Abstract
Vagus nerve stimulation (VNS) is an established adjunctive therapy for pharmacologically refractory epilepsy and depression and is currently in active clinical research for other applications. In current clinical studies, VNS is delivered in an open-loop approach, where VNS parameters are defined during a manual titration phase. However, the physiological response to a given VNS configuration shows significant inter and intra-patient variability and may significantly evolve through time. VNS closed-loop approaches, allowing for the optimization of the therapy in an adaptive manner, may be necessary to improve efficacy while reducing side effects. This paper proposes a generic, closed-loop control VNS system that is able to optimize a number of VNS parameters in an adaptive fashion, in order to keep a control variable within a specified range. Although the proposed control method is completely generic, an example application using the cardiac beat to beat interval (RR) as control variable will be developed in this paper. The proposed controller is based on a state transition model (STM) that can be configured using a partially or a fully-connected architecture, different model orders and different state-transition algorithms. The controller is applied to the adaptive regulation of heart rate and evaluated on 6 sheep, for 13 different targets, using partially-connected STM with 10 states. Also, partially and fully-connected STM defined by 30 states were applied to 7 other sheep for the same 10 targets. Results illustrate the interest of the proposed fully-connected STM and the feasibility of integrating this control system into an implantable neuromodulator.
Introduction
Vagus nerve stimulation (VNS) is an effective therapy for partial or pharmacologically refractory epilepsy [1, 2] and depression [3] and a potential therapeutic approach, under active clinical research in a number of other clinical applications, such as supraventricular arrhythmias and heart failure (HF) [4–6]. In heart failure therapy, two ways for delivering VNS exist. In one approach, VNS is delivered asynchronously, without any cardiac synchronization [7, 8]. In a second approach, VNS is timed to the cardiac cycle (synchronous VNS) [9, 10]. Although both approaches have already been clinically evaluated, they have failed to demonstrate clinical efficacy in HF patients [7–9]. This lack of clear clinical efficacy may be due to the fact that VNS parameter values are currently defined through a non-standardized manual titration phase. The interest of an automatic, closed-loop method for VNS parameter configuration is clear in this context.
Closed-loop VNS methods have been proposed for the asynchronous case, and have been experimentally evaluated [11–15]. However, these methods show a number of limitations. Firstly, as mentioned above, none of these works handle synchronous VNS. In fact, the definition of a closed-loop control for cardiac-synchronized VNS requires an adaptive discrete-time controller, able to deal with irregular sampling rates, since the cardiac period is not constant. Novel controllers have to be developed in this sense. Secondly, these controllers are restricted to the modulation of a single VNS parameter (amplitude or frequency of the delivered current), even though recent experimental studies have shown that a variety of parameters should be jointly modulated in order to correctly optimize VNS [16, 17]. Finally, the complexity of some of the previously proposed methods makes difficult a real-time integration into an implantable device.
To our knowledge, only our group has worked in overcoming these obstacles, by developing closed-loop synchronous VNS methods, evaluated in experimental conditions [18–20]. In a first approach [20], we presented an “on-off” controller, that provided a simple and robust control, but led to strong heart rate oscillations. In order to reduce these oscillations, we implemented and evaluated a proportional-integral (PI) controller [18, 19]. Control performance was significantly improved, but the obtained performance was highly dependent on the PI control parameters and optimal, subject-specific values for these parameters were difficult to define. A model-based design approach was also proposed by our group, to find the best PI parameter ranges and simplify subject-specific parameter definition [18]. However, as in other previous works, the obtained PI controller was limited to the modulation of only one VNS parameter (amplitude of the delivered current).
In this paper, we propose and characterize a novel VNS controller, based on a state transition model (STM), which is able to modulate multiple VNS parameters as a function of the patient state and has a level of complexity suitable to be embedded into an implantable medical device. In order to characterize the proposed controller, experimental evaluations on sheep are applied using different controller configurations. In particular, we evaluate the influence on control performance of the STM architecture (partially and fully-connected), the model order and the state-transition method.
The paper is organized as follows: Section 1 presents a detailed description of the proposed closed-loop control system and the evaluation methodology, which includes an experimental protocol. In section 2, results of the proposed STM-based controller are presented and the performance associated with the different configurations are compared. Discussion on the main findings and limitations of the study are given in Section 3.
1 Methods
Data for this study were obtained from healthy sheep following a protocol approved by the French local ethics committee for animal experimentation (“Comité d’éthique en matière d’expérimentation animale”, Paris Descartes, Paris, France).
1.1 General description of the proposed closed-loop control system
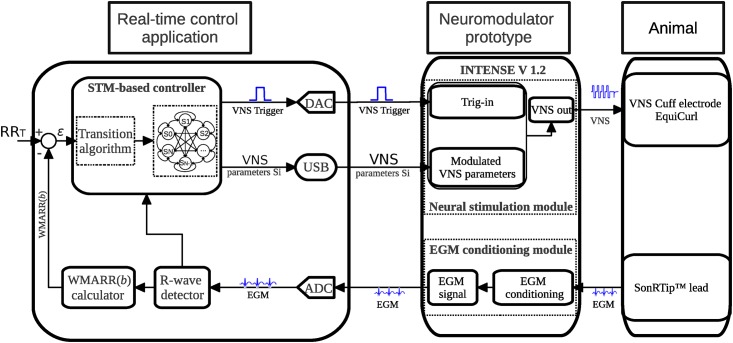
The objective of the proposed control system is to adaptively modulate the VNS parameters of a neuromodulator in order to maintain a physiological control variable, observed in real time from a patient, within a given target range. In this paper, the cardiac beat to beat (RR) interval will be used as control variable for evaluation purposes, since current studies on open loop VNS therapies use the heart rate as the main reference for titration [7–9]. Fig 1 shows the proposed closed-loop control system, combining i) a custom-made real-time control application, ii) a neuromodulator prototype (INTENSE v1.2) and iii) a sheep, for animal experimentation.
Fig 1. Proposed closed-loop control system, based on a fully-connected state transition model (STM).
The system consists of three components: a real-time control application, a neuromodulator prototype and a sheep used for animal experimentation. An intracardiac electrogram (EGM) is obtained via a cardiac lead implanted into the right ventricle of the sheep, analogically processed by the neuromodulator, and acquired via the analog to digital converter into the real-time control application. R-wave instants are detected and used for calculating a Weighted Moving Average of the RR intervals WMARR(b), which will be used as control variable. An error ϵ is obtained from the difference between the target (RRT) and WMARR(b). The STM-based controller estimates a new set of VNS parameters Si, minimizing ϵ. VNS is triggered synchronously to the R-wave, with the new set of parameters Si, to deliver VNS to the right vagus nerve of the sheep. VNS with these new parameters will modify the acquired EGM and a weighted moving average of the new RR interval is computed, closing the loop.
The control application is composed of an analog to digital converter (ADC), a digital to analog converter (DAC), a cardiac R-wave detector module, a control variable calculator (WMARR(b) calculator) and an STM-based controller. An intracardiac signal observed from the animal by the SonRTip™ lead (Sorin Group Italia, Saluggia, Italy), analogically preprocessed by the EGM conditioning module of the neuromodulator prototype, is acquired via the ADC and processed by an R-wave detector, which provides R-wave time instants for each detected beat b. The control variable calculator module computes a Weighted Moving Average of the RR interval, WMARR(b). Based on WMARR(b) and a target RR (RRT) given by the user, an error ϵ is calculated and presented as input to the proposed STM controller. This controller seeks to find the set of VNS stimulation parameters Si minimizing ϵ. The new VNS parameters Si are programmed into the neuromodulator via USB communication. After parameter modulation, the controller determines the instant of VNS activation (Pdel), using the currently detected R-wave instant as a reference. The VNS activation instant is transmitted, via a squared pulse analog signal (VNS Trigger), to the Trig-in input of the neuromodulator by means of the DAC. The new WMARR(b) is computed. More details on the WMARR(b) computation and the STM-based controller are given below.
The neuromodulator (Prototype INTENSE v1.2, Sorin CRM, Clamart, France) is composed of an EGM conditioning module and a neural stimulation module with a dynamic programmable interface, allowing for the generation of complex temporal stimulation waveforms [21]. Details on animal experimentation and how each sheep is instrumented are given in section 1.5.
1.2 VNS parameters
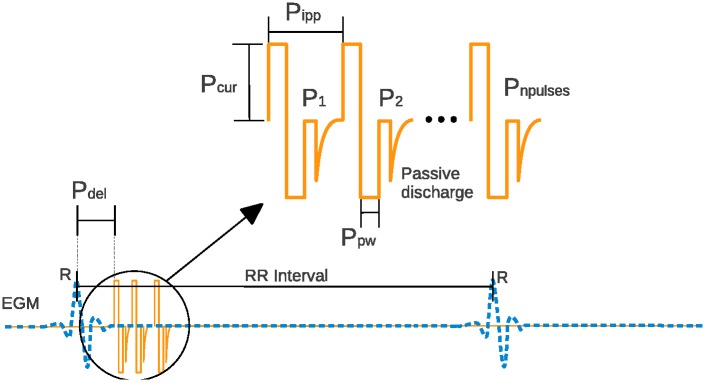
A cardiac-synchronized vagus nerve stimulation burst is composed of biphasic pulses, delivered after a programmable preset delay from the last detected R-wave, and characterized by a set of VNS parameters (Fig 2). The VNS parameters modulated by the proposed STM-based control algorithm are: the number of pulses in the train of stimulation (Pnpulses), the interpulse period (Pipp, ms), the delay between cardiac event and VNS onset (Pdel, ms), the current amplitude (Pcur, mA), and the pulse width (Ppw, ms).
Fig 2. Typical vagus nerve stimulation pattern (solid line), synchronized with the cardiac activity (dashed line).
The controller can modulate the following VNS parameters: number of pulses (Pnpulses), the interpulse period (Pipp, ms), delay (Pdel, ms), current amplitude (Pcur, mA), and pulse width (Ppw, ms).
The parameter ranges, predefined according to our previous experience on sensitivity analysis of the chronotropic effect with respect to the above-mentioned VNS parameters [16, 17], are: Pnpulses ∈ [1, 4], Pipp ∈ [23.4, 46.9] ms, Pdel ∈ [16, 156] ms, Pcur ∈ [0.2, 1.0] mA, and Ppw ∈ [0.05, 0.20] ms. These parameter ranges will be used throughout this manuscript for VNS control.
1.3 Computation of the control variable
The instantaneous RR interval (RR(b)) is constantly modulated by the autonomic nervous system and other physiological factors. Indeed, arrhythmic events may occur, inducing a wrong response of the controller. To reduce consequences of these arrhythmic events on the controller, we propose to use WMARR(b) as control variable instead of the instantaneous RR(b). WMARR(b) is a weighted moving average, computed online over the last four RR intervals, according to Eq (1).
| (1) |
The weights β0 = 0.98 > β1 = 0.40 > β2 = 0.10 > β3 = 0.01 were heuristically chosen as a good compromise between smoothness and reactivity.
1.4 State transition model-based controller
An STM consists of a set of states and behaviors [22]. The STM-based controller proposed in this paper is composed of a state-transition model associated with a state transition algorithm (STA). Only a single state can be active at a given time (current state). A transition from one state to another is performed by a triggering event or condition (transition algorithm) in order to perform different actions. A more detailed description of state transition models can be found elsewhere [23].
Two different STM-based controllers are evaluated in this study. The first one is based on a partially-connected STM, with its corresponding partially-connected STA. The second controller is based on a fully-connected STM with its corresponding fully-connected STA. The partially-connected STA allows for transitions only between neighbor states of the partially-connected STM, whereas the fully-connected STA allows for transitions between all the states of the STM.
1.4.1 State transition model
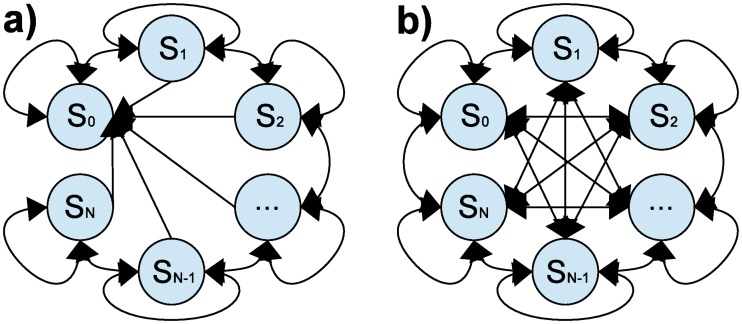
Fig 3 shows the partially- (Fig 3(a)) and the fully- (Fig 3(b)) connected STMs on which the proposed controllers are based. In both STM-based controllers, the STM is composed of N + 1 states (S0,…,SN) and each state is associated with a set of VNS parameters:
| (2) |
Fig 3.
a) Partially-connected state transition model of N + 1 states. The current state (CS(b) = Si) may transit to a neighbor state (Si-1 or Si+1) or stay at the same state (Si). All states are connected to state S0, which turns off VNS. b) Fully-connected state-transition model of N + 1 states. The current state (CS(b) = Si) may transit to any state (Si-k or Si+k) or stay at the same state (Si). All states are connected with all the states including the state S0, which turns off VNS.
The states are ranked as a function of the effect they produce on the control variable when applying the corresponding VNS parameters during a training phase (see section 1.4.3). In this sense, the effect of state i − 1 (when applying VNS parameters Si−1) on the control variable is lower than the effect of state i (when applying VNS parameters Si), for i = 0, …, N. Both STMs include a non-stimulation state (S0) allowing the controller to turn off (no-stimulation) the VNS at any moment.
The difference between the partially- and fully-connected STMs consists in the possible transitions allowed between states. In fully-connected STMs, the current state (CS(b) = Si) is allowed to transition to any state:
| (3) |
where k is obtained as a function of the error between the target RR and the observed WMARR(b). In this paper, k is defined as the integer division of the error by the constant δ defined by the user:
| (4) |
| (5) |
Partially-connected STMs correspond to the particular case where k = 1, i.e., the states are only allowed to transition to a neighbor state.
1.4.2 State transition algorithm
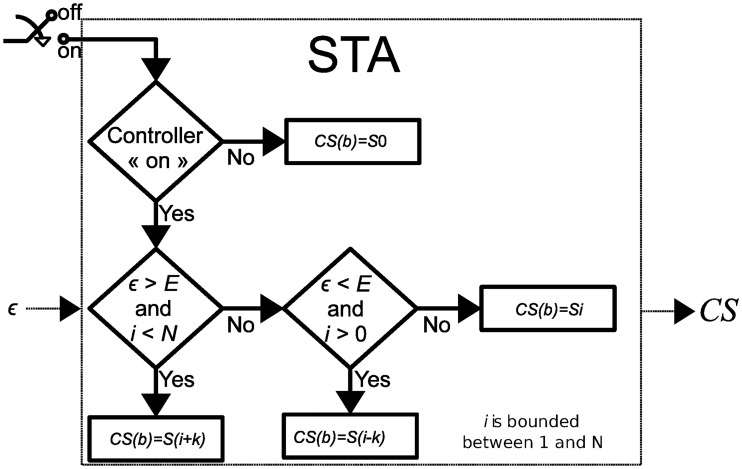
At each event b, the STA determines the state transition that minimizes the error between RRT and the control variable WMARR(b). Fig 4 summarizes the algorithm that governs the STA behavior. When the controller is operating (ON), the next state is determined as a function of user-defined thresholds E (see Fig 4) that specifies the minimal amount of acceptable error ϵ between the target RR and the control variable.
Fig 4. State-transition algorithm.
The STA determines the optimal VNS parameters Si minimizing the value ϵ, which represents the difference between the target RR and WMARR(b). Variable i is bounded between 0 and N. Parameter E defines the acceptable level of error on ϵ.
The difference between the fully- and partially-connected STAs is mainly related to the definition of parameter k, which is essential to improve the time of convergence. In fact, the partially-connected STM-based controller will take Nevents to reach the state SN whereas the fully-connected will take around (N/k)<N events to reach SN. Increasing the number of states N should lead to an improvement of the accuracy because differences between the responses associated with consecutive states are reduced.
1.4.3 Training phase
During the training phase, the STM is firstly initialized with a set of population-based VNS parameters, which were obtained from our previous studies on stimulation parameters [16, 17]. The subject-specific training phase consists of:
stimulating the vagus nerve of the subject intra-operatively, by using each set of VNS parameters of the initialized STM;
- measuring the corresponding effect (ΔRRi), evoked by VNS parameters on each state Si:
where is the mean RR interval during VNS with parameters Si, and is the mean RR interval, measured during a period of non-stimulation;(6) sorting states of the STM in ascending order, according to ΔRRi, in the range [ΔRRmin, ΔRRmax], where ΔRRmax is the effect provoked by the state SN and ΔRRmin is the effect provoked by the state S1.
1.5 Experimental evaluation
1.5.1 Experimental protocol for sheep preparation
The experimental study was conducted under the approval issued by the French ethics committee for animal experimentation. Sheep were initially anesthetized (induction) by propofol (4mg kg-1 min-1). Surgery for material implantation was performed under inhalation of isoflurane (1.5%), and control evaluation tests were performed under etomidate (100μg kg-1 min-1). A pressure and electrical probe sensor (Millar Instruments Inc., Houston, USA) was implanted into the left ventricle for pressure monitoring. A bipolar pacemaker lead containing an intracardiac accelerometer (SonRTip™ lead) was placed into the right ventricle, in order to acquire intracardiac electrograms (EGM). A quasitripolar cuff-type VNS electrode (Cuff electrode EquiCurl, Sorin Group Italia, Saluggia, Italy) was implanted on the right vagus nerve, at a cervical site. The carotid artery dissection is realized at an equal distance between the head and the trunk. The vagus nerve (VN) is gently removed from the carotid sheath and the cuff is placed around the VN. These leads were connected to the prototype neuromodulator, as shown in Fig 1.
After a verification stage of the implanted instrumentation (in particular VNS electrode impedance and EGM quality), the experimental evaluation of the proposed control system was performed. The surface ECG, the EGM, the left intra-ventricular pressure, and the body temperature were monitored during the whole procedure. Breathing was artificially controlled at 0.3 Hz (18 breaths min-1)using a Datex-Ohmeda Aestiva/5 anesthesia ventilator (GE Healthcare, Madison, Wis). This controlled ventilation also allowed us to maintain a stable respiratory sinus arrhythmia, which can be considered in this context as noise for the closed-loop control system.
1.5.2 Control evaluation tests
The proposed experimental evaluation tests aim to quantify the performance of the partially-connected and fully-connected STM-based controllers, and to evaluate the effect obtained by i) increasing the number of states in the partially-connected STM and ii) introducing the fully-connected state-transition algorithm. Three different controllers were implemented and compared:
P10: partially-connected STM with N = 10 (11 states),
P30: partially-connected STM with N = 30 (31 states) and
F30: fully-connected STM with N = 30 (31 states).
Each controller is tested in a classical control test, consisting in turning on the controller during 1 min, after 1 min of resting period, without stimulation. Performance indicators described in section 1.5.3 are computed for each control test.
In order to measure the effect of increasing the number of states in partially-connected STM, P10 and P30 controllers were compared. P10 was used to regulate heart rate on 6 sheep for 13 different targets and P30 was applied to 7 sheep, for 10 different targets. For 3 sheep of the 7 included in the present work, both the P10 and P30 controllers could be tested.
To show the improvements achieved by implementing the fully-connected STA, a P30 controller and an F30 controller were used to regulate heart rate on the same 7 sheep for the same 10 targets. The N = 30 controllers P30 and F30 were exactly the same for a given sheep. The fact of using the same number of states in both controllers to reach the same targets on a given sheep, allowed us to analyze improvements achieved by this new fully-connected STA.
1.5.3 Performance indicators
In order to measure the performance of the controller, the following indicators are computed: i) the mean squared error (MSE) during steady-state, ii) the percent overshoot (%OS), expressed as a percentage of the steady-state value, and iii) the number of beats required for the waveform to go from 10% of the final value to 90% of the final value, namely Br.
Br is equivalent to the classical performance indicator “time rise”, but is given in number of beats instead of time (in seconds). In this way, comparisons (in terms of speed of convergence) between controllers are not dependent of the instantaneous RR interval. Remember, that the proposed STM-based controller uses beat detections instants to activate a transition (synchronous VNS approach).
MSE is calculated from the beat in which the system arrived to the steady state (bs), and over a temporal support of M beats, as follows:
| (7) |
In this paper, M was set equal to 1 min in order to evaluate accuracy on a sufficiently long temporal support. In this sense, the proposed MSE is an quantitative indicator of the stability of the response, being higher for responses showing higher oscillations with respect to the expected target. Moreover, the “key performance indicator” KPI proposed in Romero et al. [18], which is a linear combination of the 3 above-mentioned indicators, is computed as follows:
| (8) |
where , , and are the mean values of the performance indicators evaluated on all sheep. Note that KPI provides a global evaluation criterion giving the same importance to the accuracy (MSE, %OS) and speed of convergence Br.
For analysis purposes, unpaired (P10 vs P30 and P10 vs F30) and paired (P30 vs F30) Wilcoxon tests were performed to measure statistical differences between the three controllers on the four performance measures. p-values between the three controllers are computed on the 4 proposed performance measures.
2 Results
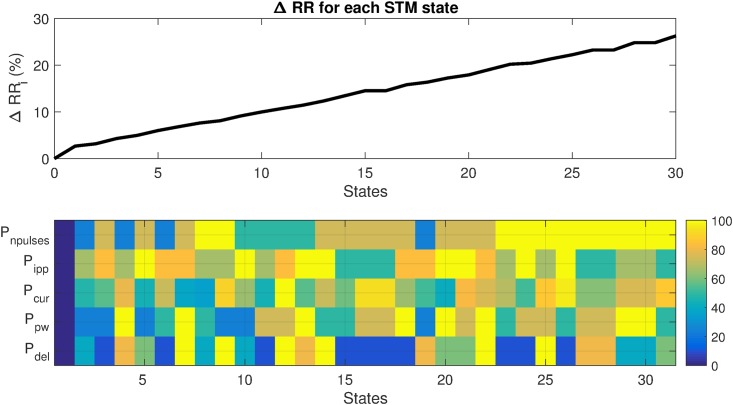
Fig 5 shows a representative example output of the training phase for one particular subject. The upper panel of Fig 5 shows the observed ΔRRi as a function of the STM states, once these states are sorted with respect to their response. The lower panel of Fig 5 displays a graphical representation of the normalized VNS parameters corresponding to each state. By definition, all parameters are set to 0 for S0 (VNS is off) leading to a ΔRRi = 0. Note that different combinations of the 5 VNS parameters are found for STM states. A similar approach has been used to define states in the case of N = 10. The output of the training phase was different for every sheep, both in the response of ΔRRi and in the distribution of VNS parameters. This is in accordance to our findings published in [17], showing the usefulness of the definition of subject specific VNS parameters.
Fig 5. Example output of the training phase for a particular sheep and N = 30.
Top panel: ΔRRi as a function of sorted states. Bottom panel: Colormap of the normalized VNS parameters associated with each state, expressed as percentage of their corresponding range values.
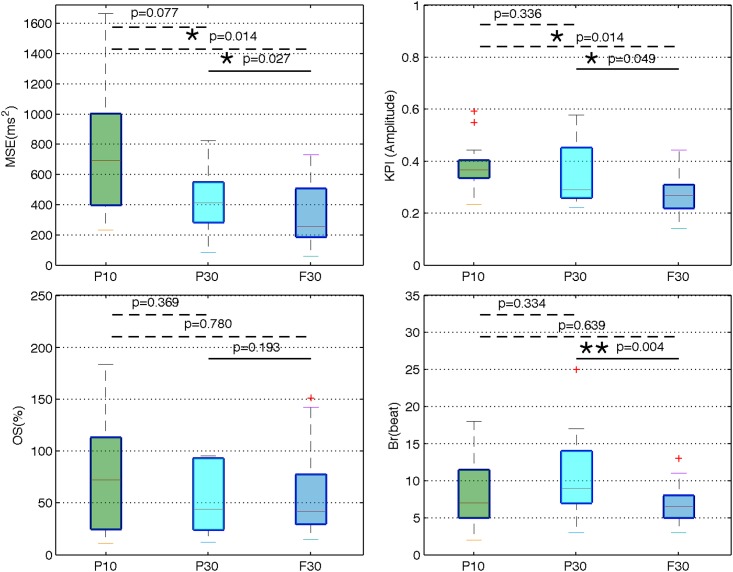
After the application of the training phase, experimental evaluation of different configurations of the proposed STM controller have been performed and the performance indicators have been calculated in each case. Fig 6 shows box plots of these performance indicators, obtained for all sheep for the 3 controller configurations. This figure shows i) the effect of the number of states in the partially-connected STM, in terms of control performance and ii) the difference, in performance, between the fully- and partially-connected STAs. The dashed line represents a Wilcoxon unpaired test, performed between the two controllers that are linked by it, whereas the solid line represents a Wilcoxon paired test. p-values less than 0.05 are marked by *. p-values less than 0.01 are marked by **. In terms of accuracy, the median MSE obtained from the P10 controller is 693 ms2, for the P30 controller is about 410 ms2, and for the F30 controller is about 250 ms2. Concerning the %OS, the P10 controller obtains a median of 72%, the P30 controller obtains 44% and the F30 controller obtains about 42%. In terms of speed of convergence, the P10, P30 and F30 controllers obtain respectively median Br values of 7, 9 and 6 beats. Finally, in terms of KPI, which is an indicator giving the same importance to the accuracy and the speed of convergence, the P10, P30 and F30 controllers obtain respectively median values of 0.36, 0.29, and 0.26.
Fig 6. Performance indicators of three STM-based controllers.
P10 is a partially-connected STM-based controller of N = 10 states, evaluated on 6 sheep for 13 different targets. P30 is a partially- connected STM-based controller of N = 30 states, evaluated on 7 sheep for 10 different targets. F30 is a fully-connected STM-based controller of N = 30 states evaluated on the same 7 sheep for the same 10 targets. The three controllers were used on sheep anesthetized by etomidate. Most of the sheep used in the experimental protocol P10 are different from the sheep used in the control tests of the P30 and F30 controllers. The sheep involved in the P30 and F30 protocols were the same. p-values are calculated between the three controllers. In the box plots, the dotted lines represent a Wilcoxon unpaired test and the solid line represents a Wilcoxon paired test. * denotes P < 0.05, and ** denotes P < 0.01.
Table 1 summarizes results on the comparison of the P10 and P30 controllers evaluated on 3 common sheep for the same targets. The mean MSE of the P10 controller is 665 ms2 whereas the P30 controller obtains a mean MSE of 227 ms2. The mean %OS of the P10 controller is 95% whereas the P30 controller obtains 82%. In terms of Br the P10 controller obtains 8 beats whereas the P30 controller obtains 12. Finally, in terms of KPI, the mean value obtained by the P10 controller is 0.49 and the mean value obtained by the P30 controller is 0.43.
Table 1. P10 vs P30.
| Sheep | Target(ms) | Controller | MSE(ms2) | %OS(%) | Br(beats) | KPI |
|---|---|---|---|---|---|---|
| 1 | 460 | P10 | 856 | 71.9 | 7 | 0.49 |
| P30 | 395 | 42.8 | 8 | 0.34 | ||
| 600 | P10 | 756 | 20.8 | 6 | 0.32 | |
| P30 | 280 | 12.2 | 10 | 0.31 | ||
| 2 | 430 | P10 | 815 | 183.4 | 11 | 0.71 |
| P30 | 180 | 197.9 | 23 | 0.80 | ||
| 3 | 430 | P10 | 274 | 104.3 | 8 | 0.37 |
| P30 | 51 | 75.3 | 7 | 0.26 |
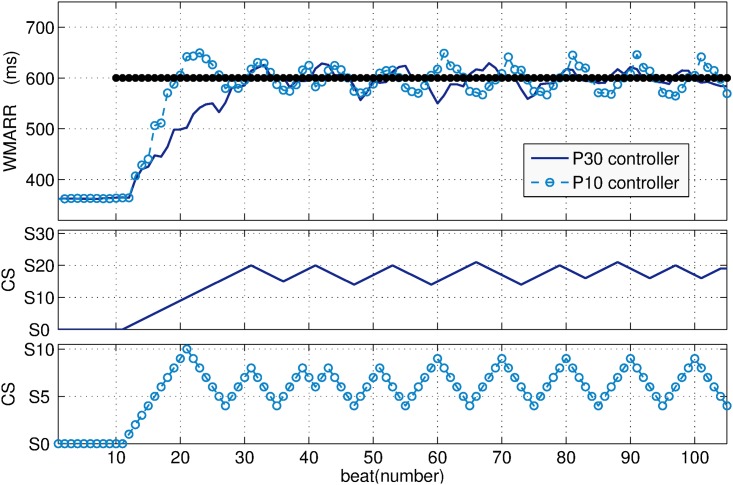
Fig 7 displays an example of the comparisons between the P10 and P30 controllers (Table 1, Sheep 1). The top panel of this figure shows the control variable WMARR(b) when the P10 (dotted line) and the P30 (solid line) controllers are used to regulate cardiac cycle length at a target RR set to 600 ms. With both controllers the target was reached. As expected, the P10 controller leads to a faster response (reduced Br), however, %OS and MSE are increased.
Fig 7.
The top of the figure shows the WMARR response when P10 (dotted line) and P30 (solid line) controllers are used to regulate RR to 600 ms. The middle shows the dynamics of the P30. The bottom shows the dynamics of the P10 controller.
The middle and bottom panels of Fig 7, respectively, show the state transitions of the P10 and P30 controllers, to reach such target. The P30 controller oscillates among states S14 and S21 (see Fig 7 (middle)), whereas the P10 controller oscillates among states S4 and S9 (see Fig 7 (bottom)). This implies that, the P30 controller requires 14 transitions to reach the minimum state leading the steady state, whereas the P10 controller only requires 4 transitions.
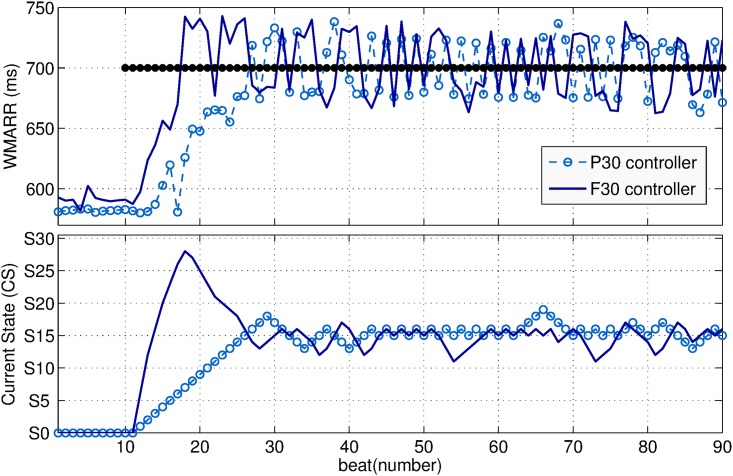
Fig 8 shows an example of the comparison between the P30 (dotted line) and F30 (solid line) controllers, for heart rate regulation on the same sheep for the target RR set to 700 ms. The top panel of this figure shows the response of the control variable WMARR. The bottom panel shows the state transitions of both controllers to reach such target. Since in both controllers the STM are composed of exactly the same N = 30 states, the current state CS tends to state S15 which may be the optimal state in this case. The F30 controller reached the target faster than the P30 controller. This was expected, since the fully-connected STA allows direct transitions of several states of the STM, differently to the partially-connected STA which only allows transitions between neighbor states.
Fig 8.
The top panel of the figure shows the WMARR response when the P30 (dotted line) and F30 (solid line) controllers are used to regulate RR to 700 ms. The bottom panel shows the dynamics of the P30 (dotted line) and F30 (solid line) controllers.
3 Discussion
Recent clinical studies on VNS for heart failure treatment have failed to show efficacy of the therapy [7–9]. One of the reasons of this failure may be that, in these studies, VNS is delivered in an open loop approach, with manually-configured stimulation parameters. We hypothesize that efficacy of VNS on heart failure, but also in other target applications may be improved by integrating a closed-loop VNS control system, allowing for an adaptive, subject-specific definition of VNS parameters. To our knowledge, the STM based controller presented in this paper is the first closed-loop VNS control systems delivering VNS synchronously to the heartbeat and modifying more than one parameter.
Comparing with control algorithms we have proposed in our previous works (on-off [20], PI [18, 19]), the STM-based controller has, by construction, several advantages: i) no preliminary tuning of control parameters is required, ii) the STM-based controller is suitable for the regulation of a discrete system with non uniform sampling rates and iii) this original approach allowed us to modulate multiple VNS parameters. Moreover, the proposed STM and the corresponding transition algorithm are suitable for the implementation in implantable devices. Indeed, the proposed controller requires limited resources in terms of computing power. Only the rules shown in Fig 4, and one integer addition have to be executed for each loop. Concerning memory usage, each state can be coded with one byte, since less than 256 VNS parameter combinations are used. Finally, the heavier calculations needed during the training phase can be performed using an external computer. These parameters will be fixed on the state table into the device afterwards. This is a straightforward implementation in comparison to a PI controller, which requires a number of floating point operations: additions for the estimation of the integral of the error, two multiplications and at least one last addition to estimate the PI output for each loop. Note also that these operations on the PI only modulate one VNS parameter.
Concerning the characterization of the proposed controller, configurations with different number of states (P10 vs P30) and transition algorithms (P30 vs F30) were experimentally evaluated. This experimental evaluation was necessary in this case, since no analytic mathematical characterization can be performed on the proposed controller, due to its non-linear and discontinuous nature and the irregularly sampling rate associated with the cardiac-synchronized function.
Figs 7 and 8 illustrate that it is not possible to obtain a perfectly stable, non-oscillating condition. This is expected in the case of living systems, which are in constant adaptation of their physiological parameters in order to maintain homeostasis through a set of physiological closed-loop systems. The objective is thus to minimize these oscillations and increasing accuracy, while keeping a short settling time. While comparing the P10 and P30 controllers (Figs 6 and 7 and Table 1), we observed that the MSE is reduced when the number of states is increased (P30), at the price of increasing Br, thus reducing KPI. In fact, according to the STM-based controller definition, all states were sorted in ascending order in the training phase (Fig 5). When the number of states of the partially-connected STM is increased, the resolution in the VNS parameter space of the controller is increased, inducing an improvement of accuracy. However, these partially-connected STM will take at least N beats to reach the state SN. If N is increased, the time of convergence is increased too. Increasing the number of states also leads to an improvement of stability (reduced MSE) because RR oscillations decrease with the P30 controller.
The influence of the state-transition algorithm was also evaluated by analyzing the performances obtained with partially- and fully-connected STMs using the same number of states (N = 30), on the same sheep for the same targets. By comparing the P30 and F30 controllers (Figs 6 and 8), we observed a significant reduction on Br with the fully-connected STA, while improving the accuracy. In fact, considering a partially- and fully-connected STMs with the same N states and Sn the state leading to the minimal error, the partially-connected STM-based controller will take N events to reach the state Sn whereas the fully-connected will take around (n/k)<n events to reach Sn.
Results displayed in Fig 6 summarize the performance associated with each controller. Increasing the number of states in a partially-connected STM-based controller (P10 vs P30) is associated with an improvement of accuracy, even though the difference is not statistically significant (p > 0.05). The comparison between P10 vs. F30 shows a significant reduction of MSE (p < 0.05). Moreover, when the number of states in the partially-connected STM-based controller is increased, from N = 10 to N = 30 states (P10 vs. P30), Br is adversely increased. Nevertheless, the implementation of the fully-connected STA showed the same speed of convergence than the P10 controller. The analysis of the performance indicator KPI, on all the controllers, confirms that the proposed F30 controller leads to the best compromise between accuracy and speed of convergence.
In addition to the above-mentioned improvements in stability and accuracy, the new fully-connected STM-based controller proposed in this paper also improves speed of convergence with respect to a partially-connected STM. As discussed above, stability and accuracy depend on N, while the speed of convergence depends on k. These parameters may be chosen during the training phase, according to the required performance and the computation capabilities of the target device. However, a limitation of increasing the number of states is that more memory (in the implantable device) will be required. The complexity of the state transition algorithm remains the same.
Limitations of the study are mainly related to the constraints associated with animal experimentation, since we consider that the analysis of the compromise between the number of states and the accuracy of the controller may be improved by testing the controller for different values of N. However, this implies more experimental time, which was limited for this study.
Another limitation of the study concerns the choice of the control variable. In this study we have chosen the RR interval as control variable, since it is the variable used for setting the VNS parameters in the current clinical studies where the VNS is delivered in open loop. However, the proposed controller may be used to regulate other physiological variables representing, for instance, the autonomic nervous system state (heart rate variability markers or sympatho-vagal balance status).
Finally, it should be noted that the proposed STM-based controller integrated a training phase in order to set the VNS parameters corresponding to a given state (Si) and to sort the states in ascending order. Although this training phase is necessary to design the controller specifically to each subject, the state transition model should evolve in order to adapt the therapy both to rapidly changing variables (seconds, minutes, etc..) and slowly changing variables (days, months, years). As a consequence, a strategy should be defined to take into account the temporal multi-resolution application of the proposed closed-loop control approach. In future studies, we plan to further evaluate the robustness of the proposed VNS controllers by activating it for longer periods of time, on non-anesthetized animals, integrating changes in the autonomic state. This will require a fully functional, implantable and chronic version of our system.
4 Conclusion
In this work we proposed, implemented and experimentally characterized a new closed-loop VNS control system, based on a state transition model, which allows for the real-time regulation of HR in sheep. By construction, the proposed STM is able to: 1) modulate multiple VNS parameters, 2) regulate a discrete system with non uniform sampling rates, 3) provide an easier subject-specific configuration and 4) be implemented into an implantable device. Results illustrate the interest of using a fully-connected STM and increasing the number of states. The proposed controller (fully-connected STM with N = 30) ensures a significant improvement of accuracy and speed of convergence with respect to other STM configurations. In a future work, we will experimentally compare the performance of the proposed controller with respect to a classical PI controller.
Supporting information
(PDF)
We confirm that there is no identifying information in these files. Datasets containing the WMARR response acquired for each sheep. The data contains: the target, the name of the controller (P10, P30 or F30), RR interval and the current state (CS). All performance indicators are also provided.
(ZIP)
Acknowledgments
The authors would like to thank Martine Rancic, Julie Piquet and Adrien Lalot for their contributions on animal experimentation.
The authors would also like to acknowledge David Andreu and David Guiraud from Inria and University of Montpellier as well as AXONIC SAS, MXM for their contribution to the prototype design, and the latter for providing the C4D3-1 cuffs.
This work was partially supported by BPIFRANCE within the framework of the French “Investments for the future” program—INTENSE project.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by BPIFRANCE within the framework of the French "Investments for the future" program (http://investissementsdavenir.bpifrance.fr) - INTENSE project. SORIN CRM SAS did not support financially the project. JLB and CH are employed by SORIN CRM SAS. No other authors received a salary or gratification from SORIN CRM SAS for their contribution to this work. The specific roles of the authors are articulated in the "author contributions" section.
References
- 1. Healy S, Lang J, Naude JTW, Gibbon F, Leach P. Vagal nerve stimulation in children under 12 years old with medically intractable epilepsy. Child’s Nervous System. 2013;29(11):2095–2099. doi: 10.1007/s00381-013-2143-3 [DOI] [PubMed] [Google Scholar]
- 2. Rychlickia F, Zamponib N, Trignania R, Ricciutia RA, Iacoangelia M, Scerratia M. Vagus nerve stimulation: Clinical experience in drug-resistant pediatric epileptic patients. Seizure. 2006;15(7):483–490. doi: 10.1016/j.seizure.2006.06.001 [DOI] [PubMed] [Google Scholar]
- 3. Akhtar H, Bukhari F, Nazir M, Anwar MN, Shahzad A. Therapeutic Efficacy of Neurostimulation for Depression: Techniques, Current Modalities, and Future Challenges. Neuroscience Bulletin. 2016;32(1):115–126. doi: 10.1007/s12264-015-0009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bilgutay A, Bilgutay IM, Merkel FK, Lillehei CW. Vagal tuning, A new concept in the treatment of supra ventricular arrhytmias, angina pectoris, and heart failure. Journal of thoracic and cardiovascular surgery. 1968;56(1):71–82. [PubMed] [Google Scholar]
- 5.Ferrari GMD, Sanzo A, Schwartz PJ. Chronic vagal stimulation in patients with congestive heart failure. In: in Engineering in Medicine and Biology Society (EMBC), 2009 Annual International Conference of the IEEE; 2009. p. 2037–2039. [DOI] [PubMed]
- 6. Kobayashi M, Massiello A, Karimov JH, Wagoner DRV, Fukamachi K. Cardiac Autonomic Nerve Stimulation in the Treatment of Heart Failure. The Annals of Thoracic Surgery. 2013;99(1):339–345. doi: 10.1016/j.athoracsur.2012.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Premchand R, Sharma K, Mittal S, Monteiro R, Dixit S, Libbus I, et al. Extended Follow-Up of Patients with Heart Failure Receiving Autonomic Regulation Therapy in the ANTHEM-HF Study. J Card Fail. 2015;pii: S1071-9164(15)01176–8. [DOI] [PubMed] [Google Scholar]
- 8. Zannad F, De Ferrari G, Tuinenburg A, Wright D, Brugada J, Butter C, et al. Chronic vagal stimulation for the treatment of low ejection fraction heart failure: results of the NEural Cardiac TherApy foR Heart Failure (NECTAR-HF) randomized controlled trial. Eur Heart J. 2015;36(7):425–33. doi: 10.1093/eurheartj/ehu345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hauptman P, Schwartz P, Gold M, Borggrefe M, Van Veldhuisen D, Starling R, et al. Rationale and study design of the increase of vagal tone in heart failure study: INOVATE-HF. Am Heart J. 2012;163(6):954–962. doi: 10.1016/j.ahj.2012.03.021 [DOI] [PubMed] [Google Scholar]
- 10. Schwartz PJ, Ferrari GMD. Vagal stimulation for heart failure: Background and first in-man study. Card Arrhythm Sudd Death Genes Prev. 2009;6:S76–S81. [DOI] [PubMed] [Google Scholar]
- 11. Buschman HP, Storm CJ, Duncker DJ, Verdouw PD, van der Aa HE, van der Kemp P. Heart Rate Control Via Vagus Nerve Stimulation. Neuromodulation. 2006;9(3):214–220. doi: 10.1111/j.1525-1403.2006.00062.x [DOI] [PubMed] [Google Scholar]
- 12. Tosato M, Yoshida K, Nekrasas ETV, Struijk JJ. Closed-loop control of the heart rate by electrical stimulation of the vagus nerve. Med Bio Eng Comput. 2006;44(3):161–169. doi: 10.1007/s11517-006-0037-1 [DOI] [PubMed] [Google Scholar]
- 13. Waninger MS, Bourland JD, Geddes LA, Schoenlein WE, Graber G, Weirigh WE, et al. Electrophysiological control of ventricular rate during atrial fibrillation. Pacing Clin Electro. 2000;23(8):1239–1244. doi: 10.1111/j.1540-8159.2000.tb00937.x [DOI] [PubMed] [Google Scholar]
- 14. Zhang Y, Mowrey KA, Zhuang S, Wallick DW, Popovi ZB, Mazgalev TN. Optimal ventricular rate slowing during atrial fibrillation by feedback AV nodal-selective vagal stimulation. Am J Physiol Heart Circ Physiol. 2002;282(3):H1102–H1110. doi: 10.1152/ajpheart.00738.2001 [DOI] [PubMed] [Google Scholar]
- 15. Zhou Y, Yuan Y, Gao J, Yang L, Zhang F, Zhu G, et al. An Implanted Closed-loop Chip System for Heart Rate Control: System Design and Effects in Conscious Rats. Journal of Biomedical Research. 2010;24(2):107–114. doi: 10.1016/S1674-8301(10)60018-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rousselet L, Rolle VL, Ojeda D, Guiraud D, Hagege A, Bel A, et al. Influence of Vagus Nerve Stimulation parameters on chronotropism and inotropism in heart failure. In: Engineering in Medicine and Biology Society (EMBC), 2014 36th Annual International Conference of the IEEE; 2014. p. 526–529. [DOI] [PubMed]
- 17. Ojeda D, Rolle VL, Romero-Ugalde HM, Gallet C, Bonnet JL, Henry C, et al. Sensitivity Analysis of Vagus Nerve Stimulation Parameters on Acute Cardiac Autonomic Responses: Chronotropic, Inotropic and Dromotropic Effects. PLoS ONE. 2016;11(9):e0163734 doi: 10.1371/journal.pone.0163734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Romero-Ugalde HM, Ojeda D, Rolle VL, Andreu D, Guiraud D, Bonnet JL, et al. Model-based design and experimental validation of control modules for neuromodulation devices. Biomedical Engineering, IEEE Transactions on. 2015;PP(99):1–1. [DOI] [PubMed] [Google Scholar]
- 19.Romero-Ugalde HM, Ojeda D, Le Rolle V, Rossel O, Bonnet JL, Karam N, et al. Model-based design of control modules for neuromodulation devices. In: Neural Engineering (NER), 2015 7th International IEEE/EMBS Conference on; 2015. p. 462–465.
- 20.Romero-Ugalde HM, Rolle VL, Bel A, Bonnet JL, Guiraud D, Mabo P, et al. On-off closed-loop control of vagus nerve stimulation for the adaptation of heart rate. In: Engineering in Medicine and Biology Society (EMBC), 2014 Annual International Conference of the IEEE; 2014. p. 6262–6265. [DOI] [PubMed]
- 21. Andreu D, Guiraud D, Souquet G. A Distributed Architecture for Activating the Peripheral Nervous System. Journal of Neural Engineering. 2009;6(2) doi: 10.1088/1741-2560/6/2/026001 [DOI] [PubMed] [Google Scholar]
- 22. Pednault EPD. ADL and the State-Transition Model of Action. J Logic Computation. 1994;4(5):467–512. doi: 10.1093/logcom/4.5.467 [Google Scholar]
- 23. Reiter R. Knowledge in action: logical foundations for specifying and implementing dynamical systems. MIT press; 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
We confirm that there is no identifying information in these files. Datasets containing the WMARR response acquired for each sheep. The data contains: the target, the name of the controller (P10, P30 or F30), RR interval and the current state (CS). All performance indicators are also provided.
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.