Abstract
Despite the occurrence of dyslipidemia and its contribution to the development of insulin resistance in obese subjects, a growing number of studies have described abnormal lipid profiles among leaner persons. For example, individuals with an abnormal paucity or distribution of fat (lipodystrophy) develop severe insulin resistance, dyslipidemia, and hepatic steatosis. Deranged adipocyte metabolism and differentiation contribute to ectopic fat deposition and consequent development of insulin resistance. Growth hormone (GH) therapy has been shown to correct body composition abnormalities in some lipodystrophy patients. However, little is known about the effects of GH-releasing peptides in this regard. Hexarelin, a GH secretagogue, has recently been shown to have beneficial effects on fat metabolism via the CD36 receptor. In this study, the effects of twice daily intraperitoneal injections of hexarelin (200 μg/kg body weight) were examined in nonobese insulin-resistant MKR mice and corresponding wild-type FVB mice for 12 days. Hexarelin treatment significantly improved glucose and insulin intolerance and decreased plasma and liver triglycerides in MKR mice. These beneficial metabolic effects could be due to the improved lipid metabolism and enhanced adipocyte differentiation of white adipose tissue with hexarelin treatment. Interestingly, although food intake of hexarelin-treated MKR mice was significantly increased, this did not change total body weight. Moreover, hexarelin treatment corrected the abnormal body composition of MKR mice, as demonstrated by a decrease in fat mass and an increase in lean mass. Our results suggest a possible application of hexarelin in treatment of lipid disorders associated with the metabolic syndrome.
Hexarelin treatment improved glucose and insulin intolerances in MKR nonobese diabetic mice through the effects on adipocyte differentiation, lipid distribution, and fatty acid oxidation.
Hyperlipidemia and ectopic fat deposition are strongly associated with insulin resistance and are well-documented risk factors for cardiovascular diseases (1, 2). Although ectopic lipid accumulation is typically observed in most obese individuals (3), it is also encountered in lean subjects, as in the lipodystrophies (4, 5). It has been suggested that adipose tissue dysfunction contributes to ectopic lipid accumulation associated with insulin resistance through different mechanisms (6). Firstly, insufficient adipose tissue mass in inherited or acquired forms of lipodystrophy leads to excessive storage of ingested fat in muscle and liver and the development of insulin resistance in these organs (7–9). Secondly, lipolysis of visceral adipose tissues is closely associated with insulin resistance (10). Thirdly, impairment in differentiation capacity of adipocytes leads to formation of large insulin-resistant adipocytes with diminished capacity to accumulate fat (11, 12). Importantly, if fat oxidation fails to adaptively compensate for the increased influx of lipid within these tissues, intracellular accumulation of lipids will occur (13, 14). The relative contribution of these factors to ectopic lipid accumulation may vary in different pathological conditions and in different tissues. In lipodystrophy, excess lipid influx would appear to be predominantly associated with ectopic lipid accumulation (15), whereas in lean insulin-resistant offspring of type 2 patients with diabetes, impaired mitochondrial fatty acid oxidation may play a major role in this process (16).
Intensive changes in lifestyle and glycemic control are unable to fully correct the metabolic aberrations in patients with lipodystrophy or lean type 2 diabetes (17, 18). Therefore, lipid-modifying therapy is warranted in patients with disorders of fat metabolism to restore the function of adipose tissue and correct dyslipidemia. Growth hormone (GH) replacement showed some promising effects for a lipodystrophy population by decreasing visceral adiposity and increasing lean mass with transient side effects, including reversible glucose intolerance (19, 20). However, the effects of GH-releasing peptides have not been well studied with respect to dyslipidemia and related metabolic disorders. Hexarelin, a synthetic peptide GH secretagogue, stimulates the release of GH through binding to GH secretagogue receptor 1a in the pituitary and hypothalamic regions (21). Owing to its anabolic effects on skeletal muscles (partially via GH), hexarelin has received attention from athletics as a performance enhancement drug (22). Moreover, the cardioprotective effects of hexarelin are well documented (23, 24). Acute intravenous administration of hexarelin induced a rapid increase in left ventricle ejection fraction, cardiac output, and cardiac index (25). Chronic administration of hexarelin to GH-deficient rats has a pronounced protective effect against ischemic and postischemic ventricular dysfunction (26).
Interestingly, there is arising evidence that hexarelin might have beneficial effects on fat metabolism. Studies by Rodrigue-Way et al. (27) showed that hexarelin might bind to a scavenger receptor class B (CD36) to enhance the activation of peroxisome proliferator-activated receptor γ (PPAR-γ) in macrophages and adipocytes. This effect is independent of GH. CD36 plays an important role in the pathogenesis of metabolic disorders (28, 29). As a fatty acid translocase, CD36 binds and internalizes long-chain fatty acids to facilitate energy production (30, 31). Moreover, CD36 expression is activated during adipocyte differentiation, and its protein levels are positively correlated with CCAAT/enhancer binding protein α and PPAP-γ, which are critical transcription factors in lipid metabolism and adipocyte differentiation (32–34). Hexarelin is proposed to act through CD36 to inhibit accumulation of oxidized low-density lipoprotein cholesterol in macrophages, resulting in decreased atherosclerotic lesions in apolipoprotein E–deficient mice fed with an atherogenic diet (35).
In the present study, we investigated the effects of hexarelin on glucose and lipid metabolism in nonobese insulin-resistant MKR mice. MKR mice with an over-expression of dominant-negative insulinlike growth factor (IGF)-1 receptor in skeletal muscle showed impaired insulin signaling pathways in skeletal muscle due to hybrid formation of the mutated IGF-1 receptor with the endogenous IGF-1 and insulin receptors (36). This defect results in insulin resistance not only in skeletal muscle but also in adipose tissue and liver, causing β cell dysfunction and hyperglycemia. Importantly, MKR mice exhibit defects in the free fatty acid (FFA) oxidation pathway, which leads to elevations in serum FFAs and triglycerides (TGs), as well as increased TG deposits in liver and muscle tissues, suggesting that hyperlipidemia and accumulated lipids in tissues may be causative factors for the progression of type 2 diabetes in MKR mice, replicating human cases with inherited lipodystrophy or dyslipidemia (37, 38). Our results showed that hexarelin reversed the abnormal lipid metabolic states of MKR mice through modulation of genes related to fatty acid uptake and oxidation and enhancement of adipocyte differentiation. Administration of hexarelin for 12 days alleviated glucose and insulin intolerance in MKR mice without affecting the levels of blood glucose, plasma insulin, or body weight (BW).
Materials and Methods
Mice
Homozygous adult MKR male mice (FVB/N background, 10 to 15 weeks old) and their corresponding age and sex-matched wild-type homozygous littermate FVB mice were used for the present study (36). All mice were bred in the Australian Institute for Bioengineering and Nanotechnology animal house within the University of Queensland and housed at 23 ± 2°C with 35% ± 4% humidity on a 12-hour light/12-hour dark cycle (dark period started at 6:00 pm). Mice had free access to water and standard rodent chow (Specialty Feeds, Glen Forrest, WA, Australia). All experiments and procedures were approved by the University of Queensland Animal Ethics Committee (SBMS/031/15/NHMRC/UQ) and performed in accordance with national guidelines. Genotyping was performed by tail biopsy and standard polymerase chain reaction (PCR) using a REDExtract-N-AMP tissue PCR kit (XNATR; Sigma-Aldrich, Castle Hill, NSW, Australia).
Peptide
Hexarelin, supplied by China Peptides (Shanghai, China), was used and prepared freshly by dissolving in physiological saline (0.9% NaCl). The drug was administered intraperitoneally (IP), at a concentration of 200 μg/kg BW. Dose was chosen according to previously published results showing that hexarelin maximally stimulated both GH secretion and food intake at a range of concentrations from 80 to 320 μg/kg (39–41). Mice were injected twice daily at 8:00 am and 18:00 pm with a dose volume of 0.1 mL/20 g BW of hexarelin or saline. Mice were divided into four groups, namely FVB mice injected with saline (FVB saline), FVB mice injected with hexarelin (FVB hex), MKR mice injected with saline (MKR saline), and MKR mice injected with hexarelin (MKR hex).
Glucose tolerance and insulin tolerance tests
A glucose tolerance test (GTT) was conducted following 6 hours of fasting (food withdrawn at 8.00 am) on day 8 of treatment. Mice were injected IP with 2 g glucose/kg BW, and blood glucose was monitored prior to administration of the glucose (0 time) and subsequently at 15, 30, 45, 60, 90, and 120 minutes after glucose injection. An insulin tolerance test (ITT) was conducted following 6 hours of fasting on day 11 of treatment. Mice were injected IP with 0.75 U insulin/kg BW (human insulin solution; Sigma-Aldrich), and blood glucose was monitored at 0, 30, 60, 90, and 120 minutes. Measurement of blood glucose was carried out using a glucometer (Nova StatStrip Xpress glucose hospital meter; Australasian Medical & Scientific Ltd., Chatswood, NSW, Australia) and glucose-test strips (42214; Australasian Medical & Scientific Ltd.).
Effects of hexarelin treatment on pulsatile GH secretion
As hexarelin is known to stimulate GH secretion, we further assessed plasma GH levels across 2 hours blood collection after injection on day 7 of treatment. Whole blood (2 μL) was withdrawn from the tail vein at −15, 0, 15, 30, 45, 60, 75, 90, 105, and 120 minutes relative to hexarelin or saline injections (0 time) and homogenized in 58 μL GH buffer [phosphate-buffered saline (PBS), 0.05% Tween 20]. GH concentrations were measured with an in-house mouse GH enzyme-linked immunosorbent assay according to our well-established method (42). Briefly, a 96-well plate (Corning, Corning, NY) was coated with 50 μL monkey anti-rat GH antibody [National Institutes of Health, Bethesda, MD; catalog no. AFP411S, research resource identifier (RRID): AB_2665538] at a dilution of 1:40,000 and incubated at 4°C overnight. To decrease nonspecific binding, each well was incubated with blocking buffer (5% skim milk powder in 0.1M PBS supplemented with 0.05% Tween 20) for 2 hours at room temperature. Following blocking, standards and samples were loaded into the precoated plate and incubated for 2 hours at room temperature. Then, 50 μL detection antibody (rabbit anti-rat GH; National Institutes of Health; catalog no. AFP5672099, RRID: AB_2629219) at a final dilution of 1:40,000 was added. The details of the antibodies used in this assay are illustrated in Table 1. Bound complex was then incubated with 50 μL horseradish peroxidase–conjugated antibody (anti-rabbit IgG; GE Healthcare, Little Chalfont, UK) at a dilution of 1:2000. The enzymatic colorimetric reaction was resulted by addition of 100 μL O-phenylenediamine (00.2003; Invitrogen, Carlsbad, CA). This reaction was stopped by the addition of 50 μL 3 M hydrochloric acid (EMD Millipore Corporation, Billerica, MA). The absorbance was read at a wavelength of 490 nm (reference wavelength set at 650 nm) on a Tecan Sunrise 96-well monochromatic microplate reader (Tecan, Männedorf, Switzerland). The concentration of GH in each well was calculated against a nonlinear regression of the standard curve.
Table 1.
Antibodies Used in This Study
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog No. | Species Raised in | Dilution Used | RRID |
|---|---|---|---|---|---|
| GH | Monkey antiserum to rat GH | A.F. Parlow, National Institutes of Health, Bethesda, MD, AFP411S | Monkey | 1:40,000 | AB_2665538 |
| GH | Rabbit anti-rat GH | A.F. Parlow, National Institutes of Health, AFP5672099 | Rabbit | 1:40,000 | AB_2629219 |
Indirect calorimetric assays
Animals were individually placed in calorimeter chambers (TSE PhenoMaster; TSE Systems, Bad Homburg, Germany) containing food and water in rooms maintained under conditions as described above. Mice were acclimatized to indirect calorimetry cages for 3 days before the start of injection. Mice received IP injection of either saline or hexarelin at day 0 of calorimeter recording. BW and food intake were recorded daily. Respiratory exchange ratios (RERs) were measured during dark and light cycles for individual mice on days 0, 5, and 10 of treatment. Locomotor activity was monitored by using a multidimensional infrared light beam system. Body composition was determined by nuclear magnetic resonance (Bruker minispec; Bruker, Billerica, MA) at the end of the experimental period (day 12).
Blood glucose and plasma hormonal analysis
At the end of treatment, all mice were culled following a single IP injection of sodium pentobarbitone (32.5 mg/mL) under fed state. Measurement of blood glucose from tail blood was carried out as described before in GTT and ITT sections. Terminal blood samples were collected via cardiac puncture into EDTA-coated tubes. Plasma was separated and stored at −80°C for assessment of insulin, c-peptide, ghrelin, GLP-1, and leptin by a Milliplex map kit (Abacus ALS Pty Ltd., Waterford, QLD, Australia) using a Luminex XMap analyzer (Magipex).
Plasma lipid and tissues TG analysis
Plasma nonesterified fatty acids were measured using a colorimetric determination kit (Wako NEFA C kit; catalog no. 279-75401; Wako Chemicals, Richmond, VA). TGs and cholesterol were measured in plasma by enzymatic colorimetric methods using a TG colorimetric assay kit (Cayman Chemical, Ann Arbor, MI) and cholesterol /cholesterol ester quantification assay kit (Abcam, UK), respectively. Hepatic content and muscle TG content were assayed as previously described (43). Briefly, tissue TGs were extracted from frozen liver and muscle by saponification in ethanoic potassium hydroxide overnight at 55°C and neutralized with MgCl2. All tissue TGs were converted to glycerol with a TG reagent (F6428; Sigma-Aldrich, Australia) and analyzed spectrophotometrically at 540 nm. The concentration of TGs was estimated from a standard glycerol curve and corrected for tissue weight.
Quantitative real-time PCR
White adipose tissue (WAT) RNA was extracted using TRIzol reagent (Ambion, Life Technology, Carlsbad, CA) and purified using a PureLink RNA mini kit (Invitrogen, Carlsbad, CA), and total RNA (1 μg) was reverse transcribed into complementary DNA (cDNA) according to the manufacturer’s instructions (iScript cDNA synthesis kit; Bio-Rad Laboratories Pty Ltd., Gladesville, NSW, Australia). For the quantitative PCR assays, cDNAs were amplified by real-time PCR in triplicate with an iTaq Universal SYBR Green Supermix (Bio-Rad Laboratories Pty Ltd.) using a QuantStudio 7 (384-well) PCR (Applied Biosystems). The forward and reverse primer sequences used in this assay are illustrated in Table 2. All optimal cycling reactions were performed in the same manner: 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Changes in cycle threshold of the genes of interest were corrected to the housekeeping gene (β-actin). The fold change value of gene expression was calculated by using the 2−ΔΔCT method as described previously (44).
Table 2.
Primer Sequences Used for Gene Expression Analysis by Quantitative PCR
| Gene | Forward (5′→3′) | Reverse (5′→3′) |
|---|---|---|
| PPAR-γ | CGGTTTCAGAAGTGCCTTG | GGTTCAGCTGGTCGATATCAC |
| PGC1α | TATGGAGTGACATAGAGTGTGCT | CCACTTCAATCCACCCAGAAAG |
| HSL | GGCTCACAGTTACCATCTCACC | GAGTACCTTGCTGTCCTGTCC |
| CD36 | GGCGTGGGTCTGAAGGACTGGAA | GGAGGCACGGGGTCTCAACCA |
| LPL | CTGCTGGCGTAGCAGGAAGT | GCTGGAAAGTGCCTCCATTG |
| Fatp1 | CGCTTTCTGCGTATCGTCTG | GATGCACGGGATCGTGTCT |
| β-actin | CTGAATGGCCCAGGTCTGA | CCCTGGCTGCCTCAACAC |
| UCP-1 | ACTGCCACACCTCCAGTCATT | CTTTGCCTCACTCAGGATTGG |
Abbreviations: CD36, fatty acid translocase; Fatp1, fatty acid transport protein 1; HSL, hormone-sensitive lipase; LPL, lipoprotein lipase; PGC1α, PPAR-γ coactivator α; UCP-1, uncoupling protein 1.
Adipose tissue histology
The left gonadal fat pads from the mice were removed following euthanasia at the end of treatment and fixed overnight in 4% paraformaldehyde in PBS buffer. The tissues were then transferred to 70% ethanol and embedded in paraffin. Sections (7 μm thick) were stained with hematoxylin and eosin and were mounted on glass slides as described previously (45). The stained sections were viewed with an Aperio XT slide scanner and analyzed for the surface area of adipocytes using ImageJ software (Java platform, Oracle).
Statistical analyses
Statistical analysis was carried out using GraphPad Prism 7 software (GraphPad Software, San Diego, CA). All data are presented as mean ± standard error of the mean (SEM). A Student t test or paired t test was used to compare the differences between two independent or dependent parameters, respectively. Statistical analysis was performed with one-way or two-way analysis of variance followed by a Tukey test for multiple comparisons. The differences were considered to be statistically significant at P < 0.05.
Results
Effects of hexarelin treatment on glucose and insulin tolerance in MKR mice
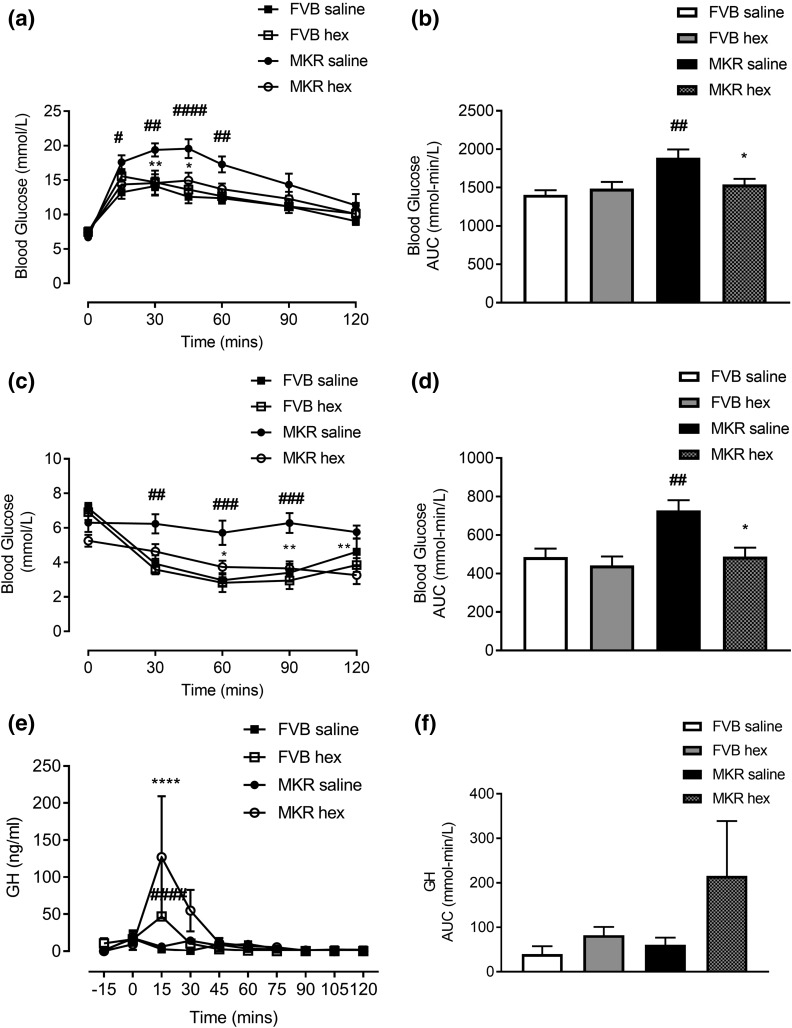
Following 8 days of treatment, GTT results showed that hexarelin significantly improved glucose excursions [Fig. 1(a)] and reduced area under the curve (AUC) of glucose in MKR mice [P = 0.0423, Fig. 1(b)]. ITTs performed on day 11 of treatment showed a significant reduction of glucose levels [Fig. 1(c)] and AUC in response to insulin injection in hexarelin-treated MKR mice [P = 0.0105, Fig. 1(d)]. Moreover, the improvement of insulin sensitivity was confirmed by comparison of ITT data from the same mice before and at day 11 of hexarelin treatment. The self-comparison analysis showed that MKR hexarelin mice had significant improvement of insulin intolerance after 11 days of the treatment (P < 0.05; Supplemental Fig. 1 (257.5KB, pdf) ). There were no changes of glucose and insulin tolerances in FVB mice following hexarelin treatment [Fig. 1(a–d)].
Figure 1.
Effects of twice daily IP injection of hexarelin (200 μg/kg BW) or saline for 12 days on (a) glucose tolerance test, (c) insulin tolerance test, and (e) pulsatile GH secretion in FVB saline (▪), FVB hex (□), MKR saline (●), and MKR hex (○). AUC of (b) GTT, (d) ITT, and (f) pulsatile GH secretion. Data are expressed as mean ± SEM (n = 5 to 6 per group). *P < 0.05, **P < 0.01, ****P < 0.0001 vs MKE saline group; #P < 0.05, ##P < 0.01, ###P < 0.001, ####P < 0.0001 vs FVB saline group.
Effects of hexarelin treatment on GH secretion
Pulsatile GH levels were significantly increased after 15 minutes of hexarelin injection in both FVB and MKR mice compared with vehicle-treated groups [P < 0.001, Fig. 1(e)]. This increase in pulsatile GH lasted for nearly 15 to 30 minutes after injections and then returned to baseline. There was no significant difference in total AUC of GH between hexarelin and vehicle treatment groups [Fig. 1(f)].
Effects of hexarelin treatment on cumulative food intake, BW, and body composition
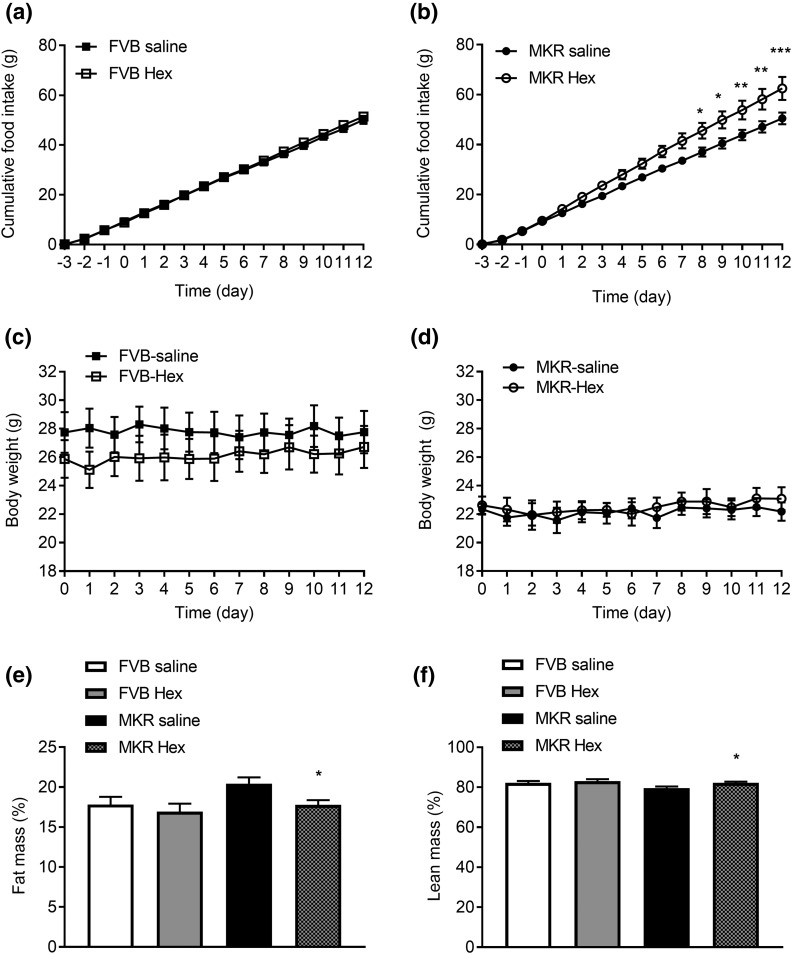
Food intake of hexarelin-treated MKR mice on days 0 to 12 of treatment was significantly increased relative to vehicle-treated MKR mice [Fig. 2(b)]. There is no difference in the food intake between FVB saline and MKR saline groups. At the end of treatment, the total cumulative food intake of hexarelin-treated MKR mice was significantly increased by 24% compared with vehicle-treated MKR mice. Interestingly, the orexigenic effect of hexarelin was not observed in FVB mice [Fig. 2(a)]. MKR mice had lower BWs compared with FVB mice. However, BWs remained unchanged in both MKR and FVB mice after hexarelin treatment [Fig. 2(c) and 2(d)]. Analysis of body composition by nuclear magnetic resonance on day 12 of treatment revealed that MKR mice tended to have higher fat mass and lower lean mass compared with FVB mice [Fig. 2(e) and 2(f)]. With hexarelin treatment, there was a 13% decrease in body fat [P = 0.0278, Fig. 2(e)] and a 3.3% increase in lean mass in MKR mice [P = 0.0278, Fig. 2(f)]. There was no significant change in body composition of hexarelin-treated FVB mice compared with vehicle-treated FVB mice.
Figure 2.
Effects of hexarelin treatment on cumulative food intake of (a) FVB mice and (b) MKR mice measured from day −3 to day 12 of treatment in indirect calorimetric cages. Change in BW of (c) FVB mice and (d) MKR mice measured from day 0 (start of treatment) to day 12 of treatment. (e) Fat mass (% BW) and (f) lean mass (% BW) measured at day 12 of treatment. Data are expressed as mean ± SEM (n = 4 to 5 per group). *P < 0.05, **P < 0.01, ***P < 0.001 vs MKR saline group.
Effects of hexarelin treatment on RER and locomotor activity
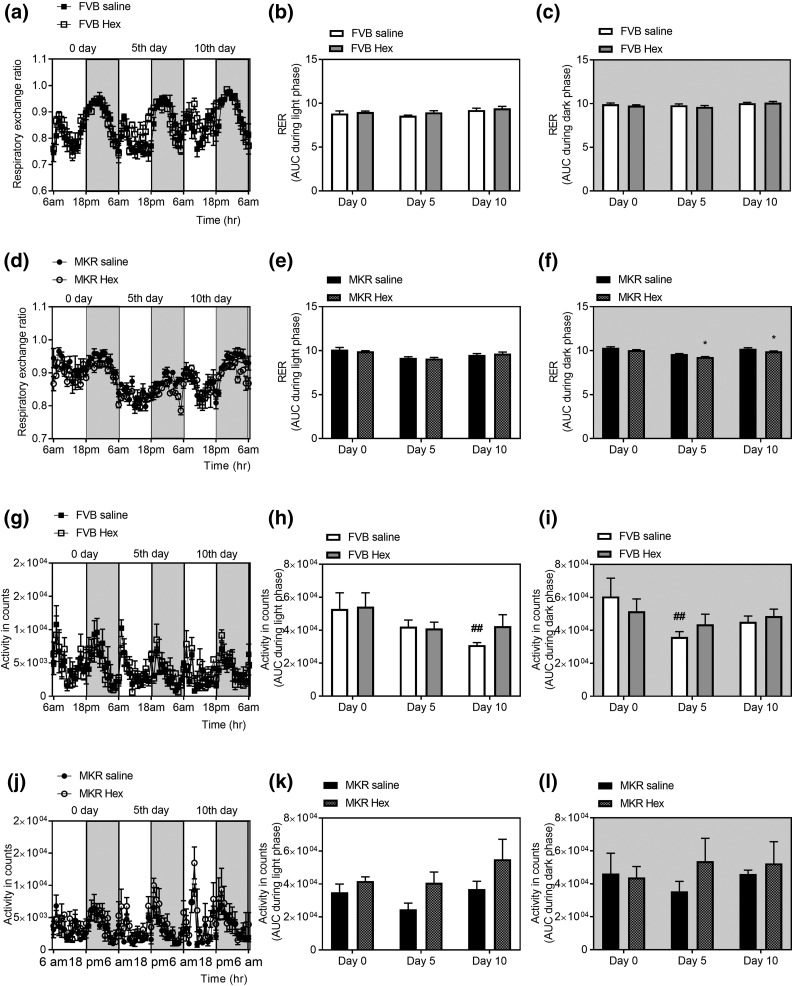
Indirect calorimetry revealed that hexarelin decreased RER of MKR mice during the dark phase but not during the light phase at days 5 and 10 of treatment (P = 0.0173 and 0.0420, respectively), demonstrating an increase in fat oxidation [Fig. 3(d–f)]. There was no change in RER in FVB mice after hexarelin treatment [Fig. 3(a–c)]. Analysis of locomotor activity showed that there was a decrease in total activity among the vehicle-treated FVB mice that was significant on day 5 during the dark phase [40.5% decrease, P = 0.0079, Fig. 3(i)] and day 10 during the light phase compared with day 0 [41% decrease, P = 0.0064, Fig. 3(h)]. This decrease was however unnoticed among hexarelin-treated FVB mice, indicating that hexarelin might have stimulatory effects on locomotor activity [Fig. 3(g–i)]. Hexarelin-treated MKR mice showed a progressive increase in the locomotor activity during the light phase, although not reaching statistical significance [Fig. 3(j–l)].
Figure 3.
Effects of hexarelin treatment on RER in (a) FVB mice, (d) RER in MKR mice, (g) total locomotor activity in FVB mice, and (j) total locomotor activity in MKR mice at days 0, 5, and 10 of treatment during dark phase (6:00 pm to 6:00 am) marked by shaded area in the graphs and light phase (6:00 am to 6:00 pm). AUC of RER during the (b) light and (c) dark phases of days 0, 5, and 10 of treatment in FVB mice. AUC of RER during the (e) light and (f) dark phases of days 0, 5, and 10 of treatment in MKR mice. AUC of locomotor activity during the (h) light and (i) dark phases of days 0, 5, and 10 of treatment in FVB mice. AUC of locomotor activity during the (k) light and (l) dark phases of days 0, 5, and 10 of treatment in MKR mice. Data are expressed as mean ± SEM (n = 4 per group). *P < 0.05 vs MKR saline group; ##P < 0.01 vs FVB saline group at day 0.
Effects of hexarelin on blood glucose and plasma hormones
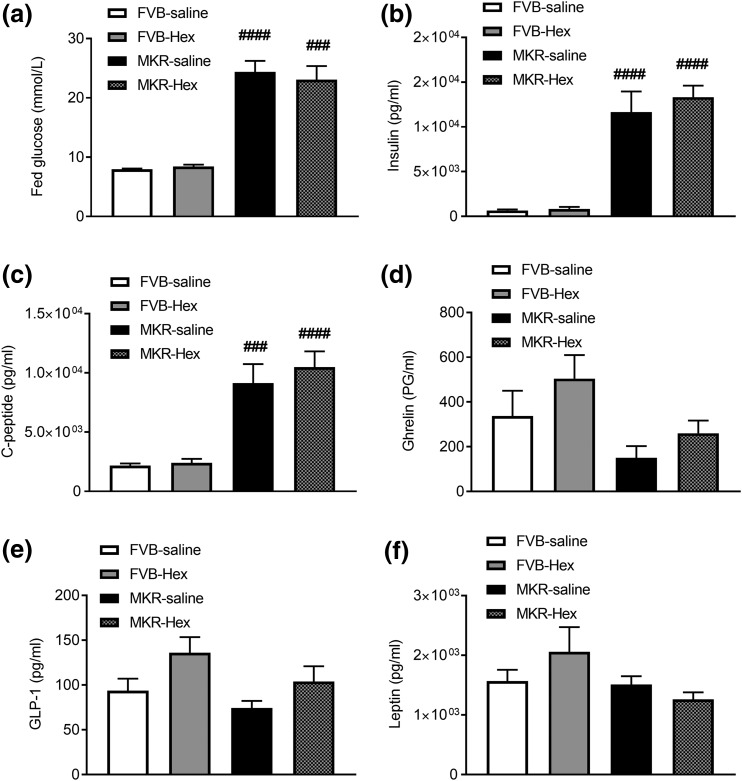
Treating MKR mice with hexarelin for 12 days did not change fed blood glucose, plasma levels of insulin, C-peptide, or leptin [Fig. 4(a–c) and 4(f), respectively]. However, plasma ghrelin and GLP-1 showed slight increases after hexarelin treatment in both FVB and MKR mice but did not reach statistical significance [Fig. 4(d) and 4(e)].
Figure 4.
The effects of hexarelin treatment on (a) blood glucose, (b) plasma concentrations of insulin, (c) C-peptide, (d) ghrelin, (e) GLP-1, and (f) leptin at the end of treatment from terminal blood samples during fed conditions. Data are expressed as mean ± SEM (n = 4 to 6 per group). ###P < 0.001, ####P < 0.0001 vs FVB saline group.
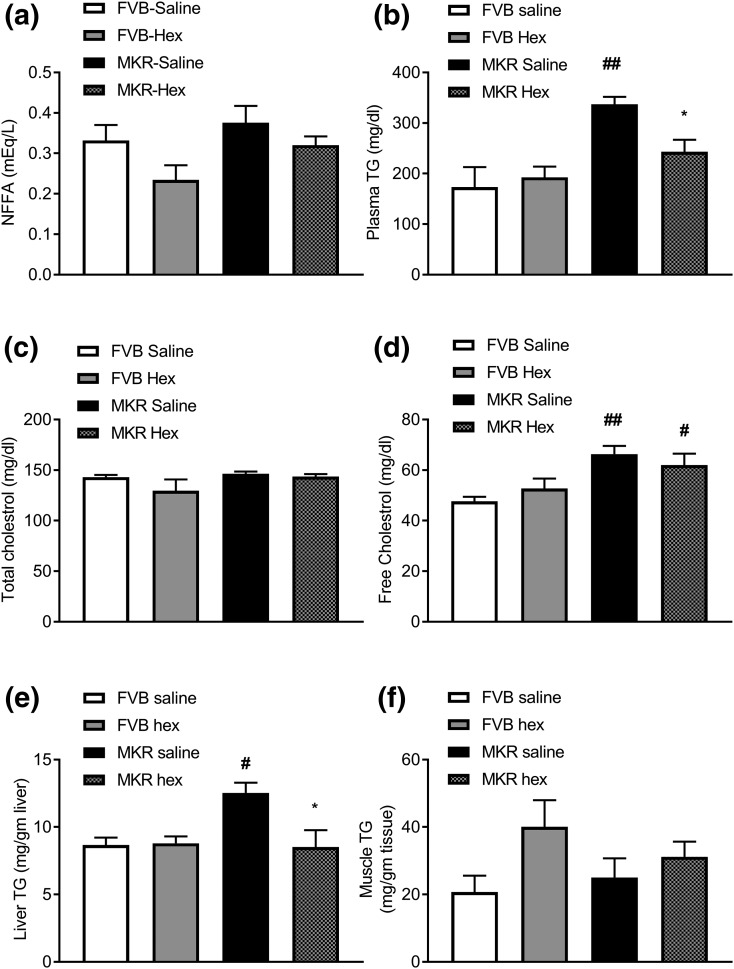
Effects of hexarelin treatment on plasma lipids and tissue TGs
Compared with FVB mice, MKR mice had nearly double plasma TGs [Fig. 5(b)], a 39% increase in free cholesterol [Fig. 5(d)], and a 44% increase in liver TGs [Fig. 5(e)], indicating the presence of hyperlipidemia and fatty liver in these mice. However, MKR mice exhibited similar plasma nonesterified fatty acid, total cholesterol, and muscle TGs to those in FVB mice [Fig. 5(a), 5(c), and 5(f), respectively]. Hexarelin treatment significantly reduced plasma TGs by 28% [P = 0.0147, Fig. 5(b)] and liver TGs by 32% [P = 0.0112, Fig. 5(e)] in MKR mice than those in vehicle-treated MKR mice. Hexarelin treatment did not change plasma FFAs, total cholesterol, or free cholesterol [Fig. 5(a), 5(c), and 5(d), respectively] in FVB and MKR mice. Thus, improved insulin sensitivity in MKR mice in response to hexarelin treatment may be due to the reduced TG levels observed in plasma and liver.
Figure 5.
Effects of hexarelin treatment on (a) plasma FFAs, (b) plasma TGs, (c) plasma total cholesterol, and (d) plasma free cholesterol. Quantitative analyses of TGs in (e) liver and (f) muscles. Data are expressed as mean ± SEM (n = 4 to 7 per group). *P < 0.05 vs MKR saline group; #P < 0.05, ##P < 0.01 vs FVB saline group.
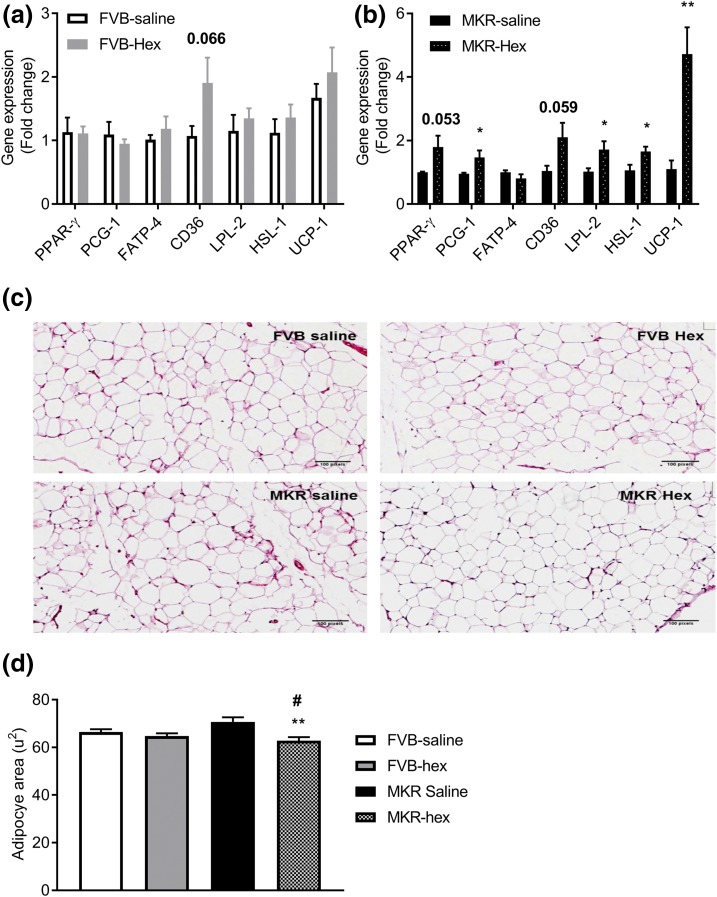
Effects of hexarelin treatment on the genes involved in fatty acid uptake and oxidative phosphorylation of WAT
Several genes required for fatty acid transport and oxidation in WAT, such as PGC-1, LPL, HSL, and UCP-1, were significantly upregulated by hexarelin treatment in MKR mice [Fig. 6(b)]. PPAR-γ and CD36 did not reach, but very close to, statistical significance (P = 0.053 and 0.059, respectively). However, in FVB mice, only CD36 was slightly increased by hexarelin treatment [P = 0.066, Fig. 6(a)]. These data, combined with indirect calorimetry data [Fig. 3(f)], further supported the conclusion that hexarelin treatment might result in the enhancement of lipid metabolism in MKR mice.
Figure 6.
Fold changes in gene expression levels of PPAR-γ, PCG-1, FATP-4, CD36, LPL, HSL, and UCP-1 of WAT of (a) FVB and (b) MKR mice after treatment. The data were calculated using the 2−ΔΔCT method. The results are presented as mean ± SEM (n = 5 to 6 per group). *P < 0.05, **P < 0.01 vs MKR saline group. (c) Hematoxylin and eosin staining of the gonadal fat pads from MKR and FVB mice treated with saline or hexarelin 12 days. Original magnification, ×10; scale bars, 100 μm. (d) Quantitative analysis of mean adipocytes area from 25 adipocytes per mouse. Data are expressed as mean ± SEM (n = 3 per group). **P < 0.01 vs MKR saline group. #P < 0.05 vs FVB saline group.
Effects of hexarelin treatment on adipocyte morphology and size
Histological examination revealed that the average adipocyte size was 8% larger in MKR mice compared with FVB mice [Fig. 6(c) and 6(d)]. Hexarelin treatment markedly reduced the size of adipocytes in MKR mice. The average size of adipocytes from hexarelin-treated MKR mice was 11% smaller than that from vehicle-treated MKR mice [Fig. 6(c) and 6(d)]. There was no significant change in the adipocyte size in FVB mice after hexarelin treatment. These results indicate a possible prodifferentiation effect of hexarelin treatment on adipocytes in MKR mice that could be linked to PPAR-γ activation.
Discussion
In this study, we demonstrated that hexarelin ameliorated the lipid metabolic abnormalities, along with improvement in glucose and insulin intolerances, in nonobese insulin-resistant MKR mice, in which lipotoxicity played a significant role in the exacerbation of insulin resistance (46). Following 12 days with hexarelin treatment, there was a significant reduction of plasma TGs in MKR mice, but total cholesterol levels remained unchanged (Fig. 5). This may be due to the increase in the ratio of high-density lipoprotein/low-density lipoprotein cholesterols. In fact, hexarelin suppressed high lipid diet and vitamin D3–induced atherosclerosis in rats, through probably an increase in the high-density lipoprotein/low-density lipoprotein ratio (47). Additionally, hexarelin treatment resulted in a decrease in hepatic TGs in MKR mice [Fig. 5(e)]. This effect may be secondary to the enhancement of fat metabolism, which prevents excessive flux of fatty acids to the liver. Additionally, direct stimulation of CD36 in the liver may lead to improved hepatic insulin sensitivity (48). Unlike the liver, TG content in muscle remained unchanged. This may be due to a lack of functional signaling pathways used by the mutated IGF-1 receptor in skeletal muscle of MKR mice. Hepatic TG content is directly correlated with fasting endogenous hepatic glucose production (49). Thus, lowering fasting blood glucose noticed during ITTs after hexarelin treatment in MKR mice could be related to improvement of liver insulin sensitivity. However, fed blood glucose depends mainly on muscle glucose uptake and insulin secretion from the pancreas (50). In this study, there were no observable changes in muscle TGs, plasma insulin, or C-peptide levels after hexarelin treatment.
It is well known that hexarelin boosts GH secretion in humans and other species (51–53). We also showed that administration of hexarelin induced a transient increase in GH secretion, in both MKR and FVB mice after injection [Fig. 1(e)]. A previous study showed that loss of muscle GH receptor signaling in MKR mice did not improve their glucose homeostasis or insulin sensitivity (54). Exogenous GH treatment was reported to improve lipid parameters in some lipodystrophy patients (55, 56). In the present study, we also reported that hexarelin treatment in MKR mice resulted in the correction of body composition abnormality as illustrated by decreasing fat mass and increasing lean mass [Fig. 2(e) and 2(f)]. However, it is not known whether hexarelin-induced GH response contributes to the correction of abnormal body composition observed in MKR mice.
According to our results, the possible mechanism by which hexarelin improved glucose and insulin tolerances is likely through enhancement of lipid metabolism via CD36 and PPAR-γ activation. In this study, activation of PPAR-γ was indicated by upregulation of its transcription coactivator (PCG-1α) and the increase in the expression of other genes that facilitate entry and acylation of FFAs in adipocytes, including CD36 and LPL [Fig. 6(b)]. In line with our results, hexarelin was reported to induce the gene expression profiles of fatty acid metabolism in cultured adipocytes and in mouse adipose tissue through CD36 (27). Additionally, the in vivo effects of hexarelin were absent in CD36-null mice, a finding that confirms that hexarelin action requires CD36 receptor (57). Interestingly, when MKR mice were crossed with mice overexpressing CD36 in skeletal muscle, the double-transgenic MKR/CD36 mice showed a normalization of hyperglycemia and hyperinsulinemia, as well as a marked improvement in muscle fatty acid metabolism (58). These findings support our hypothesis that hexarelin through activation of CD36 and PPAR-γ receptors may correct the lipid metabolic derangements associated with insulin resistance.
Additionally, hexarelin treatment enhanced thermogenic gene expression of UCP-1 [Fig. 6(b)]. UCP-1 is the hallmark of thermogenesis and browning of WAT. Recent studies showed that browning of WAT had beneficial metabolic effects (59). Transgenic mice overexpressing UCP-1 in their skeletal muscle or WAT develop a resistance to diet-induced obesity and type 2 diabetes, and they have a marked stimulation of fatty acid oxidation in muscles (60). Tiraby et al. (61) reported that the adenovirus-mediated expression of human PGC-1α increased the expression of UCP-1, respiratory chain proteins, and fatty acid oxidation enzymes in human subcutaneous white adipocytes. Thus, changes in the expression of genes in this study were consistent with browning adipocyte messenger RNA expression profile. This is inconsistent with a previous study that showed that hexarelin induced the expression of genes associated with fatty acid oxidation and browning of differentiated 3T3-L1 adipocytes (62).
Moreover, the adipose tissues from hexarelin-treated MKR mice showed a reduction in the sizes of adipocytes [Fig. 6(c)]. Recent reports have implied the correlation between adipocyte size and insulin sensitivity (63, 64). The hypertrophic adipocytes become oversaturated with lipids and exhibit changes in the secreted adipokines (65). Accordingly, lipids that cannot be stored in the engorged adipocytes may deposit ectopically in other organs such as liver and muscle. Activation of PPAR-γ reduces the size of adipocytes in WAT and increases adipocyte ability to actively take up and efficiently retain lipid (66). Consistent with our results, mature adipocytes treated with hexarelin showed a significant decrease in total lipid amount, compared with untreated cells (27). Such a decrease was comparable with cells treated with troglitazone, a specific PPAR-γ ligand (27).
The improvements in glucose and insulin tolerances with hexarelin treatment occurred without significant changes in circulating levels of fed blood glucose, plasma insulin, C-peptide, or leptin but slight increase in GLP-1 and ghrelin [Fig. 4]. In agreement with our findings, it was reported that plasma insulin and glucose concentrations did not change after hexarelin treatments in lean Zucker rats and humans (67, 68). Other studies showed that treatment of MKR mice with PPAR-γ agonists, such as rosiglitazone and pioglitazone, markedly improved lipid metabolic profiles and adipose tissue insulin sensitivity, yet failed to reverse hyperglycemia and hyperinsulinemia, similar to what observed in this study (69). Collectively, our results showed that hexarelin increased pulsatile GH secretion, reduced fat mass, and improved the lipid profile without compromising glucose metabolism in MKR mice.
Our results also demonstrated that hexarelin had potent orexigenic effects in MKR mice during the experimental period with no change in BW [Fig. 2(b) and 2(d)]. In agreement with our data, similar feeding and BW patterns have also been reported in healthy 24-month-old rats treated chronically with hexarelin for 8 weeks (39). However, note that in FVB mice, hexarelin did not show such an orexigenic effect. The underlying causes of such difference of the feeding response to hexarelin in FVB and MKR mice are not yet clear. In this study, we showed the effects of hexarelin in a nonobese insulin-resistant mouse model on energy balance and locomotor activity during chronic treatment. It was demonstrated that RER was decreased during dark cycles in hexarelin-treated MKR mice [Fig. 3(f)], further indicating an increased lipid utilization. These findings explain well that hexarelin treatment caused an increase in food intake without a related increase in BW. Additionally, hexarelin treatment tended to increase locomotor activity in both FVB and MKR mice, contributing to the energy balance by enhancing activity thermogenesis. Recent studies suggest that enhancement of spontaneous physical activity is important to counteract the sedentary lifestyle and lack of voluntary exercise, which are the major contributors of adiposity and metabolic syndrome (70).
In summary, hexarelin, a GH secretagogue as well as a CD36 ligand, could ameliorate dyslipidemia in nonobese insulin-resistant MKR mice. This was alongside improvement in glucose and insulin intolerances, as well as correction of body composition. Besides the efficacy in the prevention of heart diseases as indicated by previous studies, outcomes from the present study suggest that hexarelin can be used as a potential therapeutic drug in the treatment of dyslipidemia-associated metabolic syndrome. A limitation of this study is the use of a transgenic insulin-resistant mouse model, which may not completely replicate the human conditions. Thus, further investigations of longer duration regimens are essential to confirm hexarelin efficacy in clinical trials of relevant patient populations.
Acknowledgments
We thank Melanie Flint (PhenoMaster Facility, University of Queensland) for help with Phenomaster Experiments.
Financial Support: This work is funded by a National Health and Medical Research Council grant (to C.C.), the University of Queensland (to C.C.), and National Institutes of Health Grant 2RO1CA128799-06A1 (to D.L.). R.M. is supported by Egyptian Higher Education Ministry scholarships. Y.W. is supported by the F.G. Meade Scholarship of the University of Queensland.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BW
- body weight
- cDNA
- complementary DNA
- FFA
- free fatty acid
- FVB hex
- FVB mice injected with hexarelin
- FVB saline
- FVB mice injected with saline
- GH
- growth hormone
- GTT
- glucose tolerance test
- IGF
- insulinlike growth factor
- IP
- intraperitoneal(ly)
- ITT
- insulin tolerance test
- MKR hex
- MKR mice injected with hexarelin
- MKR saline
- MKR mice injected with saline
- PBS
- phosphate-buffered saline
- PCR
- polymerase chain reaction
- PPAR-γ
- peroxisome proliferator-activated receptor γ
- RER
- respiratory exchange ratio
- RRID
- research resource identifier
- SEM
- standard error of the mean
- TG
- triglyceride
- WAT
- white adipose tissue.
References
- 1.Krentz AJ. Lipoprotein abnormalities and their consequences for patients with type 2 diabetes. Diabetes Obes Metab. 2003;5(Suppl 1):S19–S27. [DOI] [PubMed] [Google Scholar]
- 2.Yu C, Chen Y, Cline GW, Zhang D, Zong H, Wang Y, Bergeron R, Kim JK, Cushman SW, Cooney GJ, Atcheson B, White MF, Kraegen EW, Shulman GI. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277(52):50230–50236. [DOI] [PubMed] [Google Scholar]
- 3.Adams JM II, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes. 2004;53(1):25–31. [DOI] [PubMed] [Google Scholar]
- 4.Brunetti P. The lean patient with type 2 diabetes: characteristics and therapy challenge. Int J Clin Pract Suppl. 2007;(153):3–9. [DOI] [PubMed] [Google Scholar]
- 5.Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, Sellwood J, Ariga R, Thomas S, Francis J, Rodgers C, Clarke W, Sabharwal N, Antoniades C, Schneider J, Robson M, Clarke K, Karamitsos T, Rider O, Neubauer S. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol. 2016;68(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J Clin Endocrinol Metab. 2004;89(2):463–478. [DOI] [PubMed] [Google Scholar]
- 7.Robbins DC, Danforth E Jr, Horton ES, Burse RL, Goldman RF, Sims EA. The effect of diet on thermogenesis in acquired lipodystrophy. Metabolism. 1979;28(9):908–916. [DOI] [PubMed] [Google Scholar]
- 8.Reitman ML, Mason MM, Moitra J, Gavrilova O, Marcus-Samuels B, Eckhaus M, Vinson C. Transgenic mice lacking white fat: models for understanding human lipoatrophic diabetes. Ann N Y Acad Sci. 1999;892:289–296. [DOI] [PubMed] [Google Scholar]
- 9.Rubio-Cabezas O, Puri V, Murano I, Saudek V, Semple RK, Dash S, Hyden CS, Bottomley W, Vigouroux C, Magré J, Raymond-Barker P, Murgatroyd PR, Chawla A, Skepper JN, Chatterjee VK, Suliman S, Patch AM, Agarwal AK, Garg A, Barroso I, Cinti S, Czech MP, Argente J, O’Rahilly S, Savage DB; LD Screening Consortium . Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med. 2009;1(5):280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morigny P, Houssier M, Mouisel E, Langin D. Adipocyte lipolysis and insulin resistance. Biochimie. 2016;125:259–266. [DOI] [PubMed] [Google Scholar]
- 11.Björntorp P, Berchtold P, Tibblin G. Insulin secretion in relation to adipose tissue in men. Diabetes. 1971;20(2):65–70. [DOI] [PubMed] [Google Scholar]
- 12.Weyer C, Foley JE, Bogardus C, Tataranni PA, Pratley RE. Enlarged subcutaneous abdominal adipocyte size, but not obesity itself, predicts type II diabetes independent of insulin resistance. Diabetologia. 2000;43(12):1498–1506. [DOI] [PubMed] [Google Scholar]
- 13.Dobbins RL, Szczepaniak LS, Bentley B, Esser V, Myhill J, McGarry JD. Prolonged inhibition of muscle carnitine palmitoyltransferase-1 promotes intramyocellular lipid accumulation and insulin resistance in rats. Diabetes. 2001;50(1):123–130. [DOI] [PubMed] [Google Scholar]
- 14.Seidell JC, Muller DC, Sorkin JD, Andres R. Fasting respiratory exchange ratio and resting metabolic rate as predictors of weight gain: the Baltimore Longitudinal Study on Aging. Int J Obes Relat Metab Disord. 1992;16(9):667–674. [PubMed] [Google Scholar]
- 15.Huang-Doran I, Sleigh A, Rochford JJ, O’Rahilly S, Savage DB. Lipodystrophy: metabolic insights from a rare disorder. J Endocrinol. 2010;207(3):245–255. [DOI] [PubMed] [Google Scholar]
- 16.Razak F, Anand SS. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. N Engl J Med 2004; 350: 664–71. Vasc Med. 2004;9(3):223–224. [DOI] [PubMed] [Google Scholar]
- 17.Liu C, Li C, Chen J, Liu Y, Cheng Q, Xiang X, Chen G. Effects of a very low-calorie diet on insulin sensitivity and insulin secretion in overweight/obese and lean type 2 diabetes patients. Diabetes Metab. 2015;41(6):513–515. [DOI] [PubMed] [Google Scholar]
- 18.Kohli R, Shevitz A, Gorbach S, Wanke C. A randomized placebo-controlled trial of metformin for the treatment of HIV lipodystrophy. HIV Med. 2007;8(7):420–426. [DOI] [PubMed] [Google Scholar]
- 19.Wanke C, Gerrior J, Kantaros J, Coakley E, Albrecht M. Recombinant human growth hormone improves the fat redistribution syndrome (lipodystrophy) in patients with HIV. AIDS. 1999;13(15):2099–2103. [DOI] [PubMed] [Google Scholar]
- 20.Sivakumar T, Mechanic O, Fehmie DA, Paul B. Growth hormone axis treatments for HIV-associated lipodystrophy: a systematic review of placebo-controlled trials. HIV Med. 2011;12(8):453–462. [DOI] [PubMed] [Google Scholar]
- 21.Arvat E, Ramunni J, Giordano R, Maccagno B, Broglio F, Benso A, Deghenghi R, Ghigo E. Effects of the combined administration of hexarelin, a synthetic peptidyl GH secretagogue, and hCRH on ACTH, cortisol and GH secretion in patients with Cushing’s disease. J Endocrinol Invest. 1999;22(1):23–28. [DOI] [PubMed] [Google Scholar]
- 22.Thomas A, Görgens C, Guddat S, Thieme D, Dellanna F, Schänzer W, Thevis M. Simplifying and expanding the screening for peptides <2 kDa by direct urine injection, liquid chromatography, and ion mobility mass spectrometry. J Sep Sci. 2016;39(2):333–341. [DOI] [PubMed] [Google Scholar]
- 23.Mao Y, Tokudome T, Kishimoto I. The cardiovascular action of hexarelin. J Geriatr Cardiol. 2014;11(3):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imazio M, Bobbio M, Broglio F, Benso A, Podio V, Valetto MR, Bisi G, Ghigo E, Trevi GP. GH-independent cardiotropic activities of hexarelin in patients with severe left ventricular dysfunction due to dilated and ischemic cardiomyopathy. Eur J Heart Fail. 2002;4(2):185–191. [DOI] [PubMed] [Google Scholar]
- 25.Broglio F, Guarracino F, Benso A, Gottero C, Prodam F, Granata R, Avogadri E, Muccioli G, Deghenghi R, Ghigo E. Effects of acute hexarelin administration on cardiac performance in patients with coronary artery disease during by-pass surgery. Eur J Pharmacol. 2002;448(2-3):193–200. [DOI] [PubMed] [Google Scholar]
- 26.Berti F, Muller E, De Gennaro Colonna V, Rossoni G.. Hexarelin exhibits protective activity against cardiac ischaemia in hearts from growth hormone-deficient rats. Growth Horm IGF Res. 1998;8(Suppl 2):149–152. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigue-Way A, Demers A, Ong H, Tremblay A. A growth hormone-releasing peptide promotes mitochondrial biogenesis and a fat burning-like phenotype through scavenger receptor CD36 in white adipocytes. Endocrinology. 2007;148(3):1009–1018. [DOI] [PubMed] [Google Scholar]
- 28.Garbacz WG, Lu P, Miller TM, Poloyac SM, Eyre NS, Mayrhofer G, Xu M, Ren S, Xie W. Hepatic overexpression of CD36 improves glycogen homeostasis and attenuates high-fat diet-induced hepatic steatosis and insulin resistance. Mol Cell Biol. 2016;36(21):2715–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buttet M, Poirier H, Traynard V, Gaire K, Tran TT, Sundaresan S, Besnard P, Abumrad NA, Niot I. Deregulated lipid sensing by intestinal CD36 in diet-induced hyperinsulinemic obese mouse model. PLoS One. 2016;11(1):e0145626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abumrad NA, el-Maghrabi MR, Amri EZ, Lopez E, Grimaldi PA. Cloning of a rat adipocyte membrane protein implicated in binding or transport of long-chain fatty acids that is induced during preadipocyte differentiation. Homology with human CD36. J Biol Chem. 1993;268(24):17665–17668. [PubMed] [Google Scholar]
- 31.Jayewardene AF, Gwinn T, Hancock DP, Mavros Y, Rooney KB. The associations between polymorphisms in the CD36 gene, fat oxidation and cardiovascular disease risk factors in a young adult Australian population: a pilot study. Obes Res Clin Pract. 2014;8(6):e618–e621. [DOI] [PubMed] [Google Scholar]
- 32.Qiao L, Zou C, Shao P, Schaack J, Johnson PF, Shao J. Transcriptional regulation of fatty acid translocase/CD36 expression by CCAAT/enhancer-binding protein α. J Biol Chem. 2008;283(14):8788–8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rangwala SM, Lazar MA. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol Sci. 2004;25(6):331–336. [DOI] [PubMed] [Google Scholar]
- 34.Varga T, Czimmerer Z, Nagy L.. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim Biophys Acta. 2011;1812(8):1007–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marleau S, Harb D, Bujold K, Avallone R, Iken K, Wang Y, Demers A, Sirois MG, Febbraio M, Silverstein RL, Tremblay A, Ong H. EP 80317, a ligand of the CD36 scavenger receptor, protects apolipoprotein E-deficient mice from developing atherosclerotic lesions. FASEB J. 2005;19(13):1869–1871. [DOI] [PubMed] [Google Scholar]
- 36.Fernández AM, Kim JK, Yakar S, Dupont J, Hernandez-Sanchez C, Castle AL, Filmore J, Shulman GI, Le Roith D. Functional inactivation of the IGF-I and insulin receptors in skeletal muscle causes type 2 diabetes. Genes Dev. 2001;15(15):1926–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vaitheesvaran B, LeRoith D, Kurland IJ. MKR mice have increased dynamic glucose disposal despite metabolic inflexibility, and hepatic and peripheral insulin insensitivity. Diabetologia. 2010;53(10):2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar A, Shiloach J, Betenbaugh MJ, Gallagher EJ. The β-3 adrenergic agonist (CL-316,243) restores the expression of down-regulated fatty acid oxidation genes in type 2 diabetic mice. Nutr Metab (Lond). 2015;12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bresciani E, Pitsikas N, Tamiazzo L, Luoni M, Bulgarelli I, Cocchi D, Locatelli V, Torsello A. Feeding behavior during long-term hexarelin administration in young and old rats. J Endocrinol Invest. 2008;31(7):647–652. [DOI] [PubMed] [Google Scholar]
- 40.Mao Y, Tokudome T, Kishimoto I, Otani K, Hosoda H, Nagai C, Minamino N, Miyazato M, Kangawa K. Hexarelin treatment in male ghrelin knockout mice after myocardial infarction. Endocrinology. 2013;154(10):3847–3854. [DOI] [PubMed] [Google Scholar]
- 41.Demange L, Boeglin D, Moulin A, Mousseaux D, Ryan J, Bergé G, Gagne D, Heitz A, Perrissoud D, Locatelli V, Torsello A, Galleyrand JC, Fehrentz JA, Martinez J. Synthesis and pharmacological in vitro and in vivo evaluations of novel triazole derivatives as ligands of the ghrelin receptor. 1. J Med Chem. 2007;50(8):1939–1957. [DOI] [PubMed] [Google Scholar]
- 42.Steyn FJ, Huang L, Ngo ST, Leong JW, Tan HY, Xie TY, Parlow AF, Veldhuis JD, Waters MJ, Chen C. Development of a method for the determination of pulsatile growth hormone secretion in mice. Endocrinology. 2011;152(8):3165–3171. [DOI] [PubMed] [Google Scholar]
- 43.Norris AW, Chen L, Fisher SJ, Szanto I, Ristow M, Jozsi AC, Hirshman MF, Rosen ED, Goodyear LJ, Gonzalez FJ, Spiegelman BM, Kahn CR. Muscle-specific PPARγ-deficient mice develop increased adiposity and insulin resistance but respond to thiazolidinediones. J Clin Invest. 2003;112(4):608–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmittgen TD, Lee EJ, Jiang J, Sarkar A, Yang L, Elton TS, Chen C. Real-time PCR quantification of precursor and mature microRNA. Methods. 2008;44(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berry R, Church CD, Gericke MT, Jeffery E, Colman L, Rodeheffer MS. Imaging of adipose tissue. Methods Enzymol. 2014;537:47–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao H, Yakar S, Gavrilova O, Sun H, Zhang Y, Kim H, Setser J, Jou W, LeRoith D. Phloridzin improves hyperglycemia but not hepatic insulin resistance in a transgenic mouse model of type 2 diabetes. Diabetes. 2004;53(11):2901–2909. [DOI] [PubMed] [Google Scholar]
- 47.Pang J, Xu Q, Xu X, Yin H, Xu R, Guo S, Hao W, Wang L, Chen C, Cao JM. Hexarelin suppresses high lipid diet and vitamin D3-induced atherosclerosis in the rat. Peptides. 2010;31(4):630–638. [DOI] [PubMed] [Google Scholar]
- 48.Goudriaan JR, Dahlmans VE, Teusink B, Ouwens DM, Febbraio M, Maassen JA, Romijn JA, Havekes LM, Voshol PJ. CD36 deficiency increases insulin sensitivity in muscle, but induces insulin resistance in the liver in mice. J Lipid Res. 2003;44(12):2270–2277. [DOI] [PubMed] [Google Scholar]
- 49.Ravikumar B, Gerrard J, Dalla Man C, Firbank MJ, Lane A, English PT, Cobelli C, Taylor R. Pioglitazone decreases fasting and postprandial endogenous glucose production in proportion to decrease in hepatic triglyceride content. Diabetes. 2008;57(9):2288–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jensen J, Rustad PI, Kolnes AJ, Lai YC. The role of skeletal muscle glycogen breakdown for regulation of insulin sensitivity by exercise. Front Physiol. 2011;2:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ghigo E, Arvat E, Gianotti L, Imbimbo BP, Lenaerts V, Deghenghi R, Camanni F. Growth hormone-releasing activity of hexarelin, a new synthetic hexapeptide, after intravenous, subcutaneous, intranasal, and oral administration in man. J Clin Endocrinol Metab. 1994;78(3):693–698. [DOI] [PubMed] [Google Scholar]
- 52.Rigamonti AE, Cella SG, Marazzi N, Müller EE. Six-week treatment with hexarelin in young dogs: evaluation of the GH responsiveness to acute hexarelin or GHRH administration, and of the orexigenic effect of hexarelin. Eur J Endocrinol. 1999;141(3):313–320. [DOI] [PubMed] [Google Scholar]
- 53.Guillaume V, Magnan E, Cataldi M, Dutour A, Sauze N, Renard M, Razafindraibe H, Conte-Devolx B, Deghenghi R, Lenaerts V. Growth hormone (GH)-releasing hormone secretion is stimulated by a new GH-releasing hexapeptide in sheep. Endocrinology. 1994;135(3):1073–1076. [DOI] [PubMed] [Google Scholar]
- 54.Vijayakumar A, Buffin NJ, Gallagher EJ, Blank J, Wu Y, Yakar S, LeRoith D. Deletion of growth hormone receptors in postnatal skeletal muscle of male mice does not alter muscle mass and response to pathological injury. Endocrinology. 2013;154(10):3776–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Macallan DC, Baldwin C, Mandalia S, Pandol-Kaljevic V, Higgins N, Grundy A, Moyle GJ. Treatment of altered body composition in HIV-associated lipodystrophy: comparison of rosiglitazone, pravastatin, and recombinant human growth hormone. HIV Clin Trials. 2008;9(4):254–268. [DOI] [PubMed] [Google Scholar]
- 56.Bedimo R. Growth hormone and tesamorelin in the management of HIV-associated lipodystrophy. HIV AIDS (Auckl). 2011;3:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demers A, McNicoll N, Febbraio M, Servant M, Marleau S, Silverstein R, Ong H. Identification of the growth hormone-releasing peptide binding site in CD36: a photoaffinity cross-linking study. Biochem J. 2004;382(Pt 2):417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Héron-Milhavet L, Haluzik M, Yakar S, Gavrilova O, Pack S, Jou WC, Ibrahimi A, Kim H, Hunt D, Yau D, Asghar Z, Joseph J, Wheeler MB, Abumrad NA, LeRoith D. Muscle-specific overexpression of CD36 reverses the insulin resistance and diabetes of MKR mice. Endocrinology. 2004;145(10):4667–4676. [DOI] [PubMed] [Google Scholar]
- 59.Bargut TC, Souza-Mello V, Aguila MB, Mandarim-de-Lacerda CA. Browning of white adipose tissue: lessons from experimental models [published online ahead of print 18 January 2017]. Horm Mol Biol Clin Investig. doi: 10.1515/hmbci-2016-0051. [DOI] [PubMed]
- 60.Dalgaard LT, Pedersen O. Uncoupling proteins: functional characteristics and role in the pathogenesis of obesity and type II diabetes. Diabetologia. 2001;44(8):946–965. [DOI] [PubMed] [Google Scholar]
- 61.Tiraby C, Tavernier G, Lefort C, Larrouy D, Bouillaud F, Ricquier D, Langin D. Acquirement of brown fat cell features by human white adipocytes. J Biol Chem. 2003;278(35):33370–33376. [DOI] [PubMed] [Google Scholar]
- 62.Demers A, Rodrigue-Way A, Tremblay A.. Hexarelin signaling to PPARγ in metabolic diseases. PPAR Res. 2008;2008:364784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–451. [DOI] [PubMed] [Google Scholar]
- 64.Matsubara Y, Kano K, Kondo D, Mugishima H, Matsumoto T. Differences in adipocytokines and fatty acid composition between two adipocyte fractions of small and large cells in high-fat diet-induced obese mice. Ann Nutr Metab. 2009;54(4):258–267. [DOI] [PubMed] [Google Scholar]
- 65.Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Albrektsen T, Frederiksen KS, Holmes WE, Boel E, Taylor K, Fleckner J. Novel genes regulated by the insulin sensitizer rosiglitazone during adipocyte differentiation. Diabetes. 2002;51(4):1042–1051. [DOI] [PubMed] [Google Scholar]
- 67.De Gennaro-Colonna V, Rossoni G, Cocchi D, Rigamonti AE, Berti F, Muller EE. Endocrine, metabolic and cardioprotective effects of hexarelin in obese Zucker rats. J Endocrinol. 2000;166(3):529–536. [DOI] [PubMed] [Google Scholar]
- 68.Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86(10):5083–5086. [DOI] [PubMed] [Google Scholar]
- 69.Kim H, Haluzik M, Gavrilova O, Yakar S, Portas J, Sun H, Pajvani UB, Scherer PE, LeRoith D. Thiazolidinediones improve insulin sensitivity in adipose tissue and reduce the hyperlipidaemia without affecting the hyperglycaemia in a transgenic model of type 2 diabetes. Diabetologia. 2004;47(12):2215–2225. [DOI] [PubMed] [Google Scholar]
- 70.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, Kane PH, Jensen MD, Clark MM. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307(5709):584–586. [DOI] [PubMed] [Google Scholar]