Abstract
Vasomotor symptoms (VMS; or hot flashes) plague millions of reproductive-aged men and women who have natural or iatrogenic loss of sex steroid production. Many affected individuals are left without treatment options because of contraindications to hormone replacement therapy and the lack of equally effective nonhormonal alternatives. Moreover, development of safer, more effective therapies has been stymied by the lack of an animal model that recapitulates the hot-flash phenomenon and enables direct testing of hypotheses regarding the pathophysiology underlying hot flashes. To address these problems, we developed a murine model for hot flashes and a comprehensive method for measuring autonomic and behavioral thermoregulation in mice. We designed and constructed an instrument called a thermocline that produces a thermal gradient along which mice behaviorally adapt to a thermal challenge to their core body temperature set point while their thermal preference over time is tracked and recorded. We tested and validated this murine model for VMS by administration of a TRPV1 agonist and a neurokinin B receptor agonist, capsaicin and senktide, respectively, to unrestrained mice and observed their autonomic and behavioral responses. Following both treatments, the mice exhibited a VMS-like response characterized by a drop in core body temperature and cold-seeking behavior on the thermocline. Senktide also caused a rise in tail skin temperature and increased Fos expression in the median preoptic area, a hypothalamic temperature control center. This dynamic model may be used to fully explore the cellular and molecular bases for VMS and to develop and test new therapeutic options.
Senktide administered to male and female mice on a thermal gradient induced autonomic and behavioral thermoregulation that recapitulated vasomotor symptoms and behavior observed during hot flashes.
Hot flashes are characterized by a constellation of vasomotor symptoms (VMS), which are characterized by intermittent episodes of flushing, perspiration, and tachycardia in response to highly variable or declining sex steroid levels. Moderate to severe VMS adversely affect quality of life and economic productivity. In the United States, most women who experience natural or surgical menopause are plagued with VMS, which often persist for many years (1–3). Men who are treated with inhibitors of testosterone production (e.g., for prostate cancer) also experience VMS (4, 5). Unfortunately, the underlying physiological mechanisms that drive VMS are poorly understood. However, the occurrence of VMS in both men and women suggests that the phenomenon is produced by a conserved pathophysiological mechanism that links the reproductive and thermoregulatory control systems in the brain.
Recent evidence suggests that VMS are driven by so-called KNDy neurons in the arcuate nucleus of the hypothalamus (6). KNDy neurons produce three neuropeptides: kisspeptin (Kiss1), neurokinin B (NKB), and dynorphin—hence the acronym, KNDy. These neurons are considered the central processors of reproduction, and their pulsatile activity drives the episodic secretion of gonadotropin-releasing hormone (GnRH) (7, 8). KNDy neurons sense circulating levels of sex steroids and modulate the activity of GnRH neurons, thereby governing the negative feedback control of gonadotropin [luteinizing hormone (LH) and follicle-stimulating hormone] secretion (9, 10). When circulating levels of sex steroids decline, KNDy neurons compensate by increasing their activity, amplifying gonadotropin secretion and thereby increasing sex steroid production.
In 1979, scientists observed that VMS often occur coincidentally with LH pulses (11–14); however, at the time, there was no clear physiological explanation for the phenomenon. One of the cotransmitters of KNDy neurons, NKB, can activate warm-sensing neurons (WSNs) in the median preoptic area (MnPOA) and trigger heat dissipation in rats (15). Thus, it is conceivable that following an acute decline in circulating levels of sex steroids, the increased production of NKB by hyperactivated KNDy neurons creates a state of transient thermal instability, which manifests as VMS. This event occurs in parallel with acute Kiss1-dependent activation of GnRH neurons and thus creates an LH pulse. Neuronal tracing studies have identified projections from KNDy neurons to the MnPOA (16, 17); however, there is no evidence of a direct functional connection between KNDy neurons and WSNs in the MnPOA. Although indirect evidence supports the prevailing hypothesis of VMS generation (i.e., that KNDy neurons drive the event), direct evidence has been more elusive; hence, the hypothesis remains unproven.
Several animal models have been used to interrogate the physiological basis of the VMS phenomenon following either gonadectomy or pharmacologically induced sex steroid suppression (18–24). However, the results and conclusions of these investigations have been confounded by inconsistencies in study design, age, sex, time of day, and ambient temperature. In humans, VMS are characterized by the perception of an inappropriate elevation in core body temperature (CBT), resulting in adaptive measures (e.g., vasodilating, disrobing, fanning). Mice and other small mammals also thermoregulate using a variety of adaptive measures, but behavioral adaption is the dominant strategy (25–27). For example, when mice perceive they are too hot, they move to a cooler location. We used this behavioral adaption phenomenon to develop a murine model for VMS that would approximate behaviors that closely mimic those of affected humans.
We had several experimental objectives. The first was to design a thermal gradient platform and validate its ability to reveal an animal’s changing thermal preference in response to a thermal challenge. To validate the instrument, we used capsaicin to activate TRPV1 channels to trigger a sensation of warmth and induce an appropriate behavioral response (i.e., moving to a cooler region of the thermal gradient). Second, to explore the putative role of NKB signaling in triggering VMS, we challenged mice with an NKB agonist, senktide, and assessed their thermoregulatory responses, including temperature preference on the thermocline, CBT, tail skin temperature (TST), and Fos induction in the MnPOA. We report that the response to senktide recapitulated many of the autonomic and behavioral adaptations that are characteristic of VMS.
Materials and Methods
Animals
All procedures were approved by the Institutional Animal Care and Use Committee of the University of Washington in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals. Male and female mice were housed in a temperature-controlled room (21°C to 23°C) with a 12-hour light:12-hour dark cycle with lights on at 0600 am. Housing was under specific pathogen-free conditions, and animals were provided with ad libitum access to water and chow. All mice were bred in our Kiss1-Cre-GFP transgenic colony and were wild-type littermates of mice on an F8 generation C57BL/6J background, which do not express the gene for the Cre-GFP protein. Mice born in-house were used because C57BL/6J mice shipped from Jackson Laboratories proved to be more difficult to habituate to handling and the thermocline following the stress of shipment. Heterozygous (Kiss1+/Cre) Cre-GFP−expressing mice were used only for examining Fos induction following senktide administration. Men and women experience VMS following steroid withdrawal. Therefore, we used both male (M) and female (F) mice to model the human condition.
Pain assessment following administration of various treatment doses in the mice was based on guidelines provided by our institution’s veterinary staff. Per recommendation by veterinary staff, mice were monitored for the following signs: licking/biting/scratching the injection area for more than 15 minutes, lethargy, tucked or hunched posture, failure to groom, eye discharge, poor responsiveness to stimuli, and failure to show normal patterns of inquisitiveness or alertness. None of these signs of pain was observed at the dosages of capsaicin or senktide evaluated.
Thermocline design and protocol for use
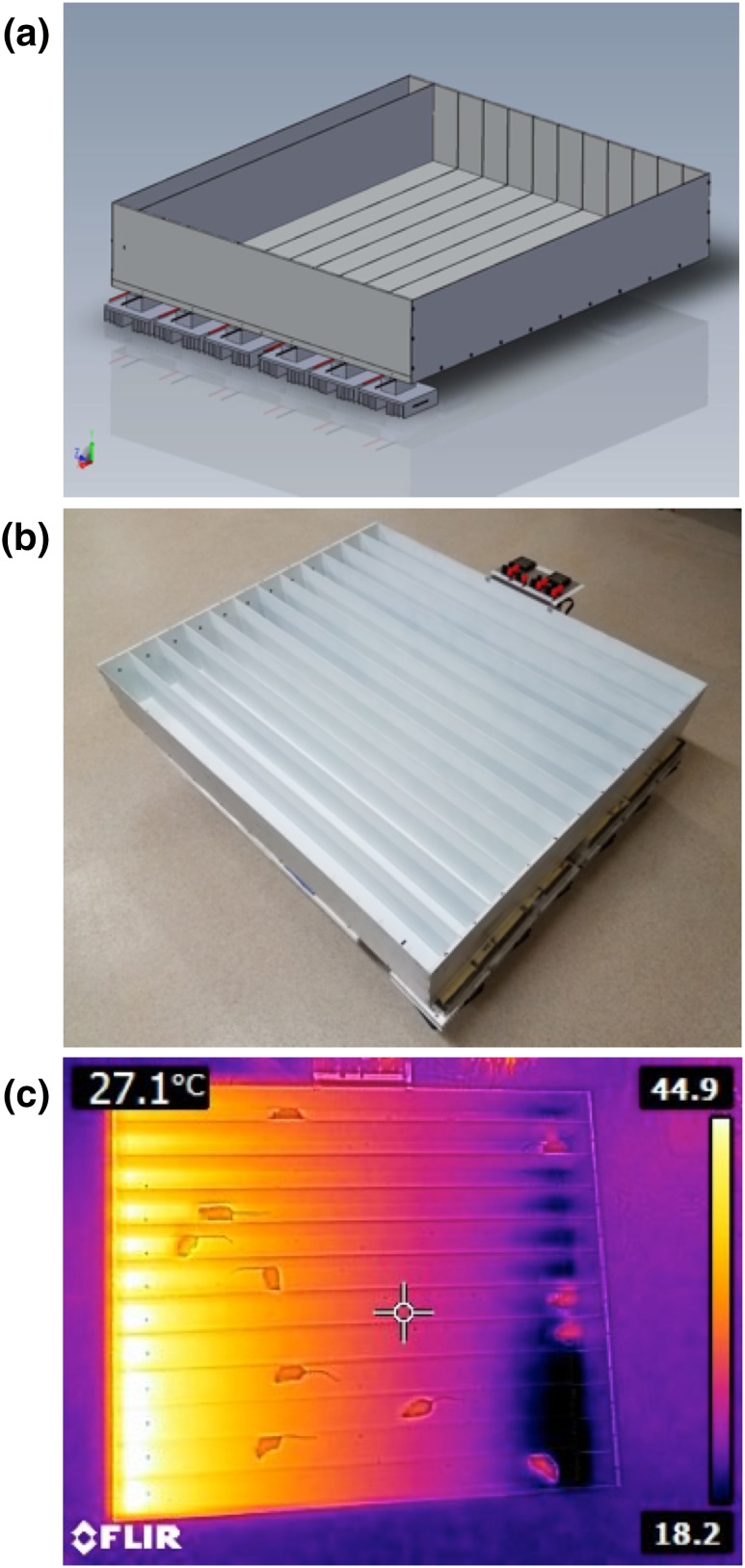
There are four components of the thermocline system (Fig. 1; patent pending): the thermocline test chamber, video camera, video streaming and recording software, and position-tracking software. The thermocline test chamber is an open-topped aluminum box (100 × 85 × 14 cm), divided into lengthwise subsections or “lanes.” The base is a ½-in-thick piece of aluminum in which a thermal gradient is generated across its length, with one end cool and the opposite end warm, ranging from 10°C to 45°C. The warm end is maintained by cartridge heaters (12v 30W) implanted in the aluminum. The cool end is maintained by solid-state refrigeration components called Peltier junctions. A Peltier junction contains semiconductor material that maintains a voltage-controlled thermal differential between its two sides. The cool side is placed against the base of the thermocline, and the opposite side has heat removed by forced air−cooled heat sinks. Keeping the hot side close to room temperature (RT) allows the cool side to drop below RT. Lane temperature is monitored via thermocouples implanted into the aluminum base. The thermocouples are connected to control circuitry, which provides a user-selectable temperature range and over-limit alarm outputs. Topping the chamber is an optically clear piece of Plexiglas that creates a consistent internal ambient temperature and allows video tracking of animals.
Figure 1.
Thermocline design. (a) Computer-generated schematic of the thermocline showing the Peltier junctions with heat sinks and slots for lane dividers. (b) Photograph of the constructed thermocline showing the control panel and twelve divided lanes, including markings to aid habituation of the mice to the temperature gradient. (c) Thermal camera−generated image of the functioning thermocline with freely behaving mice. The numbers on the right reflect the temperature range represented in the image. The number in the upper left corner reflects the temperature at the crosshairs.
When the thermocline is turned on, it creates a thermal gradient across the aluminum baseplate. Mice are placed into each lane and can freely move to the location of their choice. The position of the mouse is tracked with a USB video camera (Vimicro USB 2.0 PC Camera; Logitech HD Pro Webcam C920) mounted above the thermocline test chamber. Using VLC software (open-source software available at https://www.videolan.org/vlc), we can record a video that is streamed live to a remote location for monitoring. Positional data are analyzed with EthoVision video tracking software. The position of each animal along the thermal gradient is tracked and recorded every second. From the positional data, we can determine how each intervention influences the animal’s thermal preference along the thermocline.
Capsaicin dosing and CBT monitoring
SubCue-Mini Dataloggers (Canadian Analytical Technologies Inc., Calgary, AB, Canada) were initialized before insertion and programmed for data recording during the time window of interest. Mice (n = 30, 15 M/15 F) were anesthetized under isoflurane (3%). Dataloggers were surgically implanted via a paralumbar abdominal incision. After surgery, animals were given buprenorphine SR (0.5 mg/kg) as an analgesic and were allowed to recover for 1 week.
Capsaicin samples (M2028; Sigma Aldrich, St. Louis, MO) were prepared as a serial dilution [100 mM initial stock solution in dimethyl sulfoxide (DMSO)] with saline (0.9% sodium chloride) in dose amounts of 1.5, 1, 0.5, 0.25, and 0 mg/kg (vehicle, saline), all maintaining a constant dosing volume of 5 μL/g animal mass.
One week after surgery, the mice were habituated to injections by receiving a subcutaneous flank injection of saline once daily for 4 days. On the fifth day, the mice were divided into five groups (n = 5 or 6, with 2 or 3 M and 3 F), and mice in each group received one subcutaneous flank injection of vehicle or one of the four different doses of capsaicin. At the conclusion of the experiment, mice were euthanized under anesthesia by cervical dislocation, and the dataloggers were extracted to retrieve CBT data.
Senktide dosing and CBT monitoring
Dataloggers were inserted into 30 mice (15 M/15 F) as described previously, and animals were allowed to recover for 1 week. Senktide (Tocris Bioscience, Minneapolis, MN) samples were prepared as a serial dilution [1 mM initial senktide stock solution in 0.9% saline with 5% DMSO volume-to-volume ratio (v/v)] with vehicle (0.9% saline) in dose amounts of 0.75, 0.50, 0.25, 0.125, and 0 mg/kg senktide (vehicle, 0.9% saline with 5% DMSO), maintaining a constant dosing volume of 5 μL/g animal mass.
Similar to the capsaicin dosing protocol, 1 week after surgery, animals received a subcutaneous flank injection of saline once per day for 4 days. On the fifth day, the animals were divided into five groups (n = 5 or 6, with 2 or 3 M and 3 F) and animals in each group received one subcutaneous flank injection of either vehicle or one of the four doses of senktide. The animals were subsequently euthanized to retrieve the dataloggers and extract CBT data.
Behavior in response to capsaicin and senktide on the thermocline with thermal gradient off
For these experiments, the thermal gradient of the thermocline was turned off. Mice (n = 18, 9 M/9 F) were placed in one of 12 thermocline lanes and habituated for 4 hours per day over 5 consecutive days. Halfway through the habituation period, the mice were picked up, scruffed, momentarily touched at the injection site, and returned to the thermocline. Investigators were not present in the room except to place the mice on the thermocline, handle them briefly, and subsequently remove them from the thermocline. During the second week, each mouse was placed on the thermocline again for 4 hours on 3 separate days. Two hours after being placed in the thermocline, the mice were removed and given a subcutaneous injection of one of three different treatments: 1 mg/kg of capsaicin with 3% DMSO, 0.5 mg/kg of senktide with 3% DMSO, or vehicle only. The mice then were returned to the thermocline for another 2 hours. The vehicle used for all cases was 0.9% sodium chloride with 3% DMSO v/v. Each mouse received all three treatments one time each over the course of the experiment. Animal position on the thermocline and movement were monitored and recorded with USB cameras and VLC software and tracked using EthoVision position-tracking software. Preferred position on the thermocline as well as total movement over the course of the experiment—and exclusively postinjection—were quantified.
Behavior in response to capsaicin and senktide on the thermocline with thermal gradient on
For these experiments, the thermal gradient of the thermocline was turned on. Mice (n = 11, 5 M/6 F) were implanted with a thermal datalogger and allowed to recover for 1 week. After recovery, they were placed in one of 12 thermocline lanes and habituated for 4 hours per day over 5 consecutive days. Halfway through the habituation period, the mice were picked up, scruffed, momentarily touched at the injection site, and returned to the thermocline. Investigators were not present in the room except to place the mice in the thermocline, handle them briefly, and subsequently remove them from the thermocline. During the second week, each mouse was placed in the thermocline again for 4 hours for 3 consecutive days. Two hours after being placed in the thermocline, the mice were removed and given a subcutaneous injection of one of three different treatments: 1 mg/kg of capsaicin with 3% DMSO, 0.5 mg/kg of senktide with 3% DMSO, or vehicle only. The mice then were returned to the thermocline for another 2 hours. The vehicle used for all cases was 0.9% sodium chloride with 3% DMSO v/v. Each mouse received all three treatments one time each over the course of the experiment. Animal position on the thermocline and movement were monitored and recorded with USB cameras and VLC software. Movement was quantified and the position translated into temperature using EthoVision position-tracking software. Following all trials, the animals were euthanized, the dataloggers were removed, and the data were processed using the SubCue software.
TST in response to senktide
Mice (n = 12, 6 M/6 F) were habituated to the thermocline daily for 2 hours per day for 5 days with the thermocline at ambient temperature (off). The week following habituation, mice were again placed on the thermocline on 2 additional days for a 2-hour period each day. After 1 hour, animals were imaged with an infrared (IR) camera (FLIR E5, FLIR Systems, Wilsonville, OR) and 5 minutes later given a subcutaneous injection of either senktide (0.5 mg/kg with 3% DMSO) or vehicle in a crossover design. They were each then IR imaged at 2.5-minute intervals for 20 minutes at a distance of 6 in. above the mouse. TST was measured from the IR image by averaging the temperature of the tail along a line starting 2 cm from the base of the tail and extending for 1 cm distally.
Fos induction in MnPOA following senktide treatment
Mice (n = 24, 12 M/12 F) were divided into two groups of 12 animals (6 M/6 F) and given a subcutaneous injection of either senktide solution (0.5 mg/kg with 3% DMSO) or vehicle (0.9% saline with 3% DMSO v/v). After 2 hours, the mice were administered a ketamine/xylazine cocktail and transcardially perfused using 1× phosphate-buffered saline (PBS) and 4% paraformaldehyde in 1× PBS. Brains were postfixed in 4% paraformaldehyde overnight at 4°C and then cryoprotected in 30% sucrose solution overnight. Brains were then frozen in Tissue-Tek® O.C.T. Compound and stored at −80°C until slicing. The MnPOA of each mouse was sliced into 30-μm sections and stored in 1× PBS at 4°C. A total of 20 to 24 sections were collected, and the section closest to 0.38 mm anterior to bregma was chosen for cell quantification.
Floating sections were immune-stained for Fos with the following protocol. Sections were washed in 1× PBS and then blocked in blocking buffer (5% normal donkey serum, 0.3% Triton-X100, in 1× PBS) at RT for 60 to 90 minutes. Sections then were left on shaker in primary antibody (1:500; catalog no. sc-52, research resource identifier:AB_2106783, in blocking buffer; Santa Cruz Biotechnology, Dallas, TX) overnight at RT. Following three washes in 1× PBS, sections were left on shaker in secondary antibody solution [1:500, donkey anti-rabbit immunoglobulin G H&L (DyLight® 488; catalog no. ab96919, research resource identifier:AB_10679362, in blocking buffer; Abcam, Cambridge, MA)] for 3 to 4 hours. Slide coverslips were mounted with ProLong Diamond Antifade Mountant (Life Technologies, Carlsbad, CA) and allowed to dry overnight. Processed slides were imaged on a Leica DMLB fluorescence microscope with a ×40 objective, and Fos-expressing neurons in the MnPOA were counted by observers who were blinded to the group assignments. Images for publication were collected using a Leica SP8X Scanning Confocal microscope with a ×40/NA 1.30 oil immersion objective.
Statistical Analysis
GraphPad Prism resources (http://www.graphpad.com/quickcalcs) were used for statistical analyses. We compared the responses of mice to treatment with the responses to vehicle as seen in the animals’ CBT, temperature/position preferences, and TST over time. On the basis of preliminary dose-response studies, we identified the time-window of responses to capsaicin and senktide as 60 and 80 minutes, respectively. These time-windows following injection were used for statistical analyses of subsequent experiments. For the studies examining CBT and behavioral responses to treatment on the thermocline, we examined each mouse’s responses over time and measured the area under the response curves. We then used a two-tailed, paired t test to determine if the mean area under the response curves for each treatment group were significantly different. This same statistical strategy was applied to the data for the TST response to senktide. To determine whether senktide induced Fos expression in the MnPOA, counts of Fos-labeled cells were compared with a two-tailed t test. We used a two-way analysis of variance (thermocline status vs treatment) to evaluate whether thermocline status (on vs off) affected postinjection movement in both capsaicin- and senktide-treated animals. All data are plotted as mean ± standard error of the mean.
Results
Decreases in CBT in response to capsaicin and senktide doses
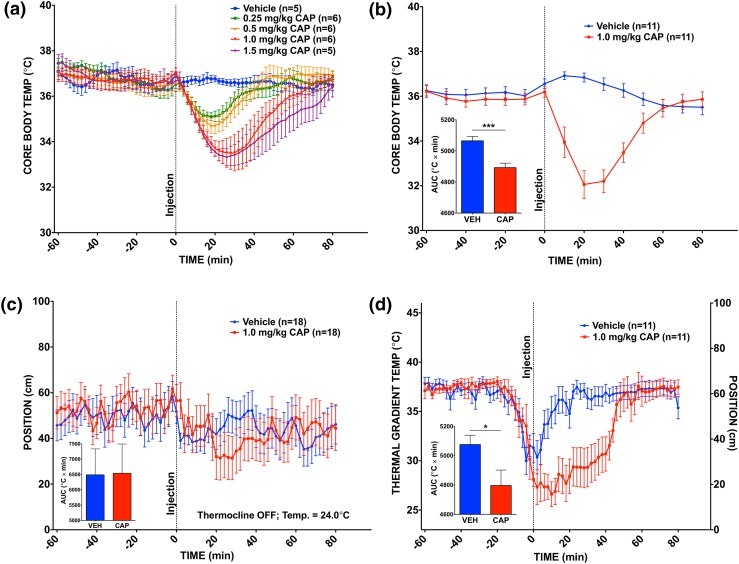
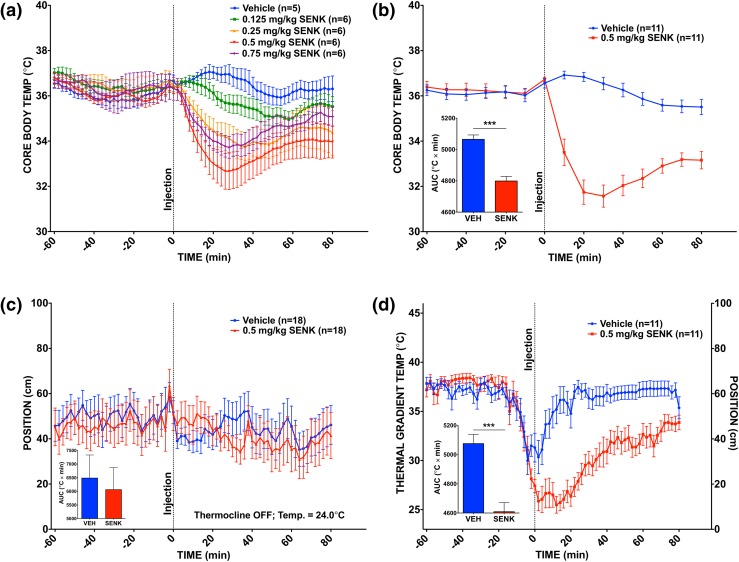
Mice injected with capsaicin and senktide exhibited dose-dependent decreases in CBT [Figs. 2(a), and 3(a)]. Vehicle-treated mice did not experience a drop in CBT. We took particular note of the response of mice given a 1.0-mg/kg dose of capsaicin or a 0.5-mg/kg dose of senktide, which provided a clear thermoregulatory response without pain (see Materials and Methods section for pain assessment details). We used these treatment doses for validation of the thermocline.
Figure 2.
CBT changes and thermocline behavior in response to capsaicin. (a) CBT response to capsaicin doses in cages (n = 5 or 6 per group). (b) CBT response in freely behaving mice administered 1.0 mg/kg capsaicin vs vehicle on the active thermal gradient. ***P < 0.001; paired t test of area under the curve (AUC); n = 11 per group. (c) Behavioral response to 1.0 mg/kg capsaicin vs vehicle on the inactive thermocline. P = 0.959, NS; paired t test of AUC; n = 18 per group. (d) Behavioral response to 1.0 mg/kg capsaicin vs vehicle treatment on the active thermocline. *P ≤ 0.05; paired t test of AUC; n = 11 per group. Dotted line at time point 0 reflects the time of the injection. Values are means ± standard error of the mean (SEM). CAP, capsaicin; NS, not significant; VEH, vehicle.
Figure 3.
CBT changes and thermocline behavior in response to senktide. (a) CBT response to senktide doses in cages (n = 5 or 6 per group). (b) CBT response in freely behaving mice administered 0.5 mg/kg senktide vs vehicle on the active thermal gradient. ***P < 0.001; paired t test of area under the curve (AUC); n = 11 per group. (c) Behavioral response to 0.5 mg/kg senktide vs vehicle on the inactive thermocline. P = 0.698, NS; paired t test of AUC; n = 18 per group. (d) Behavioral response to 0.5 mg/kg senktide vs vehicle treatment on the active thermocline. ***P < 0.001; paired t test of AUC; n = 11 per group. Dotted line at time point 0 reflects the time of the injection. Values are means ± standard error of the mean. NS, not significant; SENK, senktide; VEH, vehicle.
Response to capsaicin and senktide treatment when thermal gradient was active (on)
For observation of behavioral responses to capsaicin and senktide, mice implanted with dataloggers were placed in individual lanes of the thermocline. Two hours later, the mice were injected subcutaneously with 1.0 mg/kg of capsaicin, 0.5 mg/kg of senktide, or vehicle solution. The mice were then allowed to behaviorally thermoregulate undisturbed while CBT and positional data on the thermocline were recorded.
All mice showed an initial drop in thermal preference. However, the vehicle-treated mice quickly recovered and returned to their previously preferred temperature on the thermal gradient. In contrast, the capsaicin-treated mice maintained their position on the cooler end of the thermocline for nearly an hour following treatment [Fig. 2(d)]. The CBT of these mice dropped farther than that of the mice given capsaicin at ambient temperature during the dose-response study [nadir = 32.1°C ± 0.623°C vs 33.5°C ± 0.709°C; Fig. 2(b)]. Likewise, the senktide-treated mice preferred to remain at cooler temperatures for the duration of the study postinjection [Fig. 3(d)]. As seen with mice given capsaicin, the CBT of these mice also dropped farther than that of the mice given 0.5 mg/kg of senktide at ambient temperature during the dose-response study [nadir = 31.6°C ± 0.489°C vs 32.7°C ± 0.790°C; Fig. 3(b)].
Position preference in response to capsaicin and senktide treatment when thermal gradient was inactive (off)
We examined whether the apparent cold temperature preference of capsaicin- and senktide-treated mice was attributed to a “fear or stress response” to the injection—a response that induced the mice to seek a particular end of the thermocline—notwithstanding the presence of a thermal gradient. To examine this possibility, we evaluated the behavioral response of mice on the thermocline to the same treatments, but this time with the thermocline turned off (i.e., no thermal gradient present). We reasoned that if the injection itself or the treatment response caused the mice to prefer a specific end of the thermocline (independent of the thermal gradient), then we would expect the same location preference pattern to occur whether the thermal gradient was on or off. However, we found that when the thermocline temperature gradient was turned off, the mice did not demonstrate a placement preference (i.e., they moved randomly between ends of the thermocline) [Figs. 2(c) and 3(c)].
Comparison of movement following capsaicin and senktide treatment when thermal gradient was active vs inactive (on vs off)
We also examined whether the positional preferences of the capsaicin- and senktide-treated mice could be related to “impaired mobility” that compromised their ability to direct their movement elsewhere. To address this possibility, we used the EthoVision tracking software to quantify the total movements of mice on the active or inactive thermocline following treatment. (We focused on postinjection movement to avoid preinjection movement differences masking any potential effect of the treatments on movement.) We found that thermal gradient status (on vs off) had a significant effect on postinjection movement (P < 0.001; F = 44.75, df = 1; two-way analysis of variance), whereas treatment had no significant effect (P = 0.0904; F = 2.476, df = 2), with no significant interaction observed (P = 0.202; F = 1.630, df = 2) (Supplemental Fig. 1 (17.2MB, tif) ).
Increase in TST and Fos expression in the MnPOA in response to senktide
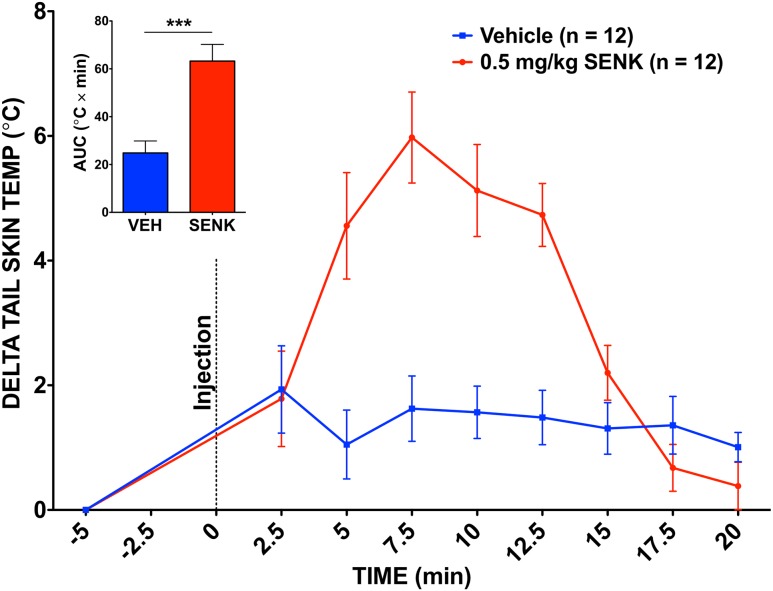
To ensure that senktide induced a physiologically normal thermoregulatory response in our animals, we also measured the TST of senktide-treated mice. Senktide-treated mice experienced a significant rise in TST compared with vehicle-treated mice (5.98°C ± 0.729°C vs 1.93°C ± 0.701°C) (Fig. 4). This rise in TST lasted about 15 minutes before subsiding, which reflects a difference in the time course of the response to senktide between the CBT and TST.
Figure 4.
TST in response to senktide. Graph shows a significant TST response to 0.5 mg/kg senktide (SENK) vs vehicle (VEH). ***P < 0.001; paired t test of area under the curve (AUC); n = 12 per group. Dotted line at time point 0 reflects the time of the injection. Values are means ± standard error of the mean.
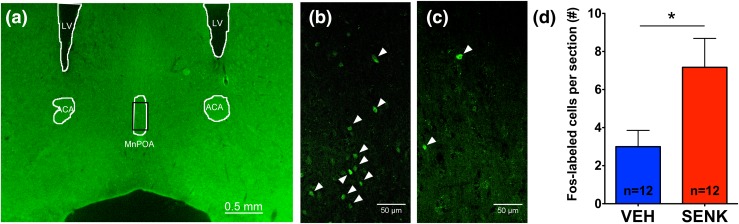
Finally, to determine whether senktide could activate neurons in the MnPOA, an important thermoregulatory region in the hypothalamus, we quantified and compared the number of Fos-expressing cells following either senktide or vehicle treatment [Fig. 5(d)]. We found that senktide treatment significantly increased the number of Fos-expressing neurons within a section of the MnPOA (P = 0.0259; two-tailed t test).
Figure 5.
Fos induction in the MnPOA in response to senktide. Senktide treatment significantly induced Fos expression in the MnPOA. (a) Representative image of the MnPOA section used for quantification, with landmarks noted for guidance. (b) Magnification (×40) image from a senktide-treated male mouse, representative of the images used for quantification. (c) Magnification (×40) image from a vehicle-treated male mouse. (b, c) White arrowheads indicate positively labeled nuclei. (d) Quantification of Fos induction by senktide treatment. *P < 0.05; two-tailed t test; n = 12 per group. Values are means ± standard error of the mean. ACA, anterior part of the anterior commissure; LV, lateral ventricle; SENK, senktide; VEH, vehicle.
Discussion
VMS are a physiological enigma that reduces the quality of life of those affected. People who experience VMS perceive an elevation in their CBT, even when little to no objective change actually occurs (28, 29); nevertheless, they respond physiologically to the actuating event that triggers the phenomenon. Scientists have struggled to devise an animal model in which to elucidate the etiology of VMS as well as test potential therapeutics. Here, we report a comprehensive approach to simultaneously and remotely measure and record physiological and behavioral adaptations by mice to a perceived thermal challenge.
Small mammals, including mice, adapt to excessive heat by seeking cooler areas that facilitate heat loss (26). We found that mice exhibited this same behavior when they were placed on a temperature gradient, a thermocline. Although thermal gradients have been used in physiological studies, our dynamic approach synergized use of a thermal gradient, constant video tracking, and measurement of various thermoregulatory responses in a convenient murine model. The thermocline gave the mice a range of ambient temperature choices and allowed recording of their adaptive movements on a second-to-second basis. As expected, when the thermal gradient was active (i.e., ON), the mice chose to sit at temperatures very close to their normal CBT (37°C). When the thermal gradient was inactive, the mice were prone to exploration, increasing their overall movement on the thermocline.
The “walled” thermocline also allowed experiments on up to 12 animals at a time while individual behavioral responses to a variety of stimuli (e.g., ambient temperature, pharmacologic agents, neural stimulation) were remotely recorded.
Once we were confident that the thermocline performed as envisioned, we addressed our second aim—to pharmacologically mimic a hot flash in mice. We induced thermoregulatory dysfunction using two compounds, capsaicin and senktide. We administered capsaicin to produce an acute sensation of hyperthermia (30, 31). We conducted the dose-response analysis of capsaicin’s effects to determine the lowest dose that would induce a thermoregulatory response without inducing any signs of pain or preventing movement, as shown by control experiments on the inactive thermocline. We observed that a dose of 1.0 mg/kg produced a significant thermoregulatory response without a coincident discernable pain response. Capsaicin caused an immediate hypothermic response that was characteristic of a mouse—or a human—experiencing VMS and lasted for about 60 minutes after injection. Capsaicin treatment caused the mice to direct their movement toward the cool end of the active thermocline, indicating their need to seek a better thermal comfort zone (i.e., cooler following the drugs) and their perception of feeling “too warm.” Such positional preferences were not observed in mice following treatment when the thermocline was turned off. This response was most likely mediated via peripherally expressed TRPV1 channels that bind capsaicin to trigger a perception of heat before the compound unbinds and can be degraded.
Although this compound enabled positive validation of the thermocline, we also wanted to test our system using senktide, a compound known to induce hypothermia and act within the central nervous system. Senktide was also chosen as a test compound because of its resemblance to NKB. Emerging evidence suggests that NKB produced by KNDy neurons is a neurochemical signal that induces menopausal VMS in women (32). Recent studies have also shown that senktide induced thermoregulatory disturbances in rats (15, 33). Senktide binds to the type 3 tachykinin receptor NK3R, which is expressed by KNDy neurons in the arcuate nucleus as well as by WSNs in the MnPOA, which may represent target neurons for the VMS phenomenon (8, 33).
We administered senktide to mice and conducted a dose-response study to determine the dose that produced a thermoregulatory response without either pain or inhibition of movement and thus gave reliable control and experimental data. We discovered that a dose of 0.5 mg/kg produced a thermoregulatory response without any signs of pain or discomfort, which is a dose previously used safely by other groups (33). Following the administration of this NKB agonist, the mice moved to a cooler region of the active thermocline, presumably to rectify their perceived elevation in CBT [Fig. 3(d)]. Again, this change in positional preferences was not observed in mice following senktide treatment when the thermocline was turned off.
It is important to note that the initial drop in thermal preference by all the mice following injection most likely reflects an initial stress response from which the vehicle-treated mice quickly recovered. Compared with capsaicin, senktide elicited a much more prolonged hypothermic response by the mice, one that lasted at least 90 minutes after injection. Also, as was seen following capsaicin treatment, the ability of the mice to move to a cooler area facilitated their thermoregulatory response to the perceived thermal threat, allowing them to more quickly and significantly drop their CBT before regaining thermoneutrality. We reason that this prolonged response—as well as a sustained perception of hyperthermia—was due to the centrally acting nature of senktide. Further studies are needed to identify senktide’s precise targets when it is given systemically. Nonetheless, our Fos results suggest that senktide induced a centrally mediated perception of heat and recruitment of heat dissipation effectors via activation of WSNs in the MnPOA.
Here, we reproduced in mice the earlier findings of hypothermia and Fos induction that occurred in response to senktide in rats (15, 33). In addition, we demonstrated that senktide caused an increase in TST in mice, demonstrating senktide’s ability to evoke tail vein vasodilation. However, the fact that the TST and behavioral responses differed significantly in their time course highlights the importance of measuring multiple readouts to derive a complete understanding of murine thermoregulatory responses. Although tail vein vasodilation may dissipate heat quickly, this method is energy sapping. Cold-seeking behavior, which rectifies temperature imbalance more slowly, requires less energy expenditure and represents a choice made by the mouse. The mouse then can choose to return to a warmer area to regain some of the heat dissipated. We assert that this willful behavioral response of the mouse may better represent how the animal is “feeling” in response to a perceived—or real—thermal threat. We deduced that the persistent choice of the senktide-treated mice to remain on the cooler end of the active thermocline reflects their sustained perception of a thermal threat (i.e., being overheated) and adds valuable information to that provided by autonomic responses alone. Overall, the effects of senktide on autonomic and behavioral thermoregulation in mice suggests that this compound can serve as a chemically induced surrogate for the VMS phenomenon and an experimental platform for testing the efficacy of drugs to block their occurrence. Indeed, new treatments are on the horizon.
At least two new potential therapies could be tested for efficacy with this technology. First, studies have shown that κ opioid receptor (KOR) agonists acting in the same manner as dynorphin can silence the KNDy neuronal activity that drives VMS (34). Moreover, KOR agonists have been engineered to be peripherally restricted [periphally restricted KOR agonists (PRKAs)], having limited access to the central nervous system. PRKAs would, in theory, obviate the side effects of nonperipherally restricted agonists (e.g., nausea, cognitive impairment, and dysphoria), yet still block VMS. Although the ideal PRKA would not cross the blood-brain barrier, it would in all likelihood inhibit the activity of KNDy neurons in the arcuate/infundibular nucleus, which resides outside the confines of the blood-brain barrier. A clinical trial conducted by our group demonstrated that a nonperipherally restricted KOR agonist can suppress the frequency of menopausal VMS (35), providing a tantalizing proof of concept for the idea.
Second, NK3R antagonists have proven efficacy in the treatment of polycystic ovarian syndrome and VMS (36). These compounds presumably work by suppressing the activity of either KNDy neurons or WSNs in the MnPOA—or both. Our results showing that senktide induced Fos expression in the MnPOA support a strategy of targeting the WSNs in the MnPOA for VMS relief. By combining neuronal stimulation on the thermocline with such treatment compounds, we could elucidate the neurobiological basis of VMS and test the efficacy of would-be blockers that could bring relief to people affected by hot flashes.
Acknowledgments
The authors thank Dr. Richard Palmiter for his work designing the transgenic mouse line used for these experiments and for his helpful guidance along the way. The authors thank Jaden Blazier, Alejandra Cabrera, and Jasmine Correa for their technical assistance with experiments and their enthusiasm. The authors are grateful to Walt Niemela for his contributions to the design and construction of the thermocline. The authors also acknowledge the W.M. Keck Center for Advanced Studies in Neural Imaging for assistance in imaging.
Financial Support: This work was supported by grants from the National Institutes of Health (RO1 HD049651 and T32 GM007108).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| FOS, human, mouse, rat | — | Rabbit anti-C-Fos polyclonal antibody, unconjugated | Santa Cruz Biotechnology, SC-52 | Rabbit; polyclonal antibody | 1:500 | AB_2106783 |
| Rabbit IgG H&L | — | Donkey anti-rabbit IgG H&L (DyLight 488) secondary antibody | Abcam, ab96919 | Donkey; polyclonal antibody | 1:500 | AB_10679362 |
Abbreviations: IgG, immunoglobulin G; RRID, research resource identifier.
Footnotes
- CBT
- core body temperature
- DMSO
- dimethyl sulfoxide
- F
- female
- IR
- infrared
- Kiss1
- kisspeptin
- KNDy
- kisspeptin, neurokinin B, and dynorphin
- KOR
- κ opioid receptor
- LH
- luteinizing hormone
- M
- male
- MnPOA
- median preoptic area
- NKB
- neurokinin B
- PBS
- phosphate-buffered saline
- PRKA
- peripherally restricted κ opioid receptor agonist
- RT
- room temperature
- TST
- tail skin temperature
- VMS
- vasomotor symptom
- v/v
- volume-to-volume ratio
- WSN
- warm-sensing neuron.
References
- 1.Avis NE, Crawford SL, McKinlay SM. Psychosocial, behavioral, and health factors related to menopause symptomatology. Womens Health. 1997;3(2):103–120. [PubMed] [Google Scholar]
- 2.Feldman BM, Voda A, Gronseth E. The prevalence of hot flash and associated variables among perimenopausal women. Res Nurs Health. 1985;8(3):261–268. [DOI] [PubMed] [Google Scholar]
- 3.Hunter MS, Gentry-Maharaj A, Ryan A, Burnell M, Lanceley A, Fraser L, Jacobs I, Menon U. Prevalence, frequency and problem rating of hot flushes persist in older postmenopausal women: impact of age, body mass index, hysterectomy, hormone therapy use, lifestyle and mood in a cross-sectional cohort study of 10,418 British women aged 54-65. BJOG. 2012;119(1):40–50. [DOI] [PubMed] [Google Scholar]
- 4.Ahmadi H, Daneshmand S. Androgen deprivation therapy: evidence-based management of side effects. BJU Int. 2013;111(4):543–548. [DOI] [PubMed] [Google Scholar]
- 5.Spetz AC, Zetterlund EL, Varenhorst E, Hammar M. Incidence and management of hot flashes in prostate cancer. J Support Oncol. 2003;1(4):263–266, 269–270, 272–273, discussion 267–268, 271–272. [PubMed] [Google Scholar]
- 6.Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34(3):211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80(4):264–272. [DOI] [PubMed] [Google Scholar]
- 8.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149(4):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Casper RF, Yen SS, Wilkes MM. Menopausal flushes: a neuroendocrine link with pulsatile luteninizing hormone secreation. Science. 1979;205(4408):823–825. [DOI] [PubMed] [Google Scholar]
- 12.DeFazio J, Meldrum DR, Laufer L, Vale W, Rivier J, Lu JK, Judd HL. Induction of hot flashes in premenopausal women treated with a long-acting GnRH agonist. J Clin Endocrinol Metab. 1983;56(3):445–448. [DOI] [PubMed] [Google Scholar]
- 13.Casper RF, SS Yen. Menopausal flushes: effect of pituitary gonadotropin desensitization by a potent luteinizing hormone-releasing factor agonist. J Clin Endocrinol Metab. 1981;53(5):1056–1058. [DOI] [PubMed] [Google Scholar]
- 14.Meldrum DR, Erlik Y, Lu JK, Judd HL. Objectively recorded hot flushes in patients with pituitary insufficiency. J Clin Endocrinol Metab. 1981;52(4):684–687. [DOI] [PubMed] [Google Scholar]
- 15.Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011;152(12):4894–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracing and monosodium glutamate lesions in the rat. Neuroscience. 2010;166(2):680–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152(6):2387–2399. [DOI] [PubMed] [Google Scholar]
- 18.Gervais NJ, Viechweg SS, Mong JA, Lacreuse A. The middle-aged ovariectomized marmoset (Callithrix jacchus) as a model of menopausal symptoms: Preliminary evidence. Neuroscience. 2016;337:1–8. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Tamura M, Hayashi M, Katsuura Y, Tanabe H, Ohta T, Komoriya K. Elevation of tail skin temperature in ovariectomized rats in relation to menopausal hot flushes. Am J Physiol Regul Integr Comp Physiol. 2000;278:R863–R869. [DOI] [PubMed] [Google Scholar]
- 20.Albertson AJ, Skinner DC. A novel animal model to study hot flashes: no effect of gonadotropin-releasing hormone. Menopause. 2009;16(5):1030–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holinka CFM, Brincat M, Coelingh Bennink HJ. Preventive effect of oral estetrol in a menopausal hot flush model. Climacteric. 2008;11(Suppl 1):15–21. [DOI] [PubMed] [Google Scholar]
- 22.Shuto H, Yamauchi A, Ikeda M, Sohda Y, Koga A, Tominaga K, Egawa T, Kataoka Y. Forced exercise-induced flushing of tail skin in ovariectomized mice, as a new experimental model of menopausal hot flushes. J Pharmacol Sci. 2005;98(3):323–326. [DOI] [PubMed] [Google Scholar]
- 23.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, McMullen NT, Rance NE. Role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons in cutaneous vasodilatation and the estrogen modulation of body temperature. Proc Natl Acad Sci USA. 2012;109(48):19846–19851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez-Alavez M, Alboni S, Conti B. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age (Dordr). 2011;33(1):89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon CJ. Behavioral and autonomic thermoregulation in mice exposed to microwave radiation. J Appl Physiol. 1983;55(4):1242–1248. [DOI] [PubMed] [Google Scholar]
- 26.Gordon CJ. Relationship between autonomic and behavioral thermoregulation in the mouse. Physiol Behav. 1985;34(5):687–690. [DOI] [PubMed] [Google Scholar]
- 27.Gaskill BN, Gordon CJ, Pajor EA, Lucas JR, Davis JK, Garner JP. Heat or insulation: behavioral titration of mouse preference for warmth or access to a nest. PLoS One. 2012;7(3):e32799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freedman RR, Norton D, Woodward S, Cornélissen G. Core body temperature and circadian rhythm of hot flashes in menopausal women. J Clin Endocrinol Metab. 1995;80(8):2354–2358. [DOI] [PubMed] [Google Scholar]
- 29.Freedman RR, Woodward S. Core body temperature during menopausal hot flushes. Fertil Steril. 1996;65(6):1141–1144. [PubMed] [Google Scholar]
- 30.Feketa VV, Zhang Y, Cao Z, Balasubramanian A, Flores CM, Player MR, Marrelli SP. Transient receptor potential melastatin 8 channel inhibition potentiates the hypothermic response to transient receptor potential vanilloid 1 activation in the conscious mouse. Crit Care Med. 2014;42(5):e355–e363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu I, Iida T, Horiuchi N, Caterina MJ. 5-Iodoresiniferatoxin evokes hypothermia in mice and is a partial transient receptor potential vanilloid 1 agonist in vitro. J Pharmacol Exp Ther. 2005;314(3):1378–1385. [DOI] [PubMed] [Google Scholar]
- 32.Jayasena CN, Comninos AN, Stefanopoulou E, Buckley A, Narayanaswamy S, Izzi-Engbeaya C, Abbara A, Ratnasabapathy R, Mogford J, Ng N, Sarang Z, Ghatei MA, Bloom SR, Hunter MS, Dhillo WS. Neurokinin B administration induces hot flushes in women. Sci Rep. 2015;5:8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mittelman-Smith MA, Krajewski-Hall SJ, McMullen NT, Rance NE. Neurokinin 3 receptor-expressing neurons in the median preoptic nucleus modulate heat-dissipation effectors in the female rat. Endocrinology. 2015;156(7):2552–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oakley AE, Steiner RA, Chavkin C, Clifton DK, Ferrara LK, Reed SD. κ Agonists as a novel therapy for menopausal hot flashes. Menopause. 2015;22(12):1328–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George JT, Kakkar R, Marshall J, Scott ML, Finkelman RD, Ho TW, Veldhuis J, Skorupskaite K, Anderson RA, McIntosh S, Webber L. Neurokinin B receptor antagonism in women with polycystic ovary syndrome: a randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4313–4321. [DOI] [PubMed] [Google Scholar]