Abstract
Pulsatile release of gonadotropin-releasing hormone (GnRH) is key to fertility. Pulse frequency is modulated by gonadal steroids and likely arises subsequent to coordination of GnRH neuron firing activity. The source of rhythm generation and the site of steroid feedback remain critical unanswered questions. Arcuate neurons that synthesize kisspeptin, neurokinin B, and dynorphin (KNDy) may be involved in both of these processes. We tested the hypotheses that action potential firing in KNDy neurons is episodic and that gonadal steroids regulate this pattern. Targeted extracellular recordings were made of green fluorescent protein–identified KNDy neurons in brain slices from adult male mice that were intact, castrated, or castrated and treated with estradiol or dihydrotestosterone (DHT). KNDy neurons exhibited marked peaks and nadirs in action potential firing activity during recordings lasting 1 to 3.5 hours. Peaks, identified by Cluster analysis, occurred more frequently in castrated than intact mice, and either estradiol or DHT in vivo or blocking neurokinin type 3 receptor in vitro restored peak frequency to intact levels. The frequency of peaks in firing rate and estradiol regulation of this frequency is similar to that observed for GnRH neurons, whereas DHT suppressed firing in KNDy but not GnRH neurons. We further examined the patterning of action potentials to identify bursts that may be associated with increased neuromodulator release. Burst frequency and duration are increased in castrated compared with intact and steroid-treated mice. The observation that KNDy neurons fire in an episodic manner that is regulated by steroid feedback is consistent with a role for these neurons in GnRH pulse generation and regulation.
Arcuate kisspeptin neurons from adult male mice display a long-term firing pattern similar to GnRH neurons in terms of frequency of peaks in activity but exhibit different steroid regulation.
Pulsatile gonadotropin-releasing hormone (GnRH) release is fundamental for the function of the hypothalamic-pituitary-gonadal axis and fertility (1). In adult males, and during most of the adult female reproductive cycle, GnRH secretion occurs in pulses ranging in interval from approximately 30 minutes to several hours and is regulated by sex steroid feedback (2–5). The source of both rhythm generation for pulsatile GnRH release and steroid feedback to regulate frequency remains incompletely understood. In vivo rhythmic volleys of hypothalamic multiunit electrical activity (MUA) that are temporally associated with pulsatile luteinizing hormone (LH) release are observed in the arcuate nucleus of primates, goats, and rats (6–8). These observations suggest that the activity of the GnRH system may arise from coordination of a neuronal population in this region, which is distant from most GnRH neuron somata. Similarly, sex steroid feedback, which regulates the GnRH pulse pattern (9), does not appear to be perceived directly by GnRH neurons because they do not express detectable levels of sex steroid receptors other than estrogen receptor β (10–13). Together these findings suggest the hypothesis that feedback and pulse generation are mediated by neuronal inputs to GnRH neurons (14).
Kisspeptin is a potent stimulator of GnRH neuron activity and release (15–17). Kisspeptin neurons of the arcuate nucleus are a strong candidate for a neuronal input that may mediate both of these processes, as they express estrogen receptor α (18), and contact GnRH neuron terminals in median eminence as well as other kisspeptin neurons (19). Arcuate kisspeptin neurons are often called kisspeptin, neurokinin B, and dynorphin (KNDy) neurons because in addition to kisspeptin, they coexpress two peptides that alter reproductive neuroendocrine function: neurokinin B (NKB; or Tac2) and dynorphin (20–26). KNDy neurons express NKB receptor type 1 and NKB receptor type 3 (NK3R) and the dynorphin receptor κ-opioid receptor (KOR), but not kisspeptin receptor 1 (kissR1), whereas GnRH neurons express kissR1, and NK3R (27–30). Consistent with this receptor expression, kisspeptin does not alter arcuate nucleus MUA but increases GnRH neuron activity, as well as GnRH and LH release, suggesting its role may be limited to output to GnRH neurons (8, 15, 31). In contrast, agonists of both NK3R and KOR alter arcuate MUA (31).
These observations have led to a model for the generation of GnRH pulses by KNDy neurons (22, 25, 32, 33). In this model, KNDy neurons are activated by NKB action on NK3R, resulting in kisspeptin output to GnRH neurons to initiate GnRH release. This is followed by subsequent release of dynorphin from KNDy neurons, which acts on KOR expressed by those cells and terminates their activity and release of kisspeptin, thus ceasing the signal for GnRH neuron activation. Elements of this model have been tested in brain slices from mice, demonstrating activating effects of NK3R agonists, inhibition by dynorphin, and modulation of these effects by sex steroids (30, 34–36). Optogenetic stimulation of KNDy neurons was recently shown to depolarize/increase firing in other KNDy neurons (37).These studies have provided important information about the short-term response of KNDy neurons to their peptides and other secretory products; however, studies of the long-term firing pattern and steroid modulation of KNDy neuron activity patterns are lacking. In the present report, we used targeted extracellular recordings of green fluorescent protein (GFP)–identified KNDy neurons to begin to fill this gap.
Materials and Methods
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless noted.
Animals
Kiss1-hrGFP transgenic mice (generous gift of Dr. Carol Elias) and Tac2-enhanced GFP transgenic mice [015495-UCD/STOCK Tg (Tac2-EGFP)381Gsat] obtained from the Mouse Mutant Regional Resource Center (www.mmrrc.org/) were propagated in our colony. GFP expression within the arcuate nucleus in both of these models is confined to KNDy neurons (30, 38). Mice were maintained under a 14-hour light, 10-hour dark photoperiod with 2916 chow (Envigo, Madison, WI) and water available ad libitum. Male mice (70-100 days old) were either left intact or castrated under isoflurane anesthesia with local bupivacaine as an analgesic. Some mice received Silastic (Dow Corning, Midland, MI) implants containing estradiol (0.625 µg) or dihydrotestosterone (DHT; 400 µg × 2) in the scapular region at the time of castration (39). Studies were conducted 5 to 10 days postsurgery; no differences were noted with time after steroid manipulation. DHT implants produce an androgen milieu capable of restoring seminal vesicle mass in castrate mice to that of intact males (39). Estradiol in the circulation 5 to 10 days after implantation provides effective negative feedback but is not able to induce an LH surge (40). The Institutional Animal Care and Use Committee at the University of Michigan approved all procedures.
Brain slice preparation
All solutions were bubbled with 95% O2/5% CO2 throughout the experiments and for at least 30 minutes before exposure to tissue. Brain slices (400 µm) were prepared through the hypothalamus as previously described (41). The brain was rapidly removed and placed in ice-cold sucrose saline solution containing (in mM): 250 sucrose, 3.5 KCl, 26 NaHCO3, 10 D-glucose, 1.25 Na2HPO4, 1.2 MgSO4, and 3.8 MgCl2. Coronal slices were cut with a Leica VT1200S (Leica Biosystems, Buffalo Grove, IL). Slices were incubated for 30 minutes at room temperature (approximately 21°C to 23°C) in 50% sucrose saline and 50% artificial cerebrospinal fluid (ACSF) containing (in mM) 135 NaCl, 3.5 KCl, 26 NaHCO3, 10 d-glucose, 1.25 Na2HPO4, 1.2 MgSO4, and 2.5 CaCl2 (pH 7.4). After this time, the slices were transferred to 100% ACSF at room temperature for 0.5 to 4.5 hours before recording.
Electrophysiological recordings
Targeted single-unit extracellular recordings were used. This configuration has minimal impact on the cell’s intrinsic properties (42, 43). Recording pipettes (2 to 4 MΩ) were pulled from borosilicate glass (Schott no. 8250; World Precision Instruments, Sarasota, FL) with a Sutter P-97 puller (Sutter Instrument, Novato, CA). Pipettes were filled with HEPES-buffered solution containing (in mM) 150 NaCl, 10 HEPES, 10 D-glucose, 2.5 CaCl2, 1.3 MgCl2, and 3.5 KCl, and low-resistance (21 ± 6.4 MΩ) seals were formed between the pipette and neuron. Recordings were made in a voltage clamp with a 0-mV pipette holding potential and signals filtered at 10 kHz using an EPC8 or one channel of an EPC10 dual patch-clamp amplifier using PatchMaster software (HEKA Elektronic, Lambrecht, Germany).
Experimental design
Slices were transferred to a recording chamber with constant perfusion of carboxygenated ACSF at 29°C to 32°C at a rate of approximately 3 mL/min. Slices were allowed to equilibrate in the chamber for 5 to 10 minutes before initiating the recording. After a 5-minute recording stabilization period, spontaneous neuronal activity was recorded from a GFP-positive cell within the arcuate nucleus. Recordings lasted from 1 to 5 hours. In some cells, a loss of recording stability was observed after 3.5 hours; analysis was thus terminated at this point for all cells, when all recordings were still of high quality. At the end of each experiment, inactive cells were treated with high-potassium ACSF (20 mM K+), 4-aminopyridine (5 mM), or senktide (300 to 500 nM; Phoenix Pharmaceuticals, Burlingame, CA). Cells that exhibited action currents in response were verified to be alive and recordable, and all data were used. For cells not responding to K+, 4-aminopyridine or senktide, data analysis was truncated at the last observed action current. No more than two cells from the same animal were included, and at least six animals were studied per group.
To compare firing patterns in different steroid milieu, four groups were studied: intact (n = 12 cells), castrated (n = 9), castrated + estradiol (n = 9), and castrated + DHT (n = 7). To test the hypothesis that NK3R activation is required for KNDy neuron activity patterns, recordings of cells from castrated mice were made with the NK3R antagonist SB222200 (10 µM; Tocris/Bio-techne, Minneapolis, MN) in the ACSF (n = 8). To test the hypothesis that activation of KOR reduces activity of KNDy neurons, recordings of cells from intact mice were made with the KOR antagonist NorBNI (10 µM; Tocris/Bio-techne) in the ACSF (intact + NorBNI; n = 7). All ACSF solutions were replaced every hour to minimize changes in osmolarity and provide fresh drug; changes in firing pattern were not associated with solution exchange.
Data analysis
The extracellular approach records action currents, which are the currents underlying action potentials. Their frequency thus reflect action potential firing rate. Action currents (events) were identified using custom software written in IgorPro (WaveMetrics, Inc., Lake Oswego, OR). To analyze the long-term pattern of the firing activity, we updated the code for Cluster8 [generously provided by Dr. Michael Johnson, University of Virginia (44)] to run in IgorPro; peak detection was verified to be the same as the native version when the same parameters were used. Error was estimated from the local standard deviation. For this analysis, firing rate was binned at 2-minute intervals and cluster sizes of 2 for peak and 2 for nadir and a T-score increase greater or equal to 2. Varying the binning interval (1 to 2.5 minutes) and the cluster sizes (1 to 3 points) yielded similar results that revealed the same statistical differences among groups. A minimum peak size of 4% of the maximal amplitude reached by the cell (or 4 events for cells with a peak under 100 events/2 min) was defined to avoid the detection of nonsubstantial elevations of firing rate as peaks. Detected peaks separated by nadirs of only 1 point (2 minutes) were manually concatenated.
In addition to the long-term pattern activity, the short-term pattern of action potentials can affect neurosecretion (45, 46). Action potential grouping [referred to here as “bursts,” for convenience, although the frequency of spikes within a burst is noticeably lower than that in other neurons (47)] was detected as previously reported (48) using software that systematically adjusted the maximum time between events (burst window) for inclusion in a burst from 0.01 to 5 seconds in 10-ms intervals. A burst window of 230 ms was chosen for comparison among groups because it is the burst window in which the maximum number of bursts was detected.
Data are reported as mean ± standard error of the mean (SEM); n indicates number of cells. Statistical analysis was performed using Prism (GraphPad, La Jolla, CA). Data were tested for normal distribution using Shapiro-Wilk normality test. Comparisons among groups were made using tests appropriate for data distribution, and number of groups are detailed in the results. Mean, median, 25th percentile, 75th percentile, and interquartile range for all data are reported in Table 1. Significance was set at P < 0.05.
Table 1.
Mean, Median, 25th Percentile, 75th Percentile, and Interquartile Range for Nonparametric Analyses
| Parameter | Mean | Median | 25th Percentile | 75th Percentile | Interquartile Range |
|---|---|---|---|---|---|
| Peaks/h | |||||
| Intact | 1.024 | 0.857 | 0.632 | 1.032 | 0.400 |
| Castrate | 2.544 | 2.804 | 1.658 | 3.095 | 1.437 |
| Castrate + estradiol | 0.739 | 0.484 | 0.000 | 1.213 | 1.213 |
| Castrate + DHT | 1.343 | 1.143 | 0.286 | 2.797 | 2.511 |
| Castrate + SB222200 | 0.813 | 0.413 | 0.075 | 1.744 | 1.669 |
| Intact + NorBNI | 1.191 | 1.023 | 0.286 | 1.899 | 1.613 |
| Mean firing rate (Hz) | |||||
| Intact | 0.262 | 0.096 | 0.043 | 0.439 | 0.396 |
| Castrate | 0.762 | 0.497 | 0.097 | 1.256 | 1.159 |
| Castrate + estradiol | 0.026 | 0.004 | 0.001 | 0.054 | 0.053 |
| Castrate + DHT | 0.143 | 0.012 | 0.001 | 0.416 | 0.415 |
| Castrate + SB222200 | 0.237 | 0.012 | 0.001 | 0.315 | 0.314 |
| Intact + NorBNI | 0.020 | 0.012 | 0.006 | 0.022 | 0.016 |
| Nadir duration (min) | |||||
| Intact | 61.480 | 61.670 | 41.170 | 83.880 | 42.710 |
| Castrate | 17.500 | 16.340 | 10.580 | 21.820 | 11.240 |
| Castrate + estradiol | 97.970 | 96.500 | 32.380 | 164.000 | 131.620 |
| Castrate + DHT | 88.430 | 75.500 | 11.000 | 193.000 | 182.000 |
| Castrate + SB222200 | 91.680 | 81.920 | 24.200 | 170.500 | 146.300 |
| Intact + NorBNI | 53.270 | 51.670 | 14.200 | 104.500 | 90.300 |
| Burst frequency (Hz) | |||||
| Intact | 0.028 | 0.010 | 0.003 | 0.052 | 0.049 |
| Castrate | 1.145 | 0.118 | 0.027 | 0.223 | 0.196 |
| Castrate + estradiol | 0.005 | 0.001 | 0.001 | 0.014 | 0.014 |
| Castrate + DHT | 0.011 | 0.007 | 0.001 | 0.026 | 0.025 |
| Castrate + SB222200 | 0.020 | 0.004 | 0.001 | 0.047 | 0.046 |
| Intact + NorBNI | 0.002 | 0.001 | 0.001 | 0.004 | 0.003 |
| Burst duration (s) | |||||
| Intact | 0.259 | 0.232 | 0.189 | 0.328 | 0.138 |
| Castrate | 0.433 | 0.393 | 0.197 | 0.619 | 0.422 |
| Castrate + estradiol | 0.165 | 0.203 | 0.064 | 0.225 | 0.160 |
| Castrate + DHT | 0.128 | 0.141 | 0.033 | 0.211 | 0.177 |
| Castrate + SB222200 | 0.160 | 0.134 | 0.083 | 0.251 | 0.168 |
| Intact + NorBNI | 0.131 | 0.151 | 0.084 | 0.169 | 0.085 |
| Spike per burst | |||||
| Intact | 3.240 | 2.991 | 2.431 | 3.931 | 1.500 |
| Castrate | 3.899 | 4.513 | 2.333 | 5.021 | 2.688 |
| Castrate + estradiol | 3.724 | 3.010 | 2.592 | 5.027 | 2.435 |
| Castrate + DHT | 2.123 | 2.078 | 2.000 | 2.266 | 0.266 |
| Castrate + SB222200 | 2.653 | 2.578 | 2.286 | 3.057 | 0.771 |
| Intact + NorBNI | 2.453 | 2.400 | 2.255 | 2.679 | 0.424 |
| Intraburst interval (s) | |||||
| Intact | 0.121 | 0.114 | 0.106 | 0.131 | 0.025 |
| Castrate | 0.127 | 0.117 | 0.099 | 0.169 | 0.070 |
| Castrate + estradiol | 0.069 | 0.070 | 0.036 | 0.103 | 0.067 |
| Castrate + DHT | 0.109 | 0.115 | 0.033 | 0.178 | 0.145 |
| Castrate + SB222200 | 0.096 | 0.117 | 0.052 | 0.130 | 0.078 |
| Intact + NorBNI | 0.090 | 0.108 | 0.054 | 0.118 | 0.064 |
| Interevent interval(s) | |||||
| Intact | 19.910 | 16.520 | 3.944 | 28.300 | 24.356 |
| Castrate | 3.177 | 2.468 | 1.593 | 3.920 | 2.327 |
| Castrate + estradiol | 200.200 | 156.300 | 16.600 | 358.500 | 341.900 |
| Castrate + DHT | 20.390 | 7.297 | 2.545 | 44.790 | 42.245 |
| Castrate + SB222200 | 50.180 | 11.440 | 3.392 | 116.300 | 112.908 |
| Intact + NorBNI | 65.680 | 64.810 | 26.530 | 105.300 | 78.770 |
Results
Spontaneous firing activity of KNDy neurons is episodic and regulated by steroid milieu
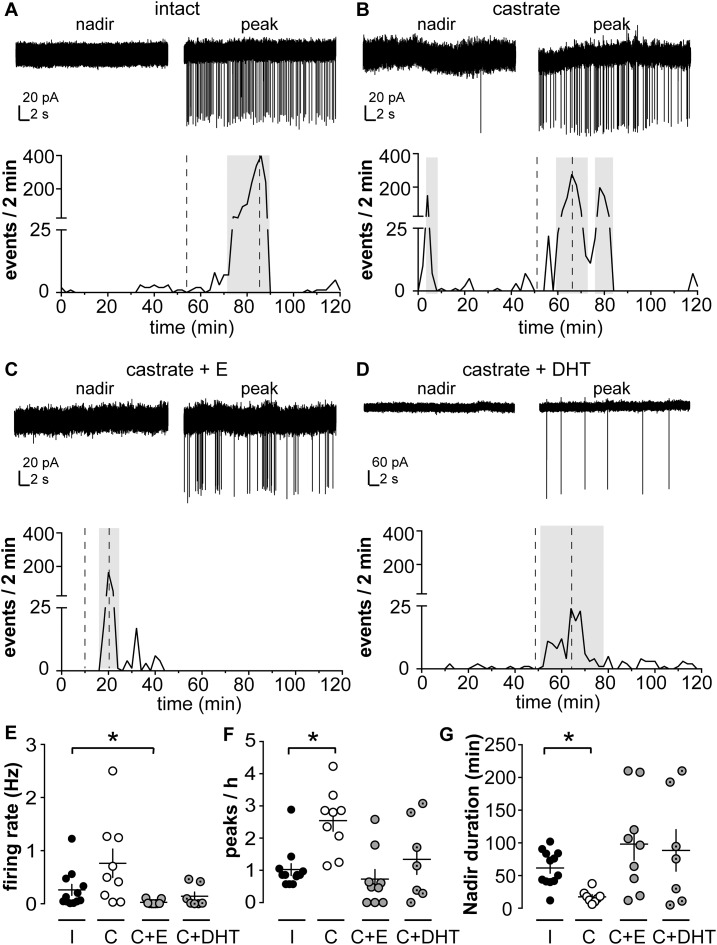
Short-term firing frequency of KNDy neurons is influenced by in vivo steroid treatment in male mice (30). Here we studied the long-term spontaneous firing pattern of KNDy neurons from intact and castrated adult male mice, as well as castrated mice supplemented with estradiol or DHT, an androgen that cannot be converted into estradiol by aromatase (49). Figure 1A–1D show representative extracellular recording traces of KNDy neurons from each group during a nadir and a peak in firing activity, as well as firing rate of that cell plotted over time. KNDy neurons from all groups exhibited a succession of peaks and nadirs in action current frequency, but the patterns were strikingly different among groups. Specifically, peaks in firing rate were more frequent in cells from castrated mice (2.5 ± 0.3 peaks/h, n = 9) than in intact mice (1 ± 0.2 peaks/h, n = 12, P < 0.05, Kruskal-Wallis/Dunn test; Fig. 1F). Duration of nadirs was also reduced in cells from castrated compared with intact mice (P < 0.05, Kruskal-Wallis/Dunn test; Fig. 1G). Both peak frequency and nadir duration were restored to levels observed in gonadal intact mice by either estradiol (n = 9) or DHT (n = 7) (Fig. 1C–1G; all P > 0.1 vs intact mice, Kruskal-Wallis/Dunn test). Mean firing rate was not different among cells from intact, castrated, and castrated + DHT groups but was reduced in castrated + estradiol mice (P < 0.05, Kruskal-Wallis/Dunn test; Fig. 1E), suggesting the negative feedback effects of estradiol may be stronger than those of DHT. Together these observations suggest both androgens and estrogens affect the long-term firing pattern of KNDy neurons.
Figure 1.
KNDy neurons exhibit spontaneous episodic activity that is regulated by steroids. (A–D) Top: representative extracellular recordings of KNDy neurons from (A) intact (I), (B) castrated (C), (C) castrated + estradiol (C+E), and (D) castrated + DHT (C+DHT) male mice during a nadir and a peak; bottom: pattern of activity plotted as number of events per 2 minutes. Note vertical axis scale change for top panel in D, as events were larger-amplitude in this recording, which was selected for the representative nature of event pattern. Amplitude is not a meaningful measure of biological changes with this type of recording as it depends on several technical, rather than biological, variables. Gray boxes show peaks detected by a version of Cluster running in IgorPro software. Dashed lines on the bottom panels indicate the timing of the traces in the top panel of each figure. (E, F, G) Individual values and mean ± SEM for (E) firing rate, (F) peaks/h, and (G) nadir duration. *P < 0.05 vs intact group, Kruskal-Wallis/Dunn test.
Burst firing activity is steroid dependent in KNDy neurons
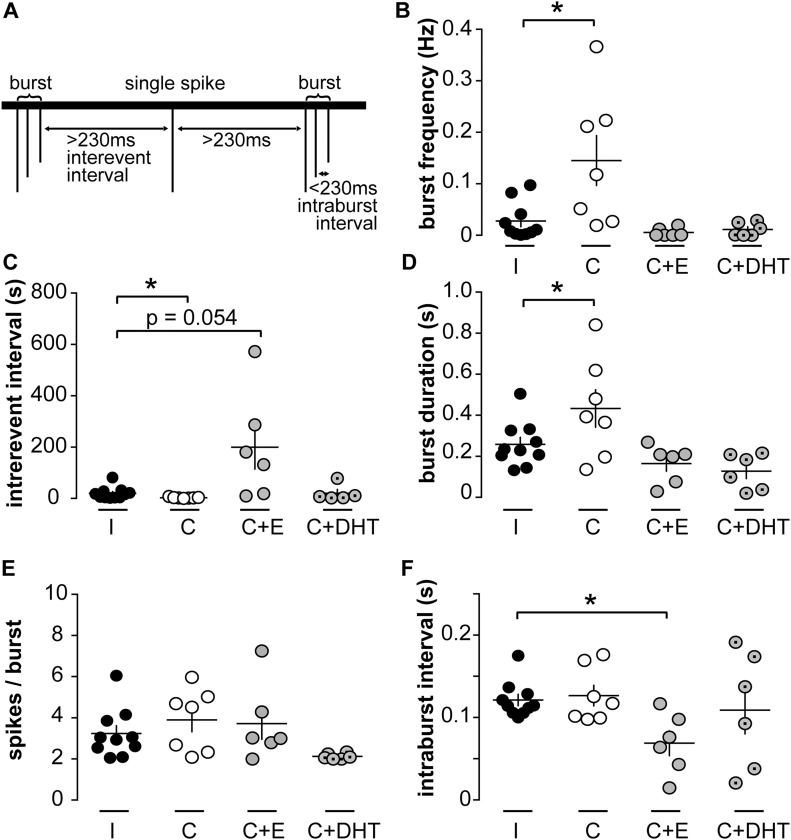
Although the long-term firing pattern is important to understand for a system that is episodically active with the period of LH release, the short-term patterns of neuronal activity with spikes occurring in rapid succession (bursts) are also likely to be associated with neurotransmitter and neuromodulator release (47, 50). The maximum time between two spikes to be considered part of the same burst varies with neuron type and has not been defined for KNDy neurons. We examined the short-term pattern of firing using burst windows ranging from 0.01 to 5 seconds at 10-ms intervals. This analysis revealed a peak in the number of bursts detectable when spikes were separated by ≤ 230 ms.
Burst characteristics were analyzed and compared between groups using this definition; neurons that did not fire bursts were excluded from this analysis (two cells from intact, two cells from castrated, three cells from castrated + estradiol, and one cell from castrated + DHT group). KNDy neurons from castrated males (n = 7) had a higher burst frequency than intact males (n = 10, P < 0.05, Kruskal-Wallis/Dunn test; Fig. 2B). Burst frequency was restored to that of cells from intact mice by either estradiol or DHT. In parallel, interevent interval, which is inversely correlated to burst frequency, was reduced in cells from castrated compared with intact mice (P < 0.05, Kruskal-Wallis/Dunn test; Fig. 2C). This was restored to intact levels by DHT. In cells from estradiol-treated mice, interevent interval appeared to be increased relative to that in cells from intact mice, but the P value is just over that accepted for significance (n = 6, P = 0.054, Kruskal-Wallis/Dunn test; Fig. 2C). The mean burst duration was increased in cells from castrated compared with intact males [P < 0.05, one-way analysis of variance (ANOVA)/Fisher least significant difference (LSD); Fig. 2D] and was rescued by either estradiol or DHT. The number of spikes per burst was not different among groups (Fig. 2E). Finally, the intraburst interval was not different among cells from intact, castrated, and castrated + DHT but was reduced in castrated + estradiol group (P < 0.05, Kruskal-Wallis/Dunn test; Fig. 2F). The observation that burst duration is increased in the castrate group with no significant change in spikes per burst or intraburst interval is likely attributed to changes in the latter two parameters that do not reach the level traditionally accepted as significant combining to generate a significant increase in burst duration. Together these data indicate that spikes are organized in bursts in KNDy neurons from male mice, that several burst parameters are regulated by gonadal status, and that estrogens and androgens may differentially regulate some of these parameters.
Figure 2.
Burst firing is regulated by gonadal status in KNDy neurons. (A) Schematic illustrating burst parameters; 230 ms was determined by systematically varying the burst window to identify the burst window with the maximum burst frequency across the majority of recordings. (B–F) Individual values and mean ± SEM of (B) burst frequency, (C) interevent interval, (D) burst duration, (E) spikes per burst, and (F) intraburst interval. *P < 0.05 vs intact group, Kruskal-Wallis/Dunn test (B, C, F) or one-way ANOVA/Fisher LSD (D, E). C, castrated; E, estradiol; I, intact.
The NK3R, but not the KOR, signaling affects episodic activity of KNDy neurons
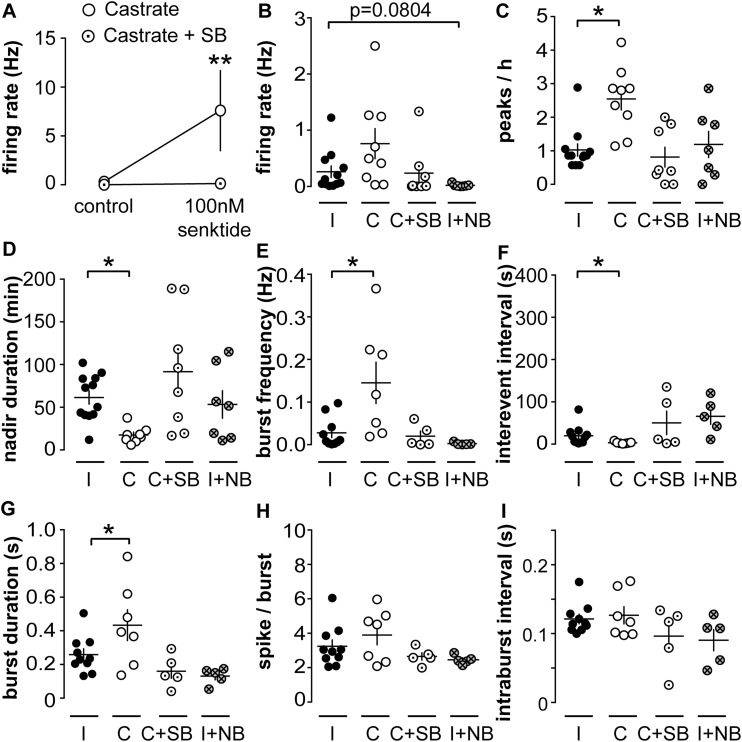
Activation of NK3R increases, whereas activation of KOR decreases, the short-term firing rate of KNDy neurons (34). These effects depend on gonadal status in male mice (35). To test the hypotheses that endogenous NKB and dynorphin regulate the episodic activity of KNDy neurons, we used pharmacology to prevent these receptors from being activated by endogenous ligand released in the slice. We first did a pilot study to determine a dose of the NK3R antagonist SB222200 that could effectively block response to the NK3R agonist senktide. Addition of 10 µM SB222200 to ACSF effectively blocked the increase in KNDy neuron firing rate in response to 100 nM senktide (control, n = 3; SB222200, n = 5; P < 0.01, two-way repeated-measures ANOVA/Sidak’s test; Fig. 3A). The KOR antagonist NorBNI was used at 10 µM, a dose previously shown to effectively block response to dynorphin (30).
Figure 3.
Blocking NK3R alters long-term activity pattern of KNDy neurons from castrated mice. (A) 10 nM SB222200 blocks activation of KNDy neuron firing rate by senktide. **P < 0.01 vs castrated group, two-way repeated-measures ANOVA/Sidak test. Individual values and mean ± SEM of mean (B) firing rate, (C) peaks per hour, (D) nadir duration, (E) burst frequency, (F) interevent interval, (G) burst duration, (H) spikes perburst, and (I) intraburst interval. *P < 0.05 vs intact group, (B, D, E, F, I) Kruskal-Wallis/Dunn test or (D, G, H) one-way ANOVA/Fisher LSD. C, castrated; I, intact; NB, NorBNI; SB, SB222200.
To test if blocking NK3R would reduce firing, we tested SB222200 effects on KNDy neurons from castrated mice because they have the highest activity. To test if blocking KOR would increase firing, we tested NorBNI effects on KNDy neurons from intact mice, which have relatively low activity. Data from both of these trials were compared with the intact and castrated data displayed in Figs. 1 and 2 as controls. Bath application of SB222200 restored peak frequency and nadir duration of cells from castrated mice to intact levels (n = 8, castrated + SB222200, P < 0.05, Kruskal-Wallis/Dunn test; Fig. 3C and 3D). Similarly, burst frequency, interevent interval, and burst duration were restored to intact levels by the SB222200 (n = 5, castrated + SB222200 cells exhibiting bursts; burst frequency and interevent interval, P > 0.5, Kruskal-Wallis/Dunn; burst duration, P > 0.2, both vs intact one-way ANOVA/Fisher LSD; Fig. 3E–3G). Spikes per burst and intraburst interval did not differ among groups (Fig. 3H and 3I). In contrast, and consistent with previous observations (30), NorBNI had no effect on either long- or short-term firing properties of cells from intact mice (intact + NorBNI, n = 7; peaks/h, P > 0.9; mean firing rate, P = 0.0804; nadir duration, P > 0.9 vs intact controls, Kruskal-Wallis/Dunn test; Fig. 3B–3F). These observations suggest activation of NK3R, but not KOR, may affect the long-term episodic and short-term activity of KNDy neurons.
Discussion
Arcuate kisspeptin neurons are an important candidate for both steroid feedback signaling to and coordination of GnRH neuron activity. In this context, a better understanding of KNDy neuron activity and its modulation by gonadal steroids is essential. Here we investigated the spontaneous long-term activity of KNDy neurons in acutely prepared brain slices from male mice in different steroid feedback conditions. KNDy neurons exhibit a long-term pattern of episodic activity, characterized by a succession of increases (peaks) and decreases (nadirs) in firing activity over time. Further, gonadal steroids in vivo and blocking NK3R in vitro modulated both the frequency of peaks in the long-term firing pattern and the short-term burst firing characteristics.
This is the first description, to our knowledge, of spontaneous succession of periods of low and high activity in KNDy neurons. These long-term shifts in firing over time may explain the variability in firing rate that has been reported in short-duration recordings of KNDy neurons from males and females (30, 35, 51, 52). That is, with a short-duration recording (5 to 15 minutes), one is more likely to capture solely a peak or a nadir depending on what phase of its activity rhythm a cell is in at the time the recording is performed. Long-term changes observed in individual KNDy neurons encompass the range of different short-term firing patterns described for these cells, such as “silent,” “irregular,” or “burst firing” (51), with transitory phases of acceleration and deceleration of the firing rate. Of interest, short-term KNDy neuron activity is also sensitive to both estradiol and DHT (35). In long-term recordings, the mean firing rate was not a parameter that discriminated effectively among groups, perhaps attributable to the higher variability observed in the castrated group, in which cells released from negative feedback may have a greater range of possible firing activity. The lack of a difference in mean firing rate is not surprising, as averaging firing rate over hours will obscure the details of peaks and nadirs.
In contrast to the effect of blocking NK3R on peak frequency, blocking KOR had no effect. This is consistent with a previous study that showed no effect of KOR antagonist on short-term activity of KNDy neurons (30). This difference may be explained by the observation that fewer KNDy neurons express KOR than NK3R (36). The effects of blocking neuropeptide receptors on firing pattern are of interest, but we urge some caution in interpretation of these data. In addition to immediate effects on activity, dynorphin and NKB could act through signaling pathways that ultimately regulate gene expression. Even with long-term recordings, it may not be possible to capture changes subsequent to blocking these processes if they have a long half-life. For these studies, we chose to compare cells with drug present for the entire recording to control cells, rather than examine a more acute drug treatment part way through a recording, for two reasons. First, this increased the observation time during treatment. Second, although individual KNDy neurons clearly show long-term changes in firing rate, the interval between peaks in firing rate within a cell is variable. This is in contrast to relative stability over several hours of GnRH and LH pulses and multiunit activity associated with LH release in vivo (5–7). One possibility is that the irregularity of peaks in the current study is an artifact of removal of important control elements in a brain slice vs the whole animal. Another possibility is that pulse generation arises as a network property in which the individual KNDy neurons contribute to a steady episodic pattern but may not themselves be active during each episodic event. Of interest in this regard, a similar irregularity in peaks of firing was observed in immortalized GnRH neurons (GT1 cells) cultured on multiunit electrode arrays. The ability to observe multiple cells simultaneously with that method revealed that when activity of individual cells was added together, peaks became much more regular (53). Continued development of methods such as calcium imaging that permit coincident measures from multiple KNDy neurons will help resolve this question.
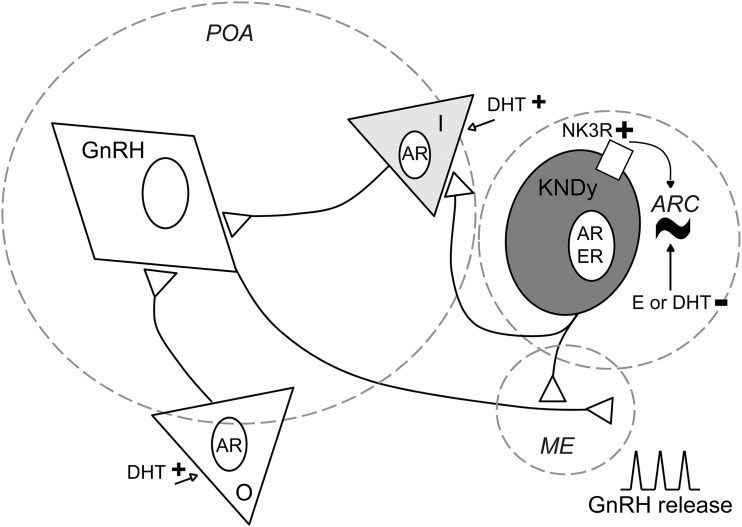
Interestingly, a similar pattern of peaks and nadirs in firing rate was observed in long-term extracellular recordings of GnRH neurons from male mice (39). The steroid modulation, however, appears to be different between these cell types. Specifically, in GnRH neurons, the number of peaks/h in castrated males is restored to normal levels by estradiol but not DHT (39), whereas both androgens and estrogens were effective in KNDy neurons. In GnRH neurons from females, estradiol negative feedback likewise reduces the frequency of peaks in firing rate (54, 55) and estradiol in combination with DHT results in activation, rather than inhibition, of GnRH neurons (39). If these cell types work together as part of a steroid-sensitive, pulse-generating network, these observations suggest a principal role for estradiol in inhibition of KNDy and GnRH peak frequency and a possible role for androgens in activating parallel pathways that may send signals to the GnRH neuron that can “override” androgen inhibition of KNDy neurons (Fig. 4). In this regard, castration in males increases γ-aminobutyric acid transmission to GnRH neurons (56), which provides an excitatory input via the γ-aminobutyric acid receptor subtype A (57, 58). KNDy neurons are primarily glutamatergic, thus the γ-aminobutyric acid afferents are likely from different cells (38).
Figure 4.
Proposed model of KNDy neurons as direct and indirect modulators of rhythmicity for episodic, steroid-dependent GnRH release. KNDy neurons in the arcuate (ARC) generate a rhythm of activity (∼). They send projections to GnRH neuron terminals in the median eminence (ME) and may also influence GnRH neuron somata in the preoptic area (POA) indirectly via intermediate (I) neurons (gray triangle). The episodic activity observed in KNDy neurons may be transmitted to GnRH neurons via either pathway. Sex steroids modulate this episodic activity. At KNDy neurons, estradiol (E) and DHT inhibit long-term episodic activity. At other populations (O; white triangle) and/or intermediate neurons that express androgen receptor (AR), DHT may initiate excitatory signals to GnRH neurons (open arrows), modifying the ultimate rhythm of GnRH release from the ME. NK3R may also be involved in the generation of this rhythm, as the modulation of NK3R signaling modifies long-term episodic activity of these cells. ER, estrogen receptor.
Despite the apparent different steroid sensitivities, the similarities in long-term firing pattern are consistent with the postulate that KNDy neurons are a source of GnRH neuron pulse generation. A likely mediator is kisspeptin, which is a strong activator of GnRH neurons via activation of kissR1. In addition to kisspeptin, activation of NK3R can also induce the release of GnRH neurons in the median eminence of male mice (59). Dynorphin is unlikely to act directly on GnRH neuron activity as KOR expression has not been detected (60, 61). The main projections from KNDy neurons to GnRH neurons are detected at the level of the terminals of these cells, in the median eminence (19). KNDy neurons also project to anteroventral periventricular (AVPV) kisspeptin neurons (19), and to other KNDy neurons on both sides of the brain. A recent study showed that optogenetic activation of KNDy neurons in one hemisphere increases KNDy neuron firing in the other (37). The postulate of KNDy neurons generating a rhythm for GnRH release does not exclude the existence of intrinsic activity of GnRH neurons, which exhibit episodic activity when fast synaptic transmission is blocked in female mice (55) and GnRH release in the absence of kisspeptin in males and females (17). The response of GnRH neurons to exogenous kisspeptin increases over the prepubertal period as organized LH pulses become detectable (62). Together these observations raise the possibility of a pulse generation signal from KNDy neurons, imposing organization and steroid responsiveness to endogenous activity of GnRH neurons.
In addition to the long-term pattern of firing activity that may be associated with the LH pulse pattern, the short-term pattern action potential grouping (bursts) is classically linked to, and perhaps required for, the release of neuropeptides such as kisspeptin (63–65). We iteratively examined how spontaneous spikes in KNDy neurons are grouped; this analysis revealed a peak in the number of bursts at a maximum intraburst interval of 230 ms. This is similar to the interval identified in current-clamp recordings of GnRH neurons when a definition of continuous depolarization from the peak of the afterhyperpolarization to the next action potential threshold was used [210 ms (66)] and to studies of GnRH neurons using the same analysis on extracellular recordings (320 ms; Phumsatitpong and Moenter, unpublished observations). The firing rate during bursts in both KNDy and GnRH neurons is lower than that in cortical and thalamic neurons and may thus be considered “nonclassical” bursts (47). Of interest, firing rates during bursts in AVPV kisspeptin neurons are also relatively low (50).
Several burst parameters were regulated by gonadal status in KNDy neurons, demonstrating a different organization of action potentials between intact and castrated mice. Burst firing is linked to neuropeptide release; thus the same number of spikes organized in bursts could trigger more neuropeptide release in castrated mice. Although a number of studies have investigated membrane properties of arcuate kisspeptin neurons (34, 67–71), relatively few have examined directly the influence of sex steroids. In females, optogenetic stimulation of firing in these cells induces a slow depolarization, the magnitude of which is enhanced in low steroid conditions (37). In males, membrane properties of KNDy neurons appeared to be regulated by gonadal status (68). This study concludes that KNDy neurons are intrinsically less excitable in the absence of steroid feedback, which is in contrast with the present findings. It is possible that the whole-cell configuration used in the previous work resulted in dialysis of factors important for mediating steroid feedback effects on neuronal activity. KNDy neurons express hyperpolarization-activated cyclic nucleotide-gated channels and the low-threshold voltage-gated calcium channel Cav.3, which often underlie burst firing (64, 69). The former is regulated by steroid feedback in AVPV kisspeptin neurons (72) but has not yet been studied in KNDy neurons. The present results indicate that KNDy neurons generate “nonclassical bursts” that are regulated by gonadal status and may contribute to steroid-dependent activity and release from these cells.
Castration augmented both the long-term and the burst-firing patterns of KNDy neurons, with both patterns restored toward intact values by either estradiol or DHT. DHT acts via androgen receptor, as it cannot be converted into estradiol by aromatase (49), and thus permits segregation of steroid action via androgen receptor or estrogen receptor, both of which are expressed by KNDy neurons (73). These data support and extend previous short-term recording studies in which estradiol and DHT restore initial firing rate and quiescence in castrated mice (35) and are consistent with studies showing both androgens and estrogens inhibit arcuate kisspeptin messenger RNA expression in mice (18, 73), suggesting steroid negative feedback acts via multiple pathways.
The present observations add to the growing body of evidence that arcuate kisspeptin neurons are likely important for both pattern generation and steroid sensitivity of GnRH pulse generation. The mechanisms underlying a “KNDy pulse generator” remain to be determined and are likely critical to the central control of reproduction.
Acknowledgments
We thank Elizabeth Wagenmaker and Laura Burger for expert technical assistance and Jacob DeFazio and Jonathon Penix for recoding Cluster and software interface development, respectively.
Financial Support: This work was supported by the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R01 HD34860 (to S.M.M.) and a Lalor Foundation Postdoctoral Fellowships (to M.R.M. and C.V.).
Current Affiliation: M. R. Moya’s current affiliation is Núcleo de Ciencias Biológicas, Facultad de Ciencias Universidad Mayor, Santiago 8550745, Chile.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ACSF
- artificial cerebrospinal fluid
- ANOVA
- analysis of variance
- AVPV
- anteroventral periventricular
- DHT
- dihydrotestosterone
- GFP
- green fluorescent protein
- kissR1
- kisspeptin receptor 1
- KNDy
- kisspeptin, neurokinin B, and dynorphin
- KOR
- κ-opioid receptor
- LH
- luteinizing hormone
- LSD
- least significant difference
- MUA
- multiunit electrical activity
- NK3R
- neurokinin type 3 receptor
- NKB
- neurokinin B
- SEM
- standard error of the mean.
References
- 1.Belchetz PE, Plant TM, Nakai Y, Keogh EJ, Knobil E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science. 1978;202(4368):631–633. [DOI] [PubMed] [Google Scholar]
- 2.Clarke IJ, Cummins JT. The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology. 1982;111(5):1737–1739. [DOI] [PubMed] [Google Scholar]
- 3.Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology. 1985;117(2):711–721. [DOI] [PubMed] [Google Scholar]
- 4.Levine JE, Duffy MT. Simultaneous measurement of luteinizing hormone (LH)-releasing hormone, LH, and follicle-stimulating hormone release in intact and short-term castrate rats. Endocrinology. 1988;122(5):2211–2221. [DOI] [PubMed] [Google Scholar]
- 5.Moenter SM, Caraty A, Locatelli A, Karsch FJ. Pattern of gonadotropin-releasing hormone (GnRH) secretion leading up to ovulation in the ewe: existence of a preovulatory GnRH surge. Endocrinology. 1991;129(3):1175–1182. [DOI] [PubMed] [Google Scholar]
- 6.Wilson RC, Kesner JS, Kaufman JM, Uemura T, Akema T, Knobil E. Central electrophysiologic correlates of pulsatile luteinizing hormone secretion in the rhesus monkey. Neuroendocrinology. 1984;39(3):256–260. [DOI] [PubMed] [Google Scholar]
- 7.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol. 2009;21(10):813–821. [DOI] [PubMed] [Google Scholar]
- 8.Kinsey-Jones JS, Li XF, Luckman SM, O’Byrne KT. Effects of kisspeptin-10 on the electrophysiological manifestation of gonadotropin-releasing hormone pulse generator activity in the female rat. Endocrinology. 2008;149(3):1004–1008. [DOI] [PubMed] [Google Scholar]
- 9.Karsch FJ, Cummins JT, Thomas GB, Clarke IJ. Steroid feedback inhibition of pulsatile secretion of gonadotropin-releasing hormone in the ewe. Biol Reprod. 1987;36(5):1207–1218. [DOI] [PubMed] [Google Scholar]
- 10.Hrabovszky E, Steinhauser A, Barabás K, Shughrue PJ, Petersen SL, Merchenthaler I, Liposits Z. Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2001;142(7):3261–3264. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Harlan RE. Absence of androgen receptors in LHRH immunoreactive neurons. Brain Res. 1993;624(1-2):309–311. [DOI] [PubMed] [Google Scholar]
- 12.Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and β-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133(2):887–895. [DOI] [PubMed] [Google Scholar]
- 13.Herbison AE, Skinner DC, Robinson JE, King IS. Androgen receptor-immunoreactive cells in ram hypothalamus: distribution and co-localization patterns with gonadotropin-releasing hormone, somatostatin and tyrosine hydroxylase. Neuroendocrinology. 1996;63(2):120–131. [DOI] [PubMed] [Google Scholar]
- 14.Wintermantel TM, Campbell RE, Porteous R, Bock D, Gröne H-J, Todman MG, Korach KS, Greiner E, Pérez CA, Schütz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52(2):271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149(4):1979–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han S-K, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25(49):11349–11356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glanowska KM, Burger LL, Moenter SM. Development of gonadotropin-releasing hormone secretion and pituitary response. J Neurosci. 2014;34(45):15060–15069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146(9):3686–3692. [DOI] [PubMed] [Google Scholar]
- 19.Yip SH, Boehm U, Herbison AE, Campbell RE. Conditional viral tract tracing delineates the projections of the distinct kisspeptin neuron populations to gonadotropin-releasing hormone (GnRH) neurons in the mouse. Endocrinology. 2015;156(7):2582–2594. [DOI] [PubMed] [Google Scholar]
- 20.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MB, Crowley WF Jr, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 21.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol. 2006;498(5):712–726. [DOI] [PubMed] [Google Scholar]
- 24.Goubillon ML, Forsdike RA, Robinson JE, Ciofi P, Caraty A, Herbison AE. Identification of neurokinin B-expressing neurons as an highly estrogen-receptive, sexually dimorphic cell group in the ovine arcuate nucleus. Endocrinology. 2000;141(11):4218–4225. [DOI] [PubMed] [Google Scholar]
- 25.Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev. 2009;30(6):713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalló I, Vida B, Deli L, Molnár CS, Hrabovszky E, Caraty A, Ciofi P, Coen CW, Liposits Z. Co-localisation of kisspeptin with galanin or neurokinin B in afferents to mouse GnRH neurones. J Neuroendocrinol. 2012;24(3):464–476. [DOI] [PubMed] [Google Scholar]
- 27.Navarro VM, Bosch MA, León S, Simavli S, True C, Pinilla L, Carroll RS, Seminara SB, Tena-Sempere M, Rønnekleiv OK, Kaiser UB. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology. 2015;156(2):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol. 2005;489(3):372–386. [DOI] [PubMed] [Google Scholar]
- 29.Herbison AE, de Tassigny Xd, Doran J, Colledge WH. Distribution and postnatal development of Gpr54 gene expression in mouse brain and gonadotropin-releasing hormone neurons. Endocrinology. 2010;151(1):312–321. [DOI] [PubMed] [Google Scholar]
- 30.Ruka KA, Burger LL, Moenter SM. Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology. 2013;154(8):2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151–4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750–2760. [DOI] [PubMed] [Google Scholar]
- 35.Ruka KA, Burger LL, Moenter SM. Both estrogen and androgen modify the response to activation of neurokinin-3 and κ-opioid receptors in arcuate kisspeptin neurons from male mice. Endocrinology. 2016;157(2):752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Ronnekleiv OK, Braun RE, Palmiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152(11):4265–4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, Rønnekleiv OK. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife. 2016;5:e16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J Jr, Atkin S, Bookout AL, Rovinsky S, Frazão R, Lee CE, Gautron L, Zigman JM, Elias CF. Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience. 2011;173:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pielecka J, Moenter SM. Effect of steroid milieu on gonadotropin-releasing hormone-1 neuron firing pattern and luteinizing hormone levels in male mice. Biol Reprod. 2006;74(5):931–937. [DOI] [PubMed] [Google Scholar]
- 40.Christian CA, Mobley JL, Moenter SM. Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proc Natl Acad Sci USA. 2005;102(43):15682–15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chu Z, Moenter SM. Endogenous activation of metabotropic glutamate receptors modulates GABAergic transmission to gonadotropin-releasing hormone neurons and alters their firing rate: a possible local feedback circuit. J Neurosci. 2005;25(24):5740–5749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nunemaker CS, DeFazio RA, Moenter SM. A targeted extracellular approach for recording long-term firing patterns of excitable cells: a practical guide. Biol Proced Online. 2003;5:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alcami P, Franconville R, Llano I, Marty A. Measuring the firing rate of high-resistance neurons with cell-attached recording. J Neurosci. 2012;32(9):3118–3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veldhuis JD, Johnson ML. Cluster analysis: a simple, versatile, and robust algorithm for endocrine pulse detection. Am J Physiol. 1986;250(4 Pt 1):E486–E493. [DOI] [PubMed] [Google Scholar]
- 45.Dutton A, Dyball RE. Phasic firing enhances vasopressin release from the rat neurohypophysis. J Physiol. 1979;290(2):433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation-coupling mechanism in the isolated rat neural lobe. J Physiol. 1985;369:45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L, Deng Y, Luo W, Wang Z, Zeng S. Detection of bursts in neuronal spike trains by the mean inter-spike interval method. Prog Nat Sci. 2009;19:229–235. [Google Scholar]
- 48.Silveira MA, Burger LL, DeFazio RA, Wagenmaker ER, Moenter SM. GnRH neuron activity and pituitary response in estradiol-induced vs proestrous luteinizing hormone surges in female mice. Endocrinology. 2017;158(2):356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mooradian AD, Morley JE, Korenman SG. Biological actions of androgens. Endocr Rev. 1987;8(1):1–28. [DOI] [PubMed] [Google Scholar]
- 50.Wang L, DeFazio RA, Moenter SM. Excitability and burst generation of AVPV kisspeptin neurons are regulated by the estrous cycle via multiple conductances modulated by estradiol action. eNeuro. 2016;3(3):ENEURO.0094-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE. Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology. 2012;153(11):5384–5393. [DOI] [PubMed] [Google Scholar]
- 52.Cholanian M, Krajewski-Hall SJ, Levine RB, McMullen NT, Rance NE. Electrophysiology of arcuate neurokinin B neurons in female Tac2-EGFP transgenic mice. Endocrinology. 2014;155(7):2555–2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nunemaker CS, DeFazio RA, Geusz ME, Herzog ED, Pitts GR, Moenter SM. Long-term recordings of networks of immortalized GnRH neurons reveal episodic patterns of electrical activity. J Neurophysiol. 2001;86(1):86–93. [DOI] [PubMed] [Google Scholar]
- 54.Nunemaker CS, Straume M, DeFazio RA, Moenter SM. Gonadotropin-releasing hormone neurons generate interacting rhythms in multiple time domains. Endocrinology. 2003;144(3):823–831. [DOI] [PubMed] [Google Scholar]
- 55.Nunemaker CS, DeFazio RA, Moenter SM. Estradiol-sensitive afferents modulate long-term episodic firing patterns of GnRH neurons. Endocrinology. 2002;143(6):2284–2292. [DOI] [PubMed] [Google Scholar]
- 56.Chen P, Moenter SM. GABAergic transmission to gonadotropin-releasing hormone (GnRH) neurons is regulated by GnRH in a concentration-dependent manner engaging multiple signaling pathways. J Neurosci. 2009;29(31):9809–9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.DeFazio RA, Heger S, Ojeda SR, Moenter SM. Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Mol Endocrinol. 2002;16(12):2872–2891. [DOI] [PubMed] [Google Scholar]
- 58.Herbison AE, Moenter SM. Depolarising and hyperpolarising actions of GABA(A) receptor activation on gonadotrophin-releasing hormone neurones: towards an emerging consensus. J Neuroendocrinol. 2011;23(7):557–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaskins GT, Glanowska KM, Moenter SM. Activation of neurokinin 3 receptors stimulates GnRH release in a location-dependent but kisspeptin-independent manner in adult mice. Endocrinology. 2013;154(11):3984–3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mitchell V, Prevot V, Jennes L, Aubert JP, Croix D, Beauvillain JC. Presence of mu and kappa opioid receptor mRNAs in galanin but not in GnRH neurons in the female rat. Neuroreport. 1997;8(14):3167–3172. [DOI] [PubMed] [Google Scholar]
- 61.Sannella MI, Petersen SL. Dual label in situ hybridization studies provide evidence that luteinizing hormone-releasing hormone neurons do not synthesize messenger ribonucleic acid for μ, κ, or δ opiate receptors. Endocrinology. 1997;138(4):1667–1672. [DOI] [PubMed] [Google Scholar]
- 62.Steyn FJ, Wan Y, Clarkson J, Veldhuis JD, Herbison AE, Chen C. Development of a methodology for and assessment of pulsatile luteinizing hormone secretion in juvenile and adult male mice. Endocrinology. 2013;154(12):4939–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van den Pol AN. Neuropeptide transmission in brain circuits. Neuron. 2012;76(1):98–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelly MJ, Zhang C, Qiu J, Rønnekleiv OK. Pacemaking kisspeptin neurons. Exp Physiol. 2013;98(11):1535–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Masterson SP, Li J, Bickford ME. Frequency-dependent release of substance P mediates heterosynaptic potentiation of glutamatergic synaptic responses in the rat visual thalamus. J Neurophysiol. 2010;104(3):1758–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chu Z, Takagi H, Moenter SM. Hyperpolarization-activated currents in gonadotropin-releasing hormone (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. J Neurosci. 2010;30(40):13373–13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Frazão R, Cravo RM, Donato J Jr, Ratra DV, Clegg DJ, Elmquist JK, Zigman JM, Williams KW, Elias CF. Shift in Kiss1 cell activity requires estrogen receptor. J Neurosci. 2013;33(7):2807–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alreja M. Electrophysiology of kisspeptin neurons. Adv Exp Med Biol. 2013;784:349–362. [DOI] [PubMed] [Google Scholar]
- 69.Gottsch ML, Popa SM, Lawhorn JK, Qiu J, Tonsfeldt KJ, Bosch MA, Kelly MJ, Rønnekleiv OK, Sanz E, McKnight GS, Clifton DK, Palmiter RD, Steiner RA. Molecular properties of Kiss1 neurons in the arcuate nucleus of the mouse. Endocrinology. 2011;152(11):4298–4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology. 2011;152(4):1503–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.True C, Verma S, Grove KL, Smith MS. Cocaine- and amphetamine-regulated transcript is a potent stimulator of GnRH and kisspeptin cells and may contribute to negative energy balance-induced reproductive inhibition in females. Endocrinology. 2013;154(8):2821–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piet R, Boehm U, Herbison AE. Estrous cycle plasticity in the hyperpolarization-activated current ih is mediated by circulating 17β-estradiol in preoptic area kisspeptin neurons. J Neurosci. 2013;33(26):10828–10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146(7):2976–2984. [DOI] [PubMed] [Google Scholar]