Abstract
Latency is a hallmark of all herpesviruses, during which the viral genomes are silenced through DNA methylation and suppressive histone modifications. When latent herpesviruses reactivate to undergo productive lytic replication, the suppressive epigenetic marks are replaced with active ones to allow for transcription of viral genes. Interestingly, by using Kaposi’s sarcoma-associated herpesvirus (KSHV) as a model, we recently demonstrated that the newly transcribed viral RNAs are also subjected to post-transcriptional N6-adenosine methylation (m6A). Blockade of this post-transcriptional event abolishes viral protein expression and halts virion production. We found that m6A modification controls RNA splicing, stability, and protein translation to regulate viral lytic gene expression and replication. Thus, our finding for the first time reveals a critical role of this epitranscriptomic mechanism in the control of herpesviral replication, which shall shed lights on development of novel strategies for the control of herpesviral infection.
Keywords: RNA m6A modification, RTA pre-mRNA splicing, KSHV lytic replication, epitranscriptomics
Kaposi’s sarcoma-associated herpesvirus (KSHV) is an oncogenic virus associated with multiple malignancies including Kaposi’s sarcoma (KS), primary effusion lymphoma (PEL), and multicentric Castleman’s disease (MCD)[1–3]. Like all herpesviruses, KSHV enters a latent phase shortly after primary infection. Under immune suppressive conditions, the latent virus reactivates to undergo lytic replication to produce new viruses. Productive lytic replication not only causes de novo infection but also plays an essential role in the development of KS and MCD[4, 5]. Previous studies demonstrate that the switch from latency to lytic replication is primarily controlled at the viral chromatin level through epigenetic mechanisms[6, 7]. Indeed, the majority of KSHV genome is silenced during latency through DNA methylation, repressive histone modifications, and other negative gene expression regulatory mechanisms[7–11]. When the latent virus reactivates, prompt epigenetic changes occur, leading to transactivation of the viral genome. However, our recent study discovered that KSHV reactivation stalls if the newly transcribed viral RNAs fail to undergo post-transcriptional N6-adenosine methylation (m6A)[12]. Our finding highlights a pivotal role of this epitranscriptomic mechanism in the control of KSHV lytic replication.
RNA N6-adenosine methylation (m6A) is one of the most abundant types of RNA modifications found in over 25% of RNA species in mammalian cells[13–15]. A complex of three methyltransferases: methyltransferase like 3 (METTL3), methyltransferase like 14 (METTL14), and Wilms tumor 1 associated protein (WTAP) acts as m6A writers and catalyze RNA m6A at specific sites with the consensus sequence (G/AGAC)[16–18]. Two demethylases, fat mass and obesity associated protein (FTO), and AlkB Homolog 5 (ALKBH5), act as m6A erasers and reverse this process[19–21]. Most m6A sites are located near the transcription start sites, exonic regions flanking splicing sites, stop codons, and the 3’untranslated region (3’UTR)[14, 22–24]. The biological functions of m6A are mediated by m6A readers. In the nucleus, for example, heterogeneous nuclear ribonucleoproteins hn-RNP-C and hn-RNP-A2/B1 selectively bind RNA at m6A sites to regulate pre-mRNA processing and alternative splicing[22, 24–27]. In addition, the YTH domain containing 1 protein (YTHDC1) binds pre-mRNA at m6A sites and preferentially recruits the serine/arginine-rich splicing factor 3 (SRSF3) over SRSF10 for exon inclusion splicing[28–31]. In the cytoplasm, three members of the YTH domain-containing family proteins, YTHDF1, YTHDF2, and YTHDF3, preferentially bind m6A-containing mRNAs to regulate RNA stability, protein translation, and RNA decay[32–35]. In addition, the eIF3, a component of 43S translation pre-initiation complex[36], directly binds m6A sites in the 5’untranslated region (5’UTR) of mRNAs to enhance protein translation[37]. Therefore, m6A represents a very important cellular mechanism for the control of gene expression at the post-transcriptional level. Interestingly, massive increases in m6A modification occur in the RNAs of human immunodeficiency virus-1 (HIV-1)[38, 39]. Blockade of m6A effectively abolishes HIV-1 protein expression and virion production, suggesting that this epitranscriptomic mechanism also controls viral gene expression.
Similar to HIV-1, most KSHV transcripts undergo m6A modification, and the level of m6A-modified mRNA of a given viral transcript increases in parallel with that of total mRNA when latently infected cells are induced by phorbol ester (TPA) or other lytic replication stimuli. Expressional knocking down of the m6A writer METTL3 substantially reduces TPA induction of KSHV lytic genes, and blockade of m6A reaction literally abolishes expression of all lytic genes examined and halts virion production. In contrast, expressional knocking down or activity inhibition of the m6A eraser FTO has the opposite effects.
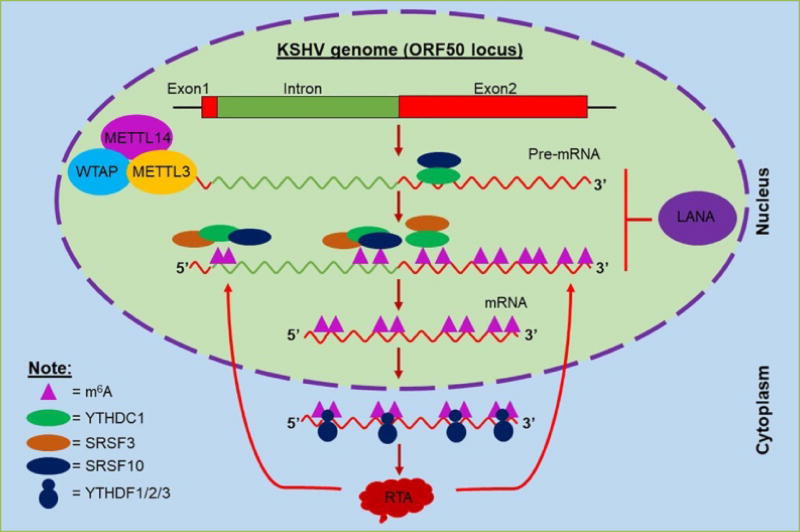
To understand how RNA methylation controls KSHV replication, we examined the effect of m6A on expression of viral regulator of transcription activation (RTA), which, encoded by open reading frame 50 (ORF50), is a key mediator of the switch from latency to lytic gene expression[40]. Due to differential splicing, the ORF50 (RTA) and ORFK8 loci produce at least three different groups of transcripts, including ORF50 /ORFK8/ORFK8.1 tricistronic mRNAs, ORFK8/ORFK8.1 bicistronic mRNAs, and monocistronic ORFK8.1 mRNAs[41]. RTA, which is expressed from the tricistronic mRNAs, consists of two exons and one intron (Fig. 1). Interestingly, blockade of m6A substantially reduces the level of TPA-induced RTA mRNA but has much less an effect on the level of RTA pre-mRNA, suggesting that m6A controls RTA pre-mRNA splicing. Indeed, multiple m6A sites are identified in RTA pre-mRNA. Data from genetic mutation assays demonstrate that the m6A sites in the intron near the two splicing sites are critical for RTA expression, and one m6A site in Exon2 near the splicing site also plays an important role in RTA pre-mRNA splicing. Data from RNA immuno-precipitation (RIP) assays confirm that these sites are indeed m6A modified. In addition, both SRSF3 and SRSF10 are present at the m6A sites in the intron near the two splicing sites, and the levels of m6A and these splicing factors increase significantly upon TPA treatment. Mutation of these m6A sites abolishes the RNA-protein interactions and RTA protein expression, thus suggesting that m6A modification of these sites is critical for recruitment of SRSF3 and SRSF10 and exclusion of the intron. In contrast, the m6A site in Exon2 near the splicing site is critical for removal of SRSF10 and Exon2 inclusion splicing. Therefore, our data highlight a pivotal role of m6A modification in RTA pre-mRNA splicing. Interestingly, when the m6A sites in both the intron and Exon2 are simultaneously mutated, the level of RTA pre-mRNA drops dramatically (un-published results), suggesting that m6A modification also contributes to stability of RTA pre-mRNA. In addition, similar to host mRNAs and HIV-1 transcripts, m6A modification may also promote RTA mRNA stability and protein translation through association with m6A readers YTHDF1, YTHDF2, and YTHDF3.
Figure 1. Post-transcriptional m6A modification controls KSHV RTA (ORF50) pre-mRNA splicing.
Multiple m6A sites are found in RTA pre-mRNA, which are methylated by m6A writers METTL3, METTL14, and WTAP. The m6A sites in the intron near the two splicing sites are critical for YTHDC1 binding and recruitment of splicing factors SRSF3 and SRSF10 while the m6A site in Exon2 near the splicing site is important for recruitment of SRSF3 and dissociation of SRSF10. Interactions between the m6A-modified RTA pre-mRNA and the different splicing factors ensure exclusion splicing of the intron to generate RTA mRNA. The other m6A sites may enhance RTA mRNA export, stability, and translation through interaction with m6A readers YTHDF1, YTHDF2, and YTHDF3. The expressed RTA protein enhances the host’s m6A machinery to increase the levels of m6A to promote its own pre-mRNA splicing and KSHV lytic gene expression. In contrast, KSHV latent protein LANA has the opposite effects on m6A and RTA pre-mRNA splicing.
Finally, we also found that expression of RTA protein increases the levels of m6A modification and promotes its own pre-mRNA splicing. RTA is known to enhance its own transcription[42]. Thus, our data for the first time demonstrate that RTA increases its own expression through both transcriptional and post-transcriptional mechanisms. Very interestingly, the KSHV latent protein LANA, which inhibits RTA expression to promote latency[43], suppresses TPA induction of RNA m6A modification and inhibits RTA pre-mRNA splicing (un-published results).
In summary, our results not only demonstrate an essential role of m6A in regulating RTA pre-mRNA splicing but also suggest that KSHV has evolved two opposite mechanisms to manipulate the host m6A machinery to its advantage in promoting lytic replication and latency respectively. This epitranscriptomic mechanism may be used by other herpesviruses as well. Our findings shall shed light on development of new strategies for the control of herpesviral infection.
Acknowledgments
This study was supported in part by grant R56DE023912 from the National Institute of Dental and Craniofacial Research of the National Institutes of Health and the CWRU/UH Center for AIDS Research through NIH grant P30 AI036219.
Abbreviations
- KSHV
Kaposi’s sarcoma-associated herpesvirus
- KS
Kaposi’s sarcoma
- PEL
primary effusion lymphoma
- MCD
multicentric Castleman’s disease
- m6A
N6-adenosine methylation
- METTL3
methyltransferase like 3
- METTL14
methyltransferase like 14
- WTAP
Wilms tumor 1 associated protein
- FTO
fat mass and obesity associated protein
- ALKBH5
AlkB Homolog 5
- 3’UTR
3’untranslated region
- 5’UTR
5’untranslated region
- YTHDC1
YTH domain containing 1 protein
- HIV-1
human immunodeficiency virus-1
- SRSF3
serine/arginine-rich splicing factor 3
- SRSF10
serine/arginine-rich splicing factor 10
- RIP
RNA immuno-precipitation
- TPA
12-O-Tetradecanoylphorbol-13-acetate
- RTA
regulator of transcription activation
- LANA
latency-associated nuclear antigen.
Footnotes
Conflicting interests
The authors have declared that no conflict of interests exist.
Author contributions
FY wrote the manuscript.
References
- 1.Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, et al. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 2.Cesarman E, Chang Y, Moore PS, Said JW, Knowles DM. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 3.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 4.Greene W, Kuhne K, Ye F, Chen J, Zhou F, Lei X, et al. Molecular biology of KSHV in relation to AIDS-associated oncogenesis. Cancer Treat Res. 2007;133:69–127. doi: 10.1007/978-0-387-46816-7_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcelin AG, Motol J, Guihot A, Caumes E, Viard JP, Dussaix E, et al. Relationship between the quantity of Kaposi sarcoma-associated herpesvirus (KSHV) in peripheral blood and effusion fluid samples and KSHV-associated disease. J Infect Dis. 2007;196:1163–1166. doi: 10.1086/521625. [DOI] [PubMed] [Google Scholar]
- 6.Ye F, Lei X, Gao SJ. Mechanisms of Kaposi's Sarcoma-Associated Herpesvirus Latency and Reactivation. Adv Virol. 2011;2011:e193860. doi: 10.1155/2011/193860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purushothaman P, Uppal T, Verma SC. Molecular biology of KSHV lytic reactivation. Viruses. 2015;7:116–153. doi: 10.3390/v7010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pantry SN, Medveczky PG. Epigenetic regulation of Kaposi's sarcoma-associated herpesvirus replication. Semin Cancer Biol. 2009;19:153–157. doi: 10.1016/j.semcancer.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu F, Stedman W, Yousef M, Renne R, Lieberman PM. Epigenetic regulation of Kaposi's sarcoma-associated herpesvirus latency by virus-encoded microRNAs that target Rta and the cellular Rbl2-DNMT pathway. J Virol. 2010;84:2697–2706. doi: 10.1128/JVI.01997-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunther T, Grundhoff A. The epigenetic landscape of latent Kaposi sarcoma-associated herpesvirus genomes. PLoS Pathog. 2010;6:e1000935. doi: 10.1371/journal.ppat.1000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toth Z, Maglinte DT, Lee SH, Lee HR, Wong LY, Brulois KF, et al. Epigenetic analysis of KSHV latent and lytic genomes. PLoS Pathog. 2010;6:e1001013. doi: 10.1371/journal.ppat.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye F, Chen ER, Nilsen TW. Kaposi's Sarcoma-Associated Herpesvirus Utilizes and Manipulates RNA N6-Adenosine Methylation To Promote Lytic Replication. J Virol. 2017;91:e00466–17. doi: 10.1128/JVI.00466-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Mason CE. The pivotal regulatory landscape of RNA modifications. Annu Rev Genomics Hum Genet. 2014;15:127–150. doi: 10.1146/annurev-genom-090413-025405. [DOI] [PubMed] [Google Scholar]
- 14.Yue Y, Liu J, He C. RNA N6-methyladenosine methylation in post-transcriptional gene expression regulation. Genes Dev. 2015;29:1343–1355. doi: 10.1101/gad.262766.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meyer KD, Jaffrey SR. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol. 2014;15:313–326. doi: 10.1038/nrm3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat Chem Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, et al. Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res. 2014;24:177–189. doi: 10.1038/cr.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534:575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 19.Feng C, Liu Y, Wang G, Deng Z, Zhang Q, Wu W, et al. Crystal structures of the human RNA demethylase Alkbh5 reveal basis for substrate recognition. J Biol Chem. 2014;289:11571–11583. doi: 10.1074/jbc.M113.546168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominissini D, Moshitch-Moshkovitz S, Schwartz S, Salmon-Divon M, Ungar L, Osenberg S, et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 2012;485:201–206. doi: 10.1038/nature11112. [DOI] [PubMed] [Google Scholar]
- 23.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linder B, Grozhik AV, Olarerin-George AO, Meydan C, Mason CE, Jaffrey SR. Single-nucleotide-resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods. 2015;12:767–72. doi: 10.1038/nmeth.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu B, Li L, Huang Y, Ma J, Min J. Readers, writers and erasers of N6-methylated adenosine modification. Curr Opin Struct Biol. 2017;47:67–76. doi: 10.1016/j.sbi.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 26.Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, et al. A majority of m6A residues are in the last exons, allowing the potential for 3' UTR regulation. Genes Dev. 2015;29:2037–2053. doi: 10.1101/gad.269415.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alarcon CR, Goodarzi H, Lee H, Liu X, Tavazoie S, Tavazoie SF. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roundtree IA, He C. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Trends Genet. 2016;32:320–321. doi: 10.1016/j.tig.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 29.Xiao W, Adhikari S, Dahal U, Chen YS, Hao YJ, Sun BF, et al. Nuclear m(6)A Reader YTHDC1 Regulates mRNA Splicing. Mol Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Xu C, Liu K, Ahmed H, Loppnau P, Schapira M, Min J. Structural Basis for the Discriminative Recognition of N6-Methyladenosine RNA by the Human YT521-B Homology Domain Family of Proteins. J Biol Chem. 2015;290:24902–24913. doi: 10.1074/jbc.M115.680389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu C, Wang X, Liu K, Roundtree IA, Tempel W, Li Y, et al. Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol. 2014;10:927–929. doi: 10.1038/nchembio.1654. [DOI] [PubMed] [Google Scholar]
- 32.Zhu T, Roundtree IA, Wang P, Wang X, Wang L, Sun C, et al. Crystal structure of the YTH domain of YTHDF2 reveals mechanism for recognition of N6-methyladenosine. Cell Res. 2014;24:1493–1496. doi: 10.1038/cr.2014.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Theler D, Dominguez C, Blatter M, Boudet J, Allain FH. Solution structure of the YTH domain in complex with N6-methyladenosine RNA: a reader of methylated RNA. Nucleic Acids Res. 2014;42:13911–13919. doi: 10.1093/nar/gku1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo S, Tong L. Molecular basis for the recognition of methylated adenines in RNA by the eukaryotic YTH domain. Proc Natl Acad Sci U S A. 2014;111:13834–13839. doi: 10.1073/pnas.1412742111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li F, Zhao D, Wu J, Shi Y. Structure of the YTH domain of human YTHDF2 in complex with an m(6)A mononucleotide reveals an aromatic cage for m(6)A recognition. Cell Res. 2014;24:1490–1492. doi: 10.1038/cr.2014.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y, Wang X, Li C, Hu S, Yu J, Song S. Transcriptome-wide N(6)-methyladenosine profiling of rice callus and leaf reveals the presence of tissue-specific competitors involved in selective mRNA modification. RNA Biol. 2014;11:1180–1188. doi: 10.4161/rna.36281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, et al. Reversible methylation of m6Am in the 5' cap controls mRNA stability. Nature. 2017;541:371–375. doi: 10.1038/nature21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lichinchi G, Gao S, Saletore Y, Gonzalez GM, Bansal V, Wang Y, et al. Dynamics of the human and viral m(6)A RNA methylomes during HIV-1 infection of T cells. Nat Microbiol. 2016;1:16011. doi: 10.1038/nmicrobiol.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tirumuru N, Zhao BS, Lu W, Lu Z, He C, Wu L. N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. Elife. 2016:5. doi: 10.7554/eLife.15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun R, Lin SF, Gradoville L, Yuan Y, Zhu F, Miller G. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci U S A. 1998;95:10866–10871. doi: 10.1073/pnas.95.18.10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng ZM. Split genes and their expression in Kaposi’s sarcomaassociated herpesvirus. Rev Med Virol. 2003;13:173–184. doi: 10.1002/rmv.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng H, Young A, Sun R. Auto-activation of the rta gene of human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus. J Gen Virol. 2000;81:3043–3048. doi: 10.1099/0022-1317-81-12-3043. [DOI] [PubMed] [Google Scholar]
- 43.Lan K, Kuppers DA, Verma SC, Robertson ES. Kaposi's sarcoma-associated herpesvirus-encoded latency-associated nuclear antigen inhibits lytic replication by targeting Rta: a potential mechanism for virus-mediated control of latency. J Virol. 2004;78:6585–6594. doi: 10.1128/JVI.78.12.6585-6594.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]