Abstract
The increased prevalence of obesity and its cardiometabolic implications demonstrates the imperative to identify novel therapeutic targets able to effect meaningful metabolic changes in this population. Antibody-mediated targeting of fibroblast growth factor receptor 1c isoform (FGFR1c) has been shown to ameliorate hyperglycemia and protect from diet- and genetically-induced obesity in rodents and nonhuman primates. However, it is currently unknown which tissue(s) contribute to this glucose-lowering effect. Thus, to elucidate this effect, we treated euglycemic mice with H7, a monoclonal antibody that selectively targets FGFR1c, and used whole-body positron emission computed tomography with a glucose tracer (18F-fluorodeoxyglucose). Treatment with H7 increased basal glucose uptake in white adipose tissue (WAT), brown adipose tissue (BAT), the brain, and liver but reduced it in the quadriceps muscles. Consequentially, blood glucose was significantly reduced in response to treatment. Under insulin-stimulated conditions, the effects of H7 were maintained in WAT, BAT, liver, and muscle. Treatment with H7 decreased triglyceride (TG) content and increased adipose TG lipase content in white adipose tissue, while increasing activation of acetyl coenzyme A carboxylase, suggesting futile cycling of TGs, albeit favoring net hydrolysis. We demonstrated, in vitro, this is a direct effect of treatment in adipose tissue, as basal cellular respiration and glucose uptake were increased in response to treatment. Taken together, these data suggest that antibody-mediated targeting of FGFR1c exerts its powerful glucose-lowering efficacy primarily due to increased glucose uptake in adipose tissue.
Targeting the FGFR1c isoform reduces blood glucose via increased uptake in adipose tissue. This is a direct effect of treatment and appears to lead to a futile (re)cycling of triglyceride content.
The fibroblast growth factor (FGF) family is a diverse array of autocrine, paracrine, and endocrine factors reported to play key roles in the development, growth, and metabolic homeostasis (1). The activity of FGFs is dependent upon engagement of tyrosine kinase FGF receptors (FGFRs), with the FGFR1c isoform being proposed to be the primary FGFR accounting for the metabolic actions of the so-called endocrine family of FGFs (2, 3). In accordance with its metabolic mode of action, the FGFR1c is expressed by several metabolically active organs, including white adipose tissue (WAT) and brown adipose tissue (BAT), as well as the hypothalamus (4), an area of the central nervous system associated with regulation of appetite. Recently, with the aim of understanding the role of the FGFR1c in the regulation of energy homeostasis, we used peripheral and central administration of a monoclonal antibody (H7) that selectively targets this isoform. We found that targeting of FGFR1c in the Siberian hamster, a natural model of adiposity, results in a profound antiobesogenic phenotype, accompanied by a substantial reduction in fed blood glucose levels and a clear suppression of daily food intake (5). In support of these data, administration of H7 to mouse models of obesity and diabetes reduces body weight, fat mass, blood glucose levels, and insulin resistance (6–8). However, it is unknown which tissue(s) are responsible for this antidiabetic activity. Mice with adipose-selective FGFR1 knockout are refractory to the antidiabetic activity of FGF21, a natural ligand for FGFR1c (8). Therefore, it appears that adipose tissue might be an important target for H7. Thus, the aim of this study was to ascertain the tissue-specific glucose uptake effects in response to peripherally targeting FGFR1c under basal and insulin-stimulated conditions, with particular emphasis on WAT and BAT utilizing in vivo and in vitro approaches. To account for any adverse effects of obesity-induced insulin resistance on tissue glucose uptake, a lean euglycemic animal model was selected to fulfill the objective of this study.
Research Design and Methods
Animals and experimental protocol
All studies were approved by the University of Nottingham Animal Welfare and Ethical Review Board and were carried out in accordance with the UK Animals (Scientific Procedures) Act of 1986 (project license PPL 40/3604). Age-matched adult male C57BL/6 euglycemic mice were randomly divided into one of two treatment groups in which they received a single subcutaneous injection of either vehicle (n = 8) or H7 (n = 8; 3 mg/kg), as previously described (6). After 24 hours, a subset of animals from each treatment group (n = 4 per group) were transferred to metabolic cages (Comprehensive Laboratory Animal Monitoring System; Linton Instrumentation, Linton, United Kingdom; Columbus Instruments, Columbus, OH) to measure daily energy expenditure. Seventy-two hours post injection, following a 4-hour fast, all animals were transferred to the nano–positron emission tomography/computed tomography (PET/CT) imaging unit (Mediso Medical Imaging Systems, Budapest, Hungary) to quantify glucose uptake in response to intraperitoneal infusion of 18F-fluorodeoxyglucose (18F-FDG) (10 MBq per animal) under basal (n = 4 per treatment) and insulin-stimulated conditions (subcutaneous injection, 0.75 U/kg, 10 minutes post 18F-FDG infusion, n = 4 per treatment). All scans lasted 60 minutes and were initiated 10 minutes post infusion of 18F-FDG. The magnitude of tissue-specific 18F-FDG uptake was expressed as the standard uptake value defined as the average 18F-FDG activity in each region of interest (in kBq/cm3) divided by the injected dose (kBq) and multiplied by the body weight of each animal (kg). Animals were subsequently euthanized, and blood was obtained, via cardiac puncture, to measure glucose (HemoCUE 201 System; HemoCUE, Angelholm, Sweden), plasma insulin, and tumor necrosis factor α (TNF-α) (Millipore, Billerica, MA). Triglyceride (TG) content of excised liver, adipose tissue, and muscle tissue was performed as previously described (9).
Mitochondrial function assay
Mouse 3T3-L1 fibroblasts (ATCC, Manassas, VA) were differentiated for 10 days and treated with vehicle, 10 nM immunoglobulin G1 (IgG1), or 10 nM H7 for 24 hours, followed by assessment of glucose uptake as previously described (10) and mitochondrial function via the mitochondrial stress test (Seahorse XF Cell Mito Stress Test Kit; part no. 103015-100; Agilent Technologies, Inc., Wilmington, DE) (n = 14 per treatment).
Western blotting
Proteins were extracted from WAT using a HEPES lysis buffer and quantified using the bicinchoninic acid protein assay (catalog no. 23227; Pierce, Thermo Fisher Scientific Inc., Waltham, MA). Western blotting was performed and resulting membranes were probed overnight at 4°C with primary antibodies (1:1000 dilution) to adipose TG lipase (ATGL; catalog no. ab109251; Abcam, Cambridge, UK), phospho-acetyl coenzyme A carboxylase (pACC; catalog no. 3661; New England Biolabs, Ipswich, MA), and cyclophilin B (CYC; 1:4000; catalog no. ab74173; Abcam). Blots were then washed and incubated for 1 hour with anti-rabbit (ATGL and pACC) and anti-mouse (CYC) horseradish peroxidase secondary antibody (Dako Denmark A/S, Glostrup, Denmark) at 1:2000 dilution. All immunoreactive proteins were detected using ECL Prime Western Blotting Detection Reagent (catalog no. RPN2232; GE Healthcare, Chicago, IL), quantified by densitometry using an Aida Image Analyzer (version 4.27), and normalized to CYC.
Statistical analysis
Descriptive statistics (mean ± standard error of the mean) were generated using GraphPad Prism (Prism 6.0; GraphPad, San Diego, CA). Body weight, food intake, and metabolic cage data were analyzed using two-way (treatment × time) repeated measures analysis of variance (ANOVA). Tissue weights and protein content, blood glucose, plasma insulin and TNF-α, tissue TG content, and 18F-FDG uptake were analyzed using a Student t test. All image analysis was performed blinded to treatment using VivoQUANT software (inviCRO, Boston, MA). Glucose uptake in adipocytes was analyzed using one-way ANOVA. Maximal cell oxygen consumption rate (OCR), analyzed using both the area under the curve and the peak values (oxidative capacity), basal cellular respiration, adenosine triphosphate (ATP) generation, and nonmitochondrial respiration were analyzed using a one-way ANOVA. Statistical significance was declared at P < 0.05.
Results
Body weight was decreased by approximately 14% compared with vehicle-treated controls (P < 0.0001). Similarly, food intake was reduced by 34% during the 72-hour experimental period (P < 0.0001), resulting in reduced visceral WAT weight (43%, P < 0.0001). Although there was a clear diurnal variation in whole-body energy expenditure in both vehicle- and H7-treated mice, there was no effect of acute treatment with H7 on energy expenditure during the final 24 hours of the experimental period.
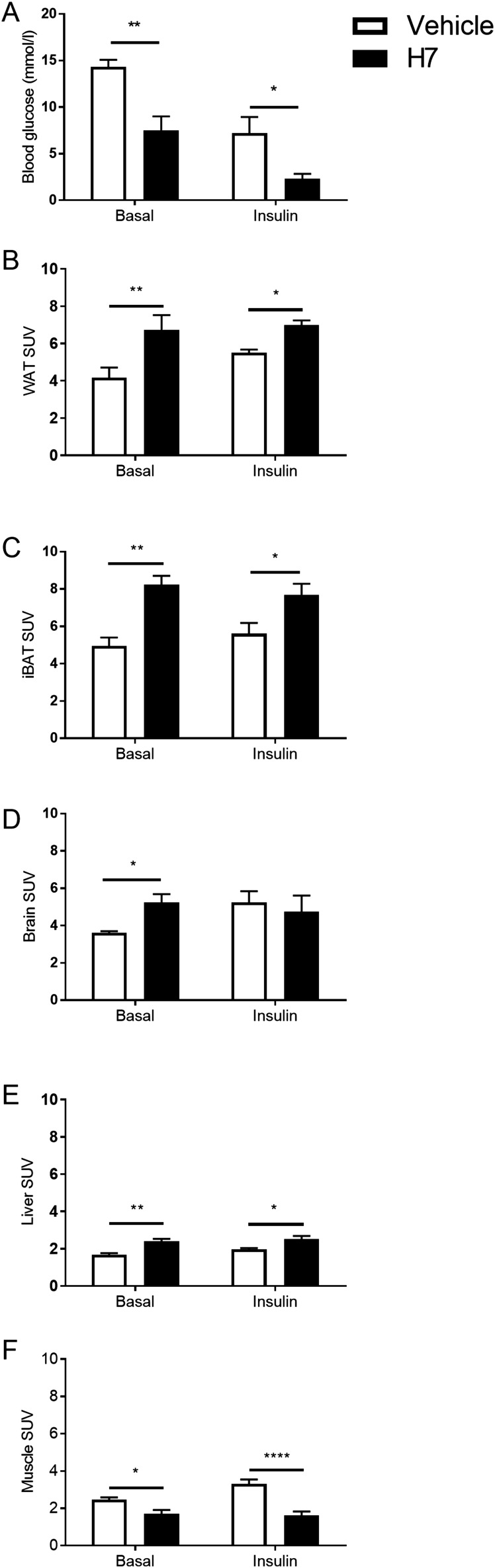
Treatment with H7 resulted in a 70% decrease in plasma insulin and a 50% decrease in TNF-α (P < 0.05, respectively). Similarly, basal blood glucose concentrations were also reduced by 53% in animals treated with H7 (Fig. 1A; P < 0.01), and they were further reduced (by 63%) in the insulin-stimulated group (Fig. 1A; P < 0.05).
Figure 1.
Effect of systemic treatment with a monoclonal antibody that targets the FGFR1c (H7) on blood glucose and basal and insulin-stimulated glucose uptake [expressed as standard uptake value (SUV)] in WAT, interscapular BAT (iBAT), brain, liver, and skeletal muscle in mice. (A) Blood glucose of adult male mice treated with vehicle or H7. Values are group mean ± standard error of the mean (SEM) (n = 4 per treatment). *P < 0.05, **P < 0.01 vs vehicle. (B–F) 18F-FDG uptake in (B) WAT, (C) iBAT, (D) brain, (E) liver, (F) and quadriceps muscles treated with vehicle or H7. Values are group mean ± SEM (n = 4 per treatment). *P < 0.05, **P < 0.01, ****P < 0.0001 vs vehicle.
In WAT and interscapular BAT, treatment increased 18F-FDG uptake compared with vehicle-treated controls (Fig. 1B and 1C; P < 0.01). This effect was maintained under insulin-stimulated conditions (Fig. 1B and 1C, P < 0.05). In the brain, under basal conditions, treatment with H7 significantly increased 18F-FDG uptake compared with vehicle-treated controls (Fig. 1D; P < 0.05). However, there was no difference under insulin-stimulated conditions (Fig. 1D). Similarly, treatment with H7 increased hepatic 18F-FDG uptake compared with vehicle-treated controls both under basal (Fig. 1E; P < 0.01) and insulin-stimulated conditions (P < 0.05). However, in the muscle, treatment with H7 reduced 18F-FDG uptake compared with vehicle-treated controls in both basal (Fig. 1F; P < 0.05) and insulin-stimulated conditions (Fig. 1F; P < 0.0001). Representative images are shown in Fig. 2.
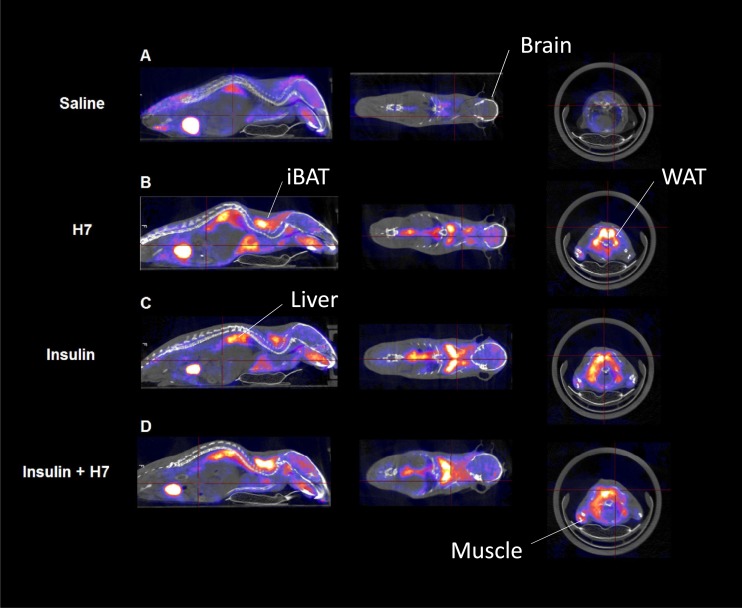
Figure 2.
Representative whole-body PET/CT images of euglycemic mice receiving 18F-FDG treated with (A) vehicle (saline), (B) H7 + vehicle (saline), (C) vehicle (saline) + insulin, and (D) H7 + insulin. iBAT, interscapular BAT.
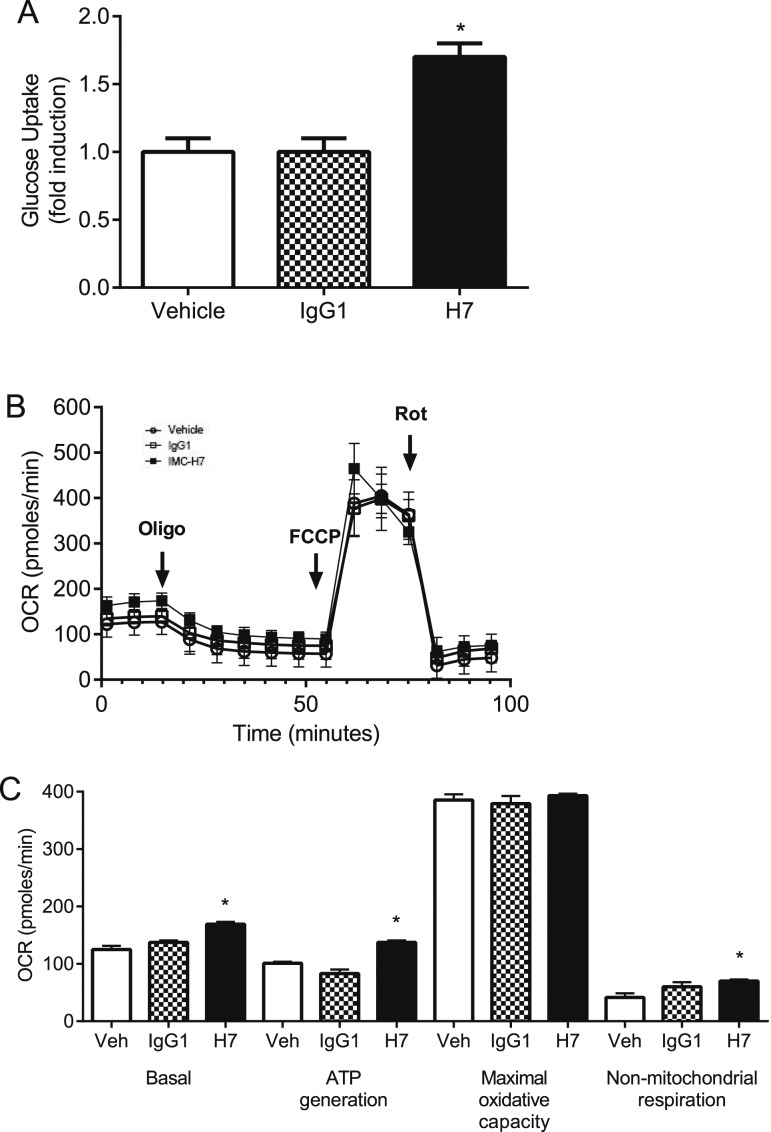
In line with in vivo data, treatment with H7 significantly increased glucose uptake into 3T3-L1 adipocytes when compared with vehicle and IgG1 (Fig. 3A; F = 16.33, P < 0.05), whereas there was no effect of treatment on maximal adipocyte OCR (oxidative capacity) (Fig. 3B and 3C). However, H7 increased (P < 0.05) basal cellular respiration, ATP generation, and nonmitochondrial respiration when compared with vehicle and IgG1 (Fig. 3C).
Figure 3.
In vitro treatment of adipocytes with a monoclonal antibody that targets the FGFR1c (H7) increases glucose uptake and basal respiration but not maximal OCR. (A) Glucose uptake in adipocytes treated with vehicle, IgG1, and H7. Values are group mean ± standard error of the mean (SEM) (n = 14 per treatment). *P < 0.05 vs vehicle/IgG1. (B) OCR in adipocytes treated with vehicle, IgG1, and H7. Values are group mean ± SEM (n = 14 per treatment). (C) Basal cellular respiration, ATP generation, maximal oxidative capacity, and nonmitochondrial respiration in adipocytes treated with vehicle, IgG1, and H7. Values are group mean ± SEM (n = 14 per treatment). *P < 0.05 vs vehicle and IgG1. FCCP, carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone; Oligo, oligomycin; Rot, rotenone plus antimycin; Veh, vehicle.
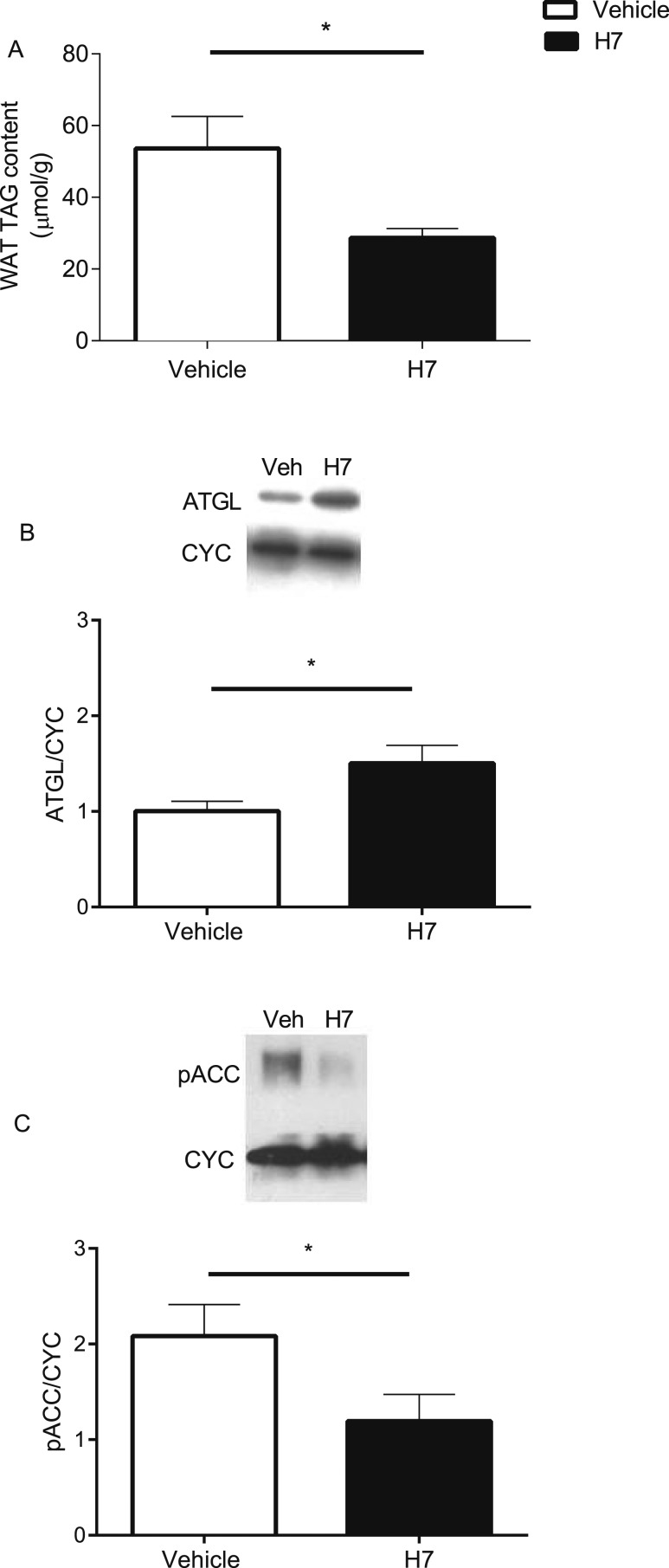
Hepatic (saline, 44.8 ± 1.7 µmol/g vs H7, 28.3 ± 1.4 µmol/g) and muscle (saline, 34.5 ± 1.1 µmol/g vs H7, 23.7 ± 1.8 µmol/g) TG content was also significantly reduced by 37% and 31%, respectively, (P < 0.05) in animals treated with H7. In WAT, TG content was significantly reduced (Fig. 4A; P < 0.05), ATGL protein content was significantly increased (Fig. 4B; P < 0.05), and pACC was significantly reduced (Fig. 4C; P < 0.05) in response to treatment with H7.
Figure 4.
Effect of systemic treatment with a monoclonal antibody that targets the FGFR1c (H7) on WAT TG and protein content. (A) WAT TG content treated with vehicle or H7. (B) ATGL and (C) pACC protein content of WAT treated with vehicle or H7. Representative blots included. Values are group mean ± SEM (n = 8 per treatment). *P < 0.05 vs vehicle.
Discussion
This study demonstrates that antibody-mediated targeting of FGFR1c exerts a potent glucose-lowering effect as a consequence of increased glucose uptake in white and brown adipose tissue. This effect is associated with increased basal cellular respiration, ATP generation, and nonmitochondrial respiration but not maximal oxygen consumption in adipocytes. However, there was no effect of treatment on whole-body energy expenditure, a possible consequence of contrasting changes in substrate utilization in other metabolically active tissues. Treatment with H7 was associated with increased markers of lipogenic (pACC) and lipolytic (ATGL) capacity in WAT, suggesting a futile (re)cycling of its TG content. Taken together, these data provide support for the notion that targeting this pathway in adipose tissue may have therapeutic potential for the treatment of metabolic syndrome (MetS).
It is now widely accepted that in addition to functioning as a daily buffer for circulating lipids, adipose tissue also plays a key role in the regulation of energy balance and glucose homeostasis via release of circulating metabolites and hormonal factors (11). Indeed, secreted adipokines have been investigated intensively for their roles in energy homeostasis (12) in an attempt to identify novel pathways for the treatment of obesity and its associated comorbidities. The endocrine FGFs have emerged as a novel family of hormonal factors that, when administered to rodents, have substantial antidiabetic activity (13). The downstream capabilities of FGF ligands are highly dependent upon engagement of FGFR1c, the predominant FGFR expressed in WAT and BAT (4). Indeed, mice with adipose-selective FGFR1 knockout were refractory to FGF21-induced improvements in glucose metabolism and body weight (8).
We previously reported that targeting FGFR1c in the Siberian hamster resulted in a profound antiobesogenic phenotype, with reduced blood glucose levels (5). Although this was supported by previous studies in mice (6), it was unknown which tissue(s) were responsible for this blood-glucose lowering effect and whether it was secondary to reduced food intake and body weight. The current study provides in vivo PET/CT data to indicate that H7-mediated lowering of blood glucose levels is predominantly associated with enhanced basal and insulin-stimulated glucose uptake in WAT and BAT. Although previous studies have shown that basal glucose uptake in WAT is low, under certain conditions (e.g., stimulation with insulin and compounds such as β-agonists), this increases (14). In WAT, the H7-mediated effect observed in the current study is presumably a consequence of accelerated rates of TG hydrolysis (as indicated by the reduction in WAT weight and TG content) coupled to resynthesis, requiring large amounts of ATP (15). This notion of futile (re)cycling is supported by the increase in the protein content of ATGL (the rate-limiting step in TG hydrolysis) and reduction in pACC (indicating increased activity of a key enzyme in de novo lipogenesis) in WAT in response to treatment with H7, albeit favoring net hydrolysis (as indicated by the reduction in TG content). This futile cycling of TG requires a significant amount of ATP, and the increase in basal cellular respiration and ATP generation in 3T3-L1 adipocytes in response to H7 treatment supports this mechanism. Furthermore, the increase in glucose uptake in vitro demonstrates a direct effect of treatment rather than a consequence of reduced food intake and body weight in vivo.
Although the primary role of BAT is to maintain body temperature during the adaptive response to cold exposure, since its discovery in adult humans, it has emerged as an attractive target organ for the treatment of MetS. BAT displays a very high rate of glucose uptake, particularly under sympathetic activation (16). Furthermore, it is known to exert anti–type 2 diabetes mellitus (T2D) effects associated with improvements of dyslipidemia and insulin secretion, as well as decreased insulin resistance in T2D (17). In the current study, basal glucose uptake in BAT was 20% higher than in WAT in response to treatment. Furthermore, treatment was associated with reduced plasma insulin and TNF-α. Increasing BAT activity has advantageous effects on body composition and insulin sensitivity in mice, which suggests that BAT is an endocrine organ that can function to improve whole-body and tissue glucose homeostasis, effects mediated by TNF-α, interleukin-6, and the glucose transporter GLUT1, which is known to regulate insulin-independent glucose uptake (18, 19). It is likely that the redirection of glucose to energy combusting BAT without further storage in skeletal muscle (which showed a reduction in glucose uptake in response to treatment with H7) may contribute to the weight loss and insulin-sensitizing effect of H7. Overall, our data provides evidence that adipose tissue plays a dominant role in mediating the effects of targeting the FGFR1c, revealing the clinical potential of this pathway for the treatment of T2D and other chronic disorders linked to MetS and obesity.
Unlike adipose tissue, liver and skeletal muscle are not likely to be direct targets tissues for the H7 actions. Indeed, severe energy restriction (that is accompanied by weight loss of similar magnitude to the one observed in this study) leads to altered hepatic glucose uptake/metabolism and a shift in substrate utilization in skeletal muscle toward fat oxidation with a concomitant reduction in the oxidation of glucose and the development of insulin resistance in that tissue (20). Future investigations are also required to determine the precise role of FGFR1c-mediated glucose uptake in the brain and possible link to H7-mediated appetite regulation.
Conclusion
The increasing prevalence of obesity and its comorbidities, including, but not limited to, T2D, are predicted to become two-thirds of the global burden of disease if current dietary and lifestyle trends continue (21). Existing therapies are unable to provide durable and robust change in metabolic health, and therefore it remains imperative to identify novel therapeutic agents able to effect meaningful change. The current study highlights the importance of adipose tissue FGFR1c as a target for an effective treatment strategy for MetS by reducing body weight, food intake, lipid accumulation, and thus lipotoxicity in adipose and nonadipose tissues, while lowering glycemia by enhancing glucose disposal primarily in adipose tissue (22–24).
Acknowledgments
Financial Support: This work was supported by the Biotechnology and Biological Sciences Research Council (Grants BB/M001555/1 and BB/M021629/1).
Acknowledgments
Disclosure Summary: R.J.S., P.J.E., and A.C.A. are employees of Eli Lilly and Company. The remaining authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (If Known) | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| ATGL | Anti-Adipose Triglyceride Lipase antibody [EPR3444(2)] | Abcam, ab109251 | Rabbit; monoclonal | 1:1000 | AB_10864772 | |
| pACC | Phospho-Acetyl-CoA Carboxylase (Ser79) antibody | New England Biolabs, 3661 | Rabbit; polyclonal | 1:1000 | AB_330337 | |
| CYC | Anti-Cyclophilin B antibody (k2E2) | Abcam, ab74173 | Mouse; monoclonal | 1:4000 | AB_1268387 |
Abbreviation: RRID, Research Resource Identifier.
Footnotes
- ANOVA
- analysis of variance
- ATGL
- adipose triglyceride lipase
- ATP
- adenosine triphosphate
- BAT
- brown adipose tissue
- CYC
- cyclophilin B
- 18F-FDG
- 18F-fluorodeoxyglucose
- FGF
- fibroblast growth factor
- FGFR
- fibroblast growth factor receptor
- FGFR1c
- fibroblast growth factor receptor 1c isoform
- IgG1
- immunoglobulin G1
- MetS
- metabolic syndrome
- OCR
- oxygen consumption rate
- pACC
- phospho-acetyl coenzyme A carboxylase
- PET/CT
- nano–positron emission tomography/computed tomography
- T2D
- type 2 diabetes mellitus
- TG
- triglyceride
- TNF-α
- tumor necrosis factor α
- WAT
- white adipose tissue.
References
- 1.Belov AA, Mohammadi M. Molecular mechanisms of fibroblast growth factor signaling in physiology and pathology. Cold Spring Harb Perspect Biol. 2013;5(6). doi:10.1101/cshperspect.a015958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams AC, Cheng CC, Coskun T, Kharitonenkov A. FGF21 requires βklotho to act in vivo. PLoS One. 2012;7(11):e49977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Suh JM, Jonker JW, Ahmadian M, Goetz R, Lackey D, Osborn O, Huang Z, Liu W, Yoshihara E, van Dijk T, Havinga R, Fan W, Yin Y-Q, Yu R, Liddle C, Atkins A, Olefsky J, Mohammadi M, Downes M, Evans R. Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature. 2014;513(7518):436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fon Tacer K, Bookout AL, Ding X, Kurosu H, John GB, Wang L, Goetz R, Mohammadi M, Kuro-o M, Mangelsdorf DJ, Kliewer SA. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24(10):2050–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samms RJ, Lewis JE, Lory A, Fowler MJ, Cooper S, Warner A, Emmerson P, Adams AC, Luckett JC, Perkins AC, Wilson D, Barrett P, Tsintzas K, Ebling FJ. Antibody-mediated inhibition of the FGFR1c isoform induces a catabolic lean state in siberian hamsters. Curr Biol. 2015;25(22):2997–3003. [DOI] [PubMed] [Google Scholar]
- 6.Sun HD, Malabunga M, Tonra JR, DiRenzo R, Carrick FE, Zheng H, Berthoud HR, McGuinness OP, Shen J, Bohlen P, Leibel RL, Kussie P. Monoclonal antibody antagonists of hypothalamic FGFR1 cause potent but reversible hypophagia and weight loss in rodents and monkeys. Am J Physiol Endocrinol Metab. 2007;292(3):E964–E976. [DOI] [PubMed] [Google Scholar]
- 7.Wu AL, Kolumam G, Stawicki S, Chen Y, Li J, Zavala-Solorio J, Phamluong K, Feng B, Li L, Marsters S, Kates L, van Bruggen N, Leabman M, Wong A, West D, Stern H, Luis E, Kim HS, Yansura D, Peterson AS, Filvaroff E, Wu Y, Sonoda J. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci Transl Med. 2011;3(113):113ra126. [DOI] [PubMed] [Google Scholar]
- 8.Foltz IN, Hu S, King C, Wu X, Yang C, Wang W, Weiszmann J, Stevens J, Chen JS, Nuanmanee N, Gupte J, Komorowski R, Sekirov L, Hager T, Arora T, Ge H, Baribault H, Wang F, Sheng J, Karow M, Wang M, Luo Y, McKeehan W, Wang Z, Véniant MM, Li Y. Treating diabetes and obesity with an FGF21-mimetic antibody activating the βKlotho/FGFR1c receptor complex. Sci Transl Med. 2012;4(162):162ra153. [DOI] [PubMed] [Google Scholar]
- 9.Samms RJ, Murphy M, Fowler MJ, Cooper S, Emmerson P, Coskun T, Adams AC, Kharitonenkov A, Ebling FJ, Tsintzas K. Dual effects of fibroblast growth factor 21 on hepatic energy metabolism. J Endocrinol. 2015;227(1):37–47. [DOI] [PubMed] [Google Scholar]
- 10.Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ, Sandusky G, Hammond L, Moyers J, Owens R, Gromada J, Brozinick J, Hawkins E, Wroblewski V, Li D-S, Mehrbod F, Jaskunas S, Shanafelt A. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115(6):1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frayn KN, Karpe F, Fielding BA, Macdonald IA, Coppack SW. Integrative physiology of human adipose tissue. Int J Obes Relat Metab Disord. 2003;27(8):875–888. [DOI] [PubMed] [Google Scholar]
- 12.Rondinone CM. Adipocyte-derived hormones, cytokines, and mediators. Endocrine. 2006;29(1):81–90. [DOI] [PubMed] [Google Scholar]
- 13.Degirolamo C, Sabbà C, Moschetta A. Therapeutic potential of the endocrine fibroblast growth factors FGF19, FGF21 and FGF23. Nat Rev Drug Discov. 2016;15(1):51–69. [DOI] [PubMed] [Google Scholar]
- 14.Park JW, Jung KH, Lee JH, Quach CH, Moon SH, Cho YS, Lee K. 18F-FDG PET/CT monitoring of β3 agonist-stimulated brown adipocyte recruitment in white adipose tissue. J Nucl Med. 2015;56(1):153–158. [DOI] [PubMed] [Google Scholar]
- 15.Mottillo EP, Balasubramanian P, Lee YH, Weng C, Kershaw EE, Granneman JG. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J Lipid Res. 2014;55(11):2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(1):277–359. [DOI] [PubMed] [Google Scholar]
- 17.Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature. 2014;510(7503):76–83. [DOI] [PubMed] [Google Scholar]
- 18.Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274(5291):1377–1379. [DOI] [PubMed] [Google Scholar]
- 19.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123(1):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsintzas K, Jewell K, Kamran M, Laithwaite D, Boonsong T, Littlewood J, Macdonald I, Bennett A. Differential regulation of metabolic genes in skeletal muscle during starvation and refeeding in humans. J Physiol. 2006;575(Pt 1):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tilman D, Clark M. Global diets link environmental sustainability and human health. Nature. 2014;515(7528):518–522. [DOI] [PubMed] [Google Scholar]
- 22.Betz MJ, Enerbäck S. Human brown adipose tissue: what we have learned so far. Diabetes. 2015;64(7):2352–2360. [DOI] [PubMed] [Google Scholar]
- 23.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122(2):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blondin DP, Labbe SM, Phoenix S, Guerin B, Turcotte EE, Richard D, Carpentier AC, Haman F. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2015;593(3):701–714. [DOI] [PMC free article] [PubMed] [Google Scholar]