Abstract
Thyroid hormone (TH) action is mediated by the products of two genes, TH receptor (THR)α (THRA) and THRβ (THRB) that encode several closely related receptor isoforms with differing tissue distributions. The vast majority of THR isoform–specific effects are thought to be due to tissue-specific differences in THR isoform expression levels. We investigated the alternative hypothesis that intrinsic functional differences among THR isoforms mediate these tissue-specific effects. To achieve the same level of expression of each isoform, we created tagged THR isoforms and tested their DNA and functional properties in vitro. We found significant homodimerization and functional differences among the THR isoforms. THRA1 was unable to form homodimers on direct repeat separated by 4 bp DNA elements and was also defective in TH-dependent repression of Tshb and Rxrg in a thyrotroph cell line, TαT1.1. In contrast, THRB2 was both homodimer sufficient and fully functional on these negatively regulated genes. Using domain exchanges and individual amino acid switches between THRA1 and THRB2, we identified three amino acids in helix 10 of the THRB2 ligand-binding domain that are required for negative regulation and are absent in THRA1.
Using DNA binding and a functional assay in cultured cells, we show the role of amino acid variations within the LBD of thyroid hormone receptors THRA and THRB in TH-mediated negative gene regulation.
Thyroid hormones (THs) are essential for regulation of development and metabolism in mammals. Whereas THs activate transcription of multiple genes in nearly all tissues, negative regulation of gene expression by triiodothyronine (T3) is critical for maintaining TH levels in a narrow range through tight regulation of the hypothalamic–pituitary–thyroid (HPT) axis (1). TH action is mediated by three predominant TH receptor (THR) isoforms, THRα1 (THRA1), THRβ1 (THRB1), and THRβ2 (THRB2), encoded by two genes, Thra and Thrb. As is true with other nuclear hormone receptors, THRs are modular in structure [Fig. 1(a)], and the most divergent domain is the N-terminal A/B domain that mediates ligand-independent transcriptional activation function (AF)-1 (2). In contrast, the DNA-binding domain (DBD) and ligand-binding domain (LBD) show >90% homology among the isoforms. The LBD performs several critical functions: (1) binds hormones with high affinity, (2) contains ligand-regulated transcriptional AF-2 necessary for recruiting various coregulatory proteins, and (3) is involved in receptor dimerization. The selectivity of the THRs for target-specific DNA response elements [TH response elements (TREs)] is encoded by the DBDs; however, the affinity of different THR complexes to various elements may also be influenced by AF-1 or LBDs (3–9).
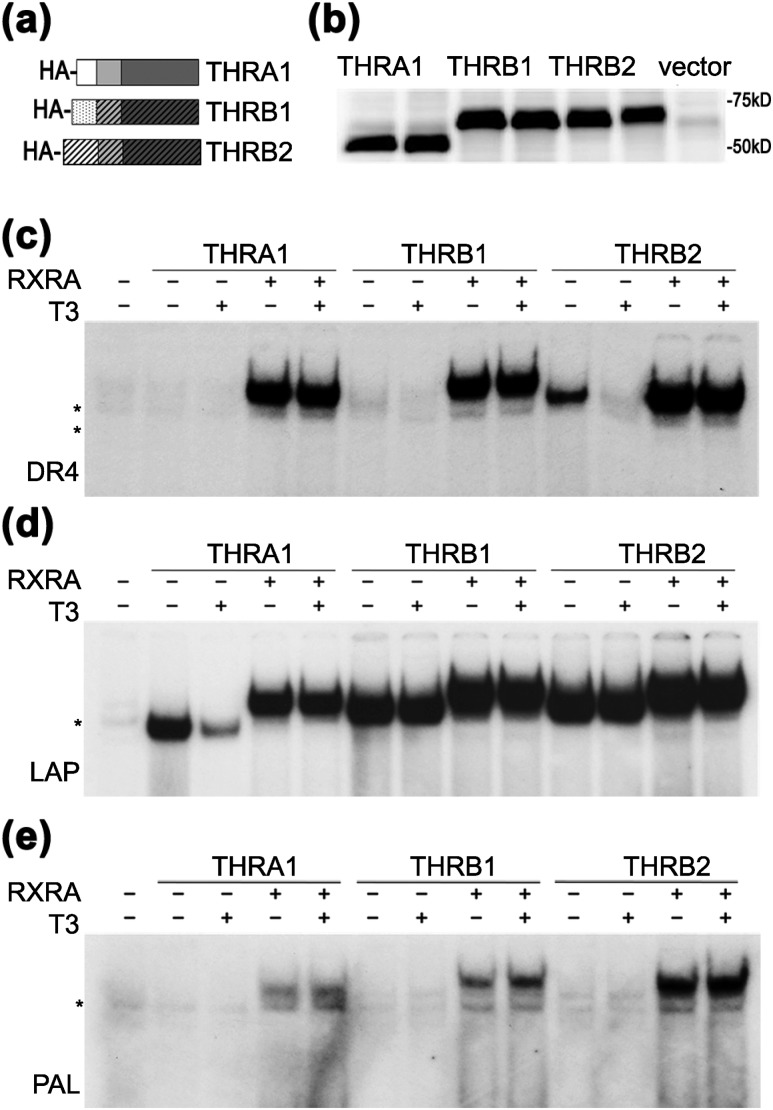
Figure 1.
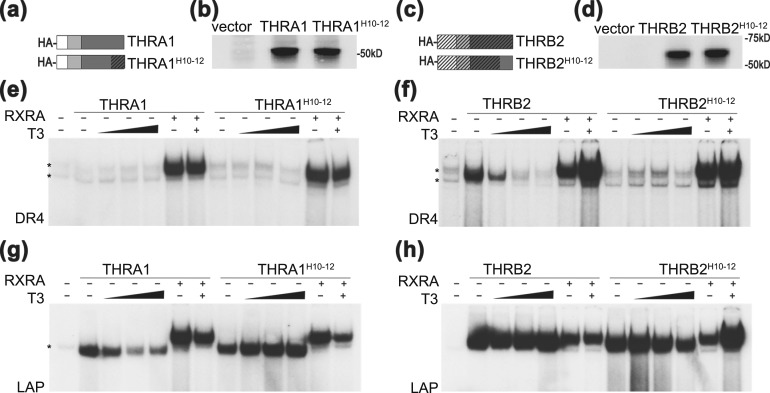
THR isoforms have different abilities to form homodimers on DNA. (a) Schematic representation of hemagglutinin (HA)-tagged THR isoforms. AF-1, DBD, and LBD domains (N to C termini) are shown by different shading. (b) In vitro transcription/translation proteins from rabbit reticulocyte lysate were analyzed in duplicate by western blot using an anti-HA antibody. pSP72 was used as an “empty” vector negative control. (c–e) Binding of wild-type THRs to DR4, LAP, and PAL in the presence or absence of retinoic X receptor α (RXRA) were tested by electrophoretic mobility shift assay. (c) On a DR4 element, THRA1 bound exclusively as a heterodimer, THRB1 showed weak homodimer formation, and THRB2 bound as homodimer in the absence of T3. All THR isoforms heterodimerized with RXRA. (d) On an LAP element, all THRs isoforms bound as homodimers and heterodimers, but only THRA1 binding was dissociated by T3. (e) On a PAL element, THRs bound exclusively in the presence of RXRA. Samples were treated with vehicle or 100 nM T3. *Nonspecific band observed in unprogrammed lysate.
The arrangement of TREs within the promoter might regulate THR action by determining THR isoform binding, THR dimerization, and coregulators binding. In the classic view of how TH and its receptor stimulate gene expression, the gene promoter contains TREs consisting of a 6-bp consensus sequence (AGGTCA) organized as a direct repeat separated by 4 bp (DR4), a palindrome without spacing (PAL), or an inverted palindrome (LAP) separated by 4 to 6 bp (10–13). THRs can bind to these sites as homodimers or as heterodimers with 9-cis retinoic acid receptor [retinoid X receptor (RXR)]. Most studies suggest a preference for heterodimerization of THRs on DR4 sites; however, THRA1, THRB1, and THRB2 appear to have different relative affinities to DR4 as well as to LAP and PAL. It has also been suggested that at least at some target genes THRB can bind DNA without RXR (14). Importantly, homodimerization appears to be an essential property of THRB isoforms, and mutations in Thrb that interrupt homodimer formation also appear to specifically affect negative gene regulation (7, 15, 16).
A model for THR–DNA binding at negatively regulated genes is less defined. On genes found in the HPT axis and regulated negatively by TH, THR has also been proposed to bind to DNA as monomers (17, 18). However, the lack of classical TRE sites in promoter regions of these genes does not exclude the possibility that chromatin structure and the associated transcriptional factors may actually be more important in determining THR–DNA binding than the sequence of the TRE.
Although functional and structural differences among THR isoforms are known, most reviews on TH action imply that the TH tissue effect is determined almost exclusively by the relative level of THR isoform expression in specific tissues, and not by their functional activity. Based on this view, organs with higher expression of THRB1 and THRB2 isoforms such as the HPT axis, liver, and inner ear are uniquely affected when these isoforms are deleted or mutated in animals. Mutations in THRB also defined classic resistance to TH (RTH) in man (RTHβ). Subsequently, RTH due to THRA1 mutations (RTHα) was described and resistance was confined to organs thought to contain predominantly THRA1 (1, 19). However, the analysis of THR isoform-specific messenger RNAs (mRNAs) suggests that few tissues predominantly express only one isoform, and the more relevant analysis of THR protein expression is lacking due to the absence of reliable THR isoform-specific antibodies. Therefore the hypothesis that the level of THR protein expression in tissues fully determines TH-dependent regulation of gene expression has yet to be rigorously proven. Alternatively, we and others have shown that the functions of THR isoforms are not completely redundant in their functional properties (20–22).
To compare the functional activities of THR isoforms, we constructed hemagglutinin (HA)-tagged versions of THRA1, THRB1, and THRB2. We established that THR isoforms vary in their ability to form homodimers, with THRA1 having the lowest and THRB2 the highest ability to form homodimers. Similarly, we tested the function of each protein in negative and positive TH gene regulation using the thyrotroph cell line, TαT.1.1. We tested two genes, Tshb and Rxrg that were repressed by TH in a concentration-dependent manner, and two genes activated by TH, Rab27 and Sema3c. We found that all THR isoforms can activate gene expression in the presence of T3, but only THRB2 is able to repress Tshb and Rxrg at physiological concentrations of T3.
Using domain exchanges between THRA1 and THRB2 proteins, we found that the isoform-specific amino acid variations within the C-terminal part of the LBD (helices 10 to 12) determine the homodimer formation on various DNA elements. Importantly, substituting helices 10 to 12 of THRB2 with the corresponding THRA1 sequence weakened the ability of THRB2 to homodimerize on DR4 and LAP, whereas performing the opposite exchange allowed THRA1 to form strong homodimers on LAP. Subsequently, we found that just three amino acids in helix 10 are responsible for both the homodimer and functional difference between THRA1 and THRB2. We suggest that these helix 10 amino acids are critical for negative regulation in THRB2 and are absent in THRA1.
Materials and Methods
THR constructs
Mouse wild-type (WT) THR complementary DNAs (cDNAs) were obtained by quantitative reverse transcription polymerase chain reaction (qRT-PCR) from liver, heart, and pituitary RNA. The cDNAs were cloned in a TOPO vector and sequenced. THR mutants with exchanged domains were chemically synthesized (GeneArt Gene Synthesis; Invitrogen/Life Technologies, Carlsbad, CA). The THRB2 H10 mutant was created using the QuickChange site-directed mutagenesis kits (Agilent Technologies, Santa Clara, CA). All WT and chimeric THR cDNAs were transferred to pENTR 2B vector, which was engineered to contain a uniform translational start site and an in-frame HA tag. All chimeric THRs were completely sequenced.
For in vitro transcription/translation, HA-tagged THRs were transferred into the pSP72 containing the T7 promoter (Invitrogen/Life Technologies, Carlsbad, CA). For THR rescue experiments, the HA-tagged cDNAs were transferred into the pAd-CMV-V5 vector. Adenovirus particles were produced according to the manufacturer’s protocol by transfection of the PacI-digested constructs into 293A cells using Lipofectamine (Invitrogen/Life Technologies, Carlsbad, CA). Viruses were amplified and purified by the Viral Vector Core Facility (University of Iowa, Ames, IA). Verification of viral genomes was performed by PCR followed by sequencing.
Electrophoretic mobility shift assay
HA-tagged WT and chimeric THRs as well as RXRα (RXRA) were synthesized using the coupled transcription/translation reticulocyte lysate system (TNT T7; Promega, Madison, WI). The relative amounts of THRs were tested by Western blotting with anti-HA antibody (Sigma-Aldrich, St. Louis, MO). Electrophoretic mobility shift assay (EMSA) was performed as described (18). Briefly, each receptor was incubated with 0.5 pmol of radiolabeled DR4, LAP, or PAL double-stranded oligonucleotide probe in the binding buffer [20 mM HEPES (pH 7.9), 50 mM KCl, 20% glycerol, 1 mM dithiothreitol, 0.05 µg/μL poly(deoxyinosinic-deoxycytidylic) acid] for 30 minutes at room temperature, and separated on nondenaturing 5% polyacrylamide gel in 0.5× TRIS borate EDTA buffer. Each experiment was repeated at least three times.
Cell culture and hormone treatments
TαT1.1 cells were plated in Dulbecco’s modified Eagle’s medium (Cellgro; Corning, Manassas, VA) containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO) and 1% antibiotic/antimycotic (Invitrogen/Life Technologies). The plates were coated with 30-fold diluted matrigel (BD Biosciences, Billerica, MA) to facilitate adhesion before the cells were seeded. Cells were maintained at 37°C, 5% CO2. T3 treatment was performed for 24 hours in Dulbecco’s modified Eagle’s medium containing 10% charcoal/dextran-stripped fetal bovine serum (Gemini Bio-Products, West Sacramento, CA).
Functional analysis of THR chimeric protein in TαT.1.1 cells
Adenoviruses expressing nonspecific scrambled short hairpin RNA (shRNA), shRNA against both THRB isoforms mRNA, and shRNA against THRA isoforms were described previously (22). Thirty-six hours after the TαT1.1 cells were plated, they were transduced with shRNA adenoviruses. Thirty-six hours later, media were changed and adenoviruses expressing THR were added. We adjusted the adenoviral amounts to obtain similar levels of protein expression of all THR isoforms in the cell cultures. HA-THRA showed a much higher level of relative protein expression when an adenoviral multiplicity of infection similar to other THR constructs was used. To address the effects of viral load and levels of THRA protein on gene regulation, we performed rescue experiments using two levels of HA-THRA expression (see Results and Supplemental Fig. 1 (692.9KB, pdf) ). Treatment with different T3 concentrations (0.1, 0.3, 1.0, 3.0, and 10 nM) was performed 12 hours after transduction. After 24 hours of T3 treatment, cultures were harvested for protein or RNA analysis. The concentration of adenoviruses was determined and the level of expression of THRs assessed by western blotting. The control scramble adenovirus and adenovirus expressing green fluorescent protein (GFP) were used at equivalent titers as the viruses of interest.
RNA was extracted using TRIzol Plus (Invitrogen/Life Technologies), and cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Real-time PCR analyses were performed using iTaq Universal SYBR Green Supermix in a CFX96 real-time PCR detection system (Bio-Rad Laboratories, Hercules, CA) according to the recommendations of the manufacturer. Relative mRNA levels were determined using the 2−ΔΔCt method. The primer efficiencies and the PCR products were assessed by relative standard curves and melting curves. Primer sequences are listed below. Knockdown (KD) and rescue experiments were performed in parallel, repeated three times, and produced similar results. The representative experiments were analyzed using a two-way analysis of variance and Student t test for assessment (GraphPad Prism; GraphPad Software, La Jolla, CA). For all analyses, statistical significance was accepted at P < 0.05.
Oligonucleotides
TRE oligonucleotide sequences used were: DR4, 5′-TCAGGTCACAGGAGGTCATC-3′; LAP-5′-GATGACCTGACGTCAGGTCAGA-3′; PAL, 5′-TCAGGTCATGACCTTC-3′. qRT-PCR primers were: Actb forward, 5′-AGCCATGTACGTAGCCATCCA-3′, reverse, 5′-TCTCCGGAGTCCATCACAATG-3′; Thrb forward, 5′-AACCAGTGCCAGGAATGTCG-3′, reverse, 5′-CTCTTCTCACGGTTCTCCTC-3′; Thra forward, 5′-GGCTGTGCTGCTAATGTCAA-3′, reverse, 5′-CTTTAGACTTCCTGATCCTCAAAG-3′; Tshb forward, 5′-GTGCTGGGTATTGTATGACACG-3′, reverse 5′-CTGGTATTTCCACCGTTCTGTAG-3′; Sema3c forward, 5′-GAACCCATGTTTGTGGACGC-3′, reverse, 5′-CCACCAGTGTCATTAGGGCA-3′; Rab27 forward, 5′-GCTGGACAAGAGCGGTTCC-3′, reverse, 5′-GCTCTGTTGACTGGTGAGGT-3′, Rxrg forward, 5′-ACAGAGTTGGTGTCCAAGATG-3′, reverse, 5′-TCTCGAAGAGTCTCCACCTC-3′; Thrb shRNA, 5′-CACCGCAAGGAAACTCAATTCTTTCCGAAGAAAGAATTGAGTTTCCTTGC-3′
Western blotting
Aliquots of protein were separated on 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and transferred to polyvinylidene difluoride membranes. Membranes were incubated with horseradish peroxidase–conjugated anti-HA antibody (1:1000, Research Resource Identifier: AB_2314622; Roche 3F10, purchased from Sigma-Aldrich). Equivalent loading was assessed by using the same amount of reticulocyte extract, protein concentration, and Ponceau S staining.
Results
THR isoforms have different properties in homodimer formation
EMSA is traditionally used to test for THR–DNA complex formation. All three major THR isoforms, THRA1, THRB1 and THRB2 [Fig. 1(a)], bind to DR4, LAP, or PAL oligonucleotides as monomers, homodimers, or heterodimers with RXR. However, the stoichiometry of the interacting molecules contributes to the kinetics and stability of each complex. Therefore, for direct comparison between THR isoforms, it is important to control for protein expression and concentrations of nonspecific proteins. To this end, we created constructs expressing HA-tagged THRs under control of the T7 promoter. THRA1, THRB1, and THRB2 were expressed in rabbit TNT reticulocyte extracts, and equal amounts of proteins [Fig. 1(b)] and TNT extract volumes were used in EMSA experiments.
On a DR4 consensus element, THRA1 was unable to form a homodimer under these assay conditions [Fig. 1(c)]. In contrast, THRB2 and, to a lesser extent,, THRB1 bound to the DR4 element as a homodimer, which dissociated after treatment with T3 [Fig. 1(c)]. These data suggest that the THRB2 N terminus may stabilize homodimer binding or that the N termini of THRA1 and THRB1 may destabilize homodimerization. All THR isoforms were able to bind to the LAP element as homodimers; however, THRA1 binding was weaker, and the homodimer was dissociated by T3 [Fig. 1(d)]. No THR isoform bound to the PAL element as a homodimer as previously reported [Fig. 1(e)] (6), and we did not pursue this element in our further experiments. All three THR isoforms were able to form heterodimers with RXRA on DR4, PAL, and LAP elements; additionally, as previously reported (23), the THR–RXR heterodimers were resistant to T3 treatment [Fig. 1(c–e)]. These results agreed well with the previous observations that THRB isoforms, especially THRB2, have greater ability than THRA1 to form homodimers (15).
Function of THR isoforms in gene repression and activation
THRB2 is a key isoform functioning in T3-mediated negative regulation of Tshb expression in pituitary thyrotrophs. Using a mouse pituitary cell line (TαT1.1), we have previously demonstrated that knockdown of Thrb using an adenoviral shRNA (Thrb KD) eliminated T3 negative regulation. We also showed that THRA1 was able to repress Tshb expression, but only at high concentrations of T3 (∼100 nM). Interestingly, THRA1 binding to Tshb in TαT1.1 cells was only detected by chromatin immunoprecipitation assay after knockdown of THRB isoforms (22).
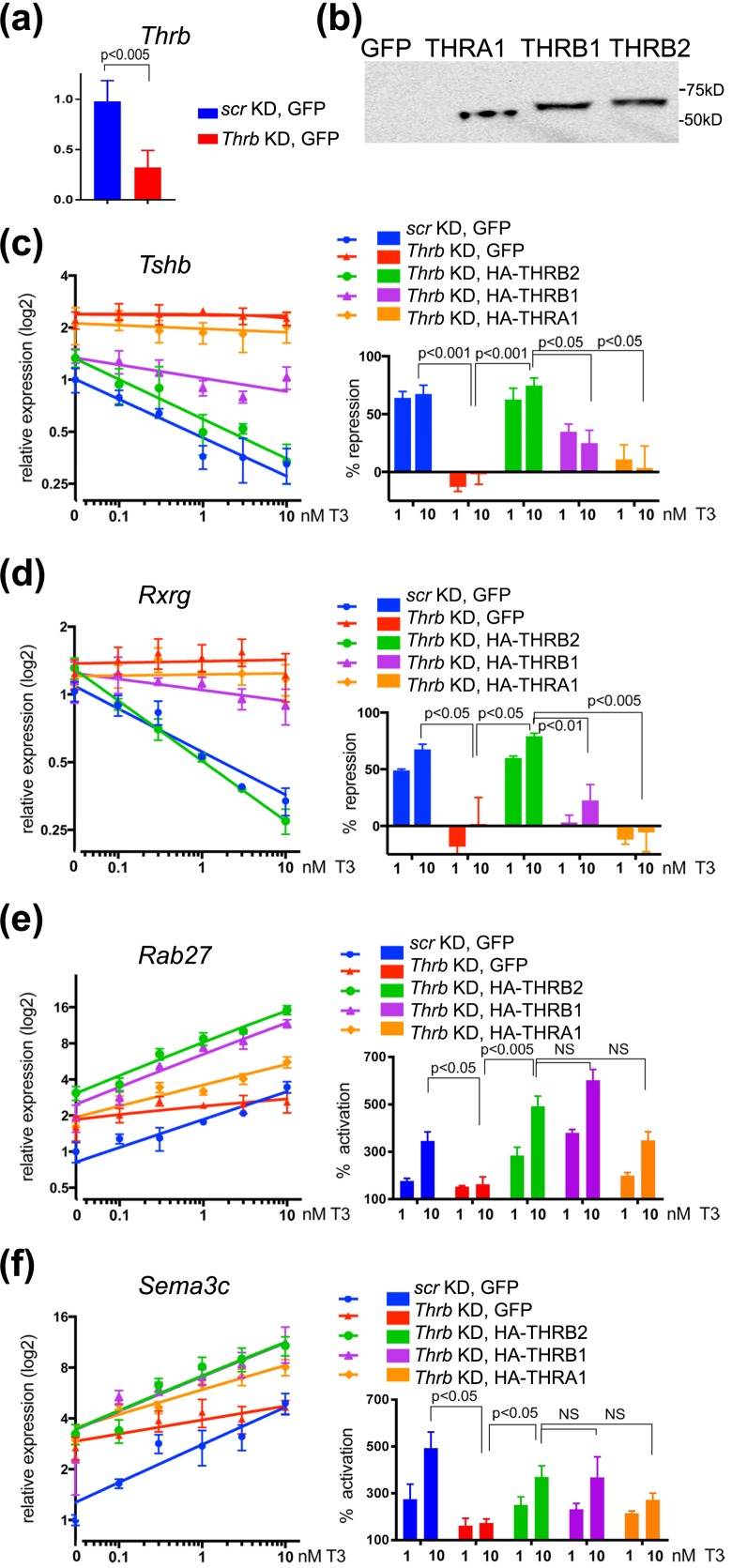
The Thrb shRNA was directed at the 3′ untranslated region of Thrb to permit reexpression of THR isoforms in an add-back (rescue) experiment (Fig. 2). Real-time PCR analysis demonstrated that Thrb1/2 knockdown with reexpression of GFP (negative control) eliminated T3 negative regulation at 0.1 to 10 nM T3 [Fig. 2(c), compare blue and red]. Interestingly, it also increased the level of Tshb expression in the absence of T3 treatment. Both basal and T3-dependent negative regulation of Tshb was restored by addition of an adenovirus expressing HA-THRB2 [Fig. 2(b) and 2(c), green]. HA-THRB2 reexpression in Thrb KD TαT1.1 cells resulted in 60% and 75% reduction in Tshb mRNA at 1 and 10 mM T3, respectively, similar to that in control cells [scrambled shRNA and GFP, Fig. 2(c)]. In an analogous experiment when both Thra and Thrb were knocked down (Supplemental Fig. 1 (692.9KB, pdf) ), reexpression of THRB2 was able to rescue T3-dependent negative regulation of Tshb, but not the basal level of Tshb mRNA [Supplemental Fig. 1(c) (692.9KB, pdf) ]. Furthermore, the basal expression of Tshb was lowered by reexpression of THRA1 at high levels [Supplemental Fig. 1(b) and 1(c) (692.9KB, pdf) ], suggesting that THRA1 may have a role in regulating basal levels of Tshb mRNA in the absence of THRB1/2; however, the physiological relevance of this effect remains unclear at this time.
Figure 2.
THR isoforms have different functions in positive and negative regulation of gene expression. (a) Relative expression of Thrb in control (scr KD GFP) and Thrb KD cells measured by qRT-PCR and normalized to Actb mRNA (see Materials and Methods). (b) Expression level of each HA-THR isoform in Thrb KD TαT1.1 cells was tested by western blotting with anti-HA antibody. Relative expression of (c and d) negatively and (e and f) positively regulated genes were measured by qRT-PCR and normalized to Actb mRNA. Relative expression [y-axis (log2)] of (c) Tshb and (d) Rxrg decreased with increased concentrations of T3 [x-axis (log10); y intersects x at 0 nM]. KD of Thrb abolishes T3-dependent repression (also see percentage of repression, left panels). Expression of HA-tagged THRB2 proteins in Thrb KD TαT1.1 cells fully reconstituted gene repression, THRB1 had weaker ability to regulate Tshb and Rxrg, and expression of THRA1 was unable to rescue Thrb KD phenotype. Relative expression [y-axis (log2)] of (e) Rab27 and (f) Sema3c increased with increased concentrations of T3 [x-axis (log10); y intersects x at 0 nM]. KD of Thrb abolishes T3-dependent gene activation (also see percentage of activation, left panels). Expression of HA-tagged THR isoforms in Thrb KD TαT1.1 cells reconstituted T3-dependent gene activation. Data points are presented as mean ± standard error of the mean. Differences were considered to be significant at P < 0.05. NS, not significant.
We also performed rescue experiments using adenoviruses expressing HA-THRB1 and HA-THRA1. HA-THRB1 only partially rescued the Thrb KD phenotype (35% and 25% of repression at 1 and 10 nM T3, respectively [Fig. 2(c); Supplemental Fig. 1(c) (692.9KB, pdf) ]. For THRA1 rescue we used two different adenoviral concentrations (1) to obtain similar levels of expression as THRB1 and THRB2 [Fig. 2(b); Supplemental Fig. 1(b) (692.9KB, pdf) ] or (2) to achieve similar multiplicity of infection resulting in higher expression of HA-THRA1 (Supplemental Fig. 1 (692.9KB, pdf) ). Importantly, the expression of HA-THRA1 in Thrb KD cells or in Thra/Thrb KD cells was unable to reestablish T3-dependent negative regulation of Tshb at either concentration.
A number of additional T3-regulated genes were identified in an RNA microarray experiment performed in TαT.1.1 cells and (unpublished data, A. Sidhaye, S. Minakhina, and F. Wondisford, 2017). Rxrg, for example, is repressed by T3 in a concentration-dependent fashion, and Thrb KD abolished this regulation [Fig. 2(d)]. We tested whether expression of HA-THRB1, HA-THRB2, or HA-THRA1 could rescue T3-dependent negative regulation of Rxrg and found that only THRB2 rescued hormone-dependent gene repression [Fig. 2(d)]. Expression of Rab27 and Sema3c was stimulated by T3 in a concentration-dependent manner [Fig. 2(e) and 2(f)]. The relative levels of mRNA were increased up to fivefold and 3.5-fold at 10 nM T3, respectively. Thrb KD significantly reduced T3-dependent gene activation, although KD did not completely abolish the regulation [Fig. 2(d) and 2(e)]. KD of both Thrb and Thra abolished positive regulation of both Rab27 and Sema3c [Supplemental Fig. 1(e) and 1(f) (692.9KB, pdf) ], suggesting that the residual activation of gene expression observed in Thrb KD cells was mediated by low levels of endogenous THRA1.
All three THR isoforms were able to rescue T3-dependent gene activation in Thrb and Thrb/Thra KD cells [Fig. 2(e) and 2(f); Supplemental Fig. 1 (692.9KB, pdf) ], suggesting that all three HA-THRs are functional in positive regulation of gene expression, at least on these two target genes. Importantly, the expression of HA-THRA1 was able to compensate for the lack of THRB in both Thrb KD and Thrb/Thra KD cells [Fig. 2(e) and 2(f); Supplemental Fig. 1 (692.9KB, pdf) ]; however, the further increase in THRA1 expression in Thrb/Thra KD cells did not further increase T3-dependent gene activation [THRA1* in Supplemental Fig. 1(e) and 1(f) (692.9KB, pdf) , right panels]. Overall, THRA1 was able to activate Rab27 and Sema3c expression in a T3-dependent manner, but it was unable to support T3-dependent negative regulation of Tshb and Rxrg.
Mapping isoform-specific amino acid variations affecting the strength of homodimer formation
All three HA-tagged THR isoforms could form heterodimers with RXR on several DNA elements [Fig. 1] and could activate gene expression in response to TH on endogenous T3-dependent genes in pituitary cells. Alternatively, THRB2 and, to a lesser extent, THRB1 were able to form homodimers on DR4 and also drive TH-dependent repression of Tshb and Rxrg at physiological concentrations of T3.
Based on this correlation, we decided to map which amino acids specify THRB homodimer formation and determine whether these same amino acids affect T3 negative gene regulation. Although THR isoforms all have different AF-1 domains, we decided to focus on the role of the LBD in homodimer formation and negative gene regulation.
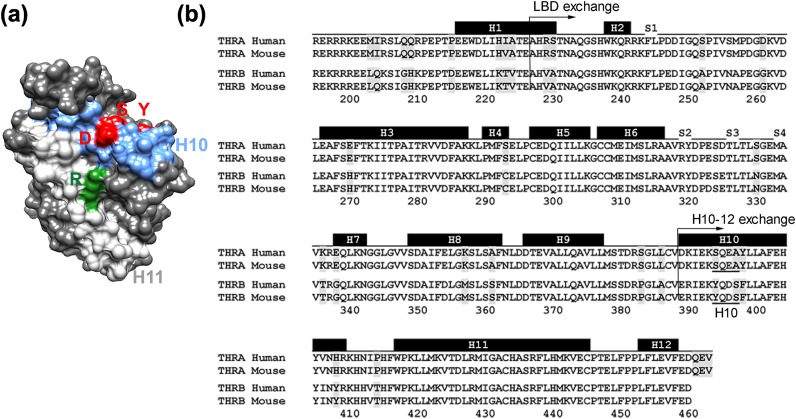
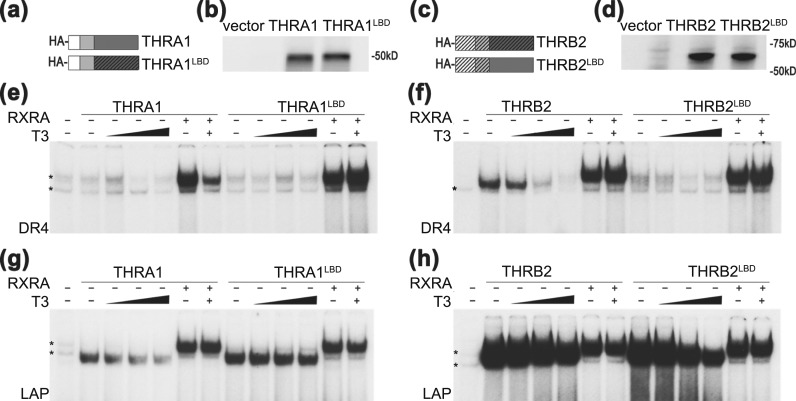
In all nuclear receptors, the LBD forms a dimerization interface [Fig. 3(a)] and amino acid variations between THRA and THRB could determine homodimerization. Previous work has focused on artificial mutations in the LBD that disrupt homodimerization and heterodimerization. We decided, in contrast, to exchange domains between THRA and THRB LBDs, which are ∼90% homologous [Fig. 3(b)]. We designed and synthesized chimeric THRs in which the LBD domains were exchanged between THRA and THRB isoforms [the exchange positions are marked in Fig. 3(b)]. Similar to WT THRA1, the THRA1 carrying THRB LBD (THRA1LBD) chimera [Fig. 4(a) and 4(b)] formed RXR heterodimers on both DR4 and LAP elements, but it was unable to bind DR4 in the absence of RXR [Fig. 4(e)]. However, the THRA1LBD homodimer on LAP was more stable compared with WT THRA1, and it did not dissociate from DNA after T3 treatment [Fig. 4(g)].
Figure 3.
Structure of THRB LBD domain. (a) Dimerization surface of THRB LBD (Protein Data Bank code 3GWS) (9). Helix 10 is shown in blue, helix 11 is in light gray. Amino acids Tyr (Y), Asp (D), and Ser (S) that were substituted in the THRBH10 chimera are shown in red, Arg (R) affected in the R429Q mutant is shown in green. (b) Alignment of LBDs of THRA and THRB from humans and mice. Positions that show nonconserved amino acid variations between mammalian α and β isoforms are shaded. Black rectangles on the top of the sequence show location of helices (H1–H12) as described in Wagner et al. (24). The position of domain exchange in THRB2LBD THRA1LBD, and in THRB2H10-12 and THRA1H10-12 are marked with arrows. The amino acids changed in THRB2 H10 are underlined.
Figure 4.
LBD domain exchange between THRA1 and THRB2 affects receptor binding to DR4 and LAP. (a and c) Schematic structures and (b and d) protein levels of in vitro–translated THRA1, THRA1LBD, THRB2, and THRB2LBD are shown. pSP72 was used as an “empty” vector negative control. (e–h) EMSA of chimeric receptors in the absence and presence of RXRA. (e) On DR4, neither THRA1 nor THRA1LBD formed a homodimer. (g) THRA1LBD formed a stronger homodimer than did WT THRA1 and did not dissociate from LAP after T3 treatment. Both THRA1 and THRA1LBD formed heterodimers with RXRA on DR4 and LAP. (f) On DR4, the THRBLBD lost the ability to form a homodimer compared with that of WT THRB2. (h) In contrast to WT THRB2, THRB2LBD partially dissociated from the LAP element after T3 treatment. THRB2 and THRB2LBD showed similar abilities to form heterodimers with RXRA on both DNA elements. Samples were treated with vehicle or increasing concentrations of T3 (1 nM, 10 nM, and 100 nM). *Nonspecific band observed in unprogrammed lysate.
When the THRB LBD was exchanged for THRA1 LBD, THRB2LBD lost its ability to homodimerize on DR4 [Fig. 4(c–f)]; additionally, THRB2LBD homodimer on LAP dissociated to some extent from the LAP element after T3 treatment, unlike WT THRB2 [Fig. 4(h)]. These data suggested that the LBD of THRB is one of the domains that defines the strength of its homodimerization on DR4 and LAP elements.
To narrow down the area within the LBD that is responsible for the differences in homodimerization, we designed chimeric THRΑ1 and THRΒ2 in which helices 10 to 12 were exchanged [THRA1H10-12 and THRB2H10-12; Fig. 3(b) and Fig. 5(a–d)]. These chimeric receptors showed similar properties as THRA1LBD and THRB2LBD. THRA1H10-12 did not form a homodimer on DR4, but its homodimer on LAP in the presence of T3 was more stable [Fig. 5(g)]. THRB2H10-12 lost its ability to form a homodimer on DR4 [Fig. 5(f)] and its homodimer on LAP in the presence of T3 was less stable [Fig. 5(h)]. Both chimeric proteins were able to form heterodimers with RXR on DR4 and LAP to an extent comparable to WT proteins. Thus, our data indicate that the amino acid differences within helices 10 to 12 of the THR LBDs are responsible for differences in homodimerization. Although the work by Ribeiro et al. (6) using artificial mutations shows that helices 10 and 11 are involved in THR homodimerization and heterodimerization, our work indicates that the amino acid variations between THRA and THRB LBDs are also responsible for structural and functional differences between isoforms.
Figure 5.
Exchange of helices 10 to 12 in THRA1 and THRB2 affects receptor binding to DR4 and LAP. (a and c) Schematic structures and (b and d) protein levels of in vitro–translated THRA1, THRA1H10-12, THRB2, and THRB2H10-12 are shown. pSP72 was used as an “empty” vector negative control. (e) Similar to Fig. 4, neither THRA1 nor THRA1H10-12 formed a homodimer on DR4 in EMSA. (g) LAP THRA1H10-12 formed a homodimer that did not dissociate from LAP after T3 treatment. (f) On DR4, THRBH10-12 lost the ability to form a homodimer and (h) partially dissociated from the LAP element after T3 treatment. All tested THRs showed similar abilities to form heterodimers with RXRA on both DNA elements. Samples were treated with vehicle or increasing concentrations of T3 (1 nM, 10 nM, and 100 nM). *Nonspecific band observed in unprogrammed lysate.
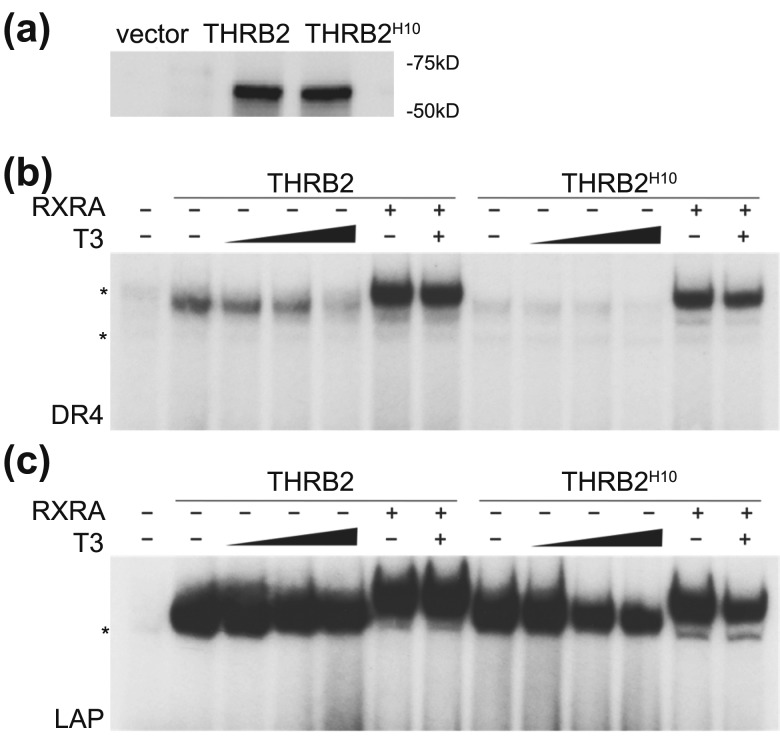
Helices 10 and 11 of THRA and THRB differ in only nine amino acids, eight of which are located in helix 10, and only three of which are nonconserved [Fig. 3(b)]. We constructed a THRB2 mutant protein where the most divergent area in helix 10 (YQDS) was converted to the sequence found in THRA1 (SQEA) (THRB2H10) [Fig. 3(b) and Fig. 6]. This small change was sufficient to abolish homodimer formation on a DR4 element, whereas heterodimer binding was unaffected [Fig. 6(b)]. Moreover, THRB2H10 dissociated to some degree from the LAP element after T3 treatment [Fig. 6(c)], which is a property found with THRA1. These data suggest that amino acid variations in helix 10 between THRA and THRB isoforms may guide isoform-specific complex formation on DNA, depending on the type of TRE element located in the promoter.
Figure 6.
The substitution of YQDS amino acid to SQEA in Thrb2H10 affects receptor homodimerization on DR4 and LAP. Three-amino-acid substitution in THRB2H10, YQDS to SQEA affects receptor homodimerization on DR4 and LAP. (a) Western blot showed equal amounts of in vitro–translated WT THRB2 and THRB2H10. (b) EMSA of THRB2 and THRB2H10 showed that similar to THRB2LBD and THRB2H10-12, THRB2H10 was unable to form a homodimer on a DR4 element, whereas heterodimer binding with RXR was not affected. (c) Similarly, THRB2H10 dissociated to some degree from the LAP element after T3 treatment. Samples were treated with vehicle or increasing concentrations of T3. *Nonspecific band observed in unprogrammed lysate.
Three amino acids within the H10 helix of the LBD are required for negative gene regulation in TαT.1.1 cells
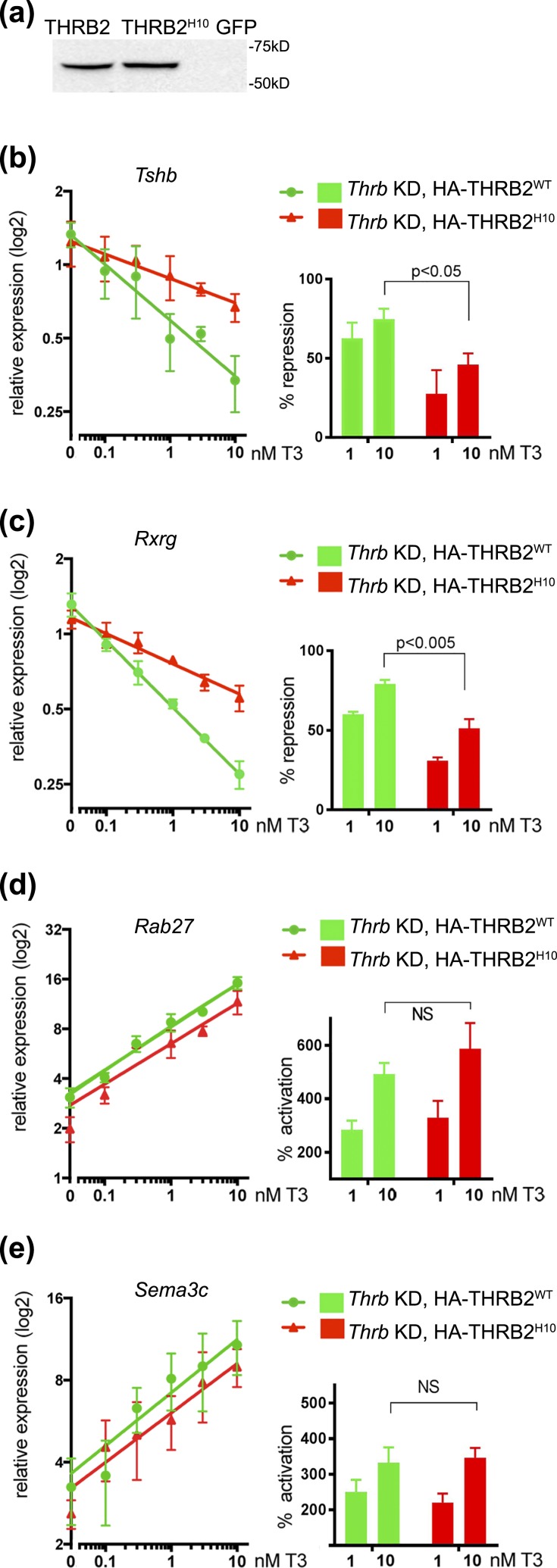
To establish whether the three amino acid change in H10 also affected the function of THRB2, we performed rescue experiments using adenovirus expressing HA-THRB2H10 as in Fig. 2. THRB2H10 and WT THRB2 were reexpressed in Thrb KD ΤαΤ1.1 cells at equal levels [Fig. 7(a)]. The cells were then exposed to different T3 concentrations, and the expression levels of negatively and positively regulated genes were measured [Fig. 7(b–e)]. Similar to the WT protein, HA-THRB2H10 was able to rescue T3-stimulated expression of Rab27 and Sema3C, increasing their expression up to 5.8-fold and 3.6-fold at 10 nM T3 [Fig. 7(d) and 7(e)], respectively. However, THRB2H10 was defective in hormone-dependent repression of Tshb and Rxrg, demonstrating 45% to 50% repression of gene expression compared with 75% to 80% that of WT, and showing no gene repression at T3 concentration <1 nM. Therefore, the THRB2H10 rescue phenotype showed similarity to that of WT THRB1 and THRA1 isoforms (Fig. 2), supporting our hypothesis that homodimerization capacity of the receptor on DR4 and LAP correlates with its function on T3 negatively regulated genes.
Figure 7.
THRB2H10 specifically affects negative regulation of gene expression. (a) Expression levels of WT THRB2 and THRB2H10 in Thrb KD TαT1.1 cells were tested by western blotting with an anti-HA antibody. Relative expression of (b and c) negatively and (d and e) positively regulated genes were measured by qRT-PCR and normalized to Actb mRNA (see Materials and Methods) and compared with controls (scrambled KD, GFP, and Thrb KD GFP, Fig. 2). Reexpression of HA-THRB2 in Thrb KD cells reconstituted (b) Tshb, and (c) Rxrg negative regulation, with the maximal percentage of repression (left panes) 75% to 80%. Expression of HA-THRB2H10 only partially rescued negative regulation of Tshb and Rxrg (45% to 50% of repression at 10 nM T3). WT THRB and THRB2H10 showed similar properties in activation of gene expression of (d) Rab 27 and (e) Sema3c. No significant differences (NS) were seen in percentage of activation (right panels) at 1 and 10 nM T3. Left panels show relative gene expression [y-axis (log2)] with increased concentration of T3 [x-axis (log10); y intersects x at 0 nM). Expression levels were normalized to those in scramled KD, GFP no T3. Data points are presented as mean ± standard error of the mean. Differences were considered to be significant at P < 0.05.
Discussion
In this work, we established that the three THR isoforms, THRA1, THRB1, and THRB2, differ in their abilities to form homodimers on DR4 and LAP DNA elements and are also functionally different when expressed in equal amounts in a mouse thyrotroph cell line (TαT.1.1). All three isoforms activated gene expression in response to increasing concentrations of T3; however, they had different abilities to repress gene expression. Expression of THRΒ2 in Thrb KD cells completely reconstituted T3-dependent repression of Tshb and Rxrg, THRB1 demonstrated reduced repressing activity, and THRA1 was unable to regulate expression of these two genes at physiological concentrations of T3 [Fig. 2(c) and 2(d)]. Furthermore, elevated expression of THRA1 in Thrb/Thra KD cells was unable to rescue T3-mediated repression of Tshb and Rxrg (Supplemental Fig. 1 (692.9KB, pdf) ), suggesting that THRA1 is “defective” in T3-dependent negative gene regulation. It remains unclear whether this applies to only a subset of negatively regulated genes expressed in thyrotrophs. T3-mediated repression occurs in tissues primarily expressing THRA1 such as brain and heart (1, 19). These tissues are not thought to express THRB2 and likely express THRB1 at low levels. Negative gene regulation in these tissues may be mediated by THRA1 partnering with tissue-specific coregulators, or THRB1 might carry out T3-mediated repression in these tissues, even though THRB1 appears less effective in negative regulation than does THRB2.
Functional differences between THRB1 and THRB2 have been previously reported and attributed to their divergent AF-1 domains (2, 8). More recently using reporter constructs, Wan et al. (20) showed that THRB2 displays an enhanced response to T3 on both positive and negative response elements. In our studies both THRB isoforms acted similar in T3-dependent activation of two endogenous genes [Rab27 and Sema3C, Fig. 2(e) and 2(f)], but THRB2 had stronger ability to mediate TH negative regulation than did THRB1 [Fig. 2(c) and 2(d)], supporting the role of the AF-1 domain in negative regulation. The differential functions of THRB1 and THRB2 agree with the differences in RTH syndromes clinically defined as generalized RTH and pituitary or central RTH (1, 16). Most mutations in THRB cause generalized RTH, which affects the HPT axis and peripheral tissues, and individuals are clinically euthyroid or hypothyroid in most tissues despite high circulating TH levels. Some mutations, however, cause central RTH, where the HPT axis is insensitive to TH but peripheral sensitivity is preserved, causing symptoms of thyrotoxicosis (7, 16, 25, 26). One such mutation (R429Q in THRB) affects arginine located in helix 11 on the dimerization surface of the LBD domain (R429 is shown green in Fig. 3), selectively blocks homodimer formation and TH-dependent negative gene regulation (7, 16). The R429Q mutation in THRB was also reported to impair the THRB2 isoform selectively (20). These findings illustrate the functional differences between THRB1 and THRB2 but also underlie the role of LBD in differential control of gene expression.
THRB1 and THRB2 contain an identical LBD domain; therefore, the LBD cannot play a direct role in the functional dissimilarity between the two isoforms. However, the amino acid variations between THRB and THRA LBDs could affect the dimerization surfaces and contribute to the differences in THR complex formation on DNA response elements. For example, THRA1 is able to bind to the 5′ flanking DNA region of Tshb only in the absence of THRB1/2, suggesting that THRA1 has lower DNA-binding affinity than THRB1/2 for the Tshb regulatory region (22). This agrees with the observation that endogenous THRA1 only represses Tshb expression in TαT.1.1 cells at very high T3 concentrations (∼100 nM) (22). To rule out that low THRA1 activity resulted from a low level of protein expression in TαT.1.1 cells, we expressed THRA1 at the same and also at higher levels than THRB1 and THRB2 in these cells. We demonstrated that compared with THRB isoforms, THRA1 was unable to mediate TH negative regulation on two endogenous genes at physiological T3 concentrations, suggesting that THRA1 is defective in negative regulation at least on certain target genes. Thus, differences in functional properties of THR isoforms, and not only their relative abundance in specific tissues, determine TH tissue effect. In part, this finding might also explain the phenotypic differences between RTHα and RTHβ syndromes. To our knowledge, this is the first direct demonstration of this finding using equivalent THR isoform expression levels and a physiological relevant cell line.
Models proposing that RTHα affects organs predominantly containing THRA1, such as brain and heart, are mostly based on mRNA studies (1, 19). However, THR mRNA levels do not always correlate with protein expression, and early studies using THR isoform-specific immunoclearance/immunoprecipitation followed by a nuclear T3 binding assay indicated that the brain and heart contained 26% and 56% THRB1, respectively (27). Such high relative expression of THRB1 in these tissues makes it highly unlikely that restricted expression of THRA1 in the brain and heart can fully explain RTHα syndrome. Similarly, RTHβ affects tissue with relatively high expression of Thrb2 mRNA, such as the brain, pituitary gland, retina, and inner ear (1, 28, 29). However, the relative distribution of THRA and THRB protein isoforms in these tissues remains unknown (30–32). Alternatively, functional differences between the isoforms could also explain these syndromes. For instance, the limited effect of THRA1 mutations on the HPT axis could be explained by defective activity of the protein on selected negative regulatory elements (e.g., Tshb).
Interestingly, the “defect” of THRA1 in negative gene regulation correlated with its decreased ability to homodimerize on DR4 and LAP. In contrast, THRB2 formed the strongest homodimer on DR4 and LAP and mediated the strongest repression of Tshb and Rxrg. The promoters of both Tshb and Rxrg lack classical DR4, DR0, or LAP motifs, suggesting that in vivo tertiary and higher order chromatin structures contribute to forming effective TRE elements. Regardless of these findings, EMSA on DR4 and LAP served as an effective method to correlate a DNA complex formation with negative gene regulation using amino acid sequence variations between THRA1 and THRB2. Homodimerization on DR4 and LAP may reflect different affinities of THR isoforms to TREs, and possibly their different abilities to interact with a partner nuclear receptor.
Although it is clear that more than one domain of THR contributes to homodimer formation as well as negative gene regulation, we focused on the LBD because it is physically involved in receptor dimerization (6). We constructed domain swaps of the LBD between WT THRs. This chimeric approach has an advantage over previous studies, which used artificial mutants of the LBD, because chimeric THR proteins should be minimally affected in their general structure and function, including ligand binding or cofactor interaction.
The first domain exchange experiment (Fig. 4) proved that the LBD from THRA in the context of THRB2 was unable to form homodimers on DR4 and formed weaker homodimers on LAP as compared with THRB2. Conversely, THRA1LBD appeared to form more stable homodimers at least on LAP elements (Fig. 4). Using EMSA, we narrowed down this homodimerization property to a small area within helix 10 of THRB (YQDS, Figs. 3 and 6). Importantly, reexpression of THRB2Η10 in Thrb KD TαT.1.1 cells demonstrated that the protein was fully functional in TH stimulation of Rab27 and Sema3c, but it was defective in TH repression of Tshb and Rxrg (Fig. 7), proving that WT THRB2 requires these three amino acids to function in negative regulation and that the corresponding amino acids in THRA1 cannot act as a substitute.
We suggest, therefore, that THRA1 contains an intrinsic defect in negative regulation. Mapping this defect to specific amino acids within the LBD domain and using chimeric design in future in vivo experiments will allow us to differentiate between relative receptor abundance and intrinsic functional differences between THR isoforms as mechanisms to explain normal TH action and complex RTH phenotypes.
Acknowledgments
We are grateful for technical assistance from K. Kalemba, and we acknowledge Drs. S. MacMillin, A. Wolfe, and S. Radovick for useful discussions.
Acknowledgments
Author contributions: F.E.W. conceived the studies. V.M.S.P. conducted EMSA experiments and analyzed the results. S.M., S.Q., and A. Sidhaye initiated, conducted, and analyzed TαT1.1 rescue experiments. V.M.S.P, S.M., A. Suhotliv, A. Sidhaye, and M.P.B. generated and analyzed constructs for EMSA and rescue experiments. S.M., V.M.S.P., and F.E.W. wrote the manuscript.
This work was supported by National Institutes of Health Grant R01-DK49126 (to F.E.W.) and by the Science Without Borders Program, CNPq (to V.M.S.P.).
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| HA | YPYDVPDYA | 3F10 | Sigma Aldrich 11058700 | Rat; monoclonal | 1:1000 | AB_2314622 |
Abbreviation: RRID, Research Resource Identifier.
Footnotes
- AF
- activation function
- cDNA
- complementary DNA
- DBD
- DNA-binding domain
- DR4
- direct repeat separated by 4 bp
- EMSA
- electrophoretic mobility shift assay
- GFP
- green fluorescent protein
- HA
- hemagglutinin
- HPT
- hypothalamic–pituitary–thyroid
- KD
- knockdown
- LAP
- inverted palindrome
- LBD
- ligand-binding domain
- mRNA
- messenger RNA
- PAL
- palindrome without spacing
- PCR
- polymerase chain reaction
- qRT-PCR
- quantitative reverse transcription polymerase chain reaction
- RTH
- resistance to thyroid hormone
- RTHα
- resistance to thyroid hormone due to thyroid hormone receptor α mutations
- RTHβ
- resistance to thyroid hormone due to thyroid hormone receptor β mutations
- RXR
- retinoic X receptor
- RXRA
- retinoic X receptor α
- shRNA
- short hairpin RNA
- T3
- triiodothyronine
- TH
- thyroid hormone
- THR
- thyroid hormone receptor
- THRA
- thyroid hormone receptor α
- THRA1LBD
- thyroid hormone receptor α 1 carrying thyroid hormone receptor β LBD
- THRA1H10-12
- thyroid hormone receptor α 1 in which helices 10 to 12 were exchanged
- THRB
- thyroid hormone receptor β
- THRB2H10
- thyroid hormone receptor β 2 mutant protein where the most divergent area in helix 10 (YQDS) was converted to the sequence found in thyroid hormone receptor α 1 (SQEA)
- THRB2H10-12
- thyroid hormone receptor β 2 in which helices 10 to 12 were exchanged
- TNT
- transcription/translation reticulocyte lysate system
- TRE
- thyroid hormone response element
- WT
- wild-type.
References
- 1.Ortiga-Carvalho TM, Sidhaye AR, Wondisford FE. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol. 2014;10(10):582–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollenberg AN, Monden T, Wondisford FE. Ligand-independent and -dependent functions of thyroid hormone receptor isoforms depend upon their distinct amino termini. J Biol Chem. 1995;270(24):14274–14280. [DOI] [PubMed] [Google Scholar]
- 3.Tian H, Mahajan MA, Wong CT, Habeos I, Samuels HH. The N-terminal A/B domain of the thyroid hormone receptor-β2 isoform influences ligand-dependent recruitment of coactivators to the ligand-binding domain. Mol Endocrinol. 2006;20(9):2036–2051. [DOI] [PubMed] [Google Scholar]
- 4.Shibusawa N, Hollenberg AN, Wondisford FE. Thyroid hormone receptor DNA binding is required for both positive and negative gene regulation. J Biol Chem. 2003;278(2):732–738. [DOI] [PubMed] [Google Scholar]
- 5.Nagaya T, Jameson JL. Thyroid hormone receptor dimerization is required for dominant negative inhibition by mutations that cause thyroid hormone resistance. J Biol Chem. 1993;268(21):15766–15771. [PubMed] [Google Scholar]
- 6.Ribeiro RC, Feng W, Wagner RL, Costa CH, Pereira AC, Apriletti JW, Fletterick RJ, Baxter JD. Definition of the surface in the thyroid hormone receptor ligand binding domain for association as homodimers and heterodimers with retinoid X receptor. J Biol Chem. 2001;276(18):14987–14995. [DOI] [PubMed] [Google Scholar]
- 7.Flynn TR, Hollenberg AN, Cohen O, Menke JB, Usala SJ, Tollin S, Hegarty MK, Wondisford FE. A novel C-terminal domain in the thyroid hormone receptor selectively mediates thyroid hormone inhibition. J Biol Chem. 1994;269(52):32713–32716. [PubMed] [Google Scholar]
- 8.Oberste-Berghaus C, Zanger K, Hashimoto K, Cohen RN, Hollenberg AN, Wondisford FE. Thyroid hormone-independent interaction between the thyroid hormone receptor β2 amino terminus and coactivators. J Biol Chem. 2000;275(3):1787–1792. [DOI] [PubMed] [Google Scholar]
- 9.Nascimento AS, Dias SM, Nunes FM, Aparício R, Ambrosio AL, Bleicher L, Figueira AC, Santos MA, de Oliveira Neto M, Fischer H, Togashi M, Craievich AF, Garratt RC, Baxter JD, Webb P, Polikarpov I. Structural rearrangements in the thyroid hormone receptor hinge domain and their putative role in the receptor function. J Mol Biol. 2006;360(3):586–598. [DOI] [PubMed] [Google Scholar]
- 10.Brent GA, Williams GR, Harney JW, Forman BM, Samuels HH, Moore DD, Larsen PR. Capacity for cooperative binding of thyroid hormone (T3) receptor dimers defines wild type T3 response elements. Mol Endocrinol. 1992;6(4):502–514. [DOI] [PubMed] [Google Scholar]
- 11.Lazar MA, Berrodin TJ, Harding HP. Differential DNA binding by monomeric, homodimeric, and potentially heteromeric forms of the thyroid hormone receptor. Mol Cell Biol. 1991;11(10):5005–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forman BM, Casanova J, Raaka BM, Ghysdael J, Samuels HH. Half-site spacing and orientation determines whether thyroid hormone and retinoic acid receptors and related factors bind to DNA response elements as monomers, homodimers, or heterodimers. Mol Endocrinol. 1992;6(3):429–442. [DOI] [PubMed] [Google Scholar]
- 13.Umesono K, Murakami KK, Thompson CC, Evans RM. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991;65(7):1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramadoss P, Abraham BJ, Tsai L, Zhou Y, Costa-e-Sousa RH, Ye F, Bilban M, Zhao K, Hollenberg AN. Novel mechanism of positive versus negative regulation by thyroid hormone receptor β1 (TRβ1) identified by genome-wide profiling of binding sites in mouse liver. J Biol Chem. 2014;289(3):1313–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velasco LF, Togashi M, Walfish PG, Pessanha RP, Moura FN, Barra GB, Nguyen P, Rebong R, Yuan C, Simeoni LA, Ribeiro RC, Baxter JD, Webb P, Neves FA. Thyroid hormone response element organization dictates the composition of active receptor. J Biol Chem. 2007;282(17):12458–12466. [DOI] [PubMed] [Google Scholar]
- 16.Machado DS, Sabet A, Santiago LA, Sidhaye AR, Chiamolera MI, Ortiga-Carvalho TM, Wondisford FE. A thyroid hormone receptor mutation that dissociates thyroid hormone regulation of gene expression in vivo. Proc Natl Acad Sci USA. 2009;106(23):9441–9446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodenner DL, Mroczynski MA, Weintraub BD, Radovick S, Wondisford FE. A detailed functional and structural analysis of a major thyroid hormone inhibitory element in the human thyrotropin beta-subunit gene. J Biol Chem. 1991;266(32):21666–21673. [PubMed] [Google Scholar]
- 18.Hollenberg AN, Monden T, Flynn TR, Boers ME, Cohen O, Wondisford FE. The human thyrotropin-releasing hormone gene is regulated by thyroid hormone through two distinct classes of negative thyroid hormone response elements. Mol Endocrinol. 1995;9(5):540–550. [DOI] [PubMed] [Google Scholar]
- 19.Moran C, Chatterjee K. Resistance to thyroid hormone due to defective thyroid receptor alpha. Best Pract Res Clin Endocrinol Metab. 2015;29(4):647–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wan W, Farboud B, Privalsky ML. Pituitary resistance to thyroid hormone syndrome is associated with T3 receptor mutants that selectively impair β2 isoform function. Mol Endocrinol. 2005;19(6):1529–1542. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Young BM, Wan W, Chan IH, Privalsky ML. A mechanism for pituitary-resistance to thyroid hormone (PRTH) syndrome: a loss in cooperative coactivator contacts by thyroid hormone receptor (TR)β2. Mol Endocrinol. 2011;25(7):1111–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiamolera MI, Sidhaye AR, Matsumoto S, He Q, Hashimoto K, Ortiga-Carvalho TM, Wondisford FE. Fundamentally distinct roles of thyroid hormone receptor isoforms in a thyrotroph cell line are due to differential DNA binding. Mol Endocrinol. 2012;26(6):926–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yen PM, Darling DS, Carter RL, Forgione M, Umeda PK, Chin WW. Triiodothyronine (T3) decreases binding to DNA by T3-receptor homodimers but not receptor-auxiliary protein heterodimers. J Biol Chem. 1992;267(6):3565–3568. [PubMed] [Google Scholar]
- 24.Wagner RL, Huber BR, Shiau AK, Kelly A, Cunha Lima ST, Scanlan TS, Apriletti JW, Baxter JD, West BL, Fletterick RJ. Hormone selectivity in thyroid hormone receptors. Mol Endocrinol. 2001;15(3):398–410. [DOI] [PubMed] [Google Scholar]
- 25.Safer JD, Langlois MF, Cohen R, Monden T, John-Hope D, Madura J, Hollenberg AN, Wondisford FE. Isoform variable action among thyroid hormone receptor mutants provides insight into pituitary resistance to thyroid hormone. Mol Endocrinol. 1997;11(1):16–26. [DOI] [PubMed] [Google Scholar]
- 26.Menzaghi C, Balsamo A, Di Paola R, Gallone G, Rossi C, Tassi V, Fonzo D, De Filippis V. Association between an R338L mutation in the thyroid hormone receptor-β gene and thyrotoxic features in two unrelated kindreds with resistance to thyroid hormone. Thyroid. 1999;9(1):1–6. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz HL, Strait KA, Ling NC, Oppenheimer JH. Quantitation of rat tissue thyroid hormone binding receptor isoforms by immunoprecipitation of nuclear triiodothyronine binding capacity. J Biol Chem. 1992;267(17):11794–11799. [PubMed] [Google Scholar]
- 28.Bradley DJ, Towle HC, Young WS III. Spatial and temporal expression of alpha- and beta-thyroid hormone receptor mRNAs, including the beta 2-subtype, in the developing mammalian nervous system. J Neurosci. 1992;12(6):2288–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley DJ, Towle HC, Young WS III. Alpha and beta thyroid hormone receptor (TR) gene expression during auditory neurogenesis: evidence for TR isoform-specific transcriptional regulation in vivo. Proc Natl Acad Sci USA. 1994;91(2):439–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iskaros J, Pickard M, Evans I, Sinha A, Hardiman P, Ekins R. Thyroid hormone receptor gene expression in first trimester human fetal brain. J Clin Endocrinol Metab. 2000;85(7):2620–2623. [DOI] [PubMed] [Google Scholar]
- 31.Wallis K, Dudazy S, van Hogerlinden M, Nordström K, Mittag J, Vennström B. The thyroid hormone receptor α1 protein is expressed in embryonic postmitotic neurons and persists in most adult neurons. Mol Endocrinol. 2010;24(10):1904–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alkemade A. Thyroid hormone and the developing hypothalamus. Front Neuroanat. 2015;9:15. [DOI] [PMC free article] [PubMed] [Google Scholar]