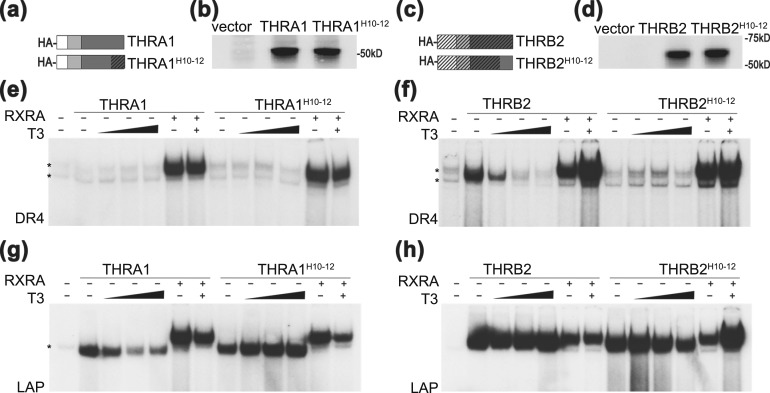
Figure 5.
Exchange of helices 10 to 12 in THRA1 and THRB2 affects receptor binding to DR4 and LAP. (a and c) Schematic structures and (b and d) protein levels of in vitro–translated THRA1, THRA1H10-12, THRB2, and THRB2H10-12 are shown. pSP72 was used as an “empty” vector negative control. (e) Similar to Fig. 4, neither THRA1 nor THRA1H10-12 formed a homodimer on DR4 in EMSA. (g) LAP THRA1H10-12 formed a homodimer that did not dissociate from LAP after T3 treatment. (f) On DR4, THRBH10-12 lost the ability to form a homodimer and (h) partially dissociated from the LAP element after T3 treatment. All tested THRs showed similar abilities to form heterodimers with RXRA on both DNA elements. Samples were treated with vehicle or increasing concentrations of T3 (1 nM, 10 nM, and 100 nM). *Nonspecific band observed in unprogrammed lysate.