liquid carrier are exposed to mechanical forces that can damage the cell membrane and result in decreased cell survival. Post-injection, cells can settle and aggregate in the defect site without the structural support to promote cell adhesion. Long-term survival and function can be diminished without mechanical cues to promote transplanted cell proliferation, migration, differentiation, and secretion. B) Cells encapsulated in a hydrogel carrier can be protected from mechanical forces exerted during the injection stage. Post-injection, cells can adhere and spread within a hydrogel support matrix throughout the defect site. Finally, long-term mechanical cues from hydrogels can support transplanted and endogenous cell migration into and out of the defect, as well as promote stem cell proliferation, differentiation, and secretion for tissue regeneration.
Injection
During syringe injection, transplanted cells are exposed to mechanical stresses that can result in membrane damage and significant loss of acute viability. Current clinical protocols using low viscosity fluids such as saline for local injection through a syringe needle have been shown to result in substantial cell death, with up to 40% of cells not surviving the injection process [15]. It has been hypothesized that this cell death is primarily caused by membrane rupture that occurs as cells are exposed to extensional flow within the syringe needle, although shear stress and high pressure are also known causes of cell death [15].
Acute Post-Injection
The therapeutic efficacy of transplanted stem cells is influenced in part by cell survival and retention during the acute post-injection stage. The local environment and structure of the transplantation site will determine the mechanical signals provided to cells following injection. Injury models including spinal cord injury, cranial defects, and stroke cavity models, which require injection into a void space, lack a three-dimensional (3D) support matrix to promote the survival of adherent cells [16] and prevent cell dispersal. In contrast, during injection into dense tissue, such as intramuscular injections, the host tissue may provide mechanical support and promote cell survival. However, injection into dense tissue requires higher injection pressures and can still result in cell leakage at the transplantation site [17]. Additionally, cells may be confronted with several other survival challenges during the acute post-injection stage that are not inherently mechanical in nature, including hypoxia, low nutrient transport, and the immune and inflammatory response [18-20]. However, these challenges may be exacerbated or diminished by the mechanical microenvironment. For example, cells can alter their growth factor secretion in response to mechanical cues [21], which may assist in surviving hypoxia or inflammation.
Long-Term Survival and Function
Long-term stem cell therapeutic efficacy can be attained by two means: (1) support of endogenous tissue regeneration through paracrine effects [22,23] or integration of transplanted cells with host tissue [24]. Much of this success is dependent on long-term stem cell retention, proliferation, migration, and/or differentiation. All of these process are known to be influenced by the mechanical microenvironment in vitro [21,25] suggesting that modification of the mechanical microenvironment in vivo may be a strategy to promote long-term transplanted cell survival and function. In therapies that rely on paracrine secretion for therapeutic efficacy, multiple cell doses over time may be necessary to maintain a sufficient level of cell-secreted therapeutic factors, thereby complicating clinical translation [26-29]. In therapies that require transplanted cell integration into the host tissue, poor differentiation into specialized cell types may limit new tissue formation and function [30] and can lead to the formation of teratomas [24,31,32]. Overcoming these challenges may require the use of structural biomaterial supports that provide instructive mechanical cues to the transplanted cells.
Material Approaches to Address Mechanical Requirements of Cell Transplantation
The different stages of the transplantation process each have unique mechanical requirements that can be addressed using biomaterial design strategies to improve stem cell transplantation efficacy. In this section, we will outline methods currently employed to provide the mechanical support and cues needed throughout the stages of transplantation (Figure 1b).
Injection
Microcarriers
Several new biomaterial approaches have been utilized to limit cell death that results from membrane damage during the injection stage of transplantation. One current approach is the use of hydrogel microcarriers, in which cells are encapsulated within small particles, typically spheres, that can be injected through a syringe needle. Cells encapsulated in microcarriers are protected from damaging mechanical forces exerted during injection, which can improve their acute survival by as much as 2-fold, and thus increase their therapeutic potential [33]. Furthermore, delivering stem cells within microcarriers enables high local cell densities, which can promote paracrine signaling and enhance differentiation that may be important for later stages of the transplantation process. Thus, the majority of studies with microcarriers load a high concentration of either single cell suspensions or cell aggregates [34,35]. The microcarrier droplets can be produced using a number of techniques including ionic crosslinking [36-38], microfluidic droplet production [39,40], water-in-oil emulsion [33,41,42], photocrosslinking [39,41], and thermal crosslinking [34]. In addition, many of these techniques can be combined to produce more complex microcarriers. For example, injectable gelatin-methacrylate (GelMA) microcarriers have been designed using microfluidic platforms to generate droplets of controllable sizes, which are then crosslinked with ultraviolet light [39,43]. Furthermore, due to their small size, microparticles have the ability to act as porous space fillers upon injection into defects, which can aid in host tissue integration [40].
Shear-thinning Hydrogels
An alternative approach to microcarrier encapsulation is the use of shear-thinning hydrogels, which allow for encapsulation of stem cells through weak dynamic interactions (e.g., hydrogen bonding, hydrophobic interactions, electrostatic attractions, and host-guest interactions) between the polymer chains prior to cell delivery [44-47]. When exposed to shear stress, as experienced during injection, these associations disassemble, resulting in a significant decrease in viscosity. Often this crosslink disassembly only occurs at the interface of the hydrogel and the syringe, resulting in “shear banding” at the interface [46,47]. This allows the rest of the hydrogel to remain intact and undergo “plug flow”, thereby protecting encapsulated cells from membrane damaging forces [15]. Several shear-thinning hydrogels have demonstrated improved cell survival post-injection including alginate hydrogels [15], protein-assembled hydrogels [45,48], supramolecular beta-hairpin hydrogels [49], and hyaluronic acid-based hydrogels [50-52]. Using protein-assembled hydrogels, acute survival of iPSC-derived endothelial cells increased 2-fold compared to saline-delivered cells [48], while encapsulation in hyaluronic acid-based hydrogels lead to an ~1.2-fold increase in survival of injected iPSC-derived neural progenitors [52]. These methods aim to improve survival of transplanted cells during the initial stage of transplantation, potentially improving overall cell engraftment.
Acute Post-injection
Several new biomaterial strategies have focused on improving cell survival and minimizing cell dispersion at the injection site and providing a cell-adhesive scaffold to promote acute cell retention within the host tissue. Three-dimensional mechanical support of transplanted cells helps prevent cell death due to anoikis, (i.e. anchorage-dependent apoptosis) and can prevent cell dispersal from the site of local injection. One approach to providing acute mechanical support after injection involves the control of hydrogel gelation kinetics. This can be accomplished through strategies including triggered gelation, or the use of shear-thinning hydrogels that are also rapidly self-healing.
Triggered Gelation
Ideally, gelation should be fast enough to promote homogenous cell distribution and acute cell retention at the transplant site, yet slow enough to prevent gelation within the syringe or catheter. Several systems have been designed to deliver cells in a viscous pre-polymer solution that will be triggered to gel in situ using biological stimuli, such as temperature [53], pH [54], ion concentration [55], or applied stimuli, such as light [56,57]. Temperature-triggered gelation has been used for a number of stem cell transplantation strategies through the incorporation of thermoresponsive polymers with a characteristic lower critical solution temperature (LCST) behavior. For example, thermoresponsive poly(N-isopropylacrylamide) (PNIPAM) has been routinely used to trigger in situ gelation in a number of hydrogel systems due to its LCST phase transition at approximately 32 °C. This results in rapid gelation at physiological temperature (37 °C), providing an effective approach to enhance cell retention [58,59]. Another material that can undergo temperature-triggered gelation is decellularized matrix hydrogels derived from native tissue [14,60,61]. In addition, the use of ECM-derived hydrogels capitalizes on the presence of tissue-specific biochemical cues and ligands to anchor adherent cells and improve cell survival. These materials have been used in several preclinical studies for stem cell transplantation based on their rapid in situ gelation. For example, the delivery of MSCs encapsulated in hydrogels derived from porcine lung tissue demonstrated increased cell retention at 24 hours following intratracheal delivery in a rat model [62].
Photopolymerization (and other mechanisms of triggered gelation using an applied stimulus) can lead to spatially controlled formation of crosslinked hydrogels at physiological pH and temperature. The incorporation of diacrylate or methacrylate functional groups has been shown to facilitate crosslinking and photo-triggered gelation in response to UV or visible light [56,57]. UV light has been used to crosslink diacrylate-modified polyethylene oxide solutions in situ resulting in increased stem cell retention following transdermal photopolymerization [63]. Similarly, other polymers including chitosan [64,65], alginate [55,66], gelatin [67,68], and hyaluronic acid (HA) [57] have all been modified with methacrylate functional groups to trigger gelation. For example, transdermal photopolymerization of methacrylated-gelatin has been shown to deliver and improve the integration of MSCs and endothelial-colony forming cells with host tissue for vascular therapies compared to non-photocrosslinked hydrogels [68].
Self-Healing Hydrogels
Shear-thinning, self-healing hydrogels have been used in a number of preclinical studies to provide protection during the injection stage and also promote acute cell retention post-transplantation. These materials undergo viscous flow when subjected to an applied shear stress and time-dependent recovery and reassembly of the hydrogel network upon relaxation [44]. When designed appropriately, these materials can demonstrate fast self-healing kinetics at the site of injection, thereby resulting in high levels of cell retention [31,50]. For example, an injectable hyaluronan/methylcellulose hydrogel demonstrated improved transplanted cell retention of iPSCs for spinal cord and retinal therapies [30,31]. In another design, hyaluronan modified to undergo rapid host-guest self-assembly was shown to improve endothelial progenitor cell retention after myocardial infarct [50]. Engineered protein-based self-assembly systems have also been shown to promote acute survival post-injection, resulting in a more than 2-fold increase in stem cell retention compared to saline-mediated delivery [48,69].
Long-Term Survival and Function
Influencing Stem Cell Differentiation
A large body of mechanotransduction research has studied the role of 2D and 3D hydrogel mechanics on stem cell differentiation and function, with substantial emphasis placed on MSC differentiation. Numerous studies have shown that substrate stiffness heavily influences stem cell fate, with compliant materials generally promoting soft tissue lineages (e.g. neural and fat cells) and stiffer materials leading to hard tissue lineages (e.g. bone cells) [25,70-73]. Substrate stiffness has been shown to play a role in stem/progenitor cell differentiation [51,74] and progenitor cell function [75]. For example, when cultured over a specific stiffness range, cardiac progenitors have enhanced electrical and contractile function [70,76,77]. Little of this work has been translated in vivo as these materials have been designed specifically for in vitro mechanotransduction studies. Complicating their direct application into clinical therapies, the mechanical cues experienced by transplanted cells may include both the mechanical properties of any engineered matrix, as well as that of the endogenous tissue. Furthermore, the mechanical cues of endogenous tissue may include aberrant signaling due to matrix stiffening (e.g. fibrosis) or matrix weakening (e.g. unchecked proteolysis) [78,79]. One recent study using injectable alginate hydrogels suggests that bulk matrix stiffness differentially promotes osteodifferentiation of transplanted MSCs and new bone formation in a cranial defect model [80], similar to results predicted by in vitro models [81]. In complementary work, improved differentiation and integration of transplanted muscle stem cells was observed when cells were transplanted on hydrogel constructs with an ideal stiffness range [74].
In addition to material stiffness, hydrogel degradation and matrix remodeling can play a significant role in stem cell behavior and differentiation [1]. MSC spreading and survival have been shown to depend on the degree of hydrogel degradation [82], which can influence stem cell fate [83]. For example, MSC-mediated degradation of a 3D matrix influences differentiation by altering the ability of cells to generate traction within the microenvironment [84]. Tuning of hydrogel degradation has been used to promote MSC differentiation towards chondrogenic lineages, resulting in improved deposition of neocartilage ECM, which may prove beneficial towards long-term integration of transplanted cells [85].
Beyond the intrinsic mechanical properties of the matrix, the dynamic mechanical microenvironment is also known to impact cell differentiation and maturation processes [86,87]. For example, several in vitro studies have demonstrated that mechanical loading of stem cells through compression, tension, or shear can lead to differentiation into osteogenic [88-90], myogenic [91-93], or vasculogenic [94-96] phenotypes. These studies suggest that dynamic in vivo mechanical cues must be considered for specific clinical applications.
Influencing Stem Cell Secretome
For many potential regenerative medicine therapies, the transplanted cells may not directly participate in regenerating the damaged tissue, but instead function through the secretion of paracrine signals that promote host tissue regeneration [22,97]. Therefore, several studies have shifted focus to the therapeutic potential of stem cells based on their secretion of pro-survival and pro-regenerative factors [22,23,26,97]. Recent work has demonstrated the use of hydrogel design strategies to enhance the secretory profile of growth factors, chemokines, and cytokines from stem cells, also known as their secretome [21,98-101].
Hydrogel mechanical properties, such as stiffness and degradation, have been suggested to influence stem cell secretion. For example, substrate stiffness has been shown to regulate MSC secretion of paracrine signals, with intermediate and stiffer substrates (10-40 kPa) leading to increased levels of pro-angiogenic factors interleukin 8, vascular endothelial growth factor, and angiogenin compared to more compliant substrates (E ~ 0.5-2 kPa) [21,99]. Similarly, hydrogels with intermediate elasticity were found to significantly increase the secretion of pro-angiogenic factors from adipose-derived stem cells [102]. Unfortunately, increasing hydrogel stiffness and crosslinking density often results in slower hydrogel degradation kinetics [85,103]. With a decrease in hydrogel degradation, there may be an associated decrease in the diffusion of secreted soluble factors, thus limiting the therapeutic benefit of transplanted stem cells [104].
Future Directions
Currently no universal material fulfills all of the mechanical needs to improve stem cell survival and functionality during all three stages of transplantation. While some material mechanical properties may be needed for enhanced long-term retention and differentiation, these same mechanical properties may limit success in the earlier transplantation stages. Therefore, a promising future research direction is the development of biomaterials that can alter their mechanical properties over time to achieve diverse mechanical requirements throughout the multiple stages of transplantation.
One approach to modify biomaterial properties over time is the use of dual-stage or multi-stage crosslinking strategies. For example, several shear-thinning and self-healing hydrogels have been designed to undergo a second stage of crosslinking, and hence mechanical stiffening, in response to various stimuli. Temperature is a common stimulus to induce secondary crosslinking in situ, since many self-assembling hydrogels can be modified to include a thermoresponsive element [30,58,105]. In this approach, cell viability is improved during the injection stage due to the shear thinning mechanical properties, acute cell retention is improved during the acute post-injection stage due to the rapid self-healing kinetics, and the temperature-triggered secondary crosslinking increases long-term cell survival due to the decreased degradation rate [58]. Alternatively, covalent crosslinking can be used as a secondary crosslinking mechanism to reinforce and strengthen injectable hydrogels [67,106,107]. For example, HA can be modified to undergo a first-stage of guest-host self-assembly followed by a second-stage of covalent crosslinking to prolong material retention and to improve integration with host tissue [67,107].
A second approach to modulating biomaterial mechanics and structure over time is to engineer complex degradation patterns into the hydrogel. For example, composite alginate hydrogels were created with regions that were fast degrading surrounded by a slower degrading material for use in MSC transplantation [80]. In situ, the fast-degrading regions created voids that enhanced cell survival through increased nutrient transport and cell migration across the host-transplant interface [80]. Meanwhile, the slow-degrading regions provided long-term mechanical support to promote osteogenic differentiation.
In the future, it is expected that creative biomaterials chemistry will be combined with novel microfabrication techniques to design a broad array of biomaterials that can stiffen and/or weaken over time at the length-scales and time-scales required to support all stages of stem cell transplantation. For example, a rich array of photoactive chemistry has already been employed in the design of in vitro biomaterials that exhibit this so-called “4D” control of mechanical properties [108,109].
Conclusion
In conclusion, a wide range of hydrogels with tunable mechanical properties are being developed to overcome the different mechanical challenges facing stem cells during each stage of transplantation: injection, acute post-injection, and long-term survival and function. While no universal material is currently capable of addressing all of the mechanical requirements, a promising future direction is the development of biomaterials that can adjust their mechanical properties for multiple transplantation stages. Thus, while current injectable biomaterials are already demonstrating that they can significantly improve transplanted stem cell viability and function, future innovation in biomaterials design is expected to even further enhance the therapeutic efficacy of transplanted stem cells.
Highlights.
Stem cell transplantation by local injection has seen limited clinical success
Different transplantation stages present different mechanical challenges
Hydrogels with tunable mechanics can overcome mechanical challenges at each stage
Acknowledgments
This work was supported by the National Institutes of Health (R21-EB020235 to S.C.H. and T32-HL098049-06 to A.F.), Stanford Bio-X (IIP-7-75), CIRM (RT3-07948), Coulter Foundation (CP-2014-4), and Stanford Geballe Laboratory for Advanced Materials Postdoctoral Fellowship (to L.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
Papers of particular interest, published within the period of review, have been highlighted as:
-
*
of special interest
-
**
of outstanding interest
- 1.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trensz F, Lucien F, Couture V, Sollrald T, Drouin G, Rouleau AJ, Grandbois M, Lacraz G, Grenier G. Increased microenvironment stiffness in damaged myofibers promotes myogenic progenitor cell proliferation. Skelet Muscle. 2015;5:5. doi: 10.1186/s13395-015-0030-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun W, Kurniawan NA, Kumar AP, Rajagopalan R, Lim CT. Effects of Migrating Cell-Induced Matrix Reorganization on 3D Cancer Cell Migration. Cellular and Molecular Bioengineering. 2014;7:205–217. [Google Scholar]
- 4.Doyle AD, Carvajal N, Jin A, Matsumoto K, Yamada KM. Local 3D matrix microenvironment regulates cell migration through spatiotemporal dynamics of contractility-dependent adhesions. Nat Commun. 2015;6:8720. doi: 10.1038/ncomms9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kass L, Erler JT, Dembo M, Weaver VM. Mammary epithelial cell: influence of extracellular matrix composition and organization during development and tumorigenesis. Int J Biochem Cell Biol. 2007;39:1987–1994. doi: 10.1016/j.biocel.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranganath SH, Levy O, Inamdar MS, Karp JM. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marquardt LM, Heilshorn SC. Design of Injectable Materials to Improve Stem Cell Transplantation. Current Stem Cell Reports. 2016;2:207–220. doi: 10.1007/s40778-016-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peppas NA, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Advanced Materials. 2006;18:1345–1360. [Google Scholar]
- 9.Tibbitt MW, Anseth KS. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol Bioeng. 2009;103:655–663. doi: 10.1002/bit.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, Huebsch N, Mooney DJ. Substrate stress relaxation regulates cell spreading. Nat Commun. 2015;6:6364. doi: 10.1038/ncomms7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malliaras K, Kreke M, Marban E. The stuttering progress of cell therapy for heart disease. Clin Pharmacol Ther. 2011;90:532–541. doi: 10.1038/clpt.2011.175. [DOI] [PubMed] [Google Scholar]
- 12.Iwasaki M, Wilcox JT, Nishimura Y, Zweckberger K, Suzuki H, Wang J, Liu Y, Karadimas SK, Fehlings MG. Synergistic effects of self-assembling peptide and neural stem/progenitor cells to promote tissue repair and forelimb functional recovery in cervical spinal cord injury. Biomaterials. 2014;35:2617–2629. doi: 10.1016/j.biomaterials.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 13.Templin C, Luscher TF, Landmesser U. Cell-based cardiovascular repair and regeneration in acute myocardial infarction and chronic ischemic cardiomyopathy-current status and future developments. Int J Dev Biol. 2011;55:407–417. doi: 10.1387/ijdb.103219ct. [DOI] [PubMed] [Google Scholar]
- 14.Singelyn JM, Christman KL. Injectable materials for the treatment of myocardial infarction and heart failure: the promise of decellularized matrices. J Cardiovasc Transl Res. 2010;3:478–486. doi: 10.1007/s12265-010-9202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguado BA, Mulyasasmita W, Su J, Lampe KJ, Heilshorn SC. Improving viability of stem cells during syringe needle flow through the design of hydrogel cell carriers. Tissue Eng Part A. 2012;18:806–815. doi: 10.1089/ten.tea.2011.0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robey TE, Saiget MK, Reinecke H, Murry CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol. 2008;45:567–581. doi: 10.1016/j.yjmcc.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian S, Liu Q, Gnatovskiy L, Ma PX, Wang Z. Heart Regeneration with Embryonic Cardiac Progenitor Cells and Cardiac Tissue Engineering. J Stem Cell Transplant Biol. 2015;1 doi: 10.19104/jstb.2015.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotaling NA, Tang L, Irvine DJ, Babensee JE. Biomaterial Strategies for Immunomodulation. Annu Rev Biomed Eng. 2015;17:317–349. doi: 10.1146/annurev-bioeng-071813-104814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emerich DF, Silva E, Ali O, Mooney D, Bell W, Yu SJ, Kaneko Y, Borlongan C. Injectable VEGF hydrogels produce near complete neurological and anatomical protection following cerebral ischemia in rats. Cell Transplant. 2010;19:1063–1071. doi: 10.3727/096368910X498278. [DOI] [PubMed] [Google Scholar]
- 20.Camci-Unal G, Alemdar N, Annabi N, Khademhosseini A. Oxygen Releasing Biomaterials for Tissue Engineering. Polym Int. 2013;62:843–848. doi: 10.1002/pi.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdeen AA, Weiss JB, Lee J, Kilian KA. Matrix composition and mechanics direct proangiogenic signaling from mesenchymal stem cells. Tissue Eng Part A. 2014;20:2737–2745. doi: 10.1089/ten.tea.2013.0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liang X, Ding Y, Zhang Y, Tse HF, Lian Q. Paracrine mechanisms of mesenchymal stem cell-based therapy: current status and perspectives. Cell Transplant. 2014;23:1045–1059. doi: 10.3727/096368913X667709. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen PK, Riegler J, Wu JC. Stem cell imaging: from bench to bedside. Cell Stem Cell. 2014;14:431–444. doi: 10.1016/j.stem.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 26.Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F, Li Z, Rollins MD, Wu JC, Gambhir SS, et al. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler Thromb Vasc Biol. 2010;30:984–991. doi: 10.1161/ATVBAHA.110.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rufaihah AJ, Huang NF, Jame S, Lee JC, Nguyen HN, Byers B, De A, Okogbaa J, Rollins M, Reijo-Pera R, et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L, Chen X, Wang WE, Zeng C. How to Improve the Survival of Transplanted Mesenchymal Stem Cell in Ischemic Heart? Stem Cells Int. 2016;2016:9682757. doi: 10.1155/2016/9682757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Donnelly EM, Lamanna J, Boulis NM. Stem cell therapy for the spinal cord. Stem Cell Res Ther. 2012;3:24. doi: 10.1186/scrt115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ballios BG, Cooke MJ, Donaldson L, Coles BL, Morshead CM, van der Kooy D, Shoichet MS. A Hyaluronan-Based Injectable Hydrogel Improves the Survival and Integration of Stem Cell Progeny following Transplantation. Stem Cell Reports. 2015;4:1031–1045. doi: 10.1016/j.stemcr.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31*.Fuhrmann T, Tam RY, Ballarin B, Coles B, Elliott Donaghue I, van der Kooy D, Nagy A, Tator CH, Morshead CM, Shoichet MS. Injectable hydrogel promotes early survival of induced pluripotent stem cell-derived oligodendrocytes and attenuates longterm teratoma formation in a spinal cord injury model. Biomaterials. 2016;83:23–36. doi: 10.1016/j.biomaterials.2015.12.032. The authors demonstrated enhanced stem cell survival and differentiation using an engineered hyaluronic acid-methylcellulose hydrogel in a spinal cord injury model. Interestingly, the use of this hydrogel carrier reduced the frequency of teratoma formation from trasnplanted cells compared to cells delivered in saline. [DOI] [PubMed] [Google Scholar]
- 32.Rubach M, Adelmann R, Haustein M, Drey F, Pfannkuche K, Xiao B, Koester A, Udink ten Cate FE, Choi YH, Neef K, et al. Mesenchymal stem cells and their conditioned medium improve integration of purified induced pluripotent stem cell-derived cardiomyocyte clusters into myocardial tissue. Stem Cells Dev. 2014;23:643–653. doi: 10.1089/scd.2013.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olabisi RM, Lazard ZW, Franco CL, Hall MA, Kwon SK, Sevick-Muraca EM, Hipp JA, Davis AR, Olmsted-Davis EA, West JL. Hydrogel microsphere encapsulation of a cell-based gene therapy system increases cell survival of injected cells, transgene expression, and bone volume in a model of heterotopic ossification. Tissue Eng Part A. 2010;16:3727–3736. doi: 10.1089/ten.tea.2010.0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hui TY, Cheung KM, Cheung WL, Chan D, Chan BP. In vitro chondrogenic differentiation of human mesenchymal stem cells in collagen microspheres: influence of cell seeding density and collagen concentration. Biomaterials. 2008;29:3201–3212. doi: 10.1016/j.biomaterials.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Wilson JL, Najia MA, Saeed R, McDevitt TC. Alginate encapsulation parameters influence the differentiation of microencapsulated embryonic stem cell aggregates. Biotechnol Bioeng. 2014;111:618–631. doi: 10.1002/bit.25121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leslie SK, Cohen DJ, Sedlaczek J, Pinsker EJ, Boyan BD, Schwartz Z. Controlled release of rat adipose-derived stem cells from alginate microbeads. Biomaterials. 2013;34:8172–8184. doi: 10.1016/j.biomaterials.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Leslie SK, Nicolini AM, Sundaresan G, Zweit J, Boyan BD, Schwartz Z. Development of a cell delivery system using alginate microbeads for tissue regeneration. Journal of Materials Chemistry B. 2016 doi: 10.1039/c6tb00035e. [DOI] [PubMed] [Google Scholar]
- 38.Markusen JF, Mason C, Hull DA, Town MA, Tabor AB, Clements M, Boshoff CH, Dunnill P. Behavior of adult human mesenchymal stem cells entrapped in alginate-GRGDY beads. Tissue Eng. 2006;12:821–830. doi: 10.1089/ten.2006.12.821. [DOI] [PubMed] [Google Scholar]
- 39.Zhao X, Liu S, Yildirimer L, Zhao H, Ding R, Wang H, Cui W, Weitz D. Injectable Stem Cell-Laden Photocrosslinkable Microspheres Fabricated Using Microfluidics for Rapid Generation of Osteogenic Tissue Constructs. Advanced Functional Materials. 2016;26:2809–2819. [Google Scholar]
- 40*.Griffin DR, Weaver WM, Scumpia PO, Di Carlo D, Segura T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat Mater. 2015;14:737–744. doi: 10.1038/nmat4294. This study displayed a high-throughput method to generate microparticles capable of forming highly-porous scaffolds that allow for cell proliferation and network formation. These porous scaffolds can be injected to form complex shapes and promote tissue regeneration in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Franco CL, Price J, West JL. Development and optimization of a dual-photoinitiator, emulsion-based technique for rapid generation of cell-laden hydrogel microspheres. Acta Biomater. 2011;7:3267–3276. doi: 10.1016/j.actbio.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Rao RR, Stegemann JP. Delivery of mesenchymal stem cells in chitosan/collagen microbeads for orthopedic tissue repair. Cells Tissues Organs. 2013;197:333–343. doi: 10.1159/000348359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi CH, Wang H, Lee H, Kim JH, Zhang L, Mao A, Mooney DJ, Weitz DA. Onestep generation of cell-laden microgels using double emulsion drops with a sacrificial ultra-thin oil shell. Lab Chip. 2016;16:1549–1555. doi: 10.1039/c6lc00261g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guvendiren M, Burdick JA. Stem cell response to spatially and temporally displayed and reversible surface topography. Adv Healthc Mater. 2013;2:155–164. doi: 10.1002/adhm.201200105. [DOI] [PubMed] [Google Scholar]
- 45.Wong Po Foo CT, Lee JS, Mulyasasmita W, Parisi-Amon A, Heilshorn SC. Two-component protein-engineered physical hydrogels for cell encapsulation. Proc Natl Acad Sci U S A. 2009;106:22067–22072. doi: 10.1073/pnas.0904851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan C, Pochan DJ. Rheological properties of peptide-based hydrogels for biomedical and other applications. Chem Soc Rev. 2010;39:3528–3540. doi: 10.1039/b919449p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glassman MJ, Chan J, Olsen BD. Reinforcement of Shear Thinning Protein Hydrogels by Responsive Block Copolymer Self-Assembly. Adv Funct Mater. 2013;23:1182–1193. doi: 10.1002/adfm.201202034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulyasasmita W, Cai L, Dewi RE, Jha A, Ullmann SD, Luong RH, Huang NF, Heilshorn SC. Avidity-controlled hydrogels for injectable co-delivery of induced pluripotent stem cell-derived endothelial cells and growth factors. J Control Release. 2014;191:71–81. doi: 10.1016/j.jconrel.2014.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49*.Sun JE, Stewart B, Litan A, Lee SJ, Schneider JP, Langhans SA, Pochan DJ. Sustained release of active chemotherapeutics from injectable-solid beta-hairpin peptide hydrogel. Biomater Sci. 2016;4:839–848. doi: 10.1039/c5bm00538h. This study demonstrated a shear-thinning, self-healing, beta-hairpin supramolecular hydrogel as a drug delivery vehicle for sustained, local delivery of chemotherapeutics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Gaffey AC, Chen MH, Venkataraman CM, Trubelja A, Rodell CB, Dinh PV, Hung G, MacArthur JW, Soopan RV, Burdick JA, et al. Injectable shear-thinning hydrogels used to deliver endothelial progenitor cells, enhance cell engraftment, and improve ischemic myocardium. J Thorac Cardiovasc Surg. 2015;150:1268–1276. doi: 10.1016/j.jtcvs.2015.07.035. Using an injectable hyaluronic acid hydrogel, the authors demonstrated improved delivery of endothelial progenitor cells to a myocardial infarct model. Enhanced ventricular function, as well as reduced pathophysiology, was observed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lam J, Carmichael ST, Lowry WE, Segura T. Hydrogel design of experiments methodology to optimize hydrogel for iPSC-NPC culture. Adv Healthc Mater. 2015;4:534–539. doi: 10.1002/adhm.201400410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52*.Lam J, Lowry WE, Carmichael ST, Segura T. Delivery of iPS-NPCs to the Stroke Cavity within a Hyaluronic Acid Matrix Promotes the Differentiation of Transplanted Cells. Adv Funct Mater. 2014;24:7053–7062. doi: 10.1002/adfm.201401483. This systematic study explored the effects of different injection parameters on stem cell survival when delivererd within an injectable hydrogel. Hydrogel transplantation of iPSCs improved cell delivery and differentiation in a rat stroke cavity model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang H, Liu Z, Li D, Guo X, Kasper FK, Duan C, Zhou J, Mikos AG, Wang C. Injectable biodegradable hydrogels for embryonic stem cell transplantation: improved cardiac remodelling and function of myocardial infarction. J Cell Mol Med. 2012;16:1310–1320. doi: 10.1111/j.1582-4934.2011.01409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garbern JC, Minami E, Stayton PS, Murry CE. Delivery of basic fibroblast growth factor with a pH-responsive, injectable hydrogel to improve angiogenesis in infarcted myocardium. Biomaterials. 2011;32:2407–2416. doi: 10.1016/j.biomaterials.2010.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Benton JA, DeForest CA, Vivekanandan V, Anseth KS. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng Part A. 2009;15:3221–3230. doi: 10.1089/ten.tea.2008.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fenn SL, Oldinski RA. Visible light crosslinking of methacrylated hyaluronan hydrogels for injectable tissue repair. J Biomed Mater Res B Appl Biomater. 2016;104:1229–1236. doi: 10.1002/jbm.b.33476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Cai L, Dewi RE, Heilshorn SC. Injectable Hydrogels with In Situ Double Network Formation Enhance Retention of Transplanted Stem Cells. Adv Funct Mater. 2015;25:1344–1351. doi: 10.1002/adfm.201403631. This study reported the design of a shear-thinning, self-healing hydrogel that undergoes secondary, thermoresponsive crosslinking. These materials provided mechanical protection of stem cells during injection and increased long-term retention of transplanted cells in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thorpe AA, Boyes VL, Sammon C, Le Maitre CL. Thermally triggered injectable hydrogel, which induces mesenchymal stem cell differentiation to nucleus pulposus cells: Potential for regeneration of the intervertebral disc. Acta Biomater. 2016;36:99–111. doi: 10.1016/j.actbio.2016.03.029. [DOI] [PubMed] [Google Scholar]
- 60.Young DA, Ibrahim DO, Hu D, Christman KL. Injectable hydrogel scaffold from decellularized human lipoaspirate. Acta Biomater. 2011;7:1040–1049. doi: 10.1016/j.actbio.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kwon JS, Yoon SM, Shim SW, Park JH, Min KJ, Oh HJ, Kim JH, Kim YJ, Yoon JJ, Choi BH, et al. Injectable extracellular matrix hydrogel developed using porcine articular cartilage. Int J Pharm. 2013;454:183–191. doi: 10.1016/j.ijpharm.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 62*.Pouliot RA, Link PA, Mikhaiel NS, Schneck MB, Valentine MS, Kamga Gninzeko FJ, Herbert JA, Sakagami M, Heise RL. Development and characterization of a naturally derived lung extracellular matrix hydrogel. J Biomed Mater Res A. 2016;104:1922–1935. doi: 10.1002/jbm.a.35726. The authors demonstrated enhanced transplantation efficacy of MSCs to the lungs using decellularized native tissue in a rat emphysema model. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma B, Williams CG, Khan M, Manson P, Elisseeff JH. In vivo chondrogenesis of mesenchymal stem cells in a photopolymerized hydrogel. Plast Reconstr Surg. 2007;119:112–120. doi: 10.1097/01.prs.0000236896.22479.52. [DOI] [PubMed] [Google Scholar]
- 64.Choi B, Kim S, Lin B, Wu BM, Lee M. Cartilaginous Extracellular Matrix-Modified Chitosan Hydrogels for Cartilage Tissue Engineering. ACS Applied Materials & Interfaces. 2014;6:20110–20121. doi: 10.1021/am505723k. [DOI] [PubMed] [Google Scholar]
- 65.Sukarto A, Yu C, Flynn LE, Amsden BG. Co-delivery of adipose-derived stem cells and growth factor-loaded microspheres in RGD-grafted N-methacrylate glycol chitosan gels for focal chondral repair. Biomacromolecules. 2012;13:2490–2502. doi: 10.1021/bm300733n. [DOI] [PubMed] [Google Scholar]
- 66.Jeon O, Bouhadir KH, Mansour JM, Alsberg E. Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials. 2009;30:2724–2734. doi: 10.1016/j.biomaterials.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 67.Lin H, Cheng AW, Alexander PG, Beck AM, Tuan RS. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng Part A. 2014;20:2402–2411. doi: 10.1089/ten.tea.2013.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin RZ, Chen YC, Moreno-Luna R, Khademhosseini A, Melero-Martin JM. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials. 2013;34:6785–6796. doi: 10.1016/j.biomaterials.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parisi-Amon A, Mulyasasmita W, Chung C, Heilshorn SC. Protein-engineered injectable hydrogel to improve retention of transplanted adipose-derived stem cells. Adv Healthc Mater. 2013;2:428–432. doi: 10.1002/adhm.201200293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Engler AJ, Carag-Krieger C, Johnson CP, Raab M, Tang HY, Speicher DW, Sanger JW, Sanger JM, Discher DE. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J Cell Sci. 2008;121:3794–3802. doi: 10.1242/jcs.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, Healy KE. Substrate modulus directs neural stem cell behavior. Biophys J. 2008;95:4426–4438. doi: 10.1529/biophysj.108.132217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seidlits SK, Khaing ZZ, Petersen RR, Nickels JD, Vanscoy JE, Shear JB, Schmidt CE. The effects of hyaluronic acid hydrogels with tunable mechanical properties on neural progenitor cell differentiation. Biomaterials. 2010;31:3930–3940. doi: 10.1016/j.biomaterials.2010.01.125. [DOI] [PubMed] [Google Scholar]
- 73.Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech. 2010;43:55–62. doi: 10.1016/j.jbiomech.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 74.Quarta M, Brett JO, DiMarco R, De Morree A, Boutet SC, Chacon R, Gibbons MC, Garcia VA, Su J, Shrager JB, et al. An artificial niche preserves the quiescence of muscle stem cells and enhances their therapeutic efficacy. Nat Biotechnol. 2016;34:752–759. doi: 10.1038/nbt.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP, Blau HM. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–1081. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Influence of substrate stiffness on the phenotype of heart cells. Biotechnol Bioeng. 2010;105:1148–1160. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- 77.Ribeiro AJ, Ang YS, Fu JD, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, Pruitt BL. Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc Natl Acad Sci U S A. 2015;112:12705–12710. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Disease Models & Mechanisms. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wells RG. Tissue Mechanics and Fibrosis. Biochimica et biophysica acta. 2013;1832:884–890. doi: 10.1016/j.bbadis.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80**.Huebsch N, Lippens E, Lee K, Mehta M, Koshy ST, Darnell MC, Desai RM, Madl CM, Xu M, Zhao X, et al. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat Mater. 2015;14:1269–1277. doi: 10.1038/nmat4407. This study demonstrated the design and use of injectable hydrogels that improve the survival and differentiation of locally-delivered stem cells through the formation of void spaces. These void spaces allowed for enhanced cell migration and neo-tissue formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nat Mater. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hudalla GA, Eng TS, Murphy WL. An approach to modulate degradation and mesenchymal stem cell behavior in poly(ethylene glycol) networks. Biomacromolecules. 2008;9:842–849. doi: 10.1021/bm701179s. [DOI] [PubMed] [Google Scholar]
- 83.Lutolf MP, Gilbert PM, Blau HM. Designing materials to direct stem-cell fate. Nature. 2009;462:433–441. doi: 10.1038/nature08602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khetan S, Guvendiren M, Legant WR, Cohen DM, Chen CS, Burdick JA. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat Mater. 2013;12:458–465. doi: 10.1038/nmat3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chung C, Beecham M, Mauck RL, Burdick JA. The influence of degradation characteristics of hyaluronic acid hydrogels on in vitro neocartilage formation by mesenchymal stem cells. Biomaterials. 2009;30:4287–4296. doi: 10.1016/j.biomaterials.2009.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Patwari P, Lee RT. Mechanical Control of Tissue Morphogenesis. Circulation research. 2008;103:234–243. doi: 10.1161/CIRCRESAHA.108.175331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shav D, Einav S. The effect of mechanical loads in the differentiation of precursor cells into mature cells. Ann N Y Acad Sci. 2010;1188:25–31. doi: 10.1111/j.1749-6632.2009.05079.x. [DOI] [PubMed] [Google Scholar]
- 88.Delaine-Smith RM, Reilly GC. Mesenchymal stem cell responses to mechanical stimuli. Muscles, Ligaments and Tendons Journal. 2012;2:169–180. [PMC free article] [PubMed] [Google Scholar]
- 89.Ye Y, Du Y, Guo F, Gong C, Yang K, Qin L. Comparative study of the osteogenic differentiation capacity of human bone marrow- and human adipose-derived stem cells under cyclic tensile stretch using quantitative analysis. Int J Mol Med. 2012;30:1327–1334. doi: 10.3892/ijmm.2012.1123. [DOI] [PubMed] [Google Scholar]
- 90.Du H, Zheng X, Wang L, Tang W, Liu L, Jing W, Lin Y, Tian W, Long J. The Osteogenic Response of Undifferentiated Human Adipose-Derived Stem Cells under Mechanical Stimulation. Cells Tissues Organs. 2012;196:313–324. doi: 10.1159/000335905. [DOI] [PubMed] [Google Scholar]
- 91.Shimko VF, Claycomb WC. Effect of mechanical loading on three-dimensional cultures of embryonic stem cell-derived cardiomyocytes. Tissue Eng Part A. 2008;14:49–58. doi: 10.1089/ten.2007.0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amin S, Banijamali SE, Tafazoli-Shadpour M, Shokrgozar MA, Dehghan MM, Haghighipour N, Mahdian R, Bayati V, Abbasnia P. Comparing the effect of equiaxial cyclic mechanical stimulation on GATA4 expression in adipose-derived and bone marrow-derived mesenchymal stem cells. Cell Biology International. 2014;38:219–227. doi: 10.1002/cbin.10194. [DOI] [PubMed] [Google Scholar]
- 93.Zhu R, Blazeski A, Poon E, Costa KD, Tung L, Boheler KR. Physical developmental cues for the maturation of human pluripotent stem cell-derived cardiomyocytes. Stem Cell Research & Therapy. 2014;5:117. doi: 10.1186/scrt507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang H, Riha GM, Yan S, Li M, Chai H, Yang H, Yao Q, Chen C. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arterioscler Thromb Vasc Biol. 2005;25:1817–1823. doi: 10.1161/01.ATV.0000175840.90510.a8. [DOI] [PubMed] [Google Scholar]
- 95.Wolfe RP, Ahsan T. Shear stress during early embryonic stem cell differentiation promotes hematopoietic and endothelial phenotypes. Biotechnol Bioeng. 2013;110:1231–1242. doi: 10.1002/bit.24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dan P, Velot É, Decot V, Menu P. The role of mechanical stimuli in the vascular differentiation of mesenchymal stem cells. Journal of Cell Science. 2015;128:2415–2422. doi: 10.1242/jcs.167783. [DOI] [PubMed] [Google Scholar]
- 97.Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med. 2010;5:121–143. doi: 10.2217/rme.09.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bakota EL, Wang Y, Danesh FR, Hartgerink JD. Injectable multidomain peptide nanofiber hydrogel as a delivery agent for stem cell secretome. Biomacromolecules. 2011;12:1651–1657. doi: 10.1021/bm200035r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seib FP, Prewitz M, Werner C, Bornhauser M. Matrix elasticity regulates the secretory profile of human bone marrow-derived multipotent mesenchymal stromal cells (MSCs) Biochem Biophys Res Commun. 2009;389:663–667. doi: 10.1016/j.bbrc.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 100.Wang Y, Bakota E, Chang BH, Entman M, Hartgerink JD, Danesh FR. Peptide nanofibers preconditioned with stem cell secretome are renoprotective. J Am Soc Nephrol. 2011;22:704–717. doi: 10.1681/ASN.2010040403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wright B, Hopkinson A, Leyland M, Connon CJ. The secretome of alginate-encapsulated limbal epithelial stem cells modulates corneal epithelial cell proliferation. PLoS One. 2013;8:e70860. doi: 10.1371/journal.pone.0070860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102*.Cai L, D R, Goldstone AB, Cohen JE, Steele AN, Woo YJ, Heilshorn Regulating Stem Cell Secretome Using Injectable Hydrogels with In situ Network Formation. Advanced Healthcare Materials. 2016 doi: 10.1002/adhm.201600497. This study demonstrated that the mechanical properties of shear-thinning and self-healing hydrogels can influence the pro-angiogenic secretome of adipose-derived stem cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lawyer T, McIntosh K, Clavijo C, Potekhina L, Mann BK. Formulation Changes Affect Material Properties and Cell Behavior in HA-Based Hydrogels. International Journal of Cell Biology. 2012;2012:9. doi: 10.1155/2012/737421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dhote V, Skaalure S, Akalp U, Roberts J, Bryant SJ, Vernerey FJ. On the role of hydrogel structure and degradation in controlling the transport of cell-secreted matrix molecules for engineered cartilage. J Mech Behav Biomed Mater. 2013;19:61–74. doi: 10.1016/j.jmbbm.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gupta D, Tator CH, Shoichet MS. Fast-gelling injectable blend of hyaluronan and methylcellulose for intrathecal, localized delivery to the injured spinal cord. Biomaterials. 2006;27:2370–2379. doi: 10.1016/j.biomaterials.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 106.Liu Z, Yao P. Injectable thermo-responsive hydrogel composed of xanthan gum and methylcellulose double networks with shear-thinning property. Carbohydr Polym. 2015;132:490–498. doi: 10.1016/j.carbpol.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 107*.Rodell CB, MacArthur JW, Dorsey SM, Wade RJ, Wang LL, Woo YJ, Burdick JA. Shear-Thinning Supramolecular Hydrogels with Secondary Autonomous Covalent Crosslinking to Modulate Viscoelastic Properties In Vivo. Adv Funct Mater. 2015;25:636–644. doi: 10.1002/adfm.201403550. This study demonstrated that the incorporation of a secondary crosslinking mechanism can tune the in vivo viscoelastic properties of an injectable hydrogel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109**.Yang C, Tibbitt MW, Basta L, Anseth KS. Mechanical memory and dosing influence stem cell fate. Nat Mater. 2014;13:645–652. doi: 10.1038/nmat3889. The authors of this study demonstrated that stem cells retain a mechanical memory of culture substrates, which influences their differentiation potential. [DOI] [PMC free article] [PubMed] [Google Scholar]


