Abstract
Growth hormone receptor (GHR) and prolactin (PRL) receptor (PRLR) are homologous transmembrane class I cytokine receptors. In humans, GH interacts with GHR homodimers or PRLR homodimers and PRL interacts with only PRLR homodimers to promote signaling. In human breast cancer cells endogenously expressing both receptors, GHR and PRLR specifically coimmunoprecipitate. We previously devised a split luciferase complementation assay to study GHR and PRLR assemblages. In this technique, firefly luciferase is split into two fragments (N- and C-terminal fragments of the luciferase), each without enzyme activity and tethered to the tails of two receptors. The fragments restore luciferase activity when brought close to each other by the receptors. Real-time ligand-induced complementation changes reflect the arrangement of receptors and indicate that GHR/PRLR is arranged as a heteromultimer comprised of GHR-GHR homodimers and PRLR-PRLR homodimers. We now dissect determinants for GHR and PRLR homodimerization versus heteroassociation. GHR and PRLR have extracellular domains comprised of the ligand-binding N-terminal subdomain 1 and a membrane-proximal subdomain 2 (S2), which fosters receptor–receptor contact. Based on previous studies of S2 versus the transmembrane domain (TMD) in GHR dimerization, we constructed GHR(PRLRS2), GHR(PRLRS2-TMD), and GHR(PRLRTMD), replacing GHR’s S2 alone, S2 plus TMD, and TMD alone with PRLR’s counterpart. We tested by complementation the ability of these chimeras and GHR or PRLR to homodimerize or heteroassociate. Comparing various combinations, we found GHR(PRLRS2) and GHR(PRLRS2-TMD) behaved as PRLR, whereas GHR(PRLRTMD) behaved as GHR regarding their dimerization partners. We conclude that S2 of GHR and PRLR, rather than their TMDs, determines their dimerization partner.
Luciferase complementation is used to explore the arrangement of GHR and PRLR within multimers. The authors conclude that extracellular subdomain 2 directs receptor dimerization within multimers.
Growth hormone (GH) receptor (GHR), a class 1 cytokine receptor superfamily member, exists largely as preformed dimers on the cell membrane (1–3). GH binds to GHR dimers and introduces a conformational change allowing receptor activation and downstream signaling (4–6). The crystal structure of human GH bound to human GHR extracellular domain (ECD) revealed a 1:2 GH:GHR stoichiometry of this ligand–receptor complex with a dimerization interface between GHR ECD subdomain 2 (S2) of the receptors (7). Consistent with the crystal structure, coimmunoprecipitation experiments suggested important contribution of S2 to GHR dimerization (2). In addition to S2, the transmembrane domain (TMD) has also been suggested to factor in the predimerization of GHR (3).
Prolactin (PRL) receptor (PRLR), also a class 1 cytokine receptor, has similarities with GHR. The human PRLR long form and human GHR share 28% sequence identity, and their folded ECD structures are very similar (8). Like GHR, PRLR is predimerized on the plasma membrane (9). The crystal structure of human PRL bound to rat PRLR ECD showed a 1:2 PRL:PRLR complex, and, similar to the GH:GHR complex, an interface between S2 of PRLR monomers is observed in the dimeric structure (10, 11). Likewise, PRLR’s TMD has also been suggested to contribute to ligand-independent dimerization (12). Thus, studies of GHR and PRLR suggest it is plausible that their S2 and TMD regions might together drive the homodimerization of each receptor.
We previously adapted the split luciferase complementation assay to study GHR-GHR dimers (6) and PRLR-PRLR dimers (13). In this assay, firefly luciferase is molecularly separated into N-terminal fragment of the luciferase (Nluc; residues 1–398) and C-terminal fragment of the luciferase (Cluc; residues 394–550), with neither fragment being enzymatically active alone. Upon molecularly fusing Nluc and Cluc, respectively, to each of two proteins of interest, luciferase activity is restored when the two proteins interact (14–16). We found strong ligand-independent complementation when GHR- and PRLR-deficient fibrosarcoma cells (γ2A-JAK2 cells) were programmed to express GHR-Nluc/GHR-Cluc or PRLR-Nluc/PRLR-Cluc. Basal GHR-Nluc/GHR-Cluc complementation was acutely augmented by GH treatment, and basal PRLR-Nluc/PRLR-Cluc complementation was acutely augmented by either GH or PRL treatment, consistent with the known propensities of human GH to bind to either human GHR or human PRLR, but human PRL to bind to only human PRLR. These data implied a similarity of receptor activation-related conformational changes between GHR homodimers and PRLR homodimers (6, 13). When cells coexpressed GHR-Nluc and PRLR-Cluc, ligand-independent complementation was also detected (13), indicating heteroassociation of GHR and PRLR, consistent with our prior findings that GHR and PRLR specifically coimmunoprecipitate from human breast cancer cells that naturally bear both receptors (17). In contrast to the acute ligand-induced augmentation of basal complementation in GHR-GHR homodimers or PRLR-PRLR homodimers, GHR-Nluc/PRLR-Cluc basal complementation declined after GH or PRL stimulation (13). Based on these and other antagonist studies, we proposed a heteromultimeric GHR-GHR/PRLR-PRLR model, in which a complex is comprised of GHR homodimers and PRLR homodimers that are in close proximity [and thus complement their luciferase (luc) fragments], but are independently activated by their ligands (13).
In the current study, we use the pattern of ligand-induced change in luciferase complementation as a signature to distinguish between dimeric versus multimeric arrangements of GHR and PRLR, asking which receptor domains determine their propensities to homodimerize versus heteromultimerize. We focus particularly on the roles of the S2 and TMD regions of each receptor, taking the approach of creating domain-swapping chimeric versions of the two receptors to preserve overall topology. Our findings strongly suggest that formation of GHR-GHR or PRLR-PRLR homodimers is determined by the identity (GHR versus PRLR) of the S2 domain rather than by the identity of the TMD.
Materials and Methods
Materials
Common molecular biology reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless otherwise noted. Restriction endonucleases were obtained from New England Biolabs (Beverly, MA). Cell culture medium, penicillin/streptomycin, and trypsin were purchased from Corning (Corning, NY). Fetal bovine serum was purchased from Atlanta Biologicals (Lawrenceville, GA). Gentamicin sulfate was purchased from RPI (Mt. Prospect, IL). Zeocin was purchased from Invitrogen (Carlsbad, CA). Recombinant human GH was provided by Eli Lilly (Indianapolis, IN). Recombinant human PRL was obtained from the National Hormone and Pituitary Program.
Antibodies
Polyclonal antibodies against GHR (anti-GHRcyt-AL47, against the rabbit GHR intracellular domain) (18), JAK2 (anti-JAK2AL33) (19), and PRLR (anti-PRLRcyt-AL84, against the human PRLR intracellular domain) (20) were developed by our laboratory and have been reported. Polyclonal anti-pJAK2 antibody detecting phospho-JAK2 (Y1007 and Y1008) was purchased from EMD Millipore (Darmstadt, Germany). Monoclonal anti-pY (4G10) was purchased from Upstate Biotechnology (Lake Placid, NY). Polyclonal anti-luc (catalog no. G7451) was purchased from Promega (Madison, WI). Anti-PRLRICD [PRL-R(H-300); catalog no. sc-20992] and anti–glutathione S-transferase [GST(B-14); catalog no. sc-138] were purchased from Santa Cruz Biotechnology (Dallas, TX). Monoclonal anti-GHRext-mAb and anti-GHRcyt-mAb were developed by our laboratory and described previously (21–25). Monoclonal anti-PRLRext-mAb was raised against a GST fusion containing the entire extracellular domain of the long form of the human PRLR. Clones were screened by enzyme-linked immunosorbent assay (positive for GST-human PRLR extracellular domain and negative for GST alone) and tested for immunoreactivity by immunoblotting and immunoprecipitation of human PRLR from T47D cells and by immunoblotting of GST fusion proteins containing either the subdomain 1 (S1) or S2 of PRLR extracellular domain (reported in this study).
Construction of the plasmids encoding GHR-PRLR chimeric receptors
The human GHR complementary DNA (cDNA) in pcDNA1 was a gift provided by R. Ross (University of Sheffield, Sheffield, UK). The human PRLR cDNA in pEF/V5/HIS was a gift provided by C. Clevenger (Virginia Commonwealth University, Richmond, VA). The full-length firefly luciferase–encoding plasmid has been described previously (16). To construct the chimeric GHR(PRLRS2-TMD), DNA fragments of GHR’s S1, PRLR’s S2, and TMD, and GHR’s intracellular domain (ICD) were generated by polymerase chain reaction (PCR) with a 21- or 23-bp overlap sequence at the junctions between each adjacent fragment and then assembled by overlap extension PCR and cloned into pcDNA3.1(+)/zeo. GHR(PRLRS2) and GHR(PRLRTMD) were assembled using the same strategy. For purposes of this study, PRLR S2 extends from the S1-S2 hinge region (IVQPDPP—a sequence shared by GHR and PRLR) to the end of ECD (spanning residues 100–210 of mature PRLR). The GHR cDNA sequence encoding S1-S2 hinge region IVQPDPP was used to construct each chimeric receptor containing PRLR S2. The cDNA of Nluc (encoding residues 1–398) or Cluc (encoding residues 394–550) with a flexible linker (AAAGSGGGGS) on the amino terminus of each fragment was created by PCR with the template being the full-length firefly luciferase cDNA. The Nluc/Cluc fragment was tethered downstream of human GHR, human PRLR, or chimeric GHR(PRLRS2-TMD), GHR(PRLRS2), and GHR(PRLRTMD) by overlap extension PCR with the stop codon of receptor cDNA being mutated to GGA (encoding a Gly), and subsequently cloned into pcDNA3.1(+)/zeo by two flanking restriction enzyme sites. ER-Cluc [estrogen receptor fragment (residues 130–398)-Cluc in the vector of pcDNA3.1/puromycin] has been described previously (26). The sequences of PCR primers are available upon request.
GST-S1 and GST-S2 are GST fusion proteins containing the S1 or S2 domain of human PRLR, respectively. cDNAs encoding PRLR S1 (residues 1–100) or PRLR S2 (residues 101–210) were inserted downstream of GST via EcoR1 and Xho1 sites in the pGEXT-4T plasmid backbone.
Cells, cell culture, and transfection
T47D cells were purchased from American Type Culture Collection (Manassas, VA; ATCC HTB-133). γ2A-JAK2 cells were generated by transfection of γ2A cells (27) (gift of G. Stark, Cleveland Clinic, Cleveland, OH) with pcDNA3.1(+)/zeo-JAK2 and carried in culture, as described previously (28, 29). γ2A-JAK2-GHR-Nluc cells were generated by cotransfection of γ2A-JAK2 cells with pcDNA3.1(+)/zeo-GHR-Nluc and a hygromycin-encoding plasmid at a weight ratio as 20:1, followed by hygromycin selection and single clone amplification. γ2A-JAK2-GHR(PRLRS2)-Nluc cells were made in the same way. All double-stable clones, γ2A-JAK2-GHR-Nluc/GHR-Cluc, γ2A-JAK2-GHR-Nluc/GHR(PRLRS2)-Cluc, γ2A-JAK2-GHR-Nluc/PRLR-Cluc, γ2A-JAK2-GHR(PRLRS2)-Nluc/PRLR-Cluc, and γ2A-JAK2-PRLR-Nluc/PRLR-Cluc, were generated by cotransfection of plasmid-encoding receptor-Nluc, plasmid-encoding receptor-Cluc, and a hygromycin-encoding plasmid at a weight ratio as 9.5:9.5:1, followed by hygromycin selection and single clone amplification. All the stable clone cells were maintained in 500 μg/mL hygromycin-containing γ2A-JAK2 cell medium. Transient expression of receptors was achieved by transfecting 0.3 pmol receptor-Cluc–encoding plasmid DNA per 6-cm2 dish, unless otherwise indicated. Lipofectamine LTX Plus (Invitrogen) was used for transfection following the manufacturer’s instructions.
Bioluminescence imaging
Transiently transfected cells or double-stable clone cells were seeded in 96-well black wall plates (3.6 × 104 cells/well). Six hours prior to experimentation, medium was replaced by serum-free medium. Then the medium was changed to imaging medium (175 μl/well; composed of phenol red-free Dulbecco’s modified Eagle medium, 1 g/L glucose, 1 mg/mL d-luciferin, 25 mM HEPES, pH 7.5, 0.1% w/v bovine serum albumin) at room temperature for 10 minutes and at 37°C for 20 minutes. Baseline bioluminescence signal (photons/s/cm2/steradian) was collected at 37°C using an IVIS 100 system (Perkin Elmer, Waltham, MA; no filter; F-stop, 1; field of view, level B; Bin: 8; exposure time: 5 minutes). GH was added in a volume of 25 μl/well to reach the final concentration of 500 ng/mL. Then the sequential bioluminescence imaging was commenced immediately thereafter with the same parameters at 5-minute intervals for 40 minutes. For the measurement of monoclonal antibodies’ effect, anti-GHRext-mAb, anti-PRLRext-mAb, or anti-GHRcyt-mAb was added into the imaging medium with the final concentration at 40 μg/mL, incubated for 30 minutes, and measured for the bioluminescence with parameters described previously.
Protein extraction, immunoprecipitation, and immunoblotting
For immunoprecipitation, T47D cells were serum starved overnight, treated with or without 500 ng/mL PRL at 37°C for 10 min, washed with ice-cold phosphate-buffered saline with Na3VO4 (400 μM), a phosphatase inhibitor, and lysed. Cell lysates were immunoprecipitated with anti-PRLRext-mAb or anti-PRLRICD. Elutes were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with indicated antibodies.
For immunoblotting, cells were plated in 96-well plates for bioluminescence imaging and 6-well plates (5.4 × 105 cells/well) for biochemical data. The cells in 6-well plates were serum starved for 6 hours, treated with or without 500 ng/mL GH at 37°C for 10 minutes, terminated from the stimulation by sitting the plates on ice, and washed with ice-cold phosphate-buffered saline with Na3VO4 (400 μM), a phosphatase inhibitor. Detergent extraction, electrophoresis, and immunoblotting of the cultured cells were performed, as previously described (22, 30).
GST-S1 and GST-S2 were expressed in Escherichia coli (BL-21) and purified, as previously described (31).
Statistical analysis and figure preparation
For bioluminescence complementation, each experimental condition was assessed in triplicate wells of 96-well plate. Each well was defined as a region of interest that generates a basal bioluminescence value expressed as total flux ± standard error (SE; photons per second). The percentage change of complementation signal was calculated by dividing the total flux value from vehicle-treated or GH-treated wells by the baseline total flux value from this same set of wells and subtracting the vehicle-induced change from the GH-induced change. Data are expressed as mean ± SE of GH-induced signal change as a percentage above baseline signal (n = 3). The split luciferase complementation data shown are in all instances representative of at least three experiments. For the bar graphs of monoclonal antibodies’ effects, bioluminescence was detected in triplicate in a 96-well plate format and normalized to the mean value from the vehicle buffer-incubated wells. Each bar represents data from three independent experiments and is displayed as mean of the fold change ± SE. Two sample t tests with equal variance and pooled SE were used for statistical analysis comparing antibody-treated cells with vehicle-treated cells. P < 0.05 was considered as significant. In figures of some immunoblots, irrelevant intervening lanes from original immunoblots have been cropped for clarity of presentation. In these cases, a space is maintained where intervening lanes were cropped. In all cases, only data from the same original blots are incorporated in figures with consistent brightness/contrast adjustment made across each blot.
Results
Domain-swapped GHR(PRLR) chimeric receptors and their luc hybrids are expressed at the cell surface and respond to ligand stimulation
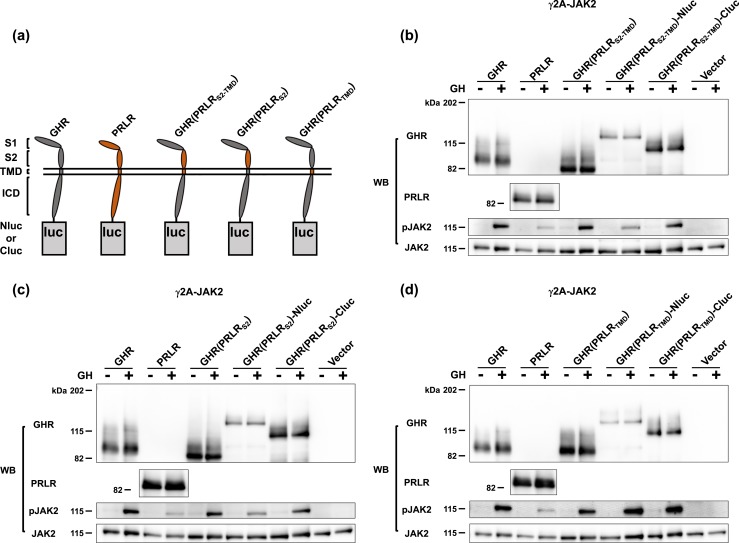
To dissect the roles of the ECD S2 and the TMD regions of GHR and PRLR in allowing receptor dimers to form, we took a domain-swapping approach to best preserve overall receptor topology and thus functional integrity. Specifically, we swapped either S2 and TMD together, S2 alone, or TMD alone of human PRLR to replace the same domains of human GHR into the backbone of the GHR. Figure 1(a) graphically represents the structures of GHR (gray) or PRLR (orange) fused at the receptor ICD C terminus to either Nluc or Cluc via a flexible 10-residue linker (AAAGSGGGGS), as previously reported (6, 13). Also graphically shown in Fig. 1(a) are the structures of three receptor-luc fusions in which the receptor portion is a chimera in which GHR domains are replaced by PRLR S2 and TMD, PRLR S2, or PRLR TMD; the receptor chimera components are, respectively, named GHR(PRLRS2-TMD), GHR(PRLRS2), and GHR(PRLRTMD).
Figure 1.
Replacement of GHR S2 or TMD with S2 or TMD of PRLR does not impair GH signaling. (a) Schematic diagram of domain-swapped human GHR/PRLR chimeras tethered with a Nluc/Cluc fragment at their cytoplasmic tails. GHR is in gray. PRLR is in orange. GHR(PRLRS2-TMD) is GHR with the S2 and TMD replaced with those of PRLR. GHR(PRLRS2) is GHR with its S2 replaced with the S2 of PRLR. GHR(PRLRTMD) is GHR with its TMD replaced with the TMD of PRLR. (b) GHR(PRLRS2-TMD), GHR(PRLRS2-TMD)-Nluc, and GHR(PRLRS2-TMD)-Cluc allow acute GH signaling. γ2A-JAK2 cells were transiently transfected with 3 μg GHR, 4.5 μg PRLR, 3 μg GHR(PRLRS2-TMD), 4.5 μg GHR(PRLRS2-TMD)-Nluc, 4 μg GHR(PRLRS2-TMD)-Cluc, or 3 μg empty vector per 6-cm dish, respectively, treated with ±GH, and analyzed by immunoblotting with anti-GHR (anti-GHRcyt-AL47, polyclonal antibody against the rabbit GHR intracellular domain), anti-PRLR (anti-PRLRcyt-AL84, polyclonal antibody against the human PRLR intracellular domain), anti-pJAK2, and anti-JAK2 (anti-JAK2AL33). (c) GHR(PRLRS2), GHR(PRLRS2)-Nluc, and GHR(PRLRS2)-Cluc allow acute GH signaling. γ2A-JAK2 cells were transiently transfected with 3 μg GHR, 4.5 μg PRLR, 3 μg GHR(PRLRS2), 4.5 μg GHR(PRLRS2)-Nluc, 4 μg GHR(PRLRS2)-Cluc, or 3 μg empty vector per 6-cm dish, respectively, treated with ±GH, and analyzed by immunoblotting with anti-GHR, anti-PRLR, anti-pJAK2, and anti-JAK2. (d) GHR(PRLRTMD), GHR(PRLRTMD)-Nluc, and GHR(PRLRTMD)-Cluc allow acute GH signaling. γ2A-JAK2 cells were transiently transfected with 3 μg GHR, 4.5 μg PRLR, 3 μg GHR(PRLRTMD), 4.5 μg GHR(PRLRTMD)-Nluc, 3 μg GHR(PRLRTMD)-Cluc, or 3 μg empty vector per 6-cm dish, respectively, split into 6-well plates, treated with ±GH, and analyzed by immunoblotting with anti-GHR, anti-PRLR, anti-pJAK2, and anti-JAK2.
To explore the structural and functional integrity of these chimeras and fusion proteins, we transiently transfected each into the GHR- and PRLR-deficient human fibrosarcoma cell line, γ2A-JAK2 (28, 29), which amply expresses JAK2, a cytoplasmic tyrosine kinase that couples to both GHR and PRLR (31–35). As anticipated, expression of GHR and PRLR was easily detected by immunoblotting of cell extracts by our antisera specific for the ICDs of each protein, and acute GH treatment resulted in phosphorylation of JAK2 for each receptor [Fig. 1(b)–1(d), left four lanes]. Likewise, expression of GHR(PRLRS2-TMD) and its Nluc and Cluc hybrids [Fig. 1(b)], GHR(PRLRS2) and its Nluc and Cluc hybrids [Fig. 1(c)], and GHR(PRLRTMD) and its Nluc and Cluc hybrids yielded specifically detected (GHR ICD-reactive) proteins of expected Mr, and each allowed acute GH-induced JAK2 tyrosine phosphorylation. As human GH binds both human GHR and PRLR (36–38) and GH binds GHR almost exclusively via the S1 domain (39), we interpret the data in Fig. 1 to indicate that each chimeric receptor was expressed at the cell surface, bound GH, and transduced GH binding to activation of JAK2. Furthermore, these trafficking, binding, and signaling capacities were retained for each chimera when fused to either Nluc or Cluc.
Basal and ligand-induced complementation of GHR-Nluc with receptor-Clucs and chimera-Clucs
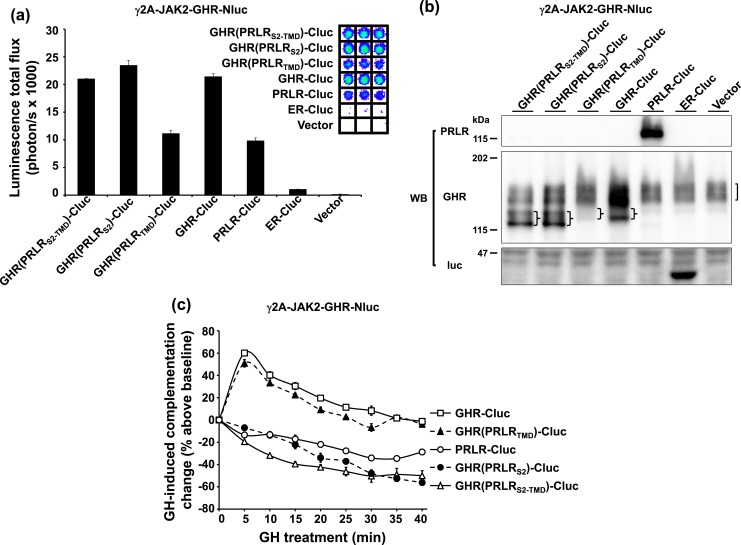
To compare the dimer/multimer arrangements of chimeric receptors with wild-type receptors, we transiently transfected each chimera-Cluc versus GHR-Cluc or PRLR-Cluc into a version of γ2A-JAK2 cells that stably expresses GHR-Nluc [so-called γ2A-JAK2-GHR-Nluc cells (6) cells]. We first examined the degree to which reconstitution of luciferase activity (complementation) was allowed between the Cluc-containing receptors and GHR-Nluc [Fig. 2(a)]. As anticipated from our previous studies (6, 13), expression of either GHR-Cluc or PRLR-Cluc yielded complementation when expressed in γ2A-JAK2-GHR-Nluc. The specificity of this complementation was validated by comparison with transfection of either vector only or a fragment of estrogen receptor fused to Cluc (ER-Cluc) (26), which is largely expressed in the cytosol and serves as a negative control. Notably, expression of each of the three receptor chimera-Cluc hybrids—GHR(PRLRS2-TMD)-Cluc, GHR(PRLRS2)-Cluc, or GHR(PRLRTMD)-Cluc—yielded basal complementation [Fig. 2(a)]. Expression of the relevant transfected proteins and of the endogenous GHR-Cluc was verified by immunoblotting of cell extracts [Fig. 2(b)]. In principle, the three chimera-Cluc hybrids and GHR-Cluc should be equally recognized by anti-GHRcyt-AL47, allowing for comparison by immunoblotting of the relative abundance of these four constructs. Notably, the hierarchy of their protein levels accurately reflected the hierarchy of their basal bioluminescence in γ2A-JAK2-GHR-Nluc cells. For example, GHR(PRLRTMD)-Cluc had both the lowest abundance among these four constructs [Fig. 2(b)] and the lowest basal complementation with GHR-Nluc [Fig. 2(a)]. Although transient transfection resulted in varied expression levels in these three chimera-Cluc hybrids, their basal complementation signals were comparable to that observed with transfection of GHR-Cluc or PRLR-Cluc and dramatically greater than with ER-Cluc transfection. Thus, like GHR-Cluc and PRLR-Cluc, chimeras with S2 and/or TMD from PRLR replacing those domains from GHR, when fused to Cluc, were similarly capable of complementation with GHR-Nluc.
Figure 2.
S2 of GHR is necessary for a chimeric receptor to dimerize with GHR. (a) Specific luciferase complementation of GHR-Nluc, a GHR-Nluc stably expressing cell line, and chimeric receptor-Clucs. γ2A-JAK2-GHR-Nluc cells were transiently transfected with GHR(PRLRS2-TMD)-Cluc, GHR(PRLRS2)-Cluc, GHR(PRLRTMD)-Cluc, GHR-Cluc, PRLR-Cluc, ER-Cluc, or empty vector, respectively, and measured for bioluminescence in triplicate in a 96-well plate format (inset shows actual signals). The quantifications are displayed graphically as mean ± SE of total flux (photons/s × 1000). (b) Transient expression of chimeric receptor-Clucs in γ2A-JAK2-GHR-Nluc. The same pools of the transfected cells in (a) were analyzed by immunoblotting with anti-PRLR, anti-GHR, and anti-luc. Stably expressed GHR-Nluc is indicated by a bracket. Transfected chimeric receptor-Clucs are indicated by braces. (c) GH-induced complementation change. After basal bioluminescence was measured (a), GH was added and bioluminescence was again serially detected at 5-minute intervals over 40 minutes. Data are displayed as mean ± SE of GH-induced signal as a percentage above baseline signal (n = 3 per condition).
We next tested the effects of treatment with GH on the complementation of each receptor-Cluc hybrid with GHR-Nluc [Fig. 2(c)]. Bioluminescence was measured serially at 5-minute intervals over 40 minutes after addition of GH to monitor changes in basal complementation that are caused by GH, and this GH-induced change was presented as the percentage over baseline signal through time. In γ2A-JAK2-GHR-Nluc cells transfected with GHR-Cluc, GH induced an acute augmentation of bioluminescence that began to decline after 5 minutes, approaching baseline after ∼30 to 40 minutes. In contrast, in γ2A-JAK2-GHR-Nluc cells transfected with PRLR-Cluc, GH induced a steady decline in bioluminescence over ∼30 to 40 minutes. These data on GHR-Nluc/GHR-Cluc and GHR-Nluc/PRLR-Cluc complementation were consistent with our previous findings (6, 13) and highlight the different GH-induced signature patterns for what we interpret are GHR-GHR homodimers versus GHR-GHR/PRLR-PRLR heteromultimers (which are comprised of GHR-GHR dimers and PRLR-PRLR dimers) (13). Interestingly, the GH-induced tracing from γ2A-JAK2-GHR-Nluc cells transfected with GHR(PRLRTMD)-Cluc mimicked that observed with GHR-Cluc transfection—acute augmentation followed by decline toward baseline—, suggesting a similar receptor dimer arrangement of GHR-Nluc/GHR(PRLRTMD)-Cluc as that of GHR-Nluc/GHR-Cluc. In contrast, transfection of chimera-Cluc hybrids that substitute either the PRLR S2 alone [GHR(PRLRS2)-Cluc] or both the PRLR S2 and PRLR TMD [GHR(PRLRS2-TMD)-Cluc] into γ2A-JAK2-GHR-Nluc cells yielded GH-induced tracings of the same pattern as that observed with PRLR-Cluc transfection—a steady decline from baseline. This suggests that the arrangements of GHR-Nluc/GHR(PRLRS2)-Cluc and GHR-Nluc/GHR(PRLRS2-TMD)-Cluc were similar to that observed for GHR-Nluc/PRLR-Cluc, and that these receptors were arranged as heteromultimers made up of homodimers, rather than as heterodimers per se. These data are consistent with the notion that, among GHRs and PRLRs, the S2 domain, rather than the TMD, is necessary for formation of dimers and that receptors containing nonhomologous S2 domains do not dimerize, but can form multimeric assemblages consisting of dimers, each partner of which bears homologous S2 domains.
Basal and ligand-induced complementation of GHR(PRLRS2)-Nluc with receptor-Clucs and chimera-Clucs
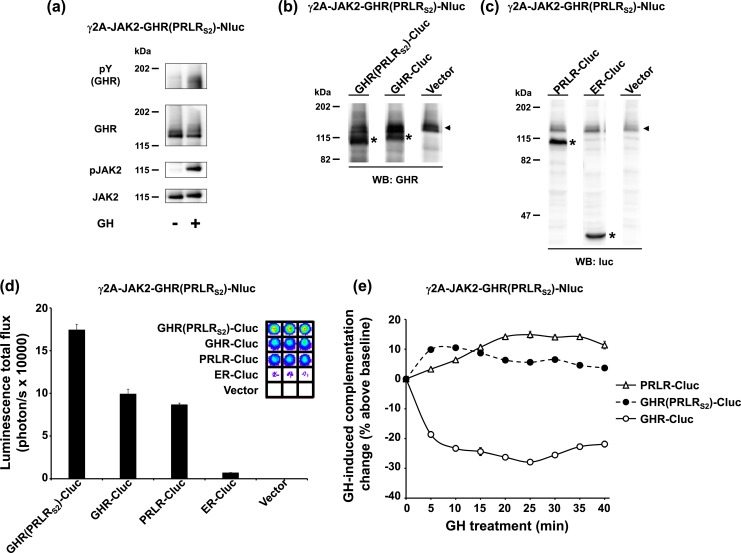
The data in Fig. 2 suggest that dimerization is conferred by the presence of GHR S2 in both receptor dimer partners. To pursue further the reciprocal requirement for PRLR S2 in dimer formation, we created a clone, γ2A-JAK2-GHR(PRLRS2)-Nluc, in which GHR(PRLRS2)-Nluc is stably expressed in γ2A-JAK2 cells. Consistent with its behavior in transiently transfected cells [Fig. 1(c)], this chimera-Nluc, when stably expressed, reached the cell surface, bound GH, and allowed JAK2 activation and phosphorylation of both the receptor and JAK2 [Fig. 3(a)]. To test complementation, we transiently transfected γ2A-JAK2-GHR(PRLRS2)-Nluc cells with GHR-Cluc, PRLR-Cluc, or GHR(PRLRS2)-Cluc, or, as negative controls, ER-Cluc or empty vector. Each transiently transfected protein, as well as endogenously stably expressed GHR(PRLRS2)-Nluc, was specifically detected in cell extracts by immunoblotting with either anti-GHR or anti-luc [Fig. 3(b) and 3(c)]. Furthermore, transiently transfected GHR-Cluc, PRLR-Cluc, and GHR(PRLRS2)-Cluc, but not ER-Cluc or vector, each complemented the stably expressed GHR(PRLRS2)-Nluc [Fig. 3(d)], indicating that, as expected, each of the three Cluc-containing hybrids specifically interacts with GHR(PRLRS2)-Nluc. The effect of ligand engagement was tested by tracking changes in complementation in response to GH treatment [Fig. 3(e)]. Both GHR(PRLRS2)-Cluc and PRLR-Cluc displayed GH-induced augmentation of basal complementation with GHR(PRLRS2)-Nluc. In contrast, GH triggered a decline in basal bioluminescence signal in γ2A-JAK2-GHR(PRLRS2)-Nluc cells transiently expressing GHR-Cluc [Fig. 3(e)]. This is consistent with the GH-induced bioluminescence profile observed with coexpression of GHR-Nluc and GHR(PRLRS2)-Cluc [Fig. 2(c)]; thus, the same findings applied independent of whether Nluc or Cluc was fused to GHR or GHR(PRLRS2) or vice versa. Again, GHR(PRLRS2) behaved more like PRLR than like GHR in both formats. The data suggest that the PRLRS2 domain is sufficient to drive its PRLR-like behavior in terms of complementation with GHR, PRLR, or GHR-PRLR chimeras, forming dimers with receptors containing another PRLRS2 domain and heteromultimers with receptors containing a GHRS2 domain.
Figure 3.
S2 of PRLR is sufficient for a chimeric receptor to dimerize with PRLR. (a) GH signaling in γ2A-JAK2-GHR(PRLRS2)-Nluc, a GHR(PRLRS2)-Nluc stably expressing cell line. γ2A-JAK2-GHR(PRLRS2)-Nluc cells were treated with ±GH (10 minutes) and analyzed by immunoblotting with anti-pY, anti-GHR, anti-pJAK2, and anti-JAK2. (b and c) Transient expression of GHR(PRLRS2)-Cluc, GHR-Cluc, PRLR-Cluc, ER-Cluc, or empty vector in γ2A-JAK2-GHR(PRLRS2)-Nluc cells. γ2A-JAK2-GHR(PRLRS2)-Nluc cells were transiently transfected with GHR(PRLRS2)-Cluc, GHR-Cluc, PRLR-Cluc, ER-Cluc, or empty vector, respectively, and analyzed by immunoblotting with (b) anti-GHR or (c) anti-luc. Stably expressed GHR(PRLRS2)-Nluc is indicated by black arrowheads. Transfected receptor-Clucs are indicated by asterisks. (d) Specific luciferase complementation of GHR(PRLRS2)-Nluc and GHR(PRLRS2)-Cluc, GHR-Cluc, or PRLR-Cluc. The same pool of the transfected cells in (b) and (c) was measured for bioluminescence in triplicate in a 96-well plate format (inset shows actual signals). The quantifications are displayed graphically as mean ± SE of total flux (photons/s × 10,000). (e) GH-induced complementation change. After basal bioluminescence was measured (d), GH was added and bioluminescence was again serially detected at 5-minute intervals over 40 minutes. Data are displayed as mean ± SE of GH-induced signal as a percentage above baseline signal (n = 3 per condition).
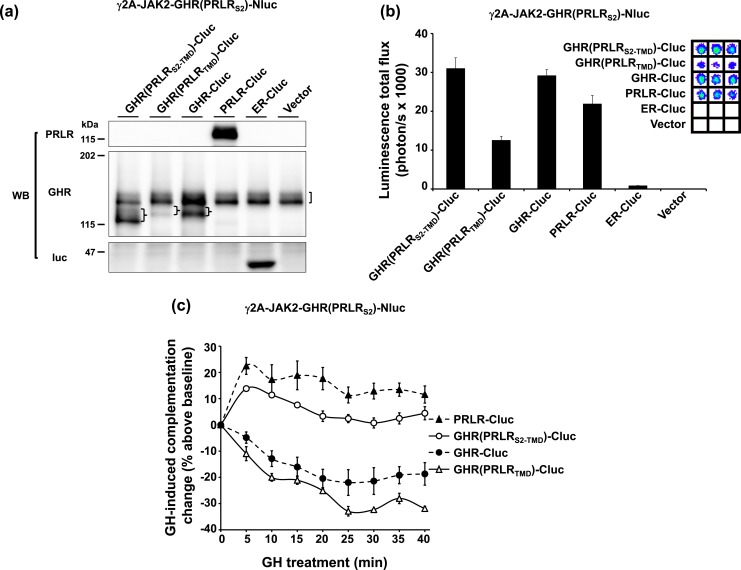
If the identity of S2 principally determines the dimerization partner, we would predict that GHR(PRLRS2-TMD)-Cluc, but not GHR(PRLRTMD)-Cluc, will dimerize with GHR(PRLRS2)-Nluc. To test these predictions, γ2A-JAK2-GHR(PRLRS2)-Nluc cells were transiently transfected with either GHR(PRLRS2-TMD)-Cluc or GHR(PRLRTMD)-Cluc or with controls including GHR-Cluc, PRLR-Cluc, ER-Cluc, and empty vector (Fig. 4). We verified expression of each of the relevant proteins by immunoblotting [Fig. 4(a)] and, as anticipated, detected robust basal complementation from GHR(PRLRS2)-Nluc with GHR(PRLRS2-TMD)-Cluc, or GHR(PRLRTMD)-Cluc, GHR-Cluc, and PRLR-Cluc, but not with ER-Cluc [Fig. 4(b)]. Note that in this transient transfection, GHR(PRLRTMD)-Cluc again displayed lower protein abundance [Fig. 4(a)] and thus a lower basal bioluminescence level [Fig. 4(b)] compared with GHR(PRLRS2-TMD)-Cluc and GHR-Cluc. However, the complementation level was still robust and specific compared with ER-Cluc transfection. As expected, GH treatment of γ2A-JAK2-GHR(PRLRS2)-Nluc cells augmented complementation when PRLR-Cluc was expressed and lessened complementation when GHR-Cluc was expressed [Fig. 4(c)]. In γ2A-JAK2-GHR(PRLRS2)-Nluc cells expressing GHR(PRLRS2-TMD)-Cluc (harboring the PRLR S2 domain), GH augmented complementation, mimicking the results with PRLR-Cluc. However, in γ2A-JAK2-GHR(PRLRS2)-Nluc cells expressing GHR(PRLRTMD)-Cluc (harboring the GHR S2 domain and the PRLR TMD), GH triggered reduced complementation in a pattern indistinguishable from that seen when GHR-Cluc was expressed. Collectively, the data in Fig. 4 emphasize that S2, and not the TMD, is most likely necessary and sufficient to determine the dimerization partner between GHR and PRLR.
Figure 4.
TMD does not alter the S2-dictated ligand-induced profile of complementation. (a) Transient expression of GHR(PRLRS2-TMD)-Cluc and GHR(PRLRTMD)-Cluc in γ2A-JAK2-GHR(PRLRS2)-Nluc cells. γ2A-JAK2-GHR(PRLRS2)-Nluc cells were transiently transfected with GHR(PRLRS2-TMD)-Cluc, GHR(PRLRTMD)-Cluc, GHR-Cluc, PRLR-Cluc, ER-Cluc, or empty vector, respectively, and analyzed by immunoblotting with anti-PRLR, anti-GHR, or anti-luc. Stably expressed GHR(PRLRS2)-Nluc is indicated by a bracket. Transfected receptor-Clucs are indicated by braces. (b) Specific luciferase complementation of GHR(PRLRS2)-Nluc and GHR(PRLRS2-TMD)-Cluc, or GHR(PRLRTMD)-Cluc. The same pool of the transfected cells in (a) was measured for bioluminescence in triplicate in a 96-well plate format (inset shows actual signals). The quantifications are displayed graphically as mean ± SE of total flux (photons/s × 1000). (c) GH-induced complementation change. After basal bioluminescence was measured (b), GH was added and bioluminescence was again serially detected at 5-minute intervals over 40 minutes. Data are displayed as mean ± SE of GH-induced signal as a percentage above baseline signal (n = 3 per condition).
Effects of anti-GHR and anti-PRLR ECD monoclonal antibodies on receptor-luc and chimera-luc complementation
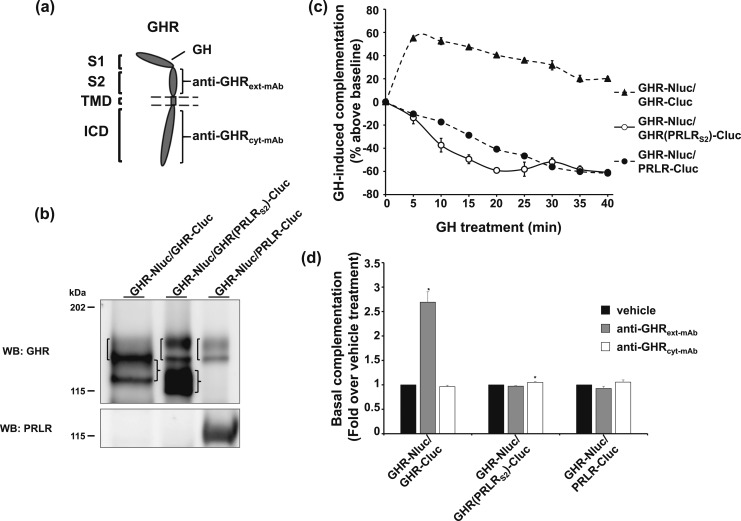
Anti-GHRext-mAb is an inhibitory monoclonal antibody (mAb) that interacts largely with the S2 of GHR’s ECD [Fig. 5(a)] (22, 23). Pretreatment with anti-GHRext-mAb inhibits subsequent GH-induced activation of GHR dimers in vitro and in vivo with little effect on GH binding; rather, this mAb appears to prevent GH-induced GHR conformational changes required for signaling (22, 23). Using our split luciferase complementation assay, we previously found that anti-GHRext-mAb treatment alone specifically enhanced basal complementation observed with coexpression of GHR-Nluc with GHR-Cluc without triggering receptor activation (6). This was interpreted as anti-GHRext-mAb introducing into GHR dimers a nonproductive conformational change (reflected in enhanced complementation signal) that prevents the GH-induced receptor conformational changes required for activation, and we took this mAb-induced receptor conformational change as a potential signature of receptor dimers.
Figure 5.
Effect of anti-GHRext-mAb suggests lack of dimerization between GHR and GHR(PRLRS2). (a) Diagram of the GHR domains that interact with anti-GHRext-mAb, anti-GHRcyt-mAb, and GH. (b) Three double-stable clones with GHR-Nluc but distinct receptor-Clucs. γ2A-JAK2-GHR-Nluc/GHR-Cluc, γ2A-JAK2-GHR-Nluc/GHR(PRLRS2)-Cluc, and γ2A-JAK2-GHR-Nluc/PRLR-Cluc cells were analyzed by immunoblotting with anti-GHR and anti-PRLR. Brackets on the left side of each lane indicate the position of GHR-Nluc, which is comprised of both the upper mature (less distinct) and lower precursor (sharper) bands. Braces on the right of lanes 1 and 2 indicate the positions of GHR-Cluc and GHR(PRLRS2)-Cluc, each comprised of both the mature and precursor bands. Note that the GHR-Nluc precursor and the GHR-Cluc mature form overlap in lane 1 to produce a more intense combined band. (c) GH-induced complementation change in three GHR-Nluc–expressing double-stable clones. γ2A-JAK2-GHR-Nluc/GHR-Cluc, γ2A-JAK2-GHR-Nluc/GHR(PRLRS2)-Cluc, and γ2A-JAK2-GHR-Nluc/PRLR-Cluc cells were measured for basal bioluminescence in triplicate in a 96-well plate format. GH was added, and bioluminescence was again serially detected at 5-minute intervals over 40 minutes. Data are displayed as mean ± SE of GH-induced signal as a percentage above baseline signal (n = 3 per condition). (d) Anti-GHRext-mAb enhances basal complementation of GHR-Nluc/GHR-Cluc, but not of GHR-Nluc/GHR(PRLRS2)-Cluc or GHR-Nluc/PRLR-Cluc. γ2A-JAK2-GHR-Nluc/GHR-Cluc, γ2A-JAK2-GHR-Nluc/GHR(PRLRS2)-Cluc, and γ2A-JAK2-GHR-Nluc/PRLR-Cluc cells were incubated with vehicle buffer, anti-GHRext-mAb, or anti-GHRcyt-mAb for 30 minutes. Bioluminescence was detected in triplicate in a 96-well plate format and normalized to the mean value from the vehicle buffer-incubated cells. Each bar represents data from three independent experiments and is displayed as mean of the fold change ± SE. *P < 0.05 when compared with respective vehicle treatment in each cell line.
To test this further, we sought to compare how anti-GHRext-mAb would affect complementation between GHR-Nluc and either GHR-Cluc, GHR(PRLRS2)-Cluc, or PRLR-Cluc. To allow adequate comparison, we created three doubly-transfected clonal cell lines that each stably expressed GHR-Nluc and one of the Cluc hybrids—these cell lines were designated γ2A-JAK2-GHR-Nluc/GHR-Cluc, γ2A-JAK2-GHR-Nluc/GHR(PRLRS2)-Cluc, and γ2A-JAK2-GHR-Nluc/PRLR-Cluc to connote that each stably expressed two different receptor-luc or chimera-luc hybrids. Immunoblotting with anti-GHR and anti-PRLR intracellular domain antisera verified expression of each protein in the three clones [Fig. 5(b)]. Each double-stable cell line displayed robust basal complementation (data not shown), and their patterns of complementation change in response to GH [Fig. 5(c)] conformed to those observed [Fig. 2(c)] when γ2A-JAK2-GHR-Nluc was transiently transfected with each of the Cluc-containing hybrids. GH induced augmentation of complementation in γ2A-JAK2-GHR-Nluc/GHR-Cluc (harboring GHR homodimers) and decline of complementation in γ2A-JAK2-GHR-Nluc/PRLR-Cluc (harboring GHR-GHR/PRLR-PRLR heteromultimers). Again, GHR(PRLRS2)-Cluc behaved like PRLR-Cluc, but not GHR-Cluc, in that GH treatment of γ2A-JAK2-GHR-Nluc/GHR(PRLRS2)-Cluc (harboring heteromultimers) caused decline of complementation nearly the same as that seen for γ2A-JAK2-GHR-Nluc/PRLR-Cluc. To evaluate their mAb response, bioluminescence of each of the three clones was monitored after treatment with either vehicle, a control antibody (anti-GHRcyt-mAb; directed at the GHR intracellular domain), or anti-GHRext-mAb [Fig. 5(d)]. As anticipated based on previous findings with transiently transfected cells (6), anti-GHRext-mAb specifically augmented complementation in γ2A-JAK2-GHR-Nluc/GHR-Cluc cells, consistent with this mAb response being a signature of GHR-GHR dimers. Furthermore, anti-GHRext-mAb failed to elicit a change in bioluminescence for either γ2A-JAK2-GHR-Nluc/PRLR-Cluc or γ2A-JAK2-GHR-Nluc/GHR(PRLRS2)-Cluc.
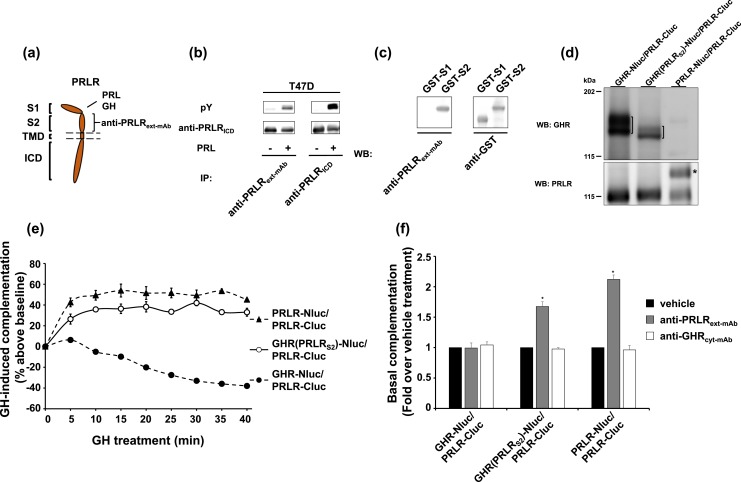
We recently raised a mouse mAb against the PRLR ECD [Fig. 6(a)], anti-PRLRext-mAb. This mAb recognizes native human PRLR, as demonstrated by immunoprecipitation of both total and PRL-induced phosphorylated receptor in T47D breast cancer cells [Fig. 6(b)]. Anti-PRLRext-mAb recognizes S2 (and not S1) of PRLR, as demonstrated by immunoblotting of GST fusion proteins incorporating either S1 or S2 [Fig. 6(c)]. Analogous to the experiments in Fig. 5 with anti-GHRext-mAb, we sought to test effects of anti-PRLRext-mAb on complementation of receptor pairs in three double-stable transfectant cell lines, each harboring PRLR-Cluc, namely: γ2A-JAK2-GHR-Nluc/PRLR-Cluc, γ2A-JAK2-GHR(PRLRS2)-Nluc/PRLR-Cluc, and γ2A-JAK2-PRLR-Nluc/PRLR-Cluc. Immunoblotting verified expression of each protein in the three clones [Fig. 6(d)]. When stimulated with GH [Fig. 6(e)], bioluminescence in γ2A-JAK2-PRLR-Nluc/PRLR-Cluc was increased [consistent with our previous findings (13)] and decreased in γ2A-JAK2-GHR-Nluc/PRLR-Cluc [consistent with our findings in the same clone in Fig. 5(c)]. In harmony with results in the transient expression system [Figs. 3(e) and 4(c)], GH caused a similar augmentation of complementation in γ2A-JAK2-GHR(PRLRS2)-Nluc/PRLR-Cluc cells as in γ2A-JAK2-PRLR-Nluc/PRLR-Cluc cells [Fig. 6(e)]. Analogous to the findings for anti-GHRext-mAb in γ2A-JAK2-GHR-Nluc/GHR-Cluc cells [Fig. 5(d)], treatment of γ2A-JAK2-PRLR-Nluc/PRLR-Cluc cells with anti-PRLRext-mAb yielded substantial and specific increase in complementation [Fig. 6(f)]. Yet, anti-PRLRext-mAb had no effect on complementation when PRLR-Nluc was replaced by GHR-Nluc (γ2A-JAK2-GHR-Nluc/PRLR-Cluc cells). However, complementation was strongly increased by anti-PRLRext-mAb treatment of γ2A-JAK2-GHR(PRLRS2)-Nluc/PRLR-Cluc cells (in which both receptors harbor the S2 domain from PRLR). The data in Fig. 6 are again consistent with the notion that both PRLR-Nluc and GHR(PRLRS2)-Nluc, but not GHR-Nluc, can dimerize with PRLR-Cluc. Of note, two clones (those harboring GHR-Nluc/PRLR-Cluc or PRLR-Nluc/PRLR-Cluc) had sister clones whose responses to anti-GHRext-mAb and/or anti-PRLRext-mAb were consistent with the clones included in Figs. 5 and 6 (data not shown). Furthermore, as to the stable clones GHR-Nluc/GHR(PRLRS2)-Cluc and GHR(PRLRS2)-Nluc/PRLR-Cluc, the results of mAb-induced changes were also observed in transient transfections (data not shown). These findings bolster those observed with the stable clones used in Figs. 5 and 6. Collectively, using the split luciferase complementation assay to analyze effects of treatment with mAbs (Figs. 5 and 6), or GH (Figs. 2–4), we revealed that the chimeric receptor GHR(PRLRS2) has a PRLR-like, not GHR-like, behavior. We accordingly conclude that the S2 of GHR or PRLR is both necessary and sufficient to determine dimerization partner of these two receptors. Dimerization results when two receptor monomers share the same S2 domain.
Figure 6.
Effect of anti-PRLRext-mAb suggests the dimerization between GHR(PRLRS2) and PRLR. (a) Diagram of the PRLR domains that interact with anti-PRLRext-mAb, GH, and PRL. (b) Anti-PRLRext-mAb was compared with commercial anti-ICD serum for immunoprecipitation (IP) of T47D cells treated ± PRL. Human breast cancer T47D cells were treated with ±PRL (500 ng/mL, 10 min), immunoprecipitated with anti-PRLRext-mAb or anti-PRLRICD, and analyzed by immunoblotting with anti-pY and anti-PRLRICD. (c) Anti-PRLRext-mAb reacts with PRLR ECD S2, but not S1. Bacterial extracts harboring GST fused to S1 (GST-S1) or S2 (GST-S2) of PRLR ECD were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted with anti-PRLRext-mAb or anti-GST. (d) Three double-stable clones with PRLR-Cluc, but distinct receptor-Nlucs. γ2A-JAK2-GHR-Nluc/PRLR-Cluc, γ2A-JAK2-GHR(PRLRS2)-Nluc/PRLR-Cluc, and γ2A-JAK2-PRLR-Nluc/PRLR-Cluc cells were analyzed by immunoblotting with anti-GHR and anti-PRLR. GHR-Nluc and GHR(PRLRS2)-Nluc are indicated by brackets. PRLR-Nluc is indicated by an asterisk. (e) GH-induced complementation change in three PRLR-Cluc–expressing double-stable clones. γ2A-JAK2-GHR-Nluc/PRLR-Cluc, γ2A-JAK2-GHR(PRLRS2)-Nluc/PRLR-Cluc, and γ2A-JAK2-PRLR-Nluc/PRLR-Cluc cells were measured for basal bioluminescence in triplicate in a 96-well plate format. GH was added, and bioluminescence was again serially detected at 5-minute intervals over 40 minutes. Data are displayed as mean ± SE of GH-induced signal as a percentage above baseline signal (n = 3 per condition). (f) Anti-PRLRext-mAb enhances basal complementation of GHR(PRLRS2)-Nluc/PRLR-Cluc or PRLR-Nluc/PRLR-Cluc, but not of GHR-Nluc/PRLR-Cluc. γ2A-JAK2-GHR-Nluc/PRLR-Cluc, γ2A-JAK2-GHR(PRLRS2)-Nluc/PRLR-Cluc, and γ2A-JAK2-PRLR-Nluc/PRLR-Cluc cells were incubated with vehicle buffer, anti-PRLRext-mAb, or anti-GHRcyt-mAb for 30 minutes. Bioluminescence was detected in triplicate in a 96-well plate format and normalized to the mean value from the vehicle buffer-incubated cells. Each bar represents data from three independent experiments and is displayed as mean of the fold change ± SE. *P < 0.05 when compared with respective vehicle treatment in each cell line.
Discussion
Our previous studies led us to conclude that GHRs and PRLRs can associate and form multimers composed of GHR-GHR homodimers and PRLR-PRLR homodimers, with each homodimer being independently activated by its ligand (13, 17). In the current study, we extended our analysis by adopting a domain-swapping approach to create GHR-PRLR chimeric receptors. We investigated their response to GH and anti-GHR or anti-PRLR mAbs in the split luciferase complementation assay and found that S2 itself is both necessary and sufficient to dictate the character of the chimeric receptor with regard to whether it forms dimers. When the chimeric receptor harbors the S2 from GHR, it behaves like GHR in terms of forming dimers with another receptor that harbors the GHR S2. When the chimeric receptor harbors the S2 from PRLR, it behaves like PRLR in that it forms dimers with another receptor that harbors the PRLR S2. Unlike S2, the origin of TMD did not alter the overall S2-dictated GHR-like or PRLR-like behavior of a particular chimeric receptor. Thus, we conclude that in a GHR-GHR/PRLR-PRLR multimeric complex, S2, and not the TMD, determines the dimerization partner of each receptor.
Our findings are consistent with our previous report that suggested an important role for S2, and not for the TMD, in GHR predimerization (2). In that study, we assessed the capacity for GHRs to associate using a coimmunoprecipitation assay. When the TMD of GHR was replaced with a heterologous TMD [that of the low-density lipoprotein receptor (LDLR)], the resulting chimeric receptor—GHRLDLR—was equally able as wild-type GHR to coimmunoprecipitate with a GHR truncated of its ICD. In contrast, a GHR mutant with a disrupted S2 region was markedly less able to coimmunoprecipitate with the truncated GHR. However, other studies that used forster resonance energy transfer (FRET) have implicated a more prominent role for the TMD in GHR dimer formation (3), in that FRET signals were detectable in either ECD-deleted or ICD-deleted GHR dimers. Collectively, these findings, in concert with those reported in this work, may suggest roles for both the ECD S2 and TMD regions in facilitating GHR dimerization with S2 required for stabilizing the mature cell surface GHR dimer and the TMD contributing to predimer formation at early stages in the secretory pathway, as previously suggested (2).
As noted previously (6), our findings of GH-induced enhancement of luciferase complementation in the setting of GHR dimers [or expression of PRLR dimers (13) or dimers that contain the same S2 region (the current study)] are at odds with some conclusions drawn from some (3, 5), but not other (4), studies using FRET/bioluminescence resonance energy transfer (BRET) methods. We note that the Waters group measured the FRET/BRET signal of activated state of GHR dimers at only one time point following GH treatment or in a constitutively activated GHR mutant and concluded that GH causes a separation of the proximal ICD of GHR (and therefore lessening of the FRET/BRET signal) as part of the activation process (5). In contrast, the Gertler group (4) measured the time course of GH-induced FRET signal changes in intact cells and found instead a transient GH-induced augmentation of signal, more similar to our luciferase complementation findings, which were obtained serially in the setting of GH stimulation of native (or subdomain-swapped and not constitutively active) receptors in intact living cells. As previously discussed, we cannot yet fully explain reasons for the apparently disparate FRET/BRET findings in the literature or between some of those findings and our luciferase complementation findings. Further biophysical studies may be helpful in this regard. In any case, our internally consistent complementation findings provided a useful signature that distinguishes between dimeric versus multimeric arrangements of GHR and PRLR and allowed further dissection of determinants for these arrangements.
Like GHR, ligand-independent (pre)dimerization of PRLR and its isoforms has been demonstrated (9, 12, 40) and neither ECD deletion nor ICD deletion of PRLR completely eliminated receptor predimerization (12). Also like GHR, the crystal structure of PRL with PRLR ECD revealed a 1:2 PRL:PRLR complex, and the interface between PRLR dimers encompassed the S2-S2 interaction (11). Again, our current data with the luciferase complementation assay and chimerized GHR/PRLR-luc fusions are consistent with a role for the S2 region in ligand-independent PRLR dimerization, in addition to its being a locus of PRLR-PRLR interaction in the setting of ligand binding.
Homomultimeric complexes, including GHRs alone or PRLRs alone, have been suggested by use of the biochemical technique of blue native gel electrophoresis of cell extracts (9, 41). Existence of such GHR or PRLR homomultimers in the intact cellular context has yet to be demonstrated by microscopy. However, emerging data suggest such assemblages for other cell surface proteins and signaling receptors (e.g., epidermal growth factor receptor) that form higher-order oligomers and/or nanoclusters in the plasma membrane and that these complexes are not likely to be artifacts of overexpression (42–45).
Our previous data (13) and that presented in this work suggest that GHR-PRLR heteromultimeric complexes also exist, but that the dimeric components of these multimers are limited to GHR-GHR and PRLR-PRLR homodimers, rather than inclusive of GHR-PRLR heterodimers. Inherent to this model is that determinants of dimerization versus multimerization may differ and be of different specificity. For example, one could envision strict specificity for dimer formation (GHR only with GHR; PRLR only with PRLR, for example), but more flexibility in terms of other receptors (perhaps also as dimers) that might be included in multimeric complexes with GHR-GHR and PRLR-PRLR dimers under some circumstances. Such arrangements could confer varying signaling outcomes and/or kinetics for GH and PRL stimulation, depending on relative receptor expression levels and/or local cellular or tissue situations. Future studies will focus on discerning more finely the determinants for dimerization and comparing them with those for multimer formation, as well as uncovering biologically relevant consequences of such arrangements.
Acknowledgments
We acknowledge helpful discussion with members of the Frank laboratory.
Financial Support: This work was supported by National Institutes of Health Grants DK58259 and DK107441 (both to S.J.F.) and a VA Merit Review award (to S.J.F.). Parts of this work were presented at the 99th Annual Endocrine Society Meeting in Orlando, Florida, 2017.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| Rabbit GHR | GHR intracellular domain | Anti-GHRcyt-AL47 | Stuart J. Frank | Rabbit; polyclonal | 1:1000 | AB_2661849 |
| Murine JAK2 | Residues 746–1129 | Anti-JAK2AL33 | Stuart J. Frank | Rabbit; polyclonal | 1:1000 | AB_2665398 |
| Human PRLR | PRLR intracellular domain | Anti-PRLRcyt-AL84 | Stuart J. Frank | Rabbit; polyclonal | 1:1000 | AB_2665406 |
| Phospho-JAK2 | Y1007 and Y1008 | Anti-pJAK2 | EMD Millipore, 07-606 | Rabbit; polyclonal | 1:1000 | AB_310746 |
| Phospho-tyrosine | Phosphotyramine-KLH | Anti-pY (4G10) | EMD Millipore, 05-321 | Mouse; monoclonal | 1:2000 | AB_309678 |
| Photinus pyralis Firefly luciferase | Full length firefly luciferase | Anti-luc | Promega, G7451 | Goat; polyclonal | 1:1000 | AB_430862 |
| Rabbit GHR | Residues 1–245 | Anti-GHRext-mAb | Stuart J. Frank | Mouse; monoclonal | Indicated amount | AB_2665407 |
| Human GHR | Residues 271–620 | Anti-GHRcyt-mAb | Stuart J. Frank | Mouse; monoclonal | Indicated amount | AB_2665408 |
| Human PRLR | PRLR extracellular domain | Anti-PRLRext-mAb | Stuart J. Frank | Mouse; monoclonal | Indicated amount | Reported in this study |
| Human PRLR | Residues 323–622 | PRL-R (H-300) | Santa Cruz, sc-20992 | Rabbit; polyclonal | 1:1000 | AB_2237692 |
| GST fusion proteins | A recombinant GST protein | GST (B-14) | Santa Cruz, sc-138 | Mouse; monoclonal | 1:1000 | AB_627677 |
Abbreviations: GHR, growth hormone receptor; GST, glutathione S-transferase; JAK2, Janus Kinase 2; KLH, keyhole limpet hemocyanin; PRLR, prolactin receptor; RRID, research resource identifier.
Footnotes
- BRET
- bioluminescence resonance energy transfer
- cDNA
- complementary DNA
- Cluc
- C-terminal fragment of the luciferase
- ECD
- extracellular domain
- FRET
- forster resonance energy transfer
- GH
- growth hormone
- GHR
- GH receptor
- GST
- glutathione S-transferase
- ICD
- intracellular domain
- luc
- luciferase
- mAb
- monoclonal antibody
- Nluc
- N-terminal fragment of the luciferase
- PCR
- polymerase chain reaction
- PRL
- prolactin
- PRLR
- PRL receptor
- S1
- subdomain 1
- S2
- subdomain 2
- SE
- standard error
- TMD
- transmembrane domain.
References
- 1.Gent J, van Kerkhof P, Roza M, Bu G, Strous GJ. Ligand-independent growth hormone receptor dimerization occurs in the endoplasmic reticulum and is required for ubiquitin system-dependent endocytosis. Proc Natl Acad Sci USA. 2002;99(15):9858–9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang N, Wang X, Jiang J, Frank SJ. Role of the growth hormone (GH) receptor transmembrane domain in receptor predimerization and GH-induced activation. Mol Endocrinol. 2007;21(7):1642–1655. [DOI] [PubMed] [Google Scholar]
- 3.Brown RJ, Adams JJ, Pelekanos RA, Wan Y, McKinstry WJ, Palethorpe K, Seeber RM, Monks TA, Eidne KA, Parker MW, Waters MJ. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol. 2005;12(9):814–821. [DOI] [PubMed] [Google Scholar]
- 4.Biener-Ramanujan E, Ramanujan VK, Herman B, Gertler A. Spatio-temporal kinetics of growth hormone receptor signaling in single cells using FRET microscopy. Growth Horm IGF Res. 2006;16(4):247–257. [DOI] [PubMed] [Google Scholar]
- 5.Brooks AJ, Dai W, O’Mara ML, Abankwa D, Chhabra Y, Pelekanos RA, Gardon O, Tunny KA, Blucher KM, Morton CJ, Parker MW, Sierecki E, Gambin Y, Gomez GA, Alexandrov K, Wilson IA, Doxastakis M, Mark AE, Waters MJ. Mechanism of activation of protein kinase JAK2 by the growth hormone receptor. Science. 2014;344(6185):1249783. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Berry PA, Zhang Y, Jiang J, Lobie PE, Paulmurugan R, Langenheim JF, Chen WY, Zinn KR, Frank SJ. Dynamic analysis of GH receptor conformational changes by split luciferase complementation. Mol Endocrinol. 2014;28(11):1807–1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ultsch M, de Vos AM, Kossiakoff AA. Crystals of the complex between human growth hormone and the extracellular domain of its receptor. J Mol Biol. 1991;222(4):865–868. [DOI] [PubMed] [Google Scholar]
- 8.Boutin JM, Edery M, Shirota M, Jolicoeur C, Lesueur L, Ali S, Gould D, Djiane J, Kelly PA. Identification of a cDNA encoding a long form of prolactin receptor in human hepatoma and breast cancer cells. Mol Endocrinol. 1989;3(9):1455–1461. [DOI] [PubMed] [Google Scholar]
- 9.Tallet E, Fernandez I, Zhang C, Salsac M, Gregor N, Ayoub MA, Pin JP, Trinquet E, Goffin V. Investigation of prolactin receptor activation and blockade using time-resolved fluorescence resonance energy transfer. Front Endocrinol (Lausanne). 2011;2:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Broutin I, Jomain JB, Tallet E, van Agthoven J, Raynal B, Hoos S, Kragelund BB, Kelly PA, Ducruix A, England P, Goffin V. Crystal structure of an affinity-matured prolactin complexed to its dimerized receptor reveals the topology of hormone binding site 2. J Biol Chem. 2010;285(11):8422–8433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Agthoven J, Zhang C, Tallet E, Raynal B, Hoos S, Baron B, England P, Goffin V, Broutin I. Structural characterization of the stem-stem dimerization interface between prolactin receptor chains complexed with the natural hormone. J Mol Biol. 2010;404(1):112–126. [DOI] [PubMed] [Google Scholar]
- 12.Gadd SL, Clevenger CV. Ligand-independent dimerization of the human prolactin receptor isoforms: functional implications. Mol Endocrinol. 2006;20(11):2734–2746. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Zhang Y, Jiang J, Lobie PE, Paulmurugan R, Langenheim JF, Chen WY, Zinn KR, Frank SJ. GHR/PRLR heteromultimer is composed of GHR homodimers and PRLR homodimers. Mol Endocrinol. 2016;30(5):504–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luker KE, Smith MC, Luker GD, Gammon ST, Piwnica-Worms H, Piwnica-Worms D. Kinetics of regulated protein-protein interactions revealed with firefly luciferase complementation imaging in cells and living animals. Proc Natl Acad Sci USA. 2004;101(33):12288–12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Villalobos V, Naik S, Piwnica-Worms D. Detection of protein-protein interactions in live cells and animals with split firefly luciferase protein fragment complementation. Methods Mol Biol. 2008;439:339–352. [DOI] [PubMed] [Google Scholar]
- 16.Paulmurugan R, Gambhir SS. Firefly luciferase enzyme fragment complementation for imaging in cells and living animals. Anal Chem. 2005;77(5):1295–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J, Zhang Y, Berry PA, Jiang J, Lobie PE, Langenheim JF, Chen WY, Frank SJ. Growth hormone signaling in human T47D breast cancer cells: potential role for a growth hormone receptor-prolactin receptor complex. Mol Endocrinol. 2011;25(4):597–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Guan R, Jiang J, Kopchick JJ, Black RA, Baumann G, Frank SJ. Growth hormone (GH)-induced dimerization inhibits phorbol ester-stimulated GH receptor proteolysis. J Biol Chem. 2001;276(27):24565–24573. [DOI] [PubMed] [Google Scholar]
- 19.Jiang J, Liang L, Kim SO, Zhang Y, Mandler R, Frank SJ. Growth hormone-dependent tyrosine phosphorylation of a GH receptor-associated high molecular WEIGHT protein immunologically related to JAK2. Biochem Biophys Res Commun. 1998;253(3):774–779. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Kleinberger JW, Takane KK, Salim F, Fiaschi-Taesch N, Pappas K, Parsons R, Jiang J, Zhang Y, Liu H, Wang P, Bender AS, Frank SJ, Stewart AF. Augmented Stat5 signaling bypasses multiple impediments to lactogen-mediated proliferation in human β-cells. Diabetes. 2015;64(11):3784–3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alele J, Jiang J, Goldsmith JF, Yang X, Maheshwari HG, Black RA, Baumann G, Frank SJ. Blockade of growth hormone receptor shedding by a metalloprotease inhibitor. Endocrinology. 1998;139(4):1927–1935. [DOI] [PubMed] [Google Scholar]
- 22.Jiang J, Wan Y, Wang X, Xu J, Harris JM, Lobie PE, Zhang Y, Zinn KR, Waters MJ, Frank SJ. Inhibitory GH receptor extracellular domain monoclonal antibodies: three-dimensional epitope mapping. Endocrinology. 2011;152(12):4777–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang J, Wang X, He K, Li X, Chen C, Sayeski PP, Waters MJ, Frank SJ. A conformationally sensitive GHR [growth hormone (GH) receptor] antibody: impact on GH signaling and GHR proteolysis. Mol Endocrinol. 2004;18(12):2981–2996. [DOI] [PubMed] [Google Scholar]
- 24.Kim SO, Jiang J, Yi W, Feng GS, Frank SJ. Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J Biol Chem. 1998;273(4):2344–2354. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Jiang J, Kopchick JJ, Frank SJ. Disulfide linkage of growth hormone (GH) receptors (GHR) reflects GH-induced GHR dimerization: association of JAK2 with the GHR is enhanced by receptor dimerization. J Biol Chem. 1999;274(46):33072–33084. [DOI] [PubMed] [Google Scholar]
- 26.Paulmurugan R, Gambhir SS. An intramolecular folding sensor for imaging estrogen receptor-ligand interactions. Proc Natl Acad Sci USA. 2006;103(43):15883–15888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohlhuber F, Rogers NC, Watling D, Feng J, Guschin D, Briscoe J, Witthuhn BA, Kotenko SV, Pestka S, Stark GR, Ihle JN, Kerr IM. A JAK1/JAK2 chimera can sustain alpha and gamma interferon responses. Mol Cell Biol. 1997;17(2):695–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loesch K, Deng L, Cowan JW, Wang X, He K, Jiang J, Black RA, Frank SJ. Janus kinase 2 influences growth hormone receptor metalloproteolysis. Endocrinology. 2006;147(6):2839–2849. [DOI] [PubMed] [Google Scholar]
- 29.Deng L, He K, Wang X, Yang N, Thangavel C, Jiang J, Fuchs SY, Frank SJ. Determinants of growth hormone receptor down-regulation. Mol Endocrinol. 2007;21(7):1537–1551. [DOI] [PubMed] [Google Scholar]
- 30.Deng L, Jiang J, Frank SJ. Growth hormone-induced JAK2 signaling and GH receptor down-regulation: role of GH receptor intracellular domain tyrosine residues. Endocrinology. 2012;153(5):2311–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frank SJ, Gilliland G, Kraft AS, Arnold CS. Interaction of the growth hormone receptor cytoplasmic domain with the JAK2 tyrosine kinase. Endocrinology. 1994;135(5):2228–2239. [DOI] [PubMed] [Google Scholar]
- 32.Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993;74(2):237–244. [DOI] [PubMed] [Google Scholar]
- 33.Brooks CL. Molecular mechanisms of prolactin and its receptor. Endocr Rev. 2012;33(4):504–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Campbell GS, Argetsinger LS, Ihle JN, Kelly PA, Rillema JA, Carter-Su C. Activation of JAK2 tyrosine kinase by prolactin receptors in Nb2 cells and mouse mammary gland explants. Proc Natl Acad Sci USA. 1994;91(12):5232–5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rui H, Kirken RA, Farrar WL. Activation of receptor-associated tyrosine kinase JAK2 by prolactin. J Biol Chem. 1994;269(7):5364–5368. [PubMed] [Google Scholar]
- 36.Cunningham BC, Bass S, Fuh G, Wells JA. Zinc mediation of the binding of human growth hormone to the human prolactin receptor. Science. 1990;250(4988):1709–1712. [DOI] [PubMed] [Google Scholar]
- 37.Fu YK, Arkins S, Fuh G, Cunningham BC, Wells JA, Fong S, Cronin MJ, Dantzer R, Kelley KW. Growth hormone augments superoxide anion secretion of human neutrophils by binding to the prolactin receptor. J Clin Invest. 1992;89(2):451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Somers W, Ultsch M, De Vos AM, Kossiakoff AA. The X-ray structure of a growth hormone-prolactin receptor complex. Nature. 1994;372(6505):478–481. [DOI] [PubMed] [Google Scholar]
- 39.de Vos AM, Ultsch M, Kossiakoff AA. Human growth hormone and extracellular domain of its receptor: crystal structure of the complex. Science. 1992;255(5042):306–312. [DOI] [PubMed] [Google Scholar]
- 40.Qazi AM, Tsai-Morris CH, Dufau ML. Ligand-independent homo- and heterodimerization of human prolactin receptor variants: inhibitory action of the short forms by heterodimerization. Mol Endocrinol. 2006;20(8):1912–1923. [DOI] [PubMed] [Google Scholar]
- 41.Sedek M, van der Velden LM, Strous GJ. Multimeric growth hormone receptor complexes serve as signaling platforms. J Biol Chem. 2014;289(1):65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Needham SR, Zanetti-Domingues LC, Hirsch M, Rolfe DJ, Tynan CJ, Roberts SK, Martin-Fernandez ML, Clarke DT. Structure-function relationships and supramolecular organization of the EGFR (epidermal growth factor receptor) on the cell surface. Biochem Soc Trans. 2014;42(1):114–119. [DOI] [PubMed] [Google Scholar]
- 43.Tynan CJ, Lo Schiavo V, Zanetti-Domingues L, Needham SR, Roberts SK, Hirsch M, Rolfe DJ, Korovesis D, Clarke DT, Martin-Fernandez ML. A tale of the epidermal growth factor receptor: the quest for structural resolution on cells. Methods. 2016;95:86–93. [DOI] [PubMed] [Google Scholar]
- 44.Goyette J, Gaus K. Mechanisms of protein nanoscale clustering. Curr Opin Cell Biol. 2017;44:86–92. [DOI] [PubMed] [Google Scholar]
- 45.Boulanger MJ, Chow DC, Brevnova EE, Garcia KC. Hexameric structure and assembly of the interleukin-6/IL-6 alpha-receptor/gp130 complex. Science. 2003;300(5628):2101–2104. [DOI] [PubMed] [Google Scholar]