Abstract
Background
Skeletal dysplasia encompasses a variety of developmental disorders of the bone and cartilage that manifest as disproportionate shortening of limbs and trunk in the neonate. Many types of skeletal dysplasia are complicated by respiratory failure at or soon after birth and require intensive care and prolonged hospitalization. Respiratory complications in these infants are complex and are characterized by airway anomalies, restrictive lung disease due to a narrow and abnormally compliant chest wall, pulmonary hypoplasia, and central apnea. Appropriate management of these unique patients requires a clear understanding of the pathophysiology and use of pulmonary function tests for early recognition and management of complications.
Conclusion
This review provides an overview of the underlying respiratory pathology and a practical guide to the newborn care provider for the diagnosis and management of respiratory complications in infants with skeletal dysplasia.
Keywords: infants, respiratory, skeletal dysplasia
Introduction
Skeletal dysplasia includes a wide variety of disorders characterized by abnormal development of bone and cartilage. To date, more than 400 distinct syndromes and subtypes have been described. It is suspected that disproportionate shortening of limbs or trunk occurs at an estimated incidence of 15.7 per 100,000 live births [1–4]. Respiratory failure due to developmental abnormalities of the airway and chest wall is the leading cause of mortality and morbidity in infants with skeletal dysplasia [5–8]. Disorders such as asphyxiating thoracic dysplasia, Ellis-van Creveld syndrome, and thanatophoric dysplasia are characterized by narrow thorax and often present with severe restrictive lung disease and respiratory failure in the immediate newborn period. Respiratory care of this distinct population poses unique challenges, and an understanding of the complex respiratory pathophysiology is crucial for appropriate diagnosis and management in these infants. The objective of this article is to describe the pathophysiology and management of respiratory failure in infants with skeletal dysplasia.
Pathophysiology
Factors contributing to lung disease in infants with skeletal dysplasia can be broadly classified into airway abnormalities, thoracic cage abnormalities, pulmonary hypoplasia, and abnormalities due to central apnea. Most infants are affected by a combination of all or some of the factors that result in respiratory compromise during the neonatal period.
Airway anomalies
Airway anomalies frequently occur in infants with skeletal dysplasia causing collapse and obstruction of the airway. Craniofacial abnormalities are commonly present in different forms of skeletal dysplasia such as achondroplasia, Apert syndrome, chondrodysplasia punctata, Ellis-van Creveld syndrome, Kniest dysplasia, Stickler syndrome, Morquio syndrome, and other mucopolysaccharidoses [1–3,9]. Common anatomical lesions that cause narrowing of the airway are narrow nasal passages, copious nasal secretions, large tongue, depressed nasal bridge, facial hypoplasia, brachycephaly, micrognathia, and stiff temperomandibular joints [10]. These externally visible obstructive lesions are often associated with internal obstruction such as narrow pharynx, larynx, and tracheal structures that are not apparent on the outside.
Laryngotracheobronchomalacia is a frequent complication in infants with type II collagen disorders such as Kniest dysplasia and spondyloepiphyseal dysplasia congenita, campomelic dysplasia, and Larsen syndrome [11–13]. Airway obstructive symptoms may arise early in the neonatal period or later in childhood, and, hence, periodic otolaryngology evaluation is necessary [2–5,8,11].
Reduced airway smooth muscle tone increases airway compliance and causes the airway to be floppy and prone to collapse [14]. Neonates are at higher risk since they have an inherently low airway smooth muscle tone [15–17]. Obstruction and limitation of air flow are accentuated during crying and coughing episodes when pleural pressure is greater than intraluminal pressure [18]. Bronchospasms due to severe tracheobronchomalacia are a serious problem in some infants, particularly in those with type II collagenopathy. These infants often require prolonged mechanical ventilation, which induces airway deformation and structural changes involving the muscle and cartilage, further worsening preexisting tracheobronchomalacia [19,20]. Frequency of episodes of bronchospasms increases as these children get older and have increased activity and periods of wakefulness. These episodes can be very severe, resulting in complete occlusion of proximal airways, and are life-threatening. Appropriate comfort measures with or without sedation to decrease the episodes of agitation and crying are critical. One of the common reasons for agitation in these infants is chronic constipation exacerbated by the use of sedatives, and, hence, these infants should be appropriately treated with laxatives [21–23].
Moreover, hypotonia of the muscles of the upper airway often occurs in infants with other underlying neurological abnormalities and generalized hypotonia. This adds to narrowing of the upper airway and is exacerbated during sleep [24,25]. Nasopharyngeal obstruction due to hypotonia and hypertrophy of the pharyngeal tissue is seen in Morquio syndrome and other mucopolysaccharidoses [26,27]. Less severe forms of obstruction mostly manifest as obstructive apnea during sleep and may be missed in the early stages. Lack of early diagnosis and treatment of obstructive sleep apnea in these infants can result in growth failure and chronic pulmonary hypertension [28].
Chest wall abnormalities and restrictive lung disease
Abnormal chemical composition of the ribs, cartilage, and spine affects the viscoelastic properties of the lung and chest wall and alters chest wall mechanics [29–31]. Functional residual capacity is determined by the equilibrium between the opposing forces of the lung and chest wall. An over-compliant chest wall offers little outward recoil to opposing forces of the elastic properties of the lung and thus leads to decreased functional residual capacity. Alternately, an excessively stiff and narrow chest causes restriction of adequate chest expansion and leads to decreased functional residual capacity. Therefore, alteration in the mechanical properties of the chest wall in infants with skeletal dysplasia, such as in Ellis-van Creveld syndrome, Jeune syndrome, diastrophic dysplasia, hypophosphatasia, and osteogenesis imperfecta type III, results in decreased functional residual capacity and respiratory insufficiency [6,32–34]. Optimal functional residual capacity is also crucial to maintain small airway and small vessel patency. Elastic components in the alveolar walls are tethered to one another and to the surrounding small bronchioles and exert a circumferential pull on the small intraparenchymal airways [35,36]. Infants with reduced total lung capacity have a characteristic rapid and shallow breathing pattern due to increased respiratory frequency and decreased tidal volume. These infants often present with tachypnea and increased work of breathing, which is a characteristic feature of short rib dysplasia syndromes. Tachypnea and work of breathing may be exacerbated by the presence of rib fractures and associated pain-related splinting of the chest wall as seen in infants with osteogenesis imperfecta. Tachypnea and increased work of breathing also increase the risk for aspiration: recurrent chronic aspiration of oral secretions exacerbates lung disease and predisposes infants to recurrent and potentially life-threatening pneumonia [31]. Additionally, concomitant presence of airway anomalies, such as tracheobronchomalacia, results in poor clearance of secretions and further contributes to atelectasis and chronic lung disease. Neonates with chest wall deformities may thus present with varying severity of lung disease. These infants also have increased frequency of hypoxemic and hypercarbic episodes during sleep when minute ventilation normally decreases [37,38].
Pulmonary hypoplasia
Since rhythmic fetal breathing movement is necessary for normal fetal lung development, infants affected by poor chest wall movement in utero often have various degrees of pulmonary hypoplasia [39–41]. Severity of lung hypoplasia varies significantly amongst different types of skeletal dysplasia. A wide spectrum of severity of lung hypoplasia, such as hypophosphatasia and osteogenesis imperfecta, also occurs within the same type of skeletal dysplasia [6,42,43]. Determining the severity of pulmonary hypoplasia prenatally by fetal magnetic resonance imaging (MRI) would provide valuable information to counsel the parents and to arrange for the delivery of the infant at a higher level neonatal intensive care unit (NICU) that has capabilities for high-frequency ventilation and inhaled nitric oxide therapy. Complex or cyanotic congenital heart disease, although uncommon, may complicate the clinical scenario and must be considered in an infant with skeletal dysplasia presenting with severe respiratory failure that is out of proportion to the degree of lung hypoplasia and chest deformity.
Central apnea
Brainstem and cranial nerve compression can occur in the neonatal period in some forms of skeletal dysplasia because of atlantoaxial instability and manifest as severe central apnea; however, this finding is rare [44,45]. These infants are also at increased risk for stridor, vocal cord paralysis, dysfunctional swallowing, and aspiration. Consultation with an otolaryngologist for direct laryngoscopy and swallow study is useful to assess the severity of vocal cord dysfunction and guide appropriate therapy. Consideration for tracheostomy or gastrostomy tube placement or both should be encouraged in neonates with severe degrees of aspiration or when aspiration is associated with severe chronic lung disease.
Congenital Heart Disease and Cor Pulmonale
Congenital heart lesions are seen in certain types of skeletal dysplasia, and, hence, all infants with disproportionate short stature should have a thorough cardiac examination and echocardiogram [1,4]. It is often challenging to differentiate respiratory distress due to primary lung pathology from congestive heart failure. Additionally, infants with chronic lung disease or obstructive sleep apnea are at high risk for development of cor pulmonale. Therefore, these infants should be evaluated and followed closely by cardiology for early identification and treatment of cor pulmonale [46,47]. Presence of a left-to-right shunt such as an atrial or ventricular septal defect and patent ductus arteriosus adds to the development of pulmonary hypertension and cor pulmonale [48]. A high index of suspicion is required since the symptoms of pulmonary hypertension often mimic exacerbations of respiratory failure. Hence, these infants require periodic echocardiograms to indirectly assess pulmonary artery pressures. In infants who show early signs of pulmonary hypertension, it is very important to minimize episodes of hypoxemia and hypercarbia to prevent further worsening of pulmonary hypertension. Early closure of left-to-right shunt across an atrial septal defect, ventricular septal defect, and patent ductus arteriosus is often necessary to prevent further worsening of pulmonary hypertension.
Special Considerations
Most infants with skeletal dysplasia are affected by a combination of all or some of the factors described above, which result in respiratory compromise during the neonatal period. For example, Morquio syndrome is a type of mucopolysaccharidoses characterized by abnormal accumulation of keratan sulfate sugar chain in various body parts including the chest wall, ribs, and spine. Deposition of mucopolysaccharides in the tracheobronchial tree causes airway obstruction, whereas deposition and infiltration into the upper airway structures can cause severe upper airway obstruction and exacerbate obstructive sleep apnea [5,8,11]. In addition, deposition of mucopolysaccharides in the lung parenchyma causes restrictive lung disease and decreased lung compliance. In diastrophic dysplasia and Kniest dysplasia, infants are affected by restrictive lung disease, tracheomalacia, and bronchomalacia.
Certain treatment modalities may also exacerbate respiratory failure in infants with skeletal dysplasia. For example, bisphosphonates, potent inhibitors of bone resorption, are used as a fracture prevention therapy in patients with osteogenesis imperfecta. Infusion of bisphosphonates is pro-inflammatory and is associated with a flu-like syndrome. It is characterized by pulmonary edema, respiratory distress, and acute exacerbation of preexisting respiratory failure. Although transient, it can occasionally manifest with severe symptoms and requires treatment with diuretics and corticosteroids [43,49–51].
Diagnostic Modalities
Various diagnostic modalities are helpful in the evaluation of the nature and severity of lung disease, particularly in complex diseases like skeletal dysplasia. Monitoring respiratory status clinically and objectively by diagnostic modalities better assists in timely diagnosis, prevention, and management of various respiratory complications.
Pulmonary function tests
Pulmonary function tests provide comprehensive pulmonary function information and help to guide clinical management. Isolated cases have been reported in the literature where results of pulmonary mechanics better assisted in optimizing appropriate ventilator settings in the management of respiratory failure in infants with skeletal dysplasia and also served as a screening tool prior to thoracic expansion surgery [33,52,53]. Although, extensive data does not exist to prove that pulmonary function tests result in improved clinical outcome compared to usual care, we have observed in our experience that these measurements assist in clinical management and therefore potentially improve outcomes.
For example, in a case report of an infant with Barnes syndrome characterized by severe thoracic dystrophy, we report how the patient was mechanically ventilated via a tracheostomy and required high peak pressures [52]. Pulmonary function testing showed a significantly reduced functional residual capacity. Total, inspiratory, and expiratory resistances were significantly increased, and the degree of thoracoabdominal asynchrony as measured by phase angle was 200% worse than normal values for age. In addition, lung compliance, total respiratory compliance, and chest wall compliance were respectively decreased compared with the predicted normal values. However, the ratio of the compliance value from the last 20% of the inspired volume to the total respiratory system compliance (C 20/C total ratio) was higher than predicted as normal as shown in Figure 1.
Figure 1.
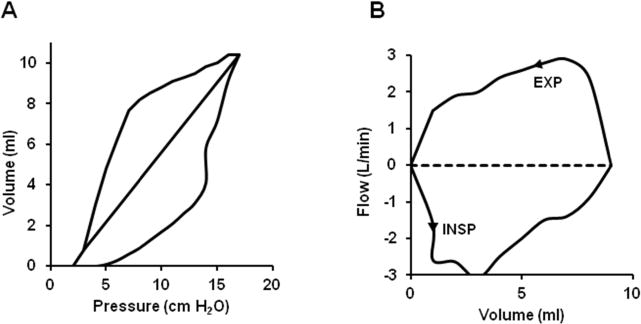
Modified pulmonary graphics of the transpulmonary pressure volume (A) and flow volume (B) relationships from the patient with Barnes syndrome. The right limb of the transpulmonary pressure-volume curve represents inspiration, and the left limb of the transpulmonary pressure-volume curve represents deflation. The C20/C total ratio represents the ratio of the slope of the last 20% of the inflation curve divided by C total as represented by the straight solid line in the middle of the transpulmonary pressure volume curve. C, compliance.
The high C 20/C total ratio indicated that the lungs were not severely hypoplastic and confirmed that the low thoracic volume due to an abnormal rib cage contributed to the low functional residual capacity and increased phase angle. On the basis of these data, ventilator management was directed toward increasing positive end expiratory pressure (PEEP) to up to 7 cm H2O to optimize functional residual capacity and maintaining at that level to ensure rib cage stability while allowing growth and maturity. It is important to note that using single surrogate parameters such as compliance alone are of limited value. However, when used in combination with other parameters of pulmonary function tests, they provide helpful adjuncts to clinical management.
Noninvasive pulmonary function testing, such as impulse oscillation system (IOS) and respiratory inductance plethysmography (RIP), offers rapid functional assessment of pulmonary mechanics and is sensitive in tracking respiratory changes and detecting early changes in lung function associated with infections and various therapeutic interventions that preceded clinical changes [33].
The IOS is a noninvasive method of pulmonary function testing that requires only normal tidal breathing and can therefore be performed independent of patient effort [53–55]. This diagnostic approach is based on the principle of using small external pressure signals (impulses) from sound waves superimposed on spontaneous breathing to measure the degree of obstruction in central and peripheral airways. The IOS is a sensitive technique and is particularly useful as an objective parameter to measure changes in disease severity, as well as identify regional disparities in disease magnitude throughout the respiratory system. Furthermore, IOS has been tested in a small group of children with skeletal dysplasia. Specifically, children with Morquio syndrome exhibited a decrease in reactance and an increase in resistance [56]. A limitation of IOS technique is that it is largely dependent on functional residual capacity and hence difficult to interpret in children with significant rib cage anomalies, since FRC measurements are very difficult to make in these patients. In such situations, RIP may be more useful.
The RIP is a simple noninvasive technique that externally measures thoracoabdominal motion and can be performed easily in infants breathing quietly in any state of arousal. It can be used to assess thoracoabdominal asynchrony by measuring the delay between the peak thoracic and abdominal excursions, the phase angle. Greater phase angle indicates worse thoracoabdominal asynchrony. As a respiratory index, phase angle has been found to correlate with changes in resistance and compliance of the lung and chest wall. Measurement of phase angle has also been shown to decrease with increasing levels of continuous positive airway pressure (CPAP) in neonates with respiratory failure. In this regard, this approach is a useful objective measure to determine the optimal level of CPAP necessary to maintain functional residual capacity and effective gas exchange [57]. It can also be used to measure the degree of rib cage and abdominal motion relative to the total tidal volume.
In a group of 17 patients affected by various types of skeletal dysplasia, RIP was evaluated to assess pulmonary function [56]. This cohort consisted of children with skeletal dysplasia including Morquio syndrome, metatropic-spondylocostal dysplasia, diastrophic dysplasia, Kniest dysplasia, cartilage hair hypoplasia, spondylometaphyseal dysplasia, and epiphyseal dysplasia at a mean age of 8 ± 5 years. At this age, the average rib cage contribution to the tidal volume (%RC) was significantly decreased compared with that of healthy controls. Phase angle and phase relation during total breath were also increased significantly compared with those of healthy subjects. Children with severe chest restrictive pattern such as in metatropic-spondylocostal dysplasia, diastrophic dysplasia, and Kniest dysplasia expressed low %RC and a high degree of thoracoabdominal asynchrony. In contrast, children with less severe restrictive lung disease, as in Morquio syndrome, exhibited %RC and phase angle measurements that were comparable with those of healthy controls. However, phase relation to total breath was significantly higher compared with that of healthy children, indicating potential early pulmonary dysfunction. The benefit of RIP during infancy in this population is relatively unknown at this time and is a potential area for future research.
As part of a multicenter trial, we performed pulmonary function tests in three patients with perinatal hypophosphatasia treated with bone-targeted human recombinant tissue non-specific isoenzyme alkaline phosphatase (TNSALP) [58] and reported the results of the first case [33]. This rare autosomal recessive disease is characterized by defective bone mineralization, rib shortening, rachitic chest wall deformity, and hypoplastic lungs. The infant’s hospital course was complicated by severe respiratory failure requiring tracheostomy and high peak inspiratory pressure through a mechanical ventilator up to 20–38 cm H2O. In addition, the infant had severe tracheomalacia confirmed by bronchoscopy. Compassionate use of recombinant human TNSALP was started at three weeks of life, and noninvasive pulmonary function tests were performed to assess the effects of the therapy on respiratory function from five weeks until 27 weeks after initiating treatment. Pulmonary function tests showed that compliance improved after 12 weeks of treatment as shown in Figure 2.
Figure 2.
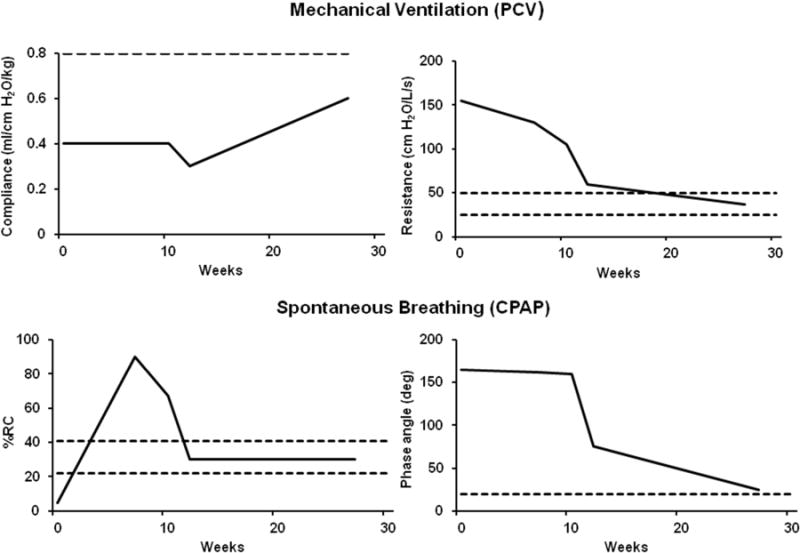
Trends in respiratory mechanics and thoracoabdominal measurements at baseline (0; 8 weeks postnatal/5 weeks on drug) and in response to ENB-0040. Solid line indicates averaged patient measurements. Dashed line indicates predicted normal values. PCV, pressure control ventilation; CPAP, continuous positive airway pressure; %RC, percentage of rib cage contribution to tidal volume excursions; phase angle in degrees. Illustrations modified from Rodriguez et al. [33].
Resistance decreased from a baseline level of 500% of normal to near normal levels between 12 to 27 weeks after initiation of treatment. Percentage of rib cage contribution to total tidal volume was only 5% at baseline but improved to normal values at approximately 12 weeks after treatment. The degree of thoracoabdominal asynchrony remained elevated at more than 900% of predicted for a prolonged period of up to 10 weeks after initiation of treatment and then steadily declined by 12 weeks and reached near normal levels by 27 weeks. Parallel to the changes in pulmonary function measurements, bone remodeling changes, such as an increase in thoracic cage dimensions and rib size with increased mineralization and areas of callus formation, were noted on chest radiographs as shown in Figure 3A and 3B.
Figure 3.
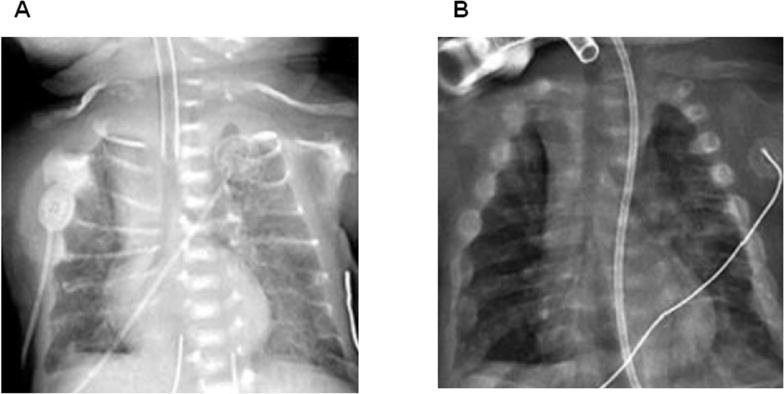
Anteroposterior chest radiographs showing progressive improvement in thoracic cage mineralization. A: Patient at baseline (3 weeks postnatal). B: Patient after 12 weeks of treatment [33]. Permission to reproduce and copyright license obtained from John Wiley and Sons, Inc., licensed content publisher.
Based on the pulmonary function measurements, ventilator management was targeted toward increasing PEEP to stabilize the chest wall, and weaning was not initiated until chest wall stabilization was evident by pulmonary function measurements, which occurred at around 12 weeks after treatment. At this time, the infant tolerated ventilator and supplemental oxygen wean and was discharged home on a portable ventilator on room air at 35 weeks of life.
Imaging techniques
Chest radiographs are a useful initial modality to look for unusual rib and spine deformities as well as to evaluate for lung hypoplasia and atelectasis. Fetal MRI obtained prenatally provides valuable information to determine the severity of pulmonary hypoplasia and is useful to counsel parents [59]. It also helps to arrange ahead of time to deliver the infant at a higher level NICU that has capabilities for high-frequency ventilation and inhaled nitric oxide. Three-dimensional computed tomography (CT) and MRI are helpful to more thoroughly evaluate the bony and airway abnormalities and help guide surgical interventions. Airway fluoroscopy and direct laryngobronchoscopy provide valuable information of airway anatomy and function.
Treatment Strategies
Neonates with mild hypoxemia without hypercarbia or increased work of breathing may be treated with supplemental oxygen via low-flow nasal cannula, whereas neonates with increased work of breathing, airway obstruction, and hypercarbia require PEEP. This may be provided noninvasively using heated humidified high-flow nasal cannula (HHFNC) or CPAP [60]. In addition, the titration of flow and CPAP can be assessed with RIP technology to provide feedback of optimum flow or CPAP levels that provide best breathing synchrony and minimum labored breathing indices. Some infants with restrictive lung disease who have mildly to moderately low lung volumes, which improve over time with growth, require a prolonged period of PEEP via nasal CPAP or HHFNC. Home high-flow oxygen delivery systems may be used in this subset of infants to facilitate discharge from the hospital while obviating the need for tracheostomy or prolonged hospital stay. In our experience, infants with restrictive lung disease due to type III osteogenesis imperfecta and Ellis-van Creveld syndrome required treatment with HHFNC up to 2 to 4 liters per minute to minimize work of breathing and optimize growth during inpatient stay. Early discharge from the hospital was facilitated by discharging on home high-flow oxygen or CPAP devices and close nursing and outpatient follow up. These infants continued to require high-flow oxygen therapy or CPAP for several weeks to months and were later able to be gradually weaned to low-flow home oxygen.
Helium is a low density gas that provides a laminar flow and lower turbulence, thereby decreasing airflow resistance and work of breathing [61,62]. Preclinical evidence suggests that heliox combined with high-flow nasal cannula decreases work of breathing and attenuates diaphragmatic injury and fatigue [63]. The role of heliox in infants with skeletal dysplasia and airway obstruction is yet to be explored and holds potential. In infants with rib fractures, and those receiving painful procedures such as pamidronate infusion, it is crucial to closely assess for pain-related splinting of the chest wall and respiratory distress and to manage pain with adequate analgesia.
Infants with more severe lung disease and airway obstruction will require intubation and long-term mechanical ventilation. These infants often require high PEEP to stabilize the chest wall to maintain adequate lung expansion and maximize synchronous breathing. Stabilization of the chest wall cannot be overemphasized, and weaning should not be started until it is stabilized, which often takes several weeks [33,52]. However, use of high PEEP is not without risk as it may exacerbate preexisting airway malacia and air trapping. In rare instances of severe upper airway compromise, an EXIT procedure would facilitate perinatal management.
Infants who require prolonged mechanical ventilation with high PEEP must have a tracheostomy. Appropriately sized tracheal tubes must be chosen since these infants often have narrow tracheal width but longer tracheal length. A tracheostomy tube of accurate length, having its tip just proximal to the carina, serves as a stent for the floppy trachea and attenuates the severity of obstructive episodes caused by tracheomalacia. Custom-made tracheal tubes are recommended after consultation with an otolaryngologist and pulmonologist. In infants with very short necks and severe pectus carinatum deformities, tracheostomy may be difficult to perform without a concomitant thoracotomy. Sometimes a tracheostomy will not always relieve the obstruction due to significant tracheal narrowing distal to the tracheostomy. In these cases, a preoperative CT or MRI of the chest may be necessary to better delineate the anatomy of the airway.
In infants with moderate-to-severe tracheobronchomalacia characterized by a floppy and collapsible trachea and bronchi, bethanechol, a cholinergic muscarinic agonist, may be used to increase tracheal smooth muscle tone and maintain airway patency and stability [64]. In a small study, two infants with moderate-to-severe tracheomalacia, confirmed by rigid bronchoscopy, received a trial of oral bethanechol for 10 days, and then pulmonary mechanics and peak expiratory flow volume curves were measured using rapid thoracoabdominal compression techniques. Measurements of spontaneous breathing during sleep in the supine position were obtained at baseline and following methacholine and bronchodilator administration, respectively.
As shown in Figure 4, in both patients, the baseline flow volume curve was concave, and maximum flow at functional residual capacity was significantly lower than those reported for children of similar age and length. Methacholine administration resulted in improvement in forced expiratory flow, whereas bronchodilator exposure resulted in a decrease in expiratory flow.
Figure 4.
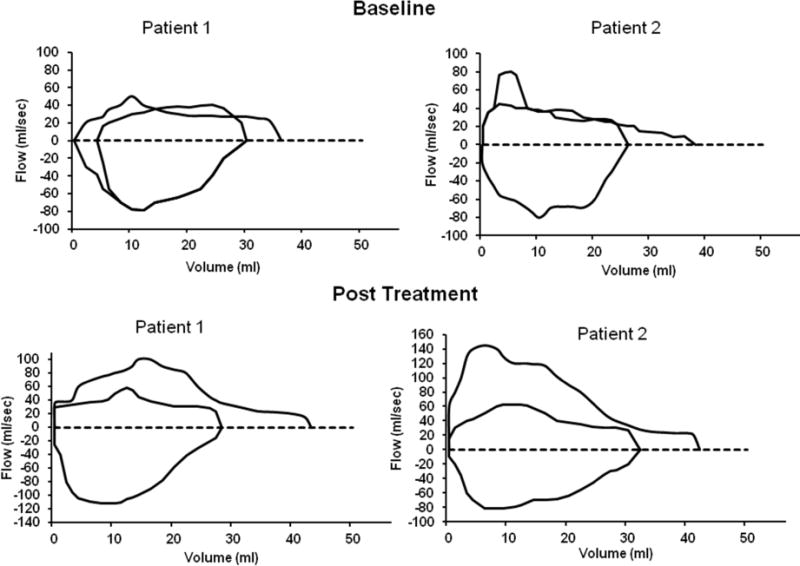
Flow volume curves for patients 1 and 2 at baseline and after 10 days of bethanechol treatment. Illustrations modified from Panitch et al. [64].
After 10 days of therapy with bethanechol, baseline flow volume curves were less concave and showed higher flows at all volumes. The maximum flow at functional residual capacity and the ratio of forced expiratory flow to expiratory tidal flow at mid tidal volume were also increased. Therefore, in situations where a high PEEP is required to maintain the patency of the airway, bethanechol may be used to increase airway smooth muscle tone and make the airway less compliant and less collapsible, thereby minimizing the requirement of positive pressure ventilation and associated lung injury.
Airway clearance and pulmonary toileting are an important aspect of respiratory care in infants with restrictive lung disease and concomitant airway malacia. However, traditional methods of chest physiotherapy may not be well tolerated in infants with osteogenesis imperfect because of increased risk of fractures and bone pain. Additionally, traditional methods of chest physiotherapy may increase agitation in older infants and exacerbate episodes of bronchospasms. In such situations, airway clearance can be better achieved using intrapulmonary percussive ventilation. Mobilization of mucous is facilitated by delivering low volumes of positive pressure to the airways at high frequencies, creating an internal percussive effect [65].
In addition to respiratory management, monitoring and optimizing growth are crucial for optimal long-term outcomes and quality of life for infants with skeletal dysplasia and respiratory comorbidities. It is important to take into consideration that these infants have short stature, and, hence, diagnosis-specific growth curves must be used rather than the healthy term infant growth curves. For those disorders that do not have predefined growth curves, targeting a growth velocity of 10 to 15 grams per day is adequate to ensure optimal growth and avoid excessive weight gain for height.
Anesthesia Management
During anesthesia, special attention is required in the management of infants with skeletal dysplasia. Upper airway obstruction at rest may be worsened with sedation or general anesthesia. Presence of concomitant cervical abnormalities, such as a short neck or cervical instability, may contribute to difficult intubation, and fiberoptic intubation is recommended in such situations. It is necessary to avoid positions of extreme flexion or extension in infants with foramen magnum stenosis or cervical instability. In addition, these complications can be reduced by placing a folded towel underneath the shoulder [66,67].
Preexisting underlying parenchymal lung disease and tracheobronchomalacia often contribute to perioperative morbidity. In infants with pulmonary hypertension, acute elevation of pulmonary artery pressure leads to a vicious cycle of right heart failure, hypoxia, and further aggravation of pulmonary hypertension; this sequence of events is a dangerous perioperative complication. Anesthesia must be planned so that anesthetic agents and stimuli that increase pulmonary vascular resistance are avoided while maintaining adequate cardiac output and coronary perfusion.
Conclusions
Respiratory disease, which varies in severity and complexity, is one of the most common causes of mortality and morbidity in infants with skeletal dysplasia. Because of the complex nature of the disease conditions, several important physiologic considerations should be taken into account for optimal respiratory management. Diagnosis of an underlying genetic condition and specific type of skeletal dysplasia may take time; however, in the meantime, appropriate respiratory management aids in stabilization and survival of the newborn infant and is required to improve both the quality of life and long-term survival in these children. This article summarizes key physiological principles and diagnostic and treatment strategies that are useful at the bedside for respiratory management of these infants. Close monitoring, clinical and objective assessments of respiratory function and multidisciplinary management improve clinical outcomes in this rare, complex group of infants.
Highlights for Review.
Skeletal dysplasia encompasses a variety of developmental disorders of the bone and cartilage and are often complicated by respiratory failure. They are characterized by airway anomalies, restrictive lung disease due to a narrow and abnormally compliant chest wall, pulmonary hypoplasia, and central apnea. Close monitoring, clinical and objective assessments of respiratory function and multidisciplinary management improve clinical outcomes in this rare, complex group of infants.
Acknowledgments
The authors acknowledge the excellent assistance of Ms. Aneesha Cheedalla, medical student, in the preparation of the figures.
Funding: This work was partially supported by NIH COBRE P30 GM 114736 (Dr. Shaffer); Institutional Development Award (IdeA) from the National Institute of General Medical Sciences of the National Institutes of Health [grant number U54-GM104941] (PI: Binder-Macleod); and The Nemours Foundation.
Abbreviations
- %RC
percentage of rib cage contribution to tidal volume excursions
- C 20/C
ration of the compliance value from the last 20% of the inspired volume to the total respiratory system compliance
- CPAP
continuous positive airway pressure
- CT
computed tomography
- HHFNC
humidified high-flow nasal cannula
- IOS
impulse oscillation system
- MRI
magnetic resonance imaging
- NICU
neonatal intensive care unit
- PEEP
positive end expiratory pressure
- RIP
respiratory inductance plethysmography
- TNSALP
tissue non-specific isoenzyme alkaline phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
References
- 1.Spranger JW, Brill PW, Poznanski A. Bone Dysplasias: An Atlas of Genetic Disorders of Skeletal Development. third. Oxford University Press; New York: 2002. [Google Scholar]
- 2.Warman ML, Cormier-Daire V, Hall C, Krakow D, Lachman R, LeMerrer M, et al. Nosology and classification of genetic skeletal disorders: 2010 revision. Am J Med Genet A. 2011;155A:943–968. doi: 10.1002/ajmg.a.33909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sillence DO, Rimoin DL, Lachman R. Neonatal dwarfism. Pediatr Clin North Am. 1978;25:453–483. doi: 10.1016/s0031-3955(16)33600-8. [DOI] [PubMed] [Google Scholar]
- 4.Hurst JA, Firth HV, Smithson S. Skeletal dysplasias. Semin Fetal Neonatal Med. 2005;10:233–241. doi: 10.1016/j.siny.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Mogayzel PJ, Marcus CL. Skeletal dysplasias and their effect on the respiratory system. Paediatr Respir Rev. 2001;2:365–371. doi: 10.1053/prrv.2001.0173. [DOI] [PubMed] [Google Scholar]
- 6.Tuysuz B, Baris S, Aksoy F, Madazli R, Ungur S, Sever L. Clinical variability of asphyxiating thoracic dystrophy (Jeune) syndrome: evaluation and classification of 13 patients. Am J Med Genet A. 2009;149A:1727–1733. doi: 10.1002/ajmg.a.32962. [DOI] [PubMed] [Google Scholar]
- 7.Stokes DC, Phillips JA, Leonard CO, Dorst JP, Kopits SE, Trojak JE, et al. Respiratory complications of achondroplasia. J Pediatr. 1983;102:534–541. doi: 10.1016/s0022-3476(83)80180-2. [DOI] [PubMed] [Google Scholar]
- 8.Mortier GR. The diagnosis of skeletal dysplasias: a multidisciplinary approach. Eur J Radiol. 2001;40:161–167. doi: 10.1016/s0720-048x(01)00397-7. [DOI] [PubMed] [Google Scholar]
- 9.Landing BH, Dixon LG. Congenital malformations and genetic disorders of the respiratory tract (larynx, trachea, bronchi, and lungs) Am Rev Respir Dis. 1979;120:151–185. doi: 10.1164/arrd.1979.120.1.151. [DOI] [PubMed] [Google Scholar]
- 10.Dinwiddie R. Congenital upper airway obstruction. Paediatr Respir Rev. 2004;5:17–24. doi: 10.1016/j.prrv.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Steven Sims H, Kempiners JJ. Special airway concerns in patients with mucopolysaccharidoses. Respir Med. 2007;101:1779–1782. doi: 10.1016/j.rmed.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Nishimura G, Haga N, Kitoh H, Tanaka Y, Sonoda T, Kitamura M, et al. The phenotypic spectrum of COL2A1 mutations. Hum Mutat. 2005;26:36–43. doi: 10.1002/humu.20179. [DOI] [PubMed] [Google Scholar]
- 13.Hicks J, De Jong A, Barrish J, Zhu SH, Popek E. Tracheomalacia in a neonate with kniest dysplasia: histopathologic and ultrastructural features. Ultrastruct Pathol. 2001;25:79–83. doi: 10.1080/019131201300004726. [DOI] [PubMed] [Google Scholar]
- 14.Bhutani VK, Koslo RJ, Shaffer TH. The effect of tracheal smooth muscle tone on neonatal airway collapsibility. Pediatr Res. 1986;20:492–495. doi: 10.1203/00006450-198606000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Laudadio RE, Wolfson MR, Shaffer TH, Driska SP. Developmental differences in the contractile response of isolated ovine tracheal smooth muscle cells. Pediatr Pulmonol. 2009;44:602–612. doi: 10.1002/ppul.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penn RB, Wolfson MR, Shaffer TH. Effect of tracheal smooth muscle tone on collapsibility of immature airways. J Appl Physiol. 1988;65:863–869. doi: 10.1152/jappl.1988.65.2.863. [DOI] [PubMed] [Google Scholar]
- 17.Koslo RJ, Bhutani VK, Shaffer TH. The role of tracheal smooth muscle contraction on neonatal tracheal mechanics. Pediatr Res. 1986;20:1216–1220. doi: 10.1203/00006450-198612000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Dinwiddie R, Pitcher-Wilmott R, Schwartz JG, Shaffer TH, Fox WW. Cardiopulmonary changes in the crying neonate. Pediatr Res. 1979;13:900–903. doi: 10.1203/00006450-197908000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Deoras KS, Wolfson MR, Bhutani VK, Shaffer TH. Structural changes in the tracheae of preterm lambs induced by ventilation. Pediatr Res. 1989;26:434–437. doi: 10.1203/00006450-198911000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Bhutani VK, Ritchie WG, Shaffer TH. Acquired tracheomegaly in very preterm neonates. Am J Dis Child. 1986;140:449–452. doi: 10.1001/archpedi.1986.02140190059026. [DOI] [PubMed] [Google Scholar]
- 21.Gordin PC. Assessing and managing agitation in a critically ill infant. M C N Am J Matern Child Nurs. 1990;15:26–32. [PubMed] [Google Scholar]
- 22.Field T, Goldson E. Pacifying effects of nonnutritive sucking on term and preterm neonates during heelstick procedures. Pediatrics. 1984;74:1012–1015. [PubMed] [Google Scholar]
- 23.Hyde BB, McCown DE. Classical conditioning in neonatal intensive care nurseries. Pediatr Nurs. 1986;12:11–13. 63. [PubMed] [Google Scholar]
- 24.Arens R, Marcus CL. Pathophysiology of upper airway obstruction: a developmental perspective. Sleep. 2004;27:997–1019. doi: 10.1093/sleep/27.5.997. [DOI] [PubMed] [Google Scholar]
- 25.Robison JG, Otteson TD. Increased prevalence of obstructive sleep apnea in patients with cleft palate. Arch Otolaryngol Head Neck Surg. 2011;137:269–274. doi: 10.1001/archoto.2011.8. [DOI] [PubMed] [Google Scholar]
- 26.Wraith JE. The mucopolysaccharidoses: a clinical review and guide to management. Arch Dis Child. 1995;72:263–267. doi: 10.1136/adc.72.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semenza GL, Pyeritz RE. Respiratory complications of mucopolysaccharide storage disorders. Medicine. 1988;67:209–219. doi: 10.1097/00005792-198807000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Katz ES, Mitchell RB, D’Ambrosio CM. Obstructive sleep apnea in infants. Am J Respir Crit Care Med. 2012;185:805–816. doi: 10.1164/rccm.201108-1455CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papastamelos C, Panitch HB, Allen JL. Chest wall compliance in infants and children with neuromuscular disease. Am J Respir Crit Care Med. 1996;154:1045–1048. doi: 10.1164/ajrccm.154.4.8887605. [DOI] [PubMed] [Google Scholar]
- 30.Papastamelos C, Panitch HB, England SE, Allen JL. Developmental changes in chest wall compliance in infancy and early childhood. J Appl Physiol. 1995;78:179–184. doi: 10.1152/jappl.1995.78.1.179. [DOI] [PubMed] [Google Scholar]
- 31.Hull D, Barnes ND. Children with small chests. Arch Dis Child. 1972;47:12–19. doi: 10.1136/adc.47.251.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baujat G, Le Merrer M. Ellis-van Creveld syndrome. Orphanet J Rare Dis. 2007;2:27. doi: 10.1186/1750-1172-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodriguez E, Bober MB, Davey L, Zamora A, Li Puma AB, Chidekel A, et al. Respiratory mechanics in an infant with perinatal lethal hypophosphatasia treated with human recombinant enzyme replacement therapy. Pediatr Pulmonol. 2012;47:917–922. doi: 10.1002/ppul.22527. [DOI] [PubMed] [Google Scholar]
- 34.Hershenson MB, Colin AA, Wohl ME, Stark AR. Changes in the contribution of the rib cage to tidal breathing during infancy. Am Rev Respir Dis. 1990;141:922–925. doi: 10.1164/ajrccm/141.4_Pt_1.922. [DOI] [PubMed] [Google Scholar]
- 35.Henschen M, Stocks J, Brookes I, Frey U. New aspects of airway mechanics in pre-term infants. Eur Respir J. 2006;27:913–920. doi: 10.1183/09031936.06.00036305. [DOI] [PubMed] [Google Scholar]
- 36.Plopper CG, Nishio SJ, Schelegle ES. Tethering tracheobronchial airways within the lungs. Am J Respir Crit Care Med. 2003;167:2–3. doi: 10.1164/rccm.2211002. [DOI] [PubMed] [Google Scholar]
- 37.Henderson-Smart DJ, Read DJ. Ventilatory responses to hypoxaemia during sleep in the newborn. J Dev Physiol. 1979;1:195–208. [PubMed] [Google Scholar]
- 38.Henderson-Smart DJ, Read DJ. Reduced lung volume during behavioral active sleep in the newborn. J Appl Physiol Respir Environ Exerc Physiol. 1979;46:1081–1085. doi: 10.1152/jappl.1979.46.6.1081. [DOI] [PubMed] [Google Scholar]
- 39.Adzick NS, Harrison MR, Glick PL, Villa RL, Finkbeiner W. Experimental pulmonary hypoplasia and oligohydramnios: relative contributions of lung fluid and fetal breathing movements. J Pediatr Surg. 1984;19:658–665. doi: 10.1016/s0022-3468(84)80349-8. [DOI] [PubMed] [Google Scholar]
- 40.Liggins GC, Vilos GA, Campos GA, Kitterman JA, Lee CH. The effect of spinal cord transection on lung development in fetal sheep. J Dev Physiol. 1981;3:267–274. [PubMed] [Google Scholar]
- 41.Fewell JE, Lee CC, Kitterman JA. Effects of phrenic nerve section on the respiratory system of fetal lambs. J Appl Physiol Respir Environ Exerc Physiol. 1981;51:293–297. doi: 10.1152/jappl.1981.51.2.293. [DOI] [PubMed] [Google Scholar]
- 42.Mornet E. Hypophosphatasia. Orphanet J Rare Dis. 2007;2:40. doi: 10.1186/1750-1172-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377–1385. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 44.Pauli RM, Scott CI, Wassman ER, Jr, Gilbert EF, Leavitt LA, Ver Hoeve J, et al. Apnea and sudden unexpected death in infants with achondroplasia. J Pediatr. 1984;104:342–348. doi: 10.1016/s0022-3476(84)81092-6. [DOI] [PubMed] [Google Scholar]
- 45.Fremion AS, Garg BP, Kalsbeck J. Apnea as the sole manifestation of cord compression in achondroplasia. J Pediatr. 1984;104:398–401. doi: 10.1016/s0022-3476(84)81103-8. [DOI] [PubMed] [Google Scholar]
- 46.Abman SH. Monitoring cardiovascular function in infants with chronic lung disease of prematurity. Arch Dis Child Fetal Neonatal Ed. 2002;87:F15–F18. doi: 10.1136/fn.87.1.F15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fike CD, Aschner JL. Looking beyond PPHN: the unmet challenge of chronic progressive pulmonary hypertension in the newborn. Pulm Circ. 2013;3:454–466. doi: 10.1086/674438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.del Cerro MJ, Sabate Rotes A, Carton A, Deiros L, Bret M, Cordeiro M, et al. Pulmonary hypertension in bronchopulmonary dysplasia: clinical findings, cardiovascular anomalies and outcomes. Pediatr Pulmonol. 2014;49:49–59. doi: 10.1002/ppul.22797. [DOI] [PubMed] [Google Scholar]
- 49.Plotkin H, Rauch F, Bishop NJ, Montpetit K, Ruck-Gibis J, Travers R, et al. Pamidronate treatment of severe osteogenesis imperfecta in children under 3 years of age. J Clin Endocrinol Metab. 2000;85:1846–1850. doi: 10.1210/jcem.85.5.6584. [DOI] [PubMed] [Google Scholar]
- 50.Alcausin MB, Briody J, Pacey V, Ault J, McQuade M, Bridge C, et al. Intravenous pamidronate treatment in children with moderate-to-severe osteogenesis imperfecta started under three years of age. Horm Res Paediatr. 2013;79:333–340. doi: 10.1159/000351374. [DOI] [PubMed] [Google Scholar]
- 51.Munns CF, Rauch F, Mier RJ, Glorieux FH. Respiratory distress with pamidronate treatment in infants with severe osteogenesis imperfecta. Bone. 2004;35:231–234. doi: 10.1016/j.bone.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Miller TL, Cox T, Blackson T, Paul D, Weiss K, Shaffer TH. Pulmonary function assessment in an infant with Barnes syndrome: proactive evaluation for surgical intervention. Pediatrics. 2006;118:e1264–e1267. doi: 10.1542/peds.2006-0135. [DOI] [PubMed] [Google Scholar]
- 53.Marotta A, Klinnert MD, Price MR, Larsen GL, Liu AH. Impulse oscillometry provides an effective measure of lung dysfunction in 4-year-old children at risk for persistent asthma. J Allergy Clin Immunol. 2003;112:317–322. doi: 10.1067/mai.2003.1627. [DOI] [PubMed] [Google Scholar]
- 54.Duiverman EJ, Clement J, van de Woestijne KP, Neijens HJ, van den Bergh AC, Kerrebijn KF. Forced oscillation technique. Reference values for resistance and reactance over a frequency spectrum of 2–26 Hz in healthy children aged 2.3–12.5 years. Bull Eur Physiopathol Respir. 1985;21:171–178. [PubMed] [Google Scholar]
- 55.Ducharme FM, Davis GM, Ducharme GR. Pediatric reference values for respiratory resistance measured by forced oscillation. Chest. 1998;113:1322–1328. doi: 10.1378/chest.113.5.1322. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez ME, Mackenzie WG, Ditro C, Miller TL, Chidekel A, Shaffer TH. Skeletal dysplasias: evaluation with impulse oscillometry and thoracoabdominal motion analysis. Pediatr Pulmonol. 2010;45:679–686. doi: 10.1002/ppul.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Locke R, Greenspan JS, Shaffer TH, Rubenstein SD, Wolfson MR. Effect of nasal CPAP on thoracoabdominal motion in neonates with respiratory insufficiency. Pediatr Pulmonol. 1991;11:259–264. doi: 10.1002/ppul.1950110313. [DOI] [PubMed] [Google Scholar]
- 58.Whyte MP, Greenberg CR, Salman NJ, Bober MB, McAlister WH, Wenkert D, et al. Enzyme-replacement therapy in life-threatening hypophosphatasia. N Engl J Med. 2012;366:904–913. doi: 10.1056/NEJMoa1106173. [DOI] [PubMed] [Google Scholar]
- 59.Weaver KN, Johnson J, Kline-Fath B, Zhang X, Lim FY, Tinkle B, et al. Predictive value of fetal lung volume in prenatally diagnosed skeletal dysplasia. Prenat Diagn. 2014;34:1326–1331. doi: 10.1002/pd.4475. [DOI] [PubMed] [Google Scholar]
- 60.Shaffer TH, Alapati D, Greenspan JS, Wolfson MR. Neonatal non-invasive respiratory support: physiological implications. Pediatr Pulmonol. 2012;47:837–847. doi: 10.1002/ppul.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moraa I, Sturman N, McGuire T, van Driel ML. Heliox for croup in children. Cochrane Database Syst Rev. 2013;(12):CD006822. doi: 10.1002/14651858.CD006822.pub4. [DOI] [PubMed] [Google Scholar]
- 62.Martinon-Torres F. Noninvasive ventilation with helium-oxygen in children. J Crit Care. 2012;27:220.e1–e9. doi: 10.1016/j.jcrc.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 63.Jassar RK, Vellanki H, Zhu Y, Hesek A, Wang J, Rodriguez E, et al. High flow nasal cannula (HFNC) with Heliox decreases diaphragmatic injury in a newborn porcine lung injury model. Pediatr Pulmonol. 2014;49:1214–1222. doi: 10.1002/ppul.23000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Panitch HB, Keklikian EN, Motley RA, Wolfson MR, Schidlow DV. Effect of altering smooth muscle tone on maximal expiratory flows in patients with tracheomalacia. Pediatr Pulmonol. 1990;9:170–176. doi: 10.1002/ppul.1950090309. [DOI] [PubMed] [Google Scholar]
- 65.Nino G, McNally P, Miske LJ, Hickey E, Panitch HB. Use of intrapulmonary percussive ventilation (IPV) in the management of pulmonary complications of an infant with osteogenesis imperfecta. Pediatr Pulmonol. 2009;44:1151–1154. doi: 10.1002/ppul.21112. [DOI] [PubMed] [Google Scholar]
- 66.Lachman RS. The cervical spine in the skeletal dysplasias and associated disorders. Pediatr Radiol. 1997;27:402–408. doi: 10.1007/s002470050156. [DOI] [PubMed] [Google Scholar]
- 67.Instability of the upper cervical spine. Skeletal Dysplasia Group. Arch Dis Child. 1989;64:283–288. doi: 10.1136/adc.64.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]


