Abstract
Trans-10, cis-12 conjugated linoleic acid (10,12 CLA) is a dietary fatty acid that promotes weight loss and disproportionate fat loss. Obese mice fed a high-fat, high-sucrose (HFHS) diet containing 10,12 CLA are resistant to weight gain and contain markedly reduced subcutaneous fat and adiponectin, with a concurrent lack of improvement in insulin sensitivity despite significant weight loss. Taken together, 10,12 CLA promotes a phenotype resembling peroxisome proliferator-activated receptor (PPAR)γ antagonism. Because thiazolidinediones such as rosiglitazone (Rosi) are used clinically to improve insulin sensitivity by activating PPARγ, with particular efficacy in subcutaneous white adipose tissue, we hypothesized that Rosi would improve glucose metabolism in mice losing weight with 10,12 CLA. Obese low-density lipoprotein receptor–deficient mice were fed a HFHS control diet, or supplemented with 1% 10,12 CLA with or without Rosi (10 mg/kg) for 8 weeks. Body composition, glucose and insulin tolerance tests, tissue gene expression, and plasma lipid analyses were performed. Mice consuming 10,12 CLA with Rosi lost weight and body fat compared with control groups, but with a healthier redistribution of body fat toward more subcutaneous adipose tissue than with 10,12 CLA alone. Further, Rosi improved 10,12 CLA–mediated insulin resistance parameters and increased plasma and subcutaneous adipose tissue adiponectin levels without adverse effects on plasma or hepatic lipids. We conclude that cotreatment of mice with 10,12 CLA and Rosi promotes fat loss with a healthier fat distribution that leads to improved insulin sensitivity, suggesting that the combination treatment strategy of 10,12 CLA with Rosi could have therapeutic potential for obesity treatment.
Obese mice supplemented with 10,12 CLA lose weight, without improved insulin sensitivity. Adding rosiglitazone maintains weight loss but also improves insulin sensitivity by increasing adiponectin.
Because obesity is reaching global epidemic proportions, novel strategies for weight reduction are urgently needed (1). A nutraceutical weight loss supplement in widespread use is conjugated linoleic acid (CLA) (2). CLA includes 28 naturally occurring isomers of linoleic acid, of which cis-9, trans-11 (9,11), and trans-10, cis-12 (10,12) CLA are the most naturally abundant. Based on evidence that CLA reduces adiposity, formulations containing equal amounts of 9,11 and 10,12 CLA are available in supplement form for weight loss. Of the two primary isomers, 10,12 CLA is responsible for reducing adiposity (3); therefore, this fatty acid is under intense investigation in the obesity community. However, 10,12 CLA has also been associated with adverse effects such as inflammation and insulin resistance (4), which lessens its appeal as a weight loss supplement.
A master regulator of adiposity, peroxisome proliferator-activated receptor (PPAR)γ, the most abundantly expressed PPAR isoform in adipose tissue, plays a major role in lipogenesis and differentiation of mature adipocytes (5). In particular, PPARγ activation directly increases gene expression of adiponectin (Adipoq), which maintains adipocyte lipid storage and improves whole-body insulin sensitivity (6, 7). PPARγ itself also plays a major role in glucose homeostasis by improving insulin sensitivity and decreasing hepatic glucose production, thereby lowering circulating glucose, fatty acids, and triglycerides (8, 9). As such, thiazolidinediones (TZDs) such as rosiglitazone (Rosi) that target and activate PPARγ are widely used for the treatment of type 2 diabetes mellitus (10). We and others have shown that 10,12 CLA reduces PPARγ expression and/or activity in cultured adipocytes by dramatically reducing triglyceride storage, mediated by decreased glucose uptake via glucose transporter 4, increased triglyceride lipolysis, and increased fatty acid oxidation (FAO), all processes that suggest PPARγ antagonism (11, 12). This raises the intriguing possibility that restoring PPARγ activity in 10,12 CLA-treated adipocytes could alleviate some adverse effects attributed to this dietary supplement.
We have extended our previous in vitro findings to show that 10,12 CLA reduces PPARγ expression in mouse adipose tissue (13), as has been suggested previously (14). In a mouse model of metabolic syndrome, mice receiving 1% 10,12 CLA on a high-fat, high-sucrose (HFHS) diet (a dose that appropriately mimics human CLA supplementation) lost considerable fat mass and gained lean mass but did not show improved insulin sensitivity over obese controls (13). In addition, these mice lost considerable subcutaneous fat mass, while visceral fat mass remained unchanged, the opposite of that seen with PPARγ agonists such as TZDs (15). By contrast, a weight-matched calorically restricted control group that did not receive 10,12 CLA lost equivalent body weight but showed improved insulin sensitivity and blood glucose levels with maintained relative mass of subcutaneous white adipose tissue (WAT) (13). Concurrently, 10,12 CLA reduced WAT Pparg and its downstream target Adipoq gene expression, which was not observed in the weight-matched control mice (13). In addition, cotreatment with mixed CLA and Rosi has been previously shown to improve insulin sensitivity in mouse models during the early stages of obesity development (16) and in obese mice not displaying CLA-mediated weight loss (17). We now expand these previous findings to examine the effect of 10,12 CLA on weight loss combined with Rosi treatment to improve insulin sensitivity in mice with established obesity and characteristics resembling a more humanoid metabolic syndrome phenotype. We hypothesized that Rosi supplementation would restore insulin sensitivity by blunting adipokine loss in HFHS-induced, obese, low-density lipoprotein receptor–deficient (Ldlr−/−) mice supplemented with 10,12 CLA. The results from this study offer an approach to the treatment of obesity with a readily accessible therapeutic option for weight loss.
Materials and Methods
In vitro experiments
CLA isomers were obtained from Nu-Check Prep (Waterville, MN) and conjugated to fatty acid- and endotoxin-free albumin (FAFA; Sigma-Aldrich, St. Louis, MO) at a 3:1 (CLA:albumin) molar ratio in 100 mM sodium hydroxide as described previously (18); 3T3-L1 fibroblasts (ATCC, Manassas, VA; mycoplasma-free) were grown to confluency and differentiated into cells resembling mature adipocytes as previously described (11). Differentiated adipocytes were treated with FAFA or CLA, +/− 1 nM Rosi (Sigma-Aldrich), replenished daily for 7 days. Cells were given Rosi 2 days prior to CLA cotreatment to ensure adequate levels of PPARγ substrate were present.
Gene expression
Total RNA (2 µg) from cells or frozen mouse tissue was reverse-transcribed into cDNA, and quantitative real-time polymerase chain reaction was performed using an Applied Biosystems (Carlsbad, CA) 7900HT system. Target genes were amplified using commercial TaqMan probe sets (Applied Biosystems), using β-2 microglobulin and glyceraldehyde-3-phosphate dehydrogenase as endogenous housekeeping controls. Gene expression was quantified using the comparative threshold cycle method and presented as a fold change from FAFA-treated control cells, or from epididymal WAT (EWAT) of mice fed the HFHS diet.
PPAR translocation assay
PPAR translocation was quantified using a PPARγ, PPARα, or PPARδ transcription factor assay kit from AbCam (Cambridge, MA) according to the manufacturer’s instructions. Briefly, nuclear protein extracts were prepared from differentiated 3T3-L1 adipocytes following 7 days of the indicated treatments. A double-stranded DNA sequence specific for the PPARγ, PPARα, or PPARδ response element was used to capture each PPAR isoform from these nuclear protein extracts, and detected using an antibody specific for PPARγ, PPARα, or PPARδ in an enzyme-linked immunosorbent assay–based fashion. Bound nuclear PPARγ is presented as optical density per milligram of nuclear protein.
Mouse study design
Ldlr−/− (C57Bl/6 background, Jackson Laboratories) mice were used due to their propensity to develop a phenotype resembling human metabolic syndrome, including insulin resistance, systemic inflammation, and obesity when fed a diet rich in saturated fat and sucrose with added cholesterol (19, 20). Male mice were used because they more reliably become obese and insulin resistant than female mice (21). Ten-week-old adult male mice were fed a HFHS (36% fat from lard, 36.2% sucrose) diet with 0.15% added cholesterol for 12 weeks to induce obesity and insulin resistance. Half the mice were then put on a HFHS diet containing Rosi maleate (purchased from Sigma-Aldrich and admixed into the HFHS diet at 10 mg/kg) for 2 weeks (Rosi pretreatment period), while the remaining mice continued on the HFHS diet. Mice were then placed on one of four diets for an additional 8 weeks: (1) HFHS diet, (2) HFHS + 10,12 CLA, (3) HFHS + Rosi, or (4) HFHS + 10,12 CLA + Rosi. Diets of 10,12 CLA replaced 1% (weight-to-weight ratio) of the lard isocalorically (Nu-Check Prep; >90% purity). Mice were randomized to test diets. All test diets were prepared by Bio-Serv (Flemington, NJ). This CLA dose appropriately mimics human CLA supplementation, based on calculations using a body surface area normalization method to compare human and mouse dose equivalencies (13, 22). Mice were housed four to five per cage in a specific pathogen-free facility with ad libitum access to food and water. Body weights were recorded weekly. Blood was sampled after 12 weeks of HFHS feeding (baseline), after 3 weeks on test diets, and at euthanasia. Following isoflurane anesthesia, harvested tissues were snap-frozen in liquid nitrogen and stored at −70°C or were fixed with 10% neutral-buffered formalin and embedded in paraffin wax. All experimental procedures were undertaken with approval from the Institution Animal Care and Use Committee of the University of Washington, according to the eighth edition of the Guide for the Care and Use of Laboratory Animals (23).
Body composition
Measurements of body lean and fat mass were determined in live, conscious animals using quantitative magnetic resonance spectroscopy (EchoMRI-700TM; Echo MRI, Houston, TX) at baseline (9 weeks of HFHS feeding) and after 5 weeks on test diets. Measurements were performed by the University of Washington Nutrition Obesity Research Center Energy Balance Core, as described previously (24).
Glucose homeostasis
An intraperitoneal glucose tolerance test (IPGTT) was performed on 4-hour-fasted mice after 10 weeks on HFHS diet (baseline), and again after 6 weeks of test diets. A glucose dose of 1.0 mg/kg lean body mass was administered followed by blood glucose measurements at 0, 15, 30, 60, and 120 minutes after injection. Area under the curve was calculated using Graph Pad Prism software. Intraperitoneal insulin tolerance testing was determined on 4-hour-fasted mice after 11 weeks on HFHS diet (baseline) and again after 7 weeks of test diets by injecting 1 U/kg lean body mass of human insulin intraperitoneally and measuring blood glucose at 0, 30, 60, and 120 minutes after injection.
Plasma analyses and hepatic lipids
Blood glucose was measured using a commercial glucometer (One Touch Ultra). Plasma insulin, adiponectin, and serum amyloid A (SAA) were measured as described previously (13). Plasma interleukin-6 (IL-6) was measured using an enzyme-linked immunosorbent assay from EMD Millipore (EZMIL6, Billerica MA), according to the manufacturer’s instructions. Nonesterified fatty acids (NEFAs) were quantified from freshly isolated fasting plasma using a commercial assay (Wako Diagnostics, Mountain View, CA). Lipoproteins were separated from pooled plasma samples by fast-phase liquid chromatography. Liver lipids were extracted using a Folch technique as described previously (25). Triglycerides and cholesterol were measured from fasting plasma, pooled plasma fast-phase liquid chromatography fractions, and extracted hepatic lipids as previously described (26). Assays were performed in duplicate.
Statistical analyses
Power calculations to determine sample sizes were performed based on our previous work that to detect twofold to threefold differences in insulin levels (at 90% power), we will need 10 mice per group. Data were analyzed using GraphPad Prism 6 software (GraphPad, La Jolla, CA) and presented as mean ± standard error of the mean. One-way analysis of variance was used to compare treatment groups. Two-way analysis of variance was used to compare the different diets at different time points, with Sidak or Tukey post hoc tests to detect differences between groups. A P value <0.05 was considered statistically significant. Data collection was performed in a blinded fashion whenever possible.
Results
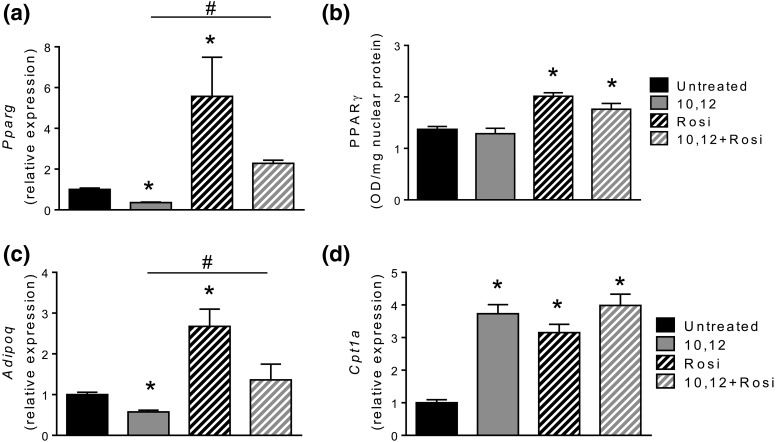
We previously showed that 10,12 CLA increases FAO while decreasing the expression of Pparg and its downstream target Adipoq in cultured adipocytes, leading to reductions in intracellular triglycerides (11). We now show that Rosi restores gene expression and nuclear activity of PPARγ and expression of Adipoq, with no effect on expression of the rate-limiting enzyme for FAO, carnitine palmitoyl transferase (Cpt)1a, in adipocytes treated with 10,12 CLA [Fig. 1(a–d)]. Notably, cytosolic activities of PPARα and PPARδ, transcription factors that control FAO, were activated by 10,12 CLA, with no effect of Rosi (not shown). These results suggest that 10,12 CLA decreases PPARγ expression and activity, which are rescued by Rosi without altering the capacity for 10,12 CLA to promote FAO.
Figure 1.
Rosi restores 10,12 CLA-blunted Pparg gene expression and improves PPARγ nuclear translocation. 3T3-L1 differentiated adipocytes were treated with FAFA (control) or 10,12 CLA, +/− 1 nM Rosi. Gene expression of (a) Pparg, (c) Adipoq, and (d) Cpt1a measured using quantitative real-time polymerase chain reaction. (b) Nuclear PPARγ protein was quantified by enzyme-linked immunosorbent assay, normalized to total nuclear protein. *P < 0.05 from control; #P < 0.05 from 10,12 CLA. Data presented are mean ± standard error of the mean from three independent experiments performed in triplicate.
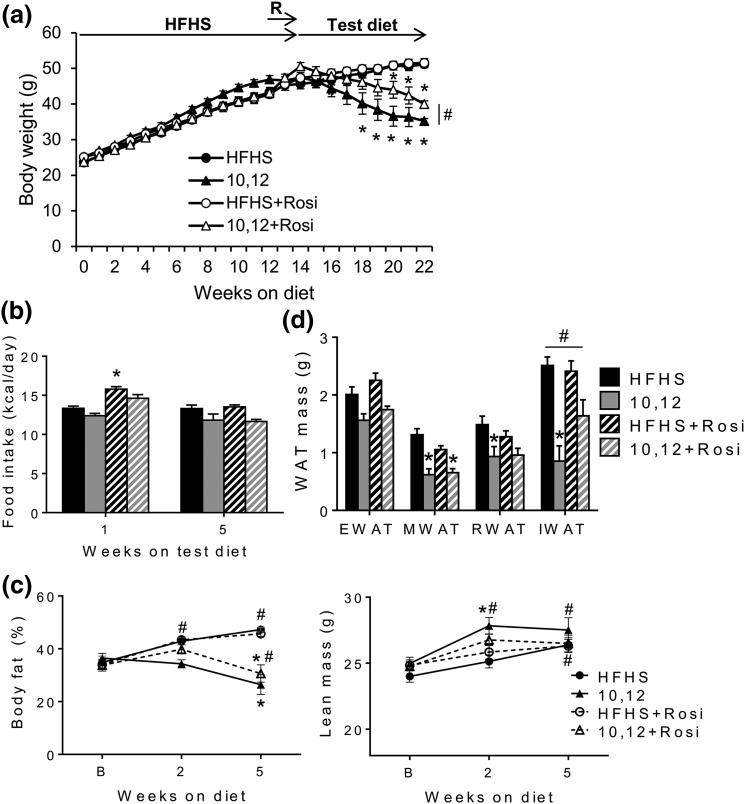
To determine whether Rosi improves 10,12 CLA-mediated insulin resistance without compromising its weight loss effects, male Ldlr−/− mice were studied, as shown in Fig. 2. After 14 weeks on a HFHS obesogenic diet, mice were continued on HFHS or switched to test diets containing 10,12 CLA, with or without Rosi, for an additional 8 weeks. As we have shown previously (13), 9,11 CLA was inert in this model (data not shown); 10,12 CLA promoted weight loss, which was maintained to a lesser degree by Rosi cotreatment [Fig. 3(a)]. While Rosi initially invoked hyperphagia, this eventually normalized [Fig. 3(b)] and could explain the delayed weight loss in combination with 10,12 CLA. From mesenteric WAT (MWAT), retroperitoneal WAT (RWAT), and subcutaneous inguinal WAT (IWAT), 10,12 CLA promoted fat loss [the latter being partially restored by Rosi cotreatment; Fig. 3(c)]. Neither 10,12 CLA nor Rosi affected visceral EWAT. Weight loss by 10,12 CLA was primarily from fat rather than lean stores, with lower levels of fat loss with Rosi cotreatment [Fig. 3(d)]. Importantly, Rosi did not promote excess weight or fat gain. These results suggest that the addition of Rosi led to a redistribution of fat stores with a higher proportion of subcutaneous WAT.
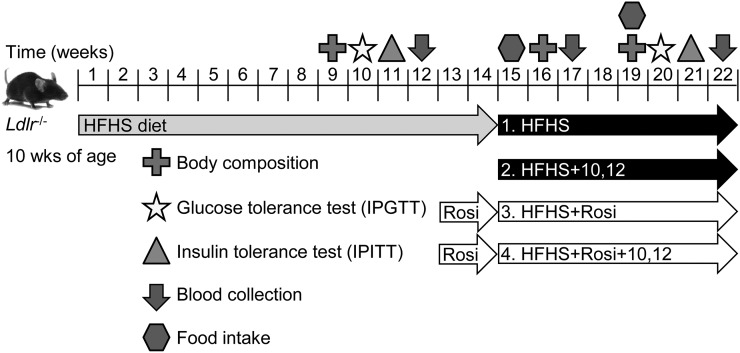
Figure 2.
Animal study design. Ldlr−/− male mice were fed a HFHS diet for 14 weeks and then switched to one of four test diets for an additional 8 weeks. Rosi groups included a 2-week lead-in prior to test diets. The dose of 10,12 CLA was 1% weight-to-weight ratio, and the Rosi dose was 10 mg/kg. Measurements were obtained as indicated. n = 8 to 12 mice per group.
Figure 3.
Rosi attenuates 10,12 CLA-induced subcutaneous fat loss. (a) Body weights measured weekly. (b) Food intake measured after 1 and 5 weeks on indicated diets (15 and 19 weeks of HFHS feeding, respectively). (c) Body composition performed at baseline (B; 9 weeks of HFHS feeding), and again after 2 and 5 weeks on the indicated diets and presented as % body fat (left) and lean body mass (right). (d) Excised fat pads weighed at euthanasia. R, Rosi lead-in. n = 8 to 12 mice per group, presented as mean ± standard error of the mean. *P < 0.05 from HFHS control; #P < 0.05 from 10,12 CLA.
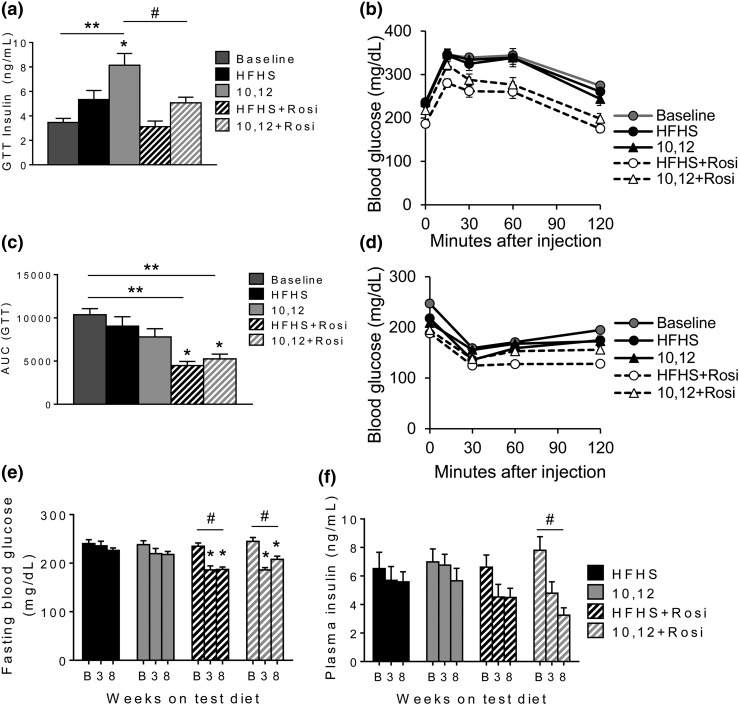
Whereas weight loss in rodents and humans is well characterized to improve glucose metabolism (27), 10,12 CLA fails to do so (13). This is clear by the increased insulin secretion in 10,12 CLA-supplemented mice that is necessary for equivalent glucose control as HFHS-fed mice during an IPGTT [Fig. 4(a) and 4(b)]. All mice given Rosi showed improved glucose tolerance, exemplified by reduced area under the curve of the IPGTT, including the 10,12 CLA + Rosi cotreatment group [Fig. 4(b) and 4(c)]. Improvements induced by Rosi were not due to enhanced insulin secretion [Fig. 4(a)]. Insulin tolerance, assessed by intraperitoneal insulin tolerance testing, was similarly improved by Rosi in all groups [Fig. 4(d)], including the 10,12 CLA + Rosi cotreatment group. The improvements in glucose and insulin tolerance by Rosi were partially attributed to improved fasting glucose levels [Fig. 4(e)]. Further, fasting insulin secretion improved with Rosi treatment, notably when combined with 10,12 CLA [Fig. 4(f)]. These results confirm that combined 10,12 CLA and Rosi treatment not only promotes weight loss, but also improves glucose metabolism.
Figure 4.
Rosi improves glucose metabolism. (a–c) Mice were subjected to an IPGTT at baseline (B; after 10 weeks of HFHS feeding) and again after 6 weeks on indicated diets. (a) Plasma insulin measured at the 30-minute time point during the IPGTT. (b) IPGTT. (c) Area under the curve (AUC) from IPGTT. (d) Intraperitoneal insulin tolerance testing performed at baseline (11 weeks of HFHS feeding) and again after 7 weeks on indicated diets. (e) Fasting blood glucose and (f) plasma insulin levels measured at baseline (12 weeks of HFHS feeding), and again after 3 and 8 weeks on indicated diets. n = 8 to 12 mice per group, presented as mean ± standard error of the mean. *P < 0.05 from HFHS control; **P < 0.05 from baseline; #P < 0.05 from 10,12 CLA.
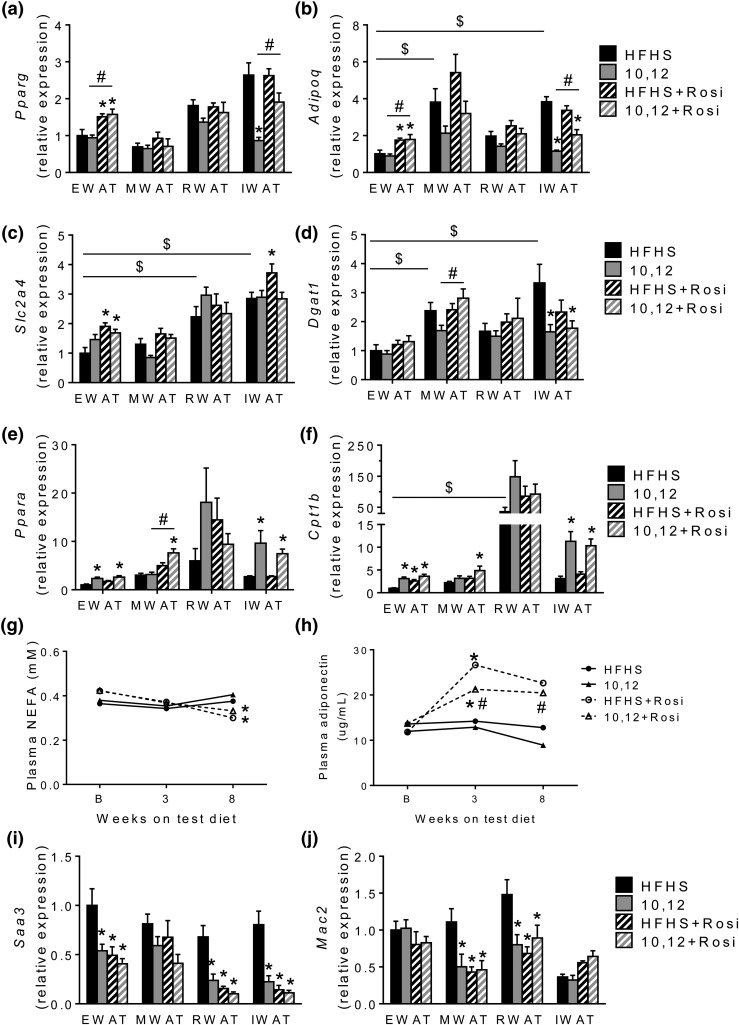
Rosi exerts its effects by activating PPARγ by unknown mechanisms (28). However, it is likely that the insulin sensitizing effects of Rosi are mediated at least in part by increased adiponectin secretion from adipocytes (29, 30). Due to the distinct loss of particular WAT depots including MWAT, RWAT, and IWAT by 10,12 CLA and the restoration of IWAT with Rosi, we measured Pparg and Adipoq expression from these WAT depots. As we reported previously (13), 10,12 CLA reduced Pparg and Adipoq expression from IWAT, with a trend toward decreased expression in MWAT and RWAT but no effect in EWAT [Fig. 5(a) and 5(b)]. However, surprisingly, Rosi had a greater effect to increase Pparg and Adipoq expression in EWAT than in other WAT depots. Moreover, Rosi treatment increased the expression of solute carrier family 2 member 4 (glucose transporter 4) from both EWAT and IWAT [Fig. 5(c)], consistent with the enhanced ability for glucose uptake. Expression of diglycerol acyltransferase (Dgat) 1, an enzyme that catalyzes the final step of triglyceride synthesis, was reduced by 10,12 CLA in IWAT [Fig. 5(d)], suggesting that reductions in IWAT mass are associated with reduced triglyceride synthesis. Rosi did not alter Dgat expression. Similarly to what we have shown in vitro, Rosi did not negatively impact the ability for 10,12 CLA to increase expression of genes essential for FAO, Ppara, and Cpt1b, in EWAT or IWAT [Fig. 5(e) and 5(f)]. In addition, plasma NEFAs were reduced by Rosi, suggesting reduced adipose tissue lipolysis in favor of lipogenesis [Fig. 5(g)]. Plasma adiponectin was reduced by 10,12 CLA and restored by Rosi to levels comparable to HFHS control mice [Fig. 5(h)]. These results suggest that Rosi restores the balance between adipogenesis and lipid catabolism necessary to promote efficient 10,12 CLA-mediated fat loss from WAT, with restoration of circulating adiponectin levels. Moreover, Rosi significantly decreased expression of Saa3 from most WAT depots [Fig. 5(i)]. SAA3 is an inflammatory chemokine expressed from both adipocytes and macrophages (31). Mac2, a general macrophage marker, decreased in only MWAT and RWAT in response to Rosi [Fig. 5(j)], suggesting that reduced EWAT and IWAT Saa3 expression is likely driven by adipocytes and not macrophages. Taken together, the increased adipokine secretion coupled with reduced WAT inflammation in Rosi-treated mice suggests improved WAT insulin sensitivity (32).
Figure 5.
Adipogenic gene expression is restored by Rosi, without altering FAO. Gene expressions of (a) Pparg, (b) Adipoq, (c) solute carrier family 2 member 4, (d) Dgat1, (e) Pparg, (f) Cpt1b, (i) Saa3, and (j) Mac2 were quantified from EWAT, MWAT, RWAT, and IWAT at euthanasia, presented normalized to EWAT HFHS controls. (g) Fasting plasma NEFA and (h) plasma adiponectin quantified at baseline (B; after 12 weeks of HFHS feeding), and again after 3 and 8 weeks on indicated diets. n = 8 to 12 mice per group, presented as mean ± standard error of the mean. *P < 0.05 from HFHS control; $P < 0.05 from EWAT; #P < 0.05 from 10,12 CLA.
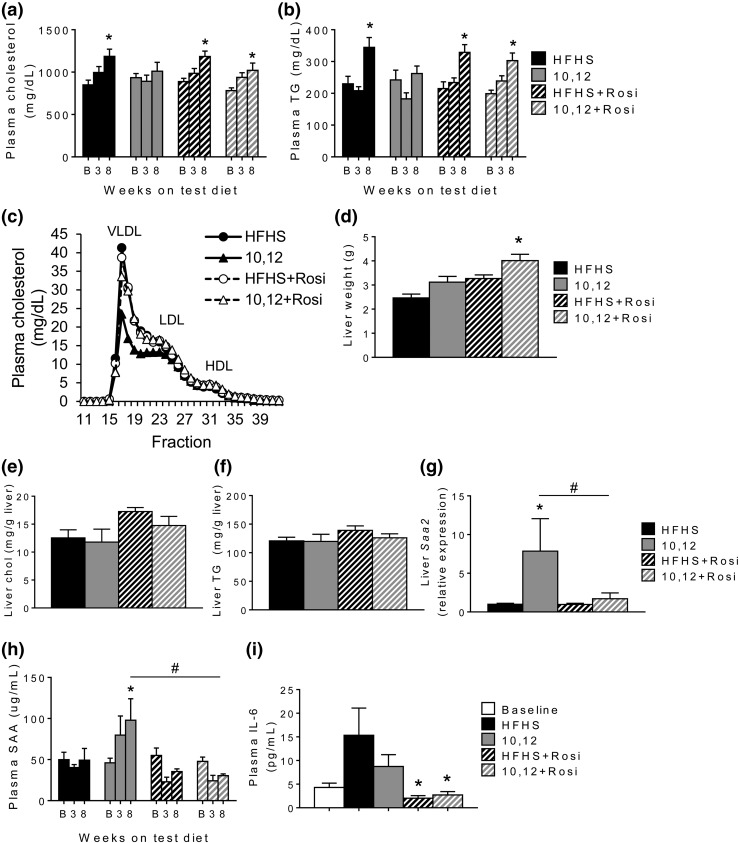
A potential mechanism of improved glucose homeostasis by Rosi is reduction in hepatic gluconeogenesis by adiponectin (9). However, expression of genes that regulate glycolysis and gluconeogenesis, including glucokinase, pyruvate kinase, and phosphoenolpyruvate carboxykinase, were unchanged by 10,12 CLA, Rosi, or the combination (Table 1), although a trend toward decreased pyruvate kinase and phosphoenolpyruvate carboxykinase was observed in groups that received Rosi. Further, there are conflicting reports concerning the effects of Rosi on plasma lipids, with some studies showing a worsening and others a beneficial effect (33, 34). Therefore, we quantified cholesterol and triglycerides from plasma and liver. Whereas all HFHS-based diets increased circulating triglycerides and cholesterol over time, Rosi did not alter this effect [Fig. 6(a) and 6(b)]. Consistent with this, plasma lipoprotein profiles were not significantly altered by Rosi, but total cholesterol in the low-density lipoprotein (LDL) and very-LDL fractions were reduced by 10,12 CLA alone [Fig. 6(c)]. Livers from mice treated with 10,12 CLA and Rosi were larger [Fig. 6(d)], but hepatic cholesterol and triglycerides were not significantly different between treatment groups [Fig. 6(e) and 6(f)]. The expression of several genes involved in hepatic lipid metabolism, including fatty acid synthase, acetyl coenzyme-A carboxylase, sterol regulatory element binding protein 1c, diglyceride acyltransferase 1, and carnitine palmitoyltansferase 1 α (Table 1), were unaffected by 10,12 CLA, Rosi, or the combination. Notably, hepatic Pparg expression did not change with Rosi treatment (Table 1). Circulating plasma SAA, a liver-derived inflammatory protein that is chronically elevated during obesity, was increased by 10,12 CLA and reduced following Rosi cotreatment [Fig. 6(h)], with a parallel effect on hepatic Saa2 gene expression [Fig. 6(g)]. Similarly, plasma IL-6 was improved by Rosi treatment [Fig. 6(i)]. Collectively, these data suggest that in the context of metabolic syndrome, Rosi improves 10,12 CLA-mediated insulin resistance and hepatic inflammation without compromising its weight loss effects and does not significantly alter plasma or hepatic lipids.
Table 1.
Expression of Hepatic Lipid Metabolism Genes
| HFHS | 10,12 CLA | HFHS + Rosi | 10,12 CLA + Rosi | |
|---|---|---|---|---|
| Glucose metabolism | ||||
| Gck | 1.00 ± 0.06 | 1.07 ± 0.08 | 0.99 ± 0.05 | 0.84 ± 0.13 |
| Pk | 1.00 ± 0.08 | 0.99 ± 0.11 | 0.81 ± 0.06 | 0.80 ± 0.07 |
| Pck | 1.00 ± 0.15 | 1.24 ± 0.26 | 0.77 ± 0.07 | 0.84 ± 0.26 |
| Lipid metabolism | ||||
| Fasn | 1.00 ± 0.17 | 1.22 ± 0.25 | 0.94 ± 0.09 | 0.74 ± 0.12 |
| Acc | 1.00 ± 0.09 | 1.25 ± 0.11 | 1.16 ± 0.10 | 0.91 ± 0.13 |
| Srebp1c | 1.00 ± 0.06 | 0.88 ± 0.09 | 0.93 ± 0.08 | 0.91 ± 0.08 |
| Dgat1 | 1.00 ± 0.14 | 0.81 ± 0.19 | 0.94 ± 0.15 | 0.90 ± 0.14 |
| Cpt1a | 1.00 ± 0.08 | 0.82 ± 0.05 | 0.94 ± 0.04 | 0.93 ± 0.07 |
| Ppara | 1.00 ± 0.06 | 0.85 ± 0.05 | 0.95 ± 0.06 | 0.90 ± 0.06 |
| Pparg | 1.00 ± 0.09 | 0.73 ± 0.06 | 0.99 ± 0.06 | 0.83 ± 0.04 |
Abbreviations: Acc, acetyl coenzyme-A carboxylase; Cpt1a, carnitine palmitoyltransferase 1 α; Fasn, fatty acid synthase; Dgat1, diglyceride acyltransferase 1; Gck, glucokinase; Pck, phosphoenolpyruvate carboxykinase; Pk, pyruvate kinase; Ppara, peroxisome proliferator-activated receptor α; Srebp1c, sterol regulatory element binding protein 1c.
Figure 6.
Rosi has little effect on plasma or liver lipids in combination with 10,12 CLA, but improves 10,12 CLA-mediated systemic inflammation. Fasting (a) plasma cholesterol and (b) triglycerides (TGs) quantified at baseline (B; after 12 weeks of HFHS feeding), and again after 3 and 8 weeks on indicated diets. (c) Lipoprotein profiles obtained by fast-phase liquid chromatography of pooled fasted plasma samples taken after 8 weeks on indicated diets. (d) Liver weight determined at euthanasia. (e) Liver cholesterol (chol) and (f) TGs quantified. (g) Hepatic gene expression of Saa2 quantified after 8 weeks on diets. Plasma (h) SAA and (i) IL-6 quantified at baseline (after 12 weeks of HFHS feeding) and again after 3 and 8 weeks on indicated diets. n = 8 to 12 mice per group, presented as mean ± standard error of the mean. *P < 0.05 from HFHS control; #P < 0.05 from 10,12 CLA. VLDL, very-low-density lipoprotein; HDL, high-density lipoprotein.
Discussion
Although supplements containing 10,12 CLA are commonly used for weight loss, their efficacy, safety, and mechanism of action are still in question. We recently showed that although 10,12 CLA promotes fat loss, it does so without the expected improvements in glucose metabolism normally associated with weight loss (13) and suggests PPARγ antagonism. We therefore hypothesized that cotreatment of obese mice with 10,12 CLA and a PPARγ -activating insulin-sensitizer such as Rosi would promote fat redistribution and improve glucose homeostasis. Rosi was chosen, as it has been shown to promote less fat gain in mice than other TZDs such as pioglitazone, with comparable improvements in glucose metabolism (35). Further, mouse and human models of PPARγ deficiency show dramatic improvements in insulin sensitivity following TZD administration (36), suggesting that this treatment strategy holds translational potential in humans. We now show that Rosi improves glucose and insulin tolerance without negative effects on plasma lipids or weight gain in 10,12 CLA-supplemented mice.
We have shown that Rosi reverses the 10,12 CLA-induced reduction in PPARγ expression in cultured adipocytes. Subsequently, gene expression of a primary downstream target of PPARγ, adiponectin, is also decreased by 10,12 CLA and reversed by Rosi treatment. However, the observation that nuclear PPARγ translocation is not decreased by 10,12 CLA suggests that its ability to initiate gene transcription of downstream targets, rather than its nuclear translocation, is impeded. Additional potential mechanisms of PPARγ activity suppression by 10,12 CLA include (1) increased activity of repressors such as nuclear receptor corepressor 1 (37); (2) inhibition of heterodimerization with nuclear retinoid X receptor, which would disable binding to PPAR response elements; or (3) altered posttranslational modifications of the PPARγ protein such as phosphorylation (38), sumoylation (39), O-GlycNAcylation (40), acetylation (41), or ubiquitination (42), any of which could impede the transactivation potential of PPARγ. A future direction is to examine the PPARγ inhibitory effects of 10,12 CLA at the molecular level.
Adiponectin is an adipokine secreted exclusively from adipocytes that is directly controlled by PPARγ. Paradoxically, circulating adiponectin is inversely correlated with fat mass (43), but closely correlated with insulin sensitivity (44). Mice lacking adiponectin fail to show PPARγ agonist-mediated improvements in insulin sensitivity (29), highlighting the critical role for adiponectin in TZD-induced improvements to glucose tolerance. Mice consuming 10,12 CLA develop progressive adiponectin deficiency (13), which, in theory, could reduce the efficacy of TZDs. However, our data shows that even small elevations in plasma adiponectin following Rosi treatment can contribute to major improvements in glucose metabolism.
Defective PPARγ signaling has been linked to lipodystrophy, as humans with dominant-negative mutations in a single allele of Pparg have partial lipodystrophy (45). In addition to reduced adipose tissue Pparg and Adipoq expression, mice consuming 10,12 CLA lose considerable subcutaneous fat mass and develop hepatic steatosis and hepatomegaly (46), consistent with a lipodystrophic-like phenotype. Rosi administration favors adipogenesis in subcutaneous rather than visceral WAT (47), which makes it an excellent candidate to reverse 10,12 CLA-induced subcutaneous lipoatrophy (13). As expected, Rosi enhanced subcutaneous IWAT mass, which likely contributed to increased plasma adiponectin levels (48). However, Rosi had a surprisingly greater effect to increase Pparg and Adipoq gene expression in visceral EWAT than subcutaneous IWAT. Baseline levels of Pparg expression were equivalent between EWAT and IWAT, but baseline Adipoq expression was threefold higher in IWAT, suggesting that baseline PPARγ activity is higher in IWAT at baseline. Therefore, we speculate that the induction potential of PPARγ by Rosi is higher in EWAT, but how this specifically impacts adipogenesis, adiponectin secretion, and subsequent whole body insulin sensitization, and how these differ between subcutaneous IWAT and visceral EWAT, remains unknown.
Adipocyte PPARγ protects nonadipose tissues such as the liver against ectopic lipid overload, which, in turn, preserves normal liver function. Activated adipocyte PPARγ promotes a balanced secretion of adipokines such as adiponectin that mediate insulin action in peripheral tissues. Therefore, appropriate PPARγ activation ensures maintenance of whole body insulin sensitivity (49). Reduced plasma NEFA following Rosi treatment suggests that Rosi tips the balance toward adipocyte lipid storage, which, in turn, protects the liver from lipid accumulation (50). Further, Rosi treatment improves systemic inflammation in humans (51), and we now show that Rosi also improves 10,12 CLA-mediated SAA and IL-6 levels. The combination of 10,12 CLA and Rosi also increased liver size without worsening hepatic cholesterol or triglycerides, which, coupled with the reduced hepatic inflammation, suggests innocuous hepatic expansion. Consistent with this, a previous study in rats presenting with a metabolic syndrome phenotype showed that Rosi had favorable hepatic effects, including improved lipid metabolism, decreased macrovesicular steatosis, and reduced oxidative stress (52).
In summary, we show that cotreatment with Rosi maintains significant weight and fat loss with a favorable redistribution of WAT toward subcutaneous sites, with concomitant improvements in insulin sensitivity and systemic SAA that were adversely affected by 10,12 CLA supplementation alone (13). Further, we show no evidence of adverse side effects such as worsened lipoprotein profiles or hepatic steatosis previously reported with Rosi treatment, suggesting that a combined therapy with 10,12 CLA and a TZD such as Rosi could be a plausible strategy to combat human obesity.
Acknowledgments
We acknowledge contributions from Mohamed Omer of the University of Washington Nutrition Obesity Research Center Energy Balance Core (P30 DK035816) in support of this work.
Acknowledgments
This work was supported by funding from the following centers and institutes of the National Institutes of Health: National Center for Complementary and Integrative Health, Career Development Award (K01 AT007177); National Institute of Diabetes and Digestive and Kidney Diseases, University of Washington Nutrition Obesity Research Center Pilot and Feasibility Award (P30 DK035816), and University of Washington Diabetes Research Center Pilot and Feasibility Award (P30 DK017047).
Acknowledgments
Author contributions: Experiments were designed by L.J.d.H. Experiments were performed by S.W., L.G., K.E.T., B.H., and L.J.d.H. Data presentation and statistical analyses were performed by S.W., K.E.T., and L.J.d.H. The manuscript was written by L.J.d.H.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 9,11 CLA
- cis-9, trans-11 conjugated linoleic acid
- 10,12 CLA
- trans-10, cis-12 conjugated linoleic acid
- Adipoq
- adiponectin gene
- CLA
- conjugated linoleic acid
- Cpt
- carnitine palmitoyl transferase
- Dgat
- diglycerol acyltransferase
- EWAT
- epididymal white adipose tissue
- FAFA
- fatty acid-free albumin
- FAO
- fatty acid oxidation
- HFHS
- high-fat, high-sucrose
- IL-6
- interleukin-6
- IPGTT
- intraperitoneal glucose tolerance test
- IWAT
- inguinal white adipose tissue
- LDL
- low-density lipoprotein
- Ldlr−/−
- low-density lipoprotein receptor–deficient
- MWAT
- mesenteric white adipose tissue
- NEFA
- nonesterified fatty acids
- PPAR
- peroxisome proliferator-activated receptor
- Pparg
- PPAR γ gene
- Rosi
- rosiglitazone
- RWAT
- retroperitoneal white adipose tissue
- SAA
- serum amyloid A
- TZD
- thiazolidinedione
- WAT
- white adipose tissue.
References
- 1.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–497. [DOI] [PubMed] [Google Scholar]
- 2.Blanck HM, Serdula MK, Gillespie C, Galuska DA, Sharpe PA, Conway JM, Khan LK, Ainsworth BE. Use of nonprescription dietary supplements for weight loss is common among Americans. J Am Diet Assoc. 2007;107(3):441–447. [DOI] [PubMed] [Google Scholar]
- 3.Park Y, Storkson JM, Albright KJ, Liu W, Pariza MW. Evidence that the trans-10,cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids. 1999;34(3):235–241. [DOI] [PubMed] [Google Scholar]
- 4.Risérus U, Vessby B, Arner P, Zethelius B. Supplementation with trans10cis12-conjugated linoleic acid induces hyperproinsulinaemia in obese men: close association with impaired insulin sensitivity. Diabetologia. 2004;47(6):1016–1019. [DOI] [PubMed] [Google Scholar]
- 5.Rosen ED. The transcriptional basis of adipocyte development. Prostaglandins Leukot Essent Fatty Acids. 2005;73(1):31–34. [DOI] [PubMed] [Google Scholar]
- 6.Tiikkainen M, Häkkinen AM, Korsheninnikova E, Nyman T, Mäkimattila S, Yki-Järvinen H. Effects of rosiglitazone and metformin on liver fat content, hepatic insulin resistance, insulin clearance, and gene expression in adipose tissue in patients with type 2 diabetes. Diabetes. 2004;53(8):2169–2176. [DOI] [PubMed] [Google Scholar]
- 7.Yamauchi T, Kamon J, Waki H, Murakami K, Motojima K, Komeda K, Ide T, Kubota N, Terauchi Y, Tobe K, Miki H, Tsuchida A, Akanuma Y, Nagai R, Kimura S, Kadowaki T. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem. 2001;276(44):41245–41254. [DOI] [PubMed] [Google Scholar]
- 8.Carey DG, Cowin GJ, Galloway GJ, Jones NP, Richards JC, Biswas N, Doddrell DM. Effect of rosiglitazone on insulin sensitivity and body composition in type 2 diabetic patients [corrected]. Obes Res. 2002;10(10):1008–1015. [DOI] [PubMed] [Google Scholar]
- 9.Gastaldelli A, Miyazaki Y, Pettiti M, Santini E, Ciociaro D, Defronzo RA, Ferrannini E. The effect of rosiglitazone on the liver: decreased gluconeogenesis in patients with type 2 diabetes. J Clin Endocrinol Metab. 2006;91(3):806–812. [DOI] [PubMed] [Google Scholar]
- 10.Mooradian AD, Chehade J, Thurman JE. The role of thiazolidinediones in the treatment of patients with type 2 diabetes mellitus. Treat Endocrinol. 2002;1(1):13–20. [DOI] [PubMed] [Google Scholar]
- 11.den Hartigh LJ, Han CY, Wang S, Omer M, Chait A. 10E,12Z-conjugated linoleic acid impairs adipocyte triglyceride storage by enhancing fatty acid oxidation, lipolysis, and mitochondrial reactive oxygen species. J Lipid Res. 2013;54(11):2964–2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy A, Chung S, LaPoint K, Fabiyi O, McIntosh MK. Trans-10, cis-12 conjugated linoleic acid antagonizes ligand-dependent PPARgamma activity in primary cultures of human adipocytes. J Nutr. 2008;138(3):455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.den Hartigh LJ, Wang S, Goodspeed L, Wietecha T, Houston B, Omer M, Ogimoto K, Subramanian S, Gowda GA, O’Brien KD, Kaiyala KJ, Morton GJ, Chait A. Metabolically distinct weight loss by 10,12 CLA and caloric restriction highlight the importance of subcutaneous white adipose tissue for glucose homeostasis in mice. PLoS One. 2017;12(2):e0172912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaRosa PC, Miner J, Xia Y, Zhou Y, Kachman S, Fromm ME. Trans-10, cis-12 conjugated linoleic acid causes inflammation and delipidation of white adipose tissue in mice: a microarray and histological analysis. Physiol Genomics. 2006;27(3):282–294. [DOI] [PubMed] [Google Scholar]
- 15.Blanchard PG, Turcotte V, Côté M, Gélinas Y, Nilsson S, Olivecrona G, Deshaies Y, Festuccia WT. PPARγ activation favors selective subcutaneous lipid deposition by coordinately regulating lipoprotein lipase modulators, fatty acid transporters, and lipogenic enzymes. Acta Physiol (Oxf). 2016;217(3):227–239. [DOI] [PubMed] [Google Scholar]
- 16.Liu LF, Purushotham A, Wendel AA, Belury MA. Combined effects of rosiglitazone and conjugated linoleic acid on adiposity, insulin sensitivity, and hepatic steatosis in high-fat-fed mice. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):G1671–G1682. [DOI] [PubMed] [Google Scholar]
- 17.Purushotham A, Wendel AA, Liu LF, Belury MA. Maintenance of adiponectin attenuates insulin resistance induced by dietary conjugated linoleic acid in mice. J Lipid Res. 2007;48(2):444–452. [DOI] [PubMed] [Google Scholar]
- 18.Yeop Han C, Kargi AY, Omer M, Chan CK, Wabitsch M, O’Brien KD, Wight TN, Chait A. Differential effect of saturated and unsaturated free fatty acids on the generation of monocyte adhesion and chemotactic factors by adipocytes: dissociation of adipocyte hypertrophy from inflammation. Diabetes. 2010;59(2):386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Subramanian S, Han CY, Chiba T, McMillen TS, Wang SA, Haw A III, Kirk EA, O’Brien KD, Chait A. Dietary cholesterol worsens adipose tissue macrophage accumulation and atherosclerosis in obese LDL receptor-deficient mice. Arterioscler Thromb Vasc Biol. 2008;28(4):685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramanian S, Goodspeed L, Wang S, Kim J, Zeng L, Ioannou GN, Haigh WG, Yeh MM, Kowdley KV, O’Brien KD, Pennathur S, Chait A. Dietary cholesterol exacerbates hepatic steatosis and inflammation in obese LDL receptor-deficient mice. J Lipid Res. 2011;52(9):1626–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stubbins RE, Holcomb VB, Hong J, Núñez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr. 2012;51(7):861–870. [DOI] [PubMed] [Google Scholar]
- 22.Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J. 2008;22(3):659–661. [DOI] [PubMed] [Google Scholar]
- 23.National Research Council Guide for the Care and Use of Laboratory Animals. 8th ed Washington, DC: National Academies Press; 2011. [Google Scholar]
- 24.Taicher GZ, Tinsley FC, Reiderman A, Heiman ML. Quantitative magnetic resonance (QMR) method for bone and whole-body-composition analysis. Anal Bioanal Chem. 2003;377(6):990–1002. [DOI] [PubMed] [Google Scholar]
- 25.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226(1):497–509. [PubMed] [Google Scholar]
- 26.Lewis KE, Kirk EA, McDonald TO, Wang S, Wight TN, O’Brien KD, Chait A. Increase in serum amyloid a evoked by dietary cholesterol is associated with increased atherosclerosis in mice. Circulation. 2004;110(5):540–545. [DOI] [PubMed] [Google Scholar]
- 27.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Group DPPR; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehmann JM, Moore LB, Smith-Oliver TA, Wilkison WO, Willson TM, Kliewer SA. An antidiabetic thiazolidinedione is a high affinity ligand for peroxisome proliferator-activated receptor gamma (PPAR gamma). J Biol Chem. 1995;270(22):12953–12956. [DOI] [PubMed] [Google Scholar]
- 29.Nawrocki AR, Rajala MW, Tomas E, Pajvani UB, Saha AK, Trumbauer ME, Pang Z, Chen AS, Ruderman NB, Chen H, Rossetti L, Scherer PE. Mice lacking adiponectin show decreased hepatic insulin sensitivity and reduced responsiveness to peroxisome proliferator-activated receptor gamma agonists. J Biol Chem. 2006;281(5):2654–2660. [DOI] [PubMed] [Google Scholar]
- 30.Combs TP, Wagner JA, Berger J, Doebber T, Wang WJ, Zhang BB, Tanen M, Berg AH, O’Rahilly S, Savage DB, Chatterjee K, Weiss S, Larson PJ, Gottesdiener KM, Gertz BJ, Charron MJ, Scherer PE, Moller DE. Induction of adipocyte complement-related protein of 30 kilodaltons by PPARgamma agonists: a potential mechanism of insulin sensitization. Endocrinology. 2002;143(3):998–1007. [DOI] [PubMed] [Google Scholar]
- 31.Meek RL, Benditt EP. Amyloid A gene family expression in different mouse tissues. J Exp Med. 1986;164(6):2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peters Harmel AL, Kendall DM, Buse JB, Boyle PJ, Marchetti A, Lau H. Impact of adjunctive thiazolidinedione therapy on blood lipid levels and glycemic control in patients with type 2 diabetes. Curr Med Res Opin. 2004;20(2):215–223. [DOI] [PubMed] [Google Scholar]
- 34.Sundaresan A, Harini R, Pugalendi KV. Ursolic acid and rosiglitazone combination alleviates metabolic syndrome in high fat diet fed C57BL/6J mice. Gen Physiol Biophys. 2012;31(3):323–333. [DOI] [PubMed] [Google Scholar]
- 35.Yang KJ, Noh JR, Kim YH, Gang GT, Hwang JH, Yang SJ, Yeom YI, Lee CH. Differential modulatory effects of rosiglitazone and pioglitazone on white adipose tissue in db/db mice. Life Sci. 2010;87(13–14):405–410. [DOI] [PubMed] [Google Scholar]
- 36.Tsai YS, Maeda N. PPARgamma: a critical determinant of body fat distribution in humans and mice. Trends Cardiovasc Med. 2005;15(3):81–85. [DOI] [PubMed] [Google Scholar]
- 37.Li P, Fan W, Xu J, Lu M, Yamamoto H, Auwerx J, Sears DD, Talukdar S, Oh D, Chen A, Bandyopadhyay G, Scadeng M, Ofrecio JM, Nalbandian S, Olefsky JM. Adipocyte NCoR knockout decreases PPARγ phosphorylation and enhances PPARγ activity and insulin sensitivity. Cell. 2011;147(4):815–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu E, Kim JB, Sarraf P, Spiegelman BM. Inhibition of adipogenesis through MAP kinase-mediated phosphorylation of PPARgamma. Science. 1996;274(5295):2100–2103. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu M, Yamashita D, Yamaguchi T, Hirose F, Osumi T. Aspects of the regulatory mechanisms of PPAR functions: analysis of a bidirectional response element and regulation by sumoylation. Mol Cell Biochem. 2006;286(1–2):33–42. [DOI] [PubMed] [Google Scholar]
- 40.Ji S, Park SY, Roth J, Kim HS, Cho JW. O-GlcNAc modification of PPARγ reduces its transcriptional activity. Biochem Biophys Res Commun. 2012;417(4):1158–1163. [DOI] [PubMed] [Google Scholar]
- 41.Qiang L, Wang L, Kon N, Zhao W, Lee S, Zhang Y, Rosenbaum M, Zhao Y, Gu W, Farmer SR, Accili D. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell. 2012;150(3):620–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floyd ZE, Stephens JM. Interferon-gamma-mediated activation and ubiquitin-proteasome-dependent degradation of PPARgamma in adipocytes. J Biol Chem. 2002;277(6):4062–4068. [DOI] [PubMed] [Google Scholar]
- 43.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. [DOI] [PubMed] [Google Scholar]
- 44.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279(13):12152–12162. [DOI] [PubMed] [Google Scholar]
- 45.Hegele RA, Cao H, Frankowski C, Mathews ST, Leff T. PPARG F388L, a transactivation-deficient mutant, in familial partial lipodystrophy. Diabetes. 2002;51(12):3586–3590. [DOI] [PubMed] [Google Scholar]
- 46.Baraldi FG, Vicentini TM, Teodoro BG, Dalalio FM, Dechandt CR, Prado IM, Curti C, Cardoso FC, Uyemura SA, Alberici LC. The combination of conjugated linoleic acid (CLA) and extra virgin olive oil increases mitochondrial and body metabolism and prevents CLA-associated insulin resistance and liver hypertrophy in C57Bl/6 mice. J Nutr Biochem. 2016;28:147–154. [DOI] [PubMed] [Google Scholar]
- 47.Blanchard PG, Turcotte V, Côté M, Gélinas Y, Nilsson S, Olivecrona G, Deshaies Y, Festuccia WT. Peroxisome proliferator-activated receptor γ activation favours selective subcutaneous lipid deposition by coordinately regulating lipoprotein lipase modulators, fatty acid transporters and lipogenic enzymes. Acta Physiol (Oxf). 2016;217(3):227–239. [DOI] [PubMed] [Google Scholar]
- 48.Fisher FM, McTernan PG, Valsamakis G, Chetty R, Harte AL, Anwar AJ, Starcynski J, Crocker J, Barnett AH, McTernan CL, Kumar S. Differences in adiponectin protein expression: effect of fat depots and type 2 diabetic status. Horm Metab Res. 2002;34(11–12):650–654. [DOI] [PubMed] [Google Scholar]
- 49.Kintscher U, Law RE. PPARgamma-mediated insulin sensitization: the importance of fat versus muscle. Am J Physiol Endocrinol Metab. 2005;288(2):E287–E291. [DOI] [PubMed] [Google Scholar]
- 50.He J, Xu C, Kuang J, Liu Q, Jiang H, Mo L, Geng B, Xu G. Thiazolidinediones attenuate lipolysis and ameliorate dexamethasone-induced insulin resistance. Metabolism. 2015;64(7):826–836. [DOI] [PubMed] [Google Scholar]
- 51.Hetzel J, Balletshofer B, Rittig K, Walcher D, Kratzer W, Hombach V, Häring HU, Koenig W, Marx N. Rapid effects of rosiglitazone treatment on endothelial function and inflammatory biomarkers. Arterioscler Thromb Vasc Biol. 2005;25(9):1804–1809. [DOI] [PubMed] [Google Scholar]
- 52.Ackerman Z, Oron-Herman M, Pappo O, Peleg E, Safadi R, Schmilovitz-Weiss H, Grozovski M. Hepatic effects of rosiglitazone in rats with the metabolic syndrome. Basic Clin Pharmacol Toxicol. 2010;107(2):663–668. [DOI] [PubMed] [Google Scholar]