Abstract
The inhibitory activities of tea catechins against carcinogenesis and cancer cell growth have been demonstrated in a large number of laboratory studies. Many mechanisms for modulating cancer signaling and metabolic pathways have been proposed based on numerous studies in cell lines with (−)-epigallocatechin-3-gallate (EGCG), the most abundant and active tea catechin. Nevertheless, the molecular basis for the proposed mechanisms and whether these mechanisms indeed contribute to the anti-cancer activities in vivo are not clearly known. This article reviews the basic redox properties of tea catechins, their binding to key enzymes and signal transduction proteins, and other mechanisms that lead to suppression of cell proliferation, increased apoptosis and inhibition of angiogenesis. More weight is put on studies in vivo over experiments in vitro. It also discusses key issues involved in extrapolating results from cell line studies to mechanistic insights in vivo.
Keywords: Tea catechins, EGCG, cancer signaling, animal models, cell lines
1. INTRODUCTION
Tea, made from the leaves of the plant Camellia sinensis, is a widely consumed beverage worldwide. In the past 25 years, the cancer preventive and other anti-cancer activities of tea preparations have been demonstrated in a variety of animal models in different laboratories (reviewed in [1–4]). Numerous studies with cell lines have also been carried out in an attempt to understand the mechanisms of the anti-cancer actions of tea constituents. Because green tea is more active than black tea, it has received more attention. Green tea, consumed mainly in China and Japan, is produced by steaming or pan-frying tea leaves, which inactivates enzymes, and the drying process stabilizes the constituents. When green tea is brewed with hot water, approximately one-third of the solid materials are water-extractable, of which about a third (by dry weight) are catechins. (−)-Epigallocatechin-3-gallate (EGCG), (−)-epigallocatechin (EGC), (−)-epicatechin-3-gallate (ECG), and (−)-epicatechin (EC) are the major polyphenols in green tea (structures showing in Figure 1). They are collectively known as tea catechins. Black tea, the major form of tea consumed in Western countries, India and other parts of the world, is produced by crushing the tea leaves to promote enzymatic oxidation and subsequent polymerization of most of the catechins to form oligomeric polyphenols (theaflavins) and polymeric polyphenols (thearubigins) [5]. These higher molecular weight black tea polyphenols have very low or no bioavailability, which may explain the lower cancer preventive activity of black tea than green tea. Green and black tea also contain 2–5% caffeine in the water-extractable materials, and caffeine has also been shown to be an active tea constituent in the prevention of skin and lung cancers in animal models [2].
Figure 1.
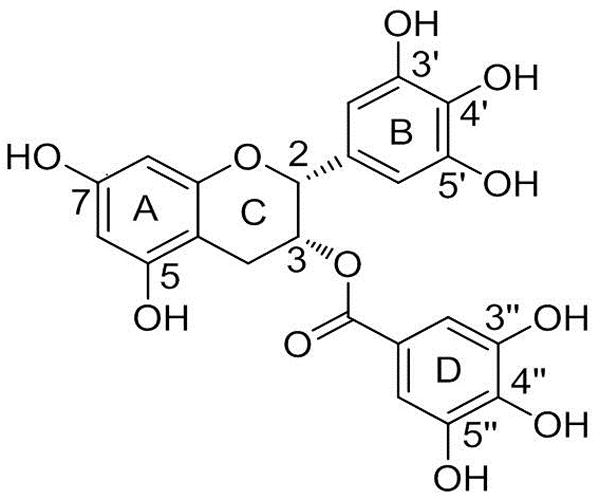
Structures of (−)-epigallocatechin-3-gallate (EGCG) and other tea catechins. For (−)-epicatechin-3-gallate (ECG), the “OH” at 3´-position is replace by “H”; for (−)-epigallocatechin (EGC), the “gallate” at 3-position is replaced by “OH”; for (−)-epicatechin (EC), the “OH” at 3´-position is replaced by “H” and “gallate” at 3-position is replaced by “OH”.
The inhibitory activities of tea catechins against carcinogenesis and the growth of cancer cells have been demonstrated in different experimental systems [1–4]. Mechanisms of action of tea catechins, especially EGCG, the most abundant and active form of catechin, have been extensively investigated in cell culture systems [2,6]. This is a very active area of research. A PubMed search in June 2014 under the title “Tea and Cancer” yielded 3621 citations (1962 to 2014), while “EGCG and cancer signaling” yielded 300 publications (1997 to 2014). EGCG has been shown to affect a variety of cancer signaling pathways. While it is surprising that a single molecule such as EGCG can have such diverse activities, it is also unclear whether these proposed mechanisms are indeed involved in the inhibition of carcinogenesis or tumor growth in animal models and humans.
This chapter reviews the chemical and biochemical properties of tea catechins and their effects on cancer signaling and metabolism. Most of the information is about EGCG, the most frequently studied catechin. Some results from our own laboratory are discussed in more details to serve as examples and to illustrate the challenges in using results from in vitro studies to gain insight into of the action of tea catechins and other natural products in animal models and humans.
2. CHEMISTRY, BIOAVAILABILITY AND BIOTRANSFORMATION OF TEA CATECHINS
2.1 Chemistry
The major catechins in green tea as shown in Figure 1 are characterized by the dihydroxyl or trihydroxyl substitutions on the B ring and the m-5,7-dihydroxyl substitutions on the A ring [5]. The B ring appears to be the principal site of antioxidant reactions, and the antioxidant activity is further increased by the trihydroxyl structure in the D ring (gallate) in EGCG and ECG [5]. The polyphenolic structure allows electron delocalization, conferring the ability to quench free radicals. Tea catechin preparations have been shown to reduce reactive oxygen species (ROS) such as superoxide radical, singlet oxygen, hydroxyl radical, peroxyl radical, nitric oxide, nitrogen dioxide and peroxynitrite [5]. Among tea catechins, EGCG is most effective in reacting with the majority of ROS. Tea polyphenols are also strong chelators of metal ions such that the chelation of free metal ions prevents the formation of ROS.
The vicinal dihydroxy or trihydroxy structures not only contribute to the antioxidant activity of tea catechins, but also increase the susceptibility of these compounds to air oxidation under alkaline or neutral pH, especially in the presence of trace amounts of cuprous or ferric ion. Auto-oxidation of EGCG generates superoxide anion and hydrogen peroxide and leads to the formation of catechin dimers, such as theasinensins, which are unstable and yield products yet to be identified [7]. We propose that this is due to superoxide anion-mediated chain reactions outside of the cells, because EGCG can be stabilized by the addition of superoxide dismutase (SOD) [7]. These reactions occur under cell culture conditions, and the ROS generated by EGCG can induce many cellular changes and may be responsible for many of the activities reported in the literature [1,2,7].
2.2 Bioavailability
The polyphenolic structure of tea polyphenols makes them good donors for hydrogen bonding. Hydrogen bonding of water molecules to EGCG forms a large hydration shell, which reduces the absorption of EGCG. The bioavailability of tea polyphenols follows the Lipinski’s Rule of Five [8] and is dependent on the molecular size, apparent size (due to the formation of a hydration shell) and polarity. For example, the bioavailabilities of EC (molecular weight 290 and 5 phenolic groups) are much higher than EGCG (molecular weight 458 and 8 phenolic groups). In humans, following the oral administration of the equivalent of two or three cups of decaffeinated green tea, the peak plasma levels of EGCG (including the conjugated forms) were usually 0.2–0.3 μM [9]. The plasma concentrations of EGC (molecular weight 306, 6 phenolic group) were higher, even though this green tea preparation contains less EGC than EGCG. With high pharmacological oral doses of EGCG, peak plasma concentrations of 2–9 μM and 7.5 μM were observed in mice and humans, respectively [9]. EGCG and other catechins are thought to enter cells through passive diffusion. However, the involvement of transporters, such as organic anion-transporting peptides (OATP) 1A2 and 1B3 [10], has been suggested. Active efflux has been shown to limit the bioavailabilities of many polyphenolic compounds, including catechins. The multidrug resistance-associated protein 2, located on the apical surface of the intestine and liver, mediates the transport of some polyphenolic compounds to the lumen and bile, respectively [11]. EGCG and its metabolites are predominantly effluxed from the enterocytes into the intestinal lumen or from the liver to the bile and excreted in the feces, with little or none of these compounds excreted in the urine [5].
2.3 Biotransformation
EGCG and other tea catechins undergo extensive biotransformations (reviewed in [9]). Because of the catechol structure, EGCG and other catechins are readily methylated by catechol-O-methyltransferase as a detoxification mechanism. In addition, catechins are glucuronidated by UDP-glucuronosyltransferases and sulfated by sulfotransferases. Multiple methylation and conjugation reactions can occur on the same molecule [5]. Tea catechins can also be degraded in the intestinal tract by microorganisms. After ingestion of tea catechins by humans, ring fission metabolites 5-(3´,4´,5´-trihydroxyphenyl)-γ-valerolactone (M4), 5-(3´,4´-dihydroxyphenyl)-γ-valerolactone (M6) and 5-(3´,5´-dihydroxyphenyl)-γ-valerolactone (M6´) have been observed in urine and plasma samples [12]. These compounds can undergo further degradation to phenylacetic and phenylpropionic acids in the intestine and are excreted in feces and urine.
3. INHIBITION OF TUMORIGENESIS BY TEA CATECHINS IN ANIMAL MODELS AND POSSIBLE MECHANISMS
Tea extracts have been demonstrated to inhibit tumorigenesis in many animal models, including those for cancers of the oral cavity, esophagus, stomach, small intestine, colon, liver, pancreas, lung, bladder, skin, prostate and mammary glands. Most of the studies were conducted with green tea or tea catechin preparations, and some were conducted with pure EGCG, administered through drinking water or diet. Some examples of these studies are as follows.
3.1 Inhibition of tumorigenesis in the digestive tract
The systemic bioavailability of gallated catechins (EGCG and ECG) is a limiting factor for their effectiveness against tumorigenesis in the internal organs. The epithelial cells in the digestive tract have the advantage of being directly exposure to the catechins that are ingested orally. Inhibitory effects of tea catechins against tumorigenesis in chemically-induced and genetic animal cancer model in the oral cavity, esophagus, stomach, small intestine, and colon have been shown in more than 30 studies. For example, we showed that administration of EGCG at 0.02%–0.32% in drinking water dose-dependently inhibited small intestinal tumorigenesis in ApcMin/+ mice, while caffeine did not have an inhibitory effect [13]. The inhibition was associated with increased levels of E-cadherin on the plasma membrane, as well as decreased levels of nuclear β-catenin, c-Myc, phospho-AKT, and phospho-ERK1/2 in the tumors [13]. Administration of green tea extracts (0.6% in drinking fluid) also inhibited the formation of azoxymethane (AOM)-induced aberrant crypt foci (ACF) in CF-1 mice on a high-fat diet [14]. Treatment of rats with 0.24% of Polyphenon (PPE, a standard tea catechin preparation containing 65% EGCG and other tea catechins) in the diet for 8 weeks decreased the total number of ACF per rat by 36.9%. In ACF with high-grade dysplasia, the inhibitory activity of PPE was associated with decreased levels of nuclear β-catenin and cyclin D1, and increased retinoid X receptor-α staining [15]. Recently, Shimizu et al. [16] demonstrated the inhibition of AOM-induced ACF formation in male C57BL/KsJ-db/db mice by EGCG (0.01% and 0.1% in drinking water) involving suppression of insulin-like growth factor 1 (IGF1) signaling. The elevated levels of IGF1 receptor (IGF1R), phospho-IGF1R, phospho-GSK3β and β-catenin in the colonic mucosa were decreased by treatment with EGCG; also decreased were the plasma levels of IGF1, insulin, triglyceride, cholesterol and leptin [16].
3.2 Inhibition of lung tumorigenesis
The inhibitory effects of tea catechins against lung tumorigenesis have been demonstrated in at least 20 studies using chemically-induced and genetic rodent models [1]. Administration of EGCG or EGC significantly decreased lung tumorigenesis in rats, mice or hamsters [1,17]. Oral administration of 0.5% PPE or 0.044% caffeine in the drinking water, to tumor-bearing A/J mice [induced by a single dose of (4-methylnitrosamino)-1-(3-pyridyl)-1-butanone 20 weeks earlier] for 32 weeks, inhibited the progression of lung adenomas to adenocarcinomas [17]. Immunohistochemical analysis showed that PPE and caffeine treatment inhibited cell proliferation, enhanced apoptosis, and decreased levels of c-Jun and phospho-ERK1/2 in adenocarcinomas. In normal lung tissues, neither agent had a significant effect on cell proliferation or apoptosis, suggesting that the action is selective against tumor tissues. These results demonstrate the broad inhibitory activity of tea catechins against lung carcinogenesis, but the mechanisms remain to be further elucidated.
3.3 Inhibition of prostate carcinogenesis
Administration of a green tea polyphenol infusion (0.1% in drinking water) to transgenic adenocarcinoma of the mouse prostate (TRAMP) mice for 24 weeks markedly inhibited prostate cancer development and distant site metastases [18,19]. The inhibition was associated with decreased cell proliferation, increased apoptosis, decreased IGF1 level, and restored IGF binding protein 3 (IGFBP3) levels in both serum and the dorso-lateral prostate [18,19]. This modulation of IGF1 and IGFBP3 levels was associated with reduced levels of phosphotidylinositol 3-kinase (PI3K) as well as phosphorylated forms of AKT and ERK1/2. The green tea polyphenol treatment also significantly decreased levels of angiogenic and metastatic markers, such as vascular endothelial growth factor A (VEGFA), matrix metalloproteinase (MMP)2 and MMP9. These results suggest that the inhibition of the IGF1 signaling, VEGFA and MMPs contributes to the cancer preventive activity of green tea polyphenols.
3.4 Human studies
In contrast to the strong evidence for the cancer preventive activity of tea constituents in animal models, results from epidemiological studies have not been consistent concerning the cancer preventive effect of tea consumption in humans [1,2]. There are only several intervention studies showing promising cancer preventive effects of tea preparations [1,2]. As for therapeutic applications, only a few clinical trials demonstrated the usefulness of EGCG. For example, in a phase 2 trial in patients with early chronic lymphocytic leukemia, oral doses of PPE (2,000 mg twice daily) caused durable declines in the absolute lymphocyte count and/or lymphadenopathy in the majority of patients [20]. Side effects observed in this study include transaminitis, abdominal pain and diarrhea.
The difference between the results from most animal and human studies is likely to be due to: 1) the lower quantities of human tea consumption as compared to the doses used in animal studies and the relatively weak cancer preventive or therapeutic effects of tea catechins in humans, and 2) confounding factors in epidemiological studies; whereas in animals studies, the conditions are controlled to maximize the opportunity to detect a cancer prevention effect. The results of human studies on tea and cancers may become more understandable after considering the quantities of tea consumption and correction for smoking and other interfering factors.
4. BIOCHEMICAL ACTIVITIES OF TEA CATECHINS
To understand the mechanisms by which EGCG and other catechins modulate cancer signaling, it is important to consider the basic biochemical activities of these compounds. The primary actions of catechins are due to their redox and physical binding activities. Using EGCG as a prototype compound, these actions are depicted in Figures 2A and discussed below. The effects of EGCG at the receptor and cellular levels are shown in Figures 2B.
Figure 2.
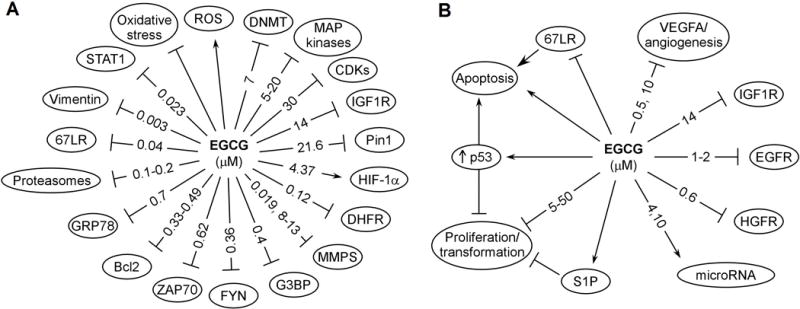
Possible targets for the cancer preventive activity of EGCG. Some of these are direct binding targets; others are affected indirectly. The reported effective concentrations, in IC50, Ki (inhibition constant) or Kd (dissociation constant) are shown in μM. All these are from studies in vitro. When two values are given, the first value is from cell-free systems and the second value is from studies in cell lines.
4.1 Antioxidant and pro-oxidative activities in vitro and in vivo
Catechins are well recognized as antioxidants, but they can also be pro-oxidants and generate ROS. ROS can alter the functions of cellular proteins, lipid and nucleic acids, and lead to different diseases [21]. Oxidative damages to DNA cause mutation and genomic instability, which are major contributing factors in the initiation, promotion and progression of carcinogenesis [22]. However, there are also suggestions that decreasing the amounts of ROS promotes tumor progression [23,24]. Therefore, the antioxidant and pro-oxidative activities of catechins have the potential to affect cancer signaling, depending on the bioavailability of catechins and context of the cellular environment. Although the antioxidant activity of tea catechins is well established in vitro [5], such activity in vivo is only observed under circumstances when the animals are under oxidative stress. For example, EGCG administration has been found to decrease the levels of lipid peroxidation and protein carbonylation in old rats, but not in young rats [25]. In animal models for carcinogenesis, ROS are induced by the treatment with carcinogens, and EGCG has been demonstrated to reduce the formation of 8-hydroxydeoxyguanosine (8-oxo-dG), a well-established marker for oxidatives DNA damage that can mispair to induce mutations [26]. As endogenously formed ROS are important in promoting carcinogenesis, tea polyphenols may have important roles in quenching these species at different stages of carcinogenesis. In human studies, administration of green tea to smokers for 4 weeks has been shown to significantly reduce the number of 8-oxo-dG-positive cells [27]. Such antioxidant actions of tea catechins may be important in the prevention of carcinogenesis.
Tea catechins can be auto-oxidized to generate ROS in cell culture medium and cause cell death [7,28]. After entering the cells, EGCG may also induce the production of ROS in the mitochondria. In our studies, oral administration of EGCG to mice bearing human lung cancer H1299 cell xenograft tumors inhibited tumor growth, enhanced tumor cell apoptosis, and produced ROS in the tumor cells [29]. The observed ROS production in tumor cells is probably due to the lack of sufficient antioxidant enzymes in H1299 cells. It remains to be demonstrated whether the production of ROS is responsible for the induction of apoptosis in vivo. At modest doses (e.g. 0.5% EGCG in the diet), although increased levels of 8-oxo-dG and phosphorylated histone 2A variant X were seen in xenograft tumors, 8-oxo-dG production and toxicity were not observed in the liver, kidney and other organs of the host mice [29]. At high doses (e.g., 750 mg/kg, i.g.), however, hepatotoxicity and ROS were observed [30]. These toxic responses are probably similar to the reported liver toxicity in individuals who took excessive amounts of tea extracts in dietary supplements used for the purpose of weight reduction [31,32].
Cellular ROS may also activate the nuclear factor erythroid 2-related factor 2 (Nrf2)-mediated signaling pathways to induce cytoprotective enzymes [33]. For example, oral gavage of EGCG (200 mg per kg) to C57BL/6J mice upregulated gene expression of γ-glutamyltransferase, glutamate cysteine ligase and haemoxygenase 1 in the liver and colon, which were most likely mediated by the activation of Nrf2 [34]. Similarly, human volunteers supplemented with 800 mg PPE per day for 4 weeks increased glutathione S-transferase P activity in lymphocytes [35]. In an intervention study in a high aflatoxin exposure area in China, supplementation with 500 or 1,000 mg green tea polyphenols per day for 3 months increased the median urinary aflatoxin B1-mercapturic acid levels by more than 10-fold compared to baseline [36]. This result is likely due to the induction of glutathione S-transferase by EGCG. It appears that levels of ROS produced by moderate doses of tea polyphenols activate Nrf2 to reduce oxidative stress, but high doses of tea polyphenols can produce high levels of ROS, which induce toxicity [31]. Thus, the biological effect of EGCG depends on the dose used and the context of the biological systems.
4.2 High affinity binding proteins as targets of EGCG
EGCG is known to bind to a variety of proteins with rather high affinities, through hydrogen bonding and binding to the hydropholic pockets of proteins. For example, a recent report on the interaction between EGCG and bovine serum albumin (BSA), studied with fluorescence lifetime and intensity, has suggested that EGCG binds to BSA with an apparent binding constant of about 4.6 × 10−5 M−1 at pH 7.4 [37]. EGCG binds with high affinity to the hydrophobic pocket between subdomains IIA and IIIA of BSA. The BSA moleucle also undergoes a binding-dependent conformational change. The multiple phenolic groups of EGCG can serve as hydrogen bond donors to many proteins and nucleic acids. In our previous work with molecular modeling, the binding of EGCG to DNA methyltransferase (DNMT) 1 has been proposed to involve 5 hydrogen bonds [38]. In an earlier study, using NMR spectroscopy, EGCG was demonstrated to directly bind to the BH3 pocket of anti-apoptotic Bcl2 proteins - with inhibition constant (Ki) of 0.33–0.49 μM [39]. However, higher EGCG concentrations (by 2 orders of magnitude) were needed to induce apoptosis.
Using an EGCG–Sepharose 4B column and 2D-gel electrophoresis, Dong et al. identified vimentin [40], IGF1R [41], FYN [42], glucose-regulated protein 78 kDa (GRP78) [43], ZAP70 [44] and Ras-GTPase-activating protein SH3 domain-binding protein 1 (G3BP1) [45] as high-affinity EGCG binding proteins (Figure 2). Functionally, for example, EGCG was shown to competitively bind to the ATP binding site of IGF1R and inhibit tyrosine phosphorylation [41]. A subsequent X-ray crystallography study by Dong’s group demonstrated the binding of EGCG to both the WW and PPIase domains of peptidyl prolyl cis/trans isomerase (Pin1) [46]. The direct binding of EGCG with Pin1 was confirmed and the binding inhibited Pin1 PPIase activity. This inhibition could have important biological consequences because Pin1 is required for full activation of AP-1, NFκB, β-catenin and other signaling pathways. Biochemical studies showed a dissociation constant of 21.6 μM for the binding of EGCG to Pin1. EGCG was shown to suppress the proliferation of cells expressing Pin1 and tumor growth in a xenograft mouse model. The binding of EGCG with Arg17 in the WW domain prevented its binding with c-Jun, a well-known Pin1 substrate. EGCG treatment also decreased cyclin D1 level and 12-O-tetradecanoylphorbol-l3-acetate-induced AP-1 or NFκB activity in cells expressing Pin1 [46].
In a recent study with surface plasmon resonance (SPR), molecular modeling and site directed mutagenesis, EGCG (and ECG) were found to tightly bind to signal transduction activator of transcription 1 (STAT1) with Kd of 23 nM in MDA-MB-231 breast cancer cells [47]. The binding involved at least three hydroxyl groups of the B ring and one hydroxyl group of the D ring of EGCG. Site-directed mutagenesis of STAT1 with H568A eliminated the high-affinity binding to EGCG. The tight binding of EGCG to STAT1 blocked its phosphorylation by Janus kinase 2 (JAK2). This could be a mechanism by which EGCG inhibits STAT1 activation and related actions on cytokines and growth factors. EGCG was also suggested to inhibit the JAK/STAT3 signaling and lead to Fas/CD95-mediated apoptosis of head and neck squamous carcinoma cells [48]. Previously, Tachibana et al. used SPR to study the binding of EGCG to the 67kDa laminin receptor (67LR) [49] and this led a series of interesting studies which will be discussed in a later section.
All of the aforementioned proteins were demonstrated to be important for the inhibitory activity of EGCG in some cell lines. However, much higher EGCG concentrations (than the Kd values) were needed to elicit a cellular response. For example, vimentin bound to EGCG with a Kd of 3.3 nM, and functional studies showed that EGCG inhibited the phosphorylation of vimentin with IC50 = 17 μM. The reason for the difference in effective concentrations is not fully understood. It is possible that EGCG binds nonspecifically to proteins and other macromolecules in the cells and therefore elevate the effective concentrations of EGCG at the targets. The discovery of the aforementioned high-affinity EGCG-binding proteins is important, and the direct involvement of these proteins in the action of EGCG in animal models and humans remains to be investigated.
4.3 Inhibition of enzyme activities
Tea polyphenols have been shown to bind and inhibit the activities of a variety of enzymes. In other studies, the inhibition of enzyme activity has been observed in cultured cells and the inhibition could be due to either direct binding or indirect actions. We previously observed that EGCG, at concentrations of 5–20 μM, inhibited the phosphorylation of JNK (Jun N-terminal kinase), c-Jun, MEK1/2, ERK1/2 and ELK1 in JB6 epidermal cell lines [50]. This inhibition was associated with the inhibition of AP1 transcriptional activity or cell transformation. Additional studies with in vitro kinase assays suggested that EGCG inhibited MEK1/2 phosphorylation by decreasing its association with the kinase RAF1 [51]. Moreover, EGCG seemed to inhibit the phosphorylation of ELK1 by competing with the binding site for ERK1/2 [52]. Recent studies also suggest that EGCG inhibited the phosphorylation of ERK1/2 and AKT in Epstein-Barr virus (EBV)-positive cells, and this could block the constitutive lytic infection of EBV at the gene transcription and translation levels [53] There were also studies showing that EGCG activated ERK1/2 and other MAP kinases through the generation of ROS, but these results could be in vitro artifacts. In lung carcinogenesis models, EGCG and a green tea polyphenol preparation have been shown to inhibit the phosphorylation of c-Jun and ERK1/2 [17].
EGCG has also been reported to inhibit the chymotryptic activity of 20S proteasomes [54], which is a key step in the degradation of many signaling proteins. The difference in the effective concentrations of EGCG in cell-free systems (IC50 = 0.09–0.2 μM) and in cell lines (IC50 = 1–10 μM) [54], is probably due to nonspecific binding to different macromolecules. MMPs are secreted by tumor cells during cancer cell invasion and metastasis. EGCG and other catechins have been shown to inhibit the activity of secreted MMP2 and MMP9 with IC50 values of 8–13 μM [55,56]. In addition, EGCG could also increase the expression of the tissue inhibitor of MMPs (TIMP1 and TIMP2) at lower concentrations (~1 μM) [56]. Recent studies demonstrated that EGCG induced TIMP3 by epigenetic mechanisms in MCF7 and MDA-MB 231 breast cancer cells, involving decreased protein levels of the enhances of zeste homolg 2 (EZH2) and class 1 histone deacetylases (HDACs) [57]. These activities may contribute to the reported inhibition of metastasis and invasion following treatment of tumor-bearing mice with green tea or EGCG [58]; however, additional in vivo studies are needed to verify this mechanism.
We reported previously that EGCG inhibited DNMT activity (Ki = 7 μM) from KYSE 510 human esophageal cancer cells, and this resulted in the demethylation and reactivation of the hypermethylated promoters of the tumor suppressor gene INK4A, retinoic acid receptorβ, as well as the DNA repair genes, MLH1 and methylguanine methyltransferase [38]. Reactivation of some of these genes was also observed in HT29 colon and PC3 prostate cancer cells. Tea catechins also reactivated GSTP1 in human prostate cancer cells by causing promoter hypomethylation and chromatin remodeling [59]. However, the induction of promoter demethyaltion by EGCG was not observed in some other hypermethylated genes. As will be discussed in a later section, EGCG has been suggested to induce other epigenetic events.
EGCG has also been reported to inhibit dihydrofolate reductase [60], glucose-6-phosphate dehydrogenase [61] glyceraldehyde-3-phosphate dehydrogenase [62], and carbonyl reductase 1 [63]. These results on enzyme inhibition are interesting biochemically. Cancer metabolism is an active area for research. Additional studies in this area are needed, especially regarding specificity to target enzymes in cancer cells versus normal cells.
5. MODULATING SIGNALING PATHWAYS AND CELL FUNCTIONS
Most of the information in this area was derived from studies in cell lines, mainly with EGCG. Some of the activities are summarized in Figure 2B. When a similar action was also observed in vivo, then the mechanism is more convincing. Some studies have also included other catechins; their activities generally follow the ranking order of EGCG > ECG > EGC > EC.
5.1 Inhibition of receptor tyrosine kinases and other receptors
Tea catechins have been shown to affect many receptor-related activities, and their inhibitory actions against receptor tyrosine kinases (RTKs) have been reviewed recently by Larsen et al. [64] and Shimizu et al. [65]. All members of the RTK family, including epidermal growth factor (EGFR), IGF1R, hepatocyte growth factor receptor (HGFR or c-Met) and vascular endothelial growth factor receptor (VEGFR), consist of an extracellular ligand-binding domain, single membranes-spanning region and a cytoplasmic protein tyrosine kinase domain. The major signaling pathways activated by RTKs are the Ras/ERK and the PI3K/AKT pathways. Some of the actions, using EGFR as an example, are depicted in Figure 3. Members of the EGFR family are frequently overexpressed in human cancers and are associated with poor prognosis [66]. Many studies have demonstrated the inhibitory effects of EGCG on the EGFR signaling pathways [7,67–70]. The different mechanisms that have been proposed for the inhibition of EGFR by EGCG include 1) interfering with the binding of EGF to EGFR and inhibiting EGFR tyrosine kinase activity [67], 2) altering lipid organization in the plasma membrane (lipid rafts) and inhibiting EGF binding to EGFR [69], and 3) inducing EGFR internalization without activation [70]. The synergistic action of EGCG and erlotinib, an EGFR tyrosine kinase inhibitor, against head and neck cancer cell growth has been reported [71].
Figure 3.
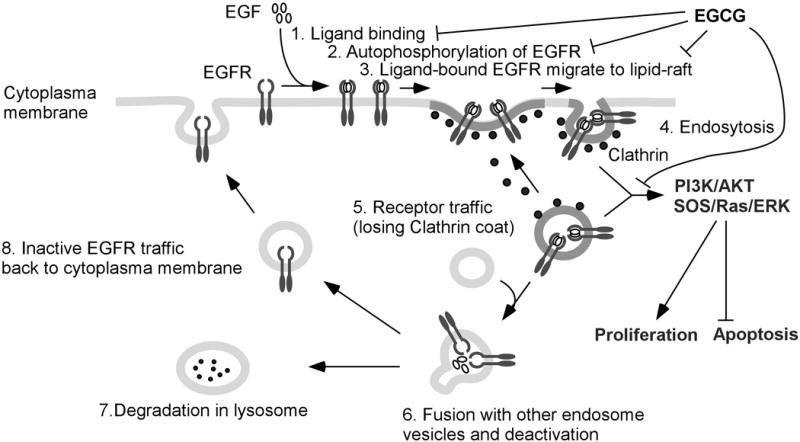
Possible mechanisms of inhibition of receptor tyrosine kinases by EGCG. Epidermal growth factor receptor (EGFR) is used as an example to illustrate the multiple intra-cellular processes in signaling. 1) Upon epidermal growth factor (EGF) binding, 2) the receptor undergoes autophosphorylation and conformational changes, transforming the EGFR to the active form on the surface of cytoplasma membrane. 3) Once activated, EGFR is in a functional membrane with unique lipid composition or mobility (often referred to as lipid raft). 4) Such a functional lipid unit of EGFR, mediated by Clathrin, internalizes and the active EGFR signaling is transduced by the activation of downstream PI3K/AKT and SOS/RAS/ERK pathways. 5) Clathrin-coated internalized vesicles is de-coated, and EGF is disassociated from the active EGFR. 6) The de-coated vesicles are fused with other intra-cellular vesicles. 7) When fused with lysosomes, EGFR is degraded. 8) When fused with vesicles from Golgi, EGFR can be recycled back to cytoplasma membrane. The signaling transduction mediated by different membrane units or sub-cellular components is also found in other receptor tyrosine kinases such as IFGR, HGFR and VEGFR. EGCG has been reported to inhibit this signaling pathway by interfering with the binding of EGF to EGFR, inhibiting EGFR kinase activity, altering lipid organization in the plasma membrane (lipid raft), and inducting EGFR internalization without activation as discussed in the text.
IGF1R activation by IGF1 can induce cell proliferation, cell survival, transformation, metastasis and angiogenesis as well as inhibit apoptosis in different cancer cell lines [72]. IGF/IGF1R axis has been reported to be targets of EGCG in human colon and hepatocellular carcinoma cells [73,74]. EGCG also inhibits IGF1R phosphorylation and increases expression of transforming growth factor-β2 (TGFβ2) in human colon cancer SW837 cells [73], which is consistent with our observations in H-Ras-transformed human bronchial epithelial 21BES cells [75]. Direct binding of EGCG to IGF1R as proposed by Li et al. [41], as discussed previously, is a likely mechanism. These results from cell line studies are consistent with those from animal studies showing that orally administrated EGCG and other tea catechins inhibited the IGF/IGF1R axis in a colon carcinogenesis model in db/db obese mice [16] and TRAMP mice [18].
Inhibition of EGFR signaling has also been shown to decrease the production of VEGFA in cancer cells [76]. EGCG (0.5–10 μM) disrupted VEGFA-induced VEGFR2 dimerization in human umbilical vein endothelial cells [77], and inhibited growth and activation of VEGF/VEGFR axis in human colorectal cancer cells [78]. We have previously observed the downregulation of VEGFA expression and suppression of angiogenesis by treatment with green tea (0.6% green tea solid in drinking fluid) in a lung tumorigenesis model [79]. In a murine gastric tumor model, EGCG suppressed VEGFA protein expression and tumor microvessel density [80].
Deregulation of HGFR pathway occurs in several types of human cancers and can lead to increased tumorigenesis and metastasis [81]. HGFR and HGF play key roles in epithelial–mesenchymal transition, which is associated with tumor invasion [82]. It has been shown in MDA-MB-231 cells that the HGF-induced phosphorylation of HGFR and AKT is completely blocked by 0.6 μM EGCG, and that cell invasion is significantly decreased by 5 μM EGCG [83]. Larsen et al. has provided evidence for the binding of EGCG to the ATP-binding site of HGFR [84]. In a study with FaDu hypopharyngeal carcinoma cells, 1 μM EGCG prevented HGF-induced motility in an in vitro wound healing assay [85]. In a series of non-small cell lung cancer cell lines, EGCG was also found to be a potent inhibitor of cell proliferation and appeared to be more effective against HGFR than against EGFR [86].
EGCG has also been suggested to transcriptionally target sphingosine-1-phosphate receptor S1P2 and prevent sphingosine-1-phosphate mediated signaling in macrophage-differentiated HL-60 promyclomoncytic leukemia cells [87].
5.2 Effects on 67kDa Laminin receptor (67LR)
Binding of EGCG to the 67LR (with a Kd value of 0.04 μM) was first observed by Tachibana et al. using a SPR assay [49]. Expression of the metastases-associated 67LR increased the responsiveness of MCF7 cells to low micromolar concentrations of EGCG [49]. RNA interference (RNAi)-mediated silencing of 67LR abrogated EGCG-induced apoptosis in multiple myeloma (MM) cells [88]. Further studies by this research group also demonstrated the critical role of 67LR in mediating anti-inflammation action of EGCG (1 μM) in macrophages [89]. Anti-67LR antibody treatment or RNAi-mediated silencing of 67LR resulted in the abrogation of the inhibitory action of EGCG on lipopolysaccharide-induced activation of TLR4 and downstream signaling of inflammation. Recent work by Kumazoe et al. [90] showed that the activation of 67LR by EGCG in primary MM cells and MM cell lines (U266, ARH-77 and RPMI 8226) resulted in elevated levels of cGMP, which initiated apoptosis through the activation of PKCδ and acid sphingomyelinase in a novel death pathway. However, EGCG alone was not very effective in killing MM U266 cells (IC50 of 23.2 μM), because these cells overexpressed phosphodiesterase 5 (PDE5), a negative regulator of cGMP. When a PDE5-selective inhibitor vardenafil was also added to cultured cells, it synergized with EGCG to reduce the IC50 of EGCG to 1.4 μM. These authors also described the synergy between EGCG and other PDE5-selective inhibitors (zaprinast, MQZ and sildenafil), and used shRNA, neutralizing antibodies and enzyme inhibitors to demonstrate the involvement of this key receptor and related enzymes in the synergistic action. This impressive synergism was also shown in MM MPC-11 cells and MDA-MB-231 breast cancer cells in a xenograft model, as well as in vitro in some gastric, pancreatic and prostate cancer cell lines. Overexpression of both 67LR and PDE5 was observed in these cell lines [90].
5.3 Inhibition of Wnt signaling
The Wnt signaling involves the nuclear translocation of β-catenin to transcriptionally activate proto-oncogenes such as c-Myc, cyclin D1 and COX-2. Our studies in Apcmin/+ mice suggested that EGCG inhibits Wnt signaling [13]. Treatment of HT29 human colon cancer cells with EGCG (20 μM) decreased nuclear levels of β-catenin as well as cellular levels of c-Myc and cyclin D1 [13]. Our recent studies in colon cancer cell lines also suggested that EGCG induced β-catenin N-terminal phosphorylation at the Ser33/37 residues and promoted its degradation in Apc mutated colon cancer cell lines (unpublished results). The EGCG-induced β-catenin phosphorylation and degradation is consistent with a similar observation by Singh et al. [91]. They also reported that EGCG downregulated the prostaglandin E2-induced levels of MMP2 and MMP9, which are downstream targets of β-catenin [91]. EGCG was also shown to inhibit the Wnt signaling in hepatoblastoma cells [92]. Interestingly, this was found to be associated with the reexpression of the silenced tumor suppressor gene, secreted frizzled-related protein (SFRP)1, which is known to modulate Wnt signaling.
5.4 Epigenetic mechanisms
Affecting epigenetic DNA methylation and histone modification
In addition to the aforementioned epigenetic changes [38,57,59,92], EGCG was reported to decrease the levels of 5-methylcytosine, DNMT activity, and expression levels of DNMT1, DNMT3a and DNMT3b in human epidermoid carcinoma A431 cells. It also decreased HDAC activity and affected levels of acetylated lysines on histones H3 and H4 [93]. Furthermore, EGCG inhibited the majority of acetyltransferase (HAT) enzymes [94] and inhibit the transcription of hTERT (human telomerase reverse transcriptase), the catalytic subunit of telomerase, through epigenetic mechanisms mediated at least partially through the inhibition of DNMT and HAT activities [95].
Effect on microRNA
MicroRNAs are small (about 22 bases) single-stranded, endogenous noncoding RNAs that negatively regulate the translation and/or stability of mRNAs [96]. MicroRNA levels could be altered by EGCG to cause subtle changes in multiple molecular targets and pathways. It has been reported that EGCG upregulated miR-16 in human hepatocellular carcinoma HepG2 cells, and this led to the downregulation of Bcl2 and induction of apoptosis [97]. In our recent work in both human and mouse lung cancer cells in culture, we found that EGCG specifically upregulated the expression of mir-210, a major microRNA regulated by HIF-1α [98]. The upregulation of mir-210 was found to be correlated with the transiently stabilized HIF-1α in lung cancer cell lines after EGCG treatment. We also demonstrated that EGCG could bind to the oxygen-dependent degradation (ODD) domain of the hypoxia-response element of HIF-1α promoter and prevented the hydroxylation-dependent ubiquitination and proteasome-mediated degradation of HIF-1α. The in vivo relevance of this observation, however, remains to be demonstrated. A recent study also showed that EGCG upregulated miR-16 in breast cancer cell line 4T1 [99]. The miR-16 could be transfected to tumor-associated macrophages (TAM) via exosomes and inhibited TAM infiltration and M2 microphage polarization. These actions were suggested to be responsible for the observed growth suppression of xenograft tumors from 4T1 cells in BALB/c mice treated with EGCG.
5.5 Other mechanisms
Modulating p53-dependent events
Many studies have demonstrated that EGCG treatment induces p53 expression and p53-dependent apoptosis. Some studies suggested that this was mediated by ROS, which are produced in the auto-oxidation of EGCG [100], while others showed this did not involve changes in intracellular levels of ROS [101]. It was also reported that the increased p53 transcription and acetylation were due to the suppression of class I HACs in LNCaP cell [102]. In another study, EGCG was found to induce the expression of p53 in HepG2 cells and blocked the cell cycle progression at the G1 phase, whereas EGCG induced apoptosis in p53-negative Hep3B cells [103]. The involvement of p53 in the biological activity of EGCG requires additional studies in vitro and in vivo.
Binding to lipids
The possibility that EGCG alters lipid organization in the plasma membrane (lipid rafts) and affect protein distribution and receptor functions has been proposed for the inhibition of the functions of EGFR [69], HGFR [104], and 67LR [105]. Although interesting, it remains to be determined whether the effects occur in normal cells, whether EGCG also alters the lipid rafts of cancer cells in vivo, and what concentrations of EGCG are required to exert an observable effect in vivo.
Binding to nucleic acids
Based on the physical binding of EGCG to nucleic acids, it has been suggested that DNA and RNA can also be targets of action of tea catechins [106]. However, the relevance of this proposed binding depends on whether the catechins can bind selectively to specific nucleic acid in the genome of cancer or premalignant cells without affecting normal cells.
6. ISSUES IN EXTRAPOPULATING STUDIES IN VITRO TO SITUATIONS IN VIVO
In relating observations in vitro to molecular events in vivo, an important issue is the difference in effective concentrations. In most animal experiments, in which an inhibitory effect of EGCG could be observed, the EGCG levels in the blood and tissues were usually in the sub-micromolar levels. How do we evaluate the relevance of an experiment using 20–100 μM EGCG in cell cultural studies? To address the relationship between the effective concentrations in vivo versus in vitro, we measured the EGCG levels in blood and xenograft tumors of H1299 lung cancer cells. At conditions when tumor growth was inhibited approximately 50% by dietary EGCG (0.5%), the EGCG concentrations was 0.52 μM in blood and 0.18 μM in tumor tissues. This value is 2-orders of magnitude lower than the IC50 values of EGCG in the inhibition of H1299 cell growth in culture [29]. One possible reason for the observed discrepancy between the cell culture system and the xenograft model is the rather short-term exposure to EGCG in cell culture studies (24 or 48 h) compared to the long-term treatment in animal models. It has been reported that prolonging the treatment period of cells in culture can reduce the effective concentration of EGCG [68]. The environment for cells in culture is also very different from that in tumors. Therefore, we cannot rule out a mechanism just because the in vitro effective concentrations of EGCG are higher than we observed in vivo. However, it is reasonable to assume that activities effected by low concentrations of EGCG are likely to be more relevant than activities that are produced only at higher concentrations. The results from very high concentrations of catechins in cell culture systems may not be relevant to cancer prevention. Similarly, events due to ROS generated by EGCG extra-cellularly (which can be prevented by the inclusion of SOD and catalase in the incubation mixture) may not occur in vivo [7]
7. CONCLUDING REMARKS
A frequently asked question is that whether all the above reviewed actions of catechins are relevant for cancer prevention or cancer therapy in vivo. Apparently, mechanisms suggested by cancer prevention studies in animal models are likely to be relevant. These include the induction of apoptosis in different animal models, inhibition of the phosphorylation of c-Jun and ERK1/2 in lung tumorigenesis models, suppression of phospho-AKT and nuclear β-catenin levels in colon cancer models, inhibition of the IGF/IGF1R axis in colon and prostate cancer models, and suppression of VEGF-dependent angiogenesis in lung and prostate cancer models [13,16,17,19,79]. It is still unclear whether these molecules are direct targets for EGCG or downstream events of the primary action. It is reasonable to assume that some of the high affinity binding proteins as discussed in Section 4.2 could serve as initial targets, but this point remains to be investigated in animal models.
Because of the broad cancer prevention activities of tea catechins in different animal models, multiple mechanisms are likely to be involved. Even in the same experimental system, one tea catechin such as EGCG may exhibit cancer inhibitory activities via more than one mechanism. The possibility that these mechanisms may work synergistically to exert the cancer preventive activity is interesting and needs to be substantiated. Because of these reasons, precise information about the mechanisms of cancer prevention by tea in humans is even more difficult to obtain. From the limited human studies that are available, action of tea constituents in reducing oxidative stress and enhancing the elimination of carcinogens [35,36] may be important.
As for therapeutic applications, even though laboratory studies with cancer cell lines generated exciting results on the effects of EGCG on cancer signaling, there are only a few clinical studies which have demonstrated the usefulness of EGCG. Although many reports indicate that EGCG act on specific targets, liver toxicity with high doses of EGCG or catechin mixtures have been well documented [30–32]. The use of lower doses of EGCG in adjuvant therapy together with other agents, such as suggested by Kumazoe et al. [90], may be more promising.
Acknowledgments
The authors thank Ms. Dorothy Wong for her assistance in the preparation of this manuscript. Our research on tea and cancer was supported by U.S. NIH grant CA120915, CA122474 and CA133021.
References
- 1.Yang CS, Wang H, Li GX, Yang Z, Guan F, Jin H. Cancer prevention by tea: Evidence from laboratory studies. Pharmacol Res. 2011;64:113–22. doi: 10.1016/j.phrs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9:429–39. doi: 10.1038/nrc2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 4.Crespy V, Williamson G. A review of the health effects of green tea catechins in in vivo animal models. J Nutr. 2004;134:3431S–3440S. doi: 10.1093/jn/134.12.3431S. [DOI] [PubMed] [Google Scholar]
- 5.Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacological research : the official journal of the Italian Pharmacological Society. 2011;64:87–99. doi: 10.1016/j.phrs.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Yang CS, Wang H. Mechanistic issues concerning cancer prevention by tea catechins. Mol Nutr Food Res. 2011;55:819–31. doi: 10.1002/mnfr.201100036. [DOI] [PubMed] [Google Scholar]
- 7.Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, et al. Mechanism of action of (−)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005;65:8049–56. doi: 10.1158/0008-5472.CAN-05-0480. [DOI] [PubMed] [Google Scholar]
- 8.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 9.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol Nutr Food Res. 2008;52(Suppl 1):S139–51. doi: 10.1002/mnfr.200700234. [DOI] [PubMed] [Google Scholar]
- 10.Roth M, Timmermann BN, Hagenbuch B. Interactions of green tea catechins with organic anion-transporting polypeptides. Drug Metab Dispos. 2011;39:920–6. doi: 10.1124/dmd.110.036640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jemnitz K, Heredi-Szabo K, Janossy J, Ioja E, Vereczkey L, Krajcsi P. ABCC2/Abcc2: a multispecific transporter with dominant excretory functions. Drug Metab Rev. 2010;42:402–36. doi: 10.3109/03602530903491741. [DOI] [PubMed] [Google Scholar]
- 12.Li C, Lee MJ, Sheng S, Meng X, Prabhu S, Winnik B, et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chemical research in toxicology. 2000;13:177–84. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- 13.Ju J, Hong J, Zhou JN, Pan Z, Bose M, Liao J, et al. Inhibition of intestinal tumorigenesis in Apcmin/+ mice by (−)-epigallocatechin-3-gallate, the major catechin in green tea. Cancer Res. 2005;65:10623–31. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 14.Ju J, Liu Y, Hong J, Huang MT, Conney AH, Yang CS. Effects of green tea and high-fat diet on arachidonic acid metabolism and aberrant crypt foci formation in an azoxymethane-induced colon carcinogenesis mouse model. Nutr Cancer. 2003;46:172–8. doi: 10.1207/S15327914NC4602_10. [DOI] [PubMed] [Google Scholar]
- 15.Xiao H, Hao X, Simi B, Ju J, Jiang H, Reddy BS, et al. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats. Carcinogenesis. 2008;29:113–9. doi: 10.1093/carcin/bgm204. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu M, Shirakami Y, Sakai H, Adachi S, Hata K, Hirose Y, et al. (−)-Epigallocatechin gallate suppresses azoxymethane-induced colonic premalignant lesions in male C57BL/KsJ-db/db mice. Cancer Prev Res (Phila) 2008;1:298–304. doi: 10.1158/1940-6207.CAPR-08-0045. [DOI] [PubMed] [Google Scholar]
- 17.Lu G, Liao J, Yang G, Reuhl KR, Hao X, Yang CS. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006;66:11494–501. doi: 10.1158/0008-5472.CAN-06-1497. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adhami VM, Siddiqui IA, Ahmad N, Gupta S, Mukhtar H. Oral consumption of green tea polyphenols inhibits insulin-like growth factor-I-induced signaling in an autochthonous mouse model of prostate cancer. Cancer Res. 2004;64:8715–22. doi: 10.1158/0008-5472.CAN-04-2840. [DOI] [PubMed] [Google Scholar]
- 20.Shanafelt TD, Call TG, Zent CS, Leis JF, LaPlant B, Bowen DA, et al. Phase 2 trial of daily, oral Polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer. 2013;119:363–70. doi: 10.1002/cncr.27719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickinson BC, Chang CJ. Chemistry and biology of reactive oxygen species in signaling or stress responses. Nat Chem Biol. 2011;7:504–11. doi: 10.1038/nchembio.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–85. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 23.DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, Frese K, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–9. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol. 2013;3:120144. doi: 10.1098/rsob.120144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Srividhya R, Jyothilakshmi V, Arulmathi K, Senthilkumaran V, Kalaiselvi P. Attenuation of senescence-induced oxidative exacerbations in aged rat brain by (−)-epigallocatechin-3-gallate. Int J Dev Neurosci. 2008;26:217–23. doi: 10.1016/j.ijdevneu.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Ho CT, Amin SG, Han C, Chung FL. Inhibition of tobacco-specific nitrosamine-induced lung tumorigenesis in A/J mice by green tea and its major polyphenol as antioxidants. Cancer Res. 1992;52:3875–9. [PubMed] [Google Scholar]
- 27.Schwartz JL, Baker V, Larios E, Chung FL. Molecular and cellular effects of green tea on oral cells of smokers: a pilot study. Mol Nutr Food Res. 2005;49:43–51. doi: 10.1002/mnfr.200400031. [DOI] [PubMed] [Google Scholar]
- 28.Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–6. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- 29.Li GX, Chen YK, Hou Z, Xiao H, Jin H, Lu G, et al. Pro-oxidative activities and dose-response relationship of (−)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: a comparative study in vivo and in vitro. Carcinogenesis. 2010;31:902–10. doi: 10.1093/carcin/bgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert JD, Kennett MJ, Sang S, Reuhl KR, Ju J, Yang CS. Hepatotoxicity of high oral dose (−)-epigallocatechin-3-gallate in mice. Food & Chem Toxicol. 2010;48:409–416. doi: 10.1016/j.fct.2009.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lambert JD, Sang S, Yang CS. Possible controversy over dietary polyphenols: benefits vs risks. Chem Res Toxicol. 2007;20:583–5. doi: 10.1021/tx7000515. [DOI] [PubMed] [Google Scholar]
- 32.Mazzanti G, Menniti-Ippolito F, Moro PA, Cassetti F, Raschetti R, Santuccio C, et al. Hepatotoxicity from green tea: a review of the literature and two unpublished cases. Eur J Clin Pharmacol. 2009;65:331–41. doi: 10.1007/s00228-008-0610-7. [DOI] [PubMed] [Google Scholar]
- 33.Hayes JD, Dinkova-Kostova AT. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci. 2014;39:199–218. doi: 10.1016/j.tibs.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Shen G, Xu C, Hu R, Jain MR, Nair S, Lin W, et al. Comparison of (−)-epigallocatechin-3-gallate elicited liver and small intestine gene expression profiles between C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Pharm Res. 2005;22:1805–20. doi: 10.1007/s11095-005-7546-8. [DOI] [PubMed] [Google Scholar]
- 35.Chow HH, Hakim IA, Vining DR, Crowell JA, Tome ME, Ranger-Moore J, et al. Modulation of human glutathione s-transferases by polyphenon e intervention. Cancer Epidemiol Biomarkers Prev. 2007;16:1662–6. doi: 10.1158/1055-9965.EPI-06-0830. [DOI] [PubMed] [Google Scholar]
- 36.Tang L, Tang M, Xu L, Luo H, Huang T, Yu J, et al. Modulation of aflatoxin biomarkers in human blood and urine by green tea polyphenols intervention. Carcinogenesis. 2008;29:411–7. doi: 10.1093/carcin/bgn008. [DOI] [PubMed] [Google Scholar]
- 37.Li M, Hagerman AE. Role of the flavan-3-ol and galloyl moieties in the interaction of (−)-epigallocatechin gallate with serum albumin. J Agric Food Chem. 2014;62:3768–75. doi: 10.1021/jf500246m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–70. [PubMed] [Google Scholar]
- 39.Leone M, Zhai D, Sareth S, Kitada S, Reed JC, Pellecchia M. Cancer prevention by tea polyphenols is linked to their direct inhibition of antiapoptotic Bcl-2-family proteins. Cancer Res. 2003;63:8118–21. [PubMed] [Google Scholar]
- 40.Ermakova S, Choi BY, Choi HS, Kang BS, Bode AM, Dong Z. The intermediate filament protein vimentin is a new target for epigallocatechin gallate. J Biol Chem. 2005;280:16882–90. doi: 10.1074/jbc.M414185200. [DOI] [PubMed] [Google Scholar]
- 41.Li M, He Z, Ermakova S, Zheng D, Tang F, Cho YY, et al. Direct inhibition of insulin-like growth factor-I receptor kinase activity by (−)-epigallocatechin-3-gallate regulates cell transformation. Cancer Epidemiol Biomarkers Prev. 2007;16:598–605. doi: 10.1158/1055-9965.EPI-06-0892. [DOI] [PubMed] [Google Scholar]
- 42.He Z, Tang F, Ermakova S, Li M, Zhao Q, Cho YY, et al. Fyn is a novel target of (−)-epigallocatechin gallate in the inhibition of JB6 Cl41 cell transformation. Mol Carcinog. 2008;47:172–83. doi: 10.1002/mc.20299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ermakova SP, Kang BS, Choi BY, Choi HS, Schuster TF, Ma WY, et al. (−)-Epigallocatechin gallate overcomes resistance to etoposide-induced cell death by targeting the molecular chaperone glucose-regulated protein. 78 Cancer Res. 2006;66:9260–9. doi: 10.1158/0008-5472.CAN-06-1586. [DOI] [PubMed] [Google Scholar]
- 44.Shim JH, Choi HS, Pugliese A, Lee SY, Chae JI, Choi BY, et al. (−)-Epigallocatechin gallate regulates CD3-mediated T cell receptor signaling in leukemia through the inhibition of ZAP-70 kinase. J Biol Chem. 2008;283:28370–9. doi: 10.1074/jbc.M802200200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shim JH, Su ZY, Chae JI, Kim DJ, Zhu F, Ma WY, et al. Epigallocatechin gallate suppresses lung cancer cell growth through Ras-GTPase-activating protein SH3 domain-binding protein 1. Cancer Prev Res (Phila) 2010;3:670–9. doi: 10.1158/1940-6207.CAPR-09-0185. [DOI] [PubMed] [Google Scholar]
- 46.Urusova DV, Shim JH, Kim DJ, Jung SK, Zykova TA, Carper A, et al. Epigallocatechin-gallate suppresses tumorigenesis by directly targeting Pin1. Cancer Prev Res (Phila) 2011;4:1366–77. doi: 10.1158/1940-6207.CAPR-11-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menegazzi M, Mariotto S, Dal Bosco M, Darra E, Vaiana N, Shoji K, et al. Direct interaction of natural and synthetic catechins with signal transducer activator of transcription 1 affects both its phosphorylation and activity. FEBS J. 2014;281:724–38. doi: 10.1111/febs.12618. [DOI] [PubMed] [Google Scholar]
- 48.Lin HY, Hou SC, Chen SC, Kao MC, Yu CC, Funayama S, et al. (−)-Epigallocatechin gallate induces Fas/CD95-mediated apoptosis through inhibiting constitutive and IL-6-induced JAK/STAT3 signaling in head and neck squamous cell carcinoma cells. J Agric Food Chem. 2012;60:2480–9. doi: 10.1021/jf204362n. [DOI] [PubMed] [Google Scholar]
- 49.Tachibana H, Koga K, Fujimura Y, Yamada K. A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol. 2004;11:380–1. doi: 10.1038/nsmb743. [DOI] [PubMed] [Google Scholar]
- 50.Dong Z, Ma W, Huang C, Yang CS. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols, (−)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997;57:4414–9. [PubMed] [Google Scholar]
- 51.Chung JY, Huang C, Meng X, Dong Z, Yang CS. Inhibition of activator protein 1 activity and cell growth by purified green tea and black tea polyphenols in H-ras-transformed cells: structure-activity relationship and mechanisms involved. Cancer Res. 1999;59:4610–7. [PubMed] [Google Scholar]
- 52.Chung JY, Park JO, Phyu H, Dong Z, Yang CS. Mechanisms of inhibition of the Ras-MAP kinase signaling pathway in 30.7b Ras 12 cells by tea polyphenols (−)-epigallocatechin-3-gallate and theaflavin-3,3′-digallate. FASEB J. 2001;15:2022–4. doi: 10.1096/fj.01-0031fje. [DOI] [PubMed] [Google Scholar]
- 53.Liu S, Li H, Chen L, Yang L, Li L, Tao Y, et al. (−)-Epigallocatechin-3-gallate inhibition of Epstein-Barr virus spontaneous lytic infection involves ERK1/2 and PI3-K/Akt signaling in EBV-positive cells. Carcinogenesis. 2013;34:627–37. doi: 10.1093/carcin/bgs364. [DOI] [PubMed] [Google Scholar]
- 54.Nam S, Smith DM, Dou QP. Ester bond-containing tea polyphenols potently inhibit proteasome activity in vitro and in vivo. J Biol Chem. 2001;276:13322–30. doi: 10.1074/jbc.M004209200. [DOI] [PubMed] [Google Scholar]
- 55.Garbisa S, Biggin S, Cavallarin N, Sartor L, Benelli R, Albini A. Tumor invasion: molecular shears blunted by green tea. Nat Med. 1999;5:1216. doi: 10.1038/15145. [DOI] [PubMed] [Google Scholar]
- 56.Garbisa S, Sartor L, Biggin S, Salvato B, Benelli R, Albini A. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer. 2001;91:822–32. doi: 10.1002/1097-0142(20010215)91:4<822::aid-cncr1070>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 57.Deb G, Thakur VS, Limaye AM, Gupta S. Epigenetic induction of tissue inhibitor of matrix metalloproteinase-3 by green tea polyphenols in breast cancer cells. Mol Carcinog. 2014 doi: 10.1002/mc.22121. [DOI] [PubMed] [Google Scholar]
- 58.Taniguchi S, Fujiki H, Kobayashi H, Go H, Miyado K, Sadano H, et al. Effect of (−)-epigallocatechin gallate, the main constituent of green tea, on lung metastasis with mouse B16 melanoma cell lines. Cancer Lett. 1992;65:51–4. doi: 10.1016/0304-3835(92)90212-e. [DOI] [PubMed] [Google Scholar]
- 59.Pandey M, Shukla S, Gupta S. Promoter demethylation and chromatin remodeling by green tea polyphenols leads to re-expression of GSTP1 in human prostate cancer cells. Int J Cancer. 2010;126:2520–33. doi: 10.1002/ijc.24988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Navarro-Peran E, Cabezas-Herrera J, Garcia-Canovas F, Durrant MC, Thorneley RN, Rodriguez-Lopez JN. The antifolate activity of tea catechins. Cancer Res. 2005;65:2059–64. doi: 10.1158/0008-5472.CAN-04-3469. [DOI] [PubMed] [Google Scholar]
- 61.Shin ES, Park J, Shin JM, Cho D, Cho SY, Shin DW, et al. Catechin gallates are NADP+-competitive inhibitors of glucose-6-phosphate dehydrogenase and other enzymes that employ NADP+ as a coenzyme. Bioorg Med Chem. 2008;16:3580–6. doi: 10.1016/j.bmc.2008.02.030. [DOI] [PubMed] [Google Scholar]
- 62.Ishii T, Mori T, Tanaka T, Mizuno D, Yamaji R, Kumazawa S, et al. Covalent modification of proteins by green tea polyphenol (−)-epigallocatechin-3-gallate through autoxidation. Free Radic Biol Med. 2008;45:1384–94. doi: 10.1016/j.freeradbiomed.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 63.Huang W, Ding L, Huang Q, Hu H, Liu S, Yang X, et al. Carbonyl reductase 1 as a novel target of (−)-epigallocatechin gallate against hepatocellular carcinoma. Hepatology. 2010;52:703–14. doi: 10.1002/hep.23723. [DOI] [PubMed] [Google Scholar]
- 64.Larsen CA, Dashwood RH, Bisson WH. Tea catechins as inhibitors of receptor tyrosine kinases: mechanistic insights and human relevance. Pharmacol Res. 2010;62:457–64. doi: 10.1016/j.phrs.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shimizu M, Adachi S, Masuda M, Kozawa O, Moriwaki H. Cancer chemoprevention with green tea catechins by targeting receptor tyrosine kinases. Mol Nutr Food Res. 2011;55:832–43. doi: 10.1002/mnfr.201000622. [DOI] [PubMed] [Google Scholar]
- 66.Lo HW, Hung MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer. 2006;94:184–8. doi: 10.1038/sj.bjc.6602941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang YC, Lin-shiau SY, Chen CF, Lin JK. Suppression of extracellular signals and cell proliferation through EGF receptor binding by (−)-epigallocatechin gallate in human A431 epidermoid carcinoma cells. J Cell Biochem. 1997;67:55–65. doi: 10.1002/(sici)1097-4644(19971001)67:1<55::aid-jcb6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 68.Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (−)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res. 2005;11:2735–46. doi: 10.1158/1078-0432.CCR-04-2014. [DOI] [PubMed] [Google Scholar]
- 69.Adachi S, Nagao T, Ingolfsson HI, Maxfield FR, Andersen OS, Kopelovich L, et al. The inhibitory effect of (−)-epigallocatechin gallate on activation of the epidermal growth factor receptor is associated with altered lipid order in HT29 colon cancer cells. Cancer Res. 2007;67:6493–501. doi: 10.1158/0008-5472.CAN-07-0411. [DOI] [PubMed] [Google Scholar]
- 70.Adachi S, Nagao T, To S, Joe AK, Shimizu M, Matsushima-Nishiwaki R, et al. (−)-Epigallocatechin gallate causes internalization of the epidermal growth factor receptor in human colon cancer cells. Carcinogenesis. 2008;29:1986–93. doi: 10.1093/carcin/bgn128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X, Zhang H, Tighiouart M, Lee JE, Shin HJ, Khuri FR, et al. Synergistic inhibition of head and neck tumor growth by green tea (−)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int J Cancer. 2008;123:1005–14. doi: 10.1002/ijc.23585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mitsiades CS, Mitsiades NS, McMullan CJ, Poulaki V, Shringarpure R, Akiyama M, et al. Inhibition of the insulin-like growth factor receptor-1 tyrosine kinase activity as a therapeutic strategy for multiple myeloma, other hematologic malignancies, and solid tumors. Cancer Cell. 2004;5:221–30. doi: 10.1016/s1535-6108(04)00050-9. [DOI] [PubMed] [Google Scholar]
- 73.Shimizu M, Deguchi A, Hara Y, Moriwaki H, Weinstein IB. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem Biophys Res Commun. 2005;334:947–53. doi: 10.1016/j.bbrc.2005.06.182. [DOI] [PubMed] [Google Scholar]
- 74.Shimizu M, Shirakami Y, Sakai H, Tatebe H, Nakagawa T, Hara Y, et al. EGCG inhibits activation of the insulin-like growth factor (IGF)/IGF-1 receptor axis in human hepatocellular carcinoma cells. Cancer Lett. 2008;262:10–8. doi: 10.1016/j.canlet.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 75.Vittal R, Selvanayagam ZE, Sun Y, Hong J, Liu F, Chin KV, et al. Gene expression changes induced by green tea polyphenol (−)-epigallocatechin-3-gallate in human bronchial epithelial 21BES cells analyzed by DNA microarray. Mol Cancer Ther. 2004;3:1091–9. [PubMed] [Google Scholar]
- 76.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350–9. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 77.Rodriguez SK, Guo W, Liu L, Band MA, Paulson EK, Meydani M. Green tea catechin, epigallocatechin-3-gallate, inhibits vascular endothelial growth factor angiogenic signaling by disrupting the formation of a receptor complex. Int J Cancer. 2006;118:1635–44. doi: 10.1002/ijc.21545. [DOI] [PubMed] [Google Scholar]
- 78.Shimizu M, Shirakami Y, Sakai H, Yasuda Y, Kubota M, Adachi S, et al. (−)-Epigallocatechin gallate inhibits growth and activation of the VEGF/VEGFR axis in human colorectal cancer cells. Chem Biol Interact. 2010;185:247–52. doi: 10.1016/j.cbi.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 79.Liao J, Yang GY, Park ES, Meng X, Sun Y, Jia D, et al. Inhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green tea. Nutr Cancer. 2004;48:44–53. doi: 10.1207/s15327914nc4801_7. [DOI] [PubMed] [Google Scholar]
- 80.Zhu BH, Zhan WH, Li ZR, Wang Z, He YL, Peng JS, et al. (−)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J Gastroenterol. 2007;13:1162–9. doi: 10.3748/wjg.v13.i8.1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peschard P, Park M. From Tpr-Met to Met, tumorigenesis and tubes. Oncogene. 2007;26:1276–85. doi: 10.1038/sj.onc.1210201. [DOI] [PubMed] [Google Scholar]
- 82.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 83.Bigelow RL, Cardelli JA. The green tea catechins, (−)-Epigallocatechin-3-gallate (EGCG) and (−)-Epicatechin-3-gallate (ECG), inhibit HGF/Met signaling in immortalized and tumorigenic breast epithelial cells. Oncogene. 2006;25:1922–30. doi: 10.1038/sj.onc.1209227. [DOI] [PubMed] [Google Scholar]
- 84.Larsen CA, Bisson WH, Dashwood RH. Tea catechins inhibit hepatocyte growth factor receptor (MET kinase) activity in human colon cancer cells: kinetic and molecular docking studies. J Med Chem. 2009;52:6543–5. doi: 10.1021/jm901330e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lim YC, Park HY, Hwang HS, Kang SU, Pyun JH, Lee MH, et al. (−)-Epigallocatechin-3-gallate (EGCG) inhibits HGF-induced invasion and metastasis in hypopharyngeal carcinoma cells. Cancer Lett. 2008;271:140–52. doi: 10.1016/j.canlet.2008.05.048. [DOI] [PubMed] [Google Scholar]
- 86.Milligan SA, Burke P, Coleman DT, Bigelow RL, Steffan JJ, Carroll JL, et al. The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells. Clin Cancer Res. 2009;15:4885–94. doi: 10.1158/1078-0432.CCR-09-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chokor R, Lamy S, Annabi B. Transcriptional targeting of sphingosine-1-phosphate receptor S1P2 by epigallocatechin-3-gallate prevents sphingosine-1-phosphate-mediated signaling in macrophage-differentiated HL-60 promyelomonocytic leukemia cells. Onco Targets Ther. 2014;7:667–77. doi: 10.2147/OTT.S62717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Umeda D, Yano S, Yamada K, Tachibana H. Green tea polyphenol epigallocatechin-3-gallate signaling pathway through 67-kDa laminin receptor. J Biol Chem. 2008;283:3050–8. doi: 10.1074/jbc.M707892200. [DOI] [PubMed] [Google Scholar]
- 89.Hong Byun E, Fujimura Y, Yamada K, Tachibana H. TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin-3-gallate through 67-kDa laminin receptor. J Immunol. 2010;185:33–45. doi: 10.4049/jimmunol.0903742. [DOI] [PubMed] [Google Scholar]
- 90.Kumazoe M, Sugihara K, Tsukamoto S, Huang Y, Tsurudome Y, Suzuki T, et al. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J Clin Invest. 2013;123:787–99. doi: 10.1172/JCI64768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh T, Katiyar SK. Green tea polyphenol, (−)-epigallocatechin-3-gallate, induces toxicity in human skin cancer cells by targeting beta-catenin signaling. Toxicol Appl Pharmacol. 2013;273:418–24. doi: 10.1016/j.taap.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Godeke J, Maier S, Eichenmuller M, Muller-Hocker J, von Schweinitz D, Kappler R. Epigallocatechin-3-gallate inhibits hepatoblastoma growth by reactivating the Wnt inhibitor SFRP1. Nutr Cancer. 2013;65:1200–7. doi: 10.1080/01635581.2013.828085. [DOI] [PubMed] [Google Scholar]
- 93.Nandakumar V, Vaid M, Katiyar SK. (−)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis. 2011;32:537–44. doi: 10.1093/carcin/bgq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Choi KC, Jung MG, Lee YH, Yoon JC, Kwon SH, Kang HB, et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009;69:583–92. doi: 10.1158/0008-5472.CAN-08-2442. [DOI] [PubMed] [Google Scholar]
- 95.Meeran SM, Patel SN, Chan TH, Tollefsbol TO. A novel prodrug of epigallocatechin-3-gallate: differential epigenetic hTERT repression in human breast cancer cells. Cancer Prev Res (Phila) 2011;4:1243–54. doi: 10.1158/1940-6207.CAPR-11-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tsang WP, Kwok TT. Epigallocatechin gallate up-regulation of miR-16 and induction of apoptosis in human cancer cells. J Nutr Biochem. 2010;21:140–6. doi: 10.1016/j.jnutbio.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 98.Wang H, Bian S, Yang CS. Green tea polyphenol EGCG suppresses lung cancer cell growth through upregulating miR-210 expression caused by stabilizing HIF-1alpha. Carcinogenesis. 2011;32:1881–9. doi: 10.1093/carcin/bgr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Jang JY, Lee JK, Jeon YK, Kim CW. Exosome derived from epigallocatechin gallate treated breast cancer cells suppresses tumor growth by inhibiting tumor-associated macrophage infiltration and M2 polarization. BMC Cancer. 2013;13:421. doi: 10.1186/1471-2407-13-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee MH, Han DW, Hyon SH, Park JC. Apoptosis of human fibrosarcoma HT-1080 cells by epigallocatechin-3-O-gallate via induction of p53 and caspases as well as suppression of Bcl-2 and phosphorylated nuclear factor-kappaB. Apoptosis. 2011;16:75–85. doi: 10.1007/s10495-010-0548-y. [DOI] [PubMed] [Google Scholar]
- 101.Lee JH, Jeong YJ, Lee SW, Kim D, Oh SJ, Lim HS, et al. EGCG induces apoptosis in human laryngeal epidermoid carcinoma Hep2 cells via mitochondria with the release of apoptosis-inducing factor and endonuclease G. Cancer Lett. 2010;290:68–75. doi: 10.1016/j.canlet.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 102.Thakur VS, Gupta K, Gupta S. Green tea polyphenols increase p53 transcriptional activity and acetylation by suppressing class I histone deacetylases. Int J Oncol. 2012;41:353–61. doi: 10.3892/ijo.2012.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Huang CH, Tsai SJ, Wang YJ, Pan MH, Kao JY, Way TD. EGCG inhibits protein synthesis, lipogenesis, and cell cycle progression through activation of AMPK in p53 positive and negative human hepatoma cells. Mol Nutr Food Res. 2009;53:1156–65. doi: 10.1002/mnfr.200800592. [DOI] [PubMed] [Google Scholar]
- 104.Duhon D, Bigelow RL, Coleman DT, Steffan JJ, Yu C, Langston W, et al. The polyphenol epigallocatechin-3-gallate affects lipid rafts to block activation of the c-Met receptor in prostate cancer cells. Mol Carcinog. 2010;49:739–49. doi: 10.1002/mc.20649. [DOI] [PubMed] [Google Scholar]
- 105.Fujimura Y, Yamada K, Tachibana H. A lipid raft-associated 67kDa laminin receptor mediates suppressive effect of epigallocatechin-3-O-gallate on FcepsilonRI expression. Biochem Biophys Res Commun. 2005;336:674–81. doi: 10.1016/j.bbrc.2005.08.146. [DOI] [PubMed] [Google Scholar]
- 106.Kuzuhara T, Sei Y, Yamaguchi K, Suganuma M, Fujiki H. DNA and RNA as new binding targets of green tea catechins. J Biol Chem. 2006;281:17446–56. doi: 10.1074/jbc.M601196200. [DOI] [PubMed] [Google Scholar]


