Abstract
Objective
Antidepressant response onset is delayed in individuals with major depressive disorder (MDD). This study compared remission rates and time to remission onset for antidepressant medication delivered adjunctive to nightly time in bed (TIB) restriction of 6 hours (6h TIB) or 8 hours (8h TIB) for the initial two weeks.
Method
Sixty-eight adults with DSM-IV diagnosed MDD (25.4 ± 6.6 years of age, 34 women) were recruited from September 2009 to December 2012 in an academic medical center. Participants received 8 weeks of open-label fluoxetine 20–40 mg and were randomized to one of three TIB conditions for the first two weeks: 8h TIB (n=19); 6h TIB with a 2-hour bedtime delay (Late Bedtime, n=24); or 6h TIB with a 2-hour rise time advance (Early Risetime, n=25). Clinicians blinded to TIB condition rated symptom severity weekly. HAMD-17 rated symptom severity, remission rates, and remission onset were the primary outcomes.
Results
Mixed effects models indicated lower depression severity for the 8h TIB compared to the 6h TIB group overall (F=2.1, df=8, 226.9, p< .05), with 63.2% of 8h TIB compared to 32.6% of 6h TIB subjects remitting by Week 8 (X2(1) = 4.9, p < .05). Remission onset occurred earlier for the 8h TIB group (hazard ratio = 0.43, 95% CI 0.20 – 0.91, p < .03), with no differences between 6h TIB conditions.
Conclusions and Relevance
Two consecutive weeks of nightly 6h TIB does not accelerate or improve antidepressant response. Further research is needed to determine whether adequate sleep opportunity is important to antidepressant treatment response.
INTRODUCTION
Major depressive disorder (MDD) affects roughly 16.5% of U.S. adults in their lifetime1 and is a leading cause of disease burden. Evidence-based pharmacotherapy is widely available, but treatment response time is delayed and failure rates are as high as 30–40%.2–4 Novel therapies are needed to accelerate and improve antidepressant response.
One night of total sleep deprivation improves mood in 60% of MDD patients;5 however, relapse following recovery sleep occurs in up to 80% of unmedicated patients (cf5,6). Serial repetition can sustain the positive mood response to total sleep deprivation, but relapse remains likely, particularly without concomitant antidepressant treatment.7 More recent studies combining total sleep deprivation with other chronotherapeutic interventions (light therapy and sleep schedule adjustments) and medication8 have shown promise, but these treatments are complex to administer.
Single-night partial sleep deprivation (PSD, 4–5 hours of sleep) has been explored as an alternative to total sleep deprivation. Studies found next-day response rates to PSD equivalent to total sleep deprivation9 with improved patient tolerance. Wakefulness during the second half of the night (late PSD, when rapid eye movement [REM] sleep predominates) is often superior to wakefulness in the first half (early PSD),10 but perhaps not if total sleep time is equivalent.11,12 Repetition of PSD during the initial 1–4 weeks of antidepressant therapy can accelerate treatment response13–15 and quality of life improvement,16 but these studies were conducted inpatient or in a laboratory setting and did not include sleep control conditions or sufficient follow-up after the PSD procedures. Ideal sleep-focused strategies would be clinically efficacious and maximize patient feasibility by allowing treatments to be carried out safely in the home environment. To date, no study has assessed the effects of a modest repeated restriction of time in bed on treatment response in outpatients with depression initiating an antidepressant treatment trial.
The primary aim of the present study was to compare the mood effects of two weeks of 6 hours time in bed (6h TIB) to 8h TIB delivered adjunctive to antidepressant therapy in outpatient adults with MDD. A secondary aim was to investigate whether the timing of the TIB restriction was important by randomizing subjects to either a two-hour delay of bedtime (Late Bedtime) or a two-hour advance of risetime (Early Risetime). We hypothesized that symptom improvement would be greater and remission onset would be earlier for MDD subjects randomized to antidepressant therapy plus 6h TIB. We expected that, relative to the 8h TIB condition, the Late Bedtime group would experience an increase in slow wave sleep, while the Early Risetime group would experience a reduction in REM sleep. Since most prior studies have shown late night sleep deprivation to be superior to early night sleep deprivation, we hypothesized that symptom improvement would be greater in the Early Risetime compared to the Late Bedtime condition.
METHOD
Participants
Participants were recruited from September 2009 to December 2012 through advertisements and clinical referrals. Inclusion criteria were: (1) 18–65 years old; (2) DSM-IV diagnosis of MDD of at least moderate severity (≥18 on the 17-item Hamilton Rating Scale for Depression, HAMD-17); and (3) habitual TIB of 7–10 hours nightly. Exclusion criteria included: (1) lifetime DSM-IV diagnosis of bipolar disorder, psychotic disorder, substance or alcohol dependence, eating disorder, post-traumatic stress disorder, or obsessive-compulsive disorder; (2) past 6-month DSM-IV alcohol abuse diagnosis; (3) medical conditions associated with depression (e.g., hypothyroidism) or interfering with sleep; (4) sleep disorder other than insomnia, based on the International Classification of Sleep Disorders, 2nd Edition (ICSD-2);17 (5) prescription or non-prescription medication for sleep or depression; (6) failed fluoxetine trial within the past six months; (7) overnight shift work; (8) pregnancy, breastfeeding, or inadequate contraception in women of childbearing potential; (9) known contraindication to fluoxetine; and (10) abnormal laboratory values. Subjects had to be free of antidepressants for ≥2 weeks (≥4 weeks for longer-acting antidepressants). Participants underwent an initial telephone screen and in-laboratory psychiatric, medical, and sleep screening. Study procedures were approved by the University of Michigan Medical School Institutional Review Board and participants provided written informed consent.
Study Design and Procedures
In this randomized, controlled parallel trial, participants received open-label fluoxetine 20–40 mg for eight weeks and were randomized (1:1:1) to one of three TIB conditions for the initial two weeks: (1) 8h TIB; (2) 6h TIB, with a two-hour delay of bedtime (Late Bedtime); or (3) 6h TIB with a two-hour advance of Risetime (Early Risetime). After enrollment but prior to the first in-laboratory night, subjects maintained a regularized 8h TIB schedule at home for 5–7 days, which was based on their self-reported preferred bedtimes and rise times. Alcohol/drug use and napping were prohibited and habitual caffeine intake was permitted before noon. Schedule compliance was confirmed with daily sleep/wake diaries and with wrist-worn actigraphy (Actiwatch-2™, Philips Respironics, Murrysville, PA).
Following the at-home 8h TIB schedule, participants spent seven nights and mornings total in the sleep laboratory: three before starting fluoxetine, two after the two-week TIB condition, and two after eight weeks of fluoxetine treatment. The first two pre-fluoxetine in-laboratory nights were adaptation and baseline nights, respectively. Subjects maintained the 8h TIB schedule and were assessed for sleep disorders on the adaptation night using standard procedures.18 Six subjects were excluded for suspicion of a sleep-related breathing disorder, based on ICSD-2 criteria. On the third pre-fluoxetine night, subjects were randomized to one of the three TIB conditions (8h TIB, Late Bedtime, Early Risetime) and maintained this schedule at home for 14.0 ± 1.6 nights until returning for two additional in-laboratory nights. Participants underwent two final in-laboratory nights following the eight-week open label antidepressant trial.
Subjects took the first 20 mg dose of fluoxetine following the first TIB condition night and then daily for eight weeks, with a possible dose increase to 40 mg after Week 4 based on clinician-rated response. Pills were counted at each in-laboratory visit to evaluate compliance.
Blinded clinician ratings of mood were completed at baseline and weekly thereafter. Subject-rated depression scales were completed at baseline and Weeks 1, 2, 4, and 8; quality of life ratings were completed at baseline and Weeks 4 and 8. A two-hour neurocognitive test battery was completed in the morning following each in-laboratory sleep assessment (results not reported herein).
Outcome Measures
The HAMD-1719,20 was the primary outcome measure. Symptom changes were evaluated with the total HAMD-17 score minus the three sleep items (range 0–46)21,22 and remission was defined as a score ≤7.21,22 Removal of the three items from the HAMD-17 scale ensures that any observed mood improvements cannot be attributable to sleep-related improvements from the TIB manipulations. The Clinical Global Impressions-Improvement subscale (CGI-I)23 was a secondary measure of clinician-rated improvement.
The 16-item Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR) was the subject-rated symptom severity measure.24 Symptom severity scores minus the sleep item (range 0–24) and remission (score ≤5) were the primary outcomes.3
The 12-item Short-Form Health Survey (SF-12) was included as a quality of life measure.25 The primary dependent variables are the physical and mental composite scores, which range from 0 to 100, with higher scores indicative of better quality of life (mean = 50.0, standard deviation = 10.0).
Polysomnography
Electrophysiological signals were collected via standard polysomnography (PSG) montage26 using the Vitaport 3 (TEMEC Instruments, The Netherlands) digital PSG acquisition system. PSG records were scored visually off-line in 30-sec epochs using standard criteria26 by sleep technicians blinded to group assignment. Changes in the following sleep variables from baseline to Week 2 were evaluated: total sleep time (total time asleep during the night); sleep efficiency (total sleep time/total recording time*100); sleep latency (time in minutes to initial sleep onset); number of arousals; % of Stages 1, 2, SWS (Stages 3 and 4), and REM; and latency to REM sleep (time in minutes to first REM episode).
Actigraphy
Actigraphs were set at a sampling rate of 1 minute and worn on the non-dominant wrist during the pre-laboratory baseline nights and during the two-week TIB condition to assess compliance. Sleep/wake activity was estimated using Actiware® – Sleep software (Version 5.0) in combination with daily sleep/wake diaries. We followed established procedures for scoring actigraphy.27 The primary outcomes were TIB and total sleep time.
Statistical Analyses
Continuous variables were analyzed in SPSS 20.0 (IBM Corporation, Armonk, NY) with linear mixed models using Akaike’s Information Criterion (AIC) to evaluate goodness of fit for covariance structures.28 The main model was parameterized to evaluate the effects of TIB Condition (6h TIB vs. 8h TIB), Visit (Baseline, Weeks 1 through 8), and their interaction (Condition by Visit), adjusting for baseline covariates. Significant main effects or interactions favoring the 6h TIB over the 8h TIB condition on mood outcomes were further evaluated with post-hoc analyses comparing the three TIB conditions separately. Because of a-priori hypothesized differences in SWS and REM between the two 6h TIB conditions, the model analyzing PSG outcomes included all three levels for TIB Condition. Differences in time to remission onset were evaluated using discrete time survival analyses using Stata 13 (StataCorp LP, College Station, TX). Data are reported as mean ± standard deviation or mean with 95% confidence interval [CI], with significance level set at 0.05.
RESULTS
Recruitment and Retention
A CONSORT diagram of participant flow through the protocol is shown in Figure 1. Overall, 58 subjects (85.2%) completed the two-week TIB condition and 54 subjects (79.4%) completed the eight-week study. Eleven of the 68 randomized subjects (16.2%) discontinued participation (3 8h TIB, 5 Late Bedtime, 3 Early Risetime) and three subjects (4.4%) were discontinued for protocol violations. Dropouts did not differ from completers on demographic or clinical variables.
Figure 1.
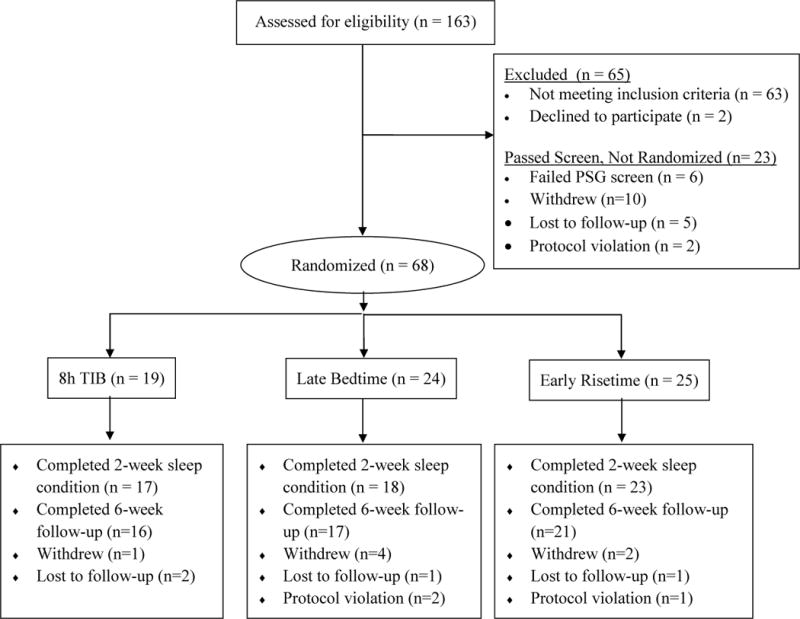
CONSORT diagram
Descriptive data for all randomized subjects are summarized in Table 1. The 8h TIB group had more years of education than the Late Bedtime and Early Risetime groups (p < .005).
Table 1.
Characteristics of randomized participants by sleep condition.
| 8h TIB (n=19) |
6h TIB (n=49)
|
Total (n=68) |
||||||
|---|---|---|---|---|---|---|---|---|
| Late Bedtime (n=24) |
Early Risetime (n=25) |
|||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 26.4 | 7.4 | 24.4 | 5.6 | 25.7 | 7.0 | 25.4 | 6.6 |
| Education (years)b | 16.0 | 2.0 | 14.4 | 1.7 | 14.5 | 1.5 | 14.9 | 1.8 |
| MDD Age of Onset (years) | 17.7 | 6.1 | 16.2 | 6.3 | 16.2 | 7.6 | 16.6 | 6.7 |
| Current episode (months) | 10.2 | 7.6 | 14.8 | 22.2 | 9.5 | 8.5 | 11.6 | 14.7 |
| n | % | n | % | n | % | n | % | |
| Sex | ||||||||
| Male | 12 | 63.1 | 11 | 45.8 | 11 | 44.0 | 34 | 50.0 |
| Female | 7 | 36.8 | 13 | 54.2 | 14 | 56.0 | 34 | 50.0 |
| Race | ||||||||
| Black/African American | 3 | 15.8 | 3 | 12.5 | 2 | 8.0 | 8 | 11.8 |
| White | 14 | 73.7 | 18 | 75.0 | 20 | 80.0 | 52 | 76.5 |
| Other | 2 | 10.5 | 3 | 12.5 | 3 | 12.0 | 8 | 11.8 |
| Marital Status | ||||||||
| Unmarried | 15 | 78.9 | 21 | 87.5 | 18 | 72.0 | 54 | 79.4 |
| Married/Partnered | 4 | 21.1 | 2 | 8.3 | 5 | 20.0 | 11 | 16.2 |
| Separated/Divorced | 0 | 0.0 | 1 | 4.2 | 2 | 8.0 | 3 | 4.4 |
| Employment Status | ||||||||
| Full-time employment | 4 | 21.1 | 4 | 16.7 | 3 | 12.0 | 11 | 16.2 |
| Part-time employment | 8 | 42.1 | 9 | 37.5 | 9 | 36.0 | 26 | 38.2 |
| Unemployed | 7 | 36.8 | 11 | 45.8 | 13 | 52.0 | 31 | 45.6 |
| Positive family history of MDD | 13 | 68.4 | 19 | 79.2 | 17 | 68.0 | 49 | 72.1 |
| MDD treatment history | ||||||||
| None | 6 | 31.6 | 8 | 33.3 | 7 | 28.0 | 21 | 30.9 |
| Medication | 5 | 26.3 | 1 | 4.2 | 3 | 12.0 | 9 | 13.2 |
| Psychotherapy | 3 | 15.8 | 2 | 8.3 | 2 | 8.0 | 7 | 10.3 |
| Both | 4 | 21.0 | 12 | 50.0 | 11 | 44.0 | 27 | 39.7 |
| Comorbidity | ||||||||
| Medical | 7 | 36.8 | 8 | 33.3 | 13 | 52.0 | 28 | 41.0 |
| Psychiatrica | 4 | 21.0 | 7 | 29.2 | 6 | 24.0 | 17 | 25.0 |
Lifetime bipolar disorder, psychotic disorder, substance or alcohol dependence, eating disorder, post-traumatic stress disorder, and obsessive-compulsive disorder and post 6-month substance or alcohol abuse were exclusionary
8h TIB group had significantly more years of education than either the Late Bedtime or Early Risetime group (p < .005)
Abbreviations
MDD = Major depressive disorder
6h TIB = 6 hours time in bed
8h TIB = 8 hours time in bed
Clinician- and Subject-Rated Symptom Changes
Summary data for clinician- and subject-rated symptom measures are shown in Table 2. Linear mixed models indicated a significant Condition by Visit interaction for the HAMD-17 (F=2.1, df=8, 226.9, p < .05). HAMD-17 ratings were significantly lower (indicating less depression) for 8h TIB compared to 6h TIB subjects at Weeks 3, 5, and 6, with trends at Weeks 2, 4, and 7. After two weeks, HAMD-17 ratings had improved by 36.0 ± 22.6% vs. 22.7 ± 31.0% for the 8h TIB and 6h TIB groups, respectively, but the proportion of subjects in remission at Week 2 did not differ. By Week 8, however, 12/19 (63.2%) 8h TIB subjects had remitted compared to 16/49 (32.6%) 6h TIB subjects (X2(1) = 4.9, p < .05). Clinician ratings on the Clinical Global Impressions – Improvement subscale indicated an overall Visit effect (F=16.1, df = 195.8, p < .001), but no TIB Condition by Visit interaction.
Table 2.
Clinician- and subject-rated scores on mood and clinical improvement outcomes by TIB condition.
| Analysis | |||||||
|---|---|---|---|---|---|---|---|
| Measure and Assessment | 8h TIB (n=19) |
6h TIB (n=49) |
β estimate§ |
SE | p value |
||
| Clinician-Rated | Mean | SD | Mean | SD | |||
| HAMD-17 (minus sleep items)a | |||||||
| Baseline | 18.2 | 2.6 | 18.0 | 2.2 | |||
| Week 1 | 13.2 | 4.0 | 15.1 | 4.1 | 2.2 | 1.6 | 0.20 |
| Week 2 | 11.3 | 3.4 | 13.9 | 5.3 | 2.9 | 1.6 | 0.08 |
| Week 3 | 6.9 | 3.5 | 11.8 | 5.0 | 5.8 | 1.6 | 0.001 |
| Week 4 | 8.5 | 5.5 | 11.0 | 5.2 | 2.9 | 1.6 | 0.07 |
| Week 5 | 7.2 | 4.7 | 10.6 | 6.4 | 3.8 | 1.6 | 0.02 |
| Week 6 | 4.9 | 4.8 | 8.7 | 5.4 | 4.4 | 1.6 | 0.006 |
| Week 7 | 6.4 | 5.5 | 8.9 | 6.0 | 2.6 | 1.6 | 0.09 |
| Week 8 | 6.1 | 6.7 | 7.1 | 4.7 | 1.2 | 1.3 | 0.35 |
| CGI - Improvement | |||||||
| Week 1 | 3.2 | 1.3 | 3.2 | 0.7 | |||
| Week 2 | 2.7 | 0.6 | 2.9 | 0.9 | 0.2 | 0.3 | 0.66 |
| Week 3 | 2.1 | 0.8 | 2.8 | 1.1 | 0.7 | 0.3 | 0.04 |
| Week 4 | 2.3 | 1.2 | 2.6 | 1.0 | 0.3 | 0.3 | 0.42 |
| Week 5 | 2.4 | 1.4 | 2.5 | 1.2 | 0.1 | 0.3 | 0.67 |
| Week 6 | 1.8 | 1.0 | 2.2 | 1.0 | 0.5 | 0.4 | 0.14 |
| Week 7 | 1.9 | 1.1 | 2.1 | 0.9 | 0.3 | 0.3 | 0.44 |
| Week 8 | 1.8 | 1.0 | 1.8 | 0.8 | 0.06 | 0.3 | 0.84 |
| Subject-Rated | |||||||
| QIDS-SR (minus sleep item)b | |||||||
| Baseline | 10.8 | 3.3 | 11.4 | 4.0 | |||
| Week 1 | 6.5 | 4.4 | 9.2 | 4.4 | 1.9 | 1.3 | 0.15 |
| Week 2 | 5.1 | 2.9 | 7.8 | 4.0 | 2.3 | 1.3 | 0.07 |
| Week 4 | 4.9 | 3.5 | 6.7 | 3.8 | 1.2 | 1.2 | 0.33 |
| Week 8 | 3.6 | 4.4 | 5.0 | 3.9 | 0.8 | 1.1 | 0.45 |
| SF-12: Physical Composite | |||||||
| Baseline | 55.8 | 5.3 | 54.7 | 10.0 | |||
| Week 4 | 56.1 | 4.9 | 51.9 | 9.2 | −1.2 | 2.5 | 0.65 |
| Week 8 | 53.7 | 6.6 | 53.3 | 8.2 | 1.5 | 2.6 | 0.56 |
| SF-12: Mental Composite | |||||||
| Baseline | 24.8 | 9.1 | 26.8 | 8.3 | |||
| Week 4 | 38.5 | 14.7 | 34.4 | 11.2 | −6.2 | 3.5 | 0.08 |
| Week 8 | 44.8 | 12.5 | 40.0 | 11.2 | −7.9 | 3.8 | 0.04 |
HAMD-17 minus sleep range is 0–46;
QIDS-SR minus sleep range is 0–24;
β estimate comparing 8h TIB vs. combined 6h TIB conditions with Baseline as reference (Visit 1 reference for CGI-I).
Note: boldface type denotes significant findings
Abbreviations
CGI=Clinical Global Impressions scale
HAMD-17= 17-item Hamilton Rating Scale for Depression
QIDS-SR=Quick Inventory of Depressive Symptomatology-Self-Rated
SF-12= 12-Item Short-Form Health Survey
6h TIB = 6 hours time in bed
8h TIB = 8 hours time in bed
No overall TIB Condition by Visit interaction was evident for QIDS-SR scores, but by Week 2, scores were improved by 49.9 ± 31.4% for 8h TIB subjects compared to 24.5 ± 43.6% in 6h TIB conditions (t=2.2, df = 56, p<.05). By Week 8, symptom improvement was equivalent between the two conditions. SF-12 mental health composite scores were significantly more improved in the 8h TIB group by Week 8 (p < .04) with a trend for significantly more improvement by Week 4.
Onset of Symptom Remission
The HAMD-17 remission survival functions for the 8h TIB and 6h TIB conditions differed significantly (hazard ratio [HR] 0.43, 95% CI 0.20 – 0.91, p < .03), with remission onset occurring after 6.4 ± 2.2 weeks for the 8h TIB group compared to 7.3 ± 1.5 weeks for the 6h TIB conditions (Figure 2). Onset of QIDS-SR remission (8h TIB: 6.7 ± 2.3 weeks vs. 6h TIB: 7.5 ± 1.8 weeks) was earlier for 8h TIB compared to 6h TIB subjects, but the survival functions were not significantly different.
Figure 2.
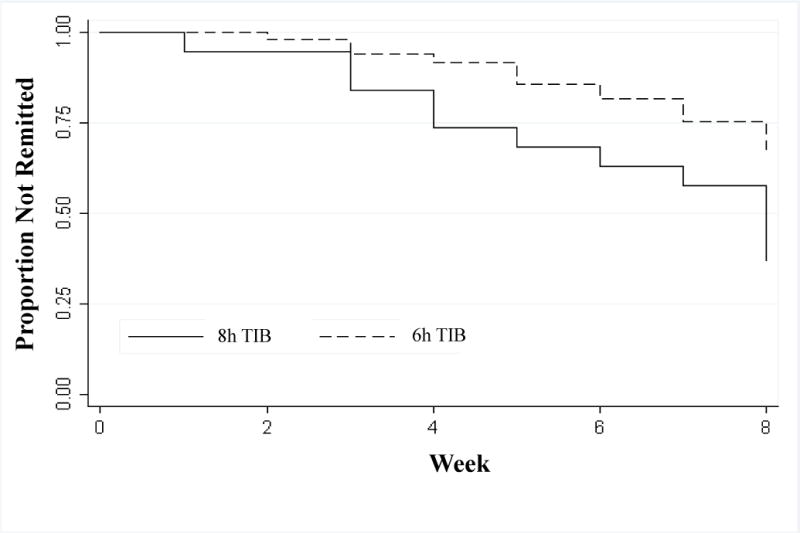
Remission survival curves across 8 weeks for adults with MDD receiving fluoxetine 20–40 mg and randomized to 8 hours time in bed (8h TIB) or 6 hours time in bed (6h TIB) during the initial two weeks of therapy.
Polysomnography
Polysomnography outcomes are displayed in Table 3. Linear mixed models indicated a significant increase in slow-wave sleep percent at Week 2 in the Late Bedtime condition compared to baseline (β = 8.4, SE = 2.4, p < .001). Post-hoc analyses indicated that, from baseline to Week 2, slow-wave sleep percent increased by 4.2 ± 11.2% for Late Bedtime subjects compared to a 4.0 ± 5.1% decrease in the 8h TIB condition (p < .002). REM sleep percent at Week 2 was significantly lower in the Early Risetime group compared to baseline (β = −5.3, SE = 2.6, p < .05). Post-hoc analyses indicated that REM sleep percent declined more in the Early Risetime compared to the 8h TIB (−6.9 ± 5.5% vs. −1.2 ± 11.3%, p<.05), but not the Late Bedtime condition. Across sleep conditions, light Stage 1 sleep was 3.2 ± 4.4% higher at Week 2 relative to baseline (β=4.4, SE=1.1, p < .001) and REM latency was 45.4 ± 59.9 minutes longer (β=31.9, SE=14.0, p < .03), consistent with the known effects of fluoxetine on sleep.
Table 3.
Polysomnography outcomes at baseline and Week 2 by TIB condition.
| 6h TIB (n=41)
|
|||||||
|---|---|---|---|---|---|---|---|
| Sleep Variable | 8h TIB (n=19) |
Late Bedtime (n=24) |
Early Risetime (n=25) |
TIB Condition by Visit p-value§ | |||
| Mean | SD | Mean | SD | Mean | SD | ||
| Bedtimes (hh:mm) | <.001 | ||||||
| Baseline | 23:42 | 1:01 | 23:42 | 1:06 | 23:57 | 1:02 | |
| Week 2 | 23:48 | 1:20 | 1:42 | 1:06 | 23:57 | 1:02 | |
| Risetimes (hh:mm) | <.001 | ||||||
| Baseline | 7:42 | 1:01 | 7:42 | 1:06 | 7:57 | 1:03 | |
| Week 2 | 7:42 | 1:01 | 7:42 | 1:06 | 5:57 | 1:03 | |
| Total sleep time (mins) | <.001 | ||||||
| Baseline | 430.1 | 60.9 | 439.2 | 36.3 | 444.0 | 19.7 | |
| Week 2 | 435.4 | 25.7 | 337.8 | 24.7 | 332.9 | 17.0 | |
| Sleep efficiency (%) | 0.70 | ||||||
| Baseline | 89.7 | 12.6 | 91.7 | 7.6 | 92.8 | 4.0 | |
| Week 2 | 90.8 | 5.3 | 93.3 | 5.0 | 92.9 | 4.7 | |
| Sleep latency (mins) | 0.64 | ||||||
| Baseline | 25.2 | 60.2 | 16.3 | 32.3 | 11.5 | 7.9 | |
| Week 2 | 17.8 | 22.2 | 6.8 | 13.4 | 8.3 | 5.2 | |
| Number of arousals | 0.17 | ||||||
| Baseline | 19.2 | 8.2 | 18.0 | 10.8 | 16.8 | 7.5 | |
| Week 2 | 22.0 | 9.2 | 17.0 | 7.6 | 14.1 | 7.0 | |
| Stage 1 (%) | 0.25 | ||||||
| Baseline | 5.0 | 3.0 | 3.9 | 3.2 | 4.5 | 3.2 | |
| Week 2 | 9.5 | 6.1 | 5.1 | 4.0 | 7.3 | 4.8 | |
| Stage 2 (%) | 0.027 | ||||||
| Baseline | 53.8 | 9.5 | 54.3 | 8.1 | 54.2 | 7.2 | |
| Week 2 | 53.1 | 8.6 | 49.5 | 9.0 | 57.8 | 8.6 | |
| Slow wave sleep (%) | 0.004 | ||||||
| Baseline | 14.4 | 9.7 | 14.6 | 6.9 | 12.8 | 8.0 | |
| Week 2 | 11.6 | 7.1 | 20.3 | 12.1 | 12.8 | 7.7 | |
| REM sleep (%) | 0.08 | ||||||
| Baseline | 22.1 | 7.6 | 23.5 | 4.7 | 25.0 | 6.1 | |
| Week 2 | 20.4 | 5.3 | 21.0 | 5.6 | 18.0 | 6.1 | |
| REM latency (mins) | 0.52 | ||||||
| Baseline | 98.4 | 42.5 | 78.1 | 33.0 | 70.3 | 21.1 | |
| Week 2 | 130.0 | 55.8 | 132.2 | 61.4 | 121.1 | 49.9 | |
TIB Condition includes all three levels (8h TIB, Late Bedtime, Early Risetime)
Note: boldface type denotes significant findings
Abbreviations
mins = minutes
REM = Rapid eye movement sleep
6h TIB = 6 hours time in bed
8h TIB = 8 hours time in bed
Compliance
Actigraphy outcomes (n=58) at baseline and during the 2-week TIB manipulation are shown in Table 4. All three groups showed good compliance with the baseline 8h TIB schedule, with no significant group differences in any actigraphy parameter. During the 2-week experimental manipulation, TIB was 8.0 ± 0.5 hours for 8h TIB subjects and 6.9 ± 1.2 hours for 6h TIB subjects (Late Bedtime 6.7 ± 1.0 hours, Early Risetime 7.0 ± 1.3 hours) (t = 3.8, df = 56, p < .001). Daily deviation from assigned TIB was 0.9 ± 29.7 minutes for the 8h TIB group, 41.9 ± 58.8 minutes for the Late Bedtime group, and 60.1 ± 80.4 minutes for the Early Risetime group (p < .01 for 8h TIB vs. Early Risetime). More 8h TIB (82.4%) than 6h TIB (53.7%) subjects were within 30 minutes of their assigned TIB schedule at the end of the two-week period (X2 = 4.2, df = 1, p < .04), however, including compliance as a covariate in analyses of primary outcomes did not change the findings. Actigraphically-measured nightly total sleep time was 6.6 ± 1.0 for the 8h TIB group and 5.9 ± 1.1 for the 6h TIB groups (Late Bedtime 5.7 ± 0.9 hours, Early Risetime 6.1 ± 1.2 hours) (t = 2.2, df = 56, p < .03). During the 2-week experimental phase, sleep latency did not differ between TIB conditions, but wake after sleep onset was significantly longer in the 8h compared to the 6h TIB condition (44.8 ± 23.2 vs. 28.3 ± 18.1 minutes, t=2.8, df=55, p < .006).
Table 4.
Actigraphy outcomes at baseline and Week 2 by TIB condition.
| 6h TIB (n=41)
|
|||||||
|---|---|---|---|---|---|---|---|
| Actigraphy Variable | 8h TIB (n=17) |
Late Bedtime (n=18) |
Early Risetime (n=23) |
8h vs. 6h TIB p-value |
|||
| Mean | SD | Mean | SD | Mean | SD | ||
| Time in Bed (hh:mm) | |||||||
| Baseline | 8:01 | 0:06 | 7:58 | 0:07 | 8:00 | 0:09 | 0.53 |
| Week 2 | 8:01 | 0:30 | 6:42 | 0:59 | 7:00 | 1:20 | <0.001 |
| Total sleep time (hh:mm) | |||||||
| Baseline | 6:20 | 0:50 | 6:27 | 0:44 | 6:39 | 0:30 | 0.31 |
| Week 2 | 6:37 | 1:00 | 5:41 | 0:53 | 6:09 | 1:10 | 0.03 |
| Sleep efficiency (%) | |||||||
| Baseline | 79.3 | 10.6 | 80.8 | 9.2 | 83.2 | 6.2 | 0.31 |
| Week 2 | 82.6 | 10.4 | 85.3 | 9.4 | 88.0 | 6.5 | 0.10 |
| Sleep latency (mins) | |||||||
| Baseline | 24.2 | 26.1 | 33.2 | 15.0 | 22.1 | 19.3 | 0.63 |
| Week 2 | 15.2 | 13.3 | 10.4 | 11.2 | 8.4 | 8.4 | 0.07 |
| Wake after Sleep Onset (mins) | |||||||
| Baseline | 52.4 | 28.0 | 43.4 | 21.4 | 41.3 | 23.2 | 0.18 |
| Week 2 | 44.8 | 23.2 | 29.3 | 17.5 | 27.5 | 18.9 | 0.006 |
Note: boldface type denotes significant findings
Abbreviations
mins = minutes
6h TIB = 6 hours time in bed
8h TIB = 8 hours time in bed
No differences were evident in medication compliance; in the percentage of participants who increased to fluoxetine 40 mg (8h TIB 42.1% vs. Late Bedtime 54.2% vs. Early Risetime 48.0%); or in the timing of fluoxetine dose increase.
DISCUSSION
This randomized controlled trial found that a 6h TIB schedule during the first two weeks of antidepressant therapy did not augment treatment response in young adults with MDD. Instead, depressed subjects who were provided an 8h TIB schedule had greater clinician-rated symptom improvement, were more likely to achieve remission after 8 weeks (75% vs. 42%), and experienced symptom remission onset one week earlier. These effects were not due to better medication compliance or to a higher medication dose in the 8h TIB group. Importantly, objective compliance monitoring indicated that subjects were compliant with the 8h TIB schedule, but subjects assigned to the 6h TIB schedule were not. To our knowledge, this study is the first to evaluate experimentally a modest repeated TIB restriction on antidepressant treatment response.
Compared to the 6h TIB condition, 8h TIB subjects experienced greater clinician-rated depression symptom improvement beginning at Week 3, 75% vs 42% remission rates at the end of treatment, and onset of remission after 6.4 vs. 7.3 weeks. The failure of TIB restriction to accelerate or augment antidepressant response contrasts with uncontrolled inpatient repeated partial sleep deprivation studies13–15 but is consistent with one of the few randomized controlled trials to evaluate whether one night of total sleep deprivation could accelerate response to paroxetine in older adults with depression.29 Given previous findings, it is conceivable that a nightly TIB restriction dose greater than 2 hours was needed or that a 6h TIB schedule of longer than two weeks was necessary to produce beneficial mood effects. However, previous studies were typically conducted in inpatient or laboratory settings, which allow for controlled and safe delivery of sleep deprivation, but which are also impractical for outpatient practice. We were fundamentally interested in evaluating a more modest TIB restriction that has been used in experimental sleep deprivation studies,30 is commonly used in behavioral sleep medicine outpatient practice,31 and that would be feasible and straightforward to deliver in outpatient psychiatric settings.
Our study is the first to demonstrate that adequate sleep opportunity may accelerate and augment treatment response, although further studies are needed to address this question directly. At a minimum, our findings raise the possibility that consideration of TIB may be relevant in the initial stages of antidepressant medication therapy. We compared the trajectory of HAMD-17 score changes in our study with a previous 8-week open label study of fluoxetine 20 mg/day in MDD outpatients.2 That study found that the onset of treatment response occurred in 26.0% of subjects; onset was defined as a decrease of at least 30% on the HAMD-17 without a subsequent increase that led to a final decrease of 50% by 8 weeks. Using a similar definition, we found that 8/19 (42.1%) 8h TIB subjects compared to only 6/48 (12.2%) 6h TIB subjects experienced an onset of response by Week 2 (X2(1) = 7.5, p < .006). These findings suggest that encouraging adequate TIB accelerated the onset of response while restricting TIB delayed it. These findings additionally complement our analyses indicating that remission onset occurred almost one week earlier in the 8h TIB group. It is notable that the 75% remission rate for the 8h TIB group after 8 weeks is higher than most randomized controlled antidepressant trials,3,32 highlighting the need for replication. In addition, while the overall and speed of treatment response on the subject-rated depression measure did not differ significantly between groups, a similar pattern of results was evident. The less robust change in self-reported depression measures has been found previously,13,33–36 but could also be reflective of the less frequent subject-rated measurement. Our findings do highlight the importance of continued clinical follow-up after any sleep manipulation has ended to assess potential longer-term or delayed mood effects.
A secondary aim was to determine the importance of sleep deprivation timing, but we did not analyze the symptom severity effects separately by TIB condition because 8h TIB subjects showed greater improvements on all major mood outcomes. Sleep architecture changes with the TIB manipulations, however, were in the expected direction based on objective polysomnography. Specifically, Late Bedtime subjects had significant increases in SWS at Week 2, while the Early Risetime group showed a reduction in REM sleep. The PSG findings for the 8h TIB group are consistent with previous studies evaluating the effects of fluoxetine on objective sleep parameters in depressed subjects after two weeks of medication.37–40 Because the 8h TIB group had a better mood response, our findings do not support SWS increase or REM sleep reduction as likely mechanisms involved in any therapeutic effects of sleep deprivation, although mechanisms associated with restricted TIB may differ from those associated with responses to total and partial sleep deprivation. The existing literature on the role of sleep-deprivation-induced sleep architecture changes in antidepressant response is mixed. For example, a landmark study by Vogel and colleagues41 found that patients deprived of REM sleep for three consecutive weeks showed more mood improvement than Non-REM-deprived patients, but these results have not been replicated. Similarly, early studies showing that restricting wakefulness to the second half of the night (when REM sleep predominates) was more effective than so-called early PSD (i.e., staying awake until 1:30 and then initiating sleep) have since been challenged.11,12,42 It is notable that the 8h TIB group was the only group to experience a reduction in slow wave sleep at Week 2 relative to baseline (4.0 ± 5.1% less). In a recent report, Landsness and colleagues43 used acoustic stimuli to reduce slow wave sleep by 54% after one night relative to baseline (without reducing total sleep time) in 17 non-medicated depressed adults. The results indicated that next-day clinician- and self-rated depression scores decreased by 27% and 10%, respectively. Thus, future experimental studies are needed to resolve whether total sleep time, specific sleep stages, the timing, and/or the quality of sleep are involved in antidepressant treatment response.
Actigraphy monitoring indicated good compliance overall with the 8h TIB schedule during the 2-week experimental phase (TIB of 8.0 ± 0.5 hours), while subjects in the 6h TIB group spent nearly an hour more TIB each night than prescribed, despite showing excellent compliance with their baseline 8h TIB schedule before antidepressant therapy initiation. Importantly, however, medication compliance was not different among the groups and remission rates for the 6h TIB conditions at the end of the 8-week trial were consistent with other studies. The TIB schedule non-compliance by 6h TIB participants may have contributed to the small group differences in total sleep time (0.7 hours difference on average), particularly between the 8h TIB and Late Bedtime groups. The actigraphy findings highlight the challenge of maintaining a restricted TIB schedule over time, raising questions about the feasibility of more intensive sleep deprivation protocols for depression (e.g., repeated wake therapy or chronotherapeutic interventions), despite recent promising findings.8 In addition to evaluating efficacy, future sleep deprivation studies in depression should monitor and report on adherence with adjunctive therapies (e.g., light therapy, sleep time stabilization) to measure the feasibility of these interventions.
The moderate sample size is a limitation as we could not perform subgroup analyses to evaluate moderators of treatment response. In addition, the sample was largely young, healthy, Caucasian men and women with depression; thus our findings may not generalize to other depressed samples. Subjects’ knowledge that they were receiving pharmacotherapy may have contributed to the higher response and remission rates. Moreover, we could not blind subjects to TIB schedule assignment; therefore subject expectancies may have influenced the results. TIB schedule assignment additionally did not take into account circadian phase information; thus the timing of the assigned TIB schedule relative to circadian preference could have affected the outcomes. In addition, differential amounts of environmental light exposure among the three groups, either during the experimental TIB manipulation or during the subsequent six weeks, could have specifically contributed to antidepressant treatment response and should be controlled more closely in future studies. Finally, we included limited measurement of sleep patterns or other potential moderators (e.g., comorbid psychiatric symptoms, diurnal mood variation) after Week 2; thus we cannot speculate on potential contributors to group differences between Weeks 2 and 8.
In summary, we found that a nightly 6h TIB schedule during the initial two weeks of antidepressant therapy did not accelerate or augment treatment response in young adults with depression; instead, our findings raise the possibility that adequate TIB duration may positively impact treatment response. Future studies that optimize and/or extend sleep duration while initiating antidepressant therapy are needed to address this question directly. In addition, more work is needed with larger, more ethnically diverse and older samples. Future treatment studies should systematically include measures to identify potentially important clinical moderators (e.g., diurnal mood variation) and sleep-related moderators (e.g., circadian preference, insomnia) of antidepressant treatment response in addition to evaluation of potential mechanisms of adjunctive depression treatments.
Clinical Points.
Effective and practical clinical strategies are critically needed to improve response and remission rates to first-line antidepressant medications.
Patients initiating a new trial of antidepressant medication should be cautioned against restricting their time in bed.
Acknowledgments
Giselle Kolenic, MA, Statistician Lead at the University of Michigan’s Center for Statistical Consultation and Research provided statistical consultation.
Funding/Support: This project was supported by the National Institutes of Mental Health (R01 MH077690, JT Arnedt, Principal Investigator) and by UL1RR024986 from the National Center for Research Resources. The content in this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. The National Center for Research Resources and the National Institutes of Health had no role in the conduct or publication of the study.
Footnotes
Previous Presentations: Preliminary findings from this study were presented at SLEEP 2013 in Minneapolis, MN on 4 June 2013.
Financial Disclosures: Dr. Armitage was a consultant for Eisai Inc. in 2010. She is now a consultant for the University of Ottawa Institute of Mental Health Research. No other conflicts were reported.
Trial Registration: Clincialtrials.gov identifier: NCT01545843
References
- 1.Kessler RC, Berglund P, Demler O, et al. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R) Journal of the American Medical Association. 2003;289(23):3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 2.Nierenberg AA, Farabaugh AH, Alpert JE, et al. Timing of Onset of Antidepressant Response With Fluoxetine Treatment. The American journal of psychiatry. 2000 Sep 1;157(9):1423–1428. doi: 10.1176/appi.ajp.157.9.1423. 2000. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi MH, Rush AJ, Wisniewski SR, et al. Evaluation of Outcomes With Citalopram for Depression Using Measurement-Based Care in STAR*D: Implications for Clinical Practice. The American journal of psychiatry. 2006 Jan 1;163(1):28–40. doi: 10.1176/appi.ajp.163.1.28. 2006. [DOI] [PubMed] [Google Scholar]
- 4.Bech P, Cialdella P, Haugh MC, et al. Meta-analysis of randomised controlled trials of fluoxetine v. placebo and tricyclic antidepressants in the short-term treatment of major depression. Br J Psychiatry. 2000 May;176:421–428. doi: 10.1192/bjp.176.5.421. [DOI] [PubMed] [Google Scholar]
- 5.Giedke H, Schwarzler F. Therapeutic use of sleep deprivation in depression. Sleep medicine reviews. 2002 Oct;6(5):361–377. [PubMed] [Google Scholar]
- 6.Wirz-Justice A, Van den Hoofdakker RH. Sleep deprivation in depression: What do we know, where do we go? Biological Psychiatry. 1999;46:445–453. doi: 10.1016/s0006-3223(99)00125-0. [DOI] [PubMed] [Google Scholar]
- 7.Kvist J, Kirkegaard C. Effects of repeated sleep deprivation on clinical symptoms and the TRH test in endogenous depression. Acta Psychiatrica Scandinavica. 1980;62:494–502. doi: 10.1111/j.1600-0447.1980.tb00638.x. [DOI] [PubMed] [Google Scholar]
- 8.Martiny K, Refsgaard E, Lund V, et al. A 9-week randomized trial comparing a chronotherapeutic intervention (wake and light therapy) to exercise in major depressive disorder patients treated with duloxetine. The Journal of clinical psychiatry. 2012 Sep;73(9):1234–1242. doi: 10.4088/JCP.11m07625. [DOI] [PubMed] [Google Scholar]
- 9.Schilgen B, Tölle R. Partial sleep deprivation as therapy for depression. Archives of General Psychiatry. 1980:267–271. doi: 10.1001/archpsyc.1980.01780160037003. [DOI] [PubMed] [Google Scholar]
- 10.Sack DA, Duncan W, Rosenthal NE, Mendelson WE, Wehr TA. The timing and duration of sleep in partial sleep deprivation therapy of depression. Acta Psychiatrica Scandinavica. 1985;77:219–224. doi: 10.1111/j.1600-0447.1988.tb05104.x. [DOI] [PubMed] [Google Scholar]
- 11.Giedke H, Geilenkirchen R, Hauser M. The timing of partial sleep deprivation in depression. Journal of affective disorders. 1992 Jun;25(2):117–128. doi: 10.1016/0165-0327(92)90074-g. [DOI] [PubMed] [Google Scholar]
- 12.Parry BL, Cover H, Mostofi N, et al. Early versus late partial sleep deprivation in patients with premenstrual dysphoric disorder. American Journal of Psychiatry. 1995;152(3):404–412. doi: 10.1176/ajp.152.3.404. [DOI] [PubMed] [Google Scholar]
- 13.Kuhs H, Farber D, Borgstadt S, Mrosek S, Tolle R. Amitriptyline in combination with repeated late sleep deprivation versus amitriptyline alone in major depression. A randomised study. Journal of affective disorders. 1996 Feb 12;37(1):31–41. doi: 10.1016/0165-0327(95)00074-7. [DOI] [PubMed] [Google Scholar]
- 14.Kuhs H, Kemper B, Lippe-Neubauer U, Meyer-Dunker J, Tolle R. Repeated sleep deprivation once versus twice a week in combination with amitriptyline. Journal of affective disorders. 1998 Jan;47(1–3):97–103. doi: 10.1016/s0165-0327(97)00123-7. [DOI] [PubMed] [Google Scholar]
- 15.Elsenga S, van den Hoofdakker RH. Clinical effects of sleep deprivation and clomipramine in endogenous depression. Journal of Psychiatric Research. 1982/1983;17:361–374. doi: 10.1016/0022-3956(82)90041-3. [DOI] [PubMed] [Google Scholar]
- 16.Caliyurt O, Guducu F. Partial sleep deprivation therapy combined with sertraline induces more rapid improvements in quality of life items in major depressive disorder. Journal of affective disorders. 2005 Sep;88(1):75–78. doi: 10.1016/j.jad.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 17.American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed.: Diagnostic and coding manual. Westchester, Illinois: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 18.Iber C, Ancoli-Israel S, Chesson A, Quan SF, for the American Academy of Sleep Medicine . The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st. Westchester, Illinois: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 19.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton M. Development of a rating scale for primary depressive illness. British Journal of Social and Clinical Psychology. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 21.Keller MB. Past, present, and future directions for defining optimal treatment outcome in depression: Remission and beyond. JAMA. 2003;289(23):3152–3160. doi: 10.1001/jama.289.23.3152. [DOI] [PubMed] [Google Scholar]
- 22.Frank E, Prien RF, Jarrett RB, et al. Conceptualization and rationale for consensus definitions of terms in major depressive disorder: Remission, recovery, relapse, and recurrence. Archives of General Psychiatry. 1991;48(9):851–855. doi: 10.1001/archpsyc.1991.01810330075011. [DOI] [PubMed] [Google Scholar]
- 23.Guy W. ECDEU assessment manual for psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976. [Google Scholar]
- 24.Rush AJ, Trivedi MH, Carmody TJ, et al. Self-reported depressive symptom measures: sensitivity to detecting change in a randomized, controlled trial of chronically depressed, nonpsychotic outpatients. Neuropsychopharmacology. 2005;30(2):405–416. doi: 10.1038/sj.npp.1300614. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Jr, Kosinski M, Keller SD. A 12-item short-form survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Los Angeles: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 27.Acebo C, Sadeh A, Seifer R, et al. Estimating sleep patterns with activity monitoring in children and adolescents: How many nights are necessary for reliable measures. Sleep. 1999;22(1):95–103. doi: 10.1093/sleep/22.1.95. [DOI] [PubMed] [Google Scholar]
- 28.Littell RC, Pendergast J, Natarajan R. Modelling covariance structure in the analysis of repeated measures data. Statistics in Medicine. 2000;19:1793–1819. doi: 10.1002/1097-0258(20000715)19:13<1793::aid-sim482>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds CF, 3rd, Smith GS, Dew MA, et al. Accelerating symptom-reduction in late-life depression: a double-blind, randomized, placebo-controlled trial of sleep deprivation. Am J Geriatr Psychiatry. 2005 May;13(5):353–358. doi: 10.1176/appi.ajgp.13.5.353. [DOI] [PubMed] [Google Scholar]
- 30.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003 Mar 15;26(2):117–126. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 31.Morin CM, Espie CA. Insomnia: A clinical guide to assessment and treatment. New York: Kluwer Academic/Plenum Publishers; 2003. [Google Scholar]
- 32.Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. The American journal of psychiatry. 2006 Nov;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 33.Carter JD, Frampton CM, Mulder RT, Luty SE, Joyce PR. The relationship of demographic, clinical, cognitive and personality variables to the discrepancy between self and clinician rated depression. Journal of affective disorders. 2010 Jul;124(1–2):202–206. doi: 10.1016/j.jad.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Cuijpers P, Li J, Hofmann SG, Andersson G. Self-reported versus clinician-rated symptoms of depression as outcome measures in psychotherapy research on depression: a meta-analysis. Clin Psychol Rev. 2010 Aug;30(6):768–778. doi: 10.1016/j.cpr.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 35.Uher R, Perlis RH, Placentino A, et al. Self-report and clinician-rated measures of depression severity: can one replace the other? Depress Anxiety. 2012 Dec;29(12):1043–1049. doi: 10.1002/da.21993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rush AJ, Hiser W, Giles DE. A comparison of self-reported versus clinician-related symptoms in depression. The Journal of clinical psychiatry. 1987 Jun;48(6):246–248. [PubMed] [Google Scholar]
- 37.Trivedi MH, Rush AJ, Armitage R, et al. Effects of fluoxetine on the polysomnogram in outpatients with major depression. Neuropsychopharmacology. 1999 May;20(5):447–459. doi: 10.1016/S0893-133X(98)00131-6. [DOI] [PubMed] [Google Scholar]
- 38.Rush AJ, Armitage R, Gillin JC, et al. Comparative effects of nefazodone and fluoxetine on sleep in outpatients with major depressive disorder. Biol Psychiatry. 1998 Jul 1;44(1):3–14. doi: 10.1016/s0006-3223(98)00092-4. [DOI] [PubMed] [Google Scholar]
- 39.Gillin JC, Rapaport M, Erman MK, Winokur A, Albala BJ. A comparison of nefazodone and fluoxetine on mood and on objective, subjective, and clinician-rated measures of sleep in depressed patients: a double-blind, 8-week clinical trial. The Journal of clinical psychiatry. 1997 May;58(5):185–192. doi: 10.4088/jcp.v58n0502. [DOI] [PubMed] [Google Scholar]
- 40.Armitage R, Yonkers K, Cole D, Rush AJ. A multicenter, double-blind comparison of the effects of nefazodone and fluoxetine on sleep architecture and quality of sleep in depressed outpatients. Journal of clinical psychopharmacology. 1997 Jun;17(3):161–168. doi: 10.1097/00004714-199706000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Vogel GW, Thurmond A, Gibbons P, Sloan K, Boyd M, Walker M. REM sleep reduction effects on depression syndromes. Archives of General Psychiatry. 1975;32:765–777. doi: 10.1001/archpsyc.1975.01760240093007. [DOI] [PubMed] [Google Scholar]
- 42.Leibenluft E, Moul DE, Schwartz PJ, Madden PA, Wehr TA. A clinical trial of sleep deprivation in combination with antidepressant medication. Psychiatry Res. 1993 Mar;46(3):213–227. doi: 10.1016/0165-1781(93)90090-4. [DOI] [PubMed] [Google Scholar]
- 43.Landsness EC, Goldstein MR, Peterson MJ, Tononi G, Benca RM. Antidepressant effects of selective slow wave sleep deprivation in major depression: a high-density EEG investigation. J Psychiatr Res. 2011 Aug;45(8):1019–1026. doi: 10.1016/j.jpsychires.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


