Abstract
A substantial amount of evidence suggests that androgen signaling through classical androgen receptors is critical for both normal and pathologic ovarian physiology. Specifically, we and others have shown that, in mouse granulosa cells, androgen actions through both extranuclear and nuclear androgen receptor signaling are critical for normal follicle development and ovulation. Here, we show that androgens through the PI3K/Akt pathway rapidly (within minutes) phosphorylate and inhibit activity of the Polycomb group protein enhancer of zeste homolog 2 (Ezh2). Over the course of 24 to 48 hours, androgens then induce expression of the microRNA miR-101, which targets Ezh2 messenger RNA (mRNA), leading to a nearly complete loss of Ezh2 protein expression. This long-term androgen-induced loss of Ezh2 actions ultimately results in sustained reduction of the H3K27me3-repressive mark in the promoter region of the Runt-related transcription factor-1 (Runx1) gene, a luteinizing hormone (LH)–induced transcription factor essential for ovulation, leading to increased Runx1 mRNA expression. Accordingly, blocking androgen-induced inhibition of Ezh2 in vivo adversely affects LH-induced Runx1 mRNA expression and subsequent ovulation. Importantly, although estrogen treatment of granulosa cells similarly causes rapid activation of the PI3K/Akt pathway and short-term phosphorylation of Ezh2, it does not induce miR-101 expression and thereby does not reduce overall Ezh2 expression, demonstrating the androgen specificity of long-term Ezh2 suppression. Thus, this study provides insight regarding how androgen-induced extranuclear kinase signaling and intranuclear transcription through Ezh2 modifications may influence the expression pattern of genes, ultimately affecting various downstream physiological processes.
During follicular development, androgens through PI3K/Akt signaling and AR-mediated transcription of an anti-Ezh2 microRNA, miR-101, regulate gene expression and downstream functions like ovulation.
In women, androgens act both as an essential adjuvant for follicular development as well as a harmful agent when present in excessive amounts. Although the global and cell-specific androgen receptor (AR) knockout (ARKO) mouse models (1) as well as various in vitro studies (2) have established the essentiality of androgens in normal female fertility, elevated androgens in females have long been associated with poor health and reduced fertility (3). These observations warrant a better understanding of the role of androgens in the regulation of folliculogenesis. Physiological actions of steroid hormones are mediated through crosstalk between steroid-induced extranuclear kinase signaling and intranuclear transcription (4–6). Previously, we reported (2, 7, 8) that androgens through membrane-localized ARs trigger matrix metalloproteinase-mediated transactivation of the epidermal growth factor receptor, which in turn leads to cytoplasmic MAPK3/1 signaling. This process is highly conserved across tissues and species (5). Moreover, we have shown that the physiological effects of androgens involve a synergistic action between these extranuclear AR signals and intranuclear transcriptional effects of ARs (2). In this study, we focus on other mechanism(s) of androgen-induced actions both inside and outside of the nucleus that lead to altered gene expression and subsequent changes in ovarian function.
ARs transcriptionally regulate the expression of a number of genes either directly through binding to AR elements (AREs) or indirectly by modulating other transcriptional regulators, with the latter being less well understood. Intriguingly, although androgens have been shown to play a major role in follicular development, very few ovarian genes have been identified as direct AR targets. This demonstrates the need to better characterize potential indirect effects of androgens on gene expression. Regulation of gene expression is controlled at a number of different levels, one of which is the accessibility of the transcription machinery to the genes and their controlling elements. This accessibility largely depends on the degree of chromatin compaction, which is influenced in part by the Polycomb group proteins. One of the Polycomb group proteins, enhancer of zeste homolog 2 (Ezh2), a histone methyltransferase, promotes histone H3 lysine 27 trimethylation (H3K27me3). This methylation event in turn restricts the ability of cofactors and enhancers to bind to specific DNA sequences, causing subsequent gene silencing (9). Accordingly, inhibition of Ezh2 prevents the placement of H3K27me3-repressive marks, thereby inducing gene expression. Chromatin remodeling via histone modification is now considered as a key control point for transcription and a primary target of signal transduction. Posttranslational modification (10) through kinase-dependent phosphorylation (11–13) is one of the major ways through which the activity of epigenetic programmers like Ezh2 can be regulated (14). However, the detailed underlying mechanisms of DNA methylation or histone modifications by acetylation or methylation, what causes these modifications, and how these changes affect downstream physiological processes are still poorly understood. Interestingly, two studies have reported regulation of gene transcription through rapid steroid receptor signaling–mediated histone modification. Xenoestrogens/endocrine-disrupting chemicals (15) through the estrogen receptor–induced PI3K/Akt pathway have been shown to regulate Ezh2 and mixed-lineage leukemia protein 1 activity, causing developmental reprogramming of genes involved in uterine myometrial cells (16) and prostate cancer (17), respectively.
Here we demonstrate that androgens, through both extranuclear and nuclear signaling, regulate gene expression in ovarian granulosa cells (GCs) through modulation of Ezh2 activity and expression. Similar to estrogens, we demonstrate in both mouse and human ovarian GCs that androgens suppress Ezh2 activity first via rapid activation of Akt-induced Ezh2 phosphorylation. Unlike estrogens, we find that androgens then suppress overall Ezh2 expression in the long term through AR-mediated transcription of an anti-Ezh2 microRNA, miR-101. These changes ultimately regulate downstream physiological functions such as ovulation.
Materials and Methods
Animals and cell culture
Mouse studies were performed in accordance with the guidelines for the care and use of laboratory animals and were approved by the University Committee on Animal Resources at the University of Rochester. Unless otherwise mentioned, mouse experiments were performed in 8- to 12-week-old C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME). Collection and culture of mouse GCs were performed as described previously (2, 7, 18–20). Mouse GCs were cultured for 24 to 48 hours prior to serum starvation and treatment. KGN cells (a human GC tumor cell line; RIKEN BioResource Center, Japan) were cultured for 18 to 24 hours in Dulbecco’s modified Eagle medium/F-12 medium containing 10% fetal bovine serum and 1% penicillin and streptomycin. Cells were serum starved for 4 hours followed by stimulation with 25 nM dihydrotestosterone (DHT) for different time points. For experiments using inhibitors (LY294002, 10 μM, and flutamide, 100 nM), cells were pretreated with the inhibitors for 30 minutes prior to DHT stimulation. For all our experiments, we used DHT instead of testosterone to avoid misinterpretation of our results due to aromatization of testosterone to estradiol.
Western blot analysis
Western blots were performed as described previously (7, 18–21). Primary antibodies used were: anti-rabbit Akt, anti-rabbit phosphorylated Akt (S473), and anti-rabbit glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Cell Signaling Technology, Danvers, MA); anti-mouse Ezh2 (Abcam, Cambridge, UK); and anti-rabbit phosphorylated (S21)Ezh2 (Abcam and Bethyl, Montgomery, TX).
RNA extraction and real-time polymerase chain reaction
RNA from ovaries or cells was isolated using the RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions, and levels of specific messenger RNA (mRNA) expression were analyzed by delta delta cycle threshold (Ct) method using One Step TaqMan gene expression assay primers (Applied Biosystems, Foster City, CA) and the Applied Biosystems StepOnePlus real-time polymerase chain reaction (RT-PCR) system. One microgram of RNA was used for all of the RT-PCR reactions. GAPDH was used as an endogenous control.
miR-101 isolation and detection
Total RNA was isolated using the standard Trizol isolation method according to the manufacturer’s instructions, and RT-PCR was performed using the TaqMan MicroRNA Reverse Transcription Kit and mouse/human miR-101 TaqMan MicroRNA Assays (Applied Biosystems). U6 was used as an endogenous control, and relative expression of miR-101 was calculated using the delta delta Ct method.
Chromatin immunoprecipitation assay
Chromatin immunoprecipitation (ChIP) was performed as described previously (7) with the MAGnify Chromatin Immunoprecipitation System (Invitrogen, Carlsbad, CA), according to manufacturer’s instructions. Chromatin fragments were immunoprecipitated with Dynabeads coupled with mouse monoclonal anti-H3K27me3 and rabbit polyclonal anti-KMT6/EZH2 ChIP-grade antibody (Abcam) and immunoglobulin G as nonspecific control. Quantitative PCR was performed using EXPRESS SYBR GreenERTM qPCR SuperMixes (Invitrogen) with previously published primers (22) designed for two regions of the human RUNX promoter (P1 and P2) [Supplemental Fig. 4(a) (10.2MB, tif) ].
Ezh2 knockdown and rescue experiments
Small interfering RNA (siRNA)–mediated Ezh2 knockdown and rescue experiments were performed as described previously (2, 7, 8). Cells were treated with nontargeting siRNA pool for mouse/human Ezh2 siRNA ON-TARGET Plus SMARTpool according to manufacturer’s instructions (catalog no. AM16708; Thermo Fisher Scientific, Waltham, MA) for 48 hours prior to 4 hours of serum starvation followed by 48 hours of DHT treatment. For rescue experiments, after siRNA, cells were transfected (FUGENE 6; Roche, Indianapolis, IN) with wild-type or mutated (S21A) Ezh2 (serine 21 converted to alanine), as described (8). After 24 hours, cells were serum starved for 4 hours followed by stimulation with or without DHT (25 nM) for 24 hours.
miR-101 inhibition in vitro
Primary mouse GCs or KGN cells were transfected with miR-101a-3p mirVana miRNA Inhibitor or with nonspecific control (Ambion). After 48 hours, GCs were serum starved for 4 hours and then stimulated with DHT. Knockdown was confirmed by measuring miR-101 expression levels. In vivo: Ovarian bursal injections were performed as described previously (2, 23, 24). miRCURY locked nucleic acid (LNA) miR-101 inhibitor (Exiqon) or vehicle control was injected into ovarian bursa (n = 3/treatment). After 3 days following surgery, animals were subjected to a superovulation regimen [5 U of pregnant mare serum gonadotropin (Sigma, St. Louis, MO) followed 48 hours later by 5 U of human chorionic gonadotropin (hCG; Sigma)]. After an additional 14 hours, oocyte/cumulus masses were surgically isolated from the oviduct and counted. Expression of miR-101, Runt-related transcription factor 1 (Runx1), and other Runx1-regulated genes were determined by RT-PCR from single ovaries isolated from individual animals.
Statistical analysis
Each in vitro experiment was repeated at least 3 times or more, and data are displayed as mean ± standard error of the mean. Statistical analysis was performed using Prism version 6 (GraphPad). Analysis of variance followed by Tukey-Kramer was used to detect differences between treatments. P ≤ 0.05 was considered significant.
Results
Androgens through PI3K/Akt signaling mediate Ezh2 serine phosphorylation
Androgens regulate follicular physiology through nuclear and extranuclear actions. Although ARs are DNA binding proteins that initiate transcriptional complexes, surprisingly few direct transcriptional targets of ARs have been identified in the ovary (25–30). Instead, genes identified so far by microarray analysis in patients who have polycystic ovary syndrome (PCOS), in prenatal androgenized animal models, or in AR null mouse models do not have AREs in their promoters. We therefore posed that, instead of directly regulating genes containing AREs, androgens may have a more indirect effect on ovarian gene expression. Previous studies (16) in the uterus show that xenoestrogens through the PI3K/Akt pathway regulate Ezh2 and thus histone methylation. Given that androgens can also trigger PI3K/Akt signaling (31) and the importance of Ezh2 in regulating transcription, we initially examined whether androgen-induced PI3K/Akt signaling influenced Ezh2 activity and/or expression in mouse ovarian GCs.
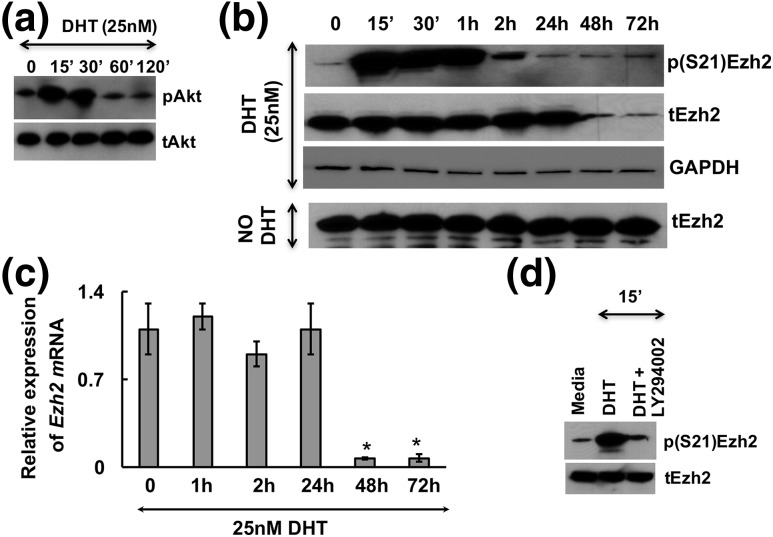
Results show that DHT treatment promotes transient phosphorylation of Akt [Fig. 1(a)] as well as phosphorylation of endogenous Ezh2 at serine 21 (S21) [Fig. 1(b)] in mouse primary GCs. Ezh2 phosphorylation occurs within 15 minutes of DHT stimulation and returns to nearly basal levels after 2 hours of treatment [Fig. 1(b)]. Interestingly, total Ezh2 levels decline by 48 to 72 hours in DHT-treated GCs, but not in untreated cells [Fig. 1(b)]. Similarly, Ezh2 mRNA levels are also downregulated by DHT stimulation at 48 hours [Fig. 1(c)]. Akt-dependent S21 phosphorylation of Ezh2 has been reported to block the methyltransferase activity of Ezh2 by disrupting the association between Ezh2 and other polycomb repressive complex 2 subunits (11, 16, 32). Therefore, to test whether the observed DHT-induced phosphorylation of Ezh2 at S21 is mediated by the DHT-induced PI3K/Akt pathway, we treated GCs with DHT in the presence or absence of a PI3K inhibitor, LY294002. Figure 1(d) shows that the DHT-induced phosphorylation of Ezh2 at S21 is inhibited by the PI3K inhibitor LY294002. These observations suggest that in GCs, androgens may regulate Ezh2 activity by two different mechanisms: first by rapid phosphorylation and inactivation of Ezh2 at S21 through PI3K/Akt signaling (11) and second by long-term suppression of Ezh2 expression. Quantitative densitometry of these immunoblots and others from repeated studies are shown in Supplemental Fig. 1 (12.8MB, tif) .
Figure 1.
Androgens through PI3K/Akt signaling mediate Ezh2 serine phosphorylation. DHT induces (a) rapid transient activation of Akt and (b) phosphorylation of endogenous Ezh2 at serine 21 in primary mouse GCs. (c) Androgen-induced Ezh2 phosphorylation is dependent on activation of PI3K/Akt signaling. GCs pretreated with the PI3K inhibitor LY294002 (10 μM) inhibit the DHT-induced (25 nM, 15-minute stimulation) Ezh2 phosphorylation. (d) Long-term effect of DHT treatment on phosphorylated [p(S21)Ezh2] and total (tEzh2) protein levels. Results are representative of three separate experiments. (e) Ezh2 mRNA levels at different time points after DHT treatment in mouse GCs. The average Ct value for Ezh2 mRNA levels declined from 21.7 (at 0 hours) to 25.0 (at 48 hours). Data are displayed as mean ± standard error of the mean (n = 3 experiments) and normalized to GAPDH levels. *P ≤ 0.05.
Androgen-induced miR-101 suppresses Ezh2 expression
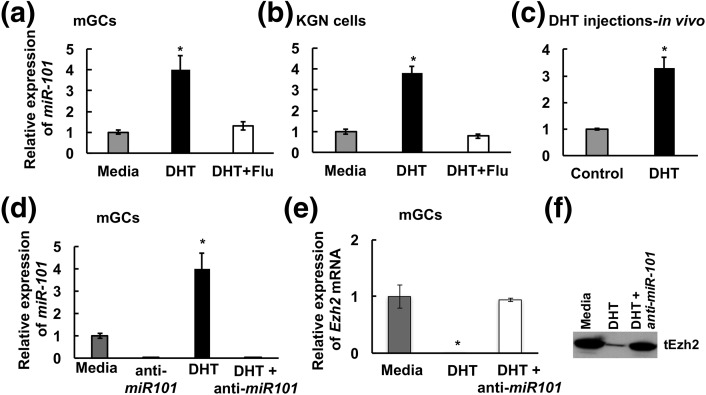
How is DHT suppressing Ezh2 mRNA and protein expression at 48 hours? Ezh2 mRNA is an established target of miR-101 (33), and sequences upstream of the miR-101 coding region have AREs (34). Moreover, studies in prostate cancer cells have shown that AR regulates miR-101 expression (18). Therefore, we examined whether miR-101 might be suppressing Ezh2 mRNA expression in response to androgens. Indeed, 24 hours of DHT treatment induces miR-101 expression in isolated primary mouse GCs [Fig. 2(a)] as well as in human KGN GCs [Fig. 2(b)]. This induction is inhibited by the AR antagonist flutamide, thereby demonstrating that this phenomenon is a direct AR function. DHT injection into female mice also promotes miR-101 expression in ovarian GCs in vivo [Fig. 2(c)]. Knockdown of miR-101 expression in primary mouse GCs [Fig. 2(d)] blocks DHT-induced (48-hour) attenuation of Ezh2 mRNA [Fig. 2(e)] expression [Fig. 2(f)]. These results confirm that miR-101 is likely regulating androgen-mediated suppression of Ezh2 mRNA expression. Notably, whereas DHT treatment significantly induces miR-101 expression at 24 hours [Fig. 2(a) and 2(b)], loss of Ezh2 mRNA and total Ezh2 protein expression does not peak until 48 hours [Fig. 1(b) and 1(c)].
Figure 2.
Androgens induce miR-101 expression and miR-101 targets Ezh2. DHT-induced (25 nM, 24 hours) expression of miR-101 in presence or absence of flutamide (100 nM) in (a) mouse primary GC (mGC) culture, (b) KGN cell line, and (C) GCs isolated from mice injected with 10 mg DHT for 3 days. The average Ct values for miR-101 in media, DHT, and DHT plus flutamide–treated samples were 26.8/24.7/26.6 (mouse primary GCs), 25.6/23.6/26.3 (KGN cells), and 25.8/24.1 (DHT injections), respectively. (d–f) Effect of downregulation of miR-101 by anti-miR-101 inhibitor in primary mouse GC culture treated with DHT (25 nM, 48 hours) on (d) miR-101, (e) Ezh2 mRNA, and (f) Ezh2 protein levels. The average Ct values for miR-101 in media and anti-miR101–treated samples were 35.2 and 36.6, respectively. Data are displayed as mean ± standard error of the mean (n = 3 experiments) and normalized to U6 levels. *P ≤ 0.05.
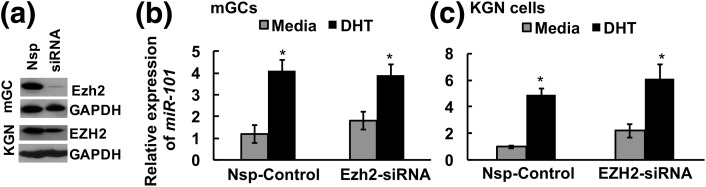
Studies (35) in hepatocarcinoma cells have reported the existence of a reciprocal negative feedback loop between Ezh2 and miR-101 whereby Ezh2 itself regulates miR-101 expression. Thus, we determined whether DHT-induced expression of miR-101 is mediated through suppression of Ezh2. In fact, knockdown of Ezh2 protein expression in mouse GCs or EZH2 in human KGN cells [Fig. 3(a)] has little effect on baseline or DHT-induced miR-101 expression [Fig. 3(b) and 3(c)], thereby suggesting that DHT directly enhances miR-101 expression independent of Ezh2.
Figure 3.
Ezh2 is not required for miR-101 expression. siRNA-mediated knockdown of (a) Ezh2 has no effect on DHT-induced miR-101 expression in (b) mouse GCs (mGCs) and (c) human KGN cells. Data are displayed as mean ± standard error of the mean (n = 3 experiments) and normalized to U6 levels. *P ≤ 0.05. Nsp, nonspecific.
Because estrogens through the PI3K/Akt pathway can also regulate Ezh2, as shown previously (16), we wanted to compare androgen- and estrogen-induced Ezh2 modulation in the ovary. In primary mouse GCs, we find that, although estrogen can also transiently phosphorylate Ezh2 at S21 through the PI3K/Akt pathway [Supplemental Fig. 2(a) and 2(b) (9.3MB, tif) ], it does not induce miR-101 expression [Supplemental Fig. 2(c) (9.3MB, tif) ]. This indicates that the long-term suppression of Ezh2 expression mediated by miR-101 is an androgen-specific action.
Runx1 is an androgen-regulated Ezh2 target gene
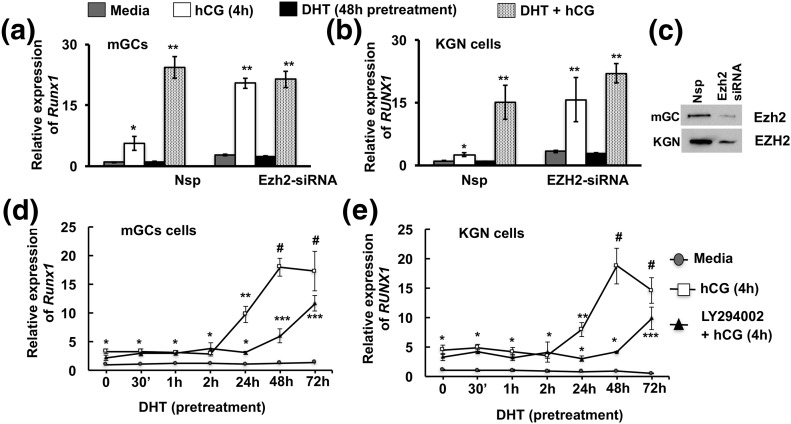
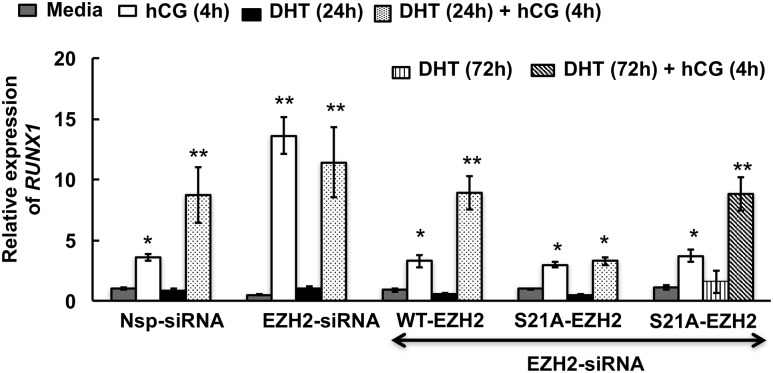
In our efforts to identify downstream targets of this androgen-mediated alteration of Ezh2 actions, we found Runx1, a transcription factor important for ovulation and regulated by luteinizing hormone (LH) or hCG in periovulatory follicles (36), to be an androgen-regulated Ezh2 target (Fig. 4). Notably, studies in prostate cancer cells (37) have also shown that Runx1 is an Ezh2-regulated gene. Results in primary mouse GCs show that hCG treatment (4 hours) significantly induces Runx1 mRNA expression [Fig. 4(a), left side]. Whereas DHT alone has no effect on Runx1 mRNA expression, priming with DHT for 48 hours followed by hCG stimulation (4 hours), causes significant induction of Runx1 mRNA expression compared with hCG treatment alone [Fig. 4(a), left side]. Knockdown of Ezh2 in mouse GCs by siRNA [Fig. 4(c)] has no effect on basal Runx1 mRNA levels; however, the induction of Runx1 mRNA expression by hCG in untreated GCs with reduced Ezh2 mimics the enhancement with DHT-primed hCG-treated GCs with normal Ezh2 endogenous expression [Fig. 4(a), right side]. These results demonstrate that, although androgens do not directly induce Runx1 expression, they play an essential indirect role in the regulation of Runx1 expression. Androgen-induced suppression of Ezh2 activity and expression might demethylate histones and open the promoter region of the Runx1 gene, making it a conducive environment for enhanced LH-induced Runx1 gene expression. Of note, the same effects of DHT and hCG on RUNX1 mRNA expression are also seen in human KGN cells [Fig. 4(b)].
Figure 4.
Runx1 is a downstream target gene for androgen-mediated modulation of Ezh2 expression and activity. Runx1 mRNA levels in (a) mouse GCs (mGCs) and (b) KGN cell line treated with nonspecific (Nsp) and Ezh2-specific siRNA and stimulated with media alone, hCG for 4 hours, or DHT (25 nM) for 48 hours or DHT priming for 48 hours followed by hCG for 4 hours. (c) Confirmation of Ezh2 knockdown. Data are displayed as mean ± standard error of the mean (n = 3 experiments) and normalized to GAPDH levels. *P ≤ 0.05, **P ≤ 0.02. (d and e) Pattern of Runx1 mRNA levels in (d) mouse GCs and (e) KGN cell line pretreated with DHT (25 nM) for different durations followed by media or hCG stimulation (4 hours) in presence or absence of the PI3K inhibitor LY294002 (10 μM). Data are displayed as mean ± standard error of the mean (n = 3 experiments) and normalized to GAPDH levels. *, **, ***P ≤ 0.05, #P ≤ 0.005 (* vs corresponding time point of media, ** vs LY294002 plus hCG, *** vs 24-hour LY294002 plus hCG, and # vs LY294002 plus hCG and 24-hour hCG treatment).
Androgen-induced regulation of Runx1 expression is mediated by both rapid (PI3K/Akt) and long-term (miR-101) modulation of Ezh2
Given that androgens regulate Ezh2 activity through rapid (PI3K/Akt-mediated) and long-term (miR-101-mediated) mechanisms, we next elucidated which of these two mechanisms is involved in enhancing Runx1 expression. Figure 4(d) represents Runx1 mRNA levels in primary mouse GCs cells primed with DHT for different time points followed by vehicle or hCG treatment of 4 hours. Results show that a minimum of 24-hour DHT priming is necessary for significant enhancement of hCG-induced Runx1 mRNA expression. Because DHT-induced reductions in Ezh2 protein occur just beyond 24 hours [Fig. 1(b)], we further investigated whether rapid DHT-induced phosphorylation of Ezh2 through of PI3K/Akt signaling contributes to hCG-induced increases in Runx1 mRNA levels. The PI3K inhibitor LY294002 does not abrogate the effects of DHT priming on hCG-induced Runx1 mRNA induction, but simply delays it by 24 to 48 hours [Fig. 4(d)]. Importantly, LY294002 has no effect on DHT-induced miR-101 expression in cells pretreated with DHT for 72 hours [Supplemental Fig. 3(a) (13.9MB, tif) ], indicating that DHT induction of miR-101 occurs independent of PI3K/Akt signaling. Finally, the same results of PI3K inhibition are seen when examining EZH2 mRNA expression in human KGN cells [Fig. 4(e)]. Together, these results suggest that the enhancement of hCG-induced Runx1 mRNA expression after DHT pretreatment occurs first rapidly due to Ezh2 inactivation in an Akt-dependent fashion, but then long term via loss of Ezh2 mRNA and Ezh2 protein by miR-101 in an Akt-independent fashion.
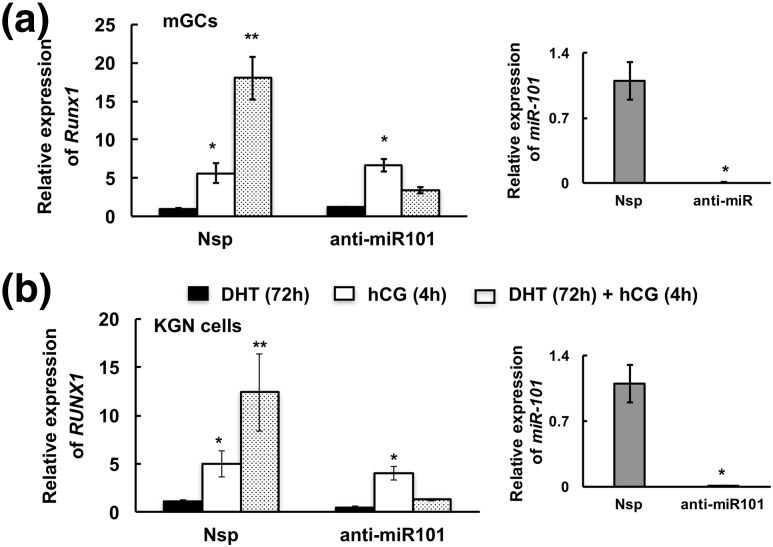
To confirm the role of DHT-induced Akt-mediated phosphorylation of Ezh2 in hCG-triggered Runx1 expression, we mutated S21 to alanine (S21A-Ezh2) in a human EZH2 complementary DNA clone. Thereafter in siRNA-mediated EZH2 knocked-down KGN cells, we overexpressed mutated or wild-type EZH2 and primed the cells with/without DHT (24 hours) followed by hCG stimulation for 4 hours. We hypothesized that without the serine phosphorylation site, EZH2 would be unaffected by Akt signaling and would therefore remain active and suppress short-term RUNX1 mRNA expression. Supplemental Fig. 3(b–d) (13.9MB, tif) shows the validation of the mutation S/A21-Ezh2 and its overexpression in the Ezh2 knockdown cells. We find that overexpression of the S/A21-EZH2 mutant in EZH2 knockdown cells abrogates the positive effects of 24 hours of DHT priming on hCG-induced RUNX1 expression (Fig. 5). However, when these same cells are primed with DHT for 72 hours (Fig. 5, last group on the right), hCG stimulation of RUNX1 mRNA expression is again enhanced. These results confirm that DHT-mediated phosphorylation of EZH2 at S21 is the primary mediator of early (24-hour) DHT effects on RUNX1 mRNA expression; however, long-term effects (e.g., 72 hours) of DHT priming on hCG-induced RUNX1 mRNA expression likely occur through miR-101-mediated loss of Ezh2 expression. Accordingly, anti-miR-101 inhibitor–mediated downregulation of miR-101 (Fig. 6) completely abolishes the long-term (72-hour) positive effects of DHT on hCG-induced RUNX1 mRNA expression in human KGN cells [Fig. 6(b)]. Anti-miR-101 treatment in primary mouse GCs similarly abrogates positive effects of DHT on hCG-induced Runx1 mRNA expression [Fig. 6(a)]. Thus, by 72 hours, the short-term effect of DHT on Ezh2 phosphorylation is lost and the effect of DHT on Runx1 mRNA expression is solely regulated through miR101-mediated modulation of Ezh2 mRNA levels.
Figure 5.
Androgen-induced phosphorylation of Ezh2 at serine 21 is necessary for Runx1 expression. Pattern of RUNX1 mRNA expression in EZH2 knockdown KGN cells overexpressing either wild-type or mutated EZH2 and stimulated with media alone or hCG for 4 hours, DHT (25 nM) for 48 hours/72 hours, or DHT priming for 48 hours/72 hours followed by hCG for 4 hours. Data are displayed as means ± standard error of the mean (n = 3 experiments) and normalized to GAPDH levels. *, **P ≤ 0.05 (* vs media and ** vs hCG). Western blot results are representative of three separate experiments.
Figure 6.
miR-101 regulates Runx1 expression. Runx1 mRNA levels in (a) mouse GCs (mGCs) and (b) KGN cells treated with nonspecific (Nsp) and anti-miR-101 inhibitor and stimulated by DHT (25 nM) for 72 hours or hCG for 4 hours or primed with DHT for 72 hours followed by 4 hours of hCG stimulation. miR-101 expression is shown on the right. Data are displayed as means ± standard error of the mean (n = 3 experiments) and normalized to GAPDH (for Runx1) and U6 (for miR-101) levels. *, **P ≤ 0.05 (* vs DHT or Nsp and ** vs hCG).
Androgens through Ezh2 modulation decrease the H3K27me3 methylation mark on the Runx1 promoter
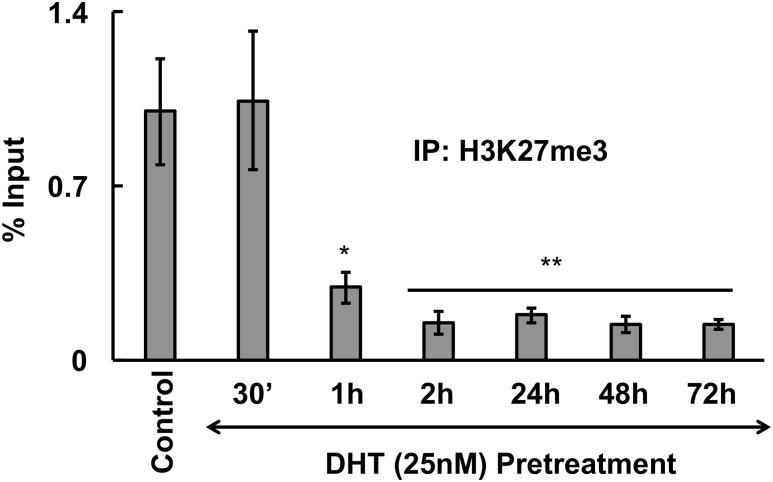
Given that Ezh2 causes methylation of H3K27me3, a negative marker for gene expression, we examined H3K27me3 levels on the RUNX1 promoter over time with respect to DHT treatment in human KGN cells. We performed ChIP assays on two regions of the RUNX1 promoter [P1 shown in Fig. 7 and P2 represented in Supplemental Fig. 4(b) (10.2MB, tif) ] with an antibody against H3K27me3. Results show [Fig. 7; Supplemental Fig. 4(b) (10.2MB, tif) ] that DHT rapidly (by 60 minutes) suppresses H3K27me3 levels on the RUNX1 promoter (most likely due to Akt-mediated inactivation of Ezh2) and the decrease in methylation continues for at least 72 hours (most likely due to miR-101-mediated loss of EZH2 protein expression). This loss in methylation may therefore be the reason for the observed increase in RUNX1 mRNA transcription. To demonstrate that this decrease in H3K27me3 levels on the RUNX1 promoter is through DHT-induced modulation of Ezh2, we performed a corresponding ChIP showing loss or decreased association of Ezh2 on RUNX1 promoter following DHT treatment [Supplemental Fig. 4(c) (10.2MB, tif) ]. Results show that association of Ezh2 with the Runx1 promoter decreases over time. In fact, at the 24-hour time point, even when p(S21)Ezh2 is dephosphorylated, the association of Ezh2 with the Runx1 promoter is significantly lower compared with controls.
Figure 7.
Androgen treatment decreases the H3K27me3 methylation mark on the Runx1 promoter. Anti-H3K27me3 ChIP assay in KGN cells showing the level of H3K27me3 on the Runx1 promoter I following DHT (25 nM) treatment of different time points. Values represent percentage input (means ± standard error of the mean, n = 3 experiments, *, **P ≤ 0.05). IP, immunoprecipitation.
DHT-regulated Ezh2-mediated modulation of Runx1 expression is essential for ovulation
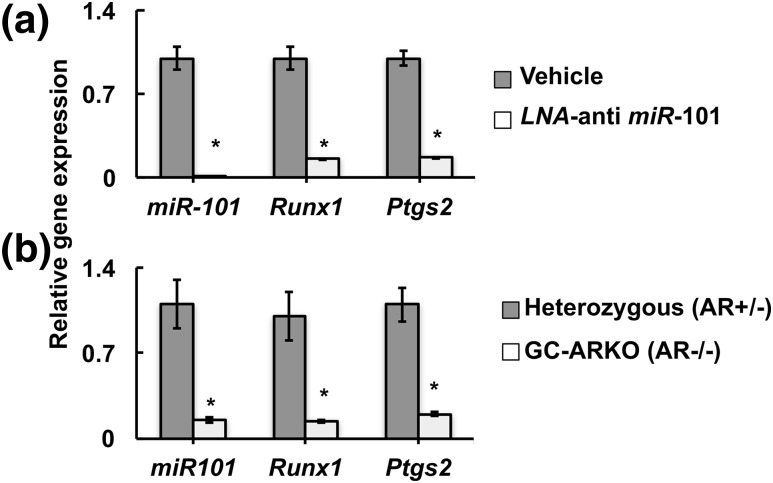
Finally, to demonstrate the physiological significance of Ezh2-mediated modulation of Runx1 expression, we knocked down miR-101 expression in vivo (Fig. 8) by injecting LNA-containing oligonucleotides targeting miR-101 or vehicle into the ovarian bursa of mice. Results show that, following superovulation, animals treated with the LNA anti-miR-101 oligonucleotides ovulated significantly fewer oocytes compared with control animals (Table 1). Quantitative PCR confirmed loss of miR-101 expression in vivo, as well as downregulation of ovarian expression of Runx1 mRNA and Runx1-regulated mRNAs encoding prostaglandin-endoperoxide synthase 2 (Ptgs2; also called cyclooxygenase-2) [Fig. 8(a)]. Ptgs2 plays (22) an obligatory role in ovulation (38, 39). To further prove that the androgen-induced modification of Runx1 promoter and therefore Runx1 expression is physiologically important, we determined the expression of miR-101, Runx1, and Ptgs2 mRNA in ovaries of 24- to 25-week-old GC-specific ARKO mice that were subjected to superovulation. Notably, we have shown previously that these GC-specific ARKO animals ovulate fewer oocytes both naturally and in response to superovulation (18). Based on our present data, it can be speculated that one of the reasons for reduced ovulation in the GC-specific ARKO mice is due to the loss of androgen-induced regulation of Ezh2 and its effect on Runx1 transcription. Supporting this hypothesis, results show [Fig. 8(b)] that indeed the expression of miR-101, Runx1, and Ptgs2 mRNA are significantly lower in ovaries of GC-specific ARKO animals compared with heterozygous littermates.
Figure 8.
In vivo knockdown of miR-101 disrupts ovulation. (a) Relative expression of miR-101, Runx1, and Ptgs2 in GCs from animals injected with LNA-containing oligonucleotides targeting miR-101 into the ovarian bursa and vehicle control. (b) Relative expression of miR-101, Runx1, and Ptgs2 in ovaries from 24- to 25-week-old GC-specific ARKO (GC-ARKO, AR–/–) and heterozygous (AR +/–) controls. Data are displayed as means ± standard error of the mean (n = 3 experiments) and normalized to U6 (for miR-101) or GAPDH (for Runx1 and Ptgs2) levels. *P ≤ 0.05.
Table 1.
Number of Superovulated Oocytes in Animals Injected With LNA-Containing Oligonucleotides Targeting miR-101 Into the Ovarian Bursa vs Vehicle Control
| Treatment | Oocyte Count |
|---|---|
| Vehicle (phosphate-buffered saline) | 20.33 ± 1.8 |
| LNA/anti-miR-101 | 10.66 ± 1.45a |
Data are displayed as means ± standard error of the mean (n = 3).
P ≤ 0.05.
Discussion
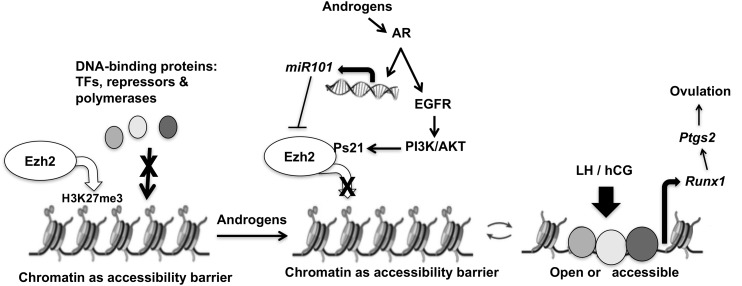
This study uncovers a unique mechanism whereby androgen-induced extracellular signals are transduced to genomic events via specific histone modifications. Here (Fig. 9) we demonstrate that androgens inactivate Ezh2 via two independent mechanisms. First, via extranuclear AR signaling, androgens promote a transient phosphorylation of Ezh2 at S21 through PI3K/Akt that is known to inhibit Ezh2 activity and reduce the Ezh2-mediated genomic H3K27me3-repressive mark. Second, by 48 hours, androgens, through intranuclear AR-mediated transcription, induce miR-101 expression. Once miR-101 is expressed, it targets Ezh2, causing a more permanent decline in H3K27me3 levels that may ultimately lead to upregulation of a myriad of genes in the ovary. One important highlight of this study is that it shows that, in GCs, androgens through modulating histone modifications may indirectly regulate gene expression to regulate ovarian function. Runx1 is one such downstream target gene where androgen-induced suppression of Ezh2 removes the H3K27me3-repressive mark from the Runx1 promoter. This significantly increases hCG-induced Runx1 expression, most likely by enabling the hCG-induced transcription machinery to access the Runx1 promoter region. Importantly, this regulation of Runx1 expression by modulation of histone methyl marks (H3K27me3) on its promoter can be seen through examining targets downstream of Runx1, including alterations in expression of Ptgs2, a gene that is essential for ovulation.
Figure 9.
Proposed model for androgen-regulated Runx1 expression through modulation of Ezh2 expression and activity. See text for details. TF, transcription factor.
In this study, we primarily focused on the androgen-induced PI3K/Akt pathway because we found that the androgen-induced MAPK pathway has no effect on phosphorylation of Ezh2 at S21. However, because nongenomic androgen-induced MAPK signaling has been shown to be critical for many genomic transcriptional androgen effects (1, 2, 7), the androgen-induced MAPK pathway may still be involved in the long-term downregulation of Ezh2 expression through regulation of miR-101. Specifically, we have reported that androgens through transactivation of the epidermal growth factor receptor can induce MAPK3/1 signaling (2, 5, 7, 8). Upon activation, MAPK3/1 promotes serine phosphorylation of the multidomain adaptor protein paxillin, resulting in the nuclear localization of phosphoserine-paxillin where it complexes with AR to retain AR in the nucleus, allowing AR-mediated transcription to occur (2, 7). This pathway is conserved in human prostate cancer (7) as well as in mouse GCs (2). Therefore, it is possible that the AR-MAPK3/1-paxillin pathway may be involved in androgen-induced expression of miR-101.
Intriguingly, our studies show that DHT-induced Akt-mediated phosphorylation of Ezh2 at S21 is transient, with p(S21)Ezh2 being dephosphorylated after 2 hours of DHT treatment. The decline in Ezh2 expression levels by miR-101 occurs only after 48 hours of DHT treatment, leaving a time gap where presumably Ezh2 reactivates. However, our ChIP studies show a loss of H3K27me3 mark on two different regions of the human RUNX1 promoter within 60 minutes of DHT treatment that continues through 72 hours. These results suggest that, although phosphorylation of Ezh2 at S21 by Akt inhibits Ezh2 activity and lowers the H3K27me3 mark, there may exist a time lag between dephosphorylation of Ezh2 at S21 and reactivation of Ezh2 to lay down the H3K27me3 mark. This concept of transient phosphorylation followed by dephosphorylation resulting in effects lasting hours to days has been reported in a myriad of other kinase signaling pathways (e.g., MAPK, Akt, RTK activation). For example, studies in mouse uteri (16) have shown that transient Ezh2 modulation by xenoestrogens via the PI3K/Akt pathway can reprogram the expression profile of estrogen-responsive genes in uterine myometrial cells with long-lasting effects, suggesting this as a potential mechanism for developmental reprogramming caused by early-life exposure to xenoestrogens (16).
Notably, in our study we find that, although estrogen treatment of GCs can cause a transient inactivation of Ezh2 through PI3K/Akt-mediated phosphorylation of Ezh2 at S21, estrogen does not induce miR-101 expression. Thus, our studies provide a unique mechanism of androgen action involving transient and long-term regulation of Ezh2 that may also serve as a potential mechanism for androgen-induced developmental reprogramming. A large volume of studies now suggests that perinatal exposure in the form of maternal/fetal pathologies, nutritional deficits/excess, environmental chemicals, lifestyle choices/stress, and medical interventions alters the developmental course of the fetus or offspring, leading to long-term harmful outcomes that often culminate in adult pathologies. In fact, fetal exposure to androgenic substances has been reported to cause developmental reprogramming via alteration of the epigenome in animal models (3, 40–46) as well as in humans in conditions such as congenital adrenal hyperplasia or after developmental exposure to endocrine-disrupting chemicals with androgenic properties like phthalates (47–50). These early androgen exposures predispose the next generation to various disease conditions like PCOS and metabolic dysfunction (51–56). Although the mechanisms behind the developmental route culminating in these animal models as well as in women who have PCOS are still unclear, this study provides a possible mechanistic understanding of androgen actions and their global effect on ovarian gene expression by regulating histone modulators rather than simply regulating genes containing AREs.
Although androgen excess is detrimental, so is a lack of androgen actions in the ovary. Previously, we (1, 18, 25) and others (57, 58) reported that, in females, androgens play a critical role in ovarian physiology and in maintaining normal fertility. In fact, knockout of AR specifically in GCs of the ovary results in female mice with impaired follicular development, lower ovulation rates, reduced number of oocytes ovulated, and development of premature ovarian failure (18). AR expression is highest in the growing preantral follicles (1, 25), and our ARKO mouse studies (2, 18) show that androgens are essential for the transition from preantral to antral stage as well as in preventing follicular atresia during normal development. Based on our present study, we propose that, at the preantral stage of follicular development, one of the underlying mechanisms of androgen actions may be mediated through the loss of Ezh2 to facilitate the induction of different genes that are important for follicular development. Here we report Runx1 as one such downstream target gene of androgen-induced Ezh2 modulation by which androgens may influence ovulation. The LH-induced Runx1 in turn regulates the expression of Ptgs2. The latter is obligatory for ovulation, as Ptgs2-deficient mice lack ovulation and are infertile (38, 39). Further studies will be necessary to identify all the genes regulated by this androgen-induced Ezh2 modulation or other genes that may be regulated by androgen-induced miR-101 and its effect on follicular development. Interestingly, a recent study by Maekawa et al. (59) shows that mRNA levels of Ezh2, as well as recruitment of Ezh2 to the promoter regions of StAR, decreases in rat GCs undergoing luteinization after ovulation induction by hCG. How LH decreases Ezh2 mRNA levels is not known. It is possible that Runx1 expression may also be influenced by LH-induced decrease in Ezh2. However, our results show that priming GCs with androgens significantly increases the induction of Runx1 expression by hCG. It is likely that, during follicular development, androgens through Ezh2 modulation increase the sensitivity of GCs to LH/hCG effects. Based on our previously published GC-specific ARKO mouse studies (18), as well as other studies (60), it is known that androgen actions during follicular development primarily occur at preantral stage, because AR expression is highest in these follicles and decreases significantly in large antral and preovulatory follicles. Therefore, the above-described priming effects of androgens through androgen-induced Ezh2 modulation may occur significantly earlier than the LH surge in preantral and small antral follicles that lack LH receptor.
In summary, we provide direct experimental evidence showing that androgens through a synergistic effect between transient membrane-initiated AR signaling and a long-term nuclear transcriptional effect promote histone modifications that control the expression of specific genes and regulate downstream physiological processes essential for female fertility. Given that androgens play a major role in various normal and pathophysiological conditions in women, results of the current study provide insights into our understanding of the androgen-induced molecular mechanisms responsible for normal ovarian physiology as well as disease conditions such as PCOS.
Acknowledgments
Acknowledgments
This work was supported by National Institutes of Health Grants RO1HD086062-01A1 (to A.S.) and RO1GM101709 (to S.R.H.) and a grant from the Foundation for Reproductive Medicine (to A.S.).
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Name of Antibody | RRID | Species Raised in; Monoclonal or Polyclonal | Dilution Used | DOI or Publication Data |
|---|---|---|---|---|
| Phospho-Akt Ser473 | AB_329825 | Rabbit; polyclonal | 1:1000 WB | Breast Cancer Res 2017;19(1):9 |
| GAPDH (14C10) | AB_561053 | Mouse; monoclonal | 1:2000 WB | Sci Rep 2016;6:37927 |
| Anti-KMT6/EZH2 | AB_2661845 | Rabbit; polyclonal | 1:1000 WB | Int J Mol Med 2016;37:1627–1635 |
| Anti-KMT6/EZH2: ChIP grade | AB_304045 | Rabbit; polyclonal | 1:100 ChIP | |
| Anti-H3K27me3: ChIP grade | AB_305237 | Mouse; monoclonal | 1:100 ChIP | |
| Phospho-EZH2-S21 | AB_1576532 | Rabbit; polyclonal | 1:1000 WB | Science 2005;310:306–310 |
| Anti-KMT6/EZH2 (phospho S21) | AB_1860363 | Rabbit; polyclonal | 1:1000 WB | |
| Akt | AB_329827 | Rabbit; polyclonal | 1:1000 WB | Breast Cancer Res 2017;19(1):9 |
Abbreviations: RRID, research resource identifier; WB, Western blot.
Footnotes
- AR
- androgen receptor
- ARE
- androgen receptor element
- ARKO
- androgen receptor knockout
- ChIP
- chromatin immunoprecipitation
- Ct
- cycle threshold
- DHT
- dihydrotestosterone
- Ezh2
- enhancer of zeste homolog 2
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GC
- granulosa cell
- H3K27me3
- histone H3 lysine 27 trimethylation
- hCG
- human chorionic gonadotropin
- LH
- luteinizing hormone
- LNA
- locked nucleic acid
- mRNA
- messenger RNA
- PCOS
- polycystic ovary syndrome
- PCR
- polymerase chain reaction
- Ptgs2
- prostaglandin-endoperoxide synthase 2
- RT-PCR
- real-time polymerase chain reaction
- Runx1
- Runt-related transcription factor 1
- siRNA
- small interfering RNA.
References
- 1.Prizant H, Gleicher N, Sen A. Androgen actions in the ovary: balance is key. J Endocrinol. 2014;222(3):R141–R151. [DOI] [PubMed] [Google Scholar]
- 2.Sen A, Prizant H, Light A, Biswas A, Hayes E, Lee HJ, Barad D, Gleicher N, Hammes SR. Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci USA. 2014;111(8):3008–3013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walters KA. Androgens in polycystic ovary syndrome: lessons from experimental models. Curr Opin Endocrinol Diabetes Obes. 2016;23(3):257–263. [DOI] [PubMed] [Google Scholar]
- 4.Hammes SR, Levin ER. Minireview: recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152(12):4489–4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sen A, Prizant H, Hammes SR. Understanding extranuclear (nongenomic) androgen signaling: what a frog oocyte can tell us about human biology. Steroids. 2011;76(9):822–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levin ER, Hammes SR. Nuclear receptors outside the nucleus: extranuclear signalling by steroid receptors. Nat Rev Mol Cell Biol. 2016;17(12):783–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen A, De Castro I, Defranco DB, Deng FM, Melamed J, Kapur P, Raj GV, Rossi R, Hammes SR. Paxillin mediates extranuclear and intranuclear signaling in prostate cancer proliferation. J Clin Invest. 2012;122(7):2469–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen A, O’Malley K, Wang Z, Raj GV, Defranco DB, Hammes SR. Paxillin regulates androgen- and epidermal growth factor-induced MAPK signaling and cell proliferation in prostate cancer cells. J Biol Chem. 2010;285(37):28787–28795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, Morey L, Van Eynde A, Bernard D, Vanderwinden JM, Bollen M, Esteller M, Di Croce L, de Launoit Y, Fuks F. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439(7078):871–874. [DOI] [PubMed] [Google Scholar]
- 10.Wei Y, Chen YH, Li LY, Lang J, Yeh SP, Shi B, Yang CC, Yang JY, Lin CY, Lai CC, Hung MC. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat Cell Biol. 2011;13(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cha TL, Zhou BP, Xia W, Wu Y, Yang CC, Chen CT, Ping B, Otte AP, Hung MC. Akt-mediated phosphorylation of EZH2 suppresses methylation of lysine 27 in histone H3. Science. 2005;310(5746):306–310. [DOI] [PubMed] [Google Scholar]
- 12.Yan J, Li B, Lin B, Lee PT, Chung TH, Tan J, Bi C, Lee XT, Selvarajan V, Ng SB, Yang H, Yu Q, Chng WJ. EZH2 phosphorylation by JAK3 mediates a switch to noncanonical function in natural killer/T-cell lymphoma. Blood. 2016;128(7):948–958. [DOI] [PubMed] [Google Scholar]
- 13.Badeaux AI, Shi Y. Emerging roles for chromatin as a signal integration and storage platform. Nat Rev Mol Cell Biol. 2013;14:211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morey L, Helin K. Polycomb group protein-mediated repression of transcription. Trends Biochem Sci. 2010;35(6):323–332. [DOI] [PubMed] [Google Scholar]
- 15.Walker CL. Minireview: epigenomic plasticity and vulnerability to EDC exposures. Mol Endocrinol. 2016;30(8):848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24(5):993–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Q, Trevino LS, Wong RL, Medvedovic M, Chen J, Ho SM, Shen J, Foulds CE, Coarfa C, O’Malley BW, Shilatifard A, Walker CL. Reprogramming of the epigenome by MLL1 links early-life environmental exposures to prostate cancer risk. Mol Endocrinol. 2016;30(8):856–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol. 2010;24(7):1393–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma X, Hayes E, Prizant H, Srivastava RK, Hammes SR, Sen A. Leptin-induced CART (Cocaine-and Amphetamine-Regulated Transcript) is a novel intra-ovarian mediator of obesity-related infertility in females. Endocrinology. 2016;157(3):1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes E, Kushnir V, Ma X, Biswas A, Prizant H, Gleicher N, Sen A. Intra-cellular mechanism of Anti-Müllerian hormone (AMH) in regulation of follicular development. Mol Cell Endocrinol. 2016;433:56–65. [DOI] [PubMed] [Google Scholar]
- 21.Evaul K, Hammes SR. Cross-talk between G protein-coupled and epidermal growth factor receptors regulates gonadotropin-mediated steroidogenesis in Leydig cells. J Biol Chem. 2008;283(41):27525–27533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Park ES, Jo M. Runt-related transcription factor 1 regulates luteinized hormone-induced prostaglandin-endoperoxide synthase 2 expression in rat periovulatory granulosa cells. Endocrinology. 2009;150(7):3291–3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, Han J. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118(5):1944–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carletti MZ, Fiedler SD, Christenson LK. MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol Reprod. 2010;83(2):286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sen A, Hammes SR. Androgens: they don’t just make a man out of you. Expert Rev Obstet Gynecol. 2011;6:19–22. [Google Scholar]
- 26.Wu YG, Bennett J, Talla D, Stocco C. Testosterone, not 5α-dihydrotestosterone, stimulates LRH-1 leading to FSH-independent expression of Cyp19 and P450scc in granulosa cells. Mol Endocrinol. 2011;25(4):656–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walters KA, Allan CM, Handelsman DJ. Androgen actions and the ovary. Biol Reprod. 2008;78(3):380–389. [DOI] [PubMed] [Google Scholar]
- 28.Walters KA, Allan CM, Jimenez M, Lim PR, Davey RA, Zajac JD, Illingworth P, Handelsman DJ. Female mice haploinsufficient for an inactivated androgen receptor (AR) exhibit age-dependent defects that resemble the AR null phenotype of dysfunctional late follicle development, ovulation, and fertility. Endocrinology. 2007;148(8):3674–3684. [DOI] [PubMed] [Google Scholar]
- 29.Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci USA. 2006;103(1):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu YC, Wang PH, Yeh S, Wang RS, Xie C, Xu Q, Zhou X, Chao HT, Tsai MY, Chang C. Subfertility and defective folliculogenesis in female mice lacking androgen receptor. Proc Natl Acad Sci USA. 2004;101(31):11209–11214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun M, Yang L, Feldman RI, Sun XM, Bhalla KN, Jove R, Nicosia SV, Cheng JQ. Activation of phosphatidylinositol 3-kinase/Akt pathway by androgen through interaction of p85alpha, androgen receptor, and Src. J Biol Chem. 2003;278(44):42992–43000. [DOI] [PubMed] [Google Scholar]
- 32.Rojanasakul Y. Linking JNK-STAT3-Akt signaling axis to EZH2 phosphorylation: a novel pathway of carcinogenesis. Cell Cycle. 2013;12(2):202–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varambally S, Cao Q, Mani RS, Shankar S, Wang X, Ateeq B, Laxman B, Cao X, Jing X, Ramnarayanan K, Brenner JC, Yu J, Kim JH, Han B, Tan P, Kumar-Sinha C, Lonigro RJ, Palanisamy N, Maher CA, Chinnaiyan AM. Genomic loss of microRNA-101 leads to overexpression of histone methyltransferase EZH2 in cancer. Science. 2008;322(5908):1695–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cao P, Deng Z, Wan M, Huang W, Cramer SD, Xu J, Lei M, Sui G. MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1alpha/HIF-1beta. Mol Cancer. 2010;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang D, Wang X, Zhuang C, Shi W, Liu M, Tu Q, Zhang D, Hu L. Reciprocal negative feedback loop between EZH2 and miR-101-1 contributes to miR-101 deregulation in hepatocellular carcinoma. Oncol Rep. 2016;35(2):1083–1090. [DOI] [PubMed] [Google Scholar]
- 36.Jo M, Curry TE Jr. Luteinizing hormone-induced RUNX1 regulates the expression of genes in granulosa cells of rat periovulatory follicles. Mol Endocrinol. 2006;20(9):2156–2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takayama K, Suzuki T, Tsutsumi S, Fujimura T, Urano T, Takahashi S, Homma Y, Aburatani H, Inoue S. RUNX1, an androgen- and EZH2-regulated gene, has differential roles in AR-dependent and -independent prostate cancer. Oncotarget. 2015;6(4):2263–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davis BJ, Lennard DE, Lee CA, Tiano HF, Morham SG, Wetsel WC, Langenbach R. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1beta. Endocrinology. 1999;140(6):2685–2695. [DOI] [PubMed] [Google Scholar]
- 39.Lim H, Paria BC, Das SK, Dinchuk JE, Langenbach R, Trzaskos JM, Dey SK. Multiple female reproductive failures in cyclooxygenase 2-deficient mice. Cell. 1997;91(2):197–208. [DOI] [PubMed] [Google Scholar]
- 40.Walters KA, Allan CM, Handelsman DJ. Rodent models for human polycystic ovary syndrome. Biol Reprod. 2012;86(5):1–12. [DOI] [PubMed] [Google Scholar]
- 41.Padmanabhan V, Manikkam M, Recabarren S, Foster D. Prenatal testosterone excess programs reproductive and metabolic dysfunction in the female. Mol Cell Endocrinol. 2006;246(1-2):165–174. [DOI] [PubMed] [Google Scholar]
- 42.Padmanabhan V, Veiga-Lopez A. Animal models of the polycystic ovary syndrome phenotype. Steroids. 2013;78(8):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67(1):155–163. [DOI] [PubMed] [Google Scholar]
- 44.Dumesic DA, Abbott DH, Padmanabhan V. Polycystic ovary syndrome and its developmental origins. Rev Endocr Metab Disord. 2007;8(2):127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87(3):1111–1119. [DOI] [PubMed] [Google Scholar]
- 46.Abbott DH, Barnett DK, Bruns CM, Dumesic DA. Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum Reprod Update. 2005;11(4):357–374. [DOI] [PubMed] [Google Scholar]
- 47.Rajesh P, Balasubramanian K. Phthalate exposure in utero causes epigenetic changes and impairs insulin signalling. J Endocrinol. 2014;223(1):47–66. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Arguelles DB, Papadopoulos V. Prenatal phthalate exposure: epigenetic changes leading to lifelong impact on steroid formation. Andrology. 2016;4(4):573–584. [DOI] [PubMed] [Google Scholar]
- 49.Ziv-Gal A, Gallicchio L, Chiang C, Ther SN, Miller SR, Zacur HA, Dills RL, Flaws JA. Phthalate metabolite levels and menopausal hot flashes in midlife women. Reprod Toxicol. 2016;60:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watkins DJ, Milewski S, Domino SE, Meeker JD, Padmanabhan V. Maternal phthalate exposure during early pregnancy and at delivery in relation to gestational age and size at birth: a preliminary analysis. Reprod Toxicol. 2016;65:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu N, Azziz R, Goodarzi MO. Epigenetics in polycystic ovary syndrome: a pilot study of global DNA methylation. Fertil Steril. 2010;94(2):781–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kokosar M, Benrick A, Perfilyev A, Fornes R, Nilsson E, Maliqueo M, Behre CJ, Sazonova A, Ohlsson C, Ling C, Stener-Victorin E. Epigenetic and transcriptional alterations in human adipose tissue of polycystic ovary syndrome. Sci Rep. 2016;6:22883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu N, Chua AK, Jiang H, Liu NA, Goodarzi MO. Early embryonic androgen exposure induces transgenerational epigenetic and metabolic changes. Mol Endocrinol. 2014;28(8):1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu N, Kwon S, Abbott DH, Geller DH, Dumesic DA, Azziz R, Guo X, Goodarzi MO. Epigenetic mechanism underlying the development of polycystic ovary syndrome (PCOS)-like phenotypes in prenatally androgenized rhesus monkeys. PLoS One. 2011;6(11):e27286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dkhil MA, Al-Quraishy S, Abdel-Baki AA, Ghanjati F, Arauzo-Bravo MJ, Delic D, Wunderlich F. Epigenetic modifications of gene promoter DNA in the liver of adult female mice masculinized by testosterone. J Steroid Biochem Mol Biol. 2015;145:121–130. [DOI] [PubMed] [Google Scholar]
- 56.Luense LJ, Veiga-Lopez A, Padmanabhan V, Christenson LK. Developmental programming: gestational testosterone treatment alters fetal ovarian gene expression. Endocrinology. 2011;152(12):4974–4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walters KA, Middleton LJ, Joseph SR, Hazra R, Jimenez M, Simanainen U, Allan CM, Handelsman DJ. Targeted loss of androgen receptor signaling in murine granulosa cells of preantral and antral follicles causes female subfertility. Biol Reprod. 2012;87(6):151. [DOI] [PubMed] [Google Scholar]
- 58.Walters KA, Simanainen U, Gibson DA. Androgen action in female reproductive physiology. Curr Opin Endocrinol Diabetes Obes. 2016;23(3):291–296. [DOI] [PubMed] [Google Scholar]
- 59.Maekawa R, Lee L, Okada M, Asada H, Shinagawa M, Tamura I, Sato S, Tamura H, Sugino N. Changes in gene expression of histone modification enzymes in rat granulosa cells undergoing luteinization during ovulation. J Ovarian Res. 2016;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walters KA. Role of androgens in normal and pathological ovarian function. Reproduction. 2015;149(4):R193–R218. [DOI] [PubMed] [Google Scholar]