Abstract
Background
Tobacco products containing menthol are widely used by youth. We used ecigarettes to conduct an experimental evaluation of the independent and interactive effects of menthol and nicotine among youth.
Procedures
Pilot chemosensory experiments with fourteen e-cigarette users identified low (barely perceptible, 0.5%) and high (similar to commercial e-liquid, 3.5%) menthol concentrations. Sixty e-cigarette users were randomized to a nicotine concentration (0 mg/ml, 6 mg/ml, 12 mg/ml) and participated in 3 laboratory sessions. During each session, they received their assigned nicotine concentration, along with one of three menthol concentrations in random counterbalanced order across sessions (0, 0.5%, 3.5%), and participated in three fixed-dose, and an ad-lib, puffing period. Urinary menthol glucuronide and salivary nicotine levels validated menthol and nicotine exposure. We examined changes in e-cigarette liking/wanting and taste, coolness, stimulant effects, nicotine withdrawal and ad-lib use.
Results
Overall, the high concentration of menthol (3.5%) significantly increased e-cigarette liking/wanting relative to no menthol (p<0.001); there was marginal evidence of nicotine* menthol interactions (p=0.06), with an increase in liking/wanting when 3.5% menthol was combined with 12 mg/ml nicotine, but not 6 mg/ml nicotine. Importantly, both 0.5% and 3.5% menthol concentrations significantly improved taste and increased coolness. We did not observe nicotine or menthol-related changes in stimulant effects, nicotine withdrawal symptoms or ad-lib use.
Conclusions
Menthol, even at very low doses, alters the appeal of e-cigarettes among youth. Further, menthol enhances positive rewarding effects of high nicotine-containing e-cigarettes among youth.
Keywords: Youth, Menthol, Nicotine, Electronic cigarettes
1. INTRODUCTION
Use of tobacco products remains a significant health concern worldwide. Regulatory measures need to focus on reducing the appeal of tobacco products (WHO-FCTC). Flavors may enhance the appeal of tobacco products, and facilitate progression from initiation to nicotine dependence (Samet et al., 2016). Scientific evidence about the influence of flavors on the appeal of tobacco products is critically needed to support regulatory efforts.
Menthol-containing tobacco products are very popular, especially among young users (Rock et al., 2010). Menthol is also a common flavor used in e-liquids (Yingst et al., 2017). Recent evidence suggests that while cigarette smoking rates are going down, use of menthol cigarettes is increasing, especially among young smokers (Villanti et al., 2016), suggesting that the presence of menthol cigarettes may be impeding decreases in cigarette use (Giovino et al., 2015).
Menthol is an organic compound that not only has a characterizing aroma but also has pharmacological effects which may interact with nicotine/tobacco. For example, menthol produces analgesic and cooling sensations via activation of the TRPM8 receptors (Hatem et al., 2006; Wasner et al., 2004), and alters irritant responses via the TRPA1 receptor, which also mediates irritant responses to nicotine (Fan et al., 2016; Karashima et al., 2007; Xiao et al., 2008). Menthol also modulates nicotinic receptor (nAChR) function (Hans et al., 2012; Ton et al., 2015). Up-regulation of nAChRs has been observed in mice following chronic exposure to menthol concentrations comparable to those in cigarette smoke (Henderson et al., 2016) and in human menthol cigarette smokers (Brody et al., 2013). Menthol may also slow nicotine metabolism (Alsharari et al., 2015; Benowitz et al., 2004; Fagan et al., 2016).
A report from the FDA’s Tobacco Product Scientific Advisory Committee (Samet et al., 2011) which concluded that menthol cigarette smoking was a risk factor for development of nicotine dependence, was challenged by tobacco industry representatives (Heck et al., 2011) who argued that there was no scientific evidence to support such causal relationships. The clinical evidence on the role of menthol in nicotine addiction is limited, and at times inconclusive. For example, while some studies suggest that menthol cigarette smokers may be more dependent and find it harder to quit smoking (Ahijevych and Garrett, 2010; Fagan et al., 2010; Foulds et al., 2010) this is not consistently observed (Hyland et al., 2002; Muscat et al., 2009).
We aimed to conduct a clinical experiment to examine if menthol has independent rewarding effects, and whether it interactively alters the reward from nicotine. We used ecigarettes which provide an ideal system for examining the inhaled effects of menthol. To achieve adequate increases in nicotine levels we adapted an e-cigarette exposure paradigm (Vansickel et al., 2010). Since menthol has been shown to disproportionately influence use by younger smokers, we conducted this study in older adolescents. Because even tobacco products that are not labeled as being mentholated may contain small concentrations of menthol (Giovino et al., 2004; Reid, 1994), we examined concentrations of menthol that were either barely perceptible or similar to levels in commercial menthol e-liquids. We hypothesized that menthol would have independent, concentration-dependent effects on liking/wanting ecigarettes, and would also interact with nicotine to alter liking/wanting e-cigarettes. Secondarily, we examined whether menthol independently or interactively (with nicotine) altered stimulant effects, nicotine withdrawal, and taste and coolness. Finally, we explored whether menthol altered e-cigarette value as well as e-cigarette use during an ad-libitum period.
2. METHODS
2.1: General Procedures
Experimental procedures were approved by the Yale School of Medicine Human Investigations Committee and followed National Advisory Council on Drug Abuse guidelines for substance use research in children and adolescents, and administration of drugs for research purposes (NACDAa, 2006; NACDAb, 2012)
Participants were recruited from local high schools, colleges, and through online advertisements and flyers. Participants had to be between 16–20 years of age, have used ecigarettes with nicotine for the past year (at least 10 days in the past month), with baseline urinary cotinine levels of > 500 ng/ml, not currently trying to quit smoking or e-cigarette use, not have any physical or psychological conditions which would increase risk of participation, and report having tried menthol-flavored tobacco products (58 of the 981 participants screened had never tried menthol products). Participants who were > 18 years old provided informed consent. Parental permission and minor assent was obtained for those < 18 years old. Participants received a physical examination (by an APRN) and a clinical evaluation (by a licensed clinical psychologist) to rule out concerning physical or psychological conditions, and substance use disorders (other than nicotine use disorder). Eligible youth participated in laboratory sessions at the John B. Pierce Laboratory in a temperature controlled and ventilated room (with air exchange 11 times/hour). Participants were asked to abstain from cigarette (confirmed by breath CO levels <10 ppm, Micro Direct, Inc., Lewiston, ME) and e-cigarette use for at least 10 hours before each session. At the end of the study participants met with a licensed clinical psychologist who encouraged them to rethink their tobacco use and explore quitting options.
2.2: Pilot Chemosensory Experiment
Fourteen participants participated in one session (See Table 1 for demographics). We™ used V2 Cigs (VMR Products LLC) with refillable cartridges which were filled with AmericaneLiquidStore® Tobacco Flavor e-liquids containing 70/30 propylene glycol (PG)/vegetable glycerin (VG) and varying concentrations of L-menthol (obtained from SigmaAldrich, USP). Two commercial American Liquid Store® menthol e-liquid flavors were also tested and concentrations of menthol in these e-liquids were determined using liquid chromatography/mass spectrometry (LC/MS/MS).
Table 1.
Demographic information participants in Pilot and Main Study
| Variable | Pilot Study (N=10) |
Main Study (N=60) |
||
|---|---|---|---|---|
| 0 mg/ml Nicotine (N=21) |
6 mg/ml Nicotine (N=22) |
12 mg/ml Nicotine (N=17) |
||
| Age, M (SD) | 18.1 (0.8) | 18.8 (0.68) | 18.8 (0.81) | 18.9 (0.83) |
| Sex, N (%) | 10 (47.6%) | 11 (50.0%) | 8 (47.1%) | |
| Male | 7 (70%) | |||
| Race/Ethnicity, N (%) | ||||
| White | 9 (90.0%) | 16 (76.2%) | 19 (86.4%) | 14 (82.4%) |
| Asian | 0 (0.0%) | 0 (0.0%) | 0 (0.00%) | 0 (0.00%) |
| Hispanic | 1 (10.0%) | 0 (0.0%) | 2 (9.09%) | 2 (11.8%) |
| Black | 0 (0.0%) | 1 (4.76%) | 0 (0.00%) | 0 (0.00%) |
| Biracial/Other | 0 (0.0%) | 4 (19.0%) | 1 (4.55%) | 1 (5.88%) |
| E-cigarette Use | ||||
| Days in past month, M (SD) | 17.4 (3.3) | 24.7 (7.4) | 22.8 (9.0) | 25.6 (5.1) |
| Menthol in e-cigarette, N (%) | 4 (40%) | 13 (61.9%) | 11 (50.0%) | 9 (52.9%) |
| Cigarette Use | ||||
| Smokers, N, (%) | 10 (100%) | 15 (71.4%) | 18 (81.8%) | 15 (88.2%) |
| Menthol cigarette smokers N (%) | 5 (50%) | 11 (52.4%) | 10 (45.5%) | 9 (52.9%) |
| Intake cotinine levels (ng/ml); M (SE) | 960.2 (76.2) | 1202 (87) | 769 (61.8) | 847 (65.1) |
Cartridges were filled with 500 μL of e-liquid and were used only in a single session. During each session, presentation of a non-menthol control trial was followed by the presentation of 5 increasing concentrations of menthol (0.5%, 1.5%, 2.5%, 3.5%, 5%, a commercial menthol flavor, and a commercial mint flavor, with 5 min breaks between trials to reduce sensitization/desensitization of menthol’s sensory effects (Cliff and Green, 1994). At the end of each trial, participants rated coolness and overall flavor intensity.
2.3: Main Study Procedures
Sixty participants who reported e-cigarette use, with or without concurrent cigarette use, (31 females, 29 males) participated in 3 laboratory sessions separated by at least 48 hours (see Table 1 for demographics).
Using randomization stratified by gender, we first assigned each participant to a single nicotine concentration [0 mg/ml, low (6 mg/ml), high (12 mg/ml)]. Combined with their assigned nicotine concentration), participants received, all three menthol concentrations [no menthol (NM)=0%, low menthol (LM)=0.5%, high menthol (HM)=3.5%], each during a separate session, in counterbalanced order. Nicotine concentrations were chosen to represent those commonly used by youth (Morean et al., 2016). The research assistants and the participants were blind to the treatment condition.
V2 Cigs™ (VMR Products LLC) with refillable tanks, and e-liquids purchased from Pace Engineering Concepts (Delafield, WI 53018), containing 70/30 PG/VG with the required combinations of nicotine and menthol (as specified above and verified using LC/MS/MS), were used. On the day of each session, the tanks were filled with 750 μL of e-liquid (delivered from the YNHH Investigational-Pharmacy). Prior to the first session, participants were trained on the puffing schedule (using tanks filled with only 70/30 PG/VG) and on completion of assessments.
Each session consisted of a half hour fixed-dose period followed by a half-hour ad-lib period. The fixed-dose period included 3 fixed puffing bouts, each separated by 10 mins. In each puffing bout, participants were asked to take 10 puffs with a 30 sec inter-puff interval [adapted from (Vansickel et al., 2010)]. Puff behavior and inter-puff interval were monitored by a research assistant who was in the room with a timer during the fixed-dose period, but not during the ad-lib period. During the ad-lib period participants were told to freely puff from the ecigarette and behavior was video-taped.
Saliva nicotine samples were obtained at baseline, after each fixed dose bout, and at the end of the ad-lib period. Urine samples for determination of menthol glucuronide (MeG) levels and creatinine were obtained at baseline and at the end of each session.
2.3: Assessments
E-cigarette Liking/Wanting, Stimulant effects and Nicotine Withdrawal
A modified version of the Drug Effects Questionnaire (Morean et al., 2013; Soria et al., 1996) was used in which participants rated acute responses to the e-cigarette on a 0 to 100 mm scale, from “not at all” to “extremely.” Following each fixed dose bout, we assessed E-cigarette Liking/Wanting (average of “I feel good e-cigarette effects”, “I want more of that e-cigarette I received”, “I feel the ecigarette strength” and “I like the e-cigarette effect”). At baseline and following each fixed-dose bout, we also assessed Stimulant effects (average of “I feel energized” and “I feel high”), and Nicotine Withdrawal (average of “I feel sleepy”, “I feel angry”, “I feel irritable”, “I am having difficulty concentrating”, “I feel restless” and “I feel hungry”).
E-cigarette Taste
Following each fixed-dose bout, we assessed how much participants liked/disliked the taste of the e-cigarette using the Labeled Hedonic Scale (LHS) (Lim et al., 2009), a category ratio scale that ranges from −100 (most disliked) to 100 (most liked).
E-cigarette Overall Intensity and Coolness
Participants rated overall intensity (pilot study only) and coolness “right now” on the Labeled Magnitude Scale [gLMS; (Bartoshuk et al., 2003; Green et al., 1993)], following each fixed-dose bout. The gLMS is a category ratio scale with 7 semantic labels: “no sensation”, “barely detectable”, “weak”, “moderate”, “strong”, “very strong”, and “strongest imaginable”, positioned quasi-logarithmically per their empirically determined semantic magnitudes, with responses coded on a 0–100 scale.
E-cigarette Value
At the end of the fixed-dose period, participants were asked to make discrete hypothetical choices between 10-puffs of the e-cigarette they had just used or a series of 44 monetary values [$0.25–$15.06; adapted Multiple Choice Procedure; MCP (Griffiths et al., 1993)]. The primary outcome was the minimum monetary value at which money was chosen over the e-cigarette puffs which provides a contingency-based estimate of e-cigarette value.
Puffing Behavior during Ad-Lib Period
Two research assistants independently coded the video-tapes to determine number of puffs taken, which was then averaged across raters. Interrater reliability was high (ICC=0.85).
Biochemical Analyses (for Main Study only)
Urine MeG was determined by LC/MS/MS employing a deuterated internal standard (Benowitz et al., 2010) and urine creatinine was determined using the Jaffe reaction to correct for urine dilution.
For salivary nicotine samples, participants rinsed their mouths with water, chewed on a sterile dental cotton roll (Salivette; Sarstedt AG and Co, Nümbrecht Germany) for 30 seconds and deposited it into a plastic tube. These tubes were centrifuged to extract the saliva and nicotine levels were determined by LC/MS/MS employing a deuterated internal standard (Sofuoglu et al., 2012) and pH was also determined.
2.4: Data analyses
After preliminary review, if needed, the raw data were log- or square-root-transformed to remove skewness.
For the chemosensory pilot study, the primary outcomes were log-transformed gLMS ratings of overall intensity and coolness. We used a repeated measures analysis of variance with Menthol (6 menthol concentrations plus 2 commercial menthol e-liquids) as the independent variable and Sensation Intensity (Overall intensity and Coolness) as the dependent variable. Post-hoc (Tukey HSD) tests investigated sources of significant interactions between the dependent variables and menthol concentrations.
For the main study, the primary outcome was fixed-dose period changes in E-cigarette Liking/Wanting. Secondary outcomes were fixed-dose period changes in e-cigarette taste, coolness, stimulant and nicotine withdrawal effects. Exploratory outcomes were MCP scores and number of puffs during the ad-lib period. We used a separate linear mixed-effects regression model for each outcome. We started with models that included categorical fixed effects for nicotine, menthol concentration, session sequence (1st, 2nd, or 3rd), time of assessment, two-way interactions of these four variables, three-way interactions of menthol*nicotine*lab and menthol*nicotine*time, and the stratification variable (gender). Cigarette use was used as a covariate. For MCP time within session was not used. The models used a random effect for each participant to model the correlations among observations obtained from the same participant, and a repeated lab-within-subject effect to model the correlations between repeated measurements. We selected the correlation structure according to BIC from the following structures: unstructured, AR(1), compound symmetry and Toeplitz. We then performed backward elimination to remove interactions that were not significant at the 0.05 level (nicotine*menthol interaction was retained to test the primary hypothesis).
We added order of menthol dose to all our models (as a 6-level categorical variable) and did not observed any significant order effects (all p’s > 0.15). For parsimony, we did not to retain this variable in our final models.
Urine MeG levels (corrected for creatinine) were examined using similar regression models. Observations with creatinine values less than 20 (23 out of 356) were excluded to correct for urine dilution. For saliva nicotine levels, the 0 mg nicotine concentration (with 84%=102/122 of values below detection limits) was excluded. The average nicotine levels obtained after puff bouts with the other doses was highly skewed and not amenable to transformations, so we used a nonparametric method for repeated measures based on the ranks of the measurements (Brunner et al., 2002); nicotine did not alter salivary pH levels.
We used a significance level of α=0.05 for the primary outcome (liking/wanting), a Bonferroni corrected α= 0.0125 for the secondary outcomes (taste, stimulant effects, nicotine withdrawal, coolness), and an uncorrected α=0.05 for the exploratory outcomes and the biochemical measures.
3. RESULTS
3.1: Pilot Chemosensory Experiment
We observed a significant interaction between Menthol Concentration and Sensation Intensity (overall intensity, coolness; [F(7, 91)=5.00), p<0.0001]); there was a main effect of menthol on both overall intensity [F(7, 91)=27.4), p<0.0001] and coolness [F(7, 91)=42.9, p<0.0001]. Posthoc Tukey HSD analyses suggested that coolness increased in response to incremental increases in menthol concentration from 0% to 0.5%,1.5% and 2.5% (p’s < 0.001). The coolness of 0.5% menthol was rated as “weak” on the gLMS, therefore, it was chosen as the low menthol concentration. Coolness did not increase significantly more among the 2.5%, 3.5% and 5% concentrations of menthol, and also did not differ from coolness ratings observed with the commercial menthol e-liquids (all p’s >0.05). Additionally, because analyses indicated that the commercial e-liquids had menthol concentrations of 2.9% and 3.1%, we chose 3.5% as the high menthol concentration.
3.2: Main study
3.2.a: Primary Outcome
E-cigarette Liking/Wanting
As summarized in Figure 1, we observed a significant main effect of menthol [(F(2,109)=5.46, p=0.006] and a trend for a nicotine*menthol interaction [(F(4,109)=2.33, p=0.06)]. Pairwise comparisons showed that, overall, liking/wanting was more for HM (43.36±3.05) than NM [34.28±3.05; t(108)=3.3; p=0.001; effect size estimate d=0.37], while LM (38.63±3.03) was not significantly different from either HM [t(109)= −1.73; p=0.09; d=0.19] or NM [t(109)=1.59; p=0.11; d=0.23]. Post hoc comparisons to explore the trend in the nicotine*menthol interaction indicated that menthol altered liking/wanting at 12 mg nicotine [F(2,108)=5.39, p=0.006] and 0 mg nicotine [F(2,110)=3.48, p=0.03], but not at 6 mg nicotine [F(2,108)=0.90, p=0.41]. At 12 mg nicotine, liking/wanting was significantly higher in the presence of HM (56.04±5.67) when compared with LM [40.61±5.67, t(108)=3.06; p=0.003; d=0.64] and NM [43.05±5.68; t(108)=2.56; p=0.01; d=0.50]; there were no significant differences between LM and NM [(t(108)= −0.48: p=0.63; d=0.15]. Further, at 0 mg nicotine, liking/wanting in the presence of NM (27.27±5.18) was significantly lower than LM [36.83±5.10; t(110)= −2.06; p=0.04; d=0.48] and HM [38.77±5.19; t(110)= −2.46; p=0.02; d=0.58]; no differences were observed between HM and LM [t(110)=0.42; p=0.68; d=0.10]. Finally, at 6 mg, no significant differences were observed between NM (32.51+4.98) and LM [38.46+4.98; t(108)= −1.34; p=0.18; d=0.26] or HM [35.28+4.97; t(108)= −0.62; p=0.54; d=0.11] or between HM and LM [t(108)= −0.72; p=0.48; d=0.21]. Since 70% (42/60) of participants reported current or past use of menthol products, we explored the influence of being a menthol user but did not find any influence of this variable on the results (p for interaction=0.68, p for main effect=0.26).
Figure 1. Mean “Liking/Wanting” E-cigarette during Fixed-Puffing Period by Nicotine (0, 6 mg/ml, 12 mg/ml) and Menthol Concentration (0, 0.5%, 3.5%).
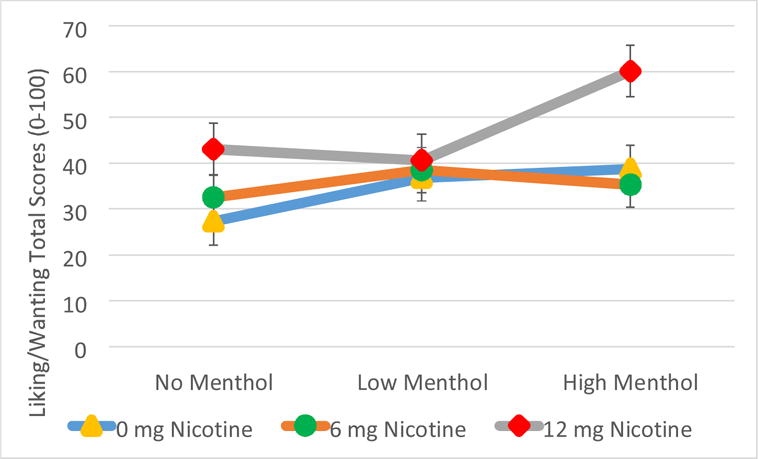
Note: Main effect of menthol [(F(2,109)=5.46, p=0.006] and a nicotine*menthol interaction [(F(4,109)=2.33, p=0.06)]. The circles (vertical lines through them) represent LS-means (± SE).
3.2.b: Secondary Outcomes
E-cigarette Taste
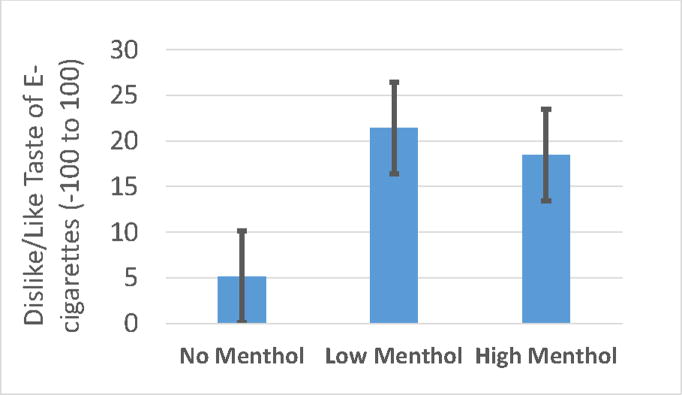
We observed a significant main effect of menthol [(F(2,111)=5.39, p=0.006]; compared to NM (5.07+4.11) both HM (18.52+4.11, p=0.013) and LM (21.40+4.12, p<0.001) improved taste (see Figure 2). With the Bonferroni correction ( =0.0125) the nicotine*menthol interaction [(F(4,111)=2.94, p=0.02)] was a trend; post hoc comparisons indicate that menthol had a significant effect on taste at 12 mg nicotine [F(2,111)=6.21, p=0.003 for 12 mg; F(2,111)=3.22, p=0.04 for 0 mg; F(2,110)=1.17, p=0.31 for 6 mg)]. At 12 mg nicotine, taste was improved in the presence of LM (22.85±7.83; trend at p=0.02) and HM (33.62±7.59; p<0.001) when compared with NM (−0.28±7.68).
Figure 2. Mean “Taste” of E-cigarette in response to No (0%), Low (0.5%) and High (3.5%) menthol.
Note: Main effect of menthol [(F(2,111)=5.39, p=0.006]. The bars represent LS-means (± SE).
Stimulant effects, Nicotine Withdrawal
We did not observe any main effects, or interactions, of nicotine or menthol. We did observe a significant main effect of time on nicotine withdrawal (F(3,170)=19.54, p<.0001); post hoc comparisons indicate that withdrawal improved from baseline (baseline: 17.19+1.45; puffing period 1: 12.76+1.33; puffing period 2: 12.03+1.32; puffing period 3: 11.71+1.32; compared to baseline all p’s <.0001).
E-cigarette Coolness (square-root transformed)
We observed a significant main effect of menthol [F(2,112)=208.14, p<.0001] and a significant menthol*time interaction (F(4,268)=4.60, p=0.001), but no nicotine main effect or a nicotine*menthol interaction. Overall, coolness increased with increasing concentrations of menthol (Figure 3; p<.0001 for all pairwise comparisons). The effect of HM on coolness was more pronounced during the first puffing period.
Figure 3. Mean “Coolness” from E-cigarette in response to No (0%), Low (0.5%) and High (3.5%) menthol.
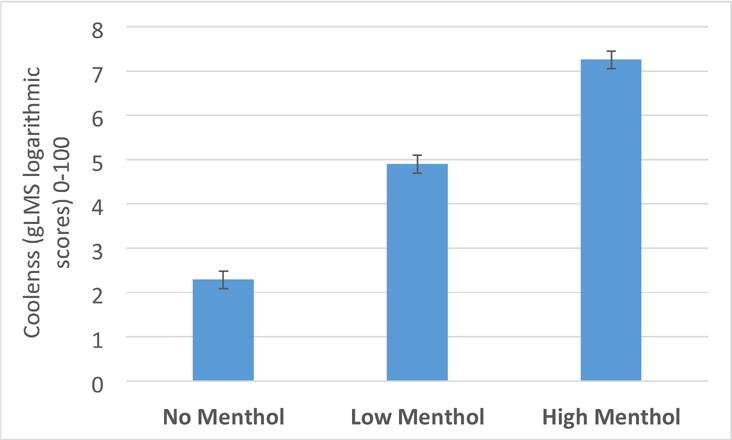
Note: Main effect of menthol [F(2,112)=208.14, p<.0001]. The bars represent LS-means (± SE) of sqrttransformed coolness.
3.2.c. Exploratory Outcomes
E-cigarette Value (MCP; log-transformed)
We did not observe any main effects of nicotine (F(2,54)=0.85, p=0.43) or menthol (F(2,101)=0.61, p=0.55), or nicotine*menthol interactions (F(4,101)=0.45, p=0.78).
Number of puffs taken during Ad-Lib Period (square-root transformed)
We did not observe any main effects of nicotine (F(2,56)=2.01, p=0.14) or menthol (F(2,106)=2.23, p=0.11), or menthol*nicotine interactions (F(4,106)=0.71, p=0.59).
3.2.d.: Biochemical Measures
Urine MeG (log-transformed)
MeG levels were low at baseline and then showed menthol concentration-dependent increases (Figure 4). We observed a significant main effect of menthol (F(2,92.4)=31.16, p<.0001) and a significant menthol*time interaction (F(2,165)=59.32, p<.0001). MeG levels did not change following NM condition (p=0.30), but significantly increased following LM and HM (p<.0001).
Figure 4.
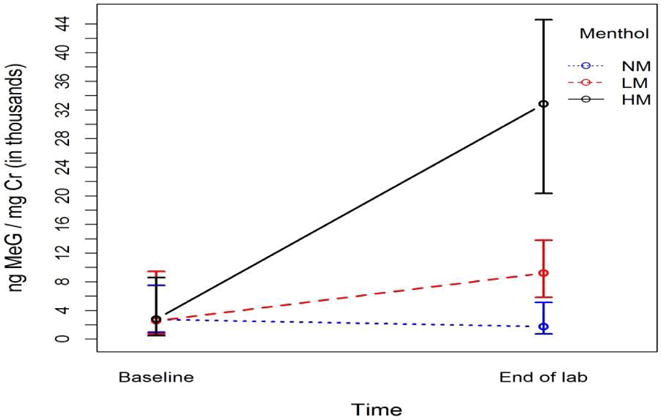
Median (IQR) urine menthol glucuronide (MeG) levels corrected for creatinine (Cr) at baseline and in pooled urine collected during and upon completion of the laboratory session, by menthol concentration (0, LM=0.5%, HM=3.5%).
Salivary Nicotine
Nicotine levels were low at baseline and evidenced nicotine-concentrationdependent increases (Figure 5). We observed a significant main effect of nicotine concentration (Anova Type Statistic ATS(1)=22.49, p<.0001) and a significant nicotine*time interaction (ATS(1.39)=8.44, p=0.001). While there were no baseline differences between 6 mg and 12 mg nicotine concentrations (p=0.12), significantly higher values were observed in the 12 mg group) at the other two time points (p<0.0001 for post-bout; p=0.001 for post ad-lib).
Figure 5.
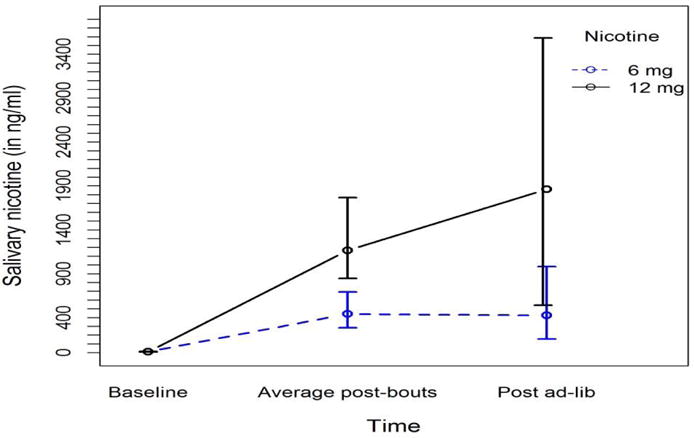
Median (IQR) salivary nicotine (ng/mL) at baseline and following the 6 and 12 mg/ml nicotine concentrations at the end of the fixed-bout period and at the end of the adlib period (no increases were observed in the 0 mg/ml nicotine condition; data not shown)
4. DISCUSSION
We examined the independent and interactive effects of inhaled menthol and nicotine, on rewarding responses among older adolescents. On our primary outcome of liking/wanting, we found that the high concentration of menthol (similar to the concentration in commercial menthol e-liquids) independently improved e-cigarette liking/wanting relative to no menthol. We also found marginal evidence of menthol interacting with nicotine (p=0.06) to improve ecigarette liking/wanting, particularly for the combination of high concentrations of menthol (3.5%) and nicotine (12 mg/ml). Our findings are strengthened by using a global measure of e-cigarette liking/wanting which assesses positive effects and liking, as well as wanting more. Prior research in humans has focused primarily on the effects of menthol on nicotine harshness and irritation from nicotine (Dessirier et al., 2001) rather than rewarding effects, and only one study has previously tested the menthol and nicotine through an inhaled route (Rosbrook and Green, 2016). The results of our study in youth suggest that high concentrations of menthol increase the rewarding effects of higher concentrations of nicotine, which we hypothesize could promote the development of nicotine dependence. Consistent with our findings, pre-clinical research has demonstrated that menthol enhances nicotine-induced reward in a conditioned place-preference test in mice (Henderson et al., 2017), perhaps via nicotine-induced upregulation of nicotinic receptors on midbrain dopamine neurons, and can also become a conditioned cue that reinforces nicotine use (Wang et al., 2014).
Menthol produced concentration-dependent improvements in the taste of e-cigarettes and cooling sensations, with improvements observed even at the low, barely perceptible concentration of menthol. Recent evidence (Paschke et al., 2017) suggests that activation of the menthol-specific receptor, TRPM8, is observed at levels of menthol that were far below the “typical” levels present in mentholated tobacco products. Taken together, these findings suggest that even a low concentration of menthol may have sufficient pharmacological effects to increase appeal of tobacco products.
There was marginal evidence of an interaction between menthol and nicotine on ecigarette liking/wanting (p=0.06). Liking/Wanting for the 12 mg nicotine concentration was significantly increased in the presence of high concentration menthol. Similar effects were not observed for the 6 mg nicotine concentration. Interestingly, menthol-related changes in liking/wanting were accompanied by improvements in taste of the e-cigarettes. Higher concentrations of nicotine are likely harsher, and the cooling effects of menthol may be needed to improve palatability and pleasure from high concentration nicotine. For example, Rosbrook and Green (2016) observed that similar high concentrations of menthol reduced irritation from a 24 mg concentration of nicotine. Interestingly, recent evidence from Perkins and colleagues (Perkins et al., 2017) also observed that menthol cigarette smokers had discrimination thresholds at higher nicotine concentrations, relative to non-menthol smokers, suggesting that menthol smokers may need higher nicotine concentrations for experience reward.
Menthol has also been shown to increase self-administration of nicotine (Biswas et al., 2016). However, we did not observe any menthol or nicotine-related differences in puffing behavior during the ad-lib period. This lack of effect may be related to the fact that we initiated our ad-lib period right after the end of the fixed-dose period when participants may have been satiated. Moreover, the ad-lib period was only half-hour in duration which may not have been sufficient to detect differences in use behaviors. Future studies need to be designed appropriately to study self-administration behaviors.
Our evidence also suggests that menthol’s effects are not related to changes in feeling stimulated or in nicotine withdrawal. Interestingly, although we observed concentrationdependent increases in salivary nicotine levels over the fixed-dose periods, we did not observe any nicotine effects on withdrawal or stimulation. Participants had high nicotine withdrawal at baseline which improved significantly during the fixed-dose period, suggesting that perhaps the behavior of using e-cigarettes alone was sufficient to reduce withdrawal, at least in this setting.
Finally, we did not observe menthol or nicotine-related changes in the value of the ecigarette, as measured using the MCP. The MCP has been used as a demand task to investigate reinforcement from many drugs in humans (Griffiths et al., 1993). However, determining the appropriate unit of measurement for each drug is critical in such demand tasks. The measurement of e-cigarette use behaviors is complex and unresolved, and other investigators have raised concerns with the use of number of puffs to quantify e-cigarette use in the development of an e-cigarette purchase task (Cassidy et al., 2017). Future studies need to develop appropriate e-cigarette quantification measures for use in demand tasks.
This study has some limitations that should be considered. While the pilot study included some 16–17 year old adolescents, we were unable to include younger adolescents in the main study due to the passing of a CT state law that disallowed the administration of ecigarettes to minors. However, since the pilot chemosensory experiment did not find differences in results between younger and older adolescents, we feel confident that our findings will generalize to a younger age group. We also cannot draw conclusions about how menthol may influence initiation of tobacco use behaviors since we studied regular e-cigarette users; exposure studies in younger non-users would be difficult to conduct due to ethical considerations. However, the adolescents in our study were not heavy users, and had only been using tobacco products for a few years (2.9 years), and therefore we can conclude that tobacco use behaviors among these less dependent users is influenced by menthol. Further, we used e-cigarettes since they could be easily manipulated to conduct concentration-ranging inhalation studies. We anticipate that the cooling and taste enhancing effects of menthol may be even more important to the appeal of combustible tobacco products, like cigarettes and cigars, that are inhaled but are harsher than e-cigarettes; however, parallel experiments using combustible products would strengthen this conclusion. Moreover, while we used structured instructions to ensure the timing and duration of puffs during the fixed-puff periods, we did not use a topography machine and therefore cannot rule out variations in e-cigarette inhalation patterns. Finally, as youth also prefer sweet flavors, the methods employed in this research should be extended to evaluate the effects of sweet flavors on appeal and reward of nicotine.
In summary, we provide human behavioral evidence that inclusion of menthol, even at very low concentrations, can increase the appeal of e-cigarettes among youth. Increased appeal could promote continued use, and transitions to higher, more addicting levels of nicotine, particularly in combination with higher concentrations of menthol comparable to those found in commercial e-liquids. Thus, inclusion of menthol alters appeal and reward from e-cigarettes among youth. Regulations to reduce appeal of e-cigarettes by removing menthol should be strongly considered.
Highlights.
Tobacco products containing menthol appeal to youth
We examined if menthol alters nicotine reward among youth
Menthol, even at low doses, independently enhanced liking/wanting for e-cigarettes
Menthol enhanced positive rewarding effects of high nicotine-containing e-cigarettes
Acknowledgments
Funding Source: This research reported in this publication was supported by NIH grants P50DA036151 (Yale TCORS) and the FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors: Dr.’s Krishnan-Sarin designed the study and interpreted the results; Dr’s Green and O’Malley were involved in the design of the study and interpretation of the results; Dr.’s Kong, Cavallo and Jatlow were involved in the design and conduct of the experiments, Dr.’s Gueorguieva and Buta were involved in the design and analyses. All authors were involved in the writing and review of the manuscript.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
Conflict of Interest: The authors have no conflicts of interest relevant to this article to disclose. Although not related to the current research study, Dr. Krishnan-Sarin reports the following: receiving donated research study medications from Astra Zeneca, Pfizer. Although not related to the current research study, Dr. O’Malley reports the following: donated research study medications from AstraZeneca, Pfizer; consultancy or advisory board membership at Alkermes, Amygdala, Cerecor, Opiant; membership in the American Society of Clinical Pharmacology Alcohol Clinical Trials Initiative supported by Ethypharm, Lilly, Lundbeck, Otsuka, Pfizer, Arbor Pharmaceuticals, Indivior.
Contributor Information
Suchitra Krishnan-Sarin, Department of Psychiatry, Yale University School of Medicine
Barry G. Green, The John B. Pierce Laboratory and Department of Surgery, Yale University School of Medicine
Grace Kong, Department of Psychiatry, Yale University School of Medicine
Dana A. Cavallo, Department of Psychiatry, Yale University School of Medicine
Peter Jatlow, Department of Laboratory Medicine, Yale University School of Medicine
Ralitza Gueorguieva, Department of Biostatistics, Yale University School of Public Health
Eugenia Buta, Department of Biostatistics, Yale University School of Public Health
Stephanie S. O’Malley, Department of Psychiatry, Yale University School of Medicine
References
- Ahijevych K, Garrett BE. The role of menthol in cigarettes as a reinforcer of smoking behavior. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2010;12(Suppl 2):S110–116. doi: 10.1093/ntr/ntq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, Zhu AZ, Tyndale RF, Kabbani N, Damaj MI. Effects of Menthol on Nicotine Pharmacokinetic, Pharmacology and Dependence in Mice. PloS one. 2015;10:e0137070. doi: 10.1371/journal.pone.0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ. Labeled scales (e.g., category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Quality & Preference. 2003;14:125–138. [Google Scholar]
- Benowitz NL, Dains KM, Dempsey D, Havel C, Wilson M, Jacob P., 3rd Urine menthol as a biomarker of mentholated cigarette smoking. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:3013–3019. doi: 10.1158/1055-9965.EPI-10-0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Herrera B, Jacob P., 3rd Mentholated cigarette smoking inhibits nicotine metabolism. The Journal of pharmacology and experimental therapeutics. 2004;310:1208–1215. doi: 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- Biswas L, Harrison E, Gong Y, Avusula R, Lee J, Zhang M, Rousselle T, Lage J, Liu X. Enhancing effect of menthol on nicotine self-administration in rats. Psychopharmacology. 2016;233:3417–3427. doi: 10.1007/s00213-016-4391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Mukhin AG, La Charite J, Ta K, Farahi J, Sugar CA, Mamoun MS, Vellios E, Archie M, Kozman M, Phuong J, Arlorio F, Mandelkern MA. Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. The international journal of neuropsychopharmacology. 2013;16:957–966. doi: 10.1017/S1461145712001022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner E, Domhof S, Langer F. Nonparametric Analysis of Longitudinal Data in Factorial Experiments. Wiley; New York: 2002. [Google Scholar]
- Cassidy RN, Tidey JW, Colby SM, Long V, Higgins ST. Initial development of an e-cigaertte purchase task: A mixed methods study. Tobacco Regulatory Science. 2017;3:139–150. doi: 10.18001/TRS.3.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff MA, Green BG. Sensory irritation and coolness produced by menthol: evidence for selective desensitization of irritation. Physiology & behavior. 1994;56:1021–1029. doi: 10.1016/0031-9384(94)90338-7. [DOI] [PubMed] [Google Scholar]
- Dessirier JM, O’Mahony M, Carstens E. Oral irritant properties of menthol: sensitizing and desensitizing effects of repeated application and cross-desensitization to nicotine. Physiology & behavior. 2001;73:25–36. doi: 10.1016/s0031-9384(01)00431-0. [DOI] [PubMed] [Google Scholar]
- Fagan P, Moolchan ET, Hart A, Jr, Rose A, Lawrence D, Shavers VL, Gibson JT. Nicotine dependence and quitting behaviors among menthol and non-menthol smokers with similar consumptive patterns. Addiction (Abingdon, England) 2010;105(Suppl 1):55–74. doi: 10.1111/j.1360-0443.2010.03190.x. [DOI] [PubMed] [Google Scholar]
- Fagan P, Pokhrel P, Herzog TA, Pagano IS, Franke AA, Clanton MS, Alexander LA, Trinidad DR, Sakuma KL, Johnson CA, Moolchan ET. Nicotine Metabolism in Young Adult Daily Menthol and Nonmenthol Smokers. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2016;18:437–446. doi: 10.1093/ntr/ntv109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Balakrishna S, Jabba SV, Bonner PE, Taylor SR, Picciotto MR, Jordt SE. Menthol decreases oral nicotine aversion in C57BL/6 mice through a TRPM8-dependent mechanism. Tobacco control. 2016;25:ii50–ii54. doi: 10.1136/tobaccocontrol-2016-053209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Hooper MW, Pletcher MJ, Okuyemi KS. Do smokers of menthol cigarettes find it harder to quit smoking? Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2010;12(Suppl 2):S102–109. doi: 10.1093/ntr/ntq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovino GA, Sidney S, Gfroerer JC, O’Malley PM, Allen JA, Richter PA, Cummings KM. Epidemiology of menthol cigarette use. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2004;6(Suppl 1):S67–81. doi: 10.1080/14622203710001649696. [DOI] [PubMed] [Google Scholar]
- Giovino GA, Villanti AC, Mowery PD, Sevilimedu V, Niaura RS, Vallone DM, Abrams DB. Differential trends in cigarette smoking in the USA: is menthol slowing progress? Tobacco control. 2015;24:28–37. doi: 10.1136/tobaccocontrol-2013-051159. [DOI] [PubMed] [Google Scholar]
- Green BG, Shaffer GS, Gilmore MM. Derivation and Evaluation of a Semantic Scale of Oral Sensation Magnitude with Apparent Ratio Properties. Chemical senses. 1993;18:683–702. [Google Scholar]
- Griffiths RR, Troisi JR, Silverman K, Mumford GK. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behavioural pharmacology. 1993;4:3–13. [PubMed] [Google Scholar]
- Hans M, Wilhelm M, Swandulla D. Menthol suppresses nicotinic acetylcholine receptor functioning in sensory neurons via allosteric modulation. Chemical senses. 2012;37:463–469. doi: 10.1093/chemse/bjr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatem S, Attal N, Willer JC, Bouhassira D. Psychophysical study of the effects of topical application of menthol in healthy volunteers. Pain. 2006;122:190–196. doi: 10.1016/j.pain.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Heck JD, Hamm LB, Lauterbach JH. The Industry Menthol Report. 2011 https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/TobaccoProductsScientificAdvisoryCommittee/UCM249320.pdf.accessed on.
- Henderson BJ, Wall TR, Henley BM, Kim CH, McKinney S, Lester HA. Menthol Enhances Nicotine Reward-Related Behavior by Potentiating Nicotine-Induced Changes in nAChR Function, nAChR Upregulation, and DA Neuron Excitability. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2017 doi: 10.1038/npp.2017.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Wall TR, Henley BM, Kim CH, Nichols WA, Moaddel R, Xiao C, Lester HA. Menthol Alone Upregulates Midbrain nAChRs, Alters nAChR Subtype Stoichiometry, Alters Dopamine Neuron Firing Frequency, and Prevents Nicotine Reward. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2016;36:2957–2974. doi: 10.1523/JNEUROSCI.4194-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland A, Garten S, Giovino GA, Cummings KM. Mentholated cigarettes and smoking cessation: findings from COMMIT. Community Intervention Trial for Smoking Cessation. Tobacco control. 2002;11:135–139. doi: 10.1136/tc.11.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karashima Y, Damann N, Prenen J, Talavera K, Segal A, Voets T, Nilius B. Bimodal action of menthol on the transient receptor potential channel TRPA1. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:9874–9884. doi: 10.1523/JNEUROSCI.2221-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Wood A, Green BG. Derivation and evaluation of a labeled hedonic scale. Chemical senses. 2009;34:739–751. doi: 10.1093/chemse/bjp054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, de Wit H, King AC, Sofuoglu M, Rueger SY, O’Malley SS. The drug effects questionnaire: psychometric support across three drug types. Psychopharmacology. 2013;227:177–192. doi: 10.1007/s00213-012-2954-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morean ME, Kong G, Cavallo DA, Camenga DR, Krishnan-Sarin S. Nicotine concentration of e-cigarettes used by adolescents. Drug and alcohol dependence. 2016;167:224–227. doi: 10.1016/j.drugalcdep.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat JE, Chen G, Knipe A, Stellman SD, Lazarus P, Richie JP., Jr Effects of menthol on tobacco smoke exposure, nicotine dependence, and NNAL glucuronidation. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2009;18:35–41. doi: 10.1158/1055-9965.EPI-08-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACDAa. Guidelines for Administration of Drugs to Human Subjects. 2006 https://www.drugabuse.gov/funding/clinical-research/nacda-guidelines-administration-drugs-tohuman-subjects.accessed on.
- NACDAb. Guidlines for Substance Abuse Research Involving Children and Adolescents. 2012 https://www.drugabuse.gov/funding/clinical-research/nacda-guidelines-substance-abuseresearch-involving-children-adolescents.accessed on.
- Paschke M, Tkachenko A, Ackermann K, Hutzler C, Henkler F, Luch A. Activation of the cold-receptor TRPM8 by low levels of menthol in tobacco products. Toxicology letters. 2017;271:50–57. doi: 10.1016/j.toxlet.2017.02.020. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, Karelitz JL. Threshold dose for behavioral discrimination of cigarette nicotine content in menthol vs. non-menthol smokers. Psychopharmacology. 2017;234:12551265. doi: 10.1007/s00213-017-4563-3. [DOI] [PubMed] [Google Scholar]
- Reid JR. A history of mentholated cigarettes: “This Spud’s for you”. Recent Advances in Tobacco Science. 1994;19:17–84. [Google Scholar]
- Rock VJ, Davis SP, Thorne SL, Asman KJ, Caraballo RS. Menthol cigarette use among racial and ethnic groups in the United States, 2004–2008. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2010;12(Suppl 2):S117–124. doi: 10.1093/ntr/ntq204. [DOI] [PubMed] [Google Scholar]
- Rosbrook K, Green BG. Sensory Effects of Menthol and Nicotine in an E-Cigarette. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2016;18:1588–1595. doi: 10.1093/ntr/ntw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JE, Benowitz NL, Clanton MS, Connolly GN, Deleeuw KL, Hamm LB, Hatsukami DK, Heck JD, Henningfield JE, Henderson PN, Lauterbach JH, Wakefield M. TPSAC-Menthol report. 2011 https://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/TobaccoProductsScientificAdvisoryCommittee/ucm247605.htm.accessed on.
- Samet JM, Pentz MA, Unger JB. Flavoured tobacco products and the public’s health: lessons from the TPSAC menthol report. Tobacco control. 2016;25:ii103–ii105. doi: 10.1136/tobaccocontrol-2016-053208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Herman AI, Nadim H, Jatlow P. Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2012;37:1509–1516. doi: 10.1038/npp.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria R, Stapleton JM, Gilson SF, Sampson-Cone A, Henningfield JE, London ED. Subjective and cardiovascular effects of intravenous nicotine in smokers and non-smokers. Psychopharmacology. 1996;128:221–226. doi: 10.1007/s002130050129. [DOI] [PubMed] [Google Scholar]
- Ton HT, Smart AE, Aguilar BL, Olson TT, Kellar KJ, Ahern GP. Menthol Enhances the Desensitization of Human alpha3beta4 Nicotinic Acetylcholine Receptors. Molecular pharmacology. 2015;88:256–264. doi: 10.1124/mol.115.098285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic "cigarettes": nicotine delivery profile and cardiovascular and subjective effects. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2010;19:1945–1953. doi: 10.1158/1055-9965.EPI-10-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villanti AC, Mowery PD, Delnevo CD, Niaura RS, Abrams DB, Giovino GA. Changes in the prevalence and correlates of menthol cigarette use in the USA, 2004–2014. Tobacco control. 2016;25:ii14–ii20. doi: 10.1136/tobaccocontrol-2016-053329. [DOI] [PubMed] [Google Scholar]
- Wang T, Wang B, Chen H. Menthol facilitates the intravenous self-administration of nicotine in rats. Frontiers in behavioral neuroscience. 2014;8:437. doi: 10.3389/fnbeh.2014.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasner G, Schattschneider J, Binder A, Baron R. Topical menthol–a human model for cold pain by activation and sensitization of C nociceptors. Brain: a journal of neurology. 2004;127:1159–1171. doi: 10.1093/brain/awh134. [DOI] [PubMed] [Google Scholar]
- WHO-FCTC. Framework Convention on Tobacco Control. http://www.who.int/fctc/WHO_FCTC_summary_January2015_EN.pdf.accessed on.
- Xiao B, Dubin AE, Bursulaya B, Viswanath V, Jegla TJ, Patapoutian A. Identification of transmembrane domain 5 as a critical molecular determinant of menthol sensitivity in mammalian TRPA1 channels. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:9640–9651. doi: 10.1523/JNEUROSCI.2772-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingst JM, Veldheer S, Hammett E, Hrabovsky S, Foulds J. A method for classifying user-reported electronic cigarette liquid flavors. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco. 2017 doi: 10.1093/ntr/ntw383. [DOI] [PMC free article] [PubMed] [Google Scholar]