Abstract
Retinoic acid (RA) is the active ingredient of vitamin A. It exerts its canonical activity by binding to nuclear RA receptors (RARs) to regulate gene expression. Increasingly, RA is also known to elicit nongenomic RAR-independent activities, most widely detected in activating extracellular regulated kinase (ERK)1/2. This study validated the functional role of cellular retinoic acid–binding protein 1 (Crabp1) in mediating nongenomic activity in RA, specifically activating ERK1/2 to rapidly augment the cell cycle by expanding the growth 1 phase and slowing down embryonic stem cell and neural stem cell (NSC) proliferation. The study further uncovered the physiological activity of Crabp1 in modulating NSC proliferation and animal behavior. In the Crabp1 knockout mouse hippocampus, where Crabp1 is otherwise detected in the subgranular zone, neurogenesis and NSC proliferation increased and hippocampus-dependent brain functions such as learning and memory correspondingly improved. This study established the physiological role of Crabp1 in modulating stem cell proliferation and hippocampus-dependent brain activities such as learning and memory.
Ablation of Crabp1 expression enhanced ESC and NSC proliferation, resulting in enhanced neurogenesis and improved learning and memory in mice.
Cellular retinoic acid–binding protein 1 (Crabp1) is a conserved cytosolic protein with a high binding affinity for retinoic acid (RA). Previous gene knockout (KO) studies showed that Crabp1 KO mice displayed no gross anatomical abnormalities in typical laboratory facilities (1, 2). However, gene overexpression studies of transgenic mice revealed interesting phenotypes related to cell growth/differentiation (3, 4). Therefore, the exact physiological role of Crabp1 has been debated for more than 2 decades (5, 6).
Studies of RA signaling pathways showed that RA can elicit certain noncanonical signaling pathways independently of RA receptors (RARs), but the mechanism was unclear (6–8). To this end, we recently showed that Crabp1 mediated the nongenomic action of RA to rapidly activate extracellular regulated kinase (ERK)1/2 to modulate cell properties specifically in the stem cell context, such as in cancers and embryonic stem cells (ESCs) (9–13). In cancer cells, the Crabp1-activated signaling pathway regulates protein phosphatase 2A activity and facilitates cell apoptosis (14). In ESCs, this pathway augments the cell cycle, slowing down proliferation (15). But whether—and how—the Crabp1-activated signaling pathway has any physiological relevance in vivo remains to be determined. We thus sought to investigate this issue in the context of whole animals by focusing on the brain, specifically the hippocampus where stem cells, such as neural stem cells (NSCs), are known to play important roles and where Crabp1 is highly expressed (16, 17). We hypothesized that Crabp1 plays a role in modulating stem cell homeostasis in the brain to affect animal behaviors/brain functions.
Cell cycle progression and lineage commitment are tightly coordinated processes in stem cells (18). Prolonging the growth 1 (G1) phase by chemical inhibition of cyclin-dependent kinase (CDK) 2−cyclin E or by RNA interference–mediated silencing of CDK4−cyclin D results in increased neurogenesis (18). In animals, there are substantial stem cell populations mostly in the brain, especially the hippocampus. NSCs reside in the subgranular zone (SGZ) of the hippocampus and in the subventricular zone (19). Importantly during NSC proliferation and differentiation into neurons and astrocytes, the intracellular Raf-ERK1/2 signaling pathway is activated (20). In adults, hippocampal neurogenesis is important for maintaining hippocampal plasticity and cognitive function. New neurons matured from NSCs incorporate into the SGZ of the dentate gyrus to support hippocampus-dependent activities such as learning and memory (21, 22). These new neurons heighten CA3 synaptic plasticity. Maintenance of the NSC pool has been strongly linked to the plastic potential and function of the brain (21). This is supported by the finding that optogenetic silencing of 4-week-old neurons impaired spatial and contextual memory retrieval (23).
In this study, we provide evidence of the physiological role of Crabp1 in regulating the NSC pool in the adult hippocampus, thereby modulating certain hippocampus-dependent brain activities in adults. Using Crabp1 KO mice, we demonstrated that Crabp1 KO enhanced NSC proliferation in the hippocampus and consequently increased neurogenesis and improved animal learning and memory performance. In the in vitro ESC-NSC differentiation system, we also demonstrated that Crabp1 specifically modulated stem cell proliferation but not early stage differentiation potential.
Materials and Methods
Animal experiments
Twenty-eight wild-type (WT) and twenty-six Crabp1 KO male mice, 6 to 8 weeks old, were used in these studies. These mice were bred in the animal facility of the University of Minnesota. The mice were housed in a temperature-controlled room (22°C ± 1°C) on a 14/10 light/dark cycle (lights on/off at 0600/2000) with ad libitum food and water. We obtained a Crabp1 KO DE3 (ES) clone that contained a hit-and-run−type vector, creating a 5-bp Not1 insertion in exon 1 (the fifth codon of the Crabp1 coding region) to ablate Crabp1 expression (1). The Crabp1 KO ES clone was injected to generate Crabp1 KO chimeric mice in the University of Minnesota transgenic facility. These mice were then backcrossed on a C57/BL6 background for 10 generations to generate Crabp1 KO C57/BL6 mice. The experimental procedures were conducted according to National Institutes of Health guidelines and were approved by the University of Minnesota Institutional Animal Care and Use Committee.
Cell culture methods
CJ7 ESCs were maintained as described (24, 25). A cell proliferation assay was conducted by labeling ESCs with 5-bromo-2′-deoxyuridine (BrdU; 10 μM; Sigma-Aldrich, St. Louis, MO) for the indicated time points. Cells were fixed with absolute ethanol and stained with fluorescein isothiocyanate anti-BrdU (347583; BD Biosciences, Franklin Lakes, NJ). For flow cytometry, ethanol-fixed cells were stained with 10 mL of phosphate-buffered saline (PBS) containing 2 mg of deoxyribonuclease-free ribonuclease A and 0.4 mL of 500-μg/mL propidium iodine at 37°C for 15 minutes and analyzed with fluorescence-activated cell sorting (FACS; BD Biosciences). For 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) thiazolyl blue assay (Sigma-Aldrich), ESCs plated at equal numbers were subjected to solubilization with dimethyl sulfoxide at indicated time points after MTT incubation for 3 hours. Absorbance was measured at 570 nm.
ESC-neuronal differentiation procedure
For the ESC-neuronal differentiation procedure (26), ESCs were seeded on gelatin-coated tissue culture dishes in Dulbecco’s modified Eagle medium [(DMEM; Gibco, Waltham, MA) supplemented with 15% fetal calf serum (Gemini Bio-Products, Sacramento, CA), 103 U/mL leukemia inhibitory factor (LIF; Millipore Sigma-Aldrich, St. Louis, MO), 2 mM l-glutamine (Gibco), 1× nonessential amino acids (Gibco), 5 mL per 500 mL of β-mercaptoethanol (Sigma-Aldrich), and 0.2% 100× penicillin/streptomycin (Gemini Bio-Products)]. Cells were trypsinized (0.05% trypsin/EDTA; Gibco), terminated by adding fresh medium, spun down, resuspended in 15 mL of medium (DMEM containing 10% fetal calf serum, 2 mM l-glutamine, 1× nonessential amino acids, 5 mL per 500 mL of β-mercaptoethanol, and 0.2% 100× penicillin/streptomycin), and transferred to bacteriological dishes to form embryoid bodies (EBs), which were transferred to 15-mL tubes to settle. Supernatant was replaced with 15 mL of EB medium supplemented with 5 μM of RA (Sigma-Aldrich). The resuspended EBs were plated on bacteriological dishes for another 4 days, and the medium was changed every 2 days. Finally, the EBs were washed and trypsinized (0.05% trypsin/EDTA), and cell suspension was spun down and resuspended in N2 medium [DMEM supplemented with 2 mM of l-glutamine, 1× N2 supplement (Gibco), and 1% 100× penicillin/streptomycin], filtered through a 40-μm nylon cell strainer, and plated on plates coated with poly-DL-ornithine hydrobromide (Sigma-Aldrich)/laminin (Thermo Fisher Scientific) (2 × 105 cells/cm2), with two changes of medium at 2 hours and 24 hours. Cells were transferred to neurobasal (NB) medium [(NB/B27) supplemented with 2 mM of l-glutamine, 1× B27 supplement (Gibco), 1% 200 mM l-glutamine, 1% 100× penicillin/streptomycin] to induce neural differentiation.
Immunohistochemistry
Mice were perfused with PBS containing 4% paraformaldehyde. Brains were removed, fixed in 4% paraformaldehyde for 24 hours, and immersed in 30% sucrose for 48 hours at 4°C. Coronal brain sections were obtained in 30-μm-thick slices. PBS-washed slices were treated with a blocking solution containing 0.2% Triton X-100, 1% bovine serum albumin, and 5% goat serum in PBS for 60 minutes at room temperature; incubated with primary antibodies, including CRABP1 [sc-10061; research resource identifier (RRID): AB_2085315; Santa Cruz], BrdU (SC-70441; RRID: AB_1119696; Santa Cruz), and doublecortin (DCX; ab18723; RRID: AB_732011; Abcam); diluted in blocking solution at 4°C overnight; and incubated with fluorochrome-conjugated secondary antibody and 4′,6-diamidino-2-phenylindole in the dark for 1 hour. Fluorescent images were acquired using an inverted fluorescence microscope (DM5500 B upright microscope; Leica, Wetzlar, Germany) and image capture system MetaMorph software (Molecular Devices, Sunnyvale, CA).
In vivo proliferation and neurogenesis in the adult hippocampus
Mice received two injections of BrdU (200 mg/kg, intraperitoneally; Sigma-Alrich) 8 hours apart and were euthanized for immunohistochemistry staining 24 hours after the last BrdU injection. Stereological scoring of BrdU- or DCX-labeled cells was performed on a series of sections of the dentate gyrus. The volume of dentate gyrus was determined by 4′,6-diamidino-2-phenylindole signal using the FluoView FV1000 confocal microscope (Olympus, Japan) with FV1000 software (Olympus). Cell proliferation was determined by the number of BrdU-positive cells. Neurogenesis was quantified using the ratio of BrdU and DCX double-positive cells over total BrdU-positive cells.
Morris water maze test
The Morris water maze (MWM) test monitors spatial learning and memory ability (27). The maze is a circular pool (diameter, 1.2 m) filled with water maintained at 22°C and supplied with nontoxic white paint. For the acquisition of spatial reference memory, mice underwent hidden-platform training for 6 consecutive days and four trials per day with visible cues. Each trial was terminated when the mouse reached the platform or after 60 seconds. Mice failing to reach the platform were guided to remain on the platform for 60 seconds. Retrieval of reference memory was performed on day 7 (24 hours after the last training trial). Mice were subjected to probe trials in which they swam for 30 seconds in the pool without a platform, as monitored and recorded with a camera mounted on the ceiling directly above the pool; results were analyzed with the Any-mazeTM (Stoelting, Wood Dale, IL). The parameters included the escape latencies, swimming speed, path length, and percentage of time spent in each quadrant of the pool during the probe trials.
Open field test
Mice were tested in an open field (Plexiglas box, 40 × 25 × 22 cm) located in a room with dim lighting. Each mouse was placed in the corner of the box and allowed free exploration for 5 minutes. Mice were monitored and recorded by a camera mounted in the ceiling directly above the box and were analyzed using the Any-mazeTM (Stoelting).
Object recognition task
For the object recognition task (28), the apparatus is a gray box (45 × 45 × 30 cm). Before the task, mice were handled for 5 minutes every day for 3 days. The task consisted of three phases (i.e., habituation, familiarization, and test) separated by 24 hours. During the habituation phase, mice were placed in the corner of the box and allowed free exploration for 5 minutes. During the familiarization phase, mice were placed in the same box with two identical objects (ceramic cylinders) for 5 minutes. After 24 hours, the mice were returned to the box with one familiar object and one novel object (a plastic building block) for 5 minutes for the test phase. The mice were monitored and recorded using a camera mounted in the ceiling directly above the box and were analyzed with the Any-mazeTM (Stoelting).
Western blotting and chemicals
Whole cell lysate was prepared as described (15). Anti-β-actin (SC-47778; RRID: AB_626632; Santa Cruz), anti-phospho-ERK1/2 (Catalog #9101; RRID: AB_2297442; Cellular Signaling), anti-ERK1 (Catalog #4372; RRID: AB_10693946; Cellular Signaling), anti-ERK2 (Catalog #9108; RRID: AB_10695610; Cellular Signaling), anti-Crabp1 (C1608; RRID: AB_258751; Sigma-Aldrich), anti-PAX6 (SC-53106; RRID: AB_630088; Santa Cruz), anti-GAPDH (SC-627679; RRID: AB_626632; Santa Cruz). RA (100 nM) was from Sigma-Aldrich.
Data analysis
Statistical differences between groups were determined by one-way or two-way repeated measure analysis of variance followed by the Bonferroni post hoc test in the MWM. Independent-sample t tests were used to compare two independent groups. Statistical analyses were performed using SPSS 17.0. All tests were performed at a significance level of P < 0.05, and data are presented as mean ± standard error of the mean.
Results
Crabp1 mediated the nongenomic activity of RA to rapidly activate ERK1/2 and expand the G1 cell cycle to suppress ESC proliferation
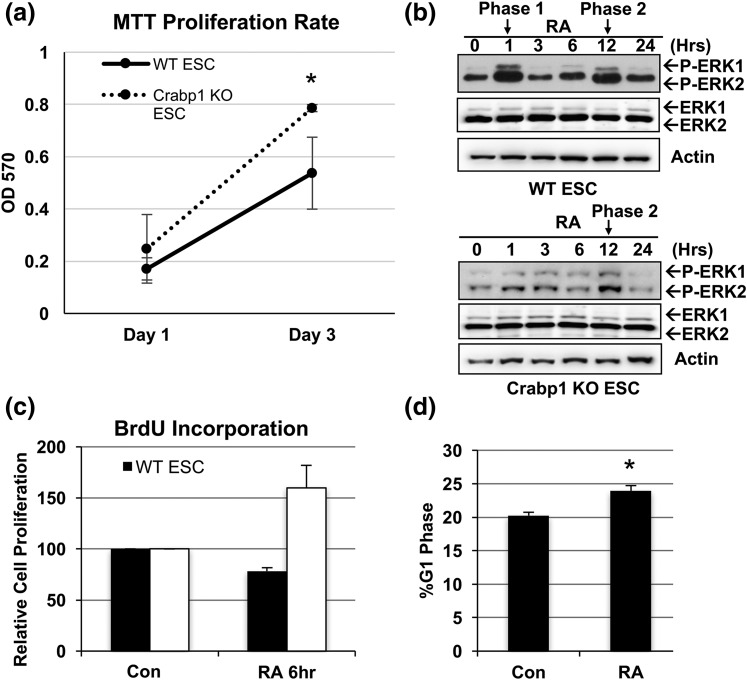
For typical ESC cultures, RA is a commonly used reagent to initiate differentiation, mostly attributed to its genomic activity mediated by RARs to regulate gene expression. Recently, our studies showed that Crabp1 can play a role in augmenting cancer cell and ESC growth (14, 15). We took this lead and examined the exact role of Crabp1 in the physiologically relevant model ESC in the current study. We first carefully compared cellular growth (cell survival) between Crabp1 KO and WT ESCs cultured in typical ESC media that contained endogenous RA. The MTT assay showed that Crabp1 KO ESCs continued proliferating [Fig. 1(a)], confirming the functional role of Crabp1 in modulating ESC growth.
Figure 1.
Crabp1 regulated the ESC cell cycle at the G1 phase. (a) Crabp1 KO ESCs proliferated more rapidly than WT ESCs, as monitored by MTT assay (P = 0.004). (b) Biphasic ERK activation by RA in WT ESCs. Phase 1 was lost in Crabp1 KO ESCs. (c) Cellular proliferation of WT ESCs slowed down after RA treatment (6 hours), as monitored by BrdU incorporation. (d) RA expanded the G1 phase. Results are presented as mean ± standard error of the mean. *P < 0.05 compared with control group (Con).
We previously showed that the addition of RA could rapidly (within 1 hour) activate ERK1/2, which is RAR independent because RAR antagonists could not block this activity, and that RAR-activated ERK1/2 was detected around 12 hours after RA treatment (15). We suspected that the immediate ERK1/2 activation by RA in ESCs might involve Crabp1. Figure 1(b) indeed shows that RA-treated WT ESCs exhibited two distinct phases of ERK1/2 activation, one immediate phase (i.e., phase 1) that was sustained over 1 hour and a later phase (i.e., phase 2) detected after 12 hours without a change in total ERK1/2 expression. Importantly, Crabp1 KO ESCs exhibited only phase 2 ERK1/2 activation (at 12 hours), validating the functional role of Crabp1 in mediating rapid activation of ERK1/2 by RA.
We then used FACS analyses to specifically monitor proliferation. WT and Crabp1 KO ESCs were labeled with BrdU for 1 hour, followed by RA treatment for 6 hours (before RA elicited genomic effects). As seen in Fig. 1(c), within 6 hours, RA suppressed WT ESC proliferation, whereas Crabp1 KO ESCs maintained their proliferation under the same treatment. This result shows that RA-Crabp1 modulated (reduced) stem cell proliferation. Using FACS analyses [Fig. 1(d)], we further showed that RA-treated (10 hours) ESCs expanded their population from 20% to 24% in the G1 phase, which occurred before phase 2 (RAR-mediated) activation of ERK1/2 (typically at 12 hours) [Fig. 1(b)]. In addition, these results corroborated our previous findings showing that short-term RA treatment expanded the G1 phase only in WT ESCs that expressed Crabp1 but not in Crabp1 KO ESCs, supporting this nongenomic function of Crabp1 (14). Together, the results confirmed the rapid (within 1 hour) nongenomic action of RA in ESCs and identified its molecular mediator as Crabp1. This Crabp1-mediated activity modulated ESC property by expanding the G1 phase to dampen proliferation.
Crabp1 KO enhanced NSC proliferation
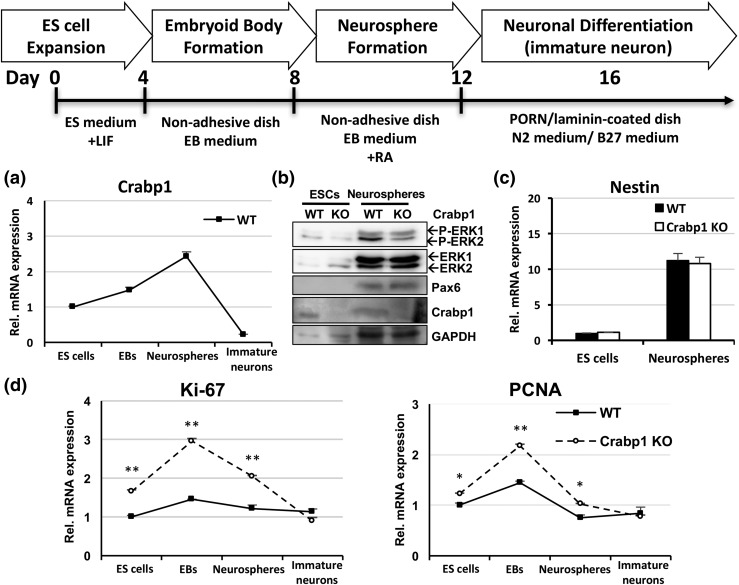
We continued to examine Crabp1 with the NSC model, a physiologically more relevant context with regard to stem cells. We used an established in vitro system (26) to monitor ESC progression to NSC as shown in Fig. 2 (top). ESCs were cultured in the presence of LIF to maintain their stemness and pluripotency or on nonadhesive dishes without LIF for 4 days to form EBs that could then progress, in the presence of RA, to NSCs in the form of neurospheres (26). Neurospheres were then cultured on poly-DL-ornithine hydrobromide/laminin–coated plates in N2/B27 medium to induce differentiation into young neurons. We first monitored the expression of endogenous Crabp1 and proliferation markers, as shown in Fig. 2. Crabp1 was readily expressed in proliferating ESCs and gradually increased in EBs. Crabp1 expression further increased in neurospheres (NSCs) but then dramatically decreased in immature neurons [Fig. 2(a)].
Figure 2.
Crabp1 negatively modulated stem cell proliferation. The top panel depicts the ESC-neuronal differentiation procedure. (a) Crabp1 messenger RNA (mRNA) expression pattern in WT ESC differentiation into immature neurons. (b) Protein levels of P-ERK1/2, ERK1/2, Pax6, Crabp1, and GAPDH in ESCs and neurospheres. (c) NSC marker nestin mRNA expression pattern in ESCs and neurospheres. (d) Expression patterns of proliferation markers Ki-67 (ES cells, P = 0.016; EBs, P < 0.001; neurospheres, P = 0.002) and PCNA (ES cells, P < 0.001; EBs, P < 0.001; neurospheres, P = 0.016). Results are presented as mean ± standard error of the mean. *P < 0.05, **P < 0.01 compared with the WT group. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PCNA, proliferating cell nuclear antigen; P-ERK1/2, phospho-extracellular regulated kinase 1/2.
In Western blot analyses, we monitored the expression of endogenous ERK activation in ESCs and neurospheres (NSCs) [Fig. 2(b)]. As predicted, Crabp1 protein was readily detected in both ESCs and NSCs of WT but not Crabp1 KO cells. Clearly, endogenous ERK was robustly activated (p-ERK) in neurospheres (NSCs) (29) of the WT but not the Crabp1 KO cells [Fig. 2(b), top panel], supporting the notion that Crabp1 modulates ERK activation.
To monitor differentiation efficiency, we detected the NSC marker Pax6 protein [Fig. 2(b), third panel from the top] and nestin [Fig. 2(c)], which indeed increased more than 10-fold in NSCs. The expression of these NSC markers, nestin and Pax6, was comparable between WT and Crabp1 KO neurospheres, suggesting that Crabp1 does not alter the progression, or differentiation, of ESCs to NSCs. Moreover, Crabp1 KO substantially elevated proliferation markers Ki-67 and proliferating cell nuclear antigen in the stages of ESCs, EBs, and neurospheres (mainly containing NSCs) [Fig. 2(c)]. These results show that Crabp1 affected at least in vitro stem cell proliferation. Deleting Crabp1 increased stem cell proliferation, such as that in the stages of ESCs, EBs, and NSCs. We then used Crabp1 KO mice to investigate the in vivo relevance of this finding.
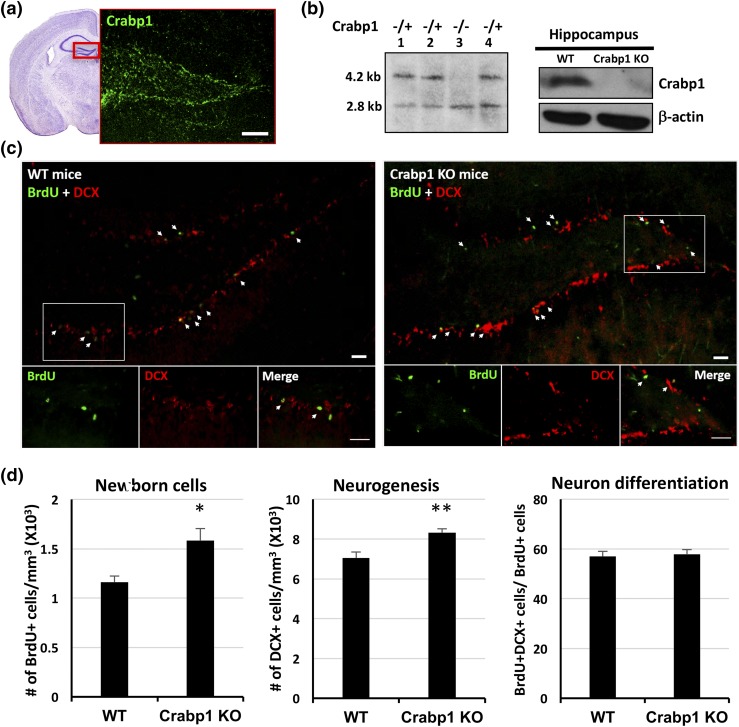
Deleting Crabp1 enhanced NSC proliferation and adult neurogenesis in the SGZ of the hippocampal dentate gyrus
We previously found prominent expression of Crabp1 in the adult hippocampus (16). Here, immunohistochemistry of 8-week-old mouse brain sections showed Crabp1 expression specifically in the SGZ of the hippocampal dentate gyrus [Fig. 3(a)], where adult neurogenesis occurs. Crabp1 KO was generated in the C57/BL6 background for this study. Figure 3(b) (left panel) shows a Southern blot analysis of digestion with Not1 (lane 3), validating Crabp1 homozygous KO (1). Figure 3(b) (right panel) shows a representative Western blot demonstrating the absence of Crabp1 protein in the Crabp1 KO hippocampus. As reported for the Crabp1 KO in the sv129 background (1), these Crabp1 KO C57/BL6 mice also exhibited no difference in gross appearance, including their coat color, hair, tail, or dental or limb morphology. Moreover, there was no difference in their body weight or food intake [(Supplemental Fig. 1(A) and 1(B) left (287.7KB, pdf) )], confirming the lack of gross abnormalities in Crabp1 KO mice.
Figure 3.
Increased cell proliferation and neurogenesis in the Crabp1 KO mouse hippocampus. (a) Confocal microscopy images showing Crabp1 expression patterns in the hippocampal dentate gyrus. Hematoxylin and eosin image shows a representative dentate gyrus according to the Mouse Brain Atlas second edition. Scale bar = 100 μm. (b) Mouse genotyped by Southern blotting and the lack of Crabp1 expression in the Crabp1 KO hippocampus is validated by Western blotting. (c) BrdU and DCX staining of the dentate gyrus, with magnified images of the boxed areas shown at the bottom. Scale bar = 20 μm. (d) Quantitative analyses showing increases in BrdU+ cells (left, P = 0.02) and DCX+ cells (middle, P = 0.003) in Crabp1 KO, but a similar fraction (∼57%) (right, P = 0.0751) of newborn cells progressed to immature neurons 1 day after BrdU injection in the WT and Crabp1 KO groups (n = 6 per group). Results are presented as mean ± standard error of the mean. *P < 0.05, **P < 0.01 compared with the WT group.
To monitor endogenous cell proliferation and neurogenesis in mice, we injected mice with BrdU to label proliferating cells and euthanized the animals 24 hours later. BrdU incorporates into replicating NSCs, which label newborn neuron progenitor cells (NPCs). The NPC marker DCX is used to monitor neurogenesis. As shown in Fig. 3(c), BrdU+ (proliferating) cells and DCX+ (NPC and immature neuron marker) cells as well as BrdU+/DCX+ cells (proliferating cells that have progressed to NPCs) were detected in the SGZ of WT and Crabp1 KO brains. Quantitative analyses showed significant increases in BrdU+ cells and DCX+ cells in the Crabp1 KO hippocampus compared with the WT hippocampus [Fig. 3(d), left and middle panels]. These in vivo data show that Crabp1 participated in the control of self-renewal of NSCs and hence affected neurogenesis. Deleting Crabp1 enhanced NSC proliferation. Thus, Crabp1 seemed to act to suppress, or negatively modulate, NSC proliferation.
To monitor differentiation efficiency, we scored the fractions of BrdU+ (proliferating) cells that had progressed (differentiated) to the DCX+ stage. The ratio of BrdU+/DCX+ cells over total BrdU+ cells reflects the differentiation efficiency. Figure 3(d) (right) shows a similar fraction (approximately 57%) of newborn cells differentiated into NPCs (24 hours after BrdU injection) in both WT and Crabp1 KO mice. This result suggests that Crabp1 was not important for early stages of neural differentiation, such as differentiation to NPCs or immature neurons.
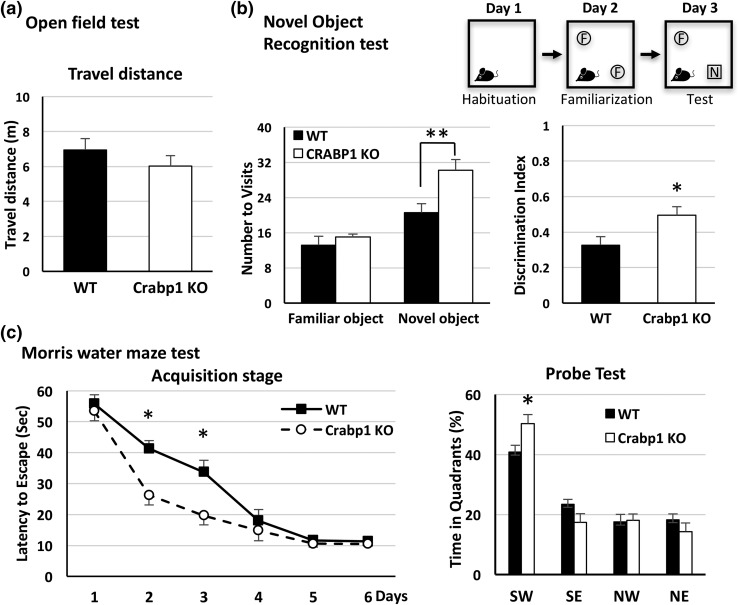
Crabp1 KO mice exhibited enhanced learning and memory
With the specific expression of Crabp1 in the SGZ of the dentate gyrus and increased NSC proliferation in Crabp1 KO mice, we speculated that deleting Crabp1 might affect hippocampal functions, such as hippocampus-dependent learning and memory. We first used an open field test to monitor their activity. The data showed no significant difference in travel distance between WT and Crabp1 KO mice [Fig. 4(a)], ruling out effects of Crabp1 KO on motor activity and exploration behavior. We used the novel object recognition test to assess their recognition memory (28) and the MWM to assess their spatial learning and memory (27). The novel object recognition task determines a rodent’s ability to recognize and remember a novel object and consists of three phases (i.e., habituation, familiarization, and test) separated by 24 hours [Fig. 4(b), top]. During the familiarization phase, the mouse spontaneously explored the open field arena with two identical objects. During the test phase, the mouse was returned to the same open field arena with one familiar object and one novel object. Data showed that compared with the WT mice, the Crabp1 KO mice significantly increased their visits to the novel object [Fig. 4(b), left]. On the contrary, there was no significant difference between the two groups in visiting the familiar object [Fig. 4(b), left]. The preference for exploring the novel object was determined by the discrimination ratio for each mouse (discrimination ratio = Tnovel − Tfamiliar/Tnovel ± Tfamiliar). Crabp1 KO mice displayed a higher discrimination ratio than the WT mice did. These data reveal an improved cognitive memory in Crabp1 KO mice.
Figure 4.
Improved hippocampal-dependent learning and memory in Crabp1 KO mice. (a) Animal movement for 5 minutes, recorded in the open field test (n = 14 per group; P = 0.401). (b) The scheme shows the 3-day procedure of a novel object recognition task (n = 12 per group) (top). Data show visit times (left; familiar object, P = 0.421; novel object, P = 0.007) and discrimination index (right; P = 0.026) during the test session. (c) The acquisition stage shows an improved learning curve in the Crabp1 KO mice compared with the WT mice (left; day 2, P = 0.014; day 3, P = 0.021). The duration spent in each zone in the probe test (right; P = 0.012) shows better memory performance for Crabp1 KO mice than for WT mice (Crabp1 KO, n = 12; WT, n = 10). Results are presented as mean ± standard error of the mean. *P < 0.05, **P < 0.01 compared with the WT group. NE, northeast; NW, northwest; SE, southeast; SW, southwest, the target zone.
During the acquisition stage of a standard MWM test, mice were trained for 6 consecutive days to find the hidden platform. The behavioral test results showed that Crabp1 KO mice required a shorter time to reach the hidden platform on days 2 and 3 of training than the WT mice required [Fig. 4(c), left], suggesting a greater learning capability in the Crabp1 KO mice. There was no significant difference in average swimming speed between the two groups [Supplemental Fig. 2(A) (287.7KB, pdf) ], further supporting the notion that deleting Crabp1 did not affect mobility or activity, as shown earlier [Fig. 4(a)].
Retrieval of spatial reference memory was assessed on day 7. The results [Fig. 4(c), right] show that Crabp1 KO mice spent a longer duration in the target zone (southwest) than the WT mice did, reflecting the better memory of Crabp1 KO mice. The heat map images of animal positions in this test are provided in Supplemental Fig. 2(B) (287.7KB, pdf) . All together, these behavioral tests show that deleting Crabp1 enhanced hippocampal functions, especially learning and memory.
Discussion
We investigated the physiological significance of Crabp1, a highly conserved cytosolic protein with unclear functional roles in vivo. We first validated the expression of Crabp1 in ESCs and the adult hippocampus, where NSCs are known to naturally exist. Using the ESC system, we validated the functional role of Crabp1 in mediating the noncanonical and rapid ERK1/2 activation pathway elicited by RA, which suppressed cell proliferation in vitro. We examined hippocampal NSCs, one physiologically relevant in vivo system, to uncover the physiological function of Crabp1 in whole animals. Our results provided in vivo evidence that Crabp1 affected stem cell properties. In the hippocampus, it specifically modulated NSC proliferation, which is important for brain function as demonstrated in hippocampus-dependent learning and memory. Conceivably, this physiological function is not vital to animal survival in a laboratory environment. This is consistent with earlier reports of Crabp1 KO mice in the sv129 background that also exhibited no gross anatomical abnormalities in the laboratory (1, 2). However, whether Crabp1 affects an animal’s survival or ability to resist challenges, such as stress, remains to be determined.
Given that Crabp1 is highly conserved, it could be predicted that deleting this gene function would harm the animals. The improved learning and memory phenotype in Crabp1 KO mice was indeed surprising. However, it is possible that Crabp1 has evolved to become involved in multiple processes that confer overall survival advantage to animals in a natural setting. The enhanced learning/memory outcome might merely represent one of the affected processes in a highly protected laboratory setting. It will be important to examine other physiological/pathological processes in addition to those modulating brain functions, such as disease vulnerability and aging processes. This current study represents a step in dissecting potential multiple biological processes that could involve Crabp1 function in whole animals.
In the context of stem cell biology, various mechanisms can alter the relationship between cell proliferation and differentiation. RA has a well-established role in inducing differentiation, which is attributable to its well-known nuclear action, mediated by RARs, in regulating the expression of numerous genes, particularly those for lineage determination/differentiation of stem cells (8). Our data demonstrated that before RA enters the nuclei, it can readily engage Crabp1 to affect a specific signaling pathway, ERK1/2, and modulate cell cycle control for proliferation. To this end, it is known that in order to maintain proliferative properties, stem cells require sustained activity of CDK2−cyclin E to ensure a short G1 phase. Rapid activation of ERK1/2 by Crabp1-RA can very quickly expand the G1 phase, which slows down the cell cycle, an event required to prepare stem cells for quiescence/differentiation. In addition, it can sensitize stem cells to stimulation by growth factors for differentiation. Thus, the presence of Crabp1 may help to orchestrate stem cell exit from the cell cycle upon stimulation by differentiation factors such as RA, ultimately modulating the stem cell pool. The absence of this cell cycle regulatory break in Crabp1 KO ESCs would slow down cells exiting the cycle, thus conserving or expanding the NSC pool (30).
Indeed, in Crabp1 KO mice, NSC proliferation in the hippocampus was increased. However, whether Crabp1 KO NPCs or young neurons mature into functional neurons as efficiently as WT cells do remains to be determined. Another intriguing question is how this negative mechanism, or break, for stem cell proliferation provides an evolutionary advantage over time. It is tempting to speculate that this negative regulation may be integrated into an intricate network of positive and negative regulatory mechanisms to maintain an optimal supply of stem cells in a normal physiological context. Finally, the classic function of Crabp1 is its tight binding to RA, potentially regulating RA metabolism in the cytoplasm (31). Boylan and Gudas (32) found that elevated expression of Crabp1 exhibited a shorter intracellular RA half-life and produced higher levels of RA metabolite, 4-oxo-retinoic acid. With these considerations, it remains to be determined whether Crabp1 KO mice have their intracellular RA metabolites altered, which may also affect the phenotypes. All these questions require further study of these Crabp1 KO animals.
Adult neurogenesis spans mainly three stages: NSC proliferation, neuronal differentiation, and cell survival. In this study, Crabp1 KO contributed to increased NSC proliferation without impacting the early stage of neuronal differentiation. It is still unclear whether Crabp1 affects neuron survival. During neurogenesis, only a small fraction of newly differentiated young neurons mature into functional neurons and integrate into the existing neuronal circuit. Most of the differentiated young neurons go on to programmed cell death (apoptosis) (33). The activated mitogen-activated protein kinase/ERK1/2 pathway is reported to be associated with apoptosis in different types of cells, including neurons (34–36). Further, RA and compounds binding to Crabp1 but not the RAR can enhance Crabp1-positive cancer cell apoptosis via activation of ERK1/2 (14). These findings suggest that Crabp1 KO may also reduce apoptosis, thus promoting the survival of neurons.
ERK1/2 can be activated by numerous inputs and in various cellular contexts. Signal duration of activated ERK1/2 can alter cellular outcomes (37–39). RA activates ERK1/2 signaling of ESCs in a biphasic manner, one immediate phase (minutes to 1 hour) involving Crabp1 and a second delayed phase (detected around 12 hours) involving RARs. The RAR-mediated phase 2 ERK1/2 activation generally alters the genomic program for differentiation (37–39). We previously found that RA-induced phase 1 ERK1/2 activation is mediated by Crabp1, which can gate the cell cycle and modulate proliferation. Changing the dynamics of the ERK1/2 signaling cascade triggered distinct cell decisions—driving proliferation or inducing differentiation (40). Our current study uncovered Crabp1 as one factor that can modulate ESCs in vitro and NSCs in vivo, specifically their proliferation. This may provide a new target in future attempts to manage stem cell pools.
Acknowledgments
We thank S. Ogokeh for technical assistance.
Acknowledgments
This work was supported by National Institutes of Health Grants DK54733 and DK60521, Dean’s Commitment, and the Distinguished McKnight Professorship of University of Minnesota (to L.-N.W.).
Acknowledgments
Author contributions: Y.-L.L., S.D.P., and L.-N.W. designed the research. Y.-L.L., S.D.P., and J.N. performed the research. L.-N.W. contributed the new reagents/tools. Y.-L.L., S.D.P., and L.-N.W. analyzed the data, and Y.-L.L., S.D.P., and L.-N.W. wrote the article.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| Crabp1 | Monoclonal anti-Crabp1 antibody | Sigma, C1608 | Mouse; monoclonal antibody | 1:1000 Western blot | AB_258751 | |
| Crabp1 | CRABP-I Antibody (S-14) | Santa Cruz, SC-10061 | Goat; polyclonal IgG | 1:100 for IHC | AB_2085315 | |
| p-ERK1/2 | Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) antibody | Cell Signaling Technology, 9101 | Mouse; monoclonal antibody | 1:1000 Western blot | AB_2297442 | |
| ERK2 | p42 MAPK (Erk2) antibody | Cell Signaling Technology, 9108 | Rabbit; polyclonal antibody | 1:1000 Western blot | AB_10695610 | |
| ERK1 | p44 MAPK (Erk1) antibody | Cell Signaling Technology, 4372 | Rabbit; polyclonal antibody | 1:1000 Western blot | AB_10693946 | |
| BrdU | BrdU antibody (3H579) | Santa Cruz, SC-70441 | Rat; monoclonal antibody | 1:200 for IHC | AB_1119696 | |
| Doublecortin | Rabbit anti-doublecortin polyclonal antibody | Abcam, AB18723 | Rabbit; polyclonal antibody | 1:400 for IHC | AB_732011 | |
| Pax6 | Pax6 antibody (AD1.5) | Santa Cruz, SC-53106 | Mouse; monoclonal antibody | 1:1000 Western blot | AB_630088 | |
| GAPDH | GAPDH antibody (6C5) | Santa Cruz, SC-32233 | Mouse; monoclonal IgG1 | 1:5000 Western blot | AB_627679 | |
| β-Actin | β-Actin antibody (C4) | Santa Cruz, SC-47778 | Mouse; monoclonal IgG1 | 1:5000 Western blot | AB_626632 |
Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Ig, immunoglobulin; IHC, immunohistochemistry; MAPK, mitogen-activated protein kinase; p-ERK1/2, phospho-extracellular regulated kinase 1/2; RRID, research resource identifier.
Footnotes
- BrdU
- 5-bromo-2′-deoxyuridine
- CDK
- cyclin-dependent kinase
- Crabp1
- cellular retinoic acid–binding protein 1
- DCX
- doublecortin
- DMEM
- Dulbecco’s modified Eagle medium
- EB
- embryoid body
- ERK
- extracellular regulated kinase
- ES
- DE3
- ESC
- embryonic stem cell
- FACS
- fluorescence-activated cell sorting
- G1
- growth 1
- KO
- knockout
- LIF
- leukemia inhibitory factor
- NPC
- neuron progenitor cell
- NSC
- neural stem cell
- MTT
- 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide
- MWM
- Morris water maze
- PBS
- phosphate-buffered saline
- RA
- retinoic acid
- RAR
- retinoic acid receptor
- RRID
- research resource identifier
- SGZ
- subgranular zone
- WT
- wild-type.
References
- 1.Gorry P, Lufkin T, Dierich A, Rochette-Egly C, Décimo D, Dollé P, Mark M, Durand B, Chambon P. The cellular retinoic acid binding protein I is dispensable. Proc Natl Acad Sci USA. 1994;91(19):9032–9036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Bruijn DR, Oerlemans F, Hendriks W, Baats E, Ploemacher R, Wieringa B, Geurts van Kessel A. Normal development, growth and reproduction in cellular retinoic acid binding protein-I (CRABPI) null mutant mice. Differentiation. 1995;58(2):141–148. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Castro AV, Tran VT, Nguyen-Huu MC. Defective lens fiber differentiation and pancreatic tumorigenesis caused by ectopic expression of the cellular retinoic acid-binding protein I. Development. 1993;119(2):363–375. [DOI] [PubMed] [Google Scholar]
- 4.Wei LN, Chang L, Lee CH. Studies of over-expressing cellular retinoic acid binding protein-I in cultured cells and transgenic mice. Transgenics. 1997;2(2):201–209. [Google Scholar]
- 5.Napoli JL. Retinoic acid biosynthesis and metabolism. FASEB J. 1996;10(9):993–1001. [DOI] [PubMed] [Google Scholar]
- 6.Rochette-Egly C. Retinoic acid signaling and mouse embryonic stem cell differentiation: cross talk between genomic and non-genomic effects of RA. Biochim Biophys Acta 2015;1851(1):66–75. [DOI] [PubMed] [Google Scholar]
- 7.Chen R, Wei L, Syme PD. Comparison of early and delayed respondents to a postal health survey: a questionnaire study of personality traits and neuropsychological symptoms. Eur J Epidemiol. 2003;18(3):195–202. [DOI] [PubMed] [Google Scholar]
- 8.Al Tanoury Z, Piskunov A, Rochette-Egly C. Vitamin A and retinoid signaling: genomic and nongenomic effects. J Lipid Res. 2013;54(7):1761–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nayak S, Shen M, Bunaciu RP, Bloom SE, Varner JD, Yen A. Arsenic trioxide cooperates with all trans retinoic acid to enhance mitogen-activated protein kinase activation and differentiation in PML-RARalpha negative human myeloblastic leukemia cells. Leuk Lymphoma. 2010;51(9):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu J, Zhou R, He Q, Li WI, Zhang T, Niu B, Zheng X, Xie J. Calmodulin kinase II activation of mitogen-activated protein kinase in PC12 cell following all-trans retinoic acid treatment. Neurotoxicology. 2009;30(4):599–604. [DOI] [PubMed] [Google Scholar]
- 11.Gupta P, Ho PC, Huq MM, Ha SG, Park SW, Khan AA, Tsai NP, Wei LN. Retinoic acid-stimulated sequential phosphorylation, PML recruitment, and SUMOylation of nuclear receptor TR2 to suppress Oct4 expression. Proc Natl Acad Sci USA. 2008;105(32):11424–11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen N, Napoli JL. All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane-associated RARalpha. FASEB J. 2008;22(1):236–245. [DOI] [PubMed] [Google Scholar]
- 13.Masiá S, Alvarez S, de Lera AR, Barettino D. Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol Endocrinol. 2007;21(10):2391–2402. [DOI] [PubMed] [Google Scholar]
- 14.Persaud SD, Park SW, Ishigami-Yuasa M, Koyano-Nakagawa N, Kagechika H, Wei LN. All trans-retinoic acid analogs promote cancer cell apoptosis through non-genomic Crabp1 mediating ERK1/2 phosphorylation [published correction appears in Sci Rep. 2016;6:27678]. Sci Rep. 2016;6:22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persaud SD, Lin YW, Wu CY, Kagechika H, Wei LN. Cellular retinoic acid binding protein I mediates rapid non-canonical activation of ERK1/2 by all-trans retinoic acid. Cell Signal. 2013;25(1):19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou FC, Wei LN. Expression of cellular retinoic acid-binding protein I is specific to neurons in adult transgenic mouse brain. Brain Res Gene Expr Patterns. 2001;1(1):67–72. [DOI] [PubMed] [Google Scholar]
- 17.Rolando C, Taylor V. Neural stem cell of the hippocampus: development, physiology regulation, and dysfunction in disease. Curr Top Dev Biol. 2014;107:183–206. [DOI] [PubMed] [Google Scholar]
- 18.Roccio M, Schmitter D, Knobloch M, Okawa Y, Sage D, Lutolf MP. Predicting stem cell fate changes by differential cell cycle progression patterns. Development. 2013;140(2):459–470. [DOI] [PubMed] [Google Scholar]
- 19.Järvinen E, Angers-Loustau A, Osiceanu AM, Wartiovaara K. Timing of the cell cycle exit of differentiating hippocampal neural stem cells. Int J Stem Cells. 2010;3(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Rheenen TE, Bryce S, Tan EJ, Neill E, Gurvich C, Louise S, Rossell SL. Does cognitive performance map to categorical diagnoses of schizophrenia, schizoaffective disorder and bipolar disorder? A discriminant functions analysis. J Affect Disord. 2016;192:109–115. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi M, Seki T, Imayoshi I, Tamamaki N, Hayashi Y, Tatebayashi Y, Hitoshi S. Neural stem cells and neuro/gliogenesis in the central nervous system: understanding the structural and functional plasticity of the developing, mature, and diseased brain. J Physiol Sci. 2016;66(3):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cameron HA, Glover LR. Adult neurogenesis: beyond learning and memory. Annu Rev Psychol. 2015;66:53–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gu Y, Arruda-Carvalho M, Wang J, Janoschka SR, Josselyn SA, Frankland PW, Ge S. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci. 2012;15(12):1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho PC, Gupta P, Tsui YC, Ha SG, Huq M, Wei LN. Modulation of lysine acetylation-stimulated repressive activity by Erk2-mediated phosphorylation of RIP140 in adipocyte differentiation. Cell Signal. 2008;20(10):1911–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chuang YS, Huang WH, Park SW, Persaud SD, Hung CH, Ho PC, Wei LN. Promyelocytic leukemia protein in retinoic acid-induced chromatin remodeling of Oct4 gene promoter. Stem Cells. 2011;29(4):660–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bibel M, Richter J, Lacroix E, Barde YA. Generation of a defined and uniform population of CNS progenitors and neurons from mouse embryonic stem cells. Nat Protoc. 2007;2(5):1034–1043. [DOI] [PubMed] [Google Scholar]
- 27.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1(2):848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdóttir HN, Stackman RW Jr. The rodent hippocampus is essential for nonspatial object memory. Curr Biol. 2013;23(17):1685–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Z, Theus MH, Wei L. Role of ERK 1/2 signaling in neuronal differentiation of cultured embryonic stem cells. Dev Growth Differ. 2006;48(8):513–523. [DOI] [PubMed] [Google Scholar]
- 30.Ruijtenberg S, van den Heuvel S. Coordinating cell proliferation and differentiation: antagonism between cell cycle regulators and cell type-specific gene expression. Cell Cycle. 2016;15(2):196–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fiorella PD, Napoli JL. Expression of cellular retinoic acid binding protein (CRABP) in Escherichia coli. Characterization and evidence that holo-CRABP is a substrate in retinoic acid metabolism. J Biol Chem. 1991;266(25):16572–16579. [PubMed] [Google Scholar]
- 32.Boylan JF, Gudas LJ. The level of CRABP-I expression influences the amounts and types of all-trans-retinoic acid metabolites in F9 teratocarcinoma stem cells. J Biol Chem. 1992;267(30):21486–21491. [PubMed] [Google Scholar]
- 33.Ryu JR, Hong CJ, Kim JY, Kim EK, Sun W, Yu SW. Control of adult neurogenesis by programmed cell death in the mammalian brain. Mol Brain. 2016;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung EC, Slack RS. Emerging role for ERK as a key regulator of neuronal apoptosis. Sci STKE. 2004;2004(251):pe45. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Chen M, Liu H, Yang L, Yang T, He G. The dual role of ERK signaling in the apoptosis of neurons. Front Biosci (Landmark Ed). 2014;19:1411–1417. [DOI] [PubMed] [Google Scholar]
- 36.Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35(6):600–604. [DOI] [PubMed] [Google Scholar]
- 37.McKay MM, Morrison DK. Integrating signals from RTKs to ERK/MAPK. Oncogene. 2007;26(22):3113–3121. [DOI] [PubMed] [Google Scholar]
- 38.Matallanas D, Birtwistle M, Romano D, Zebisch A, Rauch J, von Kriegsheim A, Kolch W. Raf family kinases: old dogs have learned new tricks. Genes Cancer. 2011;2(3):232–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albeck JG, Mills GB, Brugge JS. Frequency-modulated pulses of ERK activity transmit quantitative proliferation signals. Mol Cell. 2013;49(2):249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rauch N, Rukhlenko OS, Kolch W, Kholodenko BN. MAPK kinase signalling dynamics regulate cell fate decisions and drug resistance. Curr Opin Struct Biol. 2016;41:151–158. [DOI] [PubMed] [Google Scholar]