Abstract
High doses of estrogenic pharmaceuticals were once prescribed to women to halt lactation. Yet, the effects of low-level xenoestrogens on lactation remain poorly studied. We investigated the effects of bisphenol S (BPS), an estrogen receptor (ER) agonist, on the lactating mammary gland; the arcuate nucleus, a region of the hypothalamus important for neuroendocrine control of lactational behaviors; and nursing behavior in CD-1 mice. Female mice were exposed to vehicle, 2 or 200 µg BPS/kg/d from pregnancy day 9 until lactational day (LD) 20, and tissues were collected on LD21. Tissues were also collected from a second group at LD2. BPS exposure significantly reduced the fraction of the mammary gland comprised of lobules, the milk-producing units, on LD21, but not LD2. BPS also altered expression of Esr1 and ERα in the mammary gland at LD21, consistent with early involution. In the arcuate nucleus, no changes were observed in expression of signal transducer and activator of transcription 5, a marker of prolactin signaling, or ERα, suggesting that BPS may act directly on the mammary gland. However, observations of nursing behavior collected during the lactational period revealed stage-specific effects on both pup and maternal nursing behaviors; BPS-treated dams spent significantly more time nursing later in the lactational period, and BPS-treated pups were less likely to initiate nursing. Pup growth and development were also stunted. These data indicate that low doses of BPS can alter lactational behaviors and the maternal mammary gland. Together, they support the hypothesis that pregnancy and lactation are sensitive to low-dose xenoestrogen exposures.
Exposures to bisphenol S, a common ingredient in many consumer goods, alter the lactating mouse mammary gland and lactation behaviors in females exposed during pregnancy and lactation.
During pregnancy, the mammary gland undergoes rapid development to support lactation, including extensive ductal branching and alveolar development. The mammary epithelial cells proliferate, producing alveolar buds that differentiate into lobuloalveolar units, the functional structures that secrete milk during the lactational period (1). Furthermore, vascularization surrounds the alveoli, which multiply to fill the fat pad, leading to a decline in adipose tissue in the gland (2). Hormones, most importantly progesterone and prolactin, mediate this developmental period (3). Progesterone controls branching and alveologenesis, the process of differentiation into lobuloalveolar units. At the end of pregnancy, estrogen levels spike and progesterone levels drop, initiating parturition. The loss of progesterone allows estrogen to stimulate the secretion of prolactin, promoting the final phase of differentiation in the mammary gland as well as milk synthesis and secretion (4).
Breast milk imparts a unique set of nutritional factors and immune complements from the mother to the nursing infant (5), providing protection against a range of childhood diseases that contribute to morbidity and mortality (6). At least eight pediatric diseases have been shown to be associated with insufficient breastfeeding, including acute lymphoblastic leukemia, ulcerative colitis, lower respiratory tract infections, necrotizing enterocolitis, and sudden infant death syndrome (7–11). Meta-analyses examining the long-term benefits of breastfeeding suggest it is associated with reduced rates of noncommunicable diseases in mothers, including hypertension and cancers (12–14). A number of factors influence the likelihood that a mother will initiate and maintain breastfeeding, including biomedical issues, psychosocial influences, interactions with health care providers and lactation support services, and socioeconomic forces, such as a need to return to work (15). Within 4 months of parturition, women most commonly report that insufficient milk supply is their reason for premature cessation of breastfeeding (16).
In the 1960s and 1970s, pharmaceutical treatments were provided to suppress lactation in women who elected not to breastfeed. Double-blind placebo-controlled trials demonstrated the efficacy of several high-dose hormonal treatments (17–19). For example, diethylstilbestrol, a potent synthetic estrogen, was commonly prescribed to women for lactational suppression (20–23). Further investigation suggests a mechanism whereby very high levels of estrogen inhibit the release of prolactin, thereby reducing milk production and release. Studies of rodents exposed to environmental chemicals indicate that these compounds can also disrupt lactation. In evaluations of exposed pregnant/lactating adult female mice, exposures to dioxin or perfluorooctanoic acid (PFOA), a common surfactant, altered mammary gland morphology, expression of milk proteins, and other measures of lactogenesis (24–26). Other studies have shown that female mice exposed to environmental chemicals during perinatal development display decreased patterns of lactogenesis, altered mammary gland morphology, decreased expression of milk proteins, changes in the expression of milk enzymes, and altered milk composition (24, 27, 28). Although high doses of xenoestrogens are anticipated to affect the lactating mammary gland, little is known about the effect of low doses, including those in the range of human exposures.
Bisphenol S (BPS) is an analog of bisphenol A (BPA), with similar biological activities; BPS is an estrogen receptor (ER) agonist (29, 30), and it also alters progesterone signaling (31). BPS began to be used in consumer products because it was considered a safe replacement for BPA due to its superior thermal stability. It has been detected in canned foods, thermal paper, plastics, personal care products, food cartons, and other consumer goods (32–36). Furthermore, human biomonitoring studies have reported detectable concentrations of BPS and its metabolites in humans, with exposures that vary depending on country of residence (37, 38). Median urinary concentrations are reported at 0.299 ng/mL in the United States and 1.18 ng/mL in Japan (37), with exposures that appear to be increasing over time (38).
Only a small number of studies have examined the effects of BPS in mammals (39–41). Our recent study revealed that low doses of BPS alter maternal behavior in female mice exposed during pregnancy and lactation, as well as in their daughters exposed during early development (42). The adult BPS-treated females also had a significant increase in ERα expression in the caudal subregion of the central medial preoptic area, a region of the brain critical for maternal care. In this study, we examined the effects of BPS on the lactating mammary gland, nursing behavior, pituitary, and arcuate nucleus, an ER-rich region in the hypothalamus important for the regulation of neuroendocrine functions, including behaviors associated with lactation (43). In addition to evaluating the effect of BPS on the mother, we also examined growth and developmental endpoints in the offspring. Our results suggest that BPS alters both mammary gland morphology and nursing behaviors in exposed adult female mice and development in their exposed offspring.
Materials and Methods
Animals
Timed pregnant female CD-1 mice (Charles River Laboratories, Raleigh, NC) were housed individually in polysulfone cages with food (ProLab IsoDiet, Brentwood, MO) and water (in glass bottles with rubber stoppers) provided ad libitum, as described previously (42). The animals were maintained at the University of Massachusetts, Amherst Central Animal Facility, in temperature- and light-controlled conditions (12 hours light, 12 hours dark). All experimental procedures were approved by the University of Massachusetts Institutional Animal Care and Use Committee.
On pregnancy day 8, dams were randomly assigned to treatment groups using statistical software to give a normal distribution of body weight to each treatment group. From pregnancy day 8 until weaning [on lactational day (LD) 21], pregnant females were weighed daily. Dams were fed a small wafer (Nabisco, East Hanover, NJ) treated with BPS or vehicle alone (70% ethanol, allowed to dry before feeding) every day. Wafers were dosed with solutions to deliver 2 or 200 µg BPS/kg/d (Santa Cruz Biotechnology, Dallas, TX; purity >99%). BPS dose was adjusted for dam body weight daily. Dams delivered naturally (birth designated LD0). In one group of dams, BPS was administered from pregnancy day 9 through LD20, litters were culled to 10 pups on LD1, and pups were weaned on LD21. These dams were euthanized at LD21. In a second set of dams, BPS was administered from pregnancy day 9 through LD1, litters were culled to 10 pups on LD1, and dams were euthanized at LD2. At LD2, sample sizes were as follows: control (n = 5), 2 μg BPS/kg/d (n = 8), and 200 μg BPS/kg/d (n = 9). On LD21, sample sizes were as follows: control (n = 14), 2 μg BPS/kg/d (n = 17), and 200 μg BPS/kg/d (n = 15).
Nursing behavior assays and pup growth measurements
Nursing behaviors were observed on LD2, 7, and 14. Dams were observed without intervention for a period of 90 minutes at the beginning of the light phase. Every 3 minutes, each dam’s nursing behavior was recorded; after determining whether the dam was nursing pups, the nursing position was determined, including the following: ventral, blanket, high-crouch, and low-crouch positions (44, 45). At the end of the observational period, the dam and her pups were gently removed from the cage. Dam and pup body weights were recorded. On postnatal day (PND) 14, the pups were also observed for eye opening, a marker of development.
Evaluation of pup-initiated nursing
As described in our prior study, maternal behavior was evaluated using a standard pup retrieval assay (42). Briefly, the dams and pups were separated for short periods of time (typically 5–10 minutes) to allow nest parameters to be measured [reported in (42)]. After this time, pups were scattered in the cage at the opposite end from the nest, and the dam was returned to the cage. In addition to evaluating the dam’s ability to retrieve the pups [data already reported (42)], we observed the pups over a 10-minute period to determine whether they initiated nursing. At both LD7 and LD14, the pups were sufficiently mobile to pursue the dam and initiate nursing, even when pup retrieval was not observed.
Euthanasia and tissue collection
On LD2 or LD21, dams were euthanized via CO2 inhalation, and blood was collected immediately following decapitation. Because animals were euthanized at a particular age, estrus cycle stage was not controlled between animals or treatment groups. Blood was spun to isolate serum, which was stored at −80°C for evaluation via enzyme-linked immunosorbent assay. From every dam, the right fourth inguinal mammary gland was dissected from the skin, spread on a glass slide (Fisher Scientific, Pittsburgh, PA), and fixed in neutral buffered formalin (10%) (Fisher Scientific) overnight (standard whole mount preparation). The right fifth inguinal mammary gland was fixed in neutral buffered formalin (10%) overnight for histology. The left fourth abdominal mammary gland was dissected from the skin, flash frozen on dry ice, and stored at −80°C for quantitative reverse transcription polymerase chain reaction (qRT-PCR) analyses. Brains were collected only from dams on LD21. Brains were fixed in neutral buffered formalin (10%) using methods we optimized previously (42, 46).
Whole-mount preparation and analysis
After fixing in neutral buffered formalin, whole-mounted mammary glands were processed through an alcohol series, defatted with toluene, stained with Carmine-alum, dehydrated in an alcohol and xylene series, and preserved in k-pax heat-sealed bags (Fisher Scientific) with methyl salicylate (Acros Organics, Morris Plains, NJ) (47). Digital images of whole-mount mammary glands were obtained using a Zeiss AxioImager dissection microscope (Carl Zeiss Microscopy, Jena, Germany) at magnification of ×6 and a Zeiss high-resolution color camera. To evaluate the density of epithelial structures in the whole mount, four identical boxes (each 5 mm2) were placed to the anterior, posterior, and lateral sides of the lymph node, a central feature within the fourth inguinal mammary gland. ZEN software (Carl Zeiss Microscopy) was used to measure the intensity of the mammary tissue in each box, where higher numbers indicate brighter signals (and therefore less dense mammary tissue). Intensities were normalized for each stage (LD2, LD21) so that the average control = 1; numbers >1 indicate less epithelial tissue compared with controls, and numbers <1 indicate more epithelial tissue compared with controls.
Tissue processing and histological staining
To prepare mammary tissue for paraffin sections, the fixed excised tissue was washed in phosphate-buffered saline, dehydrated through a series of alcohols, and embedded with paraffin (Leica Biosystems, Richmond, IL) under vacuum. Five-micrometer sections were cut on a Fisher rotary microtome and mounted on positively charged slides (Fisher Scientific). These sections were used for histological and immunohistochemical analyses.
For histological evaluations, slides were deparaffinized with xylene and a series of alcohols, stained with Harris hematoxylin and eosin (Fisher Scientific), dehydrated, and mounted with permanent mounting media (Fisher Scientific). Digital images were collected using a Zeiss Axio Oberserver.Z1 inverted microscope, a ×20 objective, and a high-resolution color camera (Carl Zeiss Microscopy). First, to quantify the fraction of the mammary gland comprised of adipose tissue vs lobuloalveolar units (lobules), three images were taken at random from nonoverlapping areas of the histological section. An identical 10 × 13 grid (with 108 crosshairs, 50 µm apart) was placed on each image, and the tissue type located at each crosshair (lobule, adipose, blood vessel, other connective tissue, etc.) was recorded. Second, to quantify the average lobule size, a minimum of three nonoverlapping images was collected using a ×40 objective. For each image, an identical 5 × 5 grid (with 16 crosshairs, 60 µm apart) was placed, and all lobules that fell on crosshairs were measured for area. A minimum of 9 lobules was evaluated to calculate the average lobule size in each gland.
Mammary gland immunohistochemistry
Expression of four markers was evaluated using standard methods for immunohistochemistry and commercial antibodies, including rabbit anti-ERα (catalog no. 06-935; EMD Millipore, Temecula, CA); rabbit anti-Ki67 (catalog no. RM-9106-S1; Fisher Scientific), a marker of proliferation; rabbit anti–signal transducer and activator of transcription 5a (Stat5a; catalog no. sc-1081; Santa Cruz Biotechnology), a marker of prolactin receptor signaling; and rabbit anti-progesterone receptor (PR; catalog no. ab131486; Abcam, Cambridge, MA). See Table 1 for a summary of these antibodies. For each marker of interest, a single section cut longitudinally through the mammary gland was used for analysis. Briefly, sections were deparaffinized, hydrated through a series of alcohols, microwaved in 10 mM citrate buffer (pH 6) for antigen retrieval, and treated with hydrogen peroxide to quench endogenous peroxidases. Nonspecific binding was blocked with 1% milk protein in 5% normal goal serum (Cell Signaling Technology, Danvers, MA). Sections were incubated with primary antibodies (see Table 1 for concentrations) at 4°C for 14 to 16 hours, washed, and incubated with secondary antibody (goat anti-rabbit, catalog no. ab64256; Abcam), followed by streptavidin peroxidase complex (catalog no. ab64269; Abcam). Diaminobenzidene chromogen (catalog no. ab64238; Abcam) was used to visualize reactions. Sections were counterstained with Harris’ hematoxylin (Fisher Scientific). Each immunohistochemical run included a negative control in which the primary antibody was replaced with 5% normal goat serum.
Table 1.
Information About Antibodies
| Peptide/ Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No. | Species Raised in; Monoclonal or Polyclonal | RRID | Dilution Used |
|---|---|---|---|---|---|---|
| ERα | Anti-ERα (C1355) | Millipore, 06-935 | Rabbit; polyclonal | AB_310305 | 1:1000 (mammary); 1:20,000 (arcuate nucleus) | |
| Ki67 | Ki67 | Thermo Fisher Scientific, RM-9106-S1 | Rabbit; monoclonal | AB_149792 | 1:1000 (mammary) | |
| Stat5 | Stat5a (L-20) | Santa Cruz Biotechnology, sc-1081 | Rabbit; polyclonal | AB_632448 | 1:500 (mammary); 1:200 (arcuate nucleus) | |
| Progesterone | Anti-PR | Abcam, ab131486 | Rabbit; polyclonal | AB_11156044 | 1:500 (mammary) | |
| Secondary | Biotinylated goat anti-rabbit IgG | Abcam, ab64256 | Goat; polyclonal | AB_2661852 | Ready to use (5 μg/mL) |
Abbreviation: RRID, Research Resource Identifier.
Two nonoverlapping images were taken of each sample for each marker of interest (ERα, Ki67, Stat5) with a Zeiss Axio Observer.Z1 inverted microscope. Because previous studies have shown that PR-positive cells are not detected in the mammary gland during pregnancy and lactation in mice (48, 49), PR expression was examined in only a limited number samples from each treatment group. Expression of each marker was evaluated by counting at least 200 epithelial cells. Each marker was expressed as a percent ratio of the total number of epithelial cells evaluated.
Brain immunohistochemistry
Fixed brains were washed in phosphate-buffered saline and sectioned in the transverse plane at 40 µm thickness. The arcuate nucleus was identified using a mouse brain atlas (50). Free-floating sections were processed for immunoreactivity to ERα or Stat5 (Table 1) using methods described previously (51). Expression of markers of interest was visualized using diaminobenzidene chromogen (catalog no. ab64238; Abcam). One image per section was collected using the Zeiss AxioImager (magnification of ×150) and a Zeiss high resolution color camera. ImageJ software (National Institutes of Health, Bethesda, MD) was used to convert the image from RGB color to 8 bit, subtract background, and automatically threshold. Cells expressing ERα and Stat5 in the arcuate nucleus were counted on two anatomically matched sections per animal using ImageJ. A small number of sections was also counted manually to ensure that automated counting was accurate (within five positive cells of the automated measure). Sections incubated in blocking solution without primary antibodies were used as negative controls.
Enzyme-linked immunosorbent assay
Serum was isolated from blood samples collected from dams at LD21 at the time of necropsy. A commercially available kit (catalog no. ab108667; Abcam) was used to quantify 17β-estradiol levels in these serum samples. The absorbance of the samples was measured at 450 nm using a SpectroMax plate reader (Molecular Devices, Sunnyvale, CA). The limit of detection was 3 pg/mL. All samples were run in duplicate, and the coefficient of variation ranged from 4% to 14.8%.
Quantitative polymerase chain reaction
Total RNA was extracted from mammary glands of individual mice using TRIzol reagent (Ambion, Carlsbad, CA) and a BeadBug microtube homogenizer (Sigma-Aldrich, St. Louis, MO), according to the manufacturer’s instructions. Total RNA was quantified by UV spectrophotometry (Nanodrop 1000; Thermo Scientific). One microgram of RNA from each sample was reverse transcribed to complementary DNA using reverse transcription (Applied Biosystems, Foster City, CA). The FastStart Universal SYBR Green Master kit (Roche Diagnostics, Indianapolis, IN) was used for the quantitative polymerase chain reactions (PCRs) along with 1 μL complementary DNA and 300 nM forward and 300 nM reverse primers for each target gene. β-actin was used as a housekeeping gene. Every sample was run in duplicate for each gene target. The thermal profile was as follows: 10 minutes at 95°C; 40 cycles of 15 seconds at 95°C, 30 seconds at 60°C, and 15 seconds at 72°C; a melting-curve analysis was conducted to identify nonspecific products. Relative quantification was determined using the ΔΔ cycle threshold method to correct for differences in β-actin (52). Primer sequences are reported in Table 2.
Table 2.
Primer Sequences
| Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| ERα (Esr1) | TGC AAT GAC TAT GCC TCT GG (782–801) | CTC CGG TTC TTG TCA ATG GT (921–902) |
| ERβ (Esr2) | TGT GTG TGA AGG CCA TGA TT | TCT TCG AAA TCA CCC AGA CC |
| PR (ProgR) total | AAA GGA TCC GCA GGT TCT C | GTT CCA TCT TCC AGC GGA TA |
| PR (ProgR) B | CCC AGT TCT CAG ACC AGA CC | GTG GGA TCT CCA CCT CCT G |
| Prolactin receptor (PrlR) long | ATA AAA GGA TTT GAT ACT CAT CTG CTA GAG | TGT CAT CCA CTT CCA AGA ACT CC |
| Prolactin receptor (PrlR) total | GTG GAA TCC TGG GTC AGA TG | GGG CCA CTG GTT TGT AGT C |
| β actin (BA) | CAC ACC CGC CAC CAG TTC GC (89–108) | TTG CAC ATG CCG GAG CCG TT (162–143) |
Statistical analysis
Behavioral, morphological, histological, and immunohistochemical analyses were conducted by observers blind to the treatment groups. Data were analyzed using SPSS Version 23 (IBM Corp, Armonk, NY). Continuous variable data were analyzed using one-way analysis of variance general linear model analyses with treatment as the independent variable, followed by Bonferroni and Fisher least significant difference post hoc tests. Data that were not normally distributed were evaluated using a nonparametric independent samples median test. Categorical data (e.g., yes/no pup-initiated nursing) were evaluated with a chi square test. Data were considered statistically significant at P < 0.05. Graphs illustrate means ± standard error, unless otherwise stated.
Results
BPS alters mammary gland histoarchitecture during late lactation
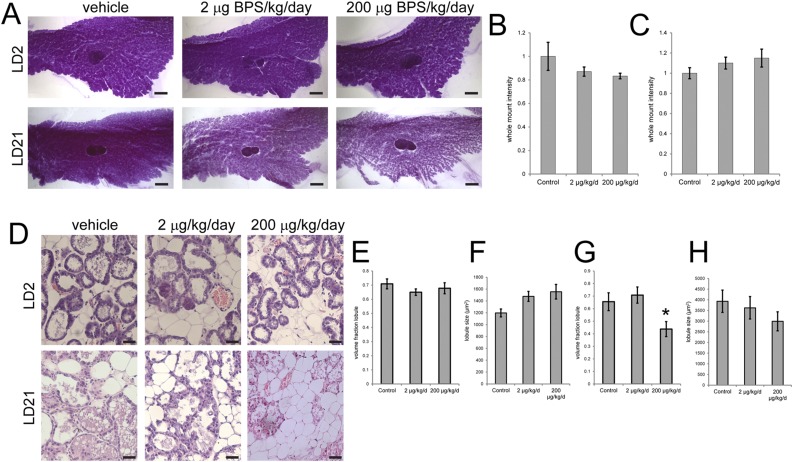
To evaluate the effect of exposure to BPS during pregnancy and lactation on the mouse mammary gland, we first examined whole-mount mammary glands collected from females at two different periods of lactation, early lactation (LD2) and just prior to natural weaning (LD21). Intensity of the mammary gland, a quantitative measure of the density of epithelial structures, was normalized at each stage of development so that BPS-treated females could be compared with vehicle-exposed animals at both stages. On LD2, BPS-treated females had mammary glands with lower intensities (corresponding to more epithelial structures), whereas at LD21, BPS-treated females had mammary glands with higher intensities (corresponding to fewer epithelial structures) (Fig. 1A–C). Due to high variability within treatment groups, these patterns were not statistically significant. However, the overall trend suggests that BPS exposure might increase the amount of functional mammary tissue in early lactation on LD2, while inducing early involution toward the end of the lactation period on LD21.
Figure 1.
Exposure to BPS alters mammary gland morphology. (A) Representative whole-mount mammary glands collected from females on LD2 and LD21. Scale bar represents 2 mm. (B) Quantification of whole-mount intensity, a measure of epithelial density, on LD2. (C) Quantification of whole-mount intensity on LD21. (D) Representative hematoxylin and eosin sections of mammary glands collected from females on LD2 and LD21. Scale bar represents 50 μm. (E) Quantification of volume fraction of lobules on LD2. (F) Quantification of average lobule size on LD2. There is a significant difference between the control and 200 µg BPS/kg/d group (P < 0.05, Fisher post hoc test), but the analysis of variance is not significant. (G) Quantification of volume fraction of lobules on LD21. *P < 0.05, Bonferroni post hoc test after significant analysis of variance. (H) Quantification of average lobule size on LD21. At LD2, sample sizes were as follows: control (n = 5), 2 μg BPS/kg/d (n = 8), 200 μg BPS/kg/d (n = 9). On LD21, sample sizes were as follows: control (n = 14), 2 μg BPS/kg/d (n = 17), 200 μg BPS/kg/d (n = 15).
To further investigate this possibility, we evaluated two histological characteristics in the lactating mammary gland on both LD2 and LD21: the volume fraction of the mammary gland comprised of lobuloalveolar structures and lobule size. At LD2, there were no statistically significant differences observed in either the volume fraction of lobules or lobule size between treatment groups (Fig. 1D–F), although a nonsignificant increase in lobule size was observed (23% in females exposed to 2 μg BPS/kg/d; 30% in females exposed to 200 μg BPS/kg/d).
In contrast, on LD21, the volume fraction of lobules was significantly reduced, and the volume fraction of adipose tissue was significantly increased, in females exposed to 200 μg BPS/kg/d (Fig. 1C, 1G); P < 0.05, Bonferroni post hoc test, and data not shown], consistent with the patterns observed in mammary gland whole mounts. Lobule size was decreased in the BPS-treated females (8% in females exposed to 2 μg BPS/kg/d; 24% in females exposed to 200 μg BPS/kg/d), but these differences were not statistically significant (Fig. 1H). Collectively, these results suggest that the histomorphology of the lactating mammary gland is disrupted by BPS exposure in late, but not early, lactation.
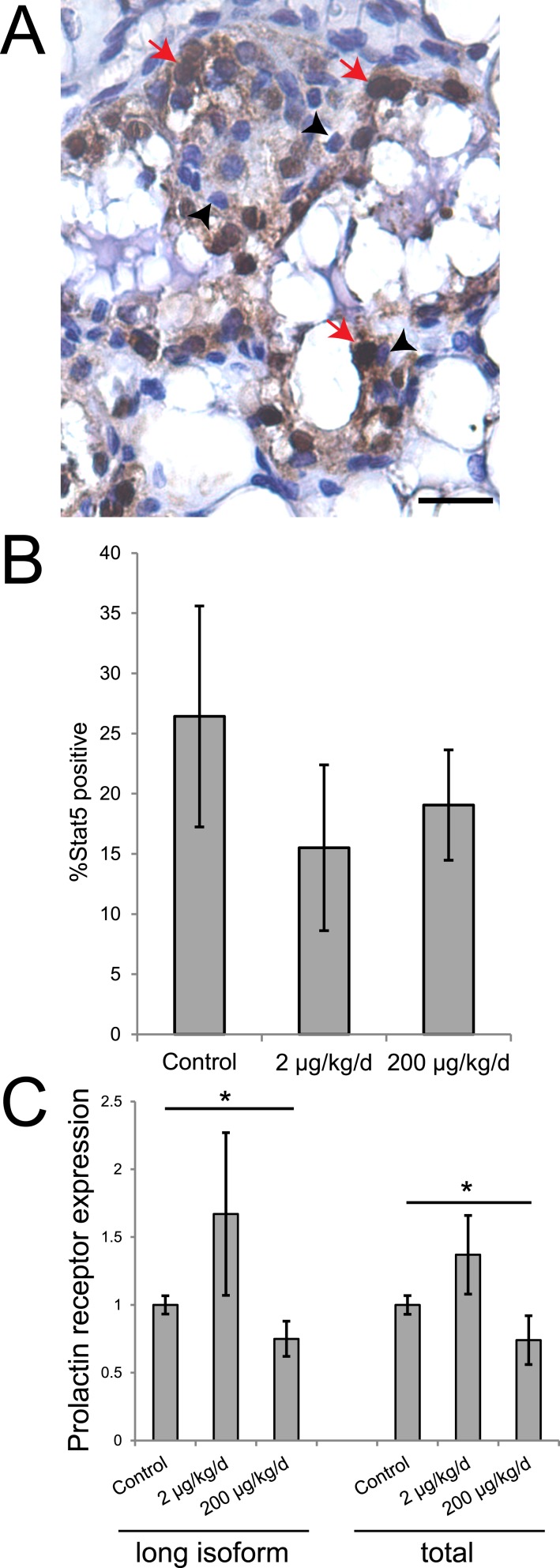
BPS alters prolactin signaling in the lactating mammary gland at LD21
In the mammary gland, expression of Stat5, a marker of prolactin signaling and mammary epithelial cell differentiation, increases during lactation. During involution, Stat5 is inactivated, halting prolactin signaling and reducing cell survival (53). To assess the effect of BPS exposure on Stat5 expression, we evaluated the presence of Stat5 in lactating mammary glands on LD21 using immunohistochemistry. Although the number of epithelial cells that were Stat5 positive was decreased in treated females compared with vehicle-exposed dams, these differences were not statistically significant (Fig. 2A, 2B). We also evaluated prolactin receptor expression using qRT-PCR. Evaluation of both the long isoform and total receptor (short plus long isoforms) revealed a similar pattern in which exposure to 2 μg BPS/kg/d increased expression of prolactin receptor and exposure to 200 μg BPS/kg/d decreased expression of prolactin receptor (Fig. 2C); P < 0.05, independent samples median test].
Figure 2.
BPS exposure alters expression of prolactin receptor in the mammary gland. (A) A mammary gland sample evaluated for Stat5 expression. Scale bar represents 20 μm; red arrows indicate positive cells; and black arrowheads indicate negative cells. (B) Quantification of the percentage of mammary epithelial cells expressing Stat5 on LD21. (C) qRT-PCR reveals significant differences in the expression of prolactin receptor (long isoform and total) between females from the three treatment groups. *P < 0.05, independent samples median test, post hoc not available. For qRT-PCR, sample sizes were as follows: control (n = 5), 2 μg BPS/kg/d (n = 6), 200 μg BPS/kg/d (n = 6). For immunohistochemistry, sample sizes were as follows: control (n = 9), 2 μg BPS/kg/d (n = 11), 200 μg BPS/kg/d (n = 10).
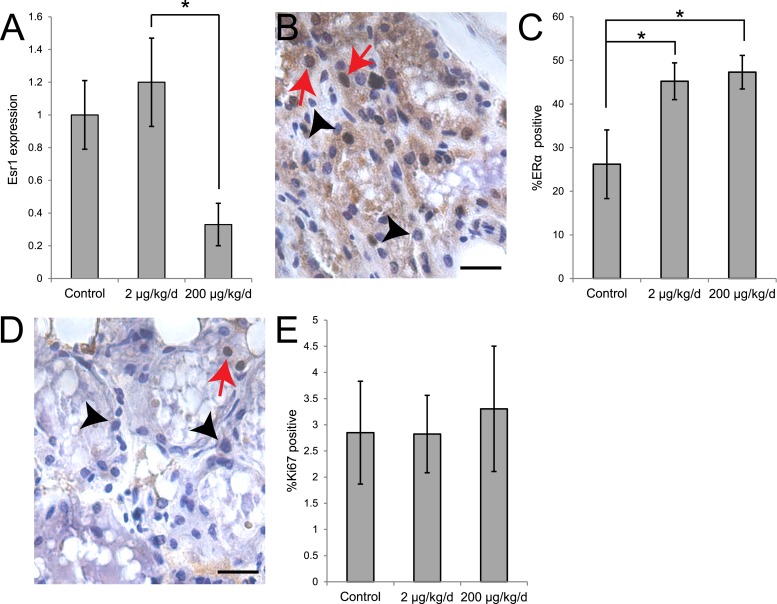
BPS alters expression of ER in the lactating mammary gland
In the mouse, ERα is expressed at relatively high levels in the adult mammary epithelium; its expression is diminished during pregnancy, and it returns to high levels of expression (of both messenger RNA and protein) during lactation (54). We next evaluated expression of Esr1, the gene encoding ERα, in the lactating mammary gland on LD21 using qRT-PCR. Expression of Esr1 was modestly increased in females exposed to 2 μg BPS/kg/d and decreased in females exposed to 200 μg BPS/kg/d (Fig. 3A); P < 0.05, Bonferroni post hoc test]. However, a different pattern was observed when we evaluated expression of ERα protein using immunohistochemistry. In this study, the percentage of epithelial cells that were ERα positive was increased in females of both BPS treatment groups relative to control animals (Fig. 3B), 3(C); P < 0.05, Bonferroni post hoc test]. Expression of Esr2, the gene encoding ERβ, was not detected in any sample at LD21, even after 40 PCR cycles (data not shown).
Figure 3.
BPS exposure alters expression of ERα in the mammary gland. (A) Expression of Esr1, the gene encoding for ERα, was significantly different between females from the two BPS treatment groups. (B) Immunohistochemistry for ERα. Scale bar represents 20 μm; red arrows indicate positive cells; and black arrowheads indicate negative cells. (C) Quantification of ERα expression in the mammary gland at LD21 reveals significant differences between controls and both BPS groups. (D) Immunohistochemistry for Ki67, a marker of proliferation. Scale bar represents 20 μm; red arrows indicate positive cells; and black arrowheads indicate negative cells. (E) Quantification of Ki67 expression in the mammary gland at LD21. In all panels, *P < 0.05, Bonferroni post hoc test after significant analysis of variance. For qRT-PCR, sample sizes were as follows: control (n = 5), 2 μg BPS/kg/d (n = 6), 200 μg BPS/kg/d (n = 6). For immunohistochemistry, sample sizes were as follows: control (n = 9), 2 μg BPS/kg/d (n = 11), 200 μg BPS/kg/d (n = 10).
We also evaluated the expression of PR using both qRT-PCR and immunohistochemistry. No PR-positive cells were observed in the epithelium of the lactating mammary gland in any of the treatment groups or in control animals, and PR messenger RNA was also not detected, even after 40 PCR cycles (data not shown). This is consistent with prior studies showing that PR is not present in the mouse mammary gland during lactation (48, 55).
BPS does not induce proliferation in the lactating mammary gland
During pregnancy, mammary alveolar buds first proliferate and then differentiate to produce lobuloalveolar structures (56). Proliferation rates are typically very low during lactation. We examined the expression of Ki67, a marker of proliferation, in the lactating mammary gland epithelium at LD2 and LD21. At LD2, the percentage of epithelial cells that were Ki67 positive was very low (<0.1%), with no significant differences between treatment groups (data not shown). BPS treatment during pregnancy and lactation also did not affect the percentage of epithelial cells that were positive for this marker of proliferation in the mammary epithelium at LD21 (Fig. 3D, 3E).
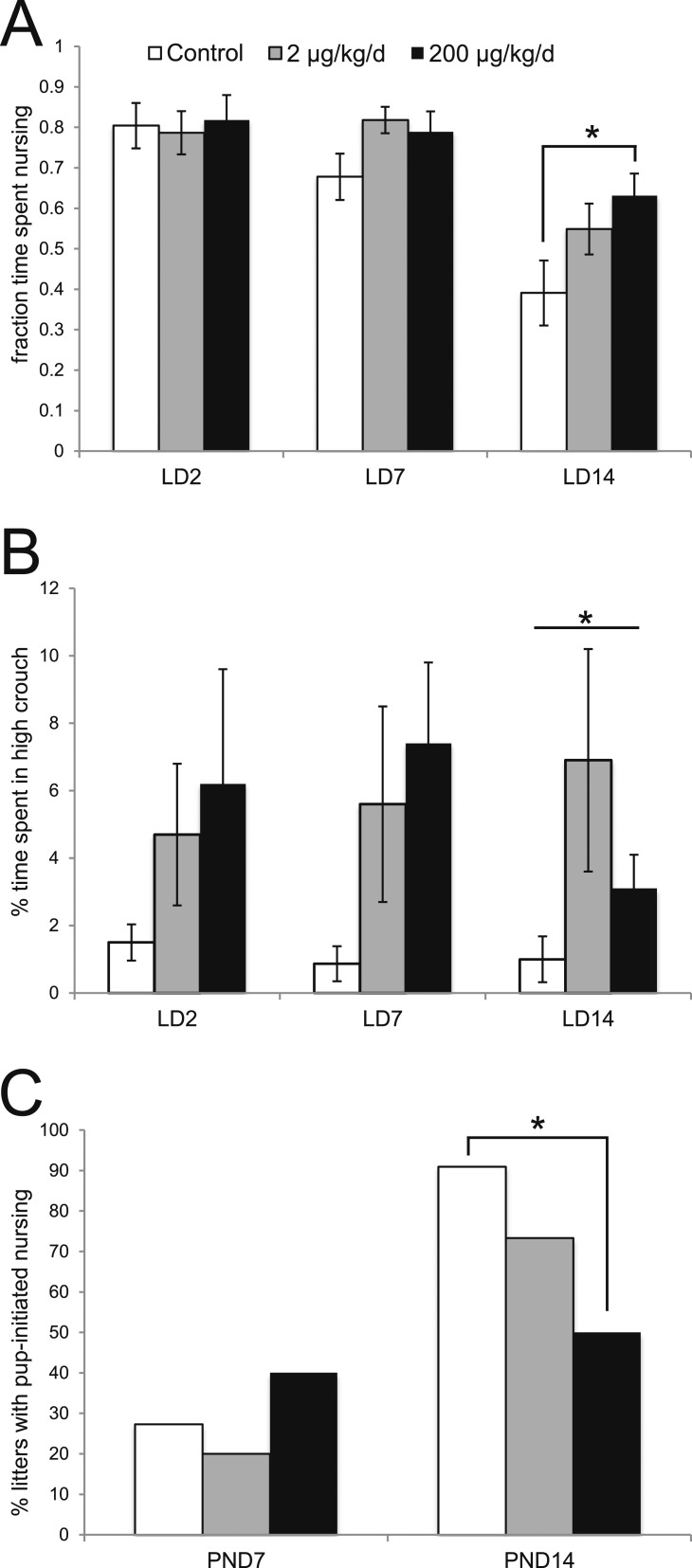
BPS treatment alters nursing behaviors in exposed dams and their pups
Our previous studies revealed that BPS exposure during pregnancy and lactation induced subtle, but significant alterations to maternal behavior; exposure to 200 μg BPS/kg/d altered the amount of time that dams spent on the nest (42). We next wanted to determine whether BPS exposure would alter time spent nursing, which can occur both on and off the nest. We also evaluated the nursing posture used by dams throughout the lactational period. During early and midlactation (L2 and LD7, respectively), we observed no significant differences in the time spent nursing based on BPS treatment. Interestingly, on LD14, when time spent nursing was decreased in control dams (with females spending only ∼40% of the observed time nursing), females treated with 200 μg BPS/kg/d spent significantly more time nursing (Fig. 4A); 200 μg BPS/kg/d, P < 0.05, Bonferroni post hoc test]. We also evaluated the percentage of observed time that females spent in a high-crouch posture, which is associated with maximal milk ejection (45). At all three lactational stages evaluated, BPS-treated dams spent more time in the high-crouch position compared with control females, with statistically significant differences observed on LD14 between controls and females treated with 2 μg BPS/kg/d (Fig. 4B); P < 0.05, independent samples median test].
Figure 4.
BPS alters nursing behaviors in exposed mothers and offspring. (A) Quantification of observations of maternal behavior. The fraction of time females exposed to 200 μg BPS/kg/d spent nursing was increased on LD14 compared with control females. *P < 0.05, Bonferroni post hoc test after significant analysis of variance. (B) At all stages of lactation evaluated, BPS-treated females spent more time in the high-crouch position. On LD14, the median time spent in high-crouch nursing was significantly different between groups. *P < 0.05, independent samples median test, post hoc not available. (C) Quantification of the percentage of litters that actively initiate nursing (pup initiated). On PND14, significantly fewer litters exposed to 200 μg BPS/kg/d successfully initiated nursing. *P < 0.05, chi square test. For behavioral assays, sample sizes were as follows: control (n = 14), 2 μg BPS/kg/d (n = 17), 200 μg BPS/kg/d (n = 15).
Finally, we evaluated pup-initiated nursing behaviors after dams were briefly separated from the litter (typically for 5 to 10 minutes) followed by a pup retrieval assay [data on pup retrieval behaviors were reported previously; see (42)]. Pup-initiated nursing requires that pups actively pursue the mother, typically outside of the nest, to suckle and nurse. On PND2, the pups are relatively immobile and do not actively initiate nursing. By PND7, 20% to 40% of litters had one or more pups actively initiate nursing, with no significant differences between treatment groups (Fig. 4C). At PND14, 91% of control litters displayed pup-initiated nursing, but only 50% of litters born to dams treated with 200 μg BPS/kg/d displayed these behaviors, a significant difference (Fig. 4C; P < 0.05, chi square test). Collectively, these results suggest that BPS induces a number of changes in nursing behaviors, including mothers that spend more time nursing later in the lactational period, mothers that spend more time in the high-crouch nursing position, and pups that are less likely to initiate nursing themselves.
BPS and the hypothalamic-pituitary-ovarian axis
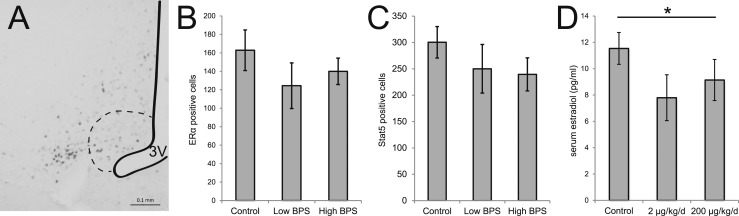
The arcuate nucleus is an ER-rich region in the hypothalamus that is important for the regulation of behaviors associated with lactation (43). To assess changes in the hormone receptor expression in this region, we examined anatomically matched brain sections containing the arcuate nucleus using immunohistochemistry. No differences between treatment groups were observed in the total number of ERα-positive cells in the arcuate nucleus (Fig. 5A, 5B). The total number of Stat5-positive cells, a marker of prolactin signaling, in the arcuate nucleus was also not affected by BPS exposure (Fig. 5C). Expression of prolactin receptor in the pituitary, evaluated using qRT-PCR, was not affected by treatment either (data not shown). Finally, we measured serum concentrations of 17β-estradiol, which is produced by the ovary and provides negative feedback to the hypothalamus and pituitary. We found that BPS-exposed dams had lower serum concentrations of 17β-estradiol, with median concentrations that were significantly lower than controls (Fig. 5D; P < 0.05, independent samples median test).
Figure 5.
BPS does not affect ERα or Stat5 expression in the arcuate nucleus. (A) A representative photomicrograph illustrating ERα expression in the arcuate nucleus (indicated by the dotted line). The third ventricle (3V) is also indicated. Scale bar represents 0.1 mm. (B) Quantification of ERα and (D) Stat5 expression revealed no effects of BPS. (d) Maternal serum concentrations of 17β-estradiol were depressed by BPS treatment. *P < 0.05, independent samples median test, post hoc not available. For immunohistochemistry, sample sizes were as follows: control (n = 6), 2 μg BPS/kg/d (n = 7), 200 μg BPS/kg/d (n = 6). For enzyme-linked immunosorbent assay, sample sizes were as follows: control (n = 6), 2 μg BPS/kg/d (n = 6), 200 μg BPS/kg/d (n = 6).
Pup growth and development are affected by BPS treatment
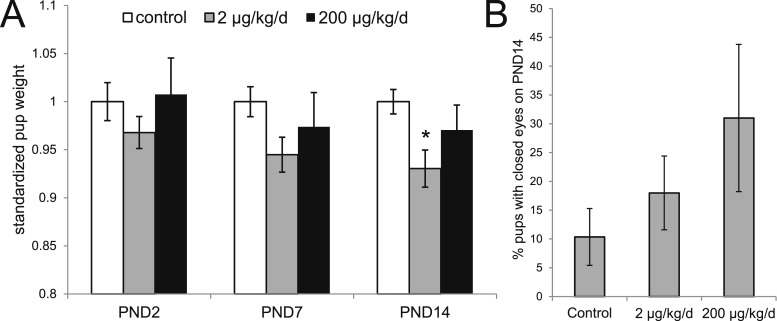
Pup weights were recorded for each litter on PND2, 7, and 14; to control for differences in litter size, only litters with 9 to 10 pups were evaluated. The average pup weight on PND2 and PND7 did not differ significantly between vehicle- and BPS-exposed animals. On PND14, pups born to mothers treated with 2 μg BPS/kg/d weighed significantly less than pups born to control mothers (control: 9.37 ± 0.12 g; 2 μg BPS/kg/d: 8.71 ± 0.18 g; P < 0.05, Fisher post hoc test). Pups born to mothers treated with 200 μg BPS/kg/d also weighed less than pups born to control dams (200 μg BPS/kg/d: 9.09 ± 0.24 g), but this decrease was not statistically significant (Fig. 6A).
Figure 6.
Pups born to BPS-exposed dams display growth and development delays. (A) Graph describing body weights across the postnatal period (PND2 through PND14), normalized to the controls at each stage to allow for comparisons across time. [Actual weights are described in the Results section.] At all ages evaluated, pups from litters exposed to 200 μg BPS/kg/d were lighter than controls, with significant differences at PND14. *P < 0.05, Fisher post hoc test after significant analysis of variance. (B) A monotonic increase in the number of pups with closed eyes on PND14 was observed, although these differences were not statistically significant. For all pup measurements, sample sizes (litters evaluated) were as follows: control (n = 14), 2 μg BPS/kg/d (n = 17), 200 μg BPS/kg/d (n = 15).
To further evaluate the effects of BPS on the developing pups, we examined pup eye opening, a standard developmental measure, at PND14. The percentage of pups in each litter with closed eyes on PND14 increased with BPS treatment, although these differences were not statistically significant (Fig. 6B). The number of litters with at least one pup with closed eyes was similar in controls and litters from the 200 μg BPS/kg/d group (4/14 and 4/15, respectively), but higher in the 2 μg BPS/kg/d group (8/17).
Discussion
In this study, we examined the lactating mammary gland and nursing behavior in female CD-1 mice exposed to BPS during pregnancy and lactation. The effects of exposures to xenoestrogens during pregnancy and lactation are not well studied, although this period is recognized for rapid development of the mammary gland and substantial changes in hormone signaling and behavior in the mother (1, 3). To date, only a small number of chemicals have been shown to affect the gland during this critical developmental stage. PFOA has been shown to decrease differentiation in the mammary gland of CD-1 mice after exposure during pregnancy, resulting in female pups with diminished growth and body weights (25). Similarly, exposure to dioxin during pregnancy and lactation induced abnormal and stunted mammary gland development and a decrease in milk protein gene expression (2, 26). These results support the current findings: mammary gland development is susceptible to chemical exposures during this stage of life.
Our data may be consistent with BPS-induced early involution in females exposed to 200 μg BPS/kg/d; these females had less developed, less dense mammary glands with nonsignificant decreases in epithelial density measured in whole mounts at LD21 and significant decreases in epithelium observed at the level of histomorphology (Fig. 1). Importantly, the fraction of the mammary gland comprised of lobuloalveolar units, the functional structures in the lactating mammary gland that secrete milk, was not different on LD2, but was significantly decreased in BPS-treated dams on LD21. Similar to what has been observed for PFOA (24, 25), this diminished function of the mammary gland was accompanied by increased time spent nursing at LD14 and increases in the time spent in high-crouch nursing, the posture associated with increased milk letdown (Fig. 4). These results suggest that the dams exposed to 200 μg BPS/kg/d may compensate for their lactational deficiencies by altering their nursing behaviors. Although this compensation appears to allow the pups in these litters to maintain almost-normal body weights, other developmental endpoints, including eye opening, suggest that the pups from these litters may be developmentally delayed. In dams treated with 2 µg BPS/kg/d, there were no significant effects observed on the dams’ mammary gland morphology or on nursing behavior, but the pups from these litters were found to have decreased body weight throughout the lactational period compared with controls (Fig. 6). These results might suggest more complex effects of BPS on the lactating mammary gland, including effects on milk protein expression or milk volume, which warrant future study. Prior studies have demonstrated that BPA can alter mammary gland differentiation and milk composition, but these effects were observed in females exposed during perinatal development (27, 28). Additional studies are needed to determine whether exposures to BPA during pregnancy and lactation induce effects on exposed adults similar to those we report in this work. Cross-fostering of litters will also help to determine whether the effects of BPS we have observed on the pups are due to direct disruptions to growth and development or indirect effects due to altered milk supply and maternal care. Prior studies suggest that BPS alters some aspects of development in exposed offspring, including development and hormone responsiveness of the female reproductive tract (41), as well as postweaning body weight and social behaviors (39).
We were also intrigued by results showing a decrease in pup-initiated nursing in litters exposed to 200 μg BPS/kg/d. Pups that are less interested in feeding would be expected to have stunted growth, and their decreased suckling may also contribute to early involution of the mammary gland in dams because this can promote accumulation of milk in the mammary gland, the first step in involution. There are a number of reasons that pups might experience problems initiating nursing, including difficulties locating the mother (e.g., abnormalities with sensory organs), problems sensing hunger and satiety, or locomotive problems. Future studies should evaluate these factors.
In addition to BPS-induced altered mammary gland morphology, the observed changes in protein expression in the lactating mammary gland are also consistent with early involution of the gland. A decrease in Stat5 activity and an increase in expression of ERα occur during the process of involution (57, 58). Although not statistically significant, we observed a decrease in the abundance of Stat5 in dams exposed to BPS compared with control animals (Fig. 2). We also observed a significant increase in the number of epithelial cells that were ERα positive in the mammary glands of females from both BPS treatment groups relative to controls (Fig. 3). These changes occurred in the absence of proliferation. Unfortunately, because of the manner in which samples were collected, we were not able to evaluate apoptosis, an additional indicator of involution.
We also tested the hypothesis that the hypothalamic-pituitary-ovarian axis may be involved in the complex loop of BPS-induced altered nursing behaviors and mammary gland involution. We examined the effects of BPS on the arcuate nucleus, an ER-rich region in the hypothalamus that is important for the regulation of behaviors associated with lactation (43). ER in the arcuate nucleus is vital in the activation of normal reproductive and maternal behavior, as well as other behaviors, including anxiety and aggression (59). ERα expression in the arcuate nucleus is suppressed by increased circulating estrogens and xenoestrogens, including BPA (60, 61). We examined both ERα and Stat5, a mediator of prolactin signaling, in the arcuate nucleus and found nonsignificant decreases in ERα expression in BPS-treated dams, similar to what has been demonstrated for BPA (Fig. 5). These results provide evidence that the mammary gland may be a direct target of BPS during lactation. We also observed decreased serum concentrations of 17β-estradiol in BPS-treated females at LD21 (Fig. 5D). Because samples were collected at LD21 when pups were weaned, and the estrus cycle stage was not evaluated in these dams, these results might indicate that control females have resumed cycling, whereas BPS-treated females have not. Importantly, lower serum estradiol concentrations in females exposed to BPS may contribute to decreases in prolactin signaling, as estrogen stimulates the secretion of prolactin, promoting milk synthesis and secretion (4).
It has been understood for several decades that pharmacological doses of estrogens can halt lactogenesis. Potent estrogenic chemicals like diethylstilbestrol and estradiol vanadate were prescribed to suppress lactation after parturition in women who were not planning to breastfeed (17–23). The question of whether low doses of xenoestrogens might also have effects on women has recently gained attention, and a small number of studies have examined associations between environmental chemical exposures and duration of breastfeeding. In a study of women from an agricultural town in northern Mexico, levels of dichlorodiphenyldichloroethylene (DDE), an estrogenic metabolite of the pesticide dichlorodiphenyltrichloroethane (DDT), measured in breast milk at parturition were inversely associated with breastfeeding duration; those with the lowest concentrations breastfed for a median of 7.5 months, whereas those from the highest group only breastfed for a median of 3 months (62). DDE exposures were also associated with decreased breastfeeding initiation (63). Similar relationships were shown in other human populations (64), but not all (65). More recent evaluations of relationships between breastfeeding and other persistent organic pollutants, including PFOA and PFOS, suggest that higher concentrations in mothers are associated with reduced breastfeeding duration and reduced exclusive breastfeeding (66–68).
We chose to study BPS due to its widespread use as a replacement for BPA in a number of consumer products; human exposure has been documented; cell-based assays have demonstrated its estrogenic properties; and its effects on mammals remain poorly studied (69). As in our previous study demonstrating alterations induced by BPS on additional measures of maternal behavior and the brain (42), the findings presented in this work further indicate that the mother is vulnerable to exposure to low doses of BPS during pregnancy and lactation. Circulating estrogens are high during pregnancy, leading many researchers to conclude that the mother is unlikely to be affected by low doses of xenoestrogens (70). The results presented in this study challenge this assumption, suggesting that pregnancy and lactation may serve as a sensitive window of development for the mother. In some cases, the effects induced by xenoestrogens on exposed mothers manifest months after exposures cease, suggesting that these effects are organizational in nature, rather than activational (71–74). Follow-up studies are needed to examine mammary glands of mice exposed to BPS during pregnancy and lactation months after the end of pregnancy and lactation to assess whether this organ experiences long-term adverse outcomes.
Breastfeeding is not only important for nourishment of the infant, but also for nonnutritional purposes. For the mother, these benefits include bonding with the child and decreased incidence of postpartum depression, which was found to be more common in women who did not breastfeed or weaned earlier (75, 76). Breastfeeding provides benefits to the child, including improved immune responses, lower contraction of respiratory tract infections, and lower incidence of other medical problems associated with infant mortality and morbidity (7–11). Environmental chemical exposures, including low-level xenoestrogens, represent an important potential entry point for clinical intervention; the effects of xenoestrogens on lactation extend beyond the mother to numerous aspects of health in her offspring, with ultimate implications for lifelong health.
Acknowledgments
Financial Support: This work was supported by the University of Massachusetts (start-up funds to L.N.V.) and National Institute of Environmental Health Sciences of the National Institutes of Health Award K22ES025811 (to L.N.V.). C.D.L. received a fellowship from the University of Massachusetts Commonwealth Honors College. M.C.C. was supported by a fellowship from the Center for Research on Families, University of Massachusetts–Amherst. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the University of Massachusetts.
Acknowledgments
Disclosure Summary: L.N.V. has received travel reimbursement from universities, governments, NGOs, and industry to speak about endocrine-disrupting chemicals. The remaining authors have nothing to disclose.
Footnotes
- BPA
- bisphenol A
- BPS
- bisphenol S
- ER
- estrogen receptor
- LD
- lactational day
- PCR
- polymerase chain reaction
- PFOA
- perfluorooctanoic acid
- PND
- postnatal day
- PR
- progesterone receptor
- qRT-PCR
- quantitative reverse transcription polymerase chain reaction
- Stat
- signal transducer and activator of transcription.
References
- 1.Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev Cell. 2001;1(4):467–475. [DOI] [PubMed] [Google Scholar]
- 2.Fenton SE. Endocrine-disrupting compounds and mammary gland development: early exposure and later life consequences. Endocrinology. 2006;147(6, Suppl)S18–S24. [DOI] [PubMed] [Google Scholar]
- 3.Macias H, Hinck L. Mammary gland development. Wiley Interdiscip Rev Dev Biol. 2012;1(4):533–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuhn NJ. Progesterone withdrawal as the lactogenic trigger in the rat. J Endocrinol. 1969;44(1):39–54. [DOI] [PubMed] [Google Scholar]
- 5.Gertosio C, Meazza C, Pagani S, Bozzola M. Breastfeeding and its gamut of benefits. Minerva Pediatr. 2016;68(3):201–212. [PubMed] [Google Scholar]
- 6.Khan J, Vesel L, Bahl R, Martines JC. Timing of breastfeeding initiation and exclusivity of breastfeeding during the first month of life: effects on neonatal mortality and morbidity--a systematic review and meta-analysis. Matern Child Health J. 2015;19(3):468–479. [DOI] [PubMed] [Google Scholar]
- 7.Amitay EL, Keinan-Boker L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 2015;169(6):e151025. [DOI] [PubMed] [Google Scholar]
- 8.Sankar MJ, Sinha B, Chowdhury R, Bhandari N, Taneja S, Martines J, Bahl R. Optimal breastfeeding practices and infant and child mortality: a systematic review and meta-analysis. Acta Paediatr. 2015;104(467):3–13. [DOI] [PubMed] [Google Scholar]
- 9.Colaizy TT, Bartick MC, Jegier BJ, Green BD, Reinhold AG, Schaefer AJ, Bogen DL, Schwarz EB, Stuebe AM; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Impact of optimized breastfeeding on the costs of necrotizing enterocolitis in extremely low birthweight infants. J Pediatr. 2016;175:100–105.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duijts L, Jaddoe VW, Hofman A, Moll HA. Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics. 2010;126(1):e18–e25. [DOI] [PubMed] [Google Scholar]
- 11.WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. Lancet. 2000;355(9202):451–455. [PubMed] [Google Scholar]
- 12.Binns C, Lee M, Low WY. The long-term public health benefits of breastfeeding. Asia Pac J Public Health. 2016;28(1):7–14. [DOI] [PubMed] [Google Scholar]
- 13.Kramer MS. “Breast is best”: the evidence. Early Hum Dev. 2010;86(11):729–732. [DOI] [PubMed] [Google Scholar]
- 14.Victora CG, Bahl R, Barros AJ, França GV, Horton S, Krasevec J, Murch S, Sankar MJ, Walker N, Rollins NC; Lancet Breastfeeding Series Group . Breastfeeding in the 21st century: epidemiology, mechanisms, and lifelong effect. Lancet. 2016;387(10017):475–490. [DOI] [PubMed] [Google Scholar]
- 15.Scott JA, Binns CW. Factors associated with the initiation and duration of breastfeeding: a review of the literature. Breastfeed Rev. 1999;7(1):5–16. [PubMed] [Google Scholar]
- 16.Li R, Fein SB, Chen J, Grummer-Strawn LM. Why mothers stop breastfeeding: mothers’ self-reported reasons for stopping during the first year. Pediatrics. 2008;122(Suppl 2):S69–S76. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz DJ, Evans PC, García CR, Rickels K, Fisher E. A clinical study of lactation suppression. Obstet Gynecol. 1973;42(4):599–606. [DOI] [PubMed] [Google Scholar]
- 18.Brun del Re R, Del Pozo E, De Grandi P, Friesen H, Hinselmann M, Wyss H. Prolactin inhibition and suppression of puerperal lactation by a Br-erocryptine (CB 154): a comparison with estrogen. Obstet Gynecol. 1973;41(6):884–890. [PubMed] [Google Scholar]
- 19.Kochenour NK. Lactation suppression. Clin Obstet Gynecol. 1980;23(4):1045–1059. [DOI] [PubMed] [Google Scholar]
- 20.Dickey RP, Stone SC. Drugs that affect the breast and lactation. Clin Obstet Gynecol. 1975;18(2):95–111. [DOI] [PubMed] [Google Scholar]
- 21.Oladapo OT, Fawole B. Treatments for suppression of lactation. Cochrane Database Syst Rev. 2012;(9):CD005937. [DOI] [PMC free article] [PubMed]
- 22.Stirrat GM, Anderson GE, Grant O. The effectiveness of stilboestrol in the suppression on postpartum lactation. J Obstet Gynaecol Br Commonw. 1968;75(3):313–315. [DOI] [PubMed] [Google Scholar]
- 23.Young PJ. Suppression of lactation. BMJ. 1969;4(5678):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White SS, Stanko JP, Kato K, Calafat AM, Hines EP, Fenton SE. Gestational and chronic low-dose PFOA exposures and mammary gland growth and differentiation in three generations of CD-1 mice. Environ Health Perspect. 2011;119(8):1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White SS, Calafat AM, Kuklenyik Z, Villanueva L, Zehr RD, Helfant L, Strynar MJ, Lindstrom AB, Thibodeaux JR, Wood C, Fenton SE. Gestational PFOA exposure of mice is associated with altered mammary gland development in dams and female offspring. Toxicol Sci. 2007;96(1):133–144. [DOI] [PubMed] [Google Scholar]
- 26.Vorderstrasse BA, Fenton SE, Bohn AA, Cundiff JA, Lawrence BP. A novel effect of dioxin: exposure during pregnancy severely impairs mammary gland differentiation. Toxicol Sci. 2004;78(2):248–257. [DOI] [PubMed] [Google Scholar]
- 27.Kass L, Altamirano GA, Bosquiazzo VL, Luque EH, Muñoz-de-Toro M. Perinatal exposure to xenoestrogens impairs mammary gland differentiation and modifies milk composition in Wistar rats. Reprod Toxicol. 2012;33(3):390–400. [DOI] [PubMed] [Google Scholar]
- 28.Altamirano GA, Muñoz-de-Toro M, Luque EH, Gómez AL, Delconte MB, Kass L. Milk lipid composition is modified by perinatal exposure to bisphenol A. Mol Cell Endocrinol. 2015;411:258–267. [DOI] [PubMed] [Google Scholar]
- 29.Viñas R, Watson CS. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect. 2013;121(3):352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molina-Molina JM, Amaya E, Grimaldi M, Sáenz JM, Real M, Fernández MF, Balaguer P, Olea N. In vitro study on the agonistic and antagonistic activities of bisphenol-S and other bisphenol-A congeners and derivatives via nuclear receptors. Toxicol Appl Pharmacol. 2013;272(1):127–136. [DOI] [PubMed] [Google Scholar]
- 31.Rosenmai AK, Dybdahl M, Pedersen M, Alice van Vugt-Lussenburg BM, Wedebye EB, Taxvig C, Vinggaard AM. Are structural analogues to bisphenol a safe alternative? Toxicol Sci. 2014;139(1):35–47. [DOI] [PubMed] [Google Scholar]
- 32.Liao C, Liu F, Guo Y, Moon HB, Nakata H, Wu Q, Kannan K. Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ Sci Technol. 2012;46(16):9138–9145. [DOI] [PubMed] [Google Scholar]
- 33.Liao C, Liu F, Kannan K. Bisphenol S, a new bisphenol analogue, in paper products and currency bills and its association with bisphenol A residues. Environ Sci Technol. 2012;46(12):6515–6522. [DOI] [PubMed] [Google Scholar]
- 34.Liao C, Kannan K. Concentrations and profiles of bisphenol A and other bisphenol analogues in foodstuffs from the United States and their implications for human exposure. J Agric Food Chem. 2013;61(19):4655–4662. [DOI] [PubMed] [Google Scholar]
- 35.Liao C, Kannan K. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Arch Environ Contam Toxicol. 2014;67(1):50–59. [DOI] [PubMed] [Google Scholar]
- 36.Simoneau C, Valzacchi S, Morkunas V, Van den Eede L. Comparison of migration from polyethersulphone and polycarbonate baby bottles. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28(12):1763–1768. [DOI] [PubMed] [Google Scholar]
- 37.Liao C, Liu F, Alomirah H, Loi VD, Mohd MA, Moon HB, Nakata H, Kannan K. Bisphenol S in urine from the United States and seven Asian countries: occurrence and human exposures. Environ Sci Technol. 2012;46(12):6860–6866. [DOI] [PubMed] [Google Scholar]
- 38.Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000-2014. Environ Sci Technol. 2015;49(19):11834–11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim B, Colon E, Chawla S, Vandenberg LN, Suvorov A. Endocrine disruptors alter social behaviors and indirectly influence social hierarchies via changes in body weight. Environ Health. 2015;14:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Castro B, Sánchez P, Torres JM, Ortega E. Bisphenol A, bisphenol F and bisphenol S affect differently 5α-reductase expression and dopamine-serotonin systems in the prefrontal cortex of juvenile female rats. Environ Res. 2015;142:281–287. [DOI] [PubMed] [Google Scholar]
- 41.Hill CE, Sapouckey SA, Suvorov A, Vandenberg LN, Schumacher U. Developmental exposures to bisphenol S, a BPA replacement, alter estrogen-responsiveness of the female reproductive tract: a pilot study. Cogent Med. 2017:1317690. [PMC free article] [PubMed]
- 42.Catanese MC, Vandenberg LN. Bisphenol (BPS) alters maternal behavior and brain in mice exposed during pregnancy/lactation and their daughters. Endocrinology. 2017;158(3):516–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Crowley WR. Neuroendocrine regulation of lactation and milk production. Compr Physiol. 2015;5(1):255–291. [DOI] [PubMed] [Google Scholar]
- 44.Myers MM, Brunelli SA, Squire JM, Shindeldecker RD, Hofer MA. Maternal behavior of SHR rats and its relationship to offspring blood pressures. Dev Psychobiol. 1989;22(1):29–53. [DOI] [PubMed] [Google Scholar]
- 45.Stern JM, Johnson SK. Ventral somatosensory determinants of nursing behavior in Norway rats. I. Effects of variations in the quality and quantity of pup stimuli. Physiol Behav. 1990;47(5):993–1011. [DOI] [PubMed] [Google Scholar]
- 46.Catanese MC, Suvorov A, Vandenberg LN. Beyond a means of exposure: a new view of the mother in toxicology research. Toxicol Res. 2015;4:592–612. [Google Scholar]
- 47.Vandenberg LN, Maffini MV, Schaeberle CM, Ucci AA, Sonnenschein C, Rubin BS, Soto AM. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol. 2008;26(3-4):210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shyamala G, Chou YC, Louie SG, Guzman RC, Smith GH, Nandi S. Cellular expression of estrogen and progesterone receptors in mammary glands: regulation by hormones, development and aging. J Steroid Biochem Mol Biol. 2002;80(2):137–148. [DOI] [PubMed] [Google Scholar]
- 49.Neville MC, McFadden TB, Forsyth I. Hormonal regulation of mammary differentiation and milk secretion. J Mammary Gland Biol Neoplasia. 2002;7(1):49–66. [DOI] [PubMed] [Google Scholar]
- 50.Franklin K, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1997. [Google Scholar]
- 51.Catanese MC, Vandenberg LN. Low doses of 17a-ethinyl estradiol alter the maternal brain and induce stereotypies in CD-1 mice exposed during pregnancy and lactation. Reprod Toxicol. 2017;73:20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 53.Baxter FO, Neoh K, Tevendale MC. The beginning of the end: death signaling in early involution. J Mammary Gland Biol Neoplasia. 2007;12(1):3–13. [DOI] [PubMed] [Google Scholar]
- 54.Gustafsson JA, Warner M. Estrogen receptor beta in the breast: role in estrogen responsiveness and development of breast cancer. J Steroid Biochem Mol Biol. 2000;74(5):245–248. [DOI] [PubMed] [Google Scholar]
- 55.Haslam SZ, Shyamala G. Progesterone receptors in normal mammary glands of mice: characterization and relationship to development. Endocrinology. 1979;105(3):786–795. [DOI] [PubMed] [Google Scholar]
- 56.Feng Z, Marti A, Jehn B, Altermatt HJ, Chicaiza G, Jaggi R. Glucocorticoid and progesterone inhibit involution and programmed cell death in the mouse mammary gland. J Cell Biol. 1995;131(4):1095–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li M, Liu X, Robinson G, Bar-Peled U, Wagner KU, Young WS, Hennighausen L, Furth PA. Mammary-derived signals activate programmed cell death during the first stage of mammary gland involution. Proc Natl Acad Sci USA. 1997;94(7):3425–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Campo Verde Arboccó F, Sasso CV, Actis EA, Carón RW, Hapon MB, Jahn GA. Hypothyroidism advances mammary involution in lactating rats through inhibition of PRL signaling and induction of LIF/STAT3 mRNAs. Mol Cell Endocrinol. 2016;419:18–28. [DOI] [PubMed] [Google Scholar]
- 59.Ogawa S, Eng V, Taylor J, Lubahn DB, Korach KS, Pfaff DW. Roles of estrogen receptor-alpha gene expression in reproduction-related behaviors in female mice. Endocrinology. 1998;139(12):5070–5081. [DOI] [PubMed] [Google Scholar]
- 60.Aloisi AM, Della Seta D, Ceccarelli I, Farabollini F. Bisphenol-A differently affects estrogen receptors-alpha in estrous-cycling and lactating female rats. Neurosci Lett. 2001;310(1):49–52. [DOI] [PubMed] [Google Scholar]
- 61.Simerly RB, Young BJ. Regulation of estrogen receptor messenger ribonucleic acid in rat hypothalamus by sex steroid hormones. Mol Endocrinol. 1991;5(3):424–432. [DOI] [PubMed] [Google Scholar]
- 62.Gladen BC, Rogan WJ. DDE and shortened duration of lactation in a northern Mexican town. Am J Public Health. 1995;85(4):504–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Karmaus W, Davis S, Fussman C, Brooks K. Maternal concentration of dichlorodiphenyl dichloroethylene (DDE) and initiation and duration of breast feeding. Paediatr Perinat Epidemiol. 2005;19(5):388–398. [DOI] [PubMed] [Google Scholar]
- 64.Rogan WJ, Gladen BC, McKinney JD, Carreras N, Hardy P, Thullen J, Tingelstad J, Tully M. Polychlorinated biphenyls (PCBs) and dichlorodiphenyl dichloroethene (DDE) in human milk: effects on growth, morbidity, and duration of lactation. Am J Public Health. 1987;77(10):1294–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Weldon RH, Webster M, Harley KG, Bradman A, Fenster L, Davis MD, Hubbard A, Barr DB, Holland N, Eskenazi B. Serum persistent organic pollutants and duration of lactation among Mexican-American women. J Environ Public Health. 2010;2010:861757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Timmermann CA, Budtz-Jorgensen E, Petersen MS, Weihe P, Steuerwald U, Nielsen F, Jensen TK, Grandjean P. Shorter duration of breastfeeding at elevated exposures to perfluoroalkyl substances. Reprod Toxicol. 2017;68:164–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal concentrations of perfluorooctanesulfonate (PFOS) and perfluorooctanoate (PFOA) and duration of breastfeeding. Scand J Work Environ Health. 2010;36(5):413–421. [DOI] [PubMed] [Google Scholar]
- 68.Romano ME, Xu Y, Calafat AM, Yolton K, Chen A, Webster GM, Eliot MN, Howard CR, Lanphear BP, Braun JM. Maternal serum perfluoroalkyl substances during pregnancy and duration of breastfeeding. Environ Res. 2016;149:239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vandenberg LN, Luthi D, Quinerly D. Plastic bodies in a plastic world: multi-disciplinary approaches to study endocrine disrupting chemicals. J Clean Prod. 2017;140(1):373–385. [Google Scholar]
- 70.Neville MC, Walsh CT. Effects of xenobiotics on milk secretion and composition. Am J Clin Nutr. 1995;61(3, Suppl):687S–694S. [DOI] [PubMed] [Google Scholar]
- 71.Alonso-Magdalena P, García-Arévalo M, Quesada I, Nadal Á. Bisphenol-A treatment during pregnancy in mice: a new window of susceptibility for the development of diabetes in mothers later in life. Endocrinology. 2015;156(5):1659–1670. [DOI] [PubMed] [Google Scholar]
- 72.Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect. 2010;118(9):1243–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Greenberg ER, Barnes AB, Resseguie L, Barrett JA, Burnside S, Lanza LL, Neff RK, Stevens M, Young RH, Colton T. Breast cancer in mothers given diethylstilbestrol in pregnancy. N Engl J Med. 1984;311(22):1393–1398. [DOI] [PubMed] [Google Scholar]
- 74.Titus-Ernstoff L, Hatch EE, Hoover RN, Palmer J, Greenberg ER, Ricker W, Kaufman R, Noller K, Herbst AL, Colton T, Hartge P. Long-term cancer risk in women given diethylstilbestrol (DES) during pregnancy. Br J Cancer. 2001;84(1):126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eidelman AI. Breastfeeding and the use of human milk: an analysis of the American Academy of Pediatrics 2012 Breastfeeding Policy Statement. Breastfeeding Med. 2012;7(5):323–324. [DOI] [PubMed] [Google Scholar]
- 76.Gartner LM, Morton J, Lawrence RA, Naylor AJ, O’Hare D, Schanler RJ, Eidelman AI; American Academy of Pediatrics Section on Breastfeeding . Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496–506. [DOI] [PubMed] [Google Scholar]