Abstract
Chorioamnionitis, premature rupture of fetal membranes (FM), and subsequent preterm birth are associated with local infection and inflammation, particularly interleukin 1 beta (IL-1β) production. While bacterial infections are commonly identified, other microorganisms may play a role in the pathogenesis. Since viral pandemics, such as influenza, Ebola and Zika are becoming more common, and pregnant women are at increased risk of associated complications, this study evaluated the impact viral infection had on human FM innate immune responses. This study shows that a herpes viral infection of FMs sensitizes the tissue to low levels of bacterial lipopolysaccharide (LPS), giving rise to an exaggerated IL-1β response. Using an ex vivo human FM explant system and an in vivo mouse model of pregnancy, we report that the mechanism by which this aggravated inflammation arises is through the inhibition of the TAM receptor, MERTK, and activation of the inflammasome. The TAM receptor ligand, growth arrest specific 6 (GAS6), re-establishes the normal FM response to LPS by restoring and augmenting TAM receptor and ligand expression, and by preventing the exacerbated IL-1β processing and secretion. These findings indicate a novel mechanism by which viruses alter normal FM immune responses to bacteria, potentially giving rise to adverse pregnancy outcomes.
Introduction
Chorioamnionitis, preterm premature rupture of membranes (PPROM) and preterm birth resulting from infection are thought to be initiated by bacteria ascending from the lower genital tract, gaining access to the fetal membranes (FMs), and activating innate immune responses (1). The pro-inflammatory cytokine, interleukin 1 beta (IL-1β) is an important mediator of PPROM and preterm birth (2–5). Normal human FMs express a range of innate immune pattern recognition receptors, such as Toll-like receptors (TLRs), Nod-like receptors (NLRs), and inflammasome family members; and can generate inflammatory responses following their activation by infectious components (6–8). Although clinical and experimental studies have correlated bacterial infection and inflammation at the maternal-fetal interface with prematurity (9–16), no single bacterium has been attributed to preterm birth (17), and identifiable bacteria associated with chorioamnionitis, PPROM and preterm birth are often common to the genital tract and the placenta (18). Moreover, while the FMs are likely the first tissue colonized by the normal flora of the lower genital tract or by an ascending pathogen (19), most FMs from normal deliveries also have bacteria present (20). Thus, bacterial stimulation of the FMs may, in it of itself, be insufficient to trigger chorioamnionitis and preterm birth.
A number of diseases are caused by polymicrobial infections, including disorders of the urogenital tract, like vaginosis (21). Thus, one potential risk factor that could contribute to bacterial-associated preterm birth may be another type of infection, such as a virus. While not all women with a viral infection during pregnancy will have complications, some viruses that are detected in the amniotic fluid or gestational tissues have been linked to an increased risk for chorioamnionitis and preterm birth. These include adenovirus, and herpes viruses, such as cytomegalovirus (CMV), Epstein-Barr virus and herpes simplex virus (HSV) (22–31). If a virus, that can infect the placenta and FMs, increases a woman’s risk for preterm birth by altering local responses to bacterial components, then the mechanisms likely involve modulation of innate immune receptors and their regulators. TLR-driven immune responses are tightly controlled by inhibitors, including the TAM tyrosine kinase receptors (32, 33). Three TAM receptors: TYRO3, AXL, and MERTK, are activated by two endogenous ligands: growth arrest specific 6 (GAS6) and Protein S1 (PROS1) (33). GAS6 activates all three TAM receptors, while PROS1 activates TYRO3 and MERTK (33). Upon ligand binding, TAM receptors trigger STAT1 phosphorylation, inducing SOCS1 and SOCS3, which broadly inhibit TLR signaling (33, 34). In this study we investigated how a polymicrobial infection could impact human FM innate immune responses and thus pregnancy outcome. Using an ex vivo human FM explant system and an in vivo mouse model of pregnancy we examined the effect a herpes virus infection had on fetal membrane responses to low levels of bacterial lipopolysaccharide (LPS), and the role of the regulatory TAM receptors.
Materials & Methods
Human fetal membrane collection and preparation
Human FMs were collected from planned uncomplicated term (37–41 weeks) cesarean deliveries without labor or known infection/inflammation, as previously described (7, 8). Tissue collection was approved by Yale University’s Human Research Protection Program. After washing the FMs with sterile PBS supplemented with penicillin (100U/ml) and streptomycin (100µg/ml) (Gibco, Grand Island, NY), adherent blood clots were removed and explants where both the chorion and amnion were intact were obtained using a 6mm biopsy punch. The FM explants were then placed in 0.4µm cell culture inserts (BD Falcon, Franklin Lakes, NJ), with 500µl Dulbecco’s Modified Eagle Medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), and these were placed in a 24-well plate containing 500µl of the same DMEM media for 24 hrs, as previously described (7, 8, 35). The next day the media was removed and replaced with serum-free OptiMEM (Gibco). After 3 hrs, treatments were initiated all in serum-free OptiMEM.
Human fetal membrane treatments
FM explants were pretreated for 24 hrs with or without either MHV-68 (1.5×104/ml PFU) (36); HSV-2 (6.4×102/ml PFU); or the viral dsRNA mimic, Poly(I:C) [High molecular weight] (20µg/ml; Invivogen, San Diego, CA). FMs were then treated with or without LPS isolated from Escherichia coli 0111:B4 (Sigma-Aldrich, St Louis, MO) at either 1ng/ml or 100ng/ml. For some experiments during the LPS treatment, FMs were also treated with or without either the caspase-1 inhibitor, Z-WEHD-FMK (1µM; R&D Systems, Minneapolis, MN) (7, 8); the NLRP3 inflammasome inhibitor, 3,4-methylenedioxy-beta-nitrostyrene (MNS; Cayman Chemical, Ann Arbor, MI) at 10µM (37); or recombinant (r) human GAS6 (50ng/ml; R&D Systems). 24 hrs later, culture supernatants and FM tissues were collected, snap frozen, and stored at −80°C until further analysis. In separate experiments, FMs were pretreated for 30 mins with blocking antibodies (0.5µg/ml) to human TYRO3 (mouse mAb #MAB859; R&D Systems), human AXL (goat polyclonal #AF154; R&D Systems) and human MERTK (goat polyclonal #AF891; R&D Systems). FMs were also pretreated with isotype control antibodies mouse IgG1 (#MAB002) and goat IgG (#AB-108-C) at the same concentrations (R&D Systems). FMs were then treated with or without LPS (1ng/ml), and after 24 hrs, culture supernatants and FM tissues were collected and stored.
Mouse Studies
All mouse studies were approved by Yale University’s Institutional Animal Care & Use Committee. Pregnant wildtype C57BL/6 or pregnant AXL−/−MERTK−/− mice (38) were injected i.p. with either PBS or low-dose LPS (20µg/kg) on E15.5 (36, 39). Pregnant wildtype C57BL/6 were also injected i.p. with either PBS or MHV-68 (1×106 PFU) on E8.5 followed by either PBS or LPS (20µg/kg) injected i.p. at E15.5. After 6hr, mice were sacrificed. FMs were collected, pooled, snap frozen, and stored at −80°C until further analysis.
Cytokine analysis
Supernatants were measured for IL-1β by ELISA (R&D Systems), and the following cytokines/chemokines were measured by multiplex analysis (BioRad, Hercules, CA): IL-6, IL-8, IL-10, IL-12, IL-17, granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor (GM-CSF), interferon gamma (IFNγ), Monocyte chemotactic protein-1 (MCP-1/CCL2), macrophage inflammatory protein-1 alpha (MIP-1α/CCL3), MIP-1β (CCL4), regulated on activation, normal T cell expressed and secreted (RANTES/CCL5), tumor necrosis factor alpha (TNFα), vascular endothelial growth factor (VEGF), growth regulated oncogene-alpha (GRO-α), and interferon gamma-induced protein 10 (IP-10/CXCL10) (7, 8). sMERTK was measured by ELISA (R&D Systems). Synergystic responses induced by combined treatments were defined as greater than the additive value of the two treatments alone.
Quantitative real time PCR
Human and mouse FMs were homogenized and total RNA extracted as previously described (6, 40). Quantitative real-time RT-PCR was performed using the KAPA SYBR Fast qPCR kit (Kapa Biosystems, Woburn, MA), and PCR amplification performed on the BioRad CFX Connect Real-time System (BioRad). Data were normalized to the housekeeping gene, GAPDH, analyzed using the Δ−ΔCT method, and presented as relative abundance. The primers used are shown in Table 1.
Table I.
Primers used for qRT-PCR
| Gene | Forward (5’ to 3’) | Reverse (5’ to 3’) |
|---|---|---|
| Human | ||
| TYRO3 | CGGTAGAAGGTGTGCCATTTT | CGATCTTCGTAGTTCCTCTCCAC |
| AXL | CCGTGGACCTACTCTGGCT | CCTTGGCGTTATGGGCTTC |
| MERTK | CTCTGGCGTAGAGCTATCACT | AGGCTGGGTTGGTGAAAACA |
| GAS6 | GGCAGACAATCTCTGTTGAGG | GACAGCATCCCTGTTGACCTT |
| PROS1 | TGCTGGCGTGTCTCCTCCTA | CAGTTCTTCGATGCATTCTCTTTCA |
| GAPDH | CAGCCTCCCGCTTCGCTCTC | CCAGGCGCCCAATACGACCA |
| Mouse | ||
| TYRO3 | CACACGCCCCAGGAGAAT | CAGGTAAAAGGTGGCACAGGA |
| AXL | ATGCCAGTCAAGTGGATTGCT | CACACATCGCTCTTGCTGGT |
| MERTK | GTAGATTTACGCACCCTCGTCAAC | GCCGAGGATGATGAACATAGAGT |
| GAS6 | ACCGTGGGCGGCATT | TCCAGGCGAGGGTTAATCG |
| PROS1 | GCACAGTGCCCTTTGCCT | CAAATACCACAATATCCTGAGACGTT |
| IL-1B | CCCAACTGGTACATCAGCAC | TCTGCTCATTCACGAAAAGG |
| GAPDH | ACCACAGTCCATGCCATCAC | CACCACCCTGTTGCTGTAGCC |
Western Blot
Human FM explants were homogenized and proteins extracted and quantified as previously described (8, 35). TYRO3, AXL, and MERTK levels were evaluated by Western blot as previously described (8) using the anti-human primary antibodies against TYRO3 (#MAB859, R&D Systems), AXL (#AF154, R&D Systems), and MERTK (#AF891, R&D Systems). IL-1β levels were evaluated using the anti-human primary antibodies against pro-IL-1β (# 12703, Cell Signaling) and the active form (# 2022, Cell Signaling). β-Actin was used as a loading control (Sigma). Images were recorded and semi-quantitative densitometry performed using the Gel Logic 100 system and Carestream software (Carestream Molecular Imaging, Woodbridge, CT). ELISA was used to measure the tissue levels of GAS6 (R&D Systems) and total PROS1 (Innovative Research Inc., Novi, MI).
Statistical analysis
Each in vitro FM treatment experiment was performed in triplicate and repeated at least three times. For in vivo studies, all FMs from each pregnant animal were pooled. All data are reported as mean ± standard error of the mean (SEM) of pooled experiments. The number of independent experiments or individual mice that data were pooled from are indicated in the figures or figure legends as "n=". Statistical significance (p<0.05) was determined by performing, for multiple comparisons one-way analysis of variance (ANOVA) or a non-parametric test; or for the comparison of two groups, t-test or the wilcoxon matched-pairs signed rank test, using Prism Software (Graphpad, Inc; La Jolla, CA).
Results
Viral infection synergistically augments human and mouse FM IL-1β production
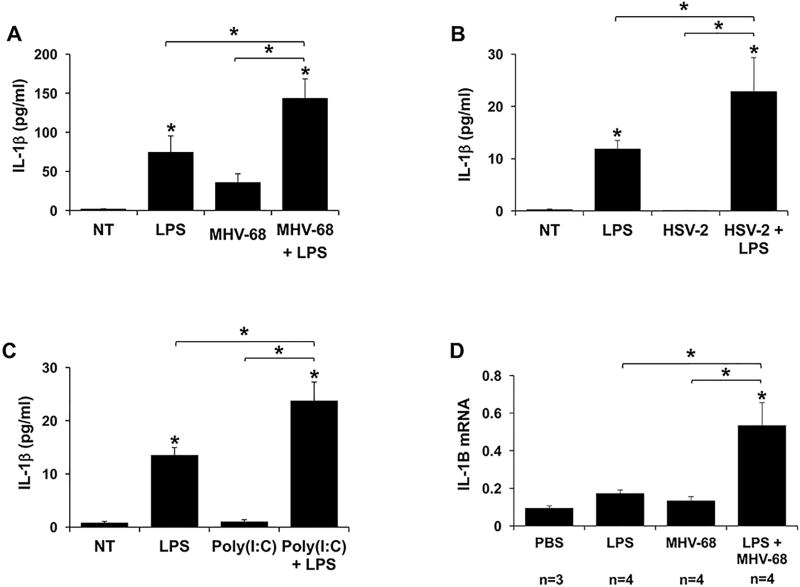
As previously reported (8), low levels of bacterial LPS significantly upregulated normal human FM explant secretion of IL-1β when compared to the no treatment (NT) control (Figure 1; A–C). Infection with the herpes virus, murine gamma herpes virus 68 (MHV-68), had no significant effect on FM IL-1β secretion compared to the no treatment (NT) control (Figure 1A). However, similarly to human placental trophoblast cells (36, 39), MHV-68 efficiently infected human FM tissues. At 24 hours post infection, the FM viral load was 7.76 × 105/500ng DNA as measured by qPCR for the MHV-68 early-late lytic gene, ORF-53 (36, 41) (data not shown). In combination with LPS, pre-treatment with MHV-68 significantly and synergistically augmented IL-1β secretion as detected by ELISA by 3.4±1.4 fold when compared to LPS alone and by 6.0±1.1 fold when compared to MHV-68 alone (Figure 1A). Western blot analysis of the culture supernatants confirmed that only the mature active form of IL-1β was released from the FM tissue; no precursor was detected in the culture media (data not shown). When FMs were pretreated with LPS followed by MHV-68 infection a similar synergistic 5.2±2.9 fold augmentation of IL-1β secretion was seen (data not shown). However, since we sought to build on previous studies that pretreated with MHV-68 prior to LPS exposure (36, 39), we continued our studies using this model.
Figure 1. Viral infection augments LPS-induced FM IL-1β.
Human FM explants were treated with (A) No treatment (NT), LPS (100ng/ml), MHV-68 (1.5×104/ml PFU) or both MHV-68 and LPS (n=7); (B) NT, LPS (100ng/ml), HSV-2 (6.4×102/ml PFU) or both HSV-2 and LPS (n=3); or (C) NT, LPS (1ng/ml), Poly(I:C) (20µg/ml) or both Poly(I:C) and LPS (n=6). (A–C) Supernatants were measured for IL-1β by ELISA. (D) Pregnant wildtype mice were injected on E8.5 with either PBS or MHV-68 (1×106 PFU), and on E15.5 with either PBS or LPS (20µg/kg). After 6hrs mice were sacrificed. The FMs were harvested and homogenized for RNA and IL-1B mRNA levels measured by qRT-PCR. *p<0.05 relative to the NT or PBS control unless otherwise indicated. Data are expressed as mean±SEM.
To validate the findings for a human viral infection, human FMs were infected with HSV-2 prior to LPS exposure. HSV-2 alone had no effect on FM IL-1β secretion when compared to the no treatment (NT) control. However, HSV-2 infection significantly and synergistically augmented IL-1β secretion by 1.9±0.4 fold when compared to LPS alone (Figure 1B). Similarly, the viral dsRNA mimic Poly(I:C) alone did not induce a FM IL-1β response, as previously reported (7). However, in combination with LPS, pretreatment with Poly(I:C) also significantly and synergistically augmented IL-1β secretion by 1.8±0.2 fold when compared to LPS alone, and by 28.8±4.5 fold when compared to Poly(I:C) alone (Figure 1B). Of note, while Poly(I:C) and HSV-2 had similar efficacies, MHV-68 was more efficient by 1.7 fold at augmenting LPS-induced IL-1β secretion by the FMs.
In order to validate our in vitro findings in vivo, pregnant wildtype mice were injected with either PBS or MHV-68 at E8.5, followed by either PBS or low dose LPS at E15.5, as previously described (36, 39). Mouse FMs exposed to either LPS alone or MHV-68 alone had no significant effect on IL-1B mRNA levels when compared to the PBS control. However, combination MHV-68 and LPS induced a significantly synergistic increase in FM IL-1B mRNA expression that was 3.1±0.7 fold higher when compared to LPS alone, and 4.0±0.9 fold higher when compared to MHV-68 alone (Figure 1D).
Viral infection augments human FM IL-1β processing and secretion in response to bacterial LPS through activation of the NLRP3 inflammasome
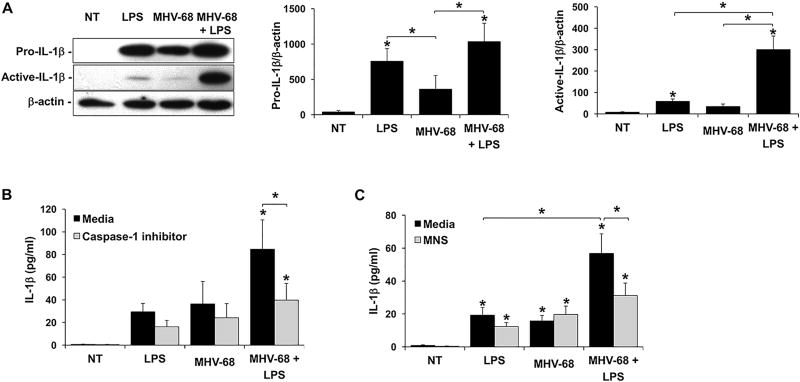
Having established in a number of systems that a viral infection or viral dsRNA sensitizes FMs to bacterial LPS by synergistically augmenting IL-1β production, we investigated the mechanism by which this response was mediated. Using the model of human FMs infected with MHV-68, first the pro- and active forms of IL-1β were measured. Under no treatment (NT) conditions, FM tissues did not express detectable levels of either form of IL-1β (Figure 2A). Treatment with LPS alone significantly induced expression of pro-IL-1β and significantly induced processing into its active form. While treatment with MHV-68 alone induced some FM pro- and active-IL-1β expression, the levels were not significantly different from the NT control (Figure 2A). MHV-68 and LPS in combination significantly induced pro-IL-1β expression to levels similar to LPS alone. Furthermore, MHV-68 and LPS in combination significantly and synergistically induced 7.9±2.3 fold more IL-1β processing into its active form when compared to LPS alone (Figure 2A). Inhibition of the common mediator of IL-1β processing, caspase-1 (42), significantly reduced FM secretion of IL-1β in response to combined MHV-68 and LPS by 53.3±8.7% (Figure 2B). Inhibition of NLRP3 inflammasome activity using the inhibitor, 3,4-methylenedioxy-beta-nitrostyrene (MNS) (37), significantly reduced FM secretion of IL-1β in response to combined MHV-68 and LPS by 43.3±11.3% (Figure 2C).
Figure 2. Viral infection augments LPS-induced FM IL-1β processing and secretion through activation of the NLRP3 inflammasome.
(A) Human FM explants were treated with (A) No treatment (NT), LPS (100ng/ml), MHV-68 (1.5×104/ml PFU) or both MHV-68 and LPS (n=7). Lysates were evaluated by Western blot for expression of pro-IL-1β (31kDa) and the 17kDa active form. Blots are from one representative experiment. Bar charts show pro- and active-IL-1β expression as determined by densitometry and normalized to β-actin (n=4–5). (B & C) Human FM explants were treated with NT, LPS, MHV-68 or both MHV-68 and LPS in the presence of either media or (B) a caspase-1 inhibitor (1µM) (n=6); or (C) the NLRP3 inhibitor, MNS (10µM) (n=5). Supernatants were measured for IL-1β by ELISA. *p<0.05 relative to the NT control unless otherwise indicated. Data are expressed as mean±SEM.
Viral infection and viral dsRNA differentially modulate the human FM cytokine/chemokine profile in response to bacterial LPS
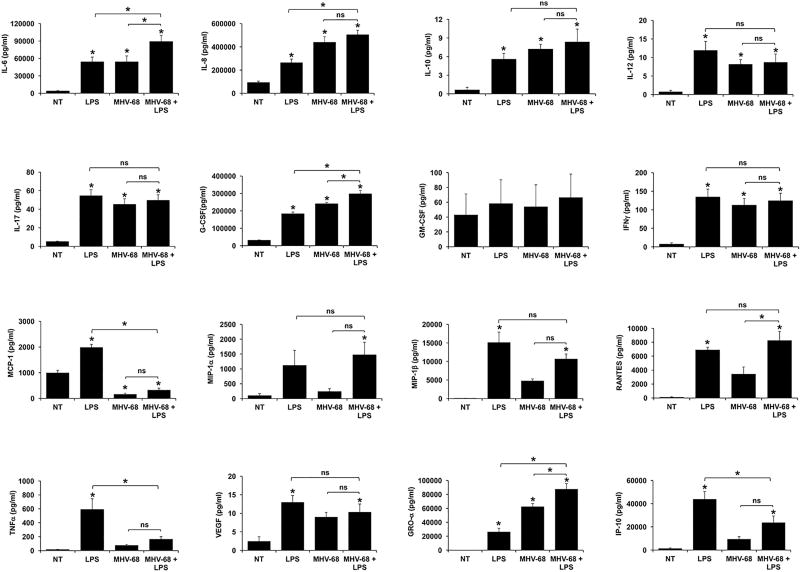
A broader range of cytokines and chemokines secreted by human FMs in response to MHV-68, HSV-2, Poly(I:C), either alone or in combination with LPS, was examined. Data from these studies have been summarized in Table 2. Treatment of FMs with LPS alone significantly increased the secretion of IL-6, IL-8, IL-10, IL-12, IL-17, G-CSF, GM-CSF, IFNγ, MCP-1, MIP-1β, RANTES, TNFα, VEGF, GRO-α and IP-10 when compared to the NT control, while having no significant effect on MIP-1α production (Figure 3–5).
Table 2.
Summary of the effect of viral infection or Poly(I:C) on human FM responses to LPS.
| Effect on LPS response |
Synergistic increase |
Additive increase |
Suppression | None |
|---|---|---|---|---|
| IL-1β | MHV-68 HSV-2 Poly(I:C) | |||
| IL-6 | MHV-68 Poly(I:C) | HSV-2 | ||
| IL-8 | MHV-68 | HSV-2 Poly(I:C) | ||
| IL-10 | MHV-68 HSV-2 Poly(I:C) | |||
| IL-12 | MHV-68 HSV-2 Poly(I:C) | |||
| IL-17 | MHV-68 HSV-2 Poly(I:C) | |||
| G-CSF | HSV-2 | MHV-68 Poly(I:C) | ||
| GM-CSF | Poly(I:C) | MHV-68 HSV-2 | ||
| IFNγ | MHV-68 HSV-2 Poly(I:C) | |||
| MCP-1 | MHV-68 HSV-2 | Poly(I:C) | ||
| MIP-1α | HSV-2 Poly(I:C) | MHV-68 | ||
| MIP-1β | MHV-68 HSV-2 Poly(I:C) | |||
| RANTES | Poly(I:C) | MHV-68 HSV-2 | ||
| TNFα | MHV-68 Poly(I:C) | HSV-2 | ||
| VEGF | Poly(I:C) | MHV-68 HSV-2 | ||
| GRO-α | HSV-2 | MHV-68 Poly(I:C) | ||
| IP10 | Poly(I:C) | MHV-68 | HSV-2 |
Figure 3. MHV-68 infection differentially modulates FM cytokine/chemokine responses to LPS.
Human FM explants were treated with no treatment (NT), LPS (100ng/ml), MHV-68 (1.5×104/ml PFU) or both MHV-68 and LPS (n=4); Supernatants were measured by multiplex analysis. *p<0.05 relative to the NT control unless otherwise indicated. Data are expressed as mean±SEM.
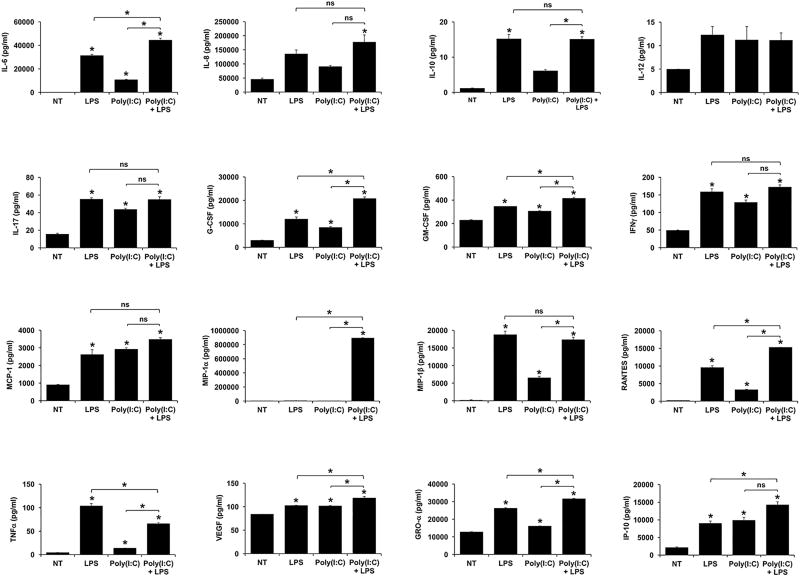
Figure 5. Poly(I:C) differentially modulates FM cytokine/chemokine responses to LPS.
Human FM explants were treated with no treatment (NT), LPS (1ng/ml), Poly(I:C) (20µg/ml) or both Poly(I:C) and LPS (n=4). Supernatants were measured by multiplex analysis. *p<0.05 relative to the NT control unless otherwise indicated. Data are expressed as mean±SEM.
As shown in Figure 3, infection of FMs with MHV-68 alone significantly increased the secretion of IL-6, IL-8, IL-10, IL-12, IL-17, G-CSF, IFNγ, and GRO-α compared to the NT control. MHV-68 infection alone significantly reduced basal FM secretion of MCP-1, and had no significant effect on FM GM-CSF, MIP-1α, MIP-1β, RANTES, TNFα, VEGF or IP-10 secretion (Figure 3). When FMs were pretreated with MHV-68 and then exposed to LPS, secretion of IL-6, IL-8, G-CSF and GRO-α was further significantly increased when compared to LPS alone, and with the exception of IL-8, when compared to MHV-68 alone, all in an additive manner. In contrast, MHV-68 infection of FMs followed by exposure to LPS significantly inhibited the LPS-induced secretion of MCP-1 by 84.7±5.2%; TNFα by 68.3±8.8%; and IP-10 by 52.9±10.0%. The secretion of IL-10, IL-12, IL-17, GM-CSF, IFNγ, MIP-1α, MIP-1β; RANTES; and VEGF were not significantly altered by combination MHV-68 and LPS when compared to LPS alone or, with the exception of RANTES, when compared to MHV-68 alone (Figure 3 & Table 2).
As shown in Figure 4, infection of FMs with HSV-2 alone had no significant effect on the FM secretion of any of the factors tested. When FMs were pretreated with HSV-2 and then exposed to LPS, FM secretion of G-CSF, MIP-1α and GRO-α was significantly and synergistically augmented by 1.3±0.1 fold, 1.2±0.1 fold, and 1.2±0.1 fold, respectively, when compared to LPS alone. Similarly to infection with MHV-68, HSV-2 significantly reduced FM secretion of MCP-1 in response to LPS by 16.0±7.3%. The secretion of IL-6, IL-8, IL-10, IL-12, IL-17, GM-CSF, IFNγ, MIP-1β, RANTES, TNFα, VEGF, or IP-10 were not significantly altered by the combination of HSV-2 and LPS, when compared to LPS alone (Figure 4 & Table 2).
Figure 4. HSV-2 infection differentially modulates FM cytokine/chemokine responses to LPS.
Human FM explants were treated with no treatment (NT), LPS (100ng/ml), HSV-2 (6.4×102/ml PFU) or both HSV-2 and LPS (n=3). Supernatants were measured by multiplex analysis. *p<0.05 relative to the NT control unless otherwise indicated. Data are expressed as mean±SEM.
A shown in Figure 5, treatment of FMs with Poly(I:C) alone significantly increased the secretion of IL-6, IL-17, G-CSF, GM-CSF, IFNγ, MCP-1, MIP-1β, RANTES, TNFα, VEGF, GRO-α and IP-10 compared to the no treatment (NT) control. Similar to infection with MHV-68, pretreatment of FMs with Poly(I:C) significantly augmented the LPS-induced secretion of IL-6, G-CSF and GRO-α when compared to LPS or Poly(I:C) alone, in an additive manner. However, additional factors where also augmented in a similar way. Poly(I:C) significantly augmented LPS-induced FM secretion of GM-CSF, VEGF and IP-10 in an additive manner when compared to LPS alone or, with the exception of IP-10, when compared to Poly(I:C) alone. Also similarly to MHV-68, pretreatment with Poly(I:C) significantly inhibited the LPS-induced FM secretion of TNFα by 36.6±1.3%. Similar to infection with HSV-2, combination Poly(I:C) and LPS significantly and synergistically augmented FM secretion of MIP-1α by 206.6±55.5 fold when compared to LPS alone and by 2563.9±179.1 fold when compared to Poly(I:C) alone. Combination Poly(I:C) and LPS also significantly and synergistically augmented FM secretion of RANTES by 1.6±0.1 fold when compared to LPS alone and by 4.7±0.2 fold when compared to Poly(I:C) alone. The secretion of IL-8, IL-10, IL-12, IL-17, IFNγ, MCP-1 and MIP-1β were not significantly altered by the combination of Poly(I:C) and LPS when compared to LPS alone, or with the exception of IL-10 and MIP-1β, when compared to Poly(I:C) alone (Figure 5 & Table 2).
Combined viral infection and LPS inhibits FM MERTK expression, which is reversed by GAS6
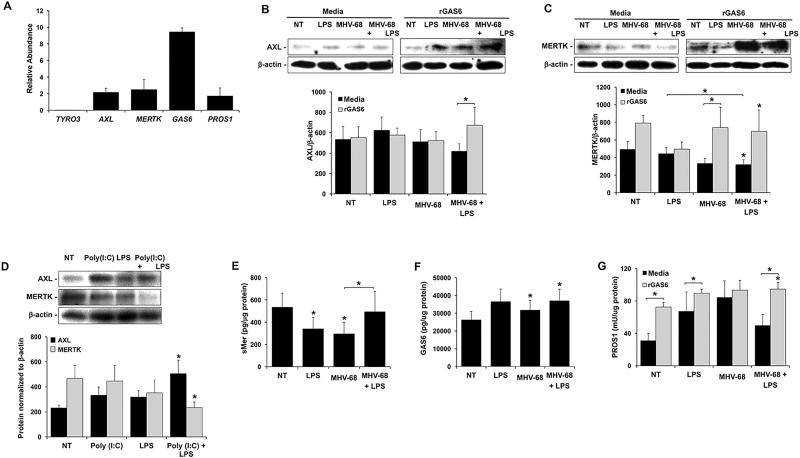
To better understand the mechanism by which viral infection of human FMs synergistically augmented the LPS-induced production of IL-1β, the expression of the TAM receptor family in these tissues was examined. Under control conditions, human FM explants expressed the TAM receptors TYRO3, AXL and MERTK, as well as their ligands GAS6 and PROS1 at the mRNA level, although TYRO3 expression was very low (Figure 6A). This was reflected at the protein level since under no treatment (NT) conditions, FMs expressed AXL (Figure 6B & D) and MERTK (Figure 6C & D), while expression of TYRO3 was undetectable (data not shown).
Figure 6. Effect of combined viral infection and LPS on FM TAM receptor and ligand expression.
(A) Untreated FM explants (n=3) were homogenized and analyzed for expression of TYRO3, AXL, MERTK, GAS6 and PROS1 mRNA by qRT-PCR. (B–C) Human FM explants were treated with no treatment (NT), LPS (100ng/ml), MHV-68 (1.5×104/ml PFU) or both MHV-68 and LPS in the presence of media or rGas6 (50ng/ml) (n=5). Tissues were homogenized for protein and Western blot performed for (B) AXL (~140kDa) and (C) MERTK (~180kDa). Blots are from one representative experiment. Bar charts show AXL and MERTK expression as determined by densitometry and normalized to b-actin. (D) FM explants were treated with NT, LPS (1ng/ml), Poly(I:C) (20µg/ml) or both Poly(I:C) and LPS (n=4). Tissues were homogenized for protein and Western blot performed for AXL and MERTK. Blots are from one representative experiment. Bar charts show AXL and MERTK expression as determined by densitometry and normalized to β-actin. (E–F) Human FM explants were treated with no treatment (NT), LPS, MHV-68 or both MHV-68 and LPS in either the presence of media or rGAS6. Tissues were homogenized for protein and ELISA performed for (E) sMERTK (n=7); (F) GAS6 (n=5), and (G) PROS1 (n=8). *p<0.05 relative to the NT control unless otherwise indicated. Data are expressed as mean±SEM.
Treatment of human FMs with MHV-68 or LPS, either alone or in combination, had no significant effect on AXL protein expression levels (Figure 6B). MERTK protein expression was significantly reduced by FMs treated with MHV-68 and LPS in combination by 42.2±5.3% when compared to the NT control, and by 34.3±9.7% when compared to LPS alone (Figure 6C). Similarly to FMs exposed to combination MHV-68 and LPS, combination Poly(I:C) and LPS significantly reduced FM MERTK protein expression by 38.7±9.2% when compared to the NT control (Figure 6D). However, combination Poly(I:C) and LPS significantly increased FM AXL protein expression by 2.1±0.3 fold compared to the NT control (Figure 6D). To assess whether the reduction of MERTK expression correlated with decoy receptor release (43), soluble (s)MERTK levels were measured. Treatment of FM explants with LPS alone or MHV-68 alone significantly reduced FM sMERTK levels by 36.4±19.4% and 44.8±19.2%, respectively compared to the NT control (Figure 6E). MHV-68 in combination with LPS significantly augmented sMERTK by 1.7±0.6-fold when compared to MHV-68 alone to near baseline levels (Figure 6E).
The presence of the common TAM receptor agonist, recombinant (r)GAS6, significantly increased AXL expression in FMs exposed to both MHV-68 and LPS by 1.6±0.2 fold (Figure 6B), and rGAS6 significantly increased MERTK expression in FMs exposed to MHV-68 alone by 1.3±0.1 fold (Figure 6C). While significance was not reached rGAS6 increased MERTK expression in FMs exposed to MHV-68 in combination with LPS by 2.4±0.7 fold (Figure 6C). FM expression of GAS6 and total PROS1 protein was also evaluated. As shown in Figure 6F, treatment of FMs with MHV-68, either alone or in combination with LPS, significantly increased GAS6 levels compared to the NT control. In contrast, PROS1 levels did not significantly change with any of the treatments (Figure 6G), however, under NT, LPS and combination MHV-68 and LPS conditions, the presence of rGAS6 significantly elevated PROS1 levels (Figure 6G).
Augmented human FM IL-1β production in response to virus and LPS is reversed by GAS6
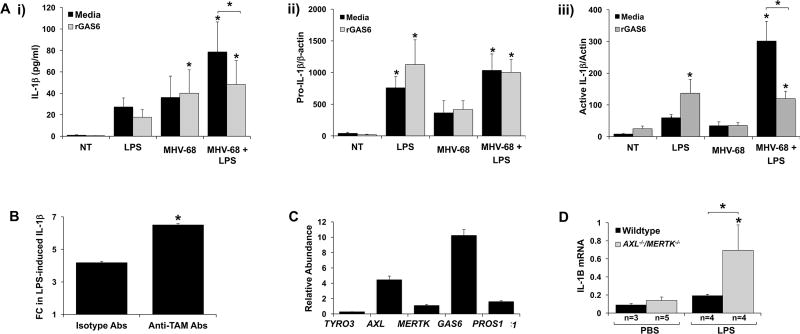
Having established that combination MHV-68 and LPS inhibited FM MERTK expression and promoted sMERTK level, and that this was reversed by rGAS6, we next sought to see if this modulation of the TAM receptor pathway played a direct role in the IL-1β response. Human FM IL-1β secretion in response to combination MHV-68 and LPS was significantly inhibited by 41.2±10.4% in the presence of rGAS6 (Figure 7A; i). To further explore the mechanism by which GAS6 regulated FM IL-1β secretion is response to combination MHV-68 and LPS, Western blot of tissue lysates for pro- and active IL-1β was performed. rGAS6 had no effect on the levels of pro-IL-1β under all conditions tested (Figure 7A; ii). However, rGAS6 significantly inhibited FM expression of active IL-1β by 52.3±5.8% when treated with combination MHV-68 and LPS (Figure 7A; iii).
Figure 7. Viral infection augments LPS-induced FM IL-1β through inhibition of the TAM receptor pathway.
(A) Human FM explants were treated with no treatment (NT), LPS (100ng/ml), MHV-68 (1.5×104/ml PFU) or both MHV-68 and LPS in the presence of media or rGas6 (50ng/ml). (i) IL-1β secretion in supernatants was measured by ELISA (n=6). Lysates were evaluated by Western blot for expression of (ii) pro-IL-1β and (iii) active IL-1β. Bar charts show pro- and active-IL-1β expression as determined by densitometry and normalized to β-actin (n=4–5) *p<0.05 relative to the NT control unless otherwise indicated. (B) Human FM explants were treated with or without LPS (1ng/ml) in the presence of blocking anti-TAM Abs or isotype control Abs. IL-1β secretion was measured and levels expressed as LPS-induced fold change (FC) relative to the NT control (n=4; *p<0.05 relative to the isotype control). (C–D) Pregnant wildtype or AXL−/−MERTK−/− mice were injected on E15.5 with either PBS or LPS (20µg/kg). After 6hrs mice were sacrificed. The FMs were harvested and homogenized for RNA. (D) TYRO3, AXL, MERTK, GAS6 and PROS1 mRNA expression levels in FMs from PBS treated wildtype mice (n=3) were measured by qRT-PCR. (E) IL-1B mRNA expression levels in FMs from PBS and LPS treated wildtype or AXL−/−MERTK−/− mice were measured by qRT-PCR. *p<0.05 relative to the control groups unless otherwise indicated. Data are expressed as mean±SEM.
Blocking TAM receptor function augments FM IL-1β production in response to bacterial LPS
To further confirm a role for the TAM receptors in modulating human FM responses to LPS, instead of using virus, their function was inhibited using blocking anti-TAM antibodies (Abs). Combination anti-TAM Abs significantly augmented LPS-induced human FM IL-1β secretion by 1.6±0.05 fold when compared to levels secreted in response to LPS in the presence of isotype antibody controls (Figure 7B). To validate the role of the TAM receptors in regulating human FM responses to LPS, an in vivo mouse model was used. Similar to human FMs, FMs collected from pregnant wildtype mice expressed the TAM receptors TYRO3, AXL and MERTK as well as their ligands GAS6 and PROS1 at the mRNA level, although again TYRO3 expression was very low (Figure 7C). Since mouse FMs predominantly expressed AXL and MERTK, we used double knockout mice as a surrogate for a viral infection (38). Thus, wildtype or AXL−/−MERTK−/− were exposed to either PBS or LPS at a dose that in wildtype mice does not induce inflammation or preterm birth (36, 39). FMs collected from pregnant wildtype mice exposed to low levels of LPS expressed similar IL-1B levels to pregnant wildtype mice exposed to PBS. However AXL−/−MERTK−/− mice exposed to low levels of LPS expressed significantly higher levels of IL-1B (5.0±2.4 fold increase) when compared to PBS-treated AXL−/−MERTK−/− mice, and significantly higher levels of IL-1B (3.6±1.7 fold increase) when compared to LPS-treated wildtype mice (Figure 7D).
Discussion
Although chorioamnionitis, PPROM, and subsequent preterm birth are associated with infection and inflammation, the underlying mechanisms involved are not fully understood. Furthermore, while local bacterial infections may be easily identified, other more difficult to detect infections may still play a role in the pathogenesis of preterm birth. Indeed, viral infections are becoming increasingly linked to pregnancy complications, and thus represent an important clinical problem. It is established that viruses able to cross the placenta and infect the fetus, such as zika virus, CMV, and rubella, often cause congenital defects and adverse pregnancy outcomes (44–46). Much less is known about viruses that may not readily vertically transmit during pregnancy, like HSV-2 and influenza H1N1. However, such viruses may still impact maternal and fetal morbidity and mortality by infecting gestational tissues and modulating local innate immune responses(47–52). The results presented in this study indicate a herpes viral infection, that infects but does not cross the placenta (36, 39), sensitizes human FM tissue to low levels of bacterial LPS, giving rise to an exaggerated inflammatory IL-1β response. Using an ex vivo human FM explant system and an in vivo mouse model of pregnancy, we have found the mechanism by which this aggravated FM inflammation arises is through the disabling of the TAM receptor pathway; and subsequent activation of the NLRP3 inflammasome.
In chorioamnionitis, PPROM, and preterm birth, the most commonly isolated microbes are bacteria, which normally colonize the lower genital tract, including E. coli, Streptococci and Ureaplasma (53–58). Mixed bacterial infections within the amniotic cavity has been demonstrated in preterm birth (53, 56) and chorioamnionitis (59), suggesting that the normal (or pathologic) bacteria of the genital tract might be an underlying cause. A recent study tested this by exposing human FM explants to a mixed bacterial culture of E. coli and S. agalactiae. However, there was no difference in the inflammatory responses generated after either mixed or single infection (60). Thus, this normal (or abnormal) bacterial flora may alone be insufficient to trigger an inflammatory response at the maternal-fetal interface that can initiate preterm birth, suggesting a role for polymicrobial infections of different types. Increasingly, infectious and inflammatory diseases are being attributed to combinations of different infectious kingdoms (21), including pregnancy complications like preterm birth (61). Furthermore, the impact viruses may have on pregnancy outcomes are beginning to be appreciated (31, 62, 63). The current study set out to test the impact a polymicrobial infection could have on human fetal membrane innate immune responses and the mechanisms involved.
Herein, we report that infection of human FM with herpes virus MHV-68 synergistically augmented the processing and secretion of mature IL-1β in response to low levels of bacterial LPS, and this was mediated by activation of the inflammasome, most likely NLRP3, which human FMs express (8). That we only detected mature IL-1β in the culture supernatants indicates that combined MHV-68 and LPS treatment of FMs promoted classical pyroptosis-associated inflammasome activation. The reduction of FM IL-1β secretion by MNS, which blocks the assembly of NLRP3 inflammasome (37), and by the caspase-1 inhibitor, a major inflammasome component, indicates that inflammasome activation, most likely NLRP3, mediated this response. However, there is the possibility that other non-NLRP3 inflammasomes might be involved since MNS can also inhibit Syk kinase signaling (37). Our finding is in keeping with recent clinical observations showing that FMs from patients with preterm birth or acute histologic chorioamnionitis have elevated IL-1β, and involvement of the inflammasome is indicated (64–66). Our in vivo observations of augmented FM IL-1β production with combination MHV-68 and LPS also complements previous reports that MHV-68 infection during pregnancy in combination with bacterial LPS trigger elevated uterine and placental inflammation and induced preterm birth (36, 39). Similarly, intrauterine delivery of viral dsRNA, which many viruses, including herpes virus produce (67), in combination with bacterial peptidoglycan (PDG) amplifies inflammation and preterm delivery (68). That we observed a similar synergistic augmentation of IL-1β secretion whether FMs were infected prior to or after LPS, demonstrates that this response is due to the polymicrobial exposure rather than the timing of virus and LPS.
While treatment of FMs with LPS induced strong pro-IL-1β expression, alone, low dose LPS was not sufficient to trigger strong IL-1β processing and secretion of active IL-1β. Furthermore, unlike the secretion of IL-1β in response to combination MHV-68 and LPS which was inhibited by MNS, IL-1β secretion by LPS alone appeared NLRP3 independent; unlike higher concentrations (69). Similarly, while MHV-68 alone induced some FM pro-IL-1β expression, there was not significant IL-1β processing. Instead, it was the combination of LPS and MHV-68 that significantly augmented FM IL-1β processing and subsequent secretion and secretion of mature IL-1β. This suggests that LPS, by activating TLR4, serves as a classical signal 1, and that MHV-68 may provide signal 2 by putatively activating NLRP3. NLRP3 can be activated by viruses (70, 71), including the herpes virus, HSV-1, although how is unclear (72). How MHV-68 may be contributing to NLRP3 inflammasome activation is an area for future study.
Both HSV-2 infection and viral dsRNA (Poly(I:C)) had a similar impact on human FM IL-1β secretion in response to LPS, and may also activate the NLRP3 inflammasome (73). Thus, the observed augmented IL-1β by virally infected human FMs in response to low levels of bacterial LPS might be common to viruses able to produce dsRNA (67) and activate the TLR3 pathway (74). However, we did note a difference in the magnitude of response. While Poly(I:C) and HSV-2 had similar efficacies, MHV-68 was more efficient at augmenting LPS-induced IL-1β secretion by the FMs. This may be due to additional mechanism(s) utilized by MHV-68. Indeed, this possibility is highlighted by the additional cytokine/chemokine data where we observed both overlapping and differential responses for MHV-68, HSV-2 and Poly(I:C). HSV-2 and Poly(I:C) both synergistically augmented LPS-induced MIP-1α, while both MHV-68 and Poly(I:C) additively augmented LPS-induced IL-6, G-CSF and GRO-α secretion by FMs; again indicating a potential role for TLR3. MHV-68 and HSV-2 both suppressed LPS-induced FM MCP-1, while MHV-68 and Poly(I:C) both reduced LPS-induced TNFα secretion. MHV-68 also reduced LPS-induced IP-10 while HSV-2 synergistically augmented LPS-induced GRO-α. The inhibition of TNFα by MHV-68 has been reported in CD8+ T cells stimulated by the viral protein encoded by ORF-61 (75). Thus, similar mechanisms may be involved in MHV-68-infected FMs for the suppression of LPS-induced TNFα, MCP-1 and IP-10. Alternatively, or in combination, the MHV-68 and LPS-induced production of IL-10 may be involved in the suppression of TNFα and IP-10 (76). Herpes simplex virus immediate-early protein ICP0 has been shown to inhibit TLR4-mediated inflammatory responses to HSV and thus, may account for HSV-2 suppression of the FM MCP-1 responses (77). Where the FM cytokine/chemokine profiles diverge in response to MHV-68, HSV-2 or Poly(I:C) combined with LPS, likely reflect the ability of different live viruses to simultaneously regulate a number of host innate immune pathways as well as producing its own immunomodulatory factors.
Since IL-1β is an important mediator of PPROM and preterm birth (2–5), and its production by human FMs was synergistically increased in our model of a polymicrobial infection for MHV-68, HSV-2 and Poly(I:C), as well as in mouse FMs, subsequent mechanistic studies focused on this inflammatory cytokine. Activation of the TAM receptors (TYRO3, AXL, MERTK) by their ligands (GAS6, PROS1) restrains TLR signaling keeping the constitutive chemokine/cytokine expression regulated (33). Since FMs constitutively express high levels of AXL, MERTK, GAS6, and PROS1 we hypothesized that MHV-68 infection removed this brake, allowing heightened TLR4-mediated IL-1β production in response to LPS. Indeed, both MHV-68 and Poly(I:C), in combination with LPS, reduced FM MERTK expression. Compared to treatment alone LPS and MHV-68 also augmented sMERTK levels which act as a decoy receptor for GAS6 (43), indicating reduced FM TAM receptor function. The increased GAS6 production under combined LPS and MHV-68 conditions may indicate a compensatory mechanism that was insufficient to restore receptor expression and function. Moreover, in the absence of viral stimulation, blocking human FM TAM receptor function also sensitized the tissue to LPS, augmenting IL-1β production. This is in contrast to studies using other enveloped viruses that through their surface expression of phosphatidylserine can bind GAS6 and activate TAM receptors (78). To further validate this finding, we turned back to an in vivo model of pregnancy using wildtype mice, which are hypo-responsive to low-dose LPS in terms of placental inflammation and pregnancy outcome (36, 39, 79, 80), and AXL−/−MERTK−/− mice, which generate hyper-responsive immune reactions to low-dose LPS (33, 81). In concert with our ex vivo human FM studies, where instead of treating with virus TAM receptor function was inhibited, FMs from AXL−/−MERTK−/− mice generated an augmented IL-1β response 6 hrs following exposure to low dose LPS when compared to FMs from wildtype mice. When mice are not sacrificed for tissues, the same dose of LPS induced preterm birth in the AXL−/−MERTK−/− mice but not in the wildtype animals (Mor, G; manuscript in preparation). Conversely, the addition of exogenous GAS6 to human FMs treated with MHV-68 and LPS not only inhibited the augmented IL-1β response, but increased FM expression of AXL, MERTK and PROS1. Thus, GAS6 may not only activate but self-regulate expression of its own signaling pathway to further enhance TAM receptor inhibition of TLR-induced inflammation. Furthermore, while rGAS6 was able to inhibit the processing and secretion of active IL-1β in response to combination MHV-68 and LPS, it was unable to alter pro-IL-1β levels, indicating that GAS6 might be suppressing virus-induced inflammasome activation. Indeed GAS6-AXL signaling may prevent NLRP3 inflammasome activation in murine macrophages (82).
One strength of our current study was the use of intact FM explants; we chose to work with a FM system in which the compartments were maintained as in vivo, since contact between the chorion and amnion likely influences each other’s response (8, 83). However, this also limits our knowledge about which cell types within the tissue (amniotic epithelial, chorionic decidual or trophoblast; resident leukocytes) are the major targets for MHV-68, HSV-2 or Poly(I:C), or the major producer of IL-1β and other cytokines/chemokines. Thus, in future studies we intent to dissect out the relative contribution of the chorion and amnion, and the specific cell types involved in the polymicrobial response.
In summary, FM inflammatory IL-1β responses to LPS become unrestrained after infection with herpes virus by reduced TAM receptor MERTK expression, and enhanced inflammasome activation. GAS6 re-establishes the normal FM response to bacterial LPS by restoring and augmenting TAM receptor and ligand expression and function, preventing the exacerbated IL-1β response. These findings suggest a novel mechanism by which viruses alter FM responses to intrauterine bacteria, giving rise to chorioamnionitis and preterm birth.
Acknowledgments
The authors would like to thank the staff of Yale-New Haven Hospital’s Labor and Birth, and the Yale University Reproductive Sciences Biobank for their help with tissue collection.
Footnotes
This study was supported in part by grants R01AI121183 (VMA) and R56AI124356 (GM) from the NIAID, NIH, and by the McKern Scholar Award for Perinatal Research (VMA).
References
- 1.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med. 2007;25:21–39. doi: 10.1055/s-2006-956773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christiaens I, Zaragoza DB, Guilbert L, Robertson SA, Mitchell BF, Olson DM. Inflammatory processes in preterm and term parturition. J Reprod Immunol. 2008;79:50–57. doi: 10.1016/j.jri.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 3.Kemp MW, Saito M, Newnham JP, Nitsos I, Okamura K, Kallapur SG. Preterm birth, infection, and inflammation advances from the study of animal models. Reprod Sci. 2010;17:619–628. doi: 10.1177/1933719110373148. [DOI] [PubMed] [Google Scholar]
- 4.Adams Waldorf KM, Rubens CE, Gravett MG. Use of nonhuman primate models to investigate mechanisms of infection-associated preterm birth. Bjog. 2011;118:136–144. doi: 10.1111/j.1471-0528.2010.02728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Menon R, Taylor RN, Fortunato SJ. Chorioamnionitis--a complex pathophysiologic syndrome. Placenta. 2010;31:113–120. doi: 10.1016/j.placenta.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 6.Abrahams VM, Potter JA, Bhat G, Peltier MR, Saade G, Menon R. Bacterial modulation of human fetal membrane Toll-like receptor expression. Am J Reprod Immunol. 2013;69:33–40. doi: 10.1111/aji.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bakaysa SL, Potter JA, Hoang M, Han CS, Guller S, Norwitz ER, Abrahams VM. Single- and double-stranded viral RNA generate distinct cytokine and antiviral responses in human fetal membranes. Mol Hum Reprod. 2014;20:701–708. doi: 10.1093/molehr/gau028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoang M, Potter JA, Gysler SM, Han CS, Guller S, Norwitz ER, Abrahams VM. Human fetal membranes generate distinct cytokine profiles in response to bacterial Toll-like receptor and nod-like receptor agonists. Biol Reprod. 2014;90:39. doi: 10.1095/biolreprod.113.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab. 2004;15:479–487. doi: 10.1016/j.tem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Pettker CM, Buhimschi IA, Magloire LK, Sfakianaki AK, Hamar BD, Buhimschi CS. Value of placental microbial evaluation in diagnosing intra-amniotic infection. Obstet Gynecol. 2007;109:739–749. doi: 10.1097/01.AOG.0000255663.47512.23. [DOI] [PubMed] [Google Scholar]
- 11.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. Bjog. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elovitz MA, Mrinalini C, Sammel MD. Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatr Res. 2006;59:50–55. doi: 10.1203/01.pdr.0000191141.21932.b6. [DOI] [PubMed] [Google Scholar]
- 13.Burd I, Bentz AI, Chai J, Gonzalez J, Monnerie H, Le Roux PD, Cohen AS, Yudkoff M, Elovitz MA. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res. 2010;88:1872–1881. doi: 10.1002/jnr.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pirianov G, Waddington SN, Lindstrom TM, Terzidou V, Mehmet H, Bennett PR. The cyclopentenone 15-deoxy-delta 12,14-prostaglandin J(2) delays lipopolysaccharide-induced preterm delivery and reduces mortality in the newborn mouse. Endocrinology. 2009;150:699–706. doi: 10.1210/en.2008-1178. [DOI] [PubMed] [Google Scholar]
- 15.Koga K, Cardenas I, Aldo P, Abrahams VM, Peng B, Fill S, Romero R, Mor G. Activation of TLR3 in the trophoblast is associated with preterm delivery. Am J Reprod Immunol. 2009;61:196–212. doi: 10.1111/j.1600-0897.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cardenas I, Mulla MJ, Myrtolli K, Sfakianaki AK, Norwitz ER, Tadesse S, Guller S, Abrahams VM. Nod1 Activation by Bacterial iE-DAP Induces Maternal-Fetal Inflammation and Preterm Labor. J Immunol. 2011;187:980–986. doi: 10.4049/jimmunol.1100578. [DOI] [PubMed] [Google Scholar]
- 17.Ganu RS, Ma J, Aagaard KM. The Role of Microbial Communities in Parturition: Is There Evidence of Association with Preterm Birth and Perinatal Morbidity and Mortality? Am J Perinatol. 2012 doi: 10.1055/s-0032-1329693. [DOI] [PubMed] [Google Scholar]
- 18.Aagaard K, Ma J, Antony KM, Ganu R, Petrosino J, Versalovic J. The placenta harbors a unique microbiome. Sci Transl Med. 2014;6:237ra265. doi: 10.1126/scitranslmed.3008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herve R, Schmitz T, Evain-Brion D, Cabrol D, Leroy MJ, Mehats C. The PDE4 inhibitor rolipram prevents NF-kappaB binding activity and proinflammatory cytokine release in human chorionic cells. J Immunol. 2008;181:2196–2202. doi: 10.4049/jimmunol.181.3.2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, Reynolds PR, Feldman RG, Sullivan MH. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005;57:404–411. doi: 10.1203/01.PDR.0000153869.96337.90. [DOI] [PubMed] [Google Scholar]
- 21.Bakaletz LO. Developing animal models for polymicrobial diseases. Nat Rev Microbiol. 2004;2:552–568. doi: 10.1038/nrmicro928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivas SK, Ma Y, Sammel MD, Chou D, McGrath C, Parry S, Elovitz MA. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am J Obstet Gynecol. 2006;195:797–802. doi: 10.1016/j.ajog.2006.05.049. [DOI] [PubMed] [Google Scholar]
- 23.Tsekoura EA, Konstantinidou A, Papadopoulou S, Athanasiou S, Spanakis N, Kafetzis D, Antsaklis A, Tsakris A. Adenovirus genome in the placenta: association with histological chorioamnionitis and preterm birth. J Med Virol. 2010;82:1379–1383. doi: 10.1002/jmv.21820. [DOI] [PubMed] [Google Scholar]
- 24.Gibson CS, Goldwater PN, MacLennan AH, Haan EA, Priest K, Dekker GA. Fetal exposure to herpesviruses may be associated with pregnancy-induced hypertensive disorders and preterm birth in a Caucasian population. Bjog. 2008;115:492–500. doi: 10.1111/j.1471-0528.2007.01653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson CS, Maclennan AH, Haan EA, Priest K, Dekker GA. Fetal MBL2 haplotypes combined with viral exposure are associated with adverse pregnancy outcomes. J Matern Fetal Neonatal Med. 2011;24:847–854. doi: 10.3109/14767058.2010.531324. [DOI] [PubMed] [Google Scholar]
- 26.Strong BS, Young SA. Intrauterine coxsackie virus, group B type 1, infection: viral cultivation from amniotic fluid in the third trimester. Am J Perinatol. 1995;12:78–79. doi: 10.1055/s-2007-994407. [DOI] [PubMed] [Google Scholar]
- 27.Chen YH, Lin HC, Lin HC. Increased risk of adverse pregnancy outcomes among women affected by herpangina. Am J Obstet Gynecol. 2010;203:49, e41–47. doi: 10.1016/j.ajog.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 28.Elefsiniotis I, Tsoumakas K, Vezali E, Glynou I, Drakoulis N, Saroglou G. Spontaneous preterm birth in women with chronic hepatitis B virus infection. Int J Gynaecol Obstet. 2010;110:241–244. doi: 10.1016/j.ijgo.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 29.Huang QT, Huang Q, Zhong M, Wei SS, Luo W, Li F, Yu YH. Chronic hepatitis C virus infection is associated with increased risk of preterm birth: a meta-analysis of observational studies. J Viral Hepat. 2015;22:1033–1042. doi: 10.1111/jvh.12430. [DOI] [PubMed] [Google Scholar]
- 30.Johansson S, Buchmayer S, Harlid S, Iliadou A, Sjoholm M, Grillner L, Norman M, Sparen P, Dillner J, Cnattingius S. Infection with Parvovirus B19 and Herpes viruses in early pregnancy and risk of second trimester miscarriage or very preterm birth. Reprod Toxicol. 2008;26:298–302. doi: 10.1016/j.reprotox.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 31.Kalu EI, Ojide CK, Chuku A, Chukwuonye, Agwu FE, Nwadike VU, Korie FC, Okafor G. Obstetric outcomes of human herpes virus-2 infection among pregnant women in Benin, Nigeria. Niger J Clin Pract. 2015;18:453–461. doi: 10.4103/1119-3077.154210. [DOI] [PubMed] [Google Scholar]
- 32.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 33.Lemke G, Rothlin CV. Immunobiology of the TAM receptors. Nat Rev Immunol. 2008;8:327–336. doi: 10.1038/nri2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Neill LA. When signaling pathways collide: positive and negative regulation of toll-like receptor signal transduction. Immunity. 2008;29:12–20. doi: 10.1016/j.immuni.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Luo G, Abrahams VM, Tadesse S, Funai EF, Hodgson EJ, Gao J, Norwitz ER. Progesterone inhibits basal and TNF-alpha-induced apoptosis in fetal membranes: a novel mechanism to explain progesterone-mediated prevention of preterm birth. Reprod Sci. 2010;17:532–539. doi: 10.1177/1933719110363618. [DOI] [PubMed] [Google Scholar]
- 36.Cardenas I, Mor G, Aldo P, Lang SM, Stabach P, Sharp A, Romero R, Mazaki-Tovi S, Gervasi M, Means RE. Placental viral infection sensitizes to endotoxin-induced pre-term labor: a double hit hypothesis. Am J Reprod Immunol. 2011;65:110–117. doi: 10.1111/j.1600-0897.2010.00908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.He Y, Varadarajan S, Munoz-Planillo R, Burberry A, Nakamura Y, Nunez G. 3,4-methylenedioxy-beta-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem. 2014;289:1142–1150. doi: 10.1074/jbc.M113.515080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosurgi L, Bernink JH, Delgado Cuevas V, Gagliani N, Joannas L, Schmid ET, Booth CJ, Ghosh S, Rothlin CV. Paradoxical role of the proto-oncogene Axl and Mer receptor tyrosine kinases in colon cancer. Proc Natl Acad Sci U S A. 2013;110:13091–13096. doi: 10.1073/pnas.1302507110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cardenas I, Means RE, Aldo P, Koga K, Lang SM, Booth CJ, Manzur A, Oyarzun E, Romero R, Mor G. Viral infection of the placenta leads to fetal inflammation and sensitization to bacterial products predisposing to preterm labor. J Immunol. 2010;185:1248–1257. doi: 10.4049/jimmunol.1000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potter JA, Garg M, Girard S, Abrahams VM. Viral single stranded RNA induces a trophoblast pro-inflammatory and antiviral response in a TLR8-dependent and -independent manner. Biol Reprod. 2015;92:17. doi: 10.1095/biolreprod.114.124032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Racicot K, Cardenas I, Wunsche V, Aldo P, Guller S, Means RE, Romero R, Mor G. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol. 2013;191:934–941. doi: 10.4049/jimmunol.1300661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martinon F, Tschopp J. Inflammatory caspases and inflammasomes: master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 43.Sather S, Kenyon KD, Lefkowitz JB, Liang X, Varnum BC, Henson PM, Graham DK. A soluble form of the Mer receptor tyrosine kinase inhibits macrophage clearance of apoptotic cells and platelet aggregation. Blood. 2007;109:1026–1033. doi: 10.1182/blood-2006-05-021634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Carvalho NS, de Carvalho BF, Doris B, Silverio Biscaia E, Arias Fugaca C, de Noronha L. Zika virus and pregnancy: An overview. Am J Reprod Immunol. 2017;77 doi: 10.1111/aji.12616. [DOI] [PubMed] [Google Scholar]
- 45.Alvarado MG, Schwartz DA. Zika Virus Infection in Pregnancy, Microcephaly, and Maternal and Fetal Health: What We Think, What We Know, and What We Think We Know. Arch Pathol Lab Med. 2017;141:26–32. doi: 10.5858/arpa.2016-0382-RA. [DOI] [PubMed] [Google Scholar]
- 46.Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral infections during pregnancy. Am J Reprod Immunol. 2015;73:199–213. doi: 10.1111/aji.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown ZA, Wald A, Morrow RA, Selke S, Zeh J, Corey L. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA. 2003;289:203–209. doi: 10.1001/jama.289.2.203. [DOI] [PubMed] [Google Scholar]
- 48.Aldo P, You Y, Szigeti K, Horvath TL, Lindenbach B, Mor G. HSV-2 enhances ZIKV infection of the placenta and induces apoptosis in first-trimester trophoblast cells. Am J Reprod Immunol. 2016;76:348–357. doi: 10.1111/aji.12578. [DOI] [PubMed] [Google Scholar]
- 49.Finger-Jardim F, Avila EC, da Hora VP, Goncalves CV, de Martinez AMB, Soares MA. Prevalence of herpes simplex virus types 1 and 2 at maternal and fetal sides of the placenta in asymptomatic pregnant women. Am J Reprod Immunol. 2017 doi: 10.1111/aji.12689. [DOI] [PubMed] [Google Scholar]
- 50.Wiley CA, Carter DM, Ross TM, Bissel SJ. Absence of fetal transmission of H1N1 despite severe maternal infection. Influenza Other Respir Viruses. 2012;6:e1. doi: 10.1111/j.1750-2659.2011.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fatemi SH, Folsom TD, Rooney RJ, Mori S, Kornfield TE, Reutiman TJ, Kneeland RE, Liesch SB, Hua K, Hsu J, Patel DH. The viral theory of schizophrenia revisited: abnormal placental gene expression and structural changes with lack of evidence for H1N1 viral presence in placentae of infected mice or brains of exposed offspring. Neuropharmacology. 2012;62:1290–1298. doi: 10.1016/j.neuropharm.2011.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beigi RH. Prevention and management of influenza in pregnancy. Obstet Gynecol Clin North Am. 2014;41:535–546. doi: 10.1016/j.ogc.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 53.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev. 2002;8:3–13. doi: 10.1002/mrdd.10008. [DOI] [PubMed] [Google Scholar]
- 54.Goldenberg RL, Andrews WW, Hauth JC. Choriodecidual infection and preterm birth. Nutr Rev. 2002;60:S19–25. doi: 10.1301/00296640260130696. [DOI] [PubMed] [Google Scholar]
- 55.Hecht JL, Onderdonk A, Delaney M, Allred EN, Kliman HJ, Zambrano E, Pflueger SM, Livasy CA, Bhan I, Leviton A. Characterization of chorioamnionitis in 2nd-trimester C-section placentas and correlation with microorganism recovery from subamniotic tissues. Pediatr Dev Pathol. 2008;11:15–22. doi: 10.2350/07-06-0285.1. [DOI] [PubMed] [Google Scholar]
- 56.DiGiulio DB. Diversity of microbes in amniotic fluid. Semin Fetal Neonatal Med. 2012;17:2–11. doi: 10.1016/j.siny.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 57.DiGiulio DB, Romero R, Amogan HP, Kusanovic JP, Bik EM, Gotsch F, Kim CJ, Erez O, Edwin S, Relman DA. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 2008;3:e3056. doi: 10.1371/journal.pone.0003056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lamont RF. Recent evidence associated with the condition of preterm prelabour rupture of the membranes. Curr Opin Obstet Gynecol. 2003;15:91–99. doi: 10.1097/00001703-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 59.Czikk MJ, McCarthy FP, Murphy KE. Chorioamnionitis: from pathogenesis to treatment. Clin Microbiol Infect. 2011;17:1304–1311. doi: 10.1111/j.1469-0691.2011.03574.x. [DOI] [PubMed] [Google Scholar]
- 60.Flores-Herrera H, Garcia-Lopez G, Diaz NF, Molina-Hernandez A, Osorio-Caballero M, Soriano-Becerril D, Zaga-Clavellina V. An experimental mixed bacterial infection induced differential secretion of proinflammatory cytokines (IL-1beta, TNFalpha) and proMMP-9 in human fetal membranes. Placenta. 2012;33:271–277. doi: 10.1016/j.placenta.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Payne MS, Bayatibojakhi S. Exploring preterm birth as a polymicrobial disease: an overview of the uterine microbiome. Front Immunol. 2014;5:595. doi: 10.3389/fimmu.2014.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71:387–390. doi: 10.1111/aji.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meijer WJ, van Noortwijk AG, Bruinse HW, Wensing AM. Influenza virus infection in pregnancy: a review. Acta Obstet Gynecol Scand. 2015;94:797–819. doi: 10.1111/aogs.12680. [DOI] [PubMed] [Google Scholar]
- 64.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, Kusanovic JP, Mittal P, Mazaki-Tovi S, Kim CJ, Kim JS, Edwin S, Nhan-Chang CL, Hamill N, Friel L, Than NG, Mazor M, Yoon BH, Hassan SS. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–616. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomez-Lopez N, Romero R, Xu Y, Plazyo O, Unkel R, Than NG, Chaemsaithong P, Chaiworapongsa T, Dong Z, Tarca AL, Abrahams VM, Yeo L, Hassan SS. A Role for the Inflammasome in Spontaneous Labor at Term with Acute Histologic Chorioamnionitis. Reprod Sci. 2016 doi: 10.1177/1933719116675058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gomez-Lopez N, Romero R, Plazyo O, Xu Y, Unkel R, Panaitescu B, Than NG, Chaiworapongsa T, Dong Z, Abrahams VM, Yeo L, Hassan SS. A Role for the Inflammasome in Spontaneous Preterm Labor with Acute Histologic Chorioamnionitis. Reproductive Sciences. 2017 doi: 10.1177/1933719116687656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ilievski V, Hirsch E. Synergy between viral and bacterial toll-like receptors leads to amplification of inflammatory responses and preterm labor in the mouse. Biol Reprod. 2010;83:767–773. doi: 10.1095/biolreprod.110.085464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lappas M. Caspase-1 activation is increased with human labour in foetal membranes and myometrium and mediates infection-induced interleukin-1beta secretion. Am J Reprod Immunol. 2014;71:189–201. doi: 10.1111/aji.12174. [DOI] [PubMed] [Google Scholar]
- 70.Jin C, Flavell RA. Molecular Mechanism of NLRP3 Inflammasome Activation. J Clin Immunol. 2010;30:628–631. doi: 10.1007/s10875-010-9440-3. [DOI] [PubMed] [Google Scholar]
- 71.Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. IL-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog. 2010;6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Johnson KE, Chikoti L, Chandran B. Herpes simplex virus 1 infection induces activation and subsequent inhibition of the IFI16 and NLRP3 inflammasomes. J Virol. 2013;87:5005–5018. doi: 10.1128/JVI.00082-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanneganti TD, Ozoren N, Body-Malapel M, Amer A, Park JH, Franchi L, Whitfield J, Barchet W, Colonna M, Vandenabeele P, Bertin J, Coyle A, Grant EP, Akira S, Nunez G. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233–236. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 74.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 75.Jennings RN, Grayson JM, Barton ES. Type I interferon signaling enhances CD8+ T cell effector function and differentiation during murine gammaherpesvirus 68 infection. J Virol. 2014;88:14040–14049. doi: 10.1128/JVI.02360-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marques CP, Hu S, Sheng W, Cheeran MC, Cox D, Lokensgard JR. Interleukin-10 attenuates production of HSV-induced inflammatory mediators by human microglia. Glia. 2004;47:358–366. doi: 10.1002/glia.20045. [DOI] [PubMed] [Google Scholar]
- 77.Daubeuf S, Singh D, Tan Y, Liu H, Federoff HJ, Bowers WJ, Tolba K. HSV ICP0 recruits USP7 to modulate TLR-mediated innate response. Blood. 2009;113:3264–3275. doi: 10.1182/blood-2008-07-168203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhattacharyya S, Zagorska A, Lew ED, Shrestha B, Rothlin CV, Naughton J, Diamond MS, Lemke G, Young JA. Enveloped viruses disable innate immune responses in dendritic cells by direct activation of TAM receptors. Cell Host Microbe. 2013;14:136–147. doi: 10.1016/j.chom.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Murphy SP, Fast LD, Hanna NN, Sharma S. Uterine NK cells mediate inflammation-induced fetal demise in IL-10-null mice. J Immunol. 2005;175:4084–4090. doi: 10.4049/jimmunol.175.6.4084. [DOI] [PubMed] [Google Scholar]
- 80.Robertson SA, Skinner RJ, Care AS. Essential role for IL-10 in resistance to lipopolysaccharide-induced preterm labor in mice. J Immunol. 2006;177:4888–4896. doi: 10.4049/jimmunol.177.7.4888. [DOI] [PubMed] [Google Scholar]
- 81.Camenisch TD, Koller BH, Earp HS, Matsushima GK. A novel receptor tyrosine kinase, Mer, inhibits TNF-alpha production and lipopolysaccharide-induced endotoxic shock. J Immunol. 1999;162:3498–3503. [PubMed] [Google Scholar]
- 82.Han J, Bae J, Choi CY, Choi SP, Kang HS, Jo EK, Park J, Lee YS, Moon HS, Park CG, Lee MS, Chun T. Autophagy induced by AXL receptor tyrosine kinase alleviates acute liver injury via inhibition of NLRP3 inflammasome activation in mice. Autophagy. 2016;12:2326–2343. doi: 10.1080/15548627.2016.1235124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zaga V, Estrada-Gutierrez G, Beltran-Montoya J, Maida-Claros R, Lopez-Vancell R, Vadillo-Ortega F. Secretions of interleukin-1beta and tumor necrosis factor alpha by whole fetal membranes depend on initial interactions of amnion or choriodecidua with lipopolysaccharides or group B streptococci. Biol Reprod. 2004;71:1296–1302. doi: 10.1095/biolreprod.104.028621. [DOI] [PubMed] [Google Scholar]