Abstract
Endoxifen, the primary active metabolite of tamoxifen, is currently being investigated as a novel endocrine therapy for the treatment of breast cancer. Tamoxifen is a selective estrogen receptor modulator that elicits potent anti–breast cancer effects. However, long-term use of tamoxifen also induces bone loss in premenopausal women and is associated with an increased risk of endometrial cancer in postmenopausal women. For these reasons, we have used a rat model system to comprehensively characterize the impact of endoxifen on the skeleton and uterus. Our results demonstrate that endoxifen elicits beneficial effects on bone in ovary-intact rats and protects against bone loss following ovariectomy. Endoxifen is also shown to reduce bone turnover in both ovary-intact and ovariectomized rats at the cellular and biochemical levels. With regard to the uterus, endoxifen decreased uterine weight but maintained luminal epithelial cell height in ovariectomized animals. Within luminal epithelial cells, endoxifen resulted in differential effects on the expression levels of estrogen receptors α and β as well as multiple other genes previously implicated in regulating epithelial cell proliferation and hypertrophy. These studies analyze the impact of extended endoxifen exposure on both bone and uterus using a Food and Drug Administration–recommended animal model. Although endoxifen is a more potent breast cancer agent than tamoxifen, the results of the present study demonstrate that endoxifen does not induce bone loss in ovary-intact rats and that it elicits partial agonistic effects on the uterus and skeleton in ovariectomized animals.
This study describes the impact of endoxifen on the skeleton and uterus of rats. Endoxifen did not induce bone loss in ovary-intact rats and elicited partial agonistic effects following ovariectomy.
Endoxifen was identified in 1986 in the breast tumor tissue of a patient taking tamoxifen therapy (1). Following this initial observation, other studies have demonstrated that endoxifen is a primary metabolite of tamoxifen that is formed by cytochrome P450 2D6 (2, 3). Endoxifen is known to elicit greater antiestrogenic effects in breast cancer models compared with its parent compound tamoxifen (4–8), and in tamoxifen-treated breast cancer patients, endoxifen concentrations are positively associated with disease-free survival (9, 10). For these reasons, endoxifen is currently being investigated as a novel endocrine therapy for the treatment of estrogen receptor (ER)α–positive breast cancer.
Endoxifen, similar to its parent compound tamoxifen, falls into the class of molecules known as selective estrogen receptor modulators (SERMs). SERMs interact with ERα and ERβ to elicit their biological functions and are defined by their ability to exert agonistic and antagonistic effects in a tissue-dependent manner (11). These diverse functions of SERMs result primarily from structural and conformational changes in the ERs upon binding, resulting in differential recruitment of transcriptional coactivators and corepressors (12–17). In addition to their use as breast cancer treatments, SERMs are also clinically used for other estrogen-related disorders such as osteoporosis and vasomotor symptoms associated with menopause (18, 19).
With regard to bone, a wealth of literature exists demonstrating that a number of SERMs can protect against bone loss following estrogen depletion in various animal models and act to preserve bone mass in postmenopausal women [reviewed in (20–22)]. In contrast, the use of tamoxifen in the premenopausal setting is known to result in bone loss (23–26). For these reasons, a number of SERMs have been approved for the treatment of postmenopausal osteoporosis (27, 28), and tamoxifen remains the drug of choice for the treatment of postmenopausal breast cancer patients with low bone mass (29). Despite these positive effects of SERMs, long-term use of compounds such as tamoxifen are also known to elicit negative side effects such as the increased risk of endometrial cancers in postmenopausal women (30–34).
Endoxifen has substantially higher affinity for the ERs compared with tamoxifen, and we and others have demonstrated that endoxifen elicits differential gene expression profiles and more potent antiestrogenic effects compared with tamoxifen (2, 7, 35, 36). For these reasons, one could speculate that endoxifen may also have differential effects in other tissues. Given that endoxifen is being investigated as a novel breast cancer therapy for which multiple clinical trials are currently ongoing (NCT02311933, NCT01327781, and NCT01273168), it is essential to determine the impact of endoxifen on other target tissues such as the bone and uterus. In the present study, we have used a rat model system to assess the effects of endoxifen on the skeleton and uterus in both ovary-intact and ovariectomized animals. Our data demonstrate that endoxifen exposure does not induce bone loss in ovary-intact rats and protects against bone loss following ovariectomy. With regard to the uterus, endoxifen reduced uterine weight in ovary-intact rats with no effect on epithelial cell height in these animals. In contrast, endoxifen had no effect on uterine weight in ovariectomized animals, but maintained epithelial cell height equivalent to that of sham controls.
Materials and Methods
Animals and experimental design
Thirty sham-operated and 20 ovariectomized 4-month-old Sprague-Dawley rats were purchased from Harlan Laboratories (Indianapolis, IN) and were housed in a temperature-controlled room (22 ± 2°C) with a light/dark cycle of 12 hours. Animals had free access to water and were fed ad libitum standard laboratory chow. Ten sham-operated rats were sacrificed at the start of the study and served as baseline controls. The remaining rats were randomized to four groups consisting of 10 animals per group as follows: (1) ovary intact (sham) plus vehicle control (0.375 mg/mL ascorbic acid), (2) ovary intact (sham) plus z-endoxifen-hydrochloride (endoxifen) (10 mg/kg/d), (3) ovariectomized plus vehicle control and, (4) ovariectomized plus endoxifen. Animals were treated with vehicle or endoxifen via oral gavage once daily for 34 days. Fresh vehicle control (0.375 mg/mL ascorbic acid) and endoxifen solutions (solubilized in 0.375 mg/mL ascorbic acid) were prepared weekly in sterile water and stored in the dark at 4°C. Purified z-endoxifen-hydrochloride was synthesized by the National Cancer Institute and obtained through the Developmental Therapeutics Program (NSC 750393). For histomorphometry, declomycin (15 mg/kg) was administered subcutaneously at start of treatment whereas calcein (15 mg/kg) was administered 8 and 2 days prior to euthanasia. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Mayo Clinic Institutional Animal Care and Use Committee approved all animal care and experimental procedures described in this study (protocol A22112).
Tissue collection
Following 34 days of treatment, rats were anesthetized using isoflurane and subsequently sacrificed using CO2. Blood was collected via terminal bleeds from all rats, and serum was separated and stored at −70°C until the time of analysis. Tibiae and fifth lumbar vertebrae were dissected out from each animal and cleaned of soft tissue. Bones were fixed in 10% neutral buffered formalin overnight, transferred to 70% ethanol, and stored at 4°C for microcomputed tomography (micro-CT) and histomorphometric evaluation.
Serum levels of endoxifen
Serum endoxifen concentrations were determined using a validated high-performance liquid chromatography assay as previously described (37).
Micro-CT
Micro-CT was used for nondestructive high-resolution three-dimensional evaluation of cortical and cancellous bone volume and architecture. Tibiae and lumbar vertebrae were scanned in 70% ethanol at a voxel size of 16 µm × 16 µm × 16 µm (55 kVp x-ray voltage, 145 µA intensity, and 200 ms integration time) using a µCT40 scanner (Scanco Medical, Basserdorf, Switzerland). Filtering parameters σ and support were set to 0.8 and 1, respectively. Bone segmentation was conducted at a threshold of 245 (scale, 0 to 1000) determined empirically. Cancellous bone in the proximal tibia metaphysis and cortical bone in the tibia diaphysis were evaluated. For assessment of cortical bone, 62 slices (992 μm in length) in the tibial midshaft diaphysis were measured and cross-sectional volume (mm3), cortical volume (mm3), marrow volume (mm3), cortical thickness (μm), periosteal perimeter (mm), and endosteal perimeter (mm) were determined. For assessment of cancellous bone, 75 slices (1200 μm in length) in the proximal tibial metaphysis, starting 150 slices (2400 μm) distal to the growth plate, were measured. Analysis of the lumbar vertebra included the entire region of secondary spongiosa between the cranial and caudal growth plates (245 ± 2 slices, 3424 ± 32 μm, mean ± standard error of the mean). Cancellous bone measurements in tibia and lumbar vertebra included cancellous bone volume fraction (bone volume/tissue volume, %), connectivity density (1/mm3), trabecular thickness (μm), trabecular number (1/mm), and trabecular spacing (μm).
Histomorphometry
Methods used for measuring static and dynamic bone histomorphometry were as previously described (38). In brief, proximal tibiae were dehydrated in a graded series of ethanol and xylene and embedded undecalcified in modified methyl methacrylate. Longitudinal sections (4 µm thick) were cut with a vertical bed microtome (Leica 2065) and affixed to slides precoated with 1% gelatin solution. One section per animal was mounted unstained for measurement of fluorochrome labels. One section per animal was stained for tartrate-resistant acid phosphatase and counterstained with toluidine blue (Sigma-Aldrich, St. Louis, MO) and used for cell-based measurements. All data were collected using the OsteoMeasure system (OsteoMetrics, Atlanta, GA). The sampling site in the proximal tibia metaphysis (cancellous bone) was located 0.5 mm distal to the growth plate and measured 2.5 ± 0.1 mm2. The sampling site in the tibial diaphysis (cortical bone) was located 4.8 to 5.5 mm distal to the growth plate. Fluorochrome-based measurements of bone formation included mineralizing perimeter (mineralizing perimeter/bone perimeter: cancellous bone perimeter covered with double plus half single label normalized to bone perimeter, %); mineral apposition rate (the distance between two fluorochrome markers that comprise a double label divided by the 34-day interlabel interval for cortical endosteum and periosteum or 6-day interlabel interval for cancellous bone, µm/d); and bone formation rate (bone formation rate/bone perimeter: calculated by multiplying mineralizing perimeter by mineral apposition rate normalized to bone perimeter, µm2/µm/y). Osteoclast perimeter (osteoclast perimeter/bone perimeter, %) was also measured and osteoclasts were identified as multinucleated (two or more nuclei) cells with acid phosphatase–positive (red-stained) cytoplasm in contact with the bone surface. All bone histomorphometric data are reported using standard two-dimensional nomenclature (39).
Biochemical markers of bone turnover
The serum levels of a bone formation marker, procollagen type 1 amino-terminal propeptide (P1NP), and a bone resorption marker, C-telopeptide of type I collagen (CTX-1), were quantitated using enzyme-linked immunosorbent assay kits from ImmunoDiagnostic Systems (Fountain Hills, AZ) as described by the manufacturer. All assays were performed in duplicate for each animal and averaged among the four treatment groups.
Uterine weight and histology
Following euthanasia, uteri were dissected from all rats and wet weights were immediately determined. Subsequently, uteri were divided in half and placed in either 10% neutral buffered formalin for fixation and histological analysis or in TRIzol for RNA extraction and gene expression analysis. Following fixation, uteri were paraffin embedded, processed using standard procedures, sectioned (5 µm), and stained with hematoxylin and eosin (H&E). Using H&E-stained sections, luminal epithelial cell height was determined for each animal using a light microscope with a total of 10 measurements acquired per specimen. Mean epithelial cell height values were calculated for individual animals and subsequently averaged among treatment groups.
RNA isolation and real-time polymerase chain reaction analysis
Immediately following dissection, half of each rat uterus was sheared in TRIzol (Invitrogen) using an Ultra-Turrax homogenizer (Laboratory Supply Network, Inc., Atkinson, NH), and total RNA was isolated as specified by the manufacturer. RNA was quantitated using a NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific, Wilmington, DE). A total of three RNA samples were generated for each treatment group by combining equal amounts of RNA originating from three individual rats. Complementary DNA was synthesized using the iScript complementary DNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Real-time polymerase chain reaction (PCR) was performed in triplicate as previously described (35), and values were normalized using TBP as a control. All PCR primers were designed using Primer3 software (http://frodo.wi.mit.edu/primer3/) and were purchased from Integrated DNA Technologies (Coralville, IA). Primer sequences are listed in Table 1.
Table 1.
Primer Sets Used in Quantitative Reverse Transcription PCR Assays
| Gene |
Primers (5′ - 3′) |
|
|---|---|---|
| Forward | Reverse | |
| rERα | TCCGGCACATGAGTAACAAA | TGAAGACGATGAGCATCCAG |
| rERβ | AAAGTAGCCGGAAGCTGACA | CTGCTGCTGGGAGGAGATAC |
| rPGR | GAGAGGCAGCTGCTTTCAGT | AAACACCATCAGGCTCATCC |
| rIGF1 | GTGATCTGAGGAGGCTGGAG | GTCTTGGGCATGTCAGTGTG |
| rIGF1R | TCCCAAGCTGTGTGTCTCTG | CTCCGTTGTTCCTGGTGTTT |
| rPCNA | GCCCTCAAAGACCTCATCAA | TGAGACGAATCCATGCTCTG |
| rSFRP4 | CACCACAGCACTCAGGAGAA | GGTGCATACATGGCACAGAG |
| rRALDH2 | ACATCAACAAGGCTCTCA | CCAAACTCACCCATTTCTC |
| rTBP | TCTAACCACAGCACCATTGC | AGAGCTCTCAGAGGCTGGTG |
Immunohistochemistry
Five-micrometer sections were cut from paraffin-embedded rat uteri for immunostaining and analysis as previously described (37). Immunohistochemistry (IHC) stains were performed on a Leica Bond-III stainer using a monoclonal ERα 1D5 antibody (1:200 dilution; Dako, Carpinteria, CA) and and a monoclonal ERβ1 PPG5/10 antibody (1:100 dilution; Thermo Scientific, Waltham, MA). Additional antibody information is listed in Table 2. Images were acquired using a light microscope.
Table 2.
Antibody Information
| Protein Target | Antibody Clone | Dilution | RRID |
|---|---|---|---|
| ERα | 1D5 | 1:200 | AB_1122655 |
| ERβ | PPG5/10 | 1:100 | AB_780510 |
Abbreviation: RRID, Research Resource Identifier.
Statistical analysis
The effects of treatment and ovarian status (surgery) were assessed using two-way analysis of variance. Where an interaction between treatment and surgery was identified, pairwise comparisons were conducted to determine whether ovariectomy, or endoxifen treatment of sham or ovariectomized rats, significantly altered a given parameter. Such significant differences are indicated on graphs by solid lines. In the absence of an interaction, no pairwise comparisons were made, but a secondary set of analyses was conducted to identify differences due to surgery or treatment of a given parameter. All graphs depict group means and standard error of the means. Significance was defined as P < 0.05.
Results
Serum endoxifen concentrations
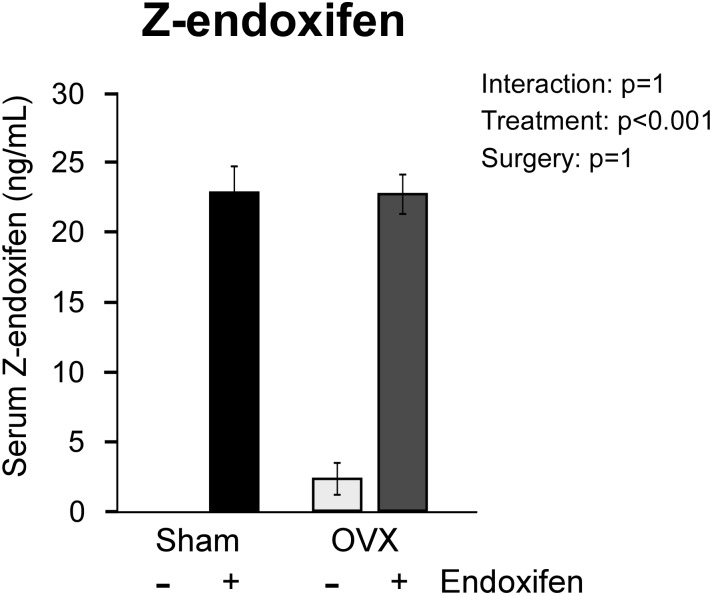
To ensure drug availability and equivalent exposure of sham and ovariectomized endoxifen-treated rats, the circulating levels of z-endoxifen were assessed in the serum using high-performance liquid chromatography. As expected, little to no endoxifen was detected in the serum of vehicle-treated rats (Fig. 1). In drug-treated rats, equivalent levels of circulating endoxifen (23 ng/mL) were detected in both sham-operated and ovariectomized animals (Fig. 1).
Figure 1.
Serum levels of z-endoxifen in ovary-intact and ovariectomized (OVX) rats following 34 days of treatment with endoxifen or vehicle. The group means ± standard error are depicted. P values for the main comparisons (surgery and treatment) are shown. There was no interaction between surgery and treatment.
Micro-CT analysis of tibiae and vertebrae
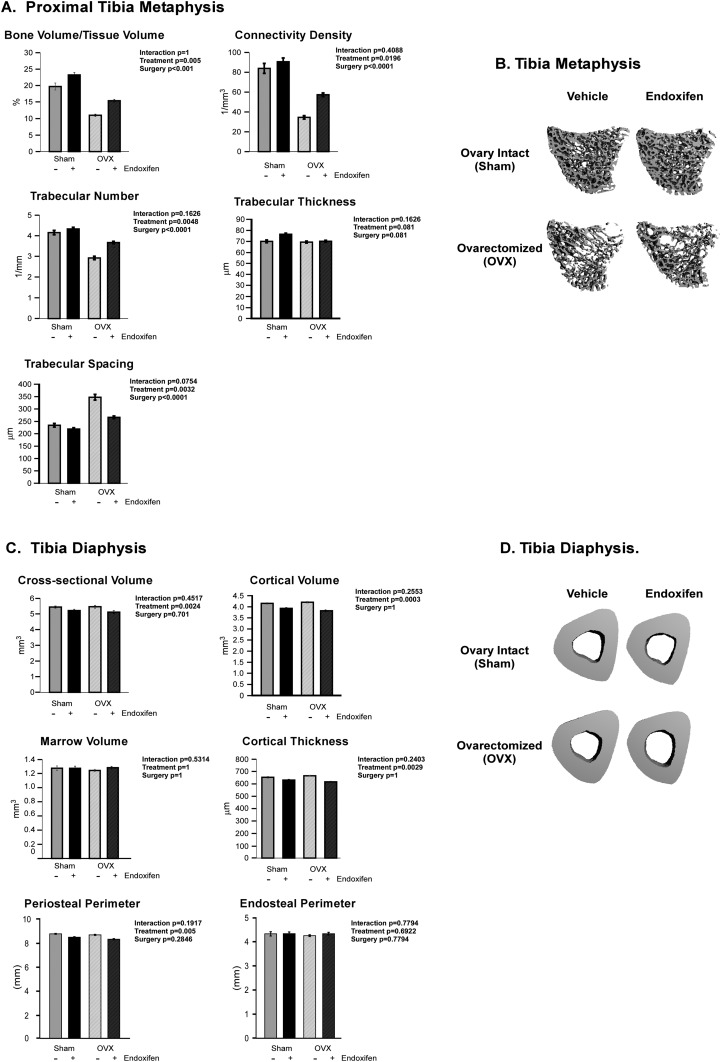
The effects of endoxifen on cancellous and cortical bone microarchitecture in the tibia, and cancellous bone in the vertebra, were first analyzed using micro-CT. Within the proximal tibia metaphysis, no significant interaction between treatment and surgery was detected for any of the parameters analyzed. Ovariectomy resulted in decreased cancellous bone volume/tissue volume (BV/TV), connectivity density, and trabecular number with a corresponding increase in trabecular spacing (Fig. 2A). Endoxifen treatment resulted in higher BV/TV, connectivity density, and trabecular number and lower trabecular spacing (Fig. 2A). Representative micro-CT images of cancellous bone in the tibial metaphysis are shown in Fig. 2B. Relative to baseline control animals, endoxifen had no effect on BV/TV in the proximal tibial metaphysis in ovary-intact rats but attenuated bone loss following ovariectomy (Supplemental Table 1 (16.5KB, xlsx) ). As with the cancellous bone measurements, there was no significant interaction between treatment and surgery for any of the cortical bone parameters analyzed at the tibial diaphysis (Fig. 2C). Cross-sectional volume, cortical volume, marrow volume, cortical thickness, periosteal perimeter, and endosteal perimeter did not differ between surgical groups (Fig. 2C). Significantly lower cross-sectional volume, cortical volume, cortical thickness, and periosteal perimeter were detected in endoxifen-treated rats compared with vehicle-treated control animals (Fig. 2C). However, these same parameters did not differ between endoxifen-treated rats and baseline control animals, indicating that endoxifen attenuates bone accrual at this site (Supplemental Table 1 (16.5KB, xlsx) ). Representative micro-CT images of cortical bone in the tibial diaphysis are shown in Fig. 2D.
Figure 2.
Micro-CT analysis of the tibia in ovary-intact and ovariectomized (OVX) rats following 34 days of treatment with endoxifen or vehicle. (A) Measurements obtained within the proximal tibial metaphysis, including BV/TV, connectivity density, trabecular number, trabecular thickness, and trabecular spacing are indicated. (B) Representative micro-CT images of cancellous bone in the tibial metaphysis for each of the four treatment groups. (C) Measurements obtained within the tibial diaphysis, including cross-sectional volume, cortical volume, marrow volume, cortical thickness, periosteal perimeter, and endosteal perimeter, are indicated. (D) Representative micro-CT images of cortical bone at the tibial diaphysis for each of the four treatment groups. The group means ± standard error are depicted. P values for the main comparisons (surgery and treatment) are shown. There was no interaction between surgery and treatment.
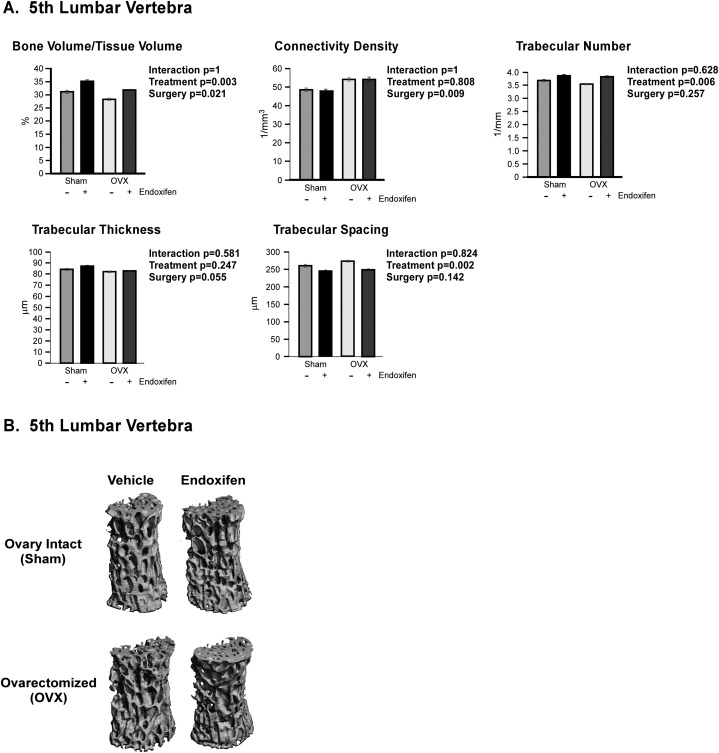
The effects of endoxifen on cancellous bone were also analyzed in the fifth lumbar vertebra (Fig. 3). As with the tibia, there were no significant interactions detected between surgery and treatment groups for any of the parameters analyzed. However, BV/TV was lower and connectivity density was higher in ovariectomized rats compared with sham-operated controls (Fig. 3A). Endoxifen-treated animals also displayed significantly higher BV/TV and trabecular number, with a concomitantly lower trabecular spacing, relative to vehicle-treated controls (Fig. 3A). Representative micro-CT images of cancellous bone in the fifth lumbar vertebra are shown in Fig. 3B. As with the proximal tibial metaphysis, endoxifen had no effect on BV/TV in ovary-intact rats and attenuated bone loss in ovariectomized rats (Supplemental Table 1 (16.5KB, xlsx) ).
Figure 3.
Micro-CT analysis of the fifth lumbar vertebrae in ovary-intact and ovariectomized (OVX) rats following 34 days of treatment with endoxifen or vehicle. (A) BV/TV, connectivity density, trabecular number, trabecular thickness, and trabecular spacing are indicated. (B) Representative micro-CT images of cancellous bone within the fifth lumbar vertebrae for each of the four treatment groups. The group means ± standard error are depicted. P values for the main comparisons (surgery and treatment) are shown. There was no interaction between surgery and treatment.
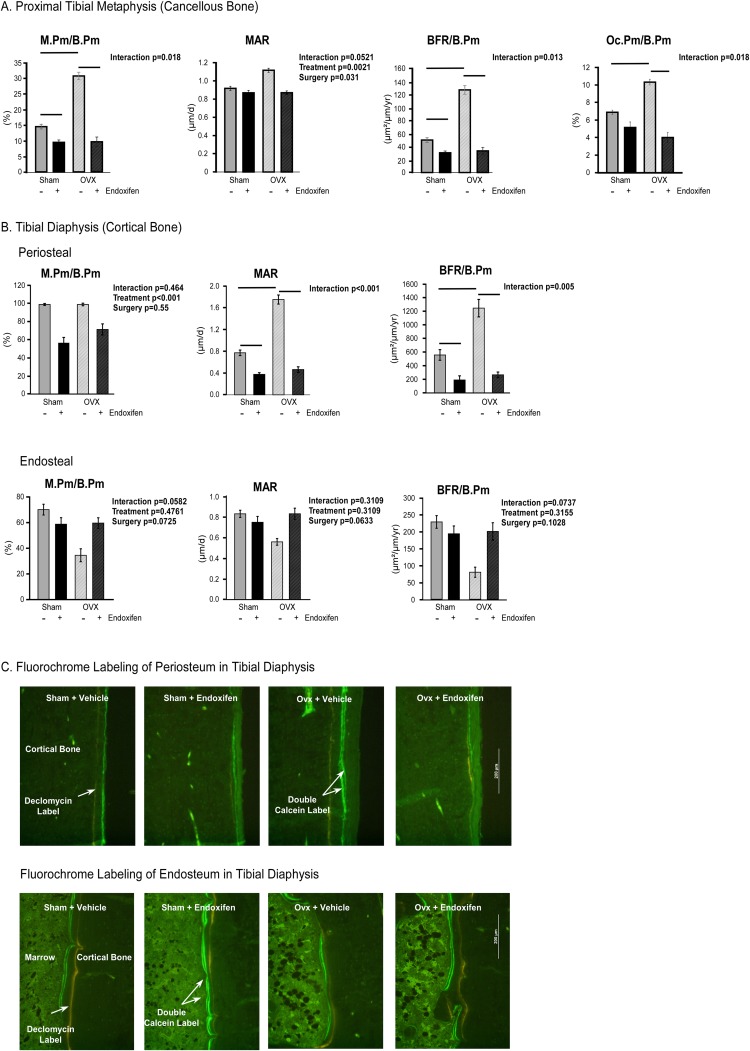
Bone histomorphometry
To explore the effects of endoxifen on the skeleton at the cellular level, we evaluated bone formation and resorption indices using static and dynamic histomorphometric analysis on cancellous bone in the tibial metaphysis (Fig. 4A). A significant interaction between treatment and surgery was detected for mineralizing perimeter per bone perimeter (M.Pm/B.Pm), bone formation rate per bone perimeter (BFR/B.Pm), and osteoclast perimeter per bone perimeter (Oc.Pm/B.Pm) (Fig. 4A). Ovariectomy resulted in significantly higher M.Pm/B.Pm, BFR/B.Pm, and Oc.Pm/B.Pm relative to sham-operated controls (Fig. 4A). Endoxifen treatment resulted in lower M.Pm/B.Pm and BFR/B.Pm in both sham-operated and ovariectomized rats as well as Oc.Pm/B.Pm in ovariectomized rats (Fig. 4A). A significant interaction between treatment and surgery was not indicated for mineral apposition rate (MAR); however, this parameter was significantly higher in ovariectomized rats and significantly lower in endoxifen-treated animals (Fig. 4A). To assess the role of endoxifen on cortical bone accrual, we evaluated bone formation at the periosteal and endosteal surfaces of the tibial diaphysis. A significant interaction between treatment and surgery was detected for periosteal MAR and BFR/B.Pm (Fig. 4B). Ovariectomy resulted in significantly higher periosteal MAR and BFR/B.Pm relative to sham-operated controls (Fig. 4B). Endoxifen treatment resulted in lower periosteal MAR and BFR/B.Pm in both sham-operated and ovariectomized rats (Fig. 4B). A significant interaction between treatment and surgery was not indicated for periosteal M.Pm/B.Pm or any of these parameters as measured on the endosteal surface. However, periosteal M.Pm/B.Pm was significantly lower in endoxifen-treated animals (Fig. 4B). Representative images for both the periosteal and endosteal surfaces of the tibial diaphysis are shown in Fig. 4C.
Figure 4.
Static and dynamic histomorphometric analysis of the tibia in ovary-intact and ovariectomized (OVX) rats following 34 days of treatment with endoxifen or vehicle. (A) M.Pm/B.Pm, MAR, BFR/B.Pm, and Oc.Pm/B.Pm for cancellous bone in the tibial metaphysis are indicated. (B) Periosteal and endosteal M.Pm/B.Pm, MAR, and BFR/B.Pm for cortical bone in the tibial diaphysis are indicated. (C) Representative images showing periosteal and endosteal fluorochrome labels (×10 magnification). The group means ± standard error are depicted. P values for the main comparisons (surgery and treatment) are indicated when the interaction was not significant. For parameters with a significant interaction, pairwise comparisons were made between individual groups, and statistically significant differences are indicated by solid lines.
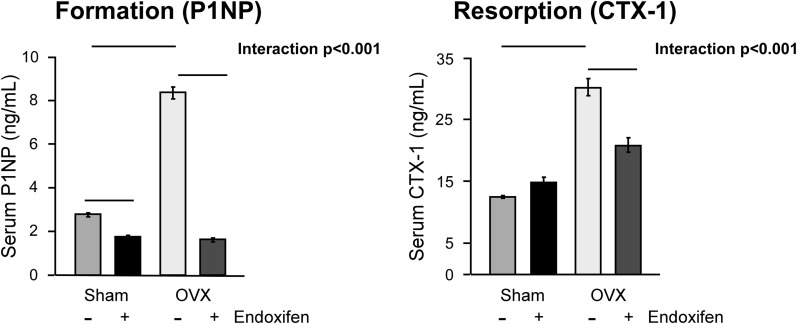
Biochemical analysis of bone turnover markers
To parallel our histomorphometric analyses, we also quantitated the serum levels of a global bone formation (P1NP) and global bone resorption (CTX-1) marker. As with the dynamic histomorphometric analyses, a significant interaction between treatment and surgery was detected for both of these serum markers (Fig. 5). Endoxifen treatment resulted in substantially lower circulating levels of P1NP in both sham-operated and ovariectomized rats (Fig. 5). Additionally, endoxifen resulted in lower CTX-1 levels in ovariectomized animals (Fig. 5). These data correlate well with the results of the histomorphometric studies described earlier and indicate that endoxifen suppresses bone turnover in ovariectomized rats.
Figure 5.
Serum levels of bone turnover markers in ovary-intact and ovariectomized (OVX) rats following 34 days of treatment with endoxifen or vehicle. Circulating levels of a bone formation marker (P1NP) and a bone resorption marker (CTX-1) were determined by enzyme-linked immunosorbent assay analysis. The group means ± standard error are depicted. Given significant interactions, pairwise comparisons were made between individual groups, and statistically significant differences are indicated by solid lines.
Uterine response to endoxifen
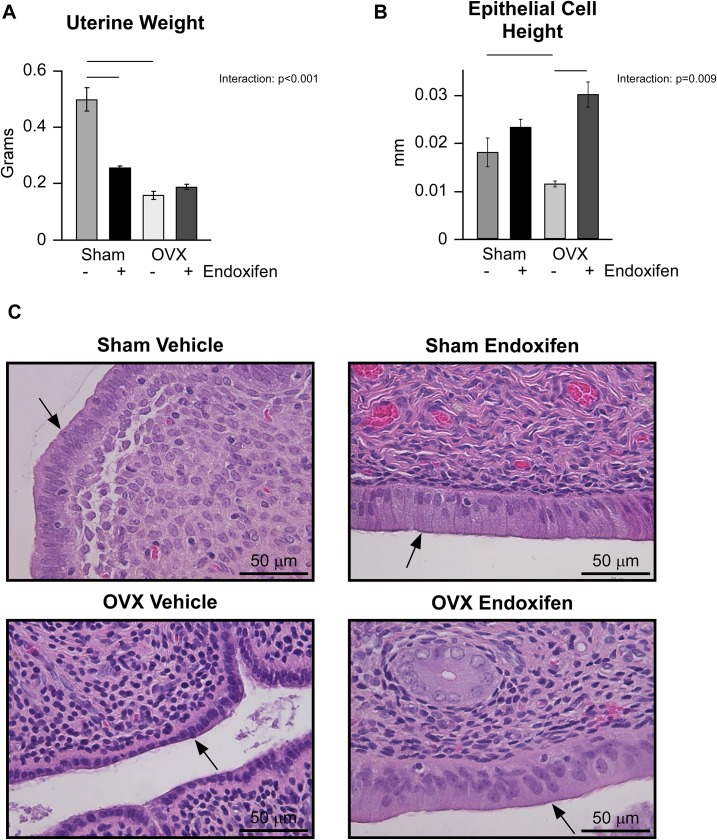
In addition to bone, SERMs are known to impact the uterus. As a first step toward elucidating the effects of endoxifen on this organ in our model system, we examined uterine weights. A significant interaction was detected between treatment and surgery groups (Fig. 6A). As expected, uterine wet weight was substantially lower in ovariectomized rats compared with sham-operated controls (Fig. 6A). Endoxifen treatment resulted in substantially lower uterine weight in intact rats, but it had no effect in ovariectomized animals (Fig. 6A). As an extension of these studies, luminal epithelial cell height was also determined. As shown in Fig. 6B, an interaction was again detected between treatment and surgical groups. Ovariectomy resulted in significantly lower epithelial cell height whereas endoxifen treatment resulted in significantly higher epithelial cell height only in ovariectomized rats (Fig. 6B). Representative H&E-stained sections of the uterus depicting the luminal epithelium are shown for each of the four animal groups in Fig. 6C.
Figure 6.
Effects of endoxifen on the rat uterus in ovary-intact and ovariectomized (OVX) animals following 34 days of treatment. Following treatment, (A) uterine wet weight and (B) luminal epithelial cell height were determined. (C) Representative H&E stain of rat uteri depicting the luminal epithelium (arrows) and surrounding endometrium. The group means ± standard error are depicted. Given significant interactions, pairwise comparisons were made between individual groups and statistically significant differences are indicated by solid lines.
Uterine expression of ERs following endoxifen treatment
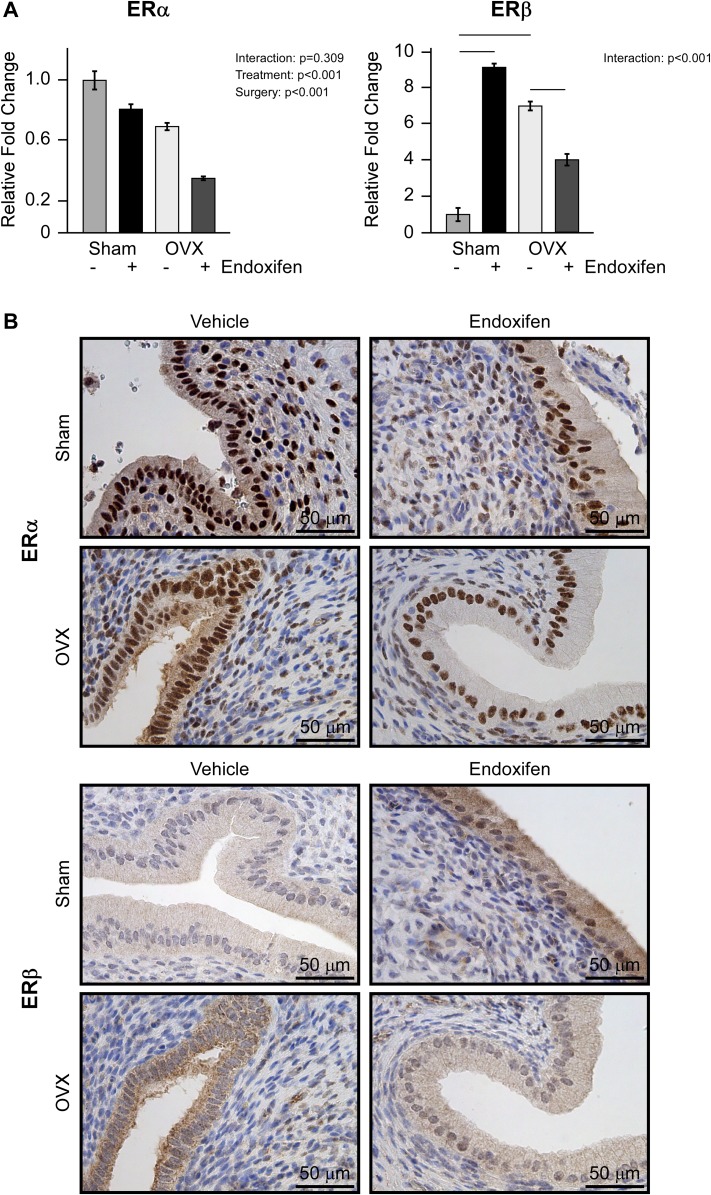
Given that SERMs elicit their effects through the actions of ERs, and because ERα and ERβ play important biological roles in the uterus (38), we examined the expression of these two nuclear receptors at the messenger RNA (mRNA) and protein level. As shown in Fig. 7A, no interaction between surgery and treatment was detected for ERα expression. However, ERα expression levels were significantly lower in ovariectomized rats compared with sham-operated animals and in endoxifen-treated rats compared with vehicle-treated controls (Fig. 7A). A significant interaction between treatment and surgery was detected for ERβ expression. Ovariectomy resulted in lower levels of ERβ mRNA compared with sham-operated controls (Fig. 7A). Endoxifen treatment resulted in higher ERβ expression in intact rats but lower expression in ovariectomized rats (Fig. 7A). These patterns of expression were observed for both ERα and ERβ at the protein level as determined by IHC analysis, and representative images are shown for each treatment group in Fig. 7B.
Figure 7.
Uterine ERα and ERβ expression following endoxifen treatment of ovary intact and ovariectomized (OVX) rats. (A) Reverse transcription PCR analysis of ERα and ERβ mRNA expression following 34 days of vehicle or endoxifen treatment. (B) Representative images depicting ERα and ERβ protein expression levels following vehicle or endoxifen treatment as detected by IHC analysis (×40 magnification, counterstained with hematoxylin). The group means ± standard error are depicted. P values for the main comparisons (surgery and treatment) are indicated when the interaction was not significant. For parameters with a significant interaction, pairwise comparisons were made between individual groups and statistically significant differences are indicated by solid lines.
Effect of endoxifen on uterine gene expression
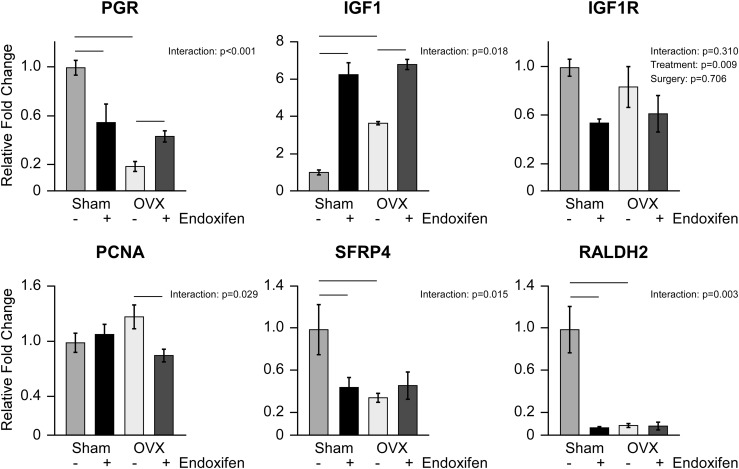
To further examine the impact of endoxifen on the uterus, we next determined the expression levels of genes known to be regulated by estrogen and/or SERMs in the organ. Significant interactions between treatment and surgery were detected for all of the genes analyzed with the exception of insulinlike growth factor 1 receptor (IGF1R) (Fig. 8). Progesterone receptor (PGR), secreted frizzled related protein (SFRP)1, and aldehyde dehydrogenase 1 family member A2 (RALDH2) expression levels were lower, whereas insulinlike growth factor 1 (IGF1) levels were higher, in ovariectomized rats compared with sham-operated animals (Fig. 8). In intact rats, the expression levels of PGR, SFRP, and RALDH2 were decreased by endoxifen treatment whereas IGF1 levels were increased (Fig. 8). In ovariectomized animals, endoxifen treatment significantly increased the expression of PGR and IGF1 and decreased the expression of proliferating cell nuclear antigen (PCNA) (Fig. 8). Although no interaction was detected for IGF1R, endoxifen-treated animals exhibited significantly lower expression levels of this gene in the uterus relative to vehicle-treated controls (Fig. 8).
Figure 8.
Gene expression analysis of ER target genes and proliferation markers in the uterus following 34 days of vehicle and endoxifen treatment. Reverse transcription PCR analysis of PGR, IGF1, IGF1R, PCNA, SFRP4, and RALDH2 in the ovary-intact and ovariectomized (OVX) rat uterus. The group means ± standard error are depicted. P values for the main comparisons (surgery and treatment) are indicated when the interaction was not significant. For parameters with a significant interaction, pairwise comparisons were made between individual groups and statistically significant differences are indicated by solid lines.
Discussion
Endoxifen is being investigated as a novel endocrine therapy for the treatment of ERα-positive breast cancer and is currently in phase I and II clinical trials (NCT02311933, NCT01327781, and NCT01273168). In this study, we used ovary-intact and ovariectomized rats to comprehensively characterize the effects of endoxifen on bone and uterus. Endoxifen treatment resulted in reduced bone turnover in ovary-intact and ovariectomized rats. These findings are consistent with prior studies evaluating SERM and estrogen impacts on bone (23). With regard to the uterus, endoxifen treatment decreased uterine weight, with no significant effects on epithelial cell height, in ovary-intact rats. In ovariectomized rats, endoxifen had no effect on uterine weight but maintained epithelial cell height at levels similar to those of ovary-intact animals. Endoxifen also regulated the expression of important genes in the uterus, including suppression of ERα expression in both ovary-intact and ovariectomized rats. Interestingly, endoxifen treatment increased expression of ERβ in ovary-intact rats and attenuated the expression of ERβ following ovariectomy.
Previously, we have examined the effects of endoxifen on bone using ovariectomized C57BL/6 mice. Our results demonstrated that an anticancer dose of endoxifen increased total body bone mineral content and bone mineral density with the largest changes observed in trabecular bone compartments (39). In this mouse model, endoxifen treatment increased the numbers and activity of both osteoblasts and osteoclasts, suggesting that endoxifen increases bone turnover (39). Although our previous studies were the first to shed light on the potential effects of endoxifen on bone, we did not examine the impact of endoxifen on other target tissues such as the uterus or determine the effects of endoxifen on the skeleton of ovary-intact animals. Furthermore, although the mouse is commonly used as a model system for characterizing the effects of novel drugs on organ systems, it does not recapitulate the effects of exogenous estrogens observed in humans. The sensitivity of mice to estrogen is well documented and as such mice are often used to test estrogenic activity of pharmacological agents. However, a seminal series of studies by Urist et al. (40) demonstrated that the skeletal response to estrogen in mice differs drastically from that of other mammals, including humans. For these reasons, we have used Sprague Dawley rats in the present study because they have accurately predicted the effects of SERMs on cancellous bone and uterus in humans (24, 41).
Tamoxifen was originally developed in 1966 as a birth control drug for women. Although it ultimately failed as an effective contraceptive in humans, tamoxifen began to gain a foothold in 1971 where the first clinical study demonstrated a promising effect of this compound as a novel anti–breast cancer agent. Tamoxifen was later shown to result in bone preservation in healthy postmenopausal women, as it increased bone mineral density in both the spine and hip (24). However, in premenopausal women, tamoxifen exposure resulted in decreased bone density (24). Nearly identical results have been observed in multiple studies examining the impact of tamoxifen on the skeleton of rats, lending confidence in the use of this animal model system for analyzing the impact of SERMs on bone (23, 24, 42). In this study, we demonstrate that endoxifen also elicits bone-sparing effects in cancellous bone compartments of ovariectomized rats. Specifically, higher BV/TV, connectivity density, and trabecular number, as well as lower trabecular spacing, were observed in the tibial metaphysis following endoxifen treatment of ovariectomized rats compared with vehicle-treated controls. The finding that connectivity density increases with loss of cancellous bone was unexpected but may arise from the selective removal of poorly connected trabeculae. Indeed, it has been shown that bone loss in the epiphysis, a site having highly connected trabeculae, is much lower than in the adjacent metaphysis that has lower connectivity (42). Within the fifth lumbar vertebrae, our data also revealed that endoxifen treatment resulted in higher BV/TV and trabecular number, and lower trabecular spacing, in ovariectomized rats. In cortical bone, endoxifen treatment resulted in decreased cross-sectional volume, cortical volume, and cortical thickness at the tibial diaphysis of ovariectomized and sham-operated rats. These effects of endoxifen resemble those previously reported for tamoxifen in rats (23, 43). Note that the lower cross-sectional volume in endoxifen-treated rats in our study was due to the suppressive effects of endoxifen on bone accrual, which would not be relevant to postmenopausal women. Rats used in this experiment (16 weeks at treatment initiation) were still growing and as such continued to accrue bone. We included cortical data to provide a more complete description of the skeletal response to endoxifen in this model. In growing rats, ovariectomy increases periosteal bone formation, and this response has been shown to be antagonized by estrogen and partial estrogen agonists such as tamoxifen (44–47). Furthermore, we found that endoxifen maintains cancellous BV/TV at baseline levels in ovary-intact rats and attenuates bone loss in ovariectomized rats (Supplemental Table 1 (16.5KB, xlsx) ).
In the ovary-intact rat, our studies revealed that endoxifen treatment resulted in higher BV/TV, connectivity density, and trabecular number as well as lower trabecular spacing. Similar trends were observed for BV/TV, trabecular number, and trabecular spacing in the fifth lumbar vertebrae of ovary-intact animals. With regard to cortical bone, cross-sectional volume, cortical volume, and cortical thickness were lower following endoxifen treatment. Similar to ovariectomized rats, the lower cortical bone parameters were due to suppression of bone accrual. These effects of endoxifen on cancellous bone in ovary-intact rats are in contrast to what is known to occur following tamoxifen treatment in rats in which substantial losses of cancellous bone, upwards of 30%, are observed (23, 48). Furthermore, tamoxifen is known to result in accelerated bone loss in premenopausal women due to suppression of estrogen signaling (24, 49, 50). Should endoxifen continue to move forward as a novel endocrine therapy for breast cancer, the present data suggest that one of the major negative side effects of tamoxifen in premenopausal patients (bone loss) may be less of a concern with the use of endoxifen.
At the cellular level, our studies indicate that endoxifen exposure results in more robust decreases in M.Pm/B.Pm, BFR/B.Pm, MAR, and Oc.Pm/B.PM in ovariectomized rats compared with sham-operated controls. These cellular changes were also reflected at the biochemical level where serum levels of a bone formation marker (P1NP) and a bone resorption marker (CTX-1) were significantly reduced following endoxifen treatment in ovariectomized rats. These effects of endoxifen on global bone formation and resorption in ovariectomized rats are similar to those previously reported for tamoxifen in preclinical rat models (23, 51).
With regard to the uterus, our data demonstrate that endoxifen treatment resulted in decreased uterine wet weight in ovary-intact rats with no effects on uterine weight in ovariectomized animals. These data are in agreement with a previous report in which short-term treatment (3 days) of ovariectomized rats with endoxifen also had no effect on uterine weight (52). However, our data contrast that of tamoxifen treatment, which is known to increase uterine wet weight in ovariectomized rat models (53–55). Although endoxifen treatment had no effect on uterine weight in ovariectomized rats, increased luminal epithelial cell height was observed. These data are in agreement with previous reports examining the impact of endoxifen and tamoxifen on luminal epithelial cell height in the uterus of ovariectomized rats (52, 54, 55). In ovary-intact animals, tamoxifen treatment has been shown to dramatically increase uterine luminal epithelial cell height (56), an effect that was not observed in the present study following endoxifen treatment. Despite the fact that endoxifen is a much more potent antiestrogen than tamoxifen in breast cancer (7, 35, 36), its effects on the uterus in the present study appear to be reduced as compared with that reported for tamoxifen.
Uterine epithelial cell hypertrophy and proliferation in response to estrogen or SERMs is mediated, at least in part, by the estrogen receptors and their impact on downstream growth factors and target genes. Following endoxifen treatment, we have demonstrated that ERα and ERβ mRNA and protein expression are reduced in ovariectomized rats, whereas ERβ expression is elevated in intact animals. The reduction in ERα levels following endoxifen treatment correlate well with previous studies from our laboratory demonstrating that endoxifen treatment of breast cancer cells also results in decreased ERα levels (7). The increased expression of ERβ in ovary-intact rats was inversely correlated with uterine weight. Given the known roles of ERβ as a growth inhibitor (36, 57) and the fact that ERβ knockout mice have increased epithelial hypertrophy (58, 59), it is possible that induction of this form of the estrogen receptor by endoxifen contributes to decreased uterine weight. It is also interesting to note that endoxifen treatment of mice resulted in increased ERβ expression in bone marrow stromal cells (39), suggesting that part of endoxifen’s mechanism of action may be through regulation of ERβ expression levels. Conversely, endoxifen treatment attenuated the increase in ERβ expression following ovariectomy. These opposing effects of endoxifen on ERβ expression may be due to differences in the hormonal milieu in intact and ovariectomized animals, a possibility that requires further investigation.
Uterine expression of the progesterone receptor, a well-characterized marker of estrogen signaling, was decreased following endoxifen treatment in ovary-intact rats but was increased in ovariectomized animals. Previous studies have demonstrated that tamoxifen induces IGF1 expression in the uterus, and it is thought that increased levels of IGF1 may contribute to the increased risk of endometrial cancer in tamoxifen-treated women (54, 60). The present data demonstrate that endoxifen enhances IGF1 expression in the rat uterus; however, expression of the IGF1 receptor was significantly decreased by endoxifen and could therefore negate the growth-promoting effects of IGF1 in this tissue. We also analyzed the expression levels of PCNA, a well-characterized cell proliferation marker in the uterus (61). Our data demonstrate that endoxifen did not affect the expression of PCNA in ovary-intact rats but significantly decreased its expression in ovariectomized animals. The data are in contrast to the effects of tamoxifen, which has been shown to induce PCNA expression in the uterus of ovariectomized rats (54), and add further evidence that endoxifen functions differently than tamoxifen in this tissue. We also examined the expression of two other estrogen regulated genes, SFRP4 and RALDH2 (62, 63), and demonstrated that endoxifen repressed the expression of these factors in the ovary-intact rat uterus to levels similar to those of estrogen depletion following ovariectomy. However, endoxifen did not induce these two genes in ovariectomized rats. These data, in combination with the uterine weight and epithelial cell height data, suggest that endoxifen functions as a partial estrogen agonist in the uterus.
Based on past work demonstrating superior anti–breast cancer effects of endoxifen compared with tamoxifen in cell lines and animal models (2, 7, 35, 36), a novel formulation of endoxifen has been developed and phase I and phase II clinical trials are ongoing at both the Mayo Clinic and National Cancer Institute. In the present study, we have used a rat model system and show that endoxifen reduces bone turnover in ovary-intact and ovariectomized animals. With regard to the uterus, endoxifen did not increase uterine wet weight in ovariectomized rats as is the case for tamoxifen, and it also resulted in suppression of a number of estrogen-regulated and proproliferative genes. Taken together, these data suggest that some of the known negative side effects of tamoxifen (bone loss in premenopausal women and increased risk of endometrial cancer in postmenopausal women) may be reduced with the use of endoxifen. The relevance of these findings is further magnified by the fact that maintenance of bone mass in breast cancer patients is of critical importance, and the identification of breast cancer therapies that either do not affect the skeleton, or elicit beneficial effects on this tissue, continues to represent a critical unmet clinical need. Future studies are required to fully elucidate the dose-dependent effects of endoxifen on the human skeleton and uterus.
Acknowledgments
We thank Dr. Thomas C. Spelsberg for excellent career mentorship, support, and helpful suggestions.
Financial Support: This work was supported by the National Cancer Institute of the National Institutes of Health Award P50CA116201 (Mayo Clinic Breast Cancer SPORE), National Institutes of Health Training Grants F32AR063596 and T32AR056950, and by a generous gift from Bruce and Martha Atwater and the Eisenberg Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BFR/B.Pm
- bone formation rate per bone perimeter
- BV/TV
- bone volume/tissue volume
- CTX-1
- C-telopeptide of type I collagen
- ER
- estrogen receptor
- micro-CT
- microcomputed tomography
- H&E
- hematoxylin and eosin
- IGF1
- insulinlike growth factor 1
- IGF1R
- insulinlike growth factor 1 receptor
- IHC
- immunohistochemistry
- MAR
- mineral apposition rate
- M.Pm/B.Pm
- mineralizing perimeter per bone perimeter
- mRNA
- messenger RNA
- Oc.Pm/B.Pm
- osteoclast perimeter per bone perimeter
- PCNA
- proliferating cell nuclear antigen
- PCR
- polymerase chain reaction
- PGR
- progesterone receptor
- P1NP
- procollagen type 1 amino-terminal propeptide
- RALDH2
- aldehyde dehydrogenase 1 family member A2
- SERM
- selective estrogen receptor modulator
- SFRP
- secreted frizzled related protein.
References
- 1.Mauvais-Javis P, Baudot N, Castaigne D, Banzet P, Kuttenn F. trans-4-Hydroxytamoxifen concentration and metabolism after local percutaneous administration to human breast. Cancer Res. 1986;46(3):1521–1525. [PubMed] [Google Scholar]
- 2.Johnson MD, Zuo H, Lee KH, Trebley JP, Rae JM, Weatherman RV, Desta Z, Flockhart DA, Skaar TC. Pharmacological characterization of 4-hydroxy-N-desmethyl tamoxifen, a novel active metabolite of tamoxifen. Breast Cancer Res Treat. 2004;85(2):151–159. [DOI] [PubMed] [Google Scholar]
- 3.Sideras K, Ingle JN, Ames MM, Loprinzi CL, Mrazek DP, Black JL, Weinshilboum RM, Hawse JR, Spelsberg TC, Goetz MP. Coprescription of tamoxifen and medications that inhibit CYP2D6. J Clin Oncol. 2010;28(16):2768–2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95(23):1758–1764. [DOI] [PubMed] [Google Scholar]
- 5.Mürdter TE, Schroth W, Bacchus-Gerybadze L, Winter S, Heinkele G, Simon W, Fasching PA, Fehm T, Eichelbaum M, Schwab M, Brauch H; German Tamoxifen and AI Clinicians Group . Activity levels of tamoxifen metabolites at the estrogen receptor and the impact of genetic polymorphisms of phase I and II enzymes on their concentration levels in plasma. Clin Pharmacol Ther. 2011;89(5):708–717. [DOI] [PubMed] [Google Scholar]
- 6.Westermann B, Dörner S, Brauch S, Schaks A, Heinke R, Stark S, van Delft FL, van Berkel SS. CuAAC-mediated diversification of aminoglycoside-arginine conjugate mimics by non-reducing di- and trisaccharides. Carbohydr Res. 2013;371:61–67. [DOI] [PubMed] [Google Scholar]
- 7.Wu X, Hawse JR, Subramaniam M, Goetz MP, Ingle JN, Spelsberg TC. The tamoxifen metabolite, endoxifen, is a potent antiestrogen that targets estrogen receptor alpha for degradation in breast cancer cells. Cancer Res. 2009;69(5):1722–1727. [DOI] [PubMed] [Google Scholar]
- 8.Wu X, Subramaniam M, Grygo SB, Sun Z, Negron V, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC, Hawse JR. Estrogen receptor-beta sensitizes breast cancer cells to the anti-estrogenic actions of endoxifen. Breast Cancer Res. 2011;13(2):R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW, Nikoloff DM, Hillman G, Fontecha MR, Lawrence HJ, Parker BA, Wu AH, Pierce JP. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89(5):718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brauch H, Schroth W, Goetz MP, Mürdter TE, Winter S, Ingle JN, Schwab M, Eichelbaum M. Tamoxifen use in postmenopausal breast cancer: CYP2D6 matters. J Clin Oncol. 2013;31(2):176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordan VC, Morrow M. Tamoxifen, raloxifene, and the prevention of breast cancer. Endocr Rev. 1999;20(3):253–278. [DOI] [PubMed] [Google Scholar]
- 12.Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—mechanisms of action and application to clinical practice. N Engl J Med. 2003;348(7):618–629. [DOI] [PubMed] [Google Scholar]
- 13.Pfaffl MW, Lange IG, Daxenberger A, Meyer HH. Tissue-specific expression pattern of estrogen receptors (ER): quantification of ERα and ERβ mRNA with real-time RT-PCR. APMIS. 2001;109(5):345–355. [DOI] [PubMed] [Google Scholar]
- 14.Tee MK, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors α and β. Mol Biol Cell. 2004;15(3):1262–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology. 1997;138(3):863–870. [DOI] [PubMed] [Google Scholar]
- 16.Webb P, Nguyen P, Kushner PJ. Differential SERM effects on corepressor binding dictate ERα activity in vivo. J Biol Chem. 2003;278(9):6912–6920. [DOI] [PubMed] [Google Scholar]
- 17.Bramlett KS, Burris TP. Effects of selective estrogen receptor modulators (SERMs) on coactivator nuclear receptor (NR) box binding to estrogen receptors. Mol Genet Metab. 2002;76(3):225–233. [DOI] [PubMed] [Google Scholar]
- 18.Pickar JH, Komm BS. Selective estrogen receptor modulators and the combination therapy conjugated estrogens/bazedoxifene: a review of effects on the breast. Post Reprod Health. 2015;21(3):112–121. [DOI] [PubMed] [Google Scholar]
- 19.Mirkin S, Pickar JH. Selective estrogen receptor modulators (SERMs): a review of clinical data. Maturitas. 2015;80(1):52–57. [DOI] [PubMed] [Google Scholar]
- 20.Migliaccio S, Brama M, Spera G. The differential effects of bisphosphonates, SERMS (selective estrogen receptor modulators), and parathyroid hormone on bone remodeling in osteoporosis. Clin Interv Aging. 2007;2(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein SR, Siddhanti S, Ciaccia AV, Plouffe L Jr. A pharmacological review of selective oestrogen receptor modulators. Hum Reprod Update. 2000;6(3):212–224. [DOI] [PubMed] [Google Scholar]
- 22.Kawai M, Modder UI, Khosla S, Rosen CJ. Emerging therapeutic opportunities for skeletal restoration. Nat Rev Drug Discov. 2011;10(2):141–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sibonga JD, Evans GL, Hauck ER, Bell NH, Turner RT. Ovarian status influences the skeletal effects of tamoxifen in adult rats. Breast Cancer Res Treat. 1996;41(1):71–79. [DOI] [PubMed] [Google Scholar]
- 24.Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14(1):78–84. [DOI] [PubMed] [Google Scholar]
- 25.Berstein LM, Yue W, Wang JP, Santen RJ. Isolated and combined action of tamoxifen and metformin in wild-type, tamoxifen-resistant, and estrogen-deprived MCF-7 cells. Breast Cancer Res Treat. 2011;128(1):109–117. [DOI] [PubMed] [Google Scholar]
- 26.Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol. 2008;26(7):1051–1057. [DOI] [PubMed] [Google Scholar]
- 27.Komm BS, Chines AA. An update on selective estrogen receptor modulators for the prevention and treatment of osteoporosis. Maturitas. 2012;71(3):221–226. [DOI] [PubMed] [Google Scholar]
- 28.Komm BS, Mirkin S. An overview of current and emerging SERMs. J Steroid Biochem Mol Biol. 2014;143:207–222. [DOI] [PubMed] [Google Scholar]
- 29.Gralow JR. Bone density in breast cancer: when to intervene? J Clin Oncol. 2007;25(22):3194–3197. [DOI] [PubMed] [Google Scholar]
- 30.Fornander T, Hellström AC, Moberger B. Descriptive clinicopathologic study of 17 patients with endometrial cancer during or after adjuvant tamoxifen in early breast cancer. J Natl Cancer Inst. 1993;85(22):1850–1855. [DOI] [PubMed] [Google Scholar]
- 31.Bernstein L, Deapen D, Cerhan JR, Schwartz SM, Liff J, McGann-Maloney E, Perlman JA, Ford L. Tamoxifen therapy for breast cancer and endometrial cancer risk. J Natl Cancer Inst. 1999;91(19):1654–1662. [DOI] [PubMed] [Google Scholar]
- 32.Iqbal J, Ginsburg OM, Wijeratne TD, Howell A, Evans G, Sestak I, Narod SA. Endometrial cancer and venous thromboembolism in women under age 50 who take tamoxifen for prevention of breast cancer: a systematic review. Cancer Treat Rev. 2012;38(4):318–328. [DOI] [PubMed] [Google Scholar]
- 33.Fischer SF, Rehm M, Bauer A, Höfling F, Kirschnek S, Rutz M, Bauer S, Wagner H, Häcker G. Toll-like receptor 9 signaling can sensitize fibroblasts for apoptosis. Immunol Lett. 2005;97(1):115–122. [DOI] [PubMed] [Google Scholar]
- 34.Chia SK, Wolff AC. With maturity comes confidence: EBCTCG tamoxifen update. Lancet. 2011;378(9793):747–749. [DOI] [PubMed] [Google Scholar]
- 35.Hawse JR, Subramaniam M, Cicek M, Wu X, Gingery A, Grygo SB, Sun Z, Pitel KS, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC. Endoxifen’s molecular mechanisms of action are concentration dependent and different than that of other anti-estrogens. PLoS One. 2013;8(1):e54613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X, Subramaniam M, Grygo SB, Sun Z, Negron V, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC, Hawse JR. Estrogen receptor-beta sensitizes breast cancer cells to the anti-estrogenic actions of endoxifen. Breast Cancer Res. 2011;13(2):R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu X, Subramaniam M, Negron V, Cicek M, Reynolds C, Lingle WL, Goetz MP, Ingle JN, Spelsberg TC, Hawse JR. Development, characterization, and applications of a novel estrogen receptor beta monoclonal antibody. J Cell Biochem. 2012;113(2):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosenfeld CS, Wagner JS, Roberts RM, Lubahn DB. Intraovarian actions of oestrogen. Reproduction. 2001;122(2):215–226. [DOI] [PubMed] [Google Scholar]
- 39.Gingery A, Subramaniam M, Pitel KS, Reese JM, Cicek M, Lindenmaier LB, Ingle JN, Goetz MP, Turner RT, Iwaniec UT, Spelsberg TC, Hawse JR. The effects of a novel hormonal breast cancer therapy, endoxifen, on the mouse skeleton. PLoS One. 2014;9(5):e98219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Urist MR, Budy AM, McLean FC. Endosteal-bone formation in estrogen-treated mice. J Bone Joint Surg Am. 1950;32A(1):143–162, illust. [PubMed] [Google Scholar]
- 41.Lelovas PP, Xanthos TT, Thoma SE, Lyritis GP, Dontas IA. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008;58(5):424–430. [PMC free article] [PubMed] [Google Scholar]
- 42.Westerlind KC, Wronski TJ, Ritman EL, Luo ZP, An KN, Bell NH, Turner RT. Estrogen regulates the rate of bone turnover but bone balance in ovariectomized rats is modulated by prevailing mechanical strain. Proc Natl Acad Sci USA. 1997;94(8):4199–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karimian E, Chagin AS, Gjerde J, Heino T, Lien EA, Ohlsson C, Savendahl L. Tamoxifen impairs both longitudinal and cortical bone growth in young male rats. J Bone Miner Res. 2008;23(8):1267–1277. [DOI] [PubMed] [Google Scholar]
- 44.Turner RT, Vandersteenhoven JJ, Bell NH. The effects of ovariectomy and 17β-estradiol on cortical bone histomorphometry in growing rats. J Bone Miner Res. 1987;2(2):115–122. [DOI] [PubMed] [Google Scholar]
- 45.Turner RT, Wakley GK, Hannon KS, Bell NH. Tamoxifen prevents the skeletal effects of ovarian hormone deficiency in rats. J Bone Miner Res. 1987;2(5):449–456. [DOI] [PubMed] [Google Scholar]
- 46.Turner RT, Backup P, Sherman PJ, Hill E, Evans GL, Spelsberg TC. Mechanism of action of estrogen on intramembranous bone formation: regulation of osteoblast differentiation and activity. Endocrinology. 1992;131(2):883–889. [DOI] [PubMed] [Google Scholar]
- 47.Turner RT, Colvard DS, Spelsberg TC. Estrogen inhibition of periosteal bone formation in rat long bones: down-regulation of gene expression for bone matrix proteins. Endocrinology. 1990;127(3):1346–1351. [DOI] [PubMed] [Google Scholar]
- 48.Moon LY, Wakley GK, Turner RT. Dose-dependent effects of tamoxifen on long bones in growing rats: influence of ovarian status. Endocrinology. 1991;129(3):1568–1574. [DOI] [PubMed] [Google Scholar]
- 49.Vehmanen L, Elomaa I, Blomqvist C, Saarto T. Tamoxifen treatment after adjuvant chemotherapy has opposite effects on bone mineral density in premenopausal patients depending on menstrual status. J Clin Oncol. 2006;24(4):675–680. [DOI] [PubMed] [Google Scholar]
- 50.Sverrisdóttir A, Fornander T, Jacobsson H, von Schoultz E, Rutqvist LE. Bone mineral density among premenopausal women with early breast cancer in a randomized trial of adjuvant endocrine therapy. J Clin Oncol. 2004;22(18):3694–3699. [DOI] [PubMed] [Google Scholar]
- 51.Aktug H, Uslu S, Terek MC, Terzi H, Turgut M, Özsener S, Bilgin O. Effects of ovariectomy and tamoxifen on rat bone tissue: histomorphometric and histopathologic study. Anal Quant Cytol Histol. 2006;28(4):207–212. [PubMed] [Google Scholar]
- 52.Schweikart KM, Eldridge SR, Safgren SL, Parman T, Reid JM, Ames MM, Goetz MP, Davis MA. Comparative uterotrophic effects of endoxifen and tamoxifen in ovariectomized Sprague-Dawley rats. Toxicol Pathol. 2014;42(8):1188–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carthew P, Edwards RE, Nolan BM, Tucker MJ, Smith LL. Compartmentalized uterotrophic effects of tamoxifen, toremifene, and estradiol in the ovariectomized Wistar (Han) rat. Toxicol Sci. 1999;48(2):197–205. [DOI] [PubMed] [Google Scholar]
- 54.Stygar D, Muravitskaya N, Eriksson B, Eriksson H, Sahlin L. Effects of SERM (selective estrogen receptor modulator) treatment on growth and proliferation in the rat uterus. Reprod Biol Endocrinol. 2003;1:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kwekel JC, Forgacs AL, Burgoon LD, Williams KJ, Zacharewski TR. Tamoxifen-elicited uterotrophy: cross-species and cross-ligand analysis of the gene expression program. BMC Med Genomics. 2009;2:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nephew KP, Osborne E, Lubet RA, Grubbs CJ, Khan SA. Effects of oral administration of tamoxifen, toremifene, dehydroepiandrosterone, and vorozole on uterine histomorphology in the rat. Proc Soc Exp Biol Med. 2000;223(3):288–294. [DOI] [PubMed] [Google Scholar]
- 57.Reese JM, Suman VJ, Subramaniam M, Wu X, Negron V, Gingery A, Pitel KS, Shah SS, Cunliffe HE, McCullough AE, Pockaj BA, Couch FJ, Olson JE, Reynolds C, Lingle WL, Spelsberg TC, Goetz MP, Ingle JN, Hawse JR. ERβ1: characterization, prognosis, and evaluation of treatment strategies in ERα-positive and -negative breast cancer. BMC Cancer. 2014;14:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wada-Hiraike O, Hiraike H, Okinaga H, Imamov O, Barros RP, Morani A, Omoto Y, Warner M, Gustafsson JA. Role of estrogen receptor β in uterine stroma and epithelium: Insights from estrogen receptor β−/− mice. Proc Natl Acad Sci USA. 2006;103(48):18350–18355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nakajima T, Tanimoto Y, Tanaka M, Chambon P, Watanabe H, Iguchi T, Sato T. Neonatal estrogen receptor β is important in the permanent inhibition of epithelial cell proliferation in the mouse uterus. Endocrinology. 2015;156(9):3317–3328. [DOI] [PubMed] [Google Scholar]
- 60.Roy RN, Gerulath AH, Cecutti A, Bhavnani BR. Effect of tamoxifen treatment on the endometrial expression of human insulin-like growth factors and their receptor mRNAs. Mol Cell Endocrinol. 2000;165(1-2):173–178. [DOI] [PubMed] [Google Scholar]
- 61.Lai MD, Lee LR, Cheng KS, Wing LY. Expression of proliferating cell nuclear antigen in luminal epithelium during the growth and regression of rat uterus. J Endocrinol. 2000;166(1):87–93. [DOI] [PubMed] [Google Scholar]
- 62.Fujita M, Ogawa S, Fukuoka H, Tsukui T, Nemoto N, Tsutsumi O, Ouchi Y, Inoue S. Differential expression of secreted frizzled-related protein 4 in decidual cells during pregnancy. J Mol Endocrinol. 2002;28(3):213–223. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Q, Shen Q, Celestino J, Milam MR, Westin SN, Lacour RA, Meyer LA, Shipley GL, Davies PJA, Deng L, McCampbell AS, Broaddus RR, Lu KH. Enhanced estrogen-induced proliferation in obese rat endometrium. Am J Obstet Gynecol. 2009;200(2):186.e1–186.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]