Abstract
Total body irradiation (TBI) damages hematopoietic cells in the bone marrow (BM) and thymus; however, the long-term effects of irradiation with aging remain unclear. In this study, we found that the impact of radiation on thymopoiesis in mice varied by sex and dose, but overall thymopoiesis remained suppressed for at least 12 months after a single exposure. Both male and female mice showed long-term dose dependent reduction of thymic ckit+ lymphoid progenitors that was maintained throughout life. Damage to hematopoietic stem cells (HSCs) in the bone marrow was dose dependent, with as little as 0.5 Gy causing a significant long-term reduction. In addition, the potential for T lineage commitment was radiation sensitive with aging. Overall, the impact of irradiation on the hematopoietic lineage was more severe in females. In contrast, the rate of decline in thymic epithelial cell numbers with age was radiation-sensitive only in males, and other characteristics including CCL25 production were unaffected. Taken together, these data suggest that long-term suppression of thymopoeisis after sub-lethal irradiation was primarily due to fewer progenitors in the BM combined with reduced potential for T lineage commitment. A single irradiation dose also caused synchronization of thymopoeisis, with a periodic thymocyte differentiation profile persisting for at least 12 months post-irradiation. This study suggests that the number and capability of HSCs for T cell production can be dramatically and permanently damaged after a single relatively low TBI dose, accelerating aging-associated thymic involution. Our findings may impact evaluation and therapeutic intervention of human TBI events.
Introduction
The thymus is the primary organ required for T cell development and maturation. In the normal steady-state thymus, different regionally restricted subsets of thymic epithelial cells (TECs) provide the required signals for thymocyte development, with T cell production dependent on the periodical importation of bone marrow derived hematopoietic progenitor cells throughout life (1–3). The newly imported progenitors, characterized as Lin−cKithi HSA+/−CD44+CD25− cells, are referred to as DN1a,b cells (4), or early thymic progenitors (ETP) (5). These cells have been shown to include the progenitors for αβ T cell receptor (TCR) T cells (4). DN1a,b cells in the thymus undergo a stepwise differentiation program to generate CD4 or CD8 single positive (SP) cells that are then exported to the periphery (6). The decline of thymocyte production is the major hallmark of aging associated thymic involution; reduction of progenitors and lost function of TECs may both mechanistically contribute to this event (7).
Ionizing irradiation is broadly used as a clinical treatment for depletion of host BM-derived cells before HSC transplantation, or as part of cancer radiotherapy after surgery and chemotherapy (8, 9). It is also widely used in the laboratory for HSC transfer experiments (10–12). Environmental exposure due to accidents (such as the recent Fukushima incident) or exposure to atomic bomb explosions (such as occurred in Hiroshima and Nagasaki during World War 2, or during bomb tests in the 1950’s) can also result in sub-lethal total body irradiation (TBI). TBI damages the DNA of cells, thus blocking their ability to divide and proliferate, but has been reported to spare the most primitive hematopoietic progenitors (13, 14), which may be protected by their residence in the bone marrow. There is a general agreement that the frequently proliferating or cycling cells, such as hematopoietic cells (including thymocytes and peripheral lymphocytes) and progenitor cells in the small intestine, epidermis, and hair follicles are considered to be radiation sensitive, while nervous, liver, muscle, and organ stromal cells, including thymic stroma, that cycle more slowly or are post-mitotic are considered to be relatively irradiation-resistant (15).
With a single sub-lethal TBI dose, most thymic lymphoid progenitors and developing thymocytes, particularly DN and DP thymocytes, are immediately damaged and undergo apoptosis at high rates, resulting in a relative increase in the frequency of CD4+ and CD8+ SP cells within a few of days after irradiation (10, 16, 17). Total thymocyte numbers recover to near-normal levels around day 9, but drop again around day 14 after irradiation in mice (11, 16, 18, 19). The recovery kinetics of total thymocyte numbers under these conditions appears to have no dose dependence between 4 and 8.5 Gy, based on published data (19). Damage to BM cells directly affects the restoration of thymocyte production due to reduction of progenitors after irradiation. Some studies have shown that the proliferation and repopulation of BM progenitors are limited because of irradiation exhaustion (20, 21), and these effects were proposed to be directly due to the irradiation itself, but not aging (22). However, this opinion has been challenged by a similar transplantation of long-term reconstituting cells (LTRCs) (12, 23). The difference between these experiments indicates that cells collected at different time periods after irradiation may exhibit differential repopulation capability, especially for LTRCs (24). In addition, a single purified HSC possesses high homing and repopulation capability sufficient to stably reconstitute lethally irradiated recipients (20, 25–28). A single lethal TBI dose (9.5 Gy) causes mice to die after day 14 in the absence of reconstitution (17). Sub-lethal TBI doses (4~8.5 Gy) reduce BM cells within a few days, and although the total number of BM cells is restored to almost untreated levels at day 28, the total number of Lin−Sca-1+cKithi (LSK) cells representing the lymphocyte progenitors in BM remain reduced 2 months after irradiation (29). Therefore, the damage–restoration relationship between the BM and thymus after a single dose of sub-lethal TBI remains unclear. Furthermore, the impact of irradiation doses lower than 4Gy has not been as thoroughly investigated, and not in the context of aging.
In the current study, male and female mice aged 2 months were exposed to γ-rays (60 Co) at a range of sub-lethal doses from 0.5 to 4 Gy, and then analyzed at age 3.5 through 18 months, to determine the long-term effects of irradiation and aging on the thymus in a sex- and dose- dependent manner. Our results suggest that a single dose of TBI at a dose of 1 Gy is sufficient to cause long-term damage to thymic T cell progenitors, characterized by the reduction of DN1a,b, and DN2 cells in both male and female mice. Several different results, including differential sensitivity of the two sexes to irradiation induced damage in hematopoietic vs. thymic epithelial cells, indicate that this reduction was due to the impairment of BM-derived progenitors rather than to changes in the thymic microenvironment. Although the initial drop in total thymocyte number was restored and even over-expanded 5–6 weeks after irradiation, the damage caused by irradiation resulted in long-term reduction of total thymocytes and specific subsets, stimulating thymocytes to undergo increased homeostatic proliferation throughout the lifespan. Damage to BM-derived HSCs was permanent, and included both fewer HSCs and a reduced potential for T lineage commitment with aging. Remarkably, a single dose of sub-lethal irradiation was sufficient to synchronize thymocyte developmental progression, resulting in waves of thymocyte maturation that were detectable even at 12 months of age. Our studies provide evidence that LSK cells are permanently damaged after even a single low dose of TBI. This long-term damage is partially compensated by the thymic microenvironment, which increases proliferation of immature thymocytes, but ultimately results in long-term declines in thymocyte production similar to accelerated aging-associated thymic involution.
Materials and Methods
Mice
C57BL6/J background mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 4, 5, 6, 7, 8 weeks. A total of 500 mice were ordered in the indicated time period and divided into female and male groups. Both groups were irradiated at age 2 months, with doses of 0, 0.5, 1, 2, and 4 Gy, and were analyzed at age of 3.5, 9, 12, and 18 months. Each dose group included 10 mice, 5 mice for thymocyte and spleen cell analysis, and 5 mice for TEC and other analysis. All mice were maintained in a specific pathogen-free facility at University of Georgia before and after irradiation. The experiments were approved by the Institutional Animal Care and Use Committee of University of Georgia.
Irradiator calibration
The irradiation exposure device used in this study was the Type 60 Co gamma (Nordion Canada), Model Gammacell 200 at UGA, and the Gammacell 40 Exactor (MDS Nordion Canada) at MSKCC. To ensure accurate and equivalent calibration of the irradiation exposure dose at both locations, OSL nanoDot dosimeters were ordered from Landauer Inc. The mice were implanted with the dosimeter and then exposed to the indicated doses of 0.5, 1, 2, and 4 Gy. The dosimeters were then sent back to Landauer Inc. for measurement. This calibration procedure was repeated every 6 months. The standard error (SE) for dose measurements was within 5% (30).
Flow cytometry and cell sorting
Gating strategies for all FACS analyses described below are shown in Supplemental Fig. 1.
Freshly isolated suspension thymocytes (1 × 106) from one thymic lobe were used for each sample. Cells were blocked with anti-CD16/32 (clone 93) before staining. For the phenotypic analysis of thymocyte subsets, antibodies specific for CD4 APC (GK1.5), CD8 FITC (53–6.7), CD44 PE (IM7), and CD25 PerCP or CD25 biotin (3C7) followed by streptavidin PerCP were used. For the profile of Lin− DN1a,b cells in total DN1 subpopulations, the PE-conjugated lineage markers anti-CD3 (145-2C11), CD4, CD8, CD11c (N418), CD19 (6D5), Gr-1(RB68-C5), TER-119 (TER-119), and NK1.1 (PK136) plus anti-CD25 PE were mixed and combined with anti-CD44 biotin followed by streptavidin PerCP to characterize the Lin−DN1 subsets; the profiles of DN1a to DN1e subsets were shown by anti-cKit-APC (2B8) and CD24-FITC (M1/69). For BM phenotypic analysis, BM suspension cells were isolated from one rear leg of 9-month-old mice, and stained with PE-conjugated antibodies for lineage markers (see above), anti-cKit APC, and Sca-1 FITC (D7). All antibodies were ordered form BioLegend. The samples were analyzed by FACS Calibur (BD) and Cyan (Beckman-Coulter) systems. The data were analyzed by CellQuest (BD) and Flowjo software (Treestar Inc).
For BrdU incorporation, 2-month-old female mice were injected intraperitoneally with 1 mg of BrdU (Sigma-Aldrich) 2 hours before sacrifice. The thymocytes were harvested, and after surface staining, the cells were fixed and permeabilized in PBS containing 1% paraformaldehyde plus 0.01% Tween-20 for 48 hours at 4°C, then incubated with 2 mg/ml DNaseI for 15 minutes in a 37°C water bath. Anti-BrdU-FITC (clone 3D4; BD Pharmingen) was used for BrdU staining according to the manufacturer’s instruction.
For TEC isolation and sorting, one thymic lobe was cut into approximately 1mm3 sections and gently washed in 2% FBS RPMI 1640 medium to remove some thymocytes. The thymic pieces were digested in 8 ml of collagenase/dispase (Roche, 1 mg/ml) plus DNase I (20 ng/mL) in 2% FBS RPMI 1640 medium, and placed in a 37°C water bath for 60–75 minutes, agitated by a plastic pipe every 20 minutes. The digested cells were filtered and then incubated with anti-CD45 APC (30-F11), EpCam PE (G8.8), MHCII FITC (M5/114.15.2), and Biotin-UEA1 (Vector, CA) or biotin-anti-Ly51 followed by streptavidin PerCP. All antibodies were ordered from BioLegend. The CD45−Epcan+MHCII+ cells were sorted as TECs by Moflo cytometry (Dako Cytomation).
CAFC assays and analysis
MS-5 cells were seeded in 12.5 cm2 tissue culture flasks in α-MEM with 10% FBS. When the cells reached confluence, medium was replaced with long-term culture (LTC) medium consisting of α-MEM, 12.5% FBS, 12.5% horse serum (HyClone Lab. Inc., UT), 10−6 M hydrocortisone (Sigma Chemical Co., MO) and 5 × 10−5 M 2-mercaptoethanol. Murine bone marrow cells (5 × 105 cells) or lineage-depleted splenocytes (1.5 × 104 cells) were added per flasks in triplicates. The cultures were weekly demidepopulated and fed with fresh LTC medium. After 3 weeks of culture, CAFCs were scored as phase-dark hematopoietic clones of at least 5 cells beneath the stromal layer using an inverted microscope.
OP9-DL1 assays and analysis
OP9-DL1, a mouse bone marrow stromal cell line of (C57BL/6 x C3H)F2-op/op origin transduced to express the Notch 1 ligand DLL1 was obtained under an MTA from Juan-Carlos Zúñiga-Pflücker, University of Toronto, Toronto, Canada. Cell culture medium consisted of αMEM (Invitrogen) 10% heat-inactivated FBS, 100 U/mL penicillin (Invitrogen) and 100 μg/mL streptomycin (Invitrogen). T cell precursors were generated in vitro as described previously with modifications (31). Briefly, freshly harvested whole bone marrow cells were seeded onto tissue-culture treated polystyrene 24-well tissue culture plates that were seeded with 4,000 OP9-DL1 cells per cm2 the day before. The tissue culture media was supplemented with 5 ng/ml murine IL-7 (Miltentyi) and 5 ng/ml murine FLT3-ligand (Miltenyi). Cultures were passaged every 3–4 days.
Flow cytometric analysis was performed at Day 28 of culture. Cells were counted and stained for analysis of the SP, DP, CD8+ SP and CD4+ SP populations. The cell stain consisted of the lineage markers CD11c FITC (HL3), CD11b FITC (M1/70), Gr1 FITC (RB6-8C5), B220 FITC (RA6-6B2), CD19 FITC (ID3), TER119 FITC (TER-110) and NK1.1 FITC (PK136) (all antibodies purchased from Pharmingen). The stain also contained CD45 PerCPCy5.5 (30-F11), CD4 PE (RM4-5), CD44 APC (IM7), CD25 APC Cy7 (PC61), c-kit V450 (2B8) and CD3 Pacific Blue (500A2) (all Pharmingen). Also included was CD8 Brilliant Violet 711 (53–6.7) (BioLegend). Samples were analyzed on an LSR-II (BD Biosciences) and analyzed with Flowjo software (Treestar Inc).
RT-PCR and real-time PCR
The mRNAs from sorted TECs were extracted using the RNeasy kit (Giangene). All mRNAs were used for RT-PCR. Ccl25 gene expression in TECs was measured by Q-PCR. The GAPDH primer/probe was used as an endogenous control. Primers/probes were ordered from Applied Biosystems. Q-PCR was performed following the kit instructions but in a 10μl volume in an AB 7500 Sequence Detector.
Statistical analyses
The Wilcoxon rank-sum test was used to compare continuous measures (e.g. absolute number) between two groups (two dose groups, female versus male, etc.). The Kruskal-Wallis rank-sum test was used to assess differential distributions and compare continuous measures across three or more dose groups as well as age groups. The Jonckheere-Terpstra (JT) test was applied to test for ordered differences in dose groups, as well as ordered differences in age groups. Relationships of variables such as age with total absolute number were evaluated using generalized linear regression models (GLM). These GLM models were used to assess relationships with all variables in the univariate setting. In addition, interactions with dose and gender were also tested in the context of these GLM models, as appropriate for the dependent variable. The Gaussian family was used as the error distribution in GLM models. As such, the regression coefficients were not scaled. Asymptotic p-values were computed for all tests (except JT test where permutation p-values were computed) and comparisons. To adjust for the multiple comparisons, p-values were also adjusted per the Benjamini-Hochberg FDR correction for the univariate analyses. All analyses were done using the R statistical program (version 3.1.1) for Linux, including the R package clinfun (version 1.0.11).
Results
The effects of irradiation and aging on total thymocytes are dose and sex dependent
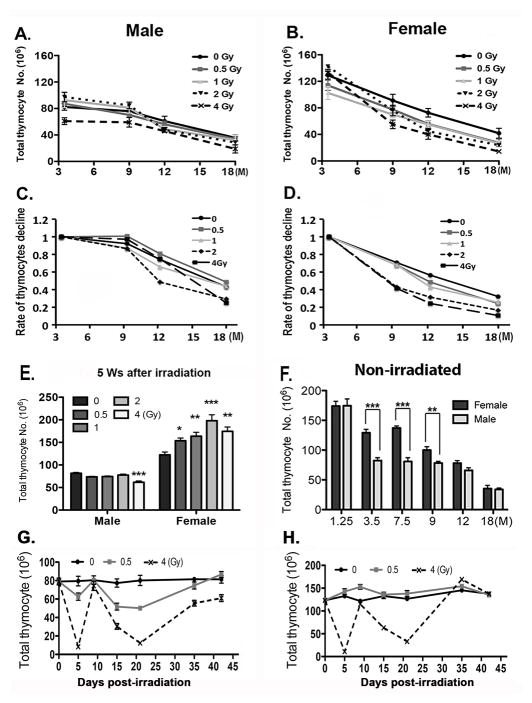
Five irradiation doses, 0, 0.5, 1, 2, and 4 Gy, were chosen for the sub-lethal TBI mouse model. Both male and female mice were exposed at age 2 months; we selected four time points at age 3.5, 9, 12, and 18 months of age, corresponding to 6 weeks, 7, 10, and 16 months after irradiation, to analyze the effects of irradiation with aging. Thymocyte numbers declined significantly with age for all doses in both genders except for males exposed at 4 Gy, which were already suppressed at the earliest 3.5 month time point assessed (Fig. 1A, B; p<0.05). By 9 months of age the 4 Gy exposed animals in particular had more dramatic declines than in controls, with females showing a significant interaction between age and dose (p < 0.001).
Figure 1. The effects of irradiation and aging on total thymocytes are dependent on irradiation dose and sex.
(A–D) Two-month-old male and female mice were irradiated at 0, 0.5, 1, 2, and 4 Gy and analyzed at ages of 3.5, 9, 12 and 18 months. Data was collected from at least 5 mice per age and dose and shown as the mean ± SE. (A, B) Total numbers of thymocytes. (C, D) Rates of thymocyte number changes, normalized to the values at 3.5 days in each dose category. (E) Total number of thymocytes from male and female mice irradiated at age 2 months, the analyzed 35 days after irradiation. (F) Total numbers of thymocytes collected from non-irradiated control mice at ages of 1.25, 3.5, 7.5, 9, 12, and 18 months are compared in the histogram. (G,H) Kinetic change of total thymocyte number within 42 days after irradiation in 2 month-old male (G) and female (H) mice exposed to 0, 0.5 and 4 Gy of irradiation and aged 5–45 days after irratiation. Student’s t-tests; P-value: *<0.05, **<0.01, ***<0.001.
A notable difference between males and females was in the effects of dose on total thymocyte numbers at 3.5 months of age. In males exposed at 4 Gy, thymocyte number at 3.5 months of age was decreased significantly below other doses (Fig. 1A; p=0.01). In females, the opposite effect was seen at 3.5 months of age, with both 2 and 4 Gy irradiated animals having higher thymocyte numbers than unexposed animals (Fig. 1B; p=0.01). These different starting points could mask the effects of irradiation on the rate of decline with aging. To more clearly compare these rates at different exposures, we further plotted these data normalizing to the starting values seen at 3.5 months of age (Fig. 1C, D). When rates of decline rather than absolute numbers were compared, both males and females showed more rapid rates of decline at the 2 and 4 Gy doses, although in males only after 9 months of age while females showed more rapid declines earlier, between 3.5 and 9 months (Fig. 1B, C; p<0.05).
To determine whether the disparity in thymocyte numbers between males and females at 6 weeks post-irradiation (3.5 months of age) was due to a difference in the timing and/or magnitude of their recovery from the acute effects of exposure, we first compared total thymocyte numbers at 5, rather than 6 weeks post-irradiation (Fig. 1E). In males, the data at 5 weeks looked similar to 6 weeks, with the 4 Gy dose suppressed and the other doses similar in total thymocyte number. However, in females all doses greater than 0 had significantly higher thymocyte numbers than unirradiated females. We then performed a more detailed time course of acute recovery comparing 0, 0.5, and 4 Gy doses in both sexes (Fig. 1G, H). Both males and females at 4 Gy showed two waves of depletion and recovery, with a dramatic cell loss at 5 days post-irradiation, recovery to levels similar to unexposed animals by 10 days, then a second loss of cellularity by 20 days post-irradiation. This second nadir of cell numbers was followed by a more gradual recovery. While the overall pattern of the acute response was similar in males and females, males clearly had a more sensitive acute response. At 4 Gy, the second ‘wave’ of thymopoiesis never fully recovered in males (Fig. 1G), while in females it actually exceeded the unirradiated controls (Fig. 1H); furthermore, males exposed even at 0.5 Gy showed a similar pattern of response, although to a lesser degree, while females exposed at 0.5 Gy did not show any reduction in cell number.
These data suggested that both males and females had long-term suppression of thymopoeisis after irradiation at a young adult age, resulting in accelerated involution at older ages.
Direct comparison of total thymocyte numbers in unirradiated males and females also identified a window of time during which female mice had consistently higher thymocyte numbers, clearly illustrating the slower involution time-course in females (Fig. 1F). While female and male mice had similar maximum thymus size at 5 and 6 weeks of age respectively (Fig. 1A, B, D (female 4Gy)), males had a rapid early decline followed by a slower rate of long-term involution, while females showed a more gradual decline, resulting in significantly higher thymocyte numbers between 3.5 and 9 months of age compared to males. This difference was temporary, as at 12 months of age and later, males and females were similar in thymocyte number. These data indicate that the differences seen in the rates of decline in total thymocyte numbers after irradiation between 3.5 and 9 months of age between males and females could also be influenced by innate differences in the rates of involution between the sexes.
Irradiation causes a significant long-term reduction of DN1a,b cells in the thymus
To further investigate the basis for these long-term effects on thymus function, we investigated the stage of thymocyte differentiation in the thymus affected by irradiation. Early T cell progenitors are highly proliferating or cycling cells and are thus more sensitive to irradiation exposure. DN1a,b cells, also called ETP, which are characterized as cKithiHSAlo/+ subpopulations in the Lin− DN1 population, represent the earliest thymic progenitors committed to the TCRαβ T cell lineage (4). The percentage of the total CD44+25− DN1 subset did show significant differences among the different dose groups at 3.5 months of age in both male and female mice, and all dose groups increased similarly with aging (Fig. 2A, B; Supplemental Fig. 1A). In contrast to total DN1, the percentage (Fig. 2C, D; Supplemental Fig. 1B) (p<0.0001) and absolute cell number (Fig. 2E, F) (p=0.043) of DN1a,b progenitors showed a significant dose-dependent reduction after irradiation in both male and female mice. There was also a significant difference in the rate of decline in DN1a,b number with dose only in females (Fig. 2G, H; p=0.005). We found a significant dose-dependent reduction in the percentage (p=0.0007) and total number (p=0.0013) of DN1a,b cells at age 3.5 months (1.5 months after irradiation). For the 1, 2, and 4 Gy doses, the reduction in the percentage of DN1a,b was similar or lower level as seen in unirradiated control mice at age 18 months for both males and females (Fig. 2C, D); this was also true for DN1a,b cell numbers at 4Gy (Fig. 2E, F) (p>0.05). More importantly, these reductions were not restored through to the age of 18 months, even though the total thymocyte number had increased by 5–6 weeks after irradiation (Fig. 1A, B, D). These results indicate that the long-term effects of reduction of thymic progenitor DN1a,b cells are dependent on dose and sex and do not recover with age, especially at the doses of 2 and 4 Gy, consistent with the effects on total thymocyte numbers.
Figure 2. Irradiation causes a significant long-term reduction in TCRαβ progenitor DN1a,b cells in the thymus.
Two-month-old mice were exposed to 0, 0.5, 1, 2, and 4 Gy of irradiation and analyzed at ages of 3.5, 9, 12 and 18 months. The thymocytes isolated from male (A, C, E) and female (B, D, F) mice are shown. (A, B). Percentages of total Lin−DN1 (Lin−CD25−CD44+). (C, D). Percentages of DN1a,b (Lin−,cKithi,CD24+/lo) subsets among total Lin−DN1 cells. (E, F). Total number of DN1a,b subsets in the thymus. (G–J) The rate of decline in the percentage and total cell number of DN1a,b cells. The percentage and total cell number for each time point and dose were plotted relative to the values at 3.5 months within each dose, to generate the rate of decline in the percentage of DN1a,b from male (G) and female (H), and in total number of DN1a,b from male (I) and female (J). Each bar represents data collected from 5 mice and is shown as the mean ± SE.
The reduction in DN1a,b progenitors is not correlated with changes in the thymic microenvironment after irradiation
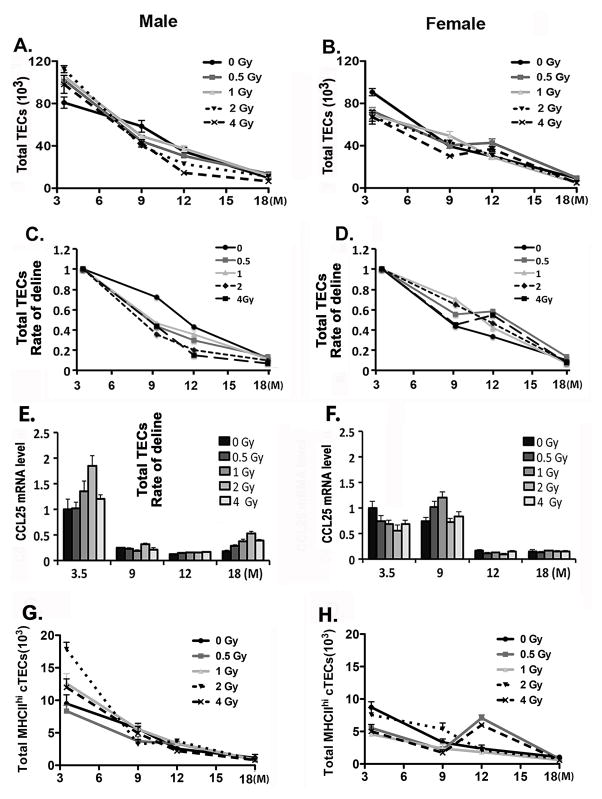
One possibility underlying the reduction of DN1a,b progenitors in the thymus is that irradiation leads to a loss of the capability of TECs to recruit progenitors into or maintain them efficiently in the thymus. To test this possibility, the long-term effects of irradiation on TECs were analyzed. The total number of TECs showed a dose-related increase in male mice at 3.5 months for all doses (6 weeks post-irradiation; Fig. 3A) (p=0.036). In contrast, in female mice at 3.5 months of age all doses showed a significant reduction in TEC numbers (p=0.027) (Fig. 3B). In both male and female mice, total TEC numbers at age 9, 12, and 18 months were not significantly different between control and irradiated groups, and showed similar declines in cell number consistent with aging-related involution (Fig. 3A, B). These differences at 3.5 months of age with a similar end point at 18 months of age led to an increased rate of involution in male mice for all doses, although in females the rates were not significantly different (Fig. 3C, D). To further test if the BM progenitor–recruiting capability of the thymus was reduced after irradiation, the gene expression of Ccl25 in total TECs (Fig. 3E, F) and the total number of MHCIIhi cTECs (Fig. 3G, H) were analyzed at all time points. Neither of these parameters showed significant reductions compared with unirradiated controls (p>0.05). Ccl25 expression did drop dramatically in both males and females with age, although the timing was earlier in males (Fig. 3E) and there was no effect of irradiation. These results suggest that the reduction of DN1a,b cells after irradiation is not correlated with changes in TEC numbers or the ability to recruit progenitors into the thymus.
Figure 3. Effects of sublethal irradiation on TEC numbers with aging.
Two-month-old mice were exposed to 0, 0.5, 1, 2, and 4 Gy of irradiation and analyzed at ages of 3.5, 9, 12 and 18 months. Stromal cells from one lobe per thymus stained with anti-CD45, Epcam, and MHCII antibodies, and gated on CD45−MHCII+Epcam+. (A, B). The numbers of total TECs in male (A) and female (B) mice. (C, D). The percentage and total cell number for each time point and dose were plotted relative to the values at 3.5 months within each dose to generate the rate of decline of TEC numbers in male (C) and female (D) mice. (E, F). Ccl25 gene expression in sorted total TECs from male (E) and female (F) mice. (G–H) Total numbers of MHCIIhi cTECs in male (G) and female (H) mice. Data were collected from 5 mice and shown as the mean ± SE.
Irradiation causes long-term reduction of functional HSC LSK populations in the BM
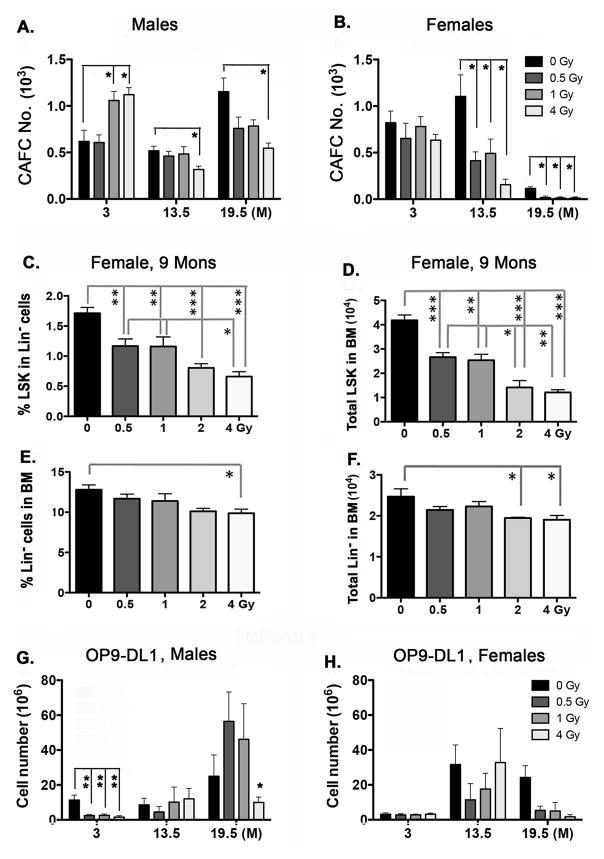
Another possibility is that the long-term reduction of DN1a,b subsets in the thymus is due to persistent reduction in or damage to hematopoietic stem cells (HSCs) in the BM after irradiation. We assessed the number of HSCs in the bone marrow by counting the number of cobblestone area forming cells (CAFC), which is a measurement of functional HSCs capable of long-term engraftment in vivo (32) (Fig. 4A, B). CAFC number did not show significant age-dependent trends in males at any dose, although at 3 months of age CAFC were higher at the 1 and 4 Gy doses (p<0.05), and the 4 Gy dose was significantly reduced at later ages (p<0.02) (Fig. 3A). In females, CAFC numbers were unaffected at any dose at 3 months, declined at all doses at 13.5 months, and then declined sharply between 13.5 and 19.5 months of age in unirradiated controls, and were undetected at any irradiation dose at 19.5 months of age (p<0.05; Fig. 3B). Thus, by this measure, HSCs in females were more sensitive to both age and irradiation than in males.
Figure 4. Irradiation causes a long-term reduction of LSK hematopoietic stem cells in the BM.
Male and female mice (as indicated) were exposed to 0, 0.5, 1, 2, and 4 Gy of irradiation and analyzed at ages of 3, 13.5, and 19.5 months (A, B, G, H) or 9 months (C–F). (A, B) Total bone marrow cells were isolated and assessed for Cobblestone Area-Forming Cells (CAFC) as a measure of primitive HSC numbers. (C) Percentage of LSK (Lin−Sca-1+cKithi) cells among Lin− BM cells. (D) Total number of LSK cells in the BM. (E) Percentage of Lin− cells in the BM. (F) Total number of Lin− cells in the BM. (G, F) Total bone marrow cells were expanded on OP9-DL1 cell layers for 28 days in MEM alpha/10% FBS/1% penicillin-streptomycin plus mIL-7 (5ng/ml) and mFlt3L (5ng/ml). Data analyzed by Wilcoxan rank sum test (A, B, G, H) or one-way ANOVA (C–F). Brackets across the top of the bars indicate significant differences between two individual samples. Data were collected from 5 mice and shown as the mean ± SE. P-values: *<0.05, **<0.01, ***<0.001.
To further investigate this female-specific effect in the CAFC assay, we analyzed the phenotypes of BM cells from female mice irradiated at 2 months of age and analyzed at 9 months of age. Both the percentages and total number of LSK cells, a functional HSC subset in the BM responsible for lymphocyte production, showed a significant and dose-dependent decrease (Fig. 4C, D; Supplemental Fig. 1D) (p<0.05). Importantly, exposure at a dose of just 0.5 Gy caused a significant decrease in LSK cells relative to controls. This change was consistent with the reduction in DN1a,b subsets in the thymus. The same profile was observed in Lin− progenitor HSCs (Fig. 4E, F), but not in granulocytes, B cells, natural killer (NK) cells, or T cells under the same conditions (P>0.05). These results indicate that by 9 months of age (7 months post-irradiation), the population of LSK cells in the BM cannot be maintained, even months after irradiation and at exposure doses as low as 0.5 Gy. As functional HSCs are required for lymphocyte production, a reduction in LSK cells could directly decrease the source of progenitors seeding the thymus.
As a final measure of the capacity of HSCs to undergo commitment to the T cell lineage, we used the OP9-DL1 in vitro assay (33). In these experiments, cells were isolated as they were for the CAFC assays, cultured for 28 days on OP9-DL1 cells with mIL-7 and mFlt3L, then the total T cell numbers were calculated. In males, total T cell numbers were suppressed at 3 months of age at all doses (p<0.01; Fig. 4G), despite the increase in CAFC number at this age at the 1 and 4 Gy doses (Fig. 4A). This suppression did not persist, as there were no significant differences at 13.5 months, and at 19.5 months only the 4 Gy dose showed a significant reduction in T cell potential (p<0.05). In females, no significant differences were seen at any age, with the exception of a trend for reduced numbers at all doses at 19.5 months (Fig. 4H). These data indicate that irradiation had some effect not only on progenitor numbers, but also on their functional capacity.
DN2 (CD44+CD25+) thymocytes are also reduced after irradiation
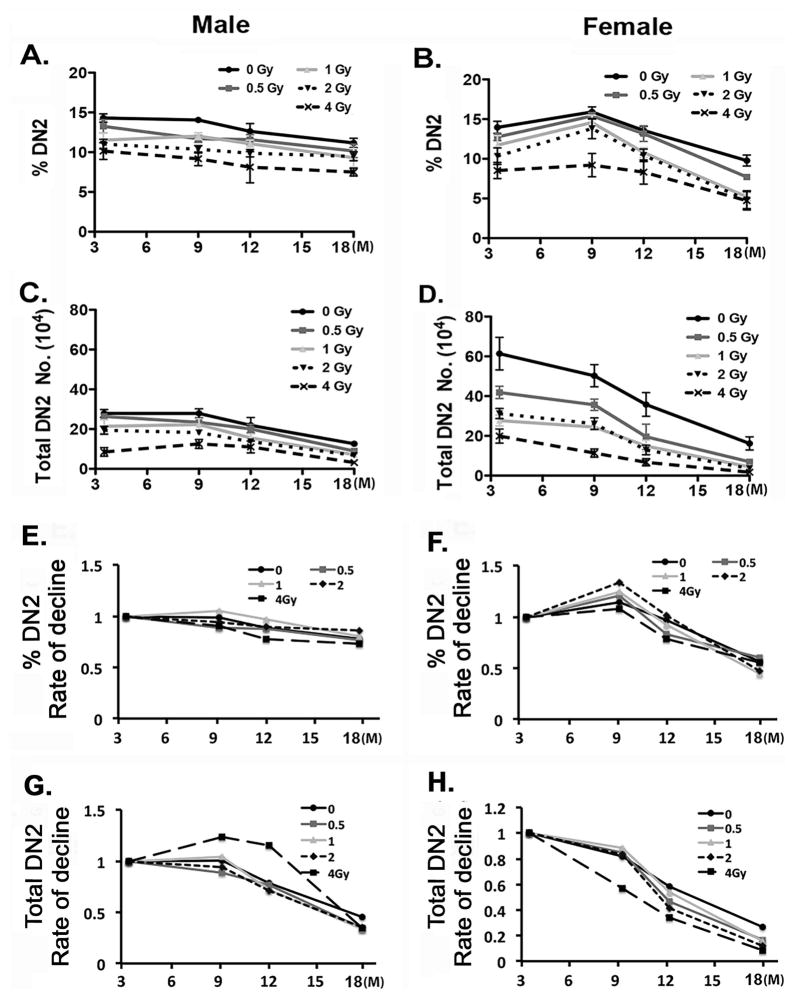
DN2 cells develop directly from DN1a,b progenitors, and consistent with the decline in DN1a,b cells with age, DN2 cells also decline in both percentage and number between 3 and 18 months of age in both males and females (Fig. 5). However, the magnitude of the decline is much less dramatic, especially in males. Irradiation flattens this decline even further, with male mice irradiated at 1, 2, and 4 Gy showing no significant trend in DN2 percentage over this time period after irradiation at 2 months. Females showed a similar result at the 4 Gy dose. DN2 cell numbers did show a significantly decreasing trend in both genders and at all doses (p<0.001), except for the 4 Gy dose in males, which were already suppressed at the 3.5 month age (Fig. 5C, D). Females showed a much wider range of values at the 3.5 month age (Fig. 5D). These results indicate that although DN2 cells are maintained at a lower level in irradiated mice than in controls, the damaging effects of irradiation on early developing thymocytes are partially compensated in the DN2 stage. However, at higher doses the decline in DN2 cell numbers appears to be permanent, consistent with the DN1a,b results.
Figure 5. Early developing DN2 (CD44+CD25+) thymocytes are reduced after irradiation.
Two-month-old mice were exposed to 0, 0.5, 1, 2, and 4 Gy of irradiation and analyzed at ages of 3.5, 9, 12 and 18 months. (A, B) Percentages of DN2 (CD44+CD25+) subsets gated on DN (CD4−CD8−) cells collected from male (A) and female (B) mice. (C, D) Total numbers of DN2 cells collected from male (C) and female (D) mice. (E–H) The percentage and total cell number for each time point and dose were plotted relative to the values at 3.5 months within each dose to generate the rate of decline. The rate of decline in the percentage of DN2 cells in total DN cells from male (E) and female (F) mice, and in the total number of DN2 cells from male (G) and female (H) mice. Data were collected from 5 mice and shown as the mean ± SE.
The thymus compensates for reductions in total thymocytes after irradiation by expanding the DN3 subset
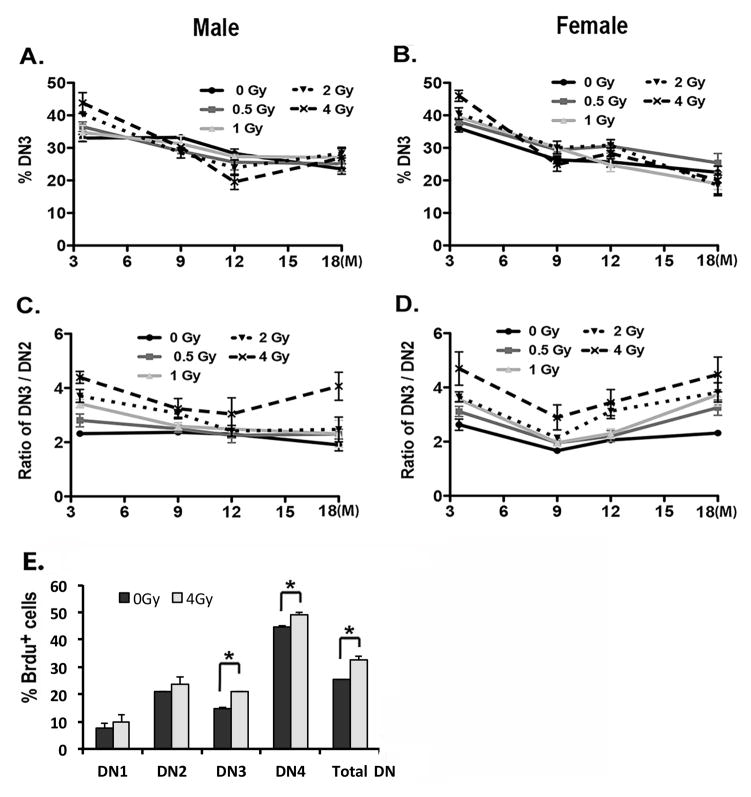
The patterns of reduction of DN1a,b and DN2 cells did not match the dose-dependent increase of total thymocyte number at 3.5 months of age, 5–6 weeks after irradiation. These results pose the question: How does the thymus compensate for the reduction of thymic lymphoid progenitors to expand the total thymocyte number after irradiation? We found that both the percentage of DN3 cells showed a dose-dependent increase 6 weeks after irradiation (Fig. 6A, B), consistent with the increase in total thymocyte numbers 5–6 weeks after irradiation. However, after this time point, the percentage of DN3 cells was similar in controls and all irradiation doses and at all ages, and thus did not match the profiles of total thymocyte numbers. Furthermore, the DN4 subset showed no consistent changes with dose, age after irradiation, or sex (p>0.05; data not shown). Interestingly, we found that the DN3/DN2 ratio increased, particularly at 3.5 and 18 months of age (Fig. 6C, D). These results were consistent with BrdU incorporation assays, results showing that proliferation was significantly increased in both DN3 and DN4 subsets and in total DN cells (Fig. 6E). Because the DN3 subset directly develops from the DN2 subset, these results are consistent with a model in which a lower level importation of DN1a,b progenitors is compensated by expansion of DN3 (and therefore DN4) subsets 5–6 weeks after irradiation. However, once the number is restored to the normal level, this compensation is limited with a progressive reduction of thymocytes occurs beginning at 5–6 weeks and continuing throughout life.
Figure 6. The proliferation of the DN3 subset is increased after irradiation, resulting in an increased ratio of DN3 to DN2 cells.
Two-month-old mice were exposed to 0, 0.5, 1, 2, and 4 Gy of irradiation and analyzed at ages of 3.5, 9, 12 and 18 months. (A, B) Percentage of DN3 subset generated from male (A) and female (B) are shown in the histograms. (C, D) Ratios of DN3 to DN2 subsets in male (C) and female (D) are shown in the histograms. (E) Total thymocytes were isolated from female mice at 6 weeks after irradiation at 4 Gy. BrdU+ cells gated on DN1–4 subsets and total DN cells are shown. Data were collected from 5 mice in each group and shown as the mean ± SE. *, p <0.05 by Student’s t-test.
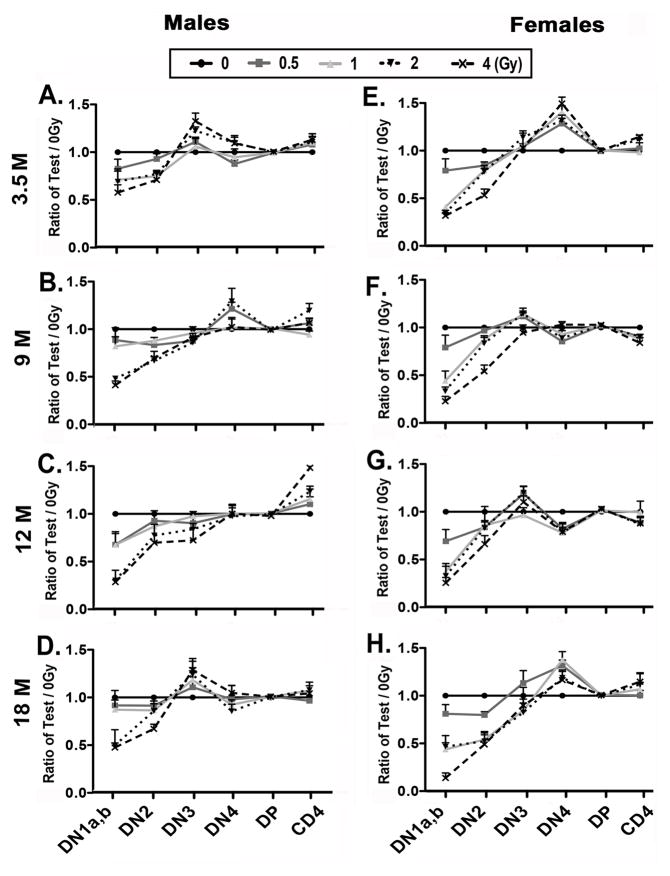
The recovery kinetics of thymocyte subsets show long-term periodicity after irradiation
In order to analyze the recovery kinetics of thymocyte subsets after irradiation with aging, we normalized the percentages of each subset, including DN1a,b, DN2, DN3, DN4, DP, and CD4 SP cells, in the irradiated groups to the control group percentages at each age of analysis (Fig. 7). At age 3.5 months, an obvious peak in the DN3 subset was observed in male mice (Fig. 7A). Interestingly, approximately 6 months later at age 9 months, the peak in DN3 had shifted to the DN4 subset, then at age 12 months appeared as an increase in CD4 SP thymocytes, recurring at the DN3 stage at age 18 months in male mice (Fig. 7A–D). In female mice, a similar “moving peak” was observed although was less dramatic, starting as a DN4 peak at 3.5 months (Fig. 7E–H). These results are consistent with a model in which sub-lethal TBI synchronized progenitor importation and thymocyte differentiation, resulting in a periodic profile that continued throughout life.
Figure 7. The kinetics of thymocyte differentiation show a periodic profile after irradiation.
Two-month-old mice were exposed to 0, 0.5, 1, 2, and 4 Gy of irradiation and analyzed at ages of 3.5, 9, 12 and 18 months. Total thymocytes were analyzed at the indicated ages after irradiation. Percentages of DN1a,b, DN2, DN3, DN4, DP, and CD4 SP subsets in each test dose were normalized to those of 0 Gy controls and shown as “ratio of Test/0 Gy” in the histograms. Data are shown separately from male (A, B, C, D) and female (E, F, G, H) mice. Data were collected from 5 mice each and shown as the mean ± SE.
Discussion
During exposure to TBI, both hematopoietic cells and their niche cells in the BM and thymus are affected simultaneously. Although many of the effects of irradiation on these cells are dose dependent, the recovery capabilities, process, and timing vary widely. Because the thymic microenvironment does not normally support the self-renewal and long-term retention of lymphocyte progenitors, the reduction of progenitors in BM directly affects thymic restoration. It is generally thought that both the reduction of progenitors and the epithelial space in the thymus are key factors that result in thymic senescence due to irradiation and aging (34, 35). However, how these changes caused by aging and exposure to different doses of sub-lethal irradiation in BM HSCs affect the intrathymic lymphoid progenitors and subsequent development of thymocytes remains unclear. In this study, by examining the long-term effects of varied sub-lethal doses of ionizing radiation at different analytic time points in a mouse model, we provide evidence for understanding the effects of irradiation and aging on thymic involution. The persistent suppression of DN1a,b progenitors in the thymus and consequent reduction in thymocyte numbers, particularly in males, could be interpreted as indicating an acceleration of aging-related reductions in thymopoiesis.
We found that the long-term effects of irradiation and aging on total thymocytes differed by sex, dose, and analytic time points. First, the thymus responds to acute irradiation-induced thymocyte loss by an immediate but transient overexpansion of the total thymocyte number within the first 10 days after irradiation (presumably mediated by resident progenitors already within the thymus) (16). This initial expansion is followed by a second reduction in thymocyte numbers that is slower to recover (presumably mediated by newly imported progenitors) (29). This second recovery reaches its peak at around 5 weeks in females and 6 weeks in males. These acute responses were dose-dependent in both males and females, but males were more sensitive, having more severe defects and a lower dose threshold, while female mice appear to have both a more sensitive response and earlier recovery potential than male mice in the first 4–6 weeks after irradiation. This early sex difference might reflect that male mice undergo thymic involution more rapidly than female mice, such that even at this relatively young age, males are already initiating involution, while females are more robust and thus might be resistant (Fig. 1F). These acute responses were followed by a dose-dependent reduction of thymocytes in both sexes that persists for at least 12 months after irradiation.
Our results suggest that the long-term reduction in total thymocytes was primarily due to a reduction in the progenitor DN1a,b stage, and that this reduction in progenitors was partially offset by a long-term increase in the proliferation of DN3 cells (Fig. 6). This conclusion is consistent with a previous report showing that DN3 and DN4 cells represent the key stages for controlling thymic size and homeostasis in non-irradiated mice (36). These data also show that the aged thymic microenvironment has the same capability for supporting the homeostatic proliferation of DN3 cells after irradiation. Finally, the dose-dependent reduction of total thymocytes at age 12 months in mice of both sexes might be due to a significant drop of DN1a,b progenitors in both control and irradiation conditions at this time point (Fig. 2). The percent and number of DN1a,b progenitors reduced in both control and irradiated mice with aging, and irradiation accelerated this process. Both the percentage and total number of DN1a,b cells at age 3.5 months (6 weeks after irradiation) was similar to the level observed in controls aged 12–18 months and never recovered, in both males and females, at doses as low as 1 Gy. Thus, the effects of TBI on these progenitors resembles premature aging.
Our data also suggest that this long-term reduction in DN1a,b cells is more likely due to the reduction of LSKs in the BM, rather than to a problem in recruiting progenitors to the thymus caused by irradiation effects on TECs. While TECs and stroma in general are generally considered radiation resistant, we documented significant declines in TEC numbers as an acute response to radiation. While this acute loss was more dose-sensitive in females, we saw a significant increase in the rate of TEC decline with aging (involution) only in males. Furthermore, the expression of CCL25 declines with age rather precipitously and with different timing in males (earlier) and females (later), but is not radiation sensitive, so is unlikely to underlie any defects seen in DN1a,b numbers. The sex-specific differences in hematopoietic cell phenotypes with aging after irradiation were usually either not sex-specific (DN1a,b decline) or were more severe in females (total thymocyte numbers, HSC number by CAFC assay). Therefore, the overall pattern suggests that the long-term intrathymic defects are most likely to be due to defects in the hematopoietic compartment, rather than in stromal cells. This conclusion is consistent with reports showing that lymphoid progenitor LSKs are reduced with age (37) or sub-lethal irradiation (6.5 Gy) within 2 months after irradiation (29, 38), or showing that irradiation-induced exhaustion of BM was induced by repeated irradiation, but not affected by aging (22). Our results extend these previous studies to show a single dose of TBI as low as 0.5 Gy produces long-term reduction in BM progenitor LSKs that persists for at least 16 months after irradiation, and that this reduction likely contributes to long-term reduction of DN1a,b progenitors in the thymus. However, we cannot exclude the possibility that long-term damage to the BM microenvironment or to other thymic stromal cell types (such as thymic mesenchyme or vacular endothelial cells) that were not assessed in our studies could influence HSC phenotypes in addition to the defects documented here.
It is worth noting that exposure to a dose of 0.5 Gy was sufficient to cause a significant reduction in LSKs, but did not affect the number of DN1a,b cells in the thymus. This difference between the BM and thymus may indicate that there is normally an excess of circulating progenitors such that small reductions in LSKs in the BM do not impact progenitor recruitment into the thymus; however, the damaging effects caused by doses of 1 Gy or higher cannot be compensated completely. Our findings indicate that the high potential of proliferation and repopulation capabilities of HSCs for lymphoid progenitors are limited after irradiation. Even a single dose of TBI at 0.5 Gy damages LSKs, which consequently accelerates thymic involution and causes a lifelong reduction of thymocytes. The variable effects of irradiation on lymphoid potential with aging in both the CAFC and OP9-Dl1 assays also suggests that HSC function, as well as number, may be impaired long-term after a single sub-lethal exposure.
Interestingly, the kinetics of thymocyte subset compensation showed a periodic profile after irradiation (Fig. 7), consistent with the reports of Foss and Goldschneider suggesting that the thymus has a periodic receptivity to importing progenitors (2, 39, 40). Our data suggest that irradiation ‘resets’ progenitor importation, and thus synchronizes thymocyte differentiation. While the actual periodicity of this cycle cannot be determined from our data, this effect is most clearly present in males, and appears to last for the life of the animal. While the functional impact of this synchronization remains to be tested, it does underscore that the effects of irradiation can impact thymopoiesis for the long-term.
Supplementary Material
Acknowledgments
We thank J. Nelson in the Center for Tropical and Emerging Global diseases Flow Cytometry Facility at the University of Georgia for cell sorting technical support.
Footnotes
This study was supported by subcontract grants of RFP-NIAID-DAIT-NIHAI2008023 to N.R.M., G. S., M.v.d.B., and M.M. from NIAID/NIH. Support for M.v.d.B. was also received from the P30 CA008748 MSK Cancer Center Support Grant/Core Grant.
Authorship and Contributions
S.X. designed the study, performed all experiments except for CAFC and OP9-Dl1, analyzed the data, and wrote the manuscript. J-H.S. performed and L.Y. analyzed the OP9-Dl1 and CAFC assays, with advice on experimental design and interpretation by M.v.d.B and M.M.. I.D.S. performed most of the statistical analysis of the data. W.Z. helped perform all experiments and took care of the mice. G.D.S. assisted with interpretation and analysis of the statistical data, and participated in study design. N.R.M. primarily designed the study, analyzed the results, and wrote the manuscript. All authors read and edited the manuscript prior to submission.
References
- 1.Donskoy E, Goldschneider I. Thymocytopoiesis is maintained by blood-borne precursors throughout postnatal life. A study in parabiotic mice. J Immunol. 1992;148:1604–1612. [PubMed] [Google Scholar]
- 2.Foss DL, Donskoy E, Goldschneider I. The importation of hematogenous precursors by the thymus is a gated phenomenon in normal adult mice. J Exp Med. 2001;193:365–374. doi: 10.1084/jem.193.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldschneider I, Komschlies KL, Greiner DL. Studies of thymocytopoiesis in rats and mice. I. Kinetics of appearance of thymocytes using a direct intrathymic adoptive transfer assay for thymocyte precursors. J Exp Med. 1986;163:1–17. doi: 10.1084/jem.163.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porritt HE, Rumfelt LL, Tabrizifard S, Schmitt TM, Zuniga-Pflucker JC, Petrie HT. Heterogeneity among DN1 prothymocytes reveals multiple progenitors with different capacities to generate T cell and non-T cell lineages. Immunity. 2004;20:735–745. doi: 10.1016/j.immuni.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Shortman K, Wu L. Early T lymphocyte progenitors. Annu Rev Immunol. 1996;14:29–47. doi: 10.1146/annurev.immunol.14.1.29. [DOI] [PubMed] [Google Scholar]
- 6.Petrie HT, Zuniga-Pflucker JC. Zoned out: functional mapping of stromal signaling microenvironments in the thymus. Annu Rev Immunol. 2007;25:649–679. doi: 10.1146/annurev.immunol.23.021704.115715. [DOI] [PubMed] [Google Scholar]
- 7.Chinn IK, Blackburn CC, Manley NR, Sempowski GD. Changes in primary lymphoid organs with aging. Seminars in immunology. 2012;24:309–320. doi: 10.1016/j.smim.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wagner JE, Huff JL, Rust WL, Kingsley K, Plopper GE. Perillyl Alcohol Inhibits Breast Cell Migration without Affecting Cell Adhesion. J Biomed Biotechnol. 2002;2:136–140. doi: 10.1155/S1110724302207020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delaney G, Jacob S, Barton M. Estimation of an optimal external beam radiotherapy utilization rate for head and neck carcinoma. Cancer. 2005;103:2216–2227. doi: 10.1002/cncr.21084. [DOI] [PubMed] [Google Scholar]
- 10.Yuuki H, Yoshikai Y, Kishihara K, Matsuzaki G, Ayukawa K, Nomoto K. The expression and sequences of T cell antigen receptor beta-chain genes in the thymus at an early stage after sublethal irradiation. J Immunol. 1989;142:3683–3691. [PubMed] [Google Scholar]
- 11.Guidos CJ, Williams CJ, Wu GE, Paige CJ, Danska JS. Development of CD4+CD8+ thymocytes in RAG-deficient mice through a T cell receptor beta chain-independent pathway. J Exp Med. 1995;181:1187–1195. doi: 10.1084/jem.181.3.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iscove NN, Nawa K. Hematopoietic stem cells expand during serial transplantation in vivo without apparent exhaustion. Current biology: CB. 1997;7:805–808. doi: 10.1016/s0960-9822(06)00341-1. [DOI] [PubMed] [Google Scholar]
- 13.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cvancarova M, Samuelsen SO, Magelssen H, Fossa SD. Reproduction rates after cancer treatment: experience from the Norwegian radium hospital. J Clin Oncol. 2009;27:334–343. doi: 10.1200/JCO.2007.15.3130. [DOI] [PubMed] [Google Scholar]
- 15.Lemischka IR, Raulet DH, Mulligan RC. Developmental potential and dynamic behavior of hematopoietic stem cells. Cell. 1986;45:917–927. doi: 10.1016/0092-8674(86)90566-0. [DOI] [PubMed] [Google Scholar]
- 16.Ayukawa K, Tomooka S, Asano T, Taniguchi K, Yoshikai Y, Nomoto K. ‘Radioresistant’ CD4−CD8− intrathymic T cell precursors differentiate into mature CD4+CD8− and CD4−CD8+ T cells. Development of ‘radioresistant’ CD4−CD8− intrathymic T cell precursors. Thymus. 1990;15:65–78. [PubMed] [Google Scholar]
- 17.Domen J, Gandy KL, Weissman IL. Systemic overexpression of BCL-2 in the hematopoietic system protects transgenic mice from the consequences of lethal irradiation. Blood. 1998;91:2272–2282. [PubMed] [Google Scholar]
- 18.Zuniga-Pflucker JC, Jiang D, Schwartzberg PL, Lenardo MJ. Sublethal gamma-radiation induces differentiation of CD4−/CD8− into CD4+/CD8+ thymocytes without T cell receptor beta rearrangement in recombinase activation gene 2−/− mice. J Exp Med. 1994;180:1517–1521. doi: 10.1084/jem.180.4.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zubkova I, Mostowski H, Zaitseva M. Up-regulation of IL-7, stromal-derived factor-1 alpha, thymus-expressed chemokine, and secondary lymphoid tissue chemokine gene expression in the stromal cells in response to thymocyte depletion: implication for thymus reconstitution. J Immunol. 2005;175:2321–2330. doi: 10.4049/jimmunol.175.4.2321. [DOI] [PubMed] [Google Scholar]
- 20.Boggs DR, Marsh JC, Chervenick PA, Cartwright GE, Wintrobe MM. Factors influencing hematopoietic spleen colony formation in irradiated mice. 3. The effect of repetitive irradiation upon proliferative ability of colony-forming cells. J Exp Med. 1967;126:871–880. doi: 10.1084/jem.126.5.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pawliuk R, Eaves C, Humphries RK. Evidence of both ontogeny and transplant dose-regulated expansion of hematopoietic stem cells in vivo. Blood. 1996;88:2852–2858. [PubMed] [Google Scholar]
- 22.Harrison DE, Astle CM, Delaittre JA. Loss of proliferative capacity in immunohemopoietic stem cells caused by serial transplantation rather than aging. J Exp Med. 1978;147:1526–1531. doi: 10.1084/jem.147.5.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valentine WN, Pearce ML, Lawrence JS. Studies on the radiosensitivity of bone marrow. II. The effect of large, repeated whole body irradiation exposure on hematopoiesis. Blood. 1952;7:14–19. [PubMed] [Google Scholar]
- 24.Magli MC, Iscove NN, Odartchenko N. Transient nature of early haematopoietic spleen colonies. Nature. 1982;295:527–529. doi: 10.1038/295527a0. [DOI] [PubMed] [Google Scholar]
- 25.Wu AM, Till JE, Siminovitch L, McCulloch EA. Cytological evidence for a relationship between normal hemotopoietic colony-forming cells and cells of the lymphoid system. J Exp Med. 1968;127:455–464. doi: 10.1084/jem.127.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abramson S, Miller RG, Phillips RA. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977;145:1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–245. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- 28.Notta F, Doulatov S, Laurenti E, Poeppl A, Jurisica I, Dick JE. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science. 2011;333:218–221. doi: 10.1126/science.1201219. [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Schulte BA, LaRue AC, Ogawa M, Zhou D. Total body irradiation selectively induces murine hematopoietic stem cell senescence. Blood. 2006;107:358–366. doi: 10.1182/blood-2005-04-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seed TM, Xiao S, Manley N, Nikolich-Zugich J, Pugh J, Van den Brink M, Hirabayashi Y, Yasutomo K, Iwama A, Koyasu S, Shterev I, Sempowski G, Macchiarini F, Nakachi K, Kunugi KC, Hammer CG, Dewerd LA. An interlaboratory comparison of dosimetry for a multi-institutional radiobiological research project: Observations, problems, solutions and lessons learned. International journal of radiation biology. 2016;92:59–70. doi: 10.3109/09553002.2015.1106024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Min D, Taylor PA, Panoskaltsis-Mortari A, Chung B, Danilenko DM, Farrell C, Lacey DL, Blazar BR, Weinberg KI. Protection from thymic epithelial cell injury by keratinocyte growth factor: a new approach to improve thymic and peripheral T-cell reconstitution after bone marrow transplantation. Blood. 2002;99:4592–4600. doi: 10.1182/blood.v99.12.4592. [DOI] [PubMed] [Google Scholar]
- 32.van Os R, Kamminga LM, de Haan G. Stem cell assays: something old, something new, something borrowed. Stem Cells. 2004;22:1181–1190. doi: 10.1634/stemcells.2004-0095. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt TM, Zuniga-Pflucker JC. Induction of T cell development from hematopoietic progenitor cells by delta-like-1 in vitro. Immunity. 2002;17:749–756. doi: 10.1016/s1074-7613(02)00474-0. [DOI] [PubMed] [Google Scholar]
- 34.Jamieson BD, Douek DC, Killian S, Hultin LE, Scripture-Adams DD, Giorgi JV, Marelli D, Koup RA, Zack JA. Generation of functional thymocytes in the human adult. Immunity. 1999;10:569–575. doi: 10.1016/s1074-7613(00)80056-4. [DOI] [PubMed] [Google Scholar]
- 35.Kohler S, Wagner U, Pierer M, Kimmig S, Oppmann B, Mowes B, Julke K, Romagnani C, Thiel A. Post-thymic in vivo proliferation of naive CD4+ T cells constrains the TCR repertoire in healthy human adults. Eur J Immunol. 2005;35:1987–1994. doi: 10.1002/eji.200526181. [DOI] [PubMed] [Google Scholar]
- 36.Prockop SE, Petrie HT. Regulation of thymus size by competition for stromal niches among early T cell progenitors. J Immunol. 2004;173:1604–1611. doi: 10.4049/jimmunol.173.3.1604. [DOI] [PubMed] [Google Scholar]
- 37.Sudo K, Ema H, Morita Y, Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao L, Feng W, Li H, Gardner D, Luo Y, Wang Y, Liu L, Meng A, Sharpless NE, Zhou D. Total body irradiation causes long-term mouse BM injury via induction of HSC premature senescence in an Ink4a- and Arf-independent manner. Blood. 2014;123:3105–3115. doi: 10.1182/blood-2013-07-515619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohmura K, Kawamoto H, Fujimoto S, Ozaki S, Nakao K, Katsura Y. Emergence of T, B, and myeloid lineage-committed as well as multipotent hemopoietic progenitors in the aorta-gonad-mesonephros region of day 10 fetuses of the mouse. J Immunol. 1999;163:4788–4795. [PubMed] [Google Scholar]
- 40.Goldschneider I. Cyclical mobilization and gated importation of thymocyte progenitors in the adult mouse: evidence for a thymus-bone marrow feedback loop. Immunol Rev. 2006;209:58–75. doi: 10.1111/j.0105-2896.2006.00354.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.