Abstract
Approximately 15% of human couples of reproductive age have impaired fertility, and the male component accounts for about half of these cases. The etiology is usually unknown, but high correlation with the increase in obesity rates is documented. In this study, we show that diet-induced and genetically obese mice display copulatory behavior comparable to controls, but the number of females impregnated by obese males is remarkably low. Screening for changes in gene expression in the male reproductive tract showed decreased Crisp4 expression in testis and epididymis of obese mice. Lack of CRISP4 in the luminal membrane of epididymal cells indicated inadequate secretion. Consistent with CRISP4 action in acrosome reaction, sperm from mice fed a high-fat diet (HFD) had decreased fertilization capacity. CRISP4 treatment of sperm from HFD mice prior to in vitro fertilization improved fertilization rate. In leptin-deficient obese and infertile mice, leptin’s effect to restore CRISP4 expression and function required gonadal hormones. Our findings indicate that the obesity-induced decline in sperm motility and fertilization capacity results in part from the disruption of epididymal CRISP4 expression and secretion.
Mating failure in obese mice is not related to impaired copulatory behavior but is related to a reduced expression of Crisp4 in testis and epididymis, which impairs the fertilization capacity.
Infertility affects ∼15% of couples of reproductive age, and the male factor accounts for 20% to 50% of all cases (1). Declines in semen quality, in the mean concentration of sperm, and sperm motility have been documented in recent years (1–3). The precise cause remains unknown, but increasing rates of obesity and metabolic syndrome have been linked to decreased testosterone levels and subfertility or infertility in men (1, 4–6). However, inconsistent findings have been reported. For example, studies focused on sperm parameters described an inverse relationship between body mass index and sperm motility, but a meta-analysis study found a weak correlation between obesity and sperm parameters in general (7, 8).
The negative impact of obesity on testosterone production is also controversial. Cases of subfertile obese subjects with either normal or decreased testosterone levels have been reported. Whereas changes in gonadal steroid levels may contribute to obesity-induced male infertility, data from assisted reproductive technology suggest that they may not be the only cause. Obesity in men is associated with decreased pregnancy rates and increased pregnancy loss in couples subjected to assisted reproductive technology (6, 9). These effects seem to be due to impaired fertilization capacity and/or blastocyst development via unknown mechanisms (6, 10). Notably, following intracytoplasmic sperm injection, the fertilization rate is dramatically improved, indicating that obesity may alter sperm maturation, capacitation, and their ability to bind and fertilize the egg (9). Consistent with findings in men, capacitation and sperm binding are both compromised in obese mice (11–15).
Increased adiposity elevates the circulating levels of the adipocyte-derived hormone leptin, which signals energy sufficiency to the brain (16, 17). Leptin signaling-deficient mice and humans are obese and infertile (18–25). Conversely, excess leptin as in obesity may lead to a resistant state, resulting in decreased activation of the hypothalamo-pituitary-gonadal (HPG) axis (16, 25–27). In previous studies, we showed that re-expression of endogenous leptin receptor in specific hypothalamic neurons of leptin receptor null infertile mice improves the reproductive function of female, but not of male mice (28). Those mice were still morbidly obese and null for the long form of leptin receptor in all other tissues, including testis. Although intriguing, this sexually dimorphic response raised the hypothesis that the high adiposity would preclude the restoration of fertility in males via actions outside the HPG axis. To test this model and to gain insights into the decreased fertilization capacity of obese males, we performed a systematic evaluation of the copulatory capacity, hormone levels, and molecular changes in the reproductive tract of diet-induced and genetically obese mice.
Materials and Methods
Ethics statement
All procedures were carried out with prior approval of the University of Michigan Institutional Committee on Use and Care of Animals (Animal Protocols: PRO04380, PRO06792), in accordance with the guidelines established by the National Research Council Guide for the Care and Use of Laboratory Animals.
Animals
Adult (8 to 10 weeks old) and prepubertal (3 weeks old) male C57BL/6 (JAX® mice, stock no. 000664), B6.Cg-Lepob/J (ob/ob; JAX mice, stock no. 000632), B6.129(Cg)-Leprtm2(cre)Rck/J (LepR-Cre; JAX mice, stock no. 008320), and B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (R26-tdTomato; JAX mice, stock no. 007914) mice were housed in the University of Michigan animal facility, in an environment controlled for light (12 hours on/off) and temperature (21 to 23°C). Mice had ad libitum access to water and food. To visualize the Lepr-expressing cells, we crossed LepR-Cre mice, a knock-in strain that expresses Cre-recombinase driven by the Lepr gene, previously described and validated (29, 30), with R26-tdTomato mice that express a red fluorescent protein following Cre recombinase excision of loxP sites. When mated to LepR-Cre mice, red fluorescence is detected only in cells that express Lepr. Obesity was induced by feeding wild-type (WT) males for 16 weeks with phytoestrogen-reduced high-fat diet (HFD; 42% of kilocalories from fat, TD.88137 Teklad Global Diet; Envigo, Madison, WI). The control group received phytoestrogen-reduced regular diet (RD; 4% of kilocalories from fat, 2016 Teklad Global 16% Protein Rodent Diet; Envigo). Phytoestrogen-reduced diets were used to avoid the effect of exogenous sex steroids. Body weight was monitored weekly during the study. Experiments were performed between 0900 and 1100 hours.
Assessment of reproductive phenotype
At 10 weeks of age, WT male mice (assigned to three independent trials, n = 9 to 10 mice/trial) were bred with WT females of proven fertility (four females/male), for 1 week (T0). Females were monitored daily for copulatory plugs (coagulated semen) as a sign of mating behavior. Males were then individually housed and randomly assigned to two groups: those on RD (n = 14; four to five mice/trial) and those on HFD (n = 14; four to five mice/trial). After 12 weeks, males were bred with WT of proven fertility (four females/male) for 1 week (T12). Mice were kept on RD or HFD for another 3 weeks (time required to confirm female pregnancy and litter size) and were decapitated under isoflurane (Fluriso; Vet One, Boise, ID) anesthesia. Blood, hypothalamus, pituitary gland, testis, epididymis, and sperm were collected for analysis. Another set of WT and leptin-deficient ob/ob male mice (8 to 10 weeks of age; n = 6 to 10 mice in two trials), fed with RD, was tested for fertility as described and was euthanized for collection of blood, testis, epididymis, and sperm.
Blood, sperm, and tissue collection
Blood was collected from the trunk, clotted for 2 hours at 4°C, and centrifuged at 3000 rpm for 20 minutes at 4°C; serum was obtained and assayed for changes in hormone levels. The mediobasal hypothalamus (MBH) was dissected (thickness: 2.0 mm) from an area 1.0 mm lateral to the midline, at the anterior border of the optic chiasm and the anterior border of the mammillary bodies. The MBH and the pituitary gland were placed in liquid nitrogen, stored at −80°C, and processed for RNA isolation. Testes and epididymides were collected and placed into 37°C HEPES-modified human tubal fluid media (Irvine Scientific, Irvine, CA) supplemented with 3 mg/mL bovine serum albumin (BSA; fatty acid free; Thermo Fisher Scientific, Waltham, MA). Testes and epididymides were separated, blotted dry, and individually weighed. One testis and one epididymis were frozen at −80°C and processed for gene expression. The contralateral testes and epididymis were processed for histology or used for sperm collection.
Assessment of sperm motility
One cauda epididymis from each animal was placed into a 35-mm dish containing 1.5 mL bicarbonate-buffered human tubal fluid media (Irvine Scientific) supplemented with 3 mg/mL BSA and overlaid with liquid paraffin (ORIGIO-MediCult, Knardrupvej, Denmark). The 35-mm dishes and their contents were equilibrated overnight in a 37°C humidified, 5% CO2, and air incubator prior to use. The epididymis was quickly ruptured using tuberculin syringes with 26G needles, and epididymal sperm was gently squeezed out of the tubules. Dishes containing dissected cauda epididymis and released sperm were incubated for 30 minutes in a 5% CO2 air incubator at 37°C, followed by removal of epididymal tissue, mixing of sperm suspension by gently swirling, and subsequent assessment of sperm motility. Sperm suspensions were diluted 1:10 in pre-equilibrated human tubal fluid media/BSA media (37°C), loaded on prewarmed glass slides (37°C), covered with a prewarmed glass coverslip, and allowed to sit for 20 seconds before analysis. Sperm motility was blind-assessed at 37°C using an ocular reticle on a Leica DM-1000 microscope at ×200 magnification. At least 200 sperm were counted in each sperm sample, within 2 minutes of removal from the incubator. The sperm motility data were recorded as number of progressive motile sperm per total number of sperm within the grid × 100.
Serum hormone analyses
Serum was assayed for determination of testosterone, aldosterone, corticosterone, 11-deoxycorticosterone, progesterone, and 17-hydroxyprogesterone levels in mice treated with RD or HFD, using tandem mass spectrometry (31, 32), at the University of Michigan Mass Spectrometry Core Services (Ann Arbor, MI). Testosterone levels of WT and ob/ob mice were assessed with a mouse/rat enzyme-linked immunosorbent assay kit (IBL International, Tecan Group, Morrisville, NC). Analysis of luteinizing hormone (LH) levels was performed using a mouse/rat LH multiplex assay, and the lowest detection limit was 0.04 ng/mL. Estradiol levels were evaluated with immunoassays (Calbiotech, Spring Valley, CA), and the lowest detection limit was 3 pg/mL. Both LH and estradiol measurements were performed by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Charlottesville, VA).
Gene expression by real-time reverse transcription polymerase chain reaction
Total RNA was isolated from MBH, pituitary gland, testis, or epididymis using RNA extraction kit, Qiazol Reagent (miRNeasy; Qiagen, Hilden, Germany), and DNase treatment (RNase-free; Qiagen). The complementary DNA was synthesized using Superscript II and random primers (Invitrogen, Carlsbad, CA), according to the manufacturer's protocol. For validation, quantitative real-time polymerase chain reaction was performed on a CFX-384 Bio-Rad reverse transcription polymerase chain reaction detection system (Hercules, CA) using SYBR® Green Gene Expression Assays, and primer pairs were synthesized by Sigma-Aldrich (St. Louis, MO) or IDT (Coralville, IA). All samples and standard curves were run in triplicate. Water instead of complementary DNA was used as a negative control, and Gapdh was used as housekeeping gene. Determination of gene transcript levels in each sample was obtained by the ΔΔ threshold cycle (Ct) method (33). The Ct of messenger RNA (mRNA) was measured and normalized to the average of the housekeeping genes (ΔCt = CtUnknown − CtHousekeeping gene). The fold change of mRNA in the unknown sample relative to control group was determined by 2−ΔΔCt, where ΔΔCt = ΔCtUnknown − ΔCtControl. Data are shown as a percentage of the relative mRNA expression to the control group. The primers are described in Table 1. The screening of the mRNA content in the testis was performed by quantitative polymerase chain reaction (qPCR) array for male infertility (PAMM-165Z RT2 profiler; Qiagen). A list of genes in the array is available at the following website: https://b2b.qiagen.com/be/shop/pcr/primer-sets/rt2-profiler-pcr-arrays?catno=PAMM165Z#geneglobe. Data from the qPCR array were represented using the heat-map function of GraphPad Prism 7 software (GraphPad, La Jolla, CA).
Table 1.
Primers Used for qPCR
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Amh | 5′-ATCTGGCTGAAGTGATATGG-3′ | 5′-CAGGGTATAGCACTAACAGG-3′ |
| Brd2 | 5′-CTTCTGTACCAGCTTTACAAC-3′ | 5′-TTTGTGATAATCCGGCAAAC-3′ |
| Catsper4 | 5′-AGTTTACTGAGACAGAAGAGG-3′ | 5′-CAAGTTCTCCTGTATTGCTTC-3′ |
| Crisp4 | 5′-TCATGAGGGAAATCATCAAG-3′ | 5′-TCCATCTTCGCAGTTATTTG-3′ |
| Cyp11a1 | 5′-GGCCCCATTTACAGGGAGAAG-3′ | 5′-CACCAGGGTACTGGCTGAAG-3′ |
| Cyp17a1 | 5′-TTCTCCCCAGACGTGGTCAT-3′ | 5′-GTCCGACAAGAGGCCTAGAG-3′ |
| Eif2b4 | 5′-AAACAGCAGAAGAAGAAACG-3′ | 5′-AGTTCTCTGATTGGGTCTTG-3′ |
| Fshb | 5′-ACTAGCACAATCGTCTGCCT-3′ | 5′-TGAGCAGCCTAACCTTGTGG-3′ |
| Fshr | 5′-TCTGGGCCAGTCGTTTTAGAC-3′ | 5′-TTGCATTCCAGTTGCATGGC-3′ |
| Gapdh | 5′-GCTCATGACCACAGTCCATGC-3′ | 5′-GTTGGGGGGGGATAGGGCCTCTCTTG-3′ |
| Insr | 5′-CTGCCCTAAGGTCTGCCAAA-3′ | 5′-GGAGCGGCGGATCTTTAGAA-3′ |
| Lep | 5′-CTTTGGTCCTATCTGTCTTATG-3′ | 5′-TCTTGGACAAACTCAGAATG-3′ |
| Lepr | 5′-TGCTGAATTATACGTGATCG-3′ | 5′-AGACGTAGGATGAATAGATGG-3′ |
| Lhb | 5′-AATTTGGAGGCCCACTCGTG-3′ | 5′-CAGGTGTCAGGGCTTAGCTG-3′ |
| Lhcgr | 5′-ACGAGACGCTTTATTCTGCCA-3′ | 5′-AGGGGTACTTTGAAGGCAGC-3′ |
| Pten | 5′-AACTTGCAATCCTCAGTTTG-3′ | 5′-CTACTTTGATATCACCACACAC-3′ |
| Ptpn1 | 5′-GAAAGGCTCGTTAAAATGTG-3′ | 5′-TCAAACCTGTGTCATCAAAG-3′ |
| Socs3 | 5′-CCAAAGAAATAACCATCCC-3′ | 5′-GATCTGCGAGGTTTCATTAG-3′ |
| Star | 5′-TGGATGGGTCAAGTTCGACG-3′ | 5′-TCCTCTGCAGGACCTTGATCT-3′ |
| Zp3 | 5′-GAGGAGAACTGGAATACTGAG-3′ | 5′-AGTCCACGATGAAGTGATAG-3′ |
Immunoblot analysis
Total protein from the epididymides was extracted using 1% Triton X-100, 25 mM Tris (pH 8.0), 1.5 mM EGTA, 0.5 mM EDTA, and protease inhibitor cocktail (PhosphoStop; Roche, Basel, Switzerland) at 4°C and 15,000g for 30 minutes. Aliquots of the lysates containing 15 µg protein were denatured in Laemmli buffer and β-mercaptoethanol (Bio-Rad) at 95°C for 5 minutes. Samples were blotted onto a nitrocellulose membrane. Nonspecific binding was prevented by immersing the membranes in blocking buffer (3% BSA in Tris-buffered saline–Tween 20) for 60 minutes at room temperature. The membranes were then exposed overnight to the primary antibodies, as follows: rabbit anti-GAPDH (1:4000, no. 5174; Cell Signaling, Danvers, MA; Table 2) and sheep anti-CRISP4 (0.2 µg/mL, AF5017; R&D Systems, Minneapolis, MN). The blots were rinsed in Tris-buffered saline–Tween 20 and incubated in horseradish peroxidase–conjugated anti-rabbit (1:4000, no. 7074; Cell Signaling) or anti-sheep antisera (1:4000, 713-035-003; Jackson Laboratories, Baltimore Pike, PA) for 1 hour at room temperature. Antibody-antigen complexes were visualized by detecting enhanced chemiluminescence using an ECL detection system (Thermo Fisher Scientific) and digital imaging with Chemi Doc XRS+ Image LaboratoryTM software (Bio-Rad). Expression of CRISP4 protein was normalized to the expression of GAPDH. Data were analyzed as relative expression (%) respective to control mouse (WT-fed RD).
Table 2.
Antibodies
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|
| GAPDH | Anti-GAPDH (D16H11) | Cell Signaling, 5174 | Rabbit; monoclonal | 1 µL/4000 µL | AB_10622025 |
| CRISP4 | Anti-CRISP4 | R&D, AF5017 | Sheep; polyclonal | 0.2 µg/mL | AB_1208020 |
| Rabbit antisera | Anti-rabbit IgG, HRP-linked | Cell Signaling, 7074 | Goat; monoclonal | 1 µL/4000 µL | AB_209923 |
| Sheep antisera | Peroxidase AffiniPure anti-sheep IgG | Jackson Laboratories, 713-035-003 | Donkey; polyclonal | 1 µL/4000 µL | AB_2340709 |
| Sheep antisera | Donkey anti-sheep IgG, AlexaFluor 488 | Thermo Fisher, A-11015 | Donkey; polyclonal | 1 µL/400 µL | AB_2534082 |
Abbreviations: HRP, horseradish peroxidase; IgG, immunoglobulin G; RRID, research resource identifier.
CRISP4 immunostaining
To assess the distribution of the LEPR in the epididymis, we used the LepR-Cre/R26-tdTomato reporter mice. CRISP4 immunoreactivity was assessed in LepR-Cre/R26-tdTomato mice, WT, and ob/ob mice (n = 3 to 4 mice/group). Mice were anesthetized using isoflurane and were transcardially perfused with saline, followed by 4% formaldehyde in 0.1 M phosphate-buffered saline (PBS). Epididymis was dissected, postfixed in the same fixative for 1 hour, placed in PBS containing 20% sucrose overnight, and sectioned in a cryostat (30-μm sections, five series) in the frontal plane. Series of epididymis sections were collected directly onto slides and processed for CRISP4 immunostaining. The tdTomato red fluorescence expressed specifically in Lepr cells does not require additional staining. Prior to antibody incubation, nonspecific binding was prevented by immersing the slides in blocking buffer (PBS, 3% normal donkey serum, and 0.25% Triton X-100) for 1 hour at room temperature. The sections were incubated for 24 hours at 25°C with sheep anti-CRISP4 primary antibody (1:1000, AF5017; R&D Systems). After rinses, sections were incubated for 2 hours at room temperature with donkey anti-sheep conjugated with AlexaFluor 488 (1:400, A-11015; Thermo Fisher Scientific). Sections were coverslipped with Fluoromount-G mounting medium (Southern Biotechnology Associates, Birmingham, AL). The CRISP4 immunoreactivity (green fluorescence) and the Lepr reporter gene (tdTomato, red) were analyzed using an Axio Imager M2 microscope (Carl Zeiss, Orbenkochen, Germany).
Superovulation and egg production for in vitro fertilization
Egg donors (4- to 6-week-old WT female mice) were intraperitoneally (IP) injected with 0.1 mL pregnant mare’s serum gonadotropin (Sigma-Aldrich) (5 IU) at 2100 hours (day 1). Forty-eight hours later (2100 hours, day 3) they were IP injected with 0.1 mL human pregnancy urine chorionic gonadotropin (Sigma-Aldrich) (5 IU). Thirteen hours after human pregnancy urine chorionic gonadotropin injection, eggs were collected from the oviducts, and 60 to 85 eggs were placed in in vitro fertilization (IVF) dish containing IVF media (K-RVFE-50; Cook Medical, Bloomington, IN) at 37°C, 5% CO2.
Mouse sperm collection and IVF
Testes, epididymides, and distal vas deferens were removed from adult RD and HFD (8 months old, n = 5) and WT and ob/ob males (10 weeks old, n = 5 to 9). Epididymis and distal vas deferens were placed in one well of an IVF dish with IVF media (K-RFVE-50; Cook Medical). After 10-minute incubation at 37°C and 5% CO2, the number of total sperm and nonmotile sperm was accessed in a Makler counting chamber at ×200 magnification (Makler 1980; Irvine Scientific). When stated, sperm from HFD or ob/ob mice was treated with vehicle (ultrapure PBS, pH 7.4; Thermo Fisher Scientific) or 1.5 µM recombinant mouse CRISP4 protein (5017-CR-050; R&D Systems), 1 hour before incubation with eggs. Concentration of CRISP4 (0.15 μM, 1.5 μM, and 15 μM) and duration of treatment (1 hour or 2 hour) were defined in pilot studies (data not shown). Equal numbers of progressive motile sperm (107/mL) from each mouse were placed with eggs from WT female mice. Sperm and eggs were incubated for 4 to 5 hours. The sperm was removed from the IVF dish using a potassium-supplemented simplex optimized medium media (ZEKS-050; Zenith Biotech, Cuilford CT), and, 24 hours later, the number of two-cell-stage embryos was assessed. Four to five days later, the number of blastocysts was estimated.
Orchidectomy
To address the effects of leptin and gonadal hormones on CRISP4 content and cellular/subcellular localization, intact or orchidectomized adult (8 to 10 weeks old) ob/ob mice (four mice/group) were treated with IP injection of leptin (2.5 µg/g/day, twice daily) for 7 days. Ten days before euthanasia, testes were removed and the epididymides remained in the pelvic cavity. Three days later, mice received IP injections of leptin for 7 days. At the day of the experiment, intact or gonadectomized ob/ob males were euthanized under anesthesia 2 hours after leptin injection, and epididymides were collected for tissue processing.
Statistical analysis
The results are expressed as means ± standard error of the mean and were analyzed using the GraphPad Prism 7 software. Two-way analysis of variance (ANOVA) followed by Newman Keuls post hoc test was used to compare fertilization rates. Comparison of body weight, mRNA expression, and hormone levels between two groups (RD and HFD or WT and ob/ob) was carried out using the unpaired two-tailed Student t test. To evaluate the net effect of HFD on the copulatory plugs, log–rank (Mantel–Cox) test was performed. Kruskall–Wallis test for one-way ANOVA was used to compare percentage of sperm motility, percentage of embryos developed to blastocysts, as well as testosterone levels in WT, ob/ob, ob/ob intact + leptin, and ob/ob gonadectomized + leptin. Ordinary one-way ANOVA followed by Tukey multiple comparisons test was used to compare Crisp4 expression in the epididymis of WT, ob/ob, and WT prepubertal mice and CRISP4 protein expression. Differences were accepted as significant at P < 0.05. Post hoc power calculation for each experiment was conducted, and type II error was considered at 20% threshold.
Results
Obesogenic diet disrupts leptin signaling in the testis
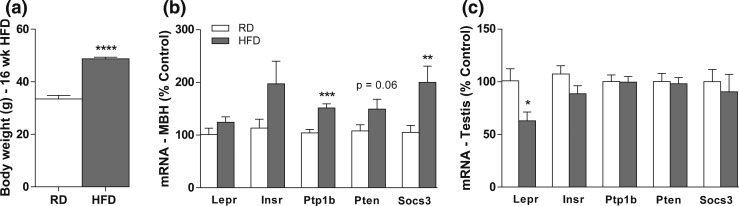
Mice fed HFD for 16 weeks showed increased body weight compared with mice fed RD (t = 9.38; df = 14; F = 6.029, P = 0.0418) [Fig. 1(a)]. No difference in the average weight of the testis (RD: 110.2 ± 2.2 mg; HFD: 104.6 ± 1.8 mg; P = 0.11) or epididymis (RD: 66.7 ± 2.7 mg; HFD: 65.2 ± 2.6 mg; P = 0.72) was detected after HFD. Leptin levels are elevated in obese individuals, favoring the development of leptin resistance (16, 17, 26, 27). In agreement with this observation, the HFD obese mice showed elevated transcripts for inhibitors of leptin signaling, such as the suppressor of cytokine signaling 3 (P < 0.05; t = 4.715; df = 14; F = 1.026) and the protein tyrosine phosphatase 1B (P < 0.05; t = 3.064; df = 14; F = 4.274) in the MBH [Fig. 1(b)]. In testis, however, mice fed HFD showed a reduction in the expression of the leptin receptor (Lepr) gene (P < 0.05; t = 2.657; df = 12; F = 1.853) but no changes in the expression of metabolism-associated intracellular signaling genes compared with controls [Fig. 1(c)].
Figure 1.
Mice fed HFD show reduced Lepr expression in the testis. (a–c) Bar graphs showing (a) the final body weight, and the mRNA expression of leptin-signaling molecules in the (b) MBH and (c) testis of mice fed RD (4% fat) or HFD (42% fat) for 16 weeks. Unpaired two-tailed Student t test was performed. Data are expressed as mean ± standard error of the mean (n = 6 to 11). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Mice on HFD show sexual behavior but have low fertility rates
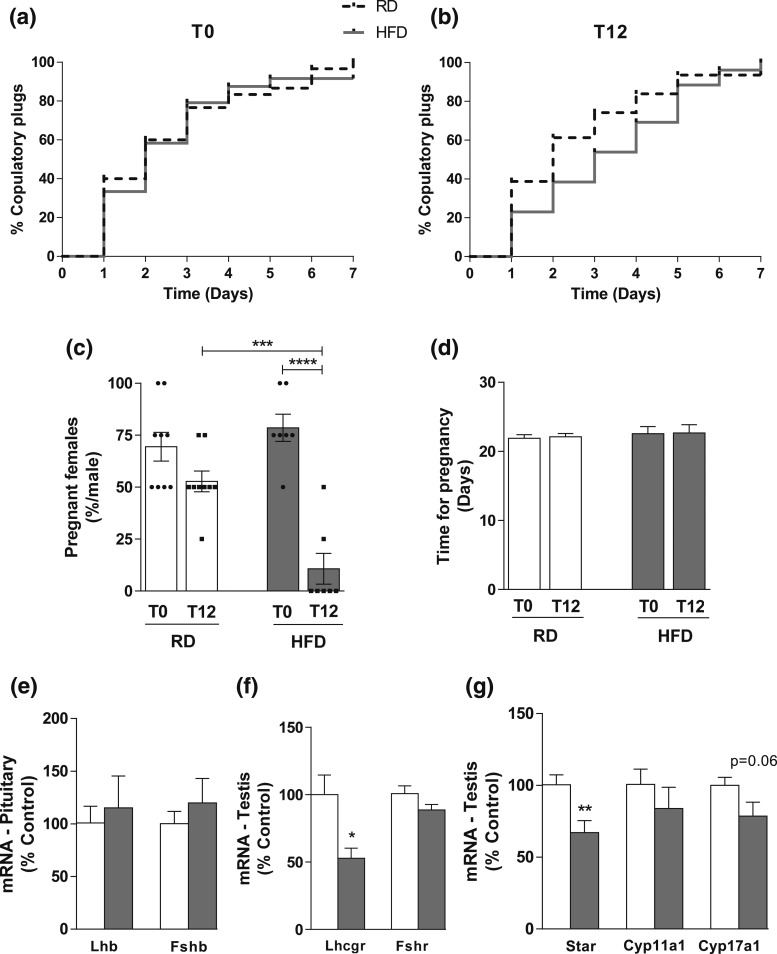
All males (8 to 10 weeks old) were initially tested for fertility for 1 week (T0, four females/male) and were assigned to two groups: RD or HFD. After 12 weeks (T12) on their respective diets, fertility testing was repeated (four females/male) to evaluate the impact of HFD on reproductive capacity. The percentage of copulatory plugs observed between the two groups at T0 and T12 was similar [Fig. 2(a) and 2(b)], suggesting that obesity does not affect sexual behavior in mice. Males fed RD had comparable pregnancy rates at T0 and T12. However, the percentage of females impregnated by males fed HFD was significantly reduced (P = 0.0005; F(1,28) = 15.39) [Fig. 2(c)]. After 12 weeks on HFD, only two of seven obese males produced offspring, whereas all males on RD produced litters. Before HFD (T0), all males impregnated at least two females (78.5% of the females: 22 from a total of 28). However, at T12, the two fertile males impregnated only three females (10.7%, from a total of 28 females). Overall, the fertile mice from both groups had comparable litter sizes (RD: 5.9 ± 0.31 pups at T0 and 6.25 ± 1.75 pups at T12; HFD: 5.9 ± 0.41 pups at T0 and 7.5 ± 0.52 pups at T12), and the latency for pregnancy and delivery was not affected (RD: t = 0.3152; df = 16; F = 1.118; P = 0.8781; HFD: t = 0.05394; df = 8; F = 1.681; P = 0.8375) [Fig. 2(d)].
Figure 2.
Diet-induced obesity does not change copulatory behavior, but impairs fertility and pituitary-testicular axis. (a and b) Graphs showing the percentage of females with copulatory plugs after mating with males in RD or HFD (a) at T0 (beginning of diet) and (b) T12 (after 12 weeks in RD or HFD). The percentage of females with copulatory plugs was similar between groups, both at T0 (RD: 100%; HFD: 91.66%) and T12 (RD: 93.54%; HFD: 100%) (four female/male; n = 7 to 9 males). Log–rank (Mantel–Cox) test was performed. (c and d) Bar graphs showing (c) the percentage of pregnant females after mating with males in RD or HFD and (d) latency for pregnancy. At T0 (before diet change) all seven HFD males impregnated at least two females (22 from a total of 28). However, at T12 only two males impregnated three females from a total of 28, demonstrating a dramatic reduction in the fertility rate after exposure to obesogenic diet. No difference in latency for pregnancy was observed. Two-way ANOVA followed by Newman Keuls post hoc test was performed. (e–g) Bar graphs showing the Lhb and Fshb mRNA expression (e) in the pituitary, (f) the Lhcgr and Fshr, and (g) the Star, Cyp11a1, and Cyp17a1 mRNA expression in the testis of mice fed RD (4% fat) or HFD (42% fat) for 16 weeks. Data are expressed as mean ± standard error of the mean (n = 5 to 9). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Pituitary–gonadal axis is disrupted in HFD males
We then assessed the hormonal and molecular changes in mice fed RD or HFD. Only the infertile HFD mice were considered for these analyses (n = 5). The expression of the β subunits of LH and follicle-stimulating hormone (Lhb and Fshb, respectively) genes in the pituitary gland was unchanged [Fig. 2(e)]. In the testes of HFD mice, the expression of follicle-stimulating hormone receptor (Fshr) gene was unaltered, but the expression of LH/choriogonadotropin receptor (Lhcgr) gene was reduced (P < 0.05; t = 2.753; df = 12; F = 4.362) [Fig. 2(f)]. We next assessed the expression of genes associated with steroidogenesis in the testis. We observed reduced expression of Star (steroidogenic acute regulatory protein) (P < 0.05; t = 3.082; df = 12; F = 1.110), no changes in Cyp11a1 (cytochrome P450, family 11, subfamily A, polypeptide 1), and a decreasing trend for Cyp17a1 (P = 0.06, cytochrome P450, family 17, subfamily A, polypeptide 1) in HFD compared with RD mice [Fig. 2(g)].
Next, we performed a comprehensive analysis of circulating levels of steroids using tandem mass spectrometry (Table 3). Testosterone was reduced in HFD males only when two cohorts were combined (P < 0.05; t = 2.708; df = 19; F = 4.561). Significance was never attained within individual cohorts (cohort 1: P = 0.058, n = 5 to 9; cohort 2: P = 0.055, n = 6 to 7). Estradiol levels were below the detection limit of the assay (3 pg/mL) in all mice regardless of diet.
Table 3.
Serum Hormone Levels in HFD- and RD-Fed Mice
| Hormones | RD | HFD | P Value |
|---|---|---|---|
| Luteinizing hormone (ng/mL) | 0.424 ± 0.135 | 0.056 ± 0.006 | <0.05 |
| Progesterone (pg/mL) | 890.2 ± 278.4 | 2135 ± 843.9 | NS |
| 17-Hydroxyprogesterone (pg/mL) | 798 ± 165.1 | 204.5 ± 94.72 | <0.05 |
| Aldosterone (pg/mL) | 218.7 ± 63.47 | 142.4 ± 93.21 | NS |
| 11-Deoxycorticosterone (pg/mL) | 2007 ± 1045 | 1816 ± 402.3 | NS |
| Corticosterone (pg/mL) | 54,586 ± 15,828 | 64,635 ± 34,609 | NS |
| Testosterone (pg/mL) | 8226 ± 1986 | 2039 ± 975.2 | <0.05 |
Abbreviation: NS, not significant.
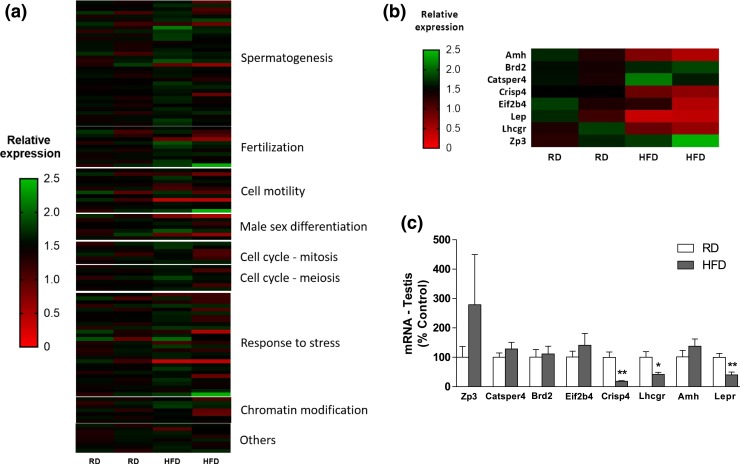
Obese infertile mice have decreased Crisp4 expression in the testis and epididymis
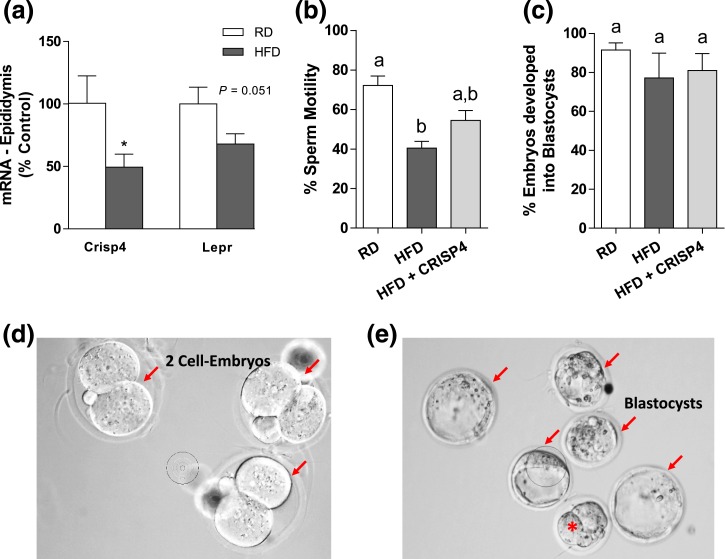
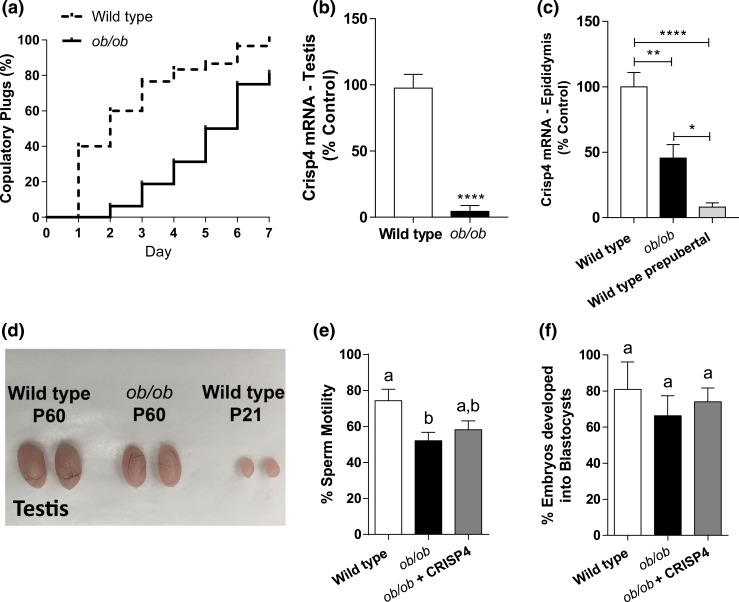
Given the negative impact of obesity on fertility despite normal copulation, we performed a screening of gonadal genes previously associated with male infertility. RNA from the testis of fertile mice fed RD and infertile mice fed HFD were isolated and hybridized to a qPCR array. From 84 genes evaluated in the array, 8 genes in the HFD group had at least a 50% change in relative expression compared with controls in RD [Fig. 3(a) and 3(b)]. The identified genes were subsequently validated using qPCR in a larger group of animals (n = 9, RD; n = 5, infertile HFD). Three of the eight candidate genes found in the array (Lepr, Lhcgr, and Crisp4) were markedly reduced in the testis of HFD mice (Crisp4: P < 0.0005; t = 3.295; df = 12; F = 165.4; Lhcgr: P < 0.04; t = 2.408; df = 10; F = 10.46; Lepr: P < 0.05; t = 3.186; df = 11; F = 2.984) [Fig. 3(c)]. A reduction in Crisp4 mRNA expression (P < 0.05; t = 2.318; df = 10; F = 3.162) and a trend toward a reduction in Lepr gene expression (P = 0.051; t = 2.165; df = 12; F = 1.511) were also found in the epididymis of mice in HFD [Fig. 4(a)].
Figure 3.
Diet-induced obesity reduced Crisp4 gene expression in the male reproductive tract. (a and b) Heat map showing (a) a qPCR array for male infertility in testis and (b) the candidate genes potentially altered by 16 weeks of HFD (42% fat) compared with RD (4% fat) (n = 2/group). (c) Graph shows mRNA expression of the candidate genes in the testis (n = 5 to 9). Unpaired two-tailed Student t test was performed. Data are expressed as mean ± standard error of the mean. *P < 0.05; **P < 0.01.
Figure 4.
Sperm from mice in HFD have reduced motility. (a–c) Bar graphs showing (a) Crisp4 and Lepr gene expression in the epididymis, (b) the percentage of motile sperm, and (c) the percentage of embryos that developed into blastocysts after IVF of WT eggs and sperm from WT mice fed RD, HFD, and HFD treated with 1.5 µM CRISP4 (HFD + CRISP4). (d and e) Images showing (d) examples of two-cell embryos and (e) blastocysts after IVF (red arrows). Red asterisk represents an abnormal developing embryo. Unpaired two-tailed Student t test was performed to compare gene expression (a). Data are expressed as mean ± standard error of the mean (n = 5 to 9). *P < 0.05. Kruskal–Wallis test of one-way ANOVA was performed to compare the sperm motility and number of two-cell embryos that developed into blastocysts. Data are expressed as mean ± standard error of the mean (n = 5 males). (a and b) Different letters = differences (P < 0.05).
The fertilization capacity of sperm from obese HFD mice was restored by in vitro CRISP4 treatment
To assess whether altered Crisp4 expression affects the motility and fertilization capacity of sperm from obese mice, we performed IVF with sperm from WT mice fed RD or HFD. Five age-matched RD and HFD mice (28 to 30 weeks old) were used as sperm donors. Sperm from HFD mice was divided into two groups, one treated with vehicle and another treated with 1.5 µM recombinant mouse CRISP4 protein. Sperm motility (percentage of motile sperm) and fertilization rates (number of eggs that undergo fertilization divided by the total number of eggs) were both reduced in HFD mice [Fig. 4(b); Table 4]. In vitro CRISP4 treatment only marginally improved the motility but restored the fertilization rate of sperm from HFD mice [Fig. 4(b); Table 4]. Of note, the percentage of two-cell embryos that developed into blastocysts was similar among groups [Fig. 4(c)].
Table 4.
Fertilization Rate of Sperm From HFD and ob/ob Mice
| Groups | No. of Eggs | No. of Two-Cell Eggs | No. Blastocystsa | Fertilization Rateb |
|---|---|---|---|---|
| RD | 386 | 215 | 187 (48%) | 56% |
| HFD | 414 | 180 | 156 (38%) | 43% |
| HFD + CRISP4 | 339 | 224 | 195 (58%) | 66% |
| WT | 229 | 149 | 126 (55%) | 65% |
| ob/ob | 760 | 243 | 195 (26%) | 32% |
| ob/ob + CRISP4 | 386 | 124 | 100 (26%) | 32% |
CRISP4 treatment restored the fertilization rate of HFD, but not of ob/ob sperm.
Parenthetical percentages = proportion of eggs that developed into blastocysts.
The fertilization rate is calculated as the number of two-cell eggs divided by the total number of eggs × 100.
Crisp4 expression is reduced in the reproductive tract of ob/ob mice
In humans and rodents, leptin-signaling deficiency causes obesity and infertility (20–24). Because of the decreased expression of the Lepr gene in the testis and epididymis of HFD mice, we assessed whether the molecular correlates for the reproductive phenotype of HFD mice were comparable to that of leptin-deficient obese mice. Ob/ob mice have reduced testis (104.8 ± 6.5 mg vs 64.02 ± 3.31 mg; P < 0.0005) and epididymis weight (54.9 ± 4.4 mg vs 32.2 ± 2.1 mg; P < 0.005), as shown by others (21, 34, 35). When mated with WT females of proven fertility, 80% of ob/ob mice showed sexual behavior with copulatory plugs in one week of fertility trial [Fig. 5(a)]. Remarkably, none of the females that mated with ob/ob males became pregnant. Similar to mice fed HFD, Lhcgr (data not shown) and Crisp4 expression were reduced (P < 0.05; t = 8.911; df = 7; F = 4.512) in the testis of ob/ob mice [Fig. 5(b)]. Because Crisp4 expression is very low in the epididymis of prepubertal mice, we assessed whether Crisp4 levels are comparable in prepubertal WT and ob/ob adult [postnatal day (P) 60] mice. Expression of Crisp4 is reduced in the epididymis of ob/ob mice compared with WT adult (P60) mice (P < 0.01), but it is increased compared with WT prepubertal (P21) mice [P < 0.05; Fig. 5(c)]. Testis weight is also reduced comparing prepubertal (31.6 ± 4.1 mg, P < 0.0001) and adult WT and ob/ob mice [Fig. 5(d)].
Figure 5.
Crisp4 mRNA expression is reduced in ob/ob mice. (a) Graph showing the percentage of copulatory plugs of WT (dashed line) and ob/ob (black line) mice when mated with WT females for 7 days (four female/male; n = 6 to 10 males). Log–rank (Mantel–Cox) test was performed. (b) Bar graph showing Crisp4 gene expression in the testis of WT and ob/ob mice. Unpaired two-tailed Student t test was performed. (c) Bar graph showing the Crisp4 expression in the epididymis of adult (P60) WT and ob/ob mice, and WT prepubertal (P21) mice. Kruskal–Wallis test of one-way ANOVA was performed. Data are expressed as mean ± standard error of the mean (n = 4 to 10). *P < 0.05; **P < 0.01; ****P < 0.0001. (d) Representative image of the testis of adult (P60) WT and ob/ob mice, and prepubertal (P21) WT mice. (e and f) Bar graphs showing (e) the percentage of motile sperm and (f) the percentage of two-cell embryos that developed into blastocysts after in vitro fertilization of WT eggs with sperm from WT, ob/ob, and ob/ob treated with 1.5 µM CRISP4 (ob/ob + CRISP4) mice. Kruskal–Wallis test of one-way ANOVA was performed. Data are expressed as mean ± standard error of the mean (n = 5 to 9 males). (a and b) Different letters = differences (P < 0.05).
In vitro CRISP4 treatment has no effect in fertilization capacity of sperm from ob/ob mice
To assess whether altered Crisp4 expression in the testis affects the motility and fertilization capacity of sperm from ob/ob mice, we performed IVF with sperm from WT mice fed RD and from obese infertile ob/ob mice. Five adult (8 to 10 weeks old) WT control mice and nine ob/ob mice were used as sperm donors. Sperm from five ob/ob mice was divided into two groups, one treated with vehicle and another treated with 1.5 µM recombinant mouse CRISP4 protein. The IVF procedure was performed as previously described. Sperm from ob/ob mice also showed reduced sperm motility [Fig. 5(e)] and fertilization rates (Table 4). As observed in HFD mice, sperm motility of ob/ob mice was only marginally, not significantly, improved by in vitro CRISP4 treatment [Fig. 5(e)]. In contrast to HFD mice, in vitro CRISP4 treatment had no effect on the fertilization rate of ob/ob sperm (Table 4). The percentage of two-cell embryos that developed into blastocysts was similar among groups [Fig. 5(f)].
Ob/ob mice show altered epididymal CRISP4 protein content and subcellular localization
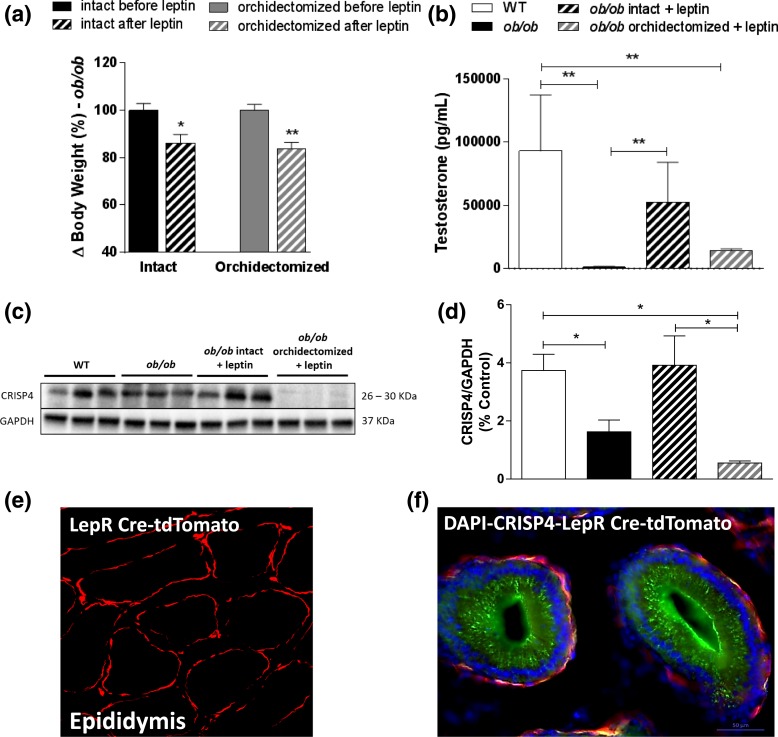
Because ob/ob mice had reduced Crisp4 mRNA expression in the epididymis compared with WT mice, we assessed CRISP4 protein expression and the effects of leptin treatment on epididymal CRISP4 of intact and castrated ob/ob mice. As expected, leptin treatment significantly reduced the body weight of both intact (P < 0.05; t = 2.989; df = 4; F = 1.497) and orchidectomized (P < 0.05; t = 4.403; df = 6; F = 1.028) ob/ob mice [Fig. 6(a)] and restored the testosterone levels of intact, but not orchidectomized ob/ob mice [Fig. 6(b)]. Ob/ob mice had reduced CRISP4 content in the epididymis, which was increased by leptin treatment [Fig. 6(c) and 6(d)]. This effect requires gonadal hormones, as leptin alone did not alter CRISP4 protein content in the epididymis of orchidectomized ob/ob mice [Fig. 6(c) and 6(d)].
Figure 6.
Gonadal hormones are required for leptin-induced increase in CRISP4 expression. (a) Bar graph showing changes in body weight (%) of intact or orchidectomized ob/ob mice before and after leptin treatment (2.5 μg/g IP) for 7 days. (b) Bar graph showing testosterone levels (pg/mL). (c and d) Image of gel and quantification of CRISP4 protein expression in the epididymis of adult WT, ob/ob, ob/ob intact + leptin, and ob/ob orchidectomized + leptin treated mice. Unpaired two-tailed Student t test was performed to compare the effect of leptin on body weight of intact and orchidectomized mice. Kruskal–Wallis test of one-way ANOVA was performed for the analysis of testosterone measurement, and ordinary one-way ANOVA followed by Tukey multiple comparison test was performed to measure CRISP4 protein expression. Data are expressed as mean ± standard error of the mean (n = 5 to 6); *P < 0.05; **P < 0.01. (e) Fluorescent image showing the Cre-induced reporter gene (tdTomato) in the epididymis of the LepR-Cre/R26 tdTomato reporter mouse fed RD. (f) Fluorescent images showing distribution of CRISP4 immunoreactivity (green), LepR-Cre/R26 tdTomato reporter gene (red), and 4′,6-diamidino-2-phenylindole (blue) staining in the epididymis. Scale bar = 50 µm.
To further define whether LEPR and CRISP4 are colocalized in epidydimal cells, we used LepR-Cre/R26-tdTomato reporter mice, which express red fluorescent protein in a Cre-dependent manner. A layer of red-fluorescent cells was observed outside the ductus epididymis, indicating that LEPR is expressed in myoid, not epithelial cells [Fig. 6(e)]. In contrast, CRISP4 immunoreactivity was dense in the luminal membrane of the epithelial cells [Fig. 6(f)].
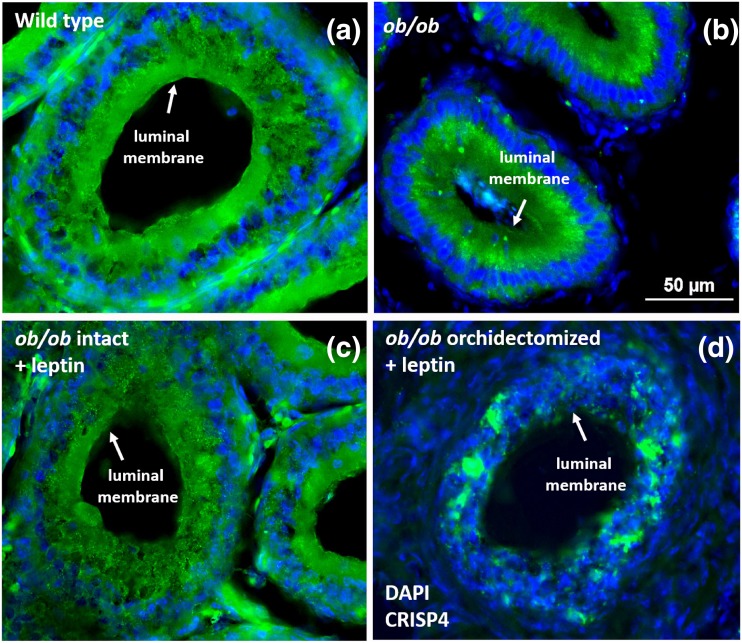
We then assessed the potential changes in CRISP4 subcellular localization. In WT mice, CRISP4 immunostaining was stronger in the apical (luminal) membrane of the epithelial cells, whereas in intact ob/ob mice, CRISP4 protein was restricted to the cytoplasm at the basal compartment of the epididymal cells. Leptin-treated ob/ob mice showed a similar pattern of CRISP4 protein localization to WT mice. Orchidectomized ob/ob mice showed abnormal epididymal histology characterized by disrupted structural organization. CRISP4 immunoreactivity was randomly localized in epididymal epithelial cells of orchidectomized ob/ob mice (Fig. 7). These findings indicate that gonadal factors are crucial for the expression and subcellular localization of CRISP4, and that leptin has an indirect effect on CRISP4 content by modulating testosterone production.
Figure 7.
CRISP4 subcellular localization in the epididymis of ob/ob mice is disrupted. (a–d) CRISP4 expression (green) and 4′,6-diamidino-2-phenylindole staining (blue) in the caput epididymis of WT, ob/ob intact, ob/ob intact + leptin (2.5 μg/g IP for 7 days), and ob/ob orchidectomized + leptin (2.5 μg/g IP for 7 days) mice. (a) In WT mice, CRISP4 expression is mostly observed at the apical (luminal) membrane of the epididymal cells. (b) In ob/ob mice, CRISP4 expression is mainly observed in the base of the cells. (c) The pattern of CRISP4 expression in ob/ob mice treated with leptin is similar to that observed in WT animals. (d) Orchidectomized ob/ob mice treated with leptin show a reduced and disorganized CRISP4 expression in the ductus epididymis. Scale bar: 50 µm.
Discussion
Diet-induced and genetically obese mice have decreased LH and low testosterone levels. These hormonal changes are not sufficient to preclude the copulatory behavior, but the fertility is dramatically reduced. Following a targeted transcriptome analysis, we found that Crisp4 expression is altered in the reproductive tract of obese mice. Decreased CRISP4 levels correlated with diminished ability of sperm to fertilize eggs. CRISP4 treatment prior to IVF restored fertilization rate of sperm from HFD mice. Our findings indicate that obesity-induced decrease in Crisp4 expression in the reproductive tract leads to decreased fertilization capacity and sub- or infertility in mice.
The proportion of overweight and obese men of reproductive age has dramatically increased in recent decades, and one of the correlates of high adiposity is reduced fertility (1, 6, 36). In rodents, the impact of obesogenic diets on testosterone production is controversial (6, 13). In humans, however, studies have reported that free testosterone is not altered in overweight men, but it is significantly decreased in severely obese men (37). These findings suggest that the degree of obesity or other associated variables (i.e., diabetes, leptin resistance, and low-grade inflammation) may influence or aggravate the infertility phenotype.
The reduced activity of the HPG axis of obese mice might indicate an impairment of sexual behavior (11). However, because no difference in copulatory plugs between lean and obese mice was observed in our study, the low fertility rate is unlikely to be caused by the disruption of sexual behavior, as also noted by others (13). The reduction in testosterone levels was not sufficient to alter sexual behavior but could disrupt the molecular pathways associated with sperm motility and fertilization capacity (12, 38). The acquisition of progressive movement by the sperm is dependent on testosterone (39, 40), but studies have also shown that diet-induced obese mice and rats had reduced sperm motility with no corresponding changes in testosterone levels (11, 13). Our data support previous reports; however, although the number of motile sperm was decreased, approximately half of the sperm showed progressive motility, suggesting that poor motility alone was not the main cause of the dramatic decrease in fertility rate.
In a search for molecular components that could add mechanistic insights, we performed a comparative screening of genes associated with male infertility using only infertile obese mice. We found a reduction in Crisp4 expression. CRISP4 is primarily secreted in the lumen of the caput epididymis, where it plays a role in the posttesticular maturation of the sperm (41–43). It acts as an inhibitor of the transient receptor potential ion channel TRPM8, which is expressed in the sperm membrane and is a modulator of both sperm function and the acrosome reaction (42). Crisp4 expression in the epididymal cells is regulated in an androgen-dependent manner, i.e., decreased after an orchidectomy and restored following testosterone treatment (44). Fertilization rates in IVF of sperm from CRISP4-deficient mice were dramatically decreased (43).
In parallel with reduced Crisp4 expression, we observed a reduction in the expression of Lepr in the epididymides of mice fed HFD. Leptin has a permissive role in reproductive function. The lack of leptin signaling causes infertility due to decreased activation of the HPG axis (21, 25, 35). Increased circulating leptin levels, as observed in obese states, impair leptin signaling, resulting in diminished gonadal steroid levels via indirect regulation of gonadotropin-releasing hormone neuronal function (16, 17, 45, 46). This phenomenon, known as leptin resistance, is characterized by increased levels of suppressor of cytokine signaling 3, protein tyrosine phosphatase 1B, and phosphatase and tensin homolog in the hypothalamus, which impair the phosphorylation of STAT3, thus reducing the overall effects of leptin (26, 27). In agreement with these reports, the diet-induced obese mice had a hypothalamic gene profile typical of leptin resistance. In the testis, however, only Lepr expression was altered, suggesting that the decrease in leptin signaling in the hypothalamus and testis of obese mice develops via distinct mechanisms. Of note, previous studies in rats have shown that leptin action in testis inhibits testosterone production via changes in the expression of steroidogenic enzymes (47, 48). Together with the increase in leptin levels typical of obese states, our finding of decreased Lepr expression in testis indicates a compensatory response. Moreover, the expression of the leptin receptor by myoid, not epithelial, cells of the ductus epididymis suggests that leptin action is not directly associated with the modulation of epididymal CRISP4 expression.
To evaluate the role of leptin in Crisp4 expression, we used leptin-deficient obese and infertile ob/ob mice. These mice have decreased LH and testosterone levels, reduced sperm motility, and deficient acrosome reaction. Leptin administration to ob/ob mice improves all reproductive parameters and restores fertility (21, 34, 35). Despite decreased testis size and low testosterone, ∼80% of obese ob/ob males showed successful copulatory behavior in 1 week of fertility testing, implying that the infertility of ob/ob males is not due to a lack of sexual behavior. Reduced sperm motility might play a role, but our observation that ∼50% of the sperm were motile may not completely explain the infertility observed in our studies. Similar to mice fed HFD, ob/ob mice show low CRISP4 expression and altered CRISP4 subcellular localization. In vitro CRISP4 treatment of sperm from obese HFD or ob/ob mice reverses the low sperm motility, but the fertilization rates are improved only in HFD group. Leptin administration to ob/ob mice restores epididymal CRISP4, but these effects require the full action of the gonadal hormones, as observed by others in different experimental designs (44). Whereas in vitro CRISP4 treatment of the sperm restored the fertilization capacity of mice fed HFD, sperm from ob/ob mice were unresponsive, indicating that factors beyond altered CRISP4 content and sperm motility contribute to the infertility of leptin deficiency.
Our study demonstrates that the obesity-induced decrease in the activity of the HPG axis in mice is not sufficient to alter sexual behavior. Also, the reduction in the amount of motile sperm does not represent a definitive explanation for the remarkably low fertility rate. Obesity disrupts epididymal CRISP4 expression and subcellular localization, leading to a decrease in the capacity of the sperm to fertilize an egg. Our findings in mice offer additional insights to the understanding of obesity-induced infertility and the decreased fertilization rate of sperm from obese subjects.
Acknowledgments
We thank Susan J. Allen and Jun Ding for expert technical support. We thank the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core for hormone assays. We also thank the Mass Spectrometry Core services, supported by Grant DK089503 from the National Institutes of Health to the University of Michigan under the Michigan Nutrition Obesity Center.
Acknowledgments
This work was supported by National Institutes of Health Grants R01-HD-069702 (to C.F.E.) and R01-GM-086596 (to R.J.A.); Sao Paulo Research Foundation fellowships FAPESP: 2013/03915-0 (to B.C.B.) and FAPESP: 2012/16536-4 (to S.d.S.C.-M.); and Brazilian National Council for Scientific and Technological Development fellowship CNPq: 203202/2014-7(to B.C.B.). The University of Virginia Ligand Core is supported by Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (National Centers for Translational Research in Reproduction and Infertility) Grant P50-HD28934.
Author contributions: B.C.B., D.G.-G., S.d.S.C.-M., G.D.S., and C.F.E. conceived the study. B.C.B. validated the study. B.C.B., X.H., G.B.G., T.L.S., S.S.H., and R.J.A. provided methodology. B.C.B., D.G.-G., and S.d.S.C.-M. performed investigations. B.C.B. wrote the original draft. C.F.E., T.L.S., S.S.H., G.D.S., S.d.S.C.-M., and R.J.A. wrote, reviewed, and edited the article. C.F.E. provided funding acquisition services. C.F.E., G.D.S., T.L.S., and R.J.A. handled the resources. C.F.E. provided supervision for the study.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- BSA
- bovine serum albumin
- Ct
- threshold cycle
- HFD
- high-fat diet
- HPG
- hypothalamo-pituitary-gonadal
- IP
- intraperitoneal
- IVF
- in vitro fertilization
- LH
- luteinizing hormone
- MBH
- mediobasal hypothalamus
- mRNA
- messenger RNA
- P
- postnatal day
- PBS
- phosphate-buffered saline
- qPCR
- quantitative polymerase chain reaction
- RD
- regular diet
- WT
- wild-type.
References
- 1.Morrison CD, Brannigan RE. Metabolic syndrome and infertility in men. Best Pract Res Clin Obstet Gynaecol. 2015;29(4):507–515. [DOI] [PubMed] [Google Scholar]
- 2.Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ. 1992;305(6854):609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auger J, Kunstmann JM, Czyglik F, Jouannet P. Decline in semen quality among fertile men in Paris during the past 20 years. N Engl J Med. 1995;332(5):281–285. [DOI] [PubMed] [Google Scholar]
- 4.Hammoud AO, Gibson M, Peterson CM, Hamilton BD, Carrell DT. Obesity and male reproductive potential. J Androl. 2006;27(5):619–626. [DOI] [PubMed] [Google Scholar]
- 5.Hammoud AO, Meikle AW, Reis LO, Gibson M, Peterson CM, Carrell DT. Obesity and male infertility: a practical approach. Semin Reprod Med. 2012;30(6):486–495. [DOI] [PubMed] [Google Scholar]
- 6.Palmer NO, Bakos HW, Fullston T, Lane M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis. 2012a;2(4):253–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kort HI, Massey JB, Elsner CW, Mitchell-Leef D, Shapiro DB, Witt MA, Roudebush WE. Impact of body mass index values on sperm quantity and quality. J Androl. 2006;27(3):450–452. [DOI] [PubMed] [Google Scholar]
- 8.MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16(3):293–311. [DOI] [PubMed] [Google Scholar]
- 9.Keltz J, Zapantis A, Jindal SK, Lieman HJ, Santoro N, Polotsky AJ. Overweight men: clinical pregnancy after ART is decreased in IVF but not in ICSI cycles. J Assist Reprod Genet. 2010;27(9-10):539–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakos HW, Henshaw RC, Mitchell M, Lane M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil Steril. 2011a;95(5):1700–1704. [DOI] [PubMed] [Google Scholar]
- 11.Ghanayem BI, Bai R, Kissling GE, Travlos G, Hoffler U. Diet-induced obesity in male mice is associated with reduced fertility and potentiation of acrylamide-induced reproductive toxicity. Biol Reprod. 2010;82(1):96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bakos HW, Mitchell M, Setchell BP, Lane M. The effect of paternal diet-induced obesity on sperm function and fertilization in a mouse model. Int J Androl. 2011b;34(5 Pt 1):402–410. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez CD, Bellentani FF, Fernandes GS, Perobelli JE, Favareto AP, Nascimento AF, Cicogna AC, Kempinas WD. Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod Biol Endocrinol. 2011;9:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallidis C, Czerwiec A, Filippi S, O’Neill J, Maggi M, McClure N. Spermatogenic and sperm quality differences in an experimental model of metabolic syndrome and hypogonadal hypogonadism. Reproduction. 2011;142(1):63–71. [DOI] [PubMed] [Google Scholar]
- 15.Palmer NO, Bakos HW, Owens JA, Setchell BP, Lane M. Diet and exercise in an obese mouse fed a high-fat diet improve metabolic health and reverse perturbed sperm function. Am J Physiol Endocrinol Metab. 2012b;302(7):E768–E780. [DOI] [PubMed] [Google Scholar]
- 16.Halaas JL, Boozer C, Blair-West J, Fidahusein N, Denton DA, Friedman JM. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc Natl Acad Sci USA. 1997;94(16):8878–8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahima RS, Saper CB, Flier JS, Elmquist JK. Leptin regulation of neuroendocrine systems. Front Neuroendocrinol. 2000;21(3):263–307. [DOI] [PubMed] [Google Scholar]
- 18.Ingalls AM, Dickie MM, Snell GD. Obese, a new mutation in the house mouse. J Hered. 1950;41(12):317–318. [DOI] [PubMed] [Google Scholar]
- 19.Swerdloff RS, Batt RA, Bray GA. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology. 1976;98(6):1359–1364. [DOI] [PubMed] [Google Scholar]
- 20.Coleman DL. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14(3):141–148. [DOI] [PubMed] [Google Scholar]
- 21.Barash IA, Cheung CC, Weigle DS, Ren H, Kabigting EB, Kuijper JL, Clifton DK, Steiner RA. Leptin is a metabolic signal to the reproductive system. Endocrinology. 1996;137(7):3144–3147. [DOI] [PubMed] [Google Scholar]
- 22.Chehab FF, Lim ME, Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat Genet. 1996;12(3):318–320. [DOI] [PubMed] [Google Scholar]
- 23.Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, Sewter CP, Digby JE, Mohammed SN, Hurst JA, Cheetham CH, Earley AR, Barnett AH, Prins JB, O’Rahilly S. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387(6636):903–908. [DOI] [PubMed] [Google Scholar]
- 24.Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341(12):879–884. [DOI] [PubMed] [Google Scholar]
- 25.Elias CF, Purohit D. Leptin signaling and circuits in puberty and fertility. Cell Mol Life Sci. 2013;70(5):841–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flak JN, Myers MG Jr. Minireview: CNS mechanisms of leptin action. Mol Endocrinol. 2016;30(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tups A. Physiological models of leptin resistance. J Neuroendocrinol. 2009;21(11):961–971. [DOI] [PubMed] [Google Scholar]
- 28.Donato J Jr, Cravo RM, Frazão R, Gautron L, Scott MM, Lachey J, Castro IA, Margatho LO, Lee S, Lee C, Richardson JA, Friedman J, Chua S Jr, Coppari R, Zigman JM, Elmquist JK, Elias CF. Leptin’s effect on puberty in mice is relayed by the ventral premammillary nucleus and does not require signaling in Kiss1 neurons. J Clin Invest. 2011;121(1):355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291(5513):2608–2613. [DOI] [PubMed] [Google Scholar]
- 30.Scott MM, Lachey JL, Sternson SM, Lee CE, Elias CF, Friedman JM, Elmquist JK. Leptin targets in the mouse brain. J Comp Neurol. 2009;514(5):518–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turcu AF, Rege J, Chomic R, Liu J, Nishimoto HK, Else T, Moraitis AG, Palapattu GS, Rainey WE, Auchus RJ. Profiles of 21-carbon steroids in 21-hydroxylase deficiency. J Clin Endocrinol Metab. 2015;100(6):2283–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sondhi V, Owen BM, Liu J, Chomic R, Kliewer SA, Hughes BA, Arlt W, Mangelsdorf DJ, Auchus RJ. Impaired 17,20-lyase activity in male mice lacking cytochrome b5 in Leydig cells. Mol Endocrinol. 2016;30(4):469–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Δ Δ C(T)) method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann A, Manjowk GM, Wagner IV, Klöting N, Ebert T, Jessnitzer B, Lössner U, Stukenborg JB, Blüher M, Stumvoll M, Söder O, Svechnikov K, Fasshauer M, Kralisch S. Leptin within the subphysiological to physiological range dose dependently improves male reproductive function in an obesity mouse model. Endocrinology. 2016;157(6):2461–2468. [DOI] [PubMed] [Google Scholar]
- 35.Mounzih K, Lu R, Chehab FF. Leptin treatment rescues the sterility of genetically obese ob/ob males. Endocrinology. 1997;138(3):1190–1193. [DOI] [PubMed] [Google Scholar]
- 36.Pasquali R, Gambineri A, Pagotto U. The impact of obesity on reproduction in women with polycystic ovary syndrome. BJOG. 2006;113(10):1148–1159. [DOI] [PubMed] [Google Scholar]
- 37.Giagulli VA, Kaufman JM, Vermeulen A. Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab. 1994;79(4):997–1000. [DOI] [PubMed] [Google Scholar]
- 38.Barratt CL, Tomlinson MJ, Cooke ID. Prognostic significance of computerized motility analysis for in vivo fertility. Fertil Steril. 1993;60(3):520–525. [PubMed] [Google Scholar]
- 39.Orgebin-Crist MC, Tichenor PL. Effect of testosterone on sperm maturation in vitro. Nature. 1973;245(5424):328–329. [DOI] [PubMed] [Google Scholar]
- 40.Henderson NA, Robaire B. Effects of PNU157706, a dual 5α-reductase inhibitor, on rat epididymal sperm maturation and fertility. Biol Reprod. 2005;72(2):436–443. [DOI] [PubMed] [Google Scholar]
- 41.Nolan MA, Wu L, Bang HJ, Jelinsky SA, Roberts KP, Turner TT, Kopf GS, Johnston DS. Identification of rat cysteine-rich secretory protein 4 (Crisp4) as the ortholog to human CRISP1 and mouse Crisp4. Biol Reprod. 2006;74(5):984–991. [DOI] [PubMed] [Google Scholar]
- 42.Gibbs GM, Orta G, Reddy T, Koppers AJ, Martínez-López P, de la Vega-Beltràn JL, Lo JC, Veldhuis N, Jamsai D, McIntyre P, Darszon A, O’Bryan MK. Cysteine-rich secretory protein 4 is an inhibitor of transient receptor potential M8 with a role in establishing sperm function. Proc Natl Acad Sci USA. 2011;108(17):7034–7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turunen HT, Sipilä P, Krutskikh A, Toivanen J, Mankonen H, Hämäläinen V, Björkgren I, Huhtaniemi I, Poutanen M. Loss of cysteine-rich secretory protein 4 (Crisp4) leads to deficiency in sperm-zona pellucida interaction in mice. Biol Reprod. 2012;86(1):1–8. [DOI] [PubMed] [Google Scholar]
- 44.Jalkanen J, Huhtaniemi I, Poutanen M. Mouse cysteine-rich secretory protein 4 (CRISP4): a member of the Crisp family exclusively expressed in the epididymis in an androgen-dependent manner. Biol Reprod. 2005;72(5):1268–1274. [DOI] [PubMed] [Google Scholar]
- 45.Quennell JH, Mulligan AC, Tups A, Liu X, Phipps SJ, Kemp CJ, Herbison AE, Grattan DR, Anderson GM. Leptin indirectly regulates gonadotropin-releasing hormone neuronal function. Endocrinology. 2009;150(6):2805–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quennell JH, Howell CS, Roa J, Augustine RA, Grattan DR, Anderson GM. Leptin deficiency and diet-induced obesity reduce hypothalamic kisspeptin expression in mice. Endocrinology. 2011;152(4):1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tena-Sempere M, Pinilla L, González LC, Diéguez C, Casanueva FF, Aguilar E. Leptin inhibits testosterone secretion from adult rat testis in vitro. J Endocrinol. 1999;161(2):211–218. [DOI] [PubMed] [Google Scholar]
- 48.Tena-Sempere M, Manna PR, Zhang FP, Pinilla L, González LC, Diéguez C, Huhtaniemi I, Aguilar E. Molecular mechanisms of leptin action in adult rat testis: potential targets for leptin-induced inhibition of steroidogenesis and pattern of leptin receptor messenger ribonucleic acid expression. J Endocrinol. 2001;170(2):413–423. [DOI] [PubMed] [Google Scholar]