Abstract
Diabetes is the greatest risk factor for the development of cardiovascular disease, which, in turn, is the most prevalent cause of mortality and morbidity in diabetics. These patients have elevations in inflammatory monocytes, a factor consistently reported to drive the development of atherosclerosis. In preclinical models of both type 1 and type 2 diabetes, studies have demonstrated that the increased production and activation of monocytes is driven by enhanced myelopoiesis, promoted by factors, including hyperglycemia, impaired cholesterol efflux, and inflammasome activation, that affect the proliferation of bone marrow precursor cells. This suggests that continued mechanistic investigations of the enhanced myelopoiesis and the generation of inflammatory monocytes are timely, from the dual perspectives of understanding more deeply the underlying bases of diabetes pathophysiology and identifying therapeutic targets to reduce cardiovascular risk in these patients.
Keywords: atherosclerosis, stem cells, diabetes, myelopoiesis
Diabetes and cardiovascular disease
Type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) are both significant risk factors for the development of cardiovascular disease (CVD), and CVD is the most prevalent cause of mortality and morbidity in diabetic patients.1,2 Over the past 30 years, the global burden of diabetes has increased significantly, from 30 million in 1985 to 382 million in 2014, with these numbers expected to rise.3 While the rates of both T1DM and T2DM are growing, T2DM, comprising 90% of diabetic cases, has a disproportionately greater contribution to the rising prevalence of diabetes. Compared to non-diabetics, the relative risk of fatal and nonfatal CVD events in men and women with diabetes are 2- to 3-fold and 3- to 4-fold higher, respectively.4–6 The mechanisms that contribute to this heightened risk are poorly understood. Both T1DM and T2DM are characterized by hyperglycemia, impaired insulin secretion, an enhanced inflammatory state, and, often, dyslipidemia and insulin resistance, especially in the case of T2DM, but also in some T1DM individuals who become obese. Each of these co-morbidities likely contributes to the increased CVD risk observed in these patients. Emerging evidence indicates that a primary factor that contributes to the development of CVD in diabetic patients is the interplay of hyperglycemia and hypercholesterolemia and their impact on the hematopoietic stem and progenitor cells (HSPCs) in the bone marrow (BM) niche. Here, we explore the mechanisms of HSPC expansion in the setting of diabetes and how myelopoiesis amplifies inflammation and is likely a contributor to heightened CVD risk in those with pre-diabetes, T1DM, and T2DM.
Leukocytosis is associated with the prevalence of CVD
Epidemiological studies and clinical evidence suggest that peripheral blood leukocyte counts, especially monocyte and neutrophil levels, and their inflammatory status are predictive of future CVD risk.7–11 The mechanisms by which leukocytosis promotes atherosclerosis development are generally poorly understood; however, preclinical models have linked hypocholesteremia to increased levels of circulating monocytes, especially inflammatory Ly6-Chi CCR2+ monocytes12,13 and neutrophils.14 These cell types are known to enter into plaques and promote lesion progression,12 with additional contributions to plaque biology further highlighted by plaque regression studies.15,16 Reductions in plasma low-density lipoprotein (LDL) cholesterol levels in humans and mice lead to decreased circulating white blood cell counts and retarded atherosclerotic lesion progression, and in many cases initiation of plaque regression.17 Both T1DM and T2DM patients have increased circulating leukocyte levels and are less responsive to lipid-lowering therapies, factors that are likely contributors to impaired regression of atherosclerosis in diabetic patients when their plasma lipid levels are controlled.16,18,19 We have used preclinical mouse models in which plasma levels of atherogenic lipoproteins were acutely lowered to carry out mechanistic studies of atherosclerosis regression.20,21 In these models, diabetic mice show markedly impaired regression compared with controls, despite similar plasma lipid lowering;16 however, the underlying mechanisms were incompletely defined.
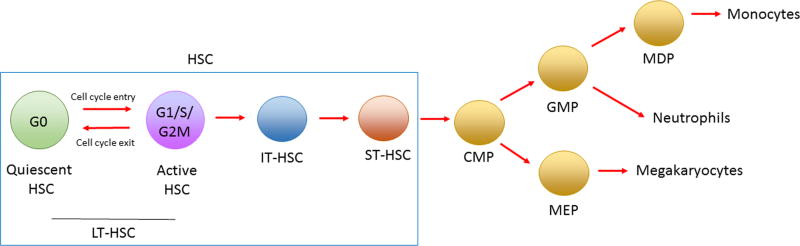
Circulating blood cells are produced via tightly regulated hematopoiesis, a process that generates billions of new leukocytes and erythrocytes each day. The classical hematopoietic ontogeny has been described as a hierarchical system originating in the hematopoietic stem cell (HSC), which differentiates in the bone marrow (BM) into myeloid, lymphoid, and erythroid–megakaryocytic lineages. Despite HSCs being a small population in the BM, accounting for around 0.01% of cells, they fulfill all characteristics of stem cells (i.e., the capacity for self-renewal and the ability to give rise to all types of blood cells). The remaining cells in the HSC BM niche are hematopoietic progenitors at various stages of maturity, nearly mature blood cells, and cells that provide key regulatory signals in the hematopoietic niche (e.g., macrophages and stromal cells that provide direct or indirect binding of stem cells and produce retention factors (e.g., CXCL12), along with hematopoietic chemokines, including thrombopoietin (TPO), stem cell factor, and colony-stimulating factors).
Within the BM, a subset of HSCs resides in a quiescent state for prolonged periods of time, a property thought to protect HSCs from functional exhaustion and cellular insults to enable lifelong hematopoietic cell production. The cell cycle is characterized by four primary phases; G1 (interphase), S (DNA synthesis phase), G2 (interphase), and M (mitosis phase). Cells that proceed past the restriction point in the G1 phase enter the S phase, whereas those that do not remain undivided. These undivided cells can withdraw from the cell cycle and enter the G0 phase, a state in which cells are quiescent or dormant.22 Such non-cycling cells in the G0 phase can either reversibly re-enter the cell cycle and divide23 or remain dormant, losing the potential to cycle and, in some cases, becoming senescent.24,25 Quiescence is thus a property that often characterizes tissue-resident stem cells and allows them to act as a dormant reserve that can replenish tissues during homeostasis. The transition from quiescence to activation and the mobilization of HSPCs from BM to blood are highly regulated by a range of intrinsic factors, extrinsic factors, HSPC receptors, and surrounding cells in the niche (Fig. 1).
Figure 1.
Circulating blood cells are produced via tightly regulated hematopoiesis. Before entry into the cell cycle, a subset of BM hematopoietic stem cells (HSCs) reside in a quiescent state (G0). The transition from quiescence to activation (long-term (LT) HSC) to intermediate (IT), and short term (ST) HSCs is a highly regulated process. Lineage-restricted differentiation of cells begins after the multipotent HSC stage of development. Clonogenic progenitors that specifically differentiate into myeloid lineages are referred to as common myeloid progenitors (CMPs), which give rise to both megakaryocyte-erythrocyte progenitors (MEPs) and granulocyte–macrophage progenitors (GMPs). MEPs give rise to all erythoid-lineage cells, including megakaryocytes and erythroid cells, whereas the commitment step to GMPs is essential for the development of myeloid-lineage cells, including granulocytes, monocytes, macrophages, and dendritic cells. A specific macrophage and dendritic cell progenitor (MDP) is essential for the production of monocytes.
Mobilization and differentiation of HSPCs in the BM has been traditionally been associated with adaptive immune responses, such as bacterial infections, or acute stresses, such as myocardial infarction; however, there is increasing appreciation for the role of chronic inflammatory stresses as modulators of HSPCs. The role that the BM plays in the etiology of metabolic diseases has been largely overlooked. Recent studies, however, suggest that pathological changes associated with metabolic dysfunction and inflammation can have repercussions on HSPC number and functional properties in the BM and thus, in turn, on production of cells released to the peripheral blood.26,27 Mounting evidence demonstrates a role for hypercholesterolemia and hyperglycemia as factors that can independently regulate HSPC function or alter the phenotype of cells within the BM niche that influence HSPC proliferation and maturation.
Myelopoiesis and metabolic disorders
Hypercholesterolemia
Before reviewing myelopoiesis and diabetes, we will review the literature concerning the contribution of hypercholesterolemia to myelopoiesis, as much of our understanding of myelopoiesis in the setting of diabetes is influenced by studies that first characterized HSPC expansion and differentiation in the presence of elevated cholesterol in the setting of CVD.
Classical monocytes, elevated in the hyperglycemic state, are derived from HPSCs in the BM. Lineage-restricted differentiation of cells begins after the multipotent HSC stage of development. Clonogenic progenitors that specifically differentiate into myeloid lineages are referred to as common myeloid progenitors (CMPs), which give rise to both megakaryocyte–erythrocyte progenitors (MEPs) and granulocyte–macrophage progenitors (GMPs).28 MEPs give rise to all erythroid lineage cells, including megakaryocytes and erythroid cells, whereas the commitment step to GMPs is essential for the development of myeloid lineage cells, including granulocytes, monocytes, macrophages, and dendritic cells.29 A specific clonogenic macrophage and dendritic cell progenitor (MDP) is essential for the production of monocytes.30,31 The development of blood monocytes from these progenitors depends on the growth factor macrophage colony-stimulating factor (M-CSF). In mice, the egress of classical Ly6-Chi monocytes from the BM to the blood is dependent on the C-C chemokine receptor 2 (CCR2) and its ligands monocyte chemoattractant protein-1 (CCL2) and monocyte chemoattractant protein-3 (CCL7).
Preclinical research into the field of HSCs and chronic inflammatory diseases has provided mechanistic insights into monocyte production during diseases, such as atherosclerosis, in which hypercholesterolemia is common. Cholesterol homeostasis in HSPCs has been shown to be dependent on the cholesterol transporters ATP-binding cassette transporter (ABCA1), ATP-binding cassette transporter subfamily G member 1 (ABCG1), scavenger receptor type B-1 (SR-B1), and the efflux factor apolipoprotein E (ApoE) on the surface of HSPCs. Deficiency of one or more of these receptors can promote HSPC proliferation and differentiation in the BM and subsequent leukocytosis in the peripheral blood.32–34 Seminal mechanistic work by Yvan-Charvet and colleagues demonstrated that a global knockout of ABCA1 and ABCG1 led to cholesterol accumulation in lipid rafts on the membrane of HSPCs, leading to increased expression of the β subunit of the IL-3/granulocyte–macrophage colony-stimulating factor (GM-CSF) receptor and enhanced proliferative responses to IL-3 and GM-CSF.34
In support of this cholesterol-dependent mechanism for HSPC proliferation, Murphy et al. found that deficiency of ApoE, a known positive regulator of cholesterol efflux, also resulted in increased membrane cholesterol accumulation and increased expression of the receptor for IL-3 and GM-CSF on HSPCs.32 Additionally, Gao et al. found that SR-B1 deficiency increased the number and proliferative capacity of HSPCs and of monocyte and macrophage progenitors in mice on a high-fat diet (HFD).33 In each of these cases, HSPC proliferation associated with deficiency of ABCA1, ABCG1, SR-B1, or ApoE could be restored to basal levels by providing cholesterol efflux factors apolipoprotein A-I (ApoA-I) or high-density lipoprotein (HDL), which at high levels can unload cells of cholesterol in a transporter-independent manner, demonstrating the importance of cholesterol homeostasis to the maintenance of HSPC quiescence. A mechanism was identified by Murphy et al.35 in ABCG4-deficient mice that present with thrombopoiesis. Following TPO binding to its receptor c-MPL, there was a failure to activate the E3-ubiquitin ligase c-CBL, key in targeting c-MPL for degradation, likely due to a trapping of the upstream LYN kinase in the cholesterol-rich plasma membrane. Importantly, this defect could be overcome by modulating cellular cholesterol levels.
Hypercholesterolemia has also been reported to increase HSPC proliferation by epigenetic regulation of pathways associated with cell proliferation.36 In an atherosclerotic model with Ldlr−/− mice, Seijkens and colleagues found that hypercholesterolemia induced loss of HSPC quiescence and suppressed expression of retinoblastoma protein (Rb). Rb is a tumor suppressor protein that plays an essential role in the regulation of cell cycle growth and is expressed in HSPC populations to control proliferation and differentiation by inhibiting cell cycle progression and maintaining HSC quiescence.37 When compared to HSPCs from normocholesterolemic mice, HSPCs from hypercholesterolemic mice had increased expression of genes that directly modulate HSPC proliferation, namely cyclins B1, C1, and D1. In competitive BM transplantation studies, they elegantly demonstrated that the hypercholesterolemic environment primes HSPCs for activation and skews their development toward myeloid lineages. Transplantation of hypercholesterolemia-primed HSPCs in a normocholesterolemic environment resulted in the increased generation of granulocytes and Ly6-Chi monocytes and promoted atherosclerosis development.36
The generation of reactive oxygen species (ROS) within the BM niche is also hypothesized to be a contributing factor to HSPC expansion under conditions of hypercholesterolemia. Under basal conditions, the hypoxic BM microenvironment is responsible for low ROS production in HSPCs, which is a significant contributing factor to the maintenance of HSPC quiescence and self-renewal, but not differentiation.38 Hypercholesterolemia causes increased ROS production in HSPCs,39 and hypercholesterolemia-induced HSPC expansion has been shown to be suppressed by treatment of mice with the ROS scavenger N-acetyl-l-cysteine (NAC). In the absence of changes to a hyperlipidemic plasma profile, NAC treatment suppresses HSPC proliferation, leukocytosis, and atherosclerosis progression in Srb1 knockout and Ldlr Apoa1 double-knockout mice.33
Overall, these studies highlight the interplay between hypercholesterolemia and ROS to promote HSPC hyperproliferation and differentiation and the subsequent generation of monocytes.
Diabetes
T1DM
Elevated white blood cell counts have been observed clinically in obese prediabetic and diabetic patients, as well as in mouse models of these diseases.40–47 Changes to the hematopoietic environment in patients with T1DM, T2DM, and obesity may be a unifying factor in each of these conditions, leading to the expansion and differentiation of HSPCs and subsequent leukocytosis. Diabetes-mediated changes to BM-derived progenitors implicated in maintaining cardiovascular homeostasis may also be a bridging mechanism linking diabetes to heightened CVD risk.
Preclinical studies performed by our lab in collaboration with others have attempted to identify the origin of leukocytosis in both diabetes and obesity and its contribution to CVD. Wild-type (C57Bl/6) mice, even when on an atherogenic diet, do not develop complex atherosclerotic plaques; thus, to study CVD in the context of diabetes, it is necessary to use atherogenic mouse models. The two most common models used for CVD research are the LDL receptor–deficient mouse (Ldlr−/−) and the ApoE-deficient mouse (Ldlr−/−),48–50 each deficient in a factor important for the clearance of cholesterol and triglyceride-rich lipoprotein particles. Ldlr−/− and Ldlr−/− mice develop complex plaques with hallmarks common to advanced human lesions (foam cells, necrotic cores, and the accumulation of smooth muscle cells), with the exception of fibrous cap thinning and spontaneous plaque rupture.51 When these models are made diabetic, atherosclerosis progression is typically accelerated, but the plaques still do not rupture. Wild-type C57BL/6 mice have low total plasma cholesterol levels, with the majority carried on HDL particles. When compared to C57Bl/6 mice, the lipoprotein profile of Ldlr−/− and Ldlr−/− mice is more similar to that of humans in that most of the plasma cholesterol is carried on non-HDL particles (chylomicrons, very-low-density lipoprotein (VLDL) and LDL particles).52 Furthermore, when diabetes is induced in these models, there is a change to their lipoprotein profiles similar to that observed in diabetic patients: high plasma triglycerides, low HDL, and increased small, dense LDL particles.53 However, it is important to note that no current mouse model accurately reflects all components of human T1DM and T2DM.
In a mouse model of T1DM, we first showed that hyperglycemia impaired atherosclerosis regression,16 consistent with the clinical finding that the CVD risk of diabetics does not decrease as much as expected from lipid lowering. To explore whether this was related to leukocytosis, we then established that T1DM mice, like patients, exhibit this phenotype. We found the leukocytosis to be driven by the neutrophil-derived S100A8/A9 calgranulin heterodimer, an inflammatory factor previously identified to be upregulated in diabetic mice.16 Among other receptors, S100A8/A9 is a ligand for the receptor for advanced glycation end products (RAGE). The stimulation of RAGE on CMPs and macrophages in the BM mediated activation of the transcription factor NF-κB, and induced M-CSF and GM-CSF production. These cytokines are known to mediate both CMP and GMP proliferation and subsequent leukocytosis.15
Notably, the increased production of neutrophil S100A8/A9 and the ensuing monocytosis was dependent on hyperglycemia. Reduction of hyperglycemia by blocking renal glucose reabsorption with a sodium–glucose cotransporter 2 (SGLT2) inhibitor, a clinically approved intervention for diabetic patients,54 reduced myelopoiesis, and monocytosis in diabetic mice.15 With regard to our original observations on atherosclerosis regression,16 hyperglycemia-induced monocytosis was also found to be a significant factor in the impaired regression in diabetic mice, as, during the lipid-lowering phase, there was a relative increase in entry of inflammatory Ly6-Chi monocytes into the plaques of diabetic mice. Both the monocytosis and the increased entry were reversed by reducing hyperglycemia with SGLT2 inhibitor treatment, thereby improving regression. This may represent the clinical scenario in diabetics whereby lipid reduction therapies are less efficacious in stabilizing pre-existing plaques.18,19
Additionally, we recently found that the T1DM milieu suppresses the expression of the cholesterol transporters ABCA1 and ABCG1 on GMP and CMP populations within the BM.55 This reduction is akin to ABCA1 and ABCG1 reduction on circulating monocytes and macrophages from diabetic patients.56–58 Given that studies in normoglycemic mice indicate that these ABC factors play critical roles in the regulation of HSPC expansion and proliferation,34 we assessed the feasibility and outcome of restoring their expression. MicroRNA (miR)-33 is a negative regulator of ABCA1 and ABCG1, and antagonism of this miR leads to the upregulation of these targets.59 We found that the restoration of Abca1 and Abcg1 expression on CMPs and GMPs in diabetic mice corrects the increased hematopoiesis in diabetic mice.55 As a consequence, miR-33 antagonism also reduces diabetes-mediated circulating leukocytosis and promotes regression of atherosclerotic lesions after lipid lowering, as inhibition suppresses the production and abundance of inflammatory Ly6-Chi monocytes, resulting in decreased entry of these cells into the plaques.
The suppression of leukocytosis in diabetic mice by antagonism of miR-33 may, however be multifaceted, given the multiple in vivo targets and the fact that miR-33 has been linked to an increase in plasma membrane lipid rafts and enhancement of TLR signaling in macrophages.60 Unpublished work from our laboratory has also shown that overexpression or infusion of ApoA-I can restore myelopoiesis in diabetic mice to that of control nondiabetic mice, highlighting the likely dependence of impaired cholesterol efflux in BM precursors to heightened hematopoiesis in the setting of diabetes. Relevant to this is the recent work by Daffu and co-workers linking the RAGE signaling axis with the suppression of macrophage ABCA1/ABCG1 expression in the setting of diabetes.61 In vivo macrophage reverse cholesterol transport (RCT) studies reveal that diabetes significantly impairs this process; however, efflux is restored when the RAGE gene is deleted.61 Given that RAGE is expressed on monocyte progenitors and macrophages in the BM, this new study supports the hypothesis that diabetes-associated leukocytosis is, in part, due to impaired cholesterol efflux within the BM niche, and attempts to correct myelopoiesis to basal levels by elevating the availability of efflux factors merit further research. Furthermore, in diabetic mice, the downregulation of ABCA1/ABCG1 leads to lower levels of the major cholesterol efflux factor HDL in diabetic mice, which can be corrected by deletion of the RAGE gene.61 Impaired cholesterol efflux by virtue of reduced HDL levels and the generation of dysfunctional HDL in diabetic patients have also been repeatedly observed clinically62,63 and represents a yet to be explored mechanism for increased myelopoiesis in diabetic patients.
T2DM and obesity
Obesity-associated inflammation is widely regarded as one of the major factors driving insulin resistance (IR) and the onset of T2DM. There are strong associations between obesity, T2DM, and leukocytosis,43,45 and clinical evidence indicates that obesity can have profound impacts on the BM niche.27 Preclinical studies from our lab and others indicate that metabolic diseases and obesity are also associated with an increased production of HSPCs and provide, in part, contributory mechanistic insight into the elevated white blood cell counts in individuals with various metabolic disorders. For example, in a mouse model of obesity, we found that chronically inflamed visceral adipose tissue (VAT) can signal to BM HSPCs to proliferate, expand, and increase the production of myeloid cells.64 Similar to the situation in T1DM, we find that S100A8/A9 plays a critical role in the maintenance of hyperproliferative HSPCs. In this case, the details diverge, in that our findings are consistent with a mechanism whereby S100A8/A9 induces adipose tissue macrophage (ATM) Toll-like receptor 4 (TLR4)/MyD88 signaling to induce the expression of the IL-1β gene, which is then processed by the NLRP3 inflammasome to produce mature IL-1β. The IL-1β then travels to the BM to induce the proliferation of hematopoietic progenitor cells via IL-1R, ultimately resulting in monocytosis and neutrophila.64
Activation of NLRP3 under diabetic conditions is not restricted to murine macrophages. In the porcine model of diabetic atherosclerosis, Cohen et al. demonstrated that cleavage processing of sterol regulatory element binding (SREBP)-1 and SREBP-2 and expression of their target genes were increased in infiltrating macrophages of both fatty streaks and of advanced lesions with fibrous caps, necrotic cores, and cholesterol crystals.65 Furthermore, NLRP3 activation has been found to not be restricted to plaque macrophages, as endothelial cells and smooth muscle cells from advanced lesions have transcriptomic upregulation of NLRP3 mediated by increased SREBP-1a expression. These changes were also observed in coronary atherosclerosis samples from diabetic human patients.66 In diabetic patients, IL-1β has been implicated as an effector molecule of inflammatory β cell destruction in the pancreas, leading to apoptosis and inhibition of their insulin-producing function.67,68 Antagonism of the IL-1β receptor has been shown to protect human β cells from glucose-induced impairment and apoptosis.68 A recent clinical trial using the IL-1β receptor antagonist Anakin improved glycemic control in T2DM patients, which is hypothesized to be due to enhanced β cell secretory function.69 In contrast to the T1DM model, glucose reduction failed to correct leukocytosis, confirming that a different process drives leukocyte production in this model of obesity/T2DM.15
An alternate mechanism for changes to leukocyte production in patients with T2DM or obesity may be associated with modifications to the microbiome. Obese mice and humans display microbiota alterations and low-grade inflammation,70 and such changes may modulate functional properties of the hematopoietic system and the immune cell function in tissues.71 A recent study by Luo and colleagues explored the possibility that the microbiota from obese mice could regulate HSC differentiation by altering the BM niche.72 To assess the effect of a high-calorie diet on HSPCs, mice were fed a high-fat diet (HFD) for 6 weeks. They found that those on a high-calorie diet had an increased abundance of HSCs in the BM compartment and that there was a shift in their differentiation pattern to favor the myeloid lineage at the expense of the lymphoid lineage. An increase in CMPs and a decrease in common lymphoid progenitor cells were observed. This shift was mediated by changes to the cell types located within the BM—mesenchymal stem cells, osteoblasts, and adipocytes—which were all more abundant as a result of increased Gram-positive bacteria in the microbiome following the HFD. In a similar model of HFD feeding, obesity was shown to alter the BM, leading to an expansion of the HSC population and increased production of myeloid precursors (GMPs), which promoted the production of proinflammatory macrophages.73 In both studies, the authors found that the quantitative expansion of HSCs is reversible upon termination of HFD exposure; however, Singer et al found that the increased capacity of HSCs to generate activated myeloid cells is sustained upon change of diet.73 This suggests that obesity may have long-term effects on inflammatory responses by altering HSC and HSPC function at an epigenetic level, or more likely that IL-1β from the obese VAT is the main driver of monocytosis in diabetes.
Epigenetic modifications have been documented in inflammation and have been shown to regulate downstream immune mediator expression in monocyte-derived macrophages.74 Diabetic conditions elicit epigenetic changes in a variety of cell types, including HSPC BM-derived monocytes and macrophages.75,76 Epigenetic changes to the cells within the BM during diabetes are likely an additional mechanism by which it promotes the inflammatory status of BM-derived monocytes and macrophages isolated from diabetic plaques, contributing to enhanced CVD.77 In a model of insulin resistance, Gallagher et al. found that a repressive histone methylation mark (H3K27me3) is decreased at the promoter of the IL-12 gene in BM progenitors, and this epigenetic signature is passed down to macrophages.77 Macrophages can be broadly separated into two major phenotypes: activated (M1) or proresolving (M2). In preclinical models of diabetes, macrophages isolated from atherosclerotic plaques are predominantly of the M1 subset, which is in contrast to macrophages isolated from nondiabetic atherosclerotic lesions,15,16,55 and the enrichment in M2 characteristics we typically observe in regressing plaques78 is also not observed.16
In contrast to these studies, however, is a recent publication in which HFD feeding of mice leads to a shift of HSPCs from quiescent to differentiating; this loss of self-renewing characteristics and decrease in proliferation results in impaired multilineage reconstitution.79 Similar to other studies, the authors find that HFD feeding induces leukocytosis; however, they report that HFD feeding induces long-term alterations in the hematopoietic system, including losses of stemness and self-renewal, which may result in a depletion of the most primitive HSPCs. Given that the diets differed in terms of carbohydrate and fat content between the studies, the contribution of each of the macronutrients to the divergent finding on HSPC maintenance and myelopoiesis would be interesting modifiers to explore.
Glucose flux: an emerging concept as a contributor to diabetes-mediated myeloid expansion
Postprandial glucose. While tight glycemic control has been demonstrated to reduce the risk of future cardiovascular events in young patients with T1DM (DCCT/EDIC),80 for patients greater than 45 years old with childhood-onset T1DM, the risk of developing CVD is significantly increased when compared with those without diabetes.2 Furthermore, when T1DM patients who had on-target glycemic control were matched with appropriate age and sex controls, their risk of death from any cause and the risk of death from CVD were twice that of the general population.81 In T2DM patients, hyperglycemia is the principal risk factor for microvascular complications; however, it represents a weak risk factor the for the development of CVD, and interventional studies that have focused on reducing plasma glucose in T2DM only report minor improvement to CVD risk.82 In one trial (EMPA-REG OUTCOME) in which there was significant improvement, this was mainly related to heart failure, suggesting unanticipated, potentially non-glycemic effects of the drug.83
In contrast to the relative paucity of evidence for hyperglycemia as a CVD risk factor, there is accumulating evidence that blood glucose variability contributes to chronic complications, including cardiovascular complications, in prediabetic and diabetic patients.84–86 Individuals with impaired glucose tolerance, the hallmark of prediabetes, are reported to have elevated white blood cell counts,45 and repetitive fluctuations in blood glucose or glucose excursions have been shown to activate monocytes and enhance monocyte adhesion to endothelium.87–89 Furthermore, recent epidemiological data have suggested postprandial hyperglycemia as a risk factor for atherosclerotic disease90–92 and a contributor to plaque rupture.93.94 A recent study in mice showed that repetitive glucose spikes can accelerate atherosclerotic lesion progression; however, the molecular mechanisms associated with these observations were not explored.95 However, given clinical evidence that glucose fluctuations are positively associated with classical (i.e., inflammation-prone) CD14brightCD16+ monocytes, underlying changes to HSCs in the BM may be a significant contributor.94
Cellular uptake of glucose. To date, the majority of research assessing the effect of either hyperglycemia or hypercholesterolemia has focused on elucidating the roles of cytokines and the microenvironment in the proliferation, mobilization, and commitment of HSPCs in diabetic and atherosclerotic models. However, recent studies indicate that intracellular glucose metabolism may be a significant contributor to myeloid cell activation under acute and chronic settings96,97 and that glucose transporter 1 (GLUT1) may play a role.
A recent study by Sarrazy and colleagues considered the importance of HSPC metabolism on their function, specifically of GLUT1, which may of significance in the context of diabetes or metabolic disorders.98 The role of GLUT1 was assessed given clinical evidence that glucose utilization can determine HSC myeloid lineage commitment99,100 and that mouse studies indicate that HSC bioenergies can influence their stemness.101,102 The authors found that, by reducing the expression of GLUT1 in BM cells via BM transplant studies, expansion of HSCs in hypercholesterolemic mice is limited. Deficiencies in GLUT1 inhibit the activation of the β subunit of the IL-3/GM-CSF receptor on HSPCs in the BM, thereby reducing glucose utilization by the mitochondria. This reduction in glucose use was found to not only reduce HSPC proliferation and differentiation but also to lead to a resultant decrease in macrophage activation and a reduction in atherosclerosis in mice.98 Furthermore, increased glycolysis induced by overexpression of GLUT1 promotes inflammatory cytokine secretion;103 however, in a mouse model of atherosclerosis, increased glucose uptake by myeloid cells did not affect the development of atherosclerosis.104 This result is perhaps not surprising given the relatively high expression of GLUT1 on myeloid cells and the fact that the enzymes in the downstream metabolic pathways are rate limiting. The relevance of these findings has yet to be determined in the setting of diabetes, although, given that cellular glucose flux can change the physiological role and phenotype of cells under the condition of hyperglycemia, this study potentially provides a new mechanism for myeloproliferation in situations of inadequately controlled glycaemia.
Concluding remarks
Hypercholesterolemia and hyperglycemia both promote leukocytosis, particularly of neutrophils and monocytes. As reviewed above, there are both overlapping and distinct mechanisms for this at the BM level. The monocytosis directly contributes to adverse effects in atherosclerosis by promoting leukocyte entry into plaques.
Our mechanistic studies have recently focused on the diabetic state in which leukocytosis is conserved between the clinical and preclinical situations. Preclinical evidence demonstrates that diabetes promotes the mobilization and differentiation of HSPCs in the BM, and this process represents a major contributor to diabetes-mediated leukocytosis. The process by which myeloproliferation occurs appears to be multifaceted and may be dependent on the type of diabetes (i.e., T1DM or T2DM) and additional risk factors (e.g., hypercholesteromia, obesity, insulin resistance) (Fig. 2). In preclinical models, persistent or transient elevations in glucose are consistent drivers of HSPC expansion and proliferation, mediated by changes to factors that regulate cell quiescence and proliferation. Furthermore, emerging evidence indicates that epigenetic mechanisms or metabolic memory may permanently alter the functionality and inflammatory status of HSCs in diabetic patients whose glucose levels are inadequately controlled.105–107
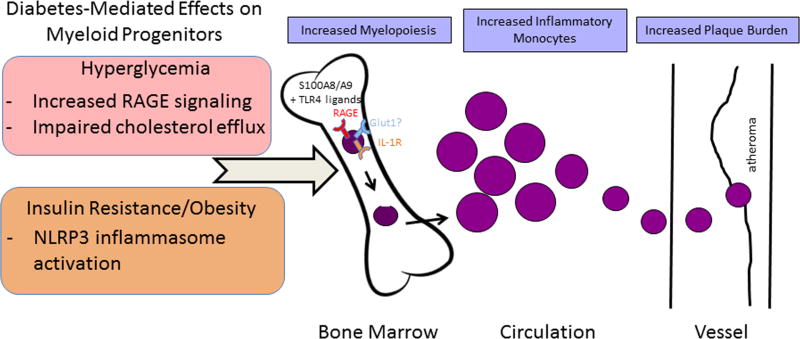
Figure 2.
Diabetes-mediated effects on myeloid progenitors and their consequences. In the setting of hyperglycemia, plasma levels of S100A8/A9 increase. In the bone marrow, this provides more S100A8/A9 to interact with RAGE on the surface of macrophages and common myeloid progenitors (CMPs), triggering the production of M-CSF and GM-CSF and increasing CMP and granulocyte–macrophage progenitors (GMPs). This subsequently accelerates the production of monocytes and neutrophils. In the setting of obesity and insulin resistance, S100A8/A9 interacts with TLR4 on adipose tissue macrophages and induces IL-1β production. Mature IL-1β then travels to the bone marrow and binds the IL-1 receptor on CMPs and GMPs, stimulating myelopoiesis. Both pathways result in the circulation of inflammation-prone monocytes (in mice, the Ly6-Chi subset), which enter the plaque in greater numbers and become macrophages. The glucose transporter GLUT1 may be a novel regulator of myelopoiesis in the diabetic state via reducing glucose utilization by hematopoietic stem and progenitor cells, thereby suppressing their proliferation and differentiation.
Studies in healthy and diabetic human populations have attempted to establish the direct relevance of the preclinical mouse studies. Peripheral blood CD34+ cells (predominantly HSCs and a small subset of endothelial progenitor cells) are often used in clinical studies as a marker of stem cell mobilization or proliferation. In healthy individuals, a mild elevation of circulating CD34+ progenitor cells, considered to be a reflection of HSPC expansion, is associated with adiposity and future metabolic deterioration in healthy individuals.108 However, diabetes has been associated with poor mobilization of CD34+ cells from the BM, with decreased circulating levels positively associated with adverse CVD outcomes.109–111 Because diabetes causes sympathetic neuropathy in the BM,111 this is likely to prevent HSC release, making the level of circulating CD34+ cells an unreliable marker of HSPC expansion. Furthermore, we have discovered in preclinical models that HSCs are largely unaffected, making their circulating levels not relevant.
Despite the need for improved diagnostic markers to bridge the preclinical mechanisms to the clinical arena, the concordance of the changes in circulating leukocyte populations in diabetic mice and humans, nevertheless, strongly (and indirectly) suggests that both enhanced myelopoiesis and elevated inflammatory status are plausible explanations for the heightened risk of CVD in diabetic patients. Further investigations in both species will likely amplify the connections and clarify the relationships between the mouse and human findings, thereby ultimately pointing to new therapeutic strategies.
Acknowledgments
T.J.B., E.A.F., and I.J.G. were supported by NIH Grants R01 DK095684, R01 HL117226, and R01 HL084312. A.J.M. is supported by NHMRC grants (APP1083138 and APP1106154), along with a career development fellowship from the NHMRC (APP1085752) and a future leader fellowship from the National Heart Foundation (100440).
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Matheus AS, et al. Impact of diabetes on cardiovascular disease: an update. Int J Hypertens. 2013;2013:653789. doi: 10.1155/2013/653789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller RG, et al. A Contemporary Estimate of Total Mortality and Cardiovascular Disease Risk in Young Adults With Type 1 Diabetes: The Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2016;39(12):2296–2303. doi: 10.2337/dc16-1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wild S, et al. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 5.Almdal T, et al. The independent effect of type 2 diabetes mellitus on ischemic heart disease, stroke, and death: a population-based study of 13,000 men and women with 20 years of follow-up. Arch Intern Med. 2004;164(13):1422–6. doi: 10.1001/archinte.164.13.1422. [DOI] [PubMed] [Google Scholar]
- 6.Stamler J, et al. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16(2):434–44. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 7.Lee CD, et al. White blood cell count and incidence of coronary heart disease and ischemic stroke and mortality from cardiovascular disease in African-American and White men and women: atherosclerosis risk in communities study. Am J Epidemiol. 2001;154(8):758–64. doi: 10.1093/aje/154.8.758. [DOI] [PubMed] [Google Scholar]
- 8.Sweetnam PM, et al. Total and differential leukocyte counts as predictors of ischemic heart disease: the Caerphilly and Speedwell studies. Am J Epidemiol. 1997;145(5):416–21. doi: 10.1093/oxfordjournals.aje.a009123. [DOI] [PubMed] [Google Scholar]
- 9.Olivares R, Ducimetiere P, Claude JR. Monocyte count: a risk factor for coronary heart disease? Am J Epidemiol. 1993;137(1):49–53. doi: 10.1093/oxfordjournals.aje.a116601. [DOI] [PubMed] [Google Scholar]
- 10.Soehnlein O, Swirski FK. Hypercholesterolemia links hematopoiesis with atherosclerosis. Trends Endocrinol Metab. 2013;24(3):129–36. doi: 10.1016/j.tem.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman GD, Klatsky AL, Siegelaub AB. The leukocyte count as a predictor of myocardial infarction. N Engl J Med. 1974;290(23):1275–8. doi: 10.1056/NEJM197406062902302. [DOI] [PubMed] [Google Scholar]
- 12.Swirski FK, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117(1):195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tacke F, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117(1):185–94. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drechsler M, et al. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122(18):1837–45. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 15.Westerterp M, et al. Deficiency of ATP-binding cassette transporters A1 and G1 in macrophages increases inflammation and accelerates atherosclerosis in mice. Circ Res. 2013;112(11):1456–65. doi: 10.1161/CIRCRESAHA.113.301086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parathath S, et al. Diabetes adversely affects macrophages during atherosclerotic plaque regression in mice. Diabetes. 2011;60(6):1759–69. doi: 10.2337/db10-0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicholls SJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365(22):2078–87. doi: 10.1056/NEJMoa1110874. [DOI] [PubMed] [Google Scholar]
- 18.Duff GL, Payne TP. The effect of alloxan diabetes on experimental cholesterol atherosclerosis in the rabbit. III. The mechanism of the inhibition of experimental cholesterol atherosclerosis in alloxan-diabetic rabbits. J Exp Med. 1950;92(4):299–317. doi: 10.1084/jem.92.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hiro T, et al. Diabetes mellitus is a major negative determinant of coronary plaque regression during statin therapy in patients with acute coronary syndrome--serial intravascular ultrasound observations from the Japan Assessment of Pitavastatin and Atorvastatin in Acute Coronary Syndrome Trial (the JAPAN-ACS Trial) Circ J. 2010;74(6):1165–74. doi: 10.1253/circj.cj-09-0766. [DOI] [PubMed] [Google Scholar]
- 20.Trogan E, et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci U S A. 2006;103(10):3781–6. doi: 10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feig JE, et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest. 2010;120(12):4415–24. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pardee AB. A restriction point for control of normal animal cell proliferation. Proc Natl Acad Sci U S A. 1974;71(4):1286–90. doi: 10.1073/pnas.71.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14(6):329–40. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120(4):513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Orlandi A, et al. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res Cardiol. 2010;105(6):703–12. doi: 10.1007/s00395-010-0109-0. [DOI] [PubMed] [Google Scholar]
- 26.Kojima H, Kim J, Chan L. Emerging roles of hematopoietic cells in the pathobiology of diabetic complications. Trends Endocrinol Metab. 2014;25(4):178–87. doi: 10.1016/j.tem.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adler BJ, Kaushansky K, Rubin CT. Obesity-driven disruption of haematopoiesis and the bone marrow niche. Nat Rev Endocrinol. 2014;10(12):737–48. doi: 10.1038/nrendo.2014.169. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26(6):726–40. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Manz MG, et al. Prospective isolation of human clonogenic common myeloid progenitors. Proc Natl Acad Sci U S A. 2002;99(18):11872–7. doi: 10.1073/pnas.172384399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fogg DK, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311(5757):83–7. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 31.Liu K, et al. In vivo analysis of dendritic cell development and homeostasis. Science. 2009;324(5925):392–7. doi: 10.1126/science.1170540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy AJ, et al. ApoE regulates hematopoietic stem cell proliferation, monocytosis, and monocyte accumulation in atherosclerotic lesions in mice. J Clin Invest. 2011;121(10):4138–49. doi: 10.1172/JCI57559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao M, et al. Regulation of high-density lipoprotein on hematopoietic stem/progenitor cells in atherosclerosis requires scavenger receptor type BI expression. Arterioscler Thromb Vasc Biol. 2014;34(9):1900–9. doi: 10.1161/ATVBAHA.114.304006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yvan-Charvet L, et al. ATP-binding cassette transporters and HDL suppress hematopoietic stem cell proliferation. Science. 2010;328(5986):1689–93. doi: 10.1126/science.1189731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murphy AJ, et al. Cholesterol efflux in megakaryocyte progenitors suppresses platelet production and thrombocytosis. Nat Med. 2013;19(5):586–94. doi: 10.1038/nm.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seijkens T, et al. Hypercholesterolemia-induced priming of hematopoietic stem and progenitor cells aggravates atherosclerosis. FASEB J. 2014;28(5):2202–13. doi: 10.1096/fj.13-243105. [DOI] [PubMed] [Google Scholar]
- 37.Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81(3):323–30. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- 38.Ito K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12(4):446–51. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 39.Tie G, et al. Hypercholesterolemia induces oxidant stress that accelerates the ageing of hematopoietic stem cells. J Am Heart Assoc. 2014;3(1):e000241. doi: 10.1161/JAHA.113.000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt MI, et al. Markers of inflammation and prediction of diabetes mellitus in adults (Atherosclerosis Risk in Communities study): a cohort study. Lancet. 1999;353(9165):1649–52. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- 41.Schipper HS, et al. Systemic inflammation in childhood obesity: circulating inflammatory mediators and activated CD14++ monocytes. Diabetologia. 2012;55(10):2800–10. doi: 10.1007/s00125-012-2641-y. [DOI] [PubMed] [Google Scholar]
- 42.Ford ES. Leukocyte count, erythrocyte sedimentation rate, and diabetes incidence in a national sample of US adults. Am J Epidemiol. 2002;155(1):57–64. doi: 10.1093/aje/155.1.57. [DOI] [PubMed] [Google Scholar]
- 43.Kullo IJ, Hensrud DD, Allison TG. Comparison of numbers of circulating blood monocytes in men grouped by body mass index (<25, 25 to <30, > or =30) Am J Cardiol. 2002;89(12):1441–3. doi: 10.1016/s0002-9149(02)02366-4. [DOI] [PubMed] [Google Scholar]
- 44.Poitou C, et al. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(10):2322–30. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 45.Ohshita K, et al. Elevated white blood cell count in subjects with impaired glucose tolerance. Diabetes Care. 2004;27(2):491–6. doi: 10.2337/diacare.27.2.491. [DOI] [PubMed] [Google Scholar]
- 46.Woo SJ, et al. Elevated systemic neutrophil count in diabetic retinopathy and diabetes: a hospital-based cross-sectional study of 30,793 Korean subjects. Invest Ophthalmol Vis Sci. 2011;52(10):7697–703. doi: 10.1167/iovs.11-7784. [DOI] [PubMed] [Google Scholar]
- 47.Jesri A, Okonofua EC, Egan BM. Platelet and white blood cell counts are elevated in patients with the metabolic syndrome. J Clin Hypertens (Greenwich) 2005;7(12):705–11. doi: 10.1111/j.1524-6175.2005.04809.x. quiz 712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plump AS, et al. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71(2):343–53. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- 49.van Ree JH, et al. Diet-induced hypercholesterolemia and atherosclerosis in heterozygous apolipoprotein E-deficient mice. Atherosclerosis. 1994;111(1):25–37. doi: 10.1016/0021-9150(94)90188-0. [DOI] [PubMed] [Google Scholar]
- 50.Ishibashi S, et al. Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J Clin Invest. 1993;92(2):883–93. doi: 10.1172/JCI116663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bentzon JF, Falk E. Atherosclerotic lesions in mouse and man: is it the same disease? Curr Opin Lipidol. 2010;21(5):434–40. doi: 10.1097/MOL.0b013e32833ded6a. [DOI] [PubMed] [Google Scholar]
- 52.Kennedy AJ, et al. Mouse models of the metabolic syndrome. Dis Model Mech. 2010;3(3–4):156–66. doi: 10.1242/dmm.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5(3):150–9. doi: 10.1038/ncpendmet1066. [DOI] [PubMed] [Google Scholar]
- 54.Rosas-Guzman J, Rosas-Saucedo J, Romero-Garcia AR. SGLT2 Inhibitors in Diabetes Mellitus Treatment. Rev Recent Clin Trials. 2016 doi: 10.2174/1574887111666160829145810. [DOI] [PubMed] [Google Scholar]
- 55.Distel E, et al. miR33 inhibition overcomes deleterious effects of diabetes mellitus on atherosclerosis plaque regression in mice. Circ Res. 2014;115(9):759–69. doi: 10.1161/CIRCRESAHA.115.304164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mauldin JP, et al. Reduction in ABCG1 in Type 2 diabetic mice increases macrophage foam cell formation. J Biol Chem. 2006;281(30):21216–24. doi: 10.1074/jbc.M510952200. [DOI] [PubMed] [Google Scholar]
- 57.Tang C, et al. Diabetes reduces the cholesterol exporter ABCA1 in mouse macrophages and kidneys. J Lipid Res. 2010;51(7):1719–28. doi: 10.1194/jlr.M003525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Passarelli M, et al. Advanced glycation end product precursors impair ABCA1-dependent cholesterol removal from cells. Diabetes. 2005;54(7):2198–205. doi: 10.2337/diabetes.54.7.2198. [DOI] [PubMed] [Google Scholar]
- 59.Rayner KJ, et al. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;328(5985):1570–3. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lai L, et al. MicroRNA-33 Regulates the Innate Immune Response via ATP Binding Cassette Transporter-mediated Remodeling of Membrane Microdomains. J Biol Chem. 2016;291(37):19651–60. doi: 10.1074/jbc.M116.723056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Daffu G, et al. RAGE Suppresses ABCG1-Mediated Macrophage Cholesterol Efflux in Diabetes. Diabetes. 2015;64(12):4046–60. doi: 10.2337/db15-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Asleh R, Levy AP. Divergent effects of alpha-tocopherol and vitamin C on the generation of dysfunctional HDL associated with diabetes and the Hp 2-2 genotype. Antioxid Redox Signal. 2010;12(2):209–17. doi: 10.1089/ars.2009.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Patel DC, et al. Type 2 diabetes is associated with reduced ATP-binding cassette transporter A1 gene expression, protein and function. PLoS One. 2011;6(7):e22142. doi: 10.1371/journal.pone.0022142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nagareddy PR, et al. Adipose tissue macrophages promote myelopoiesis and monocytosis in obesity. Cell Metab. 2014;19(5):821–35. doi: 10.1016/j.cmet.2014.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Y, et al. Activation of sterol regulatory element binding protein and NLRP3 inflammasome in atherosclerotic lesion development in diabetic pigs. PLoS One. 2013;8(6):e67532. doi: 10.1371/journal.pone.0067532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Im SS, et al. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 2011;13(5):540–9. doi: 10.1016/j.cmet.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mandrup-Poulsen T. The role of interleukin-1 in the pathogenesis of IDDM. Diabetologia. 1996;39(9):1005–29. doi: 10.1007/BF00400649. [DOI] [PubMed] [Google Scholar]
- 68.Maedler K, et al. Glucose-induced beta cell production of IL-1beta contributes to glucotoxicity in human pancreatic islets. J Clin Invest. 2002;110(6):851–60. doi: 10.1172/JCI15318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larsen CM, et al. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32(9):1663–8. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 71.Khosravi A, et al. Gut microbiota promote hematopoiesis to control bacterial infection. Cell Host Microbe. 2014;15(3):374–81. doi: 10.1016/j.chom.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Luo Y, et al. Microbiota from Obese Mice Regulate Hematopoietic Stem Cell Differentiation by Altering the Bone Niche. Cell Metab. 2015;22(5):886–94. doi: 10.1016/j.cmet.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 73.Singer K, et al. Diet-induced obesity promotes myelopoiesis in hematopoietic stem cells. Mol Metab. 2014;3(6):664–75. doi: 10.1016/j.molmet.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 75.Ahmed M, de Winther MP, Van den Bossche J. Epigenetic mechanisms of macrophage activation in type 2 diabetes. Immunobiology. 2016 doi: 10.1016/j.imbio.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 76.Hanson MA, Godfrey KM. Genetics: Epigenetic mechanisms underlying type 2 diabetes mellitus. Nat Rev Endocrinol. 2015;11(5):261–2. doi: 10.1038/nrendo.2015.31. [DOI] [PubMed] [Google Scholar]
- 77.Gallagher KA, et al. Epigenetic changes in bone marrow progenitor cells influence the inflammatory phenotype and alter wound healing in type 2 diabetes. Diabetes. 2015;64(4):1420–30. doi: 10.2337/db14-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moore KJ, Sheedy FJ, Fisher EA. Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol. 2013;13(10):709–21. doi: 10.1038/nri3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van den Berg SM, et al. Diet-induced obesity in mice diminishes hematopoietic stem and progenitor cells in the bone marrow. FASEB J. 2016;30(5):1779–88. doi: 10.1096/fj.201500175. [DOI] [PubMed] [Google Scholar]
- 80.Nathan DM, et al. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes. 2014;63(1):282–90. doi: 10.2337/db13-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lind M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371(21):1972–82. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 82.Hayward RA, et al. Follow-up of Glycemic Control and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2015;373(10):978. doi: 10.1056/NEJMc1508386. [DOI] [PubMed] [Google Scholar]
- 83.Zinman B, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 2015;373(22):2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 84.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005;54(1):1–7. doi: 10.2337/diabetes.54.1.1. [DOI] [PubMed] [Google Scholar]
- 85.Ceriello A, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes. 2008;57(5):1349–54. doi: 10.2337/db08-0063. [DOI] [PubMed] [Google Scholar]
- 86.Keating ST, Plutzky J, El-Osta A. Epigenetic Changes in Diabetes and Cardiovascular Risk. Circ Res. 2016;118(11):1706–22. doi: 10.1161/CIRCRESAHA.116.306819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Azuma K, et al. Repetitive fluctuations in blood glucose enhance monocyte adhesion to the endothelium of rat thoracic aorta. Arterioscler Thromb Vasc Biol. 2006;26(10):2275–80. doi: 10.1161/01.ATV.0000239488.05069.03. [DOI] [PubMed] [Google Scholar]
- 88.Motton DD, et al. Postprandial monocyte activation in response to meals with high and low glycemic loads in overweight women. Am J Clin Nutr. 2007;85(1):60–5. doi: 10.1093/ajcn/85.1.60. [DOI] [PubMed] [Google Scholar]
- 89.Mita T, et al. Swings in blood glucose levels accelerate atherogenesis in apolipoprotein E-deficient mice. Biochem Biophys Res Commun. 2007;358(3):679–85. doi: 10.1016/j.bbrc.2007.04.118. [DOI] [PubMed] [Google Scholar]
- 90.Temelkova-Kurktschiev TS, et al. Postchallenge plasma glucose and glycemic spikes are more strongly associated with atherosclerosis than fasting glucose or HbA1c level. Diabetes Care. 2000;23(12):1830–4. doi: 10.2337/diacare.23.12.1830. [DOI] [PubMed] [Google Scholar]
- 91.Hanefeld M, et al. Risk factors for myocardial infarction and death in newly detected NIDDM: the Diabetes Intervention Study, 11-year follow-up. Diabetologia. 1996;39(12):1577–83. doi: 10.1007/s001250050617. [DOI] [PubMed] [Google Scholar]
- 92.Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. The DECODE study group. European Diabetes Epidemiology Group. Diabetes Epidemiology: Collaborative analysis Of Diagnostic criteria in Europe. Lancet. 1999;354(9179):617–21. [PubMed] [Google Scholar]
- 93.Okada K, et al. Association between blood glucose variability and coronary plaque instability in patients with acute coronary syndromes. Cardiovasc Diabetol. 2015;14:111. doi: 10.1186/s12933-015-0275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teraguchi I, et al. Impact of glucose fluctuation and monocyte subsets on coronary plaque rupture. Nutr Metab Cardiovasc Dis. 2014;24(3):309–14. doi: 10.1016/j.numecd.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 95.Shuto Y, et al. Repetitive Glucose Spikes Accelerate Atherosclerotic Lesion Formation in C57BL/6 Mice. PLoS One. 2015;10(8):e0136840. doi: 10.1371/journal.pone.0136840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cheng SC, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345(6204):1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493(7432):346–55. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 98.Sarrazy V, et al. Disruption of Glut1 in Hematopoietic Stem Cells Prevents Myelopoiesis and Enhanced Glucose Flux in Atheromatous Plaques of ApoE(−/−) Mice. Circ Res. 2016;118(7):1062–77. doi: 10.1161/CIRCRESAHA.115.307599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oburoglu L, et al. Metabolic regulation of hematopoietic stem cell commitment and erythroid differentiation. Curr Opin Hematol. 2016;23(3):198–205. doi: 10.1097/MOH.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 100.Oburoglu L, et al. Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell Stem Cell. 2014;15(2):169–84. doi: 10.1016/j.stem.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 101.Nakada D, Saunders TL, Morrison SJ. Lkb1 regulates cell cycle and energy metabolism in haematopoietic stem cells. Nature. 2010;468(7324):653–8. doi: 10.1038/nature09571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tothova Z, et al. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell. 2007;128(2):325–39. doi: 10.1016/j.cell.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 103.Freemerman AJ, et al. Metabolic reprogramming of macrophages: glucose transporter 1 (GLUT1)-mediated glucose metabolism drives a proinflammatory phenotype. J Biol Chem. 2014;289(11):7884–96. doi: 10.1074/jbc.M113.522037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nishizawa T, et al. Testing the role of myeloid cell glucose flux in inflammation and atherosclerosis. Cell Rep. 2014;7(2):356–65. doi: 10.1016/j.celrep.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berezin A. Metabolic memory phenomenon in diabetes mellitus: Achieving and perspectives. Diabetes Metab Syndr. 2016;10(2 Suppl 1):S176–83. doi: 10.1016/j.dsx.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 107.Giacco F, et al. GLP-1 Cleavage Product Reverses Persistent ROS Generation After Transient Hyperglycemia by Disrupting an ROS-Generating Feedback Loop. Diabetes. 2015;64(9):3273–84. doi: 10.2337/db15-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fadini GP, et al. Circulating Stem Cells Associate With Adiposity and Future Metabolic Deterioration in Healthy Subjects. J Clin Endocrinol Metab. 2015;100(12):4570–8. doi: 10.1210/jc.2015-2867. [DOI] [PubMed] [Google Scholar]
- 109.Fadini GP, et al. Long-term Prediction of Cardiovascular Outcomes by Circulating CD34+ and CD34+CD133+ Stem Cells in Patients With Type 2 Diabetes. Diabetes Care. 2016 doi: 10.2337/dc16-1755. [DOI] [PubMed] [Google Scholar]
- 110.Makino H, et al. Decreased levels of circulating CD34(+) cells are associated with coronary heart disease in Japanese patients with type 2 diabetes. J Diabetes Investig. 2015;6(4):473–8. doi: 10.1111/jdi.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ferraro F, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3(104):104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]