Abstract
Partner preference, or the selective social preference for a pair mate, is a key behavioral indicator of social monogamy. Standardized partner preference testing has been used extensively in rodents but a single test has not been standardized for primates. The goal of this study was to develop a partner preference test with socially monogamous titi monkeys (Callicebus cupreus) adapted from the widely used rodent test. In Experiment 1, we evaluated the test with pairs of titi monkeys (N=12) in a three-chambered apparatus for three hours. The subject was placed in the middle chamber, with grated windows separating it from its partner on one side and an opposite sex stranger on the other side. Subjects spent a greater proportion of time in proximity to their partners’ windows than the strangers’, indicating a consistent preference for the partner over the stranger. Touching either window did not differ between partners and strangers, suggesting it is not a reliable measure of preference. Subjects chose their partner more than the stranger during catch and release sessions at the end of the test. In Experiment 2, we compared responses of females with current partners (N=12) in the preference test with other relationship types representing former attachment bonds (N=13) and no attachment bond (N=8). Only females from established pair bonds spent significantly more time near their partner’s window compared to the stranger indicating that this measure of preference is unique to current partners. Other measures of preference did not differentiate behavior toward a current partner and other relationship types. This test reproduces behavioral patterns found in previous studies in titi monkeys highlighting the accuracy of this new partner preference test. This test can be used as a standardized measure of partner preference in titi monkeys to quantitatively study pair bonding and evaluate factors influencing partner preference.
Keywords: nonhuman primate, behavior, monogamy, New World monkey, social attachment
INTRODUCTION
Only three to five percent of all mammals are classified as socially monogamous [Kleiman, 1977]. However, social monogamy is more prevalent among non-human primates and is observed in approximately 10% of primate species [Fuentes, 1998]. Studies of social monogamy have focused on a variety of species, including rodents (e.g. prairie voles and California mice) [Williams et al., 1992; Martin et al., 2006], New World primates (e.g. titi monkeys, marmosets, and owl monkeys) [Anzenberger, 1992; Smith et al., 2010; Fernandez-Duque & Huck, 2013], lesser apes (e.g. gibbons and siamangs) [Fuentes, 2000; Lappan, 2008], and humans [Hazan & Shaver, 1987]. By studying the behavior and biology of species that are capable of forming the adult male-female bonds that characterize a socially monogamous mating system, we can better understand the biological underpinnings involved in the formation and maintenance of these social bonds.
Male-female dyads in some socially monogamous species form stable adult attachments to one another, called pair bonds. Pair bonds are characterized primarily by spending time in extended physical contact and maintaining physical proximity with one’s pair mate [Carter et al., 1995]. Pair bonded animals often show aggression toward same‐sex strangers and occasionally to opposite-sex strangers, who might pose a threat to the stability of the bond [Cubicciotti & Mason, 1978; Winslow et al., 1993]. Perhaps the best studied behavioral indicator of social monogamy is partner preference, or a specific social preference displayed for the pair mate over an unfamiliar opposite-sex conspecific [Mason & Mendoza, 1998].
In order to study these pair bond related behaviors in a standardized way, laboratory assessments use a validated behavioral paradigm called the partner preference test. In rodents this test typically involves a three-chambered testing arena in which the familiar pair mate (“partner”) and an unfamiliar opposite-sex conspecific (“stranger”) are restricted to the two outside chambers. The test subject is placed into the central “neutral” chamber and then allowed to move to spend time in, or adjacent to, the two outside chambers containing each stimulus animal or to remain in the neutral portion of the apparatus [Williams et al., 1992]. The time that the test animal spends in proximity to (or in physical contact with) either of the two stimulus animals is measured, as well as any affiliative or aggressive behaviors exhibited by the test animal. The partner preference test (and its derivatives from studies of non-monogamous species, the “social preference test”) has been most widely used in rodents [Millan & Bales, 2013]. The widespread use of this behavioral paradigm across rodent laboratories and the consequent high consistency in test parameters across studies have arguably allowed this behavioral task to become as reliable and reproducible as other consistently used rodent behavior tasks, such as the Morris water maze or elevated plus maze. As a result of this reproducibility, the partner preference test has been used to establish the roles of the oxytocin, vasopressin, dopamine, and opioid systems in mediating pair bond formation in monogamous prairie voles [Williams et al., 1994; Cho et al., 1999; Gingrich et al., 2000; Young et al., 2001; Burkett et al., 2011; Resendez et al., 2012], and it has also been used to study social preferences in non-monogamous rodents like meadow voles [Parker et al., 2001; Ross et al., 2009; Beery & Zucker, 2010].
In contrast with rodents, the field of primatology has not yet adopted a standardized test to evaluate partner preference across species. Although partner preference has been studied in several primate species, a wide variety of methodological approaches have been utilized (Table I). These varied approaches likely stem from accommodating for behavioral or housing differences across species and laboratories. But even among studies of titi monkeys (Callicebus spp.), which were tested in the current study, several different methods have been used (Table I). Furthermore, partner preference tests that were previously utilized with primates differ considerably from the standardized test used with rodents. As can be seen from Table I, primate partner preference tests are variable in both duration (e.g. minutes compared to hours) and apparatus size (e.g. t-maze compared to home-cage). Furthermore, they have been utilized to study changes in partner preference under biological manipulation where baseline partner preference was not initially validated.
TABLE I.
Previous Partner Preference Tests in Socially Monogamous Rodents and Primates
| Species | Test Length | Apparatus | Stimulus Animal Access and Presentation Type1 | Preference Measure (Partner vs. stranger)2 | Length of Partner Relationship |
|---|---|---|---|---|---|
|
| |||||
| Rodents | |||||
| Prairie voles (Microtus ochrogaster) a | Exp. 1: 8 hr. Exp. 2: 3 hr. |
3 chambers | Full physical; simultaneous | Contact | Exp. 1: >24 hr. cohabitation; Exp. 2: 6 hr. cohabitation |
|
| |||||
| Primates | |||||
|
| |||||
| Titi monkeys (Callicebus cupreus) [present study] | 2.5 hr.; choice sessions | 3 chambers | Grated; simultaneous | Proximity, touching grated window, choice sessions | > 6 mo. |
| Titi monkeys (Callicebus cupreus)b | Repeated 15 min. trials | 3 chambers | Full physical; both separate and simultaneous | Proximity, social and sexual behavior | > 7 mo. |
| Titi monkeys (Callicebus cupreus)c | Repeated 30 min. trials | 1 chamber | Full physical; separate | Proximity, social and sexual behavior | > 17 mo. |
| Titi monkeys (Callicebus cupreus)d | Repeated 15 min. trials | 2 chambers | Grated; tested in pairs | Proximity, social and sexual behavior | > 2 yr. |
| Titi monkeys (Callicebus cupreus)e, 3, 4 | Repeated 5 min. trials | 3 chambers | Grated; both separate and simultaneous | Proximity | > 2 wk. |
| Titi monkeys (Callicebus cupreus)f, 3, 4 | 90 min. trials | 1 chamber | Full physical; separate | Proximity, social behavior, heart rate | > 8 wk. |
| Titi monkeys (Callicebus cupreus)g, 3, 4 | Repeated 15 min. trials | 2 chambers | Visual only; tested with pair mate in cage | Proximity, social behavior, heart rate | > 8 mo. |
| Titi monkeys (Callicebus cupreus)h, 3, 4 | Repeated 5 min. trials | V shaped | Grated; both separate and simultaneous | Proximity, agonistic behavior | > 1 yr. |
| Black-Tufted Marmosets (Callithrix penicillata)i | 20 min. | T shaped | Grated; simultaneous | Proximity, social and sexual behavior | 24 hr. or 3 wk. |
| Common Marmosets (Callithrix jacchus)j | 20 min. | T shaped | Grated; simultaneous | Proximity, social and sexual behavior | > 8 wk. |
| White-Lipped Tamarins (Saguinus labiatus)k | Repeated 30 min. trials | 3 chambers | Grated; both separate and simultaneous | Proximity, social and sexual behavior | > 6 mo. |
| Brown-Mantled Tamarins (Saguinus fuscicollis)l | Repeated 30 min. trials | 3 chambers | Grated; simultaneous | Proximity, sexual behavior | > 4 mo. |
| Golden Lion Tamarins (Leontopithecus rosalia)m | Repeated 30 min. trials | T shaped | Grated; both separate and simultaneous | Proximity, social and sexual behavior | > 6 mo. |
Note:
In cases where trials involved multiple types of access to stimulus animals (i.e. removable barriers were in place for some conditions and removed for others) the most liberal access is listed for the table.
Preference measure has been classified into broad categories to make comparison between methodologies simpler. For more detailed information about exact behaviors measured see complete citation in the references.
Study also conducted on squirrel monkeys (Saimiri).
At the time of testing and publication species was identified as Callicebus moloch but has since been updated to be Callicebus cupreus.
The first goal of the current study was to develop a partner preference test for socially monogamous primates that was adapted from the standardized rodent partner preference test in order to facilitate comparisons across mammals on this behavioral indicator of social monogamy. The second goal was to evaluate this new partner preference test by utilizing titi monkeys (Callicebus cupreus) in established pair bonds, which display characteristically similar adult attachment bonds to those of prairie voles (“Experiment 1” of the current study). The final goal was to validate this test by applying it to different relationship types. Here, we capitalized on the fact that infant titi monkeys form an attachment only to their fathers, with a distress response only observed when infants are separated from their father and not when separated from their mother [Mendoza & Mason, 1986]. Therefore, we specifically tested relationship types in adult female titi monkeys that represent former attachment bonds (i.e. former partner, father) and a relationship that presumably never constituted an attachment bond (i.e. mother) [Mendoza & Mason, 1986], to see if this preference test can differentiate between a partner preference and a more general social preference (“Experiment 2” of the current study).
Validating this partner preference test for titi monkeys will provide a standardized way to assess pair bonding in future studies across socially monogamous primate and mammalian species. For example, a partner preference paradigm can be used to determine the effect of different pharmacological manipulations on the formation or maintenance of social bonds, and to learn more about the changing dynamics of pair bonds under different social contexts or over time. Furthermore, establishing the factors that influence adult social bonds in nonhuman primates will have important implications for understanding human adult attachment relationships.
METHODS
Subjects
Experiment 1: Evaluate the partner preference test with mates in established pair bonds
In Experiment 1, 24 titi monkeys (Callicebus cupreus) were tested between February 2013 and February 2014. These subjects represented 12 current pair mates (N=12 male, N=12 female), housed at the California National Primate Research Center in Davis, California. Titi monkeys are small-bodied (approximately 1 kg) New World primates. The average age of the subjects was 8.34 ± 1.01 years (range: 2.15–23.08 years). All individuals were housed with their partner and, if applicable, offspring during the course of the study. Twenty-three of the subjects were born in captivity at the California National Primate Research Center and one was wild caught in 1990 and imported to the colony. Seventeen additional monkeys were used as stranger stimulus animals during the partner preference test sessions. Stranger animals were unfamiliar to the test animal before the study and were approximately matched with the test animal’s partner on sex, age, and weight.
Experiment 2: Validate the partner preference test by applying it to other relationship types
In Experiment 2, 13 adult female titi monkeys were tested between November 2013 and February 2014, all of which were born in captivity. Due to limited availability of animals in the colony for which these relationship types were available, only females were tested in Experiment 2. There was no overlap with the test subjects in Experiment 1. The average age of these subjects was 6.09 ± 4.5 years (range: 2.42–15.83 years). All females lived with their pair mate and, if applicable, offspring during the course of the study. All females were tested with an unfamiliar stimulus animal and a familiar stimulus animal. Familiar animals constituted the following Relationship Types: (i) former mate (N=5) representing a former adult attachment, (ii) father (N=8) representing a former infant attachment, or (iii) mother (N=8) representing no attachment at any life stage. The average length of time that the test animal had been separated from the familiar stimulus animals was 1.7 ± 1.4 years for former mates and 1.51 ± 1.17 years for parents. Sixteen additional titi monkeys were used as the stranger stimulus animals during these test sessions. As in Experiment 1, stranger animals were unfamiliar to the test animal and were approximately matched with the familiar stimulus animal on sex, age, and weight.
Housing and care
Animals in both Experiments 1 and 2 were housed indoors, in two types of cages, based on where they were located in the facility. One type of home cage measured 1.2 m ×1.2 m × 2.1 m with four horizontal perches running the width of the cage in a staggered fashion. The second type of home cage measured 1.2 m × 1.2 m × 1.8 m with four horizontal perches running the width of the cage in a staggered fashion. In both rooms animals were kept on a 12-hour light: 12-hour dark cycle (lights on at 0600 hr and off at 1800 hr). Temperature in both housing facilities was kept at approximately 21°C All animals were provided the same diet consisting of monkey chow, fruits, and rice cereal provided twice per day (at 0800 hr and 1300 hr), as well as additional availability of enrichment with water available ad libitum. All housing conditions and experimental procedures were approved by the University of California Davis Institutional Animal Care and Use Committee and adhered to the American Society of Primatologists principles for the ethical treatment of primates.
Test apparatus
Figure 1 depicts the partner preference test apparatus used in both Experiments 1 and 2. The test apparatus consisted of three adjacent cages with grated windows measuring 0.3 m × 0.3 m connecting each of the side cages to the center cage. These grated windows were made of a mesh wirework with openings of 1.3 cm × 1.3 cm and allowed full visual, olfactory and minimal physical contact between the test animal in the center and that in either of the side cages that held the stimulus animals. Two perches 1.2 m long ran the width of the cage and were measured into three equal sections, each 0.4 m, and classified as either a preference zone (the one-third of the perch closest to either grate) or a neutral zone (the middle third). All other areas of the cage were considered no preference, or neutral, zones. The two stimulus animals had visual access to one another when both animals were located near their respective grated windows at the same time. Likewise, when stimulus animals were near their grated windows they could observe the test animal interacting with the other stimulus animal. While no other animals were within the visual field of the testing animals there was auditory and olfactory contact with non-testing animals housed in the same room.
Figure 1.
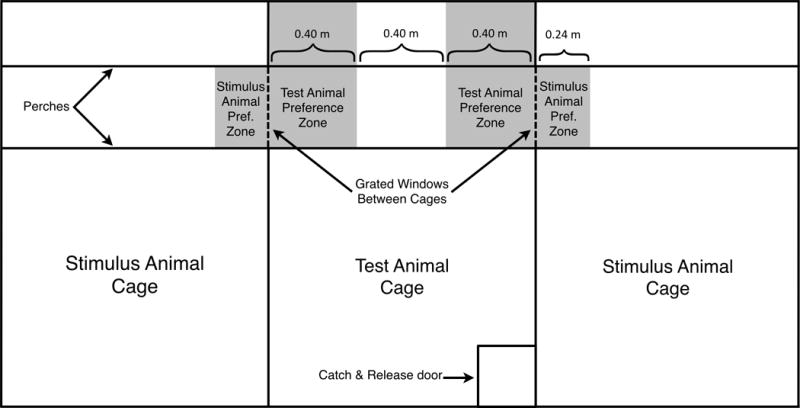
Front view of the test apparatus for the partner preference test.
Test conditions
Experiment 1: Evaluate the partner preference test with mates in established pair bonds
All tests were conducted with mates from established pair bonds with two test sessions for each pair, one with the male as the test animal and one with the female as the test animal. Pair mates’ tests took place within approximately one week of each other in order to minimize the potential effect of pair bond dynamics over time on results.
Experiment 2: Validate the partner preference test by applying it to other relationship types
In Experiment 2, animals were tested with one of three relationship types with familiar stimulus animals: (i) former partner, (ii) father, (iii) mother. These test conditions facilitated our ability to see whether this partner preference test could discriminate between a partner preference and a more general social preference. The same females were tested in condition (ii) and (iii), with approximately one week separating each test session. The female test subjects in condition (i) were distinct from those tested in condition (ii) and (iii).
Procedure
At the beginning of the test sessions in both Experiments 1 and 2, a visual block was put in front of the three‐cage apparatus to prevent visual access to other animals housed in the same room. Trained personnel caught animals in their home cages and placed them into transport cages measuring 0.6 m × 0.3 m × 0.3 m to be brought to the testing apparatus. The two stimulus animals were released into the stimulus animal cages on each side of the test animal cage. The location of the two stimulus animals was counterbalanced within each experiment, so that half of the preference test sessions had the familiar (i.e. partner, former partner, father, mother) stimulus animal located on the right of the test animal and the reverse for the remaining test sessions. Half of test sessions within each experiment took place in the morning and half in the afternoon. All animals were provided food and water throughout the duration of the test session.
Each test session consisted of 2.5 hours of focal animal observations, which were split into five consecutive 30-minute observations. Splitting observations into these 30-minute blocks allowed us to evaluate whether the animals’ preferences changed over the length of the test. The beginning of the first observation was signaled by the release of the test animal into the center cage of the apparatus. The final observation was followed by five catch and release preference sessions in which the test animal was caught from the center cage in a transport cage and immediately released again into the center cage. Following release, the test animal’s preference was recorded as the first preference zone that the test animal entered into and remained in for at least 3 seconds. If the test animal did not enter a preference zone within five minutes it was recorded as no preference. This measure of preference was included at the end of the test session when the subjects would be familiar with the location of the stimulus animals in relation to the center test cage. While all animals were familiar with transport and capture, this procedure provided a potential stressor to which the animals could respond by choosing to immediately seek proximity with one of the two stimulus animals. Finally, we included this measure to evaluate a potential simplification of the partner preference test, whereby detailed behavioral data collection would be foregone, and instead, the catch and release preference measure would be performed at the conclusion of a 2.5 hour habituation session in the test apparatus. In total, each test session lasted approximately three hours and fifteen minutes.
Behavioral data collection
Behavioral data were collected during test sessions via focal animal sampling with a defined ethogram of behaviors. The recorded behaviors focused on the physical location of the test animal including presence in the preference zone of either the familiar stimulus animal (i.e. partner, former partner, father, mother) or stranger and directly touching the grate of either stimulus animal. Behavioral data were collected using Windows tablets (Dell Windows 8 Tablet) running the Recorder module of Behavior Tracker 1.5 (www.behaviortracker.com). Multiple trained observers were validated for live focal animal sampling with greater than 90% observer reliability prior to the start of the study. Approximately 20% of observations were co-scored between two observers to ensure that observer reliability was maintained.
In Experiment 1, we recorded the proximity of both stimulus animals (i.e. partner, stranger) to the test animal in order to assess whether their social availability during the partner preference test affected the behavior of the test animal. This behavioral data, consisting of the stimulus animals’ proximity to the grated window, was collected from video recordings of the stimulus animal cages taken during Experiment 1 test sessions. This measure was similar to the preference zone designated for the test animal. These videos were scored using the DVRecorder module of Behavior Tracker 1.5. Multiple trained observers were validated for video scoring with greater than 85% observer reliability prior to independent scoring, with approximately 10% of videos co-scored in order to ensure that reliability was maintained.
Measuring Preference
We evaluated three measures of preference in order to see which measures differentiated between a partner preference for the pair mate (Experiment 1) and other types of relationships (Experiment 2). First, preference was measured by proportion of total time the test animal spent in the preference zone of his or her familiar stimulus animal (Experiment 1: partner; Experiment 2: former partner, father, mother) compared to the proportion of total time in the preference zone of the stranger stimulus animal. Second, preference was measured as the proportion of total time spent by the test animal touching the grated window that separates the center test cage from the familiar stimulus animal cage (Experiment 1: partner; Experiment 2: former partner, father, mother) compared to the proportion of total time the test animal spent touching the grated window that separates the center test cage from the stranger stimulus animal’s cage. Third, preference was measured as the test animal choosing the familiar stimulus animal (Experiment 1: partner; Experiment 2: former partner, father, mother) more often than the stranger animal during the five catch and release preference sessions at the end of each test session.
Data analysis
Experiment 1: Evaluate the partner preference test with mates in established pair bonds
We utilized a t-test and Wilcoxon Rank Sum test in R Statistical Software [version 2.15.3, R Core Development Team, 2013] for the first two measures of preference, respectively. For the first measure of preference we compared the proportion of total time the test animal spent in the partner’s preference zone to the proportion of total time the test animal spent in the stranger stimulus animal’s preference zone. For the second measure of preference we compared the proportion of time spent touching the grate of the partner to the proportion of time spent touching the grate of the stranger. For the final measure of preference, choosing the partner more than the stranger during the catch and release sessions, we utilized a Friedman’s Chi-square test.
As a measure of reliability of the partner preference test we evaluated how consistently the test measured an individual animal’s preference. To do this, we correlated the proportion of time animals spent in their partners’ preference zones when they served as the test animal with the proportion of time animals spent in the partners’ preferences zone when they served as the stimulus animals.
We utilized a dyadic mixed model in SAS PROC MIXED [version 9.2, SAS Institute, Cary, NC] to assess whether or not the proportions of time the test animal spent in the preference zone of the partner and stranger were consistent over the course of the 2.5 hour test by looking at each of the five 30-minute observations (Observation Number). With this model we also evaluated whether the proximity of the stimulus animals to the test animal (Stimulus Mate Proximity to Test Animal; Stimulus Stranger Proximity to Test Animal) influenced the proportions of time the test animal spent in the respective preference zone of these animals. Finally, with this model we also assessed the interactions between Observation Number*Stimulus Mate Proximity to Test Animal and Observation Number*Stimulus Stranger Proximity to Test Animal to see if the influence of the proximity of the stimulus animals on the test animals’ behavior was consistent through the partner preference test. We used the following model in these analyses:
where Yntk is the outcome variable for dyad n, at occasion t, for each of the two units k in the dyad. Thus, dk is a dummy variable d1 to dm (0 or 1, in our case), to indicate each unit in the dyad, β0nk is the intercept for unit k on dyad n, β1nk is the slope associated with time for each unit k on dyad n, β2nk is the slope associated with the set of predictors X, for each unit k on dyad n, and entk is the residual. In theory, the intercepts and slopes for each of the units in the dyads can be decomposed as
which indicates that, for each unit k, the intercept and slopes have fixed group means (β0k, β1k, and β2k) and random residuals (u0k, u1k, and u2k), and these residuals have variance components (σ0k2, σ1k2 and σ2k2) and could co-vary among themselves (σ01k, σ02k, and σ12k). In addition to these within-unit random effects, covariances between intercepts and slopes could also be allowed between individuals. In practice, often due to data constraints (e.g. small sample size), restrictions can be imposed as needed in the covariance matrix of individual residual variances T = cov(Ent) and in the between-individual covariance matrix Σ = cov(Ut).
This dyadic model allowed us to account for statistical interdependence between pair mates in behavioral expressions of preference. We accounted for interdependence by nesting male and female pair mates’ data within dyad-level dummy variables and regressing the dependent variables on these dummy variables [Raudenbush et al., 1995; Laurenceau & Bolger, 2005]. Using a mixed model allowed us to account for the hierarchical structure in our data (i.e. repeated observations within individuals and the individuals within dyads) and incorporate random effects [Bryk & Raudenbush, 1987]. With the dyadic mixed model, we evaluated fixed effects, as described above (Observation Number, Stimulus Mate Proximity to Test Animal, Stimulus Stranger Proximity to Test Animal, Observation Number*Stimulus Mate Proximity to Test Animal, Observation Number*Stimulus Stranger Proximity to Test Animal). The features of this model make it appropriate for evaluating preference as a behavioral characteristic that likely incorporates (but is rarely evaluated as incorporating) a dyad interdependent component.
Experiment 2: Validate the partner preference test by applying it to other relationship types
In Experiment 2, we compared across the different relationship types utilizing a General Linear Mixed Model (GLMM) [Littell et al., 1996] in SAS PROC GLM with fixed effects for Relationship Type (partner, former partner, father, mother), Stimulus Animal (familiar stimulus, stranger stimulus) and the interaction of Relationship Type*Stimulus Animal with planned comparisons for familiar stimulus vs. stranger stimulus at each level of Relationship Type. We also included a random effect for individual animal in order to account for some animals being tested for different relationship types. We applied this GLMM to the first two measures of preference, specifically the proportion of time that test animals spent in the preference zones of the stimulus animals and the proportion of time that the test animals spent touching the grates of the stimulus animals.
For the final measure of preference we utilized a GLMM with a fixed effect for Relationship Type (partner, former partner, father, mother) and a random effect for individual animal. We applied this GLMM to the proportion of choices for the familiar stimulus animal (partner, former partner, father, mother), the proportion of choices for the stranger, and the proportion of no preference choices during the catch and release sessions. For all statistical tests in Experiments 1 and 2, alpha was set at 0.05 and all tests were two-tailed.
RESULTS
Experiment 1: Evaluate the partner preference test with mates in established pair bonds
Current pair mates displayed a partner preference on two of our three measures of preference. First, subjects spent a significantly greater proportion of time in the preference zone of their partner than of the stranger (t-test: t=4.84, df=46, P < 0.001; Figure 2a). In contrast, there was no significant difference in the time subjects spent touching the grated window of their partner to that of the stranger (Wilcoxon rank sum test: W=332, P=0.37; Figure 2b). Subjects chose their partner more than the stranger during the five catch and release sessions (Friedman’s Chi-square test: χ223=23.4, P < 0.001, Figure 2c).
Figure 2.
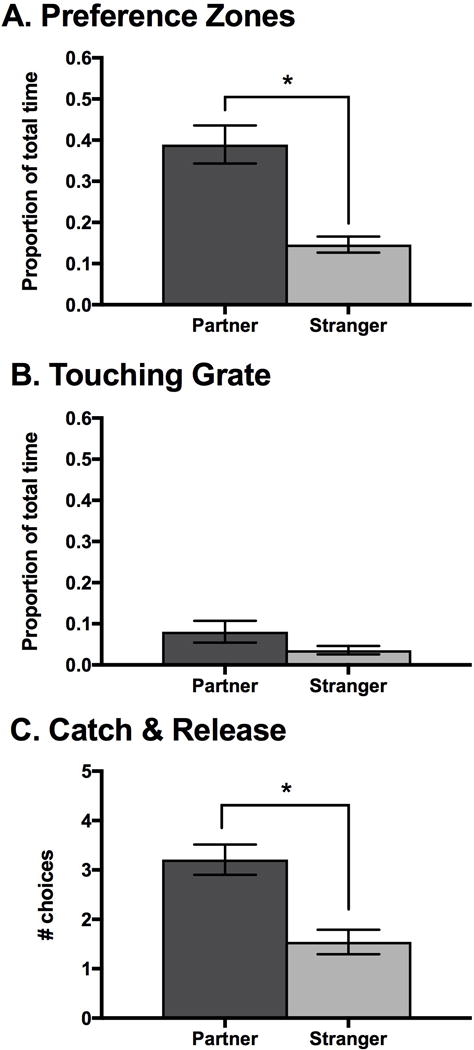
Measures of preference when applying the partner preference test to current pair bonds in Experiment 1. (a) Test animals spent a greater proportion of time in the preference zone of their mate compared to the stranger. (b) Test animals did not differentiate between their mate and the stranger with touching the grate windows separating them. (c) Test animals chose their partner more than the stranger in the catch and release sessions at the end of the test. *** P < 0.001. Error bars indicate S.E.M.
We found a significant positive correlation between the proportion of time that individuals spent in the preference zone of their partner when they were the test animal and the proportion of time the same individual spent in the stimulus animal preference zone when their partner was the test animal (Pearson correlation: r=0.64, df=22, P < 0.001). This result indicates within-individual consistency in partner preference regardless of the animal’s position in the test apparatus (Figure 3).
Figure 3.
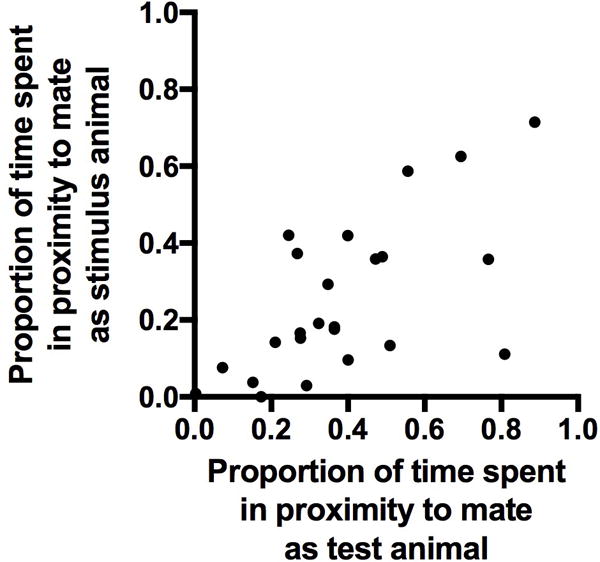
There was a positive association between the proportion of time that test animals (Experiment 1) spent in their partner’s preference zone and the proportion of time the same individual spent in the preference zone of their partner when they served as a stimulus animal for their pair mate.
Predictive parameters of spending time in the partner’s preference zone
Table II presents the unstandardized linear regression coefficients of the fixed effects for our dyadic mixed model. These coefficients are presented separately for males and females, as they were not compared directly within the dyadic structure of the model. For females (Figure 4a) and males (Figure 4b), the proportion of time spent in the partner’s preference zone was not predicted by Observation Number, indicating consistency throughout the partner preference test. Likewise, the proportion of time male and female test animals spent in the partner’s preference zone was not predicted by the Stimulus Mate Proximity to Test Animal, indicating that the test animal’s location in the partner’s preference zone was not influenced by the location of their pair mate in the stimulus animal cage (Table II). Similarly, Stimulus Stranger Proximity to Test Animal did not predict the proportion of time that test animals spent in their partners’ preference zones (Table II). Finally, there were no significant interactions between Observation Number*Stimulus Mate Proximity to Test Animal or Observation Number*Stimulus Stranger Proximity to Test Animal, suggesting there was a consistent lack of influence of the stimulus animals on all test animals’ proportion of time in the partner’s preference zone throughout the test (Table II).
TABLE II.
Partner Preference Test Parameters Predicting Proportion of Time in Preference Zone
| Coefficient (SE) | t value | Coefficient (SE) | t value | |
|---|---|---|---|---|
| Partner’s Preference Zone | Females | Males | ||
|
| ||||
| Intercept | 0.37 (0.09) | 3.96*** | 0.36 (0.08) | 4.44*** |
| Observation Number | −0.01 (0.03) | −0.42 | 0.02 (0.02) | 0.84 |
| Stimulus Mate Proximity to Test Animal | −0.06 (0.14) | −0.44 | 0.32 (0.24) | 1.35 |
| Stimulus Stranger Proximity to Test Animal | 0.15 (0.21) | 0.72 | 0.13 (0.28) | 0.49 |
| Observation Number*Stimulus Mate Proximity to Test Animal | 0.003 (0.06) | 0.06 | −0.07 (0.08) | −0.94 |
| Observation Number*Stimulus Stranger Proximity to Test Animal | −0.10 (0.09) | −1.05 | 0.01 (0.12) | 0.11 |
|
| ||||
| Stranger’s Preference Zone | Females | Males | ||
|
| ||||
| Intercept | 0.06 (0.05) | 1.37 | 0.17 (0.05) | 3.47*** |
| Observation Number | 0.05 (0.02) | 2.43* | −0.003 (0.01) | −0.24 |
| Stimulus Mate Proximity to Test Animal | 0.14 (0.09) | 1.57 | 0.12 (0.14) | 0.84 |
| Stimulus Stranger Proximity to Test Animal | 0.007 (0.13) | 0.05 | −0.22 (0.17) | −1.29 |
| Observation Number*Stimulus Mate Proximity to Test Animal | −0.07 (0.04) | −1.66 | −0.05 (0.05) | −1.11 |
| Observation Number*Stimulus Stranger Proximity to Test Animal | −0.08 (0.06) | −1.42 | 0.07 (0.07) | 0.92 |
Note:
P < 0.05.
P < 0.001.
Figure 4.
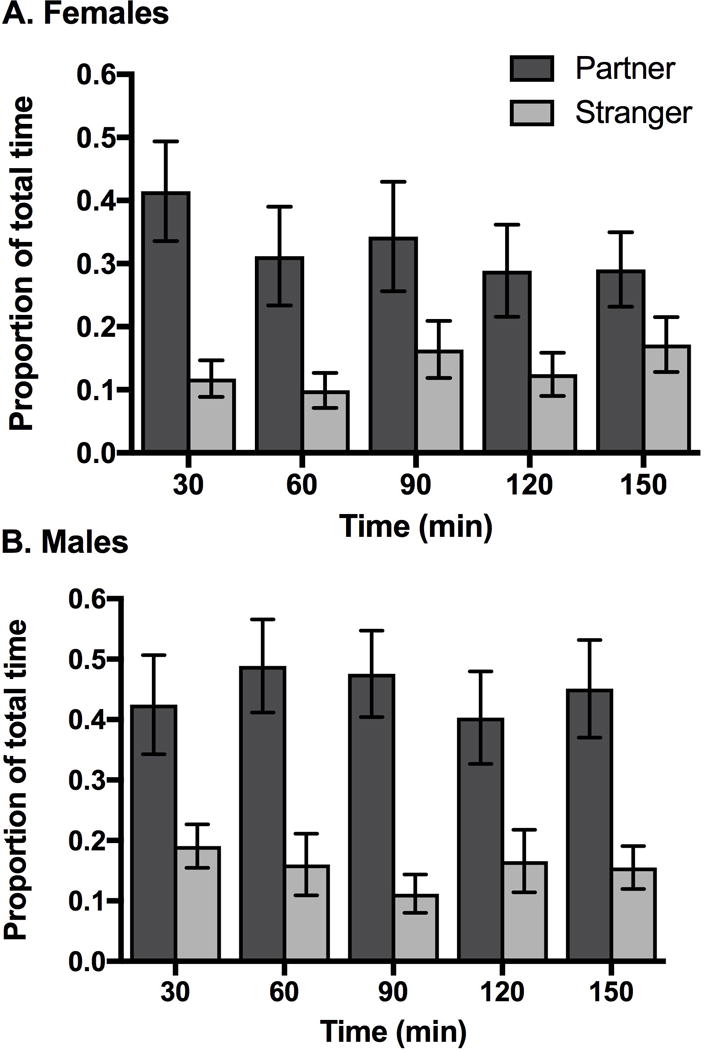
Consistency of spending time in the partner and stranger’s preference zone throughout the partner preference test for males (a) and females (b) in Experiment 1. Males and females were consistent across the test in the proportion of time spent in the partners’ preference zone. Males were consistent in the proportion of time spent in the strangers’ preference zones throughout the test. Females spent a significantly greater proportion of time in the strangers’ preference zones as the partner preference test progressed. P < 0.05. Error bars indicate S.E.M.
Predictive parameters of spending time in the stranger’s preference zone
The proportion of time that female, but not male, test animals spent in the preference zone of the stranger stimulus animal was predicted by Observation Number (Table II). Specifically, females spent a significantly greater proportion of time in the stranger’s preference zone through the course of the partner preference test (Figure 4a). In contrast, Observation Number did not predict male test animal’s proportion of time spent in the preference zone of the stranger, indicating consistency throughout the partner preference test (Figure 4b).
The proportion of time that male and female test animals spent in the preference zone of the stranger was not predicted by Stimulus Mate Proximity to Test Animal nor by Stimulus Stranger Proximity to Test Animal, suggesting that the test animals’ time spent in the preference zone of the stranger was not influenced by the location of the partner or stranger in the stimulus animal cages (Table II). Similarly there were no significant interactions for Observation Number* Stimulus Mate Proximity to Test Animal or Observation Number* Stimulus Stranger Proximity to Test Animal, meaning that this lack of influence on the location of the partner or stranger in the stimulus animal cages was consistent throughout the partner preference test (Figures 4a & 4b).
Inter-individual variation in partner preference
The dyadic model reports significant intercepts (Table 2) for both males and females along with significant variance around these intercepts (Dyadic mixed model: female variance: B=0.04, P < 0.05; male variance: B=0.04, P < 0.05) on the proportion of time spent in the partner’s preference zone. This result indicates significant individual differences in the proportion of time subjects spent in their partners’ preference zones during the first 30 minutes of the partner preference test. Combined with the non-significant effect of Observation Number for females and males, these individual differences in partner preference were consistent over time through the course of the partner preference test.
Similarly, males displayed significant inter-individual differences in the proportion of time they spent in the strangers’ preference zones during the first 30 minutes of the partner preference test, as indicated by a significant intercept (Table II) and variation around that intercept (Dyadic mixed model: B=0.01, P < 0.05). Again, combined with a non-significant effect of Observation Number, this indicates that individual differences among males were consistent throughout the test. In contrast, there was no significant intercept for females, indicating similarity across females in the proportion of time spent in the strangers’ preference zone during the first 30 minutes of the partner preference test. This result, combined with the significant effect for Observation Number, suggests that females became less similar to one another in their proportion of time in the stranger’s preference zone throughout the partner preference test.
Experiment 2: Validate the partner preference test by applying it to other relationship types
For the proportion of time spent in the preference zones, there was no significant effect for Relationship Type (GLMM: F(3, 65)=1.03, P=0.39) or the interaction of Relationship Type*Stimulus Animal (GLMM: F(3, 65)=1.02, P=0.39). However, there was a significant effect of Stimulus Animal on the proportion of time spent in the preference zones (GLMM: F(1, 65)=5.26, P < 0.05). Planned comparisons of the Relationship Type*Stimulus Animal interaction for familiar stimulus vs. stranger stimulus at each level of Relationship Type found that only in the partner condition subjects spent a significantly greater proportion of time in the preference zone of the familiar stimulus (i.e. partner) compared to the stranger (t=2.95, P < 0.01; Figure 5a).
Figure 5.
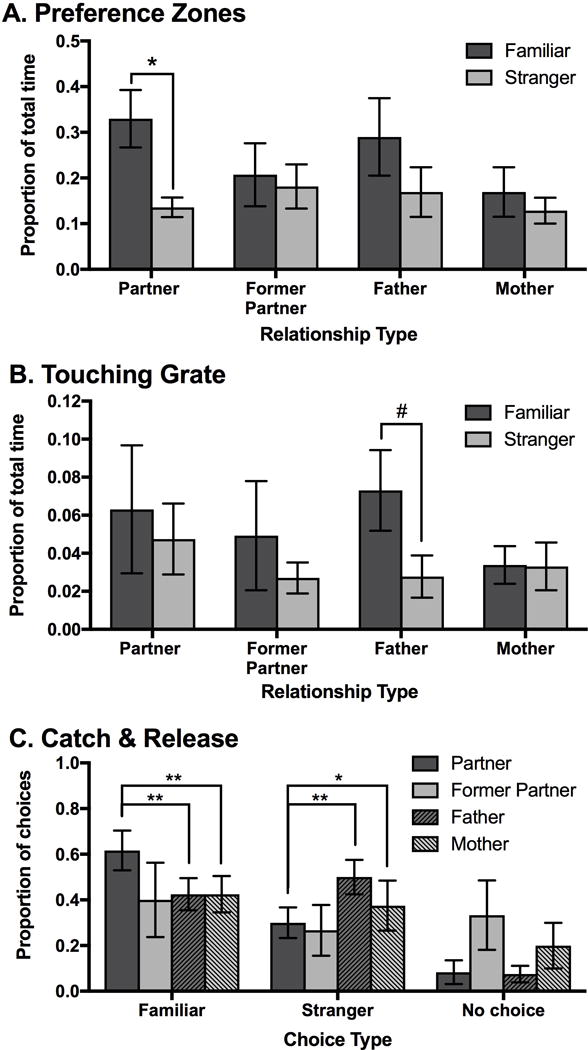
Measures of preference when applying the partner preference test across different types of relationships in Experiments 1 and 2. (a) Only in the case of a current partner did test animals spend a greater proportion of time in the preference zone of the familiar stimulus animal compared to the stranger. (b) In the case of a former infant attachment to the father, test animals displayed a trend to spend a greater proportion of time touching the grated window of the familiar compared to the stranger stimulus animals. (c) Test animals chose the familiar stimulus animal more frequently when tested with their current partner compared to when they were tested with their mother or father. Test animals also chose the stranger less frequently when tested with their current partner compared to when they were tested with their mother or father. ** P < 0.01; * P < 0.05. # P=0.07. Error bars indicate S.E.M.
For the proportion of time spent touching the grates of the stimulus animals, there were no significant fixed effects observed for Stimulus Animal (GLMM: F(1, 65)=1.84, P=0.18) or the interaction between Relationship Type*Stimulus Animal (GLMM: F(3, 65)=0.68, P=0.57). However, planned comparisons between familiar vs. stranger for the Relationship Type*Stimulus Animal interaction at each level of Relationship Type revealed a trend for females to spend a greater proportion of time touching the grate of their father compared to the stranger stimulus male (t=1.85, P=0.07; Figure 5b). The fixed effect of Relationship Type was not estimated.
The proportion of choices for the familiar stimulus animal compared to choices for the stranger stimulus animal during the catch and release sessions was significantly predicted by Relationship Type (GLMM: F(3, 23)=5.53, P < 0.05; Figure 5c). Post-hoc tests revealed that the proportion of choices for the current partner was significantly higher than choosing the father (t=−3.65, P < 0.01) or mother (t=−3.91, P < 0.01), but not different from the former partner (t =−0.31, P=0.76).
Relationship Type did not predict the proportion of choosing the stranger stimulus animal during the catch and release sessions (GLMM: F(3, 23)=2.41, P=0.13; Figure 5c). However, post-hoc tests revealed that the proportion of choices for the stranger were significantly lower when animals were tested with their current partner compared to their father (t=2.55, P < 0.05) or mother (t=2.21, P=0.05) but did not differ from the proportion of choices for the stranger when they were tested with their former partner (t= −7.33, P=0.48). Relationship Type did not predict the proportion of no preference choices during the catch and release sessions (GLMM: F(3, 23)=0.87, P=0.49; Figure 5c).
DISCUSSION
Titi monkeys and other socially monogamous primates establish pair bonds that are largely maintained by a preference for their partners over strangers, which is expressed by maintaining proximity to their partner. Our goals in the current study were to develop a primate partner preference test that was adapted from the standardized paradigm used in rodents and assess whether it would be a reliable way to measure partner preference via time spent in proximity with one’s partner in titi monkeys. Our intent in developing this new partner preference test was to further our understanding of the role of partner preference in social monogamy and to specifically facilitate more direct comparison within primates and across other socially monogamous mammals.
Our goal in Experiment 1 was to evaluate this new partner preference test using established pairs of titi monkeys. We measured partner preference via the test animal’s behavior in three ways. First, we found that titi monkeys spent significantly more time in the preference zone of their partner than that of the stranger. We observed individual differences within both males and females in the proportion of time they spent in their partners’ preference zones. However, all individuals maintained a preference for their partner throughout the course of the test. We interpret this as an advantage of this new test to detect differences across individuals and relationships in partner preference, which we intend to describe in future studies. Second, we found no difference in the proportion of time that the test subject spent touching the grated window of their partner compared to that of the stranger indicating this measure was not a reliable measure of partner preference within the test. Finally, we found that test subjects chose the preference zone of their partner significantly more than the preference zone of the stranger during the catch and release preference sessions at the end of the test. This result indicates that these sessions could be used as a simplified version of the partner preference test, but may be superfluous when combined with measuring the proportion of time spent in the preference zones. Based on these results, Experiment 1 suggested that spending time in the partner’s preference zone and choosing the partner during the catch and release sessions serve as candidate behaviors representing partner preference within this new paradigm.
Results from Experiment 1 indicated that spending time in the partner’s preference zone reliably measured individual animal’s preference for his or her partner. Specifically, we found that the proportion of time spent in the partner’s preference zone was highly correlated between trials when an individual served as the test subject and when the same individual served as the stimulus partner for his or her pair mate. We interpret this finding as an indication of high within-individual consistency in preference to remain in proximity to one’s partner, irrespective of the position of the monkey as the test subject or stimulus animal.
As a behavioral construct, one individual’s preference for his or her mate is likely influenced by the reverse preference from that mate to the first partner. Despite the bidirectionality of this construct, partner preference is typically measured as a behavioral characteristic of a single individual. We consider it a strength of the current study that we accounted for the bidirectionality of partner preference in Experiment 1 by testing both pair mates in the partner preference test and incorporating partner interdependence into our statistical model. This approach allowed us to parcel out pair level effects when evaluating which parameters of the partner preference test were predictive of spending time in the partner and strangers’ preference zones. Within this dyadic statistical approach, we found that the availability of the partner or stranger at the grate interaction window did not predict the proportion of time test animals spent in the preference zones of either stimulus animal, which indicates that the tendency of the test animals to spend more time near their partners is a valid measure of preference outside variable behavior of the stimulus animals. Taken together, these findings indicate that individual titi monkeys behave in a consistent manner regardless of whether they are the test subject or stimulus animal and that this consistent behavior is not affected by what either stimulus animal (partner or stranger) is doing.
Finally, in Experiment 1, we found a different temporal pattern across sexes in the test animal’s interaction with the opposite sex strangers. First, we found that males display consistent individual differences throughout the partner preference test in the time they spend in proximity with the female strangers. Despite these differences, all males still spent a greater proportion of time in the test near their partners compared to strangers. In contrast, female proximity to the male stranger increased over time in the partner preference test. Although these sex differences may be viewed as a limitation to the validity of the partner preference test, we interpret this finding as a presentation of species typical sex differences consistent with previous research. It has been previously demonstrated in titi monkeys that male and female pair mates respond differently to the presentation of a stranger animal of the opposite sex. Male titi monkeys tend to be more proactive in approaching unfamiliar females, both alone and in the presence of their mates, while females are more likely to rebuff stranger male attempts at contact in both settings [Anzenberger, 1988; Fernandez-Duque et al., 1997]. Thus, we interpret the temporal change for females as titi monkey females becoming less inhibited over time during the course of the test.
In Experiment 2, we applied the partner preference test to different relationship types with the goal to see if the preference test could differentiate between a partner preference and a more general social preference. Specifically, we evaluated relationships that represent former attachment bonds for adult female titi monkeys (i.e. former partner, father) and a relationship that presumably never constituted an attachment bond (i.e. mother) and compared responses to the preference test setting to those displayed when tested with a current adult attachment (i.e. partner) from Experiment 1. We found that female titi monkeys differentiated between their familiar social partner (i.e. partner, former partner, father, mother) and the stranger in time spent in preference zone—a proxy measure of proximity maintenance—only in the case of current adult attachment partner. Therefore, we interpret time spent in the preference zones of the stimulus animals in the partner preference test as a valid measure by which to differentiate a preference for a current attachment partner compared to other relationship partners for female titi monkeys.
In contrast, we found that touching the grate of the stimulus animals tended to differentiate between a former infant attachment and other social preferences. Specifically, only in the case of tests with the father did titi monkeys tend to touch the grate of the familiar stimulus more than the stranger stimulus animal. Because titi monkey fathers primarily carry infants [Mendoza & Mason, 1986], we suggest that this measure of preference may be specific to the father because this social partner is the one with which titi monkeys arguably share the longest period of physical contact.
Finally, we found that the catch and release sessions at the end of the partner preference test differentiated between adult and former infant attachment bonds. Specifically, we found that female titi monkeys chose their partners (both current and former) over strangers more frequently than choosing their mother or father over strangers. However, there was no difference between the test animal choosing the current partner over a stranger and choosing their former partner over a stranger. Moreover, titi monkeys tested with their partner (either current or former) and a stranger chose the stranger less frequently than titi monkeys tested with their mother and a stranger or their father and a stranger. Because this potential measure of preference does not differentiate between current and former adult attachment bonds, we do not recommend utilizing the catch and release session alone within this new paradigm.
In summary, across Experiments 1 and 2, a single measure of partner preference emerged for established pair bonds that did not generalize to other relationship types. Specifically, this measure was time spent in the partner’s preference zone, which broadly translates to animals’ attempting to maintain proximity with their mate. Therefore, we recommend using this measure in future uses of this partner preference test. These results are supported by a large body of literature in socially monogamous primates, including titi monkeys, that has demonstrated consistent results of a proximity preference for the partner thereby lending credibility to the accuracy of this new partner preference test [e.g. Mason, 1975; Anzenberger, 1988; Fernandez-Duque et al., 1997; Smith et al., 2010]. We propose this new partner preference test has several strengths in that it provides consistent patterns of partner preference across individuals of both sexes while still being able to detect consistent individual differences. Further, this test also detects species specific behavior patterns expected for titi monkeys based on previous research. One caveat of the current study was that we were only able to test female titi monkeys in Experiment 2. However, based on the fact that we found similar patterns of partner-related behaviors in both sexes in Experiment 1, we expect we would find similar validation of the partner preference test when applied to adult male titi monkeys.
Future studies in titi monkeys, and other socially monogamous primates, can use this paradigm to evaluate a variety of research questions. First, it would be interesting to use this paradigm to determine the effect of pair bond length on partner preference. There is some evidence that behavioral indicators of pair bond formation, such as mating, time in proximity, and time in contact with a new mate, are highly variable between pairs in the first 48 hours of pair introduction [Bales et al., 2007]. However, no studies to date have characterized how these behavioral indicators of pair bond formation change over the course of the bond (i.e. measures of pair bond maintenance) or when precisely the preference for one’s partner emerges after pairing. In addition, future studies using behavioral pharmacology in New World monkeys are now possible using this validated paradigm. For example, it is possible to design experiments analogous to those performed in prairie voles and other monogamous or highly social rodents to determine the contribution of various neuropeptide (oxytocin, vasopressin) and neurotransmitter (dopamine, serotonin, opiate) systems to pair bond formation and the expression of partner preference in nonhuman primate species.
Acknowledgments
This work was supported by a National Science Foundation Graduate Research Fellowship to ESR, HD053555, Office of Research Infrastructure Programs P51OD01107, and the Good Nature Institute. Thanks to members of the Bales lab who assisted with data collection: Benjamin Ragen, Tamara Weinstein, Rocio Arias Del Razo, Chase Nuñez, Jonathan Liu, Rachel Wu, Nancy Rebout, Louisa Radosevich, Leana Goetze, Kendall Davidek, Charlotte Blanz, and Sarah Donaldson. All research in the current study was compliant with animal care regulations and national laws.
Footnotes
The authors report no conflicts of interest.
References
- Anzenberger G, Mendoza SP, Mason WA. Comparative studies of social behavior in Callicebus and Saimiri: Behavioral and physiological responses of established pairs to unfamiliar pairs. American Journal of Primatology. 1986;11:37–51. doi: 10.1002/ajp.1350110105. [DOI] [PubMed] [Google Scholar]
- Anzenberger G. The pairbond in the titi monkey (Callicebus moloch): intrinsic versus extrinsic contributions of the pairmates. Folia Primatologica; International Journal of Primatology. 1988;50:188–203. doi: 10.1159/000156345. [DOI] [PubMed] [Google Scholar]
- Anzenberger G. Monogamous social systems and paternity in primates. In: Martin RD, Dixson AF, Wickings EJ, editors. Paternity in Primates: Genetic Tests and Theories. Basel: Karger; 1992. pp. 203–224. [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, Mendoza SP. Neural correlates of pair-bonding in a monogamous primate. Brain Research. 2007;1184:245–253. doi: 10.1016/j.brainres.2007.09.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Zucker I. Oxytocin and same-sex social behavior in female meadow voles. Neuroscience. 2010;169:665–673. doi: 10.1016/j.neuroscience.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Application of hierarchical linear models to assessing change. Psychological Bulletin. 1987;101:147–158. [Google Scholar]
- Buchanan-Smith HM, Jordan TR. An experimental investigation of the pair bond in the callitrichid monkey, Saguinus labiatus. International Journal of Primatology. 1992;13:51–72. [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, Young LJ. Activation of μ-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology. 2011;36:2200–2210. doi: 10.1038/npp.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neuroscience and Biobehavioral Reviews. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Cavanaugh J, Mustoe AC, Taylor JH, French JA. Oxytocin facilitates fidelity in well-established marmoset pairs by reducing sociosexual behavior toward opposite-sex strangers. Psychoneuroendocrinology. 2014;49:1–10. doi: 10.1016/j.psyneuen.2014.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Cubicciotti DD, III, Mason WA. Comparative studies of social behavior in Callicebus and Saimiri: male-female emotional attachments. Behavioral Biology. 1975;16:185–197. doi: 10.1016/s0091-6773(76)91296-7. [DOI] [PubMed] [Google Scholar]
- Cubicciotti DD, III, Mason WA. Comparative studies of social behavior in Callicebus and Saimiri: heterosexual jealousy behavior. Behavioral Ecology and Sociobiology. 1978;3:311–322. [Google Scholar]
- Epple G. Sex differences in partner preference in mated pairs of saddle-back tamarins (Saguinus fuscicollis) Behavioral Ecology and Sociobiology. 1990;27:455–459. [Google Scholar]
- Fernandez-Duque E, Huck M. Till death (or an intruder) do us part: intrasexual-competition in a monogamous primate. PloS ONE. 2013;8:e53724. doi: 10.1371/journal.pone.0053724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Duque E, Mason WA, Mendoza SP. Effects of duration of separation on responses to mates and strangers in the monogamous titi monkey (Callicebus moloch) American Journal of Primatology. 1997;43:225–237. doi: 10.1002/(SICI)1098-2345(1997)43:3<225::AID-AJP3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Valeggia CR, Mason WA. Effects of pair‐bond and social context on male–female interactions in captive titi monkeys (Callicebus moloch, Primates: Cebidae) Ethology. 2000;106:1067–1082. [Google Scholar]
- Fuentes A. Re‐evaluating primate monogamy. American Anthropologist. 1998;100:890–907. [Google Scholar]
- Fuentes A. Hylobatid communities: Changing views on pair bonding and social organization in hominoids. American Journal of Physical Anthropology Supplement. 2000;31:33–60. doi: 10.1002/1096-8644(2000)43:31+<33::aid-ajpa3>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, Insel TR. Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster) Behavioral Neuroscience. 2000;114:173–183. doi: 10.1037//0735-7044.114.1.173. [DOI] [PubMed] [Google Scholar]
- Hazan C, Shaver P. Romantic love conceptualized as an attachment process. Journal of Personality and Social Psychology. 1987;52:511–524. doi: 10.1037//0022-3514.52.3.511. [DOI] [PubMed] [Google Scholar]
- Inglett BJ, French JA, Dethlefs TM. Patterns of social preference across different social contexts in golden lion tamarins (Leontopithecus rosalia) Journal of Comparative Psychology. 1990;104:131–139. doi: 10.1037/0735-7036.104.2.131. [DOI] [PubMed] [Google Scholar]
- Kleiman DG. Monogamy in mammals. Quarterly Review of Biology. 1977;52:39–69. doi: 10.1086/409721. [DOI] [PubMed] [Google Scholar]
- Lappan S. Male care of infants in a siamang (Symphalangus syndactylus) population including socially monogamous and polyandrous groups. Behavioral Ecology and Sociobiology. 2008;62:1307–1317. [Google Scholar]
- Laurenceau J-P, Bolger N. Using diary methods to study marital and family processes. Journal of Family Psychology. 2005;19:86–97. doi: 10.1037/0893-3200.19.1.86. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. SAS Institute; Cary, NC: 1996. [Google Scholar]
- Martin LB, Glasper ER, Nelson RJ, DeVries AC. Prolonged separation delays wound healing in monogamous California mice, Peromyscus californicus, but not in polygynous white-footed mice, P. leucopus. Physiology & Behavior. 2006;87:837–841. doi: 10.1016/j.physbeh.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Mason WA, Mendoza SP. Generic aspects of primate attachments: parents, offspring and mates. Psychoneuroendocrinology. 1998;23:765–778. doi: 10.1016/s0306-4530(98)00054-7. [DOI] [PubMed] [Google Scholar]
- Mason WA. Comparative studies of social behavior in Callicebus and Saimiri: strength and specificity of attraction between male-female cagemates. Folia Primatologica; International Journal of Primatology. 1975;23:113–123. doi: 10.1159/000155664. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Parental division of labour and differentiation of attachments in a monogamous primate (Callicebus moloch) Animal Behaviour. 1986;34:1336–1347. [Google Scholar]
- Millan MJ, Bales KL. Towards improved animal models for evaluating social cognition and its disruption in schizophrenia: the CNTRICS initiative. Neuroscience and Biobehavioral Reviews. 2013;37:2166–2180. doi: 10.1016/j.neubiorev.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Phillips KM, Lee TM. Development of selective partner preferences in captive male and female meadow voles, Microtus pennsylvanicus. Animal Behaviour. 2001;61:1217–1226. [Google Scholar]
- Raudenbush SW, Brennan RT, Barnett RC. A multivariate hierarchical model for studying psychological change within married couples. Journal of Family Psychology. 1995;9:161–174. [Google Scholar]
- Resendez SL, Kuhnmuench M, Krzywosinski T, Aragona BJ. κ-Opioid receptors within the nucleus accumbens shell mediate pair bond maintenance. The Journal of Neuroscience. 2012;32:6771–6784. doi: 10.1523/JNEUROSCI.5779-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, et al. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. Journal of Neuroscience. 2009;29:1312–1318. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AS, Ågmo A, Birnie AK, French JA. Manipulation of the oxytocin system alters social behavior and attraction in pair bonding primates, Callithrix penicillata. Hormones and Behavior. 2010;57:255–262. doi: 10.1016/j.yhbeh.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Carter CS, Insel T. Partner preference development in female prairie voles is facilitated by mating or the central infusion of oxytocin. Annals of the New York Academy of Sciences. 1992;652:487–489. doi: 10.1111/j.1749-6632.1992.tb34393.x. [DOI] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) Journal of Neuroendocrinology. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–548. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Hormones and Behavior. 2001;40:133–138. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]


