Abstract
Activation of the renin–angiotensin–aldosterone system is common in hypertension and obesity and contributes to cardiac diastolic dysfunction, a condition for which no treatment currently exists. In light of recent reports that antihyperglycemia incretin enhancing dipeptidyl peptidase (DPP)-4 inhibitors exert cardioprotective effects, we examined the hypothesis that DPP-4 inhibition with saxagliptin (Saxa) attenuates angiotensin II (Ang II)–induced cardiac diastolic dysfunction. Male C57BL/6J mice were infused with either Ang II (500 ng/kg/min) or vehicle for 3 weeks receiving either Saxa (10 mg/kg/d) or placebo during the final 2 weeks. Echocardiography revealed Ang II–induced diastolic dysfunction, evidenced by impaired septal wall motion and prolonged isovolumic relaxation, coincident with aortic stiffening. Ang II induced cardiac hypertrophy, coronary periarterial fibrosis, TRAF3-interacting protein 2 (TRAF3IP2)-dependent proinflammatory signaling [p-p65, p-c-Jun, interleukin (IL)-17, IL-18] associated with increased cardiac macrophage, but not T cell, gene expression. Flow cytometry revealed Ang II–induced increases of cardiac CD45+F4/80+CD11b+ and CD45+F4/80+CD11c+ macrophages and CD45+CD4+ lymphocytes. Treatment with Saxa reduced plasma DPP-4 activity and abrogated Ang II–induced cardiac diastolic dysfunction independent of aortic stiffening or blood pressure. Furthermore, Saxa attenuated Ang II–induced periarterial fibrosis and cardiac inflammation, but not hypertrophy or cardiac macrophage infiltration. Analysis of Saxa-induced changes in cardiac leukocytes revealed Saxa-dependent reduction of the Ang II-mediated increase of cardiac CD11c messenger RNA and increased cardiac CD8 gene expression and memory CD45+CD8+CD44+ lymphocytes. In summary, these results demonstrate that DPP-4 inhibition with Saxa prevents Ang II–induced cardiac diastolic dysfunction, fibrosis, and inflammation associated with unique shifts in CD11c-expressing leukocytes and CD8+ lymphocytes.
Abrogation of Ang II–induced cardiac dysfunction by DPP-4 inhibition with Saxa is associated with reduced cardiac inflammation, perivascular fibrosis, and activation of CD8+ T cells in the heart.
Diastolic dysfunction is an early functional defect in a variety of cardiac diseases (1, 2) and an independent predictor of cardiac mortality (3). The prevalence of diastolic dysfunction is highest in patients with hypertension or obesity and is linked to inappropriate activation of the renin–angiotensin–aldosterone system (RAAS) (4, 5). Despite these known associations, no treatment currently exists for this common condition (6).
Clinically, dipeptidyl peptidase (DPP)-4 inhibitors have been shown to improve blood glucose control in patients with type 2 diabetes by reducing the breakdown of incretin hormones [i.e., glucagonlike peptide 1 (GLP-1), gastric inhibitory peptide (GIP)] (7). Accumulating evidence suggests however, that DPP-4 inhibition may provide direct cardiovascular protection in a number of disease states. Specifically, DPP-4 inhibition improved cardiac function in preclinical models of pressure overload by aortic banding (8), left ventricular radiofrequency ablation-induced heart failure (9), diabetic myocardial infarction (10, 11), obesity (12, 13), and isoproterenol infusion (14). However, in high-fat-fed diabetic mice, DPP-4 inhibition did not improve cardiac function and, in fact, exacerbated adverse cardiac remodeling following pressure overload (15). Furthermore, in a recent clinical trial [Saxagliptin Assessment of Vascular Outcomes Recorded in Patients with Diabetes Mellitus–Thrombolysis in Myocardial Infarction 53 (SAVOR-TIMI 53)] treatment with the DPP-4 inhibitor saxagliptin (Saxa) increased the risk of hospitalization for heart failure in diabetic patients (16). These disparate findings prompted the ongoing Saxagliptin and Cardiac Structure and Function (SCARF) trial to comprehensively examine the impact of Saxa on cardiac outcomes in diabetic patients (ClinicalTrials.gov Identifier: NCT02481479). Together, these data demonstrate mixed results of DPP-4 inhibition on cardiac function in complex disease states where the metabolic and cardiac impacts of DPP-4 inhibition can be difficult to separate. Thus, studies to better understand the impact of DPP-4 inhibition on common contributors to cardiac dysfunction in these conditions (i.e., RAAS activation), independent of the metabolic impact of DPP-4 inhibition, are warranted and necessary.
Cardiac diastolic dysfunction is characterized by cardiac inflammation and fibrosis (6). Indeed, recent evidence suggests cardiac inflammation leading to leukocyte infiltration as a proximate signal underlying RAAS-dependent cardiac fibrosis and diastolic dysfunction (17, 18) and that cardiac leukocyte infiltration corresponds with the onset of cardiac dysfunction (19, 20). In this context, DPP-4 inhibition has been shown to reduce tissue inflammation, leukocyte infiltration, and fibrosis (21). Indeed, we recently reported that DPP-4 inhibition improved obesity-associated cardiac diastolic dysfunction involving reduced inflammation and fibrosis (22). In particular, DPP-4 inhibition reduced obesity- and RAAS-dependent upregulation of cardiac inflammatory mediators including TRAF3-interacting protein 2 (TRAF3IP2), a critical regulator of cardiac inflammation (22–25). In addition, DPP-4 inhibition may modulate tissue inflammation and fibrosis via altered DPP-4–dependent immune regulation and/or increased persistence of immunomodulatory substrates of DPP-4 resulting in altered tissue leukocyte infiltration or phenotypes. Indeed, DPP-4 is an integral contributor to immune regulation, particularly T-cell activation (26), and, beyond GLP-1 and GIP, important chemokines (e.g., CCL5, CXCL12, CCL22) are DPP-4 substrates (27). Thus, whether these pleiotropic effects of DPP-4 inhibition result from reduced tissue inflammation, inhibition of leukocyte infiltration, alterations in leukocyte phenotypes, or some combination of these and their relationship to cardiac function remains unclear in the setting of RAAS activation.
In the current study, we directly addressed this gap in knowledge by evaluating the hypothesis that selective inhibition of DPP-4 with Saxa (28) abrogates angiotensin II (Ang II)–induced cardiac diastolic dysfunction. We examined this hypothesis via in vivo determination of cardiac function coupled with ex vivo molecular assays and flow cytometry to evaluate cardiac inflammation and fibrosis as well as cardiac leukocyte subpopulations.
Methods
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Missouri. Male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME) were continuously infused with Ang II (500 ng·kg−1·min−1 subcutaneously; Sigma, St. Louis, MO) or sterile saline for 3 weeks via osmotic minipumps (Alzet 1004) beginning at 13 weeks of age, as previously reported (29, 30). After one week of infusion, mice were treated with either the DPP-4 inhibitor Saxa (10 mg·kg−1·d−1, orally in peanut butter) or placebo (oral peanut butter) during the last 2 weeks. Mice were given ad libitum access to water and standard rodent chow and were housed in a temperature-controlled facility on a 12:12-hour light–dark cycle throughout the study. Animals were anesthetized with isoflurane (2% to 4% in 100% O2) and euthanized by exsanguination. The experiments were repeated twice, that is, two separate cohorts of n = 6 per group underwent the treatments described previously and analyzed as detailed later with n = 5 to 11 observations per endpoint.
Plasma chemistries
On the day of euthanasia, mice were fasted for 5 hours and anesthetized with isoflurane (2% to 4% in 100% O2); blood was collected via heart stick, processed for plasma, and frozen at –80°C. Blood glucose was determined by glucometer (AlphaTRAK 2; Zoetis, Parsippany, NJ). Plasma aldosterone levels were quantified by radioimmunoassay at the Vanderbilt Hormone Assay and Analytical Services Core (Nashville, TN) as an indication of Ang II minipump efficacy. Plasma DPP-4 activity was determined by quantification of fluorescence from H-Ala-Pro-AFC (excitation 405 nm, emission 535 nm), a substrate that emits a signal upon cleavage by DPP-4, as previously described (12). All other plasma measures were analyzed at Comparative Clinical Pathology Services (Columbia, MO).
Blood pressure
Systolic blood pressure (SBP) was determined using tail cuff plethysmography in a subset of Ang II and Ang II+Saxa mice during the last week of treatment, as previously described (30).
Echocardiography
Two-dimensional echocardiography was performed in the apical four-chamber view using a GE Vividi ultrasound instrument with a 10.5-MHz pediatric ultrasound probe. Initially, a small sample volume was positioned in the left ventricle just proximal to the mitral leaflets to acquire early and late diastolic blood flow velocities in pulse wave Doppler mode. From the pulse wave spectra we determined isovolumic relaxation time (IVRT) and isovolumic contraction time. Next, to evaluate the propagation velocity (Vp) of left ventricular inflow, a correlate of the rate of chamber relaxation, we performed color M-mode recordings of mitral inflow during early diastole at the mitral leaflets in an apical window. Finally, tissue Doppler imaging was performed by placing a sample volume at the septal annulus to acquire early and late septal annular velocities. Parameters were assessed using an average of three beats from three different Doppler spectra, and calculations were made in accordance with the American Society of Echocardiography guidelines as well as specific guidelines for rodent echocardiography. All data were acquired and analyzed offline by a single blinded observer.
Aortic pulse wave velocity
Aortic pulse wave velocity (PWV), an index of vascular stiffness, was assessed in vivo as previously described (31, 32). Briefly, mice were anesthetized with isoflurane (2% in 100% O2), and PWV assessed via Doppler ultrasound (Indus Mouse Doppler System, Webster, TX) using the foot-to-foot velocity method employing velocity waveforms obtained successively at a brief time interval, the first obtained at the top of the aortic arch and the second at a site along the abdominal aorta 35 mm distal to the arch, as previously described (31, 32). PWV was calculated by the formula PWV = D (mm) / ΔT (ms), where D is the distance between two measurement sites and ΔT is the transit time of the progressing pulse wave determined by the formula (T1 – T0). All values of T1 and T0 are gated to a heart rate of 400 bpm by multiplying the actual times by the ratio of the RR interval at 400 bpm by the actual RR interval of the sampled waveforms.
Atomic force microscopy
Aortic endothelial cortical stiffness was assessed on en face aortic preparations using atomic force microscopy (AFM), as previously described (33). Briefly, a 2-mm segment of thoracic aorta was carefully opened longitudinally, fastened (endothelial side up) to a plastic cover slip with Cell-Tak, and endothelial stiffness determined via a cell nanoindentation protocol with AFM at room temperature, as previously described (34).
Immunohistochemistry and staining
Cross-sectional cardiac slices were immersion fixed in 3% paraformaldehyde, dehydrated in ethanol, paraffin embedded, and sectioned in 5-µm slices, as previously described (30). To evaluate cardiac fibrosis, sections were stained with picrosirius red (PR) for determination of cardiac interstitial and periarterial collagen. Images were obtained using an EVOS FL Auto Imaging System and quantified using the thresholding function in ImageJ. Periarterial fibrosis was quantified as the ratio of PR-stained periarterial area to vessel luminal area. Interstitial fibrosis was quantified as percent area of myocardial PR staining. Cardiomyocyte cross-sectional area was determined in Alexa Fluor 488-tagged wheat germ agglutinin (1:100, Thermo Fisher W11261)–stained cardiac sections. Images were obtained using a Leica DMI4000b confocal microscope with quantification of cross-sectional area in ImageJ.
Immunoblots
Left ventricular cardiac tissue was homogenized in lysis buffer containing protease and phosphatase inhibitors in a TissueLyser (Qiagen, Germantown, MD), as previously described (30). Protein content of lysates was determined by bicinchoninic acid protein assay (Pierce, Rockford, IL). Electrophoresis and immunoblotting were performed, as previously described (24). The following primary antibodies were used (amount of protein per lane indicated in parentheses): CXCL12 (15 µg; 1:1000; catalog no. 3740; Cell Signaling Technology), c-Jun (20 µg; 1:1000; Cell Signaling Technology 9165), phospho-c-Jun (20 µg; Ser63, 1:1000; Cell Signaling Technology 9261), interleukin (IL)-17A (15 µg; 0.1 µg/mL; R&D Systems AF-421-SP), mature IL-18 (15 µg; 2 µg/mL; R&D Systems D048-3), phospho-p65 (20 µg; Ser536, 1:1000; Cell Signaling Technology 3031), p65 (20 µg; 1:1000; catalog no. 8242; Cell Signaling Technology ), periostin (15 µg; 0.5 µg/mL; catalog no. ab14041; Abcam), TRAF3IP2 (20 µg; 1:600; catalog no. bs-6202R; Bioss), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (15 or 20 µg; 1:1000; catalog no. sc-25778; Santa Cruz Biotechnology). Information regarding antibodies used in this study is presented in Table 1.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| c-Jun | Cell Signaling, 9165 | Rabbit; monoclonal | 1:1000 | AB_2130165 | ||
| Phospho-c-Jun Ser63 | Cell Signaling, 9261 | Rabbit; polyclonal | 1:1000 | AB_2130162 | ||
| CD4 | APC/Cy7-conjugated anti-CD4 | Biolegend, 100414 | Rat; monoclonal | 0.25 μg/106 cells | AB_312699 | |
| CD8 | Brilliant Violet 785- conjugated anti-CD8 | Biolegend, 100750 | Rat; monoclonal | 0.25 μg/106 cells | AB 2562610 | |
| CD11b | PerCP/Cy5.5-conjugated anti-CD11b | Biolegend, 101228 | Rat; monoclonal | 0.25 μg/106 cells | AB_893232 | |
| CD11c | Brilliant Violet 785- conjugated anti-CD11c | Biolegend, 117336 | Armenian hamster; monoclonal | 0.25 μg/106 cells | AB_2565268 | |
| CD44 | PerCP-Cy5.5-conjugated anti-CD44 | Biolegend, 103032 | Rat; monoclonal | 0.25 μg/106 cells | AB_2076204 | |
| CD45 | Brilliant Violet 421- conjugated anti-CD45 | BD Pharmigen, 563890 | Rat; monoclonal | 0.25 μg/106 Cells | AB 2651151 | |
| CXCL10 | R&D Systems, AF-466-NA | Goat; polyclonal | 1 μg/mL | AB_2292487 | ||
| CXCL12 | Cell Signaling, 3740 | Rabbit; polyclonal | 1:1000 | AB_2292511 | ||
| F4/80 | APC-conjugated anti- F4/80 | Biolegend, 123116 | Rat; monoclonal | 0.25 μg/106 cells | AB_893481 | |
| Fcy receptor 111/11 | eBioscience, 14-0161 | Rat; monoclonal | 0.25 μg/106 cells | AB_467132 | ||
| GAPDH | Santa Cruz, sc-25778 | Rabbit; polyclonal | 1:1000 | AB_10167668 | ||
| IL-17A | R&D Systems, AF-421-SP | Goat; polyclonal | 0.1 μg/mL | AB_354487 | ||
| IL-18 (mature) | R&D Systems, D048-3 | Rat; monoclonal | 2 μg/mL | AB_2123796 | ||
| p65 | Cell Signaling, 8242 | Rabbit; monoclonal | 1:1000 | AB_10859369 | ||
| Phospho-p65 Ser536 | Cell Signaling, 3031 | Rabbit; polyclonal | 1:1000 | AB_330559 | ||
| Periostin | Abcam, ab14041 | Rabbit; polyclonal | 0.5 μg/mL | AB_2299859 | ||
| TRAF3IP2 | Bioss, bs-6202R | Rabbit; polyclonal | 1:600 | AB_11101583 |
Abbreviation: RRID, Research Resource Identifier.
Real-time polymerase chain reaction
Left ventricular cardiac tissue was homogenized in a TissueLyser (Qiagen) and total RNA was isolated using the Qiagen RNeasy Fibrous Tissue Kit and assayed using a Nanodrop spectrophotometer (Thermo Scientific, Wilmington, DE) to assess purity and concentration, as previously described (30). First-strand cDNA was synthesized from total RNA using the Improm-II reverse transcription kit (Promega, Madison, WI) and quantitative real-time polymerase chain reaction (PCR) was performed using the CFX Connect Real-Time PCR Detection System (Biorad, Hercules, CA) using target specific primers (Supplemental Table 1 (20.9KB, docx) ). PCR reactions using iTaq Universal SYBR Green SMX (Biorad), thermal conditions, and melt curve analysis were performed as previously described (30). GAPDH was used as an internal control gene and messenger RNA (mRNA) expression values were calculated based on cycle thresholds (CTs) via the 2ΔCT, where ΔCT = GAPDH CT – gene of interest CT and are presented normalized to control mice, which were set at 1.
Flow cytometry
Animals used for flow cytometry were injected with heparin (300 IU intraperitoneal) 10 minutes prior to being anesthetized with isoflurane (2% to 4% in 100% O2). Blood was collected via heart stick and mice were perfused via the heart with ice-cold phosphate-buffered saline after which the heart was rapidly removed and placed on ice in fluorescence-activated cell sorting (FACS) buffer (eBioscience, San Diego, CA). Hearts were minced with scissors and enzymatically digested via incubation for 1 hour at 37°C in FACS buffer containing liberase (Roche; 8.7 µg/mL), collagenase XI (1250 U/mL), hyaluronidase (60 U/mL), and deoxyribonuclease (60 U/mL). Hearts were then mechanically homogenized for 1 minute (Seward Stomacher 80), filtered through a 40-µm cell strainer (50 G for 1 minute), and pelleted at 300 G for 8 minutes. Following removal of the supernatant, heart cells were resuspended in sucrose buffer and separated from cellular debris using a percoll (72%)–sucrose/percoll (36%) gradient and centrifugation at 1500 rpm for 20 minutes. Heart cells isolated in the gradient were collected, resuspended in FACS buffer, and divided for separate staining protocols described later. Peripheral blood mononuclear cells were isolated from blood following separation of plasma by three cycles of red blood cell lysis in lysis buffer for 5 minutes at room temperature followed by washing in FACS buffer, pelleting, decanting, and resuspension in FACS buffer after which cells were divided for separate staining protocols. For staining, heart cells and peripheral blood mononuclear cells were preblocked with anti-Fcγ receptor III/II antibody (clone 93, eBioscience 14-0161) at 4°C for 15 minutes, and stained in the dark at 4°C for 30 minutes with the following antibodies (Table 1): brilliant violet (BV) 421-conjugated anti-CD45 (clone 30-F11; BD Pharmigen 563890), APC-conjugated anti-F4/80 (clone BM8; Biolegend 123116), PerCP/Cy5.5-conjugated anti-CD11b (clone M1/70; Biolegend 101228), BV785-conjugated anti-CD11c (clone N418; Biolegend 117335), APC/Cy7-conjugated anti-CD4 (clone GK1.5; Biolegend 100414), BV785-conjugated anti-CD8 (clone 53-6.7; Biolegend 100750), and PerCP-Cy5.5-conjugated anti-CD44 (clone IM7; Biolegend 103032). Separate sets of cells were stained for myeloid markers (CD45, F4/80, CD11b, CD11c), lymphocyte markers (CD45, CD4, CD8, CD44), and viability (FVS510, BD Biosciences, San Jose, CA). Cell fluorescence was measured with an LSRFortessa X-20 flow cytometer (BD Biosciences) and data analysis was performed using FlowJo software (Ashland, OR). Cell isolation and flow cytometry were performed on one animal per group per day.
Statistical Analysis
Data are presented as means ± standard error. Statistical analysis was performed using Student t test for planned comparisons and one-way analysis of variance with Fisher least significant difference post hoc analysis, as appropriate, in SigmaPlot (Systat, San Jose, CA). A P value ≤ 0.05 was considered significant.
Results
DPP-4 inhibition does not impact metabolic markers and Ang II–dependent aortic stiffening or blood pressure
No treatment altered body or spleen weight, blood glucose, plasma insulin, or insulin sensitivity (homeostatic model assessment of insulin resistance) relative to control mice (Table 2). Ang II infusion (subcutaneous 500 ng·kg−1·min−1), however, resulted in elevated plasma aldosterone concentrations regardless of placebo or Saxa treatment. As expected, Saxa administration attenuated plasma DPP-4 activity ∼60% regardless of vehicle or Ang II infusion, similar to previous reports (8, 35) (Table 2). Furthermore, consistent with prior reports (8, 36, 37), Saxa did not alter SBP in Ang II-infused mice (Ang II alone: 152 ± 14 mmHg, Ang II+Saxa: 156 ± 24 mmHg). However, Ang II infusion increased aortic and endothelial cortical stiffness, assessed by PWV and AFM, respectively (Table 2). Saxa treatment abrogated endothelial cortical, but not aortic, stiffening (Table 2).
Table 2.
Phenotypic Characteristics of Study Animals by Group
| Con | Con+Saxa | Ang II | Ang II+Saxa | |
|---|---|---|---|---|
| Morphologic and plasma measures | ||||
| Body weight, g | 25.5 ± 0.6 | 25.4 ± 0.8 | 24.5 ± 0.7 | 23.9 ± 0.7 |
| Spleen weight, mg | 70 ± 6 | 66 ± 6 | 64 ± 5 | 61 ± 4 |
| Blood glucose, mg·dL–1 | 140 ± 9 | 127 ± 15 | 127 ± 11 | 116 ± 15 |
| Plasma insulin, ng·mL–1 | 0.23 ± 0.07 | 0.22 ± 0.05 | 0.20 ± 0.04 | 0.14 ± 0.01 |
| HOMA-IR | 1.00 ± 0.09 | 1.42 ± 0.46 | 1.08 ± 0.28 | 0.74 ± 0.12 |
| Plasma aldosterone, pg·mL–1 | 232 ± 44 | 184 ± 25 | 608 ± 137a | 454 ± 139a |
| Plasma DPP-4 activity, RLU rel to Con | 1.00 ± 0.12 | 0.40 ± 0.03b | 1.26 ± 0.19 | 0.42 ± 0.01b |
| Vascular measures | ||||
| Aortic PWV, m·s–1 | 3.3 ± 0.2 | 3.4 ± 0.1 | 4.1 ± 0.2a | 4.0 ± 0.3a |
| Aortic PWV adjusted,c m·s–1 | 3.1 ± 0.2 | 3.0 ± 0.3 | 4.2 ± 0.3a | 3.8 ± 0.4d |
| Endothelial cortical stiffness, kPa | 3.0 ± 0.4 | 3.9 ± 1.4 | 19.5 ± 4.9a | 3.6 ± 0.7e |
Values are mean ± standard error, n = 5 to 12.
Abbreviations: HOMA-IR, homeostatic model assessment of insulin resistance; RLU, relative light unit.
P < 0.05 vs Con/Con+Saxa.
P < 0.05 vs Con/Ang II.
Aortic PWV adjusted is adjusted to 400-bpm heart rate.
P = 0.08 vs Con+Saxa.
P < 0.05 vs Ang II.
Ang II–induced cardiac diastolic dysfunction is abrogated by DPP-4 inhibition
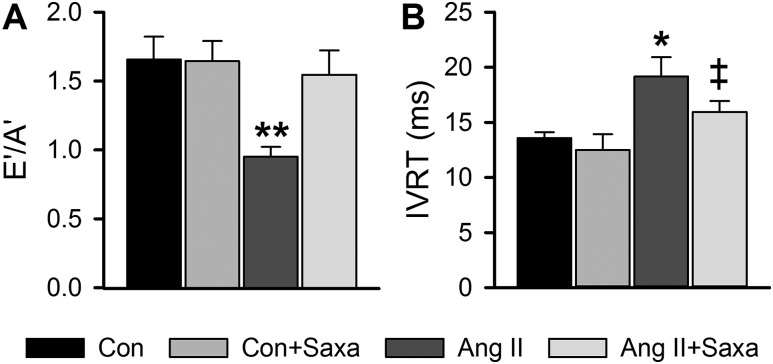
Cardiac diastolic function, assessed by echocardiography, was impaired by Ang II infusion. Specifically, Ang II reduced the ratio of early-to-late septal annulus motion in diastole, increased IVRT, and tended to reduce the Vp of mitral inflow [Vp: P = 0.06 vs Con (Fig. 1A and 1B and Supplemental Table 2 (20.9KB, docx) )]. DPP-4 inhibition with Saxa improved diastolic function indicated by normalization of early-to-late septal annulus motion in diastole and a tendency to reduce IVRT (Fig. 1A and 1B). These changes occurred independently of changes in isovolumic contraction time, an index of systolic function, or heart rate (Supplemental Table 2 (20.9KB, docx) ).
Figure 1.
DPP-4 inhibition abrogates Ang II–induced cardiac diastolic dysfunction. Indices of cardiac diastolic function, specifically ratio of (A) early-to-late septal annulus motion in diastole (E’/A’) and (B) IVRT. Values are mean ± standard error; n = 6 to 7. *P < 0.05 vs Con/Con+Saxa; **P < 0.05 vs all other groups; ‡P = 0.08 vs Ang II.
DPP-4 inhibition prevents Ang II–induced cardiac periarterial fibrosis but not hypertrophy
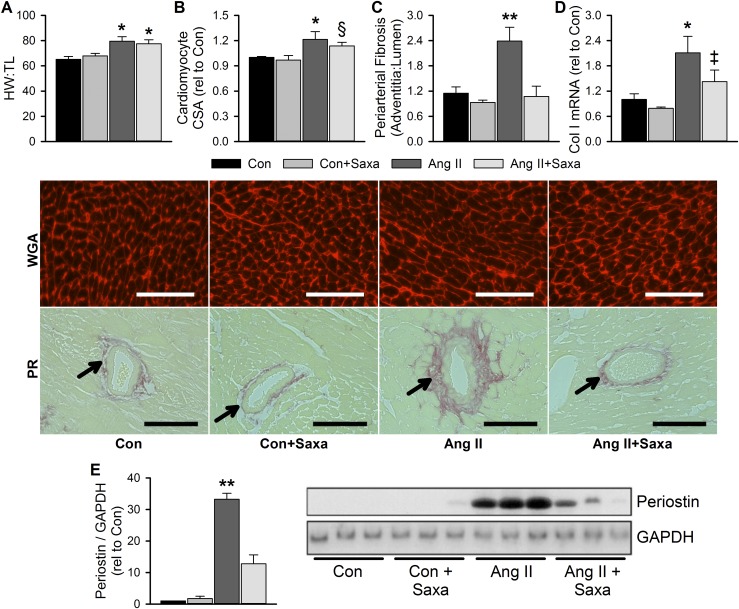
Given the link between diastolic dysfunction and cardiac fibrosis, we examined cardiac fibrosis and hypertrophy in response to Ang II and Saxa treatments. Infusion of Ang II induced cardiac and cardiomyocyte hypertrophy that was not affected by Saxa treatment (Fig. 2A and 2B). Conversely, Ang II infusion elicited coronary periarterial fibrosis, assessed by PR staining, which was abolished by Saxa treatment (Fig. 2C). Further, whereas Ang II increased cardiac collagen I mRNA expression, Saxa tended to reduce its expression in Ang II-infused mice (P = 0.09; Fig. 2D). In addition, Ang II infusion increased cardiac periostin expression, a marker of fibroblast activation, which was attenuated by Saxa (Fig. 2E). Cardiac interstitial fibrosis was unchanged by Ang II infusion or Saxa treatment (data not shown).
Figure 2.
Ang II–induced cardiac periarterial fibrosis and fibroblast activation, but not hypertrophy, is resolved by DPP-4 inhibition. Cardiac hypertrophy, assessed by (A) heart weight–to–tibia length (HW:TL) and (B) cardiomyocyte cross-sectional area (CSA), and fibrosis, assessed by (C) periarterial PR staining, (D) collagen I (Col I) mRNA expression, and (E) periostin expression by immunoblot as an index of fibroblast activation. Representative wheat germ agglutinin (WGA) and PR images for CSA and periarterial fibrosis, respectively, in the middle panel. Scale bar is 100 µm. Periarterial fibrosis quantified as the ratio of adventitial PR staining to lumen area (adventitia:lumen). Stained arterioles indicated by arrows. Representative immunoblot with matching GAPDH control in lower panel. Values are mean ± standard error; n = 4 to 10. *P < 0.05 vs Con/Con+Saxa; **P < 0.05 vs all other groups; §P = 0.06 vs Con+Saxa; ‡P = 0.09 vs Ang II.
DPP-4 inhibition ameliorates Ang II–induced cardiac fibrotic signaling and inflammation
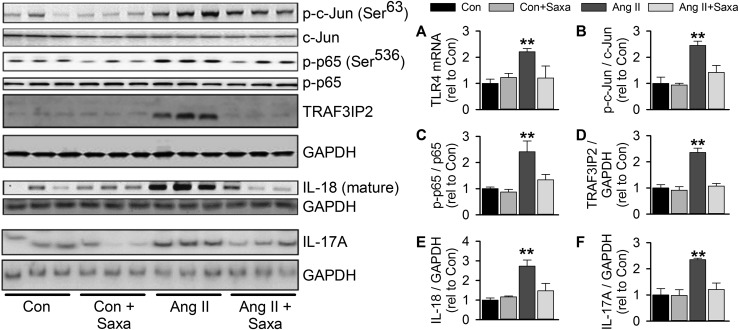
Ang II–induced cardiac fibrosis and inflammation have been attributed to activation of toll-like receptor 4 (TLR4), nuclear factor (NF)-κB, and activator protein (AP)-1 signaling as well as cardiac lymphocyte infiltration (17, 18, 38, 39). Therefore, in light of links between these processes and DPP-4 activity (40), we evaluated the impact of Ang II infusion and concomitant Saxa treatment on cardiac inflammation and profibrotic signaling. Ang II induced cardiac inflammation indicated by increased TLR4 expression that was abrogated by Saxa (Fig. 3A). Ang II induced activation of AP-1 and NF-κB in the heart, as indicated by increased phospho-c-Jun and phospho-p65 levels (Fig. 3B and 3C). Accordingly, Ang II increased cardiac expression of TRAF3IP2, an upstream activator of NF-κB and AP-1 signaling (41, 42) (Fig. 3D). Furthermore, cardiac mature IL-18 and IL-17A levels were increased by Ang II infusion (Fig. 3E and 3F). Notably, Saxa inhibited the Ang II–induced activation of these cardiac proinflammatory/profibrotic signaling intermediates (Fig. 3).
Figure 3.
Activation of cardiac inflammatory and fibrotic signaling by Ang II is ameliorated by DPP-4 inhibition. (A) Cardiac gene expression of TLR4 and (B) immunoblot assessment of cardiac AP-1 signaling by Ser63 p-c-Jun to total c-Jun, (C) NF-κB signaling by Ser536 phospho-p65 (p-p65) to total p65, (D) expression of TRAF3IP2, (E) mature IL-18, and (F) IL-17A. Representative immunoblots with matching GAPDH controls in left panel; GAPDH immunoblot for IL-17A is identical to that in Fig. 2 for periostin, as these targets were examined on the same membrane. Values are mean ± standard error; n = 4 to 5. **P < 0.05 vs all other groups.
DPP-4 inhibition differentially modulates cardiac DPP-4 substrates and Ang II–induced shifts in cardiac leukocyte subpopulations
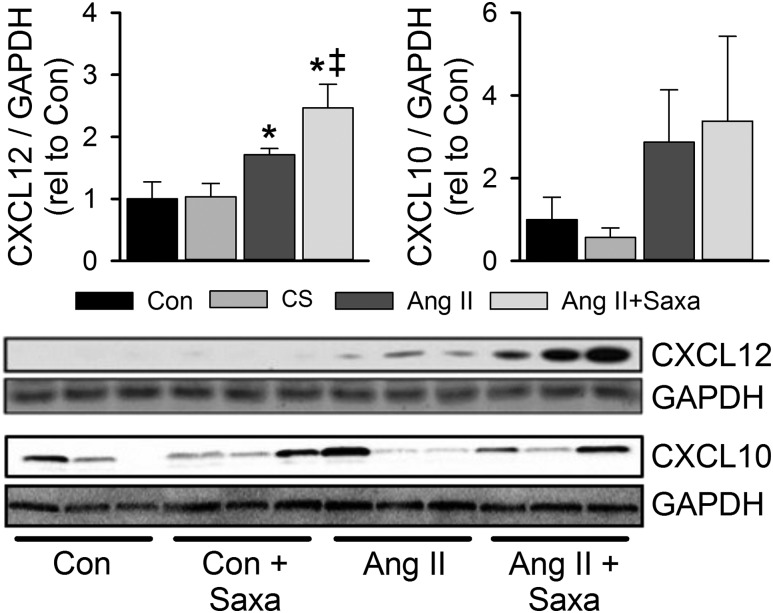
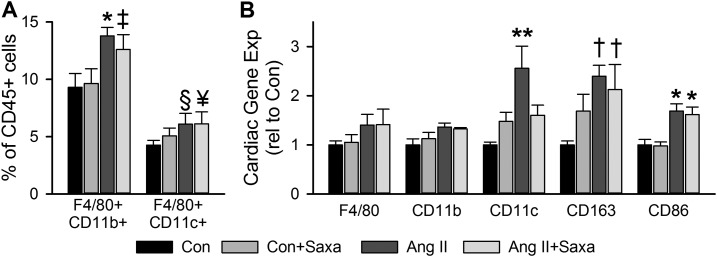
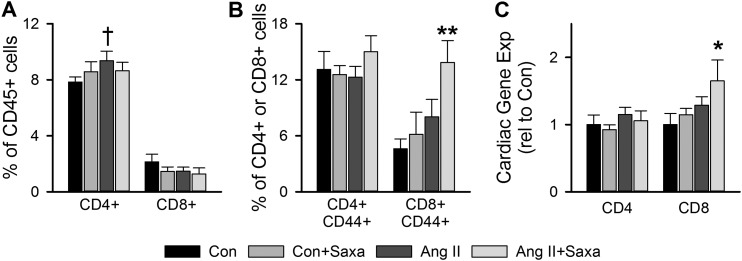
Recent evidence has revealed a role for cardiac leukocytes in cardiac dysfunction and that DPP-4 has immunomodulatory effects via its impact on important chemokines (17, 20, 26, 27, 43). Thus, we evaluated cardiac DPP-4 substrate expression by immunoblot and specific cardiac leukocyte subpopulations by flow cytometry and PCR. Infusion of Saxa alone did not alter cardiac expression of the DPP-4 substrates CXCL12 or CXCL10 (Fig. 4). Treatment with Ang II, however, increased cardiac CXCL12 but not CXCL10 and CXCL12 tended to be further increased with Saxa in the setting of Ang II infusion (P = 0.065; Fig. 4). Flow cytometry revealed Ang II–induced increases of cardiac CD45+F4/80+CD11b+ and CD45+F4/80+CD11c+ macrophages that were not affected by Saxa treatment (Fig. 5A). Furthermore, Ang II infusion increased cardiac presence of the M1-like macrophage mRNA CD11c, the M2-like macrophage mRNA CD163, and the macrophage activation mRNA CD86 despite no change in the pan-macrophage mRNA F4/80 and CD11b (Fig. 5B). Saxa treatment reduced the Ang II–induced increases in cardiac CD11c mRNA (Fig. 5B). With regard to cardiac lymphocytes, Ang II infusion resulted in increased cardiac CD45+CD4+, but not CD45+CD8+, T lymphocytes (Fig. 6A). However, although Ang II did not affect CD45+CD4+ or CD45+CD8+ lymphocyte activation, assessed by CD44+ staining, Saxa treatment increased CD45+CD8+CD44+ T cells (i.e., activated memory CD8+ lymphocytes) in the setting of Ang II infusion (Fig. 6B). Accordingly, cardiac CD8 mRNA was increased by Ang II+Saxa, whereas Ang II infusion alone did not alter cardiac presence of CD4 and CD8 mRNA (Fig. 6C). Finally, neither Ang II nor Saxa treatment altered these circulating leukocyte populations (data not shown).
Figure 4.
Impact of Ang II and DPP-4 inhibition on cardiac DPP-4 substrates. Immunoblot analysis of cardiac CXCL12 and CXCL10 expression in cardiac lysates. Representative immunoblots with matching GAPDH controls in bottom panel; GAPDH gel for CXCL12 is identical to that in Fig. 3 for IL-18, as these targets were examined on the same membrane. Values are mean ± standard error; n = 4 to 5. *P < 0.05 vs Con/Con+Saxa; ‡P = 0.065 vs Ang II.
Figure 5.
DPP-4 inhibition differentially modulates Ang II–induced shifts in cardiac myeloid populations. (A) Flow cytometric analysis of cardiac CD45+F4/80+CD11b+ and CD45+F4/80+CD11c+ macrophages and (B) gene expression of myeloid marker mRNAs assessed in cardiac tissue. Values are mean ± standard error; n = 4 to 5. *P < 0.05 vs Con/Con+Saxa; **P < 0.05 vs all other groups; †P < 0.05 vs Con; ‡P = 0.06 vs Con; §P = 0.052 vs Con; ¥P = 0.065 vs Con.
Figure 6.
DPP-4 inhibition differentially modulates Ang II–induced shifts in cardiac lymphocyte subpopulations. Flow cytometric analysis of (A) cardiac T cells, (B) activated T cells, and (C) gene expression of T cell marker mRNAs in cardiac tissue. Values are mean ± standard error; n = 4 to 6. *P < 0.05 vs Con/Con+Saxa; **P < 0.05 vs all other groups; †P < 0.05 vs Con.
Discussion
This study demonstrates that DPP-4 inhibition with Saxa abrogates Ang II–induced cardiac diastolic dysfunction. The protective cardiac effect of Saxa was associated with (1) attenuated Ang II–induced cardiac diastolic dysfunction independent of changes in SBP, aortic stiffness, and metabolic indices; (2) reduction of Ang II–induced cardiac perivascular fibrosis, but not hypertrophy; (3) abrogation of Ang II–induced activation of cardiac fibrotic/inflammatory signaling (i.e., TLR4, NF-κB, AP-1, TRAF3IP2, IL-17A, IL-18); (4) no change in cardiac macrophage recruitment, but attenuated cardiac CD11c expression; and (5) increased activation of cardiac CD8+ lymphocytes in Ang II-infused mice. Overall, these findings indicate that Saxa effectively reduces Ang II–dependent cardiac diastolic dysfunction and supports a paradigm by which DPP-4 inhibition with Saxa is cardioprotective in the setting of RAAS activation.
Cardiac diastolic dysfunction is a common condition associated with hypertension and obesity as well as an independent predictor of mortality for which no treatment currently exists (3, 5). Results of the current study are consistent with previous reports that infusion of a moderate dose of Ang II in mice induces cardiac diastolic dysfunction and vascular stiffening (i.e., increased aortic PWV) independent of changes in systemic metabolism (18, 44–48). This dysfunction is associated with cardiac inflammation (i.e., upregulation of TLR4 expression, induction of TRAF3IP2, activation of NF-κB and AP-1), leukocyte infiltration, cardiac hypertrophy, and perivascular fibrosis. Previous reports using similar doses of Ang II demonstrated that Ang II–induced cardiac leukocyte infiltration involves increased cardiac mRNA or markers of macrophages (F4/80, CD68, Mac2, Mac3) and/or T cells (CD3, CD4, CD8) (44–46, 49–51). This infiltration appears to be rapid as evidenced by increased cardiac F4/80+CD11b+ macrophages as early as 2 days of Ang II infusion with the dose used in the current study (52). Using flow cytometry, our results extend these findings by demonstrating Ang II–induced increases in cardiac F4/80+CD11b+ and F4/80+CD11c+ macrophages at 3 weeks of infusion in conjunction with increased cardiac CD4+, but not CD8+, lymphocytes. Importantly, Ang II–induced cardiac diastolic dysfunction was abrogated by DPP-4 inhibition with Saxa coincident with reduced tissue inflammation and fibrosis.
Cardiac fibrosis and chamber stiffening is a primary contributor to cardiac diastolic dysfunction (53). It has been previously reported that the early manifestation of cardiac fibrosis is coronary perivascular fibrosis that subsequently progresses to interstitial fibrosis (54). Accordingly, our data reveal that short-term Ang II infusion induced perivascular, but not interstitial, fibrosis and increased cardiac collagen I gene expression in conjunction with cardiac fibroblast activation (i.e., increased cardiac periostin expression). DPP-4 inhibition with Saxa attenuated Ang II–induced perivascular fibrosis and fibroblast activation while tending to reduce collagen I gene expression, consistent with recent reports of reduced cardiac fibrosis in a swine model of pressure overload (8) and in rats infused with Ang II (55) following DPP-4 inhibition. In addition, several reports demonstrate reduced renal fibrosis following Saxa treatment in type I diabetes and hypertension (36, 56). Importantly, in our study, the antifibrotic effect of Saxa occurred independent of changes in SBP and aortic stiffening, suggesting a local cardiac effect of Saxa to reduce fibrosis. The lack of changes in systemic metabolic markers (i.e., plasma glucose, homeostatic model assessment of insulin resistance) by Ang II in this model in the absence or presence of Saxa further supports a local impact of Saxa to reduce cardiac fibrosis, resulting in improved cardiac function.
Previous work has established that pathologic cardiac fibrosis is preceded by cardiac inflammation and leukocyte infiltration (53, 54, 57). Modulation of cardiac inflammatory pathways may be a critical component underlying the antifibrotic and cardioprotective effect of DPP-4 inhibition in RAAS-dependent cardiac diastolic dysfunction. Indeed, many DPP-4 substrates are immunomodulatory chemokines (27) and, accordingly, our data indicate that DPP-4 inhibition in the setting of Ang II infusion tended to further increase cardiac expression of CXCL12, an important contributor to leukocyte recruitment and activation. Furthermore, our data demonstrate that Saxa attenuated Ang II–induced upregulation of TLR4 gene expression and TRAF3IP2 protein expression as well as activation of cardiac NF-κB and AP-1. TLR4 signaling is a primary contributor to Ang II–induced cardiac inflammation and dysfunction, independent of angiotensin type 1 receptor activation (18, 39). In addition, TRAF3IP2 is a critical signaling intermediate in cardiac inflammatory pathways, including activation of NF-κB, AP-1, and MAPK which are established contributors to cardiac pathology (23, 42, 58). The level at which DPP-4 inhibition with Saxa interrupts these inflammatory cascades remains unclear.
Recent evidence from our group demonstrates that TRAF3IP2 (aka CIKS or Act1) physically associates with TLR4 and this association mediates, in part, lipopolysaccharide-induced cardiomyocyte dysfunction (59). Thus, it is possible that Saxa acts to disrupt the association of TLR4 and TRAF3IP2, thereby limiting Ang II–induced cardiac inflammation. It is also possible that Saxa may directly prevent the Ang II–induced upregulation of TRAF3IP2 as we recently reported that the DPP-4 inhibitor linagliptin prevented obesity-associated cardiac TRAF3IP2 upregulation in vivo as well as aldosterone-induced TRAF3IP2 upregulation in cardiac fibroblasts in vitro (22). In addition, linagliptin has been shown to reduce Ang II–induced NF-κB signaling and collagen expression in cultured cardiac fibroblasts (38). Our data would seem to more directly support, however, reduced TRAF3IP2 signaling following Saxa treatment owing to reduced cardiac IL-17A that signals exclusively via TRAF3IP2 (42, 58). Involvement of TRAF3IP2 signaling underlying Ang II–induced cardiac dysfunction and attenuation of this pathway by Saxa is further supported by the Ang II–dependent increase of cardiac IL-18 and its attenuation by Saxa. IL-18 expression is highly responsive to TRAF3IP2 signaling (60). Together, these data suggest that the cardioprotective effect of DPP-4 inhibition in RAAS-dependent cardiac inflammation may involve inhibition of TRAF3IP2 upregulation/signaling. Further studies are warranted to confirm this hypothesis, such as studies utilizing TRAF3IP2 null mice.
The Saxa-mediated attenuation of Ang II–induced cardiac inflammation and fibrosis was associated with a marked attenuation of the Ang II–induced increase of cardiac CD11c mRNA. Furthermore, evaluation of cardiac leukocyte subpopulations by flow cytometry revealed no change in Ang II–induced cardiac F4/80+CD11b+ and F4/80+CD11c+ macrophage infiltration following DPP-4 inhibition. In addition to no impact of Saxa on infiltration of these cells, it also failed to reduce the Ang II–dependent increase of cardiac CD86 expression, a marker of macrophage activation. These results suggest that a CD11c-expressing leukocyte population may be suppressed by Saxa. In this regard, it was recently revealed that ∼60% of CD11c+ cells in normal adult mouse heart are MHCII+ dendritic cells and that cardiac infiltration of these cells is increased in response to transverse aortic constriction (TAC) (61). Importantly, limiting cardiac recruitment of these cells following TAC by depletion of bone marrow-derived CD11c+ dendritic cells attenuated TAC-induced cardiac hypertrophy, fibrosis, and dysfunction (61). Together, these data suggest that Ang II–induced cardiac macrophage recruitment is not reduced by Saxa and that Saxa may reduce cardiac CD11c+ dendritic cell recruitment in the setting of Ang II infusion. Further studies are required to carefully delineate the involvement of these cell subpopulations as CD11c is also expressed by murine natural killer cells (62, 63). Mechanistically, increased endothelial cortical stiffness has been linked to increased permeability and leukocyte extravasation (64). This is consistent with the impact of Saxa to attenuate endothelial cortical stiffness in concert with reduced CD11c in the heart and suggests an important endothelial cell-dependent cardioprotective mechanism of Saxa warranting further exploration.
One of the most intriguing findings of the current study is that Saxa treatment is associated with activation of cardiac CD8+CD44+ memory T lymphocytes. We believe our data indicates activation of resident CD8+ lymphocytes in light of no change in the overall frequency of cardiac CD45+CD8+ lymphocytes (i.e., no infiltration) coupled with the increased cardiac CD8 gene expression in the Ang II+Saxa group. The latter is in agreement with the well-described upregulation of T cell receptors upon activation and corresponds to increased cardiac CXCL12 expression in this treatment group. Importantly, CXCL12 has been reported as an activator/costimulator of CD8 lymphocytes (65, 66). Whether this phenomenon of CD8 activation is consequential with regard to the reduced fibrosis and improved cardiac function following Saxa treatment in Ang II-infused mice is unclear. CD8+ memory lymphocytes are known primarily as mediators of inflammatory and cytolytic immune responses (67). Recent studies, however, have identified CD8+ memory T cell populations possessing a regulatory and immunosuppressive phenotype. Specifically, CD8+CD122+PD-1+ T cells suppress T cell proliferation and inflammatory cytokine production in vitro and in vivo (68) and CD38 expressing CD8+ memory T cells suppress CD4+ T cell activation and autoimmune responses (69). The latter effect of CD8+ T cells was interferon-γ–dependent, similar to other studies demonstrating that CD8+CD44+CD62L+ T cells inhibit the development of allergic airway inflammation (70) and that interferon-γ–expressing CD8+ T cells attenuate CD4+ T cell-dependent renal fibrosis in a model of obstructive kidney injury (71). Thus, accumulating evidence supports a role for CD8+ T cell subpopulations in the regulation and/or suppression of pathologic immune responses. The possibility that a CD8 lymphocyte-dependent mechanism may be the underlying protective effect of Saxa is further suggested by recent evidence that the onset of cardiac dysfunction in several models corresponds to (19, 20) or is dependent on (43) the infiltration of T cells, similar to the Ang II–induced increase of cardiac CD4+ T cells in the current study. Further studies to mechanistically evaluate such a paradigm in the setting of cardiac diastolic dysfunction however, are needed.
In summary, our data reveal that DPP-4 inhibition with Saxa effectively attenuates Ang II–dependent cardiac inflammation, fibrosis, and diastolic dysfunction. Importantly, this benefit of Saxa occurred independent of SBP and changes in systemic metabolic markers. It is highly likely that Saxa may interrupt local inflammatory signaling by modulating cardiac inflammatory cascades (i.e., TLR4, TRAF3IP2), by attenuation of cardiac CD11c-expressing dendritic cells, or by activation of immunosuppressant CD8+ lymphocytes. To our knowledge, this is the first study to evaluate the impact of DPP-4 inhibition on cardiac leukocytes by flow cytometry in any model. Further studies are necessary to discriminate the proximate mechanism by which Saxa acts to reduce Ang II–induced cardiac fibrosis and diastolic dysfunction. The benefit of DPP-4 inhibition in our model contrasts a recent report that DPP-4 inhibition exacerbates cardiac fibrosis and dysfunction in aged, diabetic mice following TAC (15). Notwithstanding differences in study design, these data together suggest that TAC-induced cardiac dysfunction in aged, diabetic animals may not be primarily RAAS-dependent or that other deleterious signaling cascades are activated in this complex and chronic model with multiple comorbid conditions. Thus, the increased hospitalization for heart failure reported in diabetic patients with multiple risk factors in the SAVOR-TIMI 53 trial might have occurred due to similar mechanistic complexity (72). Whether Saxa improves cardiac diastolic dysfunction in diabetic patients, similar to the impact of Saxa in the current study, awaits results of the ongoing SCARF trial (ClinicalTrials.gov Identifier: NCT02481479).
Acknowledgments
We gratefully acknowledge the assistance of Nathan Rehmer and Mona Garro-Kacher.
Financial Support: This work was supported by funding from AstraZeneca (to S.B.B. and R.N.), Dialysis Clinics Inc. (to R.N.), the Department of Veterans Affairs Biomedical Laboratory Research and Development Grant CDA-2 IK2 BX002030 (to S.B.B.) and Grant I01 BX002255 and Research Career Scientist Award (to B.C.), National Institutes of Health Grant R01 HL107910 (to J.R.S.), and the use of resources and facilities at the Harry S. Truman Memorial Veterans Hospital in Columbia, Missouri. Saxagliptin was kindly provided by AstraZeneca.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AFM
- atomic force microscopy
- Ang II
- angiotensin II
- AP
- activator protein
- BV
- brilliant violet
- DPP
- dipeptidyl peptidase
- FACS
- fluorescence-activated cell sorting
- GIP
- gastric inhibitory peptide
- GLP-1
- glucagonlike peptide 1
- IL
- interleukin
- IVRT
- isovolumic relaxation time
- mRNA
- messenger RNA
- NF
- nuclear factor
- PCR
- polymerase chain reaction
- PR
- picrosirius red
- PWV
- pulse wave velocity
- RAAS
- renin–angiotensin–aldosterone system
- Saxa
- saxagliptin
- SBP
- systolic blood pressure
- TLR4
- toll-like receptor 4
- TRAF3IP2
- TRAF3-interacting protein 2
- Vp
- propagation velocity.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–e322. [DOI] [PubMed] [Google Scholar]
- 2.Schilling JD, Mann DL. Diabetic cardiomyopathy: bench to bedside. Heart Fail Clin. 2012;8(4):619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aljaroudi W, Alraies MC, Halley C, Rodriguez L, Grimm RA, Thomas JD, Jaber WA. Impact of progression of diastolic dysfunction on mortality in patients with normal ejection fraction. Circulation. 2012;125(6):782–788. [DOI] [PubMed] [Google Scholar]
- 4.Chinnaiyan KM, Alexander D, McCullough PA. Role of angiotensin II in the evolution of diastolic heart failure. J Clin Hypertens (Greenwich). 2005;7(12):740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. [DOI] [PubMed] [Google Scholar]
- 6.Lekavich CL, Barksdale DJ, Neelon V, Wu J-R. Heart failure preserved ejection fraction (HFpEF): an integrated and strategic review. Heart Fail Rev. 2015;20(6):643–653. [DOI] [PubMed] [Google Scholar]
- 7.Ahrén B. Emerging dipeptidyl peptidase-4 inhibitors for the treatment of diabetes. Expert Opin Emerg Drugs. 2008;13(4):593–607. [DOI] [PubMed] [Google Scholar]
- 8.Hiemstra JA, Lee DI, Chakir K, Gutiérrez-Aguilar M, Marshall KD, Zgoda PJ, Cruz Rivera N, Dozier DG, Ferguson BS, Heublein DM, Burnett JC, Scherf C, Ivey JR, Minervini G, McDonald KS, Baines CP, Krenz M, Domeier TL, Emter CA. Saxagliptin and tadalafil differentially alter cyclic guanosine monophosphate (cGMP) signaling and left ventricular function in aortic-banded mini-swine. J Am Heart Assoc. 2016;5(4):e003277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arruda-Junior DF, Martins FL, Dariolli R, Jensen L, Antonio EL, Dos Santos L, Tucci PJF, Girardi ACC. Dipeptidyl peptidase IV inhibition exerts renoprotective effects in rats with established heart failure. Front Physiol. 2016;7:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connelly KA, Advani A, Zhang Y, Advani SL, Kabir G, Abadeh A, Desjardins J-F, Mitchell M, Thai K, Gilbert RE. Dipeptidyl peptidase-4 inhibition improves cardiac function in experimental myocardial infarction: role of stromal cell-derived factor-1α. J Diabetes. 2016;8(1):63–75. [DOI] [PubMed] [Google Scholar]
- 11.Sauvé M, Ban K, Momen MA, Zhou Y-Q, Henkelman RM, Husain M, Drucker DJ. Genetic deletion or pharmacological inhibition of dipeptidyl peptidase-4 improves cardiovascular outcomes after myocardial infarction in mice. Diabetes. 2010;59(4):1063–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aroor AR, Sowers JR, Bender SB, Nistala R, Garro M, Mugerfeld I, Hayden MR, Johnson MS, Salam M, Whaley-Connell A, Demarco VG. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin-resistant male Zucker obese rats. Endocrinology. 2013;154(7):2501–2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bostick B, Habibi J, Ma L, Aroor A, Rehmer N, Hayden MR, Sowers JR. Dipeptidyl peptidase inhibition prevents diastolic dysfunction and reduces myocardial fibrosis in a mouse model of Western diet induced obesity. Metabolism. 2014;63(8):1000–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ikeda J, Kimoto N, Kitayama T, Kunori S. Cardiac DPP-4 inhibition by saxagliptin ameliorates isoproterenol-induced myocardial remodeling and cardiac diastolic dysfunction in rats. J Pharmacol Sci. 2016;132(1):65–70. [DOI] [PubMed] [Google Scholar]
- 15.Mulvihill EE, Varin EM, Ussher JR, Campbell JE, Bang KWA, Abdullah T, Baggio LL, Drucker DJ. Inhibition of dipeptidyl peptidase-4 impairs ventricular function and promotes cardiac fibrosis in high fat-fed diabetic mice. Diabetes. 2016;65(3):742–754. [DOI] [PubMed] [Google Scholar]
- 16.Scirica BM, Bhatt DL, Braunwald E, Steg PG, Davidson J, Hirshberg B, Ohman P, Frederich R, Wiviott SD, Hoffman EB, Cavender MA, Udell JA, Desai NR, Mosenzon O, McGuire DK, Ray KK, Leiter LA, Raz I; SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369(14):1317–1326. [DOI] [PubMed] [Google Scholar]
- 17.Frieler RA, Mortensen RM. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation. 2015;131(11):1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han J, Zou C, Mei L, Zhang Y, Qian Y, You S, Pan Y, Xu Z, Bai B, Huang W, Liang G. MD2 mediates angiotensin II-induced cardiac inflammation and remodeling via directly binding to Ang II and activating TLR4/NF-κB signaling pathway. Basic Res Cardiol. 2017;112(1):9. [DOI] [PubMed] [Google Scholar]
- 19.Salvador AM, Nevers T, Velázquez F, Aronovitz M, Wang B, Abadía Molina A, Jaffe IZ, Karas RH, Blanton RM, Alcaide P. Intercellular adhesion molecule 1 regulates left ventricular leukocyte infiltration, cardiac remodeling, and function in pressure overload-induced heart failure. J Am Heart Assoc. 2016;5(3):e003126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nevers T, Salvador AM, Grodecki-Pena A, Knapp A, Velázquez F, Aronovitz M, Kapur NK, Karas RH, Blanton RM, Alcaide P. Left ventricular T-cell recruitment contributes to the pathogenesis of heart failure. Circ Heart Fail. 2015;8(4):776–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aroor AR, Sowers JR, Jia G, DeMarco VG. Pleiotropic effects of the dipeptidylpeptidase-4 inhibitors on the cardiovascular system. Am J Physiol Heart Circ Physiol. 2014;307(4):H477–H492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aroor AR, Habibi J, Kandikattu HK, Garro-Kacher M, Barron B, Chen D, Hayden MR, Whaley-Connell A, Bender SB, Klein T, Padilla J, Sowers JR, Chandrasekar B, DeMarco VG. Dipeptidyl peptidase-4 (DPP-4) inhibition with linagliptin reduces western diet-induced myocardial TRAF3IP2 expression, inflammation and fibrosis in female mice. Cardiovasc Diabetol. 2017;16(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Erikson JM, Valente AJ, Mummidi S, Kandikattu HK, DeMarco VG, Bender SB, Fay WP, Siebenlist U, Chandrasekar B. Targeting TRAF3IP2 by genetic and interventional approaches inhibits ischemia/reperfusion-induced myocardial injury and adverse remodeling. J Biol Chem. 2017;292(6):2345–2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yariswamy M, Yoshida T, Valente AJ, Kandikattu HK, Sakamuri SSVP, Siddesha JM, Sukhanov S, Saifudeen Z, Ma L, Siebenlist U, Gardner JD, Chandrasekar B. Cardiac-restricted overexpression of TRAF3 interacting protein 2 (TRAF3IP2) results in spontaneous development of myocardial hypertrophy, fibrosis, and dysfunction. J Biol Chem. 2016;291(37):19425–19436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Somanna NK, Yariswamy M, Garagliano JM, Siebenlist U, Mummidi S, Valente AJ, Chandrasekar B. Aldosterone-induced cardiomyocyte growth, and fibroblast migration and proliferation are mediated by TRAF3IP2. Cell Signal. 2015;27(10):1928–1938. [DOI] [PubMed] [Google Scholar]
- 26.Klemann C, Wagner L, Stephan M, von Hörsten S. Cut to the chase: a review of CD26/dipeptidyl peptidase-4's (DPP4) entanglement in the immune system. Clin Exp Immunol. 2016;185(1):1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong J, Rao X, Rajagopalan S. An emerging role of dipeptidyl peptidase 4 (DPP4) beyond glucose control: potential implications in cardiovascular disease. Atherosclerosis. 2013;226(2):305–314. [DOI] [PubMed] [Google Scholar]
- 28.Dave DJ. Saxagliptin: A dipeptidyl peptidase-4 inhibitor in the treatment of type 2 diabetes mellitus. J Pharmacol Pharmacother. 2011;2(4):230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller KB, Bender SB, Hong K, Yang Y, Aronovitz M, Jaisser F, Hill MA, Jaffe IZ. Endothelial mineralocorticoid receptors differentially contribute to coronary and mesenteric vascular function without modulating blood pressure. Hypertension. 2015;66(5):988–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bender SB, DeMarco VG, Padilla J, Jenkins NT, Habibi J, Garro M, Pulakat L, Aroor AR, Jaffe IZ, Sowers JR. Mineralocorticoid receptor antagonism treats obesity-associated cardiac diastolic dysfunction. Hypertension. 2015;65(5):1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR. Low-dose mineralocorticoid receptor blockade prevents Western diet-induced arterial stiffening in female mice. Hypertension. 2015;66(1):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bender SB, Castorena-Gonzalez JA, Garro M, Reyes-Aldasoro CC, Sowers JR, DeMarco VG, Martinez-Lemus LA. Regional variation in arterial stiffening and dysfunction in Western diet-induced obesity. Am J Physiol Heart Circ Physiol. 2015;309(4):H574–H582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Padilla J, Ramirez-Perez FI, Habibi J, Bostick B, Aroor AR, Hayden MR, Jia G, Garro M, DeMarco VG, Manrique C, Booth FW, Martinez-Lemus LA, Sowers JR. Regular exercise reduces endothelial cortical stiffness in Western diet-fed female mice. Hypertension. 2016;68(5):1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu X, Sun Z, Meininger GA, Muthuchamy M. Application of atomic force microscopy measurements on cardiovascular cells Peng X, Antonyak M. , eds. Cardiovascular Development: Methods and Protocols. Totowa, NJ: Humana Press; 2012:229–244. [DOI] [PubMed] [Google Scholar]
- 35.Wang A, Dorso C, Kopcho L, Locke G, Langish R, Harstad E, Shipkova P, Marcinkeviciene J, Hamann L, Kirby MS. Potency, selectivity and prolonged binding of saxagliptin to DPP4: maintenance of DPP4 inhibition by saxagliptin in vitro and ex vivo when compared to a rapidly-dissociating DPP4 inhibitor. BMC Pharmacol. 2012;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uchii M, Kimoto N, Sakai M, Kitayama T, Kunori S. Glucose-independent renoprotective mechanisms of the tissue dipeptidyl peptidase-4 inhibitor, saxagliptin, in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol. 2016;783:56–63. [DOI] [PubMed] [Google Scholar]
- 37.Sakai M, Uchii M, Myojo K, Kitayama T, Kunori S. Critical role of renal dipeptidyl peptidase-4 in ameliorating kidney injury induced by saxagliptin in Dahl salt-sensitive hypertensive rats. Eur J Pharmacol. 2015;761:109–115. [DOI] [PubMed] [Google Scholar]
- 38.Wang XW, Zhang FX, Yang F, Ding ZF, Agarwal N, Guo ZK, Mehta JL. Effects of linagliptin and liraglutide on glucose- and angiotensin II-induced collagen formation and cytoskeleton degradation in cardiac fibroblasts in vitro. Acta Pharmacol Sin. 2016;37(10):1349–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsuda S, Umemoto S, Yoshimura K, Itoh S, Murata T, Fukai T, Matsuzaki M, Angiotensin II. Angiotensin II activates MCP-1 and induces cardiac hypertrophy and dysfunction via toll-like receptor 4. J Atheroscler Thromb. 2015;22(8):833–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aroor AR, McKarns S, Demarco VG, Jia G, Sowers JR. Maladaptive immune and inflammatory pathways lead to cardiovascular insulin resistance. Metabolism. 2013;62(11):1543–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Venkatesan B, Valente AJ, Das NA, Carpenter AJ, Yoshida T, Delafontaine J-L, Siebenlist U, Chandrasekar B. CIKS (Act1 or TRAF3IP2) mediates high glucose-induced endothelial dysfunction. Cell Signal. 2013;25(1):359–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leonardi A, Chariot A, Claudio E, Cunningham K, Siebenlist U. CIKS, a connection to Ikappa B kinase and stress-activated protein kinase. Proc Natl Acad Sci USA. 2000;97(19):10494–10499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, Prabhu SD, Activated T. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Heart Fail. 2017;10(3):e003688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shimojo N, Hashizume R, Kanayama K, Hara M, Suzuki Y, Nishioka T, Hiroe M, Yoshida T, Imanaka-Yoshida K. Tenascin-C may accelerate cardiac fibrosis by activating macrophages via the integrin αVβ3/nuclear factor-κB/interleukin-6 axis. Hypertension. 2015;66(4):757–766. [DOI] [PubMed] [Google Scholar]
- 45.Khan NS, Song CY, Jennings BL, Estes AM, Fang XR, Bonventre JV, Malik KU. Cytosolic phospholipase A2α is critical for angiotensin II-induced hypertension and associated cardiovascular pathophysiology. Hypertension. 2015;65(4):784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.González GE, Rhaleb N-E, Nakagawa P, Liao T-D, Liu Y, Leung P, Dai X, Yang X-P, Carretero OA. N-acetyl-seryl-aspartyl-lysyl-proline reduces cardiac collagen cross-linking and inflammation in angiotensin II-induced hypertensive rats. Clin Sci (Lond). 2014;126(1):85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bersi MR, Bellini C, Wu J, Montaniel KRC, Harrison DG, Humphrey JD. Excessive adventitial remodeling leads to early aortic maladaptation in angiotensin-induced hypertension. Hypertension. 2016;67(5):890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eberson LS, Sanchez PA, Majeed BA, Tawinwung S, Secomb TW, Larson DF. Effect of lysyl oxidase inhibition on angiotensin II-induced arterial hypertension, remodeling, and stiffness. PLoS One. 2015;10(4):e0124013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.González GE, Rhaleb N-E, D’Ambrosio MA, Nakagawa P, Liao T-D, Peterson EL, Leung P, Dai X, Janic B, Liu Y-H, Yang X-P, Carretero OA. Cardiac-deleterious role of galectin-3 in chronic angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2016;311(5):H1287–H1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qi G-M, Jia L-X, Li Y-L, Li H-H, Du J. Adiponectin suppresses angiotensin II-induced inflammation and cardiac fibrosis through activation of macrophage autophagy. Endocrinology. 2014;155(6):2254–2265. [DOI] [PubMed] [Google Scholar]
- 51.Falkenham A, de Antueno R, Rosin N, Betsch D, Lee TDG, Duncan R, Légaré J-F. Nonclassical resident macrophages are important determinants in the development of myocardial fibrosis. Am J Pathol. 2015;185(4):927–942. [DOI] [PubMed] [Google Scholar]
- 52.Cardin S, Scott-Boyer M-P, Praktiknjo S, Jeidane S, Picard S, Reudelhuber TL, Deschepper CF. Differences in cell-type-specific responses to angiotensin II explain cardiac remodeling differences in C57BL/6 mouse substrains. Hypertension. 2014;64(5):1040–1046. [DOI] [PubMed] [Google Scholar]
- 53.Kai H, Kuwahara F, Tokuda K, Imaizumi T. Diastolic dysfunction in hypertensive hearts: roles of perivascular inflammation and reactive myocardial fibrosis. Hypertens Res. 2005;28(6):483–490. [DOI] [PubMed] [Google Scholar]
- 54.Kuwahara F, Kai H, Tokuda K, Takeya M, Takeshita A, Egashira K, Imaizumi T. Hypertensive myocardial fibrosis and diastolic dysfunction: another model of inflammation? Hypertension. 2004;43(4):739–745. [DOI] [PubMed] [Google Scholar]
- 55.Zhang L-H, Pang X-F, Bai F, Wang N-P, Shah AI, McKallip RJ, Li X-W, Wang X, Zhao Z-Q. Preservation of glucagon-like peptide-1 level attenuates angiotensin ii-induced tissue fibrosis by altering AT1/AT 2 receptor expression and angiotensin-converting enzyme 2 activity in rat heart. Cardiovasc Drugs Ther. 2015;29(3):243–255. [DOI] [PubMed] [Google Scholar]
- 56.Gangadharan Komala M, Gross S, Zaky A, Pollock C, Panchapakesan U. Saxagliptin reduces renal tubulointerstitial inflammation, hypertrophy and fibrosis in diabetes. Nephrology (Carlton). 2016;21(5):423–431. [DOI] [PubMed] [Google Scholar]
- 57.Sciarretta S, Paneni F, Palano F, Chin D, Tocci G, Rubattu S, Volpe M. Role of the renin-angiotensin-aldosterone system and inflammatory processes in the development and progression of diastolic dysfunction. Clin Sci (Lond). 2009;116(6):467–477. [DOI] [PubMed] [Google Scholar]
- 58.Li X, Commane M, Nie H, Hua X, Chatterjee-Kishore M, Wald D, Haag M, Stark GR. Act1, an NF-κ B-activating protein. Proc Natl Acad Sci USA. 2000;97(19):10489–10493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valente AJ, Prabhu SD, Siebenlist U, Chandrasekar B. Abstract 8420: critical role of CIKS/Act1 in lipopolysaccharide (LPS)-induced cardiac dysfunction in vivo and cardiomyocyte contractility in vitro. Circulation. 2011;124:A8420–A8420. [Google Scholar]
- 60.Valente AJ, Clark RA, Siddesha JM, Siebenlist U, Chandrasekar B. CIKS (Act1 or TRAF3IP2) mediates angiotensin-ii-induced interleukin-18 expression, and Nox2-dependent cardiomyocyte hypertrophy. J Mol Cell Cardiol. 2012;53(1):113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H, Kwak D, Fassett J, Liu X, Yao W, Weng X, Xu X, Xu Y, Bache RJ, Mueller DL, Chen Y. Role of bone marrow-derived CD11c(+) dendritic cells in systolic overload-induced left ventricular inflammation, fibrosis and hypertrophy. Basic Res Cardiol. 2017;112(3):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Burt BM, Plitas G, Stableford JA, Nguyen HM, Bamboat ZM, Pillarisetty VG, DeMatteo RP. CD11c identifies a subset of murine liver natural killer cells that responds to adenoviral hepatitis. J Leukoc Biol. 2008;84(4):1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blasius AL, Barchet W, Cella M, Colonna M. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J Exp Med. 2007;204(11):2561–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, King MR, Schaffer CB, Reinhart-King CA. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011; 3(112):112ra122.10.1126/scitranslmed.3002761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rabin RL, Park MK, Liao F, Swofford R, Stephany D, Farber JM. Chemokine receptor responses on T cells are achieved through regulation of both receptor expression and signaling. J Immunol. 1999;162(7):3840–3850. [PubMed] [Google Scholar]
- 66.Nanki T, Lipsky PE. Stimulation of T-Cell activation by CXCL12/stromal cell derived factor-1 involves a G-protein mediated signaling pathway. Cell Immunol. 2001;214(2):145–154. [DOI] [PubMed] [Google Scholar]
- 67.Samji T, Khanna KM. Understanding memory CD8(+) T cells. Immunol Lett. 2017;185:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dai H, Wan N, Zhang S, Moore Y, Wan F, Dai Z. Cutting edge: programmed death-1 defines CD8+CD122+ T cells as regulatory versus memory T cells. J Immunol. 2010;185(2):803–807. [DOI] [PubMed] [Google Scholar]
- 69.Bahri R, Bollinger A, Bollinger T, Orinska Z, Bulfone-Paus S. Ectonucleotidase CD38 demarcates regulatory, memory-like CD8+ T cells with IFN-γ-mediated suppressor activities. PLoS One. 2012;7(9):e45234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dubois A, Deruytter N, Adams B, Kanda A, Delbauve S, Fleury S, Torres D, François A, Pétein M, Goldman M, Dombrowicz D, Flamand V. Regulation of Th2 responses and allergic inflammation through bystander activation of CD8+ T lymphocytes in early life. J Immunol. 2010;185(2):884–891. [DOI] [PubMed] [Google Scholar]
- 71.Dong Y, Yang M, Zhang J, Peng X, Cheng J, Cui T, Du J. Depletion of CD8+ T cells exacerbates CD4+ T cell-induced monocyte-to-fibroblast transition in renal fibrosis. J Immunol. 2016;196(4):1874–1881. [DOI] [PubMed] [Google Scholar]
- 72.Scirica BM, Braunwald E, Raz I, Cavender MA, Morrow DA, Jarolim P, Udell JA, Mosenzon O, Im K, Umez-Eronini AA, Pollack PS, Hirshberg B, Frederich R, Lewis BS, McGuire DK, Davidson J, Steg PG, Bhatt DL; SAVOR-TIMI 53 Steering Committee and Investigators* . Heart failure, saxagliptin, and diabetes mellitus: observations from the SAVOR-TIMI 53 randomized trial. Circulation. 2014;130(18):1579–1588. [DOI] [PubMed] [Google Scholar]