Abstract
Loss-of-function or inactivating mutations in the genes coding for kisspeptin and its receptor (KISS1R) or neurokinin B (NKB) and the NKB receptor (NK3R) in humans result in a delay in or the absence of puberty. However, precise mechanisms of kisspeptin and NKB signaling in the regulation of the pubertal increase in gonadotropin-releasing hormone (GnRH) release in primates are unknown. In this study, we conducted a series of experiments infusing agonists and antagonists of kisspeptin and NKB into the stalk-median eminence, where GnRH, kisspeptin, and NKB neuroterminal fibers are concentrated, and measuring GnRH release in prepubertal and pubertal female rhesus monkeys. Results indicate that (1) similar to those previously reported for GnRH stimulation by the KISS1R agonist (i.e., human kisspeptin-10), the NK3R agonist senktide stimulated GnRH release in a dose-responsive manner in both prepubertal and pubertal monkeys; (2) the senktide-induced GnRH release was blocked in the presence of the KISS1R antagonist peptide 234 in pubertal but not prepubertal monkeys; and (3) the kisspeptin-induced GnRH release was blocked in the presence of the NK3R antagonist SB222200 in the pubertal but not prepubertal monkeys. These results are interpreted to mean that although, in prepubertal female monkeys, kisspeptin and NKB signaling to GnRH release is independent, in pubertal female monkeys, a reciprocal signaling mechanism between kisspeptin and NKB neurons is established. We speculate that this cooperative mechanism by the kisspeptin and NKB network underlies the pubertal increase in GnRH release in female monkeys.
Developmental changes in kisspeptin and neurokinin B signaling were investigated. The network formation between these two signaling mechanisms underlies the pubertal increase in GnRH release.
Genetic studies in humans and experimental approaches in animals indicate that kisspeptin and neurokinin B (NKB) and their respective receptors (KISS1R and NK3R) play a critical role in puberty and adult reproduction. Loss-of-function mutations in the genes coding for kisspeptin or its receptor in humans result in a delay in or the absence of puberty (1, 2), and inactivating mutations in the genes coding for NKB and its receptor in humans result in the failure of puberty initiation (3). Animal experiments in various species further suggest that kisspeptin and NKB neurons are involved in pulsatile release of gonadotropin-releasing hormone (GnRH) (4, 5). However, it is unclear whether kisspeptin and NKB signaling are responsible for the pubertal increase in GnRH release (6) or are involved in puberty as a component of GnRH pulse-generation.
Previous studies in this laboratory indicated that in female rhesus monkeys, (1) the pubertal increase in kisspeptin release occurs in parallel to the pubertal increase in GnRH release (7); (2) similar to the developmental increase in GnRH release (6, 8), the developmental increase in kisspeptin release is independent from ovarian steroids (9); and (3) GnRH neuron sensitivity to kisspeptin increases after puberty onset (10), indicating that, although an increased amount of kisspeptin signaling (9) contributes to the pubertal increase in GnRH release, kisspeptin signaling per se may not be responsible for puberty onset in female monkeys. Nevertheless, a report of a study of male monkeys showed NKB signaling is upstream of kisspeptin signaling (11), thus raising a possibility that circuitry formation between kisspeptin and NKB neurons in the hypothalamus is important for the pubertal increase in GnRH release, rather than respective contributions of kisspeptin and NKB signaling. Therefore, in the current study, we examined whether there is a hierarchy between kisspeptin and NKB signaling in regulation of GnRH release in developing female monkeys and, if so, whether the hierarchal relationship between kisspeptin and NKB signaling undergoes pubertal changes, using a microdialysis method.
Materials and Methods
Animals
A total of five prepubertal [mean age ± standard error of the mean (SEM), 17.2 ± 0.9 months old] and seven pubertal (mean age ± SEM, 35.6 ± 2.9 months old) ovarian intact female rhesus monkeys (Macaca mulatta) were used in this study. All animals were born and raised at the Wisconsin National Primate Research Center. They were housed in pairs (cages are 172 × 86 × 86 cm) with a 12-hour light/dark cycle and at a controlled temperature (22°C). They were fed a standard diet of Teklad Global Diets 20% Protein Primate Diet (Envigo, Madison, WI) twice per day and water was available ad libitum. Fresh fruit and other enrichment was provided daily.
Pubertal stages of female rhesus monkeys were defined as previously reported (12). Briefly, prepubertal stage animals were defined by no physical signs of puberty, low circulating levels of estradiol and luteinizing hormone (LH), and typically younger than 22 months. The pubertal stage was defined by elevated circulating levels of estradiol, clear nocturnal increases in LH, and physical signs of puberty (development of perineal sex-skin, larger nipple size, and vaginal bleeding, but no ovulation). The protocol was approved by the Animal Care and Use Committee, University of Wisconsin-Madison, in accordance with the National Institutes of Health and US Department of Agriculture guidelines.
Experimental design
To examine developmental changes in GnRH release and the NKB-induced changes in kisspeptin release, three series of in vivo microdialysis experiments were conducted in prepubertal and pubertal female rhesus monkeys. In experiment 1, to investigate whether NKB signaling to GnRH neurons or kisspeptin neurons undergoes developmental change in female monkeys, we examined the effects of two doses (0.1 µM and 10 µM) of the NKB agonist senktide (Sigma-Aldrich, St. Louis, MO) on GnRH release (experiment 1a) and kisspeptin release (experiment 1b) in prepubertal (n = 6) and pubertal (n = 6) female monkeys. The doses of senktide were based on a study by Ramaswamy et al. (11). Control data were obtained from vehicle infusion in the same prepubertal (n = 6) and pubertal (n = 6) animals. After at least 60-minute dialysate collections for assessment of baseline GnRH and kisspeptin levels, senktide or vehicle was infused into the stalk-median eminence (S-ME) for 20 minutes and dialysate sampling was continuously collected for an additional 80 minutes.
In experiment 2, to determine whether NKB signaling to GnRH neurons is mediated through kisspeptin neurons and, if so, whether this signaling network undergoes a developmental change, we examined the effects of senktide on GnRH release in the presence or absence of the kisspeptin antagonist peptide 234 (P234; kindly provided by Dr. Robert P. Millar, University of Edinburgh, Edinburgh, United Kingdom) in prepubertal (n = 6) and pubertal (n = 6) female monkeys. For the senktide dose, we used the lower dose (0.1 µM); the dose of P234 (0.1µM) was based on our previous study in prepubertal and pubertal female monkeys (10). Again, after the minimum 60-minute baseline collections for GnRH levels, P234 or vehicle was infused for 60 minutes; during the last 20 minutes of the P234 or vehicle infusion, senktide infusion was added while dialysates were continuously collected at 20-minute fractions.
In experiment 3, to determine whether kisspeptin signaling to GnRH neurons is mediated through NKB neurons and, if so, whether this signaling network undergoes a developmental change, we examined the effects of the kisspeptin agonist human kisspeptin-10 [hKP10; (112–121)-amide; Phoenix, Burlingame, CA] on GnRH release in the presence or absence of the NKB antagonist SB222200 (Sigma-Aldrich) in prepubertal (n = 6) and pubertal (n = 6) female monkeys. The dose of hKP10 (0.1 µM) was based on our previous study (10) and the dose of SB222200 (1 µM) was based on a study by Ramaswamy et al. (13). Again, after the minimum 60-minute baseline collections for GnRH levels, SB222200 or vehicle was infused for 60 minutes; during the last 20 minutes of the SB222200 or vehicle infusion, hKP10 infusion was added while dialysates were continuously collected at 20-minute fractions. As a control, the effects of SB222200 infusion alone were also tested. In experiments 2 and 3, we did not measure kisspeptin, because both hKP10 and P234 interfere with our kisspeptin assay.
Experiment 1 was conducted first and then experiments 2 and 3 were concurrently conducted. In a single microdialysis session, multiple drug (or vehicle) challenges were given at 3- to 4-hour intervals, but the same challenge was never repeated in a single microdialysis session.
Cranial pedestal implantation and guide cannula insertion
All animals were well adapted to the primate chair, experimental conditions, and researchers before experimentation. At least 1 month before experimentation, all animals were implanted with cranial pedestals under isoflurane anesthesia, as previously described (14, 15). On the morning of the experiment, monkeys were anesthetized with ketamine (10–15 mg/kg body weight) and dexmedetomidine (up to 3.0 g/kg body weight), placed in a stereotaxic apparatus, and fitted with a microdrive unit. A guide cannula with inner stylet was secured to the microdrive unit for precise placement of the stylet tip 5 mm above the base of the infundibular recess. The custom-made guide cannula (Harvard Apparatus, Holliston, MA), consisting of a stainless-steel shaft (76.0-mm long, 0.91-mm diameter) and a removable stainless steel stylet (96.0-mm long, 0.6-mm diameter). Radiographs were used to confirm the x, y, and z coordinates of the stylet tip. After confirming guide cannula position, the animal was removed from the stereotaxic apparatus and seated in the primate chair.
Microdialysis experiment
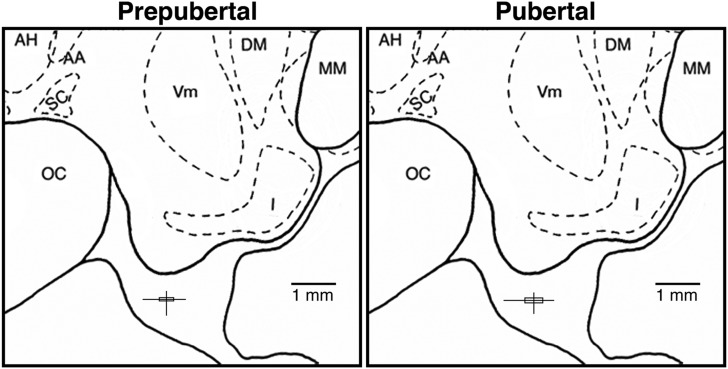
Once in the primate chair, the inner stylet was removed from the guide cannula and a microdialysis probe (stainless-steel shaft: 96.0-mm long, 0.6-mm diameter) with a polyarylethersulfone membrane (20-kDa molecular mass cutoff, 5.0-mm long, 0.5-mm diameter) was inserted into the guide cannula, such that the tip of the probe is located in the S-ME as previously described (15). The mean and range of the microdialysis probe-tip placements in each age group, assessed by the stylet-tip position, are illustrated in Fig. 1.
Figure 1.
Midsagittal diagrams of the hypothalamic S-ME showing locations of the microdialysis probe tip in prepubertal and pubertal female monkeys. The vertical and horizontal lines indicate the dorsal-ventral and anterior-posterior ranges of the sampling locations. The intersections of these lines, centering 0.5 mm below the base of the third ventricular recess, indicate the mean probe locations, and the boxes delineate the SEM. The lateral positions (from midline; mean ± SEM) were 0.4 ± 0.1 mm for the prepubertal stage and 0.6 ± 0.1 mm for the pubertal stage. Note that there is no significant difference in the distribution pattern of probe tips between the prepubertal and pubertal stages. AA, anterior hypothalamic area; AH, anterior hypothalamic nucleus; DM, dorsomedial hypothalamic nucleus; I, infundibular (arcuate) nucleus; MM, mammillary body; OC, optic chiasm; SC, suprachiasmatic nucleus; Vm, ventromedial hypothalamic nucleus.
Central nervous system perfusion fluid (artificial cerebrospinal fluid: 147 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 0.85 mM MgCl2; Harvard Apparatus) containing bacitracin (4 U/mL) was infused into the S-ME through the microdialysis probe at 2 µL/min using a 2.5-mL Hamilton syringe (Reno, NV) and a CMA/102 microdialysis pump (Harvard Apparatus). All agonists and antagonists were prepared per manufacturer’s directions and stored. On the day of experiment, they were diluted to final concentrations with artificial cerebrospinal fluid and filtered for sterility with 0.22 µm of PES filter (Millex GP, Darmstadt, Germany) before infusion. In vitro tests determined that at the same infusion rate (2 µL/min), 10% to 12% of the administered agonist or antagonist concentration diffused through the probe membrane into the S-ME (15). Dialysate collections were initiated 2 hours after probe insertion and continued for up to 10 hours. Dialysates (40 µL) were collected at 20-minute intervals with a fraction collector (model FC203B; Gilson, Middleton, WI). Samples were immediately aliquoted into two borosilicate tubes, 20 µL each, frozen on dry ice, and stored at −80°C for later assay of peptides.
During the experiment, the animals were provided monkey chow, fresh fruit, other enrichments, and water ad libitum. They were near a partner monkey for visualization and vocalization. Animals were returned to their home cage for a minimum of 3 weeks before subsequent experimentation.
Radioimmunoassay
Samples were thawed immediately before the assay. A radioimmunoassay for GnRH measurement was conducted using the R42 antiserum provided by Dr. Terry Nett (Colorado State University, Fort Collins, CO), as previously described (14). Assay sensitivity was 0.02 pg per tube. Intra- and interassay coefficients of variation were 8.3% and 10.9%, respectively. Radioimmunoassay for kisspeptin was conducted using the GQ2 antiserum provided by Dr. Stephen Bloom (Imperial College London, London, United Kingdom), as previously described (7). Assay sensitivity was 0.05 pg per tube or 1.0 pg/mL. Intra-assay and interassay coefficients of variation were 10.1% and 13.9%, respectively.
Statistical analysis
In this study, a total of five prepubertal and seven pubertal female monkeys were used. Because the three experiments were conducted successively or concurrently, most animals were used in two to three experiments. Statistical analysis was conducted with n = 6 per group in a minimum of four different animals. That is, in some experiments the data from one of the five animals had two trials or two of the four animals had two trials. The rationale for the two trials as an independent entry for statistical analysis is based on the fact that the data of each trial were obtained 2 to 5 months apart and animals are growing. That is, two trials within a stage were conducted at different developmental ages and the placement of the microdialysis probe was different for each trial. For all experiments, mean (± SEM) GnRH or kisspeptin levels were calculated. Additionally, area under the curve (AUC) after the initiation of agonist/antagonist challenge was calculated as follows: (1) In each animal, average GnRH or kisspeptin levels during the baseline period (–60 to 0 minutes) were calculated, (2) the sum of GnRH or kisspeptin levels during the 20-minute blocks up to 100 minutes after the initiation of challenge above the baseline was calculated, (3) group mean (± SEM) was calculated for each challenge, and (4) significance between doses within a developmental stage or between developmental stages (prepubertal vs pubertal) at the same dose was determined. Two-way analysis of variance with repeated measures followed by Bonferroni post hoc test were applied for statistical comparison using GraphPad Prism (San Diego, CA). Differences were considered significant at P ≤ 0.05.
Results
Developmental changes in GnRH release in response to senktide (experiment 1a)
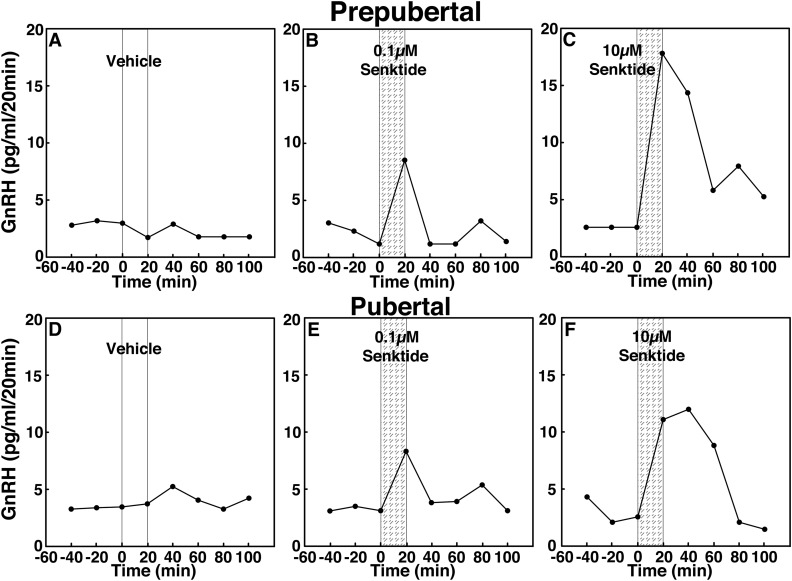
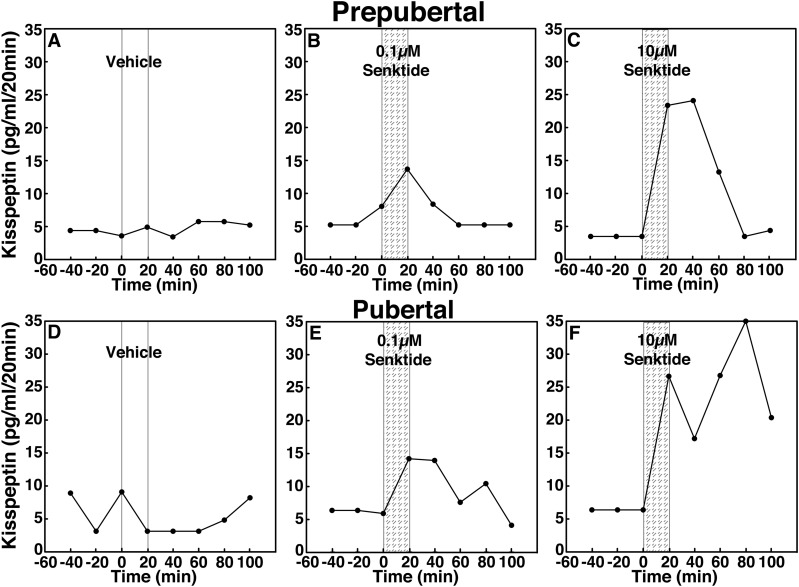
To assess the contribution of NKB signaling to GnRH release across puberty, we examined the effects of two doses of the NKB agonist senktide on GnRH release in prepubertal and pubertal female rhesus monkeys using in vivo microdialysis. In prepubertal monkeys, infusion of 0.1 and 10 µM senktide into the S-ME stimulated GnRH release in a dose-responsive manner (Fig. 2B and 2C). Similarly, in pubertal monkeys, infusion of 0.1 and 10 µM senktide stimulated GnRH release in a dose-responsive manner (Fig. 2E and 2F). Vehicle infusion did not induce any changes (Fig. 2A and 2D).
Figure 2.
Representative cases showing the effects of the NKB agonist senktide (0.1 and 10 µM), on in vivo GnRH release in (A–C) prepubertal and (D–F) pubertal female monkeys using a microdialysis method. After 60-minute baseline sampling, (A, D) vehicle, (B, E) 0.1 µM senktide, or (C, F) 10 µM senktide was infused into the S-ME for 20 minutes (vertical bars), and dialysates were continuously collected in 20-minute fractions for an additional 100 minutes. Both (B) 0.1 µM and (C) 10 µM senktide stimulated GnRH release in a dose-responsive manner in prepubertal monkeys, whereas (A) vehicle did not induce any change. Similarly, senktide at both doses [(E) 0.1 µM and (F) 10 µM] stimulated GnRH release in a dose-responsive manner in pubertal monkeys, whereas (D) vehicle did not induce any change.
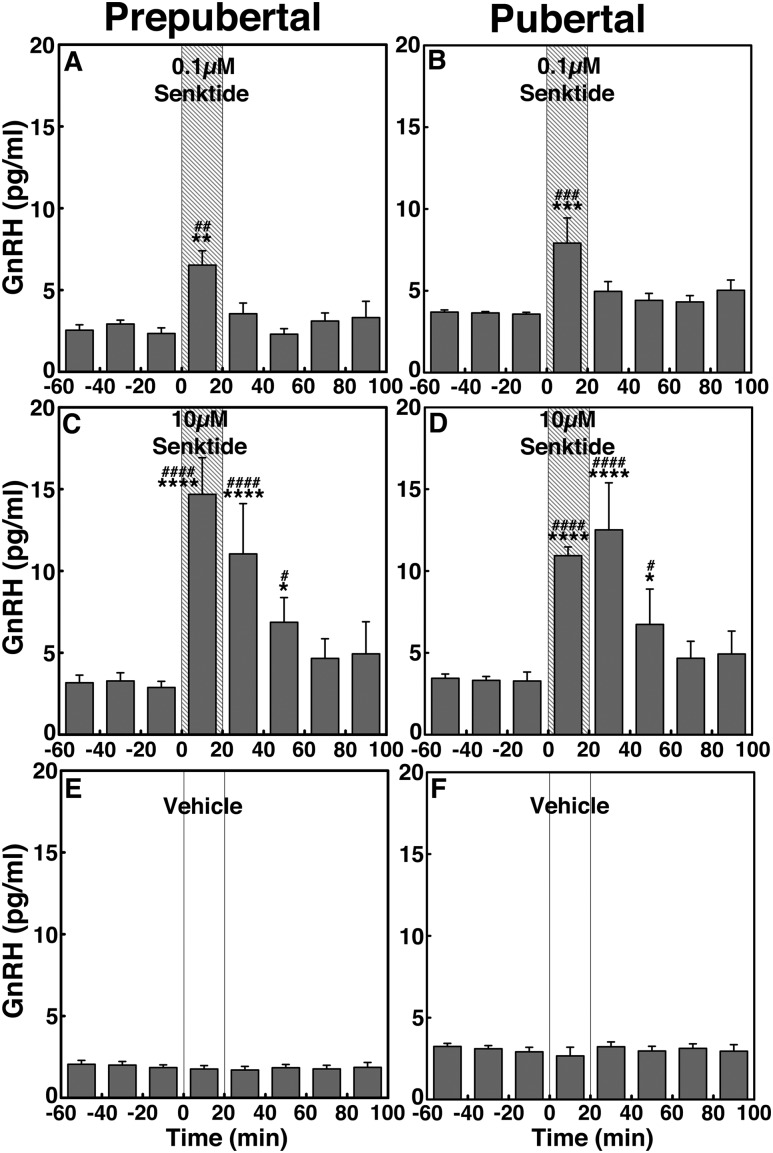
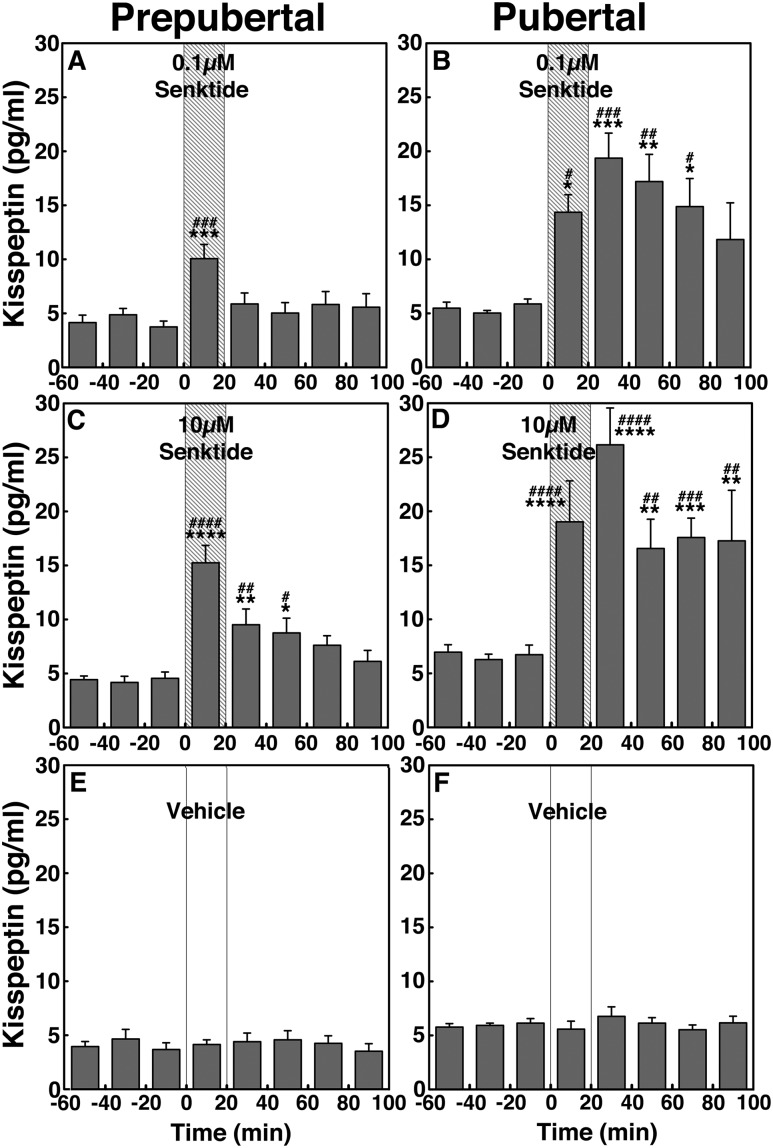
Statistical analysis indicated that senktide effects at both doses in prepubertal monkeys were significant (P < 0.01 for low-dose senktide and P < 0.0001 for high-dose senktide; Fig. 3A and 3C) when compared with vehicle infusion (Fig. 3E). Post hoc analysis indicated that the GnRH responses to senktide during the 20-minute infusion at both doses (P < 0.01 for 0.1 µM senktide and P < 0.0001 senktide for 10 µM) and the 20 to 60 minutes after the initiation of the 10-µM senktide infusion were higher (P < 0.0001 for 20- to 40-minute and P < 0.05 for 40- to 60-minute infusions) than the preinfusion levels as well as the corresponding period of vehicle control. Similarly, in pubertal female monkeys, the group data indicated that increases in GnRH release induced by senktide at both doses were significant (P < 0.001 for the low dose and P < 0.0001 for the high dose; Fig. 3B and 3D) compared with vehicle infusion (Fig. 3F). Post hoc analysis indicated that the pubertal GnRH responses to senktide during the 20-minute infusion at both doses (P < 0.001 for 0.1 µM and P < 0.0001 for 10 µM) and the 20 to 60 minutes after the initiation of the 10-µM senktide infusion were higher (P < 0.0001 for 20–40 minutes and P < 0.05 for 40–60 minutes) than the preinfusion levels as well as the corresponding period of vehicle control.
Figure 3.
Group data (mean ± SEM; n = 6 in all) showing that senktide infusion into the S-ME stimulated GnRH release in prepubertal monkeys and pubertal monkeys. Animals were treated with (A, B) 0.1 µM senktide, (C, D) 10 µM senktide, and (E, F) vehicle. In the prepubertal group, both 0.1 µM and 10 µM senktide significantly (P < 0.01 and P < 0.0001, respectively) increased GnRH release over the baseline levels and the effects of senktide at both doses were significantly larger (P < 0.01 for 0.1 µM and P < 0.0001 for 10 µM) than those in vehicle. Similarly, in pubertal monkeys, both 0.1 µM and 10 µM senktide significantly (P < 0.001 and P < 0.0001, respectively) stimulated GnRH release over the baseline levels and the effects of senktide at both doses were also significantly larger (P < 0.001 for 0.1 µM and P < 0.0001 for 1 µM) than those in vehicle. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs vehicle infusion; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs before infusion.
Additionally, calculation of the AUC for induced GnRH release indicated that senktide induced GnRH increases in a dose-responsive manner in both prepubertal (Fig. 4A) and pubertal (Fig. 4B) monkeys. Comparison between the two developmental stages indicated that the induced GnRH increase in the pubertal group was not different than that in the prepubertal group at a respective dose.
Figure 4.
Group data (mean ± SEM) showing that changes in AUC of (A, B) GnRH release induced by senktide in the presence or absence of P234, (C, D) kisspeptin release induced by senktide, and (E, F) GnRH release induced by hKP10 in the presence or absence of SB222200 in prepubertal and pubertal monkeys. ***P < 0.001, ****P < 0.0001 vs vehicle infusion; ####P < 0.0001 vs 0.1 µM senktide infusion; ++P < 0.01, ++++P < 0.0001 vs blocking experiment; aaaP < 0.001, aaaaP < 0.0001 vs prepubertal stage.
Developmental changes in kisspeptin release in response to senktide (experiment 1b)
To assess the contribution of NKB signaling to kisspeptin release across puberty, we examined the effects of senktide on kisspeptin release in prepubertal and pubertal female monkeys. Similar to GnRH release, senktide stimulated kisspeptin release in a dose-responsive manner in both prepubertal and pubertal monkeys (Fig. 5). Group data indicated that in prepubertal monkeys, 0.1 µM and 10 µM senktide stimulated kisspeptin release (P < 0.001 and P < 0.0001, respectively; Fig. 6A and 6C) when compared with vehicle control (Fig. 6E). Post hoc analysis indicated that the prepubertal kisspeptin responses to senktide during the 20-minute infusion at both doses were higher than preinfusion levels as well as with the corresponding period of vehicle control (P < 0.001 for 0.1 µM and P < 0.0001 for 10 µM). In pubertal monkeys, 0.1 µM and 10 µM senktide induced robust increases in kisspeptin release (P < 0.001 and P < 0.0001, respectively; Fig. 6B and 6D), when compared with vehicle control (Fig. 6F). Post hoc analysis indicated that the pubertal kisspeptin response to senktide at both doses was higher than preinfusion levels as well as with the corresponding period of vehicle control (P < 0.001 for 0.1 µM and P < 0.0001 for 10 µM). Senktide-induced kisspeptin increases lasted longer than the induced GnRH increases in the pubertal animals only, up to 80 and 100 minutes for the low- and high-dose, respectively.
Figure 5.
Representative cases showing the effects of the NKB agonist senktide (0.1 and 10 µM) on in vivo kisspeptin release in (A–C) prepubertal and (D–F) pubertal female monkeys, using a microdialysis method. After 60-minute baseline sampling, (A, D) vehicle, (B, E) 0.1 µM senktide, or (C, F) 10 µM senktide was infused into the S-ME for 20 minutes (vertical bars), and dialysates were continuously collected in 20-minute fractions for an additional 100 minutes. Both (B) 0.1 µM and (C) 10 µM senktide stimulated GnRH release in a dose-responsive manner in prepubertal monkeys, whereas (A) vehicle did not induce any change. Similarly, senktide at both doses [(E) 0.1 µM and (F) 10 µM] stimulated GnRH release in a dose-responsive manner in pubertal monkeys, whereas (D) vehicle did not induce any change.
Figure 6.
Group data (mean ± SEM; n = 6 in all) showing that senktide infusion into the S-ME stimulated kisspeptin release in prepubertal and pubertal monkeys. Animals were treated with (A, B) 0.1 µM senktide, (C, D) 10 µM senktide, and (E, F) vehicle. In the prepubertal group, both 0.1 µM and 10 µM senktide significantly (P < 0.01 and P < 0.0001, respectively) increased kisspeptin release over the baseline levels, and the effects of senktide at both doses were significantly larger (P < 0.01 for 0.1 µM and P < 0.0001 for 10 µM) than those in vehicle. Similarly, in pubertal monkeys, both 0.1 µM and 10 µM senktide significantly (P < 0.001 and P < 0.0001, respectively) stimulated kisspeptin release over the baseline levels, and the effects of senktide at both doses were also significantly larger (P < 0.001 for 0.1 µM and P < 0.0001 for 1 µM) than those in vehicle. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 vs vehicle infusion; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs before infusion.
Calculation of the AUC for induced kisspeptin release indicated that senktide induced kisspeptin increases in a dose-responsive manner in both prepubertal (Fig. 4C) and pubertal (Fig. 4D) monkeys. However, unlike the senktide-induced GnRH release, comparison between the two developmental stages indicated that the senktide-induced kisspeptin increase in the pubertal group was significantly larger than that in the prepubertal group at a respective dose (P < 0.001 for 0.1 µM and P < 0.0001for 10 µM).
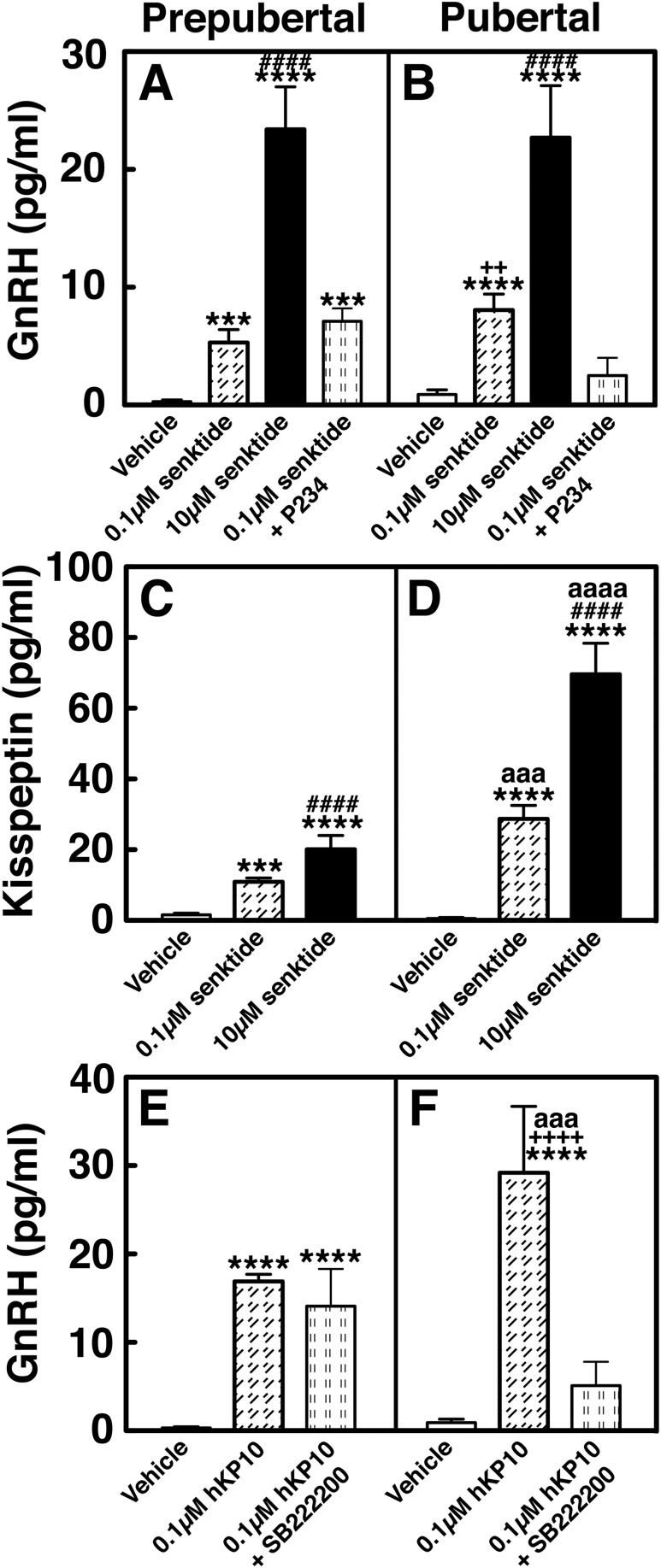
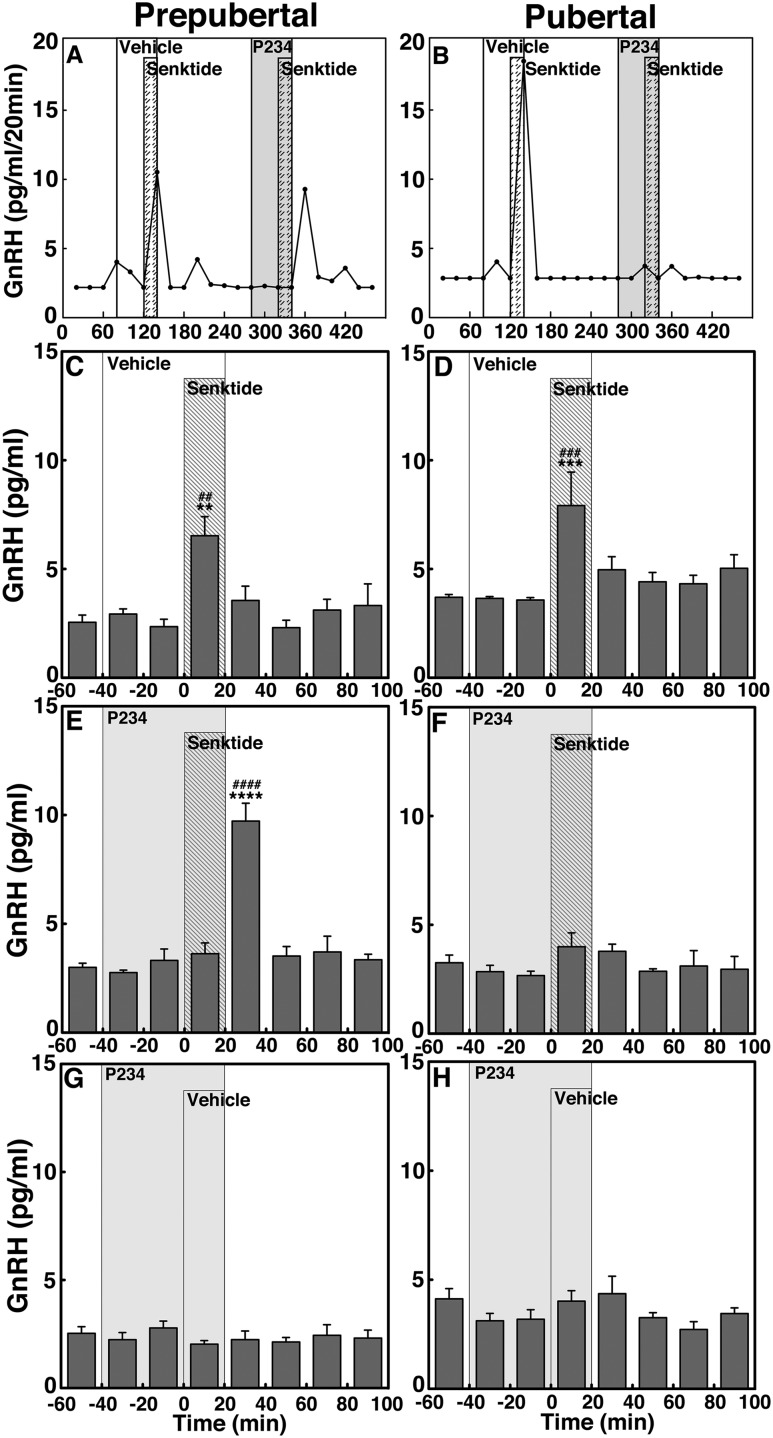
P234 blocked the senktide-induced GnRH release in pubertal but not prepubertal female monkeys (experiment 2)
To determine whether NKB signaling to GnRH release is mediated through KISS1R activation and if the signaling network between NKB and kisspeptin undergoes developmental change, we examined whether the 0.1 µM senktide-induced GnRH release occurred in the presence of the kisspeptin antagonist P234 (0.1 µM) in prepubertal and pubertal monkeys. Consistent with the results in experiment 1, senktide without P234 significantly stimulated GnRH increase in both prepubertal and pubertal monkeys (P < 0.01 and P < 0.001, respectively; Fig. 7A–D). However, senktide in the presence of P234 failed to induce GnRH increases in the pubertal group (Fig. 7B and 7F), but only delayed the senktide-induced GnRH response in the prepubertal group (Fig. 7A and 7E). That is, the senktide-induced GnRH increase did not occur during the 20-minute senktide infusion but occurred during the first 20 minutes after the senktide infusion. Although P234 infusion alone induced a small decrease in GnRH release, similar to our previous report (10), post hoc analysis indicated that P234 did not cause significant changes in GnRH release in either age group (Fig. 7G and 7H).
Figure 7.
Representative cases and group data (mean ± SEM) showing modification of the 0.1 µM senktide-induced GnRH release by the kisspeptin antagonist P234 (0.1 µM) in prepubertal and pubertal monkeys. Senktide induced GnRH release in the absence of P234 in both (C) prepubertal (P < 0.01) and (D) pubertal (P < 0.001) monkeys when compared with baseline or vehicle infusion GnRH levels. However, in the presence of P234, the senktide-induced GnRH release was absent in (F) pubertal monkeys (P > 0.05), whereas in prepubertal monkeys, senktide induced GnRH release with a 20-minute delay. The senktide-induced GnRH release in (C) prepubertal monkeys was significantly larger (P < 0.0001) than baseline GnRH levels. (G, H) In both stages, P234 alone did not alter GnRH release. **P < 0.01, ***P < 0.001, ****P < 0.0001 vs vehicle infusion; ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs before infusion.
The AUC for induced GnRH release further indicated that in the prepubertal group (Fig. 4A), the presence or absence of P234 did not change the level of senktide-induced GnRH release. In contrast, in the pubertal group (Fig. 4B), the senktide-induced GnRH release without P234 was significantly larger (P < 0.01) than with P234.
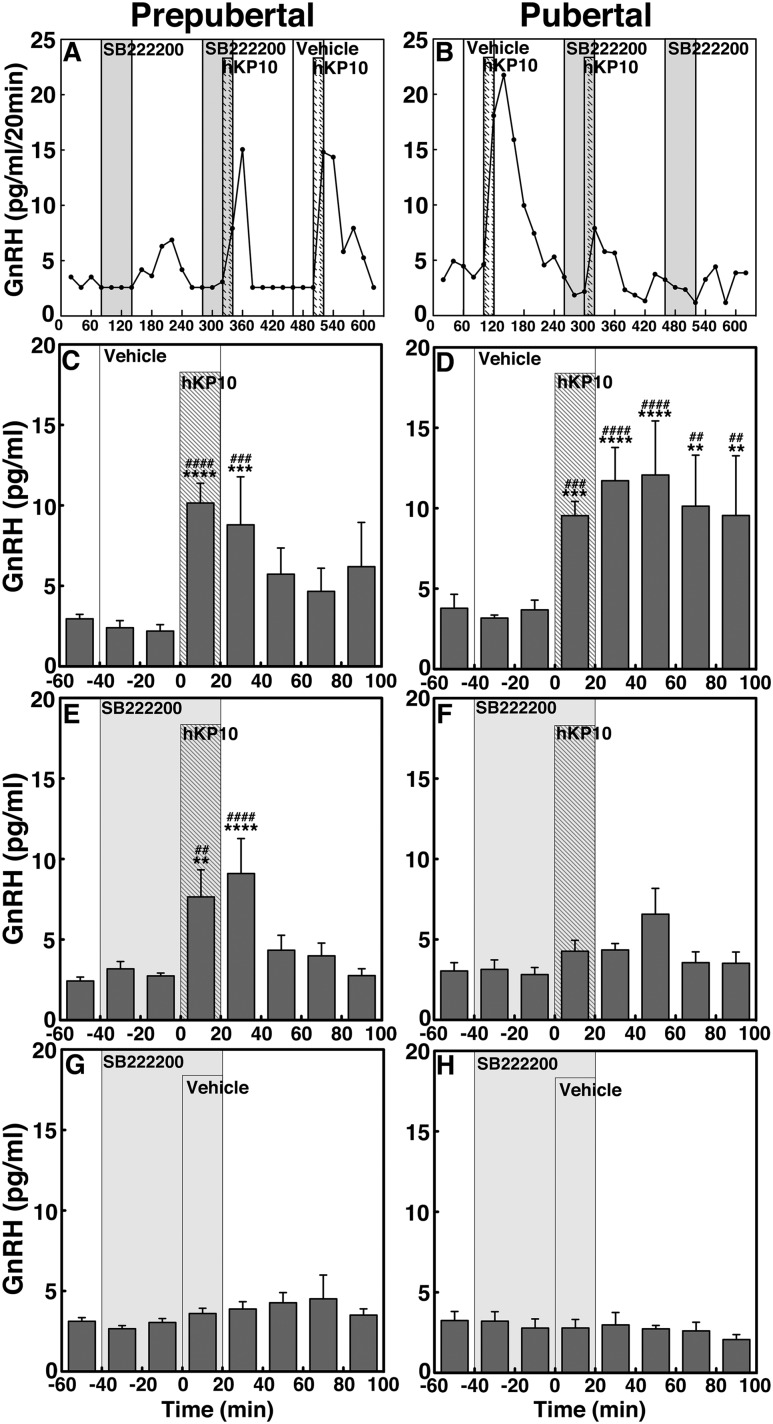
SB222200 suppressed the hKP10-induced GnRH release in pubertal but not prepubertal female monkeys (experiment 3)
We have shown that a 20-minute infusion of 0.1 µM hKP10 into the S-ME induces a developmental increase in mean GnRH release in female rhesus monkeys (10). To determine whether kisspeptin signaling to GnRH release is mediated through NK3R activation and if the signaling network between kisspeptin and NKB undergoes developmental change, we examined whether 0.1 µM hKP10-induced GnRH release occurred in the presence of the NKB antagonist SB222200 (1.0 µM) in prepubertal and pubertal monkeys. Similar to our previous report (10), hKP10 without SB222200 stimulated increases in GnRH release in both prepubertal and pubertal monkeys (P < 0.0001 for both developmental stages; Fig. 8A–D). However, the presence of SB222200 dramatically changed the profile of the hKP10-induced GnRH increase in pubertal monkeys; that is, the hKP10-induced GnRH increase was completely suppressed by SB222200 (Fig. 8F), whereas, in prepubertal females, the presence of SB222200 did not significantly alter the profile of hKP10-induced GnRH increase (Fig. 8E). SB222200 infusion alone did not significantly alter GnRH release in either age group (Fig. 8G and 8H).
Figure 8.
Representative cases and group data (mean ± SEM) showing modification of the 0.1 µM hKP-10-induced GnRH release by the NKB antagonist, SB222200 (0.1 µM) in prepubertal and pubertal monkeys. hKP10 stimulated GnRH release in the absence of SB222200 in both (C) prepubertal (P < 0.0001) and (D) pubertal (P < 0.0001) monkeys when compared with baseline or vehicle infusion GnRH levels. However, in the presence of SB222200, the hKP10-induced GnRH release was absent in (F) pubertal monkeys (P > 0.05), whereas in (E) prepubertal monkeys, hKP10-stimulated GnRH release (P < 0.0001) was unaltered. (G, H) In both stages, SB222200 alone did not significantly alter GnRH release. **P < 0.01, ***P < 0.001, ****P < 0.0001 vs vehicle infusion; ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs before infusion.
The AUC for induced GnRH release further indicated that in the prepubertal group (Fig. 4E), the presence or absence of SB222200 did not change the level of hKP10-induced GnRH release. In contrast, in the pubertal group (Fig. 4F), the hKP10-induced GnRH release without SB222200 was significantly larger (P < 0.001) than with SB222200.
Discussion
In this study, we found infusion of the NKB receptor agonist senktide into the S-ME stimulated both GnRH release and kisspeptin release in prepubertal and pubertal female monkeys in a dose-responsive manner. Interestingly, although the senktide-induced GnRH release and kisspeptin release were both dose dependent and the senktide-induced kisspeptin release in pubertal females was greater than in prepubertal females, there was no developmental difference in the senktide-induced GnRH release. Second, infusion of the kisspeptin receptor antagonist P234 blocked the senktide-induced GnRH release only in pubertal female monkeys, indicating that NKB signaling is mediated by kisspeptin neurons in pubertal, but not prepubertal, female monkeys. Third, infusion of the NKB receptor antagonist SB222200 blocked the hKP10-induced GnRH release only in pubertal female monkeys, indicating that kisspeptin signaling is mediated by NKB neurons in pubertal, but not prepubertal, female monkeys. Together, these results suggest that in female rhesus monkeys before puberty onset, kisspeptin and NKB neurons independently signal to GnRH neurons, whereas after puberty onset, they form a network resulting in a higher level of signaling to GnRH release.
Consistent with our previous study (10), 20-minute infusion of hKP10 significantly stimulated GnRH release and hKP10-induced GnRH release in pubertal females was larger than that in prepubertal females (Figs. 4 and 8). Together, the kisspeptin-induced GnRH release increases developmentally in a linear fashion and GnRH release in response to kisspeptin signaling becomes more sensitive after puberty onset (10). However, in the current study, there were no differences in the amount of the senktide-induced GnRH release between the two developmental stages (Figs. 3 and 4), despite the fact that the senktide-induced kisspeptin release was dose dependent in both developmental stages and the kisspeptin responses in pubertal females were larger than that in prepubertal females (Figs. 4 and 6). In other words, similar to the kisspeptin responses to senktide, the GnRH responses to senktide were dose dependent within the same developmental stage, but between the two developmental stages, NKB signaling to GnRH release was not equal. Although the reason for this mistranslation remains unclear, it is speculated that after puberty onset, an inhibitory signal, such as β-dynorphin and/or β-endorphin (16, 17), may be involved in the NKB and kisspeptin signaling network.
Surprisingly, we found that the hKP10-induced GnRH release was blocked in the presence of SB222200 in pubertal monkeys, but not in prepubertal monkeys. Similarly, although the senktide-induced GnRH release was absent in the presence of P234 in pubertal monkeys, in prepubertal monkeys this was not the case and senktide induced GnRH release with a 20-minute delay. The reason for this delay is unknown. Although we did not directly examine whether P234 and SB222200 are respective antagonists for hKP10 and senktide in our system, it has been shown that P234 blocks action of kisspeptin in various animal models (18) and SB222200 is a selective and competitive NK3R antagonist (19). Together, findings from the current study indicate that in prepubertal female monkeys, kisspeptin and NKB signaling modulate GnRH release independently (Fig. 9). In contrast, in pubertal monkeys, kisspeptin and NKB neurons form a network and this network influences GnRH release more effectively. The formation of this network after puberty onset allows the transmission of NKB signaling through kisspeptin neurons and kisspeptin signaling through NKB neurons (Fig. 9). Perhaps this reciprocal signaling mechanism between kisspeptin and NKB neurons further augments the pubertal increase in GnRH release.
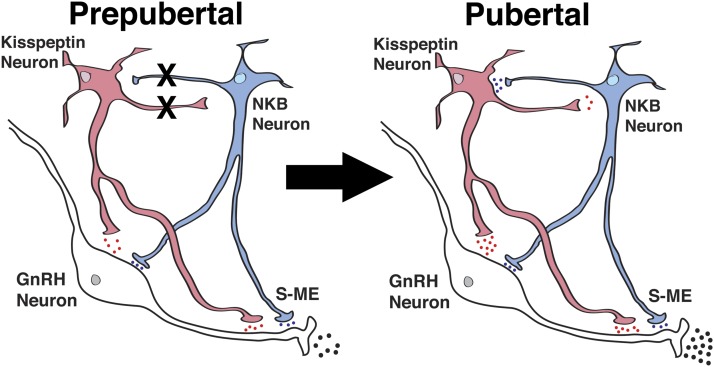
Figure 9.
Schematic diagram showing the developmental changes in kisspeptin (red) and NKB (blue) signaling to GnRH neurons in the S-ME in prepubertal and pubertal female monkeys. During the prepubertal period, kisspeptin and GnRH neurons are under tonic inhibition by substrates such as γ-amino butyric acid, neuroestradiol, MKRN3, and GATA1. X between kisspeptin and NKB neurons indicates the absence of signaling pathways, and the blue, red and black dots indicate relative amount of neuropeptide release. Kisspeptin signaling to NKB neurons is hypothetical; we did not measure the kisspeptin-induced NKB release.
One can argue that the difference between prepubertal and pubertal monkeys is due to the difference in circulating levels of estradiol, because this study was conducted in monkeys with intact ovaries. However, preliminary data indicate that in adult ovariectomized monkeys, (1) senktide (0.1 µM) induced GnRH release with a similar magnitude as ovarian-intact pubertal monkeys, (2) the senktide-induced GnRH release was not blocked by P234, and (3) senktide failed to induce kisspeptin release (Garcia and Terasawa, unpublished data). These preliminary observations are consistent with our previous study showing that hKP10 did not induce GnRH release in pubertal ovariectomized monkeys (10). Therefore, during the developmental stage, while NKB signaling to GnRH release is less dependent on the circulating estradiol, kisspeptin signaling to GnRH release is heavily dependent on circulating estradiol. Consequently, the absence of interaction between kisspeptin and NKB signaling in prepubertal female monkeys may, in part, be due to low levels of circulating estradiol. Further studies are necessary to confirm this speculation.
As described previously, the area within the hypothalamus where samples are collected and the drugs are infused with the microdialysis probe is confined (15, 20). Therefore, it is reasonable to assume that the action of senktide, SB222200, hKP10, and P234 infusions are limited to the S-ME, as shown in the mapping study of microdialysis probe tips (Fig. 1). Although a small number of cell bodies of GnRH, kisspeptin, and NKB neurons are present in the S-ME (21–23), it is likely that most interactions between GnRH neurons and kisspeptin and NKB neurons take place at terminal fibers in the S-ME. In fact, dense GnRH and kisspeptin fibers are found in the ME of mice, horses, goats, monkeys, and humans, and studies with confocal microscopy indicate a close proximity between GnRH and kisspeptin fibers (22, 24–27). Similarly, dense GnRH and NKB fibers are also present in the ME and colocalization of NK3R on GnRH fibers is also shown (23, 27). Moreover, using fast-scan cyclic voltammetry, senktide was shown to induce GnRH release in the ME in kisspeptin knockout mice (28), suggesting that stimulation of GnRH fibers in the ME by senktide, independent from kisspeptin signaling, is sufficient for GnRH release.
Based on the findings in rodents and ruminants that nearly 100% of kisspeptin neurons in the arcuate nucleus (ARC) express NKB and dynorphin (29, 30), and stimulatory kisspeptin and NKB signaling and inhibitory dynorphin signaling appear to form a GnRH pulse-generating network (5, 30–32), this group of neurons in the ARC has been named KNDy neurons (29). Interestingly, a recent study showing that kisspeptin, NKB, and dynorphin are packaged in separated vesicles in the cell bodies located in the ARC and fibers in the median eminence of adult female rats suggests that each respective neuropeptide can be released independently upon proper stimuli (33). In primates, however, the reciprocal signaling mechanism between kisspeptin and NKB neurons may not occur in KNDy neurons per se, as the coexpression rates of kisspeptin and NKB in the ARC in monkeys and humans are not as high as that seen in rodents and ruminants (13, 27). Nevertheless, the present finding suggests that collaboration of kisspeptin and NKB neurons augments the pubertal increase in pulsatile GnRH release in female monkeys.
In addition to kisspeptin and NKB neurons in the ARC, there is another group of kisspeptin neurons in the anterior ventricular periventricular nucleus (AVPV), which are sexually dimorphic in rodents (34) and indispensable for estrogen’s positive feedback effect on the GnRH/LH surge (35). Recently, the presence of kisspeptin neurons in the AVPV was identified in the monkey brain and their upregulation at the GnRH/LH surge has been reported (36, 37). Importantly, however, kisspeptin neurons in the AVPV are not sexually dimorphic in gonadally intact and gonadectomized, estrogen-treated male and female monkeys (36, 37), suggesting a clear species difference in the developmental origin, and perhaps function, of AVPV kisspeptin neurons. The role of AVPV kisspeptin neurons in puberty needs further investigation.
Despite the finding from present study showing that the establishment of a reciprocal network formation between kisspeptin and NKB neurons promotes the pubertal increase in GnRH release, it is unlikely that the pubertal changes in kisspeptin and NKB signaling are responsible for puberty onset. In primates, removal or reduction in “central inhibition” is a prerequisite for puberty onset (38, 39). GnRH neurons are active during the neonatal period, but they are suppressed by gonadal steroid-independent “central inhibition” during the prepubertal period. We previously proposed that γ-amino butyric acid and neuroestradiol could be the neural substrate for central inhibition (33, 40–42). Others also reported that makorin ring finger protein or the zinc-finger protein GATAD1 (43, 44) are possible candidates for the inhibitory neural substrate. The precise mechanism of the reduction in central inhibition and subsequent increases in stimulatory signaling for GnRH release initiating puberty are currently unknown. Nevertheless, the findings of the current study suggest that although in prepubertal female monkeys, kisspeptin and NKB signaling stimulate GnRH release independently, after puberty onset, the network between kisspeptin and NKB neurons is formed and signaling from this network further accelerates the pubertal increase in GnRH release.
Acknowledgments
We thank Dustin Richter and Lucille Kohlenberg for their technical assistance and the veterinarians and veterinary technicians of the Wisconsin National Primate Research Center.
Financial Support: This work was supported by Grant R01HD011355 (to E.T.) and Grants R01HD043341 and P50HD028138 (to S.B.S) from the Eunice Kennedy Shriver Institute of Child Health and Human Development and Grants R25GM083252 and T32HD041921 (to J.P.G.) from the National Institutes of Health (NIH) for his predoctoral training. The work was made possible by support from the NIH Office of the Director for the Wisconsin National Primate Research Center (Grant OD011106).
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| GnRH | Mammalian GnRH | R42 | Dr. Terry M. Nett (Colorado State University, Fort Collins, CO) | Rabbit; polyclonal | 1:1,200,000 | AB_2686919 |
| Kisspeptin | Human kisspeptin-54 | GQ2 | Dr. Stephen Bloom (Imperial College London, London, United Kingdom) | Sheep; polyclonal | 1:512,000 | AB_2686920 |
Abbreviation: RRID, Research Resource Identifier.
Footnotes
- ARC
- arcuate nucleus
- AUC
- area under the curve
- AVPV
- anterior ventricular periventricular nucleus
- GnRH
- gonadotropin-releasing hormone
- hKP10
- human kisspeptin-10
- KISS1R
- kisspeptin receptor
- LH
- luteinizing hormone
- NK3R
- neurokinin B receptor
- NKB
- neurokinin B
- P234
- peptide 234
- SEM
- standard error of the mean
- S-ME
- stalk-median eminence.
References
- 1.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 2.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maeda K, Ohkura S, Uenoyama Y, Wakabayashi Y, Oka Y, Tsukamura H, Okamura H. Neurobiological mechanisms underlying GnRH pulse generation by the hypothalamus. Brain Res. 2010;1364:103–115. [DOI] [PubMed] [Google Scholar]
- 5.Goodman RL, Coolen LM, Lehman MN. A role for neurokinin B in pulsatile GnRH secretion in the ewe. Neuroendocrinology. 2014;99(1):18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe G, Terasawa E. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology. 1989;125(1):92–99. [DOI] [PubMed] [Google Scholar]
- 7.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chongthammakun S, Claypool LE, Terasawa E. Ovariectomy increases in vivo luteinizing hormone-releasing hormone release in pubertal, but not prepubertal, female rhesus monkeys. J Neuroendocrinol. 1993;5(1):41–50. [DOI] [PubMed] [Google Scholar]
- 9.Guerriero KA, Keen KL, Terasawa E. Developmental increase in kisspeptin-54 release in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta). Endocrinology. 2012;153(4):1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerriero KA, Keen KL, Millar RP, Terasawa E. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication for the mechanism of puberty. Endocrinology. 2012;153(2):825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramaswamy S, Seminara SB, Plant TM. Evidence from the agonadal juvenile male rhesus monkey (Macaca mulatta) for the view that the action of neurokinin B to trigger gonadotropin-releasing hormone release is upstream from the kisspeptin receptor. Neuroendocrinology. 2011;94(3):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Werff ten Bosch JJ, Dierschke DJ, Terasawa E, Slob AK. Anterior hypothalamic lesions and pubertal development in female rhesus monkeys. Behav Brain Res. 1983;7(3):321–330. [DOI] [PubMed] [Google Scholar]
- 13.Ramaswamy S, Seminara SB, Ali B, Ciofi P, Amin NA, Plant TM. Neurokinin B stimulates GnRH release in the male monkey (Macaca mulatta) and is colocalized with kisspeptin in the arcuate nucleus. Endocrinology. 2010;151(9):4494–4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gearing M, Terasawa E. Luteinizing hormone releasing hormone (LHRH) neuroterminals mapped using the push-pull perfusion method in the rhesus monkey. Brain Res Bull. 1988;21(1):117–121. [DOI] [PubMed] [Google Scholar]
- 15.Frost SI, Keen KL, Levine JE, Terasawa E. Microdialysis methods for in vivo neuropeptide measurement in the stalk-median eminence in the Rhesus monkey. J Neurosci Methods. 2008;168(1):26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ebling FJ, Schwartz ML, Foster DL. Endogenous opioid regulation of pulsatile luteinizing hormone secretion during sexual maturation in the female sheep. Endocrinology. 1989;125(1):369–383. [DOI] [PubMed] [Google Scholar]
- 17.Terasawa E, Chongthammakun S Developmental changes in in vivo release of β-endorphin (β-END) from the stalk-median eminence (S-ME) in female rhesus monkeys. Abstracts of the 21st Annual Meeting of the Neuroscience Society, No. 361.1, 1991; New Orleans, LA. [Google Scholar]
- 18.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe HE, Somponpun SJ, Sladek CD. Role of neurokinin 3 receptors in supraoptic vasopressin and oxytocin neurons. J Neurosci. 2004;24(45):10103–10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kenealy BP, Kapoor A, Guerriero KA, Keen KL, Garcia JP, Kurian JR, Ziegler TE, Terasawa E. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33(49):19051–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldsmith PC, Lamberts R, Brezina LR. Gonadotropin-releasing hormone neurons and pathways in the primate hypothalamus and forebrain In: Norman RL, ed. Neuroendocrine Aspects of Reproduction. New York, NY: Academic Press; 1983:7–45. [Google Scholar]
- 22.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149(9):4387–4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–386. [DOI] [PubMed] [Google Scholar]
- 24.Yip SH, Boehm U, Herbison AE, Campbell RE. Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology. 2015;156(7):2582–2594. [DOI] [PubMed] [Google Scholar]
- 25.Decourt C, Tillet Y, Caraty A, Franceschini I, Briant C. Kisspeptin immunoreactive neurons in the equine hypothalamus Interactions with GnRH neuronal system. J Chem Neuroanat. 2008;36(3-4):131–137. [DOI] [PubMed] [Google Scholar]
- 26.Matsuyama S, Ohkura S, Mogi K, Wakabayashi Y, Mori Y, Tsukamura H, Maeda K, Ichikawa M, Okamura H. Morphological evidence for direct interaction between kisspeptin and gonadotropin-releasing hormone neurons at the median eminence of the male goat: an immunoelectron microscopic study. Neuroendocrinology. 2011;94(4):323–332. [DOI] [PubMed] [Google Scholar]
- 27.Hrabovszky E, Sipos MT, Molnár CS, Ciofi P, Borsay BÁ, Gergely P, Herczeg L, Bloom SR, Ghatei MA, Dhillo WS, Liposits Z. Low degree of overlap between kisspeptin, neurokinin B, and dynorphin immunoreactivities in the infundibular nucleus of young male human subjects challenges the KNDy neuron concept. Endocrinology. 2012;153(10):4978–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gaskins GT, Glanowska KM, Moenter SM. Activation of neurokinin 3 receptors stimulates GnRH release in a location-dependent but kisspeptin-independent manner in adult mice. Endocrinology. 2013;154(11):3984–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. [DOI] [PubMed] [Google Scholar]
- 30.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamura H, Tsukamura H, Ohkura S, Uenoyama Y, Wakabayashi Y, Maeda K. Kisspeptin and GnRH pulse generation. Adv Exp Med Biol. 2013;784:297–323. [DOI] [PubMed] [Google Scholar]
- 32.Grachev P, Li XF, Kinsey-Jones JS, di Domenico AL, Millar RP, Lightman SL, O’Byrne KT. Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism. Endocrinology. 2012;153(10):4894–4904. [DOI] [PubMed] [Google Scholar]
- 33.Murakawa H, Iwata K, Takeshita T, Ozawa H. Immunoelectron microscopic observation of the subcellular localization of kisspeptin, neurokinin B and dynorphin A in KNDy neurons in the arcuate nucleus of the female rat. Neurosci Lett. 2016;612:161–166. [DOI] [PubMed] [Google Scholar]
- 34.Kauffman AS, Navarro VM, Kim J, Clifton DK, Steiner RA. Sex differences in the regulation of Kiss1/NKB neurons in juvenile mice: implications for the timing of puberty. Am J Physiol Endocrinol Metab. 2009;297(5):E1212–E1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe Y, Uenoyama Y, Suzuki J, Takase K, Suetomi Y, Ohkura S, Inoue N, Maeda KI, Tsukamura H. Oestrogen-induced activation of preoptic kisspeptin neurones may be involved in the luteinising hormone surge in male and female Japanese monkeys. J Neuroendocrinol. 2014;26(12):909–917. [DOI] [PubMed] [Google Scholar]
- 37.Vargas Trujillo M, Kalil B, Ramaswamy S, Plant TM. Estradiol upregulates kisspeptin expression in the preoptic area of both the male and female rhesus monkey (Macaca mulatta): implications for the hypothalamic control of ovulation in highly evolved primates. Neuroendocrinology. 2017;105(1):77–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22(1):111–151. [DOI] [PubMed] [Google Scholar]
- 39.Plant TM. Neuroendocrine control of the onset of puberty. Front Neuroendocrinol. 2015;38:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keen KL, Burich AJ, Mitsushima D, Kasuya E, Terasawa E. Effects of pulsatile infusion of the GABA(A) receptor blocker bicuculline on the onset of puberty in female rhesus monkeys. Endocrinology. 1999;140(11):5257–5266. [DOI] [PubMed] [Google Scholar]
- 41.Kurian JR, Keen KL, Guerriero KA, Terasawa E. Tonic control of kisspeptin release in prepubertal monkeys: implications to the mechanism of puberty onset. Endocrinology. 2012;153(7):3331–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kenealy BP, Keen KL, Kapoor A, Terasawa E. Neuroestradiol in the stalk median eminence of female rhesus macaques decreases in association with puberty onset. Endocrinology. 2016;157(1):70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abreu AP, Dauber A, Macedo DB, Noel SD, Brito VN, Gill JC, Cukier P, Thompson IR, Navarro VM, Gagliardi PC, Rodrigues T, Kochi C, Longui CA, Beckers D, de Zegher F, Montenegro LR, Mendonca BB, Carroll RS, Hirschhorn JN, Latronico AC, Kaiser UB. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368(26):2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lomniczi A, Wright H, Castellano JM, Matagne V, Toro CA, Ramaswamy S, Plant TM, Ojeda SR. Epigenetic regulation of puberty via Zinc finger protein-mediated transcriptional repression. Nat Commun. 2015;6:10195. [DOI] [PMC free article] [PubMed] [Google Scholar]