Abstract
Despite recent studies that show oxidative stress–generated reactive oxygen species (ROS) regulate NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome–mediated innate immune response in various diabetic complications, the mechanism by which ROS activate innate immune response is not well understood. We have shown previously that aldose reductase (AR), besides reducing glucose, reduces lipid aldehydes and their glutathione conjugates and participates in various oxidative stress–induced inflammatory pathways. To understand the role of AR in ROS-induced innate immune response, we have investigated the mechanism(s) by which AR activates hyperglycemia-induced NLRP3 inflammsome–initiated innate immune response in Thp1 monocytes and in streptozotocin (STZ)-induced diabetic mice. In Thp1 monocytes, inhibition or ablation of AR prevented high-glucose–induced activation of NLRP3 inflammasome and caspase-1 and release of the innate immune cytokines interleukin (IL)-1β and IL-18. AR inhibition in Thp1 cells also prevented the high-glucose–induced generation of ROS, influx of Ca2+, efflux of K+, and activation of Lyn, Syk, and PI3K. Furthermore, the AR inhibitor fidarestat prevented the expression of NLRP inflammasome components in STZ-induced diabetic mouse heart and aorta, and also prevented the release of various cytokines in the serum. Collectively, our data suggest that AR regulates hyperglycemia-induced NLRP3 inflammasome–mediated innate immune response by altering the ROS/Lyn/Syk/PI3K/Ca2+/K+ signals.
This study identifies the role of aldose reductase in regulation of NLRP3 inflammasome–mediated processing of IL-1β in high-glucose–treated Thp1 cells via modulation of ROS/Ca2+/Syk/Lyn pathway.
Diabetes is involved in the pathophysiology of different forms of cardiovascular diseases such as hypertension, coronary artery disease, chronic heart failure, and peripheral artery disease (1). Further, hyperglycemia-induced inflammation constitutes a strong risk factor for developing secondary diabetic complications (2). However, it is not clearly known how high glucose levels lead to a massive and uncontrolled expression of proinflammatory mediators that initiate a chain of events that cause widespread inflammation and tissue dysfunction. Although increased formation of reactive oxygen species (ROS), macrophage infiltration, and inflammatory cytokines and chemokines in hyperglycemia are known to contribute to tissue dysfunction (3), the role of a hyperglycemia-induced innate immune response in diabetes is not well understood.
Monocytes and macrophages release various proinflammatory cytokines, which, in an autocrine/paracrine manner, cause an immune response. Interleukin (IL)-1β is one of the most prominent and early mediators of the innate immune response that mediates the pathogenesis of a number of inflammatory diseases, including diabetes, restenosis, and atherosclerosis (4). Furthermore, IL-1β can mediate its own production and also induce the expression of several proinflammatory cytokines, such as IL-6, IL-8, and tumor necrosis factor (TNF)-α, which propagate severe inflammatory response and cellular toxicity (4, 5). IL-1β, produced as a 31-kDa precursor by nuclear factor-κB (NF-κB)–mediated signalosome activation, is cleaved to a 17-kDa bioactive form. The conversion of pro-IL-1β to bioactive IL-1β is accomplished by NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome-mediated activation of caspase-1. In response to oxidative stimuli, a major NOD-like receptor family member, NLRP3, assembles in a large multiprotein complex in association with apoptosis-associated speck-like protein containing C-terminal caspase recruitment domain (ASC) and procaspase-1. This leads to autocatalytic activation of caspase-1, which is also involved in the activation of other proinflammatory cytokines, such as IL-1β and IL-18. In general, NLRP3 inflammasome assembles in response to a variety of diverse exogenous and endogenous activators, such as various microbial stimuli (e.g., bacteria, viruses), crystalline substances, and peptide aggregates (6). Furthermore, recent studies indicate that patients with diabetes have increased innate immune cytokine and elevated NLRP3 inflammasome levels (7, 8). However, the mechanism(s) of NLRP3 activation that contributes to diabetic complications is not clearly understood.
Our recent studies have firmly established that aldose reductase (AR; AKR1B1), a polyol pathway enzyme that is highly expressed during oxidative stress conditions, is the main mediator of the ROS-induced signals that contribute to the inflammatory response commonly observed in diabetes (9). We have demonstrated that AR, besides reduction of aldo sugars to corresponding polyols, reduces lipid aldehydes such as 4-hydroxynonenal (HNE) and its conjugates with glutathione to corresponding alcohols, which are the main transducers of inflammatory signals (9). ROS generated by exposure of cells to cytokines, chemokines, growth factors, and carcinogens oxidize lipids to form lipid aldehydes such as HNE. HNE and its glutathione conjugate are reduced to dihydroxynonene (DHN) or the glutathione-DHN conjugate by AR. We have further demonstrated that the glutathione -DHN conjugate activates PKC, PI3K, and IKK, which, through a series of protein kinases, activate the NF-κB signalosome that transcribes inflammatory markers such as cytokines, chemokines, and growth factors (9, 10). The small-molecule AR inhibitors such as fidarestat or AR–small interfering RNA (siRNA) prevent the NF-κB signalosome activation in in vitro and in vivo models of diabetes (11). However, the role of AR in the mediation of the hyperglycemia induced innate immune response is not known.
In this study, we examined the effect of AR inhibition on hyperglycemia-induced NLRP3 inflammasome activation, caspase-1 activation, and IL-1β release from Thp1 monocytes. AR inhibition by fidarestat or ablation by AR–siRNA substantially prevented the cleavage of caspase-1 and release of active IL-1β in Thp1 monocytes. Inhibition of AR also prevented the increase in the expression of inflammasome components in the heart and aorta, and IL-1β in the serum of streptozotocin (STZ)-induced diabetic mice. Thus, our results demonstrate that AR inhibition prevents high-glucose–induced innate immune response by regulating the NLRP3 inflammasome–mediated release of innate immune cytokines via the ROS/Lyn/Syk/PI3K/Ca2+/K+ pathway.
Materials and Methods
Materials
RPMI 1640 was purchased from Gibco. STZ, d-glucose, and adenosine triphosphate (ATP) were purchased from Sigma-Aldrich. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), phospho-Syk, Syk, phospho-Lyn, Lyn, phospho-PI3K, and caspase-1 antibodies were obtained from Cell Signaling Technology. IL-1β, IL-18, cryopyrin and ASC antibodies, and nigericin were obtained from Santa Cruz Biotechnology, as was radioimmunoprecipitation assay (RIPA) buffer. AKR1B1 siRNA, control siRNA, and Hiperfect transfection reagent were obtained from Qiagen. R406 (Syk inhibitor) was obtained from InvivoGen. Fidarestat was a gift from Livwel Therapeutics Inc. (Northridge, CA). Potassium-binding benzofuran isophthalate acetoxymethyl ester (PBFI-AM); Fura-2, AM; and 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) were obtained from Molecular Probes. The IL-1β human enzyme-linked immunosorbent assay (ELISA) kit was obtained from R&D Systems.
Cell culture studies
Human leukemia monocyte Thp1 cells were obtained from American Type Culture Collection. Thp1 monocytes were maintained in endotoxin-free RPMI 1640 medium containing 10% fetal bovine serum (FBS; Gemini Bio Products) and penicillin-streptomycin at 37°C in a humidified atmosphere of 5% CO2. Cells were treated with 25 mM glucose (19.5 mM glucose was added to the normal medium containing 5.5 mM glucose). Control (normal medium) consisted of 5.5 mM glucose. Cells treated with high glucose (HG; 25 mM) concentration were treated with or without fidarestat (10 µM) for various time periods (0–72 hours).
Animal studies
Male C57BL/6 mice, 7 weeks old, were purchased from Envigo. After 1 week of quarantine, mice were fed ad libitum and maintained in a specific pathogen-free environment with a 12-hour light/12-hour dark cycle. Mice were made diabetic by injecting a single intraperitoneal dose of STZ (165 mg/kg). Four days after STZ injection, blood glucose levels were measured by glucometer (True Metrix), and only those mice were used that had blood glucose levels >400 mg/dL. Diabetic mice were randomly divided into experimental groups without or with fidarestat. Fidarestat (10 mg/kg/d intraperitoneally) was administered to diabetic mice on day 5, and the animals were euthanized after 1, 2, 3, and 4 days.
Cell viability assay
Thp1 cells were cultured in RPMI 1640 medium. Cells (1 × 104 cells per well) were plated in 96-well plates and pretreated overnight with fidarestat (10 µM) in a medium containing 0.5% FBS. The cells were then treated with HG and IL-1β. Cell viability was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, as described previously (12). The number of cells was determined by Trypan blue exclusion method, using a hemocytometer (12).
Live cell detection
Live cells were detected by using cell-permeable dye, calcein AM (2 μM (Invitrogen, Molecular Probes). Thp1 cells (1 × 106 cells per well) were pretreated with fidarestat (10 μM) in the absence and presence of HG (25 mM) for 24 and 48 hours at 37°C in a CO2 incubator. After 24 and 48 hours, the cells (1 × 106 cells/mL) were stained with calcein AM (2 μM; 30 minutes; 37°C), washed twice in Hanks balanced salt solution (HBSS), suspended in RPMI medium 1640 containing 0.5% bovine serum, and placed in chambered coverslips. Fluorescence images were obtained using a fluorescence microscope (EVOSfl; Thermo Fisher Scientific). Calcein AM dye was excited at 495 nm and emission measured using a 517-nm filter.
Quantitative reverse-transcription polymerase chain reaction
Total RNA was isolated from Thp1 cells, using Trizol reagent. Total RNA (1 μg) was used to synthesize complementary DNA by using a Taqman reverse transcription kit (catalog no. N8080234; Life Technologies). Gene amplifications by quantitative polymerase chain reaction (PCR) were performed by using 1 µL of complementary DNA in a total volume of 20 µL using the iTaq Universal SYBR Green Supermix (1725125; Bio-Rad). The final concentration of the primers was 300 nM. Relative reverse transcription-PCR assay data were normalized with the 18S housekeeping gene and data were calculated with the 2-∆∆Ct method. All PCR assays were performed in triplicate on the ABI Prism 7500 Sequence Detection System. The primer used for PCR was IL-1β (forward: 5′-TCTGTACCTGTCCTGCGTGTTG-3′; reverse: 5′-CAAATCGCTTTTCCATCTTCTTC-3′).
ROS detection
Thp1 cells were pretreated with fidarestat followed by HG treatment. The cells (1 × 106 cells/mL) were loaded with the fluorescent dye CM-H2DCFDA (5 µM; 45 minutes), washed with and resuspended in HBSS, and placed in chambered cover slides. Fluorescence images were obtained using the fluorescence microscope. Fluorescence intensity was quantitated by measuring the CM-H2DCFDA dye-treated cells via a fluorescence plate reader [excitation: 492–495 nm; emission: 517–527 nm (13)]. Furthermore, after the incubation of the cells with HG ± fidarestat (2 hours), the cells were stained with CM-H2DCFDA for 5 minutes and fluorescence-activated cell sorting analysis was performed (LSRII Fortessa; BD Biosciences). Data were analyzed using Flow Jo software (Treestar, Ashland, OR) and represented as fold change of mean fluorescence intensity, as compared with control.
Measurement of IL-1β by ELISA
IL-1β levels in Thp1 culture media and in mice serum were measured using a commercially available human ELISA kit from R&D Systems, per the manufacturer’s instructions.
Measurement of intracellular Ca2+
Intracellular Ca2+ was measured using the cell-permeant calcium indicator Fura-2 AM (Invitrogen, Molecular Probes). Treated Thp1 cells (1 × 106 cells/mL) were loaded with Fura-2 AM (1 µM; 60 minutes; 37°C), washed twice in HBSS, resuspended in RPMI medium 1640 containing 0.5% bovine serum, and placed in chambered cover slides. Fluorescence images were obtained using the fluorescence microscope and fluorescence intensity was measured by a Biotek Synergy 2 multimode reader. Fura-2 AM dye was excited at 340/380 nm and emission measured at 510 nm, per the supplier’s instructions.
Measurement of intracellular K+
Intracellular K+ was measured using the cell-permeable potassium indicator PBFI AM (Invitrogen, Molecular Probes). HG- and HG- plus fidarestat- treated Thp1 cells (1 × 106 cells/mL) were washed with HBSS and loaded with 5 μM PBFI and 10 μM F-127 (Pluronic F-127; Molecular Probes, Inc.) for 40 minutes at 5% CO2 and 37°C in the dark. Cells were washed with HBSS three times before intensity measurement. Fluorescence images were obtained using a fluorescence microscope and fluorescence intensity was measured by the Biotek Synergy 2 multimode reader. PBFI AM dye was excited at 340/380 nm and emission was measured at 500 nm.
AR knockdown by siRNA
Thp1 cells were grown in RPMI 1640 medium containing 10% FBS and 1% penicillin/streptomycin at 37°C in a humidified atmosphere of 5% CO2. Thp1 cells (1 × 105 per well) were seeded for 24 hours before transfection in appropriate culture medium containing serum and antibiotics. Cells were incubated with AR siRNA or control siRNA with Hiperfect transfection reagent (Qiagen) to knock down AR, per the supplier’s guidelines. The cells were cultured for 48 hours at 37°C in a humidified CO2 incubator. The changes in AR expression were determined by Western blot analysis using anti-AR antibodies.
Determination of cytokine and chemokine levels
The levels of cytokines and chemokines in the mice serum were determined by using the Milliplex Map Kit (Millipore) mouse inflammatory cytokine/chemokine magnetic bead panel along with Luminex xMAP detection method per the manufacturer’s protocol in a Millipore multiplex system (Millipore) (14).
Immunoblotting
Treated Thp1 cells were washed with cold PBS and lysed in RIPA lysis buffer containing a protease inhibitor cocktail, and centrifuged to obtain cell supernatants. For heart and aorta tissues, frozen tissue sections were homogenized in RIPA buffer containing a protease inhibitor cocktail (Chem Cruz; Santa Cruz Biotechnology) with a tissue homogenizer. The tissue homogenates were centrifuged at 10,000g for 20 minutes at 4°C. Total protein in the Thp1 cell extracts and tissue homogenates was measured by using Bradford reagent (Bio-Rad protein assay). Equal amounts of proteins from Thp1 cell lysates, Thp1 cell culture media (lyophilized), and tissue homogenates were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis followed by transfer of proteins to nitrocellulose membranes and probing with the specific antibodies. The antigen-antibody complexes were detected by enhanced Super Signal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific). All blots were probed with GAPDH as loading control.
Guideline statement
All methods used in this study were in accordance with the guidelines and regulations approved by the University of Texas Medical Branch (UTMB), Galveston, Texas. Mice were maintained in a specific pathogen-free environment at the UTMB’s animal facility. The mice were randomly divided into experimental groups and the health of the animals was monitored frequently until the end of the experiments. All animal experiments were performed in accordance with relevant guidelines and protocols were approved by the Institutional Animal Care and Use Committee, UTMB, Galveston.
Statistical analysis
Data are presented as mean ± standard error of the mean, and the P values were determined using the unpaired Student t test (GraphPad Prism software; GraphPad, La Jolla, CA). One-way analysis of variance was used for multiple comparisons. P < 0.05 was considered statistically significant.
Results
Inhibition of AR prevented HG-induced Thp1 monocyte cell viability
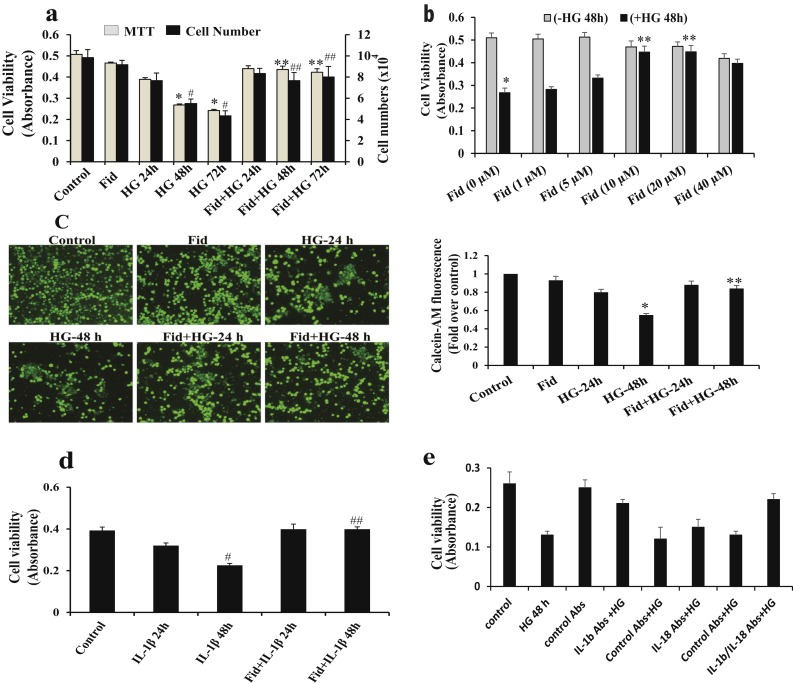
We determined the effects of AR inhibition on HG-induced Thp1 cell viability by MTT assay and cell counting. As shown in Fig. 1a, HG significantly decreased the Thp1 cell viability in a time-dependent manner and preincubation with fidarestat prevented Thp1 cell viability. Fidarestat prevented HG-induced cell viability in a dose-dependent manner (Fig. 1b). Similar results were observed when cell viability was determined by staining the live cells, using calcein AM (Fig. 1c). Because IL-1β is an important innate immune cytokine responsible for monocyte cytotoxicity (6), we next examined the effect of AR inhibition on IL-1β–induced Thp1 cell viability. Cells treated without or with fidarestat were incubated without or with IL-1β (10 ng/mL) for 24 and 48 hours and cell viability was determined. Incubation of Thp1 cells with IL-1β caused a significant (∼50%) reduction in the cell viability and fidarestat prevented the IL-1β-induced decrease in Thp1 cell viability (Fig. 1d).
Figure 1.
AR inhibition prevents HG-induced decrease in Thp1 cell viability. (a) Thp1 cells (1 × 104 cells per well in 96-well plates) were pretreated with fidarestat (10 µM) followed by incubation with HG concentration (25 mM) for 24, 48, and 72 hours. (b) Thp1 cells were pretreated with indicated concentrations of fidarestat followed by incubation with HG (25 mM) for 48 hours. Cell viability was measured by MTT assay, and cell counting was performed by Trypan blue exclusion method using a hemocytometer. (c) Thp1 cells (1 × 106 cells per well) were pretreated with fidarestat in the absence and presence of HG (25 mM) for 24 and 48 hours at 37°C in a CO2 incubator. Live cells were detected by using cell-permeable calcein AM staining (Invitrogen, Molecular Probes), and fluorescence images (×20 magnification) were obtained using a fluorescence microscope. (d) Thp1 cells (1 × 104 cells per well in 96-well plates) were pretreated with fidarestat, followed by incubation without or with IL-1β (10 ng/mL) for 24 and 48 hours. Cell viability was measured by MTT assay. (e) Cells were preincubated with IL-1β and IL-18 antibodies or respective control antibodies (5 µg/mL) before adding HG for 1 hour. HG was added and cells were further incubated for 48 hours. Cell viability was measured by MTT assay. The bars represent the mean ± standard error of the mean (n ≥ 4). *P < 0.05 and #P < 0.05 vs normal glucose concentration (5.5 mM); **P < 0.01 and ##P < 0.01 vs HG (19.5 mM glucose added to 5.5 mM normal glucose media); #P < 0.05 vs control; ##P < 0.01 vs IL-1β. Abs, absorbance; Fid, fidarestat.
To examine the role of innate immune cytokines (i.e., IL-1β and IL-18) in the HG-induced monocyte growth, we next examined the effect of IL-1β and IL-18 antibodies on HG-induced Thp1 cell viability. Thp1 cells were preincubated (1 hour) with IL-1β or IL-18 antibodies followed by incubation with HG for an additional 48 hours, and cell viability was determined by MTT assay. The results shown in Fig. 1e indicate preincubation with IL-1β antibodies, but not with IL-18 antibodies, significantly protected Thp1 cells from HG-induced decrease in the cell viability. These results suggest that IL-1β plays a major role in the mediation of HG-induced Thp1 monocyte viability. Isotype-matched control antibodies showed no effect on HG-induced cell viability, although our data suggest an approximately twofold induction in the IL-1β protein levels and IL-1β antibodies did not completely restore the HG-induced cell viability. This could be due to additional cytokines such as TNF-α that are also released in HG-treated cells. These results suggest that besides IL-1β, other cytokines or growth factors may also be involved in HG-induced cell viability.
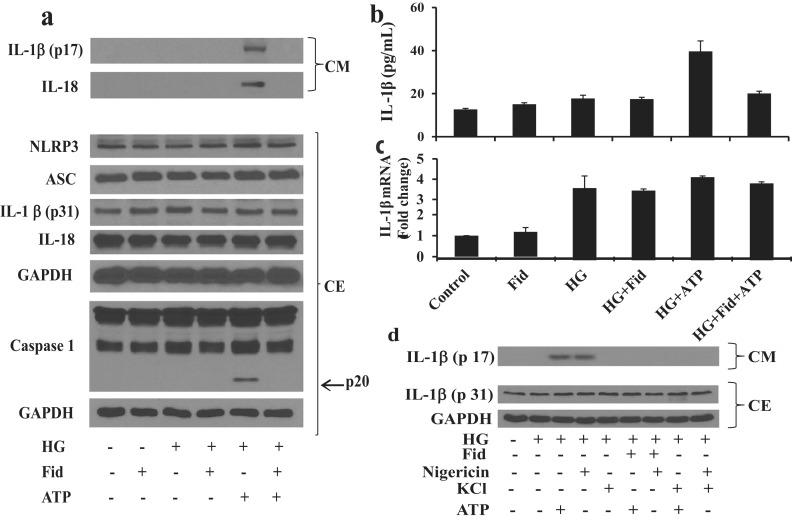
Inhibition of AR prevented HG-induced IL-1β release through NLRP3 inflammasome
Because innate immune cytokines such as IL-1β and IL-18 are released by the NLRP3 inflammasome–mediated processing (6, 7), we next examined the role of AR in the mediation of NLRP3 activation and release of active IL-1β and IL-18. Thp1 cells were incubated with HG (25 mM) for 4 hours followed by further incubation without or with fidarestat for 30 minutes and ATP (2 mM) for an additional 1 hour. Various inflammasome complex proteins such as NLRP3, ASC and caspase-1 and inactive IL-1β and IL-18 were measured in the cell extracts and the active IL-1β and IL-18 were measured in the culture media by Western blot analysis. There were no significant differences in the expression of inflammasome complex proteins such as NLRP3 and ASC, and innate cytokines such as IL-1β and IL-18 in the absence and presence of fidarestat or ATP in the Thp1 monocytes (Fig. 2a). However, in the presence of ATP, HG-treated cells showed a substantial release of active IL-1β and IL-18 in the culture media, which was prevented by fidarestat. Furthermore, under identical conditions, HG and ATP caused increased expression and activation of caspase-1, which was markedly inhibited by AR inhibition (Fig. 2a).
Figure 2.
Inhibition of AR prevented –HG-induced NLRP3 inflammasome–mediated release of innate immune cytokines in Thp1 cells. Thp1 cells were preincubated (4 hours) with HG (25 mM) and then incubated with fidarestat (10 μΜ) for 30 minutes, followed by ATP (2 mM) for 1 hour. (a) Equal amount of proteins in the cell extracts (CEs) were subjected to Western blot analysis to determine the expression of inflammasome complex proteins NLRP3, ASC, caspase-1, and innate immune cytokines IL-1β and IL-18, using specific antibodies. Equal amounts of proteins in the lyophilized culture media (CM) were also subjected to Western blot analysis for IL-1β and IL-18. (b) IL-1β release in cell culture supernatant was also determined by a specific ELISA kit. (c) IL-1β mRNA levels in Thp1 cells were determined by reverse transcription-PCR, as described in Methods. (d) Thp1 cells were incubated (4 hours) with HG (25 mM) and then incubated with fidarestat (10 μM) for 30 minutes, followed by ATP (2 mM) and nigericin (20 µM) for 1 hour without or with addition of KCl (130 mM). Equal amounts of proteins in the lyophilized CM andCEs were subjected to Western blot analysis for IL-1β. The representative cropped blots are shown. The bars represent the mean ± standard error of the mean (n ≥ 4). Fid, fidarestat.
The release of IL-1β in the culture media was further confirmed by using the specific IL-1β ELISA kit. Our results demonstrate that HG, in the presence of ATP, increased the levels of IL-1β in the culture media and fidarestat prevented it (Fig. 2b). Similarly, HG also increased the mRNA levels of IL-1β in Thp1 cells. However, fidarestat had no effect on HG-induced IL-1β mRNA levels (Fig. 2c).
The release of IL-1β was further confirmed in the presence of positive (nigericin) and negative (KCl) controls in HG-treated Thp1 cells. Our results, shown in the Fig. 2d, suggest that similar to ATP, another potassium-effluxing agent, nigericin, also released the active IL-1β in culture media and the AR inhibitor prevented it. However, treatment of cells with the potassium-efflux inhibitor KCl had no effect on the processing of IL-1β in HG-treated monocytes. These results suggest that AR inhibition prevents HG-induced NLRP3 inflammasome–mediated caspase-1 activation and release of innate immune cytokines such as IL-1β and IL-18 in Thp1 monocytes.
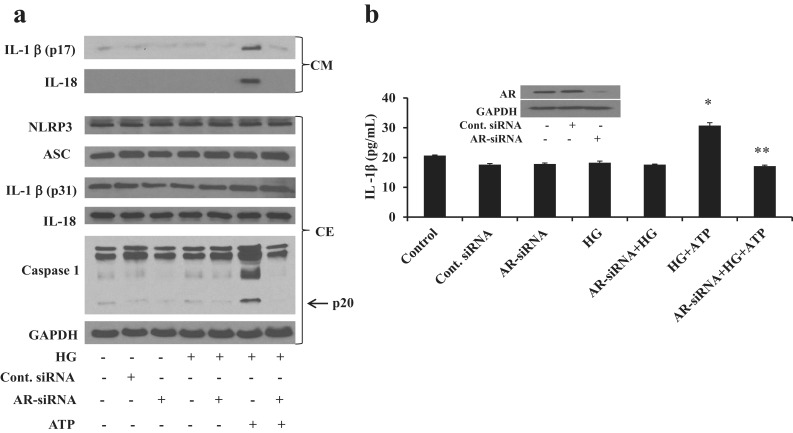
We have shown previously that fidarestat prevents cytokine- and growth factor–induced cytotoxicity in cultured cells (9–15). To further confirm the effect of fidarestat on HG-induced cell viability, we performed a dose-dependent study and found that 10 μM fidarestat substantially prevented HG-induced cell viability. To rule out any nonspecific effects of fidarestat, we selectively ablated AR in Thp1 cells by using specific AR siRNA and examined the activation of NLRP3 inflammasome and release of innate immune cytokines. As shown in the Fig. 3b inset, transfection of Thp1 cells with AR siRNA substantially (∼95%) knocked down the expression of AR, whereas, in control siRNA-transfected cells, the expression of AR was similar to that in the untransfected cells. Knockdown of AR by AR siRNA also inhibited HG- plus ATP-induced release of IL-1β and IL-18 in the culture media (Fig. 3a) and suppressed the HG- plus ATP-induced caspase-1 activation in Thp1 cells. However, no changes were observed in the expression of NLRP3, ASC, IL-1β, and IL-18 in the HG/ATP-induced as well as AR siRNA- transfected Thp1 cell extracts (Fig. 3a). Similar to Western blot analysis, ELISA results also showed that AR siRNA prevented the HG- plus ATP-induced release of IL-1β in the culture media (Fig. 3b). Our results thus suggest that AR plays an important role in HG-induced NLRP3 inflammasome-mediated innate immune response in monocytes.
Figure 3.
Knockdown of AR prevents –HG-induced NLRP3 inflammasome–mediated release of innate immune cytokines in Thp1 cells. Thp1 cells were transfected with AR siRNA or control siRNA and cultured for 48 hours at 37°C. The cells were incubated (4 hours) with HG (25 mM) followed by ATP (2 mM) for 1 hour. (a) Equal amount of proteins in the cell extracts (CEs) were subjected to Western blot analysis to determine the expression of inflammasome complex proteins NLRP3, ASC, caspase-1, and innate immune cytokines IL-1β and IL-18, using specific antibodies. Equal amount of proteins in the culture media (CM) were also subjected to Western blot analysis for IL-1β and IL-18. (b) IL-1β release in cell culture supernatant was also determined by a specific ELISA kit. Inset shows the Western blots of siRNA-transfected cells performed using specific AR antibodies. The representative cropped blots are shown. The bars represent the mean ± standard error of the mean (n ≥ 4). *P < 0.05 vs control (normal glucose concentration); **P < 0.01 vs HG plus ATP. Cont., control.
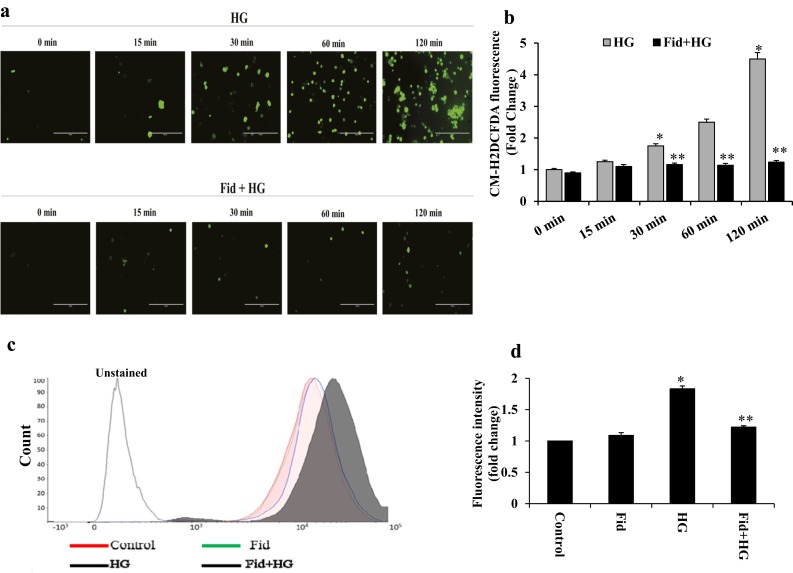
AR inhibition suppressed HG-induced ROS generation in Thp1 monocytes
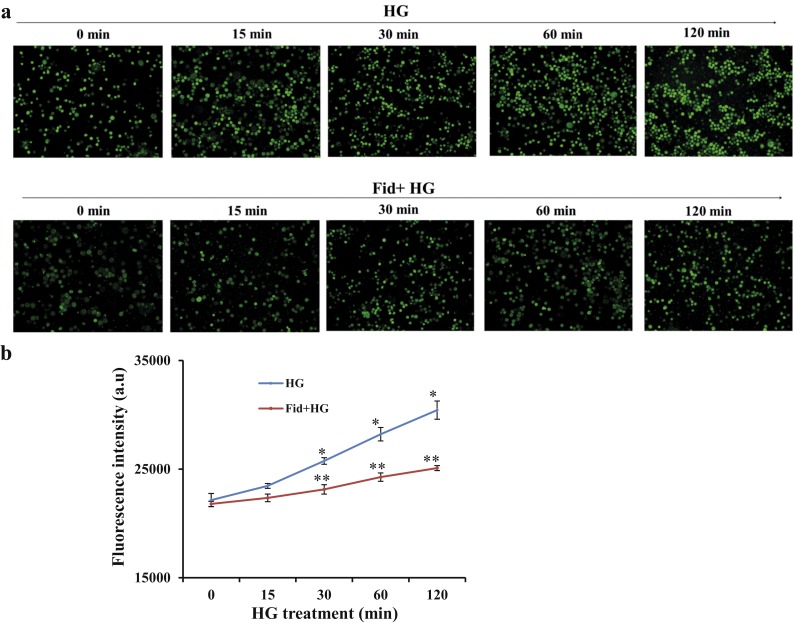
Several studies have indicated that oxidative stress activates NLRP3 inflammasome and innate immune response (16, 17); therefore, we examined the effect of AR inhibition on HG-induced generation of ROS in monocytes. Thp1 cells were pretreated with fidarestat followed by incubation with HG for 0, 15, 30, 60 and 120 minutes, and ROS levels were determined using a ROS-specific dye, CM-H2DCFDA. As shown in Fig. 4a and 4b, HG treatment of Thp1 cells increased the levels of ROS in a time-dependent manner and AR inhibition significantly prevented it. Similar results were also observed when ROS levels were quantitated by flow cytometry (Fig. 4c and 4d).
Figure 4.
AR inhibition suppressed HG-induced ROS production in Thp1 monocytes. (a) Thp1 cells (1 × 106 cells/mL) were pretreated overnight with fidarestat (10 µM), followed by incubation with HG (25 mM) for 0, 15, 30, 60, and 120 minutes. Intracellular ROS were measured using the cell-permeable dye CM-H2DCFDA (Invitrogen, Molecular Probes) and fluorescence images (×20 magnification) were obtained using a fluorescence microscope. (b) ROS levels were quantitated by determining the fluorescence intensity by using a fluorescence plate reader, as described in Methods. (c, d) Fluorescence-activated cell sorting analysis showing the CM-H2DCFDA fluorescence in Thp1 cells treated with HG with or without fidarestat for 2 hours. (c) Red, control; green, fidarestat; black (filled), HG; blue, HG plus fidarestat; black (unfilled), unstained control cells. (d) Bars show the mean fluorescence intensity. Data shown as mean ± standard deviation (n = 3). *P < 0.01 vs control; **P < 0.001 vs HG. Fid, fidarestat.
AR inhibition regulated intracellular cations responsible for inflammasome activation
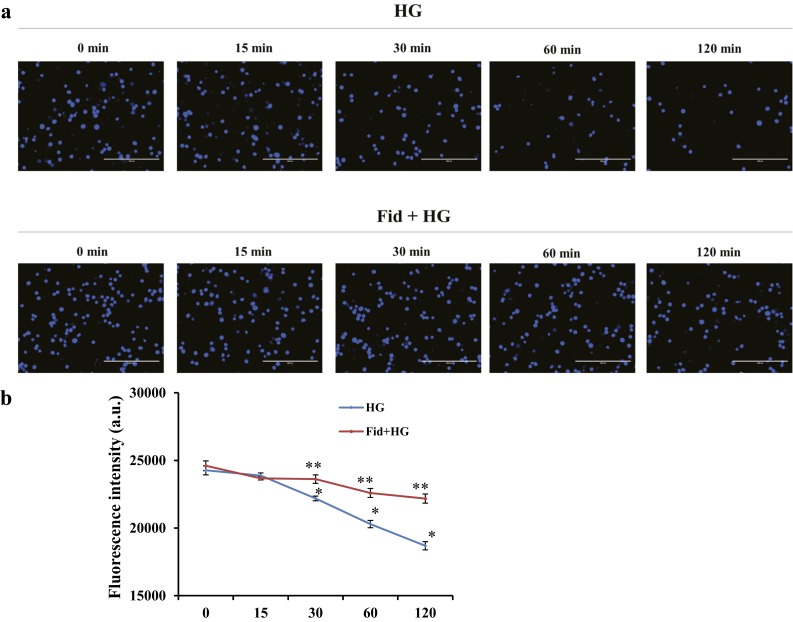
It is well established that low levels of K+ are required for the activation of NLRP3 inflammasome and various oxidants have been shown to efflux cellular K+ ions, leading to inflammasome assembly and activation of procaspase-1 (18, 19). However, the role of AR in the regulation of cellular K+ is not known. It is likely that AR inhibition prevents HG-induced K+ efflux and, thereby, prevents inflammasome activation. To examine this, Thp1 cells were pretreated with or without fidarestat followed by incubation with HG for 0, 15, 30, 60, and 120 minutes, and the levels of intracellular K+ were measured by using PBFI AM. As shown in Fig. 5a and 5b, treatment of Thp1 cells with HG caused a significant decrease in the intracellular K+ levels in a time-dependent manner and AR inhibitor significantly prevented the HG-induced efflux of K+. These results suggest that AR inhibition could prevent the activation of NLRP3 inflammasome by restoring cellular K+ levels depleted by HG.
Figure 5.
AR inhibition prevented HG-induced K+ efflux. Thp1 cells (1 × 106 cells/mL) were pretreated with fidarestat (10 µM) overnight, followed by incubation with HG (25 mM) for 0, 15, 30, 60, and 120 minutes. Intracellular K+ was measured using the cell-permeable potassium indicator PBFI AM (Invitrogen, Molecular Probes), and fluorescence images (×20 magnification) were obtained using a fluorescence microscope. Fluorescence intensity was measured by a Biotek Synergy 2 multimode reader. Data represent the mean ± standard error of the mean (n = 4). *P < 0.05 vs control (0 minutes); **P < 0.01 vs HG. a.u., arbitrary units.
It is known that increase in the Ca2+ influx could be one of the pathways that can lead to K+ efflux (20); therefore, to further understand the mechanism by which AR inhibition regulates cellular K+, we examined the effect of AR inhibition on intracellular Ca2+. As shown in Fig. 6a and 6b, HG caused a significant increase in the intracellular Ca2+ levels in a time-dependent manner in the Thp1 cells and AR inhibitor significantly prevented the HG-induced influx of Ca2+. These results suggest that AR inhibition could prevent the efflux of intracellular K+ by decreasing intracellular Ca2+ that lead to NLRP3 inflammasome activation in Thp1 monocytes.
Figure 6.
AR inhibition prevented HG-induced Ca2+ influx. Thp1 cells (1 × 106 cells/mL) were pretreated with fidarestat (10 µM) overnight, followed by incubation with HG (25 mM) for 0, 15, 30, 60, and 120 minutes. Intracellular Ca2+ was measured using the cell-permeable calcium indicator Fura-2 AM (Invitrogen, Molecular Probes), and fluorescence images (×10 magnification) were obtained using a fluorescence microscope. Fluorescence intensity was measured by a Biotek Synergy 2 multimode reader. Data represent the mean ± standard error of the mean (n = 4). *P < 0.05 vs control (0 minutes); **P < 0.01 vs HG. Fid, fidarestat.
Effect of AR inhibition on kinases that regulate intracellular cations
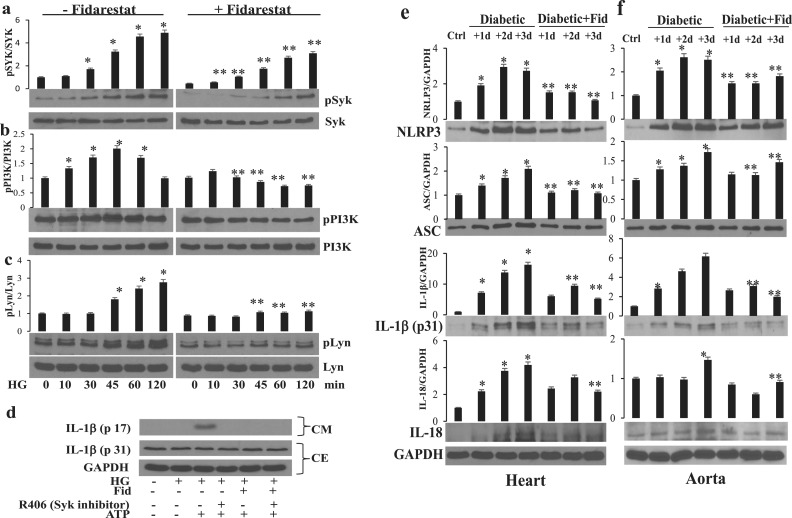
Syk is a positive effecter of oxidative stress–stimulated responses that activate Ca2+ signaling via PI3K, which generates phosphatidylinositol 3,4,5-trisphosphate, leading to Ca2+ influx (21). To further understand the mechanism by which AR regulates inflammasome activation by altering the intracellular cations, we next examined the effect of AR inhibition on HG-induced activation of Syk tyrosine kinase and its downstream PI3K. Thp1 cells were pretreated with fidarestat followed by incubation with HG for 0, 10, 30, 45, 60, and 120 minutes. The results shown in Fig. 7a and 7b suggest that HG caused a time-dependent phosphorylation of Syk and PI3K in monocytes and AR inhibition attenuated it. The nonreceptor tyrosine kinase Lyn has been shown to be upstream of Syk and is known to activate it; therefore, we next examined the effect of AR inhibition on HG-induced activation of Lyn in monocytes. HG caused phosphorylation of Lyn in a time-dependent manner and AR inhibitor prevented it (Fig. 7c).
Figure 7.
AR inhibition prevented HG-induced Lyn, Syk, and PI3K phosphorylation in Thp1 cells and NLRP3 inflammasome complex proteins in STZ-induced diabetic mice heart and aorta. Thp1 cells were pretreated with fidarestat (10 µM) overnight, followed by incubation with HG (25 mM) for 0, 10, 30, 45, 60, and 120 minutes. Western blot analyses were performed by using specific total and (a) phospho-Syk, (b) phospho-PI3K, and (c) phospho-Lyn. (d) Thp1 cells were incubated (4 hours) with HG (25 mM) and then incubated with fidarestat for 30 minutes, followed by ATP (2 mM) for 1 hour without or with pretreatment with R406 (Syk inhibitor; 2.5 µM). (e, f) Mice were made diabetic as described in Methods. The diabetic mice were treated with fidarestat (10 mg/kg/d) for 1, 2, and 3 days. The levels of NLRP3 inflammasome complex proteins as well as innate immune-response cytokines were measured in (e) heart and (f) aorta tissues by Western blot analysis using specific antibodies. The data shown are representative of three independent analyses, and representative cropped blots are shown. *P < 0.05 vs control (0 minutes); **P < 0.01 vs HG. Ctrl, control; Fid, fidarestat.
To further confirm the significance of Syk in HG-induced inflammasome activation, we treated Thp1 cells with the Syk inhibitor R406, followed by HG and ATP. Our results, shown in Fig. 7d, suggest the Syk inhibitor prevented HG- plus ATP-induced release of IL-1β. Thus, AR inhibition, by regulating the protein-tyrosine kinases, could alter the cellular K+ and Ca2+ ions responsible for inflammasome assembly and activation of innate immune response.
AR inhibition prevented NLRP3 inflammasome-mediated innate immune response in the STZ-induced diabetic mice
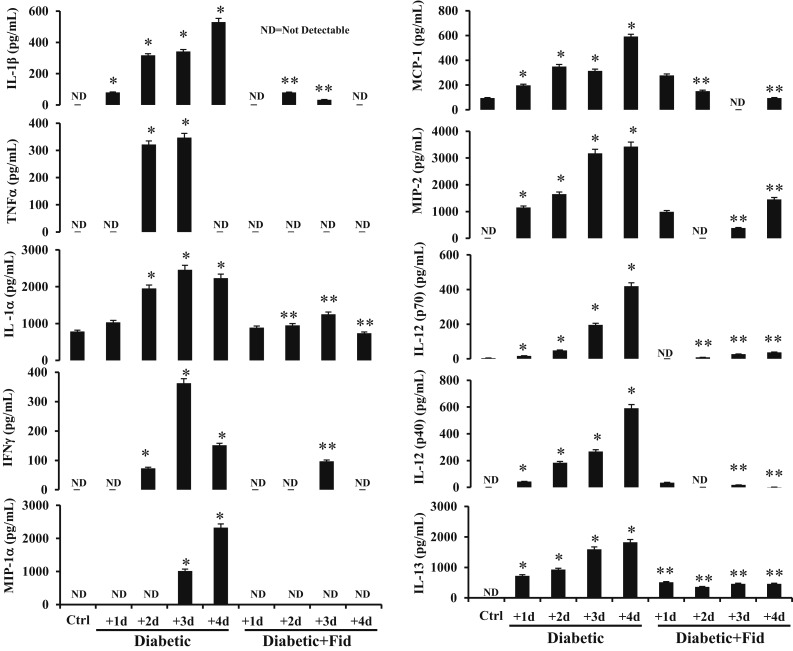
We next examined the effect of AR inhibition on NLRP3 inflammasome activation and immune response in an STZ-induced diabetic mouse model. The diabetic mice were treated with fidarestat (10 mg/kg/d) for 1, 2, and 3 days. The levels of NLRP3 inflammasome complex proteins as well as innate immune-response cytokines were measured in heart and aorta tissues. The diabetic mice showed a time-dependent increase in the expression of NLRP3, ASC, IL-1β, and IL-18 as compared with normal mice, and the treatment of diabetic mice with fidarestat significantly prevented the expression of inflammasome proteins (Fig. 7e and 7f). Similarly, there was a time-dependent increase in the release of various inflammatory cytokines such as IL-1β, TNF-α, IL-1α, interferon-γ, macrophage inflammatory protein (MIP)–1α, monocyte chemoattractant protein (MCP)–1, MIP-2, IL-12(p70), IL-12(p40), and IL-13 in the serum of diabetic mice, and treatment with fidarestat significantly prevented the release of these cytokines (Fig. 8). These results thus suggest AR inhibition prevents the activation of inflammasome-mediated immune response in diabetes.
Figure 8.
Inhibition of AR prevented inflammatory cytokines in the serum of STZ-induced diabetic mice. Mice were made diabetic as described in Methods. The diabetic mice were treated with fidarestat (10 mg/kg/d) for 1, 2, 3, and 4 days. At indicated time intervals, the levels of IL-1β, TNF-α, IL-1α, IFNγ, MIP-1α, MCP-1, MIP-2, IL-12(p70), IL-12(p40), and IL-13 were measured in the serum by using Milliplex MAP Kit mouse inflammatory cytokine/chemokine magnetic bead panel (Merck) along with Luminex xMAP detection method, per manufacturer’s protocol in a Millipore Multiplex system (Merck). ND, nondetectable levels. The bars represent the mean ± standard error of the mean (n ≥ 4). *P < 0.05 vs control; **P < 0.01 vs diabetic. Ctrl, control; Fid, fidarestat; ND, not detectable.
Discussion
The major finding of this study is that AR regulates the HG-induced innate immune response mediated by NLRP3 inflammasome in human monocytes and STZ-induced diabetic mice heart and aorta. Specifically, these observations suggest inhibition of AR could be beneficial for the control of innate immune response pathways that lead to various secondary complications in hyperglycemia.
Although hyperglycemia has been correlated with the initiation and progression of several secondary diabetic complications, such as retinopathy, neuropathy, and nephropathy (22), the precise mechanisms are not clearly understood. Recent studies indicate that activation of inflammasome-mediated immune response is involved in the pathophysiology of various secondary diabetic complications as well as diabetes-augmented inflammatory complications such as cardiovascular disease (4–6). However, the mechanisms through which hyperglycemia activates inflammasome-mediated innate immune response are not well understood.
Increased oxidative stress–generated ROS has been shown to be involved in the activation of NLRP3-mediated innate immune response in various inflammatory complications (23, 24). In diabetes, increased glucose levels have been shown to generate various free radicals and ROS, which could be the major contributor to immune response and related complications (25–27). Indeed, a 2010 study indicated hyperglycemia-generated ROS through NADPH oxidase could interact with thioredoxin-interacting protein and activate NLRP3 inflammasome (28). Furthermore, activation of adenosine 5′-monophosphate–activated protein kinase (AMPK) has been shown to reduce ROS levels by increasing the expression of thioredoxin (29, 30). The suppression of AMPK activity has been associated with increased ROS production via increased NADPH oxidase and decreased mitochondrial function in diabetes (31). AMPK activators such as metformin and resveratrol have been shown to prevent NLRP3 inflammasome activation by suppressing ROS and endoplasmic reticulum stress in diabetes (32). Furthermore, increased mitochondrial ROS could increase innate immune cytokines IL-1β and IL-18 in type 2 diabetic subjects, indicating the significance of mitochondrial ROS in the activation of NLRP3 inflammasome in hyperglycemia (7).
We and others have shown that the expression of polyol pathway enzyme AR increases significantly under oxidative stress conditions, including hyperglycemia (9, 33, 34). Increased osmotic and oxidative stress due to increased expression of AR may be responsible for diabetic complications (9, 22, 35). Furthermore, several studies using various experimental animal models have suggested the inhibition of AR prevents secondary diabetic complications (11, 36, 37). However, the association between increased ROS and AR in hyperglycemia-activated NLRP3 inflammasome is not known.
Our studies demonstrate that AR is involved in hyperglycemia-induced ROS generation, activation of NLRP3 inflammasome, and release of innate immune cytokines such as IL-1β in Thp1 monocytes. IL-1β is essential for innate immune response because it mediates the pathogenesis of a number of inflammatory diseases, including diabetes (6). Increased levels of IL-1β and IL-18 have been shown in patients with type 2 diabetes (7, 8). Production of active IL-1β involves two major steps: (1) IL-1β is synthesized as pro-IL-1β (inactive precursor) by NF-κB–mediated signalosome activation and (2) the pro-IL-1β is cleaved to an active-IL-1β by NLRP3 inflammasome (38). Activation of NLRP3 inflammasome leads to activation of caspase-1–mediated cleavage of innate immune cytokines (6). Our previous studies have shown that inhibition of AR prevents the activation of NF-κB pathway (9–12). Specifically, we have shown the inhibition of AR prevents oxidative stress signals that lead to activation of MAPK, PKC, and PLC, which, through a series of kinases, activate NF-κB and AP1, which transcribe various cytokines, chemokines, and growth factors (9, 33). Although our previous studies have shown the involvement of AR in the NF-κB–mediated production of inflammatory cytokines, the role of AR in NLRP3 inflammasome–mediated release of innate immune cytokines is not known.
Our results shown in Figs. 2 and 3 demonstrate that inhibition or ablation of AR prevents hyperglycemia-mediated assembly of inflammasome responsible for the release of innate immune cytokines, IL-1β and IL-18 in monocytes. Further, AR inhibition prevents the activation of procaspase-1 to active-caspase-1 and cleavage of inactive innate immune cytokines to biologically active innate immune cytokines. Our results are thus consistent with other studies that showed antioxidants such as N-acetylcysteine, curcumin, and genepin prevent NLRP3 inflammasome–mediated release of innate immune cytokines under oxidative stress conditions (39, 40). It has been shown that assembly of NLRP3 inflammasome complex proteins requires low levels of intracellular K+ions (18, 41). Specifically, increased ROS has been reported to be involved in the action of purinergic P2RX7 receptor-mediated activation of NLRP3 inflammasome. Agonists of P2RX7, such as ATP and nigericin, are known to enhance inflammasome assembly by inducing K+ efflux (41, 42). Our results shown in Fig. 5 demonstrate that AR inhibition substantially prevents HG-induced efflux of cellular K+, suggesting inhibition of AR could preserve intracellular K+ levels. Consistent with our studies, Lamkanfi et al. (43) have shown that glyburide, a commonly used sulfonylurea drug for the treatment of type 2 diabetes, prevents NLRP3 inflammasome activation by regulating the efflux of cellular K+. Although how AR inhibition restores cellular K+ under hyperglycemia is not obvious, we assumed that AR may modulate another cellular ionic milieu such as Ca2+ that may affect NLRP3 inflammasome activation. Indeed, our results suggest that inhibition of AR decreases hyperglycemia-mediated influx of Ca2+, indicating that AR, by regulating influx of Ca2+ in monocytes, effluxes K+ under hyperglycemic stress. Other investigators have also indicated the significance of intracellular Ca2+ in the activation of NLRP3 inflammasome and release of innate immune cytokines (44, 45).
Our studies further indicate that AR regulates HG-induced NLRP3 inflammasome by altering the dynamic ratio of intracellular K+ and Ca2+. However, the role of AR in the mediation of upstream signals of Ca2+ is not clearly understood. PI3K, besides regulating Ca2+ influx and Ca2+ signaling, promotes calcium-permeable cation channels and calcium-dependent potassium channels (46–48). Our results shown in Fig. 7b indicate that AR inhibition prevents HG-induced PI3K activation, suggesting that AR regulates phosphorylation of PI3K, which could be responsible for increased Ca2+ influx in hyperglycemia. Syk, a nonreceptor tyrosine kinase, has been associated with signaling of specific innate immune receptors like Toll-like receptors and NLRS, and is known to regulate the PI3K activity (49). Our demonstration that AR inhibition prevents HG-induced activation of Syk indicates that AR inhibition could act at or upstream of Syk tyrosine kinase. Several studies also indicate that Syk, through regulating intracellular Ca2+, probably via PI3K, could regulate inflammasome activation (50, 51). Furthermore, a recent study by Lin et al. (52) has shown Syk can directly phosphorylate ASC and promote NLRP3 inflammasome assembly. In the current study, we have also shown that fidarestat inhibited the expression of innate immune cytokines IL-1β and IL-18, and inflammasome complex proteins NLRP3 and ASC in heart and aorta tissues of STZ-induced diabetic mice. Although, STZ-induced diabetes is a well-known diabetic model in rodents, the off-target adverse effects that could cause cellular toxicity and alter inflammatory phenotype cannot be ruled out if STZ is present in the body. However, in our studies, we have used a single high dose of STZ to induce diabetes and used only those animals whose blood glucose concentrations were >400 mg/dL 4 days after STZ injection. At this time point, we assume that most of injected STZ has been eliminated from the system. Our assumption is based on published studies that indicate that within 6 hours of STZ injection, 70% of the STZ is excreted (53, 54). Furthermore, AR inhibition also prevented the release of IL-1β and various other proinflammatory cytokines in serum of STZ-induced diabetic mice. These results suggest that AR inhibition prevents the expression of inflammatory cytokines and inflammasome components probably by inhibiting the NF-κB–mediated signaling pathways as demonstrated by us and other investigators (9, 55, 56).
In summary, our studies indicate that AR inhibition prevents hyperglycemia-induced innate immune response in Thp1 monocytes as well as diabetic mouse models. Specifically, we have demonstrated that AR is a critical regulator of the ROS/Lyn/Syk/PI3K/Ca2+/K+ pathway that controls the hyperglycemia-mediated activation of NLRP3 inflammasome. Although our current data indicate that AR modulates Lyn/Syk-mediated Ca2+ influx, further studies are required to understand how AR regulates inflammasome activation by altering the redox status of the cells. Nonetheless, our findings provide an approach to control inflammasome activation in hyperglycemia, which will help in developing more effective therapies using AR inhibitors for the prevention of inflammatory complications due to uncontrolled innate immune response.
Acknowledgments
Financial Support: This work was supported by funding from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases Grant DK104786.
Author Contributions: P.B.P. performed all experiments, analyzed the data, and wrote the initial draft of the manuscript. H.S. helped P.B.P with animal models and performed multiplex analysis. K.S. contributed to immune blot experiments and read and provided input for the manuscript. S.K.S. designed the study with K.V.R and reviewed and edited the manuscript. K.V.R. designed the experimental plan, interpreted the data, and prepared the final draft of the manuscript. K.V.R. is the guarantor of this work, had full access to all the data in the study, and takes responsibility for the integrity of the data and accuracy of the data analysis.
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| IL-1β | NA | IL-1β | Santa Cruz Biotechnology, sc-7884 | Rabbit; polyclonal | 1:500 | AB_2124476 |
| IL-18 | NA | IL-18 | Santa Cruz Biotechnology, sc-7954 | Rabbit; polyclonal | 1:500 | AB_1564060 |
| ASC | NA | ASC | Santa Cruz Biotechnology, sc-22514 | Rabbit; polyclonal | 1:500 | AB_2174874 |
| NRLP3/cryopyrin | NA | NRLP3/cryopyrin | Santa Cruz Biotechnology, sc-66846 | Rabbit; polyclonal | 1:500 | AB_2152446 |
| Aldose reductase | NA | Aldose reductase | Santa Cruz Biotechnology, sc-17735 | Goat; polyclonal | 1:500 | AB_667749 |
| pSyk | NA | pSyk | Cell Signaling Technology, 2710S | Rabbit; monoclonal | 1:1000 | AB_2197222 |
| Syk | NA | Syk | Cell Signaling Technology, 2712S | Rabbit; polyclonal | 1:1000 | AB_10691458 |
| pLyn | NA | pLyn | Cell Signaling Technology, 2731S | Rabbit; polyclonal | 1:1000 | AB_2138262 |
| Lyn | NA | Lyn | Cell Signaling Technology, 2796S | Rabbit; monoclonal | 1:1000 | AB_2138391 |
| p-PI3K | NA | p-PI3K | Cell Signaling Technology, 4228S | Rabbit; polyclonal | 1:1000 | AB_659940 |
| PI3K | NA | PI3K | Cell Signaling Technology, 4292 | Rabbit; polyclonal | 1:1000 | AB_329869 |
| GAPDH | NA | GAPDH | Cell Signaling Technology), 2118 | Rabbit; monoclonal | 1:2000 | AB_561053 |
| Rabbit polyclonal IgG | NA | Control | Cell Signaling Technology, 2729S | Rabbit; polyclonal | 1:500 | AB_1031062 |
Abbreviations: NA, not applicable; RRID, Research Resource Identifier.
Footnotes
- AM
- acetoxymethyl ester
- AMPK
- adenosine 5′-monophosphate–activated protein kinase
- AR
- aldose reductase
- ASC
- apoptosis-associated speck-like protein containing C-terminal caspase recruitment domain
- ATP
- adenosine triphosphate
- CM-H2DCFDA
- 5-(and-6)-chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester
- DHN
- dihydroxynonene
- ELISA
- enzyme-linked immunosorbent assay
- FBS
- fetal bovine serum
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- HBSS
- Hanks balanced salt solution
- HG
- high glucose
- HNE
- 4-hydroxynonenal
- IL
- interleukin
- MCP
- monocyte chemoattractant protein
- MIP
- macrophage inflammatory protein
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NF-κB
- nuclear factor-κB
- NLRP3
- NOD-like receptor family pyrin domain containing 3
- PBFI
- potassium-binding benzofuran isophthalate
- PCR
- polymerase chain reaction
- RIPA
- radioimmunoprecipitation assay
- ROS
- reactive oxygen species
- siRNA
- small interfering RNA
- STZ
- streptozotocin
- TNF-α
- tumor necrosis factor-α
- UTMB
- University of Texas Medical Branch.
References
- 1.Grundy SM, Benjamin IJ, Burke GL, Chait A, Eckel RH, Howard BV, Mitch W, Smith SC Jr, Sowers JR. Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation. 1999;100(10):1134–1146. [DOI] [PubMed] [Google Scholar]
- 2.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. [DOI] [PubMed] [Google Scholar]
- 3.Mittal M, Siddiqui MR, Tran K, Reddy SP, Malik AB. Reactive oxygen species in inflammation and tissue injury. Antioxid Redox Signal. 2014;20(7):1126–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paramel Varghese G, Folkersen L, Strawbridge RJ, Halvorsen B, Yndestad A, Ranheim T, Krohg-Sørensen K, Skjelland M, Espevik T, Aukrust P, Lengquist M, Hedin U, Jansson JH, Fransén K, Hansson GK, Eriksson P, Sirsjö A. NLRP3 inflammasome expression and activation in human atherosclerosis. J Am Heart Assoc. 2016;5(5):e00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keyel PA. How is inflammation initiated? Individual influences of IL-1, IL-18 and HMGB1. Cytokine. 2014;69(1):136–145. [DOI] [PubMed] [Google Scholar]
- 6.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK. Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes. 2013;62(1):194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aribi M, Moulessehoul S, Kendouci-Tani M, Benabadji AB, Hichami A, Khan NA. Relationship between interleukin-1beta and lipids in type 1 diabetic patients. Med Sci Monit. 2007;13(8):CR372–CR378. [PubMed] [Google Scholar]
- 9.Srivastava SK, Ramana KV, Bhatnagar A. Role of aldose reductase and oxidative damage in diabetes and the consequent potential for therapeutic options. Endocr Rev. 2005;26(3):380–392. [DOI] [PubMed] [Google Scholar]
- 10.Ramana KV, Willis MS, White MD, Horton JW, DiMaio JM, Srivastava D, Bhatnagar A, Srivastava SK. Endotoxin-induced cardiomyopathy and systemic inflammation in mice is prevented by aldose reductase inhibition. Circulation. 2006;114(17):1838–1846. [DOI] [PubMed] [Google Scholar]
- 11.Ramana KV, Friedrich B, Srivastava S, Bhatnagar A, Srivastava SK. Activation of nuclear factor-kappaB by hyperglycemia in vascular smooth muscle cells is regulated by aldose reductase. Diabetes. 2004;53(11):2910–2920. [DOI] [PubMed] [Google Scholar]
- 12.Ramana KV, Chandra D, Srivastava S, Bhatnagar A, Aggarwal BB, Srivastava SK. Aldose reductase mediates mitogenic signaling in vascular smooth muscle cells. J Biol Chem. 2002;277(35):32063–32070. [DOI] [PubMed] [Google Scholar]
- 13.Saxena A, Tammali R, Ramana KV, Srivastava SK. Aldose reductase inhibition prevents colon cancer growth by restoring phosphatase and tensin homolog through modulation of miR-21 and FOXO3a. Antioxid Redox Signal. 2013;18(11):1249–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yadav UC, Aguilera-Aguirre L, Boldogh I, Ramana KV, Srivastava SK. Aldose reductase deficiency in mice protects from ragweed pollen extract (RWE)-induced allergic asthma. Respir Res. 2011;12:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oka M, Matsumoto Y, Sugiyama S, Tsuruta N, Matsushima M. A potent aldose reductase inhibitor, (2S,4S)-6-fluoro-2′, 5′-dioxospiro[chroman-4,4′-imidazolidine]-2-carboxamide (Fidarestat): its absolute configuration and interactions with the aldose reductase by X-ray crystallography. J Med Chem. 2000;43(12):2479–2483. [DOI] [PubMed] [Google Scholar]
- 16.Ives A, Nomura J, Martinon F, Roger T, LeRoy D, Miner JN, Simon G, Busso N, So A. Xanthine oxidoreductase regulates macrophage IL1β secretion upon NLRP3 inflammasome activation. Nat Commun. 2015;6:6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y, Franchi L, Núñez G. TLR agonists stimulate Nlrp3-dependent IL-1β production independently of the purinergic P2X7 receptor in dendritic cells and in vivo. J Immunol. 2013;190(1):334–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA. A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med. 2015;21(3):248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ledoux J, Werner ME, Brayden JE, Nelson MT. Calcium-activated potassium channels and the regulation of vascular tone. Physiology (Bethesda). 2006;21:69–78. [DOI] [PubMed] [Google Scholar]
- 21.Kurosaki T, Tsukada S. BLNK: connecting Syk and Btk to calcium signals. Immunity. 2000;12(1):1–5. [DOI] [PubMed] [Google Scholar]
- 22.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. [DOI] [PubMed] [Google Scholar]
- 23.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. [DOI] [PubMed] [Google Scholar]
- 25.Green K, Brand MD, Murphy MP. Prevention of mitochondrial oxidative damage as a therapeutic strategy in diabetes. Diabetes. 2004;53(Suppl 1):S110–S118. [DOI] [PubMed] [Google Scholar]
- 26.Singh R, Devi S, Gollen R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: larger-than-life. Diabetes Metab Res Rev. 2015;31(2):113–126. [DOI] [PubMed] [Google Scholar]
- 27.Schrauwen P, Hesselink MK. Oxidative capacity, lipotoxicity, and mitochondrial damage in type 2 diabetes. Diabetes. 2004;53(6):1412–1417. [DOI] [PubMed] [Google Scholar]
- 28.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11(2):136–140. [DOI] [PubMed] [Google Scholar]
- 29.Li XN, Song J, Zhang L, LeMaire SA, Hou X, Zhang C, Coselli JS, Chen L, Wang XL, Zhang Y, Shen YH. Activation of the AMPK-FOXO3 pathway reduces fatty acid-induced increase in intracellular reactive oxygen species by upregulating thioredoxin. Diabetes. 2009;58(10):2246–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hou X, Song J, Li XN, Zhang L, Wang X, Chen L, Shen YH. Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun. 2010;396(2):199–205. [DOI] [PubMed] [Google Scholar]
- 31.Bondeva T, Wolf G. Reactive oxygen species in diabetic nephropathy: friend or foe? Nephrol Dial Transplant. 2014;29(11):1998–2003. [DOI] [PubMed] [Google Scholar]
- 32.Li A, Zhang S, Li J, Liu K, Huang F, Liu B. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol Cell Endocrinol. 2016;434:36–47. [DOI] [PubMed] [Google Scholar]
- 33.Ramana KV, Friedrich B, Tammali R, West MB, Bhatnagar A, Srivastava SK. Requirement of aldose reductase for the hyperglycemic activation of protein kinase C and formation of diacylglycerol in vascular smooth muscle cells. Diabetes. 2005;54(3):818–829. [DOI] [PubMed] [Google Scholar]
- 34.Obrosova IG, Minchenko AG, Vasupuram R, White L, Abatan OI, Kumagai AK, Frank RN, Stevens MJ. Aldose reductase inhibitor fidarestat prevents retinal oxidative stress and vascular endothelial growth factor overexpression in streptozotocin-diabetic rats. Diabetes. 2003;52(3):864–871. [DOI] [PubMed] [Google Scholar]
- 35.Tang WH, Martin KA, Hwa J. Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol. 2012;3:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vedantham S, Ananthakrishnan R, Schmidt AM, Ramasamy R. Aldose reductase, oxidative stress and diabetic cardiovascular complications. Cardiovasc Hematol Agents Med Chem. 2012;10(3):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun W, Oates PJ, Coutcher JB, Gerhardinger C, Lorenzi M. A selective aldose reductase inhibitor of a new structural class prevents or reverses early retinal abnormalities in experimental diabetic retinopathy. Diabetes. 2006;55(10):2757–2762. [DOI] [PubMed] [Google Scholar]
- 38.Abais JM, Xia M, Zhang Y, Boini KM, Li PL. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid Redox Signal. 2015;22(13):1111–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rajanbabu V, Galam L, Fukumoto J, Enciso J, Tadikonda P, Lane TN, Bandyopadhyay S, Parthasarathy PT, Cho Y, Cho SH, Lee YC, Lockey RF, Kolliputi N. Genipin suppresses NLRP3 inflammasome activation through uncoupling protein-2. Cell Immunol. 2015;297(1):40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tozser J, Benko S.. Natural compounds as regulators of NLRP3 inflammasome-mediated IL-1beta production. Mediators Inflamm. 2016;2016:5460302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pétrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007;14(9):1583–1589. [DOI] [PubMed] [Google Scholar]
- 42.Yaron JR, Gangaraju S, Rao MY, Kong X, Zhang L, Su F, Tian Y, Glenn HL, Meldrum DRK. K(+) regulates Ca(2+) to drive inflammasome signaling: dynamic visualization of ion flux in live cells. Cell Death Dis. 2015;6:e1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM, Dixit VM. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187(1):61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee GS, Subramanian N, Kim AI, Aksentijevich I, Goldbach-Mansky R, Sacks DB, Germain RN, Kastner DL, Chae JJ. The calcium-sensing receptor regulates the NLRP3 inflammasome through Ca2+ and cAMP. Nature. 2012;492(7427):123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Triantafilou K, Hughes TR, Triantafilou M, Morgan BP. The complement membrane attack complex triggers intracellular Ca2+ fluxes leading to NLRP3 inflammasome activation. J Cell Sci. 2013;126(Pt 13):2903–2913. [DOI] [PubMed] [Google Scholar]
- 46.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. [DOI] [PubMed] [Google Scholar]
- 47.Saito K, Tolias KF, Saci A, Koon HB, Humphries LA, Scharenberg A, Rawlings DJ, Kinet JP, Carpenter CL. BTK regulates PtdIns-4,5-P2 synthesis: importance for calcium signaling and PI3K activity. Immunity. 2003;19(5):669–678. [DOI] [PubMed] [Google Scholar]
- 48.Viard P, Butcher AJ, Halet G, Davies A, Nürnberg B, Heblich F, Dolphin AC. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci. 2004;7(9):939–946. [DOI] [PubMed] [Google Scholar]
- 49.Kawai T, Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. Int Immunol. 2009;21(4):317–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, Olivier M. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases [published correction appears in PLoS Pathog. 2009 Sep;5(9)] PLoS Pathog. 2009;5(8):e1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mócsai A, Ruland J, Tybulewicz VL. The SYK tyrosine kinase: a crucial player in diverse biological functions. Nat Rev Immunol. 2010;10(6):387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lin YC, Huang DY, Wang JS, Lin YL, Hsieh SL, Huang KC, Lin WW. Syk is involved in NLRP3 inflammasome-mediated caspase-1 activation through adaptor ASC phosphorylation and enhanced oligomerization. J Leukoc Biol. 2015;97(5):825–835. [DOI] [PubMed] [Google Scholar]
- 53.Karunanayake EH, Hearse DJ, Mellows G. Streptozotocin: its excretion and metabolism in the rat. Diabetologia. 1976;12(5):483–488. [DOI] [PubMed] [Google Scholar]
- 54.Karunanayake EH, Hearse DJ, Mellows G. The synthesis of [14C] streptozotocin and its distribution and excretion in the rat. Biochem J. 1974;142(3):673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang B, Hodgkinson A, Oates PJ, Millward BA, Demaine AG. . High glucose induction of DNA-binding activity of the transcription factor NFkappaB in patients with diabetic nephropathy. Biochim Biophys Acta. 2008;1782(5):295–302. [DOI] [PubMed] [Google Scholar]
- 56.Zeng KW, Li J, Dong X, Wang YH, Ma ZZ, Jiang Y, Jin HW, Tu PF. Anti-neuroinflammatory efficacy of the aldose reductase inhibitor FMHM via phospholipase C/protein kinase C-dependent NF-κB and MAPK pathways. Toxicol Appl Pharmacol. 2013;273(1):159–171. [DOI] [PubMed] [Google Scholar]