Abstract
Exposure to a high-fat (HF) diet in utero is associated with increased incidence of cardiovascular disease, diabetes, and metabolic syndrome later in life. However, the molecular basis of this enhanced susceptibility for metabolic disease is poorly understood. Gene expression microarray and genome-wide DNA methylation analyses of mouse liver revealed that exposure to a maternal HF milieu activated genes of immune response, inflammation, and hepatic dysfunction. DNA methylation analysis revealed 3360 differentially methylated loci, most of which (76%) were hypermethylated and distributed preferentially to hotspots on chromosomes 4 [atherosclerosis susceptibility quantitative trait loci (QTLs) 1] and 18 (insulin-dependent susceptibility QTLs 21). Interestingly, we found six differentially methylated genes within these hotspot QTLs associated with metabolic disease that maintain altered gene expression into adulthood (Arhgef19, Epha2, Zbtb17/Miz-1, Camta1 downregulated; and Ccdc11 and Txnl4a upregulated). Most of the hypermethylated genes in these hotspots are associated with cardiovascular system development and function. There were 140 differentially methylated genes that showed a 1.5-fold increase or decrease in messenger RNA levels. Many of these genes play a role in cell signaling pathways associated with metabolic disease. Of these, metalloproteinase 9, whose dysregulation plays a key role in diabetes, obesity, and cardiovascular disease, was upregulated 1.75-fold and hypermethylated in the gene body. In summary, exposure to a maternal HF diet causes DNA hypermethylation, which is associated with long-term gene expression changes in the liver of exposed offspring, potentially contributing to programmed development of metabolic disease later in life.
Maternal high-fat diet leads to hepatic hypermethylation in offspring mice accompanied by enrichment of hypermethylated regions in cardiovascular- and diabetes-associated QTLs on chromosomes 4 and 18.
Maternal nutrition is important in determining susceptibility to metabolic diseases (1). Maternal obesity and/or consumption of a high-fat (HF) diet during pregnancy and lactation increase the risk for development of metabolic diseases in offspring (2). We have reported that differences in maternal substrate utilization can also program development of metabolic syndrome in offspring (3–5). Specifically, male offspring exposed to a maternal HF diet precociously developed metabolic syndrome.
Epigenetic mechanisms may be responsible for the programming effects of maternal nutrition (2). DNA methylation is an epigenetic mark that can potentially mediate the memory of temporally remote events to confer susceptibility to metabolic disease. Although models of fetal programming of metabolic disease have described changes in DNA methylation (6), evidence supporting this hypothesis remains limited. Thus, using our established model of in utero programmed metabolic syndrome (3), we sought to determine whether genome-wide changes in DNA methylation occur in liver of offspring exposed to a maternal HF diet. Liver was chosen because it is essential in maintaining metabolic homeostasis (7). This study shows exposure to a maternal HF diet results in genome-wide changes in hepatic DNA methylation with corresponding alterations in gene expression that persist throughout life. Hepatic hypermethylation of HF offspring is most evident on chromosomes (Chrs) 4 and 18 in quantitative trait loci (QTLs) for atherosclerosis and diabetes, respectively. These results highlight the contribution of altered hepatic DNA methylation and gene expression to development of metabolic syndrome in offspring exposed to a maternal HF diet.
Materials and Methods
Animals and experimental design
All the experiment methods involving mice were approved by the Albert Einstein College of Medicine animal use committee and conducted in accordance with the approved guidelines. Animals were housed in a barrier facility and maintained on a 14-hour to 10-hour light-dark cycle with ad libitum access to chow and water. As previously described, wild-type CD1 female mice were maintained on control (PicoLab #5058; LabDiet, St. Louis, MO) or HF (Bio-Serv #F3282; Bio-Serv, Flemington, NJ) diet 2 weeks before mating, throughout pregnancy, and throughout lactation (3). Fourteen dams per group were bred to generate all study offspring. Genotyping was performed as previously described (8). Offspring were weaned onto a low-fat (LF) diet (PicoLab #5053; LabDiet) at postnatal day 21. Wild-type offspring were studied to eliminate potential confounding effects of genetic factors related to postnatal insulin resistance previously reported in Glut4+/− mice (9, 10). Because male offspring of HF diet–fed mothers develop metabolic syndrome (3), and because there are sexual dimorphisms in gene expression patterns (11) and DNA methylation (12), the following studies used male offspring only.
DNA methylation microarray
We used a high-resolution microarray-based genome-wide hepatic DNA methylation approach (13, 14) designed to test almost a million sites throughout the mouse genome. Genomic DNA was digested with HpaII or MspI separately and ligated to different adapters. After polymerase chain reaction (PCR), the HpaII and MspI libraries were labeled with different fluorophores and cohybridized on a customized genomic microarray. Microarrays were preprocessed and subjected to quality control and quantile normalization of signal intensity as previously described (15). Log ratios of signal intensities of HpaII and MspI were then compared between groups for all 801,224 HpaII fragments throughout the mouse genome. The criteria for identifying differentially methylated regions (DMRs) was a LIMMA P value < 0.001 (Bioconductor) and an absolute log(HpaII/MspI) difference >1.0. The DMRs were defined as belonging to a promoter (−500 bp to 5 kb from the transcription start site), gene body (the remaining region within a gene), or intergenic region.
Data are available through the Gene Expression Omnibus database (GSE77430).
Bisulphite MassARRAY validation
To validate HELP assay data, bisulphite MassARRAY (Sequenom, San Diego, CA) was performed using the company’s standard protocol (16). Matched peak data were exported using EpiTYPER software (Sequenom, San Diego, CA) and quality analyzed using tools that we developed (15). Primers are listed in Supplemental Table 1 (165.7KB, docx) .
Gene expression microarray
Total RNA was extracted from liver from offspring of seven control and seven HF mice using TRIzol Reagent (Invitrogen, Carlsbad, CA). Double-stranded complementary DNA was generated (SuperScript Double-Stranded cDNA Synthesis Kit; Invitrogen) and pooled (control or HF), and then samples were hybridized onto Nimblegen Mouse Gene Expression Array (Array ID: 2006-10-26_MMUS5_60mer). Data were analyzed using DNASTAR Arraystar software (Roche Nimblegen, Madison, WI), and a stringency criterion of 1.5-fold was applied to identify significantly regulated candidates. Data are available through the Gene Expression Omnibus database (GSE77431).
Gene expression
First-strand complementary DNA was generated using SuperScript III (Invitrogen) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) was carried out in triplicate using Roche 480 Real-Time PCR System with SYBR Green Master Mix (Roche Nimblegen). Primer sets were designed using the Universal Probe Library Assay Design Center. Results were normalized to expression of the housekeeping gene 36B4 and expressed as fold change compared with control. Primers are listed in Supplemental Table 1 (165.7KB, docx) .
Ingenuity Pathway Analysis
The list of identified RefSeq genes was uploaded to Ingenuity Pathway Analysis (IPA) (QIAGEN, Hilden, Germany) and mapped to corresponding gene objects (focus genes) in the Ingenuity Pathways Knowledge Base. Core networks were constructed for direct and indirect interactions using default parameters. Then ranking score for each network was computed by a right-tailed Fisher exact test as the negative log of the probability that the number of focus genes in the network was not caused by random chance.
Hotspot analysis
To identify hotspot regions significantly enriched with hypermethylated or hypomethylated sites, a 500-kb sliding window was applied to the mouse genome, and the numbers of significant DMRs (P < 0.05) were computed. The resulting numbers of DMRs were compared with the expected numbers derived for randomly selected genomic regions of the same size for statistical significance, whereas empirical P values were determined by repetitively selecting random regions 10,000 times (17). Significant hotspots were defined as having P < 0.05. To clarify if these hotspots map to QTLs, the jaxQTL dataset, which shows approximate positions of QTLs based on reported peak LOD scores from Mouse Genome Informatics, was downloaded from Table Browser in the University of California Santa Cruz genome browser (http://genome.ucsc.edu/cgi-bin/hgTables?command=start) and merged with the hotspot data based on their genomic locations.
Evaluation of intergenic regions
Mouse liver histone H3 lysine 4 monomethylation (H3K4me1) sequencing results are provided in duplicate at mm9 genome assembly (GSM722760). We analyzed samples using MACS version 1.4.2 (Python Software Foundation, Wilmington, DE) (model-based analysis of ChIP-Seq) peak-calling algorithm against input (Gene Expression Omnibus database no. GSM722764) as previously detailed (18). Genomic features, including 3′UTR, 5′UTR, exon, intergenic region, intron, noncoding, promoters, and transcription termination sites, were evaluated. Mouse liver DMRs were liftOvered to mm9 and analyzed further by normalizing these numbers to the probe number of each category in the array.
DNA methyltransferase activity
Total DNA methyltransferase (DNMT) activity was measured with 60 µg of liver as previously described by recording the rate of radioactive labeling on poly(dI-dC) DNA in the presence of [methyl-3H] S-adenosyl-l-methionine (19).
Motif analysis
To evaluate the function of the DMR identified in Mmp9 and Camta1, motif analysis was performed using MOTIF Search (http://www.genome.jp/tools/motif/).
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Student t test was used for statistical analysis of the differences between two groups for qRT-PCR, MassARRAY, and DNMT activity. Limma P value was used for HELP assay analysis.
Detailed study design and data analysis
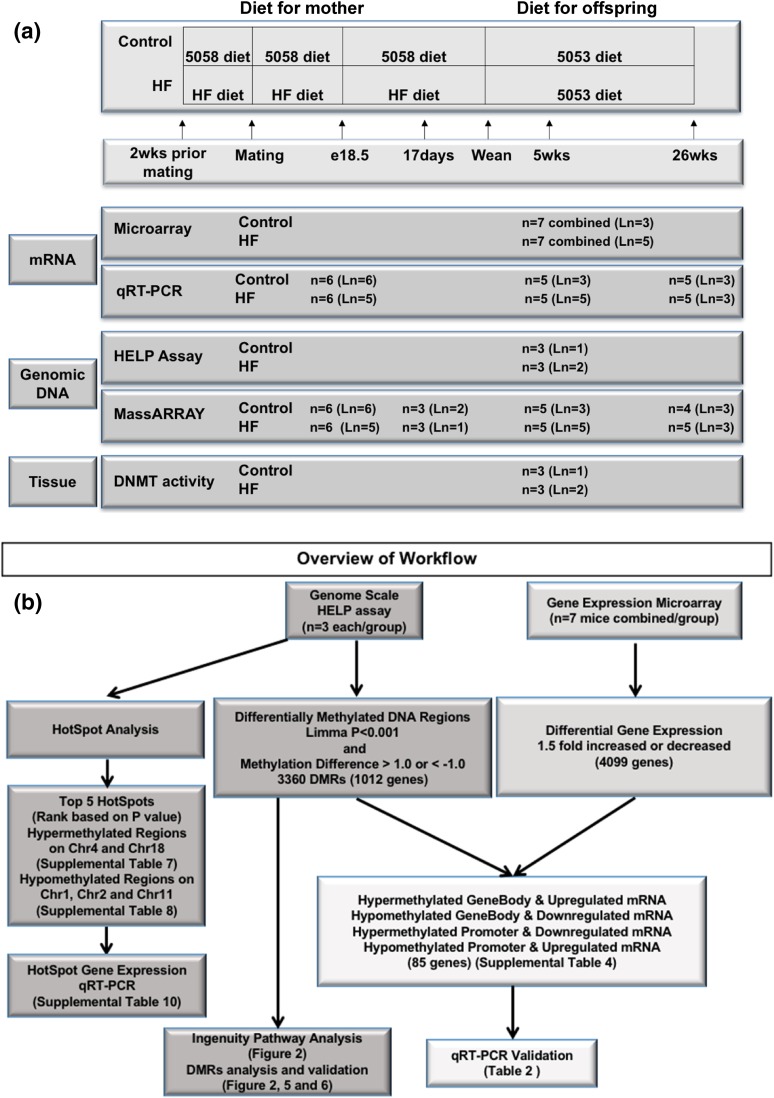
Flowcharts with the study design and processing of data are presented in Fig. 1.
Figure 1.
Flowcharts of study design and data processing. (a) Study design: female mice were maintained on a control or HF diet for 2 weeks before mating, throughout pregnancy, and throughout lactation. Offspring were weaned onto a LF diet at postnatal day 21. Wild-type offspring were analyzed at embrionic day 18.5 (e18.5), 17 days, 5 weeks, and 26 weeks of age. Ln, number of litters represented in each assay and time point. (b) Data processing scheme: genome-wide methylation status and gene expression were measured on 5-week-old liver using the HELP assay and microarray, respectively. Hot spot analysis and methylation and gene expression validation analyses were performed based on data obtained from the array experiments using MassARRAY and qRT-PCR, respectively.
Results
Genome-wide DNA hypermethylation in liver from offspring of HF diet–fed mother
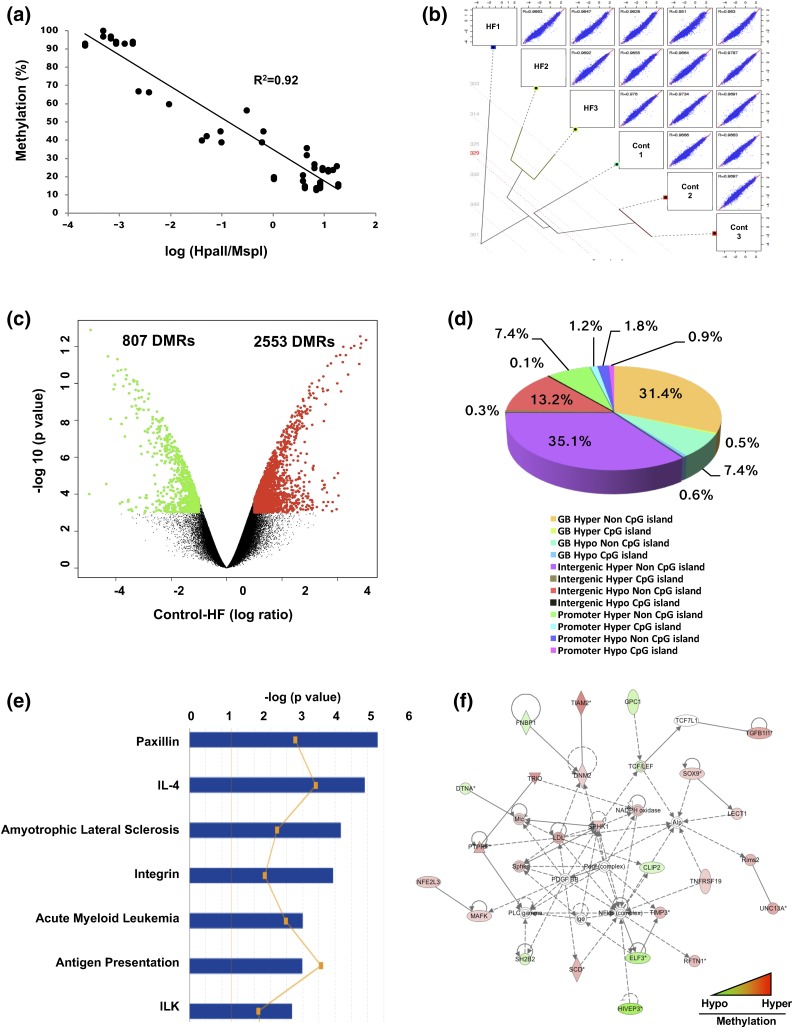
Hepatic DNA methylation profiles were generated from offspring of control or HF diet–fed mother at 5 weeks. Bisulphite MassARRAY validated the HELP assay results, whereby the methylation status and log(HpaII/MspI) were strongly inversely correlated (R2 = 0.92) [Fig. 2(a)]. The striking epigenetic distance between groups is illustrated within a hierarchical clustering dendrogram [Fig. 2(b)]. Of the 3360 DMRs identified, 2553 DMRs were hypermethylated, and 807 DMRs were hypomethylated in HF [Fig. 2(c)]. Most DMRs were in gene bodies and intergenic regions outside of CpG islands [Fig. 2(d)]. Taken together, maternal HF diet exposure led to hepatic DNA hypermethylation in all genomic contexts in 5-week offspring.
Figure 2.
Exposure to HF diet in utero induced hepatic hypermethylation. (a) Technical validation studies using bisulphite MassARRAY confirmed the data obtained from the HELP assay. Three loci were identified, each representing constitutively hypomethylated and hypermethylated sites. HELP data as log (Hpall/Mspl) ratio are shown along the x-axis, with hypermethylation toward the left and hypomethylation toward the right. MassARRAY data for the same loci are plotted along the y-axis, from 0% (hypomethylation) to 100% (hypermethylation). (b) Global correlation using the entire dataset demonstrates the difference between control and HF. A tree-pair plot was generated using R package (The R Foundation for Statistical Computing) (15). Pairwise correlations were calculated from 801,224 independent loci. R value indicates the Pearson correlation for each pair of samples (n = 3 per group). (c) Volcano plot of HF diet–induced changes in global DNA methylation of control (n = 3) vs HF diet (n = 3). Each dot corresponds to every locus showing average differences between control and HF [x-axis, below zero is hypomethylation; above zero is hypermethylation with negative log-transformed significance (P values) along the y-axis]. Larger values are more significant. (d) Analysis of 3360 DMRs were assigned by genomic context. (e) Top seven significant IPA canonical pathways of genes with DMRs. (f) Cell signaling and small molecule biochemistry and lipid metabolism interaction network. The top IPA network of genes displaying significant differences in both methylation and gene expression. An asterisk (*) indicates that a given gene is represented in the array set with multiple probes. Cont, control; GB, gene body; Hyper, hypermethylation; Hypo, hypomethylation; IL, interleukin.
Analysis of DMRs in gene-associated regions
Of the 3360 DMRs from HF, 1799 were located within either promoters or gene bodies of 1012 genes. IPA showed these genes are enriched for functions related to development and metabolism (Supplemental Table 2 (55.4KB, docx) ). Most genes containing DMRs are associated with metabolic and inflammatory diseases. The two most significantly enriched networks, based on P values, are cellular morphology, function, and maintenance, and DNA replication, recombination, and repair, respectively (Supplemental Fig. 1A and 1B) (7.6MB, pptx) .
Differentially methylated genes are found in 116 diverse canonical pathways. However, three of the top pathways are functionally related: paxillin, integrin, and integrin-linked kinase (ILK) [Fig. 2(e)]. Also in the top 25 pathways are type 2 diabetes mellitus signaling, NRF2 oxidative stress response, and Wnt/β-catenin, as well as several specific to immune cells (i.e., interleukin-4 signaling) (Supplemental Table 3 (145.7KB, docx) ). Although the differentially methylated genes in HF are involved in all aspects of cellular and organismal growth and maintenance, many play critical roles in the cross-talking paxillin, integrin, and ILK pathways involved in extracellular matrix formation, cell growth, and differentiation.
Alterations in DNA methylation and expression of cell signaling and transcriptional regulatory genes
Gene expression studies on messenger RNA (mRNA) in liver from offspring of control and HF diet–fed mother were conducted to identify genes whose expression might be affected by altered DNA methylation. There were 4099 genes differentially expressed with ~58% showing at least a 1.5-fold increase in HF. Despite being weaned onto LF diet for 2 weeks prior to sampling, the many differentially expressed genes in livers of HF offspring were linked to networks expressed in animals consuming an HF diet (i.e., hepatic dysfunction/disease and inflammation) (20, 21) (Table 1). Other networks were associated with hepatic cellular proliferation, development, and cell death, processes that have been shown to be linked to HF diet consumption (21).
Table 1.
HF Diet In Utero Affects Gene Expression
| Symbol | Entrez Gene Name | Fold Change, HF/Control |
|---|---|---|
| NFkB-related (immune response) | ||
| Tlr2 | Toll-Like Receptor 2 | 3.5 |
| Relb | V-Rel Reticuloendotheliosis Viral Oncogene Homolog B | 3.2 |
| Map3k14 | Mitogen-Activated Protein Kinase Kinase Kinase 14 | 2.5 |
| Il1r2 | Interleukin 1 Receptor, Type II | 2.4 |
| Nfkbib | NF Light Polypeptide Gene Enhancer In B-Cells Inhibitor, β | 2.1 |
| Nfkb2 | NF Kappa Light Polypeptide Gene Enhancer In B-Cells 2 (P49/P100) | 1.8 |
| Rela | V-Rel Reticuloendotheliosis Viral Oncogene Homolog A | 1.6 |
| Nfkb1 | NF Kappa Light Polypeptide Gene Enhancer In B-Cells 1 | 1.5 |
| Fibrosis-related (hepatic dysfunction) | ||
| Igfbp1 | Insulinlike Growth Factor–Binding Protein 1 | 11.5 |
| Timp1 | TIMP Metallopeptidase Inhibitor 1 | 10.9 |
| Thbs1 | Thrombospondin 1 | 5.7 |
| Mmp8 | Matrix Metallopeptidase 8 (Neutrophil Collagenase) | 3.9 |
| Cxcl2 | Chemokine (C-X-C Motif) Ligand 2 | 3.8 |
| A2m | α-2-Macroglobulin | 3.5 |
| Col4a2 | Collagen, Type IV, α2 | 2.3 |
| Cytokine (inflammatory) | ||
| Serpine1 | Serpin Peptidase Inhibitor, Clade E, Member 1 | 4.4 |
| Il-33 | Interleukin 33 | 2.4 |
| Cxcl10 | Chemokine (C-X-C Motif) Ligand 10 | 2.0 |
| Ccl3 | Chemokine (C-C Motif) Ligand 3 | 2.0 |
| Il-2 | Interleukin 2 | 1.6 |
| Il-22 | Interleukin 22 | 1.6 |
Next, we overlapped the DNA methylation and gene expression data to identify genes with dysregulation of both processes. Supplemental Fig. 2 (7.6MB, pptx) shows the strong correlation between DNA methylation and gene expression. Of 1012 genes with DMRs within the promoter or gene body, 927 genes (3227 DMRs) showed no difference in transcription. This suggested that most DMRs identified did not correspond to transcriptional changes at 5 weeks of age. However, 85 genes (133 DMRs) showed at least a 1.5-fold change in gene expression (Supplemental Table 4 (135.3KB, docx) ). These 85 genes were subjected to further IPA. The most strongly associated network is involved in cell signaling and small molecule biochemistry and lipid metabolism [Fig. 2(f)]. Many of the differentially methylated and differentially expressed genes in the top 10 networks were validated (Table 2). Interestingly, the canonical pathway containing the highest number of differentially methylated and expressed genes is the ILK pathway (Supplemental Fig. 3A) (7.6MB, pptx) . Upregulation of Itgb4, Mmp9, Sh2b2, and Tgfb1l1 was validated (Table 2). The ILK pathway shares differentially methylated and expressed genes with the paxillin and integrin pathways (Supplemental Fig. 3B) (7.6MB, pptx) . Other canonical signaling pathways with significant numbers of differentially methylated and expressed genes include the aryl hydrocarbon receptor (four genes), extracellular signal–regulated kinase/mitogen-activated protein kinase (four genes), and sphingosine-1-phosphate (three genes).
Table 2.
qRT-PCR of Genes Involved in Metabolism, Cell Signaling/Development, and Transcription Regulation in 5-Week-Old Liver
| Metabolism and Cell Signaling/Development | Control | HF | Pathway |
|---|---|---|---|
| Aldh1l2 | 1.38 ± 0.57 | 3.84 ± 0.51a | Amino acid metabolism |
| Mapkapk2 | 1.09 ± 0.20 | 2.92 ± 0.73a | Carbohydrate metabolism |
| Dusp4 | 1.15 ± 0.27 | 3.72 ± 0.78a | Cell to cell signaling |
| Tp53 (Trp53) | 1.18 ± 0.40 | 2.38 ± 0.28a | Cellular development |
| Ptprf | 1.02 ± 0.10 | 3.13 ± 0.89a | Lipid metabolism |
| Timp3 | 1.25 ± 0.46 | 5.06 ± 1.13a | Lipid metabolism |
| Trio | 1.16 ± 0.30 | 2.00 ± 0.18a | Lipid metabolism |
| ILK pathway | Control | HF | Pathway |
| Itgb4 | 1.15 ± 0.28 | 2.49 ± 0.48a | ILK pathway |
| Mmp9 | 1.34 ± 0.45 | 2.92 ± 0.43a | ILK pathway |
| Sh2b2 | 1.07 ± 0.18 | 1.65 ± 0.17a | ILK pathway |
| Tgfb1l1 | 1.04 ± 0.14 | 2.52 ± 0.60a | ILK pathway |
| Transcription regulator | Control | HF | Function |
| Etv6 | 1.08 ± 0.21 | 2.59 ± 0.54a | Cell-to-cell signaling and interaction |
| Tgfb1l1 | 1.04 ± 0.14 | 2.52 ± 0.60a | Cellular development, growth, and proliferation |
| Jdp2 | 1.05 ± 0.18 | 4.19 ± 1.33a | Hereditary disorder |
| Sox9 | 1.11 ± 0.25 | 6.34 ± 1.75a | Lipid metabolism |
| Elf3 | 1.03 ± 0.13 | 2.21 ± 0.42a | Lipid metabolism, cell signaling |
| Nfia | 1.27 ± 0.49 | 3.04 ± 0.36a | Cellular development |
Values are mean ± SEM or as otherwise indicated. Results are presented as target normalized to 36B4 in arbitrary units; n = 4 to 5 per group.
P < 0.05 using an unpaired two-tailed Student t test.
We also found 103 transcription regulators to be differentially methylated, of which 13 show altered gene expression (Apbb1, Clip2, Elf3, Etv6, Hivep3, Jdp2, Mafk, Nfe2l3, Nifa, Sox9, Tbx1, Tcf7l1, and Tgfb1l1). Six transcription regulators had a greater than twofold increase in gene expression in the HF and are involved in diverse cellular processes (Table 2).
Role of differential methylation in intergenic regions
Active enhancers are part of a chromatin landscape marked by H3K4me1 (22). To evaluate the effect of DMRs in intergenic regions on potential cell-type specific active enhancers that may confer gene regulatory information, we overlapped the DMRs with known mouse liver H3K4me1 histone marks (23). There were 764 common regions between DMRs and mouse liver H3K4me1 histone marks revealed, illustrating that 22.7% of HF-associated DMRs localize to enhancer regions. Most H3K4me1-overlapped DMRs map to introns (444) and intergenic regions (221) (Supplemental Fig. 4 (7.6MB, pptx) ).
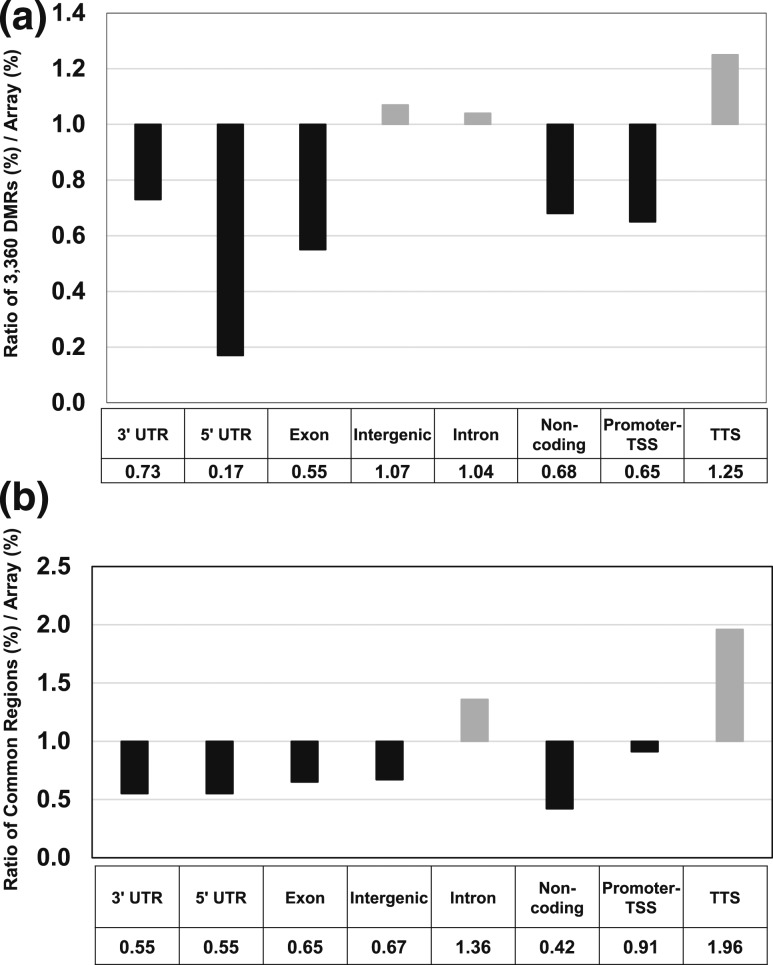
To further understand the 3360 DMRs, common regions were analyzed as described previously (18). Genomic features were examined and DMRs were enriched primarily in intergenic and intron regions [Fig. 3(a)]. Further analysis showed that intron and transcription termination site sequences were overrepresented in DMRs [Fig. 3(b)]. Sixteen of the 221 intergenic regions were located upstream of genes involved in cardiovascular disease (Adm, Atp1a1, Atp2a2, Chi3l1, Fgg, Fhl2, GDF15, Hopx, Ldlr, Mc2r, Mtpn, Ppp2ca, Smad7, Terc, Vegfa, and Wdr5). Although the role of methylation of intergenic regions is uncertain, a recent study showed that inactivation of intergenic enhancers triggered rapid transcriptional shutdown of distal genes (24).
Figure 3.
Active enhancers located in intergenic DMRs. RefSeq annotation of common regions between active enhancers (H3K4me1) and 3360 DMRs. The relative enrichment ratios of the DMRs are compared with elements of each category on the mouse methylation array. (a) Number of DMRs that overlap with H3K4me1 regions as shown. (b) Relative enrichment ratio compared with the representation of the different elements on the methylation array. TSS, transcription start site; TTS, transcription termination site; UTR, untranscribed region.
Finally, the qRT-PCR or microarray gene expression data were evaluated to identify genes containing any of the 764 DMRs. There were 67 genes with substantially different mRNA expression in differentially methylated active enhancer regions revealed (Supplemental Table 5 (77.7KB, docx) ). Of these 67 genes, 34 were upregulated. Of these, 11 genes are involved in lipid metabolism (Adm, Bag3, Cyp1a1, Cyp1a2, Dnm2, Il4r, Lect1, Prkag, Scd1, Sphk1, and Xpa).
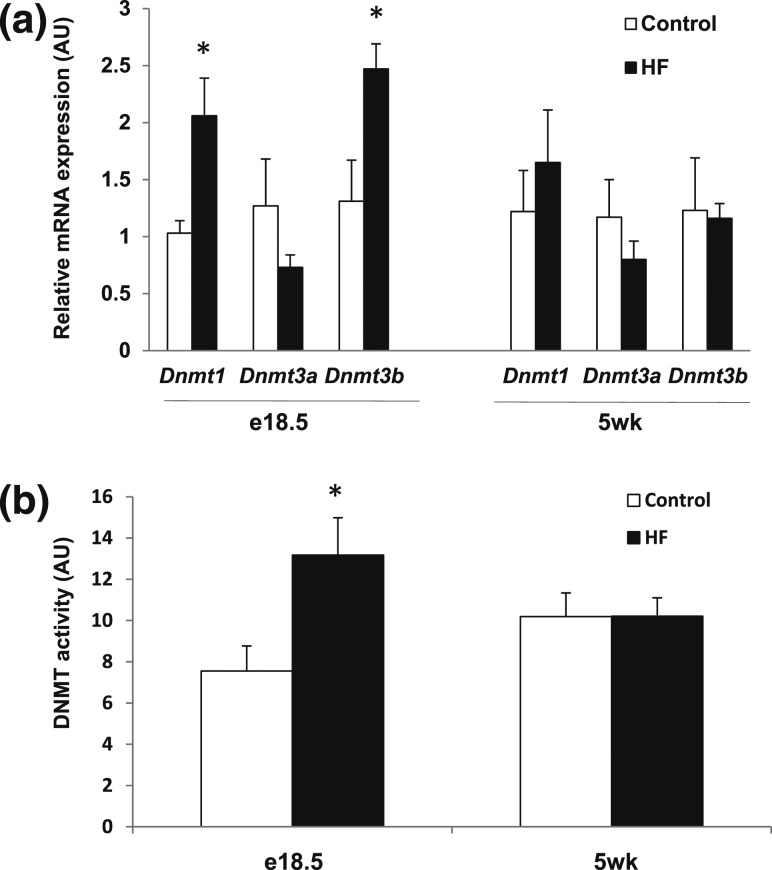
Increased DNMT mRNA and enzymatic activity in HF fetal liver
To gain insight into the mechanism of hypermethylation induced by exposure to a maternal HF diet, hepatic DNMT mRNA and enzymatic activity were measured in embrionic day 18.5 (e18.5) and 5-week livers. Dnmt1 and Dnmt3b mRNAs were increased in HF e18.5 liver [Fig. 4(a)], and likewise, fetal DNMT enzymatic activity was also significantly higher [Fig. 4(b)]. Conversely, no differences in Dnmt mRNA [Fig. 4(a)] or enzymatic activity [Fig. 4(b)] were measured in 5-week livers. These results indicate that most hepatic DNA hypermethylation brought about by exposure to a maternal HF diet occurs during fetal development. Expression of candidate DNA demethylases were not significantly different between groups (Supplemental Table 6 (56.5KB, docx) ) (25).
Figure 4.
Increased DNMT mRNA and enzymatic activity in e18.5 liver from offspring of HF. (a) DNMT mRNA expression (target normalized to 36B4 in arbitrary units; n = 4 to 5 per group) and (b) enzymatic activity (n = 3 per group) were measured in e18.5 and 5-week-old liver. *P < 0.05 using an unpaired two-tailed Student t test.
Chromosomal distribution of DMRs
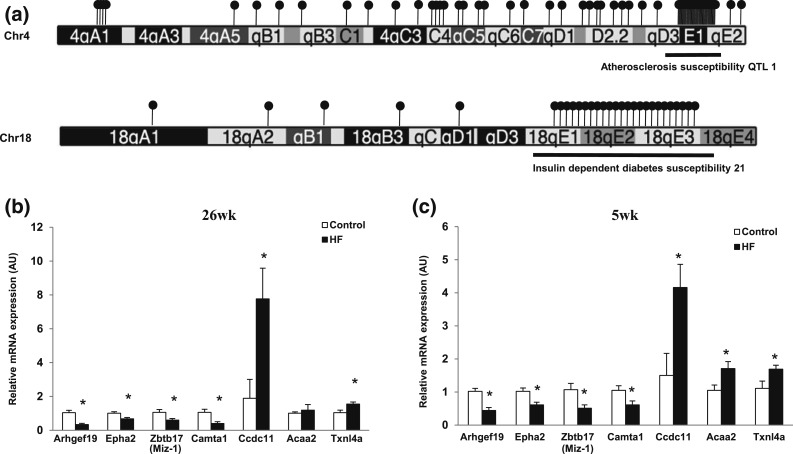
The chromosomal distribution of the 3360 DMRs was plotted (Supplemental Fig. 5 (7.6MB, pptx) ). Most of the 2553 HF hypermethylated DMRs were located on Chrs 4, 5, 6, 7, 10, 11, 17, and 18. Conversely, most of the 807 HF hypomethylated DMRs were located on Chrs 1, 2, 3, 7, 11, and 17. Because mouse QTLs often predict homologous QTLs in humans (26), we examined the distribution of DMRs that colocalized with QTLs of different traits and found that on Chr 4, 50% of the hypermethylated DMRs mapped to the atherosclerosis QTL, whereas on Chr 18, 80% mapped to the insulin-dependent diabetes susceptibility QTL 21 [Fig. 5(a)], suggesting hypermethylation of DMRs in these QTLs is involved in development of metabolic syndrome in offspring exposed to a maternal HF diet (3).
Figure 5.
Chromosomal distribution of DMRs in HF compared with (a) control, hypermethylation clusters on Chrs 4 and 18 and qRT-PCR of these hotspot hypermethylated genes in (b) 26-week- and (c) 5-week-old liver. (a) There were 3360 DMRs assigned by Chr. Exposure to HF diet induced the most hypermethylation on Chr 4. Hypermethylation DMRs are indicated by black lollipops. One lollipop represents five hypermethylated DMRs. There were 211 DMRs found on Chr 4, with 120 DMRs located in the atherosclerosis QTL 1 (black underline). There were 84 DMRs found on Chr 18, with 58 DMRs located in the insulin-dependent diabetes susceptibility 21a QTL (black underline). mRNA expression in (b) 26-week and (c) 5-week liver (target normalized to 36B4 in arbitrary units; n = 4 to 5 per group). *P < 0.05 using an unpaired two-tailed Student t test.
Identification of DNA methylation hotspots
We identified chromosomal hotspots with highly correlated differentially methylated sites using a computational method (17). In comparison with the genome-wide background, Chrs 18, 4, 14, and 17 contain the highest ranked hypermethylated hotspots (Supplemental Table 7 (182.6KB, docx) ), whereas Chrs 2, 14, 11, and 1 contain the highest-ranked hypomethylated hotspots (Supplemental Table 8 (175.1KB, docx) ). Additionally, the top 30 ranked areas from both hypermethylated and hypomethylated hotspots were analyzed for QTLs (Supplemental Table 9 (154.6KB, docx) ). Most hotspots contain QTLs related to the metabolic phenotype of maternal HF diet−exposed offspring (i.e., weight, blood glucose, insulin-dependent diabetes, and atherosclerosis) (3).
Using the most robust P values at DMRs coinciding with QTLs relevant to metabolic syndrome, we selected genes in hypermethylated hotspots on Chr 4 (atherosclerosis susceptibility QTL 1) and Chr 18 (insulin-dependent diabetes susceptibility 21a), and hypomethylated hotspots on Chr 1 (bone mineral density 5, alcohol preference QTL 1, weight 1, bone mineral density 1a) and Chr 11 (weight 6 weeks QTL 3, late-growth QTL 1, late-growth adjusted QTL 1) for expression analysis using 5-week liver (Supplemental Table 10 (106.6KB, docx) ). In the hypermethylated hotspot on Chr 4, the expression of four genes, Arhgef19, Epha2, Zbtb17/Miz-1, and Camta1, was significantly downregulated in HF diet groups. In contrast, three genes, Ccdc11, Acaa2, and Txnl4a, within the hypermethylated hotspot on Chr 18 were upregulated. There were no significant differences in gene expression in Chr 1 QTLs. However, Dstn and Banf2 were upregulated in a hypomethylated hotspot on Chr 2 (associated with type 2 diabetes mellitus 2 in SMXA RI mice and blood glucose level 1), and Gabrp was upregulated and Snrnp25 was downregulated in a hypomethylated hotspot on Chr 11 (Supplemental Table 9 (154.6KB, docx) ). Snrnp25 expression is regulated by Trafs 2 and 3, indicating a role in inflammatory metabolic disorders (27).
To assess whether differentially methylated and expressed genes within hotspot QTLs on Chrs 2, 4, 11, and 18 maintain differential expression over time, expression of these genes was measured in 26-week liver [Fig. 5(b)]. Four hypomethylated genes, Dstn, Banf2, Snrnp25, and Gabrp, showed no difference between groups. However, Arhgef19, Epha2, Zbtb17/Miz-1, and Camta1 were significantly downregulated in the HF group in 26-week [Fig. 5(b)] and 5-week liver [Fig. 5(c)]. The hypermethylation status of promoter regions of Arhgef19 and Zbtb17/Miz-1 was validated in 5-week liver (Supplemental Figs. 6 and 7 (7.6MB, pptx) ). Hypermethylation along with significant downregulation of these genes was seen in fetal liver [Arhgef19: control: 1.37 ± 0.45 vs HF: 0.43 ± 0.14; Zbtb17/Miz-1: control: 1.17 ± 0.27 vs HF: 0.43 ± 0.04 (mean ± SEM)]. These results show that altered methylation and expression of these two genes persisted from fetal stage to adulthood. Although Epha2 and Camta1 were not differentially expressed in fetal liver, both mRNAs were downregulated in 26-week liver from offspring of HF [Fig. 5(b)]. Hypermethylation was consistently measured in Camta1, transcriptional activator, from e18.5 to adulthood (Supplemental Fig. 8 (7.6MB, pptx) , see third CpG). However, the Arhgef19 and Epha2 genes did not show hypermethylation at 26 weeks. These data suggest downregulation of Arhgef19 and Epha2 may be caused by postnatal modifications of DNA other than the changes in DNA methylation.
Gene expression in 26-week liver was also determined for three genes in the QTLs hotspot on Chr 18. Ccdc11 and Txnl4a expression was upregulated in 5- and 26-week liver [Fig. 5(b) and 5(c)]. However, there was no difference in expression of Acaa2 at 26 weeks [Fig. 5(b)]. In summary, we identified six differentially methylated genes in liver from offspring of HF diet–fed mother within the hotspot QTLs associated with metabolic disease that displayed persistent gene dysregulation into adulthood. The altered methylation and expression of the QTL genes previously noted could play a role in development of metabolic syndrome in HF offspring.
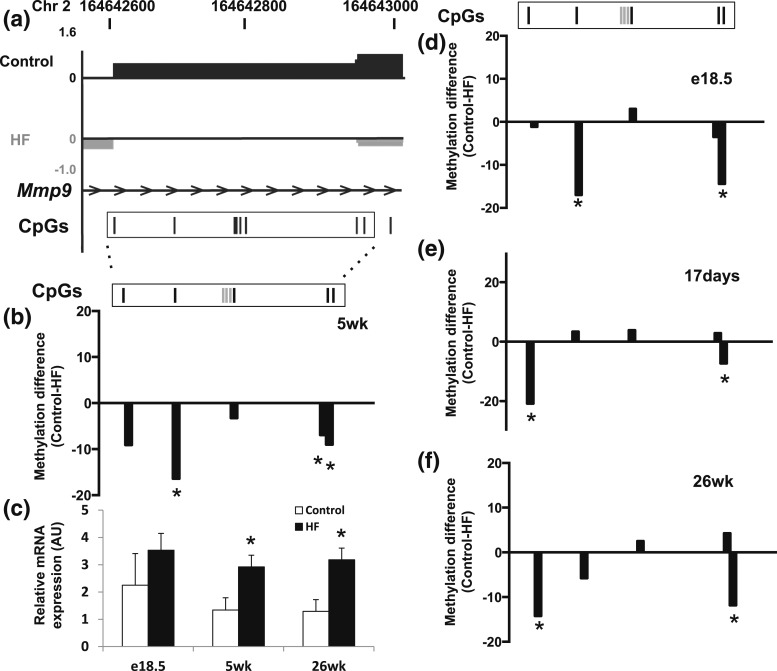
Persistent MMP9 hypermethylation throughout life
We identified a reliable epigenetic signature in Mmp9, a stable epigenetic obesity biomarker in a human cohort (28). Specifically, exposure to a maternal HF diet was associated with hypermethylation in the gene body of Mmp9 and increased Mmp9 mRNA in 5-week liver from offspring of HF [Fig. 6(a–c)]. The change in Mmp9 methylation status was assessed in e18.5, 17 days, and 26-week liver [Fig. 6(d–f)]. The fifth CpG site was consistently hypermethylated in the HF group from e18.5 to 26 weeks. Although motif analysis showed this CpG is located in a recognition site for several transcription factors, its biological function is unknown. Intriguingly, the fifth CpG is located in/close to a DNaseI hypersensitivity site identified in e14.5 liver (ENCODE/University of Washington tracks).
Figure 6.
Bisulphite MassARRAY validation and mRNA expression of Mmp9 in 5-week liver. (a) Data were uploaded into browser extensible data–formatted tracks for visualization with the USCS Genome Brower. Hypomethylated (upward peaks) and hypermethylated (downward peaks) sites are shown. (b, d–f) The degree of difference in cytosine methylation between liver from offspring of control and HF as measured by Bisulphite MassARRAY at (b) 5 weeks, (d) e18.5, (e) 17 days, and (f) 26 weeks. Gray bars in the rectangle indicate unanalyzable CpG sites (n = 3 to 6 per group). *P < 0.05 for control vs HF. (c) Mmp9 mRNA expression at different postnatal time points (target normalized to 36B4 in arbitrary units; n = 4 to 5 per group). *P < 0.05 using an unpaired two-tailed Student t test.
Discussion
This is a report of genome-wide alterations in hepatic DNA methylation and gene expression of offspring exposed to a maternal HF diet that develop metabolic syndrome in early postnatal life (3). DMRs are found predominantly in non-CpG island DNA of gene bodies and intergenic regions (29). DNA methylation can regulate gene expression (6), and gene body methylation has been shown to be positively correlated with gene expression status (30); however, its function has not yet been clarified. Maunakea et al. (31) reported that gene body DNA methylation may increase transcriptional activity by blocking the initiation of intragenic promoters. A recent study using mouse embryonic stem cells showed DNMT3B-dependent DNA methylation in the gene body is responsible for the prevention of aberrant transcription initiation events necessary to guarantee the fidelity of mRNA transcription initiation (32). This result suggests DNA methylation in the gene body might induce the gene activation; however, further studies are required to confirm this prediction.
The ENCODE project revealed that intergenic regions are also involved in transcriptional regulation (29, 33). In agreement with these studies, our results show altered gene body methylation correlated with dysregulation of gene expression. Recently, advanced technology has shown three-dimensional genome architecture is involved in regulating gene expression during development, in physiologic processes, and in disease (34). Our study focused on the relationship between DNA methylation and gene expression; however, future studies should take into consideration the three-dimensional architecture of the genome.
Many genes exhibiting differential methylation also showed altered gene expression. These genes were found in canonical pathways involved in every aspect of cellular differentiation and function. A concentration of genes with both altered methylation and gene expression was found in the paxillin, integrin, and ILK pathways that are involved in cellular differentiation and extracellular matrix maintenance (35–37). Mmp9, found in the ILK pathway, is a gene that shows upregulation in obesity (38). Here, exposure to a maternal HF diet resulted in hypermethylation in the gene body of Mmp9 and a significant increase in gene expression in 5- and 26-weeek HF liver. The Mmp9 gene body is stably hypermethylated in peripheral blood lymphocytes of adults with increased body mass index (28). Considering this fact, the persistent hypermethylation of the Mmp9 gene body in the liver of our model suggests this epigenetic biomarker may play a role in development of metabolic syndrome.
Thirteen transcription regulators with DMRs also displayed dysregulated gene expression. The altered expression was validated for six transcription regulators that control gene expression in the complex processes of development, differentiation, and proliferation in the liver. The significant overexpression of these transcription factors involved in hepatocyte proliferation and differentiation could lead to a functionally compromised liver contributing to the development of metabolic syndrome. Overexpression of these transcription regulators is involved in cellular functions that lead to the disease in the liver.
The question arises whether similar epigenetic or transcriptional changes occur in maternal livers in response to HF diet. We previously reported that consumption of HF diet during pregnancy altered the maternal metabolic milieu and significant upregulation of expression of genes associated with hepatic lipogenesis (3). In this study, offspring exposed to a maternal HF milieu displayed either downregulation or no change in expression of genes of lipogenesis in HF fetal liver. These divergent transcriptional results may reflect differences in the hepatic response to direct consumption of HF diet, as seen in the adult mothers, compared with in utero exposure to HF proinflammatory developmental milieu, as seen in the fetal offspring.
This study also defines the effect of exposure to a maternal HF diet on DNA methylation in QTLs associated with metabolic disease, particularly on Chrs 4 and 18. Studies have reported exposure to a maternal HF diet alters methylation patterns in one or more genes involved in a specific aspect of metabolism (6, 39). However, ours shows that exposure to a maternal HF diet changes the methylation patterns of specific genomic regions involved in development of metabolic syndrome. The altered expression of all hypermethylated genes in Chrs 4 and 18 QTLs suggests these genes may be involved in development of metabolic syndrome after exposure to a maternal HF diet. One of the differentially methylated and regulated genes in the hotspots, Arhgef19, plays an important role in regulating adipocyte differentiation (40). Horii et al. (40) reported that Arhgef19 mRNA is downregulated in white adipose tissue of HF diet–induced obese mice. Zbtb17/Miz-1, another hotspot gene, is a transcription factor that acts as an activator or repressor depending on its binding partners, Myc, Smad, Dnmt3a, and Dnmt3b (41). The Zbtb17/Myc complex also recruits Dnmt3a and histone deacetylases to promoters, thereby silencing gene expression (42). Zbtb17/Myc can also affect the transforming growth factor-β and Myc signaling networks that contribute to glucose homeostasis (43). We showed that Arhgef19 and Zbtb17/Miz-1 expression was decreased in e18.5 and 26-week liver. Our results show that exposure to a maternal HF diet also resulted in hypermethylation in promoter regions and downregulation of expression of genes that could contribute to the metabolic abnormalities seen in this model (3–5).
Camta1 showed the hypermethylated status from e18.5 through to adulthood (Supplemental Fig. 8 (7.6MB, pptx) , see third CpG). Motif analysis showed the third CpG is in a recognition site for transcription factors, including Pax6, suggesting hypermethylation may be involved in modulation of gene expression during development.
Why specific areas of chromatin, such as the QTLs hotspots on Chrs 4 and 18, are more affected than others is a fundamental question requiring further research. Other basic questions are (1) does the maternal environment alter DNA methylation, and (2) at what time during fetal and postnatal development do these changes occur (44)? Our results show the hypermethylation of Mmp9, Zbtb17, and Camta1 is present in the fetus and persists to 26 weeks. The results suggest in utero exposure to a maternal HF diet induced DNA methylation.
One mechanism by which the HF offspring DNA is hypermethylated could be the modified activity of DNMTs or demethylases (45). Members of this enzymatic family include DNMT3A and DNMT3B, which stimulate de novo methylation, and DNMT1, which actively maintains methylation (46). Despite extensive efforts, the factors responsible for paternal DNA demethylation remain elusive. Several enzymes with possible DNA demethylase activity have been identified (25, 45, 47). Our results show that DNMT enzymatic activity at e18.5 is increased almost twofold in HF, whereas activity at 5 weeks is similar to control. Furthermore, there is no increase in expression of these potential demethylases at 5 weeks. The increase in fetal activity of the DNMT appears to be in part responsible for the significant genome-wide hepatic DNA hypermethylation seen in HF offspring.
Using our outbred murine model of maternal overnutrition, we demonstrated that exposure to a maternal HF diet results in global hepatic DNA hypermethylation in male offspring. The significant methylation changes persist throughout life, particularly in three genes important in growth and metabolism, Arhgef19, Zbtb17/Miz-1, and Mmp9. Moreover, it was discovered that a surprisingly high percentage of hypermethylated DMRs occurred in an atherosclerosis QTL on Chr 4 and insulin-dependent diabetes susceptibility QTL on Chr 18. As we described in the Materials and Methods section, because of sexual dimorphism in gene expression patterns (11) and DNA methylation (12), this study focused on male mice. Future studies will assess whether the specific DNA methylation changes found in male offspring are also present in female offspring exposed to a maternal HF diet.
A limitation of this study is that data were derived from liver homogenates that are composed of different cell types. Epigenetic differences have been seen within the same tissue (48). Furthermore, a significant number of DMRs were in genes specific for immune cells, reflecting the inflammation present in HF, despite offspring being weaned to a LF diet. Additionally, in this study we used liver histone modification maps from mice on a control LF diet. The possibility exists that HF diet alone alters the liver histone modification map; therefore, the overlap of DMRs with gene regulatory regions may be affected by developmental dietary exposures. Presently, we are unaware of publications documenting the effect of HF diet exposure in utero on the hepatic chromatin landscape. Future studies will include characterization of DNA methylation in different hepatic cell types. Nevertheless, our results support the hypothesis that the developmental environment created by maternal HF consumption induces stable global hepatic epigenetic changes that affect gene expression and increase the risk of developing metabolic disease as previously described using this mouse model (3–5).
Acknowledgments
The authors thank the current and past members of the Charron Laboratory for fruitful discussions.
Acknowledgments
This work was supported in part by the National Institutes of Health Grant R21 DK081194 (to M.J.C. and P.M.V.), Diabetes Research Center P60 DK020541, Epigenomics, Liver and Comprehensive Cancer Centers of Albert Einstein College of Medicine) and the American Diabetes Association (1-13-CE-06, to M.J.C.).
Author contributions: Y.S., A.S.G., A.F., and Q.D. performed experiments. X.G., Y.-A.K., Y.Y., K.S., and D.Z. performed analytical methods. J.M.G. provided access to the HELP assay and analytical methods. Y.S., P.M.V., E.B.K., and M.J.C. designed experiments and cowrote the manuscript. M.J.C. conceived and oversaw all experiments. Y.S., M.S., X.G., A.S.G., P.M.V., A.F., Q.D., Y.-A.K., Y.Y., K.S., D.Z., J.M.G., E.B.K., and M.J.C. critically reviewed this manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Chr
- chromosome
- DMR
- differentially methylated region
- DNMT
- DNA methyltransferase
- e18.5
- embrionic day 18.5
- H3K4me1
- histone H3 lysine 4 monomethylation
- HF
- high fat
- ILK
- integrin-linked kinase
- IPA
- Ingenuity Pathway Analysis
- LF
- low fat
- mRNA
- messenger RNA
- PCR
- polymerase chain reaction
- qRT-PCR
- quantitative real-time polymerase chain reaction
- QTL
- quantitative trait locus
- SEM
- standard error of the mean.
References
- 1.Barker DJ. Maternal nutrition, fetal nutrition, and disease in later life. Nutrition. 1997;13(9):807–813. [DOI] [PubMed] [Google Scholar]
- 2.Williams L, Seki Y, Vuguin PM, Charron MJ. Animal models of in utero exposure to a high fat diet: a review. Biochim Biophys Acta. 2014;1842(3):507–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartil K, Vuguin PM, Kruse M, Schmuel E, Fiallo A, Vargas C, Warner MJ, Durand JL, Jelicks LA, Charron MJ. Maternal substrate utilization programs the development of the metabolic syndrome in male mice exposed to high fat in utero. Pediatr Res. 2009;66(4):368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kruse M, Seki Y, Vuguin PM, Du XQ, Fiallo A, Glenn AS, Singer S, Breuhahn K, Katz EB, Charron MJ. High-fat intake during pregnancy and lactation exacerbates high-fat diet-induced complications in male offspring in mice. Endocrinology. 2013;154(10):3565–3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vuguin PM, Hartil K, Kruse M, Kaur H, Lin CL, Fiallo A, Glenn AS, Patel A, Williams L, Seki Y, Katz EB, Charron MJ. Shared effects of genetic and intrauterine and perinatal environment on the development of metabolic syndrome. PLoS One. 2013;8(5):e63021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seki Y, Williams L, Vuguin PM, Charron MJ. Minireview: epigenetic programming of diabetes and obesity: animal models. Endocrinology. 2012;153(3):1031–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bechmann LP, Hannivoort RA, Gerken G, Hotamisligil GS, Trauner M, Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56(4):952–964. [DOI] [PubMed] [Google Scholar]
- 8.Katz EB, Stenbit AE, Hatton K, DePinho R, Charron MJ. Cardiac and adipose tissue abnormalities but not diabetes in mice deficient in GLUT4. Nature. 1995;377(6545):151–155. [DOI] [PubMed] [Google Scholar]
- 9.Stenbit AE, Burcelin R, Katz EB, Tsao TS, Gautier N, Charron MJ, Le Marchand-Brustel Y. Diverse effects of Glut 4 ablation on glucose uptake and glycogen synthesis in red and white skeletal muscle. J Clin Invest. 1996;98(3):629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenbit AE, Tsao TS, Li J, Burcelin R, Geenen DL, Factor SM, Houseknecht K, Katz EB, Charron MJ. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat Med. 1997;3(10):1096–1101. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Schadt EE, Wang S, Wang H, Arnold AP, Ingram-Drake L, Drake TA, Lusis AJ. Tissue-specific expression and regulation of sexually dimorphic genes in mice. Genome Res. 2006;16(8):995–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delahaye F, Wijetunga NA, Heo HJ, Tozour JN, Zhao YM, Greally JM, Einstein FH. Sexual dimorphism in epigenomic responses of stem cells to extreme fetal growth. Nat Commun. 2014;5:5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khulan B, Thompson RF, Ye K, Fazzari MJ, Suzuki M, Stasiek E, Figueroa ME, Glass JL, Chen Q, Montagna C, Hatchwell E, Selzer RR, Richmond TA, Green RD, Melnick A, Greally JM. Comparative isoschizomer profiling of cytosine methylation: the HELP assay. Genome Res. 2006;16(8):1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oda M, Glass JL, Thompson RF, Mo Y, Olivier EN, Figueroa ME, Selzer RR, Richmond TA, Zhang X, Dannenberg L, Green RD, Melnick A, Hatchwell E, Bouhassira EE, Verma A, Suzuki M, Greally JM. High-resolution genome-wide cytosine methylation profiling with simultaneous copy number analysis and optimization for limited cell numbers. Nucleic Acids Res. 2009;37(12):3829–3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson RF, Suzuki M, Lau KW, Greally JM. A pipeline for the quantitative analysis of CG dinucleotide methylation using mass spectrometry. Bioinformatics. 2009;25(17):2164–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ehrich M, Nelson MR, Stanssens P, Zabeau M, Liloglou T, Xinarianos G, Cantor CR, Field JK, van den Boom D. Quantitative high-throughput analysis of DNA methylation patterns by base-specific cleavage and mass spectrometry. Proc Natl Acad Sci USA. 2005;102(44):15785–15790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X, Zhang Z, Gerstein MB, Zheng D. Small RNAs originated from pseudogenes: cis- or trans-acting? PLOS Comput Biol. 2009;5(7):e1000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko YA, Mohtat D, Suzuki M, Park AS, Izquierdo MC, Han SY, Kang HM, Si H, Hostetter T, Pullman JM, Fazzari M, Verma A, Zheng D, Greally JM, Susztak K. Cytosine methylation changes in enhancer regions of core pro-fibrotic genes characterize kidney fibrosis development. Genome Biol. 2013;14(10):R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemeon I, Gutierrez JA, Ho MC, Schramm VL. Characterizing DNA methyltransferases with an ultrasensitive luciferase-linked continuous assay. Anal Chem. 2011;83(12):4996–5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S, Park NY, Valacchi G, Lim Y. Calorie restriction with a high-fat diet effectively attenuated inflammatory response and oxidative stress-related markers in obese tissues of the high diet fed rats. Mediators Inflamm. 2012;2012:984643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dudley KJ, Sloboda DM, Connor KL, Beltrand J, Vickers MH. Offspring of mothers fed a high fat diet display hepatic cell cycle inhibition and associated changes in gene expression and DNA methylation. PLoS One. 2011;6(7):e21662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bogdanovic O, Fernandez-Miñán A, Tena JJ, de la Calle-Mustienes E, Hidalgo C, van Kruysbergen I, van Heeringen SJ, Veenstra GJ, Gómez-Skarmeta JL. Dynamics of enhancer chromatin signatures mark the transition from pluripotency to cell specification during embryogenesis. Genome Res. 2012;22(10):2043–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen Y, Yue F, McCleary DF, Ye Z, Edsall L, Kuan S, Wagner U, Dixon J, Lee L, Lobanenkov VV, Ren B. A map of the cis-regulatory sequences in the mouse genome. Nature. 2012;488(7409):116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harth-Hertle ML, Scholz BA, Erhard F, Glaser LV, Dölken L, Zimmer R, Kempkes B. Inactivation of intergenic enhancers by EBNA3A initiates and maintains polycomb signatures across a chromatin domain encoding CXCL10 and CXCL9. PLoS Pathog. 2013;9(9):e1003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okada Y, Yamagata K, Hong K, Wakayama T, Zhang Y. A role for the elongator complex in zygotic paternal genome demethylation. Nature. 2010;463(7280):554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stoll M, Kwitek-Black AE, Cowley AW Jr, Harris EL, Harrap SB, Krieger JE, Printz MP, Provoost AP, Sassard J, Jacob HJ. New target regions for human hypertension via comparative genomics. Genome Res. 2000;10(4):473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28(3):391–401. [DOI] [PubMed] [Google Scholar]
- 28.Feinberg AP, Irizarry RA, Fradin D, Aryee MJ, Murakami P, Aspelund T, Eiriksdottir G, Harris TB, Launer L, Gudnason V, Fallin MD. Personalized epigenomic signatures that are stable over time and covary with body mass index. Sci Transl Med. 2010;2(49):49ra67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dunham I, Kundaje A, Aldred SF, et al. ; ENCODE Project Consortium . An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X, Han H, De Carvalho DD, Lay FD, Jones PA, Liang G. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26(4):577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D’Souza C, Fouse SD, Johnson BE, Hong C, Nielsen C, Zhao Y, Turecki G, Delaney A, Varhol R, Thiessen N, Shchors K, Heine VM, Rowitch DH, Xing X, Fiore C, Schillebeeckx M, Jones SJ, Haussler D, Marra MA, Hirst M, Wang T, Costello JF. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466(7303):253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neri F, Rapelli S, Krepelova A, Incarnato D, Parlato C, Basile G, Maldotti M, Anselmi F, Oliviero S. Intragenic DNA methylation prevents spurious transcription initiation. Nature. 2017;543(7643):72–77. [DOI] [PubMed] [Google Scholar]
- 33.Thomas RM, Sai H, Wells AD. Conserved intergenic elements and DNA methylation cooperate to regulate transcription at the il17 locus. J Biol Chem. 2012;287(30):25049–25059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz YB, Cavalli G. Three-dimensional genome organization and function in Drosophila. Genetics. 2017;205(1):5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abou Zeid N, Vallés AM, Boyer B. Serine phosphorylation regulates paxillin turnover during cell migration. Cell Commun Signal. 2006;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gagné D, Groulx JF, Benoit YD, Basora N, Herring E, Vachon PH, Beaulieu JF. Integrin-linked kinase regulates migration and proliferation of human intestinal cells under a fibronectin-dependent mechanism. J Cell Physiol. 2010;222(2):387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brizzi MF, Tarone G, Defilippi P. Extracellular matrix, integrins, and growth factors as tailors of the stem cell niche. Curr Opin Cell Biol. 2012;24(5):645–651. [DOI] [PubMed] [Google Scholar]
- 38.Unal R, Yao-Borengasser A, Varma V, Rasouli N, Labbate C, Kern PA, Ranganathan G. Matrix metalloproteinase-9 is increased in obese subjects and decreases in response to pioglitazone. J Clin Endocrinol Metab. 2010;95(6):2993–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lillycrop KA, Burdge GC. Epigenetic mechanisms linking early nutrition to long term health. Best Pract Res Clin Endocrinol Metab. 2012;26(5):667–676. [DOI] [PubMed] [Google Scholar]
- 40.Horii T, Morita S, Kimura M, Hatada I. Epigenetic regulation of adipocyte differentiation by a Rho guanine nucleotide exchange factor, WGEF. PLoS One. 2009;4(6):e5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Riggelen J, Müller J, Otto T, Beuger V, Yetil A, Choi PS, Kosan C, Möröy T, Felsher DW, Eilers M. The interaction between Myc and Miz1 is required to antagonize TGFbeta-dependent autocrine signaling during lymphoma formation and maintenance. Genes Dev. 2010;24(12):1281–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Licchesi JD, Van Neste L, Tiwari VK, Cope L, Lin X, Baylin SB, Herman JG. Transcriptional regulation of Wnt inhibitory factor-1 by Miz-1/c-Myc. Oncogene. 2010;29(44):5923–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Algamas-Dimantov A, Davidovsky D, Ben-Ari J, Kang JX, Peri I, Hertz R, Bar-Tana J, Schwartz B. Amelioration of diabesity-induced colorectal ontogenesis by omega-3 fatty acids in mice. J Lipid Res. 2012;53(6):1056–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laker RC, Wlodek ME, Connelly JJ, Yan Z. Epigenetic origins of metabolic disease: the impact of the maternal condition to the offspring epigenome and later health consequences. Food Science and Human Wellness. 2013;2:1–11. [Google Scholar]
- 45.Fuso A, Ferraguti G, Grandoni F, Ruggeri R, Scarpa S, Strom R, Lucarelli M. Early demethylation of non-CpG, CpC-rich, elements in the myogenin 5′-flanking region: a priming effect on the spreading of active demethylation. Cell Cycle. 2010;9(19):3965–3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Subramaniam D, Thombre R, Dhar A, Anant S. DNA methyltransferases: a novel target for prevention and therapy. Front Oncol. 2014;4:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mohr F, Döhner K, Buske C, Rawat VP. TET genes: new players in DNA demethylation and important determinants for stemness. Exp Hematol. 2011;39(3):272–281. [DOI] [PubMed] [Google Scholar]
- 48.Grigoriu A, Ferreira JC, Choufani S, Baczyk D, Kingdom J, Weksberg R. Cell specific patterns of methylation in the human placenta. Epigenetics. 2011;6(3):368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]