Abstract
Low thyroid hormone (TH) conditions caused by a variety of prenatal and perinatal problems have been shown to alter postnatal regulatory thyrotropin (TSH) responsiveness to TH in humans and rodents. The mechanisms underlying this pituitary TH resistance remain unknown. Here we use the evolutionarily conserved zebrafish model to examine the effects of low TH on thyrotrope development and function. Zebrafish were exposed to the goitrogen 6-propyl-2-thiouracil (PTU) to block TH synthesis, and this led to an approximately 50% increase in thyrotrope numbers and an 8- to 10-fold increase in tshb mRNA abundance in 2-week-old larvae and 1-month-old juveniles. Thyrotrope numbers returned to normal 3 weeks after cessation of PTU treatment, demonstrating that these effects were reversible and revealing substantial plasticity in pituitary-thyroid axis regulation. Using a T4 challenge assay, we found that development under low-TH conditions did not affect the ability of T4 to suppress tshb mRNA levels despite the thyrotrope hyperplasia that resulted from temporary low-TH conditions. Together, these studies show that low developmental TH levels can lead to changes in thyrotrope number and function, providing a possible cellular mechanism underlying elevated TSH levels seen in neonates with either permanent or transient congenital hypothyroidism.
Low developmental TH conditions reversibly increased thyrotrope numbers in zebrafish but did not affect the ability of T4 to suppress tshb mRNA levels.
Congenital hypothyroidism (CH), defined as thyroxine (TH) deficiency present at birth, can cause growth retardation, multiple system failure, and neurocognitive impairment (1, 2). The incidence of CH has doubled over the last three decades (3), with the increase largely attributable to the diagnosis of elevated levels of thyrotropin (TSH) in patients with intact thyroid glands (4). These TSH level elevations are operationally defined as a relatively increased set point for TH-induced TSH suppression (5, 6) and are increasingly common in premature and compromised neonates (7, 8). Delayed maturation of the hypothalamic-pituitary-thyroid (HPT) axis is proposed as one cause of this altered TSH set point (4). Mild elevations of TSH levels often persist in children born small for gestational age (SGA), possibly due to TH resistance within the TSH regulatory response (9). Interestingly, pituitary thyroid hormone resistance to therapeutic TH is observed in 43% of infants with CH, and resistance persists in 10% of these children (10). The developmental mechanisms underlying the persistence of an abnormal TSH set point in the previous scenarios are unknown.
Across vertebrate species, the pituitary responds to reduced TH levels by increasing TSH secretion (negative feedback) (11). This elevated pituitary TSH output is the result of increased TSH production within the existing thyrotrope population and an increase in thyrotrope numbers (hyperplasia). In humans, fetal levels of serum T4 and TSH indicate that thyrotropes begin to respond to T4 at 20 weeks gestation (12). Thus, developmental exposure to low TH levels could affect thyrotrope proliferation and function and lead to the abnormal TH regulation observed in some neonates with CH (10). Although the magnitude and duration of exposure to reduced TH conditions is less severe in premature and SGA infants than in those with overt CH, prolonged TSH increases have been documented in these situations as well (13), indicating long-term TSH/TH set point alterations. An understanding of the mechanism(s) responsible for TSH resistance after a transient exposure to low TH levels is critical for the proper management of neonates with overt and subtle CH. Treatment (i.e., TH supplementation) likely should be individualized to both the degree of CH severity and the ability of the system to self-correct.
Endocrine regulation is a fundamental feature of vertebrates, and the molecular and cellular components that mediate negative feedback in the thyroid axis are largely conserved across species from fish to mammals (14). The zebrafish has emerged as a new model for studying the ontogeny and functional maturation of the vertebrate HPT axis (15–17). Transgenic reporter lines allow quantification of thyrotrope populations throughout the entire life of the organism (18), and the functional consequences of altered TH levels can be determined at any time during the life cycle (19). Thyrotropes first differentiate in the developing zebrafish pituitary gland between 28 and 32 hours post fertilization (hpf), and functional negative feedback of pituitary TSH production begins coincident with TH production in the thyroid follicle (the thyroid gland analog) around 4 days post fertilization (dpf) (18). Given this rapid development, experimental accessibility, and evolutionary conservation, the zebrafish system greatly facilitates the investigation of the cellular and molecular mechanism underlying direct TH regulation of TSH production in thyrotropes, and promises to shed light on transient pituitary TH resistance in neonates.
Here we report the immediate and lasting effects of low TH levels on the developing zebrafish thyrotrope population and examine how the system responds when thyroid hormone levels are restored. We hypothesized that low developmental TH levels would cause an expansion of the thyrotrope population that would in turn lead to pituitary resistance to TH and altered suppression of tshb mRNA output. Consistent with this hypothesis, we show that thyrotrope numbers and tshb mRNA abundance increase in response to low TH levels and that thyrotrope numbers can return to normal with time. Contrary to our prediction, 6-propyl-2-thiouracil (PTU) treatment did not affect the sensitivity of thyrotropes to supplemental TH treatment, suggesting that thyrotropes can respond appropriately to a TH challenge despite hyperplasia.
Materials and Methods
Thyroid hormone level manipulations: PTU and thyroid hormone treatments
Zebrafish were maintained as described previously (20), and larvae were staged by standard length (21). Low-TH conditions were created by rearing embryos and larvae PTU (Sigma-Aldrich) (22). To produce chronic low-TH conditions, 25 to 30 zebrafish embryos and larvae were maintained in 100-mm petri dishes containing 15 mL of 0.65 mM PTU or 0.1% dimethyl sulfoxide (DMSO) (control) in embryo rearing medium (20) until 7 dpf, when they were transferred to 2-L tanks containing 1.0 L of 0.65 mM PTU or 0.1% DMSO in aerated system water. Larvae were fed rotifers starting at 5 dpf and switched to brine shrimp and dry food by 10 dpf. Tanks were kept isolated from the circulating system, but aeration was maintained with bubblers, and treatment water was fully replaced daily up to 14 dpf and then partially replaced every other day. To produce transient low-TH conditions, zebrafish embryos/larvae were raised in 0.65 mM PTU (starting at 6 hpf) for 7 days followed by a 7-day or a 21-day washout in system water. The 7-day washout experiment was repeated three times, and the 21-day washout experiment was performed twice. To verify that the PTU-induced effects were specific and due to reduced thyroid hormone levels, a T3-rescue group of embryos was exposed simultaneously to 0.65 mM PTU and 10 nM 3,3′,5-triiodo-l-thyronine (T3) (Sigma-Aldrich), and thyrotrope numbers were quantified at 7 dpf. HPT axis regulation was tested by challenging PTU-treated and control embryos with various concentrations of TH, in the form of LT4 (Sigma), or with 0.0025% DMSO (control) for 48 hours at 7 and 14 dpf and then assaying relative tshb, thrb, and dio3b mRNA levels by quantitative real-time polymerase chain reaction (RT-qPCR) (described later). LT4 was used rather than T3 because of its longer half-life and physiological relevance in zebrafish development (23).
Thyrotrope and somatotrope quantification
Thyrotropes were visualized in the Tg(tshb:EGFP)umz29 transgenic line (18) using a Zeiss apotome microscope to collect z-stacks of optical sections through the pituitary. For embryos or larvae up to 14 dpf, whole fish were sedated in tricaine (MS-222; Sigma-Aldrich), fixed in ice-cold 4% paraformaldehyde (pH 7.2) overnight, and then mounted for viewing in 25% glycerol. Zebrafish 14 dpf and older were sedated and rapidly decapitated, and heads were fixed in ice-cold 4% paraformaldehyde overnight. For mounting fish younger than 21 dpf, the lower jaw and eyes were removed to allow imaging of the pituitary gland in situ from the ventral aspect. In fish 21 dpf and older, pituitary glands were dissected away from the brain under a fluorescent dissecting microscope and individually imaged. Thyrotrope counts in larvae raised under low-TH conditions were repeated two to four times. Fluorescent cells were blindly counted in z-stacks using ImageJ software. Somatotropes were labeled by in situ hybridization using a digoxigenin-labeled growth hormone probe and manually counted as described previously (24).
RT-qPCR and green fluorescent protein intensity measurements
Relative mRNA levels were determined by RT-qPCR using the MxPro 3000P system (Stratagene) as described previously (18). Zebrafish larvae were sedated in MS-222 and rapidly decapitated, and heads were immediately submerged in 500 µL Trizol reagent (Invitrogen). Total RNA was extracted from five to seven individual heads per pooled sample, and at least six pooled samples per experimental condition were used for RT-qPCR and statistical analyses. Total RNA was extracted using a Bullet Blender homogenizer (Next Advance) and quantified using a Nanodrop spectrometer (Thermo Fisher Scientific). Total RNA (200 ng) was reverse transcribed into complementary DNA (cDNA) using a High-Capacity cDNA Reverse Transcription kit (Applied Biosystems). As a loading control, cycle threshold values were normalized to the corresponding mRNA level of the housekeeping gene ef1a (25) performed in parallel wells for each sample. Quality of RT-qPCR runs was determined by confirming the absence of amplification in the no-template control (containing SYBR green mix and primers but no cDNA) and the no–reverse transcriptase control (containing SYBR green mix, primers, and the product of a pooled mRNA sample that underwent cDNA synthesis without the addition of reverse transcription enzyme) by melting-curve analysis with single-peak verification and by ensuring that all sample cycle threshold values fell within the linear range of a serially diluted standard curve that indicated run efficiency between 90% and 100%; any run that did not meet all of these qualifications was repeated, and the original data were excluded from analyses. RT-qPCR data were analyzed using the ΔΔCt method as previously described (18). Data points falling more than 1.96 standard deviations above or below the mean were identified as outliers and omitted from the final analysis, but their omission did not alter the overall results. For each group, the mean, standard deviation, and standard error of the mean (SEM) were calculated. Primer pairs: ef1α (elongation factor 1α; forward: EF1a-F 5′-CTGGTGTCCTCAAGCCTGGTA-3′; reverse: EF1a-R 5′-ACTTGACCTCAGTGGTTACATTGG-3′); tshb (thyroid stimulating hormone β; forward: TSHb-3-FWD 5′-AACACCACCATCTGCATGGG-3′; reverse: TSHb-3-REV 5′-AAGTGCATCCCCTCTGAACAA-3′); thrb (thyroid hormone receptor β; forward: thrb-2-Fwd 5′-CATGCTGTGTTGCAGCTTCG-3′; reverse: thrb-2-Rev 5′-GCAGAGCCTTACATCCTGCC-3′); and dio3b (Deiodinase 3b; forward: Z-1Dio3b-F 5′-CGTGTCCGACAGCAACAAGATGTTCA-3′; reverse: z-1Dio3b-R 5′-TCTTGAAGAAGTCCAGCTTCTGGC-3′). Green fluorescent protein (GFP) fluorescence intensity was quantified in z-stack images using ImageJ. GFP-expressing thyrotropes were first counted using the three-dimensional object counter plugin, and cell counts were verified by eye. Raw intensity density was then measured using the measure stack plugin in a region of interest that was defined to include all thyrotropes in the confocal stack. Total pixel intensity was then divided by cell number to give the average pixel intensity per cell.
Statistics
Statistical significance of changes in cell numbers, pixel intensity/cell, and mRNA levels between groups was determined by a two-tailed Student t test assuming unequal variance or by one-way analysis of variance (ANOVA) followed by Dunnett multiple comparison test to compare every mean with a control in the T3-rescue experiment. Finally, a two-factor ANOVA was used to examine the effect of PTU, adjusting for fish size. Both factors were measured categorically. To determine whether the PTU effect differed across fish size, a group-by-size interaction was evaluated. No interaction effects were significant. Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software). Values are shown as the mean ± SEM.
Study approval
All protocols and experiments were approved by the Institutional Animal Care and Use Committee of the University of Massachusetts.
Results
Effects of low TH levels on postembryonic thyrotrope numbers and function
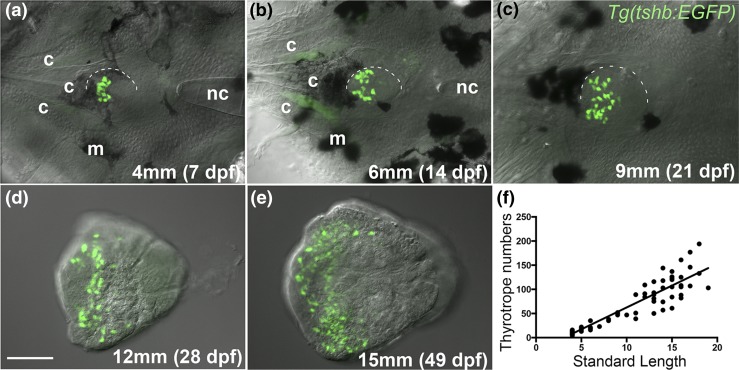
To determine the effect of low thyroid hormone levels on the development of the thyrotrope population in zebrafish, we first documented normal increases in thyrotrope numbers through larval development and into adulthood using the Tg(tshb:EGFP) transgenic line (18). Thyrotrope numbers increased through all larval stages, and the increase was linear when plotted against standard length of the fish (21) (Fig. 1). Interanimal variation in thyrotrope numbers increased after the juvenile-adult transition, which occurs at about 1 month (15 mm). Fish size was a far better predictor of thyrotrope numbers than age (data not shown), consistent with the fact that standard length has been previously shown to be the best indicator of developmental stage postembryonically (21).
Figure 1.
Increases in thyrotrope numbers through larval development. (a–e) Ventral views (anterior left) of zebrafish pituitaries in the Tg(tshb:EGFP) line showing GFP-expressing thyrotropes. Pituitaries (half outlined) were imaged in situ up to 21 dpf (9 mm standard length), with cartilage elements and melanocytes visible in these images. (d, e) In older fish, pituitaries were dissected and imaged in isolation. (f) Thyrotrope numbers plotted against standard length. Each data point represents a single individual. R2 = 0.87. Scale bar, 50 µm. c, cartilage element of the upper jaw/cranium; m, melanocyte.
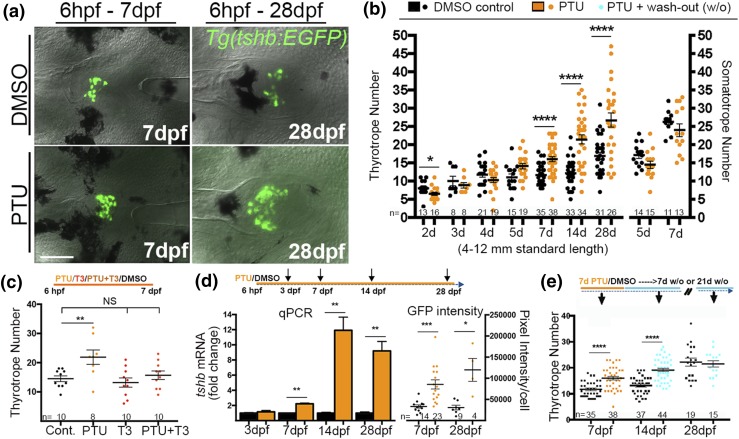
The goitrogen 6-propyl-2-thiouracil (PTU) has been previously shown to be effective in reducing TH levels in zebrafish both during larval development and in the adult (22, 26, 27). In zebrafish, TH is initially supplied maternally from the yolk, with zygotic TH production beginning in the thyroid follicles by 3 dpf (28) and significant changes in tissue levels of T4 first occurring at 5 dpf (23). To determine the effects of chronic low-TH conditions on the development of the thyrotrope population, we exposed Tg(tshb:EGFP) zebrafish to 0.65% PTU starting at 6 hpf, well before the onset of TSHb and zygotic TH production (18, 24, 29) [Fig. 2(a) and 2(b)]. PTU treatment caused a very slight decrease in thyrotrope numbers after 2 days, but no significant differences in the thyrotrope numbers were observed after 3, 4, and 5 days of growth in the presence of PTU [Fig. 2(b)]. In contrast, 7 and 14 dpf larvae grown in the presence of PTU exhibited a significant increase in thyrotrope numbers, and by 28 days the thyrotrope number had increased by approximately 50% relative to controls [Fig. 2(a) and 2(b)]. A two-way ANOVA confirmed that PTU treatments affected thyrotrope numbers independent of fish standard length (data not shown). In parallel experiments, we measured somatotrope numbers in PTU-treated larvae to test for general effects on pituitary endocrine cells. PTU treatments had no significant effect on somatotrope numbers [Fig. 2(b)], consistent with a specific effect of low-TH conditions on thyrotropes and/or thyrotrope precursors.
Figure 2.
Effects of low thyroid conditions on larval thyrotrope numbers. (a) Ventral views of GFP-expressing thyrotropes in 7 dpf (left) and 28 dpf (right) Tg(tshb:EGFP) pituitaries grown in 0.65 mM PTU or DMSO (control) starting at 6 hpf. (b) Quantification of thyrotropes (left) and somatotropes (right) in larvae grown in PTU or DMSO starting at 6 hpf. (c) Thyrotrope numbers at 7 dpf in larvae grown in PTU, a low dose of T3, or PTU+T3 starting at 6 hpf. T3 addition rescued PTU-induced thyrotrope increases, consistent with PTU treatments reducing bioactive TH levels. (d) Relative tshb mRNA levels in larvae treated starting at 6 dpf (left). Relative GFP pixel intensity/cell at 7 and 28 dpf in larvae treated with PTU or DMSO starting at 6 hpf (right). (e) Thyrotrope numbers in larvae exposed to PTU from 0 to 7 dpf followed by 7 or 14 days of growth in system water without PTU (w/o, wash-out). Increased thyrotrope numbers persisted for 7 days after removal of PTU, but the thyrotrope population was indistinguishable from controls (no PTU treatment) after 21 days. (b, d, and e) Values are shown as the mean ± SEM (Student t test). (c) ANOVA followed by Dunnett test was used to compare every mean with control. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001. Scale bar, 50 µm.
To further test the specificity of this effect, we attempted to rescue thyrotrope numbers in PTU-treated larvae by adding T3 to the treated larvae. The addition of 10 nM T3 alone did not significantly alter thyrotrope numbers [Fig. 2(c)]. However, the addition of 10 mM T3 prevented the increase in thyrotrope numbers produced by PTU treatment [Fig. 2(c)]. This finding provides strong evidence that the increased thyrotrope numbers seen after PTU treatment represents a specific response to low-TH conditions and not to other possible effects of PTU.
We next examined the thyrotrope response to PTU treatment by assaying tshb mRNA levels using RT-qPCR [Fig. 2(d)]. PTU treatment at 7 dpf led to a doubling of tshb mRNA in relation to ef1-alpha mRNA, whereas PTU treatment at 14 and 28 dpf led to an 8- to 10-fold increase in tshb mRNA [Fig. 2(d)], consistent with appropriate negative feedback regulation of TSH production by TH. We also quantified GFP fluorescence in cells carrying the tshb:EGFP transgene in both PTU- and DMSO-treated embryos and found a consistent increase in the level of fluorescence per cell after 7-day and 28-day PTU treatment [Fig. 2(d), right]. Together, these data indicate that increases in tshb gene expression within individual thyrotropes, as well as increases in thyrotrope cell numbers, could contribute to an upregulation of TSHb production under conditions of low developmental TH.
To assess the effects of transient low TH on the thyrotrope population, zebrafish larvae were raised in the presence of PTU for 7 days and then returned to drug-free water for various wash-out periods before thyrotropes were quantified. The 7-day [Fig. 2(e)] and 14-day (not shown) PTU treatments again resulted in an increase in thyrotrope numbers that persisted after 7- and 14-day wash-out periods. Thyrotrope numbers returned to normal after 21 days in drug-free water [Fig. 2(e)]. In humans, PTU has a half-life of 1.5 hours (30), suggesting that PTU may be cleared relatively early in the wash-out period. Although we do not know the kinetics of PTU elimination in these experiments, our data demonstrate that the PTU effects can be eliminated after PTU removal. Overall these data show that thyrotropes and/or thyrotrope precursors can sense and respond to low-TH conditions experienced during zebrafish development, resulting in an increase in thyrotrope numbers and increased tshb mRNA abundance. The finding that normal thyrotrope numbers are restored when PTU is removed indicates that cellular responses to altered TH conditions are reversible in zebrafish.
Maturation of TH axis negative feedback
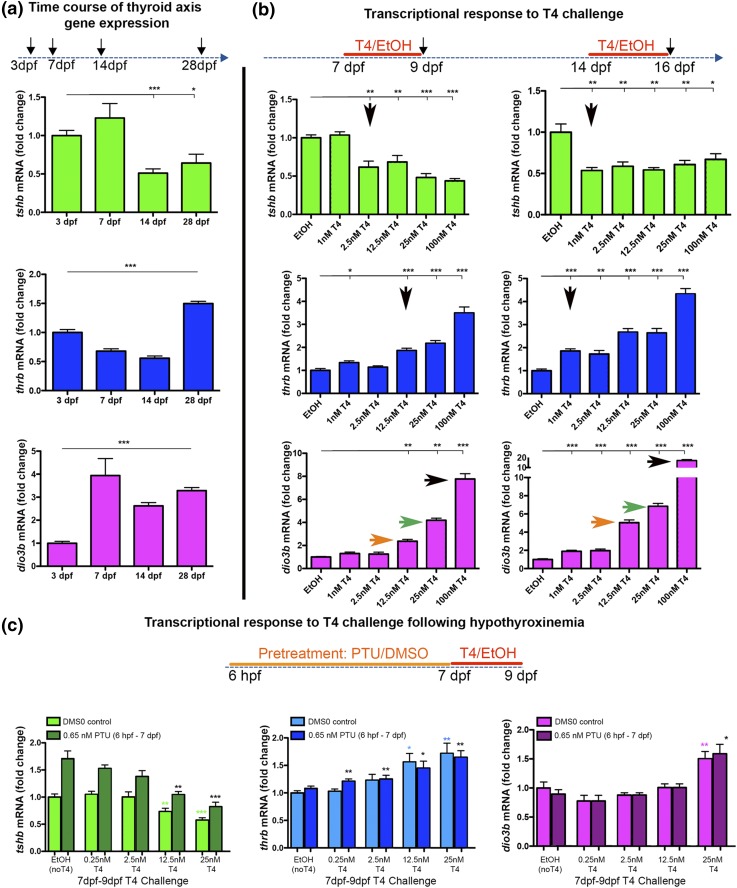
We and others previously demonstrated that thyroid axis feedback regulation, as measured by changes in tshb, thyroid hormone receptor beta (thrb), and dio3 mRNA expression in response to TH, emerges by 1 week after fertilization (18, 31), coincident with increased TH production by the thyroid follicles (23). We examined relative mRNA levels of these TH-axis genes in response to altered TH levels in an attempt to establish an assay for the maturation of thyroid axis feedback regulation. We first examined the relative changes in tshb, thrb, and dio3b mRNA levels in whole head tissue through the first month of development and found distinct profiles for each TH-regulated gene [Fig. 3(a)]. We next determined how tshb, thrb, and dio3b expression levels responded to the addition of different amounts of T4 at 7 and 14 dpf [Fig. 3(b)]. In 7 dpf larvae, exposure to 1 nM T4 had no effect on tshb mRNA abundance, whereas exposure to 2.5 nM or higher concentrations of T4 led to a 50% reduction in tshb transcripts. In contrast, in 14 dpf larvae, tshb mRNA was reduced by 50% with the addition of only 1 nM T4, indicating increased sensitivity of thyrotropes to TH levels. Higher concentrations of T4 failed to further suppress tshb mRNA levels at either age. Analysis of thrb and dio3b mRNA also revealed increased sensitivity to a TH challenge at these two ages, albeit at different concentrations. For thrb, addition of 1 nM T4 was sufficient to cause a twofold increase in mRNA at 14 dpf, whereas 12.5 nM T4 was required for a similar induction at 7 dpf. dio3b mRNA levels were affected similarly up to 25 nM T4 addition but were twofold higher in 14 dpf larvae exposed to 100 nM T4 [Fig. 3(b), colored arrows]. Together these data suggest that thyrotrope sensitivity to altered TH levels increases between 7 and 14 dpf, as indicated by changes in mRNA abundance of a subset of genes involved in thyroid axis negative feedback. Interestingly, our data point to dose dependence of transcript levels for thrb and dio3b, but not tshb, in these concentration ranges.
Figure 3.
Maturation of thyroid-axis gene regulation under altered TH conditions. (a) Changes in relative tshb, thrb, and dio3b mRNA levels during the first month of development as measured by RT-qPCR. (b) Changes in relative tshb, thrb, and dio3b mRNA levels after treatment with different doses of TH (T4 challenge). At 7 dpf (left), tshb mRNA levels responded appropriately to TH (decreased) with the addition of 2.5 nM T4, whereas thrb and dio3b mRNA levels responded appropriately (increased) with the addition of 100 nM T4 (black arrows). At 14 dpf (right), tshb mRNA levels were reduced with the addition of only 1 nM T4, suggesting increased sensitivity to TH (arrow). Similarly, thrb mRNA levels increased at lower T4 doses (arrow) at 14 dpf compared with 7 dpf. At 14 dpf, dio3b mRNA levels increased much more dramatically in response to doses of T4 higher than 12.5 nM than at 7 dpf (compare orange, green, and black arrows). (c) Effects of early PTU treatments on sensitivity to TH levels (T4 challenge). Larvae were grown in 0.65 mM PTU for 7 days prior to being challenged with 0.25 to 25 nM T4. Values are shown as the mean ± SEM. Student t test: *P < 0.05; **P < 0.01; ***P < 0.001.
Effects of transient low-TH conditions on thyroid axis gene regulation
Having established that low-TH conditions lead to increased thyrotrope numbers and tshb mRNA levels (Fig. 2) and having developed an assay that reveals developmental changes in the tshb response to TH as a proxy for changes in the TSH set point, we next examined thyroid system regulatory responses after low-TH conditions. Zebrafish larvae were again reared in PTU (or DMSO control) for 7 days and then challenged with a range of T4 concentrations for 2 days followed by RT-qPCR analysis of relative tshb, thrb, and dio3b mRNA levels [Fig. 3(c)]. As shown previously, tshb mRNA levels, as well as GFP intensity in the tshb:GFP line, were increased after 7-day PTU treatments [Fig. 2(d)]. Subsequent addition of 12.5 and 25 nM T4 reduced tshb mRNA levels in both DMSO controls and PTU-treated larvae [Fig. 3(c), left]. No significant effects on tshb mRNA levels were seen with lower doses of added T4 [Fig. 3(c), left], suggesting similar thyrotrope sensitivity to T4 in PTU-treated embryos. Importantly, the amount of T4 needed to suppress tshb expression in both PTU-treated and control larvae was close to the physiologic TH levels at this age (i.e., 10 nM) (32). In larvae grown in PTU, thrb mRNA levels increased with the addition of T4 at concentrations that were 50-fold lower than those needed to cause increases in control larvae [Fig. 3(c), middle]. We did not detect changes in sensitivity to T4 when assaying dio3b expression [Fig. 3(c), right], although we cannot rule out a differential response to TH at concentrations between 12.5 and 25 nM.
Discussion
Clinical dilemmas in CH and the zebrafish thyroid system model
Newborn screening programs designed to diagnose CH and prevent associated neurocognitive impairments were developed more than 40 years ago. This has been a major success in the industrialized world (1, 33), but there are still many unanswered clinical questions regarding both diagnosis and appropriate therapy (namely, appropriate TH supplementation doses) along the CH spectrum. These issues are difficult to address without an understanding of the mechanisms underlying the ontogeny of thyroid axis regulation at the pituitary level. Our goal was to model a wide spectrum of low-TH conditions during HPT axis development that may correspond to a full range of CH in humans. This CH spectrum includes a variety of causes and severity, from exposure to mildly decreased TH levels associated with prematurity to increasingly low TH levels experienced by 1) the euthyroid fetus of a hypothyroid mother, 2) the hypothyroid fetus of a euthyroid mother, and 3) the hypothyroid fetus of the hypothyroid mother, including cases due to endemic iodine deficiency. We used the evolutionarily conserved zebrafish model to begin to investigate the cellular consequences of altered developmental TH environment (22), focusing on changes in thyrotrope numbers and tshb expression under low-TH conditions. We document changes in thyrotrope numbers and function (Fig. 2) proportionate to the length of exposure to the goitrogen PTU. Although a limitation of these studies was a lack of direct measurements of T4 and T3 levels in larvae, the ability of T3 to rescue the effects of PTU [Fig. 2(c)] strongly supports the idea that PTU treatment lowers TH levels in these experiments, as previously reported in zebrafish (22, 26, 27). Importantly, this response appears to be evolutionarily conserved, as indicated by the thyrotrope hyperplasia seen in TRH-R1(−/−) Pax8(−/−) double-knockout mice that suffer from hypothyroidism early in development (34). Thus, thyrotrope hyperplasia, combined with increased tshb production, in embryos experiencing low-TH conditions provides a possible pituitary-centered mechanism for the findings of elevated TSH levels in infants with both mild and severe forms of CH. Overall, these data suggest that changes in the thyrotrope population in response to low developmental TH levels could underlie some mild CH cases, in particular mild persisting TSH elevations after premature birth or SGA status.
The finding that thyrotrope numbers returned to normal after removal of PTU points to considerable plasticity in the thyrotrope population. More refined wash-out experiments that include measurements of available T4 and T3 levels will provide the next step in uncovering the dynamics of thyrotrope adaptation to altered developmental TH levels. Although we did not detect changes in thyrotrope sensitivity to TH after these PTU treatments, at least as determined by measurement of tshb, thrb, or dio3b mRNA levels in larval head tissue, it is possible that nonpituitary expression of thrb and dio3b in the head could mask thyroid-axis regulatory changes in these experiments.
Pituitary TH resistance after exposure to low-TH condition was not observed in zebrafish
Although our prediction that low developmental TH levels would cause an expansion of the thyrotrope population was supported by the data, we were surprised to find that tshb mRNA levels remained responsive to T4 challenge at the same concentrations of TH as in controls. Thus, misregulation of tshb, at least at the mRNA level, may not explain the published observation of relative hyperthyrotropinemia in a subset of neonates with CH and the finding that transient neonatal exposure to abnormal TH levels resulted in long-term alterations in the TSH feedback control system in rodents (35, 36). Although species-specific differences or experimental details (e.g., length of low-TH exposure, inability to measure TSHb protein levels, and/or the masking of individual differences in pooled samples) may explain these discrepancies, our data may also suggest that TH resistance in neonates should be examined in the context of the complete HPT axis regulatory network. Establishment of a TSH set point is a complex process involving TRH and its receptor, TH transporters, TH receptor and its cofactors, and activity of iodothyronine monodeiodinases (10, 37). Given the highly conserved nature of thyroid axis components across vertebrates (17), further analysis in the zebrafish model can provide a more detailed understanding of the developmental mechanisms underlying HPT axis responses to low-TH conditions as well as potentially shedding light on variability in these responses across individuals.
Thyrotrope recovery after transient low-TH conditions
The dramatic and puzzling increase in mild and transient cases of CH in neonates with an intact thyroid gland has sparked extensive clinical research (4, 7, 38–40) and has raised multiple discussions in the pediatric endocrinology community (33, 40, 41). In rare cases, mutations in the TSH receptor can cause mild and stable elevations in TSH with normal TH levels (42). DUOX2 haploinsufficiency was found to be responsible for CH due to abnormalities in TH production (dyshormonogenesis) that improves with time (43). Both of these scenarios are rare and cannot explain the majority of infants with mild and transient CH. To date, no consensus has been reached regarding the best management strategies and whether TH supplementation should be used in neonates and children with CH in cases of modest TSH elevations and normal levels of TH. Given the serious concerns regarding neurologic deficits and the lack of indicators (markers) other than a TSH assay, the majority of endocrinologists prefer to aggressively treat patients with even mild CH, but reports of harmful long-term behavioral effects of overtreatment are concerning (44, 45). Our data reveal strong regulatory compensation during development, with increases in thyrotrope numbers and function that are proportionate to the length of exposure to low TH conditions [Fig. 2(b)]. The expanded thyrotrope population in PTU-treated larvae produced more tshb mRNA overall, with tshb gene expression also increasing within individual cells [Fig. 2(d)]. The slow restoration of normal thyrotrope numbers after transient PTU treatment (Fig. 2, data not shown) indicates that the system can respond appropriately to the restoration of normal TH levels [Fig. 2(e), data not shown]. Because we have not found evidence of increased cell death after these treatments (data not shown), this is likely due to a slowing of precursor proliferation and/or thyrotrope differentiation rates. Given the potentially long-term risks associated with overtreating infants (44, 45) with mildly abnormal but transient TSH levels, these data are consistent with a recent review suggesting that persistently elevated TSH levels may not be the best indicator of TH insufficiency at the cellular level in the brain and other tissues (40).
Overall, these findings reveal strong regulatory compensation within the thyrotrope population under low developmental TH conditions and show considerable robustness in thyrotrope negative feedback regulation by TH. These data provide a foundation for future investigation of the programming effects of developmental stressors on the regulatory TSH set point in the thyroid axis.
Acknowledgments
The authors thank Drs. Tom Zoeller, Eric Bittman, and Alan Rogol for helpful comments throughout the study; Dr. Larry Schwartz for critical reading of the manuscript; and Judy Bennett and Mary McKitrick for fish care.
Acknowledgments
This work was supported by funds from the Endocrine Fellows Foundation (to K.N.T.), the UMass/Baystate Collaborative Biomedical Research program (to K.N.T. and R.O.K.), the Tufts University Charlton Research program (to K.N.T.), and Tufts Clinical Translational Science Institute award UL1 TR000073 (to K.N.T.), and by National Institutes of Health National Institute of Neurological Disorders and Stroke Grant NS039994 (to R.O.K.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- cDNA
- complementary DNA
- CH
- congenital hypothyroidism
- dpf
- days post fertilization
- DMSO
- dimethyl sulfoxide
- GFP
- green fluorescent protein
- hpf
- hours post fertilization
- HPT
- hypothalamic-pituitary-thyroid
- PTU
- 6-propyl-2-thiouracil
- RT-qPCR
- quantitative real-time polymerase chain reaction
- SEM
- standard error of the mean
- SGA
- small for gestational age
- T3
- 3,3′,5-triiodo-l-thyronine
- TH
- thyroid hormone
- TSH
- thyrotropin.
References
- 1.Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet J Rare Dis. 2010;5(17):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LaFranchi SH. Approach to the diagnosis and treatment of neonatal hypothyroidism. J Clin Endocrinol Metab. 2011;96(10):2959–2967. [DOI] [PubMed] [Google Scholar]
- 3.Hinton CF, Harris KB, Borgfeld L, Drummond-Borg M, Eaton R, Lorey F, Therrell BL, Wallace J, Pass KA. Trends in incidence rates of congenital hypothyroidism related to select demographic factors: data from the United States, California, Massachusetts, New York, and Texas. Pediatrics. 2010;125(Suppl 2):S37–S47. [DOI] [PubMed] [Google Scholar]
- 4.Parks JS, Lin M, Grosse SD, Hinton CF, Drummond-Borg M, Borgfeld L, Sullivan KM. The impact of transient hypothyroidism on the increasing rate of congenital hypothyroidism in the United States. Pediatrics. 2010;125(Suppl 2)S54–S63. [DOI] [PubMed] [Google Scholar]
- 5.Burrow GN, Fisher DA, Larsen PR. Maternal and fetal thyroid function. N Engl J Med. 1994;331(16):1072–1078. [DOI] [PubMed] [Google Scholar]
- 6.Fisher DA. Thyroid function and dysfunction in premature infants. Pediatr Endocrinol Rev. 2007;4(4):317–328. [PubMed] [Google Scholar]
- 7.Mitchell ML, Hsu HW, Sahai I, Massachusetts Pediatric Endocrine Work G; Massachusetts Pediatric Endocrine Work Group . The increased incidence of congenital hypothyroidism: fact or fancy? Clin Endocrinol (Oxf). 2011;75(6):806–810. [DOI] [PubMed] [Google Scholar]
- 8.Oren A, Wang MK, Brnjac L, Mahmud FH, Palmert MR. Mild neonatal hyperthyrotrophinaemia: 10-year experience suggests the condition is increasingly common but often transient. Clin Endocrinol (Oxf). 2013;79(6):832–837. [DOI] [PubMed] [Google Scholar]
- 9.Lem AJ, de Rijke YB, van Toor H, de Ridder MA, Visser TJ, Hokken-Koelega AC. Serum thyroid hormone levels in healthy children from birth to adulthood and in short children born small for gestational age. J Clin Endocrinol Metab. 2012;97(9):3170–3178. [DOI] [PubMed] [Google Scholar]
- 10.Fisher DA, Schoen EJ, La Franchi S, Mandel SH, Nelson JC, Carlton EI, Goshi JH. The hypothalamic-pituitary-thyroid negative feedback control axis in children with treated congenital hypothyroidism. J Clin Endocrinol Metab. 2000;85(8):2722–2727. [DOI] [PubMed] [Google Scholar]
- 11.Salvatore D, Davies TF, Schlumberger M-J, Hay ID, Larsen PR, Melmed S, Polonsky KS, Larsen PR, Kronenberg HM. Williams Textbook of Endocrinology. 3rd ed. Philadelphia, PA: Elsevier; 2016. [Google Scholar]
- 12.Thorpe-Beeston JG, Nicolaides KH, Felton CV, Butler J, McGregor AM. Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. N Engl J Med. 1991;324(8):532–536. [DOI] [PubMed] [Google Scholar]
- 13.Franco B, Laura F, Sara N, Salvatore G. Thyroid function in small for gestational age newborns: a review. J Clin Res Pediatr Endocrinol. 2013;5(Suppl 1):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Busby ER, Roch GJ, Sherwood NM, Perry S, Ekker M, Farrell A, Brauner C, eds. Fish Physiology. Vol. 35 Philadelphia, PA: Elsevier; 2010. [Google Scholar]
- 15.McGonnell IM, Fowkes RC. Fishing for gene function: endocrine modelling in the zebrafish. J Endocrinol. 2006;189(3):425–439. [DOI] [PubMed] [Google Scholar]
- 16.Lohr H, Hammerschmidt M. Zebrafish in endocrine systems: recent advances and implications for human disease. Annu Rev Physiol. 2011;73:183–211. [DOI] [PubMed] [Google Scholar]
- 17.Heijlen M, Houbrechts AM, Darras VM. Zebrafish as a model to study peripheral thyroid hormone metabolism in vertebrate development. Gen Comp Endocrinol. 2013;188:289–296. [DOI] [PubMed] [Google Scholar]
- 18.Tonyushkina KN, Shen MC, Ortiz-Toro T, Karlstrom RO. Embryonic exposure to excess thyroid hormone causes thyrotrope cell death. J Clin Invest. 2014;124(1):321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMenamin SK, Bain EJ, McCann AE, Patterson LB, Eom DS, Waller ZP, Hamill JC, Kuhlman JA, Eisen JS, Parichy DM. Thyroid hormone-dependent adult pigment cell lineage and pattern in zebrafish. Science. 2014;345(6202):1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203(3):253–310. [DOI] [PubMed] [Google Scholar]
- 21.Parichy DM, Elizondo MR, Mills MG, Gordon TN, Engeszer RE. Normal table of postembryonic zebrafish development: staging by externally visible anatomy of the living fish. Dev Dyn. 2009;238(12):2975–3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schmidt F, and Braunbeck T.. Alterations along the hypothalamic-pituitary-thyroid axis of the zebrafish (Danio rerio) after exposure to propylthiouracil. J Thyroid Res. 2011;2011:376243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chang J, Wang M, Gui W, Zhao Y, Yu L, Zhu G. Changes in thyroid hormone levels during zebrafish development. Zoolog Sci. 2012;29(3):181–184. [DOI] [PubMed] [Google Scholar]
- 24.Devine CA, Sbrogna JL, Guner B, Osgood M, Shen MC, Karlstrom RO. A dynamic Gli code interprets Hh signals to regulate induction, patterning, and endocrine cell specification in the zebrafish pituitary. Dev Biol. 2009;326(1):143–154. [DOI] [PubMed] [Google Scholar]
- 25.Walpita CN, Van der Geyten S, Rurangwa E, Darras VM. The effect of 3,5,3′-triiodothyronine supplementation on zebrafish (Danio rerio) embryonic development and expression of iodothyronine deiodinases and thyroid hormone receptors. Gen Comp Endocrinol. 2007;152(2-3):206–214. [DOI] [PubMed] [Google Scholar]
- 26.Ji C, Jin X, He J, Yin Z. Use of TSHβ:EGFP transgenic zebrafish as a rapid in vivo model for assessing thyroid-disrupting chemicals. Toxicol Appl Pharmacol. 2012;262(2):149–155. [DOI] [PubMed] [Google Scholar]
- 27.van der Ven LT, van den Brandhof EJ, Vos JH, Power DM, Wester PW. Effects of the antithyroid agent propylthiouracil in a partial life cycle assay with zebrafish. Environ Sci Technol. 2006;40(1):74–81. [DOI] [PubMed] [Google Scholar]
- 28.Brown SB, Adams BA, Cyr DG, Eales JG. Contaminant effects on the teleost fish thyroid. Environ Toxicol Chem. 2004;23(7):1680–1701. [DOI] [PubMed] [Google Scholar]
- 29.Nica G, Herzog W, Sonntag C, Hammerschmidt M. Zebrafish pit1 mutants lack three pituitary cell types and develop severe dwarfism. Mol Endocrinol. 2004;18(5):1196–1209. [DOI] [PubMed] [Google Scholar]
- 30.Davies TF, Lauberg P, Bahn RS, Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. Philadelia, PA: Elsevier; 2016. [Google Scholar]
- 31.Liu YW, Lo LJ, Chan WK. Temporal expression and T3 induction of thyroid hormone receptors alpha1 and beta1 during early embryonic and larval development in zebrafish, Danio rerio. Mol Cell Endocrinol. 2000;159(1-2):187–195. [DOI] [PubMed] [Google Scholar]
- 32.Liu YW, Chan WK. Thyroid hormones are important for embryonic to larval transitory phase in zebrafish. Differentiation. 2002;70(1):36–45. [DOI] [PubMed] [Google Scholar]
- 33.LaFranchi SH. Increasing incidence of congenital hypothyroidism: some answers, more questions. J Clin Endocrinol Metab. 2011;96(8):2395–2397. [DOI] [PubMed] [Google Scholar]
- 34.Mittag J, Friedrichsen S, Strube A, Heuer H, Bauer K. Analysis of hypertrophic thyrotrophs in pituitaries of athyroid Pax8-/- mice. Endocrinology. 2009;150(9):4443–4449. [DOI] [PubMed] [Google Scholar]
- 35.Azizi F, Vagenakis AG, Bollinger J, Reichlin S, Braverman LE, Ingbar SH. Persistent abnormalities in pituitary function following neonatal thyrotoxicosis in the rat. Endocrinology. 1974;94(6):1681–1688. [DOI] [PubMed] [Google Scholar]
- 36.Dussault JH, Coulombe P, Walker P. Effects of neonatal hyperthyroidism on the development of the hypothalamic-pituitary-thyroid axis in the rat. Endocrinology. 1982;110(3):1037–1042. [DOI] [PubMed] [Google Scholar]
- 37.Fliers E, Unmehopa UA, Alkemade A. Functional neuroanatomy of thyroid hormone feedback in the human hypothalamus and pituitary gland. Mol Cell Endocrinol. 2006;251(1-2):1–8. [DOI] [PubMed] [Google Scholar]
- 38.Castanet M, Goischke A, Léger J, Thalassinos C, Rodrigue D, Cabrol S, Zenaty D, al-Harbi M, Polak M, Czernichow P; Fédération Parisienne pour le Dépistage et la Prévention des Handicaps de l’'Enfant (FDPHE) . Natural history and management of congenital hypothyroidism with in situ thyroid gland. Horm Res Paediatr. 2015;83(2):102–110. [DOI] [PubMed] [Google Scholar]
- 39.Aguiar L, Garb J, Reiter E, Visintainer P, Singh R, Allen H, Tonyushkina K.. Can one predict resolution of neonatal hyperthyrotropinemia? J Pediatr. 2016;174:71–77e1. [DOI] [PubMed] [Google Scholar]
- 40.Mitchell ML, Hsu HW; Massachusetts Pediatric Endocrine Working Group. Unresolved issues in the wake of newborn screening for congenital hypothyroidism. J Pediatr. 2016;173:228–231e1. [DOI] [PubMed] [Google Scholar]
- 41.Rapaport R. Congenital hypothyroidism: an evolving common clinical conundrum. J Clin Endocrinol Metab. 2010;95(9):4223–4225. [DOI] [PubMed] [Google Scholar]
- 42.Tenenbaum-Rakover Y, Almashanu S, Hess O, Admoni O, Hag-Dahood Mahameed A, Schwartz N, Allon-Shalev S, Bercovich D, Refetoff S. Long-term outcome of loss-of-function mutations in thyrotropin receptor gene. Thyroid. 2015;25(3):292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grasberger H, Refetoff S. Genetic causes of congenital hypothyroidism due to dyshormonogenesis. Curr Opin Pediatr. 2011;23(4):421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bongers-Schokking JJRW, Resing WC, de Rijke YB, de Ridder MA, de Muinck Keizer-Schrama SM. Cognitive development in congenital hypothyroidism: is overtreatment a greater threat than undertreatment? J Clin Endocrinol Metab. 2013;98(11):4499–4506. [DOI] [PubMed] [Google Scholar]
- 45.Alvarez M, Iglesias Fernández C, Rodríguez Sánchez A, Dulín Lñiguez E, Rodríguez Arnao MD. Episodes of overtreatment during the first six months in children with congenital hypothyroidism and their relationships with sustained attention and inhibitory control at school age. Horm Res Paediatr. 2010;74(2):114–120. [DOI] [PubMed] [Google Scholar]